Background:
There are indications that early-life exposure to perfluoroalkyl substances (PFAS) can impact neurodevelopment, but results are inconclusive. The objective was to investigate if high early-life exposure to primarily perfluorohexanesulfonic acid (PFHxS) and perfluorooctanesulfonic acid (PFOS) increases the risk of developmental language disorder in children up to seven years of age.
Methods:
A register-based cohort of all children born 1998–2013 in Blekinge county, Sweden, was studied. Maternal residential history, that is, with or without highly PFAS-contaminated drinking water, during the 5-year period before childbirth was used as a proxy for early-life exposure. Exposure was categorized as high (n = 646), intermediate (n = 1,650), or background (n = 9,599). We used Cox proportional hazards regression to estimate hazard ratios (HR) for (1) referral to a speech- and language pathologist after routine screening at Child Health Services, and (2) subsequent language disorder diagnosis after clinical assessment. Models were adjusted for parity, maternal age, education level, and smoking, and explored effect modification by sex.
Results:
In children from the high-exposed area, the adjusted HR for referral was 1.23 (95% CI = 1.03, 1.47) and 1.13 (95% CI = 0.97, 1.56) for subsequent diagnosis. There was no increased risk in the intermediate exposure category.
Conclusion:
Children, particularly girls, with high exposure had an increased risk of both referral and confirmed developmental language disorder. Further research is needed on PFAS in the context of general neurodevelopment, for which language development is a proxy.
Keywords: Register-based cohort study, Developmental language disorder, Early-life exposure, Perfluorohexane sulfonic acid (PFHxS), Perfluorooctane sulfonic acid (PFOS)
What this study adds
In a register-based cohort of 11,895 children, we found increased risks of both suspected and confirmed developmental language disorder, particularly among girls, after early-life exposure to high levels of primarily PFHxS and PFOS. The results emphasize the role of PFAS as a neurotoxicant and highlights a need for further research in this area. The results are also of clinical importance as they signal a potential need to increase the awareness of the association in Child Health Care services in highly exposed communities so that interventions, such as intensified screening, can be considered.
Introduction
Language development is relatively uniform across nationalities and cultures. It is also easily observed by following robust milestones in language development, that typical developing children reach within a certain age.1 However, children with developmental language disorder (DLD) fail to achieve these milestones and their language development is slow and protracted. Children with DLD have significant difficulties in one or more areas that affect spoken or written language, comprehension, and communication (i.e., social interaction). Early signs of suspected or confirmed DLD can be lack of babbling and late onset of the first spoken words.2,3 As the child grows older, DLD can manifest as the use of short and grammatically incorrect sentences, limited vocabulary, deviant speech sound production and problems understanding spoken language.4 DLD is one of the most common developmental disorders among children and affects 7%–8% of the population.5,6 If a child still meets criteria for DLD after 5 years of age, there is a risk that the condition becomes persistent with long-term effects on language and literacy development.7,8 Language development is also a well-known proxy for other neurodevelopmental domains and can predict, for example, hyperactivity, inattention, and conduct problems,5,9,10 and social and educational underachievement.5,8,11 This also includes an increased risk of psychiatric and social adaption problems through adolescence and into adult life.11
The etiology of DLD is multifactorial, including genetic, social, and environmental risk factors.4,6,12,13 Family history of language and learning difficulties, parental education, smoking, and breastfeeding are important determinants.14 Boys are more often referred to speech and language pathologists (SLP) for clinical assessment and therefore more likely to be diagnosed with DLD.5 However, sex-ratios tend to be more equal in population-based investigations.5,6,13
Sex hormones affect the developing brain, and the hormonal environment is therefore different in boys and girls.15,16 This has been associated with differences with respect to language development.17–19 Perfluoroalkyl substances (PFAS) may disrupt hormone systems that are essential for brain development.20,21 PFAS are synthetically produced chemicals that have been in use since the 1950s and are now ubiquitous in the environment. They are highly persistent, not metabolized and accumulate in humans.22–24 Transfer from mother to child during pregnancy and breastfeeding implies exposure of the fetus and the child during developmentally sensitive time windows.21,24
Background PFAS exposure generally derives from food and indoor environment. Additionally, high exposure may occur after contamination from fire-fighting foam or PFAS-manufacturing sites resulting in contaminated drinking water.25 One such “hotspot” is Ronneby in southern Sweden, where one-third of the population for decades received heavily contaminated municipal drinking water with an exposure profile dominated by perfluorohexanesulfonic acid (PFHxS) and perfluorooctanesulfonic acid (PFOS).26,27
A few studies in populations with background levels of PFAS exposure have investigated effects on children’s language development with inconsistent results, overall as well as with respect to sex differences.28–32 These studies have large variation with respect to age at assessment of both exposure and at outcome. Further, language outcomes have generally been assessed using psychometric instruments or questionnaires intended for assessment of general cognitive development, where language constitutes only a part, for example, receptive language (comprehension) or expressive language (vocabulary, sentences). At high exposures, only one study from the Mid-Ohio Valley area with industrial PFOA contamination has been published.33 Hitherto, no study has addressed clinically relevant outcomes of language and communication problems according to clinical diagnoses.
The aim of this study was to assess associations between high early-life PFAS exposure and DLD in boys and girls up to 7 years of age. We used administrative data from the regional healthcare register, which cover all health care provided in the county of Blekinge, of assessments and clinical diagnoses made by SLPs after referral from Child Health Service (CHS), where standardized language screening reaching virtually all children is routinely performed.
Methods
Setting
In December 2013, it was discovered that the drinking water from one of two municipal waterworks in Ronneby, situated in the county of Blekinge, Sweden, was heavily contaminated by PFAS. The municipality had at that time 28,000 inhabitants. The outgoing drinking water from the contaminated waterworks had supplied one-third of the households and had a sum12 PFAS concentration above 10,000 ng/L.34 This can be compared with the Swedish action limit at the time of 90 ng/L sum7 PFAS.35,36 The source of the contamination was fire-fighting foam used at a military airport since the mid-1980s. PFOS and PFHxS dominated the exposure profile, but the level of PFOA was also elevated. No analysis of PFAS in the drinking water had been performed before 2013. Notably, the sum7 PFAS concentration in water from the uncontaminated waterworks was <5 ng/L.
Study design
We used a register-based cohort to follow all children born between 1998 and 2013 in Blekinge county with respect to DLD, from birth up to 7 years of age. The mothers’ residential address history was used as a proxy for early-life exposure.
Study population
We included all children with a residential address in Blekinge for at least 1 year between birth and age 7. We excluded multiple births, children who had a reused personal identification number, and children born prematurely (<37 weeks of gestation).
Variables
Exposure assessment
Yearly information on the water supply source to each address in Ronneby between 1980 and 2013 was retrieved from the municipality and linked by Statistics Sweden to the Total Population Register,36 to retrieve information on maternal residential exposure. We limited the exposure window to the five years before childbirth because it covers 1–1.5 half-lives of PFOS, PFOA, and PFHxS, as estimated by Li et al.26,37
We categorized exposure into:
High: The mother had a residential address in Ronneby with highly contaminated water for at least one year during the 5-year period before childbirth,
Intermediate: The mother had a residential address in Ronneby with uncontaminated water for at least 1 year during the 5-year period before childbirth, and
Background: The mother had a residential address in Blekinge county at the year of birth and did not have a residential address in Ronneby municipality during the 5-year period before childbirth.
A child qualifying for more than one of the exposure categories was assigned to the category with the highest exposure.
Validation of exposure assessment
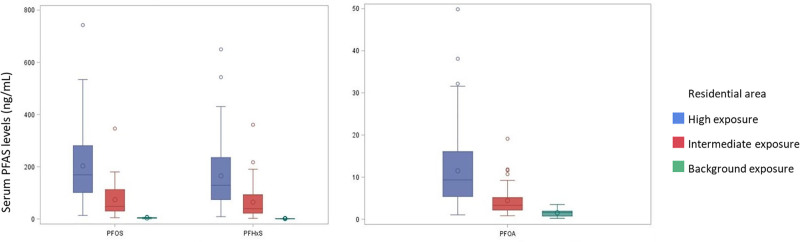
We used a cohort with measured PFAS serum concentrations from residents in Ronneby and Karlshamn, a nearby municipality with background levels of exposure, described in detail by Xu et al34 to validate our proxy measure of exposure. For women with 21–38 years of age, who were sampled in 2014 and 2016, we categorized exposure in the 5-year period before sampling according to the same exposure assessment as in the present study and compared the results with their measured serum concentrations (Figure 1).
Figure 1.
Serum concentrations (ng/mL) of PFOS, PFHxS, and PFOA in 228 women aged 21–38, participating in open serum samplings 2014–2016.34 Exposure categories were defined as high (n = 147), intermediate (n = 45), or background exposure (n = 36) according to maternal residential address during the 5-year period before sampling.
For all three PFAS, the serum concentrations were considerably higher among women in the high exposure category (median PFHxS 129 ng/mL, PFOS 169 ng/mL, and PFOA 9 ng/mL) compared with women in the intermediate (median PFHxS 40 ng/mL, PFOS 48 ng/mL, and PFOA 3 ng/mL), and the background exposure category (median PFHxS 0.8 ng/mL, PFOS 4 ng/mL, and PFOA 2 ng/mL.
Outcomes
Children with suspected DLD are primarily identified through the language screening programs performed by pediatric nurses within the CHS screening programs in Sweden since the early 1970s. Participation in screening at CHS is not selective, it is offered to all children in Sweden, free of charge and the compliance rate is almost 98%.38 The remaining 2%–2.5% have parents who decline screening because the child is already enrolled in services for children with other major neurological disorders, for example, cerebral palsy, or learning disorders.39 Thus, they were not at risk of having a referral/diagnosis registered in the register we used for outcome assessment.
The screening program includes two standardized instruments at 2.5 and 3 years of age40,41 and standardized national guidelines for language examinations at 4 years.42
Children who screen positive in the language screening programs within CHS are referred to a speech and language pathologist (SLP) for further assessments, also free of charge. SLPs diagnose DLD according to three main categories from the Swedish version of ICD-10 that is used in Europe for classification when assessing language disorders (Table S1, http://links.lww.com/EE/A207).43 Miniscalco et al.40 found that the 2.5 years screening instrument had a sensitivity of 0.69 and specificity of 0.93. Later, Schachinger-Lorentzon et al.44 found that after screening at age 2.5, out 87 of 100 referred children received a language disorder diagnosis and were followed at an SLP clinic. Thus, the language screening programme in use seems to reliably identify children with DLD.
All referred children were assessed at the SLP clinic at Blekinge hospital, which was the only caregiver for 0- to 7-year-old children with DLD during the study period. Information on all referrals from CHS to the SLP clinic with assessment by an SLP between 1998 and 2019 were retrieved from the regional health care register, together with ICD-10 language disorder diagnoses.43 At the time of data extraction, there were 18 CHS with 52 nurses employed in Blekinge county, and all followed national guidelines and used standardized screening instruments.45 It should be noted, that although the CHS is the primary source of referral to SLPs, concerned parents can contact the clinic directly.
We used two separate outcome definitions:
(1) Referral for assessment by an SLP after CHS screening: Children with ≥1 referral followed by assessment by an SLP (n = 2,173). This outcome is hereafter denoted referral.
(2) Clinical diagnosis of DLD: Children who had a clinical ICD-10 speech, language, or communication diagnosis set by an SLP at ≥2 occasions (n = 1,181). We chose the latter requirement to increase validity of the outcome assessment and reduce the risk of misclassification. This outcome is hereafter referred to as DLD.
Subgroup analysis
We explored if three specific subtypes of DLD might be differently affected by early-life PFAS exposure. Children were required to have been diagnosed with an ICD-10 diagnosis within one of the specific categories (Table S2, http://links.lww.com/EE/A207) on ≥2 occasions.
(1) Expressive language disorder, including impaired expressive grammar, semantics, phonology, articulation, and dyspraxia of speech,
(2) Mixed receptive-expressive language disorder, including impaired receptive and expressive grammar, semantics, phonology, pragmatics, and other specified and unspecified communicative abilities, and
(3) Other developmental disorders of speech and language fluency disorders such as stuttering, lisping, other specified, and unspecified abilities related to fluency.
Validation of outcomes
Two pilot studies were performed in 2019 to assess the consistency of assessments and use of diagnostic codes over time. An experienced SLP (CS) interviewed two SLPs and the head of the SLP clinic, who had been working there since 1998. The aim was to clarify the routines for assessments of patients, registration in medical charts, and reporting to regional health care registers. All aspects were found to be consistent over the study period. One or two randomly chosen medical charts per calendar year for children who had visited the SLP clinic 1998–2013 (n = 37) were scrutinized with focus on referral (i.e., age and cause) and diagnostics (i.e., use of validated test and ICD-10 codes). In addition, the charts for all children (n = 127) born between January and June 2008 that had visited the clinic were scrutinized. Most children, 81%, were referred from CHS, while the caregivers of 13% of the children had initiated contact with the SLP clinic. The reason for referral was missing for 7 children (5%). Overall, we found that the SLPs work had followed common clinical practice and that the healthcare register obtained diagnostic codes automatically from digitalized medical charts.
Confounders
We used a directed acyclic graph (DAG; Figure S1, http://links.lww.com/EE/A207) to identify potential confounders a priori through the software program DAGitty.46 The final set of confounders consisted of parity, maternal age at delivery, maternal educational attainment in the year of childbirth, and maternal smoking in early pregnancy.
The Medical Birth Register, with full coverage of all Swedish pregnancies,47 provided information on maternal age, smoking, and parity. Information on maternal education was obtained from the Longitudinal integrated database for health insurance and labor market studies.48
Statistical methods
We used Cox proportional hazards regression to estimate hazard ratios (HR) of referral and DLD for children with high and intermediate exposure relative to the reference category with background exposure. Cox regression with calendar year as the underlying time variable was used because we had different follow-up times for the children. The children were censored at the outcome, at age 7, or at the end of the study period. We ran the analyses on a complete case dataset because only 6% (n = 693) of the participants had missing data for any of the confounders. Women with more than one child in the dataset (n = 2,481) introduced correlated observations, which we accounted for by computing the robust sandwich covariance estimate.49
We investigated effect modification by sex by introducing an interaction term in the models. The confounders were treated as categorical variables: parity (1, 2, or 3+), maternal age (<25, 25–29, 30–34, or ≥35 years), maternal educational attainment (compulsory school, upper secondary school, or university), and maternal smoking in early pregnancy (yes or no).
The proportionality assumption was tested by including time dependent covariates to assess whether they were statistically significant (p < 0.05). Parity did not meet the assumption and was therefore stratified and allowed to have separate baseline hazard functions.
We explored effect modification by time period with an ad hoc cutoff in 2005,34,50,51 as we assumed exposure levels to have been highest toward the end of the study period.
In the subgroup analysis, the proportional hazards assumption was not fulfilled in the adjusted models. Thus, parity and maternal age were stratified in the model for expressive language disorder, whereas maternal education was stratified in the model for mixed receptive-expressive language disorder. Only 56 children were diagnosed with other developmental disorders of speech and language fluency disorders, and we did not consider them for further analyses.
We used SAS 9.4 for analyses (version 9.4 for Microsoft Windows; SAS Institute Inc., Cary, NC).52
Ethics
The study was approved by the Swedish Ethical Review Authority (2019-04551), and the study protocol followed the requirements of the Declaration of Helsinki. The analysis plan was preregistered at Open Science Framework, https://doi.org/10.17605/OSF.IO/3HD5N.
Results
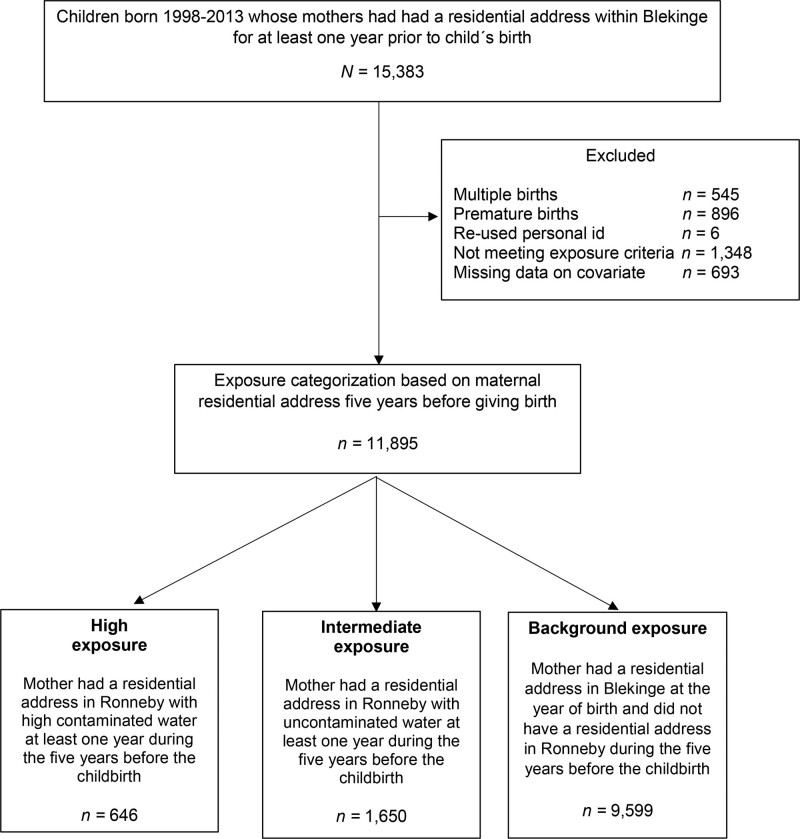
The final study population consisted of 11,895 children (Figure 2).
Figure 2.
Flowchart over the study population.
Children in the high exposure category had mothers that were younger, had lower educational attainment, and more frequently smoked in early pregnancy (Table 1). Mothers to children in the intermediate and the background exposure categories were similar with respect to these characteristics. Irrespective of exposure category, the median age for the first visit to an SLP was 4 years. Overall, more boys than girls were referred to an SLP and received a DLD diagnosis. However, the age at first visit did not differ considerably between girls and boys.
Table 1.
Description of maternal and child characteristics for the 11,895 study participants, stratified by PFAS exposure category according to maternal residential address during the 5-year period before childbirth.
| Variable | High exposure n = 646 | Intermediate exposure n = 1,650 | Background exposure n = 9,599 | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Parity | ||||||
| 1 | 316 | 48 | 856 | 50 | 4,983 | 49 |
| 2 | 225 | 34 | 626 | 37 | 3,650 | 36 |
| 3+ | 124 | 19 | 225 | 13 | 1,583 | 16 |
| Maternal age (years) | ||||||
| <25 | 181 | 27 | 310 | 18 | 1,823 | 18 |
| 25–29 | 220 | 33 | 603 | 35 | 3,470 | 34 |
| 30–34 | 172 | 26 | 552 | 32 | 3,322 | 33 |
| ≥35 | 92 | 14 | 242 | 14 | 1,601 | 16 |
| Maternal education | ||||||
| Compulsory school | 109 | 16 | 136 | 8 | 1,018 | 10 |
| Upper secondary school | 388 | 58 | 789 | 46 | 4,534 | 44 |
| University | 160 | 24 | 759 | 45 | 4,481 | 44 |
| Maternal smoking in early pregnancy (yes) | 121 | 18 | 168 | 10 | 898 | 9 |
| Sex | ||||||
| Girls | 293 | 45 | 826 | 48 | 4,455 | 46 |
| Boys | 353 | 55 | 851 | 52 | 5,141 | 54 |
| Referral to SLP | 140 | 22 | 305 | 19 | 1,728 | 18 |
| Age at first SLP visita | ||||||
| 140 | 4.1 (3.3–4.4) | 305 | 4.1 (3.2–4.4) | 1,728 | 4.1 (3.2–4.6) | |
| Girls | 52 | 4.1 (3.3–4.4) | 118 | 4.2 (3.3–4.5) | 589 | 4.2 (3.3–4.6) |
| Boys | 88 | 4.1 (3.3–4.4) | 187 | 4.1 (3.2–4.3) | 1,139 | 4.1 (3.2–4.5) |
| Diagnosis set by SLPb | 76 | 12 | 165 | 10 | 940 | 10 |
aMedian; IQR.
bAt ≥2 occasions.
Main results
There was an increased HR for referral in the high exposure category compared with the background exposure category in the crude analysis (Table 2). The strength of the association did not change after adjusting for potential confounders and the adjusted HR was 1.23 (95% CI = 1.03, 1.47). The unadjusted HR for DLD diagnosis in the high exposure category was similar to that of referral but attenuated after confounder adjustment (HR 1.13, 95% CI = 0.97, 1.56). We found no risk increase in the intermediate exposure category.
Table 2.
Crude and adjusted HR with 95% CI for referral to and DLD diagnosis from a SLP after early-life exposure to PFAS.
| Exposure classification | Events | HR Crude (95% CI) | HR Adjusteda (95% CI) |
|---|---|---|---|
| Referral to an SLP | |||
| High exposure | 140 | 1.23 (1.03, 1.47) | 1.23 (1.03, 1.47)b |
| Intermediate exposure | 305 | 1.03 (0.91, 1.17) | 1.03 (0.91, 1.17)b |
| Background exposure | 1,728 | 1.00 | 1.00 |
| DLD diagnosis | |||
| High exposure | 76 | 1.22 (0.96, 1.54) | 1.13 (0.97, 1.56) |
| Intermediate exposure | 165 | 1.02 (0.87, 1.21) | 1.04 (0.88, 1.23) |
| Background exposure | 940 | 1.00 | 1.00 |
All estimates were obtained from Cox regression. PFAS exposure was categorized according to maternal residential address during the 5-year period before childbirth.
aThe models were adjusted for parity, maternal age, maternal education, and smoking in early pregnancy.
bEstimates changed, but only in the third digit.
The adjusted associations were stronger among girls (Table 3), and, in the high exposure category, girls had an HR for referral of 1.36 (95% CI = 1.02, 1.80) and an HR of DLD diagnosis of 1.62 (95% CI = 1.12, 2.35). Although the confidence intervals spanned over 1, the point estimates for the intermediate group was suggestive of an exposure-response relationship. Among boys in the high exposure category, there was a nominally increased HR for referral but not for DLD. There was no risk increase for boys with intermediate exposure levels. We found no evidence of effect modification by time period (p = 0.55 for referral and p = 0.32 for DLD).
Table 3.
Adjusted HR with 95% CI for referral to and DLD diagnosis from a SLP in girls and boys after early-life exposure to PFAS.
| Girls | Boys | |||
|---|---|---|---|---|
| Exposure classification | Events | HRa (95% CI) | Events | HRa (95% CI) |
| Referral to an SLP | ||||
| High exposure | 52 | 1.36 (1.02, 1.80) | 88 | 1.16 (0.93, 1.44) |
| Intermediate exposure | 118 | 1.13 (0.93, 1.38) | 187 | 0.99 (0.85, 1.17) |
| Background exposure | 589 | 1.00 | 1,139 | 1.00 |
| Confirmed DLD diagnosis | ||||
| High exposure | 31 | 1.62 (1.12, 2.35) | 45 | 1.04 (0.77, 1.42) |
| Intermediate exposure | 62 | 1.18 (0.90, 1.55) | 103 | 0.99 (0.80, 1.22) |
| Background exposure | 296 | 1.00 | 644 | 1.00 |
PFAS exposure was categorized according to maternal residential address during the 5-year period before childbirth. All estimates are obtained from Cox regression.
aThe models were adjusted for parity, maternal age, maternal education, and smoking in early pregnancy, and effect modification by sex assessed through an interaction term.
Subgroup analyses
The incidence of expressive language disorder was higher than the incidence of mixed receptive-expressive language disorder (Table 4). Although not statistically significant, there was an increased HR for expressive language disorder in the high exposure category. We found no association neither between high exposure and mixed language disorder nor between an intermediate exposure level and any of the subtypes.
Table 4.
Adjusted HRs with 95% CI for expressive language disorders and mixed receptive-expressive language disorders after early-life exposure to PFAS.
| Exposure classification | Events | HRa (95% CI) |
|---|---|---|
| Expressive language disorder | ||
| High exposure | 59 | 1.25 (0.96, 1.64) |
| Intermediate exposure | 127 | 1.03 (0.85, 1.25) |
| Background exposure | 728 | 1.00 |
| Mixed receptive-expressive language disorder | ||
| High exposure | 21 | 0.98 (0.63, 1.53) |
| Intermediate exposure | 40 | 0.82 (0.59, 1.14) |
| Background exposure | 291 | 1.00 |
PFAS exposure was categorized according to maternal residential address during the 5-year period before childbirth.
aThe models were adjusted for parity, maternal age, maternal education, and smoking
in early pregnancy.
Discussion
We studied clinically relevant language-development outcomes in a large and unselected population of children with PFAS exposure levels ranging from background to very high. In the high exposure category, there were increased HRs for both referral and confirmed ICD-10 DLD diagnosis compared with children with background exposure. The association was strongest among girls. In girls, there was also a nominally increased risk in the intermediate exposure category, which can be suggestive of a sex-specific dose-response relationship.
This study is distinct from other studies that have investigated the association between PFAS and child language development. Most previous research has been conducted in birth cohorts, thus with limited sampling size and a risk of selective participation. No other study has been performed in a general population with such a large exposure contrast as in Ronneby, or with an exposure profile dominated by PFHxS and PFOS. With one exception, previous studies only considered populations with background exposure. Some of these studies suggest that PFAS might have an adverse impact on children’s language development but, overall, their findings are inconclusive.28,30–32 The only existing study at higher exposure levels found no association between PFOA exposure and the verbal scale results from the Wechsler Abbreviated Scale of Intelligence, Vocabulary and Similarities.33,53 Furthermore, previous studies usually collected data with the purpose of examine children’s overall cognitive development, and report results for language, verbal, and communication domains in standardized test batteries. In contrast, CHS uses standardized screening instruments targeting almost 98% of all eligible children directly focusing on language ability in combination with clinical SLP assessments for language disorders, resulting in ICD-10 diagnoses—that is, outcomes with highly relevant clinical outcomes.
In all exposure category, more boys than girls were referred and clinically assessed, which corresponds well with the clinical picture; although population-based studies indicate a more even distribution, ratio 1.2:1.5 We found a higher risk for DLD among high-exposed girls but not among high-exposed boys. This indicates that the effects of early-life PFAS exposure affects girls’ and boys’ developing brain differently, possibly through disruption of sex-specific hormonal pathways. Sex-dimorphic effects have been observed in studies of other neurodevelopmental outcomes (e.g., behavioral traits) after exposure to endocrine disruptors.15,16 Additionally, early language development is affected by the sex-specific hormonal environment.17–19
The association between PFAS exposure and DLD was driven by expressive language disorders. The study design likely resulted in lower detection rate of mixed receptive-expressive disorders because young children may not be able to comply with the assessment. These conditions are more severe and associated with other neurodevelopmental disorders such as autism, learning, and intellectual disorders,5,8–11 and more research on the role of high PFAS exposure in their etiology is clearly warranted. Such studies in the Ronneby population are underway.
Strengths and limitations
We avoided the risk of selection bias that potentially could have been caused by awareness of the exposure among health care personnel and caregivers by restricting our study population to children born before 2013 when the contamination was discovered. Our strict criteria for DLD of an ICD-10 diagnosis at ≥2 occasions likely improved the validity of our outcome assessment, although it obviously caused a reduction of the number of cases and hence less statistical power. The proportion of children with only one DLD diagnosis was similar across the exposure categories. We adjusted for relevant socioeconomic variables in the analyses but, as always in observational studies, residual confounding cannot be ruled out.
In Sweden, there is a strong tradition to drink tap water,54 and we used a proxy measure of exposure based on maternal residential address history. However, proxy measures can be sensitive to misclassification unless they are of decent quality. We assessed the validity of our proxy against measured serum concentrations, which confirmed clear contrasts in exposure between the exposure categories. Thus, we consider the risk of exposure misclassification to be of lesser concern.
Conclusions
Our study showed an increased risk of DLD after high exposure to primarily PFOS and PFHxS. In absolute figures, the estimated HR for girls translates into a yearly excess of 13 referrals per 1,000 individuals and an excess of 12 DLD diagnoses per 1,000 children in the high exposure category. Notably, we did not observe an increased risk among children with an intermediate level of exposure, whose mothers may have serum concentration that are more comparable with those in other international hotspot populations. The results suggest a need for increased awareness in CHS of the potential link between high PFAS exposure and DLD in highly exposed areas.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Acknowledgments
The authors are grateful to SLPs at Blekinge hospital for sharing information about their work. We are also grateful to Ying Li at University of Gothenburg and Elisabeth Bondesson at Lund University for valuable statistical consultation.
Supplementary Material
Footnotes
Published online 14 December 2022
The study has ethical approval from the Swedish Ethical Review Authority (2019-04551).
No informed consent was required because the study was based solely on registry data.
C.S. and M.E. share first authorship to this article. Also, C.N. and C.M. share the last authorship of this article.
The results herein correspond to specific aims of grant 2018-00289 to investigator CM from The Swedish Research Council for Health, Working Life and Welfare (FORTE). This work was supported by grants 2017-00896 from Formas (CN), 2017-01359 from the Swedish Research Council for Medicine (CG), and in-kind contributions from University of Gothenburg (KJ), and Silverskiöld foundation (CS).
Data contains sensitive personal information and cannot be made publicly available for legal reasons (GDPR). However, it can be accessed by other researchers after renewed ethical vetting. Any data inquiries are referred to the corresponding author.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Rescorla L. The language development survey: a screening tool for delayed language in toddlers. J Speech Hear Disord. 1989;54:587–599. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander A, Holm K, Eriksson S, Lieberman M. Observation method identifies that a lack of canonical babbling can indicate future speech and language problems. Acta Paediatr. 2017;106:935–943. [DOI] [PubMed] [Google Scholar]
- 3.Oller DK, Eilers RE, Neal AR, Cobo-Lewis AB. Late onset canonical babbling: a possible early marker of abnormal development. Am J Ment Retard. 1998;103:249–263. [DOI] [PubMed] [Google Scholar]
- 4.Bishop DVM. What causes specific language impairment in children? Curr Dir Psychol Sci. 2006;15:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norbury CF, Gooch D, Wray C, et al. The impact of nonverbal ability on prevalence and clinical presentation of language disorder: evidence from a population study. J Child Psychol Psychiatry. 2016;57:1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. J Speech Lang Hear Res. 1997;40:1245–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norbury CF, Vamvakas G, Gooch D, et al. Language growth in children with heterogeneous language disorders: a population study. J Child Psychol Psychiatry. 2017;58:1092–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stothard SE, Snowling MJ, Bishop DVM, Chipchase BB, Kaplan CA. Language-impaired preschoolers: a follow-up into adolescence. J Speech Lang Hear Res. 1998;41:407–418. [DOI] [PubMed] [Google Scholar]
- 9.Petersen IT, Bates JE, D’Onofrio BM, et al. Language ability predicts the development of behavior problems in children. J Abnorm Psychol. 2013;122:542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim F, O’Dowd J, Thompson L, et al. Language and social/emotional problems identified at a universal developmental assessment at 30 months. BMC Pediatr. 2013;13:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clegg J, Hollis C, Mawhood L, Rutter M. Developmental language disorders--a follow-up in later adult life. Cognitive, language and psychosocial outcomes. J Child Psychol Psychiatry. 2005;46:128–149. [DOI] [PubMed] [Google Scholar]
- 12.Bishop DVM, Snowling MJ, Thompson PA, Greenhalgh T. Phase 2 of CATALISE: a multinational and multidisciplinary Delphi consensus study of problems with language development: terminology. J Child Psychol Psychiatry. 2017;58:1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti-Ramsden G, Durkin K. Language development and assessment in the preschool period. Neuropsychol Rev. 2012;22:384–401. [DOI] [PubMed] [Google Scholar]
- 14.Tomblin JB, Smith E, Zhang X. Epidemiology of specific language impairment: prenatal and perinatal risk factors. J Commun Disord. 1997;30:325–43; quiz 343. [DOI] [PubMed] [Google Scholar]
- 15.Nesan D, Sewell LC, Kurrasch DM. Opening the black box of endocrine disruption of brain development: lessons from the characterization of Bisphenol A. Horm Behav. 2018;101:50–58. [DOI] [PubMed] [Google Scholar]
- 16.Palanza P, Paterlini S, Brambilla MM, et al. Sex-biased impact of endocrine disrupting chemicals on behavioral development and vulnerability to disease: of mice and children. Neurosci Biobehav Rev. 2021;121:29–46. [DOI] [PubMed] [Google Scholar]
- 17.Wermke K, Quast A, Hesse V. From melody to words: the role of sex hormones in early language development. Horm Behav. 2018;104:206–215. [DOI] [PubMed] [Google Scholar]
- 18.Quast A, Hesse V, Hain J, Wermke P, Wermke K. Baby babbling at five months linked to sex hormone levels in early infancy. Infant Behav Dev. 2016;44:1–10. [DOI] [PubMed] [Google Scholar]
- 19.Schaadt G, Hesse V, Friederici AD. Sex hormones in early infancy seem to predict aspects of later language development. Brain Lang. 2015;141:70–76. [DOI] [PubMed] [Google Scholar]
- 20.Morreale de EG, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151:U25–U37. [DOI] [PubMed] [Google Scholar]
- 21.Rappazzo KM, Coffman E, Hines EP. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health. 2017;14:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borg D, Lund BO, Lindquist NG, Håkansson H. Cumulative health risk assessment of 17 perfluoroalkylated and polyfluoroalkylated substances (PFASs) in the Swedish population. Environ Int. 2013;59:112–123. [DOI] [PubMed] [Google Scholar]
- 23.Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL. Perfluorochemicals in Pooled Serum Samples from United States Residents in 2001 and 2002. Environ Sci Technol. 2006;40:2128–2134. [DOI] [PubMed] [Google Scholar]
- 24.Piekarski D, Diaz K, McNerney M. Perfluoroalkyl chemicals in neurological health and disease: human concerns and animal models. Neurotoxicology (Park Forest South). 2020;77:155–168. [DOI] [PubMed] [Google Scholar]
- 25.Domingo JL, Nadal M. Human exposure to per-and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ Res. 2019;177:108648. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Fletcher T, Mucs D, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Li Y, Scott K, et al. Inflammatory bowel disease and biomarkers of gut inflammation and permeability in a community with high exposure to perfluoroalkyl substances through drinking water. Environ Res. 2020;181:108923. [DOI] [PubMed] [Google Scholar]
- 28.Jeddy Z, Hartman TJ, Taylor EV, Poteete C, Kordas K. Prenatal concentrations of Perfluoroalkyl substances and early communication development in British girls. Early Hum Dev. 2017;109:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liew Z, Ritz B, Bach CC, et al. Prenatal exposure to perfluoroalkyl substances and IQ scores at age 5 a study in the Danish National Birth Cohort.(Research)(Medical condition overview). Environ Health Perspect. 2018;126:067004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spratlen MJ, Perera FP, Lederman SA, et al. The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environ Pollut. 2020;263:114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Rogan WJ, Chen HY, et al. Prenatal exposure to perfluroalkyl substances and children’s IQ: the Taiwan maternal and infant cohort study. Int J Hyg Environ Health. 2015;218:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goudarzi H, Nakajima S, Ikeno T, et al. Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: the Hokkaido Study. Sci Total Environ. 2016;541:1002–1010. [DOI] [PubMed] [Google Scholar]
- 33.Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Nielsen C, Li Y, et al. Serum perfluoroalkyl substances in residents following long-term drinking water contamination from firefighting foam in Ronneby, Sweden. Environ Int. 2021;147:106333. [DOI] [PubMed] [Google Scholar]
- 35.Livsmedelsverket. Intagsberäkningar som underlag för framtagande av hälsobaserad åtgärdsgräns för perfluorerade alkylsyror (PFAA) i dricksvatten. In: Glynn A, Sand S, eds. Risk. Vol. 2014. Availalbe at: https://www.livsmedelsverket.se/globalassets/livsmedel-innehall/oönskade-amnen/pfaa/intagsberakningar-for-atgardsgrans-for-pfaa-i-dricksvatten. Accessed 13 February 2022. [Google Scholar]
- 36.Ludvigsson JF, Almqvist C, Bonamy A-KE, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31:125–136. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Andersson A, Xu Y, et al. Determinants of serum half-lives for linear and branched perfluoroalkyl substances after long-term high exposure—A study in Ronneby, Sweden. Environ Int. 2022;163:107198. [DOI] [PubMed] [Google Scholar]
- 38.Magnusson M. Rationality of routine health examinations by physicians of 18‐month‐old children: experiences based on data from a Swedish county. Acta Paediatr. 1997;86:881–887. [DOI] [PubMed] [Google Scholar]
- 39.Miniscalco C, Nygren G, Hagberg B, Kadesjö B, Gillberg C. Neuropsychiatric and neurodevelopmental outcome of children at age 6 and 7 years who screened positive for language problems at 30 months. Dev Med Child Neurol. 2006;48:361–366. [DOI] [PubMed] [Google Scholar]
- 40.Miniscalco C, Mårild S, Pehrsson N. Evaluation of a language‐screening programme for 2.5‐year‐olds at Child Health Centres in Sweden. Acta Paediatr. 2001;90:339–344. [PubMed] [Google Scholar]
- 41.Westerlund M, Sundelin C. Can severe language disability be identified in three‐year‐olds? Evaluation of a routine screening procedure. Acta Paediatr. 2000;89:94–100. [DOI] [PubMed] [Google Scholar]
- 42.National Guidelines Rikshandboken. Vol. 2021. Available at: https://www.rikshandboken-bhv.se/halsobesok/4-ar, 2021. Accessed 02 May 2022. [Google Scholar]
- 43.WHO and Socialstyrelsen W. Klassifikation av sjukdomar och hälsoproblem 1997 (International Classification of Disorders, tenth revision). Uppsala, Almquist & Wiksell, 1996. [Google Scholar]
- 44.Schachinger-Lorentzon U, Kadesjö B, Gillberg C, Miniscalco C. Children screening positive for language delay at 2.5 years: language disorder and developmental profiles. Neuropsychiatr Dis Treat. 2018;14:3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hellerfelt S, Håkansson L, Kruber K, Tell J. Barnhälsovården i Blekinge- Årsrapport. 2020. Available at: https://regionblekinge.se/halsa-och-vard/folkhalsa-i-blekinge/barnhalsovard/arsrapporter-fran-barnhalsovarden.html nhalsovard/arsrapporter-fran-barnhalsovarden.html. Accessed July 12, 2020.
- 46.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int J Epidemiol. 2016;45:1887–1894. [DOI] [PubMed] [Google Scholar]
- 47.Rydberg H, Månsson H; Socialstyrelsen. Det statistiska registrets framställning och kvalitet Medicinska födelseregistret. 1 ed. 2021:11. Available at https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2021-9-7547.pdf. Accessed 18 February 2022. [Google Scholar]
- 48.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Springer Netherlands; 1992;237-247. [Google Scholar]
- 50.Hammarstrand S, Jakobsson K, Andersson E, et al. Perfluoroalkyl substances (PFAS) in drinking water and risk for polycystic ovarian syndrome, uterine leiomyoma, and endometriosis: a Swedish cohort study. Environ Int. 2021;157:106819. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen C, Li Y, Lewandowski M, Fletcher T, Jakobsson K. Breastfeeding initiation and duration after high exposure to perfluoroalkyl substances through contaminated drinking water: a cohort study from Ronneby, Sweden. Environ Res. 2021;207:112206. [DOI] [PubMed] [Google Scholar]
- 52.SAS 9.4 for Microsoft Windows. SAS Institute Inc. C, NC. SAS/ACCESS® 9.4. Vol. 9.4. 2013. [Google Scholar]
- 53.Wechsler D. Wechsler abbreviated scale of intelligence. 1999.
- 54.Säve-Söderbergh M, Toljander J, Mattisson I, Åkesson A, Simonsson M. Drinking water consumption patterns among adults—SMS as a novel tool for collection of repeated self-reported water consumption. J Expo Sci Environ Epidemiol. 2018;28:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.