Abstract
Sensory impairment may impact individual stroke survivors’ motor recovery as well as their response to peripheral sensory stimulation treatment. The objective of this study was to determine the effect of sensory impairment level of individual stroke survivors on motor improvement with therapy and peripheral sensory stimulation. A secondary analysis of a pilot triple-blind randomized controlled trial was used. Twelve chronic stroke survivors were randomly assigned to the treatment group receiving peripheral sensory stimulation or the control group receiving no stimulation during 2-week hand task practice therapy. Sensory impairment level was quantified as the pre-intervention sensory threshold. Motor improvement was assessed as change in the Box and Block Test score from pre- to post-intervention. The association between sensory impairment level and motor improvement was examined using a regression analysis, accounting for groups. This study found that participants with better sensation (i.e., with lower sensory threshold) had better motor improvement than patients with worse sensation (i.e., with higher sensory threshold). Sensory impairment level did not alter the effect of peripheral sensory stimulation. These findings suggest that the level of sensory impairment may predict recovery potentials and direct rehabilitation treatment for stroke survivors.
Keywords: Stroke Rehabilitation, Upper Extremity, Neurologic Rehabilitation, Subliminal Stimulation, Sensory Function
Introduction
Stroke affects approximately 795,000 people per year in the U.S. and is a major cause of long-term disability (Virani et al., 2021). More than two thirds of stroke survivors have hand impairment (Lawrence et al., 2001), which limits their ability to perform activities of daily living (Stewart & Cramer, 2013). In addition to motor impairment, sensory impairment is a common after stroke. An estimated 50–85% of chronic stroke survivors have sensory impairment (Carey & Matyas, 2011; Kim & Choi-Kwon, 1996). Sensory impairment significantly hinders motor learning (Vidoni & Boyd, 2009) and thus, impedes motor recovery (Meyer, Karttunen, Thijs, Feys, & Verheyden, 2014; Tyson, Hanley, Chillala, Selley, & Tallis, 2008). However, sensory impairment is not typically considered in predicting rehabilitation outcomes (Bolognini, Russo, & Edwards, 2016).
Peripheral sensory stimulation can supplement manual rehabilitation therapy and enhance upper extremity motor recovery post-stroke (Conforto et al., 2018). Peripheral sensory stimulation not only activates the afferent pathway but also primes the motor cortex for greater motor improvement (Celnik, Hummel, Harris-Love, Wolk, & Cohen, 2007; Kaelin-Lang et al., 2002). However, sensory impairment may curtail the effect of peripheral sensory stimulation due to disruption in the afferent pathways. Yet, the impact of patients’ sensory impairment on the extent of hand functional recovery from therapy with peripheral sensory stimulation has not been examined. The objective of this study was to determine the effect of sensory impairment level of individual stroke survivors on motor improvement with therapy and peripheral sensory stimulation.
Methods
The study design is secondary analysis of a triple-blind randomized controlled trial (Seo, Woodbury, et al., 2019). Twelve chronic stroke survivors were randomly assigned to either the treatment or control group (n=6/group). Both groups received standardized hand task-practice therapy while wearing a stimulation device on the paretic wrist for 2 hours/session, 3 times/week for 2 weeks. The device delivered imperceptible random-frequency vibration at 60% of sensory threshold for the treatment group and no vibration for the control group. This vibration elevates cortical neuronal firing (Schranz, Vatinno, Ramakrishnan, & Seo, 2022; Seo, Lakshminarayanan, Bonilha, Lauer, & Schmit, 2015; Seo, Lakshminarayanan, et al., 2019) to improve sensorimotor function (Enders, Hur, Johnson, & Seo, 2013; Hur, Wan, & Seo, 2014; Lakshminarayanan, Lauer, Ramakrishnan, Webster, & Seo, 2015; Seo, Kosmopoulos, Enders, & Hur, 2014; Wang, Lakshminarayanan, Slota, Seo, & Webster, 2015) and recovery in stroke survivors (Seo, Woodbury, et al., 2019; Vatinno, Hall, et al., 2022). The device usability and feasibility have been demonstrated (Lakshminarayanan, Wang, Webster, & Seo, 2017; Seo et al., 2020). Full descriptions of this stimulation and task-practice therapy are in Seo, Woodbury, et al. (2019).
Participants were adult chronic stroke survivors (≥6 months post-stroke) with mild-to-moderate upper limb impairment (Fugl-Meyer Upper Extremity Assessment (FMA-UE) 30–60/66) with cognitive ability to participate in task-practice therapy. None of the participants had botulinum toxin injection in the paretic upper limb within 3 months of enrollment or concurrent other upper limb therapy. Participants had the mean age of 62 (SD = 8) years, time post-stroke of 5 (SD = 5) years, and FMA-UE score of 48 (SD = 8). Demographic characteristics, including age, time post-stroke, and FMA-UE, were not significantly different between groups. All participants in both groups completed the study. All participants provided informed consent approved by the local Institutional Review Board.
Sensory impairment was quantified as the sensory threshold. The sensory threshold was measured before intervention as the lowest vibration amplitude that the participant could feel on the wrist using the staircase method (Ehrenstein & Ehrenstein, 1999) and expressed as percent of the maximum amplitude (RMS velocity 68 mm/sec). Motor improvement was quantified as change in Box and Block Test scores from pre-intervention to on average 6 days after the last intervention day.
Regression was used to determine the relationship between sensory impairment and motor improvement. The regression analysis included group (treatment/control) and the interaction between sensory impairment and group. Statistical analysis was performed using SAS (SAS Institute Inc., Cary, NC, USA).
Results
Sensory impairment level significantly predicted motor improvement (p=0.006) (Figure 1). Specifically, participants with better sensation (with lower sensory threshold) had greater motor improvement than participants with worse sensation (with higher sensory threshold). The treatment group had a greater motor improvement than the control group (p=0.0117). The interaction between group and sensory impairment level was not significant (p=0.949), indicating that the motor improvement decreased with increasing sensory impairment similarly in the two groups.
Figure 1.
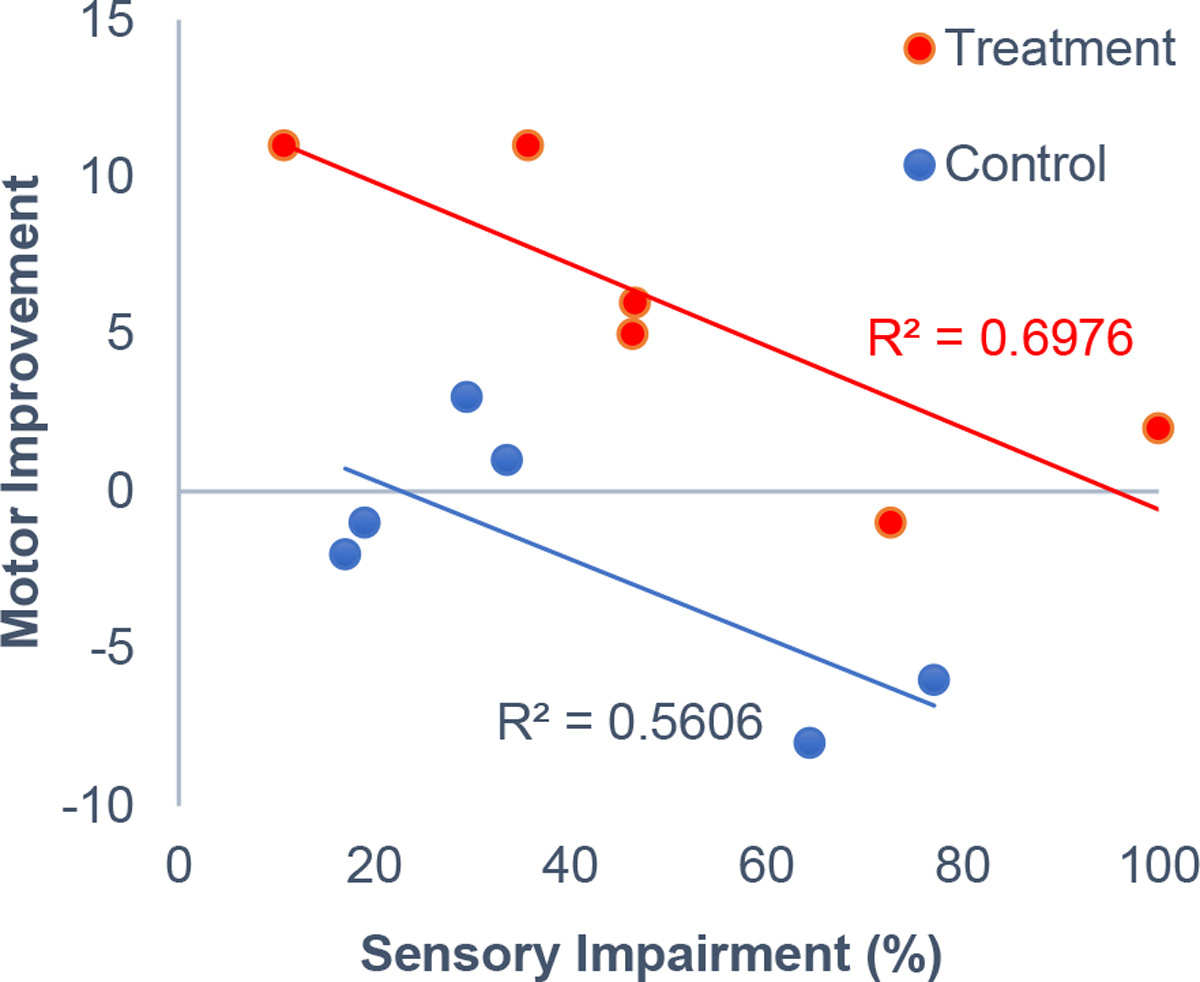
Association between sensory impairment level and motor improvement. Sensory impairment level was quantified as the pre-intervention sensory threshold. The motor improvement was quantified as change in the Box and Block Test score from pre- to post-intervention.
Discussion
Sensory impairment is rarely considered in post-stroke motor rehabilitation (Bolognini et al., 2016). However, sensation is essential to enable feedback motor control (Scott, 2004) via feedback loops from the cortical hand sensory areas to motor areas (Chen & Ashby, 1993; Matyas et al., 2010). These projections enable sensory feedback to affect motor output (Enders & Seo, 2011; Seo, Shim, Engel, & Enders, 2011). It follows that impaired sensation results in impaired motor function (Hur, Motawar, & Seo, 2014; Monzee, Lamarre, & Smith, 2003; Shim et al., 2012). Many cross-sectional studies show that stroke survivors with sensory deficits tend to have poor motor function (Blennerhassett, Matyas, & Carey, 2007; Campfens et al., 2015; Enders & Seo, 2015, 2017; Hill, Fisher, Schmid, Crabtree, & Page, 2014; Meyer et al., 2014; Tyson et al., 2008). More importantly, sensory deficits hamper motor learning (Vidoni & Boyd, 2009). Motor learning relies on the internal model to adjust motor commands appropriately per sensory feedback (Wolpert, Ghahramani, & Jordan, 1995). Sensory deficits, and thus impaired sensory feedback, likely interfere with development of an accurate internal model, thereby motor learning (Vidoni & Boyd, 2009). The significant contribution of this study is the longitudinal data suggesting that more severe sensory impairment may diminish upper extremity motor recovery in stroke survivors.
Clinically, this study suggests considering sensory impairment as prognosis for motor recovery. Improved prognosis may guide appropriate rehabilitation goals, treatment, and discharge planning. Sensory intervention (Schabrun & Hillier, 2009) may be used as a prerequisite to or in conjunction with motor intervention to promote motor recovery.
Interestingly, sensory impairment level did not alter the effect of the peripheral stimulation. All but one participant had residual sensation, as seen by the sensory threshold less than the maximum amplitude. The stimulation was adjusted to 60% of sensory threshold for individual participants. Thus, it is possible that the residual sensory pathway, together with the individually adjusted stimulation amplitude, could yield the benefit of the peripheral sensory stimulation.
The limitation of this study is that it is secondary analysis using a small sample size from the trial. Larger studies are warranted, where additional covariates of baseline motor function level, age, and time post-stroke could be examined (Seo et al., 2022). Future directions include investigation of the contributions of the sensory versus motor pathway integrity to post-stroke motor recovery and reorganization of these pathways with intervention (Vatinno, Schranz, et al., 2022).
Acknowledgements/Grants:
NIH/NICHD R01-HD094731, R01-HD094731-S2, NIH/NIGMS P20-GM109040, NIH/NCATS TL1-TR00145, NIH/NCATS UL1-TR001450, DHHS/HRSA T08-HP39300
Footnotes
Declaration of Interest: NJS is an inventor of a patent regarding the stimulation. Others have no competing interests.
Clinical Trial Registration Number: NCT02675764 registered on February 5, 2016.
References
- Blennerhassett JM, Matyas TA, & Carey LM (2007). Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabil Neural Repair, 21(3), 263–272. doi: 10.1177/1545968306295560 [DOI] [PubMed] [Google Scholar]
- Bolognini N, Russo C, & Edwards DJ (2016). The sensory side of post-stroke motor rehabilitation. Restor Neurol Neurosci, 34(4), 571–586. doi: 10.3233/RNN-150606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfens SF, Zandvliet SB, Meskers CG, Schouten AC, van Putten MJ, & van der Kooij H (2015). Poor motor function is associated with reduced sensory processing after stroke. Exp Brain Res, 233(4), 1339–1349. doi: 10.1007/s00221-015-4206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM, & Matyas TA (2011). Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. J Rehabil Med, 43(3), 257–263. doi: 10.2340/16501977-0662 [DOI] [PubMed] [Google Scholar]
- Celnik P, Hummel F, Harris-Love M, Wolk R, & Cohen LG (2007). Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil, 88(11), 1369–1376. doi: 10.1016/j.apmr.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Chen R, & Ashby P (1993). Reflex responses in upper limb muscles to cutaneous stimuli. Can J Neurol Sci, 20(4), 271–278. doi: 10.1017/s0317167100048174 [DOI] [PubMed] [Google Scholar]
- Conforto AB, Dos Anjos SM, Bernardo WM, Silva AAD, Conti J, Machado AG, & Cohen LG (2018). Repetitive Peripheral Sensory Stimulation and Upper Limb Performance in Stroke: A Systematic Review and Meta-analysis. Neurorehabil Neural Repair, 32(10), 863–871. doi: 10.1177/1545968318798943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein WH, & Ehrenstein A (1999). Psychophysical methods. In Windhorst U & Johansson H (Eds.), Modern techniques in neuroscience research (pp. 1211–1241): Springer. [Google Scholar]
- Enders LR, Hur P, Johnson MJ, & Seo NJ (2013). Remote vibrotactile noise improves light touch sensation in stroke survivors’ fingertips via stochastic resonance. J Neuroeng Rehabil, 10(1), 105. doi: 10.1186/1743-0003-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders LR, & Seo NJ (2011). Phalanx force magnitude and trajectory deviation increased during power grip with an increased coefficient of friction at the hand-object interface. J Biomech, 44(8), 1447–1453. doi: 10.1016/j.jbiomech.2011.03.020 [DOI] [PubMed] [Google Scholar]
- Enders LR, & Seo NJ (2015). Altered phalanx force direction during power grip following stroke. Exp Brain Res, 233(6), 1677–1688. doi: 10.1007/s00221-015-4241-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders LR, & Seo NJ (2017). Effects of Sensory Deficit on Phalanx Force Deviation During Power Grip Post Stroke. J Mot Behav, 49(1), 55–66. doi: 10.1080/00222895.2016.1191416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill VA, Fisher T, Schmid AA, Crabtree J, & Page SJ (2014). Relationship between touch sensation of the affected hand and performance of valued activities in individuals with chronic stroke. Top Stroke Rehabil, 21(4), 339–346. doi: 10.1310/tsr2104-339 [DOI] [PubMed] [Google Scholar]
- Hur P, Motawar B, & Seo NJ (2014). Muscular responses to handle perturbation with different glove condition. J Electromyogr Kinesiol, 24(1), 159–164. doi: 10.1016/j.jelekin.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur P, Wan YH, & Seo NJ (2014). Investigating the role of vibrotactile noise in early response to perturbation. IEEE Trans Biomed Eng, 61(6), 1628–1633. doi: 10.1109/TBME.2013.2294672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, & Cohen LG (2002). Modulation of human corticomotor excitability by somatosensory input. J Physiol, 540(Pt 2), 623–633. doi: 10.1113/jphysiol.2001.012801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, & Choi-Kwon S (1996). Discriminative sensory dysfunction after unilateral stroke. Stroke, 27(4), 677–682. doi: 10.1161/01.str.27.4.677 [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan K, Lauer AW, Ramakrishnan V, Webster JG, & Seo NJ (2015). Application of vibration to wrist and hand skin affects fingertip tactile sensation. Physiol Rep, 3(7), e12465. doi: 10.14814/phy2.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanan K, Wang F, Webster JG, & Seo NJ (2017). Feasibility and usability of a wearable orthotic for stroke survivors with hand impairment. Disabil Rehabil Assist Technol, 12(2), 175–183. doi: 10.3109/17483107.2015.1111945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, & Wolfe CD (2001). Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke, 32(6), 1279–1284. doi: 10.1161/01.str.32.6.1279 [DOI] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, . . . Petersen CC (2010). Motor control by sensory cortex. Science, 330(6008), 1240–1243. doi: 10.1126/science.1195797 [DOI] [PubMed] [Google Scholar]
- Meyer S, Karttunen AH, Thijs V, Feys H, & Verheyden G (2014). How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic review. Phys Ther, 94(9), 1220–1231. doi: 10.2522/ptj.20130271 [DOI] [PubMed] [Google Scholar]
- Monzee J, Lamarre Y, & Smith AM (2003). The effects of digital anesthesia on force control using a precision grip. J Neurophysiol, 89(2), 672–683. doi: 10.1152/jn.00434.2001 [DOI] [PubMed] [Google Scholar]
- Schabrun SM, & Hillier S (2009). Evidence for the retraining of sensation after stroke: a systematic review. Clin Rehabil, 23(1), 27–39. doi: 10.1177/0269215508098897 [DOI] [PubMed] [Google Scholar]
- Schranz C, Vatinno A, Ramakrishnan V, & Seo NJ (2022). Neuroplasticity after upper-extremity rehabilitation therapy with sensory stimulation in chronic stroke survivors. Brain Commun, 4(4), fcac191. doi: 10.1093/braincomms/fcac191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH (2004). Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci, 5(7), 532–546. doi: 10.1038/nrn1427 [DOI] [PubMed] [Google Scholar]
- Seo NJ, Enders LR, Fortune A, Cain S, Vatinno AA, Schuster E, . . . Feng W (2020). Phase I Safety Trial: Extended Daily Peripheral Sensory Stimulation Using a Wrist-Worn Vibrator in Stroke Survivors. Transl Stroke Res, 11(2), 204–213. doi: 10.1007/s12975-019-00724-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Kosmopoulos ML, Enders LR, & Hur P (2014). Effect of remote sensory noise on hand function post stroke. Front Hum Neurosci, 8, 934. doi: 10.3389/fnhum.2014.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Lakshminarayanan K, Bonilha L, Lauer AW, & Schmit BD (2015). Effect of imperceptible vibratory noise applied to wrist skin on fingertip touch evoked potentials - an EEG study. Physiol Rep, 3(11), e12624. doi: 10.14814/phy2.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Lakshminarayanan K, Lauer AW, Ramakrishnan V, Schmit BD, Hanlon CA, . . . Nagy T (2019). Use of imperceptible wrist vibration to modulate sensorimotor cortical activity. Exp Brain Res, 237(3), 805–816. doi: 10.1007/s00221-018-05465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Ramakrishnan V, Woodbury ML, Bonilha L, Finetto C, Schranz C, . . . Adams RJ (2022). Concomitant sensory stimulation during therapy to enhance hand functional recovery post stroke. Trials, 23(1), 262. doi: 10.1186/s13063-022-06241-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo NJ, Shim JK, Engel AK, & Enders LR (2011). Grip surface affects maximum pinch force. Hum Factors, 53(6), 740–748. doi: 10.1177/0018720811420256 [DOI] [PubMed] [Google Scholar]
- Seo NJ, Woodbury ML, Bonilha L, Ramakrishnan V, Kautz SA, Downey RJ, . . . Vatinno AA (2019). TheraBracelet Stimulation During Task-Practice Therapy to Improve Upper Extremity Function After Stroke: A Pilot Randomized Controlled Study. Phys Ther, 99(3), 319–328. doi: 10.1093/ptj/pzy143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Karol S, Kim YS, Seo NJ, Kim YH, Kim Y, & Yoon BC (2012). Tactile feedback plays a critical role in maximum finger force production. J Biomech, 45(3), 415–420. doi: 10.1016/j.jbiomech.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Stewart JC, & Cramer SC (2013). Patient-reported measures provide unique insights into motor function after stroke. Stroke, 44(4), 1111–1116. doi: 10.1161/STROKEAHA.111.674671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson SF, Hanley M, Chillala J, Selley AB, & Tallis RC (2008). Sensory loss in hospital-admitted people with stroke: characteristics, associated factors, and relationship with function. Neurorehabil Neural Repair, 22(2), 166–172. doi: 10.1177/1545968307305523 [DOI] [PubMed] [Google Scholar]
- Vatinno AA, Hall L, Cox H, Fluharty A, Taylor C, Wease A, . . . Seo NJ (2022). Using Subthreshold Vibratory Stimulation During Poststroke Rehabilitation Therapy: A Case Series. OTJR (Thorofare N J), 42(1), 30–39. doi: 10.1177/15394492211042275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatinno AA, Schranz C, Simpson AN, Ramakrishnan V, Bonilha L, & Seo NJ (2022). Predicting upper extremity motor improvement following therapy using EEG-based connectivity in chronic stroke. NeuroRehabilitation, 50(1), 105–113. doi: 10.3233/NRE-210171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, & Boyd LA (2009). Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct, 5, 36. doi: 10.1186/1744-9081-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, . . . Stroke Statistics, S. (2021). Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation, 143(8), e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- Wang F, Lakshminarayanan K, Slota GP, Seo NJ, & Webster JG (2015). An MRI-compatible hand sensory vibrotactile system. Physiol Meas, 36(1), N15–21. doi: 10.1088/0967-3334/36/1/N15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, & Jordan MI (1995). An internal model for sensorimotor integration. Science, 269(5232), 1880–1882. doi: 10.1126/science.7569931 [DOI] [PubMed] [Google Scholar]


