Abstract
Background
Synthesising evidence on the long-term vaccine effectiveness of COVID-19 vaccines (BNT162b2 [Pfizer–BioNTech], mRNA-1273 [Moderna], ChAdOx1 nCoV-19 [AZD1222; Oxford–AstraZeneca], and Ad26.COV2.S [Janssen]) against infections, hospitalisations, and mortality is crucial to making evidence-based pandemic policy decisions.
Methods
In this rapid living systematic evidence synthesis and meta-analysis, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, supplemented by manual searches of COVID-19-specific sources, until Dec 1, 2022, for studies that reported vaccine effectiveness immediately and at least 112 days after a primary vaccine series or at least 84 days after a booster dose. Single reviewers assessed titles, abstracts, and full-text articles, and extracted data, with a second reviewer verifying included studies. The primary outcomes were vaccine effectiveness against SARS-CoV-2 infections, hospitalisations, and mortality, which were assessed using three-level meta-analytic models. This study is registered with the National Collaborating Centre for Methods and Tools, review 473.
Findings
We screened 16 696 records at the title and abstract level, appraised 832 (5·0%) full texts, and initially included 73 (0·4%) studies. Of these, we excluded five (7%) studies because of critical risk of bias, leaving 68 (93%) studies that were extracted for analysis. For infections caused by any SARS-CoV-2 strain, vaccine effectiveness for the primary series reduced from 83% (95% CI 80–86) at baseline (14–42 days) to 62% (53–69) by 112–139 days. Vaccine effectiveness at baseline was 92% (88–94) for hospitalisations and 91% (85–95) for mortality, and reduced to 79% (65–87) at 224–251 days for hospitalisations and 86% (73–93) at 168–195 days for mortality. Estimated vaccine effectiveness was lower for the omicron variant for infections, hospitalisations, and mortality at baseline compared with that of other variants, but subsequent reductions occurred at a similar rate across variants. For booster doses, which covered mostly omicron studies, vaccine effectiveness at baseline was 70% (56–80) against infections and 89% (82–93) against hospitalisations, and reduced to 43% (14–62) against infections and 71% (51–83) against hospitalisations at 112 days or later. Not enough studies were available to report on booster vaccine effectiveness against mortality.
Interpretation
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Funding
Canadian Institutes of Health Research and the Public Health Agency of Canada.
Introduction
Mass vaccination against SARS-CoV-2 has been crucial to containing the impact of the COVID-19 pandemic.1 However, vaccine-induced antibodies are reduced at 6 months after a primary COVID-19 vaccination series (ie, two doses of a two-dose vaccine or one dose of a one-dose vaccine)2 and vaccine effectiveness, a measure of how well vaccines work relative to unvaccinated individuals,3 against infections and hospitalisations might also be reduced 2–7 months after receiving a primary vaccination series. This reduction in vaccine effectiveness might be further accentuated by the emergence of new variants of concern. However, studies on the long-term effectiveness of COVID-19 vaccines vary in study design, methodology, and quality, and have generated diverse findings, which makes it challenging for policy makers to make evidence-based decisions, such as the timing of administering COVID-19 vaccine booster doses.
To our knowledge, only one similar systematic review exists to date that offers meta-analytical evidence on the duration of COVID-19 vaccine effectiveness.4 This review, published in March, 2022, found a rapid reduction in protection against infections, but not against severe disease. However, the review only examined the vaccine effectiveness of primary vaccine series and did not report synthesised data on COVID-19-related mortality nor the omicron (B.1.1.529) variant. Given the continued evolution of the pandemic, notably the spread of the omicron variant, updated information can be invaluable to inform policy makers. As part of ongoing Canadian monitoring, this rapid living systematic evidence synthesis sought to provide information on long-term vaccine effectiveness for Canadian-licensed COVID-19 vaccines (ie, BNT162b2 [Pfizer–BioNTech], mRNA-1273 [Moderna], ChAdOx1 nCoV-19 [AZD1222; Oxford–AstraZeneca, and Ad26.COV2.S [Janssen]) for adults.5 We aimed to investigate (1) how the vaccine effectiveness of a primary series of COVID-19 vaccines against SARS-CoV-2 infections, hospitalisations, and mortality changes from shortly after completing vaccination to 112 days or more after vaccination; (2) how the vaccine effectiveness of a primary series plus a single booster dose against SARS-CoV-2 infections, hospitalisations, and mortality changes from shortly after vaccination to 84 days or more after vaccination; and (3) what the vaccine effectiveness patterns are for infections, hospitalisations, and mortality against the omicron variant.
Research in context.
Evidence before this study
Mass vaccination against the SARS-CoV-2 virus has been crucial to containing the impact of the COVID-19 pandemic. Immunogenicity research indicates that the effectiveness of vaccines might decline over time; although, it is not clear how this reduction translates to clinical vaccine effectiveness. To our knowledge, one key systematic review to date has extensively examined the duration of COVID-19 vaccine effectiveness for infections and hospitalisations. This review found that vaccine effectiveness decreased over time in response to a primary vaccine series. However, the authors did not report any data related to booster doses, COVID-19-related mortality, nor the omicron (B.1.1.529) variant—key omissions given the ongoing pandemic situation. Between Jan 1, 2020, and Dec 1, 2022, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, a comprehensive, expert-curated database covering eight publication and preprint databases, and manually searched COVID-19-specific sources for our living systematic evidence synthesis and meta-analysis. We included studies in English or French that reported vaccine effectiveness for SARS-CoV-2 infections, hospitalisations, and mortality in response to either a primary series or a booster dose of COVID-19 vaccine at baseline and in the long-term (≥112 days for the primary series or ≥84 days for the booster dose). The search strategy included key terms related to vaccination (eg, vaccine types and producers).
Added value of this study
Using three-level meta-analytic models, we found marked decreases over time in vaccine effectiveness for SARS-CoV-2 infections for both the primary series and booster, and a smaller decrease for hospitalisations and mortality. The findings for the omicron variant were similar, but had notably lower levels of vaccine effectiveness at baseline. These findings extend the previous data by showing a lower initial vaccine effectiveness response to the omicron variant with further reductions over time.
Implications of all the available evidence
Vaccination continues to be an effective measure over time to reduce COVID-19 hospitalisations and mortality, but less so for infections. Other measures (eg, face masks and physical distancing) might be necessary to control infections in the long term. Our findings provide insights for clinicians, public health-care policy makers, and researchers about the long-term vaccine effectiveness of COVID-19 vaccines, which can inform clinical and policy recommendations, such as the timing of future booster doses.
Methods
Search strategy and selection criteria
We used rapid systematic review methods for this living evidence synthesis. We initially searched for studies published until Sept 10, 2021, and updated the search on Nov 19, 2021. From Feb 25, 2022, we updated the synthesis every 4 weeks. The results reported here are from the 13th synthesis round (until Dec 1, 2022). Results from previous rounds and forthcoming updates can be accessed online at our project page. The protocol was registered with the National Collaborating Centre for Methods and Tools and is available on our project page. This report adheres to the PRISMA 2020 guidelines.6 Since the publication of the protocol, the review has been converted from a standalone review to a living review, following a request from the Public Health Agency of Canada. Additionally, we have adapted the review to account for changes in the pandemic and its management, most notably, by including studies with booster data and variant-specific information (ie, for delta [B.1.617.2] and omicron). Further, we adapted the infection outcome to combine symptomatic and asymptomatic data. When multiple infection outcomes were reported only one per study was included (ie, data on all infections was the priority, then symptomatic infections, then asymptomatic infections).
We searched the US National Institutes of Health's (NIH) iSearch COVID-19 Portfolio7 and EMBASE via OVID until Dec 1, 2022. The search strategy was tailored to each database (appendix p 7). Additionally, we manually searched COVID-19-specific sources, including the US Department of Veterans Affairs' Evidence Synthesis Program 8, 9 and by searching the reference lists of previous systematic reviews.
Controlled trials and observational studies were eligible if they reported vaccine effectiveness data on adults (aged ≥18 years) or on a predominately adult general population. Vaccine effectiveness data needed to explicitly compare participants who were fully vaccinated with unvaccinated participants. To assess the vaccine effectiveness of the primary series, fully vaccinated participants needed to have received two doses of BNT162b2, mRNA-1273, or ChAdOx1/AZD1222, or one dose of Ad26.COV2.S. For booster vaccine effectiveness, fully vaccinated participants needed to have received a full primary series and an additional dose of a Canadian-licensed COVID-19 vaccine. Studies of the vaccine effectiveness of primary series needed vaccine effectiveness data both at baseline (0–42 days after primary series) and follow-up (≥112 days after primary series). Booster-dose studies also needed baseline (0–28 days after a booster dose) and follow-up (≥84 days post booster) vaccine effectiveness data. Baselines covered the periods when vaccine effectiveness was optimal, hence the difference between primary and booster doses. All data needed to be directly extractable from the paper (eg, studies with vaccine effectiveness that was reported non-numerically as figures were excluded). Lastly, we included studies that reported original findings in English or French.
The screening was done by a small team of coders (4–6 reviewers per review round) who were included after a training–testing phase. Two stages of screening (title and abstract then full text) were done using Rayyan (Rayyan Systems, Cambridge, MA, USA), with one reviewer assigned to each record per stage. Included studies were verified by a second reviewer, with cases of uncertainty resolved in consultation with a third reviewer.
As this study is based on the extant literature, we did not require ethics approval.
Data extraction
We extracted vaccine effectiveness data for SARS-CoV-2 infections, hospitalisations, and mortality. Data were extracted for each study by a single reviewer and verified by a second reviewer. Disagreements were resolved by discussion. When vaccine effectiveness results were available both for an entire sample and for subgroups (eg, according to age), we only extracted data for the larger grouping (ie, the full sample); otherwise, we treated subgroups as separate samples. When multiple versions of confounder-adjusted vaccine effectiveness were reported, we extracted the model considered to have the lowest risk of bias. Data were extracted into a study-specific spreadsheet. We operationalised infection data as including either symptomatic or asymptomatic infections, prioritising the extraction of data combining symptomatic and asymptomatic infections over symptomatic-specific or asymptomatic-specific data, and data on symptomatic over asymptomatic infections. We also extracted descriptive characteristics of the studies (eg, publication year, author, title, country, format, and study design) and the sample characteristics, the vaccine schedule used, timing of the outcome assessments converted to days since vaccination, and information on potential stratification factors.
Adequate vaccine effectiveness response
WHO have created a definition for adequate COVID-19 vaccine effectiveness,3 in which the vaccine effectiveness against symptomatic infections should be at least 70% with a lower 95% CI of at least 50%, and the vaccine effectiveness against hospitalisations or mortality should be at least 90% with a lower 95% CI of at least 70%. In this systematic review, we assessed results in terms of statistically significant reductions in vaccine effectiveness from baseline and meeting these WHO criteria.
Quality assessment
To assess risk of bias, we used a version of the Risk of Bias in Non-randomized Studies of Interventions tool adapted to COVID-19,10, 11 which assesses seven domains of bias. The tool classified risk of bias for each study as low, moderate, serious, or critical. A single reviewer (SB) did the risk of bias assessment, verified by a second reviewer, and validated assessments against ratings from the team that developed the tool.10 Studies that were rated as having a critical risk of bias were excluded from analyses.
Data analysis
The primary outcomes were the effectiveness of COVID-19 vaccines against SARS-CoV-2 infections, hospitalisations, and mortality at various time periods since receipt of primary and booster vaccinations. Analyses reported here include all vaccines. Additional analyses on vaccine class and individual vaccines are reported in the appendix (p 44). We recorded no secondary outcomes.
We converted all extracted vaccine effectiveness estimates into log risk ratios for analyses and transformed results into percentage vaccine effectiveness for presentation.12 When extracted vaccine effectiveness values were not readily usable for computation, values were adjusted (eg, correcting for errors or adjusting values that would imply a risk ratio of 0; appendix p 13).
We pooled vaccine effectiveness estimates using three-level meta-analytic models via the rma.mv function of the metafor (version 3.0.2)13 package in R (version 4.1.2). Three-level meta-analyses allow for the explicit modelling of nested structures in data (eg, when studies provide multiple estimates each, such as for different subgroups, or timepoints), and produce more valid and reliable estimates than traditional (univariate) fixed and random effects models under such circumstances.14, 15, 16 Given our interest in vaccine effectiveness waning over time, we deemed the use of three-level models ideal for this purpose (because each study provided vaccine effectiveness data for multiple timepoints).
We specified models according to published guidelines for three-level models,17 using a restricted maximum-likelihood procedure to estimate variance components. Extracted vaccine effectiveness estimates were nested within studies, allowing for the estimation of random intercept components. Our procedure allowed us to account for within-study heterogeneity (ie, differences in vaccine effectiveness estimates between subgroups or timepoints within studies, designated as level 2), and between-study heterogeneity (differences in vaccine effectiveness estimates produced between studies, designated as level 3). To examine waning over time, we computed model-predicted vaccine effectiveness for each timepoint, and did formal moderation tests on the difference in vaccine effectiveness value at each follow-up period compared with the baseline vaccine effectiveness values. Importantly, for any given set of analyses, all timepoints were modelled simultaneously in the model (and time was specified as a moderator variable). Following recommendations to improve the reliability of meta-analytic inferences,12 we only report estimates for a given timepoint when at least four studies provided data to pool meta-analytically at that timepoint. However, for comprehensiveness, the results for timepoints examined by fewer than four studies are reported in the appendix (p 32).
We report three indices of statistical heterogeneity for each of our models. These include the I 2 and σ (ie, an estimate of τ) statistics.18, 19 Further, we also provide 95% prediction intervals for each predicted timepoint. Prediction intervals reflect the likely range within which a future observation (ie, a vaccine effectiveness estimate from a new study or a vaccine effectiveness estimate observed in a new context) would be expected to fall. This measure contrasts with a CI, which only represents the likely range in which the average population parameter (eg, across studies or contexts) might be expected to fall. Descriptions of these three indices are given in the appendix (p 14).
We used meta-regressions comparing peer-reviewed (published) versus preprint studies to assess publication bias, supplemented with funnel plots for descriptive purposes. We also assessed the effect of study design (eg, comparing case-control studies with cohort studies) and did leave-one-out analyses to assess the robustness of our analyses.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
We screened 16 696 records at the title and abstract level, appraised 832 (5·0%) full texts, and initially included 73 (0·4%) studies. Of these, we excluded five (7%) studies because of critical risk of bias,20, 21, 22, 23, 24 leaving 68 (93%) studies that were extracted for analysis (appendix p 16).
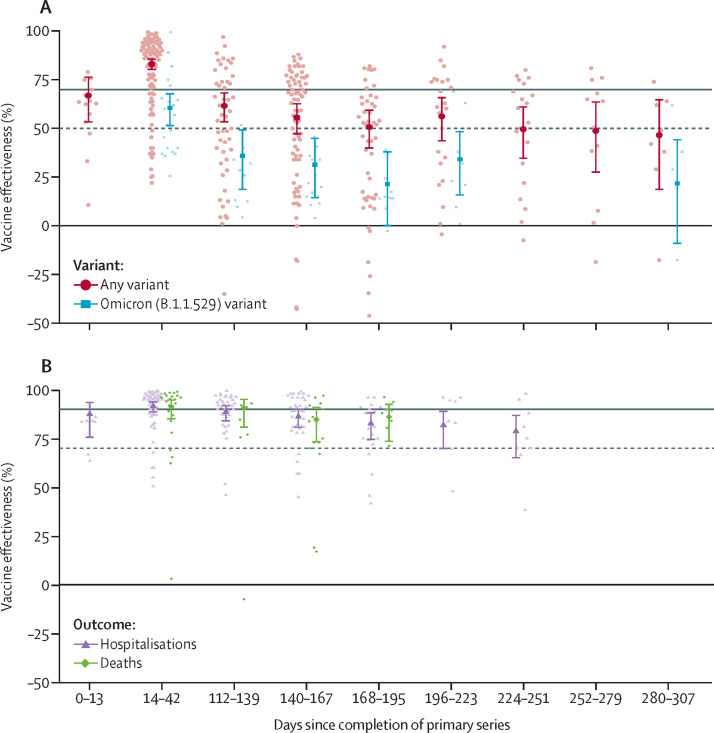
Of the 68 extracted studies, 53 (78%) were published in peer-reviewed journals and 15 (22%) were preprints. Studies recruited samples from over 23 countries. 39 (57%) studies used test-negative case-control designs, 26 (38%) studies used cohort designs, three (4%) studies were randomised controlled trials (including one open-label trial). 57 (84%) studies reported primary series data and 17 (25%) studies reported booster-dose data (table 1 ). We pooled infection data from 48 (71%) studies on the vaccine effectiveness of a COVID-19 primary vaccine series. Vaccine effectiveness met WHO guidelines for adequate protection at baseline, reaching 83% (95% CI 80–86; table 2 ). However, all follow-up periods after 112 days after vaccination showed a statistical reduction in vaccine effectiveness, none of which reached adequate protection levels (table 2; figure 1 ). We found adequate levels of baseline vaccine effectiveness for hospitalisations (92% [88–94]) from pooled data for 25 (52%) studies and for mortality (91% [85–95]) from pooled data for ten (21%) studies (table 2, figure 1). We found small but statistically significant decreases in vaccine effectiveness over time: vaccine effectiveness reduced to 79% [65–87] for hospitalisations by 224–51 days and 86% [73–93] for mortality by 168–95 days (table 2), both below WHO adequate levels.
Table 1.
Characteristics of included studies of effectiveness of COVID-19 vaccines
| Country | Vaccines | Primary series or booster | Outcome measure | Variant | |
|---|---|---|---|---|---|
| Andeweg (2022)25 | The Netherlands | mRNA-1273, BNT162b2, and Ad26.CoV2.S | Primary series and booster | Infections | Delta and omicron |
| Andrejko (2022)26 | USA | mRNA-1273 and BNT162b2 | Primary series | Infections | Non-specific |
| Andrews (2022)27 | UK | BNT162b2 and ChAdOx1 | Primary series | Infections, hospitalisations, and mortality | Delta |
| Andrews (2022)28 | England | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series | Infections | Delta and omicron |
| Baum (2022)29 | Finland | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series | Hospitalisations | Delta and omicron |
| Bedston (2022)30 | UK | BNT162b2 | Primary series | Infections | Non-specific |
| Berec (2022)31 | Czech Republic | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections, hospitalisations, and mortality | Non-specific |
| Britton (2022)32 | USA | BNT162b2, mRNA-1273, and Ad26.CoV2.S | Primary series | Infections | Delta |
| Bruxvoort (2021)33 | USA | mRNA-1273 | Primary series | Infections | Delta |
| Buchan (2022)34 | Canada | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections | Delta and omicron |
| Carazo (2022)35 | Canada | mRNA-1273 and BNT162b2 | Primary series | Infections | Omicron |
| Carazo (2022)36 | Canada | mRNA-1273 and BNT162b2 | Primary series | Infections, hospitalisations, and mortality | Omicron |
| Carazo (2022)37 | Canada | mRNA-1273 and BNT162b2 | Primary series | Infections and hospitalisations | Omicron |
| Castillo (2022)38 | France | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections and hospitalisations | Delta |
| Cerqueira-Silva (2022)39 | Brazil | BNT162b2, ChAdOx1, and Ad26.CoV2.S | Primary series | Infections | Non-specific |
| Cerqueira-Silva (2022)40 | Brazil | BNT162b2 and ChAdOx1 | Primary series | Infections | Omicron |
| Cerqueira-Silva (2022)41 | Brazil and Scotland | BNT162b2 and mRNA-1273 | Booster | Infections | Omicron |
| Cerqueira-Silva (2022)42 | Brazil | CoronaVac, BNT162b2, and ChAdOx1 | Booster | Infections, hospitalisations, and mortality | Omicron |
| Chambers (2022)43 | Canada | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections, hospitalisations, and mortality | Non-specific |
| Chemaitelly (2022)44 | Qatar | BNT162b2 | Primary series | Infections | Delta |
| Chemaitelly (2022)45 | Qatar | BNT162b2 | Primary series | Infections | Omicron |
| Chemaitelly (2022)46 | Qatar | BNT162b2 | Booster | Infections | Omicron |
| Chung (2022)47 | Canada | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Hospitalisations | Delta |
| Collie (2022)48 | South Africa | BNT162b2 | Primary series and booster | Hospitalisations | Omicron |
| Consonni (2022)49 | Italy | mRNA-1273 and BNT162b2 | Booster | Infections | Omicron |
| De Gier (2021)50 | The Netherlands | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Hospitalisations | Delta |
| El Adam (2022)51 | Canada | mRNA-1273 and BNT162b2 | Primary series | Infections | Non-specific |
| El Sahly (2021)52 | USA | mRNA-1273 | Primary series | Infections | Non-specific |
| Ferdinands (2022)53 | USA | mRNA-1273 and BNT162b2 | Primary series | Infections | Omicron |
| Florea (2021)54 | USA | mRNA-1273 | Primary series | Infections and hospitalisations | Non-specific |
| Glatman-Freedman (2022)55 | Israel | BNT162b2 | Booster | Infections, hospitalisations, and mortality | Omicron |
| Gram (2022)56 | Denmark | BNT162b2 and mRNA-1273 | Primary series and booster | Infections and hospitalisations | Delta and omicron |
| Gray (2022)57 | South Africa | BNT162b2, Ad26.COV2.S, and mRNA-1273 | Primary series | Hospitalisation | Omicron |
| Hall (2022)58 | UK | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections | Non-specific |
| Hansen (2022)59 | Denmark | BNT162b2 and mRNA-1273 | Primary series and booster | Infections and hospitalisations | Omicron |
| Horne (2022)60 | England | BNT162b2 and ChAdOx1 | Primary series | Infections, hospitalisations, and mortality | Non-specific |
| Katikireddi (2021)61 | Scotland | ChAdOx1 | Primary series | Infections | Non-specific |
| Kirsebom (2022)62 | England | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series and booster | Infections and hospitalisations | Omicron |
| Kirsebom (2022)63 | England | BNT162b2, ChAdOx1-S, and mRNA-1273 | Booster | Infections | Omicron |
| Kissling (2022)64 | Croatia, France, Ireland, the Netherlands, Portugal, Romania, Spain, England, and Scotland | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections | Delta |
| Lauring (2022)65 | USA | BNT162b2 and mRNA-1273 | Primary series | Hospitalisations | Non-specific |
| Lin (2022)66 | USA | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections, hospitalisations, and mortality | Delta and omicron |
| Lin (2022)67 | USA | BNT162b2 and ChAdOx1 mRNA-1273 | Primary series | Infections, hospitalisations, and mortality | Non-specific |
| Lind (2022)68 | USA | BNT162b2 and mRNA-1273 | Primary series | Infections | Omicron |
| Lind (2022)69 | USA | mRNA-1273 and BNT162b2 | Primary series | Infections and hospitalisations | Alpha and delta |
| Lyngse (2022)70 | Denmark | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections | Delta |
| Lytras (2022)71 | Greece | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Mortality | Non-specific |
| Machado (2021)72 | Portugal | BNT162b2 and mRNA-1273 | Primary series | Infections, hospitalisations, and mortality | Non-specific |
| Nielsen (2022)73 | Denmark | BNT162b2, Ad26.CoV2.S, ChAdOx1, and mRNA-1273 | Primary series | Infections | Omicron |
| Ng (2022)74 | Singapore | mRNA-1273 and BNT162b2 | Primary series | Infections | Delta |
| Nordstrom (2022)75 | Sweden | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series | Infections | Non-specific |
| Nyberg (2022)76 | England | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series and booster | Hospitalisations and mortality | Delta and omicron |
| Petras (2022)77 | Prague | BNT162b2 and Ad26.CoV2.S ChAdOx1 and mRNA-1273 | Primary series | Infections | Non-specific |
| Poukka (2022)78 | Finland | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series | Infections and hospitalisations | Delta |
| Richterman (2022)79 | USA | mRNA-1273 and BNT162b2 | Booster | Infections | Omicron |
| Robles-Fontan (2022)80 | Puerto Rico | BNT162b2, Ad26.CoV2.S, and mRNA-1273 | Primary series | Infections, hospitalisations, and mortality | Non-specific |
| Rosenberg (2022)81 | USA | BNT162b2, mRNA-1273, and ChAdOx1 | Primary series | Infections and hospitalisations | Non-specific |
| Skowronski (2021)82 | Canada | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series | Infections and hospitalisations | Delta |
| Sobieszczyk (2022)83 | USA, Chile, and Peru | ChAdOx1 | Primary series | Infections | Non-specific |
| Starrfelt (2022)84 | Norway | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series | Infections and hospitalisations | Non-specific |
| Stowe (2022)85 | England | BNT162b2, ChAdOx1, and mRNA-1273 | Primary series and booster | Hospitalisations | Delta and omicron |
| Suphanchaimat (2022)86 | Thailand | CoronaVac, BNT162b2, and ChAdOx1 | Booster | Infections | Delta |
| Syed (2022)87 | Qatar | BNT162b2 and mRNA-1273 | Primary series | Infections | Non-specific |
| Tartof (2022)88 | USA | BNT162b2 | Primary series | Infections and hospitalisations | Non-specific |
| Thomas (2021)89 | Global | BNT162b2 | Primary series | Infections | Non-specific |
| Thompson (2021)90 | USA | BNT162b2, Ad26.CoV2.S, and mRNA-1273 | Primary series | Hospitalisations | Non-specific |
| Tseng (2022)91 | USA | mRNA-1273 | Booster | Infections and hospitalisations | Omicron |
Table 2.
Vaccine effectiveness for any primary COVID-19 vaccine series against infections, hospitalisations, and mortality I2
|
Baseline, days |
Follow-up, days |
I2 | σ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–13 | 14–42 | 112–139 | 140–167 | 168–195 | 196–223 | 224–251 | 252–279 | 280–307 | ||||
| Any variant | ||||||||||||
| Documented infections | ||||||||||||
| Vaccine effectiveness | 67% (95% CI 53 to 77; 95% PI −20 to 91) | 83%* (95% CI 80 to 86; 95% PI 39 to 95) | 62%† (95% CI 53 to 69; 95% PI −29 to 89) | 56%† (95% CI 47 to 63; 95% PI −38 to 88) | 51%*† (95% CI 40 to 60; 95% PI −44 to 87) | 56%† (95% CI 43 to 66; 95% PI −38 to 88) | 50%*† (95% CI 34 to 61; 95% PI −46 to 86) | 49%† (95% CI 27 to 64; 95% PI −48 to 86) | 47%† (95% CI 18 to 65]; 95% PI −51 to 86) | 53, 47 | 0·48, 0·44 | |
| κ | 8 (14) | 40 (96) | 22 (50) | 31 (77) | 21 (49) | 15 (23) | 13 (21) | 8 (12) | 6 (8) | .. | .. | |
| Hospitalisations | ||||||||||||
| Vaccine effectiveness | 88% (95% CI 75 to 94; 95% PI 20 to 98) | 92% (95% CI 88 to 94; 95% PI 51 to 99) | 89%† (95% CI 84 to 92; 95% PI 33 to 98) | 86%† (95% CI 80 to 90; 95% PI 20 to 98) | 83%† (95% CI 74 to 89; 95% PI −2 to 97) | 82%† (95% CI 70 to 89; 95% PI −10 to 97) | 79%† (95% CI 65 to 87; 95% PI −23 to 97) | .. | .. | 33, 65 | 0.51, 0·72 | |
| κ | 4 (7) | 21 (55) | 11 (37) | 15 (37) | 9 (19) | 6 (8) | 7 (9) | .. | .. | .. | .. | |
| Mortality | ||||||||||||
| Vaccine effectiveness | .. | 91% (95% CI 85 to 95; 95% PI 45 to 99) | 91% (95% CI 81 to 95; 95% PI 37 to 99) | 85%† (95% CI 73 to 91; 95% PI 3 to 98) | 86% (95% CI 73 to 93; 95% PI 9 to 98) | .. | .. | .. | .. | 26, 69 | 0·46, 0·75 | |
| κ | .. | 10 (23) | 4 (7) | 8 (15) | 4 (8) | .. | .. | .. | .. | .. | .. | |
| Omicron | ||||||||||||
| Documented infections | ||||||||||||
| Vaccine effectiveness | .. | 61% (95% CI 51 to 68; 95% PI 11 to 83) | 36%† (95% CI 18 to 50; 95% PI −32 to 72) | 31%*† (95% CI 14 to 45; 95% PI −36 to 70) | 21%*† (95% CI 0 to 38; 95% PI −44 to 66) | 34%*† (95% CI 16 to 49; 95% PI −34 to 71) | .. | .. | 22%*† (95% CI −9 to 45; 95% PI −46 to 67) | 32, 68 | 0·22, 0·33 | |
| κ | .. | 12 (21) | 6 (10) | 7 (14) | 5 (10) | 6 (8) | .. | .. | 4 (4) | .. | .. | |
| Hospitalisations | ||||||||||||
| Vaccine effectiveness | .. | 71% (95% CI 58 to 80; 95% PI 32 to 88) | .. | .. | 52%† (95% CI 29 to 67; 95% PI −12 to 79) | .. | .. | .. | .. | 40, 55 | 0·23, 0·27 | |
| κ | .. | 6 (7) | .. | .. | 4 (4) | .. | .. | .. | .. | .. | .. | |
I2 is Higgin's and Thompson's I2 presented at the within-study and between-study levels. σ is an estimate of τ, the SD of effect sizes in the population, presented at the within-study and between-study levels. κ=number of studies pooled (number of cohorts or observations pooled). PI=prediction interval.
Vaccine effectiveness at this follow-up timepoint is statistically different from the vaccine effectiveness observed at baseline 1 (0–13 days).
Vaccine effectiveness at this follow-up timepoint is statistically different from the vaccine effectiveness observed at baseline 2 (14–42 days).
Figure 1.
Vaccine effectiveness of primary series
(A) Vaccine effectiveness of primary series against SARS-CoV-2 infections, by variant (κ=48). (B) Vaccine effectiveness of primary series against COVID-19-related hospitalisations (κ=25) and deaths (κ=10). The main data points with error bars represent meta-analytical estimates of vaccine effectiveness with corresponding 95% CIs. The lighter points scattered around each meta-analytical estimate represent estimates of vaccine effectiveness as extracted from individual studies. The horizontal solid grey lines represent the 90% point estimate and dotted lines represent 70% CI protection levels, as stipulated by WHO.
We pooled 14 (29%) studies for vaccine effectiveness against infections and eight (17%) studies for vaccine effectiveness against hospitalisations in response to the omicron variant, but we had too few studies to pool on mortality (κ=1). Estimates of baseline vaccine effectiveness against omicron were not considered adequate for neither infections (61% [51–68]) nor hospitalisations (71% [58–80]; table 2). The patterns of vaccine effectiveness change were similar for those against the omicron variant compared with the general data on any variant—ie, we found large reductions in vaccine effectiveness for infections but small reductions for hospitalisations (table 2).
Analyses broken down by vaccine type (ie, mRNA vaccines [BNT162b2 or mRNA-1273] and adenovirus vaccines [ChAdOx1/AZD1222 or Ad26.COV2.S]), showed similar patterns as already described; although, the baseline data for adenovirus vaccines did not meet adequate levels for hospitalisations and mortality (table 3 ). Results for individual vaccine brands can be found in the appendix (p 35).
Table 3.
Vaccine effectiveness for mRNA or adenovirus primary COVID-19 vaccine series against infections, hospitalisations, and mortality
|
Baseline, days |
Follow-up, days |
I2 | σ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–13 | 14–42 | 112–139 | 140–167 | 168–195 | 196–223 | 224–251 | 252–279 | 280–307 | ||||
| Any mRNA vaccine | ||||||||||||
| Any variant | ||||||||||||
| Documented infections | 71% (95% CI 57 to 80; 95% PI −11 to 92) | 87%* (95% CI 84 to 90; 95% PI 53 to 97) | 66%† (95% CI 57 to 74; 95% PI −20 to 91) | 57%†* (95% CI 47 to 66; 95% PI −37 to 89) | 52%†* (95% CI 39 to 63; 95% PI −44 to 87) | 52%†* (95% CI 36 to 65; 95% PI −45 to 87) | 48%†* (95% CI 31 to 62; 95% PI −49 to 86) | 48%†* (95% CI 24 to 64; 95% PI −50 to 87) | 51%† (95% CI 22 to 69; 95% PI −48 to 88) | 32, 68 | 0·37, 0·54 | |
| κ | 6 (8) | 28 (59) | 14 (28) | 24 (48) | 13 (26) | 11 (13) | 9 (14) | 5 (7) | 4 (5) | .. | .. | |
| Hospitalisations | .. | 93% (95% CI 89 to 95; 95% PI 53 to 99) | 89%† (95% CI 83 to 93; 95% PI 31 to 98) | 87%† (95% CI 80 to 91; 95% PI 14 to 98) | 84%† (95% CI 73 to 90; 95% PI −5 to 98) | 82%† (95% CI 69 to 90; 95% PI −15 to 97) | 80%† (95% CI 64 to 88; 95% PI −27 to 97) | .. | .. | 26, 73 | 0·47, 0·78 | |
| κ | .. | 18 (33) | 8 (20) | 13 (20) | 6 (9) | 5 (7) | 6 (7) | .. | .. | .. | .. | |
| Mortality | .. | 94% (95% CI (88 to 97; 95% PI 55 to 99) | .. | 87%† (95% CI 73 to 94; 95% PI −2 to 98) | .. | .. | .. | .. | .. | 25, 71 | 0·49, 0·81 | |
| κ | .. | 8 (15) | .. | 6 (8) | .. | .. | .. | .. | .. | .. | .. | |
| Omicron | ||||||||||||
| Documented infections | .. | 67% (95% CI 53 to 77; 95% PI 0 to 89) | .. | 32%† (95% CI 2 to 53; 95% PI −51 to 78) | .. | .. | .. | .. | .. | 9, 91 | 0·15, 0·49 | |
| κ | .. | 8 (12) | .. | 4 (6) | .. | .. | .. | .. | .. | .. | .. | |
| Hospitalisations | .. | 72% (95% CI 58 to 81; 95% PI 32 to 88) | .. | .. | .. | .. | .. | .. | .. | 24, 72 | 0·17, 0·31 | |
| κ | .. | 6 (6) | .. | .. | .. | .. | .. | .. | .. | .. | .. | |
| Delta | ||||||||||||
| Documented infections | .. | 91% (95% CI 88 to 93; 95% PI 78 to 96) | 73%† (95% CI 63 to 80; 95% PI 33 to 89) | 72%† (95% CI 63 to 79; 95% PI 33 to 88) | 69%† (95% CI 58 to 77; 95% PI 25 to 87) | .. | .. | .. | .. | 20, 80 | 0·18, 0·37 | |
| κ | .. | 7 (12) | 4 (6) | 7 (11) | 4 (8) | .. | .. | .. | .. | .. | .. | |
| Hospitalisations | .. | 96% (95% CI 90 to 98; 95% PI 63 to 100) | .. | 91% (95% CI 77 to 96; 95% PI 17 to 99) | .. | .. | .. | .. | .. | 48, 51 | 0·67, 0·69 | |
| κ | .. | 5 (7) | .. | 4 (5) | .. | .. | .. | .. | .. | .. | .. | |
| Any adenovirus vaccine | ||||||||||||
| Any variant | ||||||||||||
| Documented infections | .. | 69% (95% CI 60 to 75; 95% PI 18 to 88) | 56%† (95% CI 42 to 66; 95% PI −15 to 83) | 50%† (95% CI 37 to 61; 95% PI −24 to 81) | 47%† (95% CI 31 to 59; 95% PI −30 to 80) | .. | .. | .. | .. | 30, 69 | 0·26, 0·39 | |
| κ | .. | 14 (23) | 7 (12) | 12 (20) | 7 (13) | .. | .. | .. | .. | .. | .. | |
| Hospitalisations | .. | 90% (95% CI 83 to 94; 95% PI 46 to 98) | 89% (95% CI 81 to 94; 95% PI 42 to 98) | 85% (95% CI 74 to 92; 95% PI 18 to 97) | .. | .. | .. | .. | .. | 32, 66 | 0·46, 0·66 | |
| κ | .. | 9 (15) | 5 (11) | 7 (11) | .. | .. | .. | .. | .. | .. | .. | |
| Mortality | .. | 84% (95% CI 72 to 91; 95% PI 28 to 96) | .. | 75% (95% CI 53 to 86; 95% PI −14 to 94) | .. | .. | .. | .. | .. | 67, 25 | 0·57, 0·35 | |
| κ | .. | 7 (10) | .. | 5 (6) | .. | .. | .. | .. | .. | .. | .. | |
| Delta | ||||||||||||
| Documented infections | .. | 75% (95% CI 67 to 81; 95% PI 51 to 87) | .. | 58%† (95% CI 46 to 68; 95% PI 19 to 78) | .. | .. | .. | .. | .. | 23, 76 | 0·14, 0·25 | |
| κ | .. | 5 (8) | .. | 5 (8) | .. | .. | .. | .. | .. | .. | .. | |
I2 is Higgin's and Thompson's I2 presented at the within-study and between-study levels. σ is the estimate of τ, the SD of effect sizes in the population, presented at the within-study and between-study levels. κ=number of studies pooled (number of cohorts or observations pooled). PI=prediction interval.
Vaccine effectiveness at this follow-up timepoint is statistically different from the vaccine effectiveness observed at baseline 1 (0–13 days).
Vaccine effectiveness at this follow-up timepoint is statistically different from the vaccine effectiveness observed at baseline 2 (14–42 days).
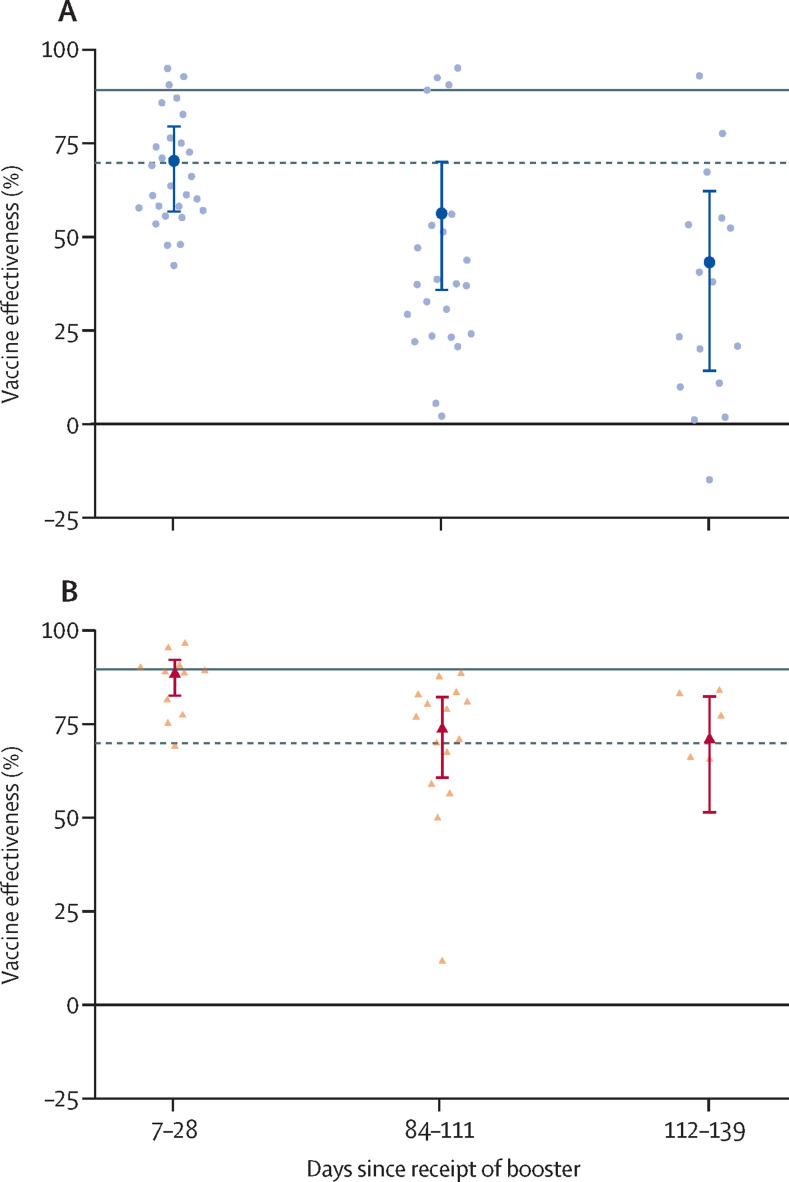
The pooled infection data from 13 (19%) booster-dose studies show that the baseline vaccine effectiveness did not meet adequate levels (70% [95% CI 56–80]) and significantly decreased over time (table 4 , figure 2 ). We pooled seven (62%) studies for hospitalisation data, with a marginally inadequate baseline response (89% [82–93]), followed by further reductions over time (table 4, figure 2). We had too few studies to report on booster vaccine effectiveness against mortality (κ=3). Findings for the vaccine effectiveness of COVID-19 booster doses against the omicron variant were largely identical to data for any variant (table 4); however, this finding was because most of our data for booster vaccine effectiveness were limited to mRNA vaccines against omicron.
Table 4.
Vaccine effectiveness for booster COVID-19 vaccine series against infections, hospitalisations, and mortality
| Baseline days (7–28) |
Follow-up days |
I2 | σ | |||
|---|---|---|---|---|---|---|
| 84–111 | 112–139 | |||||
| Any variant | ||||||
| Documented infections | 70% (95% CI 56 to 80; 95% PI −24 to 93) | 56%* (95% CI 35 to 70; 95% PI −48 to 90) | 43%* (95% CI 14 to 62; 95% PI −61 to 87) | 23, 77 | 0·35, 0·63 | |
| κ | 13 (28) | 11 (23) | 8 (16) | .. | .. | |
| Hospitalisations | 89%† (95% CI 82 to 93; 95% PI 59 to 97) | 74%* (95% CI 60 to 83; 95% PI 8 to 93) | 71%* (95% CI 51 to 83; 95% PI −6 to 92) | 34, 64 | 0·35, 0·47 | |
| κ | 7 (11) | 8 (15) | 4 (5) | .. | .. | |
| Omicron | ||||||
| Documented infections | 67% (95% CI 53 to 77; 95% PI −16 to 91) | 51%* (95% CI 30 to 66; 95% PI −44 to 87) | 40%* (95% CI 11 to 59; 95% PI −55 to 84) | 32, 68 | 0·35, 0·51 | |
| κ | 11 (24) | 9 (19) | 7 (14) | .. | .. | |
| Hospitalisations | 89%† (95% CI 82 to 93; 95% PI 59 to 97) | 74%* (95% CI 60 to 83; 95% PI 8 to 93) | 71%* (95% CI 51 to 83; 95% PI −6 to 92) | 30, 68 | 0·32, 0·48 | |
| κ | 7 (11) | 8 (13) | 4 (5) | .. | .. | |
I2 is Higgin's and Thompson's I2 presented at the within-study and between-study levels. σ is the estimate of τ, the SD of effect sizes in the population, presented at the within-study and between-study levels. κ=number of studies pooled (number of cohorts or observations pooled). PI=prediction interval.
Vaccine effectiveness at this follow-up timepoint is statistically different from the vaccine effectiveness observed at baseline 2 (14–28 days).
Vaccine effectiveness at this follow-up timepoint is statistically different from the vaccine effectiveness observed at baseline 1 (0–13 days).
Figure 2.
Vaccine effectiveness of a booster dose
(A) Vaccine effectiveness of a booster dose against SARS-CoV-2 infections (κ=13). (B) Vaccine effectiveness of a booster dose against COVID-19-related hospitalisations (κ=7). The main data points with error bars represent meta-analytical estimates of vaccine effectiveness with corresponding 95% CIs. The lighter points scattered around each meta-analytical estimate represent estimates of vaccine effectiveness as extracted from individual studies. The horizontal solid grey lines represent the 90% point estimate and dotted lines represent 70% CI protection levels, as stipulated by WHO.
Our analyses found very high levels of heterogeneity, as indicated by the I 2 and σ estimates, and the 95% prediction intervals (tables 2–4). Such heterogeneity can undermine our confidence in generalising from the estimates, even those with relatively narrow CIs. For instance, our point estimate for the vaccine effectiveness of primary series against infections (for any vaccine and any variant) was high at baseline (83% at 14–42 days) with a narrow 95% CI (80–86). This finding indicates high confidence that on average studies converge on this point estimate. However, the 95% prediction interval ranged was 38–95, indicating that predicting the results of any given new study would be difficult (eg, predicting vaccine effectiveness in a new context, such as in a country not yet represented in the data).
Overall, risk of bias was serious for most studies, which was based on the lack of adjustments for important COVID-19 prognostic factors. Three (4%) studies had a low overall risk of bias, 13 (19%) studies had moderate risk, and 52 (76%) studies had serious risk.
Because of our small sample size, analyses of publication bias were limited to the vaccine effectiveness of primary series for infections and hospitalisations (appendix p 45). Although preprint and published studies both showed similar reductions in vaccine effectiveness over time (for both outcome variables), we found a significant effect of publication type for infections such that vaccine effectiveness at baseline began at a significantly lower level for preprint studies (72% vs 84% at 14-42 days, reflecting a log odds ratio [OR] of 0·57 [95% CI 0·05–1·09]; p=0·031). We found no statistical differences between published and preprint studies in estimates of vaccine effectiveness against hospitalisations (appendix p 47). Funnel plots also showed some skewness, indicating potential publication bias in this literature (appendix p 48).
We did two sets of moderation analyses to examine the robustness of our models. The first set of analyses examined the degree to which vaccine effectiveness estimates against infections (for which we had the most data) differed across studies of different design types. We found little evidence that our results would differ if limited to either case-control or cohort-based studies (and the effect of trials on our results is mostly negligible due to their rarity; appendix p 51). Additionally, we examined whether any given study might have a disproportionate influence on our findings by generating leave-one-out analyses (ie, computing our models for primary and booster vaccines against infections and hospitalisations, leaving out one study at a time). The influence of omitting any one study at a time on our results was largely negligible (appendix p 53).
Discussion
We found that the vaccine effectiveness of the primary vaccine series against SARS-CoV-2 infections begins at an adequate level, as defined by WHO, of 83% at 14–42 days after series completion; however, vaccine effectiveness decreased significantly by 112 days after vaccination, reaching 47% by 280 days after vaccination, well below an adequate level. For COVID-19 hospitalisations and mortality, vaccine effectiveness levels were also adequate at baseline (>90%), but similarly reduced 112 days after vaccination; although, vaccine effectiveness remained high over time (>75%). When looking at omicron-only data, we found similar waning patterns, except that baseline levels of vaccine effectiveness did not reach adequate levels for infections or hospitalisations. What might be driving these omicron patterns is unclear—eg, whether a degradation in immunogenicity, changes in public health measures, variations in case numbers and general transmission, or a combination of all these. Although boosters might be promising at re-establishing some protection, our results found that the vaccine effectiveness of boosters at baseline (7–28 days after receiving the booster) were still, albeit by a small margin, below the WHO's recommended levels and in the long term, these numbers reduced further. Our booster-dose estimates predominately represent mRNA vaccines against omicron, which reflects the situation in many countries. Collectively, these data suggest that vaccines are providing reasonably stable protection against hospitalisations and mortality over the long term, but that protection against infections is more modest.
When considering different classes of vaccines for the primary series, patterns of the change in vaccine effectiveness over time were consistent, but vaccine effectiveness for adenovirus (vs mRNA) vaccines against infections was lower overall. By contrast, both vaccine classes were similar in terms of hospitalisations and mortality. However, we included fewer studies on adenovirus vaccines and few studies that directly compared mRNA with adenovirus vaccines.92 Thus, asserting that any difference exists between the two classes is difficult.
Given the marginally adequate baseline vaccine effectiveness against the omicron variant and declining vaccine effectiveness over time, other COVID-19 mitigation measures (eg, the use of face masks, physical distancing, and quarantining) might continue to be needed to reduce COVID-19 spread and reduce COVID-19-related hospitalisations and deaths.93 Growing evidence supports the utility of these measures in the management of COVID-19;94, 95, 96 however, most of this evidence has not taken vaccination status into account. Therefore, future research must study the effectiveness of these measures on vaccinated individuals.
Generally, our vaccine effectiveness findings are consistent with data on immunogenicity showing that the strong immunological response initially elicited by vaccination appears to reduce over time.97, 98 Although long-term immunological data after booster doses are scarce, Xin and colleagues99 reported substantial reductions in neutralising antibody titres around 6 months after a booster dose of CoronaVac. However, the rate of reduction in antibody titres after a booster dose might be slower than after a second dose.99, 100 Our booster-dose data were compared with that of unvaccinated individuals and did not directly compare a booster dose with the primary series itself. Richterman and colleagues79 found a baseline benefit of the two mRNA doses plus a booster dose compared with two mRNA doses (OR 0·34) for omicron infections that was only slightly decreased 112 days after booster dose (OR 0·45). By contrast, Cerqueira-Silva and colleagues42 found a benefit at baseline of the CoronaVac plus BNT162b2 booster versus CoronaVac (OR 0·41) for omicron infections with a large reduction in protection 91–120 days after the booster dose (OR 0·84). Given immunogenicity data, the relatively small proportion of unvaccinated individuals worldwide, and the fact that many countries are now giving second and third booster doses, future monitoring of the long-term vaccine effectiveness of different vaccine doses compared with each other is warranted.
Considering the highly dynamic publishing landscape, especially in the context of COVID-19, the consistency in findings across published and preprint studies was encouraging. However, the somewhat lower vaccine effectiveness values for primary series data in preprint (vs published) research highlights the importance of including such articles in systematic reviews. Although the lower vaccine effectiveness of preprints might reflect the fact that such studies were often more recent omicron-focused studies (for which vaccine effectiveness is lower compared with against previous variants), this pattern, along with the skewness we observed in funnel plots (appendix p 49), could indicate publication bias. Practices such as registering studies could attenuate the potential for such bias.
Our study has several potential limitations. As with any rapid review process, a slightly increased possibility exists that studies might be missed when compared with a full systematic review. Because of timing, we were not able to contact authors for studies that could have potentially provided data that were excluded (eg, those that reported just graphical data). We also only included findings on the four COVID-19 vaccines licensed in Canada, which do not represent all available vaccines. As such, the findings are unlikely to be generalisable to some countries such as China and India, where large parts of the population have been vaccinated using inactivated virus vaccines. We used a hierarchy when including infection data, meaning that we reviewed a mixture of symptomatic and asymptomatic data within the reported infection results; although, within any specific study all reported data over time were for the same outcome. Previous research has suggested that vaccine effectiveness of COVID-19 vaccines for symptomatic infections might be higher than for asymptomatic infections;101 although, little work has been done to examine how reductions in vaccine effectiveness operate differentially between the two outcomes. Therefore, our absolute point estimates of vaccine effectiveness might be difficult to compare with those of studies that included only symptomatic or asymptomatic infections; however, reductions in vaccine effectiveness are not likely to only affect one type of infection and not the other.
There is also ongoing debate about the effect of hybrid immunity, with factors such as the particular variant concerned, number of vaccines received, and the timing of vaccination all potentially playing a role.102 We did not explore this issue directly; although, most included studies either excluded or statistically adjusted for previous recent infection, meaning that the results generally do not reflect hybrid immunity. Data are scarce on the longer-term effects of hybrid immunity. However, data from one study by Carazo and colleagues37 suggested that, when compared with unvaccinated and uninfected individuals, there was little difference over time in the vaccine effectiveness of omicron-based reinfection between one dose and two doses of vaccines in individuals who were previously infected (ie, hybrid immunity). By contrast, we found that a benefit might exist in those with three doses (although we had only one follow-up data point in this group). Given the greater infection prevalence of the omicron variant compared with that of previous variants, future reviews should be able to explore the issue of hybrid immunity in greater depth as more data become available.
We also note that the literature itself has limitations that cannot be overcome through a systematic review alone. For instance, our supplemental analyses showed that published studies, and studies with smaller samples sizes, might produce higher baseline estimates of vaccine effectiveness than those of unpublished and larger studies. Future studies, beyond trials, might benefit from regularly registering their intended procedures and using other open-science practices to improve monitoring of bias. Another limitation of the literature is that we were only able to identify three studies that we qualified as having low risk of bias. Two were randomised controlled trials done early in the COVID-19 pandemic (with data extending up to March, 2021) that found promising results of vaccine effectiveness against infection.89, 52 The third was a more recent test-negative study from Canada that found good vaccine effectiveness against delta infections but very poor vaccine effectiveness against omicron infections across timepoints.34 Clearly, high-quality studies on vaccine effectiveness waning are needed, particularly against hospitalisations and mortality.
Last, we would like to emphasise that our review found a large degree of heterogeneity in the literature. This finding is not a limitation of our review, as documenting such heterogeneity is a valuable contribution and might reflect complex interactions between vaccination and contextual factors as they operate in the world. However, such heterogeneity does introduce challenges in predicting whether vaccination will be truly effective under a given regimen, or for a given population. This heterogeneity indicates that future studies and reviews should examine factors that predict when, where, and for whom the vaccines show differential effectiveness to address possible disparities in protection. In so doing, examining how vaccines interact with other protective measures (eg, face-mask wearing and isolation policies) might be particularly informative.
Our evidence synthesis also has additional strengths. First, we used broad search terms and a high-quality rapid review methodology, including the training of reviewers and validation of included studies and extracted data. Second, we used advanced three-level meta-analytic models to examine whether vaccine effectiveness at follow-up differed from that at baseline, taking into account both within-study and between-study variability, a technique which affords us more robust and reliable conclusions than would be available with traditional fixed and random effects models. Third, this living systematic evidence synthesis provides important updates compared with previous systematic reviews,4, 103 notably longer follow-up periods and data on the omicron variant and mortality.
Our findings provide insights for clinicians, public health-care policy makers, and researchers about the long-term vaccine effectiveness of COVID-19 vaccines, which can inform clinical and policy recommendations. Our analyses indicate that vaccine effectiveness reduces over time for both primary series and booster doses for preventing SARS-CoV-2 infections, hospitalisations, and mortality, a finding which is most pronounced for infections. Furthermore, we found similar reductions with the omicron variant, except that baseline levels of vaccine effectiveness were noticeably lower and did not meet the WHO criteria for an adequate vaccine response. Given that omicron has become the dominant variant, maintaining COVID-19 prevention behaviours might be needed (eg, face-mask wearing and physical distancing) in addition to vaccination to reduce the transmission of the virus and limit increases in infections. However, additional studies are needed to investigate the effectiveness of using multiple transmission prevention strategies simultaneously (eg, combining vaccination and face-mask wearing).
Data sharing
Extracted data are available online at https://osf.io/7uwjq/.
Declaration of interests
In the past 3 years, SLB has received consultancy and speaker fees from Resplipus, an investigator-generated educational grant from Moderna, and has served on advisory boards for Bayer, Sanofi, Respiplus, and Sojecci, none of which are related to the current Article. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The development and continued updating of this living evidence synthesis has been funded by the Canadian Institutes of Health Research (CIHR) and the Public Health Agency of Canada, through the COVID-19 Evidence Network to support Decision-making network. The members of the Montreal Behavioural Medicine Centre are supported by a variety of career and scholarship awards. SLB is supported by the CIHR-Strategy for Patient-Oriented Research (SPOR) initiative through the Mentoring Chair program (SMC-151518) and by the Fonds de recherche du Québec: Santé (FRQS) through the Chaire de recherche double en Intelligence Artificielle/Santé Numérique ET sciences de la vie program (309811). CS, NW, and KJ-D are supported by the CIHR-SPOR Mentoring Chair programme (SMC-151518). KJ-D and AMV are supported by FRQS scholarships. We would also like to acknowledge Esse Julien Atto for his contribution to statistical analysis.
Contributors
SLB, NW, and KJ-D had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SLB, NW, KJ-D, PABR, AMV, and JS conceptualised the study. SLB, NW, KJ-D, PABR, AMV, and JS curated the data. SLB, NW, KJ-D, and PABR contributed to the analyses. NW and PABR did the literature search. SLB, NW, KJ-D, PABR, AMV, JS, CS, and DY collected the data. KJ-D did the formal data analysis. SLB, NW, KJ-D, PABR, AMV, and JS designed the study methods. NW and DY administered the project. SLB supervised the project. SLB and PABR obtained funding. SLB, NW, KJ-D, PABR, and AMV contributed to the data visualisation. SLB, NW, and KJ-D drafted the manuscript. SLB, NW, KJ-D, PABR, AMV, JS, CS, and DY contributed to data interpretation and writing, reviewing, and editing this manuscript. SLB and NW were responsible for the decision to submit the manuscript for publication.
Supplementary Material
References
- 1.Harder T, Koch J, Vygen-Bonnet S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.28.2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; 2021. Vaccine efficacy, effectiveness and protection.https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection [Google Scholar]
- 4.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon S, Wu N, Joyal-Desmarais K, et al. What is the long-term effectiveness of available VID-19 vaccines for adults, including for variants of concern and over time frames beyond 112 days in those with a primary series and beyond 84 days in those with a primary series and an additional dose? McMaster Health Forum. 2022. https://www.mcmasterforum.org/networks/covid-end/resources-specific-to-canada/for-decision-makers/scan-evidence-products
- 6.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian National Institutes of Health iSearch COVID-19 Portfolio. 2022. https://icite.od.nih.gov/covid19/search/#search:searchId=621784192cbff419a54a8ae4
- 8.McMaster Health Forum COVID-END: resources to support decision-makers. 2022. [Available from: https://www.mcmasterforum.org/networks/covid-end/resources-to-support-decision-makers/inventory-of-evidence-syntheses/public-health-measures
- 9.International Vaccine Access Center. Johns Hopkins Bloomberg School of Public Health CHOICES COVID-19 vaccine evidence briefs. 2022. https://www.jhsph.edu/ivac/resources/choices-covid-19-briefs/
- 10.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio A, Little J, Linkins L, Abdelkader W, Bennett D, Lavis J. COVID-19 living evidence synthesis 6.7—what is the efficacy and effectiveness of available COVID-19 vaccines in general and specifically for variants of concern. McMaster Health Forum. 2021. https://www.mcmasterforum.org/networks/covid-end/resources-specific-to-canada/for-decision-makers/scan-evidence-products
- 12.Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64:1187–1197. doi: 10.1016/j.jclinepi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 14.Van den Noortgate W, López-López JA, Marín-Martínez F, Sánchez-Meca J. Three-level meta-analysis of dependent effect sizes. Behav Res Methods. 2013;45:576–594. doi: 10.3758/s13428-012-0261-6. [DOI] [PubMed] [Google Scholar]
- 15.Van den Noortgate W, López-López JA, Marín-Martínez F, Sánchez-Meca J. Meta-analysis of multiple outcomes: a multilevel approach. Behav Res Methods. 2015;47:1274–1294. doi: 10.3758/s13428-014-0527-2. [DOI] [PubMed] [Google Scholar]
- 16.Van Den Noortgate W, Onghena P. Multilevel meta-analysis: a comparison with traditional meta-analytical procedures. Educ Psychol Meas. 2003;63:765–790. [Google Scholar]
- 17.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Chapman and Hall; New York, NY: 2021. Doing meta-analysis with R: a hands-on guide. [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:539–558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 20.Lee LYW, Starkey T, Ionescu MC, et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 2022;23:748–757. doi: 10.1016/S1470-2045(22)00202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menni C, May A, Polidori L, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022;22:1002–1010. doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paranthaman K, Subbarao S, Andrews N, et al. Effectiveness of BNT162b2 and ChAdOx-1 vaccines in residents of long-term care facilities in England using a time-varying proportional hazards model. Age Ageing. 2022;51 doi: 10.1093/ageing/afac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stirrup O, Shrotri M, Adams NL, et al. Clinical effectiveness of SARS-CoV-2 booster vaccine against omicron infection in residents and staff of long-term care facilities: a prospective cohort study (VIVALDI) medRxiv. 2022 doi: 10.1101/2022.08.08.22278532v1. published online Aug 9. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young-Xu Y, Zwain GM, Powell EI, Smith J. Estimated effectiveness of COVID-19 messenger RNA vaccination against SARS-CoV-2 infection among older male veterans health administration enrollees, January to September 2021. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.38975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andeweg SP, de Gier B, Eggink D, et al. Protection of COVID-19 vaccination and previous infection against omicron BA.1, BA.2 and delta SARS-CoV-2 infections. Nat Commun. 2022;13 doi: 10.1038/s41467-022-31838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrejko KL, Pry J, Myers JF, et al. Waning of two-dose BNT162b2 and mRNA-1273 vaccine effectiveness against symptomatic SARS-CoV-2 infection is robust to depletion-of-susceptibles bias. medRxiv. 2022 doi: 10.1101/2022.06.03.22275958. published online June 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baum U, Poukka E, Leino T, Kilpi T, Nohynek H, Palmu AA. High vaccine effectiveness against severe COVID-19 in the elderly in Finland before and after the emergence of Omicron. medRxiv. 2022 doi: 10.1101/2022.03.11.22272140v1. published online March 13. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedston S, Akbari A, Jarvis CI, et al. COVID-19 vaccine uptake, effectiveness, and waning in 82,959 health care workers: a national prospective cohort study in Wales. Vaccine. 2022;40:1180–1189. doi: 10.1016/j.vaccine.2021.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berec L, Šmíd M, Přibylová L, et al. Protection provided by vaccination, booster doses and previous infection against covid-19 infection, hospitalisation or death over time in Czechia. PLoS One. 2022;17 doi: 10.1371/journal.pone.0270801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britton A, Fleming-Dutra KE, Shang N, et al. Association of COVID-19 vaccination with symptomatic SARS-CoV-2 infection by time since vaccination and delta variant predominance. JAMA. 2022;327:1032–1041. doi: 10.1001/jama.2022.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruxvoort KJ, Sy LS, Qian L, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Effectiveness of COVID-19 vaccines against omicron or delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carazo S, Skowronski DM, Brisson M, et al. Protection against omicron re-infection conferred by prior heterologous SARS-CoV-2 infection, with and without mRNA vaccination. medRxiv. 2022 doi: 10.1101/2022.04.29.22274455. published online May 3. (preprint). [DOI] [Google Scholar]
- 36.Carazo S, Skowronski DM, Brisson M, et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. Lancet Infect Dis. 2022;23:45–55. doi: 10.1016/S1473-3099(22)00578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carazo S, Skowronski DM, Brisson M, et al. Estimated protection of prior SARS-CoV-2 infection against reinfection with the omicron variant among messenger RNA-vaccinated and nonvaccinated individuals in Quebec, Canada. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castillo MS, Khaoua H, Courtejoie N. Vaccine effectiveness and duration of protection against symptomatic and severe Covid-19 during the first year of vaccination in France. medRxiv. 2022 doi: 10.1101/2022.02.17.22270791. published online March 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerqueira-Silva T, Andrews JR, Boaventura VS, et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case-control study. Lancet Infect Dis. 2022;22:791–801. doi: 10.1016/S1473-3099(22)00140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerqueira-Silva T, de Araújo Oliveira V, Paixão ES, et al. Protection conferred by vaccine plus previous infection (hybrid immunity) with vaccines of three different platforms during the Omicron variant period in Brazil. medRxiv. 2022 doi: 10.1101/2022.04.12.22273752. published online April 13. (preprint). [DOI] [Google Scholar]
- 41.Cerqueira-Silva T, Shah SA, Robertson C, et al. Waning of mRNA boosters after homologous primary series with BNT162b2 or ChadOx1 against symptomatic infection and severe COVID-19 in Brazil and Scotland: a test-negative design case-control study. SSRN. 2022 doi: 10.2139/ssrn.4082927. published online April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerqueira-Silva T, de Araujo Oliveira V, Paixão ES, et al. Duration of protection of CoronaVac plus heterologous BNT162b2 booster in the omicron period in Brazil. Nat Commun. 2022;13 doi: 10.1038/s41467-022-31839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers C, Samji H, Cooper CL, et al. Coronavirus disease 2019 vaccine effectiveness among a population-based cohort of people living with HIV. AIDS. 2022;36:F17–F26. doi: 10.1097/QAD.0000000000003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of protection of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 omicron infection in Qatar. medRxiv. 2022 doi: 10.1101/2022.02.07.22270568. published online Feb 8. (preprint). [DOI] [Google Scholar]
- 46.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13 doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung H, Austin PC, Brown KA, et al. Effectiveness of COVID-19 vaccines over time prior to omicron emergence in Ontario, Canada: test-negative design study. Open Forum Infect Dis. 2022;9 doi: 10.1093/ofid/ofac449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collie S, Nayager J, Bamford L, Bekker L-G, Zylstra M, Gray G. Effectiveness and durability of the BNT162b2 vaccine against omicron sublineages in South Africa. N Engl J Med. 2022;387:1332–1333. doi: 10.1056/NEJMc2210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Consonni D, Lombardi A, Mangioni D, et al. Immunogenicity and effectiveness of BNT162b2 COVID-19 vaccine in a cohort of healthcare workers in Milan (Lombardy Region, Northern Italy) Epidemiol Prev. 2022;46:250–258. doi: 10.19191/EP22.4.A513.065. [DOI] [PubMed] [Google Scholar]
- 50.de Gier B, Kooijman M, Kemmeren J, et al. COVID-19 vaccine effectiveness against hospitalizations and ICU admissions in the Netherlands, April–August 2021. medRxiv. 2021 doi: 10.1101/2021.09.15.21263613. published online Sept 17. (preprint). [DOI] [Google Scholar]
- 51.El Adam S, Zou M, Kim S, Henry B, Krajden M, Skowronski DM. SARS-CoV-2 mRNA vaccine effectiveness in health care workers by dosing interval and time since vaccination: test-negative design, British Columbia, Canada. Open Forum Infect Dis. 2022;9 doi: 10.1093/ofid/ofac178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferdinands JM, Rao S, Dixon BE, et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022;379 doi: 10.1136/bmj-2022-072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Florea A, Sy LS, Luo Y, et al. Durability of mRNA-1273 against COVID-19 in the time of Delta: interim results from an observational cohort study. PLoS One. 2022;17 doi: 10.1371/journal.pone.0267824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glatman-Freedman A, Bromberg M, Hershkovitz Y, et al. Effectiveness of BNT162b2 vaccine booster against SARS-CoV-2 infection and breakthrough complications, Israel. Emerg Infect Dis. 2022;28:948–956. doi: 10.3201/eid2805.220141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gram MA, Emborg H-D, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: a nationwide Danish cohort study. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gray G, Collie S, Goga A, et al. Effectiveness of Ad26.COV2.S and BNT162b2 vaccines against omicron variant in South Africa. N Engl J med. 2022;386:2243–2245. doi: 10.1056/NEJMc2202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen C, Schelde A, Moustsen-Helm I, et al. Vaccine effectiveness against infection and COVID-19-associated hospitalisation with the omicron (B.1.1.529) variant after vaccination with the BNT162b2 or mRNA-1273 vaccine: a nationwide Danish cohort study. medRxiv. 2021 doi: 10.1101/2021.12.20.21267966. published online Dec 23. (preprint). [DOI] [Google Scholar]
- 60.Horne EMF, Hulme WJ, Keogh RH, et al. Waning effectiveness of BNT162b2 and ChAdOx1 covid-19 vaccines over six months since second dose: OpenSAFELY cohort study using linked electronic health records. BMJ. 2022;378 doi: 10.1136/bmj-2022-071249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katikireddi SV, Cerqueira-Silva T, Vasileiou E, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399:25–35. doi: 10.1016/S0140-6736(21)02754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirsebom FCM, Andrews N, Stowe J, et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect Dis. 2022;22:931–933. doi: 10.1016/S1473-3099(22)00309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirsebom F, Andrews N, Sachdeva R, Stowe J, Ramsay M, Bernal JL. Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the omicron and delta variants in England. Nat Commun. 2022;13 doi: 10.1038/s41467-022-35168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kissling E, Hooiveld M, Martínez-Baz I, et al. Effectiveness of complete primary vaccination against COVID-19 at primary care and community level during predominant delta circulation in Europe: multicentre analysis, I-MOVE-COVID-19 and ECDC networks, July to August 2021. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.21.2101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin D-Y, Gu Y, Wheeler B, et al. Effectiveness of COVID-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386:933–941. doi: 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin D-Y, Gu Y, Xu Y, et al. Association of primary and booster vaccination and prior infection with SARS-CoV-2 infection and severe COVID-19 outcomes. JAMA. 2022;328:1415–1426. doi: 10.1001/jama.2022.17876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lind ML, Robertson A, Silva J, et al. Effectiveness of primary and booster COVID-19 mRNA vaccination against infection caused by the SARS-CoV-2 omicron variant in people with a prior SARS-CoV-2 infection. 2022. medRxiv. 2022 doi: 10.1101/2022.04.19.22274056v3. published online April 25. (preprint). [DOI] [Google Scholar]
- 69.Lind ML, Copin R, McCarthy S, et al. Use of whole genome sequencing to estimate the contribution of immune evasion and waning immunity to decreasing COVID-19 vaccine effectiveness during alpha and delta variant waves. medRxiv. 2022 doi: 10.1101/2022.08.25.22278443. published online Aug 26. (preprint). [DOI] [PubMed] [Google Scholar]
- 70.Lyngse FP, Mølbak K, Denwood M, et al. Effect of vaccination on household transmission of SARS-CoV-2 delta variant of concern. Nat Commun. 2022;13 doi: 10.1038/s41467-022-31494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lytras T, Kontopidou F, Lambrou A, Tsiodras S. Comparative effectiveness and durability of COVID-19 vaccination against death and severe disease in an ongoing nationwide mass vaccination campaign. J Med Virol. 2022;94:5044–5050. doi: 10.1002/jmv.27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Machado A, Kislaya I, Rodrigues AP, et al. COVID-19 vaccine effectiveness against laboratory confirmed symptomatic SARS-CoV-2 infection, COVID-19 related hospitalizations and deaths, among individuals aged 65 years or more in Portugal: a cohort study based on data-linkage of national registries February–September 2021. medRxiv. 2021 doi: 10.1101/2021.12.10.21267619v1. published online Dec 14. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nielsen KF, Moustsen-Helms IR, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 reinfection during periods of alpha, delta, or omicron dominance: a Danish nationwide study. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ng OT, Koh V, Chiew CJ, et al. Impact of SARS-CoV-2 Vaccination and paediatric age on delta variant household transmission. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac219. published online March 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet. 2022;399:814–823. doi: 10.1016/S0140-6736(22)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petráš M, Lesná IK, Večeřová L, et al. The Effectiveness of post-vaccination and post-infection protection in the hospital staff of three prague hospitals: a cohort study of 8-month follow-up from the start of the COVID-19 vaccination campaign (COVANESS) Vaccines (Basel) 2021;10:9. doi: 10.3390/vaccines10010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poukka E, Baum U, Palmu AA, et al. Cohort study of COVID-19 vaccine effectiveness among healthcare workers in Finland, December 2020–October 2021. Vaccine. 2022;40:701–705. doi: 10.1016/j.vaccine.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richterman A, Behrman A, Brennan PJ, O'DONNELL JA, Snider CK, Chaiyachati KH. Durability of SARS-CoV-2 mRNA booster vaccine protection against omicron among health care workers with a vaccine mandate. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac454. published online June 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robles-Fontán MM, Nieves EG, Cardona-Gerena I, Irizarry RA. Effectiveness estimates of three COVID-19 vaccines based on observational data from Puerto Rico. Lancet Reg Health Am. 2022;9 doi: 10.1016/j.lana.2022.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg ES, Dorabawila V, Easton D, et al. COVID-19 vaccine effectiveness in New York State. N Engl J Med. 2022;386:116–127. doi: 10.1056/NEJMoa2116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skowronski DM, Febriani Y, Ouakki M, et al. Two-dose severe acute respiratory syndrome coronavirus 2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from British Columbia and Quebec, Canada. Clin Infect Dis. 2022;75:1980–1992. doi: 10.1093/cid/ciac290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sobieszczyk ME, Maaske J, Falsey AR, et al. Durability of protection and immunogenicity of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine over 6 months. J Clin Invest. 2022;132 doi: 10.1172/JCI160565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Starrfelt J, Danielsen AS, Buanes EA, et al. Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, July-November 2021. BMC Med. 2022;20:278. doi: 10.1186/s12916-022-02480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stowe J, Andrews N, Kirsebom F, Ramsay M, Bernal JL. Effectiveness of COVID-19 vaccines against omicron and delta hospitalisation: test negative case-control study. Nat Commun. 2022;13 doi: 10.1038/s41467-022-33378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suphanchaimat R, Nittayasoot N, Jiraphongsa C, et al. Real-world effectiveness of mix-and-match vaccine regimens against SARS-CoV-2 delta variant in Thailand: a nationwide test-negative matched case-control study. Vaccines. 2022;10 doi: 10.3390/vaccines10071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Syed MA. A/Qotba HA, Alnuaimi AS. Effectiveness of COVID-19 vaccines. J Infect. 2022;84:e118–e119. doi: 10.1016/j.jinf.2022.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am. 2022;9 doi: 10.1016/j.lana.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 Months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of COVID-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385:1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tseng HF, Ackerson BK, Bruxvoort KJ, et al. Effectiveness of mRNA-1273 against infection and COVID-19 hospitalization with SARS-CoV-2 omicron subvariants: BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. medRxiv. 2022 doi: 10.1101/2022.09.30.22280573. published online Dec 2. (preprint). [DOI] [Google Scholar]
- 92.Brunelli SM, Sibbel S, Karpinski S, et al. Comparative Effectiveness of mRNA-based BNT162b2 vaccine versus adenovirus vector-based Ad26.COV2.S vaccine for the prevention of COVID-19 among dialysis patients. J Am Soc Nephrol. 2022;33:688–697. doi: 10.1681/ASN.2021101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Honein MA, Christie A, Rose DA, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1860–1867. doi: 10.15585/mmwr.mm6949e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta M, Gupta K, Gupta S. The use of facemasks by the general population to prevent transmission of Covid 19 infection: a systematic review. medRxiv. 2020 doi: 10.1101/2020.05.01.20087064. published online May 6. (preprint). [DOI] [Google Scholar]
- 95.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Girum T, Lentiro K, Geremew M, Migora B, Shewamare S. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Trop Med Health. 2020;48:91. doi: 10.1186/s41182-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uwamino Y, Kurafuji T, Takato K, et al. Dynamics of antibody titers and cellular immunity among Japanese healthcare workers during the 6 months after receiving two doses of BNT162b2 mRNA vaccine. Vaccine. 2022;40:4538–4543. doi: 10.1016/j.vaccine.2022.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coppeta L, Ferrari C, Somma G, et al. Reduced titers of circulating anti-SARS-CoV-2 antibodies and risk of COVID-19 infection in healthcare workers during the nine months after immunization with the BNT162b2 mRNA vaccine. Vaccines. 2022;10:141. doi: 10.3390/vaccines10020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xin Q, Wu Q, Chen X, et al. Six-month follow-up of a booster dose of CoronaVac in two single-centre phase 2 clinical trials. Nat Commun. 2022;13 doi: 10.1038/s41467-022-30864-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khoury J, Najjar-Debbiny R, Elemy A, et al. Immunity waning after COVID vaccine booster vs. infection-better than expected. Infect Dis. 2022;54:828–831. doi: 10.1080/23744235.2022.2097304. [DOI] [PubMed] [Google Scholar]
- 101.Angel Y, Spitzer A, Henig O, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325:2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.WHO Interim statement on hybrid immunity and increasing population seroprevalence rates. World Health Organization. https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates
- 103.Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29:1075–1090. doi: 10.1007/s10787-021-00839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Extracted data are available online at https://osf.io/7uwjq/.