PURPOSE
The androgen receptor (AR) is expressed (+) in a subset of salivary gland cancers (SGCs). This phase II trial evaluated the efficacy of the antiandrogen enzalutamide in AR+ SGC.
METHODS
Patients with locally advanced/unresectable or metastatic AR+ SGCs were enrolled. Enzalutamide (160 mg) was given orally once daily. The primary end point was the best overall response rate per RECIST v1.1 within eight cycles. Confirmed responses in ≥ 5 of 41 patients would be considered promising. Secondary end points were progression-free survival, overall survival, and safety.
RESULTS
Forty-six patients were enrolled; 30 (65.2%) received prior systemic therapy, including 13 (28.3%) with AR-targeted drugs. Of seven (15.2%) partial responses (PRs), only two (4.3%) were confirmed per protocol and counted toward the primary end point. Twenty-four patients (52.2%) had stable disease; 15 (32.6%) had progression of disease as best response. Twenty-six patients (56.5%) experienced tumor regression in target lesions; 18 (39.1%) had partial response/stable disease ≥ 6 months. Tumor regressions were observed in female patients (5 of 6 [83.3%]) and those who received prior AR– (6 of 13 [46.2%]) or human epidermal growth factor receptor 2–targeted therapies (5 of 8 [62.5%]). Three patients remained on treatment at data cutoff (duration, 32.2-49.8 months). The median progression-free survival was 5.6 months (95% CI, 3.7 to 7.5); the median overall survival was 17.0 months (95% CI, 11.8 to 30.0). The most common adverse events were fatigue, hypertension, hot flashes, and weight loss. Total and free testosterone levels increased by a mean of 61.2% and 48.8%, respectively, after enzalutamide.
CONCLUSION
Enzalutamide demonstrated limited activity in AR+ SGC, failing to meet protocol-defined success in part because of a lack of response durability. Strategies to enhance the efficacy of antiandrogen therapy are needed.
INTRODUCTION
Salivary duct carcinoma (SDC) is a salivary gland cancer (SGC) characterized by androgen receptor (AR) expression: 67%-98% of SDCs are AR-positive (AR+) with any degree of tumor staining by immunohistochemistry (IHC).1-5 SDC is an aggressive malignancy with a high rate of metastatic disease (50%-60%) and a low 5-year overall survival (OS; 20%-43%).5,6 Other SGCs that can express AR include adenocarcinoma, carcinoma ex pleomorphic adenoma, mucoepidermoid carcinoma, and basal cell adenocarcinoma.7,8
CONTEXT
Key Objective
This trial evaluated the hypothesis that treatment with the antiandrogen enzalutamide alone can be effective for patients with androgen receptor–positive salivary gland cancer (SGC).
Knowledge Generated
Enzalutamide alone was insufficient to produce significant, sustained tumor responses in patients with androgen receptor–positive SGC. Analysis of enzalutamide outcomes among select patient cohorts yielded hypothesis-generating insights into the impact of sex, prior therapies, tumor heterogeneity, and circulating testosterone on enzalutamide effectiveness for patients with SGC.
Relevance
Future trials should focus on combining antiandrogens with androgen deprivation and biomarker development to optimize these therapies for patients with SGC.
In 2018, Fushimi et al published the first prospective trial evaluating in 36 patients with AR+ SGC combined androgen blockade (CAB) with the gonadotropin-releasing hormone agonist leuprolide acetate (androgen deprivation therapy [ADT]) plus bicalutamide (AR antagonist; antiandrogen), reporting an overall response rate (ORR) of 42% and a median progression-free survival (PFS) of 8.8 months.9 A phase II trial of the cytochrome P450 17A1 (CYP17A1) inhibitor abiraterone acetate, a second-generation AR therapy that suppresses de novo adrenal steroidogenesis, with ADT reported activity among 24 patients with castration-resistant SGC (ORR 21%; median PFS 3.7 months),10 suggesting that prostate cancer (PCa) paradigms of continuous AR dependence after resistance to hormonal therapy is relevant in some AR+ SGCs.
Antiandrogen monotherapy possesses clinical activity in patients with PCa, potentially avoiding castration-related toxicities such as bone loss, changes in lean and fat body mass, and sexual dysfunction.11-14 Bicalutamide monotherapy has activity against AR+ SGCs in retrospective series15,16 although the efficacy observed was lower than that of CAB.9 Enzalutamide is a second-generation antiandrogen with 5- to 8-fold greater affinity for AR compared with bicalutamide. The drug also blocks AR nuclear translocation, interferes with the recruitment of transcriptional coactivators, induces conformational changes that prevent AR from binding target DNA sequences, and crosses the blood-brain barrier.17
Two phase III trials demonstrated that enzalutamide with castration improves OS in patients with castration-resistant PCa.18,19 Another phase II trial showed that enzalutamide monotherapy without castration has activity against hormone-naive PCa: 92.5% of patients had ≥ 80% decline in prostate-specific antigen and 50% with metastases had structural response,13 with a minimal impact on bone mineral density.12 By abrogating negative feedback loops in the hypothalamus-pituitary axis stimulated by testosterone, antiandrogen monotherapy also increases circulating testosterone.12-14 This includes both free testosterone that activates transcriptional programs in target tissues and the inactive, bound fraction associated with albumin and sex hormone-binding globulin. We conducted this phase II trial to test the hypothesis that enzalutamide without castration is effective in patients with AR+ SGC.
METHODS
Study Patients
This was an open-label, single-arm phase II trial of enzalutamide in patients with AR+ SGCs conducted through the Alliance for Clinical Trials in Oncology and the National Clinical Trials Network. Enrolled patients had a centrally confirmed SGC diagnosis and AR expression by IHC. AR IHC was performed with the monoclonal AR441 clone (Agilent Dako); ≥ 5% immunostaining in tumor cells was considered positive. RECIST v1.1 measurable, locally advanced/unresectable disease (determined by a surgeon), or metastatic disease was required. Any number of prior therapies were allowed, including AR-targeted approaches except for enzalutamide. Patients had to be ≥ 18 years old and have a Eastern Cooperative Oncology Group performance status of 0 or 1. Exclusion criteria included prior brain metastases, leptomeningeal disease, or seizures. Complete eligibility criteria are given in the Protocol (online only). The study was approved by the Central Institutional Review Board and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all patients. Patients were offered consent to an optional correlative tissue substudy (to be reported separately). The trial was registered at ClinicalTrials.gov (identifier: NCT02749903).
Study Procedures
Enzalutamide 160 mg orally once daily was administered in 28-day cycles. Treatment was continued until disease progression (PD), unacceptable toxicity, or patient/physician decision to withdraw. Total and free testosterone levels were measured from peripheral blood before enzalutamide, on day 1 of every even-numbered cycle up to and including cycle 6, and within 30 days after the end of treatment. Radiographic imaging was performed every 2 months for the first eight cycles and every three cycles thereafter for RECIST v1.1 assessment. Adverse events (AEs) were recorded throughout. Select AEs were solicited at every visit, including fatigue, hot flashes, diarrhea, hypertension, dizziness, seizures, edema, gynecomastia, breast pain, and weight loss. In October 2017, the Protocol was amended to allow patients previously treated with a gonadotropin-releasing hormone agonist or antagonist to continue these agents on study, recognizing that these patients may be at higher risk of failing enzalutamide monotherapy and continuing these drugs would be standard for patients with castration-resistant PCa. Three patients received ADT with enzalutamide on study.
Statistical Analysis
The primary end point was best overall response (BOR) rate by RECIST v1.1 criteria within the first eight cycles (32 weeks). BOR consisted of complete responses (CRs) and partial responses (PRs) confirmed on imaging performed > 4 weeks apart. All patients who started enzalutamide were evaluable for response. A Simon's optimal two-stage design was used to assess differences between an unacceptable 5% BOR and a desirable 20% BOR. With a one-sided type one error of 5% and a power of 90%, at least two confirmed CRs/PRs were needed among the first 21 patients in the first stage to justify a second stage accrual of 20 additional patients. ≥ 5 confirmed responses among 41 patients would be considered worthy of further investigation. Accruing an additional 10% of patients was allowed for potential ineligibility, cancellation, treatment violations, or other contingencies.
Secondary end points included PFS, OS, and AEs. The Kaplan-Meier method was used to estimate PFS (from study entry to PD or death) and OS (from study entry to death). Patients who were event-free at last follow-up or discontinued for reasons other than progression or death were censored. AEs were graded according to Common Terminology Criteria for AEs v4.0.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on April 22, 2021.
RESULTS
Patient and Disease Characteristics
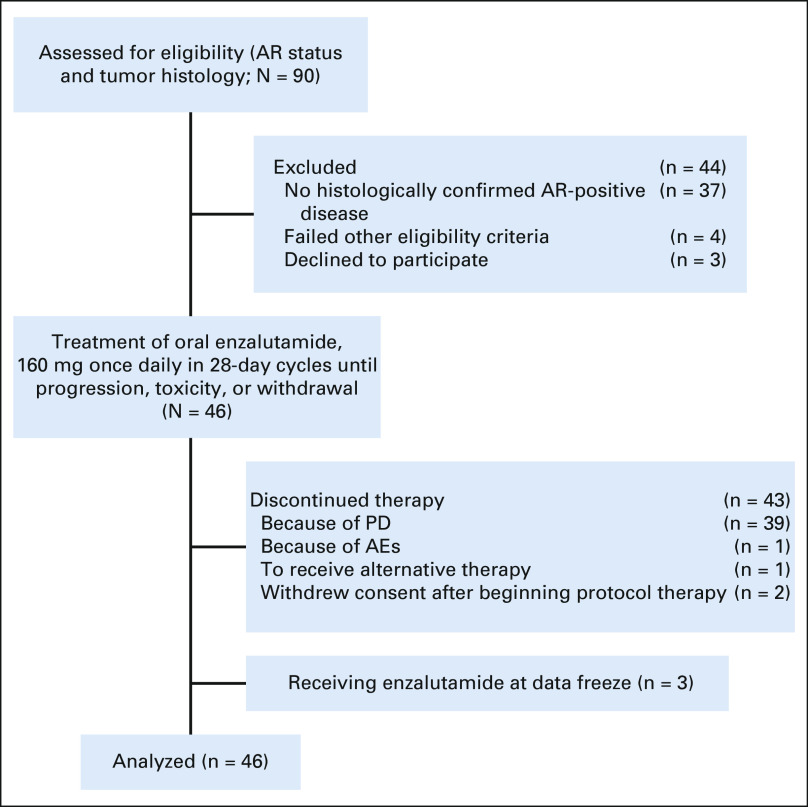
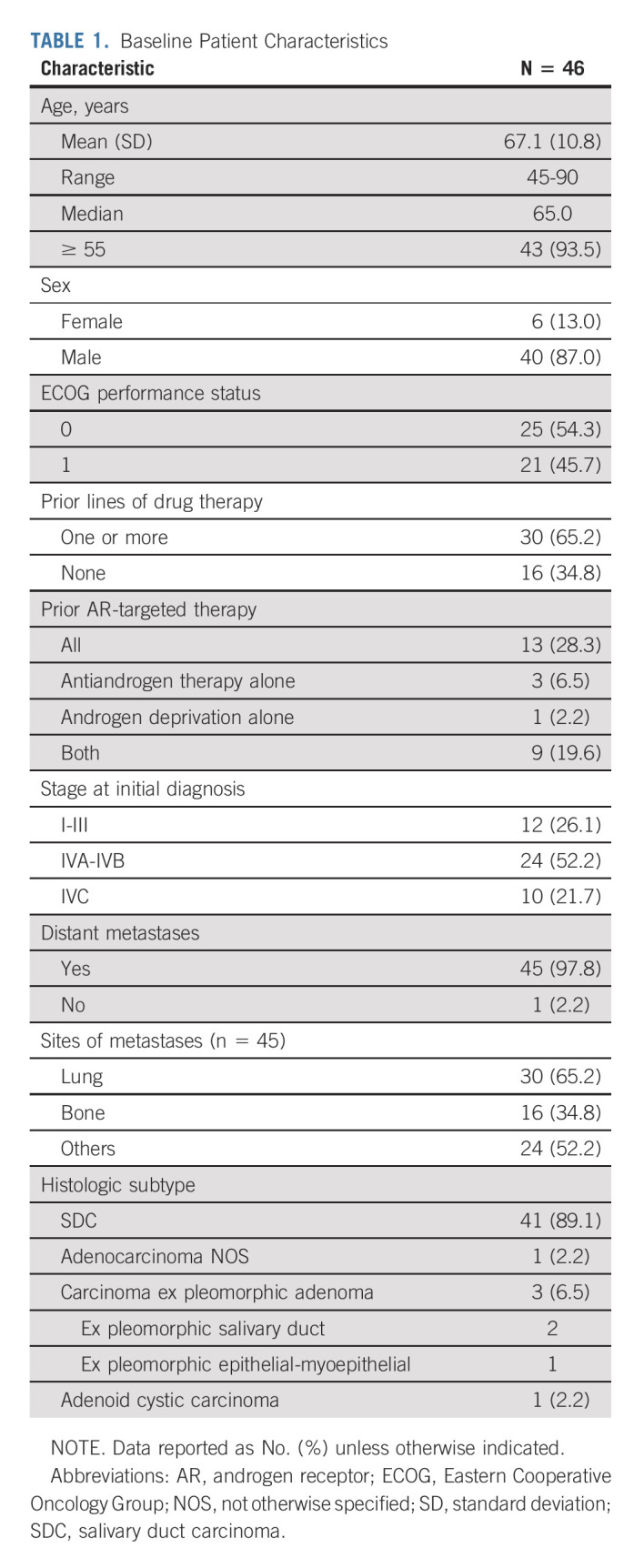
Study enrollment was completed between September 2016 and July 2018. Ninety patients were preregistered to evaluate AR and tumor histology (Fig 1). Forty-six patients met pathologic criteria and were enrolled; all were evaluable for the primary end point. Baseline patient characteristics are summarized in Table 1. The median age was 65.0 years; 93.5% were ≥ 55 years old. Six patients (13.0%) were female. Forty-one patients (89.1%) had SDC. Percent tumor cell positivity for AR by IHC and human epidermal growth factor receptor 2 (HER2) status were retrospectively assessed in a subset of patients: 71.4% of analyzed cases (n = 35) had > 70% AR+ tumor cells and 27.3% of analyzed cases (n = 33) had HER2 overexpression (IHC 3+; Data Supplement [online only]). Forty-five patients (97.8%) had metastatic disease, with lung being the most common site (65.2%). Thirty patients (65.2%) had prior systemic therapy. Thirteen (28.3%) received AR-targeted therapy before enrollment. Three patients who received androgen deprivation before enrollment continued this therapy with enzalutamide.
FIG 1.
Flow diagram. AE, adverse event; AR, androgen receptor; PD, disease progression.
TABLE 1.
Baseline Patient Characteristics
Efficacy
By January 2018, two confirmed PRs were observed among the first 21 patients, meeting the rule for second stage accrual. After completion of study accrual, re-review of the original target lesion (TL) measurements converted one of the first-stage PRs to stable disease (SD). Details of this case are given in the Data Supplement. Another confirmed PR was observed in the second stage. In total, two confirmed PRs (4.3%) were observed as of the data cutoff of April 22, 2021 (Table 2); the durations of response were 9.4 and 25.6 months. Five patients had decreased TL sizes in the RECIST PR range that were deemed unconfirmed and did not count toward the primary end point because (1) one confirmed PR was observed beyond eight cycles and (2) four other patients experienced PD before the initial PR could be confirmed. Twenty-four patients (52.2%) had SD, and 15 (32.6%) had PD as BOR (Table 2). The majority experienced tumor regression (56.5% had any decrease in TL size [Fig 2A]), and 18 (39.1%) had PR or SD for ≥ 6 months (Fig 2B). The median PFS and OS were 5.6 months (95% CI, 3.7 to 7.5) and 17.0 months (95% CI, 11.8 to 30.0), respectively (Figs 2C and 2D). Patients with a ≥ 6-month PFS (n = 18) had superior OS compared with others (n = 28; 32.7 [95% CI, 27.4 to not estimable (NE)] v 10.1 [95% CI, 6.5 to 27.6] months, log-rank P = .0004; Data Supplement). Three patients remained on enzalutamide, with a median treatment duration of 44.2 months (range, 32.2-49.8; Fig 2B), and 12 (26.1%) were alive at data cutoff. Details of the four patients on study the longest are given in the Data Supplement. Thirty-nine (84.7%) patients were removed because of PD, two (4.3%) because of consent withdrawal, one (2.2%) because of an AE (stroke/encephalopathy, unrelated to enzalutamide), and one (2.2%) because of alternative therapy (Table 2).
TABLE 2.
Study Outcomes
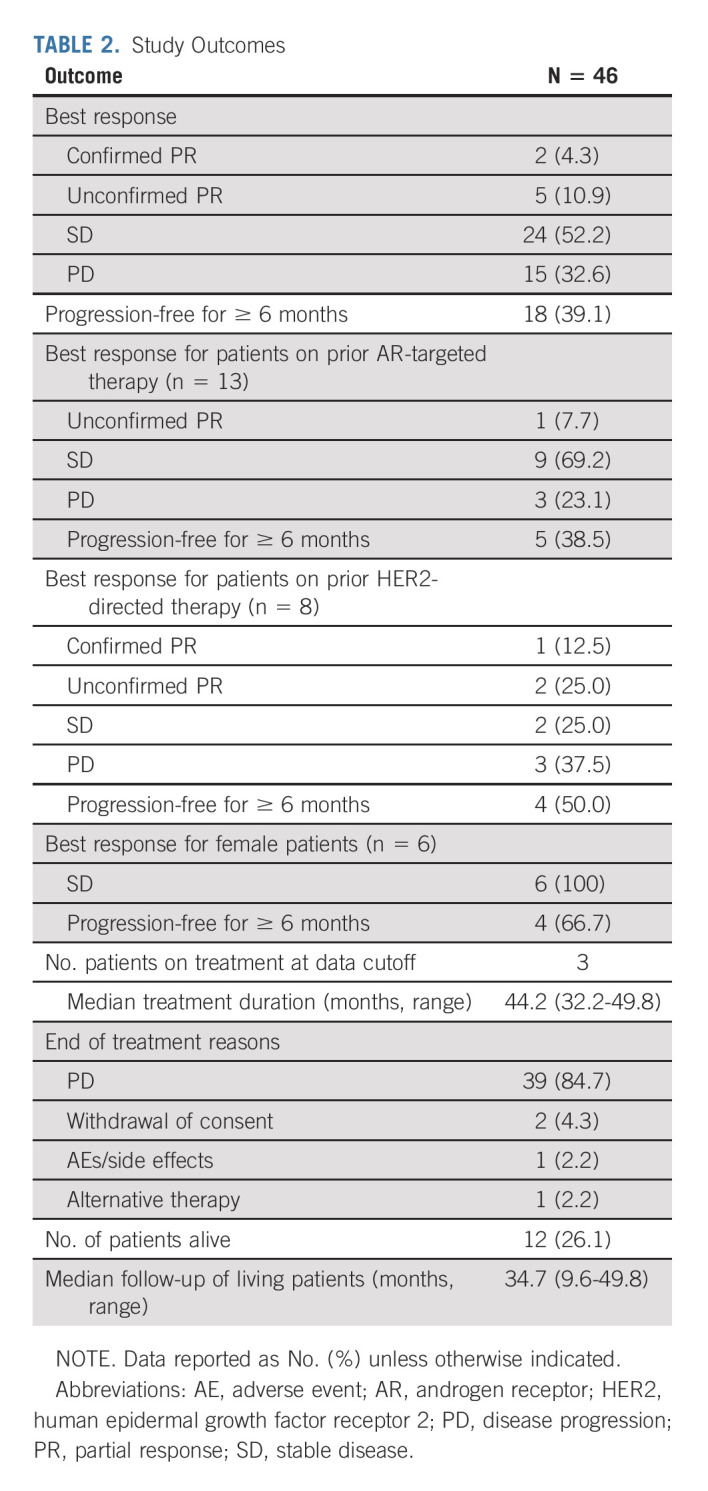
FIG 2.
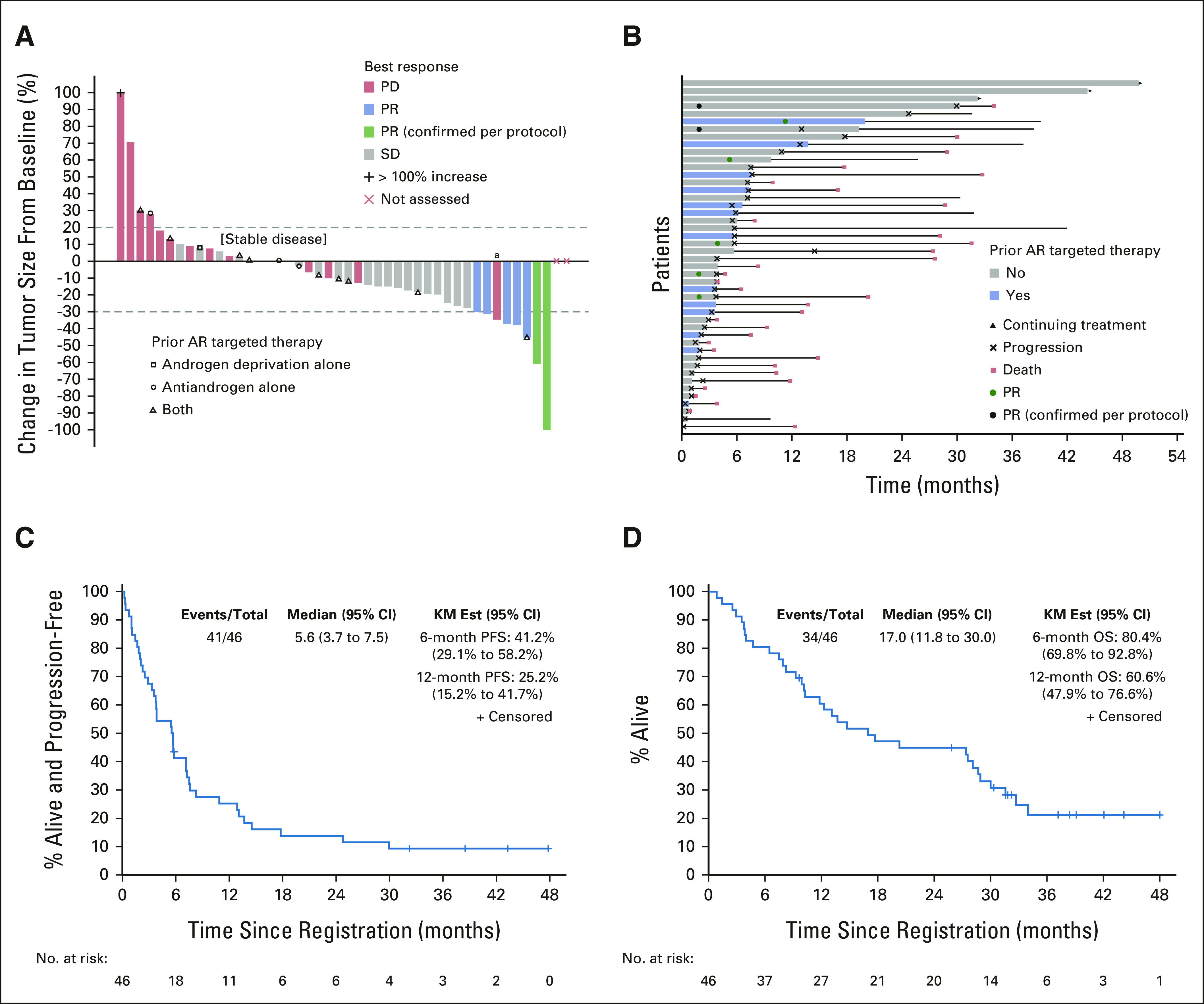
Enzalutamide efficacy in patients with AR+ SGC. (A) Waterfall plot of best percent change in tumor size from baseline by best response and prior AR-targeted therapy. (B) Swimmer's plot by prior AR targeted therapy and outcome. (C) KM curve of PFS. (D) KM curve of OS. aThough reduction of target lesions met PR criteria, this patient had a BOR of PD due to the appearance of a new tumor. AR, androgen receptor; BOR, best overall response; KM Est, Kaplan-Meier estimates; OS, overall survival; PD, disease progression; PFS, progression-free survival; PR, partial response; SD, stable disease; SGC, salivary gland cancer.
Subgroups of Interest
We performed an unplanned analysis evaluating associations between enzalutamide benefit with %AR (percentage of tumor cells positive for AR staining by IHC) and HER2 status in a subset of patients (Data Supplement). The two patients with PRs had > 70% tumor cell AR positivity; for HER2 overexpression, one was negative and the other was positive. The ≥ 6-month clinical benefit rate (CBR; defined as CR + PR + SD) was higher among patients with > 70% AR+ tumor cells (52.0% v 20.0%; P = .0045) and whose tumors lacked HER2 overexpression/amplification (45.8% v 22.2%; P = 0.0126; Data Supplement). Among 13 patients previously treated with AR therapy, six (46.2%) experienced tumor regression (Fig 2). BORs among this group were one (7.7%) unconfirmed PR (on treatment for 18+ months), nine (69.2%) SD, and three PD (23.1%). Five (38.5%) were progression-free for ≥ 6 months (Table 2 and Data Supplement). The median PFS for this subset was 5.7 months (95% CI, 3.3 to NE) compared with 5.5 months (95% CI, 2.9 to 10.9) for those without prior AR therapy (log-rank P = .8213; Data Supplement). Among eight patients who received prior HER2-directed therapy, enzalutamide produced regressions in five (62.5%), including one confirmed PR (12.5%) and two unconfirmed PRs (25.0%); four (50.0%) remained on treatment for ≥ 6 months (Data Supplement). Among the six female patients, all had SD, with five (83.3%) experiencing tumor regression (–12% to –18.8%); four patients experienced SD for ≥ 6 months (Table 2 and Data Supplement). The median PFS was 7.7 months (95% CI, 5.7 to NE) for females compared with 4.6 months (95% CI, 2.9 to 7.5) for males although the difference was not statistically significant (log-rank P = .3693; Data Supplement). One female patient remains on enzalutamide for > 49 months of treatment (Data Supplement). Among the three patients who continued ADT with enzalutamide, one experienced minor regression. Outcomes among patients (1) with versus without prior therapies and (2) with non-SDC pathologic diagnoses are given in detail in the Data Supplement.
Toxicity, Dose Reductions, and Discontinuation
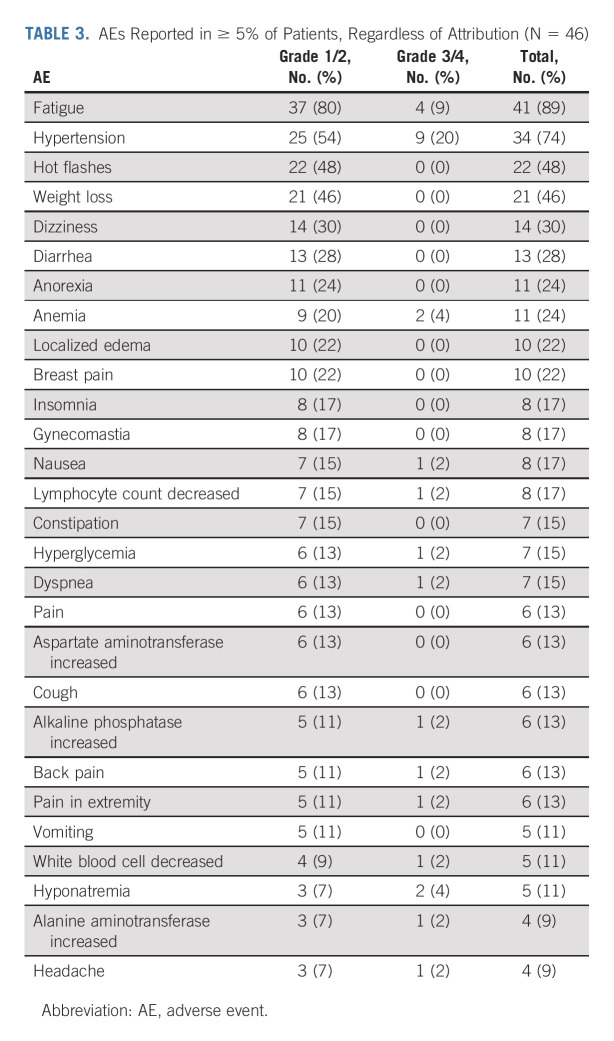
Table 3 lists the AEs observed in ≥ 5% of patients. The most common AEs of any attribution were fatigue (89%); hypertension (74%); hot flashes (48%); weight loss (46%); dizziness (30%); diarrhea (28%); anorexia and anemia (24% each); localized edema and breast pain (22% each); and insomnia, gynecomastia, nausea, and decreased lymphocyte count (17% each). Grade 3 AEs related to enzalutamide (n = 11) were hypertension and fatigue (9% each), as well as maculopapular rash, nausea, and increased alanine aminotransferase (2% each; Data Supplement). One seizure (Grade 3) was unlikely related to enzalutamide. None of the grade ≥ 4 AEs (n = 8) were related to enzalutamide (Data Supplement). One patient died on study because of respiratory failure that was unrelated to enzalutamide. Seven patients (15.2%) required dose reduction for toxicity.
TABLE 3.
AEs Reported in ≥ 5% of Patients, Regardless of Attribution (N = 46)
Total and Free Testosterone
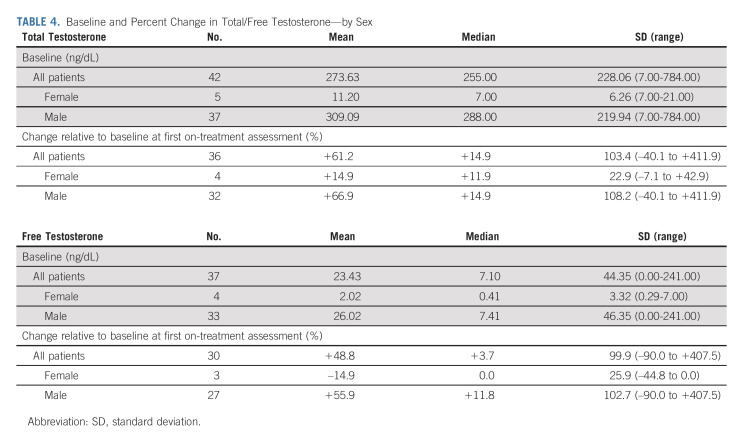
At baseline (n = 42), the mean total testosterone concentration measured among male patients (n = 37) was 309.09 ng/dL (range, 7.00-784.00 ng/dL) compared with 11.20 ng/dL (range, 7.00 to 21.00 ng/dL) among females (n = 5; Table 4). Although the female total testosterone level is considered castrate (conventionally defined as ≤ 50 ng/dL), the female baseline free testosterone mean of 2.02 ng/dL (range, 0.29-7.00 ng/dL; n = 4) is higher than the 0.17 ng/dL cutoff for castration in some studies,20 suggesting that active testosterone might have been available to engage AR.
TABLE 4.
Baseline and Percent Change in Total/Free Testosterone—by Sex
Both total (n = 36) and free testosterone levels (n = 30) increased by a mean value of +61.2% (range, –40.1% to +411.9%) and +48.8% (range, –90.0% to +407.5%), respectively, at the first on-treatment measurement relative to baseline. Among males, both total and free testosterone levels increased (total: mean +66.9% [range, –40.1% to +411.9%]; free: mean +55.9% [range, –90.0% to +407.5%]), whereas in females, the mean total testosterone level increased by +14.9% (range, –7.1% to +42.9%) although the mean free testosterone level decreased by –14.9% (range, –44.8% to 0%). For two patients on ADT, the mean total testosterone level increased by +11.9% (range, 0% to +23.8%) at week 4 although no change in free testosterone was detected (Data Supplement). Over time, testosterone levels continued to increase, except for free testosterone in females and in patients on ADT (Data Supplement). The degree of testosterone change was not associated with clinical outcomes (Data Supplement).
DISCUSSION
This trial tested the hypothesis that potent AR inhibition with enzalutamide would be sufficient to elicit responses in patients with AR therapy–naive and castration-resistant SGC. Although seven (15.2%) patients met imaging criteria for PR, only two (4.3% ORR) had confirmed PR. The failure to achieve confirmed PR was due in part to a limited duration of response. The median PFS of 5.6 months (95% CI, 3.7 to 7.5), tumor regressions in 26 (56.5%) patients, and 18 (39.1%) patients who were progression-free for ≥ 6 months suggest that some might have experienced benefit, despite the low confirmed response rate. Three patients at data cutoff were still on treatment for a median duration of 44.2 (range, 32.2-49.8) months. Regardless, in the context of superior outcomes observed with CAB9 and abiraterone,10 the failure to meet prespecified targets for efficacy suggests that use of enzalutamide monotherapy is generally not justified in patients with AR+ SGC.
We anticipated that enzalutamide monotherapy could allow patients to minimize castration-related AEs associated with ADT. However, compared with the CAB SDC study, enzalutamide produced higher rates of hot flashes (48% v 25%), gynecomastia (17% v 2.8%), and breast pain (22% v 0%).9 Our trial might have captured more AR-related AEs because the protocol mandated these toxicities be solicited at every visit. Notably, hot flashes occurred at a higher rate on this study compared with the PCa enzalutamide monotherapy trial (48% v 18%, respectively) although the rate of gynecomastia was lower (17% v 36%, respectively).13
The lower ORR observed with enzalutamide (4.3%) compared with CAB in hormone therapy–naive patients (41.7%) and with abiraterone in castration-resistant cases (21%) may reflect the importance of androgen deprivation for patients with AR+ SGC. Compared with CAB, the response rate in a retrospective series of patients with AR+ SGC treated largely with bicalutamide alone was lower (18%).15,16 The enzalutamide response rate is even lower than the bicalutamide one, possibly because of inclusion of previously treated patients on study. Enzalutamide increased total and free testosterone levels in male patients, potentially hindering AR inhibition. Whether enzalutamide plus ADT is more effective is unknown as only three patients continued ADT on study, with one experiencing minor tumor regression.
There are also differences in the characteristics of the patients enrolled on this trial compared with the CAB study.9 The enzalutamide trial included more patients with distant metastases (97.8% v 64%) and prior systemic therapy (65% v 14%). The CAB trial enrolled only hormone therapy–naive patients, whereas this study enrolled 13 with prior AR therapy (28.3%). Of these, 6 (46.2%) experienced tumor regression, including five (38.5%) who were progression-free for ≥ 6 months, suggesting a degree of continued AR dependence. The CAB trial also enrolled proportionately more patients with ≥ 70% AR+ tumor cells (83% v 71.4%). The CAB study found no statistically significant difference in outcomes among patients with ≥ 70% versus < 70% AR positivity,9 whereas the > 70% AR+ group on this trial included the two patients with PR and a higher ≥ 6-month CBR than the < 70% AR+ group (52.0% v 20.0%; P = .0045; Data Supplement). Studies with larger sample sizes are needed to more definitively establish the relevance of %AR as a biomarker of AR therapy benefit.
Castration resistance in PCa can be mediated by AR amplification/mutation/RNA splice variants, upregulation of intratumoral androgens, or co-occurring genetic alterations.21 The activity of abiraterone plus ADT in patients with castration-resistant SGC suggests that increased tumor sensitivity to castrate levels of testosterone is one form of SGC resistance.10 Given that 37.5% of patients still had PD as best response on abiraterone, other mechanisms of castration resistance may be important. SDCs can express AR-V7,22-24 an AR splice variant associated with PCa castration resistance,25,26 although the role in AR+ SGCs remains undefined.22-24,27
Approximately 29%-46% of SDCs have HER2 overexpression or amplification, typically concomitant with AR expression.6,22,28-32 AR and HER2 pathway crosstalk has been explored in PCa and breast cancer models,33-37 with some evidence that HER2 may mediate resistance to AR targeting.35,37 Observing PR in a HER2-positive patient and tumor regressions among those previously treated with HER2-directed therapies suggests that AR targeting might have some activity against HER2-overexpressed/HER2-amplified tumors. However, the ≥ 6-month CBR was higher among the HER2-negative group compared with HER2-positive (45.8% v 22.2%, P = .0126; Data Supplement), suggesting the possibility that high HER2 tumors may be less susceptible to enzalutamide. Without data establishing the optimal treatment for patients with AR+/HER2+ SGC, the high rates of response (> 60%) reported with HER2 therapeutic regimens suggest that these approaches may be prioritized over AR therapies for first-line treatment.38-40 Investigation of AR:HER2 crosstalk in SGCs is needed to identify biomarkers that could be used to optimize treatment selection.
All six female patients likely experienced some enzalutamide benefit with an overall median PFS that trended better than that among male patients (Data Supplement). Female patients had a mean concentration of bioactive free testosterone above what is considered castrate. Still, these levels were only approximately 8% of those detected in male patients and did not increase with enzalutamide. These lower testosterone levels might have provided a more favorable physiologic context for AR inhibition with enzalutamide. Alternatively, other tumor-intrinsic factors enriched in females that enhance enzalutamide susceptibility or translate to less aggressive disease may explain the study outcomes observed for this group.
Enzalutamide produced a low response rate among patients with AR+ SGC. These data suggest that maximizing antiandrogen benefit may require androgen deprivation. Developing biomarkers to predict AR therapy benefit may optimize how these drugs are used in the management of patients with AR+ SGC.
ACKNOWLEDGMENT
Editorial assistance at the Memorial Sloan Kettering Cancer Center was provided by Hannah Rice, BA, ELS, and Katharine Ollà, MA.
Alan Loh Ho
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Genzyme, Merck, Novartis, Sun Pharma, Regeneron, TRM Oncology, Ayala Pharmaceuticals, AstraZeneca, Sanofi, CureVac, CureVac, Prelude Therapeutics, Kura Oncology, McGivney Global Advisors, Elevar Therapeutics, Rgenta, AffyImmune Therapeutics, Exelixis, Cellestia Biotech, InxMed
Speakers' Bureau: Medscape, Omniprex America, Novartis
Research Funding: Lilly, Genentech/Roche, AstraZeneca, Bayer, Kura Oncology, Kolltan Pharmaceuticals, Eisai, Bristol Myers Squibb, Astellas Pharma, Novartis, Merck, Pfizer, Ayala Pharmaceuticals, Allos Therapeutics, Daiichi Sankyo, Elevar Therapeutics
Travel, Accommodations, Expenses: Janssen Oncology, Merck, Kura Oncology, Ignyta, Ayala Pharmaceuticals, KLUS Pharma
Francis Worden
Honoraria: Merck Sharp & Dohme, Eisai, Bristol Myers Squibb, Bayer, Regeneron, Exelixis
Consulting or Advisory Role: Merck, Loxo, Bristol Myers Squibb, Eisai, Bayer, Regeneron, Exelixis
Research Funding: Merck (Inst), Eisai (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), CUE Biopharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer
Douglas Adkins
Consulting or Advisory Role: Merck, CUE Biopharma, Blueprint Medicines, Exelixis, Immunitas, Kura Oncology, TargImmune Therapeutics, twoXAR, Vaccinex, Xilio Therapeutics, Boehringer Ingelheim, Eisai Europe, Coherus Biosciences
Research Funding: Pfizer (Inst), Lilly (Inst), Merck (Inst), Celgene (Inst), Novartis (Inst), AstraZeneca (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Kura Oncology (Inst), MedImmune (Inst), Exelixis (Inst), Innate Pharma (Inst), Matrix Biomed (Inst), Aduro Biotech (Inst), Sensei Biotherapeutics (Inst), CUE Biopharma (Inst), Cofactor Genomics (Inst), Shanghai Denovo (Inst), Debiopharm Group (Inst), ISA Pharmaceuticals (Inst), Gilead Sciences (Inst), BeiGene (Inst), Roche (Inst), Vaccinex (Inst), Immutep (Inst), Hookipa Biotech (Inst), Rubius Therapeutics (Inst), Epizyme (Inst)
Daniel W. Bowles
Consulting or Advisory Role: Exelixis, Blueprint Medicines
Hyunseok Kang
Honoraria: Cancer Expert Now, Axis Medical Education
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Prelude Therapeutics, Achilles Therapeutics, MitoImmune, PIN therapeutics, Exelixis, Coherus Biosciences, Tempus
Research Funding: Kura Oncology (Inst), Exelixis (Inst), Lilly (Inst), Elevar Therapeutics (Inst), PDS Biotechnology (Inst), NeoImmuneTech (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
Barbara Burtness
Consulting or Advisory Role: Merck, Debiopharm Group, CUE Biopharma, Maverick Therapeutics, Rakuten Medical, MacroGenics, ALX Oncology, IO Biotech, Genentech/Roche, Kura Oncology, Exelixis, Merck KGaA, PPD Global, Nektar, Coherus Biosciences, Arvinas, Fusion Pharmaceuticals
Speakers' Bureau: Clinical Education Alliance, Oncology Education
Research Funding: Merck (Inst), Exelixis (Inst), CUE Biopharma (Inst), Eisai, AstraZeneca, Merck
Eric Sherman
Consulting or Advisory Role: Eisai, UpToDate, Lilly, Blueprint Medicines, Exelixis
Research Funding: Plexxikon (Inst), Regeneron (Inst), Lilly (Inst), HUTCHMED (Inst), Novartis (Inst)
Luc G.T. Morris
Patents, Royalties, Other Intellectual Property: I am an inventor on IP owned by the Memorial Sloan Kettering Cancer Center and licensed to PGDx
Pamela Munster
Leadership: Alessa Therapeutics, EpiAxis Therapeutics
Stock and Other Ownership Interests: Alessa Therapeutics, OnKure, Atlas Medx
Honoraria: GlaxoSmithKline, Athenex, AstraZeneca/Merck, Alkermes, Olema Pharmaceuticals
Consulting or Advisory Role: BeiGene, Atlas Medx, RasCal, Alessa Therapeutics, Olema Pharmaceuticals
Research Funding: Merck (Inst), Pfizer (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Celgene (Inst), Sanofi (Inst), Genentech/Roche (Inst), OncoSec (Inst), Bristol Myers Squibb (Inst), Immune Design (Inst), Clovis Oncology (Inst), PMV Pharma (Inst), Tesaro/GSK (Inst), Corcept Therapeutics (Inst), Deciphera (Inst), Arch Oncology (Inst), Cyteir, H3 Biomedicine (Inst), Revolution Medicines (Inst), Actuate Therapeutics (Inst), ORIC Pharmaceuticals (Inst), Tempest Therapeutics (Inst), Relay Therapeutics (Inst), Arvinas (Inst), Bliss Biopharmaceutical (Inst), Amgen (Inst), Xynomic Pharma (Inst)
Patents, Royalties, Other Intellectual Property: UCSF patent on using silastic implants to deliver anticancer agents
Gary K. Schwartz
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: GenCirq, Bionaut Labs, January Therapeutics
Consulting or Advisory Role: Bionaut Labs, Ellipses Pharma, GenCirq, Epizyme, Array BioPharma, Apexigen, Oncogenuity, OnCusp Therapeutics, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, Sellas Life Sciences, PureTech, Killys Therapeutics
Research Funding: Astex Pharmaceuticals, Incyte (Inst), Calithera Biosciences (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Fortress Biotech (Inst), Karyopharm Therapeutics (Inst), Oxford BioTherapeutics (Inst), Astex Pharmaceuticals (Inst), TopAlliance BioSciences Inc (Inst), Adaptimmune (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Array BioPharma, Epizyme, Epizyme, Epizyme
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented at the 2019 ASCO annual meeting, virtual, June 1, 2019.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Nos U10CA180821, U10CA180882, and U24 CA196171 (to the Alliance for Clinical Trials in Oncology); UG1CA233160, UG1CA233290, UG1CA233339, and U10CA180868 (NRG); and U10CA180888 (SWOG). https://acknowledgments.alliancefound.org. This is an investigator-initiated study also supported by Astellas and Pfizer, conducted through the Alliance for Clinical Trials in Oncology. At the Memorial Sloan Kettering Cancer Center, support was also provided by NIH/NCI Cancer Center Support Grant P30 CA008748, NIH R01 DE027738-01; Geoffrey Beene Cancer Research Center; Cycle for Survival; and the Overman Fund.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Deidentified patient data may be requested from Alliance for Clinical Trials in Oncology via concepts@alliancenctn.org if data are not publicly available. A formal review process includes verifying the availability of data, conducting a review of any existing agreements that might have implications for the project, and ensuring that any transfer is in compliance with the IRB. The investigator will be required to sign a data release form prior to transfer.
AUTHOR CONTRIBUTIONS
Conception and design: Alan Loh Ho, Nathan R. Foster, Gary K. Schwartz
Administrative support: Alan Loh Ho, Pamela Munster, Gary K. Schwartz
Provision of study materials or patients: Alan Loh Ho, Katharine Price, Daniel W. Bowles
Collection and assembly of data: Alan Loh Ho, Nathan R. Foster, Katharine Price, Hyunseok Kang, Roscoe Morton, Zaineb Nadeem, Pamela Munster, Gary K. Schwartz
Data analysis and interpretation: Alan Loh Ho, Nathan R. Foster, Alex J. Zoroufy, Jordan D. Campbell, Francis Worden, Douglas Adkins, Daniel W. Bowles, Hyunseok Kang, Barbara Burtness, Eric Sherman, Luc G.T. Morris, Nora Katabi, Gary K. Schwartz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Enzalutamide for Patients With Androgen Receptor–Positive Salivary Gland Cancers (Alliance A091404)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alan Loh Ho
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Genzyme, Merck, Novartis, Sun Pharma, Regeneron, TRM Oncology, Ayala Pharmaceuticals, AstraZeneca, Sanofi, CureVac, CureVac, Prelude Therapeutics, Kura Oncology, McGivney Global Advisors, Elevar Therapeutics, Rgenta, AffyImmune Therapeutics, Exelixis, Cellestia Biotech, InxMed
Speakers' Bureau: Medscape, Omniprex America, Novartis
Research Funding: Lilly, Genentech/Roche, AstraZeneca, Bayer, Kura Oncology, Kolltan Pharmaceuticals, Eisai, Bristol Myers Squibb, Astellas Pharma, Novartis, Merck, Pfizer, Ayala Pharmaceuticals, Allos Therapeutics, Daiichi Sankyo, Elevar Therapeutics
Travel, Accommodations, Expenses: Janssen Oncology, Merck, Kura Oncology, Ignyta, Ayala Pharmaceuticals, KLUS Pharma
Francis Worden
Honoraria: Merck Sharp & Dohme, Eisai, Bristol Myers Squibb, Bayer, Regeneron, Exelixis
Consulting or Advisory Role: Merck, Loxo, Bristol Myers Squibb, Eisai, Bayer, Regeneron, Exelixis
Research Funding: Merck (Inst), Eisai (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), CUE Biopharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer
Douglas Adkins
Consulting or Advisory Role: Merck, CUE Biopharma, Blueprint Medicines, Exelixis, Immunitas, Kura Oncology, TargImmune Therapeutics, twoXAR, Vaccinex, Xilio Therapeutics, Boehringer Ingelheim, Eisai Europe, Coherus Biosciences
Research Funding: Pfizer (Inst), Lilly (Inst), Merck (Inst), Celgene (Inst), Novartis (Inst), AstraZeneca (Inst), Blueprint Medicines (Inst), Bristol Myers Squibb (Inst), Kura Oncology (Inst), MedImmune (Inst), Exelixis (Inst), Innate Pharma (Inst), Matrix Biomed (Inst), Aduro Biotech (Inst), Sensei Biotherapeutics (Inst), CUE Biopharma (Inst), Cofactor Genomics (Inst), Shanghai Denovo (Inst), Debiopharm Group (Inst), ISA Pharmaceuticals (Inst), Gilead Sciences (Inst), BeiGene (Inst), Roche (Inst), Vaccinex (Inst), Immutep (Inst), Hookipa Biotech (Inst), Rubius Therapeutics (Inst), Epizyme (Inst)
Daniel W. Bowles
Consulting or Advisory Role: Exelixis, Blueprint Medicines
Hyunseok Kang
Honoraria: Cancer Expert Now, Axis Medical Education
Consulting or Advisory Role: Bayer, GlaxoSmithKline, Prelude Therapeutics, Achilles Therapeutics, MitoImmune, PIN therapeutics, Exelixis, Coherus Biosciences, Tempus
Research Funding: Kura Oncology (Inst), Exelixis (Inst), Lilly (Inst), Elevar Therapeutics (Inst), PDS Biotechnology (Inst), NeoImmuneTech (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
Barbara Burtness
Consulting or Advisory Role: Merck, Debiopharm Group, CUE Biopharma, Maverick Therapeutics, Rakuten Medical, MacroGenics, ALX Oncology, IO Biotech, Genentech/Roche, Kura Oncology, Exelixis, Merck KGaA, PPD Global, Nektar, Coherus Biosciences, Arvinas, Fusion Pharmaceuticals
Speakers' Bureau: Clinical Education Alliance, Oncology Education
Research Funding: Merck (Inst), Exelixis (Inst), CUE Biopharma (Inst), Eisai, AstraZeneca, Merck
Eric Sherman
Consulting or Advisory Role: Eisai, UpToDate, Lilly, Blueprint Medicines, Exelixis
Research Funding: Plexxikon (Inst), Regeneron (Inst), Lilly (Inst), HUTCHMED (Inst), Novartis (Inst)
Luc G.T. Morris
Patents, Royalties, Other Intellectual Property: I am an inventor on IP owned by the Memorial Sloan Kettering Cancer Center and licensed to PGDx
Pamela Munster
Leadership: Alessa Therapeutics, EpiAxis Therapeutics
Stock and Other Ownership Interests: Alessa Therapeutics, OnKure, Atlas Medx
Honoraria: GlaxoSmithKline, Athenex, AstraZeneca/Merck, Alkermes, Olema Pharmaceuticals
Consulting or Advisory Role: BeiGene, Atlas Medx, RasCal, Alessa Therapeutics, Olema Pharmaceuticals
Research Funding: Merck (Inst), Pfizer (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Celgene (Inst), Sanofi (Inst), Genentech/Roche (Inst), OncoSec (Inst), Bristol Myers Squibb (Inst), Immune Design (Inst), Clovis Oncology (Inst), PMV Pharma (Inst), Tesaro/GSK (Inst), Corcept Therapeutics (Inst), Deciphera (Inst), Arch Oncology (Inst), Cyteir, H3 Biomedicine (Inst), Revolution Medicines (Inst), Actuate Therapeutics (Inst), ORIC Pharmaceuticals (Inst), Tempest Therapeutics (Inst), Relay Therapeutics (Inst), Arvinas (Inst), Bliss Biopharmaceutical (Inst), Amgen (Inst), Xynomic Pharma (Inst)
Patents, Royalties, Other Intellectual Property: UCSF patent on using silastic implants to deliver anticancer agents
Gary K. Schwartz
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: GenCirq, Bionaut Labs, January Therapeutics
Consulting or Advisory Role: Bionaut Labs, Ellipses Pharma, GenCirq, Epizyme, Array BioPharma, Apexigen, Oncogenuity, OnCusp Therapeutics, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, Sellas Life Sciences, PureTech, Killys Therapeutics
Research Funding: Astex Pharmaceuticals, Incyte (Inst), Calithera Biosciences (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Fortress Biotech (Inst), Karyopharm Therapeutics (Inst), Oxford BioTherapeutics (Inst), Astex Pharmaceuticals (Inst), TopAlliance BioSciences Inc (Inst), Adaptimmune (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Array BioPharma, Epizyme, Epizyme, Epizyme
No other potential conflicts of interest were reported.
REFERENCES
- 1. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: Clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38:E1838–E1847. doi: 10.1002/hed.24332. suppl 1. [DOI] [PubMed] [Google Scholar]
- 2. Masubuchi T, Tada Y, Maruya S, et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol. 2015;20:35–44. doi: 10.1007/s10147-014-0674-6. [DOI] [PubMed] [Google Scholar]
- 3. Nardi V, Sadow PM, Juric D, et al. Detection of novel actionable genetic changes in salivary duct carcinoma helps direct patient treatment. Clin Cancer Res. 2013;19:480–490. doi: 10.1158/1078-0432.CCR-12-1842. [DOI] [PubMed] [Google Scholar]
- 4. Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: The predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39:705–713. doi: 10.1097/PAS.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 5. Williams MD, Roberts D, Blumenschein GR, Jr, et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: Biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol. 2007;31:1645–1652. doi: 10.1097/PAS.0b013e3180caa099. [DOI] [PubMed] [Google Scholar]
- 6. Boon E, Bel M, van Boxtel W, et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from the Netherlands. Int J Cancer. 2018;143:758–766. doi: 10.1002/ijc.31353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Locati LD, Perrone F, Losa M, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs) Oral Oncol. 2009;45:986–990. doi: 10.1016/j.oraloncology.2009.05.635. [DOI] [PubMed] [Google Scholar]
- 8. Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am J Clin Pathol. 2003;119:801–806. doi: 10.1309/RVTP-1G0Q-727W-JUQD. [DOI] [PubMed] [Google Scholar]
- 9. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29:979–984. doi: 10.1093/annonc/mdx771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Locati LD, Cavalieri S, Bergamini C, et al. Abiraterone acetate in patients with castration-resistant, androgen receptor-expressing salivary gland cancer: A phase II trial. J Clin Oncol. 2021;39:4061–4068. doi: 10.1200/JCO.21.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iversen P, Melezinek I, Schmidt A. Nonsteroidal antiandrogens: A therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function. BJU Int. 2001;87:47–56. doi: 10.1046/j.1464-410x.2001.00988.x. [DOI] [PubMed] [Google Scholar]
- 12. Tombal B, Borre M, Rathenborg P, et al. Long-term efficacy and safety of enzalutamide monotherapy in hormone-naive prostate cancer: 1- and 2-year open-label follow-up results. Eur Urol. 2015;68:787–794. doi: 10.1016/j.eururo.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 13. Tombal B, Borre M, Rathenborg P, et al. Enzalutamide monotherapy in hormone-naive prostate cancer: Primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol. 2014;15:592–600. doi: 10.1016/S1470-2045(14)70129-9. [DOI] [PubMed] [Google Scholar]
- 14. Tyrrell CJ, Iversen P, Tammela T, et al. Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration. BJU Int. 2006;98:563–572. doi: 10.1111/j.1464-410X.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- 15. Boon E, van Boxtel W, Buter J, et al. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: A nationwide case series of 35 patients in the Netherlands. Head Neck. 2018;40:605–613. doi: 10.1002/hed.25035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: A disease entity with promising new treatment options. J Clin Oncol. 2011;29:e473–e476. doi: 10.1200/JCO.2010.32.8351. [DOI] [PubMed] [Google Scholar]
- 17. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 20. Itty S, Getzenberg RH. How do we define “castration” in men on androgen deprivation therapy? Asian J Androl. 2020;22:441–446. doi: 10.4103/aja.aja_139_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dalin MG, Desrichard A, Katabi N, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22:4623–4633. doi: 10.1158/1078-0432.CCR-16-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitani Y, Rao PH, Maity SN, et al. Alterations associated with androgen receptor gene activation in salivary duct carcinoma (SDC) of both sexes: Potential therapeutic ramifications. Clin Cancer Res. 2014;20:6570–6581. doi: 10.1158/1078-0432.CCR-14-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang RK, Zhao P, Lu C, et al. Expression pattern of androgen receptor and AR-V7 in androgen-deprivation therapy-naive salivary duct carcinomas. Hum Pathol. 2019;84:173–182. doi: 10.1016/j.humpath.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cato L, de Tribolet-Hardy J, Lee I, et al. ARv7 represses tumor-suppressor genes in castration-resistant prostate cancer. Cancer Cell. 2019;35:401–413 e6. doi: 10.1016/j.ccell.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Boxtel W, Verhaegh GW, van Engen-van Grunsven IA, et al. Prediction of clinical benefit from androgen deprivation therapy in salivary duct carcinoma patients. Int J Cancer. 2020;146:3196–3206. doi: 10.1002/ijc.32795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiosea SI, Williams L, Griffith CC, et al. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol. 2015;39:744–752. doi: 10.1097/PAS.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 29. Khoo TK, Yu B, Smith JA, et al. Somatic mutations in salivary duct carcinoma and potential therapeutic targets. Oncotarget. 2017;8:75893–75903. doi: 10.18632/oncotarget.18173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santana T, Pavel A, Martinek P, et al. Biomarker immunoprofile and molecular characteristics in salivary duct carcinoma: Clinicopathological and prognostic implications. Hum Pathol. 2019;93:37–47. doi: 10.1016/j.humpath.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 31. Takase S, Kano S, Tada Y, et al. Biomarker immunoprofile in salivary duct carcinomas: Clinicopathological and prognostic implications with evaluation of the revised classification. Oncotarget. 2017;8:59023–59035. doi: 10.18632/oncotarget.19812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K, Russell JS, McDermott JD, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res. 2016;22:6061–6068. doi: 10.1158/1078-0432.CCR-15-2568. [DOI] [PubMed] [Google Scholar]
- 33. Gregory CW, Whang YE, McCall W, et al. Heregulin-induced activation of HER2 and HER3 increases androgen receptor transactivation and CWR-R1 human recurrent prostate cancer cell growth. Clin Cancer Res. 2005;11:1704–1712. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
- 34. Liu Y, Majumder S, McCall W, et al. Inhibition of HER-2/neu kinase impairs androgen receptor recruitment to the androgen responsive enhancer. Cancer Res. 2005;65:3404–3409. doi: 10.1158/0008-5472.CAN-04-4292. [DOI] [PubMed] [Google Scholar]
- 35. Mellinghoff IK, Vivanco I, Kwon A, et al. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 36. Muniyan S, Chen SJ, Lin FF, et al. ErbB-2 signaling plays a critical role in regulating androgen-sensitive and castration-resistant androgen receptor-positive prostate cancer cells. Cell Signal. 2015;27:2261–2271. doi: 10.1016/j.cellsig.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiota M, Bishop JL, Takeuchi A, et al. Inhibition of the HER2-YB1-AR axis with Lapatinib synergistically enhances Enzalutamide anti-tumor efficacy in castration resistant prostate cancer. Oncotarget. 2015;6:9086–9098. doi: 10.18632/oncotarget.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurzrock R, Bowles DW, Kang H, et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: Results from MyPathway, a phase IIa multiple basket study. Ann Oncol. 2020;31:412–421. doi: 10.1016/j.annonc.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li BT, Shen R, Offin M, et al. Ado-trastuzmab emtansine in patients with HER2 amplified salivary gland cancers: Results from a phase 2 basket trial. J Clin Oncol. 37:2019. doi: 10.1200/JCO.2018.77.9777. suppl 15; abstr 6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi H, Tada Y, Saotome T, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. 2019;37:125–134. doi: 10.1200/JCO.18.00545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified patient data may be requested from Alliance for Clinical Trials in Oncology via concepts@alliancenctn.org if data are not publicly available. A formal review process includes verifying the availability of data, conducting a review of any existing agreements that might have implications for the project, and ensuring that any transfer is in compliance with the IRB. The investigator will be required to sign a data release form prior to transfer.