PURPOSE
Combination treatment with BRAF and MEK inhibitors has demonstrated benefits on progression-free survival (PFS) and overall survival (OS) and is a standard of care for the treatment of advanced BRAF V600–mutant melanoma. Here, we report the 5-year update from the COLUMBUS trial (ClinicalTrials.gov identifier: NCT01909453).
METHODS
Patients with locally advanced unresectable or metastatic BRAF V600–mutant melanoma, untreated or progressed after first-line immunotherapy, were randomly assigned 1:1:1 to encorafenib 450 mg once daily plus binimetinib 45 mg twice daily, vemurafenib 960 mg twice daily, or encorafenib 300 mg once daily. An updated analysis was conducted 65 months after the last patient was randomly assigned.
RESULTS
Five hundred seventy-seven patients were randomly assigned: 192 to encorafenib plus binimetinib, 191 to vemurafenib, and 194 to encorafenib. The 5-year PFS and OS rates with encorafenib plus binimetinib were 23% and 35% overall and 31% and 45% in those with normal lactate dehydrogenase levels, respectively. In comparison, the 5-year PFS and OS rates with vemurafenib were 10% and 21% overall and 12% and 28% in those with normal lactate dehydrogenase levels, respectively. The median duration of response with encorafenib plus binimetinib was 18.6 months, with disease control achieved in 92.2% of patients. In comparison, the median duration of response with vemurafenib was 12.3 months, with disease control achieved in 81.2% of patients. Long-term follow-up showed no new safety concerns, and results were consistent with the known tolerability profile of encorafenib plus binimetinib. Interactive visualization of the data presented in this article is available at COLUMBUS dashboard.
CONCLUSION
In this 5-year update of part 1 of the COLUMBUS trial, encorafenib plus binimetinib treatment demonstrated continued long-term benefits and a consistent safety profile in patients with BRAF V600–mutant melanoma.
INTRODUCTION
Approximately 50% of melanomas contain BRAF V600 mutations, which constitutively activate the mitogen-activated protein kinase pathway, driving cellular proliferation and disease progression.1,2 Combination treatment with BRAF and MEK inhibitors (BRAFi and MEKi) is now the standard of care for treating BRAF V600–mutant locally advanced or metastatic melanoma. Currently, guidelines include three combinations of BRAFi + MEKi: encorafenib plus binimetinib, vemurafenib plus cobimetinib, and dabrafenib plus trametinib.3-7
CONTEXT
Key Objective
Combination treatment of BRAF plus MEK inhibitors is now the standard of care for treating BRAF V600–mutant locally advanced or metastatic melanoma. In previously reported results of the COLUMBUS trial, encorafenib plus binimetinib extended progression-free survival and median overall survival, improved quality of life, and was well tolerated with a low discontinuation rate. This 5-year updated analysis assessed long-term efficacy and safety outcomes with encorafenib plus binimetinib in patients with unresectable or metastatic BRAF V600–mutant melanoma.
Knowledge Generated
Long-term results from the COLUMBUS trial indicate continuous benefits of encorafenib plus binimetinib and confirmed previous reports of prolonged progression-free survival and overall survival compared with vemurafenib monotherapy, consistent with those reported from other BRAF plus MEK inhibitor combinations. The burden of toxicity decreasing over time with long-term safety is consistent with previous observations.
Relevance
This 5-year update of COLUMBUS demonstrates the long-term benefits of encorafenib plus binimetinib in patients with unresectable or metastatic BRAF V600–mutant melanoma.
In phase III trials, vemurafenib plus cobimetinib and dabrafenib plus trametinib have demonstrated 5-year progression-free survival (PFS) rates of 14%-19% and overall survival (OS) rates of 31%-34%.8,9 Encorafenib plus binimetinib was evaluated in a phase Ib/II trial (ClinicalTrials.gov identifier: NCT01543698) and in the phase III COLUMBUS trial (ClinicalTrials.gov identifier: NCT01909453).10-12 Encorafenib is a highly selective ATP-competitive BRAFi.13 Its long dissociation half-life may allow for sustained target inhibition, enhanced antitumor activity, and reduced paradoxical activation of mitogen-activated protein kinase pathways in normal tissues.13-16 Binimetinib is a potent, allosteric, ATP-uncompetitive, selective MEKi with a short half-life, which may help to rapidly resolve toxicity after dose interruption.10,17
Previously, we reported results from part 1 of COLUMBUS, which compared encorafenib plus binimetinib with monotherapy with vemurafenib or encorafenib.11,18-21 Compared with vemurafenib, encorafenib plus binimetinib extended PFS (14.9 v 7.3 months; hazard ratio [HR], 0.51; 95% CI, 0.39 to 0.67) and median OS (33.6 v 16.9 months; HR, 0.61; 95% CI, 0.48 to 0.79; median follow-up for OS, 48.8 months).20 The combination was generally well tolerated; the rate of discontinuation was low (10% v 14% with vemurafenib); the burden of toxicity decreased with a longer treatment duration.20 Encorafenib plus binimetinib treatment also improved quality of life; compared with vemurafenib, postbaseline scores were 3.03 points higher (P < .0001) for FACT-M and 5.28 points higher (P = .0042) for EORTC QLQ-C30.21 In this 5-year updated analysis of COLUMBUS part 1, we assessed long-term efficacy and safety outcomes with encorafenib plus binimetinib in patients with unresectable or metastatic BRAF V600–mutant melanoma.
METHODS
Study Design and Participants
The study design and patient eligibility criteria have been published.11,18,19 Briefly, COLUMBUS (ClinicalTrials.gov identifier: NCT01909453) was a two-part, multicenter, randomized, open-label, phase III trial. Patients with locally advanced unresectable or metastatic BRAF V600–mutant melanoma who were untreated or whose cancer had progressed after first-line immunotherapy were enrolled between December 30, 2013, and April 10, 2015. In part 1 of COLUMBUS, patients were randomly assigned 1:1:1 to encorafenib 450 mg once daily plus binimetinib 45 mg twice daily, vemurafenib 960 mg twice daily, or encorafenib 300 mg once daily. Random assignment was stratified by American Joint Committee on Cancer stage (IIIB, IIIC, IVM1a, IVM1b, or IVM1c), Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1), and BRAF mutation (V600E or V600K; before Protocol amendment 2 [December 20, 2013]) or use of previous first-line immunotherapy (yes or no; after Protocol amendment 2). Patients received study treatment until disease progression (assessed by central review), death, unacceptable toxic effects, or withdrawal of consent. Dose adjustments were based on tolerability and adverse events (AEs).11 Independent ethics committees or review boards at each study site approved the study Protocol (online only) and amendments. Conduct of the study conformed with Good Clinical Practice guidelines and the ethical requirements outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients before screening procedures were initiated.
Study End Points
Updated analyses were conducted 65 months after the last patient was randomly assigned (data cutoff: September 15, 2020) for outcomes of PFS, OS, objective response rate (ORR), poststudy anticancer therapy, safety, and tolerability. In addition, PFS and OS were analyzed in subgroups, including prognostic subgroups related to lactate dehydrogenase (LDH) levels and number of organs involved as identified previously by Long et al22 and Hauschild et al.23 Outcome definitions and details of efficacy and safety assessments have been published.11,18,19
Statistical Analysis
Efficacy end points were assessed in the intent-to-treat population (defined as randomly assigned patients). Median durations of follow-up for OS and PFS were estimated by reverse Kaplan-Meier analysis. The Kaplan-Meier method was used to estimate rates of OS and PFS. Subgroup analyses of baseline variables and potential prognostic factors, including previous immunotherapy, were also specified. Because of the hierarchical testing procedure used during this study, the analyses presented here are descriptive. HRs were estimated using Cox proportional hazard regression models and presented along with 95% CIs. Safety assessments and poststudy anticancer therapy data were summarized descriptively. The safety analysis set included all patients who received at least one dose of study treatment and had one postbaseline safety assessment.20 Exposure-adjusted incidence rate (EAIR; per 100 patient-months of exposure to study treatment) was calculated for each AE as the number of patients experiencing the AE divided by the total exposure time at risk for the AE. Exposure time was the treatment duration for patients not experiencing the event and treatment duration up to the time of first onset of the AE for those experiencing the event.19 AEs of interest for encorafenib plus binimetinib were summarized by the time of onset (median and 95% CI). Detailed information on statistical analyses has been reported.11,18
RESULTS
Patients
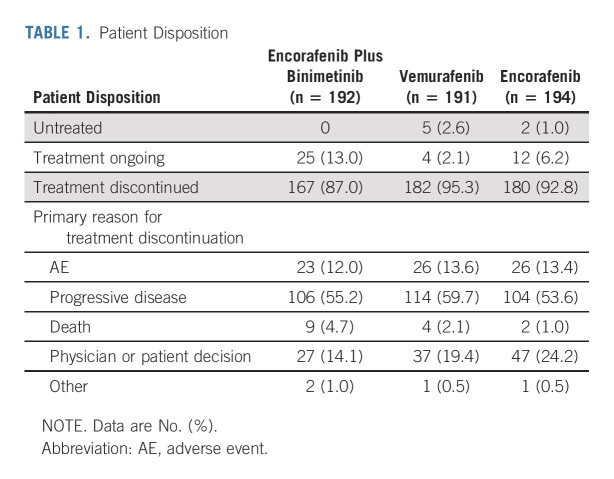
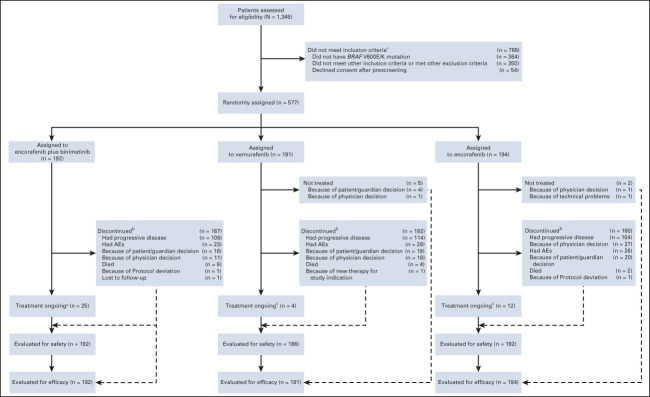
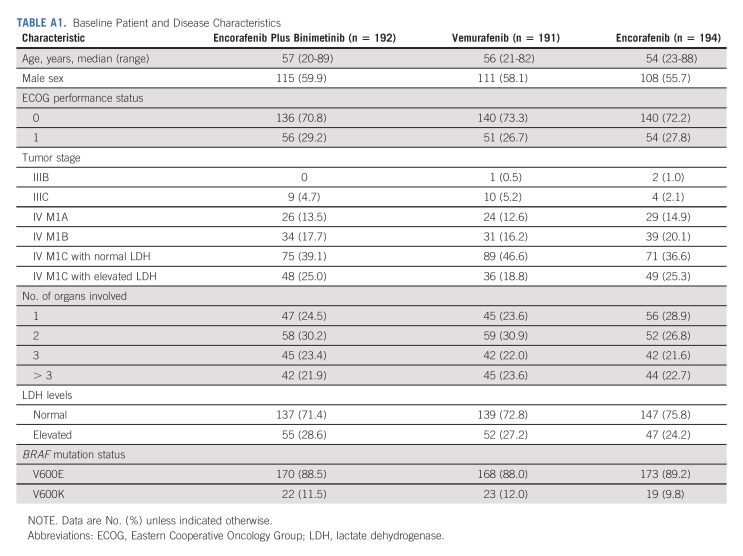
COLUMBUS part 1 randomly assigned 577 patients: 192 to encorafenib plus binimetinib, 191 to vemurafenib, and 194 to encorafenib (Appendix Fig A1, online only). Baseline characteristics were similar among treatment arms (Appendix Table A1, online only). Overall, 27% of patients had elevated LDH levels; 45% had ≥ 3 organs involved. At the time of data cutoff, treatment was ongoing in 41 patients (Table 1). Among patients treated with encorafenib plus binimetinib, 55% discontinued treatment primarily because of progressive disease; 12% discontinued primarily because of AEs.
TABLE 1.
Patient Disposition
Efficacy
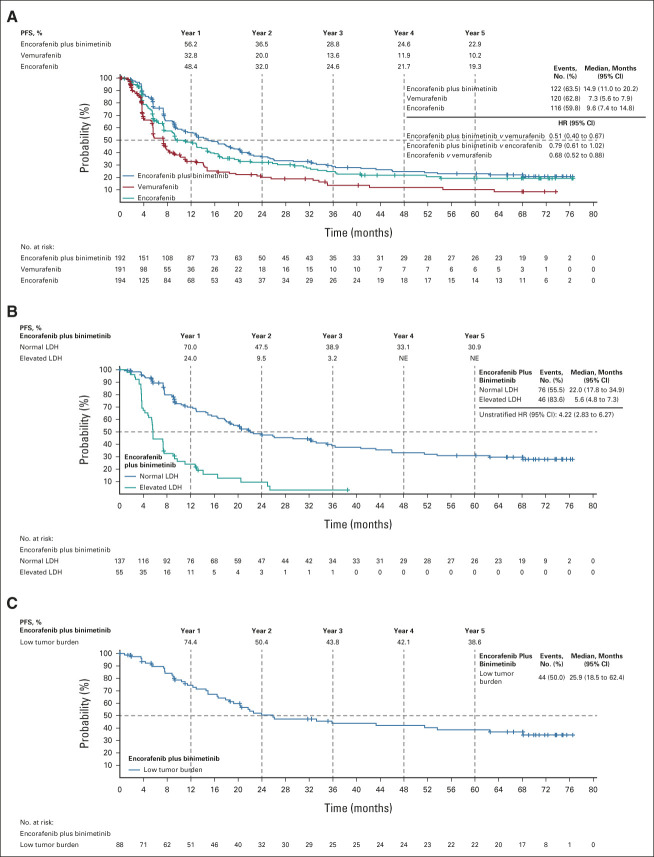
The median follow-up for PFS was 40.8 months. Median PFS in all arms was consistent with previously reported values (Fig 1). The median PFS was 14.9 months (95% CI, 11.0 to 20.2) with encorafenib plus binimetinib and 7.3 months (95% CI, 5.6 to 7.9) with vemurafenib (HR, 0.51; 95% CI, 0.40 to 0.67). The median PFS with encorafenib was 9.6 months (95% CI, 7.4 to 14.8; encorafenib plus binimetinib v encorafenib: HR, 0.79; 95% CI, 0.61 to 1.02; encorafenib v vemurafenib: HR, 0.68; 95% CI, 0.52 to 0.88). PFS rates were highest with encorafenib plus binimetinib, followed by encorafenib, at each yearly landmark. At 5 years, the PFS rates were 23% with encorafenib plus binimetinib, 10% with vemurafenib, and 19% with encorafenib. The 5-year PFS rates were 31% for patients treated with encorafenib plus binimetinib with normal LDH and 39% for those with low tumor burden (ie, normal LDH levels and < 3 organs involved) at baseline.
FIG 1.
PFS in (A) all patients, (B) patients in the encorafenib plus binimetinib arm according to baseline LDH levels, and (C) patients in the encorafenib plus binimetinib arm who had a low tumor burden (ie, normal LDH levels and < 3 organs involved) at baseline. For (A), HR was analyzed using stratified Cox regression model. HR, hazard ratio; LDH, lactate dehydrogenase; NE, not evaluable; PFS, progression-free survival.
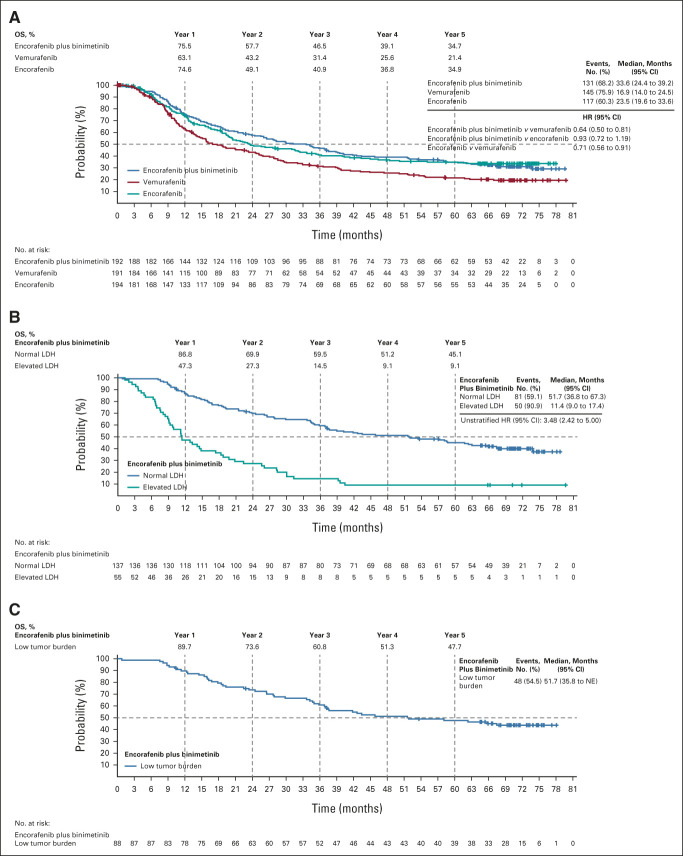
The median follow-up for OS was 70.4 months. Median OS for all arms was consistent with previously reported values (Fig 2). The median OS was 33.6 months (95% CI, 24.4 to 39.2) with encorafenib plus binimetinib and 16.9 months (95% CI, 14.0 to 24.5) with vemurafenib (HR, 0.64; 95% CI, 0.50 to 0.81). The median OS with encorafenib was 23.5 months (95% CI, 19.6 to 33.6; encorafenib plus binimetinib v encorafenib: HR, 0.93; 95% CI, 0.72 to 1.19; encorafenib v vemurafenib: HR, 0.71; 95% CI, 0.56 to 0.91). OS rates were highest in the encorafenib plus binimetinib arm, followed by the encorafenib arm, and were higher than the vemurafenib arm at each yearly landmark. Interestingly, at 1 and 5 years, the OS rates were nearly identical between the encorafenib plus binimetinib and encorafenib monotherapy arms. At 5 years, the OS rates were 35% with encorafenib plus binimetinib and encorafenib monotherapy and 21% with vemurafenib. The 5-year OS rates were 45% for patients treated with encorafenib plus binimetinib with normal LDH and 48% for those with low tumor burden at baseline.
FIG 2.
OS in (A) all patients, (B) patients in the encorafenib plus binimetinib arm according to baseline LDH levels, and (C) patients in the encorafenib plus binimetinib arm who had a low tumor burden (ie, normal LDH levels and < 3 organs involved) at baseline. For (A), HR was analyzed using a stratified Cox regression model. HR, hazard ratio; LDH, lactate dehydrogenase; NE, not evaluable; OS, overall survival.
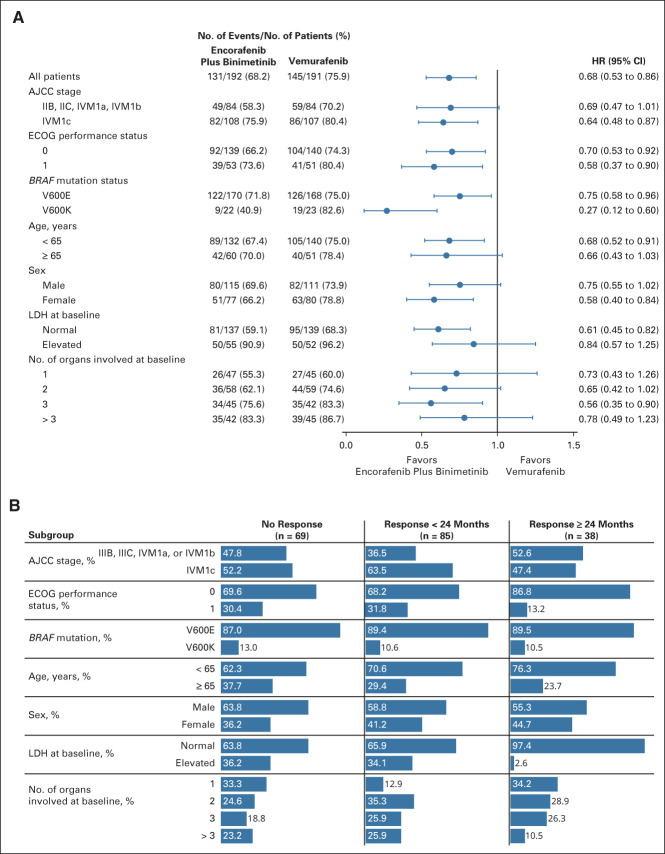
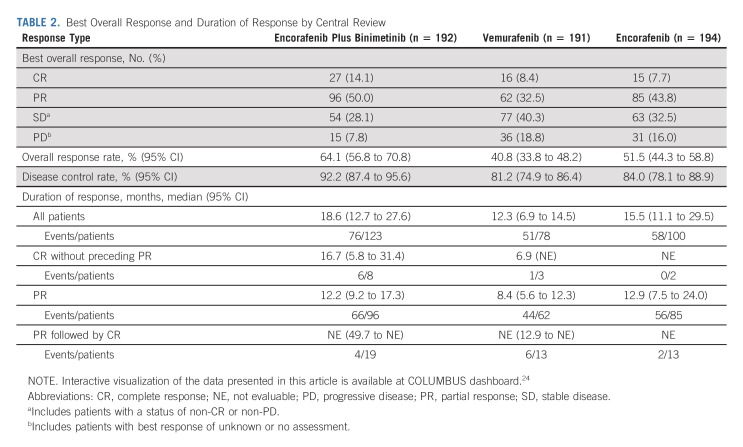
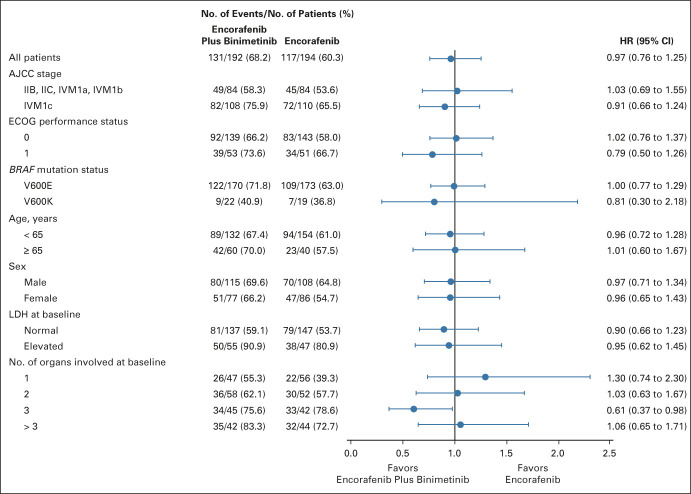
OS subgroup analyses comparing encorafenib plus binimetinib and vemurafenib showed point estimates in favor of encorafenib plus binimetinib (Fig 3A). OS subgroup analyses comparing encorafenib plus binimetinib and encorafenib did not show clear trends toward either arm, except in patients with three organs involved at baseline who had a greater OS benefit with encorafenib plus binimetinib (Appendix Fig A2, online only). Patients who had long-term response (ie, ≥ 24 months) tended to have less advanced stage of cancer, better ECOG performance status, normal LDH, and fewer organs involved at baseline (Fig 3B). By central review, 92% of patients receiving combination treatment achieved disease control (Table 2); the median duration of response among responders was 18.6 months (95% CI, 12.7 to 27.6). Complete responses (CRs) were achieved in 14% of patients in the encorafenib plus binimetinib arm and 8% each in the vemurafenib arm and encorafenib arm. Most CRs or partial responses were achieved within 6 months of encorafenib plus binimetinib treatment; median PFS and OS were similar between those with and without a response at 6 months (Appendix Fig A3, online only).
FIG 3.
(A) OS in subgroups for encorafenib plus binimetinib versus vemurafenib. The Cox proportional hazards model is unstratified. (B) Proportion of patients treated with encorafenib plus binimetinib by duration of response. AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival.
TABLE 2.
Best Overall Response and Duration of Response by Central Review
Subsequent Therapy
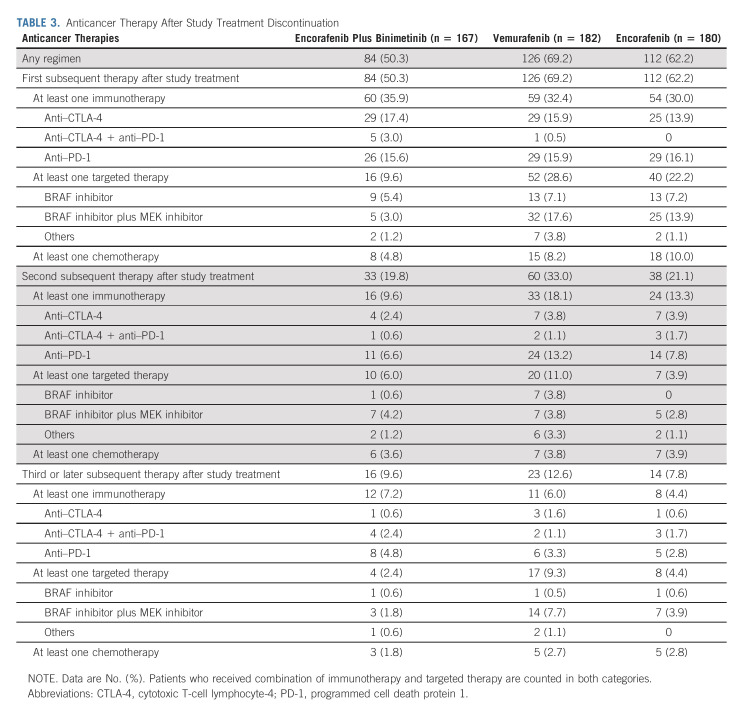
After COLUMBUS study treatment, 50% of the encorafenib plus binimetinib arm, 69% of the vemurafenib arm, and 62% of the encorafenib arm received systemic treatments. The most common subsequent treatments in all arms were anti–cytotoxic T-cell lymphocyte-4 or anti-programmed cell death protein 1 (PD-1) as monotherapy or in combination (Table 3). Immunotherapies were the most common first subsequent therapy in all arms; however, similar numbers of patients in the monotherapy arms received targeted therapies as their first treatment after COLUMBUS. Thereafter, immunotherapies were the most common second treatment in the monotherapy arms.
TABLE 3.
Anticancer Therapy After Study Treatment Discontinuation
Safety
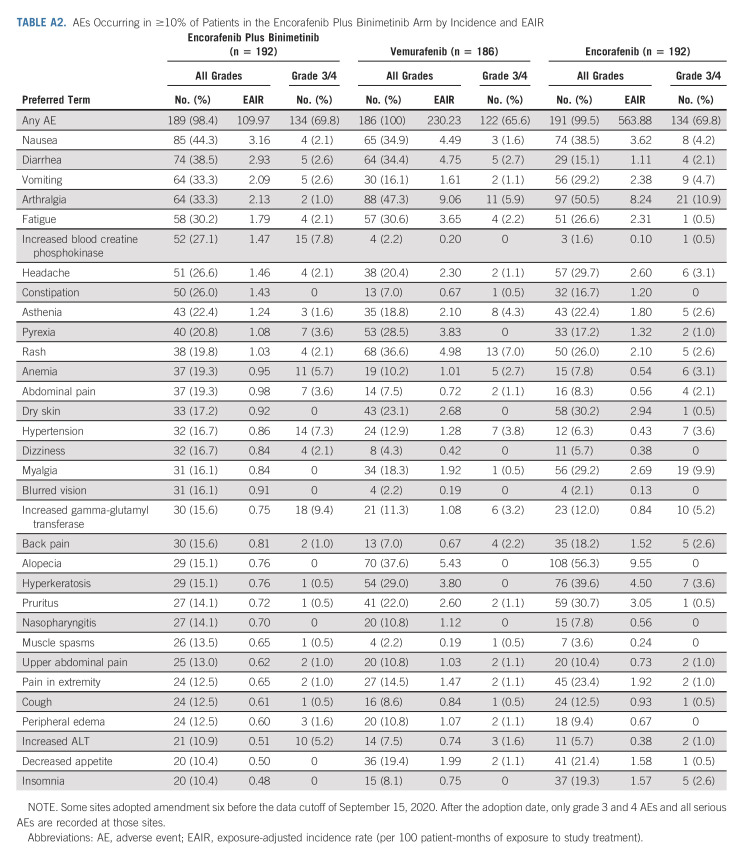
The safety profile observed with this 5-year follow-up was generally consistent with previous reports (Appendix Tables A2 and A3, online only). Grade 3/4 AEs occurred in 70%, 66%, and 70% of patients in the encorafenib plus binimetinib, vemurafenib, and encorafenib arms, respectively. For most AEs, EAIRs were lowest with encorafenib plus binimetinib. AEs led to dose adjustment or interruption in 56%, 62%, and 72% of patients in the encorafenib plus binimetinib, vemurafenib, and encorafenib arms, respectively; in the encorafenib plus binimetinib arm, these AEs were gastrointestinal disorders (17%), eye disorders (12%), pyrexia (6%), decreased ejection fraction (5%), and increased gamma-glutamyl transferase (5%). In each treatment arm, 16%-18% of patients experienced AEs, leading to study treatment discontinuation. AEs that led to discontinuation of encorafenib plus binimetinib treatment in more than one patient were increased alanine aminotransferase (n = 5; four were grade 3/4), aspartate aminotransferase (n = 4; two were grade 3/4), or blood creatinine (n = 2; one was grade 3/4); headache (n = 4; two were grade 3/4); or rash (n = 2; both were grade 3/4); three patients discontinued because of central nervous system metastases. There were 25 (13%), 20 (11%), and 16 (8%) on-treatment deaths in the encorafenib plus binimetinib, vemurafenib, and encorafenib arms, respectively; most were due to underlying disease.
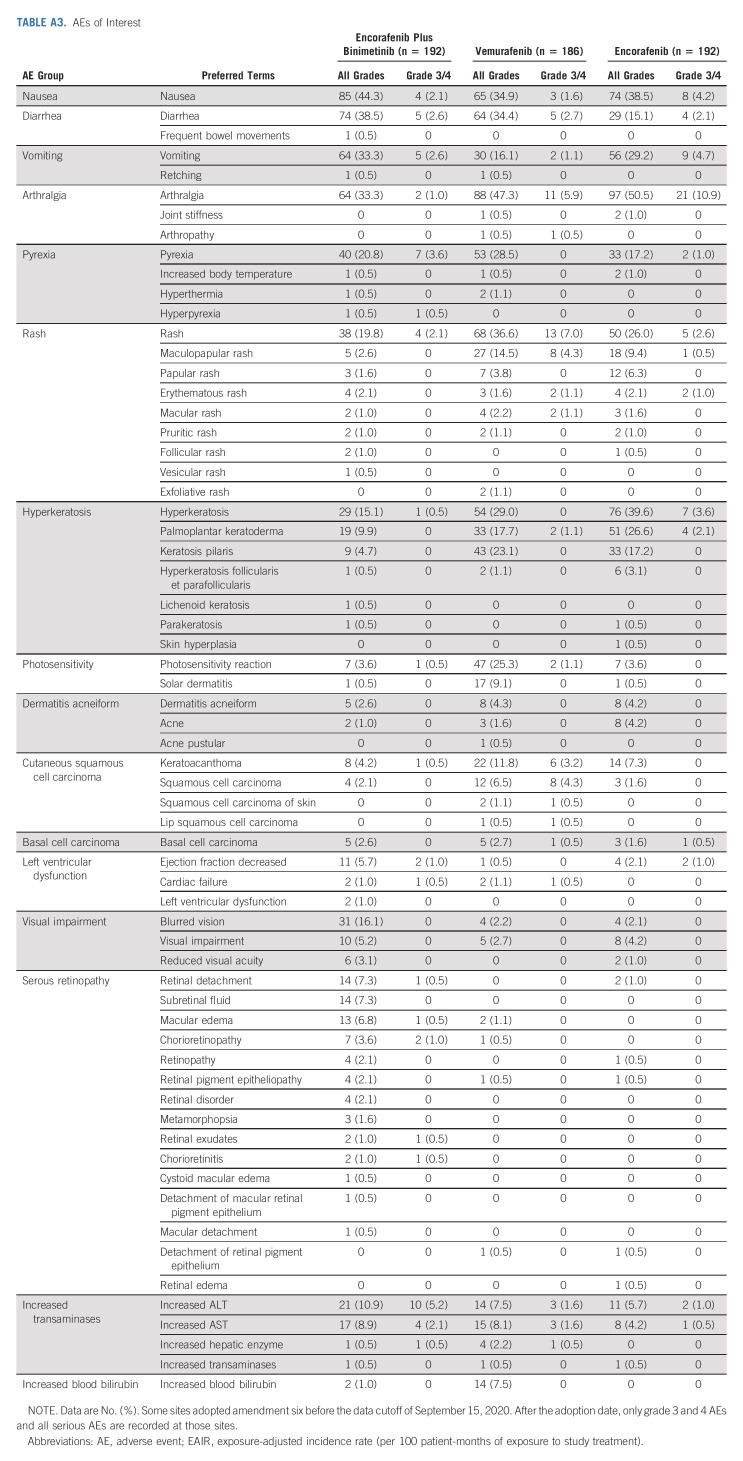
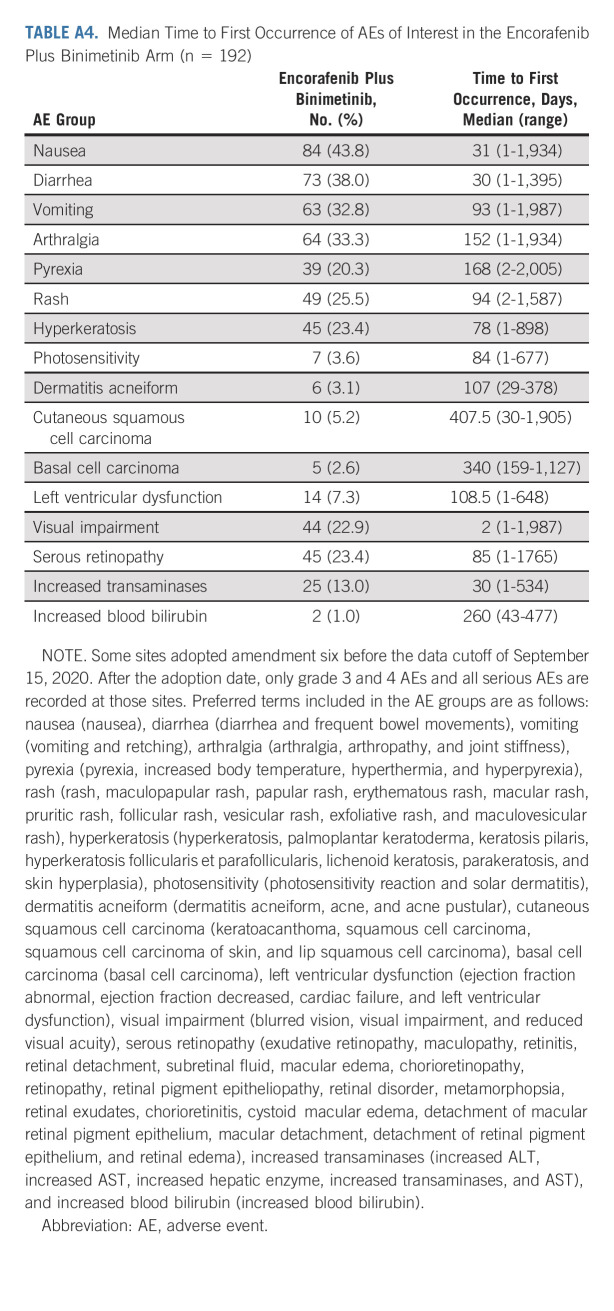
The median time to onset for most AEs of interest was within 6 months of starting treatment with encorafenib plus binimetinib (Appendix Table A4, online only). The median time to onset of nausea, diarrhea, visual impairment, and increased transaminases was within 1 month of starting treatment with encorafenib plus binimetinib. As expected, ocular AEs related to MEKi occurred in the encorafenib plus binimetinib arm (Appendix Table A3); most were mild or moderate. Blurred vision occurred in 16%; retinal detachment, subretinal fluid, and macular edema were each reported in 7%. One patient discontinued because of reduced visual acuity and retinal disorder. In the encorafenib plus binimetinib arm, left ventricular dysfunction, consisting of the AEs of abnormal or decreased ejection fraction, cardiac failure, and left ventricular dysfunction, occurred in 7% of patients (excluding censored observations); the median time to first occurrence was approximately 3.5 months (range, 0 to approximately 21 months).
Interactive visualization of the data presented in this article is available at COLUMBUS dashboard.24
DISCUSSION
Long-term results from the randomized, phase III COLUMBUS trial indicate continuous benefits of encorafenib plus binimetinib for patients with unresectable or metastatic BRAF V600–mutant melanoma. Overall, the results suggest that preclinical and pharmacologic differences between BRAFi are clinically meaningful. This 5-year update confirmed previous reports of prolonged PFS and OS with encorafenib plus binimetinib treatment compared with vemurafenib treatment.20 In patient subgroup analyses, the observed OS either favored or trended toward treatment with encorafenib plus binimetinib over vemurafenib. PFS and OS rates at each yearly landmark were higher in the encorafenib plus binimetinib arm than in the vemurafenib arm. Survival curves for encorafenib plus binimetinib began to plateau around 4 years. Similar plateaus have been observed with dabrafenib plus trametinib and vemurafenib plus cobimetinib and with immune checkpoint inhibitors in clinical trials of advanced melanoma.8,25-27 Although direct comparisons cannot be made, 5-year PFS and OS rates with encorafenib plus binimetinib treatment in COLUMBUS were consistent with those observed with other BRAFi + MEKi combinations.8,9
The 5-year OS rate was 35% for both the encorafenib plus binimetinib and encorafenib monotherapy arms; however, combination treatment demonstrated significantly longer PFS (median > 5 months) and numerically longer OS (median > 10 months). Compared with encorafenib monotherapy, the encorafenib plus binimetinib arm had numerically greater ORR, disease control rate, and duration of response. Furthermore, combination treatment improved tolerability; EAIRs for most AEs were lower with encorafenib plus binimetinib compared with encorafenib monotherapy. Patients in COLUMBUS part 1 treated with encorafenib plus binimetinib reported fewer (difference of 10% or more between arms) dermatologic AEs and arthralgia and myalgia events compared with those treated with encorafenib monotherapy. Although certain AEs occurred more commonly in the combination arm than in the encorafenib monotherapy arm (eg, diarrhea, increased blood creatine phosphokinase, and blurred vision), the rate of discontinuation because of AEs was similar between these arms. Previous studies have also shown that both efficacy and tolerability of BRAFi are improved with the addition of a MEKi.9,28-30 The contribution of binimetinib to the encorafenib plus binimetinib combination was further evaluated in part 2 of the COLUMBUS trial, which compared encorafenib 300 mg once daily plus binimetinib 45 mg twice daily with encorafenib 300 mg once daily monotherapy.12 Briefly, encorafenib plus binimetinib showed meaningful improvements in PFS by 5.5 months (HR, 0.57; 95% CI, 0.41 to 0.75) and ORR by 16%; furthermore, the combination was better tolerated than monotherapy, resulting in greater relative dose intensity, fewer grade 3/4 AEs, and fewer AEs leading to discontinuation.12
AEs with encorafenib plus binimetinib were generally manageable and consistent with previous reports; no new safety signals were observed after long-term follow-up. As reported previously in COLUMBUS and coBRIM, the overall burden of toxicity of combination treatment tends to decrease with time on treatment.20,29,31 The most common AEs observed were class effects such as gastrointestinal AEs and arthralgia; first onset of these AEs occurred within 2 months of starting treatment. Since the 3-year analysis of COLUMBUS, the proportion of patients with a rash increased by 3.7%, 6.5%, and 5.2% in the encorafenib plus binimetinib, vemurafenib, and encorafenib arms, respectively. Of note, approximately half of the newer reports of rash with vemurafenib treatment were grade 3/4 events. There was also a notable increase in pruritus of 11.2% and 8.8% in the vemurafenib and encorafenib arms, respectively, but not in the encorafenib plus binimetinib arm. Ocular toxicities, a known AE of MEKi, were routinely evaluated in COLUMBUS. Most ocular disorders were asymptomatic and managed by adjustment or interruption of the encorafenib plus binimetinib dose; discontinuation because of ocular toxicity occurred in only one patient. MEK-associated retinopathy was reported in 29% of patients treated with vemurafenib plus cobimetinib in coBRIM; approximately half of the events were symptomatic.32 Finally, left ventricular dysfunction, consisting of the AEs of abnormal or decreased ejection fraction, cardiac failure, and left ventricular dysfunction, occurred in 7.3% of patients (excluding censored observations) treated with encorafenib plus binimetinib, typically 3-4 months after treatment; no new onset was recorded after 2 years.
Within the limits of cross-trial comparisons, encorafenib plus binimetinib treatment resulted in fewer AEs of pyrexia than dabrafenib plus trametinib treatment (21% v 53%); dose adjustments or interruptions because of pyrexia in the encorafenib plus binimetinib arm were uncommon, occurring in 6% of patients.8 Furthermore, treatment with encorafenib plus binimetinib resulted in fewer AEs of rash (20% v 42% with vemurafenib plus cobimetinib) and photosensitivity (4% v 35% with vemurafenib plus cobimetinib).9 These observations are in agreement with the findings of a recent pharmacovigilance study using data from the US Food and Drug Administration Adverse Event Reporting System.33 Encorafenib plus binimetinib treatment was found to be associated with lower risks of dermatologic AEs and acute kidney injury compared with vemurafenib plus cobimetinib and lower risks of pyrexia and elevated C-reactive protein compared with dabrafenib plus trametinib.33 The same study also reported a greater likelihood of colitis, renal impairment, and seizures with encorafenib plus binimetinib than with other BRAFi + MEKi combinations; in COLUMBUS, < 10% of patients treated with encorafenib plus binimetinib experienced these AEs.
Patients treated with encorafenib plus binimetinib in COLUMBUS most commonly received immunotherapy with anti–PD-1 or anti–cytotoxic T-cell lymphocyte-4 or both after study treatment. Treatment sequence is an active area of research, as is combining targeted treatments and immunotherapy; the STARBOARD trial (ClinicalTrials.gov identifier: NCT04657991) is underway to investigate encorafenib plus binimetinib in combination with anti–PD-1 immunotherapy (pembrolizumab) for the treatment of BRAF V600–mutant melanoma.
This 5-year analysis had some limitations: it is post hoc and descriptive. OS was not a primary end point; however, OS was a key efficacy end point. The trial was not powered for OS comparisons between the encorafenib plus binimetinib arm and the encorafenib arm or vemurafenib arm. In addition, few patients with metastatic brain metastases were enrolled in COLUMBUS (n = 20). Case studies of encorafenib plus binimetinib treatment in patients with brain metastases reported promising outcomes.34-37 Patients with advanced BRAF V600–mutant melanoma and brain metastases have been enrolled in phase II trials evaluating encorafenib plus binimetinib (POLARIS [ClinicalTrials.gov identifier: NCT03911869], SWOG S2000 [ClinicalTrials.gov identifier: NCT04511013], and EBRAIN-MEL [ClinicalTrials.gov identifier: NCT03898908]).
In conclusion, 35% of patients with unresectable or metastatic BRAF V600–mutant melanoma treated with encorafenib plus binimetinib in COLUMBUS were alive after 5 years, with 23% remaining progression-free; 64% achieved CR/partial response. Among patients with normal LDH levels and < 3 organs involved at baseline, the 5-year PFS and OS rates were 39% and 48%, respectively. The safety profile observed with a longer follow-up was consistent with previous observations, and the burden of toxicity with encorafenib plus binimetinib treatment decreased over time. These data demonstrate the long-term benefits of encorafenib plus binimetinib in patients with unresectable or metastatic BRAF V600–mutant melanoma.
ACKNOWLEDGMENT
We thank the patients and their families for participating in this trial. We also thank the investigators and the clinical teams for making this trial possible. Medical writing support was provided by Raya Mahbuba at Caudex and was funded by Pfizer.
APPENDIX
FIG A1.
CONSORT diagram. aSome patients were ineligible for more than one reason. bPrimary reason. cOngoing at the time of data cutoff (September 15, 2020). AE, adverse event.
FIG A2.
OS in subgroups for encorafenib plus binimetinib versus encorafenib. The Cox proportional hazards model is unstratified. AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival.
FIG A3.
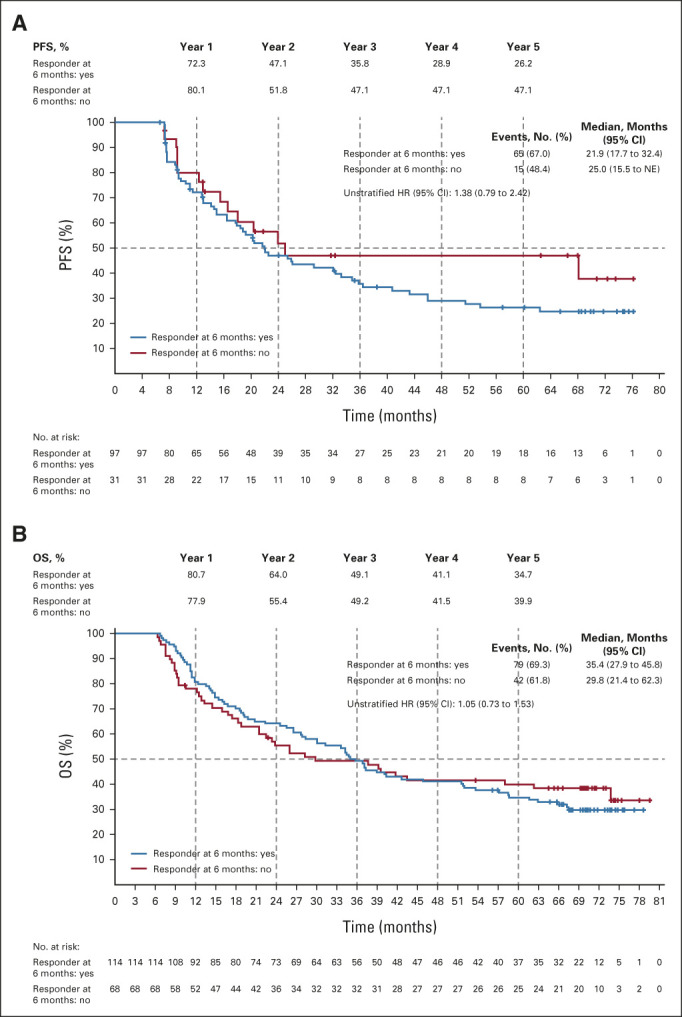
(A) PFS in the encorafenib plus binimetinib arm by response status at 6 months. (B) OS in the encorafenib plus binimetinib arm by response status at 6 months. Patients are classified on the basis of their response status at 6 months (the landmark time). The number of patients at risk at baseline excludes patients who had an event or were censored at 6 months. HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
TABLE A1.
Baseline Patient and Disease Characteristics
TABLE A2.
AEs Occurring in ≥10% of Patients in the Encorafenib Plus Binimetinib Arm by Incidence and EAIR
TABLE A3.
AEs of Interest
TABLE A4.
Median Time to First Occurrence of AEs of Interest in the Encorafenib Plus Binimetinib Arm (n = 192)
Reinhard Dummer
Honoraria: Roche, Novartis, Bristol Myers Squibb, MSD, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, CatalYm, Second Genome, Regeneron, Alligator Bioscience, MaxiVax, touchIME, T3 Pharmaceuticals, Pfizer
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, Novartis, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, CatalYm, Second Genome, Alligator Bioscience, touchIME, MaxiVax, Regeneron, Pfizer, T3 Pharmaceuticals
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), MSD (Inst), Amgen (Inst)
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FogPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Debiopharm Group
Caroline Robert
Stock and Other Ownership Interests: Ribonexus
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer
Research Funding: Novartis (Inst)
Ana Arance
Consulting or Advisory Role: BMS, Roche, Novartis, Pierre Fabre, MSD, Merck, Sanofi
Speakers' Bureau: Pierre Fabre, Novartis, MSD, BMS, Roche, Merck, Sanofi
Research Funding: Pierre Fabre (Inst), Novartis (Inst), Roche (Inst), BMS (Inst), MSD (Inst), Merck (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: BMS, MSD, Novartis, Pierre Fabre
Jan Willem B. de Groot
Consulting or Advisory Role: Bristol Myers Squibb, Pierre Fabre, Servier
Claus Garbe
Honoraria: BMS, MSD Oncology, NeraCare GmbH, Novartis, Philogen, Roche/Genentech, Sanofi, CeCaVa
Consulting or Advisory Role: BMS, MSD Oncology, NeraCare GmbH, Novartis, Philogen, Roche/Genentech, Sanofi, CeCaVa
Research Funding: BMS (Inst), Novartis (Inst), NeraCare GmbH (Inst), Roche/Genentech (Inst)
Helen J. Gogas
Honoraria: Bristol Myers Squibb, MSD Oncology, Pierre Fabre, Sanofi/Regeneron
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Amgen, Pierre Fabre, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb (Inst), Roche (Inst), MSD Oncology (Inst), Amgen (Inst), Novartis (Inst), Iovance Biotherapeutics (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Amgen, Pfizer
Ralf Gutzmer
Honoraria: Bristol Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, Novartis, Merck Serono, Almirall Hermal GmbH, Amgen, Sun Pharma, Pierre Fabre, Sanofi/Regeneron, Immunocore
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, Novartis, Almirall Hermal GmbH, 4SC, Amgen, Pierre Fabre, Merck Serono, Sun Pharma, Sanofi, Immunocore
Research Funding: Pfizer (Inst), Novartis (Inst), Johnson & Johnson (Inst), Amgen (Inst), Merck Serono (Inst), Sun Pharma (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche, Merck Serono, Pierre Fabre, Sun Pharma
Gabriella Liszkay
Consulting or Advisory Role: Roche, MSD, Novartis, Bristol Myers Squibb/Pfizer, Sanofi, Pfizer
Speakers' Bureau: Bristol Myers Squibb, MSD Oncology (Inst), Sanofi
Research Funding: Roche, Novartis (Inst), Inscite Corporation (Inst)
Carmen Loquai
Consulting or Advisory Role: Bristol Myers Squibb (Inst), MSD (Inst), BioNTech (Inst), Novartis (Inst), Sanofi (Inst), Pierre Fabre (Inst), Almirall Hermal GmbH (Inst), Sun Pharma (Inst), Merck (Inst), Roche (Inst), Kyowa Kirin International (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb (Inst), MSD (Inst), BioNTech (Inst), Novartis (Inst), Sanofi (Inst), Pierre Fabre (Inst), Almirall Hermal GmbH (Inst), Sun Pharma (Inst), Merck (Inst), Roche (Inst), Kyowa Kirin International (Inst)
Mario Mandalà
Honoraria: MSD Oncology, Novartis, Pierre Fabre, Sanofi/Aventis, Bristol Myers Squibb/Sanofi
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Novartis, Pierre Fabre
Research Funding: Novartis (Inst)
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Immunocore, Merck Serono, Array BioPharma, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Sanofi/Regeneron, NeraCare GmbH, Sun Pharma, Inflarx GmbH, Ultimovacs, Sandoz, Amgen, Daiichi Sankyo Japan, LabCorp, Nektar, Replimune
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, 4SC, Pierre Fabre, Sanofi/Regeneron, Nektar
Speakers' Bureau: Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi/Regeneron, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), MSD Oncology (Inst), Array BioPharma/Pfizer (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Bristol Myers Squibb, Merck Serono, Novartis, Merck Sharp & Dohme, Pierre Fabre, Sanofi/Regeneron
Naoya Yamazaki
Consulting or Advisory Role: Ono Pharmaceutical, Chugai Pharma, MSD
Speakers' Bureau: Ono Pharmaceutical, Bristol Myers Squibb Japan, Novartis, MSD
Research Funding: Ono Pharmaceutical (Inst), Bristol Myers Squibb Japan (Inst), Novartis (Inst), Astellas Amgen BioPharma (Inst), Merck Serono (Inst), Takara Bio (Inst)
Alessandra di Pietro
Stock and Other Ownership Interests: Pfizer
Honoraria: Pfizer
Jean Cantey-Kiser
Employment: PharPoint Research
Michelle Edwards
Employment: Arvinas, Pfizer, Merck
Stock and Other Ownership Interests: Arvinas, Merck
Paolo A. Ascierto
Stock and Other Ownership Interests: PrimeVax
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Takis Biotech, Lunaphore Technologies, Seattle Genetics, ITeos Therapeutics, Medicenna, Bio-Al Health
Research Funding: Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Array BioPharma (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
No other potential conflicts of interest were reported.
See accompanying editorial on page 4161
PRIOR PRESENTATION
Presented at the 57th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, June 6, 2021 (abstr 9507) and the 46th Annual Congress of the European Society for Medical Oncology (ESMO), Paris, France, September 16-21, 2021 (abstr 731).
SUPPORT
The COLUMBUS trial was sponsored by Array BioPharma, which was acquired by Pfizer in July 2019.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
AUTHOR CONTRIBUTIONS
Conception and design: Reinhard Dummer, Keith T. Flaherty, Caroline Robert, Dirk Schadendorf, Paolo A. Ascierto
Provision of study materials or patients: Reinhard Dummer, Keith T. Flaherty, Caroline Robert, Ana Arance, Jan Willem B. de Groot, Claus Garbe, Helen J. Gogas, Ralf Gutzmer, Ivana Krajsová, Gabriella Liszkay, Carmen Loquai, Mario Mandalà, Dirk Schadendorf, Naoya Yamazaki, Paolo A. Ascierto
Collection and assembly of data: Keith T. Flaherty, Caroline Robert, Jan Willem B. de Groot, Claus Garbe, Helen J. Gogas, Ralf Gutzmer, Ivana Krajsová, Gabriella Liszkay, Carmen Loquai, Mario Mandalà, Dirk Schadendorf, Naoya Yamazaki, Paolo A. Ascierto
Data analysis and interpretation: Reinhard Dummer, Keith T. Flaherty, Caroline Robert, Ana Arance, Jan Willem B. de Groot, Claus Garbe, Helen J. Gogas, Ralf Gutzmer, Dirk Schadendorf, Alessandra di Pietro, Jean Cantey-Kiser, Michelle Edwards, Paolo A. Ascierto
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
COLUMBUS 5-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib Plus Binimetinib Versus Vemurafenib or Encorafenib in Patients With BRAF V600–Mutant Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Reinhard Dummer
Honoraria: Roche, Novartis, Bristol Myers Squibb, MSD, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, CatalYm, Second Genome, Regeneron, Alligator Bioscience, MaxiVax, touchIME, T3 Pharmaceuticals, Pfizer
Consulting or Advisory Role: Roche, Bristol Myers Squibb, MSD, Novartis, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, CatalYm, Second Genome, Alligator Bioscience, touchIME, MaxiVax, Regeneron, Pfizer, T3 Pharmaceuticals
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), MSD (Inst), Amgen (Inst)
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FogPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Debiopharm Group
Caroline Robert
Stock and Other Ownership Interests: Ribonexus
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer
Research Funding: Novartis (Inst)
Ana Arance
Consulting or Advisory Role: BMS, Roche, Novartis, Pierre Fabre, MSD, Merck, Sanofi
Speakers' Bureau: Pierre Fabre, Novartis, MSD, BMS, Roche, Merck, Sanofi
Research Funding: Pierre Fabre (Inst), Novartis (Inst), Roche (Inst), BMS (Inst), MSD (Inst), Merck (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: BMS, MSD, Novartis, Pierre Fabre
Jan Willem B. de Groot
Consulting or Advisory Role: Bristol Myers Squibb, Pierre Fabre, Servier
Claus Garbe
Honoraria: BMS, MSD Oncology, NeraCare GmbH, Novartis, Philogen, Roche/Genentech, Sanofi, CeCaVa
Consulting or Advisory Role: BMS, MSD Oncology, NeraCare GmbH, Novartis, Philogen, Roche/Genentech, Sanofi, CeCaVa
Research Funding: BMS (Inst), Novartis (Inst), NeraCare GmbH (Inst), Roche/Genentech (Inst)
Helen J. Gogas
Honoraria: Bristol Myers Squibb, MSD Oncology, Pierre Fabre, Sanofi/Regeneron
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Amgen, Pierre Fabre, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb (Inst), Roche (Inst), MSD Oncology (Inst), Amgen (Inst), Novartis (Inst), Iovance Biotherapeutics (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Amgen, Pfizer
Ralf Gutzmer
Honoraria: Bristol Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, Novartis, Merck Serono, Almirall Hermal GmbH, Amgen, Sun Pharma, Pierre Fabre, Sanofi/Regeneron, Immunocore
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, Novartis, Almirall Hermal GmbH, 4SC, Amgen, Pierre Fabre, Merck Serono, Sun Pharma, Sanofi, Immunocore
Research Funding: Pfizer (Inst), Novartis (Inst), Johnson & Johnson (Inst), Amgen (Inst), Merck Serono (Inst), Sun Pharma (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche, Merck Serono, Pierre Fabre, Sun Pharma
Gabriella Liszkay
Consulting or Advisory Role: Roche, MSD, Novartis, Bristol Myers Squibb/Pfizer, Sanofi, Pfizer
Speakers' Bureau: Bristol Myers Squibb, MSD Oncology (Inst), Sanofi
Research Funding: Roche, Novartis (Inst), Inscite Corporation (Inst)
Carmen Loquai
Consulting or Advisory Role: Bristol Myers Squibb (Inst), MSD (Inst), BioNTech (Inst), Novartis (Inst), Sanofi (Inst), Pierre Fabre (Inst), Almirall Hermal GmbH (Inst), Sun Pharma (Inst), Merck (Inst), Roche (Inst), Kyowa Kirin International (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb (Inst), MSD (Inst), BioNTech (Inst), Novartis (Inst), Sanofi (Inst), Pierre Fabre (Inst), Almirall Hermal GmbH (Inst), Sun Pharma (Inst), Merck (Inst), Roche (Inst), Kyowa Kirin International (Inst)
Mario Mandalà
Honoraria: MSD Oncology, Novartis, Pierre Fabre, Sanofi/Aventis, Bristol Myers Squibb/Sanofi
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Novartis, Pierre Fabre
Research Funding: Novartis (Inst)
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Immunocore, Merck Serono, Array BioPharma, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Sanofi/Regeneron, NeraCare GmbH, Sun Pharma, Inflarx GmbH, Ultimovacs, Sandoz, Amgen, Daiichi Sankyo Japan, LabCorp, Nektar, Replimune
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, 4SC, Pierre Fabre, Sanofi/Regeneron, Nektar
Speakers' Bureau: Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi/Regeneron, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), MSD Oncology (Inst), Array BioPharma/Pfizer (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Bristol Myers Squibb, Merck Serono, Novartis, Merck Sharp & Dohme, Pierre Fabre, Sanofi/Regeneron
Naoya Yamazaki
Consulting or Advisory Role: Ono Pharmaceutical, Chugai Pharma, MSD
Speakers' Bureau: Ono Pharmaceutical, Bristol Myers Squibb Japan, Novartis, MSD
Research Funding: Ono Pharmaceutical (Inst), Bristol Myers Squibb Japan (Inst), Novartis (Inst), Astellas Amgen BioPharma (Inst), Merck Serono (Inst), Takara Bio (Inst)
Alessandra di Pietro
Stock and Other Ownership Interests: Pfizer
Honoraria: Pfizer
Jean Cantey-Kiser
Employment: PharPoint Research
Michelle Edwards
Employment: Arvinas, Pfizer, Merck
Stock and Other Ownership Interests: Arvinas, Merck
Paolo A. Ascierto
Stock and Other Ownership Interests: PrimeVax
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Takis Biotech, Lunaphore Technologies, Seattle Genetics, ITeos Therapeutics, Medicenna, Bio-Al Health
Research Funding: Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Array BioPharma (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
No other potential conflicts of interest were reported.
REFERENCES
- 1.Krauthammer M, Kong Y, Bacchiocchi A, et al. : Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet 47:996-1002, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, et al. : Mutations of the BRAF gene in human cancer. Nature 417:949-954, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Seth R, Messersmith H, Kaur V, et al. : Systemic therapy for melanoma: ASCO guideline. J Clin Oncol 38:3947-3970, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Michielin O, van Akkooi ACJ, Ascierto PA, et al. : Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1884-1901, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Keilholz U, Ascierto PA, Dummer R, et al. : ESMO consensus conference recommendations on the management of metastatic melanoma: Under the auspices of the ESMO Guidelines Committee. Ann Oncol 31:1435-1448, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Michielin O, van Akkooi A, Lorigan P, et al. : ESMO consensus conference recommendations on the management of locoregional melanoma: Under the auspices of the ESMO Guidelines Committee. Ann Oncol 31:1449-1461, 2020 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Cutaneous Melanoma (Version 3.2022). NCCN.org, 2022 [Google Scholar]
- 8.Robert C, Grob JJ, Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Ascierto PA, Dreno B, Larkin J, et al. : 5-year outcomes with cobimetinib plus vemurafenib in BRAF (V600) mutation-positive advanced melanoma: Extended follow-up of the coBRIM study. Clin Cancer Res 27:5225-5235, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan RJ, Weber J, Patel S, et al. : A phase Ib/II study of the BRAF inhibitor encorafenib plus the MEK inhibitor binimetinib in patients with BRAFV600E/K-mutant solid tumors. Clin Cancer Res 26:5102-5112, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Dummer R, Ascierto PA, Gogas HJ, et al. : Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 19:603-615, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Dummer R, Ascierto PA, Gogas H, et al. : Results of COLUMBUS Part 2: A phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus ENCO in BRAF-mutant melanoma, Presented at 42nd Annual Congress of the European Society for Medical Oncology, Madrid, Spain, September 8-12, 2017 (abstr 1215O)
- 13.Koelblinger P, Thuerigen O, Dummer R: Development of encorafenib for BRAF-mutated advanced melanoma. Curr Opin Oncol 30:125-133, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holderfield M, Merritt H, Chan J, et al. : RAF inhibitors activate the MAPK pathway by relieving inhibitory autophosphorylation. Cancer Cell 23:594-602, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Stuart DD, Li N, Poon DJ, et al. : Preclinical profile of LGX818: A potent and selective RAF kinase inhibitor, Presented at 103rd Annual Meeting of the American Association for Cancer Research, Chicago, IL, March 31-April 4, 2012 (abstr 3790)
- 16.Delord JP, Robert C, Nyakas M, et al. : Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Cancer Res 23:5339-5348, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Ascierto PA, Schadendorf D, Berking C, et al. : MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: A non-randomised, open-label phase 2 study. Lancet Oncol 14:249-256, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Dummer R, Ascierto PA, Gogas HJ, et al. : Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19:1315-1327, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Gogas HJ, Flaherty KT, Dummer R, et al. : Adverse events associated with encorafenib plus binimetinib in the COLUMBUS study: Incidence, course and management. Eur J Cancer 119:97-106, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Ascierto PA, Dummer R, Gogas HJ, et al. : Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 126:33-44, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Gogas H, Dummer R, Ascierto PA, et al. : Quality of life in patients with BRAF-mutant melanoma receiving the combination encorafenib plus binimetinib: Results from a multicentre, open-label, randomised, phase III study (COLUMBUS). Eur J Cancer 152:116-128, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Long GV, Grob JJ, Nathan P, et al. : Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol 17:1743-1754, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Hauschild A, Larkin J, Ribas A, et al. : Modeled prognostic subgroups for survival and treatment outcomes in BRAF V600-mutated metastatic melanoma: Pooled analysis of 4 randomized clinical trials. JAMA Oncol 4:1382-1388, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical Trials Dashboard: COLUMBUS trial 5-year results. https://clinical-trials.dimensions.ai/columbus
- 25.Ascierto PA, Del Vecchio M, Mackiewicz A, et al. : Overall survival at 5 years of follow-up in a phase III trial comparing ipilimumab 10 mg/kg with 3 mg/kg in patients with advanced melanoma. J Immunother Cancer 8:e000391, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribas A, Daud A, Pavlick AC, et al. : Extended 5-year follow-up results of a phase Ib study (BRIM7) of vemurafenib and cobimetinib in BRAF-mutant melanoma. Clin Cancer Res 26:46-53, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Ascierto PA, McArthur GA, Dreno B, et al. : Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17:1248-1260, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Long GV, Flaherty KT, Stroyakovskiy D, et al. : Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert C, Karaszewska B, Schachter J, et al. : Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30-39, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Dréno B, Ribas A, Larkin J, et al. : Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann Oncol 28:1137-1144, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Booth AEC, Hopkins AM, Rowland A, et al. : Risk factors for MEK-associated retinopathy in patients with advanced melanoma treated with combination BRAF and MEK inhibitor therapy. Ther Adv Med Oncol 12:1758835920944359, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meirson T, Asher N, Bomze D, et al. : Safety of BRAF+MEK inhibitor combinations: Severe adverse event evaluation. Cancers (Basel) 12:1650, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holbrook K, Lutzky J, Davies MA, et al. : Intracranial antitumor activity with encorafenib plus binimetinib in patients with melanoma brain metastases: A case series. Cancer 126:523-530, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukushima H, Iwata Y, Saito K, et al. : Successful rechallenge therapy for BRAF/MEK inhibitor-resistant multiple brain metastases of melanoma. J Dermatol 48:1291-1295, 2021 [DOI] [PubMed] [Google Scholar]
- 36.Khullar K, Hanft S, Mehnert JM, et al. : Complete response of brainstem metastasis in BRAF-mutated melanoma without stereotactic radiosurgery after initiation of encorafenib and binimetinib. Melanoma Res 31:393-396, 2021 [DOI] [PubMed] [Google Scholar]
- 37.McLoughlin EM, Fadul CE, Patel SH, et al. : Clinical and radiographic response of leptomeningeal and brain metastases to encorafenib and binimetinib in a patient with BRAF V600E-mutated lung adenocarcinoma. J Thorac Oncol 14:e269-e271, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.