Abstract
Objectives
Vaccination has been effective in ameliorating the impact of COVID-19. Here, we report vaccine effectiveness (VE) of the nationally available COVID-19 vaccines in Mexico.
Methods
Retrospective analysis of a COVID-19 surveillance system to assess the VE of the BNT162b2, messenger RNA (mRNA)-12732, Gam-COVID-Vac, Ad5-nCoV, Ad26.COV2.S, ChAdOx1, and CoronaVac vaccines against SARS-CoV-2 infection, COVID-19 hospitalization, and death in Mexico. The VE was estimated using time-varying Cox proportional hazard models in vaccinated and unvaccinated adults, adjusted for age, sex, and comorbidities. VE was also estimated for adults with diabetes, aged ≥60 years, and comparing the predominance of SARS-CoV-2 variants B.1.1.519 and B.1.617.2.
Results
We assessed 793,487 vaccinated and 4,792,338 unvaccinated adults between December 24, 2020 and September 27, 2021. The VE against SARS-CoV-2 infection was the highest for fully vaccinated individuals with mRNA-12732 (91.5%, 95% confidence interval [CI] 90.3-92.4) and Ad26.COV2.S (82.2%, 95% CI 81.4-82.9); for COVID-19 hospitalization, BNT162b2 (84.3%, 95% CI 83.6-84.9) and Gam-COVID-Vac (81.4% 95% CI 79.5-83.1), and for mortality, BNT162b2 (89.8%, 95% CI 89.2-90.2) and mRNA-12732 (93.5%, 95% CI 86.0-97.0). The VE decreased for all vaccines in adults aged ≥60 years, people with diabetes, and periods of Delta variant predominance.
Conclusion
All the vaccines implemented in Mexico were effective against SARS-CoV-2 infection, COVID-19 hospitalization, and death. Mass vaccination with multiple vaccines is useful to maximize vaccination coverage.
Keywords: COVID-19, Vaccine effectiveness, SARS-CoV-2, Mortality, Hospitalization
Introduction
COVID-19 has imposed a significant public health challenge worldwide. In Mexico, the mitigation of COVID-19 has imposed a significant challenge due to the large impact of cardiometabolic diseases and long-standing sociodemographic and health care inequalities in the region [1], [2], [3]. The most significant contributing factor to decreasing the impact of COVID-19 has been the availability of vaccines to prevent symptomatic and severe forms of SARS-CoV-2 infections [4,5]. Available COVID-19 vaccines are effective in preventing symptomatic SARS-CoV-2 infection and COVID-19-related hospitalization and death [6], [7], [8], [9], [10], [11], [12]. Despite the availability of randomized clinical trials to inform vaccine efficacy, population-based studies to estimate vaccine effectiveness (VE) are required to evaluate and inform future vaccine rollouts, assess and prioritize eligibility for vaccine boosters, and reduce vaccine hesitancy [13]. With the emergence of new SARS-CoV-2 variants of interest with varying rates of immune evasion and the occurrence of increasingly complex forms of hybrid immunity, information on the effectiveness of vaccines remains of high relevance as more vaccine boosters become available [14].
The COVID-19 vaccination program in Mexico sequentially implemented seven nationally available vaccines in its initial rollout: BNT162b2, messenger RNA (mRNA)-12732, Gam-COVID-Vac, Ad5-nCoV, Ad26.COV2.S, ChAdOx1, and CoronaVac. To date, population-based studies have reported population-wide effectiveness of the BT162b2 vaccine [15], [16], [17], ChAdOx1 [18], [19], [20], mRNA-12732 [21,22], Ad26.COV2.S [23,24], and the rAd26 component of the Gam-COVID-Vac vaccine [25]. Nevertheless, studies in Latin America have been limited, with only two reports on the efficacy of the CoronaVac vaccine [26,27] and recent reports of programs with multiple vaccine programs in Argentina and Colombia [28,29]. The Mexican COVID-19 vaccination program offers a unique opportunity to evaluate the simultaneous effectiveness and impact of a large set of available COVID-19 vaccines against SARS-CoV-2 infection and COVID-19-related hospitalization and death, and report the estimates for the effectiveness of the Gam-COVID-Vac and Ad5-nCoV vaccines, which have had limited population-based evidence beyond clinical trials [7,12,30]. Furthermore, older adults and patients with conditions which may lead to impaired immune response to COVID-19 vaccines and who may benefit from prioritization for booster allocation are often under-represented in clinical trials; evaluating the effectiveness of COVID-19 vaccines in these groups remains a public health priority [31]. In this study, we sought to provide the first nationwide evaluation for the effectiveness of nationally available COVID-19 vaccines for SARS-CoV-2 infections and COVID-19 hospitalization and death and the impact of circulating variants, older age, and comorbidities, with a particular focus on diabetes, in modifying the effectiveness for the seven nationally available COVID-19 vaccines implemented within the initial rollout of the Mexican vaccination program during the 2020-2021 period.
Methods
Study population and design
We conducted a retrospective study using data from a national cohort within a SARS-CoV-2 surveillance program to evaluate the VE for the nationally available COVID-19 vaccines in Mexico. We included adults aged 18 years or older who were evaluated within the Sistema de Vigilancia Epidemiológica de Enfermedades Respiratorias (SISVER) database of the General Directorate of Epidemiology of the Mexican Ministry of Health for suspected SARS-CoV-2 infection. SISVER is a nationwide sentinel surveillance system that collects clinical and epidemiological information of suspected COVID-19 cases in Mexico at the point of testing and updates clinical and sociodemographic information pertaining to clinical outcomes and vaccination status during epidemiological follow-up of all suspected cases as more information becomes available [1,2]. Vaccination status, date, and specific vaccine product were self-reported by the evaluated persons as part of epidemiological follow-up of suspected COVID-19 cases and were ascertained as part of epidemiological follow-up, whenever possible, with official proof of vaccination. The data are collected and its quality is evaluated in each individual facility by trained personnel and then pooled and verified in its entirety on a daily basis by personnel from the General Directorate of Epidemiology of the Mexican Ministry of Health. For this analysis, we included cases of individuals aged ≥18 years with suspected SARS-CoV-2 infection, evaluated in SISVER from December 24, 2020, the start of the vaccination rollout program, and until September 27, 2021 to account for the primary rollout of vaccines before the initiation of booster vaccination, which implemented complex homologous and heterologous vaccination protocols and were analyzed elsewhere [14]. We compared the cases with at least one dose of any available vaccine in Mexico with unvaccinated cases during the same period. As per the World Health Organization recommendations and given previous evidence on immune response to COVID-19 vaccination, we only considered fully or partially vaccinated cases as those with ≥14 days after receiving at least one vaccine dose and those with less than the specified time were considered as unvaccinated or partially vaccinated, as required [27,31].
COVID-19 vaccination in Mexico
Mexico approached its vaccination strategy by incorporating multiple vaccines to maximize the national vaccination coverage. The SARS-CoV-2 vaccines, which were applied in Mexico during the primary vaccination rollout, included BNT162b2, mRNA-12732, Gam-COVID-Vac, Ad5-nCoV, Ad26.COV2.S, ChAdOx1, and CoronaVac [32]. The primary vaccination protocols for most vaccines included a two-dose regimen as recommended by manufacturers, with the exemption of one-dose vaccines for Ad5-nCoV and Ad26.COV2.S. Fully vaccinated individuals were considered if they completed the vaccination protocol for two- or one-dose vaccines and partially vaccinated individuals were considered if they only completed one of a two-dose vaccine protocol. All vaccines received emergency authorization for their use during the COVID-19 pandemic in Mexico by the Federal Commission for the Protection against Sanitary Risks, the Mexican health regulatory agency, after demonstrating the efficacy in phase III studies [33]. The primary vaccination rollout for the population aged ≥18 years started for essential health care workers on December 24, 2020 and continued with adults aged over 60 years and the rest of the population starting in February 2021, with an estimated vaccine rollout of 44.63 million adults with a full vaccination protocol and 18.85 million with a partial vaccination protocol successfully completed as of September 27, 2021, accounting for a coverage of 49% of the population with at least one dose [34]. Additional details on nationally available COVID-19 vaccines in Mexico are reported in Supplementary Material. This report excludes information regarding booster vaccination in Mexico, which started its rollout in December 2021, coinciding with the spread of the SARS-CoV-2 Omicron variant in Mexico [35].
Outcomes
We calculated the VE based on three primary outcomes (i) confirmed SARS-CoV-2 infections, including both symptomatic and asymptomatic individuals, defined as laboratory-confirmed SARS-CoV-2 infections with either rapid antigen test or reverse transcription-polymerase chain reaction. All the diagnostic tests used to identify SARS-CoV-2 infections were certified by the Federal Commission for the Protection against Sanitary Risks and implemented as per the World Health Organization recommendations [36]; (ii) COVID-19-related hospitalization; and (iii) COVID-19-related death, classified as all-cause mortality. We also further stratified the VE based on the following criteria:
-
a.
Predominant SARS-CoV-2 variant—the relevant variants of interest in Mexico were screened by the Mexican Genomic Surveillance Consortium (COVIDGen-Mex) [37]. During the evaluated period, COVIDGen-Mex reported the predominance of the B.1.1.519 variant before July 1, 2021 and the B.1.617.2 (Delta) variant after this period. To evaluate the changes in the VE based on the predominant variant circulation, we stratified the analyses based on the date of symptom onset before July 1, 2021 as the B.1.1.519 predominance and after that period, the Delta predominance. Infection was assumed to be most likely caused by the predominant variant based on the date of symptom onset.
-
b.
Age strata—Given the under-representation of older adults in vaccine clinical trials and observed inequities on the impact of COVID-19 on this population in Mexico [2], we evaluated the VE using 60 years as the cut-off for age.
-
c.
Comorbidities—The prevalence of diabetes and obesity in Mexican population are high and the impact of their impact on the course of the COVID-19 pandemic in Mexico has been widely reported [1,38,39]. To this end, we also evaluated the VE for individuals with diabetes and, secondarily, obesity.
Statistical analyses
Given that the vaccination rollout was sequential and that rates of infection in vaccinated and unvaccinated changes over time, we included a time-varying component into the VE estimation. Briefly, the VE was estimated using a Cox proportional hazard regression model, which accounts for the time-varying covariate of time from the beginning of follow-up until vaccination, as previously described [27,40]. The model was specified as follows:
Where is the baseline risk function for individuals at the beginning of the study, without assumption of an underlying distribution, represents a vector of fixed covariates, and represents the time-varying vaccination status for all evaluated persons. The exposure person-time for all subjects was calculated from December 24, 2020 until the onset of each evaluated outcome or until the last follow-up, whichever occurred first. The vaccination status was evaluated as a time-dependent covariate for each evaluated vaccine, calculated as the time from study entry to application of the first dose of any received vaccine. The VE was estimated for each evaluated vaccine product, where effectiveness is defined as 1 minus the hazard ratio of the resulting Cox model. For each evaluated outcome, each individual vaccine product was compared with the unvaccinated population; this was also conducted for the subgroup analyses for age, predominant circulating variant, and comorbidities. We fitted all models for each vaccine product adjusted for age, sex, comorbidities and stratified them per municipality of origin to account for region-level variation in pandemic dynamics, including all stratified analyses. All statistical analyses were conducted using R version 4.1.2.
Results
Study population
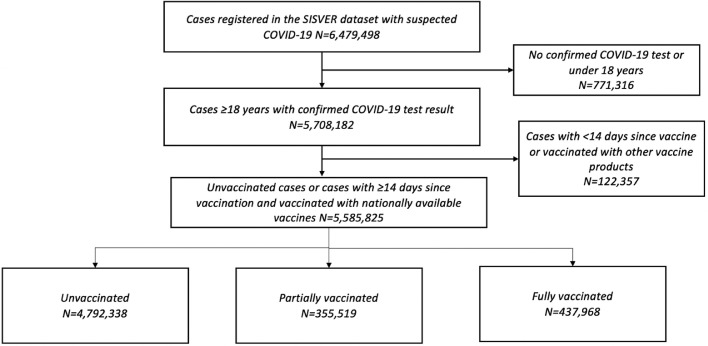
We evaluated 793,487 vaccinated persons aged 18 or older and with ≥14 days from vaccination who were evaluated for suspected SARS-CoV-2 infection from December 24, 2020 until September 27, 2021. Overall, 437,968 cases were fully vaccinated with either one- or two-dose regimens and 355,519 were partially vaccinated. The most frequent vaccines with at least one dose were ChAdOx1 (n = 292,800), followed by BNT162b2 (n = 250,806), Ad5-nCoV (n = 87,585), Gam-COVID-Vac (n = 79,551), CoronaVac (n = 62,474), Ad26.COV2.S (n = 14,870), and mRNA-12732 (n = 5401). During the study period 4,792,338 unvaccinated individuals aged ≥18 years were assessed and included as controls in the study. The comparison of demographic characteristics and clinical outcomes disaggregated by vaccination status and type are presented in Supplementary Materials. Of interest, most vaccinated cases were in the 30-59 years age group. Regarding vaccine distribution, the Ad26.COV2.S vaccine was used in a younger population and the Gam-COVID-Vac and CoronaVac vaccines were used in a generally older population. Cases with diabetes more frequently reported vaccination with the BT162b2, Gam-COVID-Vac, and the CoronaVac vaccines. A full flowchart of evaluated individuals and the distribution of vaccines within the SISVER registry and included in the study is provided in Figure 1 .
Figure 1.
Flow diagram of individuals registered in the SISVER dataset from December 24, 2020 until September 27, 2021 after consideration of exclusion criteria for inclusion in our study.
SISVER, Sistema de Vigilancia Epidemiológica de Enfermedades Respiratorias.
Incidence of COVID-19 and related outcomes during the study period
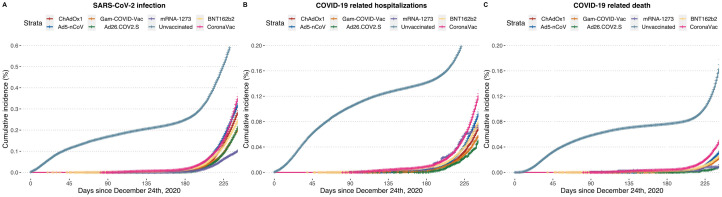
During the study period, a total of 1,700,212 confirmed SARS-CoV-2 cases were reported. Overall, unvaccinated individuals accounted for 549,685,635 person-days of follow-up, with 80,621,913 person-days for partially vaccinated and 98,631,832 person-days for fully vaccinated individuals. The incidence rates for SARS-CoV-2 infection in these subgroups were 1.19 cases per 1000 person-days for fully vaccinated individuals, followed by 1.41 cases per 1000 person-days for partially vaccinated individuals and 2.67 cases per 1000 person-days for unvaccinated individuals. We identified 230,780 COVID-19-related hospitalizations and 112,234 COVID-19 confirmed deaths. The cumulative incidence of SARS-CoV-2 infection and COVID-19-related hospitalizations and deaths were lower for vaccinated individuals for all evaluated vaccine products (Figure 2 ).
Figure 2.
Cumulative incidence plots for laboratory confirmed SARS-CoV-2 infection, COVID-19-related hospitalization and death for unvaccinated individuals compared with individuals with at least one-dose of all evaluated nationally available vaccines after the start of follow-up from December 24, 2020.
mRNA, messenger RNA.
VE for nationally available two-dose vaccines
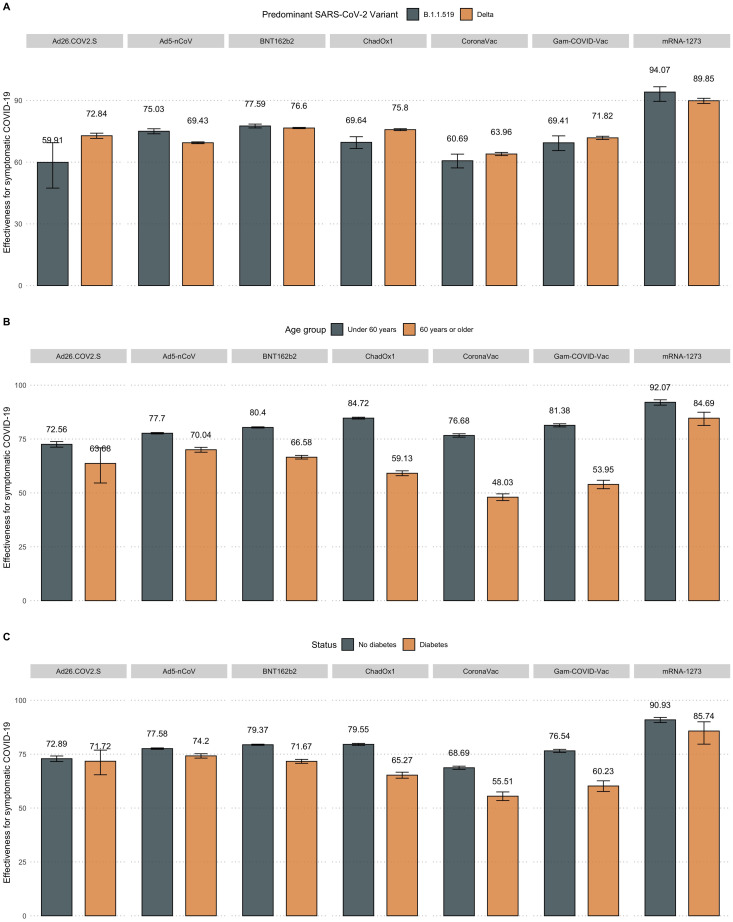
The detailed effectiveness for SARS-CoV-2 infection and COVID-19-related hospitalization and mortality are available in Table 1 and stratified by subgroups in Figure 3 . For the BNT162b2 mRNA vaccine, the effectiveness against SARS-CoV-2 infection in partially vaccinated individuals was 63.59% (95% confidence interval [CI] 62.87-64.3) and increased with a two-dose regimen to 80.34% (95% CI 80.11-80.57). The protection for fully vaccinated individuals was 84.26% (95% CI 83.61-84.89) for hospitalization and 89.83% (95% CI 89.16-90.46) for mortality. In fully vaccinated individuals, a decreased effectiveness for SARS-CoV-2 infection and COVID-19-related mortality was observed for individuals aged ≥60 years or those with diabetes. The protection against the predominant Delta variant was decreased for SARS-CoV-2 infection but slightly increased against COVID-19-related mortality than the period with predominance of the B.1.1.519 variant (Supplementary Material).
Table 1.
Estimated vaccine effectiveness for all nationally available COVID-19 vaccines in Mexico compared to unvaccinated adults (≥18 years) from December 24, 2020, until September 27, 2021. Effectiveness was estimated against laboratory-confirmed SARS-CoV-2 infection, COVID-19-related hospitalization and death. Partial and fully vaccinated status were considered for individuals with ≥14 days from vaccination until outcome.
| COVID-19 Vaccine | Number vaccinated | Status | Incident SARS-CoV-2 infection (95% CI) |
COVID-19 Hospitalization (95% CI) | COVID-19 Mortality (95% CI) |
|---|---|---|---|---|---|
| BNT162b2 | 53,728 | Partial | 63.59 (62.87-64.3) | 72.08 (70.54-73.53) | 79.62 (77.76-81.33) |
| 197,078 | Complete | 80.34 (80.11-80.57) | 84.26 (83.61-84.89) | 89.83 (89.16-90.46) | |
| ChAdOx1 | 232,550 | Partial | 61.49 (61.14-61.83) | 73.9 (73.13-74.65) | 81.02 (80.03-81.96) |
| 60,250 | Complete | 80.79 (80.43-81.14) | 80.23 (79.29-81.13) | 86.81 (85.89-87.67) | |
| Gam-COVID-Vac | 39,599 | Partial | 67.73 (66.88-68.57) | 82.71 (80.66-84.53) | 87.14 (84.45-89.36) |
| 39,952 | Complete | 78.75 (78.17-79.31) | 81.38 (79.45-83.13) | 87.7 (85.82-89.33) | |
| CoronaVac | 28,005 | Partial | 53.41 (52.41-54.39) | 69.47 (67.59-71.24) | 76.14 (73.71-78.34) |
| 34,469 | Complete | 71.93 (71.35-72.51) | 73.76 (72.49-74.96) | 80.38 (79.04-81.64) | |
| messenger RNA-1273 | 1637 | Partial | 87.48 (85.13-89.45) | 85.07 (76.25-90.62) | 93.1 (81.55-97.42) |
| 3764 | Complete | 91.45 (90.34-92.43) | 78 (69.01-84.38) | 93.46 (85.96-96.95) | |
| Ad5-nCoV | 87,585 | Complete | 70.5 (70.09-70.9) | 72.31 (71.1-73.47) | 79.93 (78.52-81.24) |
| Ad26.COV2.S | 14,870 | Complete | 82.18 (81.39-82.94) | 77.33 (72.91-81.03) | 85.79 (80.1-89.86) |
CI, confidence interval
Figure 3.
Estimated vaccine effectiveness against SARS-CoV-2 infection for nationally available COVID-19 vaccines stratified by predominant circulating SARS-CoV-2 variant (B.1.1.519 vs B.1.617.2, a), age group (over vs under 60 years, b) and diabetes status (c).
mRNA, messenger RNA.
For the ChAdOx1 vaccine, the effectiveness against SARS-CoV-2 infection in partially vaccinated individuals was 61.49% (95% CI 61.14-61.83), which increased in fully vaccinated individuals with a two-dose regimen to 80.79% (95% CI 80.43-81.14). Regarding hospitalization, the ChAdOx1 vaccine reached an effectiveness of 80.23% (95% CI 79.29-81.13) against COVID-19-related hospitalization and 86.81% (95% CI 85.89-87.67) for mortality. A lower effectiveness was observed for adults aged ≥60 years and those with diabetes or obesity, as well as during the predominance of the Delta variant, for both SARS-CoV-2 infection and COVID-19-related mortality (Figure 3, Supplementary Material).
The rAd26 first-dose component of the Gam-COVID-Vac vaccine reached an effectiveness of 67.73% (95% CI 66.88-68.57) against SARS-CoV-2 infection and increased with the second rAd5 component to 78.75% (95% CI 78.17-79.31). The effectiveness for hospitalization in fully vaccinated individuals was 81.38% (95% CI 79.45-83.13) and 87.7% (95% CI 85.82-89.33) for mortality. The effectiveness in fully vaccinated individuals with the Gam-COVID-Vac vaccine against SARS-CoV-2 infection markedly decreased for adults aged ≥60 years and those with obesity or diabetes or during the periods of Delta variant predominance (Figure 3a). A decreased effectiveness was also observed for COVID-19 mortality for these evaluated categories, except for periods of Delta predominance (Supplementary Material).
The inactivated SARS-CoV-2 CoronaVac vaccine reported an effectiveness of 53.41% (95% CI 52.41-54.39) for SARS-CoV-2 infection in partially vaccinated and 71.93% (95% CI 71.35-72.51) in fully vaccinated individuals. The protection against COVID-19-related hospitalization in fully vaccinated individuals was 73.76% (95% CI 72.49-74.96) and 80.38% (95% CI 79.04-81.64) for mortality. CoronaVac showed a decreased effectiveness in adults aged >60 years with obesity or those with diabetes for both SARS-CoV-2 infection and COVID-19-related death. Nevertheless, this vaccine did not display a decreased effectiveness during the periods of predominance of the Delta variant (Figure 3a).
Finally, for the mRNA-1273 vaccine, we observed the highest effectiveness against SARS-CoV-2 infection in partially vaccinated individuals with 87.48% (95% CI 85.13-89.45), which increased to 91.45% (95% CI 90.34-92.43) in fully vaccinated individuals. For the protection against COVID-19-related hospitalization, the mRNA-1273 vaccine reached 78.0% (95% CI 69.01-84.38) effectiveness and 93.46% (95% CI 85.96-96.95) for mortality in fully vaccinated individuals. A decreased effectiveness was only observed for adults aged ≥60 years for SARS-CoV-2 infection, with stable effectiveness for all other evaluated categories (Figure 3b, Supplementary Material).
VE for nationally available one-dose vaccines
Regarding the Ad5-nCoV vaccine, we observed an effectiveness for SARS-CoV-2 infection of 70.5% (95% CI 70.09-70.9), 72.31% (95% CI 71.1-73.47) for hospitalization, and 79.93% (95% CI 78.52-81.24) for mortality (Table 1). The effectiveness against SARS-CoV-2 infection decreased for adults aged ≥60 years, those with diabetes, and during predominance of the Delta variant (Figure 3); however, against COVID-19-related death, this reduction was only observed in adults aged ≥60 years or cases with diabetes, with stable effectiveness during periods with predominance of the Delta compared with the B.1.1.519 variant (Supplementary Material).
Finally, the Ad26.COV2.S vaccine yielded the second highest effectiveness against SARS-CoV-2 infection, with 82.18% (95% CI 81.39-82.94), 77.33% (95% CI 72.91-81.03) for hospitalization, and 85.79% (95% CI 80.1-89.86) for mortality. For SARS-CoV-2 infection, this vaccine displayed stable effectiveness for all evaluated categories, with decreased effectiveness only for individuals with diabetes against COVID-19-related mortality.
Discussion
In this study of 793,487 vaccinated individuals aged ≥18 years compared with 4,792,338 unvaccinated controls, we provided the estimates of the effectiveness of seven COVID-19 vaccines for the prevention of laboratory-confirmed SARS-CoV-2 infection, as well as COVID-19-related hospitalization and all-cause mortality, implemented within the primary national COVID-19 vaccination in Mexico. Overall, all vaccines implemented in the Mexican National Vaccination Program proved to be effective in protecting against all evaluated outcomes in fully vaccinated adults; VE for SARS-CoV-2 infection was reduced in for adults aged ≥60 years, people living with diabetes, and during periods with predominance of the Delta compared with the B.1.1.519 variants of SARS-CoV-2 in Mexico. To the best of our knowledge, ours is the first study to additionally report estimates of effectiveness for the heterologous Gam-COVID-Vac and the Ad5-nCOV vaccines, which were widely used in Mexico to rapidly increase vaccine coverage, which proved to be effective against all evaluated outcomes as previously observed for the Gam-COVID-Vac vaccine in Argentina [28]. Notably, the effectiveness of COVID-19 vaccines in Mexico has been reported to be sustained for booster vaccination and provide additional protection in individuals with previous SARS-CoV-2 infection [14].
The VE estimates in Mexico were similar to those reported in other countries and settings [23,[25], [26], [27], [28], [29],40], with similar reductions of effectiveness for periods of increased circulation of the Delta variant of SARS-CoV-2 [41], [42], [43]. Notably, all estimates of VE observed in our study coincide with the circulation of two main SARS-CoV-2 variants, the B.1.1.519, which was highly prevalent in Mexico from December 2020 until July 2021 [44,45], and the Delta variant (B.1.617.2) [46]. The estimates for VE for SARS-CoV-2 infection for all vaccines were generally lower for Delta than the B.1.1.519 variant. Interestingly, the effectiveness against COVID-19-related mortality was surprisingly lower for B.1.1.519, confirming previous data suggesting increased severity for cases infected with this variant, which comprised up to 89% of cases in Mexico by February 2021 [44].
We also report on a reduction in the VE against SARS-CoV-2 infection and COVID-19-related hospitalization and death for most evaluated vaccines in older adults and persons living with diabetes. Previous data had shown that the waning of vaccine-induced immunity was associated with increased age and comorbidity [47]. Our observations of reduced VE in people living with diabetes also supports previous evidence, which reported that reduced neutralizing antibody response and immunogenicity were observed in people with diabetes [48]. These results are particularly relevant in Mexico, where diabetes is a leading cause of morbidity, increasing the risk of severe COVID-19 and COVID-19 mortality [1,38,49]. Reductions in the VE against SARS-CoV-2 infection were most pronounced for the ChAdOx1, Gam-COVID-Vac, CoronaVac, and BNT162b2 vaccines, with more stability for the Ad26.COV2.S, Ad5-nCoV, and mRNA-1273 vaccines both for older adults and persons living with diabetes. Of note, partial vaccination for the Gam-COVID-Vac vaccine can also be considered a primary full dose of the Sputnik Light vaccine, which has also proved to be effective at a population level [25]. These results should be considered when evaluating prioritization and eligibility for vaccine boosters, which should be allocated to higher-risk individuals.
Our study had some strengths and limitations. Among the strengths, we highlight the use of a nationwide surveillance system to monitor SARS-CoV-2 infections, which can capture the dynamics of COVID-19 transmission at the municipal and even local level, allowing the evaluation of vaccination status and clinical follow-up of all suspected, confirmed, and negative COVID-19 cases in Mexico within the SISVER registry. This allowed us to evaluate the three relevant outcomes for VE, including SARS-CoV-2 infection, COVID-19-related hospitalization, and all-cause death at a national level [31]. Furthermore, by evaluating a national vaccination campaign, which incorporated multiple vaccines, we were able to assess the feasibility of incorporating multiple vaccines simultaneously to maximize vaccination coverage, which will also be helpful when evaluating policies to implement the heterologous schemes for vaccine boosters in the future. Finally, by incorporating a time-varying component to the vaccination status, we were able to capture shifts in exposure risks through the follow-up time to allow more precise estimates of the VE for all evaluated outcomes. We would also like to acknowledge some limitations, which should be considered to adequately interpret our results. First, the SISVER dataset is a sentinel surveillance system, which is designed to evaluate high-risk symptomatic cases, which are assessed and tested for COVID-19 [36]; because of its design, the SISVER primarily identifies cases with moderate to severe COVID-19, which may skew our observations toward more severe cases and under-represented mild and asymptomatic infections, which represent most SARS-CoV-2 cases; therefore, we are unable to distinguish the VE in symptomatic versus asymptomatic SARS-CoV-2 infections [50]. This may also lead to a health care seeking bias, whereby the identification of cases who did not seek testing or in-hospital care may be under-represented in our estimation [31]. Second, the information regarding vaccination status and date was collected during the epidemiological characterization of SARS-CoV-2 cases and relies on accurate self-reporting by cases, which precludes precise evaluation of VE waning through time, which should be evaluated in further studies and which may influence the precision of the VE estimates. Third, the estimates of hospitalization should be taken with caution, particularly considering the effect of hospital saturation in admittance of COVID-19 cases in Mexico during the second and third waves of the COVID-19 pandemic, which are included in the study and that our study was not designed to distinguish cases, which were hospitalized due to other causes but had comorbid COVID-19 [3,51,52]. Fourth, because the vaccination rollout in Mexico has been sequential and was based on age categories, representation of vaccinated cases of patients aged under 29 years has been low in comparison to the incidence of COVID-19 in this age group, which is heavily represented in the unvaccinated group. Even though all our models were adjusted for age, the possibility of residual confounding in our estimations cannot be formally ruled out. When we analyzed the influence of the circulation of predominant SARS-CoV-2 variants, our primary assumption was that the infection was most likely caused by the dominant variant during each evaluated period, a method which has been previously implemented to analyze evasion of circulating variants at the population level [44]. Despite being a widely used method, our approach does not have the degree of precision of genomic sequencing and cannot allow to make precise inferences on the degree of immune evasion or decreased protection for all evaluated vaccines. Finally, because of the period we evaluated in our study, we were unable to estimate the effectiveness during periods of increased circulation of the Omicron SARS-CoV-2 variant in Mexico, which had reported increased immune evasion and reduced VE for infection in different settings and which also presented an increased risk of reinfection and a wider diversity of hybrid immunity phenotypes [14,53,54]. Further evaluation is required to assess the impact of the Omicron variant in VE in Mexico and the potential role of vaccine boosters in increasing protection against infection and severe forms of COVID-19 associated with this variant, as well as the impact of previous infection in modifying VE [55].
In conclusion, all the nationally available vaccines in Mexico were protective against SARS-CoV-2 infection and COVID-19-related hospitalization and death. This is the first report to simultaneously evaluate the effectiveness of seven different vaccines and among the first population-based reports for the effectiveness of the Gam-COVID-Vac and Ad5-nCoV vaccines. The VE against SARS-CoV-2 infection was lower in periods of predominant circulation of the Delta variant than periods of B.1.1.519 predominance; however, most vaccines were more protective against COVID-19-related death during periods of high Delta circulation than B.1.1.519. Similarly, the VE against SARS-CoV-2 infection and COVID-19-related death was lower for adults ≥60 years and people living with diabetes for most evaluated vaccine products, which should be taken into consideration when evaluating eligibility for further vaccine rollout and booster allocation.
Declaration of competing interest
SECL, GCS, CAZJ, GGR, RCA, and HLG works at the Mexican Ministry of Health and oversee the monitoring SARS-CoV-2 epidemiological surveillance and designing COVID-19 vaccination policies in Mexico. Data analysis and interpretation were primarily conducted by members of the team independent of these duties.
Acknowledgments
Funding
This research was supported by Instituto Nacional de Geriatría.
Ethical approval
This study was approved by the Research and Ethics Committee at Instituto Nacional de Geriatría, project number DI-PI-006/2020. Due to the retrospective nature of the study and the use of anonymized registry data, written informed consent from the study populations is not formally required.
Acknowledgments
This project was registered and approved by the Research Committee at Instituto Nacional de Geriatría, project number DI-PI-006/2020. NEAV and CAFM are enrolled at the PECEM Program of the Faculty of Medicine at National Autonomous University of Mexico. NEAV is supported by CONACyT. The authors would like to acknowledge the invaluable work of all of Mexico's health care community in managing the COVID-19 pandemic; their participation in the COVID-19 surveillance program alongside with open data sharing has made this work a reality; the authors are thankful for their effort.
Author contributions
Research idea and study design: OYBC, NEAV, SIVF, CAFM, LFC, DRG, LMGR; data acquisition: JAMG, SECL, SECL, CAZJ, GCS, GGR, RCA, HLG, LMGR; analysis/interpretation: OYBC, NEAV, SIVF, CAFM, LFC, DRG; statistical analysis: OYBC, NEAV; manuscript drafting: OYBC, NEAV, CAFM, LFC, DRG, SIVF, LMGR, JMG, AKG, JAMG,SECL, HLG, LMGR, JAAF, CHZG, GRT; supervision or mentorship: OYBC, LMGR, SECL, CAZJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
DATA AVAILABILITY: Code and materials are available for reproducibility of results at https://github.com/oyaxbell/covid_vaccines/. Data are available upon request to the General Directorate of Epidemiology of the Mexican Ministry of Health.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.01.040.
Appendix. Supplementary materials
References
- 1.Bello-Chavolla OY, Bahena-López JP, NE Antonio-Villa, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105:2752–2761. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bello-Chavolla OY, González-Díaz A, NE Antonio-Villa, Fermín-Martínez CA, Márquez-Salinas A, Vargas-Vázquez A, et al. Unequal impact of structural health determinants and comorbidity on COVID-19 severity and lethality in older Mexican adults: considerations beyond chronological aging. J Gerontol A Biol Sci Med Sci. 2021;76:e52–e59. doi: 10.1093/gerona/glaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NE Antonio-Villa, Fernandez-Chirino L, Pisanty-Alatorre J, Mancilla-Galindo J, Kammar-García A, Vargas-Vázquez A, et al. Comprehensive evaluation of the impact of sociodemographic inequalities on adverse outcomes and excess mortality during the COVID-19 pandemic in Mexico City. Clin Infect Dis. 2021;74:785–792. doi: 10.1093/cid/ciab577. [DOI] [PubMed] [Google Scholar]
- 4.Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021;73:2257–2264. doi: 10.1093/cid/ciab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . WHO Regional Office for the Western Pacific; Geneva: 2020. Regional office for the western Pacific. Routine immunization services during the COVID-19 pandemic. [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med. 2022;386:847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21:1654–1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laine C, Cotton D, Moyer DV. COVID-19 vaccine: promoting vaccine acceptance. Ann Intern Med. 2021;174:252–253. doi: 10.7326/M20-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montes-González JA, Zaragoza-Jiménez CA, NE Antonio-Villa, Fermín-Martínez CA, Ramírez-García D, Vargas-Vázquez A, et al. Protection of hybrid immunity against SARS-CoV-2 reinfection and severe COVID-19 during periods of Omicron variant predominance in Mexico. medRxiv. 03 December 2022 doi: 10.3389/fpubh.2023.1146059. https://www.medrxiv.org/content/10.1101/2022.12.02.22282981v1 [accessed DD Month YYYY] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Raddad LJ, Chemaitelly H, Butt AA. National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21:1529–1538. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on Covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and moderna COVID-19 vaccines among health care personnel - 33 U.S. Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:753–758. doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative effectiveness of moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions - United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corchado-Garcia J, Zemmour D, Hughes T, Bandi H, Cristea-Platon T, Lenehan P, et al. Analysis of the effectiveness of the Ad26.COV2.S Adenoviral Vector Vaccine for Preventing COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naranbhai V, Garcia-Beltran WF, Chang CC, Berrios Mairena C, Thierauf JC, Kirkpatrick G, et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J Infect Dis. 2022;225:1141–1150. doi: 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González S, Olszevicki S, Salazar M, Calabria A, Regairaz L, Marín L, et al. Effectiveness of the first component of Gam-COVID-Vac (Sputnik V) on reduction of SARS-CoV-2 confirmed infections, hospitalisations and mortality in patients aged 60–79: a retrospective cohort study in Argentina. EClinicalmedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranzani OT, Hitchings MDT, Dorion M, D'Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of Covid-19 in Brazil: test negative case-control study. BMJ. 2021;374:n2015. doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rearte A, Castelli JM, Rearte R, Fuentes N, Pennini V, Pesce M, et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. Lancet. 2022;399:1254–1264. doi: 10.1016/S0140-6736(22)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arregocés-Castillo L, Fernández-Niño J, Rojas-Botero M, Palacios-Clavijo A, Galvis-Pedraza M, Rincón-Medrano L, et al. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the Esperanza cohort. Lancet Healthy Longev. 2022;3 doi: 10.1016/S2666-7568(22)00035-6. e242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson VL, Camacho Franco MA, Bautista Márquez A, Martínez Valdez L, Castro Ceronio LE, Cruz Cruz V, et al. Vaccine effectiveness of CanSino (Adv5-nCoV) coronavirus disease 2019 (COVID-19) vaccine among childcare workers-Mexico, March–December 2021. Clin Infect Dis. 2022;75:S167–S173. doi: 10.1093/cid/ciac488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel MK, Bergeri I, Bresee JS, Cowling BJ, Crowcroft NS, Fahmy K, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the World Health Organization. Vaccine. 2021;39:4013–4024. doi: 10.1016/j.vaccine.2021.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salud Nicio. Información de la vacuna – vacuna Covid, http://vacunacovid.gob.mx/wordpress/informacion-de-la-vacuna/; n.d. [accessed 17 February 2022].
- 33.Gobieeno De Mexico, Sanitarios CF para la P contra R. COFEPRIS emite autorización para uso de emergencia a vacuna moderna, gob.mx, http://www.gob.mx/cofepris/articulos/cofepris-emite-autorizacion-para-uso-de-emergencia-a-vacuna-moderna?idiom=es; n.d. [accessed 21 February 2022].
- 34.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus pandemic (COVID-19) Our World Data. 2020 https://ourworldindata.org/coronavirus [accessed 21 February 2022] [Google Scholar]
- 35.Olaiz-Fernández G, Vicuña de Anda FJ, Diaz-Ramirez JB, Fajardo Dolci GE, Bautista-Carbajal P, Angel-Ambrocio AH, et al. Effect of Omicron on the prevalence of COVID-19 in international travelers at the Mexico City international airport. December 16th, 2021 to January 31st, 2022. Travel Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bello-Chavolla OY, NE Antonio-Villa, Fernández-Chirino L, Guerra EC, Fermín-Martínez CA, Márquez-Salinas A, et al. Diagnostic performance and clinical implications of rapid SARS-CoV-2 antigen testing in Mexico using real-world nationwide COVID-19 registry data. PLoS One. 2021;16 doi: 10.1371/journal.pone.0256447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MexCoV2. Consorcio Mexicano de Vigilancia Genómica (CoViGen-Mex). 2022. http://mexcov2.ibt.unam.mx:8080/COVID-TRACKER/ [accessed 18 February 2022].
- 38.Vargas-Vázquez A, Bello-Chavolla OY, Ortiz-Brizuela E, Campos-Muñoz A, Mehta R, Villanueva-Reza M, et al. Impact of undiagnosed type 2 diabetes and pre-diabetes on severity and mortality for SARS-CoV-2 infection. BMJ Open Diabetes Res Care. 2021;9 doi: 10.1136/bmjdrc-2020-002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta R, Bello-Chavolla OY, Mancillas-Adame L, Rodriguez-Flores M, Pedraza NR, Encinas BR, et al. Epicardial adipose tissue thickness is associated with increased COVID-19 severity and mortality. Int J Obes (Lond) 2022;46:866–873. doi: 10.1038/s41366-021-01050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin DY, Gu Y, Wheeler B, Young H, Holloway S, Sunny SK, et al. Effectiveness of Covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386:933–941. doi: 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nanishi E, Levy O, Ozonoff A. Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: a rapid review. Hum Vaccin Immunother. 2022;18 doi: 10.1080/21645515.2022.2045857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murillo-Zamora E, Sánchez-Piña RA, Trujillo X, Huerta M, Ríos-Silva M, Mendoza-Cano O. Independent risk factors of COVID-19 pneumonia in vaccinated Mexican adults. Int J Infect Dis. 2022;118:244–246. doi: 10.1016/j.ijid.2022.02.003. S1201-9712(22)00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cedro-Tanda A, Gómez-Romero L, Alcaraz N, de Anda-Jauregui G, Peñaloza F, Moreno B, et al. The evolutionary landscape of SARS-CoV-2 variant B.1.1.519 and its clinical impact in Mexico City. Viruses. 2021;13:2182. doi: 10.3390/v13112182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-Maldonado AP, Vázquez-Pérez JA, Cedro-Tanda A, Taboada B, Boukadida C, Wong-Arámbula C, et al. Emergence and spread of the potential variant of interest (VOI) B.1.1.519 of SARS-CoV-2 predominantly present in Mexico. Arch Virol. 2021;166:3173–3177. doi: 10.1007/s00705-021-05208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Péré H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Lorière E, Rey FA, Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 47.Fabiani M, Puopolo M, Morciano C, Spuri M, Spila Alegiani S, Filia A, et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe Covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soetedjo NNM, Iryaningrum MR, Lawrensia S, Permana H. Antibody response following SARS-CoV-2 vaccination among patients with type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr. 2022;16 doi: 10.1016/j.dsx.2022.102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bello-Chavolla OY, Rojas-Martinez R, Aguilar-Salinas CA, Hernández-Avila M. Epidemiology of diabetes mellitus in Mexico. Nutr Rev. 2017;75:4–12. doi: 10.1093/nutrit/nuw030. [DOI] [PubMed] [Google Scholar]
- 50.Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Fermín-Martínez CA, Márquez-Salinas A, Bahena-López JP. Profiling cases with nonrespiratory symptoms and asymptomatic severe acute respiratory syndrome coronavirus 2 infections in Mexico City. Clin Infect Dis. 2021;72:e655–e658. doi: 10.1093/cid/ciaa1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antonio-Villa NE, Bello-Chavolla OY, Fermín-Martínez CA, Aburto JM, Fernández-Chirino L, Ramírez-García D, et al. Socio-demographic inequalities and excess non-COVID-19 mortality during the COVID-19 pandemic: a data-driven analysis of 1 069 174 death certificates in Mexico. Int J Epidemiol. 2022;51:1711–1721. doi: 10.1093/ije/dyac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bello-Chavolla OY, NE Antonio-Villa, Fermín-Martínez CA, Fernández-Chirino L, Vargas-Vázquez A, Ramírez-García D, et al. Diabetes-related excess mortality in Mexico: a comparative analysis of national death registries between 2017–2019 and 2020. Diabetes Care. 2022;45:2957–2966. doi: 10.2337/dc22-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.