Abstract
Background: Patients with coronavirus disease 2019 (COVID-19) undergoing maintenance hemodialysis have a poor prognosis and limited treatment options. Methods: This paper outlines the impact of COVID-19, its treatment, and the efficacy of vaccines in Japanese patients undergoing hemodialysis with a review of the literature. Results: Patients undergoing dialysis in dialysis facilities are at greater risk of exposure to severe acute respiratory syndrome coronavirus 2 than the general population due to limited isolation capabilities. Therefore, vaccines are expected to be effective for patients undergoing dialysis. In addition, effective use of available medications is important because treatment options are limited. Conclusions: Efforts should be made to prevent the spread of the infection to high-risk patients undergoing dialysis while ensuring the effective use of vaccines.
Keywords: COVID-19, hemodialysis, vaccination
1. Introduction
In December 2019, an outbreak of unknown viral pneumonia was reported among patients in Wuhan, Hubei Province, People’s Republic of China. Over a short period, infection by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread worldwide. SARS-CoV-2 has been identified as an animal-derived coronavirus and is the same pathogen responsible for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). On 11 March 2020, the World Health Organization (WHO) declared a pandemic status, and SARS-CoV-2 was linked to a severe acute respiratory condition. SARS-CoV-2 has been reported to be stable on environmental surfaces for approximately 3 days. Therefore, preventing infection among staff is crucial in medical institutions.
Initial coronavirus disease 2019 (COVID-19) symptoms are similar to those of influenza, including fever, cough, malaise, and dyspnea. The median hospitalization time was 7 days. Diarrhea and taste and smell disorders may occur; however, they are not inevitable. Data from the COVID-19 Registry Japan (COVIREGI-JP), which is a Japanese registry of patients with COVID-19, showed that 60% of hospitalized patients did not require oxygen administration, whereas 30% required oxygen administration, 9% required ventilation or extracorporeal membrane oxygenation (ECMO), and 7.5% died [1]. Therefore, predicting which patients will become critically ill is important for using limited medical resources [2].
In severe cases, COVID-19 causes respiratory tract infection symptoms, such as acute respiratory distress syndrome and cytokine release syndrome (CRS)-like symptoms because of excessive inflammation. Endothelial cell damage and disruption of the immunomodulatory system lead to multiple organs failure. During the COVID-19 pandemic, the number of dialysis cases in the hospital was also reported to have increased greatly, partly due to the involvement of acute kidney injury [3]. Therefore, in addition to antiviral drugs, various therapies have been investigated to suppress excess cytokines, such as steroids, neutralizing antibody therapy, and some blood purification therapies [4,5]. However, patients undergoing dialysis are prone to severe disease, and their treatment options are limited because of renal dysfunction. This manuscript outlines the current impact of COVID-19 and its treatment in Japanese patients undergoing dialysis.
2. Number and Severity of Patients with COVID-19 Undergoing Dialysis in Japan
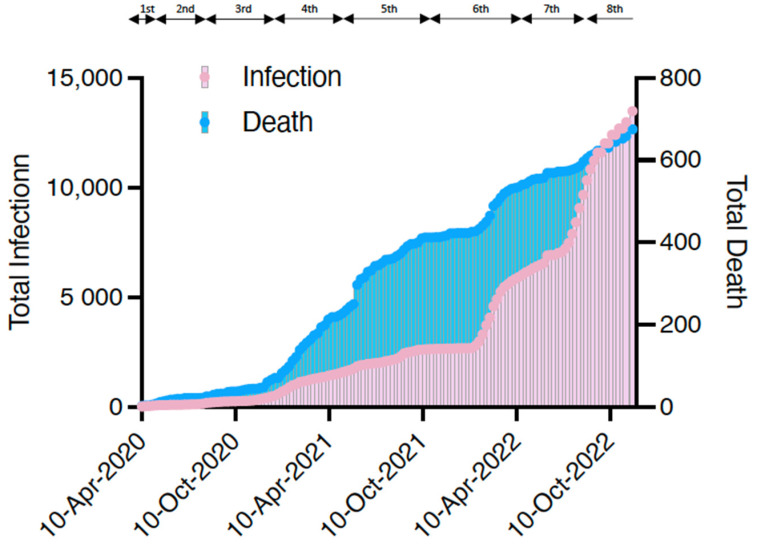
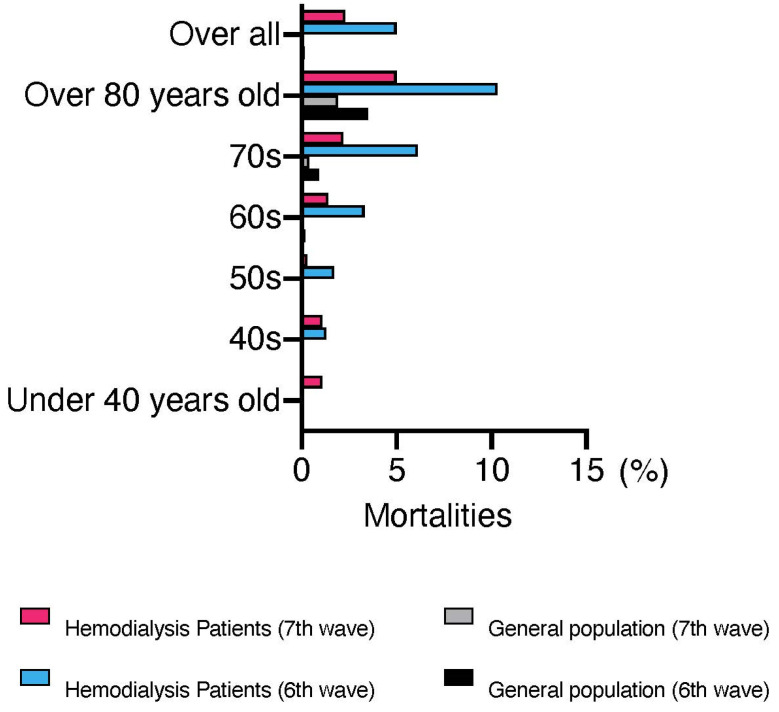
The first case of COVID-19 in a patient undergoing dialysis was reported in Japan on 1 March 2020 [6]. The Japanese Association of Dialysis Physicians, the Japanese Society for Dialysis Therapy, and the Japanese Society of Nephrology established the Joint Committee on Countermeasures against SARS-CoV-2 infection in Dialysis Patients to monitor the infection status of patients undergoing dialysis in Japan. As shown in Figure 1, the number of infected patients on dialysis continues to increase in Japan. Although the number of deaths appears to have decreased compared with the past, partly due to the spread of vaccines, continued attention should be given in the future. As of November 2022, the total number of infected patients was 12,978, and the infection rate was 3.8% of the total number of patients on maintenance dialysis in Japan (approximately 340,000). Overall, 658 confirmed deaths have been recorded due to COVID-19 among patients undergoing dialysis, with a mortality rate of 5.1% higher than that in the general population (0.2%). Figure 2 shows that even after the virus mutated to Omicron, the mortality rate among patients undergoing dialysis remained higher than that of the general population, particularly among those aged <60 years.
Figure 1.
The total number of infected patients undergoing dialysis in Japan and the number of deaths. The periods of the first to eighth waves (the 8th wave is ongoing) are also shown. Data were taken from the website of the Japanese Association of Dialysis Physicians (http://www.touseki-ikai.or.jp/, accessed on 7 December 2022) and plotted.
Figure 2.
Mortalities in the general population and patients undergoing dialysis after the sixth wave. This figure was drawn from the 9 November 2022 report of the Advisory Board for New Coronavirus Infections (https://www.mhlw.go.jp/content/10900000/001010896.pdf, accessed on 7 December 2022) and the “Report on COVID-19 Infection Cases at Dialysis Facilities” jointly published by the Japanese Association of Dialysis Physicians, the Japanese Society for Dialysis Therapy, and the Japanese Society of Nephrology. This figure is accurate as of 7 December 2022.
3. Efficacy of COVID-19 Vaccination in Patients with End-Stage Renal Disease
During the first to fourth waves, vaccines had not yet been developed and disseminated in Japan; however, they became widespread during the fifth wave. The weakening of the virus may have played a role in the significant decrease in severe cases and deaths among patients undergoing dialysis. However, these patients remain at high risk compared with the general population, as shown above.
Several studies have analyzed the efficacy and safety of the COVID-19 vaccine among patients undergoing hemodialysis [7,8]. A study of 148 and 20 patients undergoing hemodialysis and peritoneal dialysis, respectively, reported similar efficacy of COVID-19 mRNA vaccination [9]. Although caution must be exercised during interpretation due to the heterogeneous study design, in most studies, humoral responses were lower than that in the control group. In contrast, seroconversion rates and the number of patients in whom S-protein reactive T-cell immunity was detected, were very high [10]. On 15 October 2021, the American Society of Nephrology released a statement on the need for vaccines for patients undergoing dialysis [11]. The report emphasizes the importance of vaccination in patients with end-stage renal disease (ESRD) to reduce the increased risk of complications and death secondary to COVID-19 infection. In addition, patients with end-stage kidney disease and kidney transplantation have a reduced antibody response to the COVID-19 vaccine; however, antibody production has been shown to increase with the third and fourth doses [12]. Multivariate logistic regression analysis was used to examine post-infection oxygen demand in patients with post-vaccinated infection and breakthrough infection in Japan [13]. The odds ratio (OR) 0.197 (95% confidence interval [CI]: 0.120–0.322), p < 0.001, showed that patients with breakthrough infection had lower oxygen demand. The prognosis of breakthrough-infected patients was also better than that of unvaccinated patients.
4. Infection Control and Hospitalization of Patients Undergoing Dialysis in Japan
Patients receiving dialysis at the center were at greater risk of exposure to SARS-CoV-2 than the general population because of their limited isolation capabilities [14]. They were initially required by national policy to be hospitalized because of the high mortality rate associated with COVID-19 [6]. A report from Canada showed that the rate of hospitalization, 30-day mortality, and overall mortality were all significantly lower in patients receiving home dialysis, including patients undergoing peritoneal dialysis, than in those undergoing outpatient hemodialysis [15]. However, an analysis by Kikuchi et al. based on Japanese registries comparing patients receiving peritoneal dialysis and hemodialysis in terms of overall survival and length of hospitalization showed no significant difference between the two groups [16]. Therefore, caution should be exercised because overly strict infection precautions increase the burden on staff, increase healthcare costs, and make compliance more challenging. Infection control in patients undergoing dialysis has traditionally been well-established in Japan, and the 5th edition of the guidelines was issued in 2020 [17]. The following is a list of infection control measures that are in place at dialysis facilities in Japan regularly:
(1) Personal protective equipment (PPE) is recommended for the medical staff in the dialysis unit.
Before performing procedures such as puncture, hemostasis, catheter access and management, and wound care, hand hygiene should be performed by washing hands with soap and running water or using a quick-drying hand sanitizer, and unused disposable gloves should be worn. In addition, wear a disposable nonpermeable gown or plastic apron, surgical mask, goggles, or face shield when performing procedures such as puncture, hemostasis, catheter access and management, and wound care.
(2) Environmental hygiene in the dialysis unit.
Linens (sheets, pillowcases, and blanket covers) should be changed for each patient. The exterior of the dialysis machine, bed rails, and over tables should be cleaned at the end of each dialysis session. Stethoscopes, thermometers, and blood pressure cuffs should be cleaned after each use. Instruments in the dialysis room should be cleaned and disinfected with either 0.05–0.1% sodium hypochlorite, potassium hydrogen peroxymonosulfate, or alcohol-based disinfectants. Forceps and trays, among others, should be disinfected with hot water (80 °C for 10 min) or thoroughly pre-cleaned with a cleaning agent before each use, immersed in 0.1% sodium hypochlorite for 30 min, and then thoroughly rinsed with water. The above infection control measures are recommended in normal times, and the use of PPE and environmental sanitation are also preventive measures against contact and droplet infection of COVID-19.
A survey of dialysis facilities in Japan [18] revealed that several infection prevention measures were implemented during the COVID-19 pandemic, including health checks of staff and patients, wearing of masks before and after hemodialysis, and disinfection of frequently contacted areas. The implementation rate of these measures was significantly improved compared with that of the pre-pandemic rate, reaching ˃90%. However, because of the high risk of infectious disease transmission in the hospital setting during a pandemic, alternative end-stage renal failure management methods may need to be considered, such as a temporary switch to peritoneal dialysis or the implementation of a home dialysis program [19].
As noted above, the Japanese government recommended that patients who tested positive be hospitalized because of the high mortality rate associated with COVID-19, particularly patients receiving maintenance dialysis and those with a definite need for regular dialysis. However, after experiencing a delta surge, a strategic shift to outpatient care for mildly ill or asymptomatic patients and increased emergency preparedness was necessary. In response to the rise in the Omicron variant, the Tokyo Metropolitan Government opened a temporary medical facility with a dialysis center in January 2022, providing more beds and access to hemodialysis [20]. The hospital ran a smooth ward operation and reduced the number of complications with new patients with positive COVID-19 test results that required treatment and could not be hospitalized.
5. Current Treatment of COVID-19 in Patients Undergoing Dialysis
As of November 2022, the antivirals approved in Japan for treating COVID-19 include remdesivir, molnupiravir, nirmatrelvir/ritonavir, and the recently approved ensitrelvir (Table 1).
Table 1.
Current COVID-19 treatment for patients undergoing dialysis.
| Type | Antiviral Drug | Immunity Suppressants/Regulators | Neutralizing Antibody | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Remdesivir | Molnupiravir | Nirmatrelvir/ Ritonavir |
Ensitrelvir | Dexamethasone | Baricitinib | Tocilizumab | Sotrovimab | Casirivimab–imdevimab |
| Severity to be administered | Mild, Moderate Severe |
Mild, Moderate | Mild, Moderate |
Mild, Moderate | Moderate, Severe |
Moderate, Severe |
Moderate, Severe |
Mild | Mild |
| Response to Omicron | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Route of administration | Intravenous | Oral | Oral | Oral | Intravenous, oral | Oral | Intravenous | Intravenous | Intravenous |
| Length of treatment | 3–10 days | 5 days | 5 days | 5 days | 10 days | 14 days | Single dose | Single dose | Single dose |
| Dosage in dialysis patients | 100 mg 4 h before dialysis initiation. Approximately 6 doses |
No adjustment required | No administration to dialysis patients | No clinical trials have been conducted in patients with renal dysfunction | No adjustment required | No administration to dialysis patients | No adjustment required | No adjustment required | No adjustment required |
Remdesivir is recommended for mild-to-moderate disease within 7 days of onset. Japanese patients with COVID-19 undergoing hemodialysis enrolled by 19 June 2020, with (N = 98) and without (N = 294) remdesivir, were studied using propensity matching [16]. Patients receiving remdesivir had a significantly better prognosis than those not receiving it. In addition, the remdesivir-treated group had a shorter hospital stay. In a retrospective study of 486 patients (407 on hemodialysis and 79 on peritoneal dialysis) in the United States, 112 (23%) received remdesivir [21]. The estimated 30-day mortality rate was 0.74 (95% confidence interval, 0.52–1.05) in the remdesivir-treated group compared with the non-treated group. These results suggest that remdesivir is an effective treatment option for patients undergoing maintenance hemodialysis.
Molnupiravir was the first oral antiviral drug approved in Japan to treat COVID-19 [22]. It does not require dosage adjustment according to renal function or volume adjustment in patients undergoing hemodialysis, making it easy to use in outpatient settings [23]. However, the disadvantage is that the capsule formulation is large and challenging to take internally. Nirmatrelvir/ritonavir is another oral antiviral drug approved in Japan [24]. As ritonavir inhibits drug metabolism in CYP3A to maintain drug blood levels, it increases the blood levels of drugs metabolized by CYP3A. Calcium channel blockers and statins are typical examples, but many other drugs, such as tranquilizers, are also affected. Dose adjustment is required in patients with moderately impaired renal function, and administration is not recommended for patients with severe renal dysfunction, including those on maintenance dialysis. Clinical trials have not been conducted on ensitrelvir in patients with renal dysfunction, and its efficacy in those undergoing hemodialysis requires further study.
Omicron strains have been classified into five strains (BA.1, BA.2, BA.3, BA.4, and BA.5). The BA.2 strain has been the primary epidemic strain; however, since July 2022, the BA.2 strain has been rapidly replaced by the BA.5 strain in many countries, including Japan. The inhibitory effects of different antibodies and antiviral drugs on Omicron strains isolated from clinical specimens are being investigated [25]. The neutralizing activity of sotrovimab and casirivimab–imdevimab [26] was significantly lower against all strains after BA.2 than the effect against the conventional stress (from Wuhan). The efficacy of tixagevimab and cilgavimab was similarly reduced. In contrast, bebtelovimab showed a high neutralizing activity against BA.2.12.1, BA.4, and BA.5 strains. Furthermore, the efficacy of the three antiviral drugs (remdesivir, molnupiravir, and nirmatrelvir) was subsequently analyzed, and they were found to effectively inhibit the growth of BA.2.12.1, BA.4, and BA.5 strains.
6. Conclusions
This paper outlines the impact and treatment of COVID-19 on patients undergoing hemodialysis, which has not yet reached a global consensus. Therefore, it is important to continue to elucidate the pathogenesis of severe disease in patients with hemodialysis, leading to expanded vaccination and the establishment of more effective treatment strategies.
Author Contributions
D.K.; writing—original draft preparation, K.K.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the National Center for Global Health and Medicine (NCGM-G-003616-00, Approval Date: 7 August 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Grants-in-Aid for Research from the National Center for Global Health and Medicine, grant number 21A-2002.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Matsunaga N., Hayakawa K., Terada M., Ohtsu H., Asai Y., Tsuzuki S., Suzuki S., Toyoda A., Suzuki K., Endo M., et al. Clinical Epidemiology of Hospitalized Patients With Coronavirus Disease 2019 (COVID-19) in Japan: Report of the COVID-19 Registry Japan. Clin. Infect. Dis. 2021;73:e3677–e3689. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katagiri D., Ishikane M., Asai Y., Kinoshita N., Ota M., Moriyama Y., Ide S., Nakamura K., Nakamoto T., Nomoto H., et al. Evaluation of Coronavirus Disease 2019 Severity Using Urine Biomarkers. Crit. Care Explor. 2020;2:e0170. doi: 10.1097/CCE.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mureșan A.V., Russu E., Arbănași E.M., Kaller R., Hosu I., Arbănași E.M., Voidăzan S.T. Negative Impact of the COVID-19 Pandemic on Kidney Disease Management-A Single-Center Experience in Romania. J. Clin. Med. 2022;11:2452. doi: 10.3390/jcm11092452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katagiri D. For safe and adequate blood purification therapy in severe COVID-19—What we have learned so far. Glob. Health Med. 2022;4:94–100. doi: 10.35772/ghm.2022.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katagiri D., Ishikane M., Asai Y., Izumi S., Takasaki J., Katsuoka H., Kondo I., Ide S., Nakamura K., Nakamoto T., et al. Direct hemoperfusion using a polymyxin B-immobilized polystyrene column for COVID-19. J. Clin. Apher. 2021;36:313–321. doi: 10.1002/jca.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuchi K., Nangaku M., Ryuzaki M., Yamakawa T., Hanafusa N., Sakai K., Kanno Y., Ando R., Shinoda T., Nakamoto H., et al. COVID-19 of dialysis patients in Japan: Current status and guidance on preventive measures. Ther. Apher. Dial. 2020;24:361–365. doi: 10.1111/1744-9987.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashby D.R., Caplin B., Corbett R.W., Asgari E., Kumar N., Sarnowski A., Hull R., Makanjuola D., Cole N., Chen J., et al. Severity of COVID-19 after Vaccination among Hemodialysis Patients: An Observational Cohort Study. Clin. J. Am. Soc. Nephrol. CJASN. 2022;17:843–850. doi: 10.2215/CJN.16621221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunelli S.M., Sibbel S., Karpinski S., Marlowe G., Walker A.G., Giullian J., Van Wyck D., Kelley T., Lazar R., Zywno M.L., et al. Comparative Effectiveness of mRNA-based BNT162b2 Vaccine versus Adenovirus Vector-Based Ad26.COV2.S Vaccine for the Prevention of COVID-19 among Dialysis Patients. J. Am. Soc. Nephrol. 2022;33:688–697. doi: 10.1681/ASN.2021101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frittoli M., Cassia M., Barassi A., Ciceri P., Galassi A., Conte F., Cozzolino M.G. Efficacy and Safety of COVID-19 Vaccine in Patients on Renal Replacement Therapy. Vaccines. 2022;10:1395. doi: 10.3390/vaccines10091395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babel N., Hugo C., Westhoff T.H. Vaccination in patients with kidney failure: Lessons from COVID-19. Nat. Rev. Nephrol. 2022;18:708–723. doi: 10.1038/s41581-022-00617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blake P.G., Hladunewich M.A., Oliver M.J. COVID-19 Vaccination Imperatives in People on Maintenance Dialysis: An International Perspective. Clin. J. Am. Soc. Nephrol. 2021;16:1746–1748. doi: 10.2215/CJN.07260521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno N.F., McAdams R., Goss J.A., Galvan N.T.N. COVID-19 Vaccine Efficacy and Immunogenicity in End-Stage Renal Disease Patients and Kidney Transplant Recipients. Curr. Transplant. Rep. 2022;9:174–184. doi: 10.1007/s40472-022-00366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi K., Nangaku M., Ryuzaki M., Yamakawa T., Yoshihiro O., Hanafusa N., Sakai K., Kanno Y., Ando R., Shinoda T., et al. Effectiveness of SARS-CoV-2 vaccines on hemodialysis patients in Japan: A nationwide cohort study. Ther. Apher. Dial. 2022;27:19–23. doi: 10.1111/1744-9987.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahalingasivam V., Su G., Iwagami M., Davids M.R., Wetmore J.B., Nitsch D. COVID-19 and kidney disease: Insights from epidemiology to inform clinical practice. Nat. Rev. Nephrol. 2022;18:485–498. doi: 10.1038/s41581-022-00570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perl J., Thomas D., Tang Y., Yeung A., Ip J., Oliver M.J., Blake P.G. COVID-19 among Adults Receiving Home versus In-Center Dialysis. Clin. J. Am. Soc. Nephrol. CJASN. 2021;16:1410–1412. doi: 10.2215/CJN.04170321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi K., Nangaku M., Ryuzaki M., Yamakawa T., Yoshihiro O., Hanafusa N., Sakai K., Kanno Y., Ando R., Shinoda T., et al. Survival and predictive factors in dialysis patients with COVID-19 in Japan: A nationwide cohort study. Ren. Replace. Ther. 2021;7:59. doi: 10.1186/s41100-021-00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Japanese Associations of Dialysis Physicians . Guidelines for Standard Hemodialysis Procedure and Prevention of Infection in Maintenance Hemodialysis Facilities. 5th ed. The Japanese Associations of Dialysis Physicians; Tokyo, Japan: 2020. [Google Scholar]
- 18.Sugawara Y., Iwagami M., Kikuchi K., Yoshida Y., Ando R., Shinoda T., Ryuzaki M., Nakamoto H., Sakai K., Hanafusa N., et al. Infection prevention measures for patients undergoing hemodialysis during the COVID-19 pandemic in Japan: A nationwide questionnaire survey. Ren. Replace. Ther. 2021;7:27. doi: 10.1186/s41100-021-00350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behlul S., Artac Ozdal M. Risk of COVID-19 and Cost Burden in End-Stage Renal Disease Patients and Policy Implications for Managing Nephrology Services during the COVID-19 Pandemic. Healthcare. 2022;10:2351. doi: 10.3390/healthcare10122351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito K., Kikuchi K., Watanabe Y., Narita T. Implementation of two novel schemes for patients on dialysis as a response to the COVID-19 surge in Tokyo. Glob. Health Med. 2022;4:253–258. doi: 10.35772/ghm.2022.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaki K.E., Huang C.W., Zhou H., Chung J., Selevan D.C., Rutkowski M.P., Sim J.J. Comparison of safety and outcomes related to remdesivir treatment among dialysis patients hospitalized with COVID-19. Clin. Kidney J. 2022;15:2056–2062. doi: 10.1093/ckj/sfac185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poznański P., Augustyniak-Bartosik H., Magiera-Żak A., Skalec K., Jakuszko K., Mazanowska O., Janczak D., Krajewska M., Kamińska D. Molnupiravir When Used Alone Seems to Be Safe and Effective as Outpatient COVID-19 Therapy for Hemodialyzed Patients and Kidney Transplant Recipients. Viruses. 2022;14:2224. doi: 10.3390/v14102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takashita E., Yamayoshi S., Simon V., van Bakel H., Sordillo E.M., Pekosz A., Fukushi S., Suzuki T., Maeda K., Halfmann P., et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022;387:468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terakawa K., Katagiri D., Shimada K., Sato L., Takano H. Safety of casirivimab/imdevimab administration in a SARS-CoV-2 positive maintenance dialysis patient in Japan. CEN Case Rep. 2022;11:328–332. doi: 10.1007/s13730-021-00671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.