Abstract
Fecal calprotectin (FC) levels correlate with the disease activity of inflammatory bowel diseases (IBD); however, the utility of FC in predicting IBD relapse remains to be determined. We aim to evaluate the efficacy of fecal calprotectin in predicting the relapse of inflammatory bowel disease. We searched Pubmed (MEDLINE), Embase, Web of Science, and the Cochrane library databases up to 7 July 2021. Our study estimated the pooled sensitivity and specificity, summary receiver operating characteristic (SROC) curve, and the optimal cut-off value for predicting IBD relapse using a multiple threshold model. A total of 24 prospective studies were included in the meta-analysis. The optimal FC cut-off value was 152 μg/g. The pooled sensitivity and specificity of FC was 0.720 (0.528 to 0.856) and 0.740 (0.618 to 0.834), respectively. FC is a useful, non-invasive, and inexpensive biomarker for the early prediction of IBD relapse. An FC value of 152 μg/g is an ideal threshold to identify patients with a high relapse probability.
Keywords: Fecal calprotectin, inflammatory bowel diseases, biomarker, diagnosis
1. Introduction
Inflammatory bowel diseases (IBD) are chronic gastrointestinal disorders with a remitting and relapsing course and are associated with multiple complications. IBD incidence has increased in industrialized countries with increased healthcare expenditure and poor quality of life [1]. Ulcerative colitis (UC) and Crohn’s disease (CD) represent the two main types of IBD. Since the clinical course of IBD remains unpredictable, there is an urgent need to develop serum and fecal biomarkers to help predict relapse to take appropriate measures to reduce complications [2,3].
Endoscopy plays an essential role in the diagnosis, management, prognosis, and surveillance of IBD [4,5]. However, in routine practice, endoscopic evaluations of disease severity are relatively expensive and invasive. In addition, endoscopic monitoring is the least acceptable for of monitoring from the patients’ perspectives [6]. Accurate tests that are practical, non-invasive, and inexpensive would be ideal. Several promising serologic and fecal biomarkers have emerged that could fulfill this role, including fecal calprotectin (FC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) [7]. CRP and ESR are useful to confirm ongoing mucosal inflammation but are of less value to predict a future relapse since elevated levels of these markers have not been found to precede a clinical flare [7]. Furthermore, there is considerable heterogeneity in CRP generation based on the genetics of individual patients [8]. These limitations have encouraged the development of alternative tests, specifically stool biomarkers with higher specificity for intestinal inflammation.
FC is an excellent marker of intestinal inflammation. Calprotectin is a calcium and zinc-binding protein formed by a heteromeric complex of two subunits, S100A8 and S100A9. It is derived from human neutrophils and monocytes and represents around 60% of soluble cytosol proteins in human neutrophil granulocytes [9]. Calprotectin is a heterocomplex of the S100 proteins S100A8 and S100A9 (also called myeloid-related protein 8 and MRP14) [10]. It has been classically considered an abundant innate immune protein due to its antimicrobial activity depriving microorganisms of transition metals [11]. In addition, it has been associated with antiproliferative and immunomodulatory effects [12].
FC is currently incorporated as a routine test to aid in diagnosing and monitoring IBD [13]. Though increasing evidence has been published about the usefulness of FC in predicting IBD relapse, the optimal cut-off of FC has been controversial [14]. This systematic review and meta-analysis aimed to evaluate the efficacy of FC as a predictor of IBD relapse in adult patients in remission and to obtain a cut-off value to help in clinical practice.
2. Materials and Methods
2.1. Search Strategy
The meta-analysis followed the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplementary Table S1). We searched the articles published in Pubmed (MEDLINE), Embase, Web of Science, and the Cochrane library databases from inception until July 2021. The following search terms were used: “Calprotectin”, “IBD”, “UC”, and “CD”. The search strategy was noted in Supplementary Table S2. References of eligible articles were also screened. All meeting abstracts were excluded because of insufficient data to reconstruct the 2 × 2 table. We restricted our search to studies published in English only.
2.2. Study Selection
The eligible articles were initially screened independently by three reviewers (JTS, NC, and JX) based on their titles and abstracts. Full manuscripts of the potentially eligible articles were reviewed while removing the duplicates. Three reviewers (JTS, NC, and JX) independently assessed articles if they met the including criteria: ① diagnostic cohort studies, ② prospective studies using FC to predict IBD relapse, ③ FC level was measured at baseline, ④ patient’s baseline status was in remission, ⑤ sufficient data to reconstruct 2 × 2 table, ⑥ relapse was confirmed by clinical symptoms and endoscopic results, and ⑦ studies conducted on adult’s patients with IBD. All disagreements were resolved through discussion with the authors (HG and HGX). Studies that did not meet our prespecified criteria were excluded.
2.3. Data Extraction and Quality Assessment
Three reviewers (JTS, NC, and JX) extracted the following characteristics from each article independently: name of the study, country, authors, publication year, age, gender, FC cut-off value, data for the construction of 2 × 2 table, follow-up time, type of FC assay, reference standard, medical treatment, and funding sources. The quality of included articles was assessed by JX using the QUADAS-2 (A revised tool for the Quality Assessment of Diagnostic Accuracy Studies) [15]. All disagreements were resolved through discussion with three authors (JTS, NC, and JX). The specific criteria were explained in Supplementary Table S3.
2.4. Data Synthesis and Analysis
To obtain the summary receiver operating characteristic (SROC) curve and an optimal cut-off for predicting IBD relapse, we applied the multiple thresholds model, which included multiple cut-off values with the results of true positive, true negative, false positive, and false negative. The multiple threshold model is a new approach for the meta-analysis of diagnostic test accuracy studies where several studies reported more than one threshold and the corresponding sensitivity and specificity values. The approach is based on the idea of estimating the distribution functions of the biomarker with the nondiseased and diseased individuals using a common parametric assumption (normal or logistic) for the distribution of a continuous biomarker. This was achieved using a mixed effects model with the study as a random factor [16]. The optimal cut-off was defined as the point where the Youden index (sensitivity + specificity − 1) was maximized [17]. We used the inverse variance weight to measure the mean value in order to represent the weight of individual studies. The model that minimized the restricted maximum likelihood criterion was chosen as the best. In addition, we used random effects bivariate models to calculate pooled sensitivity and specificity, and the same is true for subgroup analysis. We also created forest plots for each study.
To explore the clinical utility of FC for the prediction of the relapse of IBD, we performed a Fagan nomogram. The relationship between the prior probability, the likelihood ratio, and the posterior test probability is portrayed graphically by comparing 25, 50, and 75% prior probabilities [18]. The likelihood ratios obtained represented three clinical application scenarios: ① low suspicion of relapse for IBD: 25%; ② high suspicion of relapse for IBD: 75%; and ③ worst-case scenario: 50%.
Additionally, we calculated the positive predictive values (PPVs) and negative predictive values (NPVs) related to different cut-off values under varying levels of relapse rate by using a linear mixed-effects model for multiple thresholds model [19].
Although a funnel plot is the basic graphical method to detect publication bias, it is not recommended to be used in the diagnostic meta-analysis because of the multiple thresholds, so we did not explore publication bias [20].
As the thresholds can vary for each study, it was essential to see how close the observed results are to the receiver operating characteristic (ROC) curve rather than how dispersed they are in the ROC space [21]. The magnitude of heterogeneity is best accessed by a graph, which can be observed by the dispersion of points and the closeness between the 95% prediction region and 95% confidence region in the SROC curve [22]. We performed subgroup analysis related to the type of diseases, follow-up time, reference standard, and FC assay.
All data analyses were performed using STATA version 15, “DIAGMETA” package of R language for windows (Version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria) and MetaDisc version 1.4 [16,19,23].
3. Results
3.1. Selection, Characteristics, and Quality of Studies
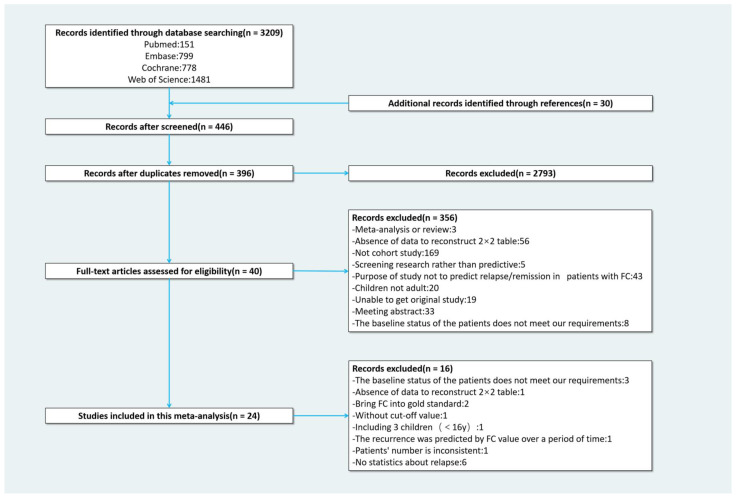
Our initial search yielded 3209 papers. Additionally, we added 30 papers from the review of relevant literature references. After removing duplicates and screening titles and abstracts, 396 studies were selected for full-text review. Of these, 356 were initially excluded. After data extraction and discussion, another 16 studies were excluded. The reasons for the exclusion of each study were listed in the Table 1. Finally, 24 studies were included with a total of 2260 patients of whom 715 relapsed (Figure 1).
Table 1.
List of excluded studies and reasons for their exclusion.
| Author | Year | Title | Reason for Exclusion | |
|---|---|---|---|---|
| 1 | Boschetti G | 2015 [24] | Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn’s disease. | The baseline status of the patients does not meet our requirements. |
| 2 | Lasson A | 2013 [25] | Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. | The baseline status of the patients does not meet our requirements. |
| 3 | Yamamoto T | 2015 [26] | Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis. | The baseline status of the patients does not meet our requirements. |
| 4 | Brooks AJ | 2017 [27] | Outcome of elective withdrawal of anti-tumour necrosis factor-α therapy in patients with Crohn’s disease in established remission. | Absence of data to reconstruct 2 × 2 table. |
| 5 | De Vos M | 2011 [28] | Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. | Bring FC into gold standard. |
| 6 | Sollelis E | 2019 [29] | Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. | Bring FC into gold standard. |
| 7 | Reinisch W | 2019 [30] | Fecal Calprotectin Responses Following Induction Therapy With Vedolizumab in Moderate to Severe Ulcerative Colitis: A Post Hoc Analysis of GEMINI 1. | Without cut-off value. |
| 8 | Mooiweer E | 2015 [31] | Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: a plea for deep remission. | Including children (<16 y). |
| 9 | Molander P | 2014 [32] | Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? | The recurrence was predicted by FC value over a period of time. |
| 10 | Garcia-Planella E | 2018 [33] | Serial semi-quantitative measurement of fecal calprotectin in patients with ulcerative colitis in remission. | Patients’ numbers are inconsistent. |
| 11 | Tursi A | 2019 [34] | Vedolizumab is effective and safe in real-life treatment of inflammatory bowel diseases outpatients: A multicenter, observational study in primary inflammatory bowel disease centers. | No statistics about relapse. |
| 12 | Bertani L | 2020 [35] | Fecal Calprotectin Predicts Mucosal Healing in Patients With Ulcerative Colitis Treated With Biological Therapies: A Prospective Study. | No statistics about relapse. |
| 13 | Bertani L | 2020 [36] | Serum oncostatin M at baseline predicts mucosal healing in Crohn’s disease patients treated with infliximab. | No statistics about relapse. |
| 14 | Guidi L | 2014 [37] | Faecal calprotectin assay after induction with anti-Tumour Necrosis Factor α agents in inflammatory bowel disease: Prediction of clinical response and mucosal healing at one year. | No statistics about relapse. |
| 15 | Beswick L | 2018 [38] | Exploration of Predictive Biomarkers of Early Infliximab Response in Acute Severe Colitis: A Prospective Pilot Study. | No statistics about relapse. |
| 16 | Reinisch W | 2020 [39] | Association of Biomarker Cutoffs and Endoscopic Outcomes in Crohn’s Disease: A Post Hoc Analysis From the CALM Study. | No statistics about relapse. |
Figure 1.
Flow chart of selection process.
Table 2 summarizes the characteristics of the included studies. All studies used a prospective study design and enrolled patients with quiescent IBD at baseline. In included studies, 7/24 (29.2%) of them [40,41,42,43,44,45,46] solely involved patients with CD, while 7/24 (29.2%) of them [47,48,49,50,51,52,53] involved only patients with UC. The remaining 10/24 (41.7%) studies [7,54,55,56,57,58,59,60,61,62] included patients with both UC and CD. FC was measured at baseline. The IBD relapse was identified with clinical symptoms and/or endoscopic findings on follow-up over a period of time. The follow-up period varied between studies, as shown in Table 2. The definitions of relapse in each study were listed in the Table 3. Since the definition of recurrence varies from study to study, for the sake of analysis, we divided them into two broad categories: clinical relapse and endoscopic relapse. A total of 5/24 (20.8%) studies used endoscopy as a reference; 19/24 (79.2%) studies used clinical symptoms or therapy change. Cut-off values for predicting relapse ranged from 50 to 500 μg/g, and most of them were mainly in the range of 100–250 μg/g.
Table 2.
Summary of findings and raw data of studies included in the meta-analysis.
| Study | Country | Age | Male (%) |
Disease Type (N) | Follow-Up Time | TP | FP | FN | TN | Cut-Off (μg/g) |
Reference Standard | Medication | FC-Assay ELISA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Jauregui-Amezaga 2014 [47] | Spain | 46 (13.6 relapse); 46 (15.9 no relapse) |
46.88 | UC (82) | 12 (m) | 9 | 14 | 8 | 33 | 100 | 1, 2, 4 | Mixed | Cerba Internacional |
| 7 | 7 | 10 | 40 | 250 | |||||||||
| Buisson 2019 [62] | America | 36 (16.3) | 50.63 | IBD (160) | 12 (m) | 53 | 9 | 35 | 63 | 100 | 1 | Mixed | Genova Diagnostics |
| F.Costa 2005 [54] | Italy | 56.96 | IBD (79) | 12 (m) | 30 | 17 | 4 | 28 | 150 | 1, 3 | Mixed | Calprest | |
| 41.2 (12.7) | UC (41) | 17 | 4 | 2 | 18 | ||||||||
| 35.7 (11.6) | CD (38) | 13 | 13 | 2 | 10 | ||||||||
| D. Naismith 2014 [40] | UK | 18–83 | 35.87 | CD (97) | 12 (m) | 8 | 21 | 2 | 61 | 240 | 2, 3, 4 | Mixed | Buhlmann |
| D’Inca 2008 [55] | Italy | 15–80 | 51.85 | IBD (162) | 12 (m) | 39 | 35 | 18 | 70 | 130 | 1 | Mixed | Calprest |
| UC (97) | 26 | 18 | 11 | 42 | |||||||||
| CD (65) | 13 | 17 | 7 | 28 | |||||||||
| Ferreiro-Iglesias 2018 [56] | Spain | 18–78 | 49.47 | IBD (106) | 12 (m) | 30 | 13 | 0 | 52 | 130 | 1 | anti-TNF | Buhlmann |
| Gisbert 2009 [57] | Spain | 43 (13) | 51.30 | IBD (163) | 12 (m) | 18 | 43 | 8 | 94 | 150 | 1 | Mixed | PhiCal |
| 18 | 34 | 8 | 103 | 167 | |||||||||
| UC (74) | 9 | 20 | 4 | 41 | 150 | ||||||||
| 9 | 16 | 4 | 45 | 164 | |||||||||
| CD (89) | 9 | 23 | 4 | 53 | 150 | ||||||||
| 9 | 18 | 4 | 58 | 169 | |||||||||
| Hosseini 2015 [48] | Iran | 20–83 | 51.30 | UC (157) | 12 (m) | 59 | 9 | 15 | 71 | 341 | 1, 3 | NA | Buhlmann |
| Kallel 2010 [41] | Tunis | 15–66 | 43.40 | CD (53) | 12 (m) | 8 | 4 | 2 | 39 | 340 | 1, 3 | Mixed | Calprest |
| Keshteli 2017 [49] | Canada | 42.7 (14.8) | 45.00 | UC (20) | 12 (m) | 5 | 2 | 2 | 11 | 124 | 1 | Mixed | Buhlmann |
| Kostas 2017 [58] | Greece | 17–76 | 51.68 | IBD (149) | 6 (m) | 41 | 15 | 6 | 87 | 261 | 1, 3 | NA | Buhlmann |
| L. Ye 2017 [42] | China | 24 (23–43.5 relapse); 28 (19–42.5no relapse) |
64.52 | CD (62) | 24 (m) | 27 | 9 | 2 | 6 | 225 | 1, 2, 3 | Mixed | Buhlmann |
| Ferreiro-Iglesias 2016 [43] | Spain | 18–68 | 47.17 | IBD (53) | 2 (m) | 11 | 7 | 1 | 34 | 160 | 1 | IFX | Buhlmann |
| UC (20) | 2 (m) | 4 | 3 | 0 | 13 | 198 | |||||||
| CD (33) | 2 (m) | 7 | 4 | 1 | 21 | 160 | |||||||
| R.Ferreiro-Iglesias 2016 [59] | Spain | 24–64 | 43.33 | CD (30) | 4 (m) | 9 | 3 | 0 | 18 | 204 | 1 | ADA | Buhlmann |
| S. Monteir 2019 [44] | Portugal | 38.4 (12.2) | 45.83 | CD (144) | 6 (m) | 12 | 23 | 1 | 108 | 327 | 2,3 | Mixed | Buhlmann |
| Shimoyama 2018 [50] | Japan | NA | NA | UC (196) | 12 (m) | 26 | 7 | 8 | 39 | 114 | 1 | Mixed | Cell Sciences |
| Theede 2017 [51] | Denmark | 39.3 (13.92) | 72.86 | UC (70) | 12 (m) | 7 | 8 | 8 | 47 | 321 | 3 | Mixed | Buhlmann |
| Tibble 2000 [7] | UK | 33 (16–77 CD); 49 (21–72 UC) |
45.00 | IBD (80) | 12 (m) | 40 | 6 | 4 | 30 | 50 | 1, 3 | Mixed | NA |
| V. García-Sánchez 2009 [60] | Spain | 57.04 | IBD (135) | 12 (m) | 29 | 31 | 10 | 65 | 150 | 1 | Mixed | Calprest | |
| 40.4(13.1) | UC (69) | 17 | 18 | 4 | 30 | 120 | |||||||
| 36.9(9.2) | CD (66) | 14 | 17 | 4 | 31 | 200 | |||||||
| Y. Zhulina 2016 [61] | Sweden | 50 (42–61 relapse)/ 58 (41–64 no relapse) |
23.08 | IBD (130) | 3 (m) | 5 | 17 | 3 | 79 | 500 | 3 | Mixed | Buhlmann |
| 6 | 37 | 2 | 59 | 250 | |||||||||
| 12 (m) | 10 | 12 | 14 | 68 | 500 | ||||||||
| 15 | 28 | 9 | 52 | 250 | |||||||||
| 24 (m) | 11 | 11 | 26 | 56 | 500 | ||||||||
| 18 | 25 | 19 | 42 | 250 | |||||||||
| Yamamoto 2013 [45] | Japan | 32 (1.6) | 60.00 | CD (20) | 12 (m) | 5 | 1 | 1 | 13 | 170 | 1 | Mixed | Cell Sciences |
| Yamamoto 2014 [52] | Japan | 35.1 (0.8) | 61.25 | UC (80) | 12 (m) | 16 | 14 | 5 | 45 | 170 | 1, 2 | Mixed | Cell Sciences |
| Yamamoto 2018 [53] | Japan | 35 (31–39) | 61.59 | UC (164) | 12 (m) | 38 | 22 | 8 | 96 | 115 | 1 | Mixed | Cell Sciences |
| D. Laharie 2011 [46] | France | 27(17–65 relapse); 31(15–68 no relapse) |
32.00 | CD (65) | 38 (m) | 14 | 14 | 9 | 13 | 130 | 1,3 | Mixed | Buhlmann |
| 10 | 11 | 13 | 16 | 250 |
Age: Median (IQR) or mean (SD) or age range; disease type (No of patients); TP: true positive; FP: false positive; FN: false negative; TN: true negative; NA: not available. PhiCal, Bühlmann, Cell Sciences, Calprest, Genova Diagnostics, and Cerba Internacional are different fecal calprotectin test kits. Reference standard: 1: Clinic; 2: Endoscopy; 3: change in therapy or surgery; and 4: histopathology.
Table 3.
Definitions of relapse in each study included.
| Study | Disease Type | Definition of Relapse |
|---|---|---|
| A. Jauregui-Amezaga 2014 [47] | UC | Presence of blood in stool and MES ≥ 3 with histologic confirmation. |
| Buisson 2019 [62] | UC | Reappearance of clinical manifestation (SCCAI > 2 with subscore > 1 for at least one item among stool frequency and rectal bleeding) leading to medication intensification, hospitalization, or colectomy. |
| CD | Reappearance of clinical manifestation (HBI > 4) leading to therapeutic intensification, hospitalization, or CD-related surgery. | |
| F.Costa 2005 [54] | UC | Worsening of symptoms, accompanied by an increase in the UCAI score to >4, sufficient to require a change in therapy (addition of steroids, immunosuppressors, surgery, etc.). |
| CD | Worsening of symptoms, accompanied by an increase in the CDAI score to >150, sufficient to require a change in therapy (addition of steroids, immunosuppressors, surgery, etc.). | |
| D. Naismith 2014 [40] | CD | An unplanned escalation in therapy, progression of disease phenotype by the Montreal classification or hospitalisation and/or emergency surgery for active CD. |
| D’Inca 2008 [55] | UC | ET scores exceeding 4 and requiring additional treatment. |
| CD | CDAI exceeding 150, with an increment of more than 50 points over the baseline score (75 points in resected patients) and requiring additional treatment. | |
| Ferreiro-Iglesias 2018 [56] | UC | PMS > 2. |
| CD | HBI > 4. | |
| Gisbert 2009 [57] | UC | Truelove modified index > 11 points. |
| CD | CDAI > 150. | |
| Hosseini 2015 [48] | UC | Elevated Seo activity index higher than 220 or worsening of symptoms (including abdominal pain, diarrhea with or without blood and rectal bleeding) sufficient to require a change in therapy (increasing the dose, changing the current drug (s), addition of steroids, hospitalization or surgery). |
| Kallel 2010 [41] | CD | CDAI > 150 or an increase of more than 100 from the inclusion value and was sufficiently severe to warrant treatment. |
| Keshteli 2017 [49] | UC | PMS ≥ 3. |
| Kostas 2017 [58] | UC | (1) Significant increase in respective clinical activity indices above accepted cut-offs for remission in UC (Simple Colitis Activity Index ≥ 3) and/or (2) step up in the patient’s therapeutic regimen, including surgery for intractable disease-related symptoms. |
| CD | (1) Significant increase in respective clinical activity indices above accepted cut-offs for remission in CD (HBI ≥ 5) and/or (2) step up in the patient’s therapeutic regimen, including surgery for intractable disease-related symptoms. | |
| L. Ye 2017 [42] | CD | Worsening symptoms requiring intensified therapy or surgery or a CDAI score > 150, with confirmation by ileocolonoscopy. |
| Ferreiro-Iglesias 2016 [43] | UC | PMS > 3. |
| CD | HBI > 4. | |
| R.Ferreiro-Iglesias 2016 [59] | CD | HBI > 4. |
| S. MONTEIR 2019 [44] | CD | An unexpected escalation in therapy, hospitalization or surgery for active CD with ileocolonoscopy and inflammatory activity assessed by SES-CD or Rutgeerts score. |
| Shimoyama 2018 [50] | UC | The sum of ‘stool frequency’ score (0–3) and ‘rectal bleeding’ score (0–3) in the Mayo scoring system exceeding 0. |
| Theede 2017 [51] | UC | The symptoms of active UC demanding adjustment of actual or initiation of new UC therapy. |
| Tibble 2000 [7] | UC | HBI > 4 and an increase of >2 from the inclusion value. All relapses were of sufficient severity to warrant a change in treatment. |
| CD | CDAI > 150 with an increase of >100 from the inclusion value. All relapses were of sufficient severity to warrant a change in treatment. | |
| V. García-Sánchez 2009 [60] | UC | Worsening of the symptoms, accompanied by or a modified TW score of ≥11 points. |
| CD | Worsening of the symptoms, accompanied by a CDAI score of ≥150 points. | |
| Y. Zhulina 2016 [61] | IBD | Increasing symptoms necessitating intensified medical therapy or surgery. |
| Yamamoto 2013 [45] | CD | CDAI > 150 with an increase of ≥70 points. |
| Yamamoto 2014 [52] | UC | Worsening of stool frequency and/or rectal bleeding with an endoscopic score of 2 or 3. |
| Yamamoto 2018 [53] | UC | Worsening of stool frequency and/or rectal bleeding with the MES of 2 or 3. |
| D. Laharie 2011 [46] | CD | Increasing symptoms (CDAI > 250 within 2 weeks or CDAI > 150 with an at least 70 points of increase as compared with CDAI at week 14) or the need for an additional steroid or IFX course or for a surgical resection. |
HBI: Harvey-Bradshaw index; PMS: partial Mayo score; MES: Mayo endoscopic score; UCAI: ulcerative colitis activity index; CDAI: Crohn’s disease activity index; ET score: Edwards and Truelove (ET) scores; SCAI: Simple Colitis Activity Index; SES-CD: Simple Endoscopic Score for Crohn Disease; and IFX: Infliximab.
Overall, the quality of the included studies was good (see the results of QUADAS-2 in Supplementary Table S4). Eleven studies [42,44,45,47,48,51,52,53,56,58,62] did not mention whether the patients enrolled were consecutive or not. Blinding of reference standard results was reported in all but one study [61]. Four studies [46,47,50,51,61] reported the blinding of index test results, while others did not mention it.
3.2. Performance of FC at the Optimal Cut-Off Value
3.2.1. Primary Outcome
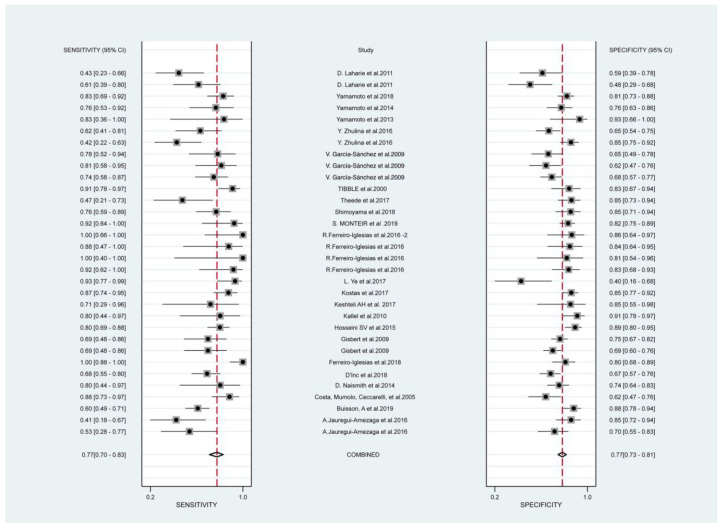
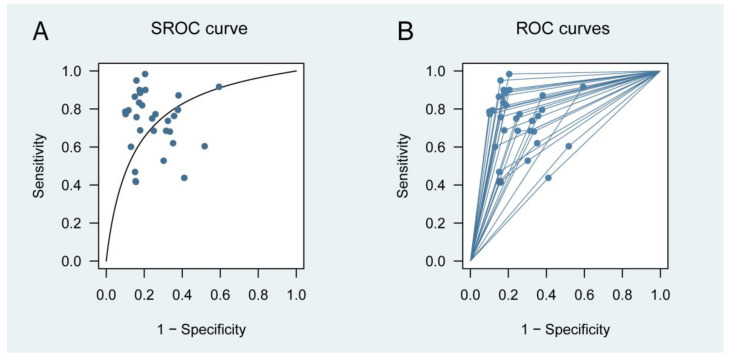
Figure 2 presents the forest plots of sensitivity (true positive rate) and 1 − specificity (false positive rate) for the 24 studies. Combining all available data from the 24 studies using the multiple thresholds model, the resulting SROC curve is shown in Figure 3. An optimal cut-off value of 152 μg/g was identified. At 152 μg/g, the Youden index reached its maximum (Supplementary Figure S1). Its corresponding sensitivities and specificities were 0.720 (0.528 to 0.856) and 0.740 (0.618 to 0.834), respectively. The area under the SROC curve (AUC) for predicting IBD relapse was found to be 0.794.
Figure 2.
Forest plots of pooled sensitivity and 1 − specificity of Fecal Calprotectin at remission for predicting relapse in inflammatory bowel disease. Plots display diagnostic probabilities of included studies and their corresponding 95% confidence intervals [7,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62].
Figure 3.
Test performance for predicting relapse in patients with IBD. (A) Multiple threshold SROC curve; (B) multiple threshold ROC curves based on the multiple thresholds model. Circles represent information on sensitivity and specificity. ROC, receiver operating characteristic; and SROC, summary receiver operating characteristic.
Furthermore, the bivariate model was also applied to evaluate the diagnostic performance of FC by using the data from just one cut-off reported for each study. Based on the multiple threshold model results, if a study reported multiple cut-off values, then the option closest to 152 μg/g was selected. The cut-off values ranged from 50 to 340 μg/g. Its corresponding sensitivities, specificities, and AUC were 0.80 (0.73 to 0.85), 0.78 (0.73 to 0.82), and 0.85 (0.82 to 0.88), respectively. The SROC for the bivariate model can be found in Supplementary Figure S2.
3.2.2. Post-Test Probability of Relapse
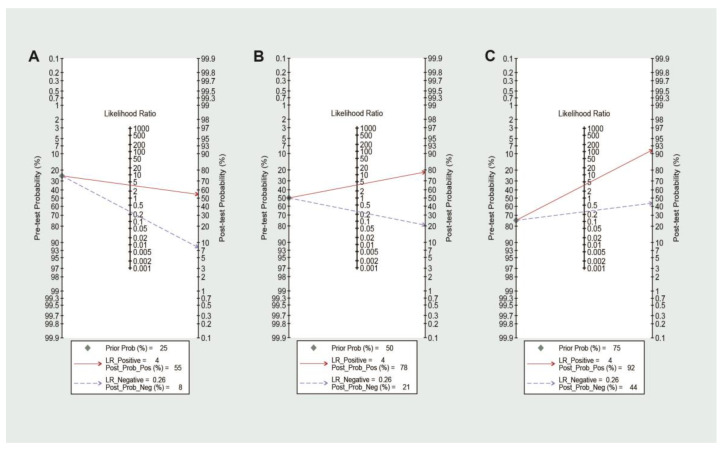
In clinical practice, there is a need to understand the probability that a patient with quiescent IBD will relapse or not when an FC test result exceeds a certain threshold. The PPV and NPV varied for various relapse rates of IBD because these are related to the disease prevalence. Therefore, it was addressed with a multiple thresholds model, with a calculation of PPVs and NPVs related to the optimal and other common cut-off values for different levels of relapse rate (Supplementary Table S5). Employing an FC threshold of 152 μg/g, the highest NPV of 0.98 was observed when using the test in a low-relapse rate setting, i.e., when the relapse rate was no more than 5%. The highest PPV of 0.893 was observed in a high-relapse rate setting (72.5%). To further improve the analysis of the predictive effect of FC on relapse with the threshold of 152 μg/g, we additionally calculated the post-test probability with three different levels of relapse rate. The Fagan nomogram showed that FC testing changed the post-test probability of IBD (Figure 4). In the low suspicion of IBD relapse, the results showed that a negative post-test probability of 8% could be considered sufficient to exclude the high possibility of relapse. On the other hand, in the high suspicion of IBD relapse, a positive post-test probability of 92% could be considered sufficient to warn of the relapse within 24 months.
Figure 4.
Fagan nomogram analysis evaluating the clinical utility of FC for predicting IBD relapse according to different pretest probabilities (prior). (A) Pretest probability = 25%; (B) pretest probability = 50%; and (C) pretest probability = 75%.
3.3. Subgroups Analysis
In order to determine whether disease types (CD or UC), follow-up time (<1 year or ≥1 year), reference standard (clinic or endoscopy), and FC-assay (BÜHLMANN fCAL® ELISA, Calprest® or Human Calprotectin ELISA Kit, Cell Sciences Inc., Newburyport, MA, USA) were sources of heterogeneity, we performed subgroup analyses. Analyses showed similar summary performance for all subgroups (Supplementary Table S6).
4. Discussion
Our meta-analysis aimed to obtain an ideal cut-off value for predicting IBD relapse suitable for clinical use. Although we intended to stratify patients with UC and CD and tried to calculate a threshold unique for CD and UC using the multiple threshold model, there were not enough studies. However, by performing further subgroup analysis, we found that the sensitivity and specificity results did not change when the disease type was stratified into UC vs. CD using the random-effects bivariate models. Thus, we calculated the cut-off value for the IBD group (including patients with CD and UC). It was found that the pooled sensitivity of FC is 0.720 (95% CI 0.528–0.856) and the pooled specificity is 0.740 (0.618–0.834) with an AUC of 0.794 at the cut-off value of 152 μg/g. The estimated DOR is 14, indicating that FC is a useful biomarker in predicting the relapse of IBD.
4.1. Implications of Key Findings
Heida A et al. [63] suggested that FC levels in remission should be used to predict recurrence trends in patients with IBD. FC is inexpensive and non-invasive and has better specificity than CRP. FC is remarkably stable in stools for up 7 days at room temperature, enabling sample collection at home even in patients’ remote locations. These characteristics of FC may make monitoring IBD patients convenient and practical. Based on our results, we suggest that 152 μg/g was an appropriate threshold for monitoring IBD. Patients with higher FC levels (>152 μg/g) should be warned of the possibility of relapse within 24 months.
We used the comprehensive GRADE approach [64] to examine the validity of our results (Table 4). Recognition of the accuracy of FC as a substitute for outcomes important to patients is central to this approach. Detection of FC levels to predict relapse of patients with IBD will be valuable only if the FC monitoring improves the care of patients with IBD. Therefore, we inferred from the pooled sensitivity and specificity for the effect of the FC test on patient monitoring for IBD relapse. The key question is whether the numbers of false negatives (cases that the risk of recurrence was underestimated) and false positives (cases that the risk of recurrence was overestimated) are acceptable in this context.
Table 4.
Effect of pooled sensitivity and specificity of fecal calprotectin on patients.
| Test Result | Number of Participants (Studies) | Number of Results per 100 Patients Tested (95% CI) | Importance (Grade) * |
Comments | ||
|---|---|---|---|---|---|---|
| Prevalence 25% | Prevalence 50% | Prevalence 75% | ||||
| True positive (TP) |
2457 (24) |
18 (13 to 21) | 36 (26 to 43) | 54 (40 to 64) | 8 | Benefit from early identification of relapse. |
| False negative (FN) |
7 (4 to 12) | 14 (7 to 24) | 21 (11 to 35) | 9 | Detriment from delays in identification of relapse and treatment. | |
| True negative (TN) |
56 (46 to 63) | 37 (31 to 42) | 19 (15 to 21) | 8 | Benefit from reassurance and relief of economic costs. | |
| False positive (FP) |
19 (12 to 29) | 13 (8 to 19) | 6 (4 to 10) | 7 | Detriment from undertake unnecessary psychological burden and financial expenditure. | |
CI: Confidence interval. * GRADE recommends classifying patient-important outcomes on a 9-point scale: 7–9: critical for decision making; 4–6: essential but not critical for decision making; and 1–3: of lower importance to patients.
In a hypothetical population of 100 IBD adults in remission (given an overall recurrence rate of 25%), eighteen patients should increase the frequency of FC testing. Additionally, they need to be monitored continuously and, if necessary, endoscopically examined to confirm recurrence. Fifty-six percent of patients have a low risk of recurrence in the future and only need to be observed according to the original plan. FC testing reduces the psychological burden of those patients. Seven patients will be missed. A false negative FC result can delay determining the patient’s risk of recurrence and delay treatment. Nineteen percent will be diagnosed as false positives, which results in inconvenience and unnecessary financial expense. There will also be a certain amount of stress on the mental side. We also hope that when determining whether the FC results are reliable, the factors that will produce false positives and false negatives should be excluded (Table 5). Based on the comprehensive analysis, FC is still recommended to predict recurrence in patients with IBD.
Table 5.
Causes of abnormal results for fecal calprotectin other than inflammatory bowel disease.
| Type of the Causes | Specific Reasons |
|---|---|
| Infections | Giardia lamblia |
| Bacterial dysentery | |
| Viral gastroenteritis | |
| Helicobacter pylori gastritis | |
| Clostridium difficile | |
| HIV | |
| Malignancies | Colorectal cancer |
| Gastric carcinoma | |
| Intestinal lymphoma | |
| Pancreatic cancer | |
| Polyposis intestinalis | |
| Drugs | NSAIDs |
| PPIs | |
| Other gastrointestinal diseases | Gastro-oesophageal reflux disease |
| Cystic fibrosis | |
| Coeliac disease (untreated) | |
| Diverticular disease | |
| Protein losing enteropathy | |
| Colorectal adenoma | |
| Juvenile polyp | |
| Autoimmune enteropathy | |
| Microscopic colitis | |
| Liver cirrhosis | |
| Gastrointestinal bleeding | |
| IBS | |
| Proctitis after radiation therapy | |
| Colon inflammation bag | |
| Pancreatitis | |
| Lifestyle | Obesity |
| Physical inactivity | |
| Age | <9 years |
| >65 years | |
| Others | Bowel preparation for colonoscopy |
| Rheumatologic diseases | |
| Perianal disease | |
| Stoma | |
| Immune deficiency | |
| Intestinal transplant | |
| Proteolysis | |
| Food allergy (untreated) |
HIV: human immunodeficiency virus; NSAIDs: nonsteroidal anti-inflammatory drugs; PPIs: proton pump inhibitors; and IBS: irritable bowel syndrome.
4.2. Comparison with Other Reviews
Previous meta-analyses have evaluated the performance of FC for relapse in patients with IBD [64,65,66,67]. However, an ideal cut-off value for FC was never determined. This is the first meta-analysis evaluating the FC level and obtaining an excellent cut-off value to distinguish whether patients would relapse in the near future, which is more helpful for clinical practice. YS Tham et al. [67] suggested that an FC cut-off value of 150 µg/g is associated with optimal diagnostic accuracy for postoperative endoscopic recurrence in CD. However, the performance for an FC level of 135 μg/g was not examined. Although it was the optimal cut-off value for the largest cohort they included, the value appeared in only one cohort and was not sufficient to obtain a pooled performance. Li et al. [64] showed that in patients with UC, the accuracy of FC was better in studies with a cut-off of ≥150 µg/g but did not discuss the optimal cut-off value. However, we used a novel multiple threshold model to obtain the ideal cut-off value by maximizing the Youden index rather than comparing it with other cut-off values. Also, this is the first cut-off value that takes IBD as a whole, and subgroup analysis proves that the results of UC and CD are similar. Therefore, this cut-off value is more convenient to be used in clinical practice.
Additionally, the diagnostic performance results obtained by the multiple threshold model would be a little lower but more realistic than by traditional approaches. This is because the multiple threshold model uses all the available information and results in an estimation of the performance of the biomarker, which avoids the drawback of using a single cut-off value. Previous meta-analyses only used one pair of sensitivity and specificity per study, which may lead to an overestimation of the SROC curve because there would be cut-off selection bias, and the ‘optimal’ point would be generally chosen [68].
4.3. Limitations of Study
We would also like to report some of the limitations of this study. The reference standard for the diagnosis of IBD relapse is still controversial; however, the reference standards used in the included studies are currently recommended. Also, QUADAS-2 is not a quality assessment method for prognostic tests, but it is still the most suitable method. We have deleted some items that are not applicable, which may lead to some deviation in the results. Additionally, due to the lack of data, we could not perform a subgroup analysis of medications used in these patients. The threshold may vary slightly when faced with different patients, and sometimes clinicians need to consider the extent of involvement and past history of the patient on the basis of the given threshold. In addition, a recent study reported that FC may increase with age, even 3–4 times [13], which reminded us it is possible that cut-off values vary in different age groups. Despite these limits, our analysis is rigorous and will further increase interest in performing high-quality studies using FC in predicting relapse in patients with IBD.
5. Conclusions
In conclusion, regularly measuring FC levels in IBD remission is a useful tool for the early prediction of relapse. The FC value of 152 μg/g is an ideal threshold for identifying patients with a high probability of relapse, suggesting careful follow-up and adjusting medications. Moreover, this noninvasive monitoring method will be better received by the patients without any preparation for colonoscopies and with high sensitivity and specificity. Further prospective high-quality trials are needed to determine the optimal FC measurement interval and cut-off value for the FC trend. In addition, it will also be useful to study further the predictive performance of combining markers, such as CRP and FC, for the relapse of patients with IBD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12031206/s1, Figure S1: Youden Index of FC for predicting IBD relapse; Figure S2: Receiver operating characteristic graph of fecal calprotectin test in fecal calprotectin at remission for predicting relapse in inflammatory bowel disease, with 95% confidence region and 95% prediction regions; Table S1: The PRISMA checklist; Table S2: The search strategies of four databases; Table S3: Specific criteria of QUADAS-2; Table S4: Quality assessment of included studies; Table S5: Calculated sensitivities and specificities at cut-offs of 160, 50, 150 in predicting relapse and their corresponding PPVs and NPVs for different prevalences using the multiple thresholds model; and Table S6: Assessment of diagnostic accuracy in subgroup analysis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This research is a meta-analysis and all data have been uploaded along with the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Natural Science Foundation of Jiangsu Province of China (BK20181492), the National Key Clinical Department of Laboratory Medicine of China in Nanjing, the Key Laboratory for Laboratory Medicine of Jiangsu Province (ZDXKB2016005), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wang X.P., Mao M.J., He Z.L., Zhang L., Chi P.D., Su J.R., Dai S.Q., Liu W.L. A retrospective discussion of the prognostic value of combining prothrombin time(PT) and fibrinogen(Fbg) in patients with Hepatocellular carcinoma. J. Cancer. 2017;8:2079–2087. doi: 10.7150/jca.19181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ordás I., Eckmann L., Talamini M., Baumgart D.C., Sandborn W.J. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart D.C., Sandborn W.J. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 4.Stange E.F., Travis S.P., Vermeire S., Reinisch W., Geboes K., Barakauskiene A., Feakins R., Flejou J.F., Herfarth H., Hommes D.W., et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J. Crohn’s Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Annese V., Daperno M., Rutter M.D., Amiot A., Bossuyt P., East J., Ferrante M., Gotz M., Katsanos K.H., Kiesslich R., et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohn’s Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Buisson A., Gonzalez F., Poullenot F., Nancey S., Sollellis E., Fumery M., Pariente B., Flamant M., Trang-Poisson C., Bonnaud G., et al. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017;23:1425–1433. doi: 10.1097/MIB.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 7.Tibble J.A., Sigthorsson G., Bridger S., Fagerhol M.K., Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 8.Guo X., Huang C., Xu J., Xu H., Liu L., Zhao H., Wang J., Huang W., Peng W., Chen Y., et al. Gut Microbiota Is a Potential Biomarker in Inflammatory Bowel Disease. Front. Nutr. 2021;8:818902. doi: 10.3389/fnut.2021.818902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caviglia G.P., Ribaldone D.G., Rosso C., Saracco G.M., Astegiano M., Pellicano R. Fecal calprotectin: Beyond intestinal organic diseases. Panminerva Med. 2018;60:29–34. doi: 10.23736/S0031-0808.18.03405-5. [DOI] [PubMed] [Google Scholar]
- 10.Hurnakova J., Zavada J., Hanova P., Hulejova H., Klein M., Mann H., Sleglova O., Olejarova M., Forejtova S., Ruzickova O., et al. Serum calprotectin (S100A8/9): An independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2015;17:252. doi: 10.1186/s13075-015-0764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakeman C.A., Moore J.L., Noto M.J., Zhang Y., Singleton M.D., Prentice B.M., Gilston B.A., Doster R.S., Gaddy J.A., Chazin W.J., et al. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun. 2016;7:11951. doi: 10.1038/ncomms11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radin J.N., Kelliher J.L., Parraga Solorzano P.K., Kehl-Fie T.E. The Two-Component System ArlRS and Alterations in Metabolism Enable Staphylococcus aureus to Resist Calprotectin-Induced Manganese Starvation. PLoS Pathog. 2016;12:e1006040. doi: 10.1371/journal.ppat.1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laserna-Mendieta E.J., Lucendo A.J. Faecal calprotectin in inflammatory bowel diseases: A review focused on meta-analyses and routine usage limitations. Clin. Chem. Lab. Med. 2019;57:1295–1307. doi: 10.1515/cclm-2018-1063. [DOI] [PubMed] [Google Scholar]
- 14.Chen F., Hu Y., Fan Y.H., Lv B. Clinical Value of Fecal Calprotectin in Predicting Mucosal Healing in Patients With Ulcerative Colitis. Front. Med. 2021;8:679264. doi: 10.3389/fmed.2021.679264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiting P., Rutjes A., Westwood M., Mallett S., Deeks J., Reitsma J., Leeflang M., Sterne J., Bossuyt P., QUADAS-2 Group QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 16.Steinhauser S., Schumacher M., Rücker G. Modelling multiple thresholds in meta-analysis of diagnostic test accuracy studies. BMC Med. Res. Methodol. 2016;16:97. doi: 10.1186/s12874-016-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rupprecht T.A., Manz K.M., Fingerle V., Lechner C., Klein M., Pfirrmann M., Koedel U. Diagnostic value of cerebrospinal fluid CXCL13 for acute Lyme neuroborreliosis. A systematic review and meta-analysis. Clin. Microbiol. Infect. 2018;24:1234–1240. doi: 10.1016/j.cmi.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Hellmich M., Lehmacher W. A ruler for interpreting diagnostic test results. Methods Inf. Med. 2005;44:124–126. [PubMed] [Google Scholar]
- 19.Schneider A., Linde K., Reitsma J., Steinhauser S., Rücker G. A novel statistical model for analyzing data of a systematic review generates optimal cut-off values for fractional exhaled nitric oxide for asthma diagnosis. J. Clin. Epidemiol. 2017;92:69–78. doi: 10.1016/j.jclinepi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Bürkner P., Doebler P. Testing for publication bias in diagnostic meta-analysis: A simulation study. Stat. Med. 2014;33:3061–3077. doi: 10.1002/sim.6177. [DOI] [PubMed] [Google Scholar]
- 21.Macaskill P., Gatsoni C., Deeks J., Harbord R., Takwoingi Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 1.0. The Cochrane Collaboration; London, UK: 2010. [Google Scholar]
- 22.Koziarz A., Sne N., Kegel F., Nath S., Badhiwala J.H., Nassiri F., Mansouri A., Yang K., Zhou Q., Rice T., et al. Bedside Optic Nerve Ultrasonography for Diagnosing Increased Intracranial Pressure: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019;171:896–905. doi: 10.7326/M19-0812. [DOI] [PubMed] [Google Scholar]
- 23.Zamora J., Abraira V., Muriel A., Khan K., Coomarasamy A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boschetti G., Garnero P., Moussata D., Cuerq C., Préaudat C., Duclaux-Loras R., Mialon A., Drai J., Flourié B., Nancey S., et al. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn’s disease. Inflamm. Bowel Dis. 2015;21:331–336. doi: 10.1097/MIB.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 25.Lasson A., Simrén M., Stotzer P.O., Isaksson S., Ohman L., Strid H. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm. Bowel Dis. 2013;19:576–581. doi: 10.1097/MIB.0b013e31827e78be. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T., Shimoyama T., Matsumoto K. Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis. Aliment. Pharmacol. Ther. 2015;42:549–558. doi: 10.1111/apt.13308. [DOI] [PubMed] [Google Scholar]
- 27.Brooks A.J., Sebastian S., Cross S.S., Robinson K., Warren L., Wright A., Marsh A.M., Tsai H., Majeed F., Lobo A.J., et al. Outcome of elective withdrawal of anti-tumour necrosis factor-α therapy in patients with Crohn’s disease in established remission. J. Crohns Colitis. 2017;11:1456–1462. doi: 10.1016/j.crohns.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 28.De Vos M., Dewit O., D’Haens G., Baert F., Fontaine F., Vermeire S., Franchimont D., Moreels T., Staessen D., Terriere L., et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J. Crohns Colitis. 2012;6:557–562. doi: 10.1016/j.crohns.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Sollelis E., Quinard R.M., Bouguen G., Goutte M., Goutorbe F., Bouvier D., Pereira B., Bommelaer G., Buisson A. Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. World J. Gastroenterol. 2019;25:2354–2364. doi: 10.3748/wjg.v25.i19.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinisch W., Bressler B., Curtis R., Parikh A., Yang H., Rosario M., Røseth A., Danese S., Feagan B., E Sands B., et al. Fecal Calprotectin Responses Following Induction Therapy With Vedolizumab in Moderate to Severe Ulcerative Colitis: A Post Hoc Analysis of GEMINI 1. Inflamm. Bowel Dis. 2018;25:803–810. doi: 10.1093/ibd/izy304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooiweer E., Severs M., Schipper M.E., Fidder H.H., Siersema P.D., Laheij R.J., Oldenburg B. Low Fecal Calprotectin Predicts Sustained Clinical Remission in Inflammatory Bowel Disease Patients: A Plea for Deep Remission. J. Crohn’s Colitis. 2014;9:50–55. doi: 10.1093/ecco-jcc/jju003. [DOI] [PubMed] [Google Scholar]
- 32.Molander P., Färkkilä M., Ristimäki A., Salminen K., Kemppainen H., Blomster T., Koskela R., Jussila A., Rautiainen H., Nissinen M., et al. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J. Crohn’s Colitis. 2014;9:33–40. doi: 10.1016/j.crohns.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Planella E., Mañosa M., Chaparro M., Beltrán B., Barreiro-De-Acosta M., Gordillo J., Ricart E., Bermejo F., García-Sánchez V., Piqueras M., et al. Serial semi-quantitative measurement of fecal calprotectin in patients with ulcerative colitis in remission. Scand. J. Gastroenterol. 2017;53:152–157. doi: 10.1080/00365521.2017.1410219. [DOI] [PubMed] [Google Scholar]
- 34.Tursi A., Mocci G., Faggiani R., Allegretta L., Della Valle N., de Medici A., Forti G., Franceschi M., Ferronato A., Gallina S., et al. Vedolizumab is effective and safe in real-life treatment of inflammatory bowel diseases outpatients: A multicenter, observational study in primary inflammatory bowel disease centers. Eur. J. Intern. Med. 2019;66:85–91. doi: 10.1016/j.ejim.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Bertani L., Blandizzi C., Mumolo M.G., Ceccarelli L., Albano E., Tapete G., Svizzero G.B., Zanzi F., Coppini F., de Bortoli N., et al. Fecal Calprotectin Predicts Mucosal Healing in Patients With Ulcerative Colitis Treated With Biological Therapies: A Prospective Study. Clin. Transl. Gastroenterol. 2020;11:e00174. doi: 10.14309/ctg.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertani L., Fornai M., Fornili M., Antonioli L., Benvenuti L., Tapete G., Svizzero G.B., Ceccarelli L., Mumolo M.G., Blandizzi C., et al. Serum oncostatin M at baseline predicts mucosal healing in Crohn’s disease patients treated with infliximab. Aliment. Pharmacol. Ther. 2020;52:284–291. doi: 10.1111/apt.15870. [DOI] [PubMed] [Google Scholar]
- 37.Guidi L., Marzo M., Andrisani G., Felice C., Pugliese D., Mocci G., Nardone O., De Vitis I., Papa A., Rapaccini G., et al. Faecal calprotectin assay after induction with anti-Tumour Necrosis Factor α agents in inflammatory bowel disease: Prediction of clinical response and mucosal healing at one year. Dig. Liver Dis. 2014;46:974–979. doi: 10.1016/j.dld.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Beswick L., Rosella O., Rosella G., Headon B., Sparrow M.P., Gibson P.R., Van Langenberg D.R. Exploration of Predictive Biomarkers of Early Infliximab Response in Acute Severe Colitis: A Prospective Pilot Study. J. Crohns Colitis. 2017;12:289–297. doi: 10.1093/ecco-jcc/jjx146. [DOI] [PubMed] [Google Scholar]
- 39.Reinisch W., Panaccione R., Bossuyt P., Baert F., Armuzzi A., Hébuterne X., Travis S., Danese S., Sandborn W.J., Schreiber S., et al. Association of Biomarker Cutoffs and Endoscopic Outcomes in Crohn’s Disease: A Post Hoc Analysis From the CALM Study. Inflamm. Bowel Dis. 2020;26:1562–1571. doi: 10.1093/ibd/izaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naismith G.D., Smith L.A., Barry S.J., Munro J.I., Laird S., Rankin K., Morris A.J., Winter J.W., Gaya D.R. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn’s disease. J. Crohn’s Colitis. 2014;8:1022–1029. doi: 10.1016/j.crohns.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Kallel L., Ayadi I., Matri S., Fekih M., Mahmoud N.B., Feki M., Karoui S., Zouari B., Boubaker J., Kaabachi N., et al. Fecal calprotectin is a predictive marker of relapse in Crohn’s disease involving the colon: A prospective study. Eur. J. Gastroenterol. Hepatol. 2010;22:340–345. doi: 10.1097/MEG.0b013e32832bab49. [DOI] [PubMed] [Google Scholar]
- 42.Ye L., Chen B.Q., Wang S.D., Shi H., Yang Z., Wang F.Y. Fecal calprotectin is a strong predictive marker of relapse in Chinese patients with Crohn’s disease: A two-year prospective study. Scand. J. Gastroenterol. 2017;52:1113–1119. doi: 10.1080/00365521.2017.1346704. [DOI] [PubMed] [Google Scholar]
- 43.Ferreiro-Iglesias R., Barreiro-de Acosta M., Lorenzo-Gonzalez A., Dominguez-Munoz J.E. Usefulness of a rapid faecal calprotectin test to predict relapse in Crohn’s disease patients on maintenance treatment with adalimumab. Scand. J. Gastroenterol. 2016;51:442–447. doi: 10.3109/00365521.2015.1115546. [DOI] [PubMed] [Google Scholar]
- 44.Monteiro S., de Castro F.D., Leite S., Moreira M.J., Cotter J. Low fecal calprotectin predicts clinical remission in Crohn’s disease patients: The simple answer to a challenging question. Scand. J. Gastroenterol. 2019;54:49–54. doi: 10.1080/00365521.2018.1549683. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T., Shiraki M., Bamba T., Umegae S., Matsumoto K. Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn’s disease after ileocolonic resection: A prospective pilot study. United Eur. Gastroenterol. J. 2013;1:368–374. doi: 10.1177/2050640613501818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laharie D., Mesli S., El Hajbi F., Chabrun E., Chanteloup E., Capdepont M., Razaire S., de Ledinghen V., Zerbib F. Prediction of Crohn’s disease relapse with faecal calprotectin in infliximab responders: A prospective study. Aliment. Pharmacol. Ther. 2011;34:462–469. doi: 10.1111/j.1365-2036.2011.04743.x. [DOI] [PubMed] [Google Scholar]
- 47.Jauregui-Amezaga A., Lopez-Ceron M., Aceituno M., Jimeno M., Rodriguez de Miguel C., Pino-Donnay S., Zabalza M., Sans M., Ricart E., Ordas I., et al. Accuracy of advanced endoscopy and fecal calprotectin for prediction of relapse in ulcerative colitis: A prospective study. Inflamm. Bowel Dis. 2014;20:1187–1193. doi: 10.1097/MIB.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 48.Hosseini S.V., Jafari P., Taghavi S.A., Safarpour A.R., Rezaianzadeh A., Moini M., Mehrabi M. Fecal Calprotectin is an Accurate Tool and Correlated to Seo Index in Prediction of Relapse in Iranian Patients With Ulcerative Colitis. Iran. Red Crescent Med. J. 2015;17:e22796. doi: 10.5812/ircmj.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keshteli A.H., van den Brand F.F., Madsen K.L., Mandal R., Valcheva R., Kroeker K.I., Han B., Bell R.C., Cole J., Hoevers T., et al. Dietary and metabolomic determinants of relapse in ulcerative colitis patients: A pilot prospective cohort study. World J. Gastroenterol. 2017;23:3890–3899. doi: 10.3748/wjg.v23.i21.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimoyama T., Yamamoto T., Umegae S., Matsumoto K. Faecal calprotectin level for assessing endoscopic activity and predicting future clinical course in patients with moderately active ulcerative colitis undergoing granulomonocytapheresis: A prospective cohort study. BMC Gastroenterol. 2018;18:120. doi: 10.1186/s12876-018-0853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theede K., Holck S., Ibsen P., Kallemose T., Nordgaard-Lassen I., Nielsen A.M. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm. Bowel Dis. 2016;22:1042–1048. doi: 10.1097/MIB.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T., Shiraki M., Bamba T., Umegae S., Matsumoto K. Fecal calprotectin and lactoferrin as predictors of relapse in patients with quiescent ulcerative colitis during maintenance therapy. Int. J. Color. Dis. 2014;29:485–491. doi: 10.1007/s00384-013-1817-3. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T., Shimoyama T., Umegae S., Matsumoto K. Endoscopic score vs. fecal biomarkers for predicting relapse in patients with ulcerative colitis after clinical remission and mucosal healing. Clin. Transl. Gastroenterol. 2018;9:e136. doi: 10.1038/s41424-018-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costa F., Mumolo M.G., Ceccarelli L., Bellini M., Romano M.R., Sterpi C., Ricchiuti A., Marchi S., Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Inca R., Dal Pont E., Di Leo V., Benazzato L., Martinato M., Lamboglia F., Oliva L., Sturniolo G.C. Can calprotectin predict relapse risk in inflammatory bowel disease? Am. J. Gastroenterol. 2008;103:2007–2014. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 56.Ferreiro-Iglesias R., Barreiro-de Acosta M., Lorenzo-Gonzalez A., Dominguez-Munoz J.E. Accuracy of Consecutive Fecal Calprotectin Measurements to Predict Relapse in Inflammatory Bowel Disease Patients Under Maintenance With Anti-TNF Therapy: A Prospective Longitudinal Cohort Study. J. Clin. Gastroenterol. 2018;52:229–234. doi: 10.1097/MCG.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 57.Gisbert J.P., Bermejo F., Perez-Calle J.L., Taxonera C., Vera I., McNicholl A.G., Algaba A., Lopez P., Lopez-Palacios N., Calvo M., et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm. Bowel Dis. 2009;15:1190–1198. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 58.Kostas A., Siakavellas S.I., Kosmidis C., Takou A., Nikou J., Maropoulos G., Vlachogiannakos J., Papatheodoridis G.V., Papaconstantinou I., Bamias G. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J. Gastroenterol. 2017;23:7387–7396. doi: 10.3748/wjg.v23.i41.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreiro-Iglesias R., Acosta M.B.-D., Santiago M.O., Gonzalez A.L., De La Peña C.A., Estevez A.J.B., Dominguez-Munoz J.E. Fecal Calprotectin as Predictor of Relapse in Patients With Inflammatory Bowel Disease Under Maintenance Infliximab Therapy. J. Clin. Gastroenterol. 2016;50:147–151. doi: 10.1097/MCG.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Sanchez V., Iglesias-Flores E., Gonzalez R., Gisbert J.P., Gallardo-Valverde J.M., Gonzalez-Galilea A., Naranjo-Rodriguez A., de Dios-Vega J.F., Muntane J., Gomez-Camacho F. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J. Crohn’s Colitis. 2010;4:144–152. doi: 10.1016/j.crohns.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Zhulina Y., Cao Y., Amcoff K., Carlson M., Tysk C., Halfvarson J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment. Pharmacol. Ther. 2016;44:495–504. doi: 10.1111/apt.13731. [DOI] [PubMed] [Google Scholar]
- 62.Buisson A., Mak W.Y., Andersen M.J., Lei D., Kahn S.A., Pekow J., Cohen R.D., Zmeter N., Pereira B., Rubin D.T. Faecal Calprotectin Is a Very Reliable Tool to Predict and Monitor the Risk of Relapse After Therapeutic De-escalation in Patients With Inflammatory Bowel Diseases. J. Crohn’s Colitis. 2019;13:1012–1024. doi: 10.1093/ecco-jcc/jjz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heida A., Park K.T., van Rheenen P.F. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm. Bowel Dis. 2017;23:894–902. doi: 10.1097/MIB.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J., Zhao X., Li X., Lu M., Zhang H. Systematic Review with Meta-Analysis: Fecal Calprotectin as a Surrogate Marker for Predicting Relapse in Adults with Ulcerative Colitis. Mediat. Inflamm. 2019;2019:2136501. doi: 10.1155/2019/2136501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mao R., Xiao Y.L., Gao X., Chen B.L., He Y., Yang L., Hu P.J., Chen M.H. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: A meta-analysis of prospective studies. Inflamm. Bowel Dis. 2012;18:1894–1899. doi: 10.1002/ibd.22861. [DOI] [PubMed] [Google Scholar]
- 66.Qiu Y., Mao R., Chen B.L., He Y., Zeng Z.R., Xue L., Song X.M., Li Z.P., Chen M.H. Fecal calprotectin for evaluating postoperative recurrence of Crohn’s disease: A meta-analysis of prospective studies. Inflamm. Bowel Dis. 2015;21:315–322. doi: 10.1097/MIB.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 67.Tham Y.S., Yung D.E., Fay S., Yamamoto T., Ben-Horin S., Eliakim R., Koulaouzidis A., Kopylov U. Fecal calprotectin for detection of postoperative endoscopic recurrence in Crohn’s disease: Systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2018;11:1756284818785571. doi: 10.1177/1756284818785571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rucker G., Schumacher M. Summary ROC curve based on a weighted Youden index for selecting an optimal cutpoint in meta-analysis of diagnostic accuracy. Stat. Med. 2010;29:3069–3078. doi: 10.1002/sim.3937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research is a meta-analysis and all data have been uploaded along with the Supplementary Materials.