Abstract
In vertebrates, mainly single genes with an allele ratio of 1:1 trigger sex-determination (SD), leading to initial equal sex-ratios. Such genes are designated master-key regulators (MKRs) and are frequently associated with DNA structural variations, such as copy-number variation and null-alleles. Most MKR knowledge comes from fish, especially cichlids, which serve as a genetic model for SD. We list 14 MKRs, of which dmrt1 has been identified in taxonomically distant species such as birds and fish. The identification of MKRs with known involvement in SD, such as amh and fshr, indicates that a common network drives SD. We illustrate a network that affects estrogen/androgen equilibrium, suggesting that structural variation may exert over-expression of the gene and thus form an MKR. However, the reason why certain factors constitute MKRs, whereas others do not is unclear. The limited number of conserved MKRs suggests that their heterologous sequences could be used as targets in future searches for MKRs of additional species. Sex-specific mortality, sex reversal, the role of temperature in SD, and multigenic SD are examined, claiming that these phenomena are often consequences of artificial hybridization. We discuss the essentiality of taxonomic authentication of species to validate purebred origin before MKR searches.
Keywords: sex-determination, master key regulator, sex-ratio, mono-factorial, hybridization, validation
1. Sex Determination
Sex determination (SD) is a fundamental biological process that drives the optimal sex ratio for reproduction and for defective allele purging in natural populations [1,2,3]. In birds and mammals, SD mechanisms are mainly genetic (GSD), whereas lower vertebrates show a variety of SD mechanisms, such as genetic, environmental (ESD), social factors, and their combinations [4].
Mainly performed on fish species, most studies show that a single genetic sequence, which could be a gene or any other genomic element, triggers SD for each species (Table 1), resulting in a dichotomic segregation of sexes and an initial even sex ratio. Such factors are designated master-key regulators (MKRs) of SD [5]. However, some researchers prefer to denote only a protein-producing gene as the causative regulator and do not include other genomic elements, which could be considered a terminology problem, e.g., amhΔy vs. amhy in Nile tilapia (discussed in Section 4) [6]. Many studies have mapped a region for SD in different species, and suggest multiple candidates from the mapped region. However, this review considers only those candidate MKRs that were suggested after isolating a specific DNA sequence variation, which was then confirmed by recombination boundaries, multiple species conservation, or functional studies (Table 1). MKRs are not universal, and closely related species may utilize different MKR genes for SD [7,8,9]. In some species, sex ratios in adults are significantly influenced by temperature during early development. However, as shown in avian species, ratios can also be modulated by sex-specific survival rates [10]. Thus, deviation from the equal sex ratio is not always an indication of the number and type of factors involved in SD for an examined species, since sex-specific mortality can mimic environmental SD [11,12].
Two common scenarios have been observed for vertebrate MKR genes. In a minority of cases, the MKR is a sex-specific gene with two different allele variants for males and females. However, in the majority of cases, MKRs are associated with copy-number variation (CNV), in which a unique variant evolved (on the Y or W chromosome) into an SD regulator [9]. When the MKR is present in a single copy, it may be regarded as a CNV involving a null allele, as in the case of dmrt1 in birds (Table 1). The existence of common MKRs in taxonomically distant species indicates that the number of MKRs is limited to a set of factors which belong to a conserved regulatory SD pathway [13,14]. This pathway controls the SD cascade, and triggers primary differentiation of the bi-potential gonad up to the stage of the sex-specific pattern of steroid hormone synthesis [15,16]. An extremely well-observed example of this is in the paralogs of amh and dmrt1, which serve as MKRs of SD in many distant fish species (Table 1): an amh copy was found to be the initiator of only the XY/XX SD mechanism, whereas dmrt1 was found to initiate both WZ/ZZ and XY/XX SD mechanisms. Moreover, currently, only two additional MKR genes have been found as the initiators of WZ/WW mechanisms: hsd17b and banf2 (Table 1). The fact that specific MKRs initiate a certain type of SD mechanism i.e., either the XX/XY or the ZZ/WZ, or both systems, is an indication of the existence of conserved specialization and hierarchy of factors in the SD pathway.
The model of interaction of GSD and ESD in lower vertebrates is based on two principles. First, the gonads appear to be morphologically identical in both sexes (bi-potential gonad), but through sex-hormone synthesis, the MKR induces differentiation into the ovary or testis [17]. This process of puberty can take place at different ages, depending on the species. Second, depending on the type of hormones secreted, which could be affected by environment, morphological differentiation might be terminated or altered completely, causing sex-reversal [17,18]. Furthermore, the genes of SD MKRs are frequently expressed beyond the period of embryonic development, indicating that they are not involved only in SD initiation, but also in sex development and maintenance. For example, the human MKR, SRY, is expressed in different human tissues up to adolescence, independent of gonadal hormone levels [19]. There are also surprising discoveries of MKRs, such as the immune-related gene (sdY) of salmonids [20,21], showing that a complex regulation mechanism of SD may have evolved [22]. Yet, a recent study has found sdY integration with the classical SD cascade by interaction with foxl2, which is associated with ovarian development [23]. However, many central factors of the SD pathway, such as sox9, wt1, dax1, sf1, wnt4, foxl2 and cyp19a1a/b were not observed to have a natural MKR function in any fish species studied so far (Table 1). This is puzzling because transgenic experiments have demonstrated that knockouts in some of these central genes may lead to sex reversal [24,25]. It is possible that these genes are conserved because of their critical role in development, and thus sex specific variation might adversely affect their function.
Fish demonstrate a wide variety of SD MKRs and are, therefore, an excellent model for the study of SD and the sex differentiation cascade. Moreover, SD of closely related species can be controlled by different MKRs [26]. In such cases, interactions between different MKRs may be examined in the following generations, if the interspecific hybrids are viable [27]. Many fish have large populations with short generation intervals, thus facilitating effective genetic studies. Particularly, tilapia has been used in many SD studies to investigate the diversity of SD genes and their functional characteristics [28,29,30].
Environmental and social factors have been shown to be involved in SD of many low vertebrate species, and can be dominant over genetic factors [31]. However, in some cases, cryptic genetic factors may be involved together with environmental SD. For example, in the protandrous hermaphrodite gilthead seabream (Sparus aurata), a strong quantitative trait locus (QTL) was detected for SD, which does not overlap with a QTL for weight [32]. It may be speculated that in such species, progeny that are initially all-males or all-females, segregate for a cryptic genetic SD system that contributes to the propensity for sex reversal [33]. In addition, high temperatures have been shown to affect SD, resulting in skewed sex ratios [34]. However, sex-specific larval and adult mortality may also contribute to skewed sex ratios, which is not always taken into consideration in association studies [11,12]. Specifically in O. niloticus and O. aureus, high incubation temperatures (34–37 °C) may cause female to male sex-reversal [34]. It was shown that O. aureus is sensitive to temperature treatment, whereas O. niloticus stocks demonstrate a lesser degree of sensitivity to temperature sex-reversal induction [34]. Moreover, different levels of sensitivity were detected amongst families of the same population. A QTL for thermo-sensitivity was mapped to LG20 in the Stirling tilapia strain [35]. However, a later investigation of the appearance of multiple SD systems in different strains of O. niloticus raises the question of whether they stem from O. niloticus × O. aureus hybridization [36], and whether the temperature sensitivity originated from O. aureus. Our long-term study of reproductive activity and spawning of O. aureus in a natural temperature regime in Israel detected a significant decrease, or a complete retention of spawning in July to August, when temperatures exceed 29 °C [37]. In species with an XX/XY SD mechanism, this may be a natural mechanism to avoid the appearance of XX neo-males, and of Y chromosome loss, which could occur following subsequent reproduction. Recent analysis of purebred O. niloticus and of O. aureus populations of Uganda and Israel, respectively, with markers in MKR genes, detected 0–4% of sex reversed males. Therefore, the influence of temperature on SD in purebred tilapia populations, and perhaps in other species with GSD, is possibly restricted in natural conditions. Nonetheless, the detection of a QTL for temperature sensitivity on LG20 needs further investigation to determine the underlying genetic factors.
Table 1.
Identification of Master Key Regulators (MKRs) of Sex Determination (SD) and their paralogs.
| Human Ortho/Paralog |
SD System |
MKR | Organism/Species | Type 1 | References/ Chr 2 |
|---|---|---|---|---|---|
|
SRY-Box Transcription Factor 3 (SOX3) |
XX/XY |
sox3Y
sry |
Oryzias dancena, O. marmoratus, O. profundicola | SV | [26,38]/LG10 |
| mammals (most) | MSD | [39,40]/ChrY | |||
|
Anti-Müllerian Hormone Receptor 2 (AMHR2) |
XX/XY | amhr2y | Takifugu rubripes, Plecoglossus altivelis | SV | [41,42]/Chr19 |
| Perca flavescens, Phyllopteryx taeniolatus, Syngnathoides biaculeatus, Pangasiidae | MSD | [43]/Chr4 [44]/Chr9 [45,46]/Chr4 |
|||
| no ortholog | XX/XY | gsdfY | Oryzias luzonensis, Hippoglossus hippoglossus, Anoplopoma fimbria | SV | [47]/LG12 [48]/Chr13 [49]/LG14 |
|
Hydroxysteroid 17-Beta Dehydrogenase 1 (HSD17B1) |
WZ/ZZ | hsd17b1w | Seriola genus, Trachinotus anak | SV | [50,51]/Chr24 |
|
Bone Morphogenetic Protein Receptor Type 1B (BMPR1B) |
XX/XY | bmpr1bbY | Clupea harengus | MSD | [52]/Chr8 |
|
Double-sex and Mab-3 Related Transcription Factor 1 (DMRT1) |
XX/XY |
dmrt1Y
dmrt1bY dmW dmrt1z |
Oryzias latipes | MSD | [53,54]/LG1 [55,56]/ChrW [57,58,59] |
| XX/XY | Scatophagus argus | ||||
| WZ/ZZ | Xenopus laevis (amphibians) | FSD | |||
| WZ/ZZ | Gallus gallus (birds), Cynoglossus semilaevis | ||||
|
Interferon Regulatory Factor 9
(IRF9) |
XX/XY | sdY | Salmonids | MSD | [21]/BT18, RT01 |
|
Growth Differentiation Factor 6 (GDF6) |
XX/XY |
gdf6aY
gdf6b |
Nothobranchius furzeri, Astyanax mexicanus | SV | [60,61]/ChrB |
| no ortholog | XX/XY | zkY | Gadus morhua | MSD | [62]/LG11 |
|
Anti-Müllerian Hormone (AMH) |
XX/XY | amhΔy | Odontesthes hatcheri, Oreochromis niloticus, Ophiodon elongates, Hypoatherina tsurugae, Esox lucius, Gasterosteus aculeatus, Sebastes schlegelii, Culaea inconstans | MSD | [63] [64]/LG23 [65,66] [67]/LG24 [68,69] [70]/Chr10 |
|
Barrier- to-Autointegration- Factor-like protein 2 (BANF2) |
WZ/ZZ | banf2w | Oreochromis aureus, Oreochromis urolepis hornorum, Pelmatolapia mariae | FSD | [71]/LG3 |
|
Follicle Stimulating Hormone Receptor (FSHR) |
XX/XY | fshry | Mugil cephalus | SV | [9]/LG9 |
|
Folliculogenesis Specific BHLH Transcription Factor (FIGLA) |
XX/XY |
figla
-like |
Oreochromis mossambicus, Coptodon zillii, Sarotherodon galilaeus | MSD | [36]/LG1 |
1 Sequence variation (SV) and structural sequence variation, including copy number variation (CNV) of male (MSD) or female (FSD) specific gene duplications. 2 Sex chromosome annotation for mapped MKRs (Chr).
2. Search for MKRs
The search for SD MKRs relies on linkage disequilibrium between genetic markers and the causative gene. Thus, genetic maps that are based on linkage between adjacent markers are being used for such studies [72,73]. Recently, genome sequence data are being used for genome-wide association studies (GWAS) [74,75]. Although sex is a categorical trait, which is usually controlled by a single gene, many studies analyze sex as a QTL [76,77,78,79]. Non-recombining regions are part of the characteristics of many sex chromosomes, which may cause extensive association with SD throughout the sex chromosome [80]. This has hampered fine-mapping studies of QTLs in tilapia [71]. Recent studies have shown that regional non-recombining blocks of purebred species can be broken down following hybridization, aiding in the fine genetic mapping of MKRs for SD [27,71,81,82]. Multiple sex determination loci that may segregate in a single strain may also contribute to the complexity of MKR searches [27,83]. Sex specific mortality [11,12] and sex reversal [34] also affect the sex phenotype, and may involve multiple genetic loci.
Although the traditional candidate gene approach may be used for MKR detection, it has been considered unproductive in comparison with GWAS [84]. Moreover, results of the candidate gene approach in the analysis of the genetic control of human diseases have been “woefully inadequate” [85]. Nevertheless, since the number of MKRs of SD seems to be limited, the identification of MKRs (Table 1) may indicate candidate genes for future searches for MKRs in additional species, using heterologous sequences without prior genetic mapping.
3. Identification of SD MKRs
The discovery of the mammalian Sry [39,40] uncovered an extra-numeral copy in males that evolved from sox3 [86,87,88,89]. This was followed by the identification of other SD MKRs in Oryzias latipes dmrt1 [53,54] and O. niloticus amh [90]. With the development of the technology of deep sequencing in recent years, the number of vertebrates with characterized SD genes has significantly increased, revealing that amh and dmrt1 have similar functions in several species (Table 1). Yet, genes known to be involved in sex differentiation frequently adopt an MKR role in SD. These genes usually affect sex-hormone synthesis, directly or indirectly. In fish, estrogens and androgens are critical for ovarian and testicular differentiation and maintenance, respectively [17,91]. Estrogens are produced by the conversion of androgens through cytochrome P450 aromatase [92], which is encoded in teleost fish by cyp19a1a/b. The cyp19a1 a and b genes are mainly expressed in the ovary and the brain, respectively [93]. Thus, cyp19a1a is the key gene which is essential for estrogen synthesis and androgen depletion in the ovary. The female gene pathway determines ovarian development by up-regulating cyp19a1a expression [17,91]. Whereas, conversely, male-pathway genes determine testicular development by repressing cyp19a1a expression.
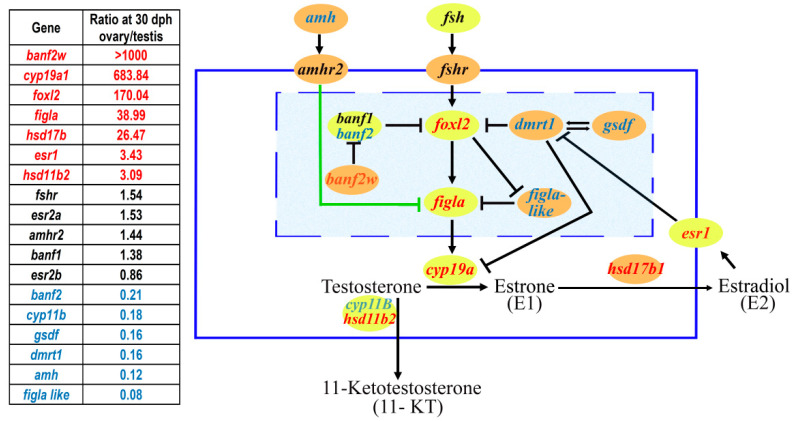
More than 10 MKRs of SD have currently been identified (Table 1). Most of these discoveries were made in fish, specifically in Oreochromis species (amh, banf2w and figla-like). With an emphasis on these species, we here propose a schematic pathway for sex-initiation and maintenance based on the major players/genes and their related functional effects. Integrating the MKR listed below, the proposed scheme is shown in Figure 1. It is noteworthy that, as shown (Table 1, Figure 1), the SD MKR role is often engaged by polymorphic downstream genes in the SD cascade (e.g., hsd17b1). Yet, regulatory feedback allows these genes to control upstream genes.
Figure 1.
A proposed schematic model of the sex determination (SD) pathway in tilapia, based on current knowledge of master key regulators (MKRs) of SD and their effects on estrogen/androgen (E2/11-KT) equilibrium. Identifications of MKRs of SD from different fish species are colored orange and other factors are yellow. The table on the left indicates the RPKM (Reads Per Kilobase of transcript per Million mapped reads) of ovary/testis expression-values of genes in Oreochromis aureus at 30 days post hatching (dph) (BioProject accession number: PRJNA609616). Genes with male and female biased expression values are colored in blue and red, respectively. Arrows (↓) represent up- regulation/production, whereas blocked arrows (⊥) mark down-regulation/production. The green lines represent the SMAD signaling pathway. MKRs of SD without a clear function or relevance to SD in tilapia are not displayed (gdf6, sdY, dmy, sox3, bmpr1bby and zkY) in the figure. However, gdf6 and bmpr1bby are members of the TGF-beta superfamily and may affect SMAD signaling, and thus may have similar functions to those of amh and gsdf. Other undepicted SD MKRs are the sdY gene that interacts with foxl2 in Salmonids, sox3 that regulates gsdf, and zkY with unknown functions. The blue solid line represents the cell membrane.
Sry and sox3: Sry is the SD gene in most mammals, and is thought to have evolved from the X linked gene Sox3 [86,87,88,89]. This assumption was strengthened by evidence from transgenic mice lines and individual mutations in humans, which showed that Sox3 can replace the role of Sry and cause sex-reversal [88,89]. In some Oryzias species, sox3 plays a similar MKR role [7,38], and it has been suggested that sox3 triggers sex by up-regulation of gsdf in Oryzias [38]. In Nile tilapia, sox3 was recently shown to be involved in oogenesis [94]. However, there is no literature connecting it to SD in tilapia species. In mammals, Sry controls Sox9 which is involved in testis development [95]. In teleost fish, sox9 is duplicated [96] and in O. niloticus, sox9b and sox9a show significantly higher expression in XY gonads at 30 days post hatch (dph), and in XX gonads at 5 and 10 dph, respectively [97]. However, in medaka, sox9b is thought to be involved in maintenance of testis differentiation, without a role in initiating SD [98].
Hsd17b1: The gene hsd17b1 was found as an SD MKR in the Seriola genus and in Trachinotus anak [50,51]. In humans, Hsd17b1 is responsible for interconversion between the estrogen precursor estrone (E1) and the extremely potent estradiol (E2), together with other functions in steroid biosynthesis [50,99]. As in humans, in Seriola species, functional experiments suggested that the Z linked hsd17b1 gene decreases the conversion of E1 to E2 [50]. The comparison of XY and XX gonads from O. niloticus shows that this gene is almost exclusively expressed in XX gonads from 5 to 35 days after hatching [100].
Gsdf: This gene is a member of the TGF-β superfamily, which is found mainly in teleost fish [101] and is an SD MKR in several fish species [26,47,48,49]. In O. niloticus, both over-expression of gsdf in XX fish [102] and its knockdown in XY fish cause sex-reversal [103]. As in medaka and rainbow trout, in O. niloticus, gsdf is distinctly and predominantly expressed in XY undifferentiated gonads; additionally, it was found to be expressed earlier than any other testis-differentiation-related gene, apart from dmrt1 [102]. The function of gsdf is important for maintaining dmrt1 expression, and inhibiting estrogen production in the gonad; whereas, dmrt1 is thought to be upstream to gsdf, and controls gsdf expression [103]. Like amh, gsdf may affect the SMAD signaling pathway by stimulating a TGF-β receptor. However, gsdf may also function in a cytoplasmic protein network [104,105]. A recent study in mature stages (0.5–2.0 years) of XX O. niloticus concluded that similar receptors are targets of gsdf and amh, and that both genes control similar downstream genes [105]. Gene knockout of gsdf did not affect serum levels of E2, and probably does not control sex hormone synthesis directly. However, it has been suggested that gsdf may influence pituitary fsh gene expression in fish [105]. The expressions of amh, amhr2, fshr, sox9a, and hsd17b1 were up-regulated in the ovaries of XX gsdf knockout fish [105]. However, these differences in XX gonads do not seem to reflect the differences of gene expression of XX and XY gonads which are relevant for SD. This is evident through comparison of the gene expression profiles of XX and XY gonads (at a control temperature of 28 °C), showing that amh, gsdf, dmrt1, and sox9a have higher expression levels in XY gonads than in XX gonads across a wide range of ages (20–180 dpf) [106].
Dmrt1: This gene is an MKR of SD in different vertebrate species, including teleost fish [53,54,55,56,57,58,59]. In Nile tilapia, dmrt1 is involved in testicular development. Different studies in zebrafish and Nile tilapia suggest that dmrt1 is down-regulated by cyp19a1a expression [24,107,108,109,110] and that in turn, in Nile tilapia, dmrt1 represses cyp19a1a [111] and foxl2 [112]. The activity of foxl2 involves many different proteins that allow estrogen production, and influence granulosa cell activity [113,114]. In tilapia, foxl2 up-regulates aromatase expression in vivo. Moreover, mutation of foxl2 in XX tilapia fish decreased aromatase gene expression and serum estrogen levels [17,24]. Thus, repression of foxl2 down-regulates the production of E2 [112]. It is also thought that part of the involvement of dmrt1 in testis development is due to its ability to up-regulate two genes, sox9b and sox30, which are known for their involvement in testis development [96,115].
Amh and AMH singling: The gene amh and its receptor amhr2 are known MKRs in teleost fish [63,64,65,66,67,68]. Specifically, in Nile tilapia, amh was found to be an MKR of SD [6,64]. In humans, amh is involved in hormone steroidogenesis in granulosa cells where it inhibits cyp19a1 up-regulation by follicle-stimulating hormone (FSH) and luteinizing hormone (LH) [116]. In the medaka (hotei) mutant fish, amh signaling is responsible for down-regulation of cyp19a1a expression levels [17]. A recent study in Nile tilapia has shown that amh down-regulates cyp19a1a expression through SMAD protein phosphorylation [110]. A similar pathway was suggested in Atlantic herring (Clupea harengus), where a male specific gene, bmpr1bby, is a candidate MKR which enhances amh signaling and SMAD phosphorylation [52]. In Nile tilapia, amh is important for follicular development, and its knockdown causes a decrease in levels of LH, FSH, and E2 [117]. This seems in contrast to its function in SD, where its high expression is correlated with low cyp19a1a expression [6]. Nonetheless, it may function in the opposite way at the onset of SD, when the gonads’ fate is not yet determined.
Fshr: This gene was suggested as a candidate MKR in M. cephalus [9]. Fshr involvement in SD is well-established in fish and amphibians [9]. Fshr is presumed to be involved in functions that are related to SD through control of cyp19a1 [9]. Specifically, in Nile tilapia, at very young ages (6–25 dph) fshr is highly expressed in XX gonads where fshr signaling may induce cyp19a1a expression [118].
Figla and figla-like: The gene figla is known to be associated with femaleness. Figla plays an essential role in the development and maintenance of the ovary, and in the suppression of spermatogenesis [119,120]. In O. niloticus, it was suggested that figla terminates the male gonadal differentiation, either by manipulating steroid production or by the meiotic regulation of spermatocytes [119,120]. A homolog gene, figla-like, is a candidate MKR in multiple tilapia species [36]. In tongue sole (Cynoglossus semilaevis), a figla homolog has a role in spermatogenesis, and in the regulation of the synthesis of steroid hormones, which are required for male determination [121]. Thus, figla homologs, including figla-like, have the potential of participating in the control of steroid hormone synthesis. Figla-like was down-regulated in foxl2 knockdown fish, which suggests a role for foxl2 in its regulation [24].
Banf2: A banf2 homolog, designated as banf2w, was proposed as an MKR of SD in multiple cichlids [71]. It was suggested that banf2w regulates foxl2 by repression of banf1, which in turn is a repressor of foxl2’s activity in gonad development [122,123,124,125].
sdY: In salmonids, an immune-system-related SD MKR (sdY) has been found [20]. The gene sdY encodes a protein with similarities to interferon regulatory factor 9, which mediates the interferon antiviral response [20]. However, a recent study has found that sdY integrates into the canonical SD cascade by interaction with foxl2, which is associated with ovarian development [23].
Gdf6: This growth differentiation factor is an SD MKR in killifish (Nothobranchius furzeri) and presumably in Mexican tetra (Astyanax mexicanus) [60,61]. Its involvement in SD is poorly characterized; nonetheless, as with amh and gsdf, it is part of the TGF-β superfamily [60,61].
ZkY: The gene zinc knuckle on the Y chromosome (zkY) was indicated as an MKR of SD in Atlantic cod (Gadus morhua) [62]. However, the target of this putative RNA-binding protein and its function in SD is still unknown.
4. From Identification to Validation of MKRs of SD
The process of validation has been termed as “winning by points rather than knockout” [126]. This concept applies to the validation of MKRs because there is no single test or knock-out experiment that proves the functional role of a specific gene. As noted by [127] ‘the only option… is to collect multiple pieces of evidence, no single one of which is convincing, but which together consistently point to a candidate gene’. Genetic mapping has proven a powerful tool for identifying SD loci and candidate genes in many studies [72,73,75,128]. In some cases, the sex chromosome is not morphologically different from its homologue, and only a very small region is associated with sex. In such cases, analysis of multiple strains or populations with a different recombination history may fine- map the SD region towards localization of the MKR. Thus, the conservation of SD regions among multiple species with similar SD systems presents strong evidence supporting the findings of SD MKRs [7,36,71,129,130].
The actual validation of an MKR of SD is usually performed by functional studies [67,131]. The candidate gene is manipulated by different techniques and the resulting phenotype is considered as proof of function. These methods use transgenic fish manipulated by genomic editing with CRISPR/Cas9, or other methods such as TALEN and antisense RNA [6,67,131,132]. However, manipulations of genes that are part of the sex cascade often alter SD, even though they are not the causative variation. Many functional studies in Nile tilapia (Oreochromis niloticus), including manipulation of different genes such as cyp19a1a and gsdf, resulted in sex-reversal [24,101]. Yet, these genes are not the SD MKRs in this species [36]. Moreover, many central genes of the SD cascade, such as sox9 and dax1, have so far not been identified as MKRs of SD (Table 1), although variations and manipulations of these genes indicate that they may control sex [25,133]. For example, it is unclear whether amhy or amhΔy is the MKR in Nile tilapia. Initially, genetic studies localized the SD genomic region by positional mapping [64,90,134,135], and recognized a unique variant copy of amh in males with a 233 bp deletion in exon 7, thus highlighting it as the candidate MKR [64]. Performed on the Swansea strain of O. niloticus, a follow up study showed that this form, with additional alterations, encodes a truncated protein due to an insertion of 5 bp into exon 6. This form was designated amhΔy [6]. In addition, this study found that amhΔy is in tandem with a regular amh gene (amhy) on the Y chromosome, and based on functional studies and transgenesis, it has been suggested that an SNP in exon 2 of amhy controls sex. According to this study amhΔy is a pseudogene with no clear function [6]. Later studies found that this exotic SNP is specific to the Swansea strain of Nile tilapia, and is not found in any other strains of this species that have the same SD system. However, including an insertion and deletion on exons 6 and 7, the existence and structure of amhΔy are conserved among different strains of O. niloticus [37]. Thus, it was concluded that amhΔy may be the initiator of SD in Nile tilapia [37].
A recent, related study failed to prove that amhΔy is not functional because of the high similarity of amh/amhy/amhΔy sequences, and the difficulty of knockdown of a whole specific copy [110]. Moreover, it was discovered that the Swansea strain possessed two MKRs, on LG1 and 23 [35,64], thus proving it is a hybrid of O. niloticus and O. aureus [36]. Hence, knockout of amhΔy on LG23 in some of the males from this strain may not cause sex reversal due to the existence of an additional MKR (figla-like) on LG1. Sequence analysis for conserved elements in hybrids can also result in false conclusions due to the intra-specific sequence variability of amh/amhy/amhΔy between species. The truncated amhΔy is expressed at sex initiation [6] and may affect expression of both amhy and amh. Generally, tandem duplicates give rise to altered expression often greater than twofold; this may be caused by different mechanisms that may involve chromatin remodeling, DNA looping, frequent transcription factor binding, or other synergistic effects, which are caused by the actual duplicated sequence, and are not fully understood [136,137]. Moreover, a large deletion in the amhy promoter [6,110] raises the possibility that it is not functional, and that both amhΔy and amhy act as one unit under the control of the amhΔy promoter. The pronounced effect of the distance between promoter elements and genes was discussed for the mammalian sox9 [138]. In addition, there is a possibility that alternative splicing may overcome the stop code on exon 6 of the amhΔy gene. Thus, it has not been rejected that amhΔy itself may produce a functional protein. This study eventually attributed the non-functionality of amhΔy through an analysis of conservation in different strains [110], since a strain of Nile tilapia from Lake Koka in Ethiopia lacks the amhΔy 233-bp deletion on exon 7 [139]. In addition, the studies that analyzed the SD region of tilapia from the lakes in Ethiopia suggest a rapid turnover of SD loci in O. niloticus wild populations [139,140]. This is based on the observation that in some cases, these wild populations did not segregate for the O. niloticus SD locus on LG23, and in other cases, the amh polymorphism between males and females was different from that found in other O. niloticus populations [139,140]. However, detailed analysis of the cox1 sequences from these libraries (SRA accession numbers: SRX8948078, SRX8948077 and SRX14028757), which is used as a DNA barcode standard for species assignment, revealed 2–4% differences from the O. niloticus barcode references. Thus, none of these libraries are bona fide O. niloticus, as 1% is considered the threshold that indicates species divergence in the cox1 reference fragment [141,142,143,144,145,146]. Hence, analysis of SD in these species and populations provides important data for exotic tilapias, but is not relevant for the study of SD initiation by amhΔy in O. niloticus. Moreover, in recent studies, we showed cases of taxonomic classification errors of tilapia species [36,147]. Therefore, although multiple functional studies have been performed, it is not yet clear what the causative polymorphism of SD on LG23 of Nile tilapia is, and most evidence is still based on the conservation of elements in the SD locus among multiple strains. Considering all the evidence, it seems that the structural variation of amhΔy may have an MKR role by inducing over-expression of the amhy gene that affects the estrogen/androgen equilibrium.
5. The Significance of Taxonomic Authentication of Species Using DNA Barcoding for MKRs Search
The identification of taxonomy errors is common in the tilapia species as there are many species in this genus that are frequently hybridized for aquaculture purposes and then escape to the wild, affecting native tilapia populations in natural water resources [148,149]. Mass production of tilapia is based on hybridization of two or more tilapia species, usually O. niloticus, O. aureus, O. mossambicus, and O. urolepis hornorum, in which three different MKRs govern SD. The MKRs of O. niloticus and O. mossambicus were mapped on LGs 23 and 14, respectively [37,150]; both O. aureus and O. urolepis hornorum have the same MKR i.e., banf2w on LG3 [30,71,151,152]. Consequently, commercial strains may segregate for the original and the de novo SD loci which emerged following hybridization [36,37,147]. This additional complexity of SD of hybrid species stems from the confluence of different alleles of loci which had no effect on SD in their species of origin. As a result of hybridization, these alleles may segregate and influence SD. The first generations of such hybrids, which are reared in commercial ponds, have strong advantages due to fast growth, high reproductive levels, and excellent adaptation to a wide range of temperatures and salinities in rearing ponds [153,154,155]. However, having high invasive characteristics, hybrids are less affected by natural reproductive barriers between species, and therefore endanger native tilapia populations [154,156]. Different explanations have been given for the decline in size of tilapia populations in natural sources which have been polluted by commercial hybrids; these include the transfer of pathogens and parasites from ponds, weaknesses of commercial traits in the wild, and accumulated chromosomal aberrations driven by faulty crossing-over of homologous chromosomes [148].
In vertebrate mitochondrial oxidative phosphorylation, 13 mitochondrial proteins have evolved with more than 70 nuclear-coding proteins of inner mitochondrial membranes [157]. These proteins cooperate in ATP production through oxidative phosphorylation co-adapting during evolution [158]. Consequently, there is an expected variability of mitochondrial and nuclear sequences, even between closely related species such as O. niloticus and O. aureus, which can be attributed to the differentiation of the species [159,160]. Thus, reports of discordance between genomic and mitochondrial sequence variants in O. niloticus and O. aureus tilapia populations suggest that hybridization has been caused by relatively recent aquaculture pollution [148,149]. In our opinion, the destructive influence of commercial activity might have given rise to the erroneous scientific hypothesis of “rapid turnover” of MKRs of SD [140], which in turn may have legitimized negative commercial activity. Thus, it is essential that the taxonomy of a species is initially verified before studying its SD mechanism. The BOLD taxonomy system provides an infrastructure for such preliminary tests. The latest progress in DNA recovery from formalin-fixed samples [161] could be utilized to examine these hypotheses through the analysis of the thousands of cichlids, collected and preserved over the last 120 years, which are kept in numerous museums, private collections, and laboratories.
6. Single vs. Polygenic SD Systems
Currently, most of the findings in species with a genetic SD system show that the sex trait is categorical, and controlled by a single gene (Table 1). Moreover, a single-factor SD system is not only limited to species where a sex-biased ratio is considered disadvantageous for its fitness. This phenomenon could be explained by genomic conflicts between parents and offspring [162], and is demonstrated by closely related species of the Oryzias genus, which have seven different SD genes, even though SD in each species is controlled by a single MKR [7].
Nonetheless, there are cases with polygenic SD systems. This is especially common in commercial and laboratory stocks, which may differ from the natural population which segregates for a stable mono-factorial SD system. In zebrafish (Danio rerio), domesticated strains have lost the natural SD region on chromosome 4 [163]. In fighting fish (Betta splendens), it was suggested that a polygenic SD system evolved through hybridization and selection, and that the complexity of SD in different populations is dependent on the chances of admixture [164]. Laboratory stocks of guppy, defined as Poecilia reticulata, are possibly hybrids of two species, P. reticulata and P. wingei [81]. In contrast to tilapia species, both Poecilia species have morphologically different sex chromosomes with a common region on LG12; consequently, their hybrids still segregate for a single SD locus with varying recombination patterns in the two strains [82,165].
Although the loss of stability of the SD systems in commercial stocks is common, the evidence of novel sex chromosome systems following hybridization is rare because of slow evolutionary processes [166]. A 30-year experiment on swordtail fish demonstrated how the hybridization of two different species can cause the evolvement of a new SD system on a new chromosome [166]. In this example, the SD system was translocated from one of the species to a new genetic locus in the examined hybrid.
In tilapia, multiple SD MKRs have frequently been found in different families of the same species [36]. However, tilapia aquaculture involves hybridization, and therefore commercial stocks of fish may present SD systems governed by several segregating loci as a result of these practices [27,36]. Nile tilapia has an SD system on LG23 which is controlled by the amh gene [6,37,64]. However, different studies have found that Nile tilapia also segregates for an SD system on LG1 [35]. A recent study showed the hybrid origin of two stocks with an LG1 SD system, and explained how LG1 evolves as an SD gene in these hybrids [36]. It was suggested that SD MKR on LG1 is lacking in Nile tilapia, whereas it is autosomal in O. aureus. Thus, a cross of the two species causes segregation of this locus in the same way as a natural SD MKR of an XY SD system [36]. This can explain how the mono-factorial SD system on LG23 of purebred O. niloticus dispersed to additional loci on LGs 1 and 3 in hybrid strains, such as Chitralada, Amherst, Stirling, and Swansea [35,36,37,147]. Thus, mitochondrial-nuclear genome concordance, and mono-factorial SD systems are important indicators of the purebred origin of stocks and populations. Multiple SD loci, which have been reported for other African cichlid species [83,167], need to be further validated by testing if these are of purebred origin.
Author Contributions
Conceptualization, A.Y.C., A.S., M.R. and E.S.; methodology, A.Y.C., A.S. and E.S.; formal analysis, A.Y.C., A.S. and E.S.; investigation, A.Y.C., A.S. and E.S.; writing—original draft preparation, A.Y.C., A.S. and E.S.; supervision, M.R. and E.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was partially funded by the Fisheries Department of the Israel Ministry of Agriculture and Rural Development.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.West S.A., Reece S.E., Sheldon B.C. Sex ratios. Heredity. 2002;88:117–124. doi: 10.1038/sj.hdy.6800018. [DOI] [PubMed] [Google Scholar]
- 2.West S.A., Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- 3.Sarre S.D., Georges A., Quinn A. The ends of a continuum: Genetic and temperature-dependent sex determination in reptiles. BioEssays. 2004;26:639–645. doi: 10.1002/bies.20050. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya I., Modi D. Recent Updates in Molecular Endocrinology and Reproductive Physiology of Fish. Springer; Singapore: 2021. Sex Determination in Teleost Fish; pp. 121–138. [Google Scholar]
- 5.Harrison D.A. Sex Determination: Controlling the master. Curr. Biol. 2007;17:R328–R330. doi: 10.1016/j.cub.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Li M., Sun Y., Zhao J., Shi H., Zeng S., Ye K., Jiang D., Zhou L., Sun L., Tao W., et al. A tandem duplicate of Anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 2015;11:e1005678. doi: 10.1371/journal.pgen.1005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda M., Sakaizumi M. Evolution of the sex-determining gene in the teleostean genus Oryzias. Gen. Comp. Endocrinol. 2016;239:80–88. doi: 10.1016/j.ygcen.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Gammerdinger W.J., Kocher T.D. Unusual diversity of sex chromosomes in african cichlid fishes. Genes. 2018;9:480. doi: 10.3390/genes9100480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curzon A.Y., Dor L., Shirak A., Meiri-Ashkenazi I., Rosenfeld H., Ron M., Seroussi E. A novel c.1759T>G variant in follicle-stimulating hormone-receptor gene is concordant with male determination in the flathead grey mullet (Mugil cephalus) G3 Genes Genomes Genet. 2021;11:jkaa044. doi: 10.1093/g3journal/jkaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Göth A., Booth D.T. Temperature-dependent sex ratio in a bird. Biol. Lett. 2005;1:31–33. doi: 10.1098/rsbl.2004.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirak A., Golik M., Lee B.-Y., Howe A.E., Kocher T.D., Hulata G., Ron M., Seroussi E. Copy number variation of lipocalin family genes for male-specific proteins in tilapia and its association with gender. Heredity. 2008;101:405–415. doi: 10.1038/hdy.2008.68. [DOI] [PubMed] [Google Scholar]
- 12.Shirak A., Palti Y., Cnaani A., Korol A., Hulata G., Ron M., Avtalion R.R. Association between loci with deleterious alleles and distorted sex ratios in an inbred line of tilapia (Oreochromis aureus) J. Hered. 2002;93:270–276. doi: 10.1093/jhered/93.4.270. [DOI] [PubMed] [Google Scholar]
- 13.Marshall Graves J.A., Peichel C.L. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 2010;11:205. doi: 10.1186/gb-2010-11-4-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall Graves J.A. How to evolve new vertebrate sex determining genes. Dev. Dyn. 2013;242:354–359. doi: 10.1002/dvdy.23887. [DOI] [PubMed] [Google Scholar]
- 15.Capel B. Vertebrate sex determination: Evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 2017;18:675–689. doi: 10.1038/nrg.2017.60. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y., Capel B. Balancing the bipotential gonad between alternative organ fates: A new perspective on an old problem. Dev. Dyn. 2006;235:2292–2300. doi: 10.1002/dvdy.20894. [DOI] [PubMed] [Google Scholar]
- 17.Li M., Sun L., Wang D. Roles of estrogens in fish sexual plasticity and sex differentiation. Gen. Comp. Endocrinol. 2019;277:9–16. doi: 10.1016/j.ygcen.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Tenugu S., Senthilkumaran B. Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal. Aquac. Fish. 2022;7:525–539. doi: 10.1016/j.aaf.2022.02.007. [DOI] [Google Scholar]
- 19.Turner M.E., Ely D., Prokop J., Milsted A. Sry, more than testis determination? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R561–R571. doi: 10.1152/ajpregu.00645.2010. [DOI] [PubMed] [Google Scholar]
- 20.Yano A., Guyomard R., Nicol B., Jouanno E., Quillet E., Klopp C., Cabau C., Bouchez O., Fostier A., Guiguen Y. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 2012;22:1423–1428. doi: 10.1016/j.cub.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Yano A., Nicol B., Jouanno E., Quillet E., Fostier A., Guyomard R., Guiguen Y. The sexually dimorphic on the Y-chromosome gene (sdY) is a conserved male-specific Y-chromosome sequence in many salmonids. Evol. Appl. 2013;6:486–496. doi: 10.1111/eva.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herpin A., Schartl M. Plasticity of gene-regulatory networks controlling sex determination: Of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015;16:1260–1274. doi: 10.15252/embr.201540667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertho S., Herpin A., Branthonne A., Jouanno E., Yano A., Nicol B., Muller T., Pannetier M., Pailhoux E., Miwa M., et al. The unusual rainbow trout sex determination gene hijacked the canonical vertebrate gonadal differentiation pathway. Proc. Natl. Acad. Sci. USA. 2018;115:12781–12786. doi: 10.1073/pnas.1803826115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Li M., Ma H., Liu X., Shi H., Li M., Wang D. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile Tilapia. Endocrinology. 2017;158:2634–2647. doi: 10.1210/en.2017-00127. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes-Freitas I., Milona A., Murphy K.G., Dhillo W.S., Owen B.M. Live birth in sex-reversed XY mice lacking the nuclear receptor Dax1. Sci. Rep. 2020;10:1703. doi: 10.1038/s41598-020-58788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myosho T., Takehana Y., Hamaguchi S., Sakaizumi M. Turnover of Sex Chromosomes in Celebensis Group Medaka Fishes. G3 Genes Genomes Genet. 2015;5:2685–2691. doi: 10.1534/g3.115.021543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curzon A.Y., Shirak A., Zak T., Dor L., Benet-Perlberg A., Naor A., Low-Tanne S.I., Sharkawi H., Ron M., Seroussi E. All-male production by marker-assisted selection for sex determining loci of admixed Oreochromis niloticus and Oreochromis aureus stocks. Anim. Genet. 2021;52:361–364. doi: 10.1111/age.13057. [DOI] [PubMed] [Google Scholar]
- 28.Li M., Dai S., Liu X., Xiao H., Wang D. A detailed procedure for CRISPR/Cas9-mediated gene editing in tilapia. Hydrobiologia. 2021;848:3865–3881. doi: 10.1007/s10750-020-04414-8. [DOI] [Google Scholar]
- 29.Yan L., Feng H., Wang F., Lu B., Liu X., Sun L., Wang D. Establishment of three estrogen receptors (esr1, esr2a, esr2b) knockout lines for functional study in Nile tilapia. J. Steroid Biochem. Mol. Biol. 2019;191:105379. doi: 10.1016/j.jsbmb.2019.105379. [DOI] [PubMed] [Google Scholar]
- 30.Tao W., Xu L., Zhao L., Zhu Z., Wu X., Min Q., Wang D., Zhou Q. High-quality chromosome-level genomes of two tilapia species reveal their evolution of repeat sequences and sex chromosomes. Mol. Ecol. Resour. 2021;21:543–560. doi: 10.1111/1755-0998.13273. [DOI] [PubMed] [Google Scholar]
- 31.Adolfi M.C., Fischer P., Herpin A., Regensburger M., Kikuchi M., Tanaka M., Schartl M. Increase of cortisol levels after temperature stress activates dmrt1a causing female-to-male sex reversal and reduced germ cell number in medaka. Mol. Reprod. Dev. 2019;86:1405–1417. doi: 10.1002/mrd.23177. [DOI] [PubMed] [Google Scholar]
- 32.Franch R., Louro B., Tsalavouta M., Chatziplis D., Tsigenopoulos C.S., Sarropoulou E., Antonello J., Magoulas A., Mylonas C.C., Babbucci M., et al. A genetic linkage map of the hermaphrodite teleost fish Sparus aurata L. Genetics. 2006;174:851–861. doi: 10.1534/genetics.106.059014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dor L., Shirak A., Gorshkov S., Ron M., Hulata G. Development of genetic markers for the white grouper (Epinephelus aeneus) Aquaculture. 2014;420–421:S104–S110. doi: 10.1016/j.aquaculture.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baroiller J.F., D’Cotta H., Bezault E., Wessels S., Hoerstgen-Schwark G. Tilapia sex determination: Where temperature and genetics meet. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009;153:30–38. doi: 10.1016/j.cbpa.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Taslima K., Khan M.G.Q., McAndrew B.J., Penman D.J. Evidence of two XX/XY sex-determining loci in the Stirling stock of Nile tilapia (Oreochromis niloticus) Aquaculture. 2021;532:735995. doi: 10.1016/j.aquaculture.2020.735995. [DOI] [Google Scholar]
- 36.Curzon A.Y., Shirak A., Benet-Perlberg A., Naor A., Low-Tanne S.I., Sharkawi H., Ron M., Seroussi E. Absence of figla-like gene is concordant with femaleness in cichlids harboring the LG1 sex-determination system. Int. J. Mol. Sci. 2022;23:7636. doi: 10.3390/ijms23147636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curzon A.Y., Shirak A., Dor L., Zak T., Perelberg A., Seroussi E., Ron M. A duplication of the Anti-Müllerian hormone gene is associated with genetic sex determination of different Oreochromis niloticus strains. Heredity. 2020;125:317–327. doi: 10.1038/s41437-020-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takehana Y., Matsuda M., Myosho T., Suster M.L., Kawakami K., Shin-I T., Kohara Y., Kuroki Y., Toyoda A., Fujiyama A., et al. Co-option of Sox3 as the male-determining factor on the y chromosome in the fish Oryzias dancena. Nat. Commun. 2014;5:4157. doi: 10.1038/ncomms5157. [DOI] [PubMed] [Google Scholar]
- 39.Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 40.Sinclair A.H., Berta P., Palmer M.S., Hawkins J.R., Griffiths B.L., Smith M.J., Foster J.W., Frischauf A.-M., Lovell-Badge R., Goodfellow P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 41.Kamiya T., Kai W., Tasumi S., Oka A., Matsunaga T., Mizuno N., Fujita M., Suetake H., Suzuki S., Hosoya S., et al. A trans-species missense SNP in amhr2 is associated with sex determination in the tiger Pufferfish, Takifugu rubripes (Fugu) PLoS Genet. 2012;8:e1002798. doi: 10.1371/journal.pgen.1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamoto M., Uchino T., Koshimizu E., Kuchiishi Y., Sekiguchi R., Wang L., Sudo R., Endo M., Guiguen Y., Schartl M., et al. A Y-linked anti-Müllerian hormone type-II receptor is the sex-determining gene in ayu, Plecoglossus altivelis. PLoS Genet. 2021;17:e1009705. doi: 10.1371/journal.pgen.1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu M., Liu Y., Zhang Y., Wan S., Ravi V., Qin G., Jiang H., Wang X., Zhang H., Zhang B., et al. Seadragon genome analysis provides insights into its phenotype and sex determination locus. Sci. Adv. 2021;7:5196–5214. doi: 10.1126/sciadv.abg5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feron R., Zahm M., Cabau C., Klopp C., Roques C., Bouchez O., Eché C., Valière S., Donnadieu C., Haffray P., et al. Characterization of a Y-specific duplication/insertion of the anti-Mullerian hormone type II receptor gene based on a chromosome-scale genome assembly of yellow perch, Perca flavescens. Mol. Ecol. Resour. 2020;20:531–543. doi: 10.1111/1755-0998.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen M., Pan Q., Jouanno E., Montfort J., Zahm M., Cabau C., Klopp C., Iampietro C., Roques C., Bouchez O., et al. An ancient truncated duplication of the anti-Müllerian hormone receptor type 2 gene is a potential conserved master sex determinant in the Pangasiidae catfish family. Mol. Ecol. Resour. 2022;22:2411–2428. doi: 10.1111/1755-0998.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nacif C.L., Kratochwil C.F., Kautt A.F., Nater A., Machado-Schiaffino G., Meyer A., Henning F. Molecular parallelism in the evolution of a master sex-determining role for the anti-Mullerian hormone receptor 2 gene (amhr2) in Midas cichlids. Mol. Ecol. 2022:1–13. doi: 10.1111/mec.16466. [DOI] [PubMed] [Google Scholar]
- 47.Myosho T., Otake H., Masuyama H., Matsuda M., Kuroki Y., Fujiyama A., Naruse K., Hamaguchi S., Sakaizumi M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics. 2012;191:163–170. doi: 10.1534/genetics.111.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edvardsen R.B., Wallerman O., Furmanek T., Kleppe L., Jern P., Wallberg A., Kjærner-Semb E., Mæhle S., Olausson S.K., Sundström E., et al. Heterochiasmy and the establishment of gsdf as a novel sex determining gene in Atlantic halibut. PLoS Genet. 2022;18:e1010011. doi: 10.1371/journal.pgen.1010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herpin A., Schartl M., Depincé A., Guiguen Y., Bobe J., Hua-Van A., Hayman E.S., Octavera A., Yoshizaki G., Nichols K.M., et al. Allelic diversification after transposable element exaptation promoted gsdf as the master sex determining gene of sablefish. Genome Res. 2021;31:1366–1380. doi: 10.1101/gr.274266.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koyama T., Nakamoto M., Morishima K., Yamashita R., Yamashita T., Sasaki K., Kuruma Y., Mizuno N., Suzuki M., Okada Y., et al. A SNP in a steroidogenic enzyme is associated with phenotypic sex in seriola fishes. Curr. Biol. 2019;29:1901–1909.e8. doi: 10.1016/j.cub.2019.04.069. [DOI] [PubMed] [Google Scholar]
- 51.Fan B., Xie D., Li Y., Wang X., Qi X., Li S., Meng Z., Chen X., Peng J., Yang Y., et al. A single intronic single nucleotide polymorphism in splicing site of steroidogenic enzyme hsd17b1 is associated with phenotypic sex in oyster pompano, Trachinotus anak. Proc. R. Soc. B. 2021;288:20212245. doi: 10.1098/rspb.2021.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rafati N., Chen J., Herpin A., Pettersson M.E., Han F., Feng C., Wallerman O., Rubin C.-J., Péron S., Cocco A., et al. Reconstruction of the birth of a male sex chromosome present in Atlantic herring. Proc. Natl. Acad. Sci. USA. 2020;117:24359–24368. doi: 10.1073/pnas.2009925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda M., Nagahama Y., Shinomiya A., Sato T., Matsuda C., Kobayashi T., Morrey C.E., Shibata N., Asakawa S., Shimizu N., et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 54.Nanda I., Kondo M., Hornung U., Asakawa S., Winkler C., Shimizu A., Shan Z., Haaf T., Shimizu N., Shima A., et al. A duplicated copy of dmrt1 in the sex-determining region of the Y chromosom of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA. 2002;99:11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimoto S., Okada E., Umemoto H., Tamura K., Uno Y., Nishida-Umehara C., Matsuda Y., Takamatsu N., Shiba T., Ito M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA. 2008;105:2469–2474. doi: 10.1073/pnas.0712244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith C.A., Roeszler K.N., Ohnesorg T., Cummins D.M., Farlie P.G., Doran T.J., Sinclair A.H. The avian Z-linked gene dmrt1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- 57.Chen S., Zhang G., Shao C., Huang Q., Liu G., Zhang P., Song W., An N., Chalopin D., Volff J.N., et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014;46:253–260. doi: 10.1038/ng.2890. [DOI] [PubMed] [Google Scholar]
- 58.Cui Z., Liu Y., Wang W., Wang Q., Zhang N., Lin F., Wang N., Shao C., Dong Z., Li Y., et al. Genome editing reveals dmrt1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis) Sci. Rep. 2017;7:42213. doi: 10.1038/srep42213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mustapha U.F., Jiang D.-N., Liang Z.-H., Gu H.-T., Yang W., Chen H.-P., Deng S.-P., Wu T.-L., Tian C.-X., Zhu C.-H., et al. Male-specific dmrt1 is a candidate sex determination gene in spotted scat (Scatophagus argus) Aquaculture. 2018;495:351–358. doi: 10.1016/j.aquaculture.2018.06.009. [DOI] [Google Scholar]
- 60.Reichwald K., Petzold A., Koch P., Downie B.R., Hartmann N., Pietsch S., Baumgart M., Chalopin D., Felder M., Bens M., et al. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell. 2015;163:1527–1538. doi: 10.1016/j.cell.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 61.Imarazene B., Du K., Beille S., Jouanno E., Feron R., Pan Q., Torres-Paz J., Lopez-Roques C., Castinel A., Gil L., et al. A supernumerary “B-sex” chromosome drives male sex determination in the Pachón cavefish, Astyanax mexicanus. Curr. Biol. 2021;31:4800–4809.e9. doi: 10.1016/j.cub.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirubakaran T.G., Andersen Ø., de Rosa M.C., Andersstuen T., Hallan K., Kent M.P., Lien S. Characterization of a male specific region containing a candidate sex determining gene in Atlantic cod. Sci. Rep. 2019;9:116. doi: 10.1038/s41598-018-36748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hattori R.S., Murai Y., Oura M., Masuda S., Majhi S.K., Sakamoto T., Fernandino J.I., Somoza G.M., Yokota M., Strussmann C.A. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA. 2012;109:2955–2959. doi: 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eshel O., Shirak A., Dor L., Band M., Zak T., Markovich-Gordon M., Chalifa-Caspi V., Feldmesser E., Weller J.I., Seroussi E., et al. Identification of male-specific amh duplication, sexually differentially expressed genes and microRNAs at early embryonic development of Nile tilapia (Oreochromis niloticus) BMC Genom. 2014;15:774. doi: 10.1186/1471-2164-15-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rondeau E.B., Laurie C.V., Johnson S.C., Koop B.F. A PCR assay detects a male-specific duplicated copy of Anti-Müllerian hormone (amh) in the lingcod (Ophiodon elongatus) BMC Res. Notes. 2016;9:230. doi: 10.1186/s13104-016-2030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bej D.K., Miyoshi K., Hattori R.S., Strüssmann C.A., Yamamoto Y. A duplicated, truncated amh gene is involved in male sex determination in an old world Silverside. G3 Genes Genomes Genet. 2017;7:2489–2495. doi: 10.1534/g3.117.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan Q., Feron R., Yano A., Guyomard R., Jouanno E., Vigouroux E., Wen M., Busne J.M., Bobe J., Concordet J.P., et al. Identification of the master sex determining gene in Northern pike (Esox lucius) reveals restricted sex chromosome differentiation. PLoS Genet. 2019;15:e1008013. doi: 10.1371/journal.pgen.1008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song W., Xie Y., Sun M., Li X., Fitzpatrick C.K., Vaux F., O’Malley K.G., Zhang Q., Qi J., He Y. A duplicated amh is the master sex-determining gene for Sebastes rockfish in the Northwest Pacific. Open Biol. 2021;11:210063. doi: 10.1098/rsob.210063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peichel C.L., McCann S.R., Ross J.A., Naftaly A.F.S., Urton J.R., Cech J.N., Grimwood J., Schmutz J., Myers R.M., Kingsley D.M., et al. Assembly of the threespine stickleback Y chromosome reveals convergent signatures of sex chromosome evolution. Genome Biol. 2020;21:177. doi: 10.1186/s13059-020-02097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeffries D.L., Mee J.A., Peichel C.L. Identification of a candidate sex determination gene in Culaea inconstans suggests convergent recruitment of an amh duplicate in two lineages of stickleback. J. Evol. Biol. 2022;35:1683–1695. doi: 10.1111/jeb.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curzon A.Y., Shirak A., Benet-Perlberg A., Naor A., Low-Tanne S.I., Sharkawi H., Ron M., Seroussi E. Gene variant of barrier to autointegration factor 2 (banf2w) is concordant with female determination in Cichlids. Int. J. Mol. Sci. 2021;22:7073. doi: 10.3390/ijms22137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu F., Sun F., Li J., Xia J.H., Lin G., Tu R.J., Yue G.H. A microsatellite-based linkage map of salt tolerant tilapia (Oreochromis mossambicus × Oreochromis spp.) and mapping of sex-determining loci. BMC Genom. 2013;14:58. doi: 10.1186/1471-2164-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun C., Niu Y., Ye X., Dong J., Hu W., Zeng Q., Chen Z., Tian Y., Zhang J., Lu M. Construction of a high-density linkage map and mapping of sex determination and growth-related loci in the mandarin fish (Siniperca chuatsi) BMC Genom. 2017;18:446. doi: 10.1186/s12864-017-3830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M., Li L., Lin H., Zhou Z., Liu B., Zhong J., Pu F., Shi Y., Zhou T., Xu P. Genome-wide association study identifies genomic loci of sex determination, gonadal weight and gonadosomatic index traits in Takifugu bimaculatus. Aquaculture. 2022;546:737389. doi: 10.1016/j.aquaculture.2021.737389. [DOI] [Google Scholar]
- 75.Martínez P., Robledo D., Taboada X., Blanco A., Moser M., Maroso F., Hermida M., Gómez-Tato A., Álvarez-Blázquez B., Cabaleiro S., et al. A genome-wide association study, supported by a new chromosome-level genome assembly, suggests sox2 as a main driver of the undifferentiatiated ZZ/ZW sex determination of turbot (Scophthalmus maximus) Genomics. 2021;113:1705–1718. doi: 10.1016/j.ygeno.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Lv J., Sun D., Huan P., Song L., Liu P., Li J. QTL mapping and marker identification for sex-determining: Indicating XY sex determination system in the swimming crab (Portunus trituberculatus) Front. Genet. 2018;9:337. doi: 10.3389/fgene.2018.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feulner P.G.D., Schwarzer J., Haesler M.P., Meier J.I., Seehausen O. A dense linkage map of Lake Victoria cichlids improved the Pundamilia genome assembly and revealed a major QTL for sex-determination. G3 Genes Genomes Genet. 2018;8:2411–2420. doi: 10.1534/g3.118.200207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou Y., Liu H., Wang X., Fu B., Yu X., Tong J. QTL fine mapping for sex determination region in Bighead Carp (Hypophthalmichthys nobilis) and comparison with Silver Carp (iHypophthalmichthys molitrix) Mar. Biotechnol. 2020;22:41–53. doi: 10.1007/s10126-019-09929-3. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Z.X., Lin Y.L., Ai C.H., Xiong Y.Y., Huang D.D., Yao Y.Y., Liu T.D., Chen C.H., Lin H.R., Xia J.H. First identification of two co-existing genome-wide significant sex quantitative trait loci (QTL) in red tilapia using integrative QTL mapping. Zool Res. 2022;43:205. doi: 10.24272/j.issn.2095-8137.2021.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bachtrog D. A dynamic view of sex chromosome evolution. Curr. Opin. Genet. Dev. 2006;16:578–585. doi: 10.1016/j.gde.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 81.Dor L., Shirak A., Kohn Y.Y., Gur T., Weller J.I., Zilberg D., Seroussi E., Ron M. Mapping of the sex determining region on linkage group 12 of guppy (Poecilia reticulata) G3 Genes Genomes Genet. 2019;9:3867–3875. doi: 10.1534/g3.119.400656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charlesworth D., Bergero R., Graham C., Gardner J., Yong L. Locating the sex determining region of linkage group 12 of guppy (Poecilia reticulata) G3 Genes Genomes Genet. 2020;10:3639–3649. doi: 10.1534/g3.120.401573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kocher T.D., Behrens K.A., Conte M.A., Aibara M., Mrosso H.D.J., Green E.C.J., Kidd M.R., Nikaido M., Koblmüller S. New sex chromosomes in Lake Victoria cichlid fishes (Cichlidae: Haplochromini) Genes. 2022;13:804. doi: 10.3390/genes13050804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duncan L.E., Ostacher M., Ballon J. How genome-wide association studies (GWAS) made traditional candidate gene studies obsolete. Neuropsychopharmacology. 2019;44:1518–1523. doi: 10.1038/s41386-019-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Altshuler D., Daly M.J., Lander E.S. Genetic mapping in human disease. Science. 2008;322:881–888. doi: 10.1126/science.1156409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foster J.W., Graves J.A. An SRY-related sequence on the marsupial X chromosome: Implications for the evolution of the mammalian testis-determining gene. Proc. Natl. Acad. Sci. USA. 1994;91:1927–1931. doi: 10.1073/pnas.91.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sreenivasan R., Gonen N., Sinclair A. SOX genes and their role in disorders of sex development. Sex Dev. 2022;16:80–91. doi: 10.1159/000524453. [DOI] [PubMed] [Google Scholar]
- 88.Haines B., Hughes J., Corbett M., Shaw M., Innes J., Patel L., Gecz J., Clayton-Smith J., Thomas P. Interchromosomal insertional translocation at Xq26.3 alters SOX3 expression in an individual with XX male sex reversal. J. Clin. Endocrinol. Metab. 2015;100:E815–E820. doi: 10.1210/jc.2014-4383. [DOI] [PubMed] [Google Scholar]
- 89.Sutton E., Hughes J., White S., Sekido R., Tan J., Arboleda V., Rogers N., Knower K., Rowley L., Eyre H., et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J. Clin. Investig. 2011;121:328–341. doi: 10.1172/JCI42580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shirak A., Seroussi E., Cnaani A., Howe A.E., Domokhovsky R., Zilberman N., Kocher T.D., Hulata G., Ron M. Amh and dmrta2 genes map to tilapia (Oreochromis spp.) linkage group 23 within quantitative trait locus regions for sex determination. Genetics. 2006;174:1573–1581. doi: 10.1534/genetics.106.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guiguen Y., Fostier A., Piferrer F., Chang C.F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010;165:352–366. doi: 10.1016/j.ygcen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Simpson E.R., Mahendroo M.S., Means G.D., Kilgore M.W., Hinshelwood M.M., Graham-Lorence S., Amarneh B., Ito Y., Fisher C.R., Michael M.D., et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/EDRV-15-3-342. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y., Zhang S., Lu H., Zhang L., Zhang W. Genes encoding aromatases in teleosts: Evolution and expression regulation. Gen. Comp. Endocrinol. 2014;205:151–158. doi: 10.1016/j.ygcen.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 94.Li Y., Tang Y., Wang L., Li X., Deng L., Deng W., Zheng Y., Wang D., Wei L. Transcription factor sox3 is required for oogenesis in the teleost fish Nile tilapia. Int. J. Biol. Macromol. 2022;222:2639–2647. doi: 10.1016/j.ijbiomac.2022.10.046. [DOI] [PubMed] [Google Scholar]
- 95.Vining B., Ming Z., Bagheri-Fam S., Harley V., Barrionuevo Jimenez J., Burgos M., Jiménez R. Diverse regulation but conserved function: SOX9 in vertebrate sex determination. Genes. 2021;12:486. doi: 10.3390/genes12040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei L., Li X., Li M., Tang Y., Wei J., Wang D. Dmrt1 directly regulates the transcription of the testis-biased Sox9b gene in Nile tilapia (Oreochromis niloticus) Gene. 2019;687:109–115. doi: 10.1016/j.gene.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 97.Wei L., Yang C., Tao W., Wang D. Genome-wide identification and transcriptome-based expression profiling of the sox gene family in the Nile tilapia (Oreochromis niloticus) Int. J. Mol. Sci. 2016;17:270. doi: 10.3390/ijms17030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakamoto M., Suzuki A., Matsuda M., Nagahama Y., Shibata N. Testicular type sox9 is not involved in sex determination but might be in the development of testicular structures in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 2005;333:729–736. doi: 10.1016/j.bbrc.2005.05.158. [DOI] [PubMed] [Google Scholar]
- 99.Heinosalo T., Saarinen N., Poutanen M. Role of hydroxysteroid (17beta) dehydrogenase type 1 in reproductive tissues and hormone-dependent diseases. Mol. Cell. Endocrinol. 2019;489:9–31. doi: 10.1016/j.mce.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 100.Ijiri S., Kaneko H., Kobayashi T., Wang D.-S., Sakai F., Paul-Prasanth B., Nakamura M., Nagahama Y. Sexual dimorphic expression of genes in gonads during early differentiation of a Teleost fish, the Nile tilapia Oreochromis niloticus. Biol. Reprod. 2008;78:333–341. doi: 10.1095/biolreprod.107.064246. [DOI] [PubMed] [Google Scholar]
- 101.Hsu C.W., Chung B.C. Evolution, expression, and function of gonadal somatic cell-derived factor. Front. Cell Dev. Biol. 2021;9:1600. doi: 10.3389/fcell.2021.684352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaneko H., Ijiri S., Kobayashi T., Izumi H., Kuramochi Y., Wang D.S., Mizuno S., Nagahama Y. Gonadal soma-derived factor (gsdf), a TGF-beta superfamily gene, induces testis differentiation in the teleost fish Oreochromis niloticus. Mol. Cell. Endocrinol. 2015;415:87–99. doi: 10.1016/j.mce.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 103.Jiang D.N., Yang H.H., Li M.H., Shi H.J., Zhang X.B., Wang D.S. Gsdf is a downstream gene of dmrt1 that functions in the male sex determination pathway of the Nile tilapia. Mol. Reprod. Dev. 2016;83:497–508. doi: 10.1002/mrd.22642. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X., Chang Y., Zhai W., Qian F., Zhang Y., Xu S., Guo H., Wang S., Hu R., Zhong X., et al. A potential role for the Gsdf–eEF1α complex in inhibiting germ cell proliferation: A protein-interaction analysis in Medaka (Oryzias latipes) from a proteomics perspective. Mol. Cell. Proteom. 2021;20:100023. doi: 10.1074/mcp.RA120.002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang D.N., Peng Y.X., Liu X.Y., Mustapha U.F., Huang Y.Q., Shi H.J., Li M.H., Li G.L., Wang D.S. Homozygous mutation of gsdf causes infertility in female Nile tilapia (Oreochromis niloticus) Front. Endocrinol. 2022;13:135. doi: 10.3389/fendo.2022.813320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu J., Li W., Hu R., Zhou Y., Fei Y., Zhang Y., Zhai W., Chen L. Molecular and morphological changes in Nile tilapia (Oreochromis niloticus) gonads during high-temperature-induced masculinization. Aquac. Res. 2022;53:921–931. doi: 10.1111/are.15633. [DOI] [Google Scholar]
- 107.Romano S., Kaufman O.H., Marlow F.L. Loss of dmrt1 restores zebrafish female fates in the absence of cyp19a1a but not rbpms2a/b. Development. 2020;147:dev190942. doi: 10.1242/dev.190942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu K., Song W., Zhang Z., Ge W. Disruption of dmrt1 rescues the all-male phenotype of the cyp19a1a mutant in zebrafish—A novel insight into the roles of aromatase/estrogens in gonadal differentiation and early folliculogenesis. Development. 2020;147:dev182758. doi: 10.1242/dev.182758. [DOI] [PubMed] [Google Scholar]
- 109.Webster K.A., Schach U., Ordaz A., Steinfeld J.S., Draper B.W., Siegfried K.R. Dmrt1 is necessary for male sexual development in zebrafish. Dev. Biol. 2017;422:33–46. doi: 10.1016/j.ydbio.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu X., Dai S., Wu J., Wei X., Zhou X., Chen M., Tan D., Pu D., Li M., Wang D. Roles of anti-Müllerian hormone and its duplicates in sex determination and germ cell proliferation of Nile tilapia. Genetics. 2022;220:iyab237. doi: 10.1093/genetics/iyab237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang D.S., Zhou L.Y., Kobayashi T., Matsuda M., Shibata Y., Sakai F., Nagahama Y. Doublesex- and mab-3-related transcription factor-1 repression of aromatase transcription, a possible mechanism favoring the male pathway in tilapia. Endocrinology. 2010;151:1331–1340. doi: 10.1210/en.2009-0999. [DOI] [PubMed] [Google Scholar]
- 112.Dai S., Qi S., Wei X., Liu X., Li Y., Zhou X., Xiao H., Lu B., Wang D., Li M. Germline sexual fate is determined by the antagonistic action of dmrt1 and foxl3 /foxl2 in tilapia. Development. 2021;148:dev199380. doi: 10.1242/dev.199380. [DOI] [PubMed] [Google Scholar]
- 113.Kim S.Y., Weiss J., Tong M., Laronda M.M., Lee E.J., Jameson J.L. Foxl2, a forkhead transcription factor, modulates nonclassical activity of the estrogen receptor-α. Endocrinology. 2009;150:5085–5093. doi: 10.1210/en.2009-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Georges A., L’Hôte D., Todeschini A.L., Auguste A., Legois B., Zider A., Veitia R.A. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells. Elife. 2014;3:1–19. doi: 10.7554/eLife.04207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang Y., Li X., Xiao H., Li M., Li Y., Wang D., Wei L. Transcription of the sox30 Gene Is Positively Regulated by dmrt1 in Nile Tilapia. Int. J. Mol. Sci. 2019;20:5487. doi: 10.3390/ijms20215487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sacchi S., D’Ippolito G., Sena P., Marsella T., Tagliasacchi D., Maggi E., Argento C., Tirelli A., Giulini S., la Marca A. The anti-Müllerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J. Assist. Reprod. Genet. 2016;33:95–100. doi: 10.1007/s10815-015-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qiang J., Cao Z.-M., Zhu H.-J., Tao Y.-F., He J., Xu P. Knock-down of amh transcription by antisense RNA reduces FSH and increases follicular atresia in female Oreochromis niloticus. Gene. 2022;842:146792. doi: 10.1016/j.gene.2022.146792. [DOI] [PubMed] [Google Scholar]
- 118.Yan H., Ijiri S., Wu Q., Kobayashi T., Li S., Nakaseko T., Adachi S., Nagahama Y. Expression patterns of gonadotropin hormones and their receptors during early sexual differentiation in nile tilapia Oreochromis niloticus. Biol. Reprod. 2012;87:116–117. doi: 10.1095/biolreprod.112.101220. [DOI] [PubMed] [Google Scholar]
- 119.Qiu Y., Sun S., Charkraborty T., Wu L., Sun L., Wei J., Nagahama Y., Wang D., Zhou L. Figla favors ovarian differentiation by antagonizing spermatogenesis in a teleosts, Nile Tilapia (Oreochromis niloticus) PLoS ONE. 2015;10:e0123900. doi: 10.1371/journal.pone.0123900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou L., Qiu Y. Functional studies of Figla in the ovarian differentiation and maintenance in Nile tilapia (Orechromis niloticus) J. Fish China. 2016;40:665–672. [Google Scholar]
- 121.Li H., Xu W., Zhang N., Shao C., Zhu Y., Dong Z., Wang N., Jia X., Xu H., Chen S. Two Figla homologues have disparate functions during sex differentiation in half-smooth tongue sole (Cynoglossus semilaevis) Sci. Rep. 2016;6:28219. doi: 10.1038/srep28219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Margalit A., Segura-Totten M., Gruenbaum Y., Wilson K.L. Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc. Natl. Acad. Sci. USA. 2005;102:3290–3295. doi: 10.1073/pnas.0408364102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tifft K.E., Segura-Totten M., Lee K.K., Wilson K.L. Barrier-to-autointegration factor-like (BAF-L): A proposed regulator of BAF. Exp. Cell Res. 2006;312:478–487. doi: 10.1016/j.yexcr.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 124.Penrad-Mobayed M., Perrin C., Herman L., Todeschini A., Nigon F., Cosson B., Caburet S., Veitia R.A. Conventional and unconventional interactions of the transcription factor FOXL2 uncovered by a proteome-wide analysis. FASEB J. 2020;34:571–587. doi: 10.1096/fj.201901573R. [DOI] [PubMed] [Google Scholar]
- 125.L’Hôte D., Georges A., Todeschini A.L., Kim J.H., Benayoun B.A., Bae J., Veitia R.A. Discovery of novel protein partners of the transcription factor FOXL2 provides insights into its physiopathological roles. Hum. Mol. Genet. 2012;21:3264–3274. doi: 10.1093/hmg/dds170. [DOI] [PubMed] [Google Scholar]
- 126.Ron M., Weller J.I. From QTL to QTN identification in livestock—Winning by points rather than knock-out: A review. Anim. Genet. 2007;38:429–439. doi: 10.1111/j.1365-2052.2007.01640.x. [DOI] [PubMed] [Google Scholar]
- 127.Mackay T.F.C. The genetic architecture of quantitative traits. Annu. Rev. Genet. 2001;35:303–339. doi: 10.1146/annurev.genet.35.102401.090633. [DOI] [PubMed] [Google Scholar]
- 128.Dor L., Shirak A., Curzon A.Y., Rosenfeld H., Ashkenazi I.M., Nixon O., Seroussi E., Weller J.I., Ron M. Preferential mapping of sex-biased differentially-expressed genes of larvae to the sex-determining region of Flathead Grey Mullet (Mugil cephalus) Front. Genet. 2020;11:839. doi: 10.3389/fgene.2020.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuhl H., Guiguen Y., Höhne C., Kreuz E., Du K., Klopp C., Lopez-Roques C., Yebra-Pimentel E.S., Ciorpac M., Gessner J., et al. A 180 Myr-old female-specific genome region in sturgeon reveals the oldest known vertebrate sex determining system with undifferentiated sex chromosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021;376:20200089. doi: 10.1098/rstb.2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Curzon A.Y., Shirak A., Meerson A., Degani G., Hurvitz A., Ben-Naim N., Domovitz R., Ron M., Seroussi E. Cross-species conservation of a transposase-linked element enables genetic sexing of commercial populations of Russian sturgeon (Acipenser gueldenstaedtii) Anim. Genet. 2022;53:441–446. doi: 10.1111/age.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bao L., Tian C., Liu S., Zhang Y., Elaswad A., Yuan Z., Khalil K., Sun F., Yang Y., Zhou T., et al. The Y chromosome sequence of the channel catfish suggests novel sex determination mechanisms in teleost fish. BMC Biol. 2019;17:6. doi: 10.1186/s12915-019-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ventura T., Sagi A. The insulin-like androgenic gland hormone in crustaceans: From a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol. Adv. 2012;30:1543–1550. doi: 10.1016/j.biotechadv.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 133.Gonen N., Futtner C.R., Wood S., Alexandra Garcia-Moreno S., Salamone I.M., Samson S.C., Sekido R., Poulat F., Maatouk D.M., Lovell-Badge R. Sex reversal following deletion of a single distal enhancer of Sox9. Science. 2018;360:1469–1471. doi: 10.1126/science.aas9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eshel O., Shirak A., Weller J.I., Slossman T., Hulata G., Cnaani A., Ron M. Fine-mapping of a locus on linkage group 23 for sex determination in Nile tilapia (Oreochromis niloticus) Anim. Genet. 2011;42:222–224. doi: 10.1111/j.1365-2052.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- 135.Eshel O., Shirak A., Weller J.I., Hulata G., Ron M. Linkage and physical mapping of sex region on LG23 of Nile tilapia (Oreochromis niloticus) G3 Genes Genomes Genet. 2012;2:35–42. doi: 10.1534/g3.111.001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Loehlin D.W., Carroll S.B. Expression of tandem gene duplicates is often greater than twofold. Proc. Natl. Acad. Sci. USA. 2016;113:5988–5992. doi: 10.1073/pnas.1605886113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loehlin D.W., Kim J.Y., Paster C.O. A tandem duplication in Drosophila melanogaster shows enhanced expression beyond the gene copy number. Genetics. 2022;220:iyab231. doi: 10.1093/genetics/iyab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ridnik M., Schoenfelder S., Gonen N. Cis-regulatory control of mammalian sex determination. Sex Dev. 2021;15:317–334. doi: 10.1159/000519244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Triay C., Conte M.A., Baroiller J.F., Bezault E., Clark F.E., Penman D.J., Kocher T.D., D’cotta H. Structure and sequence of the sex determining locus in two wild populations of Nile tilapia. Genes. 2020;11:1017. doi: 10.3390/genes11091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Triay C., Courcelle M., Caminade P., Bezault E., Baroiller J.-F., Kocher T.D., D’Cotta H. Polymorphism of sex determination amongst wild populations suggests its rapid turnover within the Nile tilapia species. Front. Genet. 2022;13:345. doi: 10.3389/fgene.2022.820772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Landi M., Dimech M., Arculeo M., Biondo G., Martins R., Carneiro M., Carvalho G.R., Lo Brutto S., Costa F.O. DNA Barcoding for species assignment: The case of Mediterranean marine fishes. PLoS ONE. 2014;9:e106135. doi: 10.1371/journal.pone.0106135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lowenstein J.H., Amato G., Kolokotronis S.O. The real maccoyii: Identifying Tuna Sushi with DNA barcodes—Contrasting characteristic attributes and genetic distances. PLoS ONE. 2009;4:e7866. doi: 10.1371/journal.pone.0007866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shirak A., Cohen-Zinder M., Barroso R.M., Seroussi E., Ron M., Hulata G. DNA barcoding of Israeli indigenous and introduced cichlids. Isr. J. Aquac. 2009;61:83–88. [Google Scholar]
- 144.Zhang J., Hanner R. Molecular approach to the identification of fish in the South China sea. PLoS ONE. 2012;7:e30621. doi: 10.1371/journal.pone.0030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shirak A., Dor L., Seroussi E., Ron M., Hulata G., Golani D. DNA barcoding of fish species from the Mediterranean coast of Israel. Mediterr. Mar. Sci. 2016;17:459–466. doi: 10.12681/mms.1384. [DOI] [Google Scholar]
- 146.Keskİn E., Atar H.H. DNA barcoding commercially important fish species of Turkey. Mol. Ecol. Resour. 2013;13:788–797. doi: 10.1111/1755-0998.12120. [DOI] [PubMed] [Google Scholar]
- 147.Curzon A.Y., Shirak A., Dor L., Zak T., Benet-Perelberg A., Seroussi E., Ron M. Hybrid origin of the Thai-Chitralada tilapia strain using DNA barcoding and microsatellite analysis. Isr. J. Aquac. 2019;71:20988. doi: 10.46989/001c.20988. [DOI] [Google Scholar]
- 148.Geraerts M., Vangestel C., Artois T., de Oliveira Fernandes J.M., Jorissen M.W.P., Chocha Manda A., Danadu Mizani C., Smeets K., Snoeks J., Sonet G., et al. Population genomics of introduced Nile tilapia Oreochromis niloticus (Linnaeus, 1758) in the Democratic Republic of the Congo: Repeated introductions since colonial times with multiple sources. Mol. Ecol. 2022;31:3304–3322. doi: 10.1111/mec.16479. [DOI] [PubMed] [Google Scholar]
- 149.Blackwell T., Ford A.G.P., Ciezarek A.G., Bradbeer S.J., Gracida Juarez C.A., Smith A.M., Ngatunga B.P., Shechonge A., Tamatamah R., Etherington G., et al. Newly discovered cichlid fish biodiversity threatened by hybridization with non-native species. Mol. Ecol. 2021;30:895–911. doi: 10.1111/mec.15638. [DOI] [PubMed] [Google Scholar]
- 150.Gammerdinger W.J., Conte M.A., Sandkam B.A., Penman D.J., Kocher T.D. Characterization of sex chromosomes in three deeply diverged species of Pseudocrenilabrinae (Teleostei: Cichlidae) Hydrobiologia. 2019;832:397–408. doi: 10.1007/s10750-018-3778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu H., Liu Z., Lu M., Gao F., Ke X., Ma D., Huang Z., Cao J., Wang M. Screening and identification of a microsatellite marker associated with sex in Wami tilapia, Oreochromis urolepis hornorum. J. Genet. 2016;95:283–289. doi: 10.1007/s12041-016-0653-y. [DOI] [PubMed] [Google Scholar]
- 152.Wu X., Zhao L., Fan Z., Lu B., Chen J., Tan D., Jiang D., Tao W., Wang D. Screening and characterization of sex-linked DNA markers and marker-assisted selection in blue tilapia (Oreochromis aureus) Aquaculture. 2021;530:735934. doi: 10.1016/j.aquaculture.2020.735934. [DOI] [Google Scholar]
- 153.Mtaki K., Limbu S.M., Mmochi A.J., Mtolera M.S.P. Hybrids production as a potential method to control prolific breeding in tilapia and adaptation to aquaculture climate-induced drought. Aquac. Fish. 2022;7:647–652. doi: 10.1016/j.aaf.2021.04.005. [DOI] [Google Scholar]
- 154.Geletu T.T., Zhao J. Genetic resources of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in its native range and aquaculture. Hydrobiologia. 2022:1–21. doi: 10.1007/s10750-022-04989-4. [DOI] [Google Scholar]
- 155.Nwachi O., Irabor A. Diallel Hybridization of Oreochromis niloticus (Nile Tilapia) to Oreochromis aureus (Blue Tilapia) Yuzuncu Yıl Univ. J. Agric. Sci. 2022;32:280–285. doi: 10.29133/yyutbd.1035192. [DOI] [Google Scholar]
- 156.Stauffer J.R., Chirwa E.R., Jere W., Konings A.F., Tweddle D., Weyl O. Nile Tilapia, Oreochromis niloticus (Teleostei: Cichlidae): A threat to native fishes of Lake Malawi? Biol. Invasions. 2022;24:1585–1597. doi: 10.1007/s10530-022-02756-z. [DOI] [Google Scholar]
- 157.Brown K.H. Fish mitochondrial genomics: Sequence, inheritance and functional variation. J. Fish Biol. 2008;72:355–374. doi: 10.1111/j.1095-8649.2007.01690.x. [DOI] [Google Scholar]
- 158.Anderson L., Camus M.F., Monteith K.M., Salminen T.S., Vale P.F. Variation in mitochondrial DNA affects locomotor activity and sleep in Drosophila melanogaster. Heredity. 2022;129:225–232. doi: 10.1038/s41437-022-00554-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou Y., Zhang X., Xu Q., Yan J., Yu F., Wang F., Xiao J., Luo Y., Zhong H. Nonadditive and allele-specific expression of insulin-like growth factor 1 in Nile tilapia (Oreochromis niloticus, ♀) × blue tilapia (O. aureus, ♂) hybrids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019;232:93–100. doi: 10.1016/j.cbpb.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 160.Avella M., Berhaut J., Bornancin M. Salinity tolerance of two tropical fishes, Oreochromis aureus and O. niloticus. I. Biochemical and morphological changes in the gill epithelium. J. Fish Biol. 1993;42:243–254. doi: 10.1111/j.1095-8649.1993.tb00325.x. [DOI] [Google Scholar]
- 161.Shiozaki T., Itoh F., Hirose Y., Onodera J., Kuwata A., Harada N. A DNA metabarcoding approach for recovering plankton communities from archived samples fixed in formalin. PLoS ONE. 2021;16:e0245936. doi: 10.1371/journal.pone.0245936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Uller T., Pen I., Wapstra E., Beukeboom L.W., Komdeur J. The evolution of sex ratios and sex-determining systems. Trends Ecol. Evol. 2007;22:292–297. doi: 10.1016/j.tree.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 163.Wilson C.A., High S.K., McCluskey B.M., Amores A., Yan Y.L., Titus T.A., Anderson J.L., Batzel P., Carvan M.J., Schartl M., et al. Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics. 2014;198:1291–1308. doi: 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Panthum T., Jaisamut K., Singchat W., Ahmad S.F., Kongkaew L., Wongloet W., Dokkaew S., Kraichak E., Muangmai N., Duengkae P., et al. Something fishy about Siamese fighting fish (Betta splendens) sex: Polygenic sex determination or a newly emerged sex-determining region? Cells. 2022;11:1764. doi: 10.3390/cells11111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fraser B.A., Whiting J.R., Paris J.R., Weadick C.J., Parsons P.J., Charlesworth D., Bergero R., Bemm F., Hoffmann M., Kottler V.A., et al. Improved reference genome uncovers novel sex-linked regions in the guppy (Poecilia reticulata) Genome Biol. Evol. 2020;12:1789–1805. doi: 10.1093/gbe/evaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Franchini P., Jones J.C., Xiong P., Kneitz S., Gompert Z., Warren W.C., Walter R.B., Meyer A., Schartl M. Long-term experimental hybridisation results in the evolution of a new sex chromosome in swordtail fish. Nat. Commun. 2018;9:5136. doi: 10.1038/s41467-018-07648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moore E.C., Ciccotto P.J., Peterson E.N., Lamm M.S., Albertson R.C., Roberts R.B. Polygenic sex determination produces modular sex polymorphism in an African cichlid fish. Proc. Natl. Acad. Sci. USA. 2022;119:e2118574119. doi: 10.1073/pnas.2118574119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.