Abstract
Long-term clinical repair of articular cartilage remains elusive despite advances in cartilage tissue engineering. Only one cartilage repair therapy classified as a “cellular and gene therapy product” has obtained Food and Drug Administration (FDA) approval within the past decade although over 200 large animal cartilage repair studies were published. Here, we identify the challenges impeding translation of strategies and technologies for cell-based cartilage repair, such as the disconnect between university funding and regulatory requirements. Understanding the barriers to translation and developing novel solutions to address them will be critical for advancing cell therapy products for cartilage repair to clinical use.
One sentence summary:
Bottlenecks in preclinical research on cell therapy products for cartilage repair must be addressed to bridge the gap to clinical application.
INTRODUCTION
Despite major advances in cell and tissue research in recent decades, to date, the FDA has approved only 23 cellular and gene therapy products (1). While the Agency provides guidance documents toward developing cell therapy products (2, 3), interpreting such documents can be difficult given the lack of predicate therapies. When researchers seek to obtain regulatory approval for a product, they must navigate guidance documents that apply to a broad spectrum of therapies, determine what is applicable for their specific product, and design preclinical studies to support an application for clinical trials. This task can be daunting to researchers without a regulatory background. The objective of this review is to discuss the main bottlenecks that hinder the translation of cell therapy products from basic research to clinical trials, with a specific focus on the regulatory guidance for cartilage repair therapies.
In the early 1990s when the term tissue engineering came to denote the fabrication of tissues using cells, scaffolds, and bioactive signals, it was predicted that cartilage would be one of the first tissues to be successfully regenerated (4). Twenty years later, we published a review highlighting the many challenges of regenerating cartilage despite these predictions (5). Today, nearly three decades later, only one cellular and gene therapy product is approved for cartilage repair (matrix-induced autologous chondrocyte implantation, MACI, Vericel Corp) despite a wealth of basic science research within the field of cartilage repair and the clear clinical need for additional treatments.
Hyaline articular cartilage is a highly specialized connective tissue that facilitates joint movement by providing lubrication between bones, reducing friction, distributing forces, and preventing wear within the articulation (6). It is composed of water (70–80%), collagen type II (~20%), proteoglycans (~5%), chondrocytes (~2%), and trace amounts of minor collagen subtypes (7, 8). Articular cartilage is susceptible to injury and osteoarthritis (OA); in the United States alone, about 78 million adults will be diagnosed with arthritis by 2040 (9). Current treatment options for early-stage articular cartilage defects and OA include allografts, autografts, microfracture, and MACI. The use of cartilage allografts and autografts is restricted by tissue scarcity and donor-site morbidity, respectively (10–12). Microfracture induces fibrocartilage repair tissue with inadequate mechanical properties and a propensity to degenerate (13, 14). MACI, a therapy that uses expanded autologous chondrocytes seeded on a porcine collagen type I and III membrane, is limited in that it requires two surgeries and may cause donor-site morbidity (15–18). Because these treatment options are not ideal, the development and translation of new strategies to treat cartilage ailments would have a notable impact on public health.
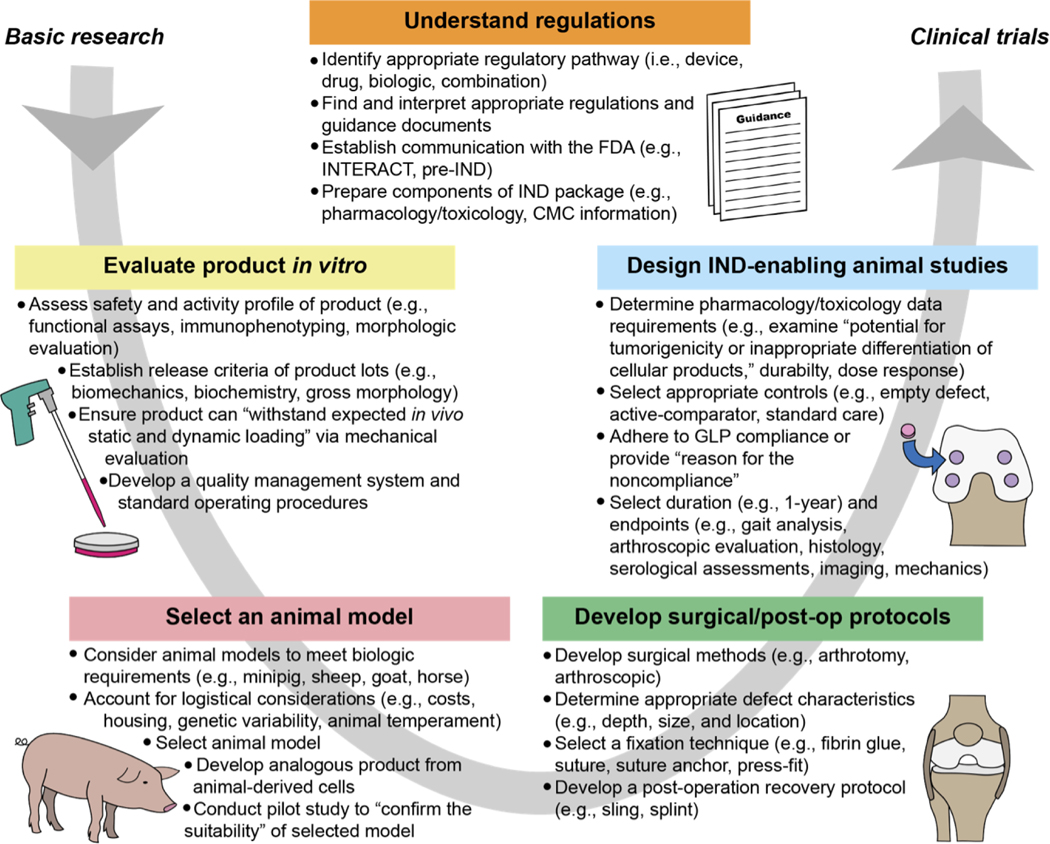
In this review, we will provide context and perspective on the main barriers to clinical translation of articular cartilage repair products, as overviewed in Figure 1. First, the regulatory structure of the FDA with respect to review of cartilage repair products will be presented, followed by a description of in vitro evaluations to conduct before proceeding with animal studies. Next, large animal models for cartilage repair studies will be discussed, including species-specific advantages and disadvantages, key considerations for the surgical approach in these animal models, and in vivo experimental designs. Finally, we will provide perspective on the challenges that the field faces and steps that may be taken to increase the chances of future translation of cartilage repair products.
Fig. 1. Bottlenecks in translating biologic cartilage repair products from basic research to clinical trials.
Researchers must navigate the many challenges within this process at the stages of in vitro research, animal model selection, surgical development, and the conduct of IND-enabling animal studies. All stages should be conducted in accordance with appropriate regulations and guidance. This figure captures the salient aspects of this process, but for additional information guidance documents should be consulted. All quotations within this figure were taken from guidance documents (2, 3). Abbreviations: INitial Targeted Engagement for Regulatory Advice on CBER producTs (INTERACT), Chemistry, Manufacturing, and Controls (CMC), Good Laboratory Practice (GLP), Investigational New Drug (IND).
FDA REGULATION OF CARTILAGE REPAIR PRODUCTS
FDA structure.
In the United States, the FDA regulates all medical products including cartilage-repair products. Three of the FDA’s centers are particularly relevant for cartilage products: the Center for Biologics Evaluation and Research (CBER), the Center for Drug Evaluation and Research (CDER), and the Center for Devices and Radiological Health (CDRH) (19). Products classified as a biologic, drug, or device will be assigned to CBER, CDER, and CDRH, respectively. If assigned to CDRH, the product will also be subject to device classifications (i.e., Class I, II, and III) that depend on the risk the device poses to the patient (20). Products may be combination products (e.g., biologic and device) in which case the primary mechanism of action will be used by the Office of Combination Products to determine assignment to a center with primary jurisdiction. Each center has a different set of applications that must be completed to obtain approval for clinical studies and commercialization, as depicted in Figure 2A. For example, a product classified as a biologic will be regulated by CBER and will require an Investigational New Drug (IND) application before clinical studies and a Biologics License Application (BLA) before market approval.
Fig. 2. FDA organizational structure as it relates to biologic cartilage repair products.
(A) The sequence of studies, applications, and meetings that products proceeding through Center for Biologics Evaluation and Research (CBER), the Center for Drug Evaluation and Research (CDER), and the Center for Devices and Radiological Health (CDRH) regulatory pathways must follow. Before clinical trials, an Investigational New Drug (IND) application or Investigational Device Exemption (IDE) application must be filed. Upon completion of clinical trials and before marketing a Premarket Approval (PMA), Biologics License Application (BLA), or New Drug Application (NDA) must be filed. (B) The two pathways of human cell, tissue, and cellular and tissue-based product (HCT/P) approval and cartilage repair products that have utilized these mechanisms. The 361 HCT/P pathway includes minimally processed products for homologous use. All other products must follow the 351 HCT/P pathway, which requires preclinical and clinical data.
Meetings with the FDA are held at specific times along the regulatory pathway. Optional informal meetings are offered by each center, e.g., INitial Targeted Engagement for Regulatory Advice on CBER producTs (INTERACT) for CBER, to obtain initial guidance during development. The first formal meeting within the CBER and CDER pathways is the pre-IND meeting. Other key meetings during the regulatory process are depicted in Figure 2A. There are several expedited programs that can be considered when applying for biologic or drug approval: Fast Track designation, Breakthrough Therapy designation, regenerative medicine advance therapy (RMAT) designation, priority review, and accelerated review (Table 1) (21). While each expedited program has specific criteria for acceptance and varying benefits, these programs, overall, are intended to reduce the time to market and may allow for more directed guidance from the Agency. Navigating the regulatory process for cellular and gene therapy products may be challenging because the research on these products is rapidly evolving and there has been little historical precedent on this matter.
Table 1.
Expedited programs that are offered by the FDA for products classified as biologics
| Program | Requirements | Benefits |
|---|---|---|
|
| ||
| Fast Track designation | • Treats a serious or life-threatening condition • Addresses an unmet clinical need or provides an advantage over available therapies |
• Close communication with FDA throughout development • Rolling review of BLA • Eligibility for priority review and accelerated approval of BLA |
| Breakthrough Therapy designation | • Treats a serious or life-threatening condition • Provides substantial improvement over available therapies |
• All the benefits of Fast Track designation • Intensive FDA guidance • Organizational involvement of senior management in facilitating product’s development |
| RMAT (regenerative medicine advanced therapy) designation | • Is a regenerative medicine therapy • Treats a serious or life-threatening condition • Preliminary clinical evidence indicates the product has potential to address unmet clinical needs |
• All the benefits of Breakthrough Therapy designation |
| Priority review | • Previously received Fast Track, Breakthrough, or RMAT designation • Treats a serious or life-threatening condition • Provides a significant improvement in safety or effectiveness of the treatment of a condition |
• BLA application will be reviewed within 6 months |
| Accelerated review | • Treats a serious or life-threatening condition • Provides therapeutic advantage over available therapies • Demonstrates effect on a surrogate endpoint or clinical endpoint that can be measured earlier than irreversible morbidity and mortality |
• Approval based on surrogate endpoint |
Summary of approved cellular and gene therapy products.
To date, the FDA has approved 23 cellular and gene therapy products (1). Of these products, eight are allogeneic cord blood cell therapy products, six are T cell therapy products, and three are viral therapies. Other products include Laviv (autologous fibroblasts to reduce the appearance of nasolabial folds), Rethymic (human allogeneic thymus tissue to treat congenital athymia), Gintuit (allogeneic cultured keratinocytes and fibroblasts for treatment of mucogingival conditions), Stratagraft (allogeneic cultured keratinocytes and dermal fibroblasts for treatment of deep partial-thickness burns), and MACI. Table 2A provides details on each product and the preclinical studies performed for each product as found within their FDA documentation (note cord blood cell therapies were excluded).
Table 2.
Preclinical data for cell and gene therapy products including MACI.
| A. Summarized preclinical data submitted by FDA-approved cellular and gene therapy products . CAR, Chimeric antigen receptor. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Company PRODUCT NAME BLA approval date | Product description | Indication | Prior clinical use | Number of preclinical studies In vitro/in vivo | Animal model(s) | Pharmacology tests | Toxicology tests | Activity tests | Analogous animal product | Control groups | Study duration | Sex | GLP | Refs |
|
| ||||||||||||||
| CELLULAR PRODUCTS (TISSUE ENGINEERING) | ||||||||||||||
|
| ||||||||||||||
| MACI Vericel Corp. Dec. 13, 2016 | Autologous cultured chondrocytes on a porcine collagen membrane | Repair of single or multiple symptomatic, full-thickness cartilage defects of the knee with or without bone involvement | Yes | 4 0/4 |
Rabbit Horse |
No | Yes | Yes | Yes | Empty defect, ACI-Maix, micro-fracture |
12–53 wks | M + F |
No | (104) |
|
GINTUIT Organogenesis Inc. Mar. 9, 2012 |
Allogeneic cultured keratinocytes and fibroblasts in bovine collagen | Topical (non-submerged) application to a surgically created vascular wound bed in the treatment of mucogingival conditions | Yes | 26 ≥12/≥9 |
Mouse | Yes | Yes | Yes | ■ | ■ | 7–28 days or 3–6 mos |
■ | No† | (105) |
|
STRATAGRAFT Stratatech Corp June 15, 2021 |
Allogeneic cultured keratinocytes and dermal fibroblasts in murine “collagen-dsat” | Treatment of thermal burns containing intact dermal elements for which surgical intervention is clinically indicated (deep partial-thickness burns) | No | 18 ≥8/≥4 |
Mouse | No | Yes | No | ■ | No controls | 7–421 days | M + F |
No | (106) |
|
LAVIV Fibrocell Technologies, Inc. June 21, 2011 |
Autologous cellular product | Improvement of the appearance of moderate to severe nasolabial fold wrinkles | Yes | 0 | No preclinical studies performed due to previous human experience, the autologous nature of the product, and the lack of a proper animal model | (116) | ||||||||
|
| ||||||||||||||
|
RETHYMIC Enzyvant Therapeutics GmbH Oct. 8, 2021 |
Allogeneic processed thymus tissue | Immune reconstitution in pediatric patients with congenital athymia | No | 2 1/1 |
Rat | Yes | No | Yes | Yes | Non-trans-planted | 2–294 days | M | ■ | (117) |
|
| ||||||||||||||
| CELLULAR PRODUCTS (IMMUNOTHERAPY) | ||||||||||||||
|
| ||||||||||||||
|
ABECMA Celgene Corporation Mar. 26, 2021 |
B cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy | Treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy* | No | 12 ≥6/≥6 |
Mouse | Yes | Yes | Yes | No | Vehicle, Positive (Borte-zomib), Negative (anti-CD19 CAR T cells) |
23–85 days | F† | ■ | (107) |
|
BREYANZI Juno Therapeutics, Inc. Feb. 5, 2021 |
CD19-directed genetically modified autologous T cell immunotherapy | Treatment of patients with relapsed or refractory large B cell lymphoma after two or more lines of systemic therapy* | No | 28 22/6 |
Mouse | Yes | Yes | Yes | ■ | Vehicle, Mock trans-duced cells, No control |
14–100 days | F† | No† | (108) |
|
CARVYKTI Janssen Biotech Inc. Feb. 28, 2022 |
B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy |
Treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy |
No | 22 19/3 |
Mouse NHP |
Yes | Yes | Yes | No |
UnT cells, Salt solution (HBSS), or none |
55–98 days | M + F |
No | (118) |
|
KYMRIAH Novartis Pharmaceuticals Corp. Aug. 30, 2017 |
CD19-directed genetically modified autologous T cell resolution immunotherapy | Treatment of acute lymphoblastic leukemia and relapsed or refractory large B cell lymphoma | No | ■ | Mouse | Yes | Yes | Yes | ■ | ■ | ■ | ■ | ■ | (119) |
|
PROVENGE Dendreon Corp. Apr. 29, 2010 |
Autologous cellular immunotherapy | Treatment of asymptomatic or minimally symptomatic metastatic castrate resistant (hormone refractory) prostate cancer | No | 6 1/5 |
Mouse Rat |
Yes | No | Yes | Yes | ■ | ■ | M | No | (120) |
|
TECARTUS Kite Pharma, Inc. Oct. 1, 2021 |
CD19-directed genetically modified autologous T cell immunotherapy | Treatment of patients with relapsed or refractory mantle cell lymphoma and patients with relapsed or refractory B cell precursor acute lymphoblastic leukemia | No | 6 3/3 |
Mouse | Yes | No | Yes | Yes | Untrea-ted or ■ | ■ | ■ | ■ | (121) |
|
YESCARTA Kite Pharma, Inc. Oct. 18, 2017 |
CD19-directed genetically modified autologous T cell immunotherapy | Treatment of patients with relapsed or refractory large B cell lymphoma and patients with relapsed or refractory follicular lymphoma | No | 4 3/1 |
Mouse | Yes | No | Yes | Yes | Non-trans-duced T cells | ■ | ■ | ■ | (122) |
|
| ||||||||||||||
| GENE THERAPY PRODUCTS | ||||||||||||||
|
| ||||||||||||||
|
IMLYGIC Amgen, Inc. Oct. 27, 2015 |
Genetically modified oncolytic viral therapy | Local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma | No | 56 8/48 |
Mouse Rat Dog |
Yes | Yes | Yes | Yes | ■ | 3–85 days | M/ F† |
No† | (123) |
|
LUXTURNA Spark Therapeutics, Inc. Dec. 19, 2017 |
Adeno-associated virus vector-based gene therapy | Treatment of patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy | No | 15 3/12 |
Mouse Dog NHP |
Yes | Yes | Yes | Yes | No injection, Sham injection |
≤6.5 mos | M/ F |
No† | (124) |
|
ZOLGENSMA Novartis Gene Therapies, Inc. May 24, 2019 |
Adeno-associated virus vector-based gene therapy | Treatment of pediatric patients less than 2 years of age with spinal muscular atrophy with biallelic mutations in the survival motor neuron 1 (SMN1) gene | No | 3 0/3 |
Mouse | Yes | Yes | Yes | No | Vehicle or ■ | 3–12 wks | M/ F† |
Yes† | (125) |
| B. Preclinical data submitted by MACI. (104) | |||||
|---|---|---|---|---|---|
|
| |||||
| Preclinical studies | Pharmacology assessment | Toxicology assessment | Activity assessment | Duration | Endpoints |
|
| |||||
| Cellular cartilage repair product | |||||
|
| |||||
| Four studies: Two studies in rabbits (S1 and S2); two studies in horses (S3 and S4). Experiment: implantation of analogous products (same membrane as MACI with rabbit or horse cells) in critical-size defects in the hind limbs. |
Yes. The four preclinical in vivo studies were used to determine safety and/or activity. Pharmacokinetics assessed in S4. No mechanism of action. Please see BLA reviewer’s comment below regarding only considering the horse study for overall safety and activity. |
Yes, S4, plus one additional genotoxicity study (not disclosed), one additional in vitro toxicity study (of fibrin glue on chondrocytes, details not disclosed), and 13 additional biocompatibility studies (not disclosed). No developmental and reproductive toxicity studies and no carcinogenicity/ tumorigenicity studies were performed (due to the avascular local implantation, the terminally differentiated sate of the cells, and the lack of systemic distribution of MACI). |
Yes. S1 and S2 reported an improvement in the histopathology assessment (in-house scoring system) compared to empty defect (S1) and microfracture controls (S2). S3 and S4 report a trend toward improved cartilage repair compared to empty and ACI-Maix controls. S4 further reports near native cartilage appearance, increased GAG, and improved mechanical testing outcomes of the repair tissue. | S1 and S2 (in rabbit): 12 and 24 weeks. S3 and S4 studies (in horse): 24 and 53 weeks. |
For S1: Histopathology For S2: Histopathology, clinical observation, body weights. For S3: Lameness score, clinical pathology, second-look arthroscopy, synovial fluid analysis, synovial membrane histology, gross and microscopic exams, collagen type II IHC. For S4: Clinical observation, mortality, lameness, clinical pathology, synovial fluid analysis, second-look arthroscopy, and terminal assessments (gross pathology, histology, scoring of the repair tissue, glycosaminoglycan, and collagen content, DNA measure, and biomechanical testing). |
|
|
|||||
| Comments: “This reviewer cannot make any definitive conclusions from the study summary provided (S1). The safety and activity profile of MACI will thus be entirely based on the 53-week horse study (Study #4) and the clinical data.” | |||||
Data not disclosed or not available
Predominantly; M, male; F, female. For other FDA-approved Cellular and Gene Therapy (CGT) products please refer to table S1.
Predicate cartilage therapies.
Human cell, tissue, and cellular and tissue-based products (HCT/Ps) are regulated under Section 361 of the Public Health Service (PHS) Act (for minimally manipulated, homologous use products) and Section 351 of the PHS Act (all other products), as illustrated in Figure 2B (22, 23). The 361 HCT/P pathway is intended for minimally processed products that perform the same basic function in the recipient and donor, and these products are subject to relatively less burdensome regulation (e.g., clinical trials not required). Cartilage repair products that have utilized the 361 HCT/P pathway are DeNovo NT, ProChondrix, and Cartiform, all of which consist of minimally manipulated allograft tissue. A product that is not minimally processed must utilize the 351 HCT/P pathway, which requires clinical trials to market. MACI is the only cartilage product that has obtained approval through the 351 HCT/P pathway. It should be noted that, before its approval in 2016, MACI had already been marketed and used in the European Union (EU). However, more stringent requirements for animal testing in the EU have been implemented in recent years (24, 25). Therefore, future products may not be able to follow the same pathway for regulatory approval as MACI. For specific details on MACI preclinical data, see Table 2B. In addition to MACI, NeoCart (Histogenics) pursued FDA approval via the 351 HCT/P pathway. However, this product did not meet the primary endpoints during Phase 3 clinical trials (26) and has not moved forward. While the 361 HCT/P pathway is less financially and technically burdensome to develop than the 351 HCT/P pathway, it does not increase the likelihood that insurance companies will accept the procedure for reimbursement (27). Thus, products that pursued the 361 pathway, such as DeNovo NT, still need to report clinical trial data to increase the likelihood of acceptance by insurance companies (27).
Historically, the Office of Combination Products has determined CBER to have primary jurisdiction over cell therapy products for cartilage repair. With this precedent, future tissue-engineered cartilage repair products will likely be considered primarily as biologics under Section 351 of the PHS, and secondarily a device (Class III). A sponsor (i.e., a person or organization responsible for a clinical investigation) will need to file an IND/Investigational device exemption (IDE) application to begin clinical trials and, eventually, to file a BLA/Premarket Approval (PMA) to request marketing approval. The FDA has issued guidance documents for the types of data and assays that will be required before first-in-human testing can proceed; the next section presents the salient scientific considerations for in vitro preclinical studies.
SCIENTIFIC AND ETHICAL CONSIDERATIONS PRIOR TO CONDUCTING LARGE ANIMAL STUDIES
In vitro development is the first step toward advancing through the FDA regulatory framework, and aspects of in vitro research will be included in the preparation of IND and BLA submissions. Before proceeding with animal studies, it is prudent to conduct a thorough in vitro characterization of the product, perform initial safety evaluations, consider manufacturing requirements of a definitive (i.e., IND-enabling) animal study, and uphold the ethical principles of animal use.
In vitro functional characterization.
Inasmuch as FDA guidance specifically calls for the inclusion of mechanical testing data (2), it is important that tissue engineered products undergo mechanical evaluation. It should be noted, however, that in vitro mechanical testing would not be applicable to products that only consist of a cell slurry. Mechanical characterization of the product aims to determine its biomimicry and “ability to withstand the level of static and dynamic loading expected in vivo” (2). Guidance recommends that static mechanical behavior should be assessed (e.g., Young’s modulus, aggregate modulus) as well as the dynamic properties (e.g., complex shear modulus G*) (2). Although not stated in the guidance document, tribological testing can also be considered to provide friction and lubrication (28). As acknowledged in the guidance, certain types of products such as soft scaffolds and membranes are not capable of withstanding such mechanical tests prior to implantation, for which case the characterization will have to be made at a preclinical or clinical study stage following repair tissue maturation (2). Large animal studies should be preceded with clear hypotheses on how a therapeutic agent would behave mechanically, remain in place, and persist within the in vivo mechanical environment.
The FDA-recommended preclinical assessments for medical devices, pharmaceuticals, and biologics are summarized in Table 3A and cartilage-specific recommendations are presented in Table 3B. While not specified in FDA guidance, a functionality index (FI) can be used to combine multiple outcome measures or endpoints into a single value, as previously described (29, 30). The FI is a weighted average of salient functional outputs directly compared to native tissue properties. The closer the FI value is to 1, the closer the functional properties are to native tissue. Having an FI to distill multiple functional properties into a single value could be useful to establish release criteria for a particular implant design (in in vitro research) or implant batch (in large animal studies, clinical studies, and implant manufacturing upon regulatory approval). Overall, ensuring that the implant’s functional characteristics mimic those of native tissue will likely improve the durability of an implant in vivo.
Table 3. Recommended assessments and measurements/assays for IND applications of cartilage cellular therapy products.
(A) General FDA guidance for medical devices, pharmaceuticals, and biologics. (B) Guidance specific for cartilage cellular therapy products. (C) Literature-based recommendations for cartilage cellular therapy products. ASTM, the American Society for Testing and Materials; H&E, hematoxylin and eosin; DMMB dimethylmethylene blue; GAG, glycosaminoglycan; HPLC, high-performance liquid chromatography; ELISA, enzyme-linked immunoassay.
| Assessment | Measurement / Assay | In vitro | In vivo | Refs |
|---|---|---|---|---|
|
| ||||
| A. Selected preclinical assessments recommended by the FDA for medical devices, pharmaceuticals, and biologics | ||||
|
| ||||
| CMC safety and quality testing | Per requirements of 21 CFR 610, including potency, sterility, purity, identity, stability, mycoplasma, endotoxin, and other adventitious agents | × | - | (3, 126, 127) |
| Genotoxicity | Standard genetic toxicology. Measure chromosomal aberrations using in vitro and in vivo (for mutagenic assessment) tests | × | × | (3, 128, 129) |
| Carcinogenicity | Evaluation of carcinogenic and tumorigenic potential. Early stage in vitro assays (e.g., cell transformation) can be performed. In vivo studies are recommended (e.g., rodent study) | × | × | (3, 128, 130) |
| Toxicity | Chronic toxicity testing (in rodents and non-rodents) | - | × | (3, 128, 131) |
| Toxicokinetics, systemic exposure | Generation of pharmacokinetic data to describe the systemic exposure (in vivo) and its relationship to dose and the time course of the toxicity study | - | × | (128, 132) |
| Safety pharmacology | Identify, evaluate, and investigate the mechanism of any adverse pharmacodynamic effect of a therapy product (i.e. substance) that may have relevance to its human safety | × | × | (133) |
| Developmental and Reproductive toxicity (DART) | Assess the effect of a therapeutic product (i.e., pharmaceutical) on reproduction and development (if applicable). Complete life cycle (in vivo) studies are recommended. Alternative assays (e.g., in vitro, ex vivo) may defer or replace (in certain circumstances) conventional in vivo studies | × | × | (128, 134) |
| Immunotoxicity | Identify compounds which have the potential to be immunotoxic. Assays include T cell-dependent antibody response (TDAR), immunophenotyping, and cell/function activation assays | × | × | (128, 135) |
| Cytotoxicity | Agar diffusion cell culture screening | × | × | (41) |
| Degradation | In vivo degradation assessment recommended for absorbable therapy products | × | (41) | |
|
| ||||
| B. Cartilage-specific recommended assessments (from FDA and ASTM) | ||||
|
| ||||
| Mechanical data | Address the product’s ability to withstand loading, the fixation method, and the propensity to generate wear debris. Compressive testing is specifically recommended to quantify aggregate modulus (HA), shear modulus (μ), permeability (k), dynamic modulus (G*) | × | × | (2) |
| Biological response | Biological activity, including proof-of-concept (treatment feasibility) and safety data | - | × | (2) |
| Durability | Length of time needed to assess repair tissue formation and wear resistance. Large animal models recommended | - | × | (2) |
| Toxicology | Assessment of local and systemic toxicity | - | × | (2) |
| Dose response | Characterization of the therapy product’s components that may affect repair tissue formation. Large animal models recommended | - | × | (2) |
| Arthroscopy and/or MRI | Interim arthroscopic assessments and/or MRI evaluations in the animal studies (to reduce number of animals euthanized at each time) | - | × | (2) |
| Tissue structure | Histological evaluation (macroscopic and microscopic analysis and scoring) | × | × | (111) |
| General biochemical characterization | Biochemical quantification of proteins and proteoglycans in repair tissue compared to native cartilage is recommended but not specified | × | × | (111) |
|
| ||||
| Part C. Recommended assessments for cartilage cellular therapy products (from scientific literature and guidance documents) | ||||
|
| ||||
| Compressive mechanics | Aggregate modulus, shear modulus, and permeability via creep indentation; or instantaneous modulus, relaxation modulus, and coefficient of viscosity via stress-relaxation | × | × | (136, 137) |
| Tensile mechanics | Young’s modulus, ultimate tensile strength, and toughness via uniaxial tensile loading | × | × | (138) |
| Tribology | Coefficient of friction (μ) via tribometer | × | × | (139, 140) |
| Mechanics of fixation | Fixation strength via pull-apart or push-through or lap shear tests | × | - | (141) |
| Mechanics of integration | Interface strength via pull-apart or push-through tests | - | × | (142) |
| Chromosome instability and genetic alterations | Karyotype analysis of cells (for extensively passaged cells and stem cell products) | × | - | (41, 143) |
| Tumor formation and cell malignancy | Soft agar colony formation, tumor sphere assay, colony forming/clonogenic assay, immunodeficient rodent tumorigenicity assay (for stem cell products) | × | × | (41) |
| Abnormal cell differentiation | FACS, qRT-PCR (for stem cell products) | × | - | (41) |
| Tissue structure | Histological staining for general structure and integration (H&E), collagen (picrosirius red), and glycosaminoglycan (safranin-O) | × | × | (144) |
| Biochemical composition | Collagen (e.g., hydroxyproline) Glycosaminoglycan (e.g., DMMB Blyscan GAG) DNA (e.g., Picogreen) Crosslinks (e.g., mass spectrometry or HPLC-based) |
× | × | (145–148) |
| Activity | Pre- and post-operative activity measurement via activity monitors | - | × | (149) |
| Repair tissue collagens | Collagen types via mass spectrometry or immunohistochemistry or ELISA (at least types I and II) | - | × | (148) |
| Midpoint assessments | Arthroscopic evaluation, synovium analysis | - | × | (66) |
| Animal observation | Lameness, behavior, and eating and drinking habits | - | × | (82) |
| Additional terminal assessments | Necropsy, gross pathology, histological evaluation and scoring, synovium analysis, immunohistochemistry of immune cells | - | × | (82) |
In vitro safety evaluation.
Early safety assessments of the product may be obtained through in vitro assays (Table 3, A to C). The safety profile of the product must be provided within an IDE or an IND, and products categorized as biologics may use in vitro assays to screen for abnormal cell differentiation, cell malignancy, or tumorigenicity (31). A karyotype analysis can determine the existence of genetic alterations, which can appear during extensive subculture or differentiation steps (32). Similarly, fluorescence-activated cell sorting (FACS) and qRT-PCR can be used to determine the existence of abnormally differentiated cells (33). The effects of genotype alterations can also be studied using tumorigenicity assays such as the soft agar colony formation assay. Short-term degradation kinetics for the product can be evaluated in vitro (34), as a degradation profile should be provided for scaffold-based approaches (3). Finally, though less common and often restricted to immune therapy products, in vitro immunogenicity assays can test for potential adverse immune reaction based on specific cell type activation (e.g., T cell response, secreted cytokine) or establish the type of reaction based on cell population recruitment (immunophenotyping) (35, 36).
In products that utilize biomaterials, toxicity testing must be considered. Biocompatibility evaluation endpoints are selected based on device category and duration of time the material is intended to be in contact with the body (37). The FDA refers to the consensus standard ISO 10993–1 for general guidelines on testing for cytotoxicity, sensitization, hemocompatibility, pyrogenicity, implantation, genotoxicity, carcinogenicity, reproductive and developmental toxicity, and degradation assessments (38). Though there is ubiquitous use of various biomaterials such as polylactides, polyglycolides, polycaprolactones, and their copolymers (39, 40), the FDA assesses risk based on the final finished device. The FDA does not clear or approve individual materials used in the devices. Thus, the risk assessment includes not only the device, but the materials that make up the device as well as any residual materials from manufacturing (41). If novel biomaterials that have not been previously used in legally US-marketed medical devices, additional evaluations beyond ISO 10993–1 may be required. In this case, further clarification on testing requirements can be found within an FDA guidance document developed to supplement ISO 10993–1 (41). Assessing these safety considerations before proceeding with animal studies will both mitigate problems that can arise during preclinical studies and provide initial pharmacology/toxicology data.
Chemistry, manufacturing, and controls.
As in vitro development of a product advances, thought should be given to the Chemistry, Manufacturing, and Controls (CMC) of a product. For a cellular therapy to be tested in animals, an analogous animal product must be developed for the animal species used for testing (3). If passaged cells are used in the generation of a cellular therapy, a cell bank system should be considered, which will also be subject to safety testing (42). Because CMC information is a required component of an IND application, CMC should be considered early within the product development.
FDA applications require Good Laboratory Practice (GLP) or a justification for why GLP was not followed (2, 43). In the latter case, a quality assurance statement must be provided, and an independent overseer must provide a statement that Standard Operating Procedures (SOPs) were followed. Although not all in vitro data can or should be collected under GLP, preclinical safety and toxicology studies are governed by GLP regulations, and considering GLP implementation early on will help ease the transition to IND-enabling research (44). Variability within the product manufacturing process or the product itself will negatively affect a study’s outcome and, thus, a quality management system (QMS) should be implemented (45–47). Likewise, the development of SOPs, for example, should occur before performing large animal studies to ensure reproducibility in the manufacturing process and outcome measures (48). However, GLP-level scientific rigor can be expensive due to the necessity to adapt the existing infrastructure to meet GLP standards, to prepare and maintain extensive documentation, and to provide appropriate training to all personnel (49). Interestingly, out of the 23 FDA-approved, most did not comply with GLP in most or all their preclinical data (Table 2A). Overall, considering how and when GLP will be implemented will be a crucial decision within the translation of a product.
Ethics of animal research.
Before proceeding with in vivo experimentation, researchers should fully optimize and characterize their product in vitro. The “principles of the 3Rs,” which focus on replacement, reduction, and refinement strategies within animal studies, were established to assess the necessity of animal research, to ensure that only the minimum number of animals is used, and to improve animal welfare (3, 50). Replacement of animal experimentation emphasizes the use of in vitro studies when alternatives exist or can be developed (3). Reduction and refinement focus on the model selection, reducing the number of animals used, and how the animals will be managed to minimize suffering to the fullest extent possible (51). Reduction and refinement include, for example, using a single species and a single study to collect both pharmacological and toxicological data and using non-terminal evaluations, such as magnetic resonance imaging (MRI) to maximize data acquisition. Appointed Institutional Animal Care and Use Committees (IACUCs) oversee the proper use of animals in research, in accordance with the Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals (52, 53). Therefore, all in vitro data should be evaluated critically and a careful review of supporting literature should be performed to establish a strong rationale for any proposed animal study.
RATIONALE FOR SELECTING PRECLINICAL ANIMAL MODELS IN CARTILAGE REPAIR
While the FDA recognizes “there is no perfect animal model of articular cartilage injury” (2), several models have been widely used for cartilage repair studies. The International Cartilage Repair Society (ICRS) specifically recommends the pig, horse, sheep, and goat for preclinical animal studies (54). While ICRS does not specify pig breed, minipigs (e.g., Yucatan, Göttingen) are used more commonly than farm pigs because their size is more manageable and they are temperamentally more docile (55). Researchers have also utilized the canine (dog) as a preclinical model for cartilage repair (56, 57). Small and intermediate animal models (e.g., mouse or rabbit) do not provide sufficient evidence to support translation of a cartilage-repair product due to their capacity for spontaneous cartilage healing, thin articular cartilage, and gait patterns that differ significantly from those of humans (56). When selecting an animal model, one must consider the i) analogy of the animal model’s anatomy, cartilage, and biology to the human and ii) experimental requirements and logistical considerations. An overview of characteristics of key animal models is provided in Table 4.
Table 4.
Biologic and practical considerations of common large animal models for cartilage repair. ROM, range of motion.
| Consideration | Sheep | Goat | Pig | Horse | Dog |
|---|---|---|---|---|---|
|
| |||||
| Passive ROM | 40° to 147° (59) 72° to 145° (60) |
45° to 146° (59) | 42° to 144° (59) | 30° to 160° (61) | 34° to 160° (59) |
| Cartilage thickness | 0.4 to 1.2 mm (54, 62) | 0.7 to 1.5 mm (54, 62) | 1.5 to 3.2 mm (54, 62, 63) | 1.5 to 3.2 mm (54, 62, 63) | 0.95 to 1.3 mm (56) |
| Subchondral cyst formation | Yes (73) (74) | Yes (75) (76) | No | No | No |
| Facilities required | Large animal facilities | Large animal facilities | Large animal facilities | Equine facilities | Large animal facilities |
| Purpose for breeding | Agriculture | Agriculture | Research | Companion animal | Companion animal |
| Temperament | Instinct to flee upon awaking from surgery | Instinct to flee upon awaking from surgery | Relatively docile upon awaking from surgery | Instinct to flee upon awaking from surgery | Relatively docile upon awaking from surgery |
Analogy of the model’s joint anatomy, cartilage characteristics, and biology.
An animal model’s cartilage, joint, and mechanism of osteochondral healing should mimic those of human cartilage to the greatest extent possible. Because the pig, horse, sheep, and goat are quadrupeds, there are inherent differences between the animal model and human that cannot be reconciled regardless of choice of animal model.
The FDA has acknowledged that range of motion and joint anatomy are important for animal model selection (58). The goat, sheep, and pig knee (stifle) joint have similar ranges of motion ranging from about 45° to 145°, whereas dog and horse range from about 30° to 160° (see Table 4 for animal-specific ranges) (59–61). Because the human range of motion is 2.5° to 137.5° (59), no widely used quadrupedal animal model replicates the human range of motion. In terms of cartilage thickness, the pig and the horse most closely mimic the human (Table 4) (54, 62, 63). Joint proportions in the pig, horse, sheep, and goat are similar to humans, but ligaments may have different attachment sites than in the human (59). Knee joint anatomy does not preclude these animal models from “second look” arthroscopic evaluation (64–66). Although there are differences between quadruped models and the human, in terms of anatomy, all proposed quadrupedal large animal models are relatively comparable.
Although the mechanical properties of the cartilage should be considered when selecting an animal model, direct comparison of the mechanical properties of knee cartilage in minipigs, sheep, goats, and horses has not been performed. Previous interspecies characterization of human, cow, dog, monkey, and rabbit found that compressive stiffness of distal femur cartilage was similar between the examined species (67). Analogous studies are needed for the major translational models used for cartilage repair research. For other anatomical locations, it has been found that minipig and human cartilage have similar functional properties. For example, in the human and minipig temporomandibular joint (TMJ) disc, mechanical properties were reported to be comparable with a compressive 20% instantaneous modulus of 1.12 MPa in the minipig and 1.32 MPa in the human (68). In the facet joint, the aggregate modulus of minipig cartilage was reported to be similar to primate (Rhesus Macaque) cartilage (69). Similar interspecies comparisons in the stifle joint of minipigs, goats, sheep, horses, and dogs are needed.
The healing response to cartilage injury of a chosen animal model should mimic that of humans. Ovine or caprine models have a tendency to form subchondral cysts during cartilage repair studies (70), which is not representative of the human situation in which subchondral cysts are considered to be a precursor to OA (71, 72). Osteochondral grafts implanted into sheep have been reported to induce cystic lesion formation in the subchondral bone (73). Subchondral drilling in sheep was reported to induce subchondral bone cyst formation in 63% of cases and intralesional osteophytes in 26% of cases (74). Similarly, in the goat, cavitation of the subchondral bone, a persistent central cyst, and, ultimately, structural collapse of the underlying bone was observed after full-thickness defects were generated (75). Goats implanted with osteochondral allografts developed bone cysts, subchondral bone channels, and subchondral bone roughening, which were not present in the non-operated contralateral control joints (76). Because ovine or caprine models have a greater propensity to exhibit abnormal healing patterns, they appear to have a different healing response than that of humans, and, therefore, may not be as suitable for animal studies as other large animals.
Overall, many of the anatomic and functional characteristics of the most common quadruped models are relatively similar, with the notable exception of cyst formation in the goat and sheep. Additionally, the pig and the horse have slightly thicker cartilage layers that more accurately resemble human anatomy, and, thus, may be favorable for cartilage repair studies.
Animal model logistical considerations and experimental requirements.
Logistics of conducting a definitive animal must also be considered including resource requirements, genetic variability, and animal temperament. Because of the significant capital investment of conducting IND-enabling animal research, costs and the availability of facilities are of great importance when designing a large animal study. Although the horse has been used in previous preclinical studies, its use has been limited due to the high costs associated with purchase, husbandry, surgery, and recovery as compared to the other large animal models discussed above. Moreover, horses require specialized equine facilities to house, which are rare, especially in GLP settings. There are several GLP-compliant animal facilities that frequently use the minipig animal model (e.g., Absorption Systems in San Diego, CA, BioSurg in Winters, CA, Sinclair Research in Auxvasse, MO). While the size of an animal does not necessarily preclude a species from use, the selection of a smaller animal (e.g., minipig and dog) may reduce personnel required and housing costs, thus, reducing costs overall. Therefore, unless the sponsor resides at an institution that specializes in equine research, the minipig and dog are considerably more feasible than the horse.
Another practical consideration when designing a study is the genetic variability of the species or herd that is chosen for the study. Of the large animal models recommended by ICRS for preclinical research, the minipig is the only model that is bred specifically for research purposes, and, thus, specific parameters can be controlled (animal number, sex, and age). It is difficult to obtain a homogenous population of horses for research purposes, limiting the interpretability of data gathered from horse studies. However, as companion animals with a propensity to develop focal cartilage defects and OA (77), horses may be useful for providing predictive proof-of-concept data or veterinary clinical trial to support clinical translation (78). Similarly, as companion animals, dogs can be enrolled in veterinary clinical trial studies to generate safety and efficacy data for a given therapy (78). Because goats and sheep are bred for agricultural purposes, these species also have more genetic variability than the minipig (70). Therefore, to obtain a homogenous population of animals for the completion of a proposed definitive preclinical study, the minipig may be the most accessible.
The temperament of the animals used for an in vivo study can dictate the success or failure of a study. In our experience, we have worked with horses (79), goats (80), sheep (81), and minipigs (66, 82) for large animal studies. We have found goats and sheep to be problematic from a behavioral standpoint because of their tendency to panic and jump upon waking from surgery, imparting super-physiological and biomechanically aberrant stresses to the joint. Similarly, immediately post-operatively the horse poses a danger to itself because of its tendency to flee. The post-surgery recovery of these animals, thus, does not replicate a human surgery where the patient can be informed of the importance of avoiding any extreme movements post-operatively to allow the implant time to integrate into the host tissue. On the other hand, other groups have noted that dogs are amenable to post-operative procedures that mimic those used for human cartilage repair (56, 57). Likewise, minipigs are relatively docile, especially when recovered in a sling or small pen (66). We have carried out successful studies in the minipig (66, 82), and have found minipigs to have the appropriate temperament for a study of this nature.
Perspectives on the minipig.
The porcine species has been increasingly recognized as an important animal model for translational research due to its similarity to the human in terms of size, immunology, genome, and physiology, as recently reviewed (83). Within the context of cartilage repair, the minipig has been recognized as a “clinically-relevant large animal model” (84) and has been used in many cartilage repair studies (84–89). In 2011, the FDA released guidance for conducting cartilage repair preclinical animal studies (2). This document did not specifically list the minipig as a model although it should be noted that the FDA does not limit models to those listed and allows for investigators to provide appropriate justification for their chosen model, for example through the Animal Model Qualification Program. Despite one report suggesting that the pig’s range of motion may be inappropriate for cartilage repair studies (58), literature reports that the stifle joint of the pig is comparable to all other animal models listed within the guidance document (59). The many advantages of using the minipig described in this review elevate this animal model to, in our opinion, the foremost animal model for cartilage repair studies and should be included within a revised FDA guidance document.
SURGICAL APPROACH AND REHABILITATION
Once an animal model has been selected, the surgical approach, defect parameters, fixation technique, and post-surgical rehabilitation and recovery protocols must be developed before IND-enabling animal studies can be conducted. Although surgical and rehabilitation methods for animal models will have inherent differences from the human situation, they should strive to mimic human surgical and rehabilitation methods wherever possible.
Surgical approach.
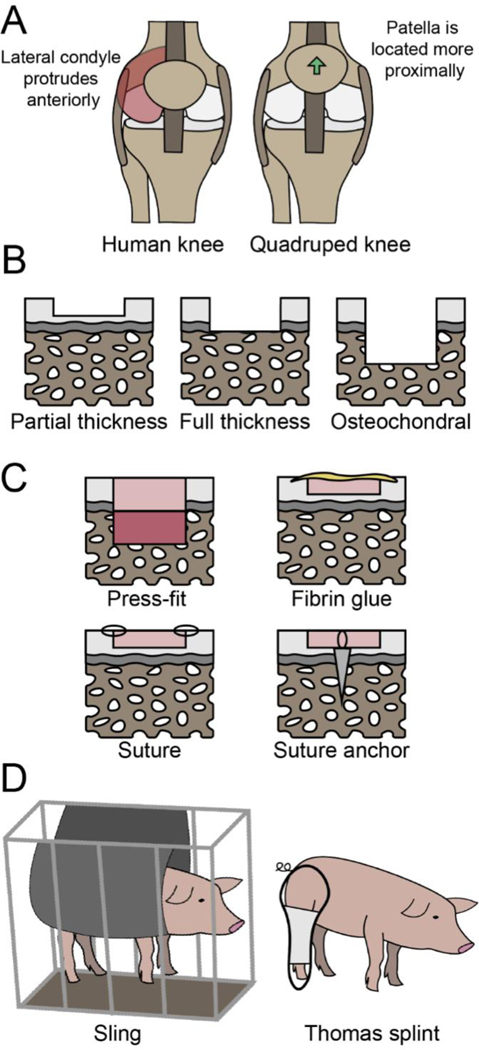
The technical aspects of the surgical approach, crucial for the success of the therapeutic, need to be tailored to the specific animal model utilized. Specifically, patellar maltracking, which can cause mechanical abrasion of the cartilage and intra-articular inflammation, is a major concern when translating surgical techniques from the human to a quadrupedal model. In the human, open surgical exposure of the knee joint is performed by medial arthrotomy, which requires cutting the medial retinaculum, an important stabilizer of the patella. Although this approach rarely causes problems in humans, a medial arthrotomy in animals can lead to patellar maltracking and lateral patellar instability, even after meticulous repair of the medial retinaculum (90). Both medial and lateral patellar subluxation are well-reported problems in large animals (90, 91), which are likely due to two major differences in anatomy between the human and quadruped models (illustrated in Figure 3A). First, compared to animal models, in the human the lateral condyle of the femur and trochlear ridge extend more anteriorly, which helps prevent lateral patellar instability. Second, in the animal the patella is located more proximally than in the human, which places it at the shallow portion of the trochlea, thereby predisposing it to patellar instability (92, 93). Due to this risk in large animals, retinaculum-sparing approaches that limit the extent of the proximal arthrotomy to the apex of the patella, as well as transpatellar approaches, have been recommended (90, 94, 95). In short, due to the differences between humans and animal models, one of the major considerations is how the surgery would affect the patella, which, if displaced, can lead to cartilage degeneration and failure of the experiment.
Fig. 3. Surgical and rehabilitation protocols for cartilage repair in animal models.
(A) The major anatomical differences between the human and animal knee anatomy that can lead to patellar maltracking. (B) Depth of defects that may be considered when designing surgical techniques. (C) Fixation methods that are currently utilized in cartilage repair. (D) Potential rehabilitation regimens that can be customized for quadruped models.
Defect parameters.
The depth, size, and location of the defect must reflect the intended indication in the human and, thus, these decisions should be incorporated into the surgical planning of preclinical research. Defect depth may be partial-thickness (leaving calcified cartilage layer intact), full-thickness (removing the calcified cartilage but leaving the subchondral bone intact), or osteochondral (penetrating into the subchondral bone), as depicted in Figure 3B. It is traditionally thought that partial-thickness defects do not heal, whereas osteochondral defects that penetrate the subchondral bone more regularly exhibit filling of the defect with reparative tissue due to egress of the underlying marrow elements (such as in microfracture). Based on a study investigating the healing response to isolated microfracture in horses (96), the current clinical paradigm argues for removal of the calcified cartilage layer during cartilage repair treatment. However, if a treatment does not utilize the underlying marrow elements, it may be beneficial to preserve the calcified cartilage and subchondral bone layers. Defect size in animals should mimic either a small or large lesion in humans because lesion size typically dictates the type of clinical treatment. Defect size can be calculated as a proportion of the surface area of the hemicondyle or trochlea in the animal model being utilized, with 2–4 cm2 as the typical threshold differentiating between small versus large defects in humans (97, 98). Although some have described a “critical size” that is needed for lesions to exhibit a limited repair process, in most skeletally mature large animals, even small lesions do not exhibit healing (99). With regard to location, the extensor digitorum longus is an intra-articular structure unique to quadrupeds that originates at the craniolateral aspect of the stifle joint (60). If left intact, this tendon can mechanically abrade any defects or implants placed on the lateral femoral condyle. Overall, the defect parameters selected should mimic the human indication while taking into consideration the anatomy and size of the selected animal model.
Fixation technique.
Adequate fixation of cartilage implants in large animal models is challenging. The four main fixation methods currently employed are depicted in Figure 3C. Although osteochondral cartilage repair implants can be press-fit into surgically created defects, chondral implants are much more difficult to retain in the defect. The maximum thickness of the articular cartilage in skeletally mature large animals is typically no more than 2 mm (54, 62, 63), making it difficult to adhere constructs within partial-thickness defects. Based on our experience, fibrin sealant, which is the gold standard fixation method for cartilage repair in humans, is typically not strong enough to retain implants in the large animal stifle. Therefore, many investigators have used sutures or suture anchors to fix implants (95, 100). However, these methods are not without their own set of dilemmas. Suture fixation, which secures the implant to the surrounding native articular cartilage rim, is technically challenging, causes iatrogenic perforation injury to both the implant and native cartilage, and can cause abrasive wear from suture knots between the articulating surfaces. Moreover, suture anchor methods, which naturally perforate the subchondral bone, can produce unwanted bleeding, along with carrying the same disadvantages as suture fixation. In addition to robust initial fixation, recession of the implant within the defect seems to be crucial for its retention to avoid any catching of a prominent lip that would lift off the implant. Alternative fixation methods that do not cause damage to the cartilage implant or surrounding native articular cartilage warrant further investigation.
Post-surgery rehabilitation and recovery protocols.
Design of an analogous rehabilitation regimen for animals, compared to humans, is challenging. Animals, upon wakening, can exhibit erratic behaviors that jeopardize the success of the surgery (101). These behaviors are practically guaranteed in some species, such as the goat and sheep, where the animal will display a strong run-and-hide response, sometimes jumping energetically regardless of the knee condition. Strategies such as casting, external fixator immobilization, and botulinum toxin paralysis are typically not well tolerated by animals and can introduce other unintended consequences. For example, botulinum toxin-induced muscle paralysis and the resulting underloading of bone structures has been associated with loss of bone density (102). The use of slings and splints may minimize the load placed on the operated joint immediately after surgery (Figure 3D) (54). The Thomas splint, for example, diverts the load experienced during ambulation from the knee to the hip. This method, however, is prone to failure given the physiognomy of most animal models’ knees. Other options could include bodyweight suspension out of surgery to prevent violent loaded motions and thrashing upon awaking from anesthesia, although one must be cognizant of the potential for the development of pressure sores (66). Common clinical rehabilitation programs after knee cartilage procedures include continuous passive motion and progressive weight-bearing to protect the cartilage while healing (103). Many of the current reports for cartilage repair do not include descriptions of post-operative rehabilitation; this is troublesome because it either means rehabilitation is not performed or, if it was, a lack of reporting limits other researchers in replicating and building upon successful methods. Protecting the repair cartilage immediately after surgery in animal studies may contribute to the success of the study and should be a key element in the development and reporting of animal studies.
IND-ENABLING PRECLINICAL ANIMAL STUDIES
Once an animal model has been selected and a surgical approach devised, IND-enabling in vivo studies are required to “demonstrate an acceptable safety profile” and to obtain “data sufficient to establish scientific support for clinical investigation” (2). While the FDA has released guidance documents to aid in this process, guidance is intentionally flexible to be applicable to a wide range of potential products. Thus, it may be difficult for a sponsor to surmise the appropriate study design for a specific product under development. Guidance documents represent the Agency’s current thinking on a subject, but an alternative approach may be deemed acceptable if a sponsor can justify the approach scientifically and it abides by applicable statutes and regulations. Discussion of the preclinical animal studies should occur with the FDA at the stage of the pre-IND meeting. In this section, the design of the preclinical animal studies for cartilage repair will be discussed. The primary guidance documents referred to in this discussion include “Guidance for Industry Preclinical Assessment of Investigational Cellular and Gene Therapy Product” (3) and “Guidance for Industry Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage” (2).
Overview of animal study objectives.
FDA guidance for cartilage repair products specifically states that animal studies should assess the dose response, biological response/activity, durability, and toxicology of a product (2). Proof-of-concept (POC) studies should be used to assess feasibility, establish product administration/dosing parameters, and determine a putative mechanism of action (3). Once POC studies have been completed, definitive preclinical studies should be used to assess toxicology and biological activity. In the case of cartilage repair, this will likely consist of a large animal study that assesses both safety and activity within the same study design. For reference, MACI’s Pharmacology Toxicology Review lists four studies (two rabbit studies, a pilot horse study, and a definitive horse study). Of these, the safety and activity profile of MACI was entirely based on the definitive horse study (104).
Study duration.
Durability of a cartilage repair product is critical “to resist wear, degradation, [and] withstand physiological relevant loads over time” and, thus, has been highlighted as a primary concern within guidance for cartilage repair products (2). The Agency has released guidance stating that long-term (i.e., 12-month) in vivo studies are required to demonstrate durability of effect for products intended for cartilage repair (2, 3). Accordingly, a 53-week large animal study was conducted for MACI (104). For perspective, other tissue engineering products such as Gintuit and Stratagraft reported studies up to 6 months and 14 months in duration, respectively (105, 106). Approval of immunotherapy products such as Abecma and Breyanzi only required studies lasting up to 85 days and 100 days, respectively (107, 108). However, considering the mechanical requirements of joint repair, sponsors of cartilage repair products may anticipate a year-long duration for the definitive animal study.
Control selection.
Inasmuch as the use of controls in large animal preclinical studies for cartilage treatment is essential, FDA guidance suggests the use of untreated controls (defect generated but no therapeutic treatment applied), sham-surgery controls (surgery performed but no defect or therapeutic agent applied), active-comparator controls (defect generated and another therapeutic treatment applied), or standard of care controls (defect generated and microfracture applied) (2, 3). It should be noted that MACI’s definitive in vivo study used empty defects and collagen only membranes (ACI-Maix) as controls (104). MACI’s lack of active-comparator controls or standard of care controls is consistent with what has been submitted with other cellular and gene therapy products. Furthermore, Stratagraft and Breyanzi received BLAs without controls in some of their animal studies after providing sufficient rationale (106, 108). Although active-comparator controls and standard of care controls may be beneficial for assessing efficacy in clinical studies, as discussed under Perspectives, such controls may be inappropriate for use in animal models. Thus, based on guidance and the precedent set by previously approved products, empty defects may be the most appropriate controls in the case of cartilage repair studies.
Endpoints.
Endpoint selection is crucial to the success of an IND application and should be discussed with the Agency in a pre-IND meeting. Broadly, endpoints are used to assess safety and/or activity of the product. In terms of safety, FDA guidance states that standard endpoints include “mortality (with cause of death determined, if possible), clinical observations, body weights, physical examinations, food consumption/appetite, water consumption (as applicable), clinical pathology (serum chemistry, hematology, coagulation, urinalysis), organ weights, gross pathology, and histopathology” (3). Cell fate post-administration should be considered, and, if appropriate (e.g., in stem cell products), issues such as tumorigenicity should be assessed within the study design (3). Overall, the assays selected should demonstrate that all constituents of the product (e.g., cells, biomaterials) are reasonably safe to administer in future clinical investigations.
The purpose of activity endpoints in animal testing is to obtain “data sufficient to establish scientific support for clinical investigation” (2). In clinical studies for cartilage repair, efficacy is determined by reduced pain and improved physical function using scoring systems such as the Knee Injury and Osteoarthritis Outcome Score (KOOS) or Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (2). Because these assessments cannot be used in animals, surrogate measures of efficacy (i.e., biological activity) must be utilized and should “mirror” the endpoints of the clinical study (2). Guidance also suggests the use of arthroscopic and/or magnetic resonance imaging (MRI) evaluations to assess animals in a non-terminal manner (2). Other potential measures that could be utilized in the future include activity monitors, which have been used in dogs to assess movement after biologic resurfacing of a cartilage defect (57), and clinical evaluation of distress or pain via evaluating facial expressions of animals (109, 110). Although preclinical biologic activity is not equivalent to clinical efficacy, endpoints should be selected to provide scientific support that the treatment would be efficacious in human patients.
Mechanical testing is requested within guidance for cartilage repair products. Specifically, the FDA has categorized mechanical testing into three areas: i) mechanical characterization of the tissue (as discussed in the in vitro evaluation section), ii) evaluation of the fixation method, and iii) degradation behavior (2). Mechanical properties should be tested at discrete time points after implantation into an animal. Degradation behavior is important for degradable scaffolds and synthetic biomaterials, but it may not be directly applicable to cell-based products.
GLP compliance.
The Code of Federal Regulations, Title 21, part 58, specifies that GLP work is required for IND-enabling studies, including personnel, facilities, protocols, and all equipment and materials used (44). It is expected that all preclinical safety studies are performed consistent with GLP, and that a statement of compliance is included within the final study report (2, 43). However, the FDA can consider exceptions on a case-by-case basis where compliance is not possible (e.g., animal models of disease/injury with unique care requirements). For such cases, a statement of the reasons for the noncompliance should be provided if the study was not conducted according to GLP regulation, which includes the areas of deviation and how these affected the study outcome (3, 44). Similarly, an exemption from GLP provisions can be discussed with the FDA prior to the preclinical studies to ensure that the nonconformance can be justified and that provisions are made to ensure data integrity (43, 44). In the preclinical MACI research, both the small and large animal studies were deemed to be not GLP-compliant (104). As many of the resources needed for conducting preclinical animal research may not be available in GLP facilities, discussing GLP requirements with the Agency at the pre-IND meeting is advised.
Sex as a biologic variable (SABV).
Sex is an important source of biological variability and is associated with biologic function, and, therefore, must be factored into the study design where relevant. The ASTM guide for preclinical studies of articular cartilage establishes that sex should be considered in data analysis due to the impact of circulating steroids on cartilage and bone metabolism and regeneration, and that sex be the same within the cohort, implying that one sex can be used in a study design (111). These recommendations were reconsidered in 2015 by the National Institutes of Health (NIH), with expectations that sex would be factored into the research design, analysis, and reporting in studies of vertebrate animals without calling for doubling of research animals (112, 113). However, the most pertinent directive is from the Cellular and Gene Therapy Products Guidance which states that adequate numbers from each sex should be included and randomized to each group (3). With a few exceptions, both male and female animals were utilized in most of the approved cellular and gene therapy products, including MACI.
PERSPECTIVES
In this review, we have described the main barriers to clinical translation of cartilage repair products. Through these discussions, we have identified six major bottlenecks that hinder translation at the preclinical stage: i) obtaining FDA guidance early within preclinical development, ii) conducting GLP research to support FDA applications within the infrastructure at research universities, iii) developing an appropriate rehabilitation protocol, iv) selecting appropriate in vivo controls, v) ensuring that the primary outcome measures for activity are relevant to the human condition, and vi) defining standardized endpoints. Here, we provide perspectives on these challenges.
A major bottleneck in translating cell therapy products for cartilage repair is navigating the regulatory process. Regulatory guidance provided by the FDA is intentionally flexible to accommodate a range of products that may be developed. However, this flexibility can allow for various interpretations of the guidance documents, and there are few relevant, approved cell therapy products to use as a blueprint for this process. Guidance documents “recommend early and ongoing communication with [the Office of Cellular, Tissue and Gene Therapies] Pharmacology/Toxicology staff during product development”(3). However, in practice, this is not possible. The FDA offers, as its first official interaction with the sponsor, the pre-IND meeting, but for many, this may be too late in the product development process. INTERACT meetings are meant to offer an opportunity for early dialog between sponsors and the Agency, but even these meetings require pharmacology and toxicology data for a meeting request to be accepted. Thus, we advocate for a move toward increased and early dialog between translational research groups and regulatory agencies. Such interactions may be facilitated by professional organizations or societies, where common, general issues can be identified through surveys and conferences, and then for these issues to be addressed by the Agency. Moreover, as the regulatory environment of cell therapy matures, more nuanced guidance for specific applications (e.g., meniscus, TMJ disc, facet joint) should also be developed. Increased regulatory dialog would greatly enhance the ability of researchers to translate cartilage repair products and cellular and gene therapy products in general.
A large subset of cellular and gene therapy research is conducted at research universities and academic centers. The infrastructure at such institutions is not conducive to conducting GLP research. For example, we estimate that conducting a year-long study with 24 minipigs would cost about $50,000 in procurement, surgery, husbandry, care of animals, and necropsy costs within the University of California research system (which is not GLP) but over $700,000 when outsourced to a GLP facility. Funding mechanisms through the NIH and National Science Foundation (NSF), which fund research on cellular and gene therapy products, rarely would provide enough support to conduct a GLP-level study of this magnitude. Therefore, there is a disconnect between the funding and resources that researchers have access to and the regulatory requirements of translating cellular and gene therapy products. When we embarked on investigating the documentation of approved cellular and gene therapy products, it was surprising for us to see that most of the 23 products that have obtained regulatory approval did not conduct animal research in GLP facilities, even though it appears to be requested by the FDA. Given that there are only 23 translated products, with the majority of them not following GLP, GLP requirements may be too stringent to facilitate effective translation of cellular and gene therapy research from universities to clinical use.
Developing appropriate post-operative and rehabilitation protocols for animal models is difficult but necessary, and animal care can have an outsized influence on study results. In the human case, post-surgical immobilization or directed rest after cartilage repair surgeries can be understood by the patient, but in the animal situation rehabilitation strategies cannot be employed in the same manner. No standardized method for post-surgery rehabilitation in large animal models exists due to the wide variety of indications that researchers seek to address. For this reason, investigators would do well in using pilot studies to develop these protocols before commencing animal work at a large scale. For example, for treating defects in knee articular cartilage, we have had success in using a sling to elevate minipigs as they recover from anesthesia to ensure that violent postoperative movements are minimized (66). Toward the end of developing effective post-operative and rehabilitation protocols, researchers should strive to devise protocols that are both relevant to the human situation and tolerable to the animal.
Selecting appropriate controls for definitive preclinical studies is challenging. The FDA suggests the use of active-comparator controls, standard of care controls, or sham-surgery controls within the guidance for the development of cartilage repair products (2). However, the use of active-comparator controls and/or standard of care controls presents a new layer of challenges to a study for cell therapies. If the active comparator is another cell therapy, then it would either have to be xenogeneic or a new, analogous animal product would have to be devised, making such comparisons unusually burdensome for the sponsor. For example, purchasing MACI for xenogeneic use in animals would increase the cost of a study substantially not to mention that such an approach is probably scientifically ill-advised. Similarly, the applicability of microfracture developed in the human knee may not be directly equivalent to such an approach in the animal. Due to the challenges of other controls, empty defects may be the best comparator option for preclinical studies, although active comparators can still be employed in clinical trials.
Selecting endpoints in animal models that mirror the clinical endpoints is difficult, but recent advancements in technology may enable outcome measures that better replicate the human situation. In humans, the primary endpoint of cartilage repair is the reduction in pain, which is in stark contrast to animal studies in which repair is typically evaluated after euthanasia. To simulate the human situation, non-terminal endpoints should be incorporated into animal studies such as non-invasive imaging or arthroscopic protocols. With the emergence of more robust imaging modalities (e.g., near-infrared spectroscopy, arthroscopic optical coherence tomography) we can envision the development of surrogate measures for determining the quality of cartilage repair (114). Moreover, utilizing outcome measures that assess activity and behavior may be beneficial for indicating that a treatment is efficacious. Function can be measured indirectly in animals via an attached activity monitor (57). Additionally, with the advancement of artificial intelligence, in the future, behavioral measures may be able to be quantified in an unbiased manner, similar to poultry monitoring systems currently in development for agricultural purposes (115). Going forward, researchers should take advantage of these technological advancements to enhance the collection of meaningful primary outcome measures in animal studies.
The guidance document on cartilage repair products calls for mechanical testing (2), but it is unclear what data should be collected at the in vitro stage and what endpoint data should be collected upon euthanasia during IND-enabling animal studies. For in vitro-stage research on a product that is a tissue-engineered implant, we suggest that at minimum compressive data (e.g., aggregate modulus, shear modulus, and permeability), tensile data (e.g., Young’s modulus, ultimate tensile strength), and tribological data (e.g., coefficient of friction) be collected. Upon euthanasia of an animal, we suggest that the same parameters be measured in the repair tissue along with the integration strength between the native tissue and implant. Standardizing specific mechanical endpoints within FDA guidance would facilitate experimental design development and allow for comparison between studies conducted by different research groups. For a comprehensive list of recommended in vitro and in vivo assessments and measurements/assays, see Table 3C based on our thorough review of the literature and regulatory guidance.
In conclusion, this review sought to demystify the process and challenges of translating biologic cartilage products from basic research to clinical trials. As appropriate to the technologies under translation, researchers should consider the issues of navigating regulatory pathways, designing in vitro studies that support in vivo success, selecting an appropriate animal model, adapting surgical and rehabilitation strategies to animal models, and determining what in vivo data are required to support an application to conduct clinical trials. Increased dialog between regulatory agencies and research groups is necessary to overcome barriers to clinical translation and to ensure the development of cogent therapies.
Supplementary Material
Acknowledgements:
This work was supported by two grants from the NIH (Grant # R01 AR067821 and R01 AR078389). This work was also supported by NIH TL1 TR001415 (RCN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Approved Cellular and Gene Therapy Products | FDA (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products).
- 2.U.S. Food and Drug Administration, Guidance for Industry: Preparation of IDEs and INDs for Products Intented to Repair or Replace Knee Cartilage, www.FDA.gov , 45 (2011).
- 3.US Food and Drug Administration, FDA/CBER, Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products (2013; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preclinical-assessment-investigational-cellular-and-gene-therapy-products).
- 4.Langer R, Vacanti JP, Tissue Engineering, Science (80-. ). 260, 920–926 (1993). [DOI] [PubMed] [Google Scholar]
- 5.Huey DJ, Hu JC, Athanasiou KA, Unlike bone, cartilage regeneration remains elusive, Science 338, 917–921 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Athanasiou K, Darling E, Hu J, DuRaine G, Reddi H, Articular Cartilage, Second Edition (2017). [Google Scholar]
- 7.Responte DJ, Natoli RM, Athanasiou KA, Collagens of articular cartilage: Structure, function, and importance in tissue engineering Crit. Rev. Biomed. Eng 35, 363–411 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Bielajew BJ, Hu JC, Athanasiou KA, Collagen: quantification, biomechanics and role of minor subtypes in cartilage, Nat. Rev. Mater. 2020 510 5, 730–747 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthritis-Related Statistics | Data and Statistics | Arthritis | CDC (available at https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm).
- 10.Wang D, Rebolledo BJ, Dare DM, Pais MD, Cohn MR, Jones KJ, Williams RJ, Osteochondral Allograft Transplantation of the Knee in Patients with an Elevated Body Mass Index, Cartilage 10, 214–221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaul F, Tírico LEP, McCauley JC, Bugbee WD, Long-term Follow-up of Revision Osteochondral Allograft Transplantation of the Ankle, Foot Ankle Int. 39, 522–529 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Pareek A, Reardon PJ, Maak TG, Levy BA, Stuart MJ, Krych AJ, Long-term Outcomes after Osteochondral Autograft Transfer: A Systematic Review at Mean Follow-up of 10.2 YearsArthrosc. - J. Arthrosc. Relat. Surg 32, 1174–1184 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Pellegrino M, Trinchese E, Bisaccia M, Rinonapoli G, Meccariello L, Falzarano G, Medici A, Piscitelli L, Ferrara P, Caraffa A, Long-term outcome of grade III and IV chondral injuries of the knee treated with Steadman microfracture technique, Clin. Cases Miner. Bone Metab 13, 237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erggelet C, Vavken P, Microfracture for the treatment of cartilage defects in the knee joint – A golden standard? J. Clin. Orthop. Trauma 7, 145–152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemeyer P, Pestka JM, Salzmann GM, Südkamp NP, Schmal H, Influence of cell quality on clinical outcome after autologous chondrocyte implantation, Am. J. Sports Med 40, 556–561 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Dugard MN, Kuiper JH, Parker J, Roberts S, Robinson E, Harrison P, Richardson JB, Development of a Tool to Predict Outcome of Autologous Chondrocyte Implantation, Cartilage 8, 119–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert JR, Smith A, Edwards PK, Hambly K, Wood DJ, Ackland TR, Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint, Am. J. Sports Med 41, 1245–1254 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Ebert JR, Fallon M, Wood DJ, Janes GC, A Prospective Clinical and Radiological Evaluation at 5 Years after Arthroscopic Matrix-Induced Autologous Chondrocyte Implantation, Am. J. Sports Med 45, 59–69 (2017). [DOI] [PubMed] [Google Scholar]
- 19.McGowan KB, Stiegman G, Regulatory Challenges for Cartilage Repair Technologies, Cartilage 4, 4–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.V Sweet B, Schwemm AK, Parsons DM, Review of the Processes for FDA Oversight of Drugs, Medical Devices, and Combination Products (; www.amcp.org). [DOI] [PMC free article] [PubMed]
- 21.Fda Cder, Reese S, Guidance for Industry Expedited Programs for Serious Conditions-Drugs and Biologics Guidance for Industry, (2014) (available at http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htmand/orhttp://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm).
- 22.Lazar J, Caressi G, A Product and Pipeline Analysis of the Global Knee Cartilage Repair Market. A New Standard of Care May Be on the Horizon. (2014; https://cds.frost.com/p/67239#!/ppt/c?id=NE07-01-00-00-00&hq=cartilage regenerative). [Google Scholar]
- 23.Kwon H, Brown WE, Lee CA, Wang D, Paschos N, Hu JC, Athanasiou KA, Surgical and tissue engineering strategies for articular cartilage and meniscus repair, Nat. Rev. Rheumatol 15, 550–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.REGULATION (EC) No 1394/2007 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004 (Text with EEA relevance).
- 25.Kleiderman E, Boily A, Hasilo C, Knoppers BM, Overcoming barriers to facilitate the regulation of multi-centre regenerative medicine clinical trials Stem Cell Res. Ther 9 (2018), doi: 10.1186/s13287-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Histogenics, NeoCart Phase 3 Clinical Trial Results Call (2018; www.sec.gov.).
- 27.Yanke AB, Tilton AK, Wetters NG, Merkow DB, Cole BJ, DeNovo NT Particulated Juvenile Cartilage Implant, (2015) (available at www.sportsmedarthro.com). [DOI] [PubMed]
- 28.Link JM, Salinas EY, Hu JC, Athanasiou KA, The tribology of cartilage: Mechanisms, experimental techniques, and relevance to translational tissue engineering Clin. Biomech (2019), doi: 10.1016/j.clinbiomech.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elder BD, Athanasiou KA, Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs, Osteoarthr. Cartil 17, 114–123 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacBarb RF, Chen AL, Hu JC, Athanasiou KA, Engineering functional anisotropy in fibrocartilage neotissues, Biomaterials 34, 9980–9989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito E, Miyagawa S, Takeda M, Kawamura A, Harada A, Iseoka H, Yajima S, Sougawa N, Mochizuki-Oda N, Yasuda S, Sato Y, Sawa Y, Tumorigenicity assay essential for facilitating safety studies of hiPSC-derived cardiomyocytes for clinical application, Sci. Rep 9 (2019), doi: 10.1038/s41598-018-38325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stultz BG, McGinnis K, Thompson EE, Lo Surdo JL, Bauer SR, Hursh DA, Chromosomal stability of mesenchymal stromal cells during in vitro culture, Cytotherapy 18, 336–343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuroda T, Yasuda S, Kusakawa S, Hirata N, Kanda Y, Suzuki K, Takahashi M, Nishikawa S-I, Kawamata S, Sato Y, Chaum E, Ed. Highly Sensitive In Vitro Methods for Detection of Residual Undifferentiated Cells in Retinal Pigment Epithelial Cells Derived from Human iPS Cells, PLoS One 7, e37342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams DF, Challenges With the Development of Biomaterials for Sustainable Tissue Engineering, Front. Bioeng. Biotechnol 7, 127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lock A, Cornish J, Musson DS, The role of in vitro immune response assessment for biomaterials J. Funct. Biomater 10 (2019), doi: 10.3390/jfb10030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joubert MK, Deshpande M, Yang J, Reynolds H, Bryson C, Fogg M, Baker MP, Herskovitz J, Goletz TJ, Zhou L, Moxness M, Flynn GC, Narhi LO, Jawa V, Use of In Vitro Assays to Assess Immunogenicity Risk of Antibody-Based Biotherapeutics, PLoS One 11 (2016), doi: 10.1371/JOURNAL.PONE.0159328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biocompatibility Evaluation Endpoints by Device Category | FDA (available at https://www.fda.gov/medical-devices/biocompatibility-assessment-resource-center/biocompatibility-evaluation-endpoints-device-category#implant-tissuebone).
- 38.ISO - ISO 10993–1:2018 - Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process (available at https://www.iso.org/standard/68936.html).
- 39.Elmowafy EM, Tiboni M, Soliman ME, Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles, J. Pharm. Investig . 2019 494 49, 347–380 (2019). [Google Scholar]
- 40.Marin E, Briceño MI, Caballero-George C, Critical evaluation of biodegradable polymers used in nanodrugs, Int. J. Nanomedicine 8, 3071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Food and Drug Administration, Use of International Standard ISO 10993–1, “Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process,” (2020) (available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and).
- 42.FDA, Guidance for Industry Cell Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications, , 1–50 (2010). [Google Scholar]
- 43.Guidance for Industry, Investigators, and Reviewers Exploratory IND Studies Contains Nonbinding Recommendations Guidance for Industry, Investigators, and Reviewers Exploratory IND Studies (2006; http://www.fda.gov/cder/guidance/index.htm).
- 44.CFR - Code of Federal Regulations Title 21, (available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=610.18).
- 45.<au>FDA, Manual, MDSAP QMS P0001.004: Quality System (2019). [Google Scholar]
- 46.Jarvis MF, Williams M, Irreproducibility in Preclinical Biomedical Research: Perceptions, Uncertainties, and Knowledge GapsTrends Pharmacol. Sci 37, 290–302 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Karp NA, Reproducible preclinical research—Is embracing variability the answer?, PLOS Biol. 16, e2005413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Putten M, Aartsma-Rus A, Grounds MD, Kornegay JN, Mayhew A, Gillingwater TH, Takeda S, Rüegg MA, De Luca A, Nagaraju K, Willmann R, in Journal of Neuromuscular Diseases, (IOS Press, 2018), vol. 5, pp. 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adamo JE, Bauer G, Berro M, Burnett BK, Hartman KA, Masiello LM, Moorman-White D, Rubinstein EP, Schuff KG, A roadmap for academic health centers to establish good laboratory practice-compliant infrastructure, Acad. Med 87, 279–284 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell W, Burch R, The principles of humane experimental technique (1959; http://117.239.25.194:7000/jspui/bitstream/123456789/1342/1/PRILIMINERY AND CONTENTS.pdf).
- 51.Allen MJ, Hankenson KD, Goodrich L, Boivin GP, von Rechenberg B, Ethical use of animal models in musculoskeletal research, J. Orthop. Res 35, 740–751 (2017). [DOI] [PubMed] [Google Scholar]
- 52.PHS Policy on Humane Care and Use of Laboratory Animals | OLAW (available at https://olaw.nih.gov/policies-laws/phs-policy.htm).
- 53.USDA Animal Care: Animal Welfare Act and Animal Welfare Regulations, (available at www.ascr.usda.gov/fling-program-discrimination-).
- 54.Hurtig MB, Buschmann MD, Fortier LA, Hoemann CD, Hunziker EB, Jurvelin JS, Mainil-Varlet P, McIlwraith CW, Sah RL, Whiteside RA, Preclinical studies for cartilage repair: Recommendations from the international cartilage repair society, Cartilage 2, 137–152 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cone SG, Warren PB, Fisher MB, Rise of the Pigs: Utilization of the Porcine Model to Study Musculoskeletal Biomechanics and Tissue Engineering During Skeletal Growth, Tissue Eng. Part C Methods 23, 763–780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook JL, Hung CT, Kuroki K, Stoker AM, Cook CR, Pfeiffer FM, Sherman SL, Stannard JP, Animal models of cartilage repair, Bone Joint Res. 3, 89–94 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estes BT, Enomoto M, Moutos FT, Carson MA, Toth JM, Eggert P, Stallrich J, Willard VP, Veis DJ, Little D, Guilak F, Lascelles BXD, Biological resurfacing in a canine model of hip osteoarthritis, Sci. Adv 7, 5918–5933 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Committee BRMA, Briefing Document Cellular, Tissue, and Gene Therapies Advisory Committee (2005). [Google Scholar]
- 59.Proffen BL, McElfresh M, Fleming BC, Murray M, A Comparative Anatomical Study of the Human Knee, Knee 19, 493–499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen MJ, Houlton JEF, Adams SB, Rushton N, The surgical anatomy of the stifle joint in sheepVet. Surg. 27, 596–605 (1998). [DOI] [PubMed] [Google Scholar]
- 61.Fowlie JG, Arnoczky SP, Stick JA, Pease AP, Meniscal translocation and deformation throughout the range of motion of the equine stifle joint: An in vitro cadaveric study, Equine Vet. J 43, 259–264 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Frisbie DD, Cross MW, McIlwraith CW, A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee, Vet. Comp. Orthop. Traumatol 19, 142–146 (2006). [PubMed] [Google Scholar]
- 63.Chu CR, Szczodry M, Bruno S, Animal models for cartilage regeneration and repair, Tissue Eng. B, Rev 16, 105–115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan Y, Li Z, Xie T, Chu CR, Hand-held arthroscopic optical coherence tomography for in vivo high-resolution imaging of articular cartilage, J. Biomed. Opt 8, 648 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Zelle S, Zantop T, Schanz S, Petersen W, Arthroscopic Techniques for the Fixation of a Three-Dimensional Scaffold for Autologous Chondrocyte Transplantation: Structural Properties in an In Vitro Model, Arthrosc. - J. Arthrosc. Relat. Surg 23, 1073–1078 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Wang D, Cubberly M, Brown WE, Kwon H, Hu JC, Athanasiou KA, Diagnostic Arthroscopy of the Minipig Stifle (Knee) for Translational Large Animal Research, Arthrosc Tech 10, e297–e301 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC, Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage, J. Orthop. Res 9, 330–340 (1991). [DOI] [PubMed] [Google Scholar]
- 68.Vapniarsky N, Aryaei A, Arzi B, Hatcher DC, Hu JC, Athanasiou KA, The Yucatan Minipig Temporomandibular Joint Disc Structure–Function Relationships Support Its Suitability for Human Comparative Studies, Tissue Eng. Part C Methods 23, 700–709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Leary SA, Link JM, Klineberg EO, Hu JC, Athanasiou KA, Characterization of facet joint cartilage properties in the human and interspecies comparisons, Acta Biomater. 54, 367–376 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Moran CJ, Ramesh A, Brama PAJ, O’byrne JM, O’brien FJ, Levingstone TJ, The benefits and limitations of animal models for translational research in cartilage repair, (2011), doi: 10.1186/s40634-015-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonilla AG, Equine bone cysts: What do we know about them and their treatment?, (2019), doi: 10.1111/eve.13212. [DOI] [Google Scholar]
- 72.Audrey HX, Bin Abd Razak HR, Andrew THC, The Truth Behind Subchondral Cysts in Osteoarthritis of the Knee, Open Orthop. J 8, 7–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinszky K, Kutter A, Poole AR, Auer JA, Changes in subchondral bone in cartilage resurfacing - An experimental study in sheep using different types of osteochondral grafts, Osteoarthr. Cartil 11, 265–277 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Orth P, Goebel L, Wolfram U, Ong MF, Gräber S, Kohn D, Cucchiarini M, Ignatius A, Pape D, Madry H, Effect of subchondral drilling on the microarchitecture of subchondral bone: Analysis in a large animal model at 6 months, Am. J. Sports Med 40, 828–836 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Jackson DW, Lalor PA, Aberman HM, Simon TM, Spontaneous repair of full-thickness defects of articular cartilage in a goat model, J Bone Jt. Surg 23-A, 53–64 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Pallante-Kichura AL, Cory E, Bugbee WD, Sah RL, Bone Cysts After Osteochondral Allograft Repair of Cartilage Defects in Goats Suggest Abnormal Interaction Between Subchondral Bone and Overlying Synovial Joint Tissues, (2013), doi: 10.1016/j.bone.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McIlwraith CW, Frisbie DD, Kawcak CE, The horse as a model of naturally occurring osteoarthritis, Bone Joint Res. 1, 297–309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kol A, Arzi B, Athanasiou KA, Farmer DL, Nolta JA, Rebhun RB, Chen X, Griffiths LG, Verstraete FJM, Murphy CJ, Borjesson DL, Companion animals: Translational scientist’s new best friends, Sci. Transl. Med 7, 308ps21–308ps21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murray RC, Zhu CF, Goodship AE, Lakhani KH, Agrawal CM, Athanasiou KA, Exercise affects the mechanical properties and histological appearance of equine articular cartilage, J. Orthop. Res 17, 725–731 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Athanasiou K, Korvick D, Schenck R, Biodegradable Implants for the Treatment of Osteochondral Defects in a Goat Model, https://home.liebertpub.com/ten 3, 363–373 (1997). [Google Scholar]
- 81.Van Der Meulen MCH, Beaupré GS, Lane Smith R, Giddings VL, Allen WA, Athanasiou KA, Fang Zhu C, Mandell JA, Song Y, Poser RD, Goodman SB, Factors influencing changes in articular cartilage following hemiarthroplasty in sheep, J. Orthop. Res 20, 669–675 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Vapniarsky N, Huwe LW, Arzi B, Houghton MK, Wong ME, Wilson JW, Hatcher DC, Hu JC, Athanasiou KA, Tissue engineering toward temporomandibular joint disc regeneration, Sci. Transl. Med 10, eaaq1802 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lunney JK, Van Goor A, Walker KE, Hailstock T, Franklin J, Dai C, Importance of the pig as a human biomedical model, Sci. Transl. Med 13 (2021), doi: 10.1126/SCITRANSLMED.ABD5758. [DOI] [PubMed] [Google Scholar]
- 84.Sennett M, Friedman J, Ashley B, Stoeckl B, Patel J, Alini M, Cucchiarini M, Eglin D, Madry H, Mata A, Semino C, Stoddart3 M, Moutos FT, Estes BT, Guilak F, Mauck R, Dodge G, Long Term Outcomes of Biomaterial-Mediated Repair of Focal Cartilage Defects in a Large Animal Model, Ors2019 , 2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gotterbarm T, Breusch SJ, Schneider U, Jung M, The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: Retrospective analysis of 180 defects, Lab. Anim 42, 71–82 (2008). [DOI] [PubMed] [Google Scholar]
- 86.Fisher MB, Belkin NS, Milby AH, Henning EA, Bostrom M, Kim M, Pfeifer C, Meloni G, Dodge GR, Burdick JA, Schaer TP, Steinberg DR, Mauck RL, Cartilage Repair and Subchondral Bone Remodeling in Response to Focal Lesions in a Mini-Pig Model: Implications for Tissue Engineering, Tissue Eng. Part A 21, 850–860 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfeifer CG, Fisher MB, Saxena V, Kim M, Henning EA, Steinberg DA, Dodge GR, Mauck RL, Age-Dependent Subchondral Bone Remodeling and Cartilage Repair in a Minipig Defect Model, Tissue Eng. Part C Methods 23, 745–753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T, Breusch SJ, An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects, Biomaterials 27, 3387–3395 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Jung M, Kaszap B, Redöhl A, Steck E, Breusch S, Richter W, Gotterbarm T, Enhanced early tissue regeneration after matrix-assisted autologous mesenchymal stem cell transplantation in full thickness chondral defects in a minipig model, Cell Transplant. 18, 923–932 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Orth P, Madry H, A low morbidity surgical approach to the sheep femoral trochlea, BMC Musculoskelet. Disord 14 (2013), doi: 10.1186/1471-2474-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Dona F, Della Valle G, Fatone G, Patellar luxation in dogs, Vet. Med. Res. Reports Volume 9, 23–32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson AL, Broaddus KD, Hauptman JG, Marsh S, Monsere J, Sepulveda G, Vertical patellar position in large-breed dogs with clinically normal stifles and large-breed dogs with medial patellar luxation, Vet. Surg 35, 78–81 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Mostafa AA, Griffon DJ, Thomas MW, Constable PD, Proximodistal alignment of the canine patella: Radiographic evaluation and association with medial and lateral patellar luxation, Vet. Surg 37, 201–211 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Bonadio MB, Friedman JM, Sennett ML, Mauck RL, Dodge GR, Madry H, A retinaculum-sparing surgical approach preserves porcine stifle joint cartilage in an experimental animal model of cartilage repair, J. Exp. Orthop 4 (2017), doi: 10.1186/s40634-017-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Christensen BB, Foldager CB, Olesen ML, Vingtoft L, Rölfing JHD, Ringgaard S, Lind M, Experimental articular cartilage repair in the Göttingen minipig: the influence of multiple defects per knee, J. Exp. Orthop 2, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW, Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses, Am. J. Sports Med (2006), doi: 10.1177/0363546506289882. [DOI] [PubMed] [Google Scholar]
- 97.Mandelbaum BR, † Md, Browne JE, Fu F, Micheli L, Mosely JB, Erggelet C, Minas T, Peterson L, Current Concepts Articular Cartilage Lesions of the Knee, (1998). [DOI] [PubMed] [Google Scholar]
- 98.Hinckel BB, Thomas D, Vellios EE, Hancock KJ, Calcei JG, Sherman SL, Eliasberg CD, Fernandes TL, Farr J, Lattermann C, Gomoll AH, Algorithm for Treatment of Focal Cartilage Defects of the Knee: Classic and New Procedures, Cartilage 13, 473S–495S (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hunziker EB, The elusive path to cartilage regeneration, Adv. Mater 21, 3419–3424 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedman JM, Sennett ML, Bonadio MB, Orji KO, Neuwirth AL, Keah N, Carey JL, Moutos FT, Estes BT, Guilak F, Madry H, Mauck RL, Dodge GR, Comparison of Fixation Techniques of 3D-Woven Poly(ϵ-Caprolactone) Scaffolds for Cartilage Repair in a Weightbearing Porcine Large Animal Model, Cartilage 9, 428–437 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eisenach JC, Warner DS, Houle TT, Reporting of preclinical research in anesthesiology transparency and enforcement Anesthesiology 124, 763–765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rafferty KL, Liu ZJ, Ye W, Navarrete AL, Nguyen TT, Salamati A, Herring SW, Botulinum toxin in masticatory muscles: Short- and long-term effects on muscle, bone, and craniofacial function in adult rabbits, Bone 50, 651–662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sparks DS, Saifzadeh S, Savi FM, Dlaska CE, Berner A, Henkel J, Reichert JC, Wullschleger M, Ren J, Cipitria A, McGovern JA, Steck R, Wagels M, Woodruff MA, Schuetz MA, Hutmacher DW, A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction, Nat. Protoc 15, 877–924 (2020). [DOI] [PubMed] [Google Scholar]
- 104.US Food and Drug Administration, MACI ® (autologous cultured chondrocytes on porcine collagen membrane) Cellular sheet for autologous implantation, (2021) (available at https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142569.pdf).
- 105.US Food and Drug Administration, GINTUIT (Allogeneic Cultured Keratinocytes and Fibroblasts in Bovine Collagen) (2018) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/gintuit-allogeneic-cultured-keratinocytes-and-fibroblasts-bovine-collagen).
- 106.US Food and Drug Administration, STRATAGRAFT (2021) (available at https://www.fda.gov/vaccines-blood-biologics/stratagraft).
- 107.US Food and Drug Administration, ABECMA (idecabtagene vicleucel) (2021) (available at https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel).
- 108.US Food and Drug Administration, BREYANZI (lisocabtagene maraleucel) (2021) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi-lisocabtagene-maraleucel).
- 109.McLennan KM, Miller AL, Dalla Costa E, Stucke D, Corke MJ, Broom DM, Leach MC, Conceptual and methodological issues relating to pain assessment in mammals: The development and utilisation of pain facial expression scales, Appl. Anim. Behav. Sci 217, 1–15 (2019). [Google Scholar]
- 110.Ison SH, Eddie Clutton R, Di Giminiani P, Rutherford KMD, A review of pain assessment in pigs, Front. Vet. Sci 3, 1–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.International ASTM, Standard Guide for in vivo Assessment of Implantable Devices Intended to Repair or Regenerate Articular Cartilage F2451 − 05 (Withdrawn 2019), ASTM Int. (2010) (available at https://www.astm.org/f2451-05r10.html). [Google Scholar]
- 112.Clayton JA, Studying both sexes: A guiding principle for biomedicine, FASEB J. 30, 519–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.NOT-OD-15–102: Consideration of Sex as a Biological Variable in NIH-funded Research (available at https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html).
- 114.Castro NJ, Babakhanova G, Hu J, Athanasiou KA, Nondestructive testing of native and tissue-engineered medical products: adding numbers to pictures, Trends Biotechnol. 40, 194–209 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Okinda C, Nyalala I, Korohou T, Okinda C, Wang J, Achieng T, Wamalwa P, Mang T, Shen M, A review on computer vision systems in monitoring of poultry: A welfare perspective, Artif. Intell. Agric 4, 184–208 (2020). [Google Scholar]
- 116.US Food and Drug Administration, LAVIV (Azficel-T) (2018) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/laviv-azficel-t).
- 117.US Food and Drug Administration, RETHYMIC (2021) (available at https://www.fda.gov/vaccines-blood-biologics/rethymic).
- 118.US Food and Drug Administration, CARVYKTI (2022) (available at https://www.fda.gov/vaccines-blood-biologics/carvykti). [Google Scholar]
- 119.US Food and Drug Administration, KYMRIAH (tisagenlecleucel) (2021) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel).
- 120.US Food and Drug Administration, PROVENGE (sipuleucel-T) (2019) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/provenge-sipuleucel-t).
- 121.US Food and Drug Administration, TECARTUS (brexucabtagene autoleucel) (2021) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus-brexucabtagene-autoleucel).
- 122.US Food and Drug Administration, YESCARTA (axicabtagene ciloleucel) (2022) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel).
- 123.US Food and Drug Administration, IMLYGIC (2021) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/imlygic).
- 124.US Food and Drug Administration, LUXTURNA (2018) (available at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna).
- 125.US Food and Drug Administration, ZOLGENSMA (2021) (available at https://www.fda.gov/vaccines-blood-biologics/zolgensma).
- 126.US Food and Drug Administration, Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs) (2008) (available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/content-and-review-chemistry-manufacturing-and-control-cmc-information-human-somatic-cell-therapy).
- 127.US Food and Drug Administration, 21 CFR Part 610 -- General Biological Products Standards, (1973) (available at https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-610).
- 128.International Conference on Harmonisation, Preclinical Safety Evaluation Of Biotechnology-Derived Pharmaceuticals S6(R1) (2011) (available at https://www.ich.org/page/safety-guidelines). [PubMed]
- 129.International Conference on Harmonisation, Guidance On Genotoxicity Testing And Data Interpretation For Pharmaceuticals Intended For Human Use S2(R1) (2011) (available at https://www.ich.org/page/safety-guidelines). [PubMed]
- 130.International Conference on Harmonisation, Testing For Carcinogenicity Of Pharmaceuticals S1B (1997) (available at https://www.ich.org/page/safety-guidelines).
- 131.International Conference on Harmonisation, Duration Of Chronic Toxicity Testing In Animals (Rodent And Non Rodent Toxicity Testing) S4 (1998) (available at https://www.ich.org/page/safety-guidelines).
- 132.International Conference on Harmonisation, Note For Guidance On Toxicokinetics: The Assessment Of Systemic Exposure In Toxicity Studies S3A (1994) (available at https://www.ich.org/page/safety-guidelines).
- 133.International Conference on Harmonisation, Safety Pharmacology Studies For Human Pharmaceuticals S7A (2000) (available at https://www.ich.org/page/safety-guidelines). [PubMed]
- 134.International Conference on Harmonisation, Detection Of Reproductive And Developmental Toxicity For Human Pharmaceuticals S5(R3), (2020) (available at https://www.ich.org/page/safety-guidelines).
- 135.International Conference on Harmonisation, Immunotoxicity Studies For Human Pharmaceuticals S8 (2005) (available at https://www.ich.org/page/safety-guidelines).
- 136.Athanasiou KA, Niederauer GG, Schenck RC Jr., Biomechanical topography of human ankle cartilage, Ann Biomed Eng 23, 697–704 (1995). [DOI] [PubMed] [Google Scholar]
- 137.Mow VC, Kuei SC, Lai WM, Armstrong CG, Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments, J Biomech Eng 102, 73–84 (1980). [DOI] [PubMed] [Google Scholar]
- 138.Murphy MK, Arzi B, Hu JC, Athanasiou KA, Tensile characterization of porcine temporomandibular joint disc attachments, J Dent Res 92, 753–758 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Espinosa G, Otarola G, Hu JC, Athanasiou KA, Cartilage assessment requires a surface characterization protocol: roughness, friction, and function, Tissue Eng. Part C Methods , ten.TEC.2020.0367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nordberg RC, Espinosa MG, Hu JC, Athanasiou KA, A Tribological Comparison of Facet Joint, Sacroiliac Joint, and Knee Cartilage in the Yucatan Minipig., Cartilage , 19476035211021904 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Knecht S, Erggelet C, Endres M, Sittinger M, Kaps C, Stussi E, Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts, J Biomed Mater Res B Appl Biomater 83, 50–57 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Link JM, Hu JC, Athanasiou KA, Chondroitinase ABC Enhances Integration of Self-Assembled Articular Cartilage, but Its Dosage Needs to Be Moderated Based on Neocartilage Maturity, Cartilage 13, 672S–683S (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trimborn M, Endres M, Bommer C, Janke U, Kruger JP, Morawietz L, Kreuz PC, Kaps C, Karyotyping of human chondrocytes in scaffold-assisted cartilage tissue engineering, Acta Biomater 8, 1519–1529 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Calvert JW, Brenner K, DaCosta-Iyer M, Evans GR, Daniel RK, Histological analysis of human diced cartilage grafts, Plast Reconstr Surg 118, 230–236 (2006). [DOI] [PubMed] [Google Scholar]
- 145.Cissell DD, Link JM, Hu JC, Athanasiou KA, A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content, Tissue Eng Part C Methods 23, 243–250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Farndale RW, Sayers CA, Barrett AJ, A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures, Connect Tissue Res 9, 247–248 (1982). [DOI] [PubMed] [Google Scholar]
- 147.Singer VL, Jones LJ, Yue ST, Haugland RP, Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation, Anal Biochem 249, 228–238 (1997). [DOI] [PubMed] [Google Scholar]
- 148.Bielajew BJ, Hu JC, Athanasiou KA, Methodology to Quantify Collagen Subtypes and Crosslinks: Application in Minipig Cartilages, Cartilage 13, 1742S-1754S (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Netukova S, Duspivova T, Tesar J, Bejtic M, Baxa M, Ellederova Z, Szabo Z, Krupicka R, Instrumented pig gait analysis: State-of-the-art, J. Vet. Behav 45, 51–59 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.