1. Introduction
Vaccine durability has been a matter of significant global public and research interest since the rapid development and deployment of successful vaccines for SARS-CoV-2. The continuing evolution of SARS-CoV-2 variants has emphasized the distinction between protection from infection and from severe disease. A variety of vaccine platforms, including mRNA, adenovirus-vectored, adjuvanted protein subunit, and whole inactivated virus, have been employed in the SARS-CoV-2 pandemic, with varying efficacy and durability. Protection may be defined by multiple clinical and immunological endpoints and vaccination against different viral pathogens requires different levels of protection. For example, a successful preventative vaccine for HIV, with its vast genetic diversity and ability to integrate into the human genome, requires a particularly high bar: durable sterilizing immunity. Immunologically, protection is most often measured by levels of binding or neutralizing antibodies in the serum, which can be convenient surrogates but do not capture the full picture of memory. The virus, vaccine platform, and scientific or clinical endpoint will determine which measures are most relevant.
Mark Slifka (Oregon Health and Science University) discussed protective thresholds; durable protection requires maintenance of immune responses over this threshold. A declining immune response may be protective as long as it remains above this threshold. Data presented by Mark Slifka and Matthew Snape (University of Oxford) indicate that peak serum antibody responses tend to correlate in magnitude with plateau levels of antibody,1 but in quantity is not always sufficient. Experimental vaccines for HIV elicit high titer, long-lived serum antibody responses, but do not provide protection from infection.2 The quality of the antibody response matters; epitope specificity, breadth, and isotype are important determinants of protection.
How to instruct a durable, protective immune response is coming into focus. Deepta Bhatacharya (University of Arizona) and Bali Pulendran (Stanford University) emphasized that early innate and adaptive signals are critical to establish long-lived immunity.3 Long-lived germinal center (GC) responses can program durable adaptive immune responses, and long-lived GCs have been successfully induced using several strategies discussed below. The tools and technology to engineer durable vaccines are advancing, but we need a clearer understanding of the rules for achieving durable protection. To address this timely topic, the National Institute of Allergy and Infectious Diseases (NIAID) organized a workshop, on July 27 and 28, 2022, with the objective of identifying knowledge gaps in the research on vaccine durability.
In contrast to the unprecedented development and licensure of COVID-19 vaccines, the development of HIV vaccines has been long and challenging. Of the eight vaccine efficacy trials conducted so far, only one, RV144, has shown moderate efficacy, and the follow-up trial, HVTN 702, failed to replicate this result.4 The focus in the field has shifted to experimental medicine trials, enabled and expedited by use of mRNA platforms licensed for use with COVID vaccines (NCT05217641). There is no doubt that HIV presents a much greater challenge for vaccine developers than COVID-19. Iterative experimental medicine will determine whether further optimization of Env immunogens, together with advances in vaccine delivery and composition, will overcome this challenge. For HIV, SARS-CoV-2, and other viruses, monitoring long-term vaccine-specific durability in real-world settings, where viral diversity affects efficacy and multiple clinical outcomes are measured, is crucial for developing vaccine policy.
2. Vaccine Platforms
Waning serum antibody titers to SARS-CoV-2 vaccination have been observed, though protection against severe disease remains high. David Kaslow (PATH) discussed multiple factors affecting vaccine efficacy, including viral incubation period and force of infection (the rate of acquisition of infection).5 Longer incubation periods allow an effective recall response, and protection from symptomatic disease. Shorter incubation periods do not allow sufficient time for a protective recall response, and vaccine efficacy decreases.5 This effect has been widely observed in the SARS-CoV-2 pandemic, where protection from infection decreased as viral incubation times decreased. Force of infection also affects vaccine efficacy. Vaccine efficacy tends to be higher in areas with lower viral transmission, or force of infection, as observed with a pentavalent rotavirus vaccine with real-world efficacy ranging from 45%-90%.5 Protective efficacy and durability are influenced by these factors, as we have witnessed in the current pandemic.5 Other factors unique to each virus, such as the tremendous viral diversity of HIV and its ability to integrate into the genome and to hide in immune privileged sites, also affect efficacy and durability.5
Two vaccine platforms dominated the initial SARS-CoV-2 vaccine rollout in the US: Adenovirus-26 (Ad26) and mRNA. These two platforms induced immune responses with different kinetics. Dan Barouch (Ragon Institute) presented data on Janssen’s Ad26-based vaccine for SARS-CoV-2. A single dose of this vaccine induced binding and neutralizing antibodies, CD4, and CD8 T cells, all detectable through 8 months, though peak titers were significantly with lower than after mRNA vaccines.6 Protection against hospitalization by Ad26 vaccination for adults under age 65 remained consistently over 90% through the Delta wave. In contrast, mRNA vaccines induce high peak serum antibody responses that waned by 6 months, although binding antibodies, CD4 and CD8 T cells, and GCs persist as long as 6 months after vaccination, highlighted by Andrea Carfi (Moderna).6 While protection from infection by mRNA vaccines has decreased with time, protection from severe disease remains around 90%.7, 8 Mark Slifka emphasized that antibody responses to many vaccines plateau by 3 years,9 therefore understanding the true durability of mRNA vaccines will require time.
Boosting with heterologous vectors is commonly thought to enhance immune responses. Matthew Snape presented data from the Com-COV2 study, conducted in the United Kingdom, demonstrating that heterologous and homologous regimens resulted in varied peak titers, and similar rates of serum antibody decline through 6 months.1 In the related COV-BOOST trial and the US MixNMatch trial, a third dose of adenovirus (AstraZeneca’s ChAdOx1 or Janssen’s Ad26, respectively) vaccine in Pfizer or Moderna mRNA-primed recipients resulted in higher titers of binding antibody.10 John Beigle (NIAID) stressed that the MixNMatch study was not designed to assess superiority of heterologous vaccine regimens. This study found that all combinations of mRNA and Ad26 did increase WA-1 and D614G binding and neutralizing antibody responses through 6 months post-boost,11 a relevant observation as immunity in the population becomes increasingly heterologous.
The predictive value of commonly used serum antibody and neutralizing antibody titers varies. In the COVE phase III efficacy trial, serum neutralizing and binding antibody titers at days 29 and 57 were highly predictive of protection from infection by mRNA-1273 vaccination.12 In contrast, experimental vaccines for HIV, among others, induce high antibody titers,13 but fail to protect from infection. Throughout workshop discussions, Andrea Carfi, Bali Pulendran, Galit Alter (Ragon Institute), and Shane Crotty (La Jolla Institute for Immunology) remarked that recruiting sometimes rare B cells with potential for protective specificities and optimal Fc-effector functions is critical. Serum antibodies are the most accessible measure but may not reflect the full picture of immunity at the site of infection. Though more labor- and time-intensive to measure, T cell responses are an important component of protection, both as direct effectors in the control of viral replication, and to induce durable, long-lived antibody responses.9
The protective antibody threshold has increased with SARS-CoV-2 viral drift, as Dan Barouch observed. Protective serum antibody thresholds are defined for some pathogens, like hepatitis viruses,9 and even for the VRC01 class of HIV neutralizing antibodies.14 Adjuvants may enhance plateau levels by increasing peak antibody responses, with mixed effects on durability. Mark Slifka presented data from on an AS04-adjuvanted HPV vaccine that elicited higher peak and plateau neutralizing titers than alum-adjuvanted HPV vaccines.9 In contrast the administration of various adjuvants had a limited impact on reshaping HIV-specific antibody durability.15 Thus, engaging specific TLRs and other innate receptors with adjuvants, singly or in combination, may instruct a durable immune response, but optimizing the effects of adjuvants requires more research.
3. Engineering Durable Vaccines
To engineer durable vaccines, we must first define the rules and features of durable protection. These rules may differ for each virus, each platform, and between prime and boost responses. Knowledge of immune signals driving development of long-lived plasma cells (LLPCs) and key factors impacting persistence of GC responses remains insufficient to allow engineering of durable immunity. Shane Crotty presented data from non-human primate models of HIV, in which an osmotic pump for sustained delivery of antigen or sequential escalating doses of immunogen, induced long-lived GCs, increased epitope breadth, and enhanced affinity maturation of B cell lineages following a single prime.16 In humans, whether dose escalation of antigen and adjuvant can prolong GC reactions, resulting in increased somatic hypermutation and neutralizing antibody responses, is being tested, based on this NHP data (NCT05471076). Rama Amara (Emory University) demonstrated that route of administration matters as well; intradermal administration (ID), in contrast to intramuscular (IM), of an MVA-vectored vaccine to non-human primates increased antigen retention in the lymph nodes, resulting in higher and more durable activation of antigen-specific GC T and B cells.17 ID administration resulted in better protection against BG505 SHIV challenge (Amara, unpublished), highlighting the impact of different vaccination regimens and routes of immunization.
Deepta Bhattacharya and Eun-Hyung Lee (Emory University) emphasized that more investigation is needed to identify how initial B cell activation imprints key survival programs of LLPCs and which early signals predict a durable immune response.3 Neil King (University of Washington) observed that infections and replicating viral vectors engage multiple receptors and inflammatory pathways, in contrast to unadjuvanted platforms or those with a single adjuvant. These data suggest that early innate signals, which vary by pathogen or platform, as surrogate endpoints to expedite vaccine development, and to enable engineering of more durable vaccines. Initial antigen encounter similarly affects durability. Antigen presentation formats similarly affects durability. Neil King further illustrated that higher antigen valence and sustained antigen delivery recruit B cells with a larger breadth of affinities, and more effectively drive B cells into the LLPC pool.3, 9, 18 GC interactions ultimately dictate the quality and longevity of the antibody response generated following vaccination, and each step in the process could be exploited to improve durable humoral immune responses.
4. Measurement of Vaccine Durability
Sophisticated tools and sampling methods are being applied to profile immunity in mice, NHPs, and humans with great precision and detail. Draining lymph nodes can be sampled using ultrasound-guided fine needle aspirates. Using this technique, the Ali Ellebedy (Washington University) presented data showing high frequencies of S-binding GC B cells and antibody-producing plasmablasts in draining lymph nodes at least 29 weeks after the second immunization.19, 20 These studies demonstrated that SARS-CoV-2 mRNA vaccination induces a persistent GC B cell response, and robust humoral immunity comparable to seasonal influenza vaccination. Lymph node and bone marrow (BM) aspirates can be used to follow B cell maturation by applying single-cell sequencing to track B cell clones and somatic hypermutation.20 In BM, CD19− CD38hi CD138+ LLPCs are the cellular basis of durable antibody responses. Single cell omics analysis of human BM aspirates uncovered significant heterogeneity in B lineage compartments in the BM.21 Eun-Hyung Lee explained that generation of LLPC is not fully understood but likely involves antibody secreting cell (ASC) differentiation in GC, with T cell help, followed by migration and further LLPC adaptation to the hostile, hypoxic BM niche.21 To explore further, the Lee lab has developed in vitro BM cultures that mimic the unique BM microenvironment needed to sustain LLPCs.21 Application of advanced culture techniques may uncover environmental and autologous signals guiding development of LLPCs.
ASCs are a heterogenous effector cell population, due to differences in memory B cell precursors and cytokine milieus. Much of our understanding about memory B cells is derived from studies of responses to systemic antigens in lymphoid organs, while initial protective responses occur in mucosal tissues. Troy Randall (University of Alabama) presented research on a novel population of murine flu-specific, lung-resident memory B cells (BRM cells) that rapidly give rise to plasmablasts, reside in bronchus-associated lymphoid tissue, are induced in response to intranasal vaccination, and provide a significant degree of protection from infection.22 Ignacio Sanz (Emory University) stated that the heterogeneity of the memory B cell compartment in humans remains poorly understood, though antigen-specific B cells can be detected in CD27+ and CD27− B cell populations 6 months after 3 dose immunization against SARS-CoV-2. It remains unclear which B cell populations are most likely to re-enter the germinal center during a recall response.23 The requirements for stimulation and maintenance of memory B cell populations remain to be determined.
Similarly, Susan Kaech (Salk Institute) described distinct memory T cell subsets (effector memory (TEM), central memory (TCM), stem-cell memory, tissue-resident memory (TRM), and peripheral memory (TPM)) which vary in their migratory and effector properties. These populations form a layered system of protection, with tissue-resident memory T cells serving as the first line of defense. Indeed, pathogen specific TRMs are found in the affected organs and coordinate recall responses: hepatitis-specific T cells tend to be localized to the liver.24 Antigen-specific memory T cells may be measured using several assays—peptide MHC tetramer staining, activation induced marker (AIM) assays, and ELISPOT—but the difficulty in detecting and following localized T cell responses remains a significant limitation. In humans, tissue resident lymphocytes may be studied in clinical biopsies and donated organs, though following immune responses over time remains a challenge.
Systems biology has been employed to uncover signatures predicting durability and mechanistic correlates. The Pulendran laboratory has shown that live attenuated Yellow Fever vaccine induces extremely long-lived protection, engaging multiple Toll like receptors (TLRs) and inflammasome activators.25 Interestingly, the Pfizer BioNTech mRNA vaccine does not engage TLRs or inflammasome activators. It does, however, engage MDA-5, an RNA associated with T cell responses.26 Knowledge of these pathways has been applied in combining TLR-activating adjuvants with subunit or nanoparticle vaccines to induce strong, durable responses in non-human primates and in clinical trials.27, 28 Mechanistic knowledge of the immune system, sampling of difficult-to-access lymphoid tissues, and powerful computational methods are now being combined to predict and engineer durable immune responses. To understand durability, it will be necessary to uncover more signals required to move an ASC to the BM, to survive in the hostile environment of the BM, and to become an LLPC.
Heterogeneity in memory cell subsets, both T and B-lineage, is a feature of the immune system, providing multiple layers of defense and adaptability. Protective immune responses require layered, tissue-specific defenses; it’s necessary to induce both serologic memory and tissue-specific memory. Mucosal responses differ in their potential to generate immunity and memory and are distinct from systemic responses. Kanta Subbarao (University of Melbourne) and Chris Chiu (Imperial College, London) posited that the short-half-life of IgA in the nasal mucosa is likely a major factor in the lack of protection from re-infection by respiratory viruses. In contrast, long-lived serum IgG is accessible to the lower respiratory tract and protects against severe disease from respiratory infections.29 Combining different platforms and routes of delivery has potential to induce superior protection. In animal models, intranasal prime with live attenuated influenza virus followed by IM boost with a subunit vaccine and IM mRNA-lipid nanoparticle prime followed by intranasal protein boost induced robust immune responses to influenza and SARS-CoV-2, respectively.30 Understanding the adaptive immune cells in their changing microenvironments is critical to enhancing vaccine durability. Bali Pulendran and Eun-Hyung Lee echoed that understanding which lymph node plasmablasts acquire the lifespans of LLPCs upon residence in the BM is essential, since they likely acquire distinct intrinsic signals and molecular programs in the microniche.21
Ali Ellebedy raised the limitation that spatial information is not captured by techniques like lymph node aspirates; developing a method of visualizing T and B cells in situ would allow interrogation of the microenvironment and cellular interactions. Kanta Subbarao and Chris Chiu also noted that studying mucosal immunity in humans is challenging, in part due to significant variability in sampling, particularly for the respiratory tract. As serum responses are not a reliable correlate of mucosal immunity, understanding and measuring mucosal immune memory remains a gap.
5. Conclusions
Investigators participating in the workshop, particularly Bali Pulendran and Julie McElrath (Fred Hutchinson Cancer Research Center), championed a need for standardized human studies that directly compare vaccine platforms, including traditional recombinant protein with or without adjuvants, replicating and nonreplicating viral vectors, and nucleic acid DNA and mRNA approaches. There is precedent in the harmonized COVID-19 vaccine efficacy trials conducted by the NIAID-funded Coronavirus Prevention Network (CoVPN), and in human clinical vaccine trials comparing multiple adjuvants with a common stabilized HIV Env trimer immunogen (NCT04177355). Valid comparisons require similar or identical qualified or validated assays to profile the innate and the adaptive response, with in depth sampling of not just in the blood, but also mucosa, lymph nodes, and bone marrow. Standardized clinical trials and assays will enhance the ability to define vaccine durability and correlates of protection, speeding licensure for vaccines and uncovering the requirements for durable protection.
While there is abundant information about serum antibody responses to vaccination, many mechanistic protective responses remain unclear. There is a need to define biomarkers of a durable protective response, and the rules for induction of a durable protective response at the site of infection, as Troy Randall and Kanta Subburao emphasized. Advances in bioengineering have provided the tools necessary to engineer durable vaccines once the parameters are known. With nanoparticle and virus-like particle vaccines, antigen valence, density, and pathogen variants can be tightly controlled. Advances in biomaterials can allow controlled, sustained delivery of antigen. An ever-expanding panel of novel adjuvants can be employed to activate specific innate immune pathways. The tools exist. What remains is to define the rules of durability for a particular pathogen, platform, and population. Uncovering these rules will require rigorous, standardized human experimental medicine.
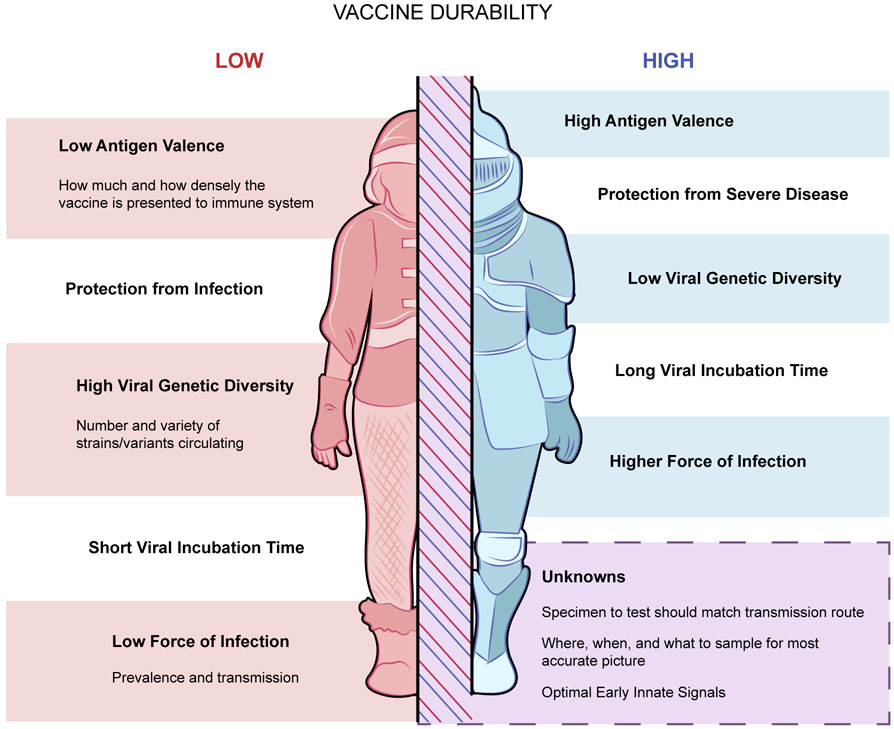
Figure 1.
Factors that decrease vaccine durability are listed on the left in red; factors that promote vaccine durability are listed on the right in blue. Current unknowns are shown in the box at the bottom.
Acknowledgements
The authors wish to thank the workshop speakers and panelists for their contributions and enthusiasm: Rama Amara, Dan Barouch, John Beigel, Deepta Bhattacharya, Andrea Carfi, Chris Chiu, Sue Kaech, David Kaslow, Neil King, Mary Marovich, Troy Randall, Iñaki Sanz, Mark Slifka, Matthew Snape, and Kanta Subbarao. Thank you to NIAID Meet for their assistance, Ian Anglin for notetaking and Rose Perry-Gottschalk of Visual and Medical Arts at Rocky Mountain Laboratories, NIAID.
Footnotes
Competing Interests
G.A. is a founder and/or equity holder in Systems Seromyx and Leyden Labs, and since October 2022 is an employee of Moderna Therapeutics. S.C. has consulted for GSK, JP Morgan, Citi, Morgan Stanley, Avalia NZ, Nutcracker Therapeutics, University of California, California State Universities, United Airlines, Adagio, and Roche. B.P. serves on the External Immunology Network of GSK, and the scientific advisory board of Medicago. M.P.D., M.C.L., M.L., C.M., M.J.M., A.C.P. and A.S. declare no competing interests.
References
- 1.Shaw RH et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: exploratory analyses of Com-COV, a randomised control trial. The Lancet Respiratory Medicine (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitisuttithum P & Marovich MA Prophylactic HIV vaccine: vaccine regimens in clinical trials and potential challenges. Expert Review of Vaccines 19, 133–142 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya D Instructing durable humoral immunity for COVID-19 and other vaccinable diseases. Immunity 55, 945–964 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray GE et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C gp120–MF59 in Adults. New England Journal of Medicine 384, 1089–1100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaslow DC Force of infection: a determinant of vaccine efficacy? NPJ Vaccines 6, 51 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier AY et al. Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines. N Engl J Med 385, 2010–2012 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews N et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. New England Journal of Medicine 386, 340–350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKeigue PM et al. Vaccine efficacy against severe COVID-19 in relation to delta variant (B.1.617.2) and time since second dose in patients in Scotland (REACT-SCOT): a case-control study. The Lancet Respiratory Medicine 10, 566–572 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slifka MK & Amanna IJ Role of Multivalency and Antigenic Threshold in Generating Protective Antibody Responses. Frontiers in Immunology 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: Three month analyses of the COV-BOOST trial. Journal of Infection 84, 795–813 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atmar RL et al. Homologous and Heterologous Covid-19 Booster Vaccinations. New England Journal of Medicine 386, 1046–1057 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert PB et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 375, 43–50 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barouch DH et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). The Lancet 392, 232–243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert PB et al. Neutralization titer biomarker for antibody-mediated prevention of HIV-1 acquisition. Nature Medicine (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francica JR et al. Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Advances 1, 2329–2342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH et al. Long-lasting germinal center responses to a priming immunization with continuous proliferation and somatic mutation. bioRxiv, 2021.2012.2020.473537 (2021). [Google Scholar]
- 17.Styles TM et al. V2 hotspot optimized MVA vaccine expressing stabilized HIV-1 Clade C envelope Gp140 delays acquisition of heterologous Clade C Tier 2 challenges in Mamu-A*01 negative Rhesus Macaques. Frontiers in Immunology 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walls AC et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 183, 1367–1382.e1317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner JS et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 596, 109–113 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim W et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature 604, 141–145 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen DC et al. Plasma cell survival: The intrinsic drivers, migratory signals, and extrinsic regulators. Immunological Reviews 303, 138–153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allie SR et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nature Immunology 20, 97–108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner JS et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature 586, 127–132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg SP, Ural BB & Farber DL Tissue-specific immunity for a changing world. Cell 184, 1517–1529 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Querec TD et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nature Immunology 10, 116–125 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol 23, 543–555 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasturi SP et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci Immunol 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arunachalam PS et al. Durable protection against the SARS-CoV-2 Omicron variant is induced by an adjuvanted subunit vaccine. Sci Transl Med 14, eabq4130 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuman PD et al. Influenza-specific ELISA IgA and IgG predict severity of influenza disease in subjects prescreened with hemagglutination inhibition. Antiviral Res 13, 103–110 (1990). [DOI] [PubMed] [Google Scholar]
- 30.Jegaskanda S et al. Intranasal Live Influenza Vaccine Priming Elicits Localized B Cell Responses in Mediastinal Lymph Nodes. Journal of Virology 92, e01970–01917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]