Supplemental Digital Content is Available in the Text.
Paraventricular hypothalamic CaMKIIα-positive neurons input to ventral of lateral septal CaMKIIα-positive neurons controls chronic visceral pain.
Keywords: Irritable bowel syndrome, Visceral pain, Paraventricular hypothalamic, Ventral of lateral septal, Neural circuit
Abstract
Irritable bowel syndrome is a functional gastrointestinal disorder characterized by chronic visceral pain with complex etiology and difficult treatment. Accumulated evidence has confirmed that the sensitization of the central nervous system plays an important role in the development of visceral pain, whereas the exact mechanisms of action of the neural pathways remain largely unknown. In this study, a distinct neural circuit was identified from the paraventricular hypothalamic (PVH) to the ventral of lateral septal (LSV) region. This circuit was responsible for regulating visceral pain. In particular, the data indicated that the PVH CaMKIIα-positive neurons inputs to the LSV CaMKIIα-positive neurons were only activated by colorectal distention rather than somatic stimulations. The PVH-LSV CaMKIIα+ projection pathway was further confirmed by experiments containing a viral tracer. Optogenetic inhibition of PVH CaMKIIα+ inputs to LSV CaMKIIα-positive neurons suppressed visceral pain, whereas selective activation of the PVH-LSV CaMKIIα+ projection evoked visceral pain. These findings suggest the critical role of the PVH-LSV CaMKIIα+ circuit in regulating visceral pain.
1. Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disease characterized by abdominal pain and changes in bowel habits, with a high incidence in the population.9,33 Accumulating evidence has shown that subjects with IBS are more likely to undergo trauma and psychological stress, notably, early life stress compared with healthy subjects.11,14 Early life stress may contribute to long-term changes in brain function and visceral sensitivity to stimuli.5 Neonatal maternal deprivation (NMD) is a typical early life stress that has been shown to induce visceral pain behavior.18,27 At present, most research studies on visceral pain have focused on the investigation of the molecular events underlying the peripheral nervous system and the spinal cord.29,41,47 However, the studies that have focused on the brain are very limited, and the role of the brain in regulating visceral pain remains largely unclear. The NMD-induced visceral pain may be better explained from the perspective of the brain circuit.
The lateral septal (LS) is generally divided into LS dorsal, LS intermediate, and LS ventral parts. The lateral septum is known to be critical for processing emotional information and for regulating behavioral responses to stress.12,34 Increasing evidence suggests that LS plays an important role in fear,4 cognition,22 addiction,15 reward,20 and other neurological disorders, whereas the role of visceral pain is not clear. Previous studies have shown that the noxious stimulation of the viscera leads to visceral pain and enhances the regional cerebral blood flow of LS in rats,17 suggesting that LS may have a potential regulatory role in visceral hyperalgesia. In addition, previous studies have shown that LSV receives projections from the paraventricular hypothalamic (PVH) region and regulates feeding and hypersomnia behaviors.2,42 Studies have shown that the occurrence of IBS may be related to the functional changes of the hypothalamic–pituitary–adrenal (HPA) axis,10 and the increase in visceral sensitivity is the main feature of IBS, which can cause the functional changes of the HPA axis.31 As part of the HPA axis, the PVH plays a crucial role in coordinating behavioral, neuroendocrine, and autonomic responses to stress in response to a variety of physiological and psychological stressors, including pain behaviors.26 Although PVH has been reported to play an important role in the regulation of somatic pain,26 its role in visceral pain has not been fully clarified. In addition, certain studies have shown a direct structural link between colons and PVH,24 suggesting that the regulation of the activity of PVH neurons can change the progression of colorectal cancer32 and that PVH may be a potential intervention target for the treatment of IBS.
In this study, we showed that activities of CaMKIIα-positive neurons in the LSV region in response to visceral pain were greatly elevated after NMD. Moreover, our analysis demonstrated that the regulation of LSV CaMKIIα-positive neurons could alter visceral pain behaviors. It is important that we showed that the effect of LSV was mediated by the PVH-LSV pathway. This study identified a functionally distinct neural circuit acting from the PVH to the LSV, which was responsible for mediating visceral pain in mice. This finding is expected to provide a novel therapeutic strategy for the clinical treatment of visceral pain in patients with IBS.
2. Materials and methods
2.1. Animals
Male wild-type (C57BL/6) mice aged 6 to 12 weeks were housed at a constant temperature of 24 ± 2°C, in the presence of 40 to 60% relative humidity. The animals were maintained at a 12-hour light/dark cycle in a clean-level animal facility at the Experimental Animal Center of Soochow University. All mice were obtained from the Experimental Animal Center of Soochow University and had free access to food and water. All experimental procedures were approved by the Animal Care Committee of the Soochow University and adhered to the guidelines of the International Association for the Study of Pain.
2.2. Induction of chronic visceral pain
Chronic visceral pain was induced by neonatal maternal deprivation (NMD), as previously described.8,27 In brief, from day 2 to day 15 after birth, the newborn C57BL/6 male mouse pups of the NMD group were placed in a separate box with an electric blanket for 3 hours. After the separation period, the pups were placed back in their dams. The control pups and their dam were placed in the same cage without handling. On the 21st day after birth, the pups were weaned and separated from their dams. The experiments were initiated at 6 weeks of age.
2.3. Abdominal electrode implantation and electromyography recordings
To record abdominal electromyography (EMG) activities, the abdominal electrode implantation was performed, as previously described.21,45 Briefly, the mice were deeply anesthetized with isoflurane. The sterilized 2 nickel-chrome electrodes were implanted into the abdominal external oblique muscles, using aseptic techniques, and the incision was closed with a 4–0 silk suture. At 5 to 7 days after the surgery, colorectal distention was used to evaluate visceral pain by EMG recording.
2.4. Visceral pain tests
Visceral hypersensitivity was determined by colorectal distention (CRD) and EMG, as previously described.40,45 In brief, the mice were lightly anesthetized with isoflurane and a homemade flexible polypropylene balloon (2 cm) was inserted into the colorectum 3 cm from the mouse anus. The balloon was made by attaching the fingers of a surgical glove to an infusion hose. The pressure-sensitive tape was used to attach the infusion hose at the end of the balloon firmly to the mouse's tail to prevent it from slipping or falling off. The mice were allowed to acclimate in a separate clear box for 30 to 60 minutes before colorectal distention. The latter was progressively increased in 20 mm Hg steps, starting from 20 to 80 mm Hg. Each step lasted 20 seconds with 2 to 3 minutes of nondistension periods in between. During the distension periods, the EMG activities were recorded under different pressure conditions. A Biopac electromyogram (EMG)-150C amplifier (Biopac Systems, Inc, Goleta, CA) continuously recorded the EMG, and its traces were analyzed via Acknowledge (Biopac Systems, Inc). The response to visceral pain was defined as an increase in EMG activity above baseline during the 20-second visceral pain period. The data were reported as the area under the curve (AUC) of the integrated EMG following baseline subtraction. All behavioral tests were performed in a blinded manner.
2.5. c-fos experiments
To minimize stress caused by environmental stimuli, the mice were placed in the test environment for at least 3 days before performing the behavioral tests. All behavioral experiments were conducted at the same temperature and humidity conditions. For habituation, each mouse was placed in a separate clear box and allowed to acclimate for 1 hour. After animal adaptation, CRD or von Frey stimuli were applied. For the CRD-induced c-fos test, the force of the CRD stimuli required to induce nocifensive behaviors (eg, abdomen withdrawal) was 60 mm Hg. To avoid colorectal tissue damage, the CRD stimuli were applied every 3 minutes over a 15-minute test period. Therefore, 5 trials of repeated CRD stimuli were applied to the animal. The procedures for the von Frey–induced c-fos test were the same, as previously described.39 In brief, the force of the von Frey fiber to induce nocifensive behaviors (eg, paw withdrawal) was 1.0 g. To avoid tissue damage caused by von Frey fiber stimuli, the von Frey filaments were applied every 3 minutes over a 1.5-hour test period. After 1.5-hour CRD or von Frey fiber stimulation, the mice were deeply anesthetized, and cardiac perfusion was performed. The mice were transcardially perfused with 0.9% normal saline and 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.2-7.4), and the brain was removed and postfixed for 2 hours. The brains of the mice were sliced into 30-μm sections using a freezing microtome (Leica, CM3050 S, Germany). The sections were stored at −20°C in cryoprotectant solution for immunofluorescence staining experiments.
2.6. Immunofluorescence staining
Immunofluorescent staining was performed by washing the brain sections with PBS 3 times (10 minutes each time) and incubating them with blocking buffer containing 7% normal donkey serum, 0.3% Triton X-100, and 0.05% sodium azide at room temperature for 1 hour. The sections were subsequently incubated with primary antibodies diluted in blocking buffer overnight at 4°C. After incubation, the brain sections were rinsed 3 times (10 minutes each time) with PBS and incubated with a fluorescent dye–conjugated secondary antibody for 1 hour at room temperature. After 3 washes (10 minutes each time) with PBS, the brain sections were incubated with DAPI. The primary antibodies used were the following: anti-c-fos (1:200, Rabbit, Cell Signaling Technology, Danvers, MA), anti-c-fos (1:500, Mouse, Santa Cruz Biotechnology), anti-CaMKIIα (1:200, Mouse, Cell Signaling Technology, Danvers, MA), anti-GABA (1:500, Rabbit, Sigma-Aldrich), and anti-NeuN (1:100, Rabbit, Cell Signaling Technology, Danvers, MA). The secondary antibody included antirabbit Alexa Fluor 488 (1:500, Invitrogen) or antimouse Alexa Fluor 555 (1:500, Invitrogen).
2.7. Stereotaxic injection and optical fiber implantation
During surgery and viral injection, the mice were deeply anesthetized with isoflurane and placed in a stereotaxic apparatus (RWD, 71,000-M, Shenzhen, China). The skin was disinfected with iodophor solution and carefully removed using sterile surgical instruments. The skull above the target area was slowly and subtly removed with a dental drill. A 10-μL syringe (Gaoge, Shanghai, China) connected to a glass micropipette with a 15-μm-diameter tip was used to perform the injections. Syringe pumps (Longer Pump, TJ-2A, Baoding, China) were used to inject the viruses for volume and speed control. At each location, 200 nL of the virus was injected at a rate of 30 nL/minute. At each injection site, the needle was left in place for 10 to 15 minutes after the completion of the injection to allow the viral spread, and subsequently, it was slowly withdrawn. Because unilateral injection of the virus can change pain behavior fully35,37 and bilateral injections might cause more damage to the brain tissue than unilateral injection, the unilateral injection (right hemisphere) was performed in this study.
For fiber photometry, 200 nL AAV2/9-hSyn-GCaMP6f (from Gene Biotechnology, Shanghai, China, titer: 3.42 × 1012 genome copies/mL), AAV2/9-CaMKIIα-GCaMP6f (from Taitool Bioscience, Shanghai, China, titer: 3.6 × 1012 genome copies/mL), AAV2/9-VGAT1-GCaMP6m (from BrainVTA Wuhan, China, titer: 3.14 × 1012 genome copies/mL), AAV2/9-hSyn-EGFP (from BrainVTA, Wuhan, China, titer: 5.12 × 1012 genome copies/mL), and AAV2/9-CaMKIIα-EYFP (from BrainVTA, Wuhan, China, titer: 5.33 × 1012 genome copies/mL) were unilaterally injected into the LSV (AP, 0.74 mm; ML, ± 0.6 mm; DV, 3.75 mm) or PVH (AP, −0.6 mm; ML, ± 0.2 mm; DV, 4.75 mm).
For local selective optical activation or inhibition of LSV or PVH CaMKIIα+ neurons, LSV or PVH were unilaterally injected with AAV2/9-CaMKIIα-hChR2-EYFP (from Gene Biotechnology, Shanghai, China, titer: 5.2 × 1012genome copies/mL), AAV2/9-CaMKIIα-eNpHR3.0-EYFP (from Gene Biotechnology, Shanghai, China, titer: 6.27 × 1012 genome copies/mL), or AAV2/9-CaMKIIα-EYFP (from BrainVTA, Wuhan, China, titer: 5.33 × 1012 genome copies/mL).
Selective optical activation or inhibition of PVH CaMKIIα+ neuron-projecting LSV axon terminal and chemogenetic manipulation of CaMKIIα+ neuronal activity of the LSV region were performed using a dual-virus strategy. AAV2/9-CaMKIIα-hChR2-EYFP (from Gene Biotechnology, Shanghai, China, titer: 5.2 × 1012genome copies/mL) or AAV2/9-CaMKIIα-eNpHR3.0-EYFP (from Gene Biotechnology, Shanghai, China, titer: 6.27 × 1012 genome copies/mL) was unilaterally injected into PVH. Concomitantly, AAV2/9-CaMKIIα-hM4Di-mCherry (from BrainVTA, Wuhan, China, titer: 2.44 × 1012 genome copies/mL) or AAV2/9-CaMKIIα-hM3Dq-mCherry (from BrainVTA, Wuhan, China, titer: 5.40 × 1012 genome copies/mL) was unilaterally injected into LSV.
For retrograde tracing, the AAV2/R-hSyn-EGFP (from BrainVTA, Wuhan, China, titer: 5.56× 1012 genome copies/mL) was unilaterally injected into the LSV region. For anterograde tracing, the AAV2/9-hSyn-EGFP (from BrainVTA, Wuhan, China, titer: 5.12× 1012 genome copies/mL) was unilaterally injected into the LSV region. For monosynaptic anterograde tracing, the AAV2/1-CaMKIIα-Cre (from BrainVTA, Wuhan, China, titer: 1.05 × 1013 genome copies/mL) was injected into the PVH region, and the AAV2/9-EF1α-DIO-EYFP (from BrainVTA, Wuhan, China, titer: 5.24 × 1012 genome copies/mL) was unilaterally injected into the LSV region.
The local fiber photometry and optical activation or inhibition were performed in awake behaving mice at 2 to 3 weeks after viral injection as follows: the optical fibers (200 μm O.D., 0.37 NA, Thinker Tech Nanjing Bioscience, Nanjing, China) were unilaterally implanted into the LSV or PVH regions (100-200 μm above the viral injection coordinates). To allow for optogenetic manipulation of the PVH-LSV circuit axon terminals, the optical fibers were implanted 100 to 200 μm above the LSV viral injection coordinates. All fibers were attached to the skull with bone screws and dental cement. The mice were allowed to recover for at least 1 week after implantation and were habituated for 30 to 60 minutes after connection to a laser source. Subsequently, the behavioral tests were performed. The lasers were applied at the wavelengths of 473 nm (blue) or 589 nm (yellow) and controlled with an optogenetic system (Alpha Omega Engineering, Nazareth, Israel) at 5 mW for yellow illumination (589 nm, continuous mode) and at 3 mW for blue illumination (473 nm, 20 Hz, 5 ms pulse width) in eNpHR3.0, hChR2. The tests were performed in EYFP-expressing mice.39,48
For the behavioral experiments, the mice were allowed to recover 1 week after fiber implantation. They were handled and habituated to the environment of the behavioral tests for 3 days. In all the experiments performed, the mice with incorrect injection sites were excluded from further data analysis.
2.8. Fiber photometry system
To evaluate the neuronal activity of the LSV and PVH neurons, AAV2/9-hSyn-GCaMP6f, AAV2/9-hSyn-EGFP, AAV2/9-CaMKIIα-GCaMP6f, AAV2/9-VGAT1-GCaMP6m, or AAV2/9-CaMKIIα-EYFP was injected into the LSV and PVH regions of the C57BL/6 mice. After 2 to 3 weeks, an optical fiber (200 μm OD, 0.37 NA) was implanted into the LSV or PVH region, and the mice were allowed to recover for 1 week before recording. The mice were handled and habituated to the environment of the behavioral tests for 3 days. The fiber photometry system (ThinkerTech Nanjing Bioscience Inc.) has been previously described.28,39 In brief, a 488-nm laser beam was reflected off a dichroic mirror, focused with a 10x objective lens, and coupled to an optical commutator to record the calcium signals. The commutator and implanted fiber were connected by a 2-m optical fiber (200 μm O.D., 0.37 NA). The laser power was adjusted at the tip of the optical fiber to 10 to 20 μW to decrease laser bleaching, and the analog voltage signals were digitalized at 100 Hz and recorded by the fiber photometry system. The recording of the responses of GCaMP to the stimuli in awake mice was performed using fluorescence values obtained before the stimuli and during the assessment by CRD, von Frey, pinch, and brush (-2-0 s). They were also obtained after the stimuli (0-10 s). For the von Frey used for the photometry experiments, the force of the von Frey fiber to induce nocifensive behavior (eg, paw withdrawal) was 1.0 g, and 9 trials of repeated von Frey stimuli were applied to the mouse during calcium signal recording. For the pinch used in the photometry experiments, an alligator clip was applied to the tail to induce noxious pain behavior (eg, tail withdrawal), and 9 trials of repeated tail pinch stimuli were applied to the mouse during calcium signal recording. For the brush used in the photometry experiments, a soft brush gently stroked the mouse's tail to produce a noninvasive behavior, and 9 trials of repeated tail brush stimuli were applied to the mouse during calcium signal recording. The data were segmented based on the behavioral events within the individual trials. ΔF/F of the 2-s before stimulation was obtained as the baseline. The photometry data were analyzed with custom-written MATLAB codes (MATLAB R2017b, MathWorks).
2.9. Data analyses
All data are presented as mean ± SEM. The error bars in the figures indicate SEM. Statistical analysis was performed using GraphPad Prism 8 and Origin 8 with appropriate inferential methods. Normality was initially assessed for all data before analysis. Significance was determined using the Mann–Whitney test, the two-sample t test, and the one-way analysis of variance (ANOVA) followed by the Tukey test for multiple comparisons. A two-way ANOVA was also used and was followed by Bonferroni test for multiple comparisons. P < 0.05 was considered to indicate statistically significant differences.
3. Results
3.1. c-fos expression in the lateral septal ventral region is significantly increased by colorectal distention stimulation but not by von Frey fiber stimulation of the hindpaw of neonatal maternal deprivation mice
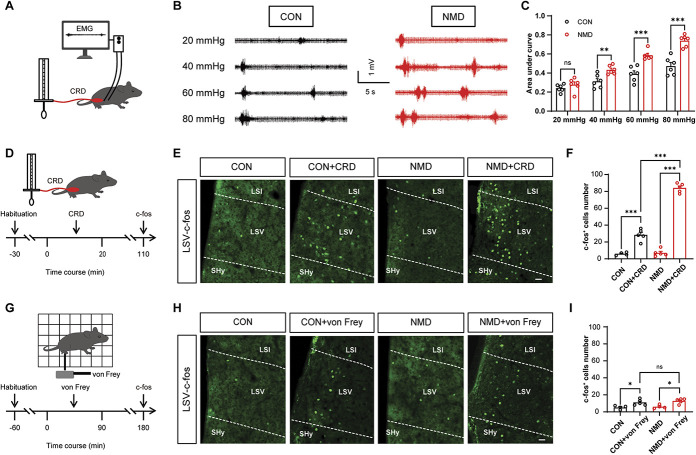
The pups following NMD were subjected to CRD combined with EMG. The records confirmed that visceral pain was successfully induced in adult animals (6 weeks). The schematic diagram of CRD combined with EMG recording is shown in Figure 1A. Figure 1B indicates the representative EMG traces of CON and NMD mice at a CRD pressure of 20, 40, 60, and 80 mm Hg, respectively. The area under the curve (AUC) of the EMG in NMD mice did not change at 20 mm Hg compared with that of the CON mice, whereas it was significantly increased at 40, 60, and 80 mm Hg, respectively. (Fig. 1C, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test). To evaluate the effects of visceral pain on the brain, CRD stimulation was performed. The steps of CRD stimulation are shown in Figure 1D. As indicated, the c-fos expression increased sharply after repeated CRD stimuli and especially in LSV. In addition, it was also found that the c-fos expression of LSV in NMD mice was increased more obviously than that of CON mice (Figs. 1E and F, ***P < 0.001, one-way ANOVA followed by Tukey post hoc).
Figure 1.
The expression levels of c-fos in the LSV region are significantly increased after CRD stimulation. (A) Schematic of EMG recording for evaluating visceral pain in mice. (B) Representative traces of EMG records. (C) The area under the curve of EMG at 20, 40, 60, and 80 mm Hg in CON and NMD mice (F (3, 15) = 83.68, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (D) Schematic of c-fos expression evoked by visceral stimulation. (E) Representative images of c-fos–expressed neurons in the LSV region after CRD stimulation. Scale bar, 50 μm. (F) Total number of c-fos–expressed neurons of LSV after CRD stimulation (F (3, 15) = 250.6, ***P < 0.001, one-way ANOVA followed by Tukey post hoc test, n = 4 brain sections from 3 mice for CON, n = 5 brain sections from 3 mice for CON + CRD, NMD and NMD + CRD). (G) Schematic of c-fos expression evoked by mechanical stimulation. (H) Representative images of c-fos–expressed neurons in LSV after von Frey fiber stimulation. Scale bar, 50 μm. (I) Total number of c-fos–expressed neurons of the LSV region after von Frey fiber stimulation (F (3, 14) =8.093, *P < 0.05, one-way ANOVA followed by Tukey post hoc test, n = 4 brain sections from 3 mice of the CON and NMD groups, n = 5 brain sections from 3 mice of the CON + von Frey and NMD + von Frey groups). n.s. indicates nonsignificant differences, P > 0.05. ANOVA, analysis of variance; CRD, colorectal distention; EMG, electromyography; LSV, lateral septal ventral; NMD, neonatal maternal deprivation.
To further assess the specificity of LSV in visceral hyperalgesia, the von Frey–induced c-fos test was performed, and the procedure is shown in Figure 1G. After repeated von Frey filament stimulation, a negligible amount of c-fos expression was noted in the LSV region of CON and NMD mice, whereas no significant difference was noted between the CON and NMD groups (Figs. 1H and I, *P < 0.05, one-way ANOVA followed by Tukey post hoc). This suggested that noxious somatic stimulation may slightly but not specifically activate LSV. It is noteworthy that c-fos expression of LSV was dramatically increased by CRD stimulation compared with that noted after von Frey filament stimulation. These data suggest that LSV was primarily activated by visceral rather than somatic nociceptive stimulation.
3.2. Visceral rather than somatic stimulation significantly enhances calcium activity of lateral septal ventral neurons
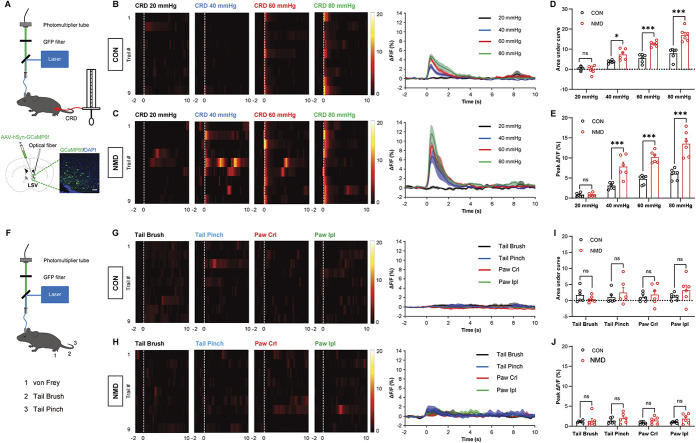
To quantify the physiological activity of LSV neurons during visceral and somatic stimulation, fiber photometry was used to record the in vivo physiological calcium signal changes from these neurons in awake behaving mice (Fig. 2A, top). Wild-type mice were stereotactically injected with AAV2/9-hSyn-GCaMP6f. The injection was performed into their LSV neurons (Fig. 2A, bottom). The area covered by transduced cells was 0.25 to 0.30 mm2. GCaMP6f recording of LSV neurons during CRD, pinch, brush, and von Frey fibers indicated sharp or slightly dynamic fluctuations. However, these dynamics were absent and unaffected by movement and laser lighting originating from the neuronal expression of GFP, indicating that they were calcium-dependent GCaMP6f signals (Fig. S1A-S1D, available at http://links.lww.com/PAIN/B695). A harmful CRD stimulation caused visceral pain and induced a sharp increase in Ca2+ activity in the LSV region of both CON and NMD mice. However, the Ca2+ activity of NMD mice was significantly higher than that of CON mice (Figs. 2B–E,*P < 0.05, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test).
Figure 2.
CRD stimulation induces a sharp increase in calcium signals in LSV neurons. (A) Schematic of the recording system for evaluating calcium signals with fiber photometry and visceral pain behavior evoked by CRD stimuli (top) and representative image of GCaMP6f expression in LSV (bottom). Scale bar, 50 μm. (B and C) Heatmap and average Ca2+ transients of LSV neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively. (D) The area under the curve of calcium activity of LSV neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively (F (3, 15) = 82.26, *P < 0.05, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). € Averaged peak ΔF/F of calcium activity of LSV neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively (F (3, 15) = 113.8, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (F) Schematic of the recording system for evaluating calcium signals with fiber photometry and somatic stimulation evoked by von Frey fiber, tail pinch, and tail brush. (G and H) Heatmap and average Ca2+ transients of LSV neurons in CON and NMD mice receiving tail brush, tail pinch, and von Frey filament (Crl and Ipl) stimulation, respectively. (I) The area under the curve of calcium activity of LSV neurons in CON and NMD mice receiving tail brush, tail pinch, and von Frey filament (Crl and Ipl) stimulation, respectively (F (3, 20) = 0.5363, P > 0.05, two-way ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (J) Averaged peak ΔF/F of calcium activity of LSV neurons in CON and NMD mice receiving tail brush, tail pinch, and von Frey filament (Crl and Ipl) stimulation, respectively (F (3, 20) = 0.7553, P > 0.05, two-way ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). Crl, contralateral; Ipl, ipsilateral. n.s. indicates nonsignificant differences, P > 0.05. See also Figure S1, available at http://links.lww.com/PAIN/B695. ANOVA, analysis of variance; CRD, colorectal distention; LSV, lateral septal ventral; NMD, neonatal maternal deprivation.
To further assess the specificity of the LSV neuronal response to visceral stimulation, somatic stimulation was performed (Fig. 2F). The results indicated that pinch, brush, and von Frey fibers did not cause significant fluctuation of Ca2+ signals in LSV neurons (Figs. 2G–J), suggesting that LSV neurons were insensitive to somatic stimulation. These results suggest that LSV is mainly activated by CRD-evoked visceral pain rather than somatic stimulation, which is consistent with our previous results of c-fos expression.
3.3. Visceral pain mainly activates lateral septal ventral CaMKIIα-positive neurons
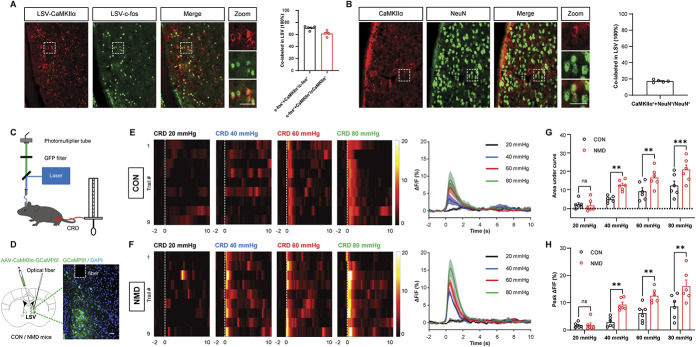
To verify the types of LSV neurons activated by visceral stimulation, immunofluorescence was performed. Immunostaining showed that c-fos–positive cells in the LSV region were mainly co-expressed with the Ca2+/calmodulin–dependent protein kinase Iiα (CaMKIIα). The ratio of CaMKIIα + c-fos to c-fos was 70.73%, and the ratio of CaMKIIα + c-fos to CaMKIIα was 61.29% in LSV brain region (Fig. 3A). In addition, we performed immunostaining to determine the percentage of CaMKIIα-positive neurons in the LSV. We showed that the ratio of CaMKIIα + NeuN to NeuN was 17.24% (Fig. 3B), suggesting that the number of CaMKIIα-positive neurons in LSV may not be a predominant population. We also examined the colocalization of GABA with c-fos in LSV. The ratio of GABA+c-fos to c-fos was 22.61% (Fig. S2A, available at http://links.lww.com/PAIN/B695), suggesting that CaMKIIα-positive neurons were the main type of neurons that were activated by CRD stimulation in NMD mice.
Figure 3.
LSV CaMKIIα-positive neurons are activated in visceral pain behaviors. (A) Representative images of c-fos (green) and CaMKIIα (red) coexpression in LSV (left) and coexpression ratios (right). Scale bar, 50 μm. (B) Representative images of CaMKIIα (red) and NeuN (green) coexpression in LSV (left) and coexpression ratio (right). Scale bar, 50 μm. (C) Schematic of a recording system for evaluating calcium signals with fiber photometry and visceral pain behavior caused by CRD stimuli. (D) Representative image of GCaMP6f expression in LSV CaMKIIα-positive neurons. Scale bar, 50 μm. (E and F) Heatmap and average Ca2+ transients of LSV CaMKIIα-positive neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively. (G) The area under the curve of calcium activity of LSV CaMKIIα-positive neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively (F (3, 15) = 40.44, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (H) Averaged peak ΔF/F of calcium activity of LSV CaMKIIα-positive neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively (F (3, 15) = 51.93, **P < 0.01, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). White dotted frame indicates the optical fiber trace. n.s. indicates nonsignificant differences, P > 0.05. See also Figure S2, available at http://links.lww.com/PAIN/B695. ANOVA, analysis of variance; CRD, colorectal distention; LSV, lateral septal ventral; NMD, neonatal maternal deprivation.
To further characterize the types of neurons activated in the LSV region after visceral stimulation, fiber photometry was used to record the in vivo Ca2+ signaling changes (Figs. 3C and S2B, available at http://links.lww.com/PAIN/B695). AAV2/9-CaMKIIα-GCaMP6f and AAV2/9-VGAT1-GCaMP6m were injected into the LSV to infect CaMKIIα-positive neurons and GABAergic neurons, respectively (Figs. 3D and S2C, available at http://links.lww.com/PAIN/B695). The data indicated that CRD induced a sharp increase in Ca2+ activity in LSV CaMKIIα-positive neurons in CON and NMD mice, whereas Ca2+ activity in NMD mice stimulated by the same level of CRD was significantly higher than that of CON mice (Figs. 3E–H, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test). Although a small percentage of co-expression was noted between c-fos–positive cells and GABAergic neurons, CRD stimulation did not significantly increase Ca2+ activity in GABAergic neurons (Fig. S2D-S2G, available at http://links.lww.com/PAIN/B695). These data suggested that CaMKIIα-positive neurons in LSV were activated by visceral pain.
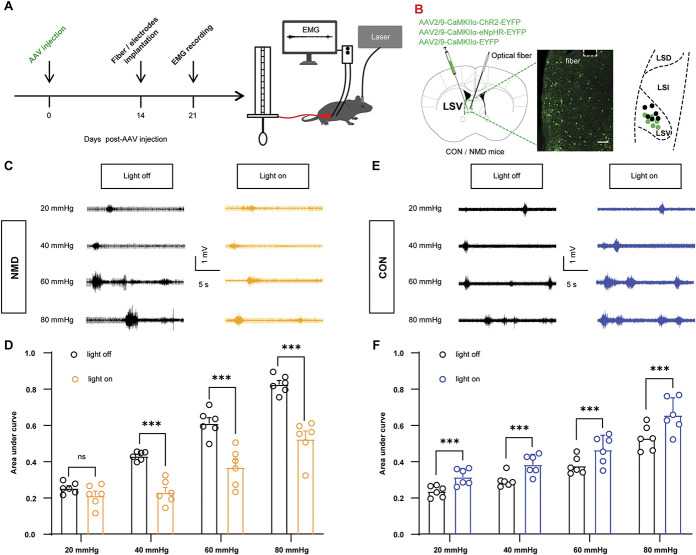
3.4. Optosilencing of lateral septal ventral CaMKIIα-positive neurons attenuates colorectal distention–evoked visceral pain in neonatal maternal deprivation mice, whereas optoactivation of lateral septal ventral CaMKIIα-positive neurons promotes colorectal distention-evoked visceral pain behaviors in normal animals
To further characterize the precise role of LSV CaMKIIα-positive neurons in visceral pain behaviors, we performed optogenetic manipulations of these neurons. Visceral pain was evaluated by CRD combined with EMG records (Fig. 4A). AAV2/9-CaMKIIα-ChR2-EYFP, AAV2/9-CaMKIIα-NpHR-EYFP, and AAV2/9-CaMKIIα-EYFP were injected into the LSV region to infect CaMKIIα-positive neurons (Fig. 4B). The area covered by transduced cells was 0.25 to 0.30 mm2. NpHR-dependent and ChR2-dependent optogenetic manipulations were performed. Optosilencing of LSV CaMKIIα-positive neurons attenuated CRD-evoked visceral pain responses in NMD mice (Figs. 4C and D, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test), whereas optoactivation of LSV CaMKIIα-positive neurons promoted adaptive CRD-evoked visceral pain responses in CON mice (Figs. 4E and F, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test). However, neither optosilencing nor optoactivation of control virus–infected CaMKIIα-positive neurons could alter CRD-evoked visceral pain responses in NMD (Fig. S3A and S3B, available at http://links.lww.com/PAIN/B695) or CON mice (Fig. S3C and S3D, available at http://links.lww.com/PAIN/B695). Therefore, these results suggest that LSV CaMKIIα-positive neurons can modulate visceral pain behaviors.
Figure 4.
Optosilencing of LSV CaMKIIα-positive neurons attenuates visceral hyperalgesia, whereas optoactivation of LSV CaMKIIα-positive neurons promotes adaptive visceral pain behavior. (A) Experimental procedure of optogenetic manipulation of LSV CaMKIIα-positive neurons (left) and schematic of EMG recording for evaluating visceral pain (right). (B) Representative image of viral expression in LSV CaMKIIα-positive neurons (left) and summary view of fiber tip and injection site (right). Black dots and green dots indicate the fiber tip and injection site, respectively. Scale bar, 200 μm. (C) The representative EMG traces of NMD mice were recorded at 20, 40, 60, and 80 mm Hg, respectively. (D) The area under the curve of the EMG at 20, 40, 60, and 80 mm Hg in NMD mice (F (3, 15) = 121.3, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (E) Representative EMG traces of CON mice were recorded at 20, 40, 60, and 80 mm Hg, respectively. (F) The area under the curve of EMG at 20, 40, 60, and 80 mm Hg in CON mice (F (3, 15) = 117.0, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). White dotted frame indicates the optical fiber trace. n.s. indicates nonsignificant differences, P > 0.05. See also Figure S3, available at http://links.lww.com/PAIN/B695. ANOVA, analysis of variance; CRD, colorectal distention; EMG, electromyography; LSV, lateral septal ventral; NMD, neonatal maternal deprivation.
3.5. Paraventricular hypothalamic CaMKIIα-positive neurons project to the lateral septal ventral region, and c-fos expression is significantly increased in the paraventricular hypothalamic region after colorectal distention stimulation
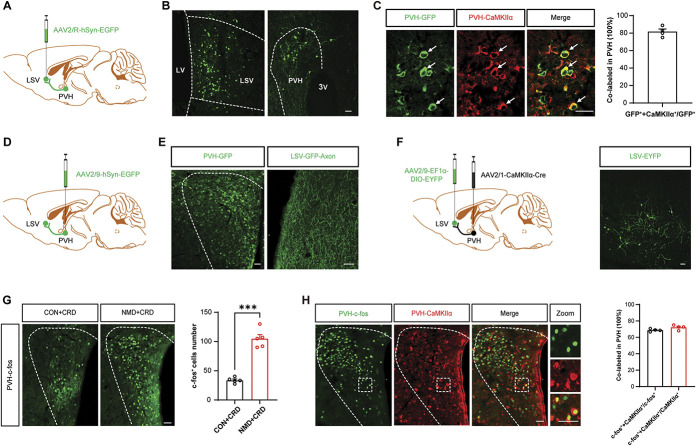
Our previous data suggest that LSV CaMKIIα-positive neurons can regulate visceral pain behaviors. To investigate the functional changes of LSV CaMKIIα-positive neurons in NMD mice, circuit tracing was conducted. Stereotactic injection of a retrograde tracer virus (AAV2/R-hSyn-EGFP) was performed into LSV (Fig. 5A) region, and the apparent GFP signal was observed in the PVH region (Fig. 5B). The area covered by transduced cells was 0.20 to 0.25 mm2. In the retrograde tracer experiments, we also showed that GFP label signals were expressed in prefrontal cortex, anterior cingulate cortex, and hippocampus, which was consistent with a previous study.7 Immunofluorescence staining further revealed that the GFP signal in the PVH region was mainly co-expressed with CaMKIIα (Fig. 5C, left), and the colocalization ratio of CaMKIIα with GFP was 81.8% (Fig. 5C, right), which indicated that PVH exhibited anterograde projection of CaMKIIα-positive neurons to the LSV region. To verify that the PVH-LSV pathway was involved in this process, an anterograde tracer virus (AAV2/9-hSyn-EGFP) was injected into the PVH region (Fig. 5D), and a large number of axon terminals were observed in the LSV (Fig. 5E), which demonstrated that the PVH region exhibited anterograde projection to the LSV region. In anatomic tracer experiments, the projections were seen bilaterally, but ipsilateral projections seem to be more frequent.
Figure 5.
Dissection of the PVH-LSV circuit. (A) Schematic of the retrograde virus tracing strategy. (B) Typical images of viral expression within the LSV and PVH. Scale bars, 50 µm. (C) Representative images of GFP (green, viral expression) and CaMKIIα (red) coexpression (left) and coexpression ratio in PVH (right). Scale bars, 50 µm. (D) Schematic of the anterograde viral tracing strategy. (E) Typical images of viral expression within the PVH and LSV. Scale bars, 50 µm (left) and 20 µm (right). (F) Schematic of monosynaptic anterograde tracing (left) and representative images of viral expression in LSV. Scale bars, 50 µm. (G) Representative images of c-fos–expressed neurons of PVH after CRD stimulation in CON and NMD mice (left) and the total number of c-fos–expressed neurons (right) (***P < 0.001, unpaired t test, n = 5 brain sections from 3 mice per group). (H) Representative images of c-fos (green) and CaMKIIα (red) coexpression in PVH (left) and coexpression ratios (right, n = 4 brain sections from 3 mice). Scale bars, 50 µm. ANOVA, analysis of variance; CRD, colorectal distention; LSV, lateral septal ventral; NMD, neonatal maternal deprivation; PVH, paraventricular hypothalamic.
To further explore the cell types of PVH anterograde projection to LSV, a dual-virus model that selectively expressed EYFP was used in a projection-specific manner. A monosynaptic anterograde virus (AAV2/1-CaMKIIα-Cre) was injected into the PVH region, and a locally expressed virus (AAV2/9-EF1α-DIO-EYFP) was injected into the LSV region (Fig. 5F, left). A large number of EYFP signatures were noted in the LSV region, which further supported the notion that PVH exhibited an anterograde projection to LSV CaMKIIα-positive neurons (Fig. 5F, right). These results suggest that the CaMKIIα-positive neurons of the PVH region may possess a potential regulatory role on the LSV CaMKIIα-positive neurons. Therefore, we evaluated c-fos expression in the PVH region after CRD stimulation. Consistent with the results of the LSV experiments, the data indicated that c-fos expression in the PVH region was sharply increased after CRD stimulation (Fig. 5G, left). It is noteworthy that the expression levels of c-fos in NMD mice were significantly higher than those in the CON mice (Fig. 5G, right, ***P < 0.001, two-sample t test). Additional immunofluorescence staining indicated that c-fos was mainly co-expressed with CaMKIIα. The ratio of CaMKIIα + c-fos to c-fos was 68.74%, and the ratio of CaMKIIα + c-fos to CaMKIIα was 72.08% in PVH brain region (Fig. 5H). These data suggested that the CaMKIIα-positive neurons of the PVH region exhibited an anterograde projection to the LSV CaMKIIα-positive neurons, whereas the CRD stimulation could also activate the CaMKIIα-positive neurons of the PVH region.
3.6. Paraventricular hypothalamic CaMKIIα-positive neurons modulate visceral pain behaviors
To further characterize the activation of the CaMKIIα-positive neurons of the PVH region during CRD-evoked visceral pain responses, fiber photometry was used to record the in vivo Ca2+ signaling changes (Fig. 6A, top). AAV2/9-CaMKIIα-GCaMP6f was stereotactically injected into the PVH region to infect CaMKIIα-positive neurons (Fig. 6A, bottom). The area covered by transduced cells was 0.25∼0.30 mm2. It was found that CRD induced a significant Ca2+ elevation in both CON and NMD mice, whereas the Ca2+ activity in the NMD mice stimulated by the same level of CRD was significantly higher than that of the CON mice (Figs. 6B–E,*P < 0.05, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test). These data suggested that the CaMKIIα-positive neurons in the PVH region were activated by visceral stimulation.
Figure 6.
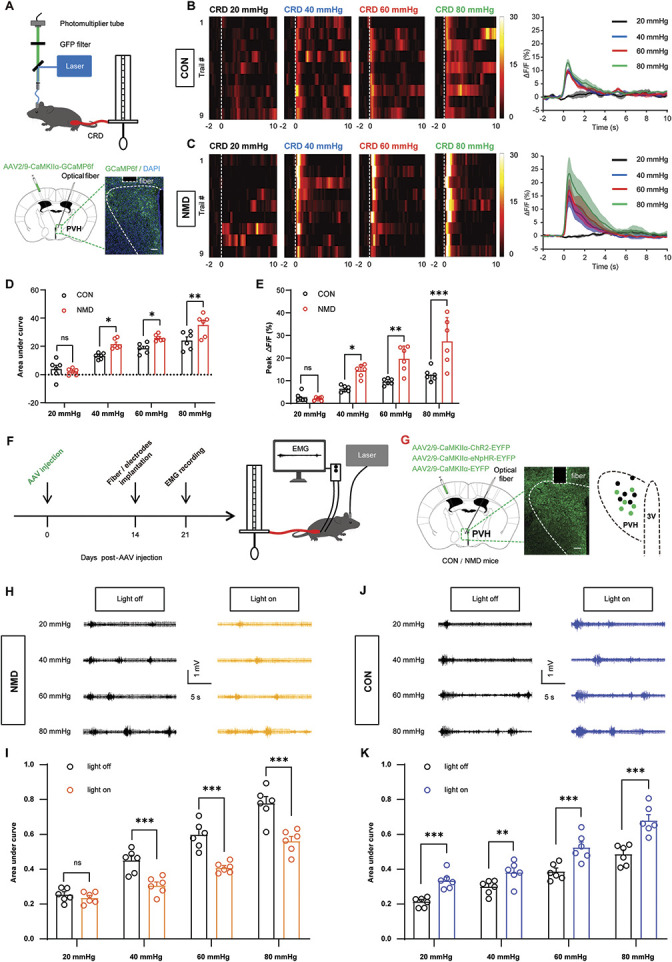
PVH CaMKIIα-positive neurons are involved in regulating visceral hyperalgesia. (A) Schematic of the recording system for evaluating calcium signals with fiber photometry and visceral pain behavior caused by CRD stimuli (top) and representative image of GCaMP6f expression in PVH CaMKIIα-positive neurons (bottom). Scale bar, 50 μm. (B and C) Heatmap and average Ca2+ transients of PVH CaMKIIα-positive neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively. (D) The area under the curve of calcium activity of PVH CaMKIIα-positive neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively (F (3, 15) = 62.90, *P < 0.05, **P < 0.01, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (E) Averaged peak ΔF/F of calcium activity of PVH CaMKIIα-positive neurons in CON and NMD mice receiving CRD stimulation at 20, 40, 60, and 80 mm Hg, respectively (F (3, 15) = 72.94, *P < 0.05, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (F) Experimental procedure of optogenetic manipulation of PVH CaMKIIα-positive neurons (left) and schematic of EMG recording for evaluating visceral pain (right). (G) Representative image of viral expression in PVH CaMKIIα-positive neurons (left) and summary view of fiber tip and injection site (right). Black dots and green dots indicate the fiber tip and injection site, respectively. Scale bar, 50 μm. (H) Representative EMG traces of NMD mice were recorded at 20, 40, 60, and 80 mm Hg, respectively. (I) The area under the curve of EMG at 20, 40, 60, and 80 mm Hg in NMD mice (F (3, 15) = 57.67, ***P < 0.001, two-way repeated measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). (J) Representative EMG traces of CON mice were recorded at 20, 40, 60, and 80 mm Hg, respectively. (K) The area under the curve of EMG at 20, 40, 60, and 80 mm Hg in CON mice (F (3, 15) = 43.84, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). White dotted frame indicates the optical fiber trace. n.s. indicates nonsignificant differences, P > 0.05. See also Figure S4, available at http://links.lww.com/PAIN/B695. ANOVA, analysis of variance; CRD, colorectal distention; LSV, lateral septal ventral; NMD, neonatal maternal deprivation; PVH, paraventricular hypothalamic.
To further evaluate the role of the PVH CaMKIIα-positive neurons in visceral pain behaviors, optogenetic manipulation was performed in both CON and NMD mice. Visceral pain was assessed by CRD combined with EMG recording (Fig. 6F). AAV2/9-CaMKIIα-ChR2-EYFP, AAV2/9-CaMKIIα-NpHR- EYFP, and AAV2/9-CaMKIIα-EYFP were injected into the PVH region to infect the CaMKIIα-positive neurons (Fig. 6G). We performed NpHR-dependent and ChR2-dependent optogenetic manipulations in PVH CaMKIIα-positive neurons. The data demonstrated that optosilencing of PVH CaMKIIα-positive neurons significantly attenuated visceral pain behaviors in NMD mice (Figs. 6H and I, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test), whereas optoactivation of PVH CaMKIIα-positive neurons contributed to adaptive visceral pain behaviors in CON mice (Figs. 6J and K, **P < 0.01, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test). However, the same optogenetic manipulation of control virus–infected CaMKIIα-positive neurons did not induce changes in the CRD-evoked visceral pain responses of NMD (Fig. S4A and S4B, available at http://links.lww.com/PAIN/B695) or CON mice (Fig. S4C and S4D, available at http://links.lww.com/PAIN/B695). These data suggest that PVH CaMKIIα-positive neurons can regulate visceral pain behaviors.
3.7. Inhibition of the paraventricular hypothalamic–lateral septal ventral pathway attenuates colorectal distention–evoked visceral pain responses, whereas activation of the paraventricular hypothalamic–lateral septal ventral pathway contributes to adaptive colorectal distention–evoked visceral pain responses
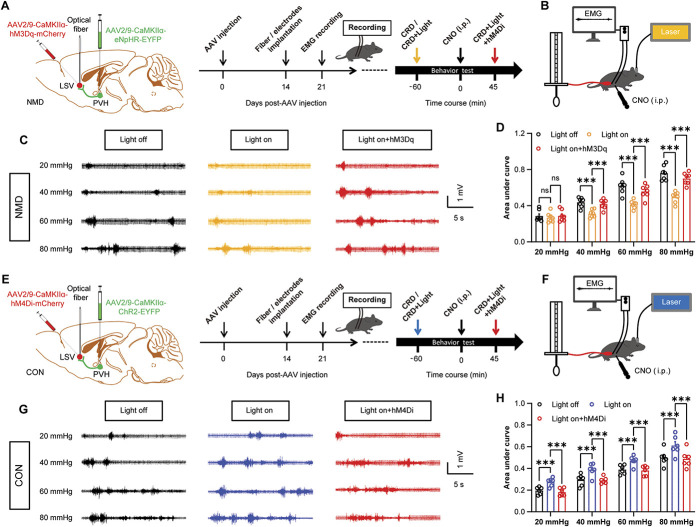
Our previous results suggested that CaMKIIα-positive neurons in the LSV and PVH regions modulated visceral pain behaviors and that PVH exerted an anterograde projection of CaMKIIα-positive neurons to the LSV region. However, the role of the PVH-LSV pathway in visceral pain remains unknown. To further investigate the role of the PVH–LSV pathway in visceral pain behaviors, a dual-virus strategy was used that aimed to specifically regulate the activity of CaMKIIα-positive neurons in the PVH–LSV pathway by combining optogenetic with chemogenetic factors. AAV2/9-CaMKIIα-NpHR-EYFP and AAV2/9-CaMKIIα-hM3Dq-mCherry were injected into the PVH and LSV regions in NMD mice, respectively (Fig. 7A). The time course and evaluation methods of the visceral pain behavior are shown in Figure 7B. Optosilencing of the PVH CaMKIIα-positive neurons axon terminals that project to LSV significantly alleviated visceral pain in NMD mice. However, this effect was blocked by CNO-activation of the LSV CaMKIIα-positive neurons (Figs. 7C and D, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test). This result suggests that activation of the PVH-LSV pathway is necessary for visceral pain behaviors. Furthermore, AAV2/9-CaMKIIα-ChR2-EYFP and AAV2/9-CaMKIIα-hM4Di-mCherry were injected into the PVH and LSV regions in CON mice, respectively (Fig. 7E). The time process and detection methods of the visceral pain behavior are shown in Figure 7F. The results indicated that optoactivation of the PVH CaMKIIα-positive neurons axon terminals that project to LSV promoted adaptive visceral pain behaviors in CON mice, whereas this effect was eliminated by CNO-inhibition of LSV CaMKIIα-positive neurons (Figs. 7G and H, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test). This result further suggested that the activation of the PVH–LSV pathway was sufficient to promote visceral pain behaviors. These data indicated that the PVH–LSV pathway was sufficient and necessary to modulate visceral pain behaviors.
Figure 7.
Inhibition of PVH–LSV pathway reduces visceral hyperalgesia, whereas activation of PVH–LSV pathway contributes to adaptive visceral pain behavior. (A) Experimental procedure of a dual-virus strategy that specifically regulates the activity of CaMKIIα-positive neurons of the PVH–LSV pathway in NMD mice by combining optogenetic with chemogenetic (left) and schematic of experimental timelines for optogenetic and chemogenetic manipulation (right). (B) Schematic EMG recording for evaluating visceral pain under optogenetic and chemogenetic manipulation. (C) Representative EMG traces of NMD mice were recorded at 20, 40, 60, and 80 mm Hg, respectively. (D) The area under the curve of EMG at 20, 40, 60, and 80 mm Hg in NMD mice (F (3, 18) = 86.90, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 7 mice per group). (E) Experimental procedure of a dual-virus strategy that specifically regulates the activity of CaMKIIα-positive neurons of the PVH–LSV pathway in CON mice by combining optogenetic with chemogenetic (left) and schematic of experimental timelines for optogenetic and chemogenetic manipulation (right). (F) Schematic EMG recording for evaluating visceral pain after optogenetic and chemogenetic manipulation. (G) The representative EMG traces of CON mice were recorded at 20, 40, 60, and 80 mm Hg, respectively. (H) The area under the curve of EMG at 20, 40, 60, and 80 mm Hg in CON mice (F (3, 15) = 101.1, ***P < 0.001, two-way repeated-measure ANOVA followed by Bonferroni post hoc test, n = 6 mice per group). n.s. indicates nonsignificant differences. P > 0.05. ANOVA, analysis of variance; CRD, colorectal distention; LSV, lateral septal ventral; NMD, neonatal maternal deprivation; PVH, paraventricular hypothalamic.
4. Discussion
The identification of the central mechanisms underlying chronic visceral pain has been a major challenge and key step forward for the treatment of chronic visceral pain in patients with IBS. In this study, it was shown for the first time that LSV CaMKIIα-positive neurons and the PVH–LSV CaMKIIα-positive circuit specifically controlled visceral pain in a mouse model of IBS. Using both gain-of-function and loss-of-function approaches, evidence is provided to confirm that the PVH–LSV circuit is necessary and sufficient for visceral pain control. In particular, activation of LSV CaMKIIα-positive neurons and the PVH–LSV circuit induces visceral pain, whereas th inhibition of the circuit suppresses visceral analgesia. These data suggest that manipulation of the PVH–LSV circuit exerts a critical effect on visceral pain behaviors, whereas the LSV region may be a key factor in regulating the visceral pain process.
4.1. Lateral septal ventral is a distinct center for visceral pain but not for the somatic pain process
Although no significant difference was noted in the expression levels of c-fos in the LSV region between NMD and control mice, CRD stimulation induced a dramatic increase in c-fos expression in the LSV region of NMD mice compared with that noted in the control mice (Figs. 1D–F), suggesting that LSV could be activated by visceral pain stimulation. This conclusion was further confirmed by in vivo calcium imaging indicating that CRD stimulation significantly enhanced LSV neural activation compared with that of the control mice (Figs. 2A–E). It is worth noting that von Frey fiber stimulation failed to induce a great amount of c-fos expression in the LSV region (Figs. 1G–I) and did not produce the fluctuation of calcium signals in NMD mice (Figs. 2F–J). These data strongly suggested that the LSV region served as 1 of the key elements in regulating visceral hyperalgesia, which provides the possibility of a precise therapeutic strategy for the treatment of visceral pain in the brain.
4.2. CaMKIIα-positive neurons in the lateral septal ventral region mediate visceral pain
To identify which types of neurons of the LSV region are involved in the visceral pain process, multiple techniques, including optogenetics and viral tracing, were used. In this study, we demonstrated for the first time that LSV CaMKIIα-positive neurons could specifically regulate visceral pain and play an important role in its development because most c-fos–positive neurons were CaMKIIα-positive neurons. More importantly, this finding suggests that CaMKIIα-positive neurons that are present in low percentages in the LSV region play a major role in mediating visceral pain, demonstrating an endogenous antinociceptive role in visceral pain. The endogenous antinociceptive brain regions in visceral pain need to be further investigated. This study provided sufficient evidence indicating that LSV CaMKIIα-positive neurons were specifically activated by CRD stimulation. LSV CaMKIIα-positive neurons play a role in promoting visceral hyperalgesia, which can be caused by excessive excitation. CaMKIIα-positive neurons of the LSV region serve as key elements in regulating visceral hyperalgesia. Local regulation of the excitability of CaMKIIα-positive neurons of the LSV region suppressed visceral hyperalgesia, which provided the possibility of precise drug therapy in the brain. The neurotransmitter properties of CaMKIIα-positive neurons in LSV brain region may be complex, whether they are excitatory, inhibitory, or a mix of both is unknown, which will be further investigated in future studies.
4.3. A paraventricular hypothalamic CaMKIIα-positive projection to lateral septal ventral CaMKIIα-positive neurons regulates visceral pain
One of the very important findings of this study is the identification of the specific neural circuit PVH–LSV, which mediates visceral pain. Using a dual-virus strategy, a neural connection between the PVH and LSV regions was found. This is in agreement with the data reported in a previous report indicating that CaMKIIα-positive neurons in the PVH region directly project to the LSV region. This connection is considered to be very important for sleeping and feeding.2,42 However, the neuronal cell types present in the LSV region were not specified regarding the CaMKIIα+ projection from the PVH region. In this study, we provided evidence to show a CaMKIIα+–CaMKIIα+ connection between these 2 brain regions. The role of this pathway in the visceral pain process was subsequently investigated. Our analysis further demonstrated that this pathway was necessary and sufficient for the visceral pain process. This conclusion was based on the following observations: First, CaMKIIα-positive neurons of the PVH region were activated by CRD stimulation and were shown to play a role in regulating visceral pain (Figs. 5G and H, Figs. 6A–K). Second, activation of the PVH–LSV CaMKIIα+ pathway produced visceral hyperalgesia, whereas inhibition of the PVH–LSV CaMKIIα+ pathway suppressed visceral hyperalgesia. Third, the regulatory effect of the PVH CaMKIIα-positive neuron activities on visceral pain was almost completely blocked by the activities of the LSV CaMKIIα-positive neurons (Figs. 7A–H). However, additional studies are required to investigate the molecular mechanism of the PVH–LSV pathway. It is also worthy to study the effect of photogenetic inhibition of CON mice on visceral pain behaviors because it will help to define the endogenous function of these circuits at baseline or NMD states.
Glutamatergic neurons are the major components of the PVH, whereas GABAergic neurons are more scarcely represented.38,42,43,49 Previous studies have shown that PVH glutamatergic neurons coexpress corticotropin-releasing hormone (CRH)44,46 and oxytocin (OT).16,23 CRH neurons in the PVH region coordinate stress-related behavior and respond to stress in a behavioral manner.6,25,30 This plays an important role in the regulation of somatic pain.13 In addition, OT neurons also play an important role in the regulation of pain.1,26 It is therefore reasonable to speculate that CRH or OT released from PVH glutamatergic neurons may be an important molecular mechanism underlying visceral pain. It is noteworthy that the experiments in this study were only performed in male mice and that the results from male mice may not be fully applicable to female mice. Previous studies have shown that estrogen has an important effect on visceral function and pain.3,19,36 In addition, the menstrual cycle of female mice may affect brain function. Therefore, only male mice were used in this study to avoid the effects of estrogen on visceral pain. Using male mice alone may prevent estrogen from interfering with the results, thus giving more accurate results.
In conclusion, the results of this study demonstrated the critical role of the CaMKIIα-positive neuronal connection between the PVH–LSV regions in visceral hyperalgesia. Targeting this unique pathway may provide a potential and powerful approach for the clinical treatment of visceral pain in patients with IBS.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B695.
Supplemental video content
A video abstract associated with this article can be found at http://links.lww.com/PAIN/B696.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (81920108016 and 31730040), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_2966), and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Care and handling of the animals were approved by the Institutional Animal Care and Use Committee of Soochow University and were in accordance with the guidelines of the International Association for the Study of Pain.
Author contributions: Y.-C. Li performed experiments, analyzed data, and prepared figures and the manuscript. Q. Wang analyzed data and edited the manuscript. M.-G. Li and S.-F. Hu performed experiments and analyzed data. G.-Y. Xu designed experiments, supervised the experiments, and finalized the manuscript. All the authors have read and approved the paper.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Y.-C. Li and Q. Wang contributed equally to this work.
Contributor Information
Yong-Chang Li, Email: ycli123@stu.suda.edu.cn.
Qian Wang, Email: q_wang0302@suda.edu.cn.
Meng-Ge Li, Email: 20204250142@stu.suda.edu.cn.
Shu-Fen Hu, Email: sfhu@suda.edu.cn.
References
- [1].Bharadwaj VN, Tzabazis AZ, Klukinov M, Manering NA, Yeomans DC. Intranasal administration for pain: oxytocin and other polypeptides. Pharmaceutics 2021;13:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen CR, Zhong YH, Jiang S, Xu W, Xiao L, Wang Z, Qu WM, Huang ZL. Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice. eLife 2021;10e69909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen JH, Sun Y, Ju PJ, Wei JB, Li QJ, Winston JH. Estrogen augmented visceral pain and colonic neuron modulation in a double-hit model of prenatal and adult stress. World J Gastroenterol 2021;27:5060–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen YH, Wu JL, Hu NY, Zhuang JP, Li WP, Zhang SR, Li XW, Yang JM, Gao TM. Distinct projections from the infralimbic cortex exert opposing effects in modulating anxiety and fear. J Clin Invest 2021;131:e145692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol 2008;103:765–74. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dedic N, Kuhne C, Jakovcevski M, Hartmann J, Genewsky AJ, Gomes KS, Anderzhanova E, Pohlmann ML, Chang S, Kolarz A, Vogl AM, Dine J, Metzger MW, Schmid B, Almada RC, Ressler KJ, Wotjak CT, Grinevich V, Chen A, Schmidt MV, Wurst W, Refojo D, Deussing JM. Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nat Neurosci 2018;21:803–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deng K, Yang L, Xie J, Tang H, Wu GS, Luo HR. Whole-brain mapping of projection from mouse lateral septal nucleus. Biol Open 2019;8:bio043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Du WJ, Hu S, Li X, Zhang PA, Jiang X, Yu SP, Xu GY. Neonatal maternal deprivation followed by adult stress enhances adrenergic signaling to advance visceral hypersensitivity. Neurosci Bull 2019;35:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilic-Stojanovic M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fuentes IM, Christianson JA. The influence of early life experience on visceral pain. Front Syst Neurosci 2018;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grad S, Grad C, Baban A, Dumitrascu D. Child abuse in the irritable bowel syndrome. Rom J Intern Med = Revue roumaine de medecine interne 2014;52:183–8. [PubMed] [Google Scholar]
- [12].Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci 2013;16:1185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hagiwara H, Sakimura K, Abe M, Itoi K, Kamiya Y, Akema T, Funabashi T. Sex differences in pain-induced modulation of corticotropin-releasing hormone neurons in the dorsolateral part of the stria terminalis in mice. Brain Res 2021;1773:147688. [DOI] [PubMed] [Google Scholar]
- [14].Halland M, Almazar A, Lee R, Atkinson E, Larson J, Talley NJ, Saito YA. A case-control study of childhood trauma in the development of irritable bowel syndrome. Neurogastroenterology Motil : official J Eur Gastrointest Motil Soc 2014;26:990–8. [DOI] [PubMed] [Google Scholar]
- [15].Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, Schneider M, Klugmann M. Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology. official Publ Am Coll Neuropsychopharmacol 2015;40:1969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He Z, Young L, Ma XM, Guo Q, Wang L, Yang Y, Luo L, Yuan W, Li L, Zhang J, Hou W, Qiao H, Jia R, Tai F. Increased anxiety and decreased sociability induced by paternal deprivation involve the PVN-PrL OTergic pathway. eLife 2019;8:e44026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Holschneider DP, Guo Y, Mayer EA, Wang Z. Early life stress elicits visceral hyperalgesia and functional reorganization of pain circuits in adult rats. Neurobiol Stress 2016;3:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hu S, Sun Q, Du WJ, Song J, Li X, Zhang PA, Xu JT, Xu GY. Adult stress promotes purinergic signaling to induce visceral pain in rats with neonatal maternal deprivation. Neurosci Bull 2020;36:1271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J pain : official J Am Pain Soc 2007;8:494–502. [DOI] [PubMed] [Google Scholar]
- [20].Jiang JX, Liu H, Huang ZZ, Cui Y, Zhang XQ, Zhang XL, Cui Y, Xin WJ. The role of CA3-LS-VTA loop in the formation of conditioned place preference induced by context-associated reward memory for morphine. Addict Biol 2018;23:41–54. [DOI] [PubMed] [Google Scholar]
- [21].Kamphuis JBJ, Guiard B, Leveque M, Olier M, Jouanin I, Yvon S, Tondereau V, Riviere P, Gueraud F, Chevolleau S, Noguer-Meireles MH, Martin JF, Debrauwer L, Eutamene H, Theodorou V. Lactose and fructo-oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology 2020;158:652–63 e656. [DOI] [PubMed] [Google Scholar]
- [22].Khakpai F, Nasehi M, Haeri-Rohani A, Eidi A, Zarrindast MR. Septo-hippocampo-septal loop and memory formation. Basic Clin Neurosci 2013;4:5–23. [PMC free article] [PubMed] [Google Scholar]
- [23].King CE, Griffin WC, Lopez MF, Becker HC. Activation of hypothalamic oxytocin neurons reduces binge-like alcohol drinking through signaling at central oxytocin receptors. Neuropsychopharmacol : official Publ Am Coll Neuropsychopharmacol 2021;46:1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, Azulay-Debby H, Zalayat I, Avishai E, Hajjo H, Schiller M, Haykin H, Korin B, Farfara D, Hakim F, Kobiler O, Rosenblum K, Rolls A. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021;184:5902–15 e5917. [DOI] [PubMed] [Google Scholar]
- [25].Li SB, Borniger JC, Yamaguchi H, Hedou J, Gaudilliere B, de Lecea L. Hypothalamic circuitry underlying stress-induced insomnia and peripheral immunosuppression. Sci Adv 2020;6:eabc2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li XH, Matsuura T, Xue M, Chen QY, Liu RH, Lu JS, Shi W, Fan K, Zhou Z, Miao Z, Yang J, Wei S, Wei F, Chen T, Zhuo M. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep 2021;36:109411. [DOI] [PubMed] [Google Scholar]
- [27].Li YC, Tian YQ, Wu YY, Xu YC, Zhang PA, Sha J, Xu GY. Upregulation of spinal ASIC1 and NKCC1 expression contributes to chronic visceral pain in rats. Front Mol Neurosci 2020;13:611179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Z, Le Q, Lv Y, Chen X, Cui J, Zhou Y, Cheng D, Ma C, Su X, Xiao L, Yang R, Zhang J, Ma L, Liu X. A distinct D1-MSN subpopulation down-regulates dopamine to promote negative emotional state. Cell Res 2022;32:139-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lucarini E, Coppi E, Micheli L, Parisio C, Vona A, Cherchi F, Pugliese AM, Pedata F, Failli P, Palomino S, Wahl J, Largent-Milnes TM, Vanderah TW, Tosh DK, Jacobson KA, Salvemini D, Ghelardini C, Di Cesare Mannelli L. Acute visceral pain relief mediated by A3AR agonists in rats: involvement of N-type voltage-gated calcium channels. PAIN 2020;161:2179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 2015;87:605–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res 2010;214:465–9. [DOI] [PubMed] [Google Scholar]
- [32].Pan S, Yin K, Tang Z, Wang S, Chen Z, Wang Y, Zhu H, Han Y, Liu M, Jiang M, Xu N, Zhang G. Stimulation of hypothalamic oxytocin neurons suppresses colorectal cancer progression in mice. eLife 2021;10:e67535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Raskov H, Burcharth J, Pommergaard HC, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut microbes 2016;7:365–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Singewald GM, Rjabokon A, Singewald N, Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 2011;36:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Song Y, Meng QX, Wu K, Hua R, Song ZJ, Song Y, Qin X, Cao JL, Zhang YM. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology 2020;117:104690. [DOI] [PubMed] [Google Scholar]
- [36].Sun LH, Zhang WX, Xu Q, Wu H, Jiao CC, Chen XZ. Estrogen modulation of visceral pain. J Zhejiang Univ Sci B 2019;20:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tian YQ, Li JH, Li YC, Xu YC, Zhang PA, Wang Q, Li R, Xu GY. Overexpression of GRK6 alleviates chronic visceral hypersensitivity through downregulation of P2Y6 receptors in anterior cingulate cortex of rats with prenatal maternal stress. CNS Neurosci Ther 2022;28:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vong L, Ye C, Yang Z, Choi B, Chua S, Jr., Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011;71:142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang H, Dong P, He C, Feng XY, Huang Y, Yang WW, Gao HJ, Shen XF, Lin S, Cao SX, Lian H, Chen J, Yan M, Li XM. Incerta-thalamic circuit controls nocifensive behavior via cannabinoid type 1 receptors. Neuron 2020;107:538–51 e537. [DOI] [PubMed] [Google Scholar]
- [40].Wang HJ, Xu X, Zhang PA, Li M, Zhou YL, Xu YC, Jiang XH, Xu GY. Epigenetic upregulation of acid-sensing ion channel 1 contributes to gastric hypersensitivity in adult offspring rats with prenatal maternal stress. PAIN 2020;161:989-1004. [DOI] [PubMed] [Google Scholar]
- [41].Xu X, Li YC, Wu YY, Xu YC, Weng RX, Wang CL, Zhang PA, Zhang Y, Xu GY. Upregulation of spinal ASIC1 by miR-485 mediates enterodynia in adult offspring rats with prenatal maternal stress. CNS Neurosci Ther 2021;27:244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu Y, Lu Y, Cassidy RM, Mangieri LR, Zhu C, Huang X, Jiang Z, Justice NJ, Xu Y, Arenkiel BR, Tong Q. Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses. Nat Commun 2019;10:3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xu Y, Wu Z, Sun H, Zhu Y, Kim ER, Lowell BB, Arenkiel BR, Xu Y, Tong Q. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab 2013;18:860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yuan Y, Wu W, Chen M, Cai F, Fan C, Shen W, Sun W, Hu J. Reward inhibits paraventricular CRH neurons to relieve stress. Curr Biol 2019;29:1243–51 e1244. [DOI] [PubMed] [Google Scholar]
- [45].Zhang HH, Hu J, Zhou YL, Hu S, Wang YM, Chen W, Xiao Y, Huang LY, Jiang X, Xu GY. Promoted interaction of nuclear factor-kappaB with demethylated cystathionine-beta-synthetase gene contributes to gastric hypersensitivity in diabetic rats. J Neurosci 2013;33:9028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang X, Lei B, Yuan Y, Zhang L, Hu L, Jin S, Kang B, Liao X, Sun W, Xu F, Zhong Y, Hu J, Qi H. Brain control of humoral immune responses amenable to behavioural modulation. Nature 2020;581:204–8. [DOI] [PubMed] [Google Scholar]
- [47].Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, Croce C, Verne GN. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2016;65:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, Mao Y, Wang L, Xie W, Zhong H, Zhang N, Luo MH, Tao W, Wang H, Li J, Li J, Qiu BS, Zhou JN, Li X, Xu H, Wang K, Zhang X, Liu Y, Richter-Levin G, Xu L, Zhang Z. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci 2019;22:1649–58. [DOI] [PubMed] [Google Scholar]
- [49].Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol 2005;484:43–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B695.
A video abstract associated with this article can be found at http://links.lww.com/PAIN/B696.