Abstract
Oxidative stress (OS) results in many disorders, of which degenerative musculoskeletal conditions are no exception. However, the interaction between OS and ligamentum flavum (LF) hypertrophy in lumbar spinal canal stenosis is not clearly understood. The first research question was whether OS was involved in LF hypertrophy, and the second was whether the antioxidant N-acetylcysteine (NAC) was effective on LF hypertrophy. In total, 47 LF samples were collected from patients with lumbar spinal disorders. The cross-sectional area of LF was measured on axial magnetic resonance imaging. Immunohistochemistry of 8-OHdG and TNF-α were conducted on human LF samples. A positive association was found between 8-OHdG or TNF-α expression and cross-sectional area of LF. Flow cytometry analysis showed that H2O2, buthionine sulfoximine, and TNF-α treatment significantly increased intracellular reactive oxygen species in primary LF cells. NAC inhibited the induction of LF hypertrophy markers by OS or TNF in a real-time reverse transcriptase polymerase chain reaction and enzyme-linked immunosorbent assay. Western blotting analysis indicated that p38, Erk, and p65 phosphorylation were involved in intracellular OS signaling in LF cells. In conclusion, our results indicated that OS could be a therapeutic target for LF hypertrophy. Although this study included no in vivo studies to examine the longitudinal efficacy of NAC on LF hypertrophy, NAC may have potential as a therapeutic agent against lumbar spinal canal stenosis.
Keywords: lumbar spinal canal stenosis, ligamentum flavum hypertrophy, oxidative stress, reactive oxygen species, N-acetylcysteine
1. Introduction
Lumbar spinal canal stenosis (LSS) is a highly prevalent degenerative musculoskeletal disease, which leads to a limitation of functional activity for patients with pain in the low back and lower extremities. With the increasing proportion of the geriatric population, LSS has started to show an upward trend in incidence along with financial burden and social participation restrictions [1,2,3,4]. Presently, LSS treatment is limited to conservative treatment to control low back and lower extremity pain or surgical treatment for enlargement of the lumbar spinal canal and fixation of spinal instability [5,6,7,8]. The pathological changes associated with LSS are commonly due to ligamentum flavum (LF) hypertrophy, degenerative changes in the intervertebral disc (IVD), and facet joint osteophyte formation. These changes can potentially cause chronic cauda equina and nerve root impingement. The changes associated with LF hypertrophy are elastin fiber proportion reduction and an increase in collagen fibers, particularly type I and type III collagen, which increase proportionately with LF hypertrophy [9,10,11]. Meanwhile, a recent histological study showed that the total cell number was significantly higher in the hypertrophied LFs than in the non-hypertrophied LFs [12]. Therefore, the pathology of LF hypertrophy includes both hyperplasia and an increase in extracellular matrix.
Regarding the LF hypertrophy mechanisms, mechanical loading, macrophage infiltration, and chronic inflammation were documented to be involved [13,14,15,16,17,18]. In LF hypertrophy, there is a particular increase in proinflammatory cytokines like tumor necrosis factor (TNF)-α and interleukin (IL) 1-β, which can disturb the normal collagen and elastin fiber proportion [15,16]. However, the molecular aspect is not clearly understood and explored even after a good research volume on LF hypertrophy [13,14,15,16,17,18].
As per the free-radical theory, which defines the aging changes, oxidative stress (OS) caused by reactive oxygen species (ROS) causes a functional decline in the aging population [19]. OS causes many disorders, among which are degenerative musculoskeletal conditions like osteoarthritis and osteoporosis [20,21,22]. Several studies have reported that IVD degeneration, one of the etiological factors of LSS, is also related to OS [23,24,25]. We also previously reported that OS was involved in IVD degeneration progression and that N-acetylcysteine (NAC), one of the antioxidants, may inhibit IVD degeneration progression [26]. NAC is a vital drug for treating acetaminophen overdose and a widely used dietary supplement. Meanwhile, a limited number of papers have analyzed the interaction between OS and LF hypertrophy [27,28]. Thus, in this study, the first research question was whether OS was involved in LF hypertrophy, and the second was whether NAC is effective in LF hypertrophy.
2. Materials and Methods
2.1. Human LF Samples
This study was performed with 47 LF tissue samples obtained from participants who were surgically treated for lumbar spinal disorders after obtaining informed consent to utilize them for our research. The ethics committee of our institution approved this study. A portion of the samples have been used in previous studies [29,30]. Table 1 presents the participants’ baseline characteristics.
Table 1.
Baseline characteristics of human surgical samples.
| Samples | n = 47 | |
|---|---|---|
| Patients | n = 39 | |
| Gender | Male | 18 |
| Female | 21 | |
| Age (years) | ≥80 | 3 |
| 60–80 | 20 | |
| 40–60 | 10 | |
| <40 | 6 | |
| Level | L2/3 | 2 |
| L3/4 | 9 | |
| L4/5 | 24 | |
| L5/S | 12 | |
| Diagnosis | LSS | 32 |
| LDH | 15 | |
LSS, lumbar spinal canal stenosis; LDH, lumbar disc herniation.
Magnetic Resonance Imaging
According to previous studies [29], preoperative lumbar magnetic resonance imaging (MRI) was used to measure the LF’s cross-sectional area (CSA) at the level from which LF samples were obtained during surgery (Figure 1a).
Figure 1.
Correlation between 8-OHdG or TNF-α expression and ligamentum flavum (LF) hypertrophy. (A) Measurement of the cross-sectional area (CSA) of LF under axial MRI. (B,C) Representative immunohistochemistry data for TNF-α (A) and 8-OHdG (B) in human LF samples. Scale bars, 50 μm. (D,E) Correlation between the positive cell number of TNF-α (C) or 8-OHdG (D) and the LF cross-sectional area on axial lumbar magnetic resonance imaging. (F) Correlation of the positive cell number of TNF-α and 8-OHdG.
2.2. Immunostaining
Antibodies for TNF-α (diluted 200-fold; Novus Biologicals, Centennial, CO, USA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) (diluted 200-fold; Japan Institute for the control of Aging, Shizuoka, Japan) were used for immunohistochemistry. A peroxidase-labeled anti-rabbit antibody (Histofine Simplestain Max PO, Nichirei, Tokyo, Japan) was followed as the secondary antibody.
2.3. Human LF Cells
Human LF cells were isolated, as reported earlier [30]. The cells were put into 6-well plates before treatment with reagents at a density of 1 × 105 cells/well and 10 cm dishes at a density of 5 × 105 cells/dish for real-time reverse transcriptase polymerase chain reaction (RT-PCR) and Western blotting (WB), respectively. In the in vitro experiments, the original samples were not used, and LF cells were extracted from the surgical LF samples of a 68-year-old woman diagnosed with lumbar disc herniation (LDH) at L 4/5 level. The isolated LF cells were used in five passages for in vitro analyses.
2.4. Measurement of Intracellular ROS
The dissociated cells were loaded with Mitotracker Orange CMH2TM ROS (Thermo Fisher Scientific, Waltham, MA, USA) and incubated on a shaker at 37 °C for 30 min. FACSAria was used (Becton, Dickinson, and Company, Franklin Lakes, NJ, USA) for intracellular ROS measurement.
2.5. Treatment
Primary LF cells were treated with H2O2 (100 μM; Wako, Tokyo, Japan), buthionine sulfoximine (BSO) (1 mM; Sigma-Aldrich, St. Louis, MO, USA), which is a glutathione synthesis suppressor that initiates OS, recombinant human TNF-α (50 ng/mL; Thermo Fisher Scientific, Waltham, MA, USA) and NAC (100 uM; Sigma-Aldrich) for 24 h. The LF cells were cultured with TNF α (50 ng/mL) and BSO (1 mM) for a duration of 30 min in association with NAC pretreatment (100 uM, 10 min) to assess the phosphorylation capacity of intracellular signaling. These experiments were conducted independently in triplicate.
2.6. Real-Time RT-PCR
Real-time RT-PCR was conducted according to the previous report [30]. Type I and type III collagen, TNF-α, and β-actin primers were used and the results were quantified using the ddCt.
2.7. WB Analysis
The following antibodies were utilized: nuclear factor (NF)-κB p65, phosphorylated p65, extracellular signal-regulated kinases (Erk), phosphorylated Erk, c Jun amino-terminal kinase (JNK), phosphorylated JNK, p38, and phosphorylated p38. All these antibodies were bought from Cell Signaling Technology (Danvers, MA, USA), which were diluted 1000-fold.
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
Human Pro-Collagen I alpha 1 ELISA Kit (Bio-Techne, Minneapolis, MN, USA) was used for quantitative type I collagen measurement in cell culture supernatants.
2.9. Statistical Analysis
The mean ± standard deviation was the central tendency, and dispersion was preferred to specify the data. A parametric independent t-test, nonparametric Mann–Whitney U test, parametric analysis of variance, or nonparametric Kruskal–Wallis were used for the corresponding data type. A significance level of p < 0.05 was used for the study. Pearson’s correlation coefficient was used to analyze the relationship between the CSA of LF on MRI and the total positive cell number of TNF-α or 8-OHdG in 100 fields.
3. Results
3.1. Expression of TNF-α and 8-OHdG in LF Tissues
To evaluate the chronic inflammation and OS level in hypertrophied LF, TNF-α, and 8-OHdG immunohistochemistry was conducted in LF samples, respectively. Subsequently, the positive TNF-α or 8-OHdG cells in 100 fields of each section were counted. Figure 1b,c shows high protein expression of TNF-α and 8-OHdG in hypertrophied LF, respectively. Because even patients who were diagnosed with LDH may have LF hypertrophy clinically, we did not compare LF samples of LDH and LSS but rather we put these samples together and analyzed the correlation between their values in the present study. The correlation between the positive cell numbers of TNF-α and 8-OHdG with LF CSA out of the 47 samples was examined (Figure 1d,e). Both TNF-α and 8-OHdG showed a significant but weak correlation with the CSA of LF (TNF-α; r = 0.29, p = 0.046; 8-OHdG; r = 0.33, p = 0.024). Furthermore, there was a moderate correlation between TNF-α and 8-OHdG positive cell numbers (r = 0.48, p < 0.01) (Figure 1f).
3.2. Intracellular ROS Level in LF Cells
The intracellular ROS level of LF cells cultured with H2O2, BSO, and TNF-α were analyzed using flow cytometry. Figure 2a–c shows representative data for the analysis of intracellular ROS. Figure 2d shows that H2O2, BSO, and TNF-α treatment significantly increased intracellular ROS levels in LF cells compared with the control.
Figure 2.
Flow cytometry analysis for the intracellular level of reactive oxygen species (ROS) in ligamentum flavum (LF) cells. (A–C) The horizontal axis represents the fluorescence intensity of the ROS probe per cell, and the vertical axis represents the relative cell number at each fluorescence intensity. Histograms filled in gray represent fluorescence data for untreated control cells, while solid black lines represent H2O2-treated (A), buthionine sulfoximine (BSO)-treated (B), and TNF-α-treated cells (C). (D) Treatment of H2O2, BSO, and TNF-α significantly increased intracellular ROS levels in the cells. * <0.05.
3.3. Interaction between OS and Expression of LF Hypertrophy Markers in LF Cells
To find the influence of OS on LF hypertrophy in vitro, type I and type III collagen and TNF-α mRNA expression were probed, which were greatly induced in LF hypertrophy, following BSO treatment of primary LF cells. For the suppressing experiment, NAC was added to BSO-treated LF cells. Figure 3a shows that BSO-mediated mRNA expression induction of type I and type III collagen and TNF-α was significantly reduced by NAC treatment in LF cells. TNF-α was administered to the LF cells with or without NAC, considering that TNF-α treatment induced the LF cells’ intracellular ROS level. Expectedly, TNF-α-mediated mRNA expression induction of type I and type III collagen was significantly reduced by NAC treatment in LF cells (Figure 3b). TNF-α-mediated induction of TNF-α mRNA expression was also reduced, albeit not significantly, by NAC treatment.
Figure 3.
Real-time RT-PCR analysis to assess oxidative stress impact on ligamentum flavum (LF) cells. (A) Buthionine sulfoximine (BSO)-mediated induction of type I and type III collagen and TNF-α mRNA expression was significantly neutralized by N-acetylcysteine (NAC) treatment in LF cells. (B) NAC treatment significantly reduced TNF-α-mediated induction of type I and type III collagen mRNA expression in LF cells. These experiments were conducted independently in triplicate. Data are presented as the mean ± standard deviation. * <0.05.
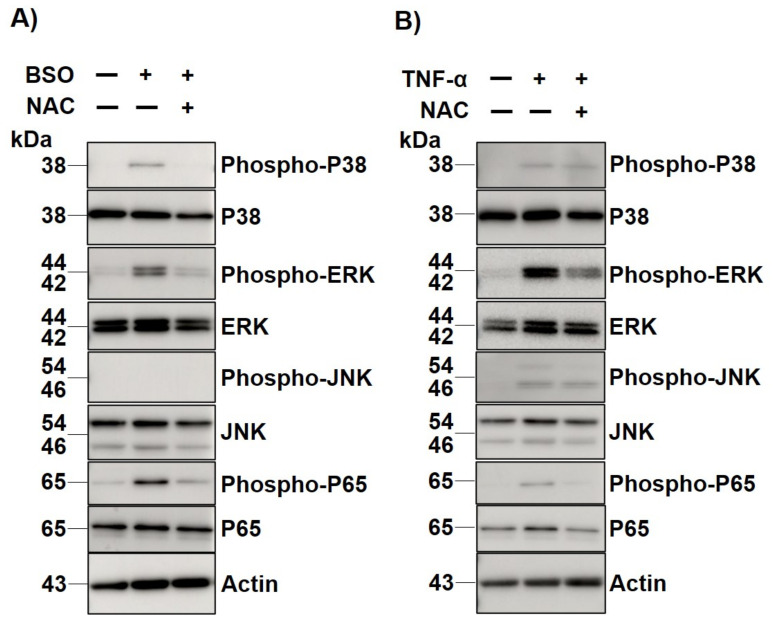
3.4. Intracellular Signaling of OS in LF Cells
The p38, Erk, JNK, and p65 phosphorylation status was evaluated in human LF cells following 30 min of BSO treatment with and without 10 min of NAC pretreatment to identify the intracellular signaling associated with the OS of LF hypertrophy. WB analysis clarified that p38, Erk 1/2, and p65 phosphorylation were enhanced by BSO treatment (Figure 4a). Apart from this, it was also observed that the phosphorylation of these molecules was suppressed when NAC was added (Figure 4a). Next, this study evaluated the phosphorylation status of TNF-α treated LF cells with/without NAC to determine whether TNF α mediated intracellular signaling is modulated by OS. WB analysis revealed that TNF-α mediated phosphorylation of Erk 1/2 and p65 was clearly neutralized by NAC treatment (Figure 4b).
Figure 4.
Western blot analysis for BSO- or TNF-α-mediated MAPKs and NF-κb p65 phosphorylation. (A) MAPKs phosphorylation, including p38, Erk 1/2, and p65, was enhanced after buthionine sulfoximine (BSO) treatment, but was suppressed by additional N-acetylcysteine (NAC) treatment. (B) TNF-α mediated Erk 1/2 and p65 phosphorylation was neutralized by NAC treatment, but not p38 and JNK.
3.5. Interaction between the OS and the Release of Type I Collagen in LF Cells
Finally, this study quantitatively measured the protein level of pro-collagen I alpha 1 in LF cell culture supernatants without or with BSO, or with BSO and NAC, to identify OS involvement in type I collagen release in human LF cells. ELISA showed that the BSO mediated induction of pro-collagen I alpha 1 was significantly reduced with NAC treatment (Figure 5).
Figure 5.
Enzyme-linked immunosorbent assay for quantitative pro-collagen I alpha 1 measurement in LF cell culture supernatants. Buthionine sulfoximine (BSO)-mediated induction of pro-collagen I alpha 1 was significantly reduced with N-acetylcysteine (NAC) treatment. These experiments were conducted independently in triplicate. The data are presented as the mean ± standard deviation. * < 0.05.
4. Discussion
In this study, a positive association was established between 8-OHdG or TNF-α expression and LF hypertrophy. The functional outcome illustrated that treatment using NAC neutralized the OS-mediated induction of the mRNA expression of LF hypertrophy markers. WB analysis proved that p38, Erk, and p65 were the key components in OS in LF cells as a predominant intracellular signal agent. NAC treatment suppressed all the signaling phosphorylation. Additionally, NAC partially neutralized the TNF-α-mediated induction of the mRNA expression of LF hypertrophy markers and phosphorylation of intracellular signaling in LF cells. Finally, NAC reduced the OS mediated release of type I collagen in LF cells.
Our analysis showed a weak but significant positive correlation, between OS and LF hypertrophy, indicating that OS was potentially involved in LF hypertrophy. In this analysis, there were some concerns about reproducibility. Firstly, the LF CSA on MRI was measured at the most severely stenosed level from which the LF sample was taken, but it may not be completely consistent with the degree of LF hypertrophy because the LF CSA is expected to depend on other factors, such as the IVD level, the subject’s physique and gender, and pathological condition. Previous studies have used LF thickness on axial MRI as an LF parameter for hypertrophy, which has a similar problem [31]. In the currently available image modality, no parameter may be found that can completely represent LF hypertrophy. Under these situations, LF CSA on MRI may be the most common LF hypertrophy parameter, as used in past studies by our group and others [29,30,32]. Second, it should be noted that the 8-OHdG protein expression level in LF was measured semi-quantitatively from the immunostaining results, and thus this data is not a completely accurate representation of the OS amount. A method of quantitatively and reproducibly evaluating OS in the proteins directly extracted from LF tissues has not yet been established. Therefore, in the future, these results will have to be verified by further increasing the number of cases after matching the IVD level, gender, physique, and pathological condition.
Considering that there was a significant positive correlation between the expression levels of 8-OHdG and TNF-α in LF tissues and that TNF-α, as well as H2O2 and BSO, increased the intracellular LF cells’ ROS level in flow cytometry analysis, TNF-α can induce OS in LF cells. In real-time RT-PCR and WB analysis, the antioxidant NAC partially neutralized the influence caused by TNF-α, supporting these hypotheses. Conversely, it has been reported that OS induces chronic inflammation in other cells [26,33], suggesting that OS and chronic inflammation may also form a positive feedback loop. Furthermore, inflammatory cytokines, such as TNF-α and IL 1β, have also interacted with OS in another spine tissue, the IVD [26], suggesting that chronic inflammation and OS may be closely related throughout the LSS based on IVD degeneration and LF hypertrophy.
NAC has been used to treat a variety of diseases and is widely used as an antioxidant supplement [34,35]. Our results, although an in vitro experiment, also suggested that NAC was effective in suppressing LF hypertrophy. Recently, Hsu et al. also reported that NAC administration regressed the fibrogenic and proinflammatory OS effects in hypertrophic LF cells [28]. In their in vitro experiments, the concentration of NAC was 10 mM, while in our study, we used NAC at 100 μM. Given the similar results in the two studies, our results are of clinical significance, showing that even a low concentration of NAC is sufficiently effective. In addition, using animal models, OS was previously reported to induce IVD degeneration, and NAC has an inhibitory effect [26]. Combining present and past results, it is believed that NAC may have potential as a preventive or therapeutic agent against LSS, which is mainly caused by IVD degeneration and LF hypertrophy (Figure 6). NAC is a drug that is both medically and economically easy to prescribe for patients because it has few side effects and is inexpensive. However, because we have a number of concerns that need to be addressed, such as the dose, duration, and timing of oral administration, further preclinical and clinical evaluations are warranted.
Figure 6.
Schematic model showing the potential of N-acetylcysteine (NAC) as a preventive or therapeutic agent for lumbar spinal canal stenosis.
The present study has several limitations. First, the number of human LF samples was limited. Second, as stated above, a technical bias was identified in the LF CSA measurements on MRI and the counting of 8-OHdG- or TNF-α-positive cells in the immunostained section. Lastly, no in vivo studies have been conducted to determine whether OS is involved in LF hypertrophy or whether NAC can suppress LF hypertrophy.
5. Conclusions
Our human sample analysis and in vitro analysis indicated that OS could be a therapeutic target for LF hypertrophy. Although the present study included no in vivo studies to examine the longitudinal efficacy of NAC on LF hypertrophy, NAC may have potential as a preventive or therapeutic agent against LSS.
Acknowledgments
We thank Kaori Tajima for technical support.
Author Contributions
Conceptualization, N.F.; data curation, K.T. and S.K. (Shinjiro Kaneko); formal analysis, K.I., H.K., S.S., S.N., K.H., H.T., S.K. (Soya Kawabata), D.I., K.T. and S.K. (Shinjiro Kaneko); funding acquisition, N.F.; investigation, K.I., H.K., S.S., S.N., K.H., H.T., S.K. (Soya Kawabata), D.I. and K.T.; methodology, K.T.; supervision, S.K. (Shinjiro Kaneko) and N.F.; writing—original draft, N.F.; writing—review and editing, K.I., H.K., S.S., S.N., K.H., H.T., D.I., K.T. and S.K. (Shinjiro Kaneko). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval for this study was granted by the Fujita Health University Ethics Committee (Approval No. HM20-055).
Informed Consent Statement
We obtained written informed consent from each patient for the experimental use of surgical samples.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to limitations of ethical approval involving the patient data and anonymity but are available from the corresponding author on reasonable request.
Conflicts of Interest
There are no conflicts of interest to declare.
Funding Statement
This work was supported by JSPS KAKENHI grant numbers 19K09634.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Szpalski M., Gunzburg R. Lumbar spinal stenosis in the elderly: An overview. Eur. Spine J. 2003;12:S170–S175. doi: 10.1007/s00586-003-0612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishimoto Y., Yoshimura N., Muraki S., Yamada H., Nagata K., Hashizume H., Takiguchi N., Minamide A., Oka H., Kawaguchi H., et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Osteoarthr. Cartil. 2012;20:1103–1108. doi: 10.1016/j.joca.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Yabuki S., Fukumori N., Takegami M., Onishi Y., Otani K., Sekiguchi M., Wakita T., Kikuchi S., Fukuhara S., Konno S. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: A population-based study. J. Orthop. Sci. 2013;18:893–900. doi: 10.1007/s00776-013-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buser Z., Ortega B., D’Oro A., Pannell W., Cohen J.R., Wang J., Golish R., Reed M., Wang J.C. Spine degenerative conditions and their treatments: National trends in the United States of America. Glob. Spine J. 2018;8:57–67. doi: 10.1177/2192568217696688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.North American Spine Society Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis. 2011. [(accessed on 27 January 2021)]. Available online: http://www.spine.org/Documents/LumbarStenosis11.pdf.
- 6.Marcolina A., Vu K., Annaswamy T.M. Lumbar spinal stenosis and potential management with prostaglandin E1 analogs. Am. J. Phys. Med. Rehabil. 2021;100:297–302. doi: 10.1097/PHM.0000000000001620. [DOI] [PubMed] [Google Scholar]
- 7.Turner J.A., Ersek M., Herron L., Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Williams M.G., Wafai A.M., Podmore M.D. Functional outcomes of laminectomy and laminotomy for the surgical management lumbar spine stenosis. J. Spine Surg. 2017;3:580–586. doi: 10.21037/jss.2017.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda T., Baba I., Fujimoto Y., Tanaka N., Sumida T., Manabe H., Hayashi Y., Ochi M. The pathology of ligamentum flavum in degenerative lumbar disease. Spine. 2004;29:1689–1697. doi: 10.1097/01.BRS.0000132510.25378.8C. [DOI] [PubMed] [Google Scholar]
- 10.Kosaka H., Sairyo K., Biyani A., Leaman D., Yeasting R., Higashino K., Sakai T., Katoh S., Sano T., Goel V.K., et al. Pathomechanism of loss of elasticity and hypertrophy of lumbar ligamentum flavum in elderly patients with lumbar spinal canal stenosis. Spine. 2007;32:2805–2811. doi: 10.1097/BRS.0b013e31815b650f. [DOI] [PubMed] [Google Scholar]
- 11.Zhong Z.M., Zha D.S., Xiao W.D., Wu S.H., Wu Q., Zhang Y., Liu F.Q., Chen J.T. Hypertrophy of ligamentum flavum in lumbar spine stenosis associated with the increased expression of connective tissue growth factor. J. Orthop. Res. 2011;29:1592–1597. doi: 10.1002/jor.21431. [DOI] [PubMed] [Google Scholar]
- 12.Habibi H., Suzuki A., Hayashi K., Salimi H., Terai H., Hori Y., Tamai K., Orita K., Ohyama S., Yabu A., et al. Expression and function of FGF9 in the hypertrophied ligamentum flavum of lumbar spinal stenosis patients. Spine J. 2021;21:1010–1020. doi: 10.1016/j.spinee.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Saito T., Yokota K., Kobayakawa K., Hara M., Kubota K., Harimaya K., Kawaguchi K., Hayashida M., Matsumoto Y., Doi T., et al. Experimental mouse model of lumbar ligamentum flavum hypertrophy. PLoS ONE. 2017;12:e0169717. doi: 10.1371/journal.pone.0169717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito T., Hara M., Kumamaru H., Kobayakawa K., Yokota K., Kijima K., Yoshizaki S., Harimaya K., Matsumoto Y., Kawaguchi K., et al. Macrophage infiltration is a causative factor for ligamentum flavum hypertrophy through the activation of collagen production in fibroblasts. Am. J. Pathol. 2017;187:2831–2840. doi: 10.1016/j.ajpath.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Sairyo K., Biyani A., Goel V.K., Leaman D.W., Booth R., Jr., Thomas J., Ebraheim N.A., Cowgill I.A., Mohan S.E. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine. 2007;32:E340–E347. doi: 10.1097/01.brs.0000263407.25009.6e. [DOI] [PubMed] [Google Scholar]
- 16.Park J.O., Lee B.H., Kang Y.M., Kim T.H., Yoon J.Y., Kim H., Kwon U.H., Lee K.I., Lee H.M., Moon S.H. Inflammatory cytokines induce fibrosis and ossification of human ligamentum flavum cells. J. Spinal Disord. Tech. 2013;26:E6–E12. doi: 10.1097/BSD.0b013e3182698501. [DOI] [PubMed] [Google Scholar]
- 17.Sun C., Zhang H., Wang X., Liu X. Ligamentum flavum fibrosis and hypertrophy: Molecular pathways, cellular mechanisms, and future directions. FASEB J. 2020;34:9854–9868. doi: 10.1096/fj.202000635R. [DOI] [PubMed] [Google Scholar]
- 18.Yabu A., Suzuki A., Hayashi K., Hori Y., Terai H., Orita K., Habibi H., Salimi H., Kono H., Toyoda H., et al. Periostin increased by mechanical stress upregulates interleukin-6 expression in the ligamentum flavum. FASEB J. 2023;37:e22726. doi: 10.1096/fj.202200917RR. [DOI] [PubMed] [Google Scholar]
- 19.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 20.Basu S., Michaëlsson K., Olofsson H., Johansson S., Melhus H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001;288:275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 21.Banfi G., Iorio E.L., Corsi M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008;46:1550–1555. doi: 10.1515/CCLM.2008.302. [DOI] [PubMed] [Google Scholar]
- 22.Loeser R.F. Aging and osteoarthritis. Curr. Opin. Rheumatol. 2011;23:492–496. doi: 10.1097/BOR.0b013e3283494005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poveda L., Hottiger M., Boos N., Wuertz K. Peroxynitrite induces gene expression in intervertebral disc cells. Spine. 2009;34:1127–1133. doi: 10.1097/BRS.0b013e31819f2330. [DOI] [PubMed] [Google Scholar]
- 24.Dimozi A., Mavrogonatou E., Sklirou A., Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cell Mater. 2015;30:89–102. doi: 10.22203/eCM.v030a07. [DOI] [PubMed] [Google Scholar]
- 25.Nasto L.A., Robinson A.R., Ngo K., Clauson C.L., Dong Q., St Croix C., Sowa G., Pola E., Robbins P.D., Kang J., et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J. Orthop. Res. 2013;31:1150–1157. doi: 10.1002/jor.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki S., Fujita N., Hosogane N., Watanabe K., Ishii K., Toyama Y., Takubo K., Horiuchi K., Miyamoto T., Nakamura M., et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res. Ther. 2015;17:316. doi: 10.1186/s13075-015-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang H.C., Tsai K.L., Tsai K.J., Tu T.Y., Shyong Y.J., Jou I.M., Hsu C.C., Shih S.S., Liu Y.F., Lin C.L. Oxidative stress mediates age-related hypertrophy of ligamentum flavum by inducing inflammation, fibrosis, and apoptosis through activating Akt and MAPK pathways. Aging. 2020;12:24168–24183. doi: 10.18632/aging.104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu Y.C., Chuang H.C., Tsai K.L., Tu T.Y., Shyong Y.J., Kuo C.H., Liu Y.F., Shih S.S., Lin C.L. Administration of N-acetylcysteine to regress the fibrogenic and proinflammatory effects of oxidative stress in hypertrophic ligamentum flavum cells. Oxid. Med. Cell Longev. 2022;2022:1380353. doi: 10.1155/2022/1380353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda H., Nagai S., Ikeda D., Kaneko S., Tsuji T., Fujita N. Collagen profiling of ligamentum flavum in patients with lumbar spinal canal stenosis. J. Orthop. Sci. 2021;26:560–565. doi: 10.1016/j.jos.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Nagai S., Hachiya K., Takeda H., Ikeda D., Kawabata S., Watanabe K., Kaneko S., Fujita N. Impact of oxidized LDL/LOX-1 system on ligamentum flavum hypertrophy. J. Orthop. Sci. 2022 doi: 10.1016/j.jos.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Wang A.Y., Saini H., Tingen J.N., Sharma V., Flores A., Liu D., Olmos M., McPhail E.D., Safain M.G., Kryzanski J., et al. The relationship between wild-type transthyretin amyloid load and ligamentum flavum thickness in lumbar stenosis patients. World Neurosurg. 2022;164:e113–e118. doi: 10.1016/j.wneu.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Sakai S., Ito T., Hida K., Ito A., Harada K., Watanabe K. Clinical outcome of lumbar spinal stenosis based on new classification according to hypertrophied ligamentum flavum. J. Orthop. Sci. 2017;22:27–33. doi: 10.1016/j.jos.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Ansari M.Y., Ahmad N., Haqqi T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020;129:110452. doi: 10.1016/j.biopha.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadowska A.M., Manuel-Y-Keenoy B., De Backer W.A. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm. Pharmacol. Ther. 2007;20:9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Santus P., Corsico A., Solidoro P., Braido F., Di Marco F., Scichilone N. Oxidative stress and respiratory system: Pharmacological and clinical reappraisal of N-acetylcysteine. COPD. 2014;11:705–717. doi: 10.3109/15412555.2014.898040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to limitations of ethical approval involving the patient data and anonymity but are available from the corresponding author on reasonable request.