Abstract
Stilbenoids are anti-inflammatory and antioxidant compounds, with resveratrol being the most investigated molecule in this class. However, the actions of most other stilbenoids are much less studied. This study compares five monomeric (resveratrol, piceatannol, pterostilbene, pinostilbene, and trimethoxy-resveratrol) and two dimeric (dehydro-δ-viniferin and trans-δ-viniferin) stilbenoids for their capability to modulate the production of bacteria-induced cytokines (IL-12, IL-10, and TNF-α), as well as lipopolysaccharide (LPS)-induced reactive oxygen species (ROS), in murine bone marrow-derived dendritic cells. All monomeric species showed dose-dependent inhibition of E. coli-induced IL-12 and TNF-α, whereas only resveratrol and piceatannol inhibited IL-10 production. All monomers, except trimethoxy-resveratrol, inhibited L. acidophilus-induced IL-12, IL-10, and TNF-α production. The dimer dehydro-δ-viniferin remarkably enhanced L. acidophilus-induced IL-12 production. The contrasting effect of resveratrol and dehydro-δ-viniferin on IL-12 production was due, at least in part, to a divergent inactivation of the mitogen-activated protein kinases by the two stilbenoids. Despite having moderate to high total antioxidant activity, dehydro-δ-viniferin was a weak inhibitor of LPS-induced ROS formation. Conversely, resveratrol and piceatannol potently inhibited LPS-induced ROS formation. Methylated monomers showed a decreased antioxidant capacity compared to resveratrol, also depending on the methylation site. In summary, the immune-modulating effect of the stilbenoids depends on both specific structural features of tested compounds and the stimulating bacteria.
Keywords: monomeric stilbenoids, dimeric stilbenoids, immune modulating, ROS formation, dendritic cells
1. Introduction
Stilbenoids, which include resveratrol (3,4′,5-trihydroxy-trans-stilbene) and its hydroxylated (e.g., piceatannol) and methylated (e.g., pterostilbene and pinostilbene) derivatives, are a group of naturally occurring phenolic compounds found in various plant species. Stilbenoids possess several biological activities, including anti-inflammatory, antioxidative, and anticarcinogenic properties [1]. The bioavailability of the different stilbenoid compounds differs substantially and may depend on multiple factors, such as solubility, lipophilicity, and metabolic and chemical stability [2]. Substitution of hydroxy with methoxy groups increases lipophilicity, so that pterostilbene is more bioavailable than resveratrol [1]. Furthermore, resveratrol-derived dimers, such as δ-viniferin and its dehydro analogue, are also characterized by different bioavailability and biological activities compared to their monomers. For these reasons, information on the relative potency of stilbenoids, including whether a relationship exists between the anti-inflammatory and antioxidant ability, is still fuzzy.
Macrophages and dendritic cells constitute key cell types involved in inflammation and in the initiation of immune responses. In particular, dendritic cells play an essential role in the conduction of immune responses, e.g., by activating different subtypes of T-cells through the production of cytokines in response to signals provided by microbial organisms [3]. Regarding bacteria-induced cytokine production, it has been previously demonstrated that Gram-negative and Gram-positive bacteria induce responses through different pathways [4,5,6].
The antioxidant effect of stilbenoids has often been assumed to explain most of the other observed bioactivities, not least the anti-inflammatory effect [7]. However, stilbenoids have also demonstrated the ability to act on a broad range of cellular molecules involved in inflammatory signaling [8]. Most studies on the antioxidative or anti-inflammatory properties of stilbenoids were conducted on resveratrol, whereas only marginal attention has been placed on its hydroxylated and methylated analogues [8]. Additionally, assessment of bioactivities has generally been performed in a limited experimental setup without considering that the modulation exerted by these molecules is dependent on the nature of the stimulating agent [4,5].
To our knowledge, no systematic study comparing the immune-modulating and antioxidant effects of the various stilbenoid subclasses has been conducted so far. Here, a comparative and inclusive study has been performed to assess the immune-modulating effect of monomeric (resveratrol, piceatannol, pterostilbene, pinostilbene, and trimethoxy-resveratrol) and dimeric (dehydro-δ-viniferin and trans-δ-viniferin) stilbenoids [9] on the production of bacterially induced cytokines (IL-12, IL-10, and TNF-α) in murine bone marrow-derived dendritic cells (bmDCs). The study also included the possible effects on the lipopolysaccharide (LPS)-induced production of reactive oxygen species (ROS) in the same experimental system. In this frame, the relationship between the aforementioned effects and the antioxidant capacity of the individual molecules (as evaluated by ABTS and DPPH assays) was also investigated to provide a comprehensive vision of these systems.
2. Results
2.1. Methylation of Resveratrol Diminishes Its Inhibitory Effect on the L. Acidophilus NCFM-Induced Cytokine Response
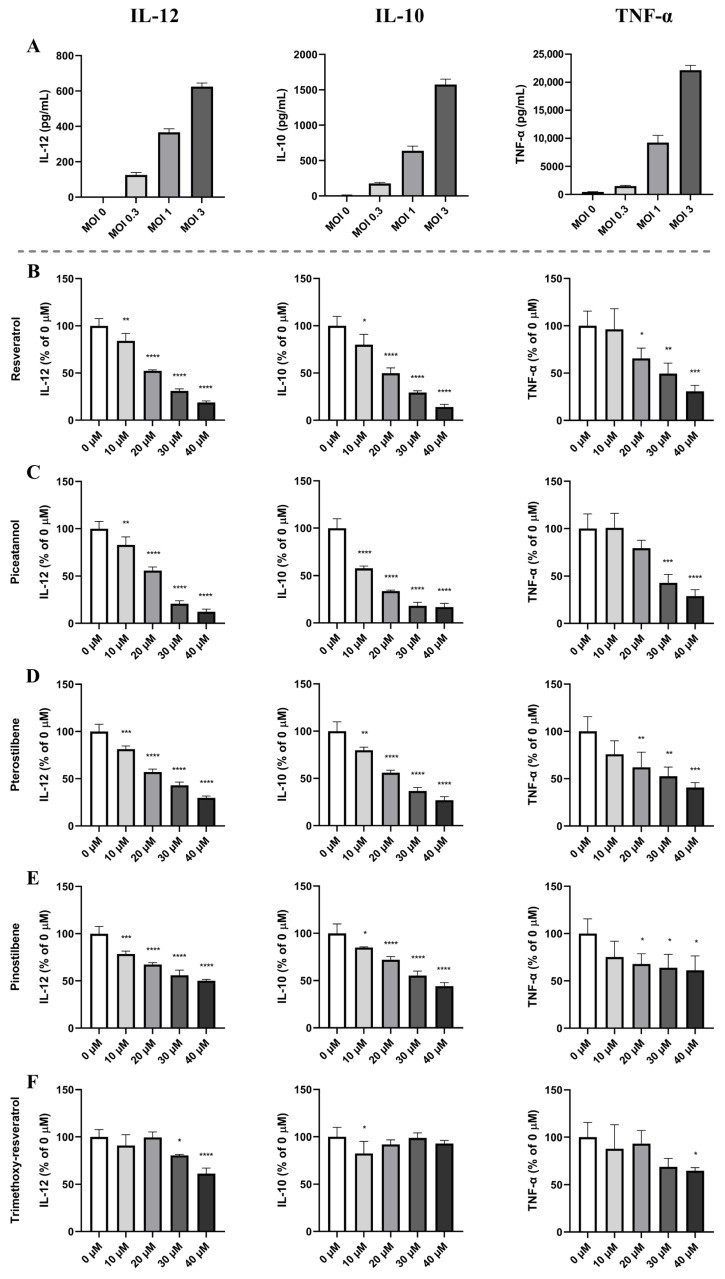
Murine bmDCs stimulated with an increasing multiplicity of infection (MOI) of L. acidophilus NCFM responded with a dose-dependent production of IL-12, IL-10, and TNF-α (Figure 1A). An increasing concentration of either stilbenoid resulted in a dose-dependent inhibition of all three cytokines, leading to at least a 70% decrease in their production at the highest stilbenoid concentration.
Figure 1.
Resveratrol and piceatannol hold vigorous cytokine-inhibiting roles in L. acidophilus NCFM-stimulated bmDCs, and methylation of resveratrol weakens the inhibitory capabilities. Murine bmDCs were stimulated with L. acidophilus NCFM at multiplicity of infection (MOI) at rates of 0.3, 1, and 3 (A) and incubated for 20 h. To assess the effect of monomers on the cytokine response, bmDCs were incubated with increasing concentrations (0–40 µM) of resveratrol (B), piceatannol (C), pterostilbene (D), pinostilbene (E), and trimethoxy-resveratrol (F) for 30 min before L. acidophilus NCFM (MOI 1) was added to the samples. Cytokine levels in the supernatant for stilbenoid-treated samples (B–F) are shown as percentages of samples with no stilbenoid treatment (0 µM). The depicted data are representative of at least three experiments. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
Methylated analogues demonstrated a decreased inhibition capacity compared to resveratrol. More specifically, methylation of two hydroxy groups in resveratrol (i.e., pinostilbene and pterostilbene) resulted in a less decreased cytokine production, while methylation of all three hydroxy groups in resveratrol (i.e., trimethoxy-resveratrol) almost entirely abrogated the inhibitory capacity (Figure 1, panels D–F). The inhibitory effect on cytokine production relates to the substitution pattern. Whereas methylation of the two hydroxyl groups at C3 and C5 (pterostilbene, Figure 1D) gave only a slightly lower inhibition than resveratrol, methylation of the hydroxy groups at C3 and C4′ (pinostilbene, Figure 1E) resulted in a substantially weakened inhibition of cytokine release (40 µM pinostilbene gave ≈ 50% inhibition of IL-12 production compared to ≈81% inhibition for 40 µM resveratrol).
2.2. Monomer Derivatives Show Potent IL-12 Inhibition but Weak Inhibition of IL-10 and TNF-α Production in bmDCs Stimulated with E. Coli Nissle 1917
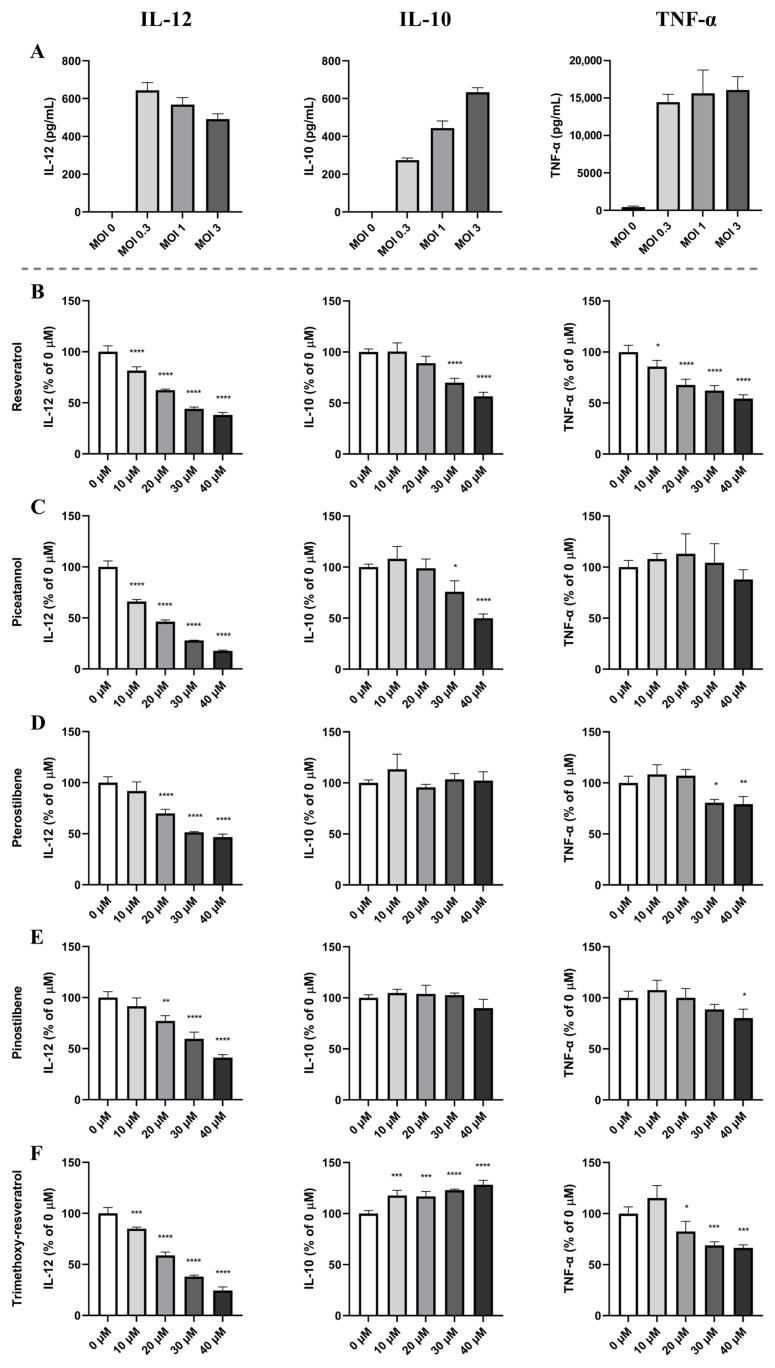
Stimulation with increasing MOI (0.3–3) of the Gram-negative E. coli Nissle 1917 resulted in a dose-dependent increase for IL-10, a slight decrease for IL-12, and no effects on TNF-α production (Figure 2A). All monomers significantly inhibited the E. coli-induced production of IL-12 in a dose-dependent manner (Figure 2, panels B–F). Piceatannol was the most potent inhibitor of IL-12 production (≈82% at 40 µM, Figure 2C). Trimethoxy-resveratrol also displayed more potent inhibition of IL-12 than resveratrol (75% inhibition vs. ≈61% for resveratrol, both at 40 µM, Figure 2, panel F vs. panel B). Only resveratrol and piceatannol displayed significant inhibition of IL-10 production, but only at high concentrations (30 μM and 40 μM, Figure 2, panels B,C). Only the trimethylated analogue and resveratrol showed potent inhibition of the E. coli-induced TNF-α production (Figure 2, panels B,F).
Figure 2.
Methylation of resveratrol weakens the IL-10 inhibitor activity in E. coli Nissle 1917-stimulated bmDCs. Murine bmDCs were stimulated with E. coli Nissle 1917 at multiplicity of infection (MOI) rates of 0.3, 1, and 3 (A) and incubated for 20 h. To assess the effect of monomers on the cytokine response, bmDCs were incubated with increasing concentrations (0–40 µM) of resveratrol (B), piceatannol (C), pterostilbene (D), pinostilbene (E), and trimethoxy-resveratrol (F) for 30 min before E. coli Nissle 1917 (MOI 1) was added to the samples. Cytokine levels in the supernatant for stilbenoid-treated samples (B–F) are shown as percentages of samples without stilbenoid treatment (0 µM). The depicted data are representative of at least three experiments. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
2.3. Effect of Dimeric Stilbenoids on the Bacterially Induced Cytokine Response
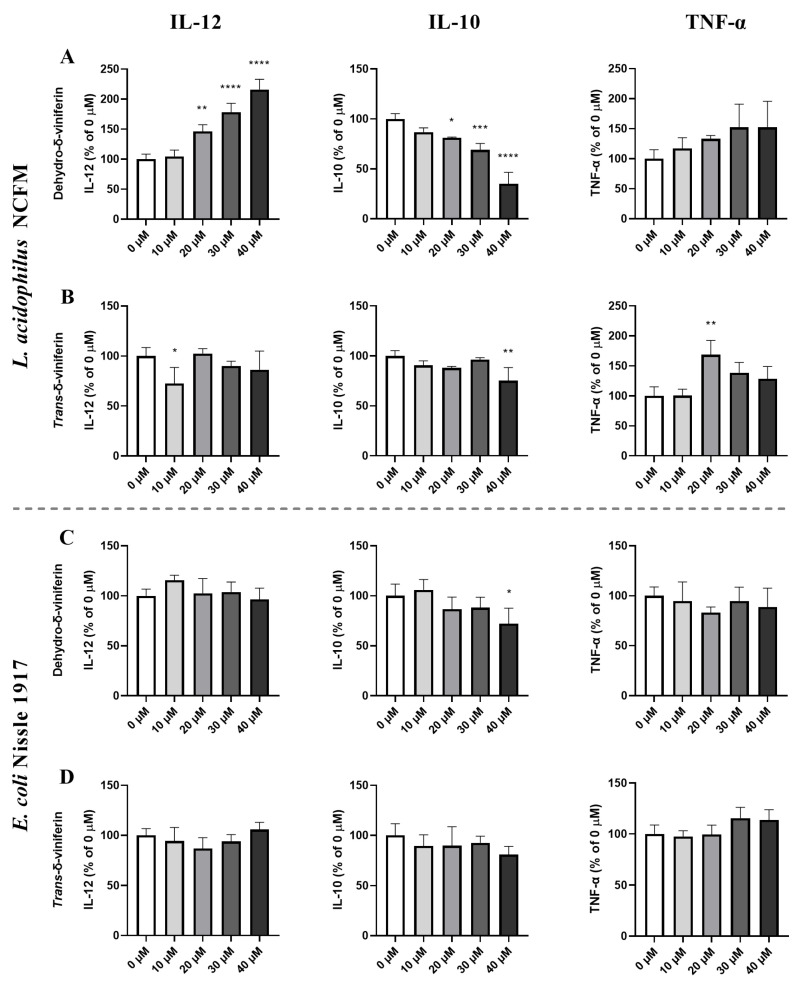
The stilbenoid dimers dehydro-δ-viniferin and trans-δ-viniferin were used in the same molar concentration range of stilbenoid monomers (0–40 μM) to assess whether dimerization affected bacterially induced cytokine response. Dehydro-δ-viniferin enhanced L. acidophilus-induced IL-12 response in a dose-dependent manner, whereas the IL-10 response showed a dose-dependent inhibition, and TNF-α gave a non-significant increase (Figure 3A). Treatment with trans-δ-viniferin did not affect the L. acidophilus-induced cytokine response pattern (Figure 3B). Neither dehydro-δ-viniferin nor trans-δ-viniferin affected the E. coli-induced production of any of the examined cytokines (Figure 3, panels C,D).
Figure 3.
Effect of stilbenoid dimers on the bacterially induced cytokine response depends on the bacterial strain. Murine bmDCs were incubated with increasing concentrations (0–40 µM) of dehydro-δ-viniferin (A,C) and trans-δ-viniferin (B,D) for 30 min before stimulation with L. acidophilus NCFM (multiplicity of infection (MOI) 1) (A,B) or E. coli Nissle 1917 (MOI 1, C,D) and incubated for 20 h. Cytokine levels in the supernatant for the stilbenoid treated samples are shown as percentages of samples without stilbenoid treatment (0 µM). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
2.4. Number and Position of Hydroxy Groups Affect Stilbenoids Inhibition of Intracellular ROS
The antioxidant effect of the stilbenoid structures in the bmDCs was investigated by measuring their influence on the microbially induced intracellular ROS formation, as determined by oxidation of 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA).
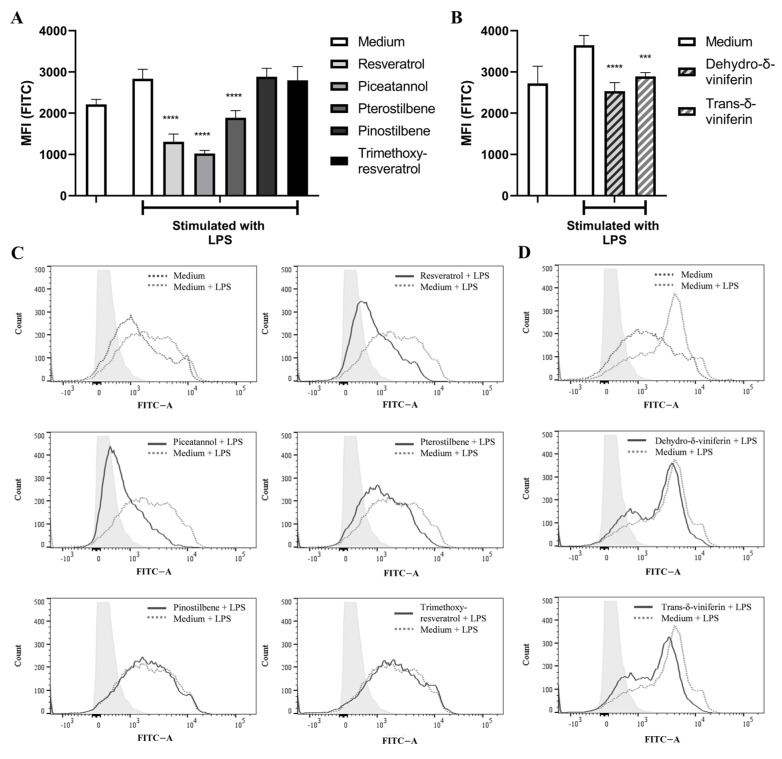
LPS-induced ROS formation was diminished for cells treated with the stilbenoid monomers resveratrol, piceatannol, and, to a lesser extent, pterostilbene (Figure 4A,C). Cells treated with pinostilbene or trimethoxy-resveratrol did not show a significant decrease of ROS formation, whereas dehydro-δ-viniferin and trans-δ-viniferin decreased LPS-induced ROS formation (Figure 4B,D) and dehydro-δ-viniferin led to slightly greater inhibition of ROS formation than trans-δ-viniferin.
Figure 4.
Effect of stilbenoid monomers and dimers on LPS-induced ROS formation in bmDCs. Murine bmDCs were administered carboxy-H2DCFDA and incubated in the absence or in the presence of the given stilbenoids (30 µM) for 30 min before stimulation with LPS (100 ng/mL). After 4 h, ROS formation in bmDCs was determined by using flow cytometry to quantitate formation of oxidized carboxy-H2DCFDA. The mean fluorescence index (MFI) is shown in panels (A) (monomers) and (B) (dimers). The original flow cytometry results for monomers and dimers are grouped in (C,D), respectively. *** p ≤ 0.001, **** p ≤ 0.0001.
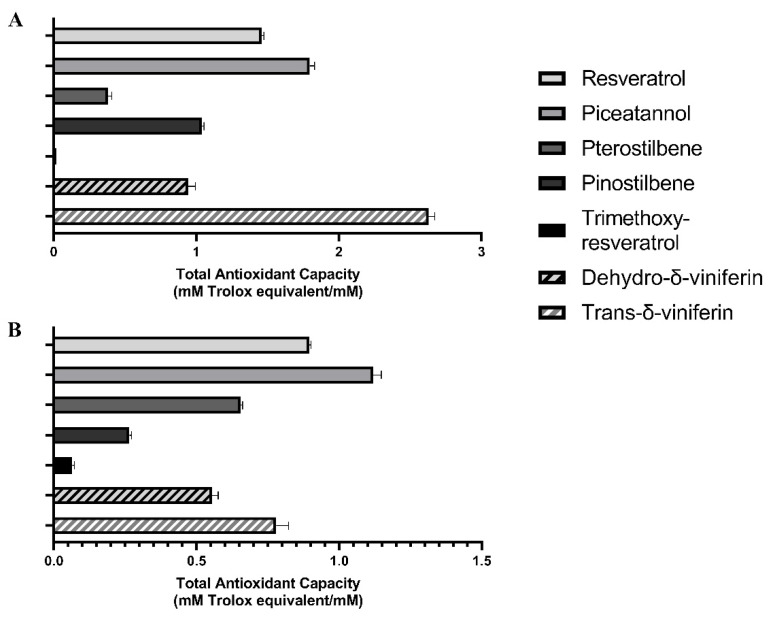
2.5. Determination of the Total Antioxidant Capacity of Resveratrol and Resveratrol Derivatives
The antioxidant capacity of the monomeric and dimeric resveratrol derivatives was further examined by determining their total antioxidant capacity (TAC), as measured with both the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay.
In agreement with data on intracellular ROS formation, piceatannol and resveratrol showed the highest TAC rates in ABTS assays. Pterostilbene’s TAC was lower than that of pinostilbene. As expected, trimethoxy resveratrol displayed no detectable TAC. Indeed, a significant correlation (r = 0.9339; R2 = 0.8722; p < 0.05) was observed between the number of hydroxyl groups present in the chemical structure of each monomer and their TAC, as measured by the ABTS assay. As for stilbenoid dimers, the highest TAC was observed for trans-δ-viniferin, whereas the TAC of dehydro-δ-viniferin was comparable to that of pinostilbene (Figure 5A). Again, a strong positive correlation was evident between the number of hydroxy groups in all stilbenoids (monomers and dimers) and the TAC evaluated by the ABTS assay (r = 0.7776; R2 = 0.6047; p < 0.05).
Figure 5.
Total antioxidant capacity (TAC) in stilbenoid monomers and dimers. The TAC rates of the five monomeric stilbenoids and the two dimeric stilbenoids were determined using ABTS (A) and DPPH assay (B). TAC values were calculated for stilbenoids at an equimolar concentration (1 mM) and are expressed as mM Trolox equivalent/mM stilbenoids.
The DPPH assay confirmed that piceatannol and resveratrol had the highest TAC rates and trimethoxy-resveratrol the lowest. However, when using DPPH, pterostilbene displayed a higher TAC than pinostilbene, with values for pterostilbene similar to those measured for dehydro-δ-viniferin and trans-δ-viniferin (Figure 5B). By using the DPPH assay, a significant correlation (r = 0.9371; R2 = 0.8781; p < 0.05) was observed between TAC and the number of hydroxy groups present in the chemical structure of monomers.
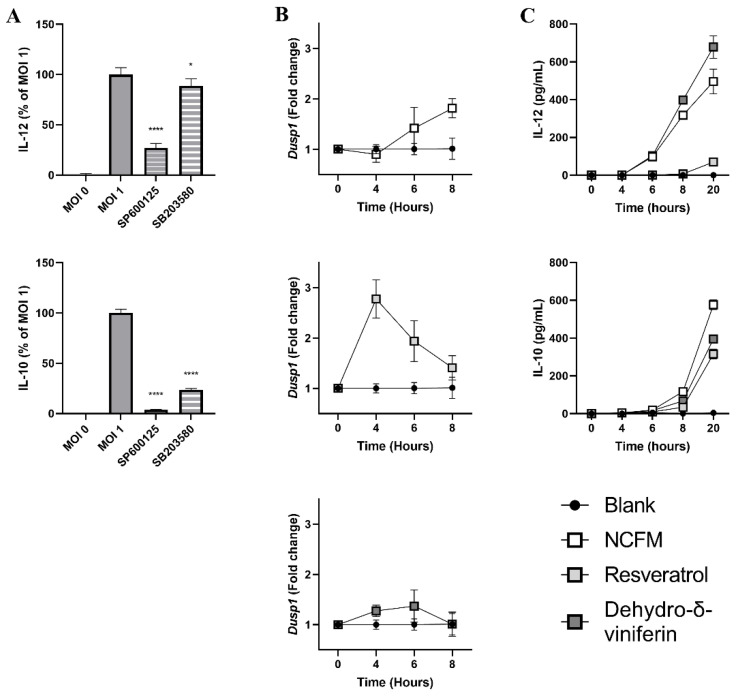
2.6. Resveratrol and Dehydro-δ-Viniferin Affect Map Kinases Divergently
The striking difference in L. acidophilus NCFM-induced IL-12 production elicited by resveratrol and dehydro-δ-viniferin prompted us to investigate the mechanisms underlying the divergent activities of the two stilbenoids. Accordingly, it has been investigated how inhibitors of the Jun N-terminal kinase (JNK) and p38 affected the IL-12 and IL-10 responses to stimulation with L. acidophilus NCFM and how L. acidophilus (used alone or after a resveratrol or dehydro-δ-viniferin pretreatment) affected the expression of dual specificity phosphatase 1 (Dusp1) (Figure 6). While JNK inhibition gave a potent inhibition of IL-12 (73%) and IL-10 (96%) production, inhibition of p38 inhibition had only minor effects on IL-12 (11% inhibition), but strongly impaired IL-10 production (76% inhibition, Figure 6A). Thus, JNK activation seems indispensable for L. acidophilus NCFM-induced effects on IL-12 production.
Figure 6.
Resveratrol and dehydro-δ-viniferin interfere differently with MAP kinase pathways. Murine bmDCs were treated with or without SP600125 (JNK-inhibitor, 25 µM) or SB203580 (p38-inhibitor, 10 µM) for 30 min before stimulation with L. acidophilus NCFM (MOI 1) and incubated for 20 h (A). Cytokine levels in the supernatant for stilbenoid-treated samples are shown as percentages of samples without stilbenoid treatment (MOI 1). The expression rates of Dusp1 in bmDCs (0 to 8 h) upon stimulation with L. acidophilus NCFM with or without 30 min pre-incubation with resveratrol (30 µM) or dehydro-δ-viniferin (30 µM) are shown (B). Dusp1 expression is shown as fold change relative to unstimulated cells (black) with Actb as the reference gene. The cytokine responses in the supernatants of bmDCs stimulated with L. acidophilus NCFM over time (white) with or without resveratrol (30 µM) (light grey) or dehydro-δ-viniferin (30 µM) (dark grey) added 30 min prior to stimulation are shown (C). * p ≤ 0.05, **** p ≤ 0.0001.
To investigate whether resveratrol and dehydro-δ-viniferin affect the induction of the Dusp1 expression, bmDCs were incubated for 30 min, with or without resveratrol (30 µM) or dehydro-δ-viniferin (30 µM), prior to stimulation with L. acidophilus NCFM (MOI 1). At multiple time points, cells were lysed and RNA extracted. bmDCs stimulated with L. acidophilus NCFM displayed increased Dusp1 expression after 6 h, with a further increase at 8 h (Figure 6B). For cells incubated with resveratrol, the Dusp1 expression showed a threefold increase at 4 h and then dropped gradually at 6 and 8 h. Cells incubated with dehydro-δ-viniferin showed only a very weak increase in Dusp1 expression at 6 h, after which it dropped to a background level at 8 h (Figure 6B). The cytokine response in the supernatant from the stilbenoid-treated cells was also examined (Figure 6C). At 8 h after L. acidophilus NCFM stimulation, the IL-12 concentration in the supernatants of cells treated with dehydro-δ-viniferin increased more compared to untreated cells, and for resveratrol-treated cells, the IL-12 concentration remained close to zero for 8 h, then a subtle increase appeared. The production of IL-10 was almost zero for 8 h, with a drastic increase from 8 to 20 h. Treatment with the both stilbenoids led to a decreased IL-10 production.
3. Discussion
Resveratrol and its natural derivatives are regarded as anti-oxidative and anti-inflammatory compounds, but how their individual structures affect these activities is much less clear. Here, monomeric and dimeric derivatives of resveratrol have been used to scrutinize the effect of each single molecule by comparing their impact on the oxidative status of microbially stimulated dendritic cells, as well as on bacterially induced cytokine production in the same cell system. This latter approach also allowed us to take into appropriate consideration the distinct signaling pathways and cytokine production involved in the response of bmDCs towards different bacteria [10].
The disparate effects of stilbenoids on the cytokine production induced by the two bacterial strains have not been demonstrated before, but may be expected when considering the different signaling pathways induced by the two bacteria [6]. The Gram-negative E. coli Nissle 1917 induces pathways involving MyD88, as well as TIR domain-containing adaptor-inducing interferon-β (TRIF)-induced TLR signaling, depending on whether stimulation takes place from the plasma or the endosomal membrane. In contrast, the Gram-positive L. acidophilus NCFM exclusively stimulates from the endosomal membrane and only through MyD88 [6], in a process strictly dependent on endosomal degradation [11]. These differences cause the activation of diverse signaling pathways employing various signaling molecules and acting to a different extent and with different kinetics [12,13].
Kinases at large, and in the particular mitogen-activated protein (MAP) kinases, represent a group of signaling molecules that reportedly are inhibited by stilbenoids and other polyphenols [11,14,15], but bacteria exploit different activation patterns for kinases. For example, it was previously demonstrated that IL-12 induction by the Gram-positive bacteria L. acidophilus and S. aureus is strictly dependent on JNK and less on p38 [16,17]. In contrast, for Gram-negative bacteria E. coli, signaling (through TRIF) is dependent on p38 signaling [18]. In addition, the rate of endosomal degradation of the bacteria may affect the relative contribution of different MAP kinases. Indeed, recent reports have indicated that the kinetics of p38 activation involved in the signaling to some Gram-positive bacteria depends on how readily bacteria are degraded in endosomes [16,19]. Hence, the effect of specific stilbenoids may depend on their ability to bind to one or more MAP-kinases, as well on which kinases are most relevant to cytokine production.
This work also reports high variation of cytokine production in bmDCs depending on the stilbenoid’s structure. The most striking variation was the diverging effect of resveratrol and the resveratrol dimer, dehydro-δ-viniferin, on L. acidophilus NCFM-induced IL-12 production. Whereas resveratrol, similarly to other stilbenoids, inhibited the L. acidophilus NCFM-induced production of IL-12, dehydro-δ-viniferin enhanced it. L. acidophilus NCFM-induced IL-12 is strictly dependent on the MAP kinase JNK [17] and an early transcription of the p38-induced Dusp1 was reported to lead to a decrease in IL-12 production [16]. The results reported here indicate that dehydro-δ-viniferin almost completely suppresses L. acidophilus NCFM-induced Dusp1 expression, whereas resveratrol leads to an earlier and stronger Dusp1 expression. As DUSP1 inactivates both JNK and p38 [20], inhibition of Dusp1 expression by dehydro-δ-viniferin indicates that p38 activation is abrogated, resulting in persistent phosphorylation and activity of JNK. Conversely, the earlier enhanced induction of Dusp1 expression and the almost full abrogation of IL-12 during the first 8 h in the presence of resveratrol could indicate that JNK was inhibited. Here, it is relevant that some of the kinase signaling pathways may activate both JNK and p38, and that their actions may be redundant, at contrast with other activation pathways that are highly specific and involve only one of the two MAP kinases [20,21].
The cytokine-modulating capacity of each stilbenoid may be influenced by several properties of the molecule. Firstly, the capacity of the stilbenoid to pass through the plasma membrane may affect the proportion of the stilbenoid that actually reaches intracellular targets. Methylated monomers, as well as the two dimers, more readily pass the hydrophobic membrane due to their higher lipophilicity compared to the parent compounds. In support of this, Dias et al. [22] demonstrated a 40 times higher serum concentration of trimethoxy-resveratrol than resveratrol in mice after oral administration. The documented differences in pharmacokinetics have been suggested to account for the higher biological activity of pterostilbene and other methylated compounds over the parental compound resveratrol [23]. Given the comparable effects of resveratrol and trimethoxy-resveratrol in E. coli-stimulated bmDCs, the differences between these stilbenoids observed in vivo seem more likely to be caused by the transformation of resveratrol into other molecules. As a matter of fact, resveratrol was demonstrated to be transformed (e.g., by oxidation by the gut commensals [24]) in the digestive tract.
When investigating the capacity of each stilbenoid to affect LPS-induced ROS formation in the dendritic cells, methylated monomers were found to be much less efficient than resveratrol, as expected. Surprisingly, the stilbenoid dimers showed weak ROS-inhibiting activity in the LPS-stimulated cells and also showed a reduced antioxidant capacity when TAC was determined by DPPH assay. This suggests that the lower antioxidant capacity of the dimers is related to their lower anti-radical activity rather than to poor cellular uptake or to molecular interaction with intracellular targets involved in the activation of ROS formation, e.g., nuclear factor erythroid-derived 2-like pathway [25,26,27], contributing to the final effect on counteracting ROS formation [28].
Although the strength of the antioxidant effect of polyphenols has been described and depends on their structural properties, such as the Bors criteria [29], this study makes evident a linear correlation between TAC and the number of hydroxyl groups in stilbenoids, especially for their monomeric forms. This is in line with a recent paper by Platzer et al., who studied several phenolic compounds and found that the number of hydroxyl groups influenced the antioxidant activity more accurately than the Bors criteria [30]. Although the ABTS and DPPH assays are both single electron transfer-based assays and determined the anti-radical capacity of the molecules in the sample, slight differences were found between the two methods. These discrepancies may be due to the stilbenoids’ solubility in the reaction media (water and hydro-methanolic solution in ABTS and DPPH assay, respectively) and electron transfer kinetic issues [31,32], compared to the ROS formation measured in LPS-stimulated dendritic cells.
Endosomal ROS formation is required for efficient degradation of some phagocytosed bacteria and is often, but not always, required to release ligands for the TLRs and, thus, for cytokine production. This makes it relevant to assess if there is a relationship between ROS formation, the requirement of bacterial degradation, and cytokine production. Efficient endosomal degradation of L. acidophilus NCFM is a prerequisite for cytokine production, in particular for IL-12 [6,11], as it is for many other Gram-positive bacteria [33,34]. In contrast, many Gram-negative bacteria, such as E. coli Nissle 1917, shed TLR-stimulating LPS from the surface and are, therefore, less dependent on endosomal degradation to induce cytokine production [35,36]. The stilbenoids affected the cytokine production induced by L. acidophilus and E. coli differently with the most pronounced effects on the L. acidophilus-induced cytokine production. Resveratrol and piceatannol exhibited the most potent inhibitory effects in L. acidophilus-induced cytokines; a part of the actions of these two stilbenoids might be due to an inhibition of the ROS formation. This is, however, purely speculative.
Interestingly, in contrast to the monomeric stilbenoids, the dimeric stilbenoid dehydro-δ-viniferin enhanced the IL-12 production induced by L. acidophilus. Notably, IL-12 treatment of WT mice before pulmonary challenge with MRSA protected mice against bacterial growth and increased survival [37].
The IL-12-enhancing capacity reported here has not been shown, to our best knowledge, for any stilbenoids or for other polyphenols. Considering the rather potent antimicrobial activity towards Gram-positive pathogens, dehydro-δ-viniferin may represent a dual-pronged strategy for the treatment of infections by Gram-positive bacteria such as Staphylococcus aureus, by acting directly on the pathogen as well as indirectly, through an enhanced cellular immune response against the pathogen.
4. Materials and Methods
4.1. Generation of Murine Bone Marrow-Derived Dendritic Cells (bmDCs)
The bmDCs were generated as described previously [4,38]. In brief, the femur and tibia were removed from C57BL/6NTac mice (Taconic, Lille Skensved, Denmark). The bone marrow cells were cultivated at 3 × 105 cells/mL in RPMI medium containing 10% fetal calf serum and 15 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF). After eight days of incubation, non-adherent bmDCs were used. All animals used as sources of bone marrow cells were housed under conditions approved by the Danish Animal Experiments Inspectorate (Forsøgdyrstilsynet) according to The Danish Animal Experimentation Act; LBK no. 474 from 15 May 2014, and experiments were carried out in accordance with the guidelines of ‘The Council of Europe Convention European Treaty Series (ETS)123 on the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes’.
4.2. Bacterial Strains and Stilbenoid Synthesis
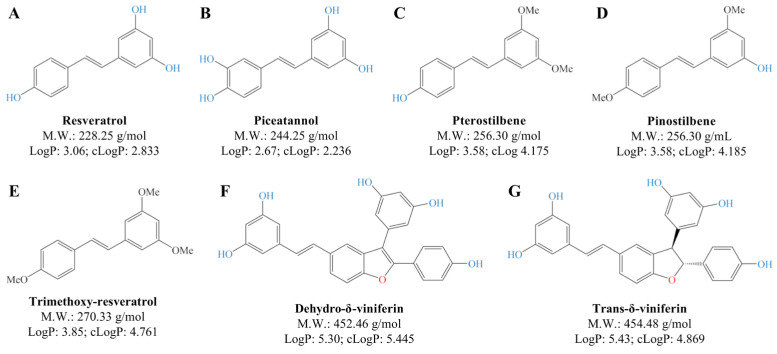
The Gram-positive bacterium L. acidophilus NCFM (Danisco, Copenhagen, Denmark) was grown anaerobically overnight at 37 °C in de Man Rogosa Sharp broth (Merck, Darmstadt, Germany). The Gram-negative bacterium E. coli Nissle 1917 O6:K5:H1 (Statens Serum Institute, Copenhagen, Denmark) was grown aerobically overnight at 37 °C in Luria-Bertani broth. The bacteria were subcultured twice, harvested by centrifugation at 1250× g for 10 min, and washed twice in sterile PBS. The bacteria were seeded in Petri dishes, killed with UV pulsation, and the bacterial viability was verified. Stilbenoids (Figure 7) were synthesized as previously described [9], solubilized in DMSO to a concentration in excess of 10 mM (6.38 mM for trans-δ-viniferin), and further diluted in PBS or growth media as appropriate. Final DMSO concentrations below the 0.5% (v/v) thresholds did not lead to significant changes of the cytokine response in the system used in this study.
Figure 7.
Chemical structures of the utilized stilbenoid monomers and dimers. Overview of the examined stilbenoids, listing the structure, molecular weight, LogP values, and cLogP values for resveratrol (A), piceatannol (B), pterostilbene (C), pinostilbene (D), trimethoxy-resveratrol (E), dehydro-δ-viniferin (F), and trans-δ-viniferin (G).
4.3. Assessment of Bacteria-Induced Cytokine Response
For determination of the appropriate MOI to be used in further tests, bmDCs (2 × 106 cells/mL) were stimulated with increasing concentration (MOI 0.3–3) of L. acidophilus NCFM or E. coli Nissle 1917 and incubated for 20 h at 37 °C, 5% CO2 before the supernatant was harvested. For examinations of the stilbenoids’ effect on the cytokine response, bmDCs (2 × 106 cells/mL) were incubated for 30 min at 37 °C, 5% CO2 in the presence of increasing concentrations (0–40 μM) of the individual stilbenoid, prior to stimulation with L. acidophilus NCFM (MOI 1) or E. coli Nissle 1917 (MOI 1). For assessing the effects of inhibition of MAP kinases, SP600125 (a JNK inhibitor, 25 µM) and SB203580 (a p38 inhibitor, 10 µM) were added to bmDCs and incubated for 30 min at 37 °C, 5% CO2 prior to stimulation with L. acidophilus NCFM. After 20 h of incubation, the culture supernatants were harvested and stored at −20 °C until analysis. The cytokine concentration in the culture supernatants was assessed using the Duoset™ ELISA kits for mouse IL-12p70 (DY419), IL-10 (DY417), and TNF-α (DY410). All kits were from R&D Systems® (Minneapolis, MN, USA) and used according to the manufacturer’s instructions.
4.4. Assessment of Intracellular ROS Formation
Murine bmDCs (2 × 106 cells/mL) were treated with 5 μM carboxy-H2DCFDA (Invitrogen™, Carlsbad, CA, USA) before the addition of resveratrol-derived stilbenoid monomers or dimers (30 μM, final concentration) and incubated for 30 min at 37 °C and 5% CO2. Samples were then stimulated with 100 ng/mL LPS (E. coli O26:B6, Sigma Aldrich, St Louis, MO, USA) and incubated for another 4 h at 37 °C and 5% CO2. Controls included samples without stilbenoids and LPS as well as samples with stilbenoids but without LPS and samples not treated with carboxy-H2DCFDA. Assessment of the intracellular ROS formation was carried out by measuring the intensity of the cellular fluorescence generated by oxidation of the internalized carboxy-H2DCFDA on a BD FACS Diva flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). All events counted (20,000/sample) were included in the analysis. Comparison of the intracellular ROS formation in various samples was based on the mean fluorescent intensity (MFI) of treated bmDCs. The flow cytometry results were analyzed using the FlowJo™ software (version 10.6.2, BD Life Sciences, Ashland, OR, USA).
4.5. In Vitro Total Antioxidant Capacity (TAC) Determination
TAC was assessed by the ABTS and DPPH assays [39] on the basis of the ability of the antioxidant molecules to reduce the radical cation of 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH). Ten microliters of a 1 mM stock solution of each species were added to 990 μL of 80μM ABTS+ and 100 μM·DPPH and the quenching of the absorbance at 734 nm for 1 min and at 517 nm for 30 min for ABTS·+ and DPPH·, respectively, was monitored. Values obtained for each sample were compared to the concentration–response curve of the standard Trolox solution and expressed as mM of Trolox equivalent (TE).
4.6. Assessment of Fold Change in Gene Expression in bmDC
Murine bmDCs (2 × 106 cells/mL) were stimulated with L. acidophilus NCFM (MOI 1) and incubated at 37 °C and 5% CO2. To determine the effect of stilbenoids on the expression of specific genes in bmDCs, cells were incubated with 30 µM of the examined stilbenoids for 30 min at 37 °C and 5% CO2 prior to stimulation. Supernatant from stimulated bmDCs was removed and mRNA was extracted using MagMAX-96 Total RNA Isolation Kit (AM1830, Applied Biosystems, Foster City, CA, USA). The extracted RNA (500 ng for each sample) was converted to cDNA using Applied Biosystems™ High-Capacity cDNA Reverse Transcription Kit. Applied Biosystems™ TaqMan™ qPCR with a specific TaqMan MGB probe and primer sequences were then used to estimate the expression of Dusp1 (Mm00457274_g1). Actb (Mm00607939_s1) was used as the reference gene to define ΔCt for each sample (ΔCttarget = Cttarget − Ctreference). Fold change expression was estimated using the ΔΔCt method, where first the ΔCt of the blank (unstimulated cells) from each time point is subtracted from the ΔCt of a target sample (stimulated cells) at each time point (ΔΔCt = ΔCttarget − ΔCtcontrol), with fold change in gene expression being defined by 2(−(ΔΔCt).
4.7. Statistical Analysis
Statistical analysis was performed by using the GraphPad Prism software (version 9.3.1, GraphPad Software, San Diego, CA, USA). Unless otherwise specified, results are illustrated as means ± SD. The results were analyzed by one-way analysis of variance (ANOVA) to determine significance. p values less than 0.05 were considered statistically significant and indicated by asterisks. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
5. Conclusions
This work demonstrates that the potential of stilbenoids to affect the cytokine production induced by bacteria in murine dendritic cells depends on the specific structure of the stilbenoid, as well as on the type of microbial stimulant. Specific knowledge of stilbenoid molecular determinants modulating the cytokine response of dendritic cells, i.e., the cells orchestrating the type of immune response against pathogens, may be exploited in outlining future strategies for development of new drugs and nutraceuticals.
Acknowledgments
The authors wish to acknowledge the project “One Health Action Hub: University Task Force for the resilience of territorial ecosystems” supported by Università degli Studi di Milano—PSR 2021—GSA—Linea 6, and the “Transition Grant 2015-2017—Linea 1A” of the University of Milan. The Authors thanks Francesco Bonomi for reading and commenting the manuscript.
Author Contributions
Conceptualization, P.R.J., A.P., S.D. and H.F.; methodology, P.R.J., C.P., L.M., M.B.S. and M.D.N.; software, P.R.J.; validation, P.R.J. and M.D.N.; formal analysis, P.R.J. and M.D.N.; investigation, P.R.J.; data curation, P.R.J. and M.D.N.; writing—original draft preparation, P.R.J. and H.F.; writing—review and editing, M.D.N., S.I., S.D., A.P. and H.F.; supervision, S.I., S.D., A.P. and H.F.; funding acquisition, A.P., S.D. and H.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Danish Animal Experiments Inspectorate (Forsøgdyrstilsynet) according to The Danish Animal Experimentation Act (protocol code LBK no. 474 from 15 May 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Independent Research Fund Denmark, grant number 1032-00221B (H.F.), and by Italian Piano di Sostegno della Ricerca 2022-Azione A (Linea 2) (M.D.N.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Remsberg C.M., Yáñez J.A., Ohgami Y., Vega-Villa K.R., Rimando A.M., Davies N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008;22:169–179. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- 2.Teuscher K.B., Zhang M., Ji H. A Versatile Method to Determine the Cellular Bioavailability of Small-Molecule Inhibitors. J. Med. Chem. 2017;60:157–169. doi: 10.1021/acs.jmedchem.6b00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman R.M., Nussenzweig M.C. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathiesen R., Eld H.M.S., Sørensen J., Fuglsang E., Lund L.D., Taverniti V., Frøkiær H. Mannan Enhances IL-12 Production by Increasing Bacterial Uptake and Endosomal Degradation in L. acidophilus and S. aureus Stimulated Dendritic Cells. Front. Immunol. 2019;10:2646. doi: 10.3389/fimmu.2019.02646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkelsen M.S., Jespersen B.M., Mehlsen A., Engelsen S.B., Frøkiær H. Cereal β-glucan immune modulating activity depends on the polymer fine structure. Food Res. Int. 2014;62:829–836. doi: 10.1016/j.foodres.2014.04.021. [DOI] [Google Scholar]
- 6.Weiss G., Maaetoft-Udsen K., Stifter S.A., Hertzog P., Goriely S., Thomsen A.R., Paludan S.R., Frøkiær H. MyD88 drives the IFN-β response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3, and IRF7. J. Immunol. 2012;189:2860–2868. doi: 10.4049/jimmunol.1103491. [DOI] [PubMed] [Google Scholar]
- 7.Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016;2016:5276130. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorakova M., Landa P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017;124:126–145. doi: 10.1016/j.phrs.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Mattio L.M., Dallavalle S., Musso L., Filardi R., Franzetti L., Pellegrino L., D’Incecco P., Mora D., Pinto A., Arioli S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019;9:19525. doi: 10.1038/s41598-019-55975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeuthen L.H., Christensen H.R., Frøkiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clin. Vaccine Immunol. 2006;13:365–375. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boye L., Welsby I., Lund L.D., Goriely S., Frøkiaer H. Plasma membrane Toll-like receptor activation increases bacterial uptake but abrogates endosomal Lactobacillus acidophilus induction of interferon-β. Immunology. 2016;149:329–342. doi: 10.1111/imm.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saikh K.U. MyD88 and beyond: A perspective on MyD88-targeted therapeutic approach for modulation of host immunity. Immunol. Res. 2021;69:117–128. doi: 10.1007/s12026-021-09188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciesielska A., Matyjek M., Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021;78:1233–1261. doi: 10.1007/s00018-020-03656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao X., Tian S., Fu M., Li Y., Sun Y., Liu J., Liu Y. Resveratrol protects human bronchial epithelial cells against nickel-induced toxicity via suppressing p38 MAPK, NF-κB signaling, and NLRP3 inflammasome activation. Environ. Toxicol. 2020;35:609–618. doi: 10.1002/tox.22896. [DOI] [PubMed] [Google Scholar]
- 15.Fu S., Lv R., Wang L., Hou H., Liu H., Shao S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. Saudi J. Biol. Sci. 2018;25:259–266. doi: 10.1016/j.sjbs.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eld H.M.S., Nielsen E.M., Johnsen P.R., Marengo M., Kamper I.W., Frederiksen L., Bonomi F., Frees D., Iametti S., Frøkiær H. Cefoxitin treatment of MRSA leads to a shift in the IL-12/IL-23 production pattern in dendritic cells by a mechanism involving changes in the MAPK signaling. Mol. Immunol. 2021;134:1–12. doi: 10.1016/j.molimm.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Weiss G., Christensen H.R., Zeuthen L.H., Vogensen F.K., Jakobsen M., Frøkiær H. Lactobacilli and bifidobacteria induce differential interferon-β profiles in dendritic cells. Cytokine. 2011;56:520–530. doi: 10.1016/j.cyto.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira-Coelho M., Guedes J., Ferreirinha P., Howes A., Pedrosa J., Rodrigues F., Lai W.S., Blackshear P.J., O’Garra A., Castro A.G., et al. Differential post-transcriptional regulation of IL-10 by TLR2 and TLR4-activated macrophages. Eur. J. Immunol. 2014;44:856–866. doi: 10.1002/eji.201343734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eld H.M.S., Johnsen P.R., Nielsen E.M., Jørgensen F.Z., Lindstrøm-Svendsen M., Baldry M., Ingmer H., Frøkiær H. Soluble C-Type Lectin-Receptor Ligands Stimulate ROS Production in Dendritic Cells and Potentiate Killing of MRSA as Well as the MRSA Induced IL-12 Production. Front. Immunol. 2022;13:845881. doi: 10.3389/fimmu.2022.845881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arthur J.S., Ley S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 21.Huang G., Shi L.Z., Chi H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine. 2009;48:161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias S.J., Li K., Rimando A.M., Dhar S., Mizuno C.S., Penman A.D., Levenson A.S. Trimethoxy-Resveratrol and Piceatannol Administered Orally Suppress and Inhibit Tumor Formation and Growth in Prostate Cancer Xenografts. Prostate. 2013;73:1135–1146. doi: 10.1002/pros.22657. [DOI] [PubMed] [Google Scholar]
- 23.Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today. 2010;15:757–765. doi: 10.1016/j.drudis.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y., Chen D., Zheng P., Yu J., He J., Mao X., Yu B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. Biomed. Res. Int. 2019;2019:5403761. doi: 10.1155/2019/5403761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H., Jiang N., Liang B., Liu Q., Zhang E., Peng L., Deng H., Li R., Li Z., Zhu H. Pterostilbene protects against UVB-induced photo-damage through a phosphatidylinositol-3-kinase-dependent Nrf2/ARE pathway in human keratinocytes. Redox Rep. 2017;22:501–507. doi: 10.1080/13510002.2017.1329917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soeur J., Eilstein J., Léreaux G., Jones C., Marrot L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic. Biol. Med. 2015;78:213–223. doi: 10.1016/j.freeradbiomed.2014.10.510. [DOI] [PubMed] [Google Scholar]
- 27.Wahdan S.A., Azab S.S., Elsherbiny D.A., El-Demerdash E. Piceatannol protects against cisplatin nephrotoxicity via activation of Nrf2/HO-1 pathway and hindering NF-κB inflammatory cascade. Naunyn Schmiedebergs Arch. Pharmacol. 2019;392:1331–1345. doi: 10.1007/s00210-019-01673-8. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bors W., Heller W., Michel C., Saran M. Radical chemistry of flavonoid antioxidants. Adv. Exp. Med. Biol. 1990;264:165–170. doi: 10.1007/978-1-4684-5730-8_25. [DOI] [PubMed] [Google Scholar]
- 30.Platzer M., Kiese S., Tybussek T., Herfellner T., Schneider F., Schweiggert-Weisz U., Eisner P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022;9:882458. doi: 10.3389/fnut.2022.882458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platzer M., Kiese S., Herfellner T., Schweiggert-Weisz U., Miesbauer O., Eisner P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules. 2021;26:1244. doi: 10.3390/molecules26051244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao Z., He L., Hou X., Wei J., Ma X., Gao Z., Yuan Y., Xiao J., Li P., Yue T. Relationships between Structure and Antioxidant Capacity and Activity of Glycosylated Flavonols. Foods. 2021;10:849. doi: 10.3390/foods10040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charrel-Dennis M., Latz E., Halmen K.A., Trieu-Cuot P., Fitzgerald K.A., Kasper D.L., Golenbock D.T. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe. 2008;4:543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund L.D., Ingmer H., Frøkiær H. D-Alanylation of Teichoic Acids and Loss of Poly-N-Acetyl Glucosamine in Staphylococcus aureus during Exponential Growth Phase Enhance IL-12 Production in Murine Dendritic Cells. PLoS ONE. 2016;11:e0149092. doi: 10.1371/journal.pone.0149092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackowiak P.A. Relationship between growth temperature and shedding of lipopolysaccharides by gram-negative bacilli. Eur. J. Clin. Microbiol. 1984;3:406–410. doi: 10.1007/BF02017360. [DOI] [PubMed] [Google Scholar]
- 36.Zanoni I., Ostuni R., Marek L.R., Barresi S., Barbalat R., Barton G.M., Granucci F., Kagan J.C. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen Q.T., Furuya Y., Roberts S., Metzger D.W. Role of Interleukin-12 in Protection against Pulmonary Infection with Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2015;59:6308–6316. doi: 10.1128/AAC.00968-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen H.R., Frøkiaer H., Pestka J.J. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 39.Valli V., Gómez-Caravaca A.M., Di Nunzio M., Danesi F., Caboni M.F., Bordoni A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J. Agric. Food Chem. 2012;60:12508–12515. doi: 10.1021/jf304416d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.