Abstract
Several COVID-19 patients frequently experience with happy hypoxia. Sometimes, the level of nitric oxide (NO) in COVID-19 patients was found to be greater than in non-COVID-19 hypoxemics and most of the cases lower. Induced or inhaled NO has a long history of usage as a therapy for hypoxemia. Excessive production of ROS and oxidative stress lower the NO level and stimulates mitochondrial malfunction is the primary cause of hypoxia-mediated mortality in COVID-19. Higher level of NO in mitochondria also the cause of dysfunction, because, excess NO can also diffuse quickly into mitochondria or through mitochondrial nitric oxide synthase (NOS). A precise dose of NO may increase oxygenation while also acting as an effective inhibitor of cytokine storm. NOS inhibitors may be used in conjunction with iNO therapy to compensate for the patient's optimal NO level. NO play a key role in COVID-19 happy hypoxia and a crucial component in the COVID-19 pathogenesis that demands a reliable and easily accessible biomarker to monitor.
Keywords: COVID-19, Nitric oxide, Happy hypoxia, Mitochondrial dysfunction
1. Introduction
The impact of the SARS-CoV-2 pandemic and COVID-19 has been widely explored, and numerous strategies have been adopted to improve the immune systems of patients [[1], [2], [3]]. Few vaccines are available with efficacy rates ranging from 60 to 95% and endurance durations are yet to be determined.
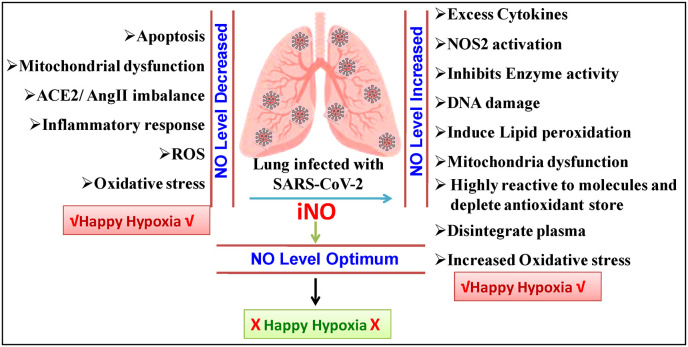
When oxygen levels in blood drop unnaturally, one of the key life-threatening symptoms is happy or silent hypoxia. The hypoxic situation damage the important organs in a sequential manner, and they are unable to recover, resulting in silent death. However, the mechanism by which the virus produces silent hypoxia has yet to be investigated. Surprisingly, some COVID-19 patients can continue to live despite severe hypoxia [4]. COVID-19 patients had a substantially higher amount of NO inside their RBCs than non-COVID-19 hypoxemic individuals [5]. It demonstrates that a greater NO level in the blood and happy hypoxia have a direct association. On the other hand, in contrast to healthy controls, NO and H2S availability is decreased in COVID-19 patients (Fig. 1 ). Reduced NO levels are accompanied by an increase in nitrotyrosine, a sign of oxidative stress, in COVID-19 patients [6].
Figure: 1.
Schematic illustration of nitric oxide dynamics in COVID-19 patients and possible happy hpoxia.
Nitric oxide (NO) is a versatile chemical that regulates physiological processes at low concentrations but is harmful to host cells at higher concentrations. At appropriate cellular concentrations of NO stimulate the immune and inflammatory systems to aid in the eradication of harmful microorganisms and also kill tumour cells [7]. Overproduction of nitric oxide (NO•)- in cells causes cytotoxicity. Various cytotoxic effects have been observed including mitochondrial damage, DNA synthesis inhibition, loss of cell membrane integrity, and DNA strand breakage [8]. NO•- induced cytotoxicity is a complicated signalling cascade that leads to cell death by apoptosis through a complex signalling cascade. Apoptosis is a highly controlled form of cell death in which mitochondria play an important role [9]. Mitochondria cause cell death by releasing proteins such as cytochrome c. The Bcl-2 family proteins Bax and Bid increase cytochrome c release into the cytosol by binding to adaptor molecules such apoptotic protease activating factor-1 and activating pro-caspase-9, which then activates pro-caspase-3 and -7. The primary actors in NO-induced cell death are mitochondria [10].
2. NO in hypoxic vasodilatation
Under normal conditions, NO plays a key role in controlling cardiac functions and blood flow by primarily acting as a signalling molecule. NO binds to soluble Guanyl Cyclase and cGMP regulates many ion channels for muscle or endothelial cell relaxation. When a patient has extremely low oxygen levels in their blood but no signs or symptoms of dyspnea, this is referred as “happy/quiet hypoxemia" in COVID-19 [11]. Hypoxic vasodilation tends to increase blood flow in order to maintain oxygen transfer [12]. The response of hypoxic vasodilation was remarkably decreased after providing oxyhemoglobin, which potentially controls NO scavenging activity. The amount of NO in Tibetan plateau residents was found to be ten times greater than in sea-level residents [13] which confirms the increased blood flow in the fore arm (which was in a relaxed state) and counteracts the effects of high altitude O2 deficit.
NO was reported to play key role in COVID-19 patients following four common mechanisms such as vasodilation, anti-coagulation, anti-inflammation, and antiviral. NO serves as a good pulmonary vasodilator, having bronchodilator effects, anti-thrombotic activity, and enhanced blood flow to the alveoli, and an essential immune regulator, including anti-inflammatory and microbicidal activities [14]. The rate of generation and the appropriate concentration are also important factors. The use of inhaled nitric oxide (iNO) for patients with severe hypoxemia has been shown to be critical in reducing hypoxic development in COVID-19 patients.
The Nitric Oxide Synthase (NOS) gene is expressed at a high level in healthy para-nasal sinus epithelial cells, resulting in continual high-level NO generation. Other than being used in the treatment of several life-threatening diseases, such as acute respiratory distress syndrome, including myocardial or cerebral ischemia, cerebral malaria, and sickle cell disease, and ischemia-reperfusion injury, iNO was approved by the FDA in 1999 as a clinical adjuvant therapy for persistent pulmonary hypertension in newborn babies. iNO can also be utilized in critical surgery or organ transplants in a few circumstances. After iNO therapy, no noticeable adverse effects were recorded.
3. NO induced silent death
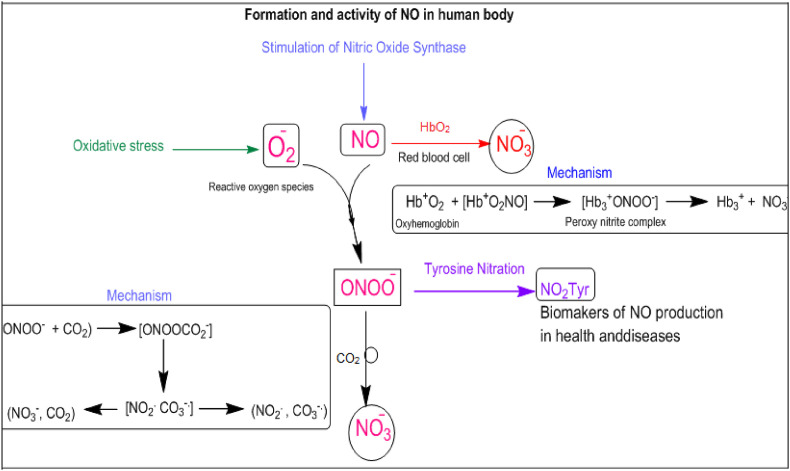
NO production causes small vessel vasodilation, allowing oxygen to flow freely to tissues. A recent research found that intracellular NO level is much higher in COVID-19 patients than in healthy control group, suggesting that this may allow for the release of oxygen to tissues, leading to the clinical presentation of silent hypoxia in these individuals [15]. Tobin and colleagues recently described three instances of silent hypoxemia with PaO2 values ranging from 36 to 45 mmHg and no increased alveolar ventilation [16]. Nitrite and nitrate have long been thought to be physiologically inert and have been utilized as NO production monitors. NO production (e.g., denitrification) to the existing NO synthesis route via NOS. Clearly, l-arginine oxidation with its sophisticated substrate is a complex process. It is obvious that these simple nitrogen oxyanions are more than just NO breakdown products; they have biological actions of their own. In haemoglobin red blood cells, reactive oxygen species and nitric oxide generated nitrate anion (Fig. 2 ).
Fig. 2.
Schematic cellular mechanism of NO formation and reactivity.
In 1950s, an evidence pointed to the biological formation of oxygen radicals associated with cell redox metabolism starting with the monovalent reduction of molecular oxygen by electron transfer proteins or endogenous reductants to yield O2 to O2•−: O2+e−→O2∙−.
First, O2•− can react with •NO in aqueous solution to yield peroxynitrite anion
| O2∙−+N∙O→ONOO− |
Recent studies have also demonstrated that heme-peroxynitrite complexes occur when heme compounds interact with •NO and O2. The oxidation of •NO to NO−3, NO−3 by oxyhemoglobin in the following reaction is a remarkable and biological example of this sort of structure.
| Hb2+O2+N∙O→[Hb2+O2NO]→[Hb3+ONOO−] →Hb3++NO−3 |
The intermediate [Hb3+ONOO−] complex readily isomerizes to NO−3, NO−3 by an “in-cage” recombination of •NO2 with an oxo-ferryl (Fe4+=O) intermediate. Very small amounts of •NO2 leak out of the cage and a minor percentage of oxy-Hb becomes nitrated (<1% yield) in the presence of •NO (Fig. 2). This marginal but continuous “radical leakage” from the [Hb3+ONOO−] complex (a process that may extend to other transition metal-containing centres) likely contributes to hemoglobin tyrosine nitration in normal blood cells (at α-Tyr24, α-Tyr42, β-Tyr130). Peroxynitrite itself can cross the erythrocyte membranes by anion channels and react with oxyHb to mostly isomerize to NO−3; the reaction also yields ∼10% of oxo-ferryl intermediates, •NO2, and protein radicals which can also contribute to hemoglobin nitration. Oxidative modifications of hemoglobin in vivo including tyrosine nitration are greatly enhanced in smokers and type-2 diabetes patients conditions that disrupt vascular •NO and redox metabolism.
Furthermore, overindulgence level of NO in cytoplasm readily diffuses into mitochondria, and mitochondrial specific nitric oxide synthase (mtNOS) regulates NO generation. It was also shown that neuronal nitric oxide synthase (nNOS) alpha isoform is the key regulator of NO generation in mitochondria. mtNOS was shown to be modulated in response to lower O2 availability and hypoxia [17].
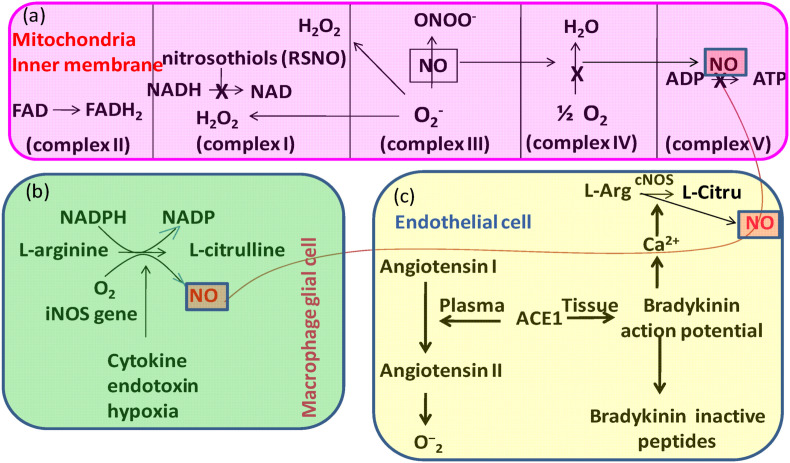
NO produced in mitochondria and NO in cytoplasm diffuses into mitochondria increased several folds NO level that interact and inhibit mitochondrial function in several ways such as- (i) NO activates soluble guanylate cyclase (sGC) and inhibits mitochondrial cytochrome c oxidase in vascular smooth muscles that regulates the blood flow and discontinues mitochondrial O2 consumption, (ii) NO interacts with available O2 .- in mitochondria to form ONOO−, which initiates a destructive cascade by inactivating the Mn-SOD and Fe–S centre of aconitase, (iii) NO directly nitrosylate critical cysteine threonine, (iv) NO directly nitrosylate critical cysteine threonine [18], (v) NO directly nitrosylate cysteine, (v) At low concentrations of NO, it is sufficient to limit electron transport by reversibly binding to heme a3 in cytochrome oxidase and directly interacting with cytochrome c oxidase (complex IV), whereas peroxynitrite inhibits mitochondrial complex I by S-nitrosation (Fig. 3 ) [19]. Reactive oxygen species such as NO and ONOO−, on the other hand, stimulate cell defence response mechanisms that activate nuclear factor B (NF–B) and AMP kinase at extremely low concentrations (nM).
Fig. 3.
Nitric oxide (NO) and reactive nitrogen species (RNS) have major effects on mitochondria (a). They cause an increase in superoxide radical (O2) generation by over-reducing upstream redox centres. NO inhibits cytochrome oxidase (complex IV) selectively and reversibly; RNS inactivate numerous respiratory complexes (I, II, IV), as well as the ATP synthetase (ATPase). The newly produced O2 is either effectively dismutated by mitochondrial Mn-SOD or interacts with available NO to create ONOO under certain conditions. Complex I is inactivated by nitrosothiols, but peroxynitrite inhibits aconitase and activates the proton leak and permeability transition pore (ANT-PTP), which may contribute to NO-induced cell death. NO produced by inducible NOS (iNOS) in macrophage glial cells regulates mitochondrial ATP production (b). Constitutive forms of NOS (cNOS) generated from the action of ACE inhibitors (ACE-I) to improve the equilibrium between nitric oxide (NO) and superoxide (O2) and control mitochondrial respiration to correct endothelial dysfunction (c).
Low O2• levels, as well as greater NO• and ONOO− radical concentrations, are shown to enhance the expression of hypoxia inducible factor-1 (HIF-1). The NF-κB pathway is completely responsible for mitochondrial dynamics [20]. Increased affinity of NO for oxygen may be a key source of physiological and/or pathological symptoms of happy hypoxia, as it entirely stops electron transport and ATP generation. Prior to invasive therapy, inhaled NO has been recommended as an alternate rescue strategy, particularly for the treatment of hypoxemia. However, among patients with extensive mechanical ventilation who had developed persistent hypoxemia, NO is unable to reverse oxygenation [21,22].
The levels of erythrocytic iron-nitrosyl complex (HbNO) in the blood were compared by Montiel and colleagues (2022) among 30 patients with severe COVID-19 and 15 healthy controls who were matched for cardiovascular risk factors using electron paramagnetic spectroscopy. Patients with severe COVID-19 had lower levels of HbNO and lower levels of nitrite/nitrate, by-products of NO metabolism, as well as higher levels of lipid peroxides is the indicator of oxidative stress [23]. Therefore, endothelial oxidative stress with a consequent decrease in NO bioavailability appears to be a likely pathogenic factor of endothelial dysfunction in the intensive care unit COVID-19 patient. Even though iNO administered at 20–40 ppm for a short period of time does not always improve oxygenation in patients with severe COVID-19, it may have other positive effects when administered at higher concentrations or for longer durations, even outside of the pulmonary vasculature [24,25].
4. Conclusion and perspectives
High amount of intracellular NO may neutralise the effects of silent hypoxia4, according to a recent investigation on COVID-19 patients. It is hypothesised that increasing NO levels by external NO treatment will downregulate NOS expression, reducing illness severity. Additional research is needed to determine whether NO therapy is effective in the treatment of silent hypoxia or not. L-NMMA (L-N(G) methyl arginine hydrochloride) is a nitric oxide synthase inhibitor that limits increased blood flow during hypoxia. Although NO bioavailability may be reduced by the release of circulating cell-free haemoglobin, NO release normally counteracts hypoxia circumstances and so enhances hypoxic vasodilation. Mitochondrial breakdown causes harmed tissue in the case of acute hypoxia conditions. Surprisingly, in the case of COVID-19 patients, there is no substantial increase in carbon dioxide partial pressure during the most critical intervals [26].
Furthermore, before the onset of a respiratory insufficiency, the percentage of PaO2/FiO2 in the lung of COVID-19 patients is rather low [11]. Interestingly, pre-incubation of the RBC solution with the NOS inhibitor l-NAME, prevented vasodilation. These findings back with the theory that RBC-derived NO plays a role in local blood flow control [27]. Exogenous NO therapy may be beneficial in COVID-19 individuals since NO is a pulmonary vasodilator and also has antiviral efficacy against coronavirus strains. Although there is no evidence that direct oxygen therapy is effective in the treatment of severe COVID-19 patients with breathlessness, our findings imply that NO therapy may be beneficial in COVID-19 patients with hypoxia [28]. As a result, it is required to begin alternative therapy by treating iNO at an optimum dose while continuously monitoring met-myoglobin, nitrogen dioxide (NO2), and blood coagulation levels. In COVID patients, iNO may not only enhance oxygenation but also act as a powerful inhibitor of cytokine storm. The NOS inhibitor may be an appropriate choice in conjunction with iNO therapy to compensate for the patient's NO level, however NOS signalling must be stopped for a few hours until the patient is released from silent hypoxia. Further study on the subject is necessary given the success of NO gas as a therapeutic option and the development of evidence linking NO to COVID-19 severity. Only optimum level of NO is helpful with a reliable and easily accessible biomarker.
Data availability
No data was used for the research described in the article.
References
- 1.Pal S., Mandal S.M., et al. Brief survey on phytochemicals to prevent COVID-19. J. Indian Chem. Soc. 2021;99 100244-100244. [Google Scholar]
- 2.Manna S., Mandal S.M., et al. Probiotics-derived peptides and their immunomodulatory molecules can play a preventive role against viral diseases including COVID-19. Probiotics Antimicrob. Proteins. 2021;13:611–623. doi: 10.1007/s12602-020-09727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paria K., Mandal S.M., et al. Synergy of melanin and vitamin-D may play a fundamental role in preventing SARS-CoV-2 infections and halt COVID-19 by inactivating furin protease. Transl. Med. Commun. 2020;5:21. doi: 10.1186/s41231-020-00073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serebrovska Z.O., et al. Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020;41:1539–1546. doi: 10.1038/s41401-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortazet E., et al. Silent hypoxia: higher NO in red blood cells of COVID-19 patients. BMC Pulm. Med. 2020;20:269. doi: 10.1186/s12890-020-01310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominic P., et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdan C. The multiplex function of nitric oxide in (auto) immunity. J. Exp. Med. 1998;187:1361–1365. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdan C. In: Handbook of Experimental Pharmacology. Mayer B., editor. Springer; Heidelberg: 2000. The function of nitric oxide in the immune system. Nitric Oxide. [Google Scholar]
- 9.Peng H., et al. Nitric oxide inhibits endothelial cell apoptosis by inhibiting cysteine-dependent SOD1 monomerization. FEBS Open Bio. 2022;12:538–548. doi: 10.1002/2211-5463.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson J.D., Orrenius S. Role of mitochondria in toxic cell death. Toxicology. 2002;27:181–182. doi: 10.1016/s0300-483x(02)00464-x. [DOI] [PubMed] [Google Scholar]
- 11.Archer S.L., Sharp W.W., Weir E.K. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications. Circulation. 2020;142:101–104. doi: 10.1161/CIRCULATIONAHA.120.047915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pohl U., Busse R. Hypoxia stimulates release of endothelium-derived relaxant factor. Am. J. Physiol. Heart Circ. Physiol. 1989;256:H1595–H1600. doi: 10.1152/ajpheart.1989.256.6.H1595. [DOI] [PubMed] [Google Scholar]
- 13.Beall C.M., et al. Pulmonary nitric oxide in mountain dwellers. Nature. 2001;414:411–412. doi: 10.1038/35106641. [DOI] [PubMed] [Google Scholar]
- 14.Saunders N.R., Dinenno F.A., Pyke K.E., Rogers A.M., Tschakovsky M.E. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H214–H220. doi: 10.1152/ajpheart.00762.2004. [DOI] [PubMed] [Google Scholar]
- 15.McKeown D.J., McNeil C.J., Brotherton E.J., Simmonds M.J., Kavanagh J.J. Severe acute hypoxia impairs recovery of voluntary muscle activation after sustained submaximal elbow flexion. J. Physiol. 2021;599:5379–5395. doi: 10.1113/JP281897. [DOI] [PubMed] [Google Scholar]
- 16.Couzin-Frankel J. The mystery of the pandemic's 'happy hypoxia. Science. 2020;368:455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 17.Lacza Z., et al. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free Radic. Biol. Med. 2001;31:1609–1615. doi: 10.1016/s0891-5849(01)00754-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 19.Sun J., Steenbergen C., Murphy E., S-nitrosylation NO-related redox signaling to protect against oxidative stress. Antioxidants Redox Signal. 2006;8:1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laforge M., et al. NF-κB pathway controls mitochondrial dynamics. Cell Death Differ. 2016;23:89–98. doi: 10.1038/cdd.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavazzi G., et al. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit. Care. 2020;17:508. doi: 10.1186/s13054-020-03222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garfield B., et al. Potential for personalized application of inhaled nitric oxide in COVID-19 pneumonia. Br. J. Anaesth. 2021;126:e72–e75. doi: 10.1016/j.bja.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari M., Protti A. Nitric oxide in COVID-19: too little of a good thing? EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamenshchikov N.O., et al. Therapeutic effects of inhaled nitric oxide therapy in COVID-19 patients. Biomedicines. 2022;10:369. doi: 10.3390/biomedicines10020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shei R.J., Baranauska M.N. More questions than answers for the use of inhaled nitric oxide in COVID-19. Nitric Oxide. 2022;124:39–48. doi: 10.1016/j.niox.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y., et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellsworth M.L., et al. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology. 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pimentel-Muiños F.X., Seed B. Regulated commitment of TNF receptor signalling: a molecular switch for death or activation. Immunity. 1999;11:783–793. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.