Abstract
Purpose
In the critically ill, hospital-acquired bloodstream infections (HA-BSI) are associated with significant mortality. Granular data are required for optimizing management, and developing guidelines and clinical trials.
Methods
We carried out a prospective international cohort study of adult patients (≥ 18 years of age) with HA-BSI treated in intensive care units (ICUs) between June 2019 and February 2021.
Results
2600 patients from 333 ICUs in 52 countries were included. 78% HA-BSI were ICU-acquired. Median Sequential Organ Failure Assessment (SOFA) score was 8 [IQR 5; 11] at HA-BSI diagnosis. Most frequent sources of infection included pneumonia (26.7%) and intravascular catheters (26.4%). Most frequent pathogens were Gram-negative bacteria (59.0%), predominantly Klebsiella spp. (27.9%), Acinetobacter spp. (20.3%), Escherichia coli (15.8%), and Pseudomonas spp. (14.3%). Carbapenem resistance was present in 37.8%, 84.6%, 7.4%, and 33.2%, respectively. Difficult-to-treat resistance (DTR) was present in 23.5% and pan-drug resistance in 1.5%. Antimicrobial therapy was deemed adequate within 24 h for 51.5%. Antimicrobial resistance was associated with longer delays to adequate antimicrobial therapy. Source control was needed in 52.5% but not achieved in 18.2%. Mortality was 37.1%, and only 16.1% had been discharged alive from hospital by day-28.
Conclusions
HA-BSI was frequently caused by Gram-negative, carbapenem-resistant and DTR pathogens. Antimicrobial resistance led to delays in adequate antimicrobial therapy. Mortality was high, and at day-28 only a minority of the patients were discharged alive from the hospital. Prevention of antimicrobial resistance and focusing on adequate antimicrobial therapy and source control are important to optimize patient management and outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06944-2.
Keywords: bloodstream infection, bacteremia, hospital-acquired, antibiotic resistance
Take-home message
| In this study, hospital-acquired bloodstream infections were frequently caused by Gram-negative, carbapenem resistant or with difficult to treat resistance pathogens. Antibiotic resistance was associated with delays to antimicrobial therapy. Mortality was 37% at day-28. |
Introduction
Hospital-acquired bloodstream infections (HA-BSI) are the healthcare-associated infection causing the highest burden in disability-adjusted life years [1]. They are relatively frequent in intensive care unit (ICU) patients and are associated with 36–42% mortality [2–5]. In 2012, the EUROBACT-1 international cohort study highlighted the prevalence of multidrug-resistant organisms and its association with higher risk of death in ICU patients with HA-BSI. In recent years, worrisome increases in antimicrobial resistance have been highlighted by agencies and scientific societies worldwide [6–8]. Indeed, antimicrobial resistance is associated with delays to adequate antimicrobial therapy, increased mortality, resource utilisation and costs [2, 9, 10]. It leads to considerable increases in the use of broad-spectrum antimicrobials which in turn exacerbates the problem by selecting antimicrobial resistant micro-organisms. Given the frequency of sepsis, septic shock, and the high mortality in ICU patients with HA-BSI, large international studies are essential to identify potentially modifiable factors of poor prognosis. These data may inform patient care, the development of guidelines, and the design of clinical trials.
The EUROBACT-2 study was designed to update the epidemiology and main factors associated with day-28 mortality in ICU patients with HA-BSI by prospectively collecting granular center, patient, pathogen, treatment, and outcome data from ICUs worldwide.
Methods
Study design
EUROBACT-2 was a prospective international cohort study, registered with ClinicalTrials.org (NCT03937245) and reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [11]. The study was conducted across the first year of the pandemic caused by coronavirus disease 2019 (COVID-19). We reported the differences in the epidemiology of HA-BSI in patients with COVID-19 separately [12]. Initial ethical approval as a low-risk research project with waiver of individual consent was granted by the Human Research Ethics Committee of the Royal Brisbane & Women's Hospital, Queensland, Australia (LNR/2019/QRBW/48376). Each study site then obtained ethical and governance approvals according to national and/or local regulations.
Setting
Endorsement, financial, and logistical support were obtained from the European Society of Intensive Care Medicine (ESICM) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) study Group for Infections in Critically Ill Patients (ESGCIP). The operational committee (AT, NB, FB, SR, QS, CD, JFT) oversaw study operations under the responsibility of the primary investigator (AT). Logistics were provided by the OUTCOMEREA non-profit research group (Paris, France). National coordinators recruited participating ICUs, applied for ethical and regulatory approvals, and facilitated communication within their country.
Participants
We included adult (≥ 18 years of age) patients with a HA-BSI treated in the ICU.
HA-BSI was defined as a positive blood culture sampled more than 48 h after hospital admission. Treatment in the ICU was defined as the blood culture having been either sampled in the ICU or the patient having been transferred to the ICU for the treatment of the HA-BSI. Detailed definitions are available in the electronic supplemental material (ESM).
For usually considered as common contaminants (list provided in the ESM), at least 2 blood cultures with the same antimicrobial susceptibility profile, or strong clinical grounds that it was not a contaminant (e.g., intravascular catheters or other infected material proven as a source for the HA-BSI) were mandatory. All possible contaminants were carefully reviewed for eligibility by the operational committee in collaboration with the local investigators and excluded if the above criteria were not met.
Data collection
Centers prospectively recruited patients between the 1st of June 2019 and the 30th of January 2021, with a minimum of 10 consecutive patients or for a 3-month period, which on request could be extended. Hospital and ICU characteristics were recorded. Patient data were retrieved from the hospital charts without additional tests or interventions. Demographic data, the main diagnosis at ICU admission, and comorbidities were collected. Geographical regions and income categories were defined using the United Nations M49 standard [13]. Severity of illness was assessed at ICU admission by the Simplified Acute Physiology Score II (SAPS II) [14], and at HA-BSI diagnosis by the Sequential Organ Failure Assessment (SOFA) score [15]. Given all included patients had an infection, sepsis was defined at HA-BSI diagnosis according to Sepsis III criteria by a SOFA score ≥ 2, and septic shock as sepsis plus vasopressor use plus lactate > 2 mmol/L [16]. We focused on each patient’s first episode of HA-BSI, collected pathogen with antibiogram, date and time of blood culture sampling and followed patients for 28 days, until hospital discharge, or death. Blood culture sampling represented the time zero of the study from which all timings were calculated (e.g., time to adequate antimicrobial therapy). Sources of HA-BSI were recorded in order of clinical likelihood according to the treating clinician. Primary HA-BSI was defined as no clear portal of entry or source of infection. Antimicrobials were collected from 2 days prior to HA-BSI to ICU discharge or day-28 follow-up. Carbapenem resistance for Enterobacterales was defined as resistance to at least one carbapenem [17]. Difficult-to-treat resistance (DTR) was defined as resistance to all first line antimicrobials [18], and pan-drug-resistance (PDR) as resistance to all tested antimicrobials. To avoid over-reporting DTR and PDR for pathogens with incompletely reported antibiograms, the assessment required availability of antimicrobial susceptibility testing for at least one fluoroquinolone, one cephalosporin, one carbapenem, plus polymyxins for PDR. DTR and PDR were assessed for Enterobacterales, Pseudomonas spp., and Acinetobacter spp. Adequate antimicrobial therapy was defined as receiving at least 1 antimicrobial with in-vitro activity for the pathogen at the considered timepoint, with adequacy of antimicrobial selection, dosing and administration manually reviewed for all infections and sources of HA-BSI. Time to adequate antimicrobial therapy was defined as the time between sampling of the first positive blood culture and receipt of at least one adequate antibiotic for each pathogen. Source control was reported according to the source and intervention, with adequacy assessed by the investigator.
Statistical analysis
As detailed in the ESM, and to ensure consistency, database lock was made on the 12/08/2021 after answering of all queries by the investigators, crosschecking with electronic controls, and careful reading of all the case-report forms by the operational committee.
Linearity to the logit for continuous variables was checked with generalized additive models. Non-linear variables were discretised into categorical variables based on quartiles. Continuous variables were expressed as medians (interquartile range [IQR]) and categorical variables as absolute frequencies and percentage. Differences were tested by the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
To identify factors associated with day-28 death, we built a three-tiered hierarchical logistic mixed model and a subdistribution hazard frailty model that considered ICU discharge as a competing risk, as suggested by Fine and Gray [19]. Both are presented in the ESM as exploratory analyses, alongside sensitivity analysis excluding COVID-19 patients and investigating the role of carbapenem resistance in place of DTR. All analyses were two-sided with p values less than 0.05 deemed statistically significant. Statistical analysis was done using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC, USA) and R project version 4.04.
Results
Study population
We enrolled 2600 patients from 333 ICUs in 52 countries or territories (ESM eFigures 1–2, Table 1 and eTables 2–3). Most ICUs were in public (83.8%), teaching (82.6%) hospitals, with a mixed medical-surgical (79.5%) and general case mix (91.7%). Median [IQR] ICU size was 14 [10; 21] ventilator-equivalent beds with wide variability in infrastructure and factors related to antimicrobial stewardship.
Table 1.
Characteristics of participating ICUs and association with day-28 (D28) patient mortality
| Characteristics* | All ICUs (n = 333)* |
All patients (n = 2600) | Dead on D28 (n = 966) | Alive on D28 (n = 1634) | OR [95% CI] |
p value |
|---|---|---|---|---|---|---|
| Geographic region | 0.824 | |||||
| Europe and Central Asia | 184 (55.3) | 1775 (68.3) | 689 (71.3) | 1086 (66.5) | 1 | |
| East Asia and Pacific | 69 (20.7) | 412 (15.8) | 127 (13.1) | 285 (17.4) | 0.83 [0.54; 1.27] | |
| Middle East and North Africa | 48 (14.4) | 268 (10.3) | 91 (9.4) | 177 (10.8) | 0.96 [0.61; 1.54] | |
| South Asia | 14 (4.2) | 54 (2.1) | 24 (2.5) | 30 (1.8) | 1.29 [0.52; 3.2] | |
| Latin America and the Caribbean | 11 (3.3) | 52 (2) | 17 (1.8) | 35 (2.1) | 0.78 [0.33; 1.85] | |
| Sub-Saharan Africa | 5 (1.5) | 20 (0.8) | 6 (0.6) | 8 (0.5) | 1.7 [0.55; 5.21] | |
| North America | 2 (0.6) | 19 (0.7) | 8 (0.8) | 11 (0.7) | 1.34 [0.34; 5.31] | |
| National income | 0.145 | |||||
| High-income | 202 (60.7) | 1479 (56.9) | 485 (50.2) | 994 (60.8) | 1 | |
| Upper-middle-income | 80 (24) | 870 (33.5) | 393 (40.7) | 477 (29.2) | 1.42 [0.99; 2.04] | |
| Low and lower-middle-income** | 51 (14.1) | 251 (9.2) | 88 (9.1) | 163 (10) | 1.11 [0.72; 1.72] | |
| Academic status of the hospital | ||||||
| Teaching hospital | 270 (82.6) | 2207 (85.4) | 823 (85.4) | 1384 (85.4) | 1 | 0.122 |
| Non-teaching hospital | 57 (17.4) | 378 (14.6) | 141 (14.6) | 237 (14.6) | 1.28 [0.94; 1.76] | |
| Type of ICU | ||||||
| Mixed (medical-surgical) | 260 (79.5) | 2040 (78.9) | 732 (75.9) | 1308 (80.7) | 1 | 0.048 |
| Medical | 41 (12.5) | 383 (14.8) | 180 (18.7) | 203 (12.5) | 1.49 [1.07; 2.09] | |
| Surgical | 26 (8) | 162 (6.3) | 52 (5.4) | 110 (6.8) | 0.9 [0.57; 1.43] | |
| Number of ventilator equivalent beds in the ICU ≥ 15 | 176 (53.82) | 1464 (56.63) | 499 (51.8) | 965 (59.5) | 0.81 [0.65; 1.03] | 0.081 |
| Nurse to ventilator-bed ratio | 2 [1.3; 2.9] | 2.2 [1.6; 2.8] | 2.2 [1.6; 2.8] | 2.2 [1.5; 2.9] | 0.99 [0.97; 1] | 0.106 |
| Senior doctor to ventilator-bed ratio | 6.7 [4.3; 10] | 6 [4; 9.5] | 6 [4; 9] | 6.3 [4.3; 9.5] | 0.99 [0.97; 1.01] | 0.213 |
| Senior medical cover is available 24/7 | 304 (93.3) | 2355 (91.5) | 869 (90.3) | 1486 (92.1) | 0.81 [0.53; 1.23] | 0.319 |
| General surgery is available 24/7 | 321(98.2) | 2556 (98.9) | 951 (98.7) | 1605 (99) | 0.8 [0.31; 2.05] | 0.637 |
| Infectious diseases specialist or clinical microbiologist are consulted | ||||||
| 24/7 | 170 (54.8) | 1479 (60) | 566 (60.9) | 913 (59.4) | 1 | 0.57 |
| During business hours | 114 (36.8) | 855 (34.7) | 313 (33.7) | 542 (35.3) | 1.06 [0.82; 1.38] | |
| Never or sporadically | 26 (8.4) | 131 (5.3) | 50 (5.4) | 81 (5.3) | 1.29 [0.8; 2.09] | |
| Clinical pharmacists are consulted | ||||||
| 24/7 | 82 (25.5) | 636 (25.1) | 188 (19.9) | 448 (28.2) | 1 | 0.007 |
| During business hours | 129 (40.1) | 811 (32) | 284 (30.1) | 527 (33.2) | 1.32 [0.99; 1.78] | |
| Never or sporadically | 111 (34.5) | 1084 (42.8) | 471 (49.9) | 613 (38.6) | 1.64 [1.21; 2.24] | |
| TDM of aminoglycosides is available | ||||||
| Everyday | 171 (52.5) | 1249 (48.5) | 383 (39.8) | 866 (53.7) | 1 | 0.002 |
| At least once a week | 30 (9.2) | 213 (8.3) | 81 (8.4) | 132 (8.2) | 1.32 [0.88; 1.98] | |
| Not available | 125 (38.3) | 1113 (43.2) | 498 (51.8) | 615 (38.1) | 1.6 [1.23; 2.09] | |
| TDM of vancomycin is available | ||||||
| Everyday | 200 (61.3) | 1419 (55.1) | 462 (48) | 957 (59.3) | 1 | 0.012 |
| At least once a week | 43 (13.2) | 319 (12.4) | 120 (12.5) | 199 (12.3) | 1.07 [0.74; 1.53] | |
| Not available | 83 (25.5) | 837 (32.5) | 380 (39.5) | 457 (28.3) | 1.55 [1.15; 2.07] | |
| TDM of β-lactams is available | ||||||
| Everyday | 35 (10.7) | 256 (9.9) | 87 (9) | 169 (10.5) | 1 | 0.255 |
| At least once a week | 51 (15.6) | 408 (15.8) | 117 (12.2) | 291 (18) | 0.81 [0.52; 1.27] | |
| Not available | 240 (73.6) | 1911 (74.2) | 758 (78.8) | 1153 (71.5) | 1.1 [0.75; 1.62] | |
Full report of center characteristics is available in ESM eTable 3. Results reported as n (%) for categorical variables and median [IQR] for continuous variables
*All center data were missing for 6 ICUs and 7–12 did not provide staffing or stewardship data
**There were 4 ICUs and 11 patients in the low-income category. 24/7: 24 h a day, 7 days a week. ICU: intensive care unit. TDM: therapeutic drug monitoring. Ventilator equivalent beds refers to the maximum number of ventilated patients the ICU can accommodate at one time
ICUs recruited a median [IQR] of 6 [3, 10] patients. Most patients were males (63.7%), median [IQR] age was 64 [52;73] years, and 74.8% had at least one comorbidity (Table 2 and eTable 2). Most common ICU admission diagnoses were non-COVID-19-related respiratory failure (21.2%), sepsis or septic shock (20.4%), and COVID-19 (12.9%).
Table 2.
Baseline (admission to the ICU) patient characteristics and day-28 (D28) mortality
| Variable | All patient (n = 2600) |
Dead on D28 (n = 966) | Alive on D28 (n = 1634) | OR [95% CI] |
p value |
|---|---|---|---|---|---|
| Age (years) | < 0.001 | ||||
| < 52 | 649 (25) | 175 (18.1) | 474 (29) | Ref | |
| [52–64] | 691 (26.6) | 256 (26.5) | 435 (26.6) | 1.59 [1.25; 2.04] | |
| [65–73] | 618 (23.8) | 223 (23.1) | 395 (24.2) | 1.53 [1.19; 1.97] | |
| ≥ 74 | 642 (24.7) | 312 (32.3) | 330 (20.2) | 2.46 [1.91; 3.16] | |
| SAPS II score on ICU admission (age excluded)a | < 0.001 | ||||
| < 26 | 585 (22.5) | 186 (19.3) | 399 (24.4) | Ref | |
| [26–35] | 708 (27.2) | 227 (23.5) | 481 (29.4) | 1.09 [0.84; 1.39] | |
| [36–47] | 618 (23.8) | 223 (23.1) | 395 (24.2) | 1.37 [1.06; 1.77] | |
| ≥ 48 | 689 (26.5) | 330 (34.2 | 359 (22) | 2.28 [1.78; 2.93] | |
| Male gender | 1657 (63.7) | 596 (61.7) | 1061 (64.9) | 0.89 [0.75; 1.06] | 0.192 |
| Body Mass Index (kg per m2) | |||||
| < 18.5 | 98 (3.8) | 32 (3.3) | 66 (4) | 1 | 0.771 |
| [18.5; 30] | 1845 (71.1) | 687 (71.3) | 1158 (71) | 1.13 [0.71; 1.78] | |
| ≥ 30 | 652 (25.1) | 245 (25.4) | 407 (25) | 1.18 [0.73; 1.9] | |
| Charlson comorbidity index | |||||
| 0 | 792 (30.5) | 223 (23.1) | 569 (34.8) | 1 | < 0.001 |
| 1–2 | 935 (36) | 371 (38.4) | 564 (34.5) | 1.59 [1.28; 1.97] | |
| > 2 | 873 (33.6) | 372 (38.5) | 501 (30.7) | 1.83 [1.47; 2.28] | |
| Solid tumor, no metastasis | 242 (9.3) | 88 (9.1) | 154 (9.4) | 0.99 [0.74; 1.32] | 0.931 |
| Solid tumor, with metastasis | 159 (6.1) | 76 (7.9) | 83 (5.1) | 1.54 [1.09; 2.17] | 0.013 |
| Haematological malignancy | 159 (6.1) | 71 (7.3) | 88 (5.4) | 1.55 [1.1; 2.2] | 0.013 |
| Type of ICU admission | |||||
| Medical | 1922 (73.9) | 777 (80.4) | 1145 (70.1) | 1 | < 0.001 |
| Surgical elective | 186 (7.2) | 56 (5.8) | 130 (8) | 0.69 [0.49; 0.97] | |
| Surgical emergency | 492 (18.9) | 133 (13.8) | 359 (22) | 0.6 [0.47; 0.76] | |
| Primary ICU admission diagnosisb | |||||
| Sepsis or septic shock | 530 (20.4) | 189 (19.6) | 341 (20.9) | 1 | < 0.001 |
| Respiratory admissionb | 550 (21.2) | 232 (24) | 318 (19.5) | 1.14 [0.88; 1.48] | |
| COVID-19b | 336 (12.9) | 195 (20.2) | 141 (8.6) | 2.07 [1.5; 2.85] | |
| Post-operative admission | 258 (9.9) | 83 (8.6) | 175 (10.7) | 0.84 [0.6; 1.17] | |
| Other admission diagnoses | 926 (35.6) | 267 (27.6) | 659 (40.3) | 0.68 [0.53; 0.87] | |
Continuous variables are presented as median [IQR]. Categorical variables are presented as n (%). CI: confidence interval. Closed brackets [;] denote inclusive of the end of the range and open brackets]; [denote the exclusion of the end of the range. ICU: Intensive care unit, SAPS II: Simplified Acute Physiology Score II
aThe SAPS II score was calculated excluding age-related points to avoid collinearity
bRespiratory admission refers to admission for respiratory failure other than COVID-19 that has been categorized separately. A full list of co-morbidities as defined in Charlson score and admission diagnosis can be found in the electronic supplement eTable 3
Median [IQR] time from hospital admission to HA-BSI was 13 [8;25] days. Most HA-BSI (78.5%) were ICU-acquired (median [IQR] time from ICU admission to diagnosis, 10 [5; 18] days). The median [IQR] SOFA score was 8 [5; 11] at HA-BSI diagnosis, with 4% of the patients not meeting the criteria for sepsis, while 64.2% and 31.7% met the criteria for sepsis and septic shock, respectively (Table 3).
Table 3.
Patient characteristics at diagnosis of hospital-acquired bloodstream infection and day-28 mortality
| Characteristics | All patients (N = 2600) | Dead on D28 (n = 966) | Alive on D28 (n = 1634) | OR [95% CI] | p value |
|---|---|---|---|---|---|
| Time from ICU admission to HA-BSI | |||||
| Acquired prior to ICU admission | 558 (21.5) | 188 (19.5) | 370 (22.6) | 1.03 [0.82; 1.29] | 0.017 |
| Early ICU-acquired (≤ 7 days) | 810 (31.2) | 327 (33.9) | 483 (29.6) | 1.32 [1.08; 1.6] | |
| Late ICU-acquired (> 7 days) | 1232 (47.4) | 451 (46.7) | 781 (47.8) | 1 | |
| Maximum temperature | |||||
| < 38.2 °C | 1412 (54.5) | 588 (61.2) | 824 (50.6) | 1 | < 0.001 |
| ≥ 38.2 °C | 1179 (45.5) | 373 (38.8) | 806 (49.4) | 0.72 [0.6; 0.86] | |
| Sepsis or septic shock | |||||
| No sepsis or sepsis without shock | 1776 (68.5) | 538 (55.9) | 1238 (76) | 1 | < 0.001 |
| Septic shock—no steroids | 446 (17.2) | 213 (22.1) | 233 (14.3) | 2.38 [1.89; 2.99] | |
| Septic shock—steroids administered | 370 (14.3) | 211 (21.9) | 159 (9.8) | 3.85 [2.98; 4.97] | |
| SOFA score | 8 [5; 11] | 10 [7; 13] | 7 [5; 10] | 1.21 [1.19; 1.24] | < 0.001 |
| Ventilation status | |||||
| Low flow oxygen or no oxygen | 493 (19) | 104 (10.8) | 389 (23.8) | 1 | < 0.001 |
| High flow oxygen nasal canula | 163 (6.3) | 50 (5.2) | 113 (6.9) | 1.69 [1.11; 2.57] | |
| Non-invasive mechanical ventilation or CPAP | 153 (5.9) | 50 (5.2) | 103 (6.3) | 1.84 [1.2; 2.81] | |
| Invasive Mechanical Ventilation | 1791 (68.9) | 762 (78.9) | 1029 (63) | 2.81 [2.18; 3.61] | |
| ECMO (VA or VV) | 41 (1.6) | 21 (2.2) | 20 (1.2) | 1.92 [0.99; 3.72] | 0.053 |
| Vasopressors (adrenaline or noradrenaline) | 1376 (52.9) | 614 (63.6) | 762 (46.6) | 2.44 [2.04; 2.93] | < 0.001 |
| Vasopressin | 113 (4.3) | 61 (6.3) | 52 (3.2) | 2.89 [1.89; 4.4] | < 0.001 |
| Gram-negative bacteriaa | 1623 (62.4) | 608 (62.9) | 1015 (62.1) | 0.98 [0.82; 1.17] | 0.823 |
| DTR Gram-negative | 350 (13.5) | 185 (19.2) | 165 (10.1) | 1.71 [1.33; 2.21] | < 0.001 |
| Gram-positive bacteriaa | 859 (33) | 312 (32.3) | 547 (33.5) | 0.98 [0.82; 1.17] | 0.821 |
| Resistant Gram-positive (MRSA, MRSE or VRE) | 323 (12.4) | 112 (11.6) | 211 (12.9) | 0.86 [0.66; 1.11] | 0.248 |
| Fungusa | 227 (8.7) | 102 (10.6) | 125 (7.6) | 1.39 [1.04; 1.86] | 0.026 |
| Strict anaerobe bacteriaa | 57 (2.2) | 15 (1.6) | 42 (2.6) | 0.76 [0.41; 1.41] | 0.382 |
| Polymicrobial blood culture | 290 (11.2) | 106 (11) | 184 (11.3) | 1 [0.77; 1.32] | 0.973 |
| Source of HA-BSI | |||||
| Intravascular catheter | 686 (26.4) | 239 (24.7) | 447 (27.4) | 1 | 0.027 |
| Intra-abdominal | 392 (15.1) | 145 (15) | 247 (15.1) | 1.33 [1; 1.76] | |
| Other | 217 (8.3) | 69 (7.1) | 148 (9.1) | 1.01 [0.71; 1.44] | |
| Primary | 425 (16.3) | 169 (17.5) | 256 (15.7) | 1.26 [0.96; 1.65] | |
| Respiratory | 694 (26.7) | 288 (29.8) | 406 (24.8) | 1.39 [1.09; 1.77] | |
| Urinary | 186 (7.2) | 56 (5.8) | 130 (8) | 0.9 [0.62; 1.3] | |
| More than 1 possible source of infection | 853 (32.8) | 322 (33.3) | 531 (32.5) | 1.14 [0.94; 1.37] | 0.191 |
| Time to adequate antimicrobial therapy | |||||
| ≤ 24 h, n (%) | 1339 (51.5) | 463 (47.9) | 876 (53.6) | 1 | < 0.001 |
| ]24;48] hours, n (%) | 336 (12.9) | 117 (12.1) | 219 (13.4) | 1.03 [0.79; 1.34] | |
| ]48;120] hours, n (%) | 396 (15.2) | 134 (13.9) | 262 (16) | 0.96 [0.74; 1.23] | |
| > 120 h, n (%) | 125 (4.8) | 38 (3.9) | 87 (5.3) | 0.72 [0.47; 1.09] | |
| Never, n (%) | 403 (15.5) | 214 (22.2) | 189 (11.6) | 1.98 [1.55; 2.53] | |
| Source control | |||||
| Not required | 1235 (47.5) | 488 (50.5) | 747 (45.7) | 1 | < 0.001 |
| Required, achieved | 1117 (43) | 321 (33.2) | 796 (48.7) | 0.63 [0.52; 0.76] | |
| Required, but NOT achieved | 248 (9.5) | 157 (16.3) | 91 (5.6) | 2.6 [1.92; 3.51] |
Continuous variables are presented as median [IQR] and categorical variables as n (%).Closed brackets [;] denote inclusive of the end of the range and open brackets]; [ denote the exclusion of the end of the range. HA-BSI: hospital-acquired blood stream infection, CPAP: continuous positive airway pressure ECMO: extra-corporeal membrane oxygenation, VA: venoarterial, VV: venovenous. DTR: Difficult to treat resistance MRSA: methicillin-resistant Staphylococcus aureus, MRSE: methicillin-resistant Staphylococcus epidermidis and includes all coagulase negative staphylococcus reported as non-susceptible to methicillin, VRE: vancomycin-resistant Enterococcus
aSum of percentages exceed 100 because a patient may have had several pathogens in the blood culture, referring to the 11.2% polymicrobial blood cultures
Sources of infection were predominantly respiratory (pneumonia) (26.7%) and intravascular catheters (26.4%), followed by the abdomen (15.1%). While primary HA-BSI were common (16.3%), one third of the patients (32.8%) had more than one possible source of HA-BSI.
Pathogens
Most (88.8%) blood cultures were mono-microbial, with 10% containing two, and 1.2% more than two pathogens, resulting in a total of 2927 bacterial and fungal isolates. Pathogens were most commonly Gram-negative (1726/2927; 59%), with a predominance of Klebsiella spp. (482/1726; 27.9%), Acinetobacter spp. (350/1726; 20.3%), Escherichia coli (272/1726; 15.8%) and Pseudomonas spp. (247/1726; 14.3%) (Table 4 and ESM eFigure 3). Carbapenem resistance was encountered in 37.8% (182/482) Klebsiella spp., 84.6% (296/350) Acinetobacter spp., 7.4% (20/272) Escherichia coli and 33.2% (82/247) Pseudomonas spp. When analysing Enterobacterales, Pseudomonas spp. and Acinetobacter spp., DTR was present in 23.5% (351/1492) and PDR in 1.5% (23/1492). Gram-positive pathogens (910/2972; 31.1%) were mainly Enterococcus spp. (314/910, 34.5%) and coagulase-negative staphylococci (273/910, 30%). Of the 27.6% (251/910) Staphylococcus aureus, 37.1% (93/251) were methicillin-resistant Staphylococcus aureus (MRSA). There were 2.1% (61/2927) strict anaerobe bacteria, and 7.9% (230/2927) fungi of which 39.6% (91/230) were Candida albicans, 57.8% (133/230) non-albicans Candida spp., and 6 (2.6%) other fungi.
Table 4.
Characteristics of the pathogens in the initial blood culture in EUROBACT-2 and comparison with EUROBACT-1 and EPIC III studies
| Pathogens | EUROBACT-2 n = 2927 (%) |
EUROBACT-1 (n = 1317)* |
EPIC III BSI (n = 1239)** |
|---|---|---|---|
| Gram-negative bacteria | 1726 (59) | 759 (57.6) | 515 (44.6) |
| Klebsiella spp. | 482 (27.9) | 156 (20.1) | 144 (28) |
| Carbapenem resistant | 182 (37.8) | 59 (37.8) | 86 (59.7) |
| DTR* | 133 (27.6) | . | . |
| PDR* | 11 (2.3) | 3 (1.9) | . |
| Escherichia coli | 272 (15.8) | 98(12.9) | 116 (22.5) |
| Carbapenem resistant | 20 (7.4) | 1(1) | 32 (27.6) |
| DTR* | 9 (3.3) | . | . |
| PDR* | 0 (0) | 0(0) | . |
| Enterobacter spp. | 141 (8.2) | 88 (11.6) | . |
| Carbapenem resistant | 31 (22) | 5 (5.7) | . |
| DTR* | 8 (5.7) | . | . |
| PDR* | 0 (0) | 0(0) | . |
| Pseudomonas spp. | 247 (14.3) | 150 (19.7) | 67 (13) |
| Carbapenem resistant | 82 (33.2) | 56 (37.3) | 10 (14.9) |
| DTR* | 25 (10.1) | . | . |
| PDR* | 4 (1.6) | 0(0) | . |
| Acinetobacter spp. | 350 (20.3) | 160 (21.1) | 68 (13.2) |
| Carbapenem resistant | 296 (84.6) | 110 (68.7) | 53 (77.9) |
| DTR* | 176 (50.3) | . | . |
| PDR* | 8 (2.3) | 1 (0.6) | . |
| Other Gram-negative bacteria | 234 (13.6) | 107 (14.1) | 177 (34.4) |
| Carbapenem resistant | 24 (12.5) | . | . |
| Gram-positive bacteria | 910 (31.1) | 440 (33.4) | 494 (42.7) |
| Enterococcus spp. | 314 (34.5) | 144 (32.7) | 58 (11.7) |
| Enterococcus faecium | 156 (49.7) | 70 (48.6) | . |
| VRE | 37 (23.7) | 16 (22.9) | . |
| Coagulase-negative Staphylococcus | 273 (30) | 141(32) | 182 (36.8) |
| MRSE | 200 (73.3) | . | 73 (40.1) |
| Staphylococcus aureus | 251 (27.6) | 119 (27) | 180 (36.4) |
| MRSA | 93 (37.1) | 57 (47.9) | 54 (30) |
| Other Gram-positive bacteria | 72 (7.9) | 36 (8.2) | 40 (8.1) |
| Strict anaerobe bacteria | 61 (2.1) | 20 (1.5) | 19 (1.6) |
| Bacteroides | 29 (47.5) | . | . |
| Other anaerobes | 32 (52.5) | . | . |
| Fungi | 230 (7.9) | 98 (7.4) | 126 (10.9) |
| Candida albicans | 91 (39.6) | 56 (57.1) | 71 (56.3) |
| Candida non-albicans spp. | 133 (57.8) | 39 (39.8) | 53 (42.1) |
| Other fungi | 6 (2.6) | 4 (3.2) |
Percentages shown for the relevant pathogen or category. *denotes unavailable or not comparable data. MRSA and MRSE denotes the % of Staphylococcus aureus and Coagulase negative Staphylococcus resistant to methicillin, VRE the % of enterococcus faecium resistant to vancomycin. Carbapenem resistant is defined as at least one carbapenem has been tested and the isolate is resistant to all the carbapenems that have been tested. DTR: Difficult to treat resistance. PDR: Pan-drug resistant (resistant to all tested antibiotics). DTR status is determined on Enterobacteriaceae, Pseudomonas and Acinetobacter species and requires require antibiogram results for ≥ 1 carbapenem, ≥ 1 extended-spectrum cephalosporin, and ≥ 1 fluoroquinolone. Candida unknown species have been classified in non-albicans. All PDR pathogens are DTR, and all DTR are carbapenem-R, thus the count and proportion of DTR and carbapenem-R micro-organisms includes that of the more resistant categories. EUROBACT-1 reported susceptibilities on monomicrobial infections. EPIC III reported the pathogens from 1154 bacterial or fungal bloodstream infections, not restricted to hospital-acquired infections. Sum of percentages exceeds 100 because patients may have had more than 1 infection
Antimicrobial therapy and source control
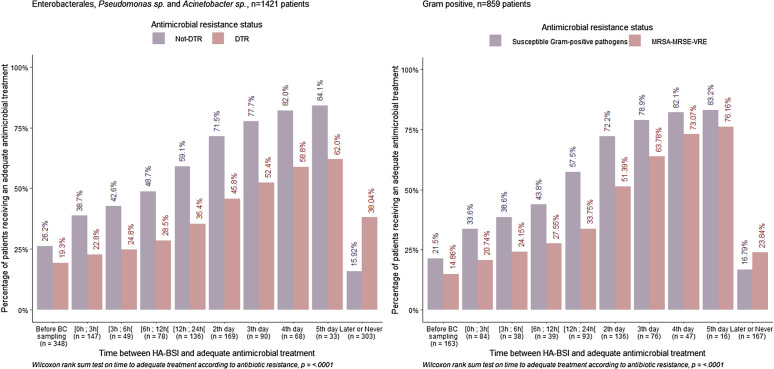
Adequate antimicrobial therapy was received by 51.5% within 24 h of blood culture sampling. As shown in Fig. 1, time to adequate antimicrobial therapy increased with antimicrobial resistance (p < 0.0001). The 3 antimicrobials most frequently administered in the 24 h following HA-BSI diagnosis included meropenem 463/2600 (17.8%), piperacillin/tazobactam 380/2600 (14.6%), and vancomycin 266/2600 (10.2%). They were deemed adequate in 275/463 (59.4%), 244/380 (64.2%), and 132/266 (49.6%) prescriptions, respectively. Source control was deemed to be required for 52.5% of the patients and was effectively achieved in 81.8% of these, after a median of 24.5 [IQR 1;72] hours.
Fig. 1.
Relationship between resistance and timing of adequate antimicrobial therapy. Cumulative percentage of patients receiving at least one adequate antimicrobial, on each time-period before and after the date of collection of the first positive blood culture, shown by antimicrobial resistance status. MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis includes all methicillin resistant coagulase-negative staphylococcus, VRE: vancomycin-resistant Enterococcus. Closed brackets [;] denote inclusive of the end of the range and open brackets]; [ denote the exclusion of the end of the range
Mortality
By day-28, 966 (37.1%) patients had died, 91% in the ICU and 9% after ICU discharge. Death was preceded by a decision to withhold or withdraw life-sustaining treatment for 268 (27.7%). At that time point, 38.7% of the survivors were still in the ICU, 35.7% had been discharged from the ICU, and 25.6% had been discharged from the hospital, which represents 16.1% of the total cohort.
Multiple factors were associated with day-28 mortality in the univariable analysis (Tables 1, 2, 3). At center level these included medical ICUs, lower availability of clinical pharmacists and of therapeutic drug monitoring (TDM) for aminoglycosides or vancomycin. Mortality was higher in patients with co-morbidities, medical and COVID-19 admissions, and those with higher severity of illness, including requirements for organ supportive therapy. Higher mortality was found in early ICU-acquired HA-BSI, respiratory sources, DTR Gram-negative bacteria or fungus, and patients who did not receive adequate antimicrobials or for whom source control was required but not achieved. There was no statistically significant association between time to adequate antimicrobial therapy and day-28 mortality.
Factors associated with death in the multivariable hierarchical logistic model and with an increased subdistribution hazard ratio (sHR) of death at day-28 in a competitive risk model are shown in eTable 5. In summary, factors that were statistically significant in both models included infrequent clinical pharmacist consultation, older age, severity of illness at HA-BSI, DTR Gram negative bacteria, and not achieving source control for patients who required an intervention. Conversely, achieving source control was protective in both analyses.
Discussion
EUROBACT-2 provides an update on the epidemiology and prognostic factors of HA-BSI in the ICU by including 2600 patients from 333 ICUs in 5 continents. We report substantial day-28 mortality, especially in HA-BSI caused by DTR pathogens, patients with septic shock, and those who never received adequate antibiotics or source control. There was a broad range of sources of infection and pathogens. Gram-negative bacteria were frequently carbapenem resistant or DTR. Antibiotic resistance was associated with longer delays to adequate antibiotics. Center data showed important variability of service availability including for the variables related to antimicrobial stewardship.
To our knowledge, the EUROBACT-2 study represents the largest international study of HA-BSI s in ICU patients. Few large international studies have investigated this population, which limits possibilities for direct comparisons with our data. We conducted the EUROBACT-1 study in 2010, with a similar methodology but a smaller group of ICUs [2]. The EPIC III point prevalence study investigated the prevalence and outcomes of ICU patients with infections in 2017 and was not limited to hospital-acquired or bloodstream infections [3]. As shown in Table 4, the two EUROBACT studies showed a predominance of Gram-negative bacteria. In comparison, bloodstream pathogens from the EPIC III cohort showed a higher proportion of Gram-positive bacteria, with more Staphylococcus spp. but less Enterococcus spp. The European Centre for Disease Prevention and Control (ECDC) epidemiological report of hospital-acquired infections in the ICU, computed from 2017 data, showed a predominance of Gram-positive pathogens in HA-BSI. There were 23.6% coagulase-negative staphylococci and 14.9% Enterococcus spp., followed by 12.4% Klebsiella spp. [20].While some of these differences may be explained by the inclusion of community-acquired infections in EPIC III, the lower proportion coagulase-negative staphylococci in our study is probably secondary to the careful review of each case and discussion with the investigators, leading to the exclusion of all potential blood culture contaminants that did not meet the inclusion criteria. Between EUROBACT-1 and 2, the proportion of MRSA has decreased by 10%, and the proportion of vancomycin-resistant Enterococcus (VRE) has remained stable. Interestingly, there has been an increase in the proportion of non-albicans Candida spp., which have now become dominant. Carbapenem resistance has substantially increased, especially for Enterobacter spp. and Acinetobacter spp., leading to a substantial proportion of DTR in Gram-negative pathogens, and up to 1.6% PDR for Pseudomonas spp. and 2.3% for Acinetobacter spp. In keeping with previous reports, and as shown in eTables 5 and 7, carbapenem resistance and DTR in Gram-negative bacteremia were associated with mortality, highlighting the importance of strategies aimed at preventing and treating infections caused by multidrug resistant pathogens [2, 18, 21, 22]. A detailed description of the of the pathogens causing HA-BSI in the COVID-19 population is reported separately [12].
Ten years after the first EUROBACT-2 study, we observed comparable delays to adequate antimicrobial therapy as around half of the patients received such within 24 h of blood culture sampling. Antimicrobial resistance was associated with delays. In the setting of widespread resistance to broad-spectrum antibiotics, molecular rapid diagnostic testing may be a key for earlier adequate antimicrobial treatment [23, 24]. That delays to adequate antimicrobial therapy were not associated with day-28 mortality may be subsequent to multiple confounders and should be interpreted with caution. Indeed, the relationship between time to antimicrobial therapy and mortality in observational research is complicated [25]. On the one hand, the clinical impression of severity may be a driver for earlier administration of broader spectrum antimicrobials to patients with an increased risk of death. Moreover, a non-negligible proportion of patients with sepsis may inexorably die, regardless of the antibiotic treatment. Others may have died before antibiogram results could be acted upon, eliminating an opportunity for antimicrobial adequacy. On the other hand, patients identified at lower risk may have been treated later, when positive microbiology was reported [26]. Another source of immortal-time bias may be present as some patients with HA-BSI may have never been diagnosed or included in the study. Some may have died before they could be transferred to the ICU, underestimating mortality, while others may have rapidly improved, before ICU admission, overestimating mortality of HA-BSI. These findings do not challenge the recommendation for early adequate antimicrobial therapy for patients with sepsis or septic shock [27]. Indeed, while we need to avoid antibiotic overuse and its associated harms [28], early adequate antimicrobial therapy is one of the most important interventions for HA-BSI [27].
How can these observations improve clinical practice? The exploratory analysis suggests a protective effect of source control and a possible detrimental effect of infrequent clinical pharmacist consultation. These highlight the importance of a multidisciplinary approach for managing critically ill patients with HA-BSI, and by extension, severe infections. Hospitals require integrated pathways, protocols, and educational programs targeting recognition, diagnosis, and treatment of sepsis, including prediction of antimicrobial resistance, antimicrobial prescription, and source control [27, 29, 30]. The optimisation of antimicrobial therapy in critically ill patients involves a multifaceted approach. Pharmacodynamic/pharmacokinetic optimisation and adequate exposure at the source of infection requires optimal dosing and delivery, considering potential interactions, modified volume of distribution, and decreased or augmented renal clearance [31]. Integrated antimicrobial stewardship programs may facilitate clinically relevant advice and recommendations on antibiotic choice, dosing, mode of delivery, indications for therapeutic drug monitoring, and a discussion on source control [6, 27, 32].
There are important limitations to this study. Firstly, ICUs were predominantly from the Europe and Central Asia and the East Asia and Pacific regions, and from high-income and upper-middle-income countries, thus limiting the generalizability of our results. Second, we started data collection before and continued during the first year of the COVID-19 pandemic. This likely influenced the patient population, microorganism distribution, antimicrobial resistance and mortality [33, 34]. Some ICUs were unable to start or complete the study, leading to multiple exclusions. However, we report similar patient severity, pathogen distribution, and mortality to the EUROBACT-1 study, validating the current report. Thirdly, pathogen identification and antimicrobial susceptibility testing relied on each laboratory, with possible differences in interpretation leading to inconsistencies. The patients at risk of late onset BSI had to stay in the ICU for more than 7 days to be exposed to this risk, leading to potential selection bias. The method used for the multivariable analysis led to poor calibration, which is now presented in the ESM. Moreover, data collection was performed by individual investigators in 330 ICUs, without on-site monitoring. We improved the risk of inconsistencies with online checks through the electronic case report file, and by closely monitoring data quality and coherence for each case-report.
Interpretation
HA-BSI in ICU patients was mainly caused by Gram-negative bacteria, with widespread carbapenem resistance and DTR. Antibiotic resistance was associated with longer delays to adequate antimicrobial therapy. HA-BSI was associated with 37.1% mortality, and by day-28 only 16.1% of the patients had been discharged alive from the hospital. Multifaceted programs to decrease multidrug resistance as well as prevent, recognize, and manage HA-BSI, with a focus on antimicrobial adequacy and source control are suggested to improve patient management and outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The EUROBACT-2 study was endorsed by the European Society of Intensive Care Medicine (ESICM), the infection section of the ESCIM and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) study Group for Infections in Critically Ill Patients (ESGCIP), with scientific input of the OUTCOMEREA network. The members of the Eurobact 2 Study Group are: Alexis Tabah, Hamish Pollock, Ben Margetts, Meredith Young, Neeraj Bhadange, Steven Tyler, Anne Ledtischke, Mackenzie Finnis, Anne Ledtischke, Mackenzie Finnis, Jyotsna Dwivedi, Manoj Saxena, Vishwanath Biradar, Natalie Soar, Vineet Sarode, David Brewster, Adrian Regli, Elizabeth Weeda, Samiul Ahmed, Cheryl Fourie, Kevin Laupland, Mahesh Ramanan, James Walsham, Jason Meyer, Edward Litton, Anna Maria Palermo, Timothy Yap, Ege Eroglu, Antony George Attokaran, C'havala Jaramillo, Khalid Mahmood Khan Nafees, Nurhikmahtul Aqilah Haji Abd Rashid, Haji Adi Muhamad Ibnu Walid, Tomas Mon, P. Dhakshina Moorthi, Shah Sudhirchandra, Dhadappa Damodar Sridharan, Qiu Haibo, Xie Jianfeng, Lu Wei-Hua, Wang Zhen, Chuanyun Qian, Jili Luo, Xiaomei Chen, Hao Wang, Peng Zhao, Juan Zhao, Qiu Wusi, Chen Mingmin, Lei Xu, Chengfen Yin, Ruilan Wang, Jinfeng Wang, Yongjie Yin, Min Zhang, Jilu Ye, Chungfang Hu, Suming Zhou, Min Huang, Jing Yan, Yan Wang, Bingyu Qin, Ling Ye, Xie Weifeng, Li Peije, Nan Geng, Yoshiro Hayashi, Toshiyuki Karumai, Masaki Yamasaki, Satoru Hashimoto, Koji Hosokawa, Jun Makino, Takeo Matsuyoshi, Akira Kuriyama, Hidenobu Shigemitsu, Yuka Mishima, Michio Nagashima, Hideki Yoshida, Shigeki Fujitani, Koichiro Omori, Hiroshi Rinka, Hiroki Saito, Kaori Atobe, Hideaki Kato, Shunsuke Takaki, M. Shahnaz Hasan, Muhamad Fadhil Hadi Jamaluddin, Lee See Pheng, Sheshendrasurian Visvalingam, Mun Thing Liew, Siong Ling Danny Wong, Kean Khang Fong, Hamizah Bt Abdul Rahman, Zuraini Md Noor, Lee Kok Tong, Abd. Hamid Azman, Mohd Zulfakar Mazlan, Saedah Ali, Kyeongman Jeon, Sang-Min Lee, Sunghoon Park, Seung Yong Park, Sung Yoon Lim, Qing Yuan Goh, Shin Yi Ng, Sui An Lie, Andrea Lay Hoon Kwa, Ken Junyang Goh, Andrew Yunkai Li, Caroline Yu Ming Ong, Jia Yan Lim, Jessica Lishan Quah, Kangqi Ng, Louis Xiang Long Ng, Yu Chang Yeh, Nai-Kuan Chou, Cong-Tat Cia, Ting-Yu Hu, Li-Kuo Kuo, Shih-Chi Ku, Phunsup Wongsurakiat, Yutthana Apichatbutr, Supattra Chiewroongroj, Rashid Nadeem, Ashraf El Houfi, Adel Alsisi, Amr Elhadidy, Mina Barsoum, Nermin Osman, Tarek Mostafa, Mohamed Elbahnasawy, Ahmed Saber, Amer Aldhalia, Omar Elmandouh, Ahmed Elsayed, Merihan A. Elbadawy, Ahmed K. Awad, Hanan M. Hemead, Farid Zand, Maryam Ouhadian, Seyed Hamid Borsi, Zahra Mehraban, Davood Kashipazha, Fatemeh Ahmadi, Mohsen Savaie, Farhad Soltani, Mahboobeh Rashidi, Reza Baghbanian, Fatemeh Javaherforoosh, Fereshteh Amiri, Arash Kiani, Mohammad Amin Zargar, Ata Mahmoodpoor, Fatemeh Aalinezhad, Gholamreza Dabiri, Golnar Sabetian, Hakimeh Sarshad, Mansoor Masjedi, Ramin Tajvidi, Seyed Mohammad Nasirodin Tabatabaei, Abdullah Khudhur Ahmed, Pierre Singer, Ilya Kagan, Merav Rigler, Daniel Belman, Phillip Levin, Belal Harara, Adei Diab, Fayez Abilama, Rebecca Ibrahim, Aya Fares, Ahmad Buimsaedah, Marwa Gamra, Ahmed Aqeelah, Almajdoub Ali Mohammed Ali, Ahmed Gaber Sadik Homaidan, Bushray Almiqlash, Hala Bilkhayr, Ahmad Bouhuwaish, Ahmed Sa Taher, Eman Abdulwahed, Fathi A. Abousnina, Aisha Khaled Hdada, Rania Jobran, Hayat Ben Hasan, Rabab Shaban Ben Hasan, Issam Serghini, Rachid Seddiki, Brahim Boukatta, Nabil Kanjaa, Doumiri Mouhssine, Maazouzi Ahmed Wajdi, Tarek Dendane, Amine Ali Zeggwagh, Brahim Housni, Oujidi Younes, Abdelhamid Hachimi, A. Ghannam, Z. Belkhadir, Sarah Amro, Mustafa Abu Jayyab, Ali Ait Hssain, Abdurahaman Elbuzidi, Edin Karic, Marcus Lance, Shaikh Nissar, Hend Sallam, Omar Elrabi, Ghaleb A. Almekhlafi, Maher Awad, Ahmed Aljabbary, Mohammad Karam Chaaban, Natalia Abu-Sayf, Mohammad Al-Jadaan, Lubna Bakr, Mounir Bouaziz, Olfa Turki, Walid Sellami, Pablo Centeno, Lic Natalia Morvillo, José Oscar Acevedo, Patricia Mabel Lopez, Rubén Fernández, Matías Segura, Dra Marta Aparicio, Microbiologa Irene Alonzo, Yanina Nuccetelli, Pablo Montefiore, Luis Felipe Reyes, Luis Felipe Reyes, Silvio A. Ñamendys-Silva, Juan P. Romero-Gonzalez, Mariana Hermosillo, Roberto Alejandro Castillo, Jesús Nicolás Pantoja Leal, Candy Garcia Aguilar, Mara Ocotlan Gonzalez Herrera, Missael Vladimir Espinoza Villafuerte, Manuel Lomeli-Teran, Jose G. Dominguez-Cherit, Adrian Davalos-Alvarez, Silvio A. Ñamendys-Silva, Luis Sánchez-Hurtado, Brigitte Tejeda-Huezo, Orlando R. Perez-Nieto, Ernesto Deloya Tomas, Liesbet De Bus, Jan De Waele, Isabelle Hollevoet, Wouter Denys, Marc Bourgeois, Sofie F. M. Vanderhaeghen, Jean-Baptiste Mesland, Pierre Henin, Lionel Haentjens, Patrick Biston, Cindérella Noel, Nathalie Layos, Benoît Misset, Nicolas De Schryver, Nicolas Serck, Xavier Wittebole, Elisabeth De Waele, Godelive Opdenacker, Pedja Kovacevic, Biljana Zlojutro, Aida Custovic, Ina Filipovic-Grcic, Radovan Radonic, Ana Vujaklija Brajkovic, Jasminka Persec, Sanja Sakan, Mario Nikolic, Hrvoje Lasic, Marc Leone, Charlotte Arbelot, Jean-François Timsit, Juliette Patrier, N. Zappela, P. Montravers, Thierry Dulac, Jérémy Castanera, Johann Auchabie, Anthony Le Meur, A. Marchalot, M. Beuzelin, Alexandre Massri, Charlotte Guesdon, Etienne Escudier, Philippe Mateu, Jérémy Rosman, Olivier Leroy, Serge Alfandari, Alexandru Nica, Bertrand Souweine, Elisabeth Coupez, Thibault Duburcq, Eric Kipnis, Perrine Bortolotti, Mathieu Le Souhaitier, Jean-Paul Mira, Pierre Garcon, Matthieu Duprey, Martial Thyrault, Rémi Paulet, François Philippart, Marc Tran, Cédric Bruel, Emmanuel Weiss, Sylvie Janny, Arnaud Foucrier, Pierre-François Perrigault, Flora Djanikian, François Barbier, Marc Gainnier, Jérémy Bourenne, Guillaume Louis, Roland Smonig, Laurent Argaud, Thomas Baudry, Armand Mekonted Dessap, Keyvan Razazi, Pierre Kalfon, Gaëtan Badre, Romaric Larcher, Jean-Yves Lefrant, Claire Roger, Benjamine Sarton, Stein Silva, Sophie Demeret, Loïc Le Guennec, Shidasp Siami, Christelle Aparicio, Guillaume Voiriot, Muriel Fartoukh, Claire Dahyot-Fizelier, Nadia Imzi, Kada Klouche, Hendrik Bracht, Sandra Hoheisen, Frank Bloos, Daniel Thomas-Rueddel, Sirak Petros, Bastian Pasieka, Simon Dubler, Karsten Schmidt, Antje Gottschalk, Carola Wempe, Philippe Lepper, Carlos Metz, Dmitriy Viderman, Yerlan Ymbetzhanov, Miras Mugazov, Yelena Bazhykayeva, Zhannur Kaligozhin, Baurzhan Babashev, Yevgeniy Merenkov, Talgat Temirov, Kostoula Arvaniti, Dimitrios Smyrniotis, Vasiliki Psallida, Georgios Fildisis, Vasiliki Soulountsi, Evangelos Kaimakamis, Cristina Iasonidou, Sofia Papoti, Foteini Renta, Maria Vasileiou, Vasiliki Romanou, Vasiliki Koutsoukou, Mariana Kristina Matei, Leora Moldovan, Ilias Karaiskos, Harry Paskalis, Kyriaki Marmanidou, M. Papanikolaou, C. Kampolis, Marina Oikonomou, Evangelos Kogkopoulos, Charikleia Nikolaou, Anastasios Sakkalis, Marinos Chatzis, Maria Georgopoulou, Anna Efthymiou, Vasiliki Chantziara, Aikaterini Sakagianni, Zoi Athanasa, Eirini Papageorgiou, Fadi Ali, Georges Dimopoulos, Mariota Panagiota Almiroudi, Polychronis Malliotakis, Diamantina Marouli, Vasiliki Theodorou, Ioannis Retselas, Vasilios Kouroulas, Georgios Papathanakos, Giorgia Montrucchio, Gabriele Sales, Gennaro De Pascale, Luca Maria Montini, Simone Carelli, Joel Vargas, Valentina Di Gravio, Daniele Roberto Giacobbe, Angelo Gratarola, Elisa Porcile, Michele Mirabella, Ivan Daroui, Giovanni Lodi, Francesco Zuccaro, Maria Grazia Schlevenin, Paolo Pelosi, Denise Battaglini, Andrea Cortegiani, Mariachiara Ippolito, Davide Bellina, Andrea Di Guardo, Lorella Pelagalli, Marco Covotta, Monica Rocco, Silvia Fiorelli, Antonella Cotoia, Anna Chiara Rizzo, Adam Mikstacki, Barbara Tamowicz, Irmina Kaptur Komorowska, Anna Szczesniak, Jozef Bojko, Anna Kotkowska, Paulina Walczak-Wieteska, Dominika Wasowska, Tomasz Nowakowski, Hanna Broda, Mariusz Peichota, Iwona Pietraszek-Grzywaczewska, Ignacio Martin-Loeches, Alessandra Bisanti, Nuno Cartoze, Tiago Pereira, Nádia Guimarães, Madalena Alves, Ana Josefina Pinheiro Marques, Ana Rios Pinto, Andriy Krystopchuk, Ana Teresa, António Manuel Pereira de Figueiredo, Isabel Botelho, Tiago Duarte, Vasco Costa, Rui Pedro Cunha, Elena Molinos, Tito da Costa, Sara Ledo, Joana Queiró, Dulce Pascoalinho, Cristina Nunes, José Pedro Moura, Énio Pereira, António Carvalho Mendes, Liana Valeanu, Serban Bubenek-Turconi, Ioana Marina Grintescu, Cristian Cobilinschi, Daniela Carmen Filipescu, Cornelia Elena Predoi, Dana Tomescu, Mihai Popescu, Alexandra Marcu, Ioana Grigoras, Olguta Lungu, Alexey Gritsan, Anastasia Anderzhanova, Yulia Meleshkina, Marat Magomedov, Nadezhda Zubareva, Maksim Tribulev, Denis Gaigolnik, Aleksandr Eremenko, Natala Vistovskaya, Maria Chukina, Vladislav Belskiy, Mikhail Furman, Ricard Ferrer Rocca, Maria Martinez, Vanessa Casares, Paula Vera, Matias Flores, Joaquin Amador Amerigo, Maria Pilar Gracia Arnillas, Rosana Munoz Bermudez, Fernando Armestar, Beatriz Catalan, Regina Roig, Laura Raguer, María Dolores Quesada, Emilio Diaz Santos, Gemma Gomà, Alejandro Ubeda, Dra Maria Salgado, Lorena Forcelledo Espina, Emilio Garcia Prieto, Dra Mj Asensio, Dra M. Rodriguez, Emilio Maseda, Alejandro Suarez De La Rica, J. Ignacio Ayestaran, Mariana Novo, Miguel Angel Blasco-Navalpotro, Alberto Orejas Gallego, Fredrik Sjövall, Dzana Spahic, Carl Johan Svensson, Michael Haney, Alicia Edin, Joyce Åkerlund, Lina De Geer, Josef Prazak, Stephan Jakob, Jl Pagani, S. Abed-Maillard, Murat Akova, Abdullah Tarik Aslan, Arif Timuroglu, Sesin Kocagoz, Hulya Kusoglu, Selcuk Mehtap, Solakoğlu Ceyhun, Neriman Defne Altintas, Leyla Talan, Bircan Kayaaslan, Ayşe Kaya Kalem, Ibrahim Kurt, Murat Telli, Barcin Ozturk, Çiğdem Erol, Emine Kubra Dindar Demiray, Sait Çolak, Türkay Akbas, Kursat Gundogan, Ali Sari, Canan Agalar, Onur Çolak, Nurcan N. Baykam, Ozlem O. Akdogan, Mesut Yilmaz, Burcu Tunay, Rumeysa Cakmak, Nese Saltoglu, Ridvan Karaali, Iftihar Koksal, Firdevs Aksoy, Ahmet Eroglu, Kemal Tolga Saracoglu, Yeliz Bilir, Seda Guzeldag, Gulden Ersoz, Guliz Evik, Hulya Sungurtekin, Cansu Ozgen, Cem Erdoğan, Yunus Gürbüz, Nilgün Altin, Yasar Bayindir, Yasemin Ersoy, Senay Goksu, Ahmet Akyol, Ayse Batirel, Sabahat Cagan Aktas, Andrew Conway Morris, Matthew Routledge, Andrew Conway Morris, Ari Ercole, David Antcliffe, Roceld Rojo, Kate Tizard, Maria Faulkner, Amanda Cowton, Melanie Kent, Ashok Raj, Artemis Zormpa, George Tinaslanidis, Reena Khade, Tomasz Torlinski, Randeep Mulhi, Shraddha Goyal, Manan Bajaj, Marina Soltan, Aimee Yonan, Rachael Dolan, Aimee Johnson, Caroline Macfie, James Lennard, Maie Templeton, Sonia Sousa Arias, Uwe Franke, Keith Hugill, Hollie Angell, Benjamin J. Parcell, Katherine Cobb, Stephen Cole, Tim Smith, Clive Graham, Jaroslav Cerman, Allison Keegan, Jenny Ritzema, Amanda Sanderson, Ashraf Roshdy, Tamas Szakmany, Tom Baumer, Rebecca Longbottom, Daniel Hall, Kate Tatham, S. Loftus, A. Husain, E. Black, S. Jhanji, R. Rao Baikady, Peter Mcguigan, Rachel Mckee, Santhana Kannan, Supriya Antrolikar, Nicholas Marsden, Valentina Della Torre, Dorota Banach, Ahmed Zaki, Matthew Jackson, Moses Chikungwa, Ben Attwood, Jamie Patel, Rebecca E. Tilley, Miss Sally K. Humphreys, Paul Jean Renaud, Anton Sokhan, Yaroslava Burma, Wendy Sligl, Nadia Baig, Lorena McCoshen, Demetrios J. Kutsogiannis, Wendy Sligl, Patricia Thompson, Tayne Hewer, Raihan Rabbani, Shihan Mahmud Redwanul Huq, Rajib Hasan, Mohammad Motiul Islam, Mohan Gurjar, Arvind Baronia, Nikhil Kothari, Ankur Sharma, Saurabh Karmakar, Priya Sharma, Janardan Nimbolkar, Pratit Samdani, R. Vaidyanathan, Noor Ahmedi Rubina, Nikhilesh Jain, Madhumati Pahuja, Ritu Singh, Saurav Shekhar, Syed Nabeel Muzaffar, Ahmad Ozair, Suhail Sarwar Siddiqui, Payel Bose, Avijatri Datta, Darshana Rathod, Mayur Patel, M. K. Renuka, Sailaja K. Baby, Carol Dsilva, Jagadish Chandran, Pralay Ghosh, Sudipta Mukherjee, Kaladhar Sheshala, Krushna Chandra Misra, Saidu Yusuf Yakubu, Euphemia Mgbosoro Ugwu, John O. Olatosi, Ibironke Desalu, Gabriel Asiyanbi, Motunrayo Oladimeji, Olusola Idowu, Fowotade Adeola, Melanie Mc Cree, Ali Adil Ali Karar, Elfayadh Saidahmed, Hytham K. S. Hamid
Author contributions
Alexis Tabah, Niccolò Buetti, Jean-François Timsit, Quentin Staiquly, and Stéphane Ruckly had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All the authors approved the manuscript in its final format. Concept and design: Alexis Tabah, Jean-François Timsit, Jan De Waele, Jeffrey Lipman, and Jose Artur Paiva. Coordination: Alexis Tabah, Caroline Dallongeville, Quentin Staiquly, Stéphane Ruckly, Jean-François Timsit, Guy Francois, Murat Akova, Abdullah Tarik Aslan, Marc Leone, Andrew Conway Morris, Matteo Bassetti, Kostoula Arvaniti, Ricard Ferrer, Haibo Qiu, Jose Artur Paiva, Liesbet De Bus, Guy Francois, Farid Zand, Mohan Gurjar, Adel Alsisi, Khalid Abidi, Hendrik Bracht, Yoshiro Hayashi, Adam Mikstacki, Alexey Gritsan, Kyeongman Jeon, Liana Valeanu, Helmi Sulaiman, Tony Yeh, Muhammed Elhadi, Mounir Bouaziz, Khalid Mahmood Khan Nafees, Gabriela Vidal, Qing Yuan Goh, Dmitriy Viderman, Silvio A. Namendys-Silva, Josef Prazak, Phunsup Wongsurakiat, Wendy Sligl, Pierre Singer, Ali Aithssain, Fredrik Sjovall, Pedja Kovacevic, Bashir Kamal Eldin Hamid el Sanousi, Mario Arias, Aaron Mark Hernandez, Ignacio Martin-Loeches, Ina Filipovic-Grcic, Fayez Abillama, Raihan Rabbani, Mervyn Mer, Lowell Ling, Oyebola Olubodun Adekola. Communication, centre registration and coordination: Caroline Dallongeville. Quality control, data curation, analysis, and interpretation: Alexis Tabah, Niccolò Buetti, François Barbier, Caroline Dallongeville, Quentin Staiquly, Stéphane Ruckly, and Jean-François Timsit. Statistical analysis: Quentin Staiquly, Stéphane Ruckly, and Jean-François Timsit. Drafting of the manuscript: Alexis Tabah. Initial revision of the manuscript: Niccolò Buetti, François Barbier, Jean-François Timsit and Jeffrey Lipman. Revision of the manuscript for important intellectual content: All authors. Administrative, technical, or material support: Caroline Dallongeville, Quentin Staiquly, Stéphane Ruckly, and Guy Francois. Group Information: The EUROBACT-2 national coordinators, scientific committee and investigators are listed in the electronic supplement. Additional Contributions: We thank Guy Francois, Division of Scientific Affairs and Research of the ESICM for his unvaluable assistance in recruiting participating ICUs. We thank the health care workers at each study site, laboratory, and beyond that contributed to the EUROBACT-2 study and acknowledge their work during the initial waves of the COVID-19 pandemic. We thank the patients who participated in the EUROBACT-2 study. We thank Professor Andrea Marshall, PhD, and Doctor Mahesh Ramanan, MBBS, FCICM for proofreading and editing the manuscript.
Funding
JdW is a senior clinical investigator funded by the Research Foundation Flanders (FWO, Ref. 1881020N). ACM is supported by a Medical Research Council Clinician Scientist Fellowship (MR/ V006118/1). NB received a fellowship grant (Grant number: P4P4PM_194449) from the Swiss National Science Foundation. Research grants were obtained from the European Society of Intensive Care Medicine (ESICM), the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) study Group for Infections in Critically Ill Patients (ESGCIP), the Norva Dahlia foundation and the Redcliffe Hospital Private Practice Trust Fund.
Availability of data and material
The datasets used and/or analysed during the current study are available from the OUTCOMEREA organisation on reasonable request.
Declarations
Conflict of interest
AT has nothing to disclose, NB has nothing to disclose, QS has nothing to disclose, SR has nothing to disclose, MA reports honoraria paid to his university for educational activities by Pfizer, Sanofi, MSD and Astra Zeneca, ATA has nothing to disclose, ML reported consulting and lecture fees from Amomed Pharma, Aspen, LFB and Gilead, ACM has received payment for speaking on behalf of Boston Scientific and sits on the Scientific Advisory Board of Cambridge Infection Diagnostics, a start-up seeking to develop novel diagnostics for infectious diseases, MB received advisory board, speaker activities from Angelini, Bayer, Biomerieux, Cidara, Gilead, Menarini, MSD, Pfizer, Roche, Shionogi, study grants from: Angelini, Shionogi, Cidara, Gilead, Pfizer, and MSD, KA has nothing to disclose, JL has received lecture fees and honoraria from MSD, RF reports Payment for lectures, speakers bureaus or advisory boards from Grifols, MSD, Pfizer, Gilead, Shionogi, Thermofisher, Hill Rom, AOP Health, BD, HQ has nothing to disclose, JAP reports consulting, advisory boards or lectures fees and honoraria for MSD, Pfizer, Astra-Zeneca, Gilead, Jansen, Cepheid, AOP Orphan Pharmaceuticals, PP reported advisory boards participation for Gilead, Technophage and Sanofi, lectures fees from MSD, Gilead and Pfizer, and research grant from Abionic, LDB has nothing to disclose, JdW has consulted for Pfizer, MSD (honoraria paid to institution), FZ has nothing to disclose, MG has nothing to disclose, AA has nothing to disclose, KA has nothing to disclose, HB has nothing to disclose, YH has nothing to disclose, KJ has nothing to disclose, ME has nothing to disclose, FB reported consulting and lecture fees, conference invitation from MSD and lecture fees from BioMérieux, J-FT reported advisory boards participation for Merck, Gilead, Beckton-Dickinson, Pfizer, Shinogi, Medimune, Paratek, research grants from Merck, Pfizer, Thermofischer.
Footnotes
The members of the EUROBACT-2 study group are listed in the Acknowledgement section of the manuscript.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexis Tabah, Email: a.tabah@uq.edu.au.
on behalf of the EUROBACT-2 Study Group, ESICM, ESCMID ESGCIP and the OUTCOMEREA Network:
Alexis Tabah, Hamish Pollock, Ben Margetts, Meredith Young, Neeraj Bhadange, Steven Tyler, Anne Ledtischke, Mackenzie Finnis, Anne Ledtischke, Mackenzie Finnis, Jyotsna Dwivedi, Manoj Saxena, Vishwanath Biradar, Natalie Soar, Vineet Sarode, David Brewster, Adrian Regli, Elizabeth Weeda, Samiul Ahmed, Cheryl Fourie, Kevin Laupland, Mahesh Ramanan, James Walsham, Jason Meyer, Edward Litton, Anna Maria Palermo, Timothy Yap, Ege Eroglu, Antony George Attokaran, C’havala Jaramillo, Khalid Mahmood Khan Nafees, Nurhikmahtul Aqilah Haji Abd Rashid, Haji Adi Muhamad Ibnu Walid, Tomas Mon, P. Dhakshina Moorthi, Shah Sudhirchandra, Dhadappa Damodar Sridharan, Qiu Haibo, Xie Jianfeng, Lu Wei-Hua, Wang Zhen, Chuanyun Qian, Jili Luo, Xiaomei Chen, Hao Wang, Peng Zhao, Juan Zhao, Qiu Wusi, Chen Mingmin, Lei Xu, Chengfen Yin, Ruilan Wang, Jinfeng Wang, Yongjie Yin, Min Zhang, Jilu Ye, Chungfang Hu, Suming Zhou, Min Huang, Jing Yan, Yan Wang, Bingyu Qin, Ling Ye, Xie Weifeng, Li Peije, Nan Geng, Yoshiro Hayashi, Toshiyuki Karumai, Masaki Yamasaki, Satoru Hashimoto, Koji Hosokawa, Jun Makino, Takeo Matsuyoshi, Akira Kuriyama, Hidenobu Shigemitsu, Yuka Mishima, Michio Nagashima, Hideki Yoshida, Shigeki Fujitani, Koichiro Omori, Hiroshi Rinka, Hiroki Saito, Kaori Atobe, Hideaki Kato, Shunsuke Takaki, M. Shahnaz Hasan, Muhamad Fadhil Hadi Jamaluddin, Lee See Pheng, Sheshendrasurian Visvalingam, Mun Thing Liew, Siong Ling Danny Wong, Kean Khang Fong, Hamizah Bt Abdul Rahman, Zuraini Md Noor, Lee Kok Tong, Abd. Hamid Azman, Mohd Zulfakar Mazlan, Saedah Ali, Kyeongman Jeon, Sang-Min Lee, Sunghoon Park, Seung Yong Park, Sung Yoon Lim, Qing Yuan Goh, Shin Yi Ng, Sui An Lie, Andrea Lay Hoon Kwa, Ken Junyang Goh, Andrew Yunkai Li, Caroline Yu Ming Ong, Jia Yan Lim, Jessica Lishan Quah, Kangqi Ng, Louis Xiang Long Ng, Yu Chang Yeh, Nai-Kuan Chou, Cong-Tat Cia, Ting-Yu Hu, Li-Kuo Kuo, Shih-Chi Ku, Phunsup Wongsurakiat, Yutthana Apichatbutr, Supattra Chiewroongroj, Rashid Nadeem, Ashraf El Houfi, Adel Alsisi, Amr Elhadidy, Mina Barsoum, Nermin Osman, Tarek Mostafa, Mohamed Elbahnasawy, Ahmed Saber, Amer Aldhalia, Omar Elmandouh, Ahmed Elsayed, Merihan A. Elbadawy, Ahmed K. Awad, Hanan M. Hemead, Farid Zand, Maryam Ouhadian, Seyed Hamid Borsi, Zahra Mehraban, Davood Kashipazha, Fatemeh Ahmadi, Mohsen Savaie, Farhad Soltani, Mahboobeh Rashidi, Reza Baghbanian, Fatemeh Javaherforoosh, Fereshteh Amiri, Arash Kiani, Mohammad Amin Zargar, Ata Mahmoodpoor, Fatemeh Aalinezhad, Gholamreza Dabiri, Golnar Sabetian, Hakimeh Sarshad, Mansoor Masjedi, Ramin Tajvidi, Seyed Mohammad Nasirodin Tabatabaei, Abdullah Khudhur Ahmed, Pierre Singer, Ilya Kagan, Merav Rigler, Daniel Belman, Phillip Levin, Belal Harara, Adei Diab, Fayez Abilama, Rebecca Ibrahim, Aya Fares, Ahmad Buimsaedah, Marwa Gamra, Ahmed Aqeelah, Almajdoub Mohammed AliAli, Ahmed Gaber Sadik Homaidan, Bushray Almiqlash, Hala Bilkhayr, Ahmad Bouhuwaish, Ahmed Sa Taher, Eman Abdulwahed, Fathi A. Abousnina, Aisha Khaled Hdada, Rania Jobran, Hayat Ben Hasan, Rabab Shaban Ben Hasan, Issam Serghini, Rachid Seddiki, Brahim Boukatta, Nabil Kanjaa, Doumiri Mouhssine, Maazouzi Ahmed Wajdi, Tarek Dendane, Amine Ali Zeggwagh, Brahim Housni, Oujidi Younes, Abdelhamid Hachimi, A. Ghannam, Z. Belkhadir, Sarah Amro, Mustafa Abu Jayyab, Ali Ait Hssain, Abdurahaman Elbuzidi, Edin Karic, Marcus Lance, Shaikh Nissar, Hend Sallam, Omar Elrabi, Ghaleb A. Almekhlafi, Maher Awad, Ahmed Aljabbary, Mohammad Karam Chaaban, Natalia Abu-Sayf, Mohammad Al-Jadaan, Lubna Bakr, Mounir Bouaziz, Olfa Turki, Walid Sellami, Pablo Centeno, Lic Natalia Morvillo, José Oscar Acevedo, Patricia Mabel Lopez, Rubén Fernández, Matías Segura, Dra Marta Aparicio, Microbiologa Irene Alonzo, Yanina Nuccetelli, Pablo Montefiore, Luis Felipe Reyes, Luis Felipe Reyes, Silvio A. Ñamendys-Silva, Juan P. Romero-Gonzalez, Mariana Hermosillo, Roberto Alejandro Castillo, Jesús Nicolás Pantoja Leal, Candy Garcia Aguilar, Mara Ocotlan Gonzalez Herrera, Missael Vladimir Espinoza Villafuerte, Manuel Lomeli-Teran, Jose G. Dominguez-Cherit, Adrian Davalos-Alvarez, Silvio A. Ñamendys-Silva, Luis Sánchez-Hurtado, Brigitte Tejeda-Huezo, Orlando R. Perez-Nieto, Ernesto Deloya Tomas, Liesbet De Bus, Jan De Waele, Isabelle Hollevoet, Wouter Denys, Marc Bourgeois, Sofie F. M. Vanderhaeghen, Jean-Baptiste Mesland, Pierre Henin, Lionel Haentjens, Patrick Biston, Cindérella Noel, Nathalie Layos, Benoît Misset, Nicolas De Schryver, Nicolas Serck, Xavier Wittebole, Elisabeth De Waele, Godelive Opdenacker, Pedja Kovacevic, Biljana Zlojutro, Aida Custovic, Ina Filipovic-Grcic, Radovan Radonic, Ana Vujaklija Brajkovic, Jasminka Persec, Sanja Sakan, Mario Nikolic, Hrvoje Lasic, Marc Leone, Charlotte Arbelot, Jean-François Timsit, Juliette Patrier, N. Zappela, P. Montravers, Thierry Dulac, Jérémy Castanera, Johann Auchabie, Anthony Le Meur, A. Marchalot, M. Beuzelin, Alexandre Massri, Charlotte Guesdon, Etienne Escudier, Philippe Mateu, Jérémy Rosman, Olivier Leroy, Serge Alfandari, Alexandru Nica, Bertrand Souweine, Elisabeth Coupez, Thibault Duburcq, Eric Kipnis, Perrine Bortolotti, Mathieu Le Souhaitier, Jean-Paul Mira, Pierre Garcon, Matthieu Duprey, Martial Thyrault, Rémi Paulet, François Philippart, Marc Tran, Cédric Bruel, Emmanuel Weiss, Sylvie Janny, Arnaud Foucrier, Pierre-François Perrigault, Flora Djanikian, François Barbier, Marc Gainnier, Jérémy Bourenne, Guillaume Louis, Roland Smonig, Laurent Argaud, Thomas Baudry, Armand Mekonted Dessap, Keyvan Razazi, Pierre Kalfon, Gaëtan Badre, Romaric Larcher, Jean-Yves Lefrant, Claire Roger, Benjamine Sarton, Stein Silva, Sophie Demeret, Loïc Le Guennec, Shidasp Siami, Christelle Aparicio, Guillaume Voiriot, Muriel Fartoukh, Claire Dahyot-Fizelier, Nadia Imzi, Kada Klouche, Hendrik Bracht, Sandra Hoheisen, Frank Bloos, Daniel Thomas-Rueddel, Sirak Petros, Bastian Pasieka, Simon Dubler, Karsten Schmidt, Antje Gottschalk, Carola Wempe, Philippe Lepper, Carlos Metz, Dmitriy Viderman, Yerlan Ymbetzhanov, Miras Mugazov, Yelena Bazhykayeva, Zhannur Kaligozhin, Baurzhan Babashev, Yevgeniy Merenkov, Talgat Temirov, Kostoula Arvaniti, Dimitrios Smyrniotis, Vasiliki Psallida, Georgios Fildisis, Vasiliki Soulountsi, Evangelos Kaimakamis, Cristina Iasonidou, Sofia Papoti, Foteini Renta, Maria Vasileiou, Vasiliki Romanou, Vasiliki Koutsoukou, Mariana Kristina Matei, Leora Moldovan, Ilias Karaiskos, Harry Paskalis, Kyriaki Marmanidou, M. Papanikolaou, C. Kampolis, Marina Oikonomou, Evangelos Kogkopoulos, Charikleia Nikolaou, Anastasios Sakkalis, Marinos Chatzis, Maria Georgopoulou, Anna Efthymiou, Vasiliki Chantziara, Aikaterini Sakagianni, Zoi Athanasa, Eirini Papageorgiou, Fadi Ali, Georges Dimopoulos, Mariota Panagiota Almiroudi, Polychronis Malliotakis, Diamantina Marouli, Vasiliki Theodorou, Ioannis Retselas, Vasilios Kouroulas, Georgios Papathanakos, Giorgia Montrucchio, Gabriele Sales, Gennaro De Pascale, Luca Maria Montini, Simone Carelli, Joel Vargas, Valentina Di Gravio, Daniele Roberto Giacobbe, Angelo Gratarola, Elisa Porcile, Michele Mirabella, Ivan Daroui, Giovanni Lodi, Francesco Zuccaro, Maria Grazia Schlevenin, Paolo Pelosi, Denise Battaglini, Andrea Cortegiani, Mariachiara Ippolito, Davide Bellina, Andrea Di Guardo, Lorella Pelagalli, Marco Covotta, Monica Rocco, Silvia Fiorelli, Antonella Cotoia, Anna Chiara Rizzo, Adam Mikstacki, Barbara Tamowicz, Irmina Kaptur Komorowska, Anna Szczesniak, Jozef Bojko, Anna Kotkowska, Paulina Walczak-Wieteska, Dominika Wasowska, Tomasz Nowakowski, Hanna Broda, Mariusz Peichota, Iwona Pietraszek-Grzywaczewska, Ignacio Martin-Loeches, Alessandra Bisanti, Nuno Cartoze, Tiago Pereira, Nádia Guimarães, Madalena Alves, Ana Josefina Pinheiro Marques, Ana Rios Pinto, Andriy Krystopchuk, Ana Teresa, António Manuel Pereira de Figueiredo, Isabel Botelho, Tiago Duarte, Vasco Costa, Rui Pedro Cunha, Elena Molinos, Tito da Costa, Sara Ledo, Joana Queiró, Dulce Pascoalinho, Cristina Nunes, José Pedro Moura, Énio Pereira, António Carvalho Mendes, Liana Valeanu, Serban Bubenek-Turconi, Ioana Marina Grintescu, Cristian Cobilinschi, Daniela Carmen Filipescu, Cornelia Elena Predoi, Dana Tomescu, Mihai Popescu, Alexandra Marcu, Ioana Grigoras, Olguta Lungu, Alexey Gritsan, Anastasia Anderzhanova, Yulia Meleshkina, Marat Magomedov, Nadezhda Zubareva, Maksim Tribulev, Denis Gaigolnik, Aleksandr Eremenko, Natala Vistovskaya, Maria Chukina, Vladislav Belskiy, Mikhail Furman, Ricard Ferrer Rocca, Maria Martinez, Vanessa Casares, Paula Vera, Matias Flores, Joaquin Amador Amerigo, Maria Pilar Gracia Arnillas, Rosana Munoz Bermudez, Fernando Armestar, Beatriz Catalan, Regina Roig, Laura Raguer, María Dolores Quesada, Emilio Diaz Santos, Gemma Gomà, Alejandro Ubeda, Dra Maria Salgado, Lorena Forcelledo Espina, Emilio Garcia Prieto, Dra Mj Asensio, Dra M. Rodriguez, Emilio Maseda, Alejandro Suarez De La Rica, J. Ignacio Ayestaran, Mariana Novo, Miguel Angel Blasco-Navalpotro, Alberto Orejas Gallego, Fredrik Sjövall, Dzana Spahic, Carl Johan Svensson, Michael Haney, Alicia Edin, Joyce Åkerlund, Lina De Geer, Josef Prazak, Stephan Jakob, Jl Pagani, S. Abed-Maillard, Murat Akova, Abdullah Tarik Aslan, Arif Timuroglu, Sesin Kocagoz, Hulya Kusoglu, Selcuk Mehtap, Solakoğlu Ceyhun, Neriman Defne Altintas, Leyla Talan, Bircan Kayaaslan, Ayşe Kaya Kalem, Ibrahim Kurt, Murat Telli, Barcin Ozturk, Çiğdem Erol, Emine Kubra Dindar Demiray, Sait Çolak, Türkay Akbas, Kursat Gundogan, Ali Sari, Canan Agalar, Onur Çolak, Nurcan N. Baykam, Ozlem O. Akdogan, Mesut Yilmaz, Burcu Tunay, Rumeysa Cakmak, Nese Saltoglu, Ridvan Karaali, Iftihar Koksal, Firdevs Aksoy, Ahmet Eroglu, Kemal Tolga Saracoglu, Yeliz Bilir, Seda Guzeldag, Gulden Ersoz, Guliz Evik, Hulya Sungurtekin, Cansu Ozgen, Cem Erdoğan, Yunus Gürbüz, Nilgün Altin, Yasar Bayindir, Yasemin Ersoy, Senay Goksu, Ahmet Akyol, Ayse Batirel, Sabahat Cagan Aktas, Andrew Conway Morris, Matthew Routledge, Andrew Conway Morris, Ari Ercole, David Antcliffe, Roceld Rojo, Kate Tizard, Maria Faulkner, Amanda Cowton, Melanie Kent, Ashok Raj, Artemis Zormpa, George Tinaslanidis, Reena Khade, Tomasz Torlinski, Randeep Mulhi, Shraddha Goyal, Manan Bajaj, Marina Soltan, Aimee Yonan, Rachael Dolan, Aimee Johnson, Caroline Macfie, James Lennard, Maie Templeton, Sonia Sousa Arias, Uwe Franke, Keith Hugill, Hollie Angell, Benjamin J. Parcell, Katherine Cobb, Stephen Cole, Tim Smith, Clive Graham, Jaroslav Cerman, Allison Keegan, Jenny Ritzema, Amanda Sanderson, Ashraf Roshdy, Tamas Szakmany, Tom Baumer, Rebecca Longbottom, Daniel Hall, Kate Tatham, S. Loftus, A. Husain, E. Black, S. Jhanji, R. Rao Baikady, Peter Mcguigan, Rachel Mckee, Santhana Kannan, Supriya Antrolikar, Nicholas Marsden, Valentina Della Torre, Dorota Banach, Ahmed Zaki, Matthew Jackson, Moses Chikungwa, Ben Attwood, Jamie Patel, Rebecca E. Tilley, Miss Sally K. Humphreys, Paul Jean Renaud, Anton Sokhan, Yaroslava Burma, Wendy Sligl, Nadia Baig, Lorena McCoshen, Demetrios J. Kutsogiannis, Wendy Sligl, Patricia Thompson, Tayne Hewer, Raihan Rabbani, Shihan Mahmud Redwanul Huq, Rajib Hasan, Mohammad Motiul Islam, Mohan Gurjar, Arvind Baronia, Nikhil Kothari, Ankur Sharma, Saurabh Karmakar, Priya Sharma, Janardan Nimbolkar, Pratit Samdani, R. Vaidyanathan, Noor Ahmedi Rubina, Nikhilesh Jain, Madhumati Pahuja, Ritu Singh, Saurav Shekhar, Syed Nabeel Muzaffar, Ahmad Ozair, Suhail Sarwar Siddiqui, Payel Bose, Avijatri Datta, Darshana Rathod, Mayur Patel, M. K. Renuka, Sailaja K. Baby, Carol Dsilva, Jagadish Chandran, Pralay Ghosh, Sudipta Mukherjee, Kaladhar Sheshala, Krushna Chandra Misra, Saidu Yusuf Yakubu, Euphemia Mgbosoro Ugwu, John O. Olatosi, Ibironke Desalu, Gabriel Asiyanbi, Motunrayo Oladimeji, Olusola Idowu, Fowotade Adeola, Melanie Mc Cree, Ali Adil Ali Karar, Elfayadh Saidahmed, and Hytham K. S. Hamid
References
- 1.Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank H-P, Ducomble T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. doi: 10.1371/journal.pmed.1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38(12):1930–1945. doi: 10.1007/s00134-012-2695-9. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487. doi: 10.1001/jama.2020.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adrie C, Garrouste-Orgeas M, Ibn Essaied W, Schwebel C, Darmon M, Mourvillier B, et al. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. 2017;74(2):131–141. doi: 10.1016/j.jinf.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Prowle JR, Echeverri JE, Ligabo EV, Sherry N, Taori GC, Crozier TM, et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care. 2011;15(2):R100. doi: 10.1186/cc10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Waele JJ, Akova M, Antonelli M, Canton R, Carlet J, De Backer D et al (2018) Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med 44(2):189–196 [DOI] [PubMed]
- 7.Bezabih YM, Bezabih A, Dion M, Batard E, Teka S, Obole A, et al. Comparison of the global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli between healthcare and community settings: a systematic review and meta-analysis. JAC Antimicrob Resist. 2022;76(1):22–29. doi: 10.1093/jacamr/dlac048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (2021) Global antimicrobial resistance and use surveillance system (GLASS) report: 2021
- 9.Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Suppl 2):S82–89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Buetti N, Tabah A, Loiodice A, Ruckly S, Aslan AT, Montrucchio G, et al. Different epidemiology of bloodstream infections in COVID-19 compared to non-COVID-19 critically ill patients: a descriptive analysis of the Eurobact II study. Crit Care. 2022;26(1):319. doi: 10.1186/s13054-022-04166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UN Statistics Division. Standard country and area codes for statistical use. https://unstats.un.org/unsd/methodology/m49/. Accessed 1 July 2022
- 14.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22(7):707–710 [DOI] [PubMed]
- 16.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for disease control and prevention C (2019) Carbapenem-resistant enterobacterales (CRE): CRE technical information. https://www.cdc.gov/hai/organisms/cre/technical-info.html#Definition. Accessed 1 July 2022
- 18.Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67(12):1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 20.European Centre for Disease Prevention and Control (2019) Healthcare-associated infections acquired in intensive care units. In: ECDC. Annual epidemiological report for 2017. https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-intensive-care-units-annual-epidemiological-1. Accessed 1 July 2022
- 21.Bonnet V, Dupont H, Glorion S, Aupee M, Kipnis E, Gerard JL, et al. Influence of bacterial resistance on mortality in intensive care units: a registry study from 2000 to 2013 (IICU Study) J Hosp Infect. 2019;102(3):317–324. doi: 10.1016/j.jhin.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Huh K, Chung DR, Ha YE, Ko JH, Kim SH, Kim MJ, et al. Impact of difficult-to-treat resistance in gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data. Clin Infect Dis. 2020;71(9):e487–e496. doi: 10.1093/cid/ciaa084. [DOI] [PubMed] [Google Scholar]
- 23.Giacobbe DR, Giani T, Bassetti M, Marchese A, Viscoli C, Rossolini GM. Rapid microbiological tests for bloodstream infections due to multidrug resistant Gram-negative bacteria: therapeutic implications. Clin Microbiol Infect. 2020;26(6):713–722. doi: 10.1016/j.cmi.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61(7):1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberger J, Rhee C, Klompas M. A critical analysis of the literature on time-to-antibiotics in suspected sepsis. J Infect Dis. 2020;222(Suppl 2):S110–S118. doi: 10.1093/infdis/jiaa146. [DOI] [PubMed] [Google Scholar]
- 26.Hranjec T, Rosenberger LH, Swenson B, Metzger R, Flohr TR, Politano AD, et al. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: a quasi-experimental, before and after observational cohort study. Lancet Infect Dis. 2012;12(10):774–780. doi: 10.1016/S1473-3099(12)70151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curran J, Lo J, Leung V, Brown K, Schwartz KL, Daneman N, et al. Estimating daily antibiotic harms: an umbrella review with individual study meta-analysis. Clin Microbiol Infect. 2022;28(4):479–490. doi: 10.1016/j.cmi.2021.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Goodman KE, Lessler J, Cosgrove SE, Harris AD, Lautenbach E, Han JH, et al. A clinical decision tree to predict whether a bacteremic patient is infected with an extended-spectrum beta-lactamase-producing organism. Clin Infect Dis. 2016;63(7):896–903. doi: 10.1093/cid/ciw425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 31.Heffernan AJ, Mohd Sazlly Lim S, Lipman J, Roberts JA. A personalised approach to antibiotic pharmacokinetics and pharmacodynamics in critically ill patients. Anaesth Crit Care Pain Med. 2021;40(6):100970. doi: 10.1016/j.accpm.2021.100970. [DOI] [PubMed] [Google Scholar]
- 32.Tabah A, Lipman J, Barbier F, Buetti N, Timsit J-F. Use of antimicrobials for bloodstream infections in the intensive care unit, a clinically oriented review. Antibiotics. 2022;11(3):362. doi: 10.3390/antibiotics11030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160(2):454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buetti N, Ruckly S, de Montmollin E, Reignier J, Terzi N, Cohen Y, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180–187. doi: 10.1007/s00134-021-06346-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the OUTCOMEREA organisation on reasonable request.