Abstract
Background
Children are at increased risk from transfusion-related medical errors. Clinical decision support (CDS) can enhance pediatric providers’ decision-making regarding transfusion practices including indications, volume, rate, and special processing instructions. Our objective was to use CDS in a pediatric health system to reduce:
blood product-related safety events from ordering errors;
special processing ordering errors for patients with T-cell dysfunction, sickle cell disease (SCD), or thalassemia;
transfusions administered faster than 5 mL/kg/h.
Materials and methods
In this single-center before and after quality improvement study, we evaluated how user-centered design of pediatric blood product orders influenced pediatric transfusion practices and outcomes. Safety events were identified through active and passive surveillance. Other clinically relevant outcomes were identified through electronic health record queries.
Results
Blood product-related safety events from ordering errors did not change significantly from the baseline period (6 events, 0.4 per month, from 1/1/2018–3/27/2019) to the intervention period (1 event, 0.1 per month, from 3/28/2019–12/31/2019; rate ratio: 0.27 [0.01–2.25]). Packed red blood cell (PRBC) and platelet orders for patients with T-cell dysfunction that did not specify irradiation decreased significantly from 488/12,359 (3.9%) to 204/6,711 (3.0%, risk ratio: 0.77 [0.66–0.90]). PRBC orders for patients with SCD or thalassemia that did not specify phenotypically similar units fell from 386/2,876 (13.4%) to 57/1,755 (3.2%, risk ratio: 0.24 [0.18–0.32]). Transfusions administered faster than 5 mL/kg/h decreased from 4,112/14,641 (28.1%) to 2,125/9,263 (22.9%, risk ratio: 0.82 [0.78–0.85]).
Discussion
User-centered design of CDS for pediatric blood product orders significantly reduced special processing ordering errors and inappropriate transfusion rates. Larger studies are needed to evaluate the impact on safety events.
Keywords: clinical decision support, user-centered design, Patient Blood Management, medical errors, quality improvement
INTRODUCTION
Medical errors in blood transfusion practice are a common source of transfusion-related morbidity and mortality1. Children are at a higher risk of adverse outcomes from such errors than adults2,3. Due to their small size, errors in transfusion volume and/or rate are more likely to lead to inadequate treatment from under-transfusion or complications of over-transfusion such as transfusion-associated circulatory overload (TACO). Children are also more susceptible to errors in special processing of blood products. For example, preterm and term infants are relatively immunocompromised; many with immune dysfunction disorders involving T-cell immunity which manifest in the first 6 months to 1 year of life. Non-irradiated blood products administered to these populations increase the risk of rare, but severe and potentially fatal complications such as transfusion associated graft vs host disease (TA-GVHD)4. Additionally, administration of packed red blood cells (PRBCs) to children with sickle cell disease (SCD) without selecting phenotypically similar units (minor RBC antigen matching to a certain degree) can lead to production of minor RBC antigen alloantibodies, increasing the risk of hemolysis during future transfusions and complicating the ability to be transfused for the rest of the child’s life5,6.
Ordering blood products is a complex task for health providers who are often not trained sufficiently in Transfusion Medicine7–9. To make appropriate decisions, ordering providers must not only weigh the risks and benefits of a transfusion, but also decide on the appropriate volume and transfusion rate2,10, select special processing requirements based on the patient’s medical condition(s)4, and effectively communicate with the blood bank medical technologists and nurses to ensure timely therapeutic interventions (e.g., preparing blood for immediate use vs future transfusion during a procedure)11. In the setting of this complexity, safety events from transfusion practice errors related to human factors and information technology have been frequently reported in transfusion surveillance systems12.
Clinical decision support systems (CDS) can reduce blood product ordering errors through just-in-time education and patient-specific contextual knowledge. Numerous studies have demonstrated the effectiveness of CDS systems to promote restrictive transfusion strategies in adults and children13–20. However, to our knowledge, no studies have examined the role of CDS in improving transfusion volume, rate, and special processing requests in pediatric settings.
After reviewing transfusion-related safety events, a Failure Modes and Effects Analysis (FMEA)21 at our institution identified ordering errors as the most likely source of serious patient blood management errors. We re-designed the blood product ordering process through a combination of design by expert committee and user-centered design through formative usability testing. The resulting ordering process led to fewer severe errors in simulated scenarios during summative usability testing22. In this study, we evaluate the impact of implementing this new ordering process on pediatric transfusion practice and patient outcomes.
Primary objective
Reduce blood product safety events attributed to ordering errors (as detected from hospital incident reports plus active surveillance by a transfusion safety specialist).
Secondary objectives
-
Reduce the proportion of transfusion orders with special processing errors defined as:
PRBC or platelet transfusion orders for patients with T-cell dysfunction that did not specify irradiation;
PRBC transfusion orders for patients with SCD or thalassemia that did not specify to use phenotypically similar units.
Reduce the proportion of all flowsheet-tracked transfusions faster than 5 mL/kg/h.
Balance objective
To determine if our interventions for the primary and secondary objectives led to new problems in other parts of the system, we measured the proportion of transfusion orders with special processing (i.e., irradiation or phenotypically similar units) requested.
MATERIALS AND METHODS
Context
This study was performed at a large, urban pediatric health system in which the primary blood bank serves 2 freestanding children’s hospitals as well as high volume hematology and oncology clinics with over 10,000 blood transfusions per year. The study was conducted from 1/1/2018–12/31/2019. The study start date was chosen to provide a baseline to the interventions implemented on 3/28/2019 (see below) and the end date was selected prior to a new set of blood transfusion workflow changes implemented in early 2020 as part of Electronic Health Record (EHR) upgrades. In addition to ad-hoc transfusions, the patient blood management program supports specialized transfusion workflows for bone marrow transplant (BMT), chronic transfusion therapy, cardiac surgery, solid organ transplant, extracorporeal membrane oxygenation (ECMO), and apheresis. Transfusion orders are placed by providers using an enterprise EHR (Epic Systems©, Verona, WI, USA) which interfaces with a separate laboratory information system containing an FDA-approved blood banking module (Sunquest LaboratoryTM, Sunquest Information Systems, Tucson, AZ, USA).
Transfusion orders consisted of Prepare and Transfuse orders. The Prepare order signaled the blood bank to allocate a specific volume of blood product(s) to a patient and also allowed the ordering provider to request special processing including irradiation, washed cells, phenotypically similar units, and cytomegalovirus (CMV) seronegative units. The Transfuse order signaled the nurse to transfuse the blood product and specified the duration. Of note, a Transfuse order was not required in workflows in which an anesthesiologist or other physician would administer the blood transfusion directly. All transfusion orders were embedded in order sets, collections of related orders to improve physician efficiency and reduce the cognitive workload of individually searching for specific orders. The majority of blood product orders were placed from dedicated blood transfusion order sets consisting of pre-transfusion testing orders, Prepare orders, and Transfuse orders. However, a substantial fraction of blood product orders originated from disease or workflow-specific order sets (e.g., post-operative cardiac surgery) in which blood product orders were a single section embedded within a larger order set.
Interventions
Blood transfusion orders and order sets were re-designed through formative usability testing described elsewhere22. Briefly, a multi-disciplinary expert committee reviewed the FMEA results and recommended a new design. The design was then adjusted through formative usability testing, in which front-line providers were provided a scenario appropriate to their clinical specialty and asked to “think aloud”23 as they ordered blood products in an EHR test environment that had identical functionality to the production EHR environment except for re-designed blood orders. Based on comments and errors in simulated performance, iterative adjustments were made to blood product orders and order sets in the EHR test environment until 5 unique providers made no errors in 10 scenarios and had no new suggestions for design improvement. The new user-centered design was evaluated in summative usability testing and demonstrated significant reductions in severe ordering errors in simulation.
The user-centered design process led to adjustments in the Prepare and Transfuse orders as well as dedicated blood transfusion order sets (Online Supplementary Content, Figure S1). To implement these changes across the enterprise, the authors also reviewed 52 disease or workflow-specific order sets that included blood product orders and either retired or adjusted the order sets to conform to the new design. During this process, stakeholders also had the opportunity to adjust order set defaults. In particular, cardiac surgery and ECMO order sets were re-designed such that the number of units of each blood product would automatically calculate based on the patient’s weight.
Additionally, a novel workflow was developed for BMT patients. Prior to the intervention BMT providers would place a nursing communication order denoting specific parameters for transfusion (e.g. “transfuse 1 unit irradiated PRBCs if hemoglobin <8”), and then nurses would enter the Prepare and Transfuse orders themselves into a dedicated blood transfusion order set and sign under the provider’s name. After the intervention, in addition to the nursing communication order the BMT provider would place conditional blood product Prepare and Transfuse orders that specified the volume and special processing instructions. The nurse would then release the orders when the patient met criteria specified in the communication order, but the nurse did not have to enter a new order themselves for each transfusion (Online Supplementary Content, Figure S2).
Measures
All Prepare orders were extracted using EHR procedure codes associated with PRBC, platelet, FFP, and cryoprecipitate orders. Transfusion administrations were extracted from flowsheet rows entered by nurses. Of note, flowsheet data elements were not reliably entered for transfusions administered by physicians (e.g. by anesthesiology in the operating room or bedside procedures in the neonatal intensive care unit), emergency situations (e.g. massive transfusion protocol, emergency release), or for apheresis procedures. Thus, transfusions without volumes documented in flowsheet entries were excluded from measures of transfusion administrations. Additionally, no explicit link existed in the EHR during the study period between Prepare orders and Transfuse orders as this study was conducted prior to implementation of the Epic Blood Product Administration Module. Thus, order questions in the Prepare order (e.g. special processing requests) could not always be linked to the actual transfusion administration records generally created by nurses, which contained the rate of transfusion, time of transfusion, and other elements related to the administration.
Safety events related to patient blood management were detected through a combination of passive surveillance through incident reports as well as active surveillance by a dedicated transfusion safety nurse (JJ) who rotated systematically through hospital units providing nursing education and eliciting errors. Safety events were reported as all events per month and events attributed to ordering errors per month. Transfusion orders for patients with T-cell dysfunction were identified as follows:
(1) a list of the most common diagnosis codes in patients receiving PRBC or platelet transfusions was reviewed by a pediatric hematologist/oncologist and transfusion medicine specialist (MR), and diagnoses meriting irradiated PRBCs or platelets outside of emergencies were labelled (Table I); (2) patients were classified as having T-cell dysfunction if any of these diagnoses were present during the encounter where the transfusion took place; (3) all patients <6 months of age were assumed to be at risk for T-cell dysfunction. Transfusion orders for patients with SCD or thalassemia were identified by a pediatric hematologist/oncologist reviewing the most common diagnosis codes in patients receiving PRBC transfusions (Table II).
Table I.
Frequency of transfusion orders and administrations by blood product
| Blood product | Baseline (1/1/18 – 3/27/19) | Intervention (3/28/19 – 12/31/19) | ||
|---|---|---|---|---|
| Orders (%) | Administrations with documented volume | Orders (%) | Administrations with documented volume | |
| PRBCs | 14,819 (58) | 9,339 (64) | 8,999 (61) | 6,098 (69) |
| Platelets | 6,372 (25) | 3,960 (27) | 3,372 (23) | 2,079 (23) |
| FFP | 2,958 (12) | 1,090 (7) | 1,557 (11) | 588 (7) |
| Cryoprecipitate | 1,235 (5) | 247 (2) | 775 (5) | 114 (1) |
PRBCs: packed red blood cells; FFP: fresh, frozen plasma.
Table II.
Proportion of transfusions administered at a rate >5 mL/kg/h
| Blood product | Baseline | Intervention | Fast Transfusions Averted* |
|---|---|---|---|
| PRBCs | 1,262/9,339 (14) | 735/6,098 (12) | 89 |
| Platelets | 2,151/3,960 (54) | 1,105/2,463 (45) | 233 |
| FFP | 608/1,090 (56) | 268/588 (46) | 60 |
| Cryoprecipitate | 89/247 (36) | 17/114 (15) | 24 |
Fast transfusions averted was calculated by (1) estimating the expected number of fast transfusions by multiplying the baseline rate for each blood product by the number of transfusions during the intervention period and (2) subtracting the actual number of fast transfusions.
PRBCs: packed red blood cells; FFP: fresh, frozen plasma.
Transfusions administered faster than 5 mL/kg/h were identified by comparing the maximum documented rate of transfusion in mL/h per flowsheet entries to the most recently documented weight for the same patient taken prior to the transfusion start time.
Study of the interventions
Adjustments to blood product orders, dedicated blood transfusion order sets, other order sets that included blood product orders, and the new BMT workflow were all implemented simultaneously on 28 March 2019. We compared the measures described above during the baseline period (01/01/2018–03/27/2019) to the intervention period (03/28/2019–12/31/2019) after implementation. The intervention period was truncated at the end of 2019 due to unrelated interventions implemented in early 2020.
Analysis
Safety events were compared as Poisson rates per month. All other measures were compared visually using statistical process control charts (p-charts) and analytically using chi-square tests of proportions.
Statistical analyses were performed using R, version 3.5.1 (R Foundation, Vienna, Austria)24.
Ethical considerations and reporting guidelines
This study was determined to be Non-Human Subjects Research as a local quality improvement study by the Children’s Healthcare of Atlanta Institutional Review Board. This study is reported according to the SQUIRE 2.0 guidelines for quality improvement reports and the Safety-related EHR Research (SAFER) Reporting Framework for safety-related EHR interventions25,26.
RESULTS
A total of 25,384 unique Prepare orders were placed during the baseline period and 14,703 Prepare orders during the intervention period with PRBC orders comprising the majority (Table I). We identified 14,636 blood product administrations during the baseline period and 9,263 during the intervention period that had a documented transfusion rate entered in the flowsheets. The discrepancy between the number of Prepare orders and administrations with a documented transfusion rate resulted from (1) blood products ordered for the operating room (OR) in case of bleeding and never transfused, (2) apheresis orders where volume and rates were not consistently documented, (3) Prepare orders for sickle cell disease patients ordered in anticipation of potential long turnaround times in case of an acute need for transfusion that were never administered, and (4) cancelled orders. Nonetheless all Prepare orders were included in the analysis of ordering errors since they could have been administered to the patient.
Primary outcome
During the baseline period, we identified 0.9 safety events per month (13 total) related to patient blood transfusion practices, of which 0.4 per month (6 total) were attributed to ordering errors. During the intervention period, we identified 0.4 safety events per month (6 total) related to patient blood management (rate ratio 0.76, 95% confidence interval [CI]: 0.24–2.13, p=0.643), of which 0.1 per month (1 total) were attributed to ordering errors (rate ratio 0.27, 95% CI: 0.01–2.25, p=0.265).
Secondary outcomes
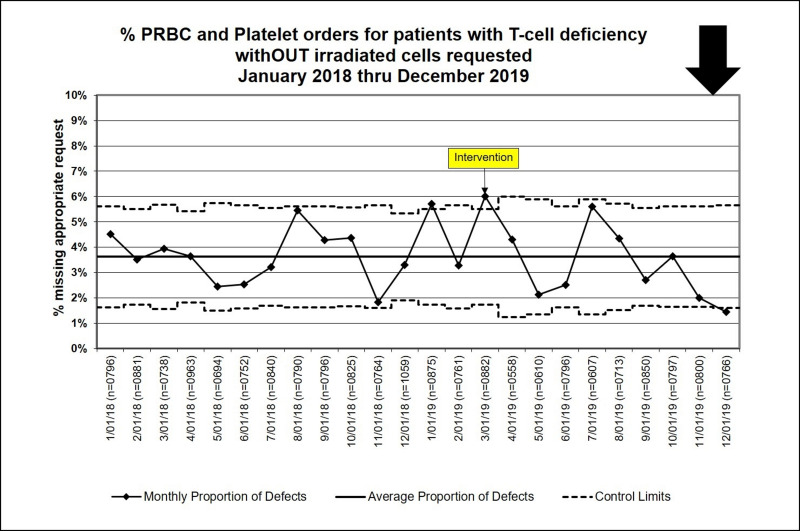
During the baseline period, 488/12,359 (3.9%) of PRBC and platelet Prepare orders placed for a patient with at least 1 visit diagnosis indicative of T-cell dysfunction or for a patient <6 months old did not have a special processing request for irradiation. In the intervention period, 204/6,711 (3.0%) such orders did not have a request for irradiation (risk ratio: 0.770, 95% CI: 0.66–0.90, p=0.001). While error rates peaked just prior to the intervention, the p-chart did not demonstrate special cause variation (Figure 1).
Figure 1.
Statistical Process Control Chart (p-chart) for the proportion of PRBC and platelet orders that should have been irradiated, but irradiation was not requested in the Prepare order
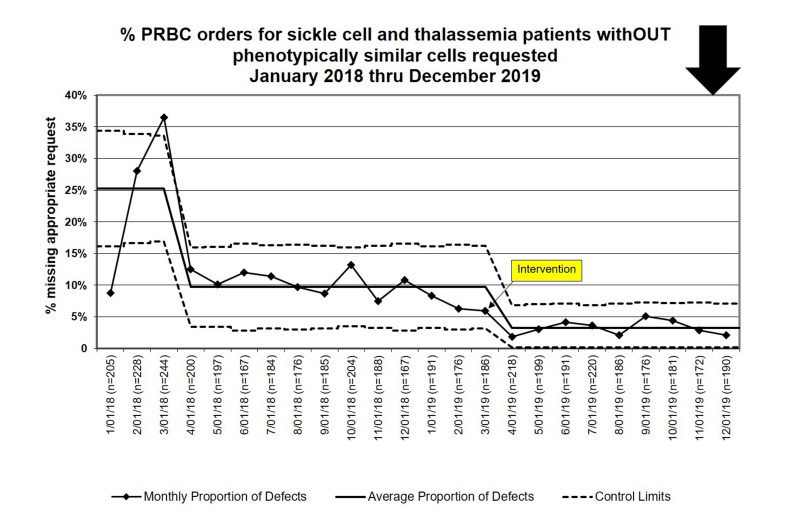
During the baseline period, 386/2,876 (13.4%) of PRBC Prepare orders placed for patients with SCD or thalassemia did not have a request for phenotypically similar units. In the intervention period, 57/1,755 (3.2%) such orders did not have a request for phenotypically similar units (risk ratio: 0.24, 95% CI: 0.18–0.32, p<0.001). The p-chart demonstrated special cause variation temporally associated with intervention implementation at the end of March, 2019 and sustained through the rest of the study period (Figure 2).
Figure 2.
Statistical Process Control Chart (p-chart) for the proportion of PRBC orders that should have been from phenotypically similar units, but phenotypically similar units were not requested in the Prepare order
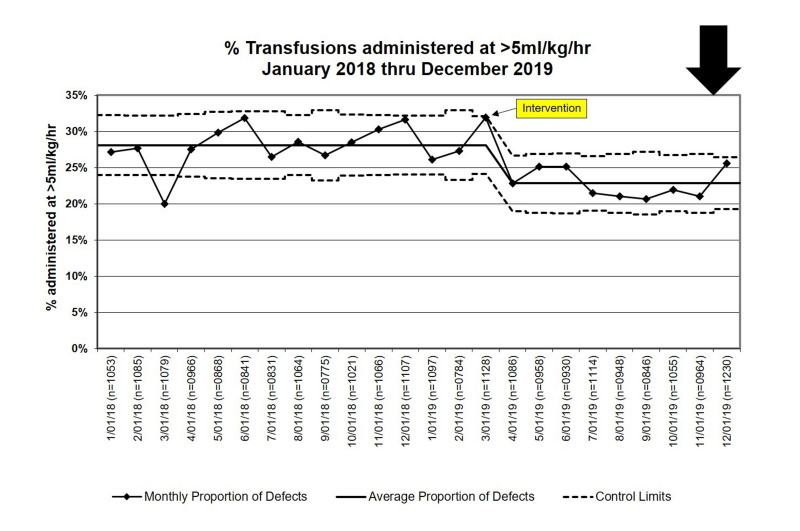
During the baseline period, 4,112/14,641 (28.1%) of all transfusions were administered faster than 5 mL/kg/h, compared to 2,125/9,263 (22.9%, risk ratio: 0.82, 95% CI: 0.78–0.85, p<0.001) in the intervention period, with special cause variation apparent in the p-chart at the time of the intervention in March, 2019 (Figure 3). If baseline rates of fast transfusions persisted, we would have expected an additional 477 transfusions at a rate >5 mL/kg/h. The greatest reductions were seen in platelet transfusions, which accounted for 233 (49%) of the 477 transfusions >5 mL/kg/h averted (Table II).
Figure 3.
Statistical Process Control Chart (p-chart) for the proportion of blood product transfusions administered at a rate >5 mL/kg/h
Balancing outcome
We reviewed the proportion of blood product orders requesting irradiation and phenotypically similar units. The proportion of PRBCs and platelets ordered as irradiated decreased slightly from 61.6 to 57.3% (p<0.001). The proportion of PRBCs where phenotypically similar units were requested increased slightly from 17.8 to 20.1% (p<0.001).
SAFER framework
We reviewed the 8 sociotechnical dimensions (i.e. combination of technology and human elements) of patient safety for EHR interventions and described pre-intervention issues, what sociotechnical changes were made, why they were felt to be effective, and how they could be applied in other settings (Table III).
Table III.
Sociotechnical interventions described using the SAFER Reporting Framework for Safety Related EHR Research26
| Sociotechnical Dimension | Pre-Intervention Issues | What* | Why† | How‡ |
|---|---|---|---|---|
| Hardware & software |
|
|
|
|
| Clinical content |
|
|
|
|
| Human-computer interface |
|
|
|
|
| Workflow & communication |
|
|
|
|
| People |
|
|
|
|
| Internal organizational features |
|
|
|
|
| External rules & regulations |
|
|
|
|
| Measuring & monitoring |
|
|
|
|
BMT: Bone Marrow Transplant; ECMO: Extracorporeal Membrane Oxygenation; EHR: electronic health record; FDA: Food & Drug Administration; IT: Information Technology; VS: vital signs;
What sociotechnical changes were made to implement an EHR-related intervention to improve patient safety.
Why the intervention did or did not lead to safety improvements.
How the intervention can be applicable or exported to others.
DISCUSSION
User-centered design of blood product orders and order sets led to reductions in ordering errors for special processing, transfusion rate, and total volume. However, there was no significant reduction in total blood product safety events, as detected from hospital incident reports and/or active surveillance by a transfusion safety specialist or those attributed specifically to ordering errors. This finding may be due to inadequate power as safety events are rare, inadequate surveillance for safety events, or because special processing, transfusion rate, and volume errors account for only a small proportion of blood product safety events. In contrast, for patients with SCD or thalassemia the new design led to dramatic improvements in the proportion of orders requesting phenotypically similar PRBCs, preventing “near-misses” that may lead to hospital safety events. Among patients at risk for T-cell dysfunction, the new design led to significantly more requests for irradiated PRBCs and platelets, although the effect size was not as large as progress seen for SCD and thalassemia patients. Nonetheless, this improvement was achieved in the context of fewer requests for irradiated blood products across the system. Finally, the proportion of transfusions administered faster than the recommended 5 mL/kg/h for non-emergent transfusions improved significantly, particularly for platelets.
The new blood product orders did not ask ordering providers to enter specific special processing requests, instead asking for indications for special processing and then cascading to the appropriate processing request. There exist fewer indications for phenotypically similar units than for irradiated blood products, and less diagnostic uncertainty for SCD and thalassemia compared to some diagnoses associated with T-cell dysfunction. This combination may explain the more dramatic reductions in ordering errors for phenotypically similar units. Many CDS systems have demonstrated improved adherence to evidence-based practices in patient blood management27. In adult settings, order sets and alerts promoting restrictive transfusion strategies have repeatedly shown reductions in transfusions for patients with hemoglobin >7g/dL13,17–20. In pediatrics, the evidence base for specific patient blood management strategies is not as well defined2. Nonetheless, CDS has demonstrated improved utilization of PRBCs, plasma, and platelets in children15,16,28,29. However, to our knowledge this is the first pediatric study demonstrating the effectiveness of CDS to improve special processing requests and transfusion rate.
Limitations
This is a single center study using one EHR instance from Epic Systems. Thus, the conclusions from this study may depend on organizational culture, behavioral habits prior to the intervention, change management structures, the existence of safety events at our institution prioritizing specific goals, and other factors that reduce its generalizability. While we have attempted to capture important sociotechnical elements using the SAFER reporting framework, these assessments are likely not comprehensive, and other factors may lead to different outcomes when applied to other health systems. Additionally, safety events associated with pediatric patients are thankfully very rare, and while the raw frequency decreased from the baseline to the intervention period, we were unable to demonstrate statistically significant improvement. Finally, our secondary study outcomes were limited to process measures. While the relevant transfusion reactions may have been detectable through incident reports, we had no reliable measures to determine if interventions changed patient outcome measures such as TA-GVHD, development of RBC alloimmunization and subsequent hemolytic reactions, or delays in transfusion.
CONCLUSIONS
User-centered design of blood product orders and order sets can improve pediatric blood transfusion practices, but its impact on safety events and patient outcomes remains unclear. Asking ordering providers for clinical indications that drive the need for special processing instead of the specific special processing requests themselves led to substantial improvements in ordering for SCD and thalassemia patients as well as improvements in patients with T-cell dysfunction. Blood product orders occur in the context of a complex sociotechnical system with changes to orders potentially affecting a wide variety of workflows. Future studies examining the impact of CDS on additional evidence-based practices and through multicenter initiatives would demonstrate the generalizability of this approach to optimize pediatric patient blood management.
Supplementary Information
ACKNOWLEDGMENTS
We would like to acknowledge the front-line nurses and physicians who participated in usability testing leading to the clinical decision support design described in this study. We also recognize the significant contributions to this work of Children’s Healthcare of Atlanta Information Services & Technology teams who implemented the clinical decision support described in this study into our electronic health record.
Footnotes
FUNDING AND RESOURCES
EWO and SK’s participation in this work was supported in part by the National Blood Foundation Early Career Investigator Award (NBF 20-15).
AUTHORSHIP CONTRIBUTIONS
EWO, MR, JJ, JB, ABC, and CDJ designed the study; MR, JJ, and CDJ collected data on safety events; EWO, MR, and SK collected and validated electronic health record data; EWO and SK performed the statistical analysis; EWO, MR, JJ, SK, JB, ABC, and CDJ wrote the manuscript.
DISCLOSURE OF CONFLICTS OF INTEREST
EWO has equity in Phrase Health© a clinical decision support analytics company. He does not receive any direct revenue. ABC is a paid faculty member of the American Medical Informatics Association Clinical Informatics Board Review Course. All other Authors declare no conflicts of interest.
REFERENCES
- 1.AABB Building a Better Patient Blood Management Program Identifying Tools, Solving Problems and Promoting Patient Safety Building a Better Patient Blood Management Program. 2015. [Accessed on 30/10/2019]. Available at: https://www.aabb.org/docs/default-source/default-document-library/resources/aabb-pbm-whitepaper.pdf?sfvrsn=4578189.
- 2.Goel R, Cushing MM, Tobian AAR. Pediatric Patient Blood Management programs: not just transfusing little adults. Transfus Med Rev. 2016;30:235–241. doi: 10.1016/j.tmrv.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Vossoughi S, Perez G, Whitaker BI, Fung MK, Rajbhandary S, Crews N, et al. Safety incident reports associated with blood transfusions. Transfusion. 2019;59:2827–2832. doi: 10.1111/trf.15429. [DOI] [PubMed] [Google Scholar]
- 4.Zantek ND, Parker RI, van de Watering LM, Josephson CD, Bateman ST, Valentine SL, et al. Recommendations on selection and processing of RBC components for pediatric patients from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med. 2018;19(9S Suppl 1):S163–169. doi: 10.1097/PCC.0000000000001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kangiwa U, Ibegbulam O, Ocheni S, Madu A, Mohammed N. Pattern and prevelence of alloimmunization in multiply transfused patients with sickle cell disease in Nigeria. Biomark Res. 2015;3:26. doi: 10.1186/s40364-015-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou ST, Alsawas M, Fasano RM, Field JJ, Hendrickson JE, Howard J, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4:327–355. doi: 10.1182/bloodadvances.2019001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Haspel RL. Transfusion medicine education for non-transfusion medicine physicians: a structured review. Vox Sang. 2017;112:97–104. doi: 10.1111/vox.12499. [DOI] [PubMed] [Google Scholar]
- 8.Haspel RL, Lin Y, Mallick R, Tinmouth A, Cid J, Eichler H, et al. Internal medicine resident knowledge of transfusion medicine: results from the BEST-TEST international education needs assessment. Transfusion. 2015;55:1355–1361. doi: 10.1111/trf.12968. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien KL, Champeaux AL, Sundell ZE, Short MW, Roth BJ. Transfusion medicine knowledge in Postgraduate Year 1 residents. Transfusion. 2010;50:1649–1653. doi: 10.1111/j.1537-2995.2010.02628.x. [DOI] [PubMed] [Google Scholar]
- 10.New HV, Berryman J, Bolton-Maggs PHB, Cantwell C, Chalmers EA, Davies T, et al. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol. 2016;175:784–828. doi: 10.1111/bjh.14233. [DOI] [PubMed] [Google Scholar]
- 11.Jones H, Reeve K. Transfusion guidelines in children: II. Anaesth Intensive Care Med. 2017;21:625–629. doi: 10.1016/j.mpaic.2020.10.006. [DOI] [Google Scholar]
- 12.Rowley M. Bolton-Maggs PHB, Poles D, editors. Chapter 13: Errors Related to Information Technology (IT) The 2017 Serious Hazards of Transfusion (SHOT) Report. 2018. [Accessed on dd/mm/yyyy]. pp. 104–5. Available from: https://www.shotuk.org/wp-content/uploads/myimages/Chapter-13-Errors-Related-to-Information-Technology-IT-2017.pdf.
- 13.Mcgreevey JD. Order sets in electronic health records principles of good practice. Chest. 2013;143:228–235. doi: 10.1378/chest.12-0949. [DOI] [PubMed] [Google Scholar]
- 14.Michetti CP, Prentice HA, Lita E, Wright J, Ng E, Newcomb AB, et al. Reducing transfusions in critically injured patients using a restricted-criteria order set. J Trauma Acute Care Surg. 2016;8:18–21. doi: 10.1097/TA.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 15.Adams ES, Longhurst CA, Pageler N, Widen E, Franzon D, Cornfield DN. Computerized physician order entry with decision support decreases blood transfusions in children. Pediatrics. 2011;127:e1112–9. doi: 10.1542/peds.2010-3252. [DOI] [PubMed] [Google Scholar]
- 16.Adams E, Longhurst C. Clinical decision support for pediatric blood product prescriptions. J Pediatr Intensive Care. 2015;5:108–112. doi: 10.1055/s-0035-1569996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassakian SZ, Yackel TR, Deloughery T, Dorr DA. Clinical decision support reduces overuse of red blood cell transfusions: interrupted time series analysis. Am J Med. 2016;129:636.e13–20. doi: 10.1016/j.amjmed.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Goodnough LT, Shieh L, Hadhazy E, Cheng N, Khari P, Maggio P. Improved blood utilization using real-time clinical decision support. Transfusion. 2014;54:1358–1365. doi: 10.1111/trf.12445. [DOI] [PubMed] [Google Scholar]
- 19.Rana R, Afessa B, Keegan MT, Whalen FX, Nuttall GA, Evenson LK, et al. Evidence-based red cell transfusion in the critically ill: quality improvement using computerized physician order entry. Crit Care Med. 2006;34:1892–1897. doi: 10.1097/01.CCM.0000220766.13623.FE. [DOI] [PubMed] [Google Scholar]
- 20.Fernández Pérez ER, Winters JL, Gajic O. The addition of decision support into computerized physician order entry reduces red blood cell transfusion resource utilization in the intensive care unit. Am J Hematol. 2007;82:631–633. doi: 10.1002/ajh.20888. [DOI] [PubMed] [Google Scholar]
- 21.Institute for Healthcare Improvement. QI Essentials Toolkit: Failure Modes and Effects Analysis (FMEA) 2017. [Accessed on 15/09/2019]. pp. 1–8. Available at: http://www.ihi.org/resources/Pages/Tools/Quality-Improvement-Essentials-Toolkit.aspx.
- 22.Orenstein EW, Boudreaux J, Rollins M, Jones J, Bryant C, Karavite D, et al. Formative usability testing reduces severe blood product ordering errors. Appl Clin Inform. 2019;10:981–990. doi: 10.1055/s-0039-3402714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumacher RM, Lowry SZ, Locke G, Gallagher PD. NIST Guide to the processes approach for improving the usability of electronic health records. 2010. [Accessed on dd/mm/yyyy]. Available at: https://www.nist.gov/publications/nistir-7741-nist-guide-processes-approach-improving-usability-electronic-health-records.
- 24.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; [Accessed on 01/10/2019]. p. 2019. Available at: https://www.r-project.org/ [Google Scholar]
- 25.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 ( Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process: Table 1. BMJ Qual Saf. 2015. [Accessed on 20/02/2020]. bmjqs-2015-004411. Available at: http://qualitysafety.bmj.com/lookup/doi/10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed]
- 26.Singh H, Sittig DF. A sociotechnical framework for safety-related electronic health record research reporting: the SAFER Reporting Framework. Ann Intern Med. 2020;172(11 Suppl):S92–100. doi: 10.7326/M19-0879. [DOI] [PubMed] [Google Scholar]
- 27.Hibbs SP, Nielsen ND, Brunskill S, Doree C, Yazer MH, Kaufman RM, et al. The Impact of electronic decision support on transfusion practice: a systematic review. Transfus Med Rev. 2015;29:14–23. doi: 10.1016/j.tmrv.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Baer VL, Henry E, Lambert DK, Stoddard RA, Wiedmeier SE, Eggert LD, et al. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: A pre-post analysis within a multihospital health care system. Transfusion. 2011;51:264–269. doi: 10.1111/j.1537-2995.2010.02823.x. [DOI] [PubMed] [Google Scholar]
- 29.Yarahuan JW, Billet A, Hron JD. A quality improvement initiative to decrease platelet ordering errors and a proposed model for evaluating clinical decision support effectiveness. doi: 10.1055/s-0039-1693123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.