Abstract
Background
The number of articles evaluating the efficacy of platelet-rich plasma (PRP) in androgenetic alopecia (AGA) and alopecia areata (AA) has increased exponentially during the last years. This systematic review and meta-analysis is aimed at evaluating the benefit of PRP in the treatment of alopecia.
Material and methods
We searched MEDLINE (through PUBMED), Embase, and CENTRAL for relevant data. Treatment effect was described by mean difference (MD) and risk difference with 95% confidence intervals (CI). The GRADE system was used to assess the certainty of the body of evidence.
Results
We found 27 controlled trials (1,117 subjects) that met our inclusion criteria: 18 trials (713 subjects) in patients with AGA, and 9 (404 subjects) in patients with AA. Eleven studies had a split head design. There was heterogeneity in types of PRP (e.g., activated and non-activated) and administration schedules. PRP was compared to saline injections (18 studies), local steroid injections (4 studies) and other comparators (5 studies). Most commonly reported outcomes were hair density and hair regrowth. It was not possible to pool all outcome data because of heterogeneity in reporting, and because reporting was often limited to a single study. Compared to saline injections, PRP injections increased hair density over a medium-term follow-up (MD, 25.6 hairs/cm2; 95 % CI: 2.62–48.57), but the evidence was rated as low quality due to inconsistency and risk of bias. In individuals with AA, it is unclear whether PRP injection compared with triamcinolone injection increase the rate of subjects with hair regrowth (very-low quality of evidence due to inconsistency, imprecision, and risk of bias). There were no serious adverse events related to PRP injection or control treatments.
Conclusions
There is limited evidence showing benefit of PRP for treatment of alopecia, and most of this evidence is of low quality.
Keywords: platelet-rich plasma, alopecia, treatment, systematic review, meta-analysis
INTRODUCTION
Platelet-rich plasma (PRP) is an autologous blood product with a high concentration of platelets. Numerous systems have been developed to concentrate autologous whole blood into platelet-rich products1. PRP contains some inflammatory cells (e.g. monocytes and polymorphonuclear neutrophils) and abundant quantities of proteins, including platelet-derived growth factor, transforming growth factor beta, vascular endothelial growth factor, epithelial growth factor, and cell adhesion molecules (e.g. fibrin, fibronectin and vitronectin)2–4. The growth factors and inflammatory cells promote cell recruitment, proliferation and angiogenesis, and may be involved in tissue regeneration and healing5–8. As a result of these biological regenerative properties, PRP has found applications in different fields of medicine, such as orthopaedics, sports medicine, oro-maxillo-facial surgery, ocular surface disorders and dermatology, to stimulate the regeneration and healing of wounds9–14.
In dermatology PRP is being used as a promising option for the treatment of several dermatological conditions including tissue regeneration, scar revision, wound healing, and some forms of alopecia, such as androgenetic alopecia (AGA), a genetically predetermined disorder due to an excessive response to androgens, and alopecia areata (AA), an autoimmune condition that causes inflammation-induced hair loss15–18. For the purpose of hair restoration, PRP is applied by intradermal injections to affected areas of skin, although its use has not been approved in either the USA or the European Union19–21. At present, patterned hair loss treatment includes topical minoxidil, finasteride, dutasteride (approved by the Food and Drug Administration for the treatment of benign prostatic hyperplasia), topical ketoconazole, anti-androgens and oestrogens (for female hair loss pattern), and bonding of hair follicle units22. Current first-choice therapies in use for the treatment of AA consist of topical corticosteroids or intralesional injections; however, unsatisfactory outcomes and risks for patients constitute limitations to the use of these treatments23. Surgical options include follicular unit transplant and follicular unit extraction techniques, which are outpatient procedures with excellent reported outcomes24. Currently, the evidence to support the clinical efficacy of PRP in pattern hair loss is controversial19,25–27. The number of primary studies and systematic reviews in this area has increased substantially over the years. However, we have observed variability in the studies included, differences in eligibility criteria, in types of studies selected, in statistical methods, or even subjective interpretation of otherwise similar results in most of the available systematic reviews19,26–35.
Moreover, assessment of the methodological quality of included studies and of evidence quality, which are key methodological procedures when conducting systematic reviews, were performed infrequently. For these reasons we have updated the systematic review of PRP for treatment of alopecia, including new primary studies and grading the quality of the available evidence endorsing Cochrane guidance for methodology. The studies included in this systematic review used a single or double spin procedure for the preparation of the PRP. Many of the protocols used required an activation step, before PRP administration, which commonly involves adding thrombin and/or calcium chloride (CaCl2); the clinical improvements, in patients treated with calcium gluconate-activated PRP, may be attributed to the release and concentration of α-granule proteins, including growth factors and cytokines, which promote cellular proliferation and differentiation36.
MATERIAL AND METHODS
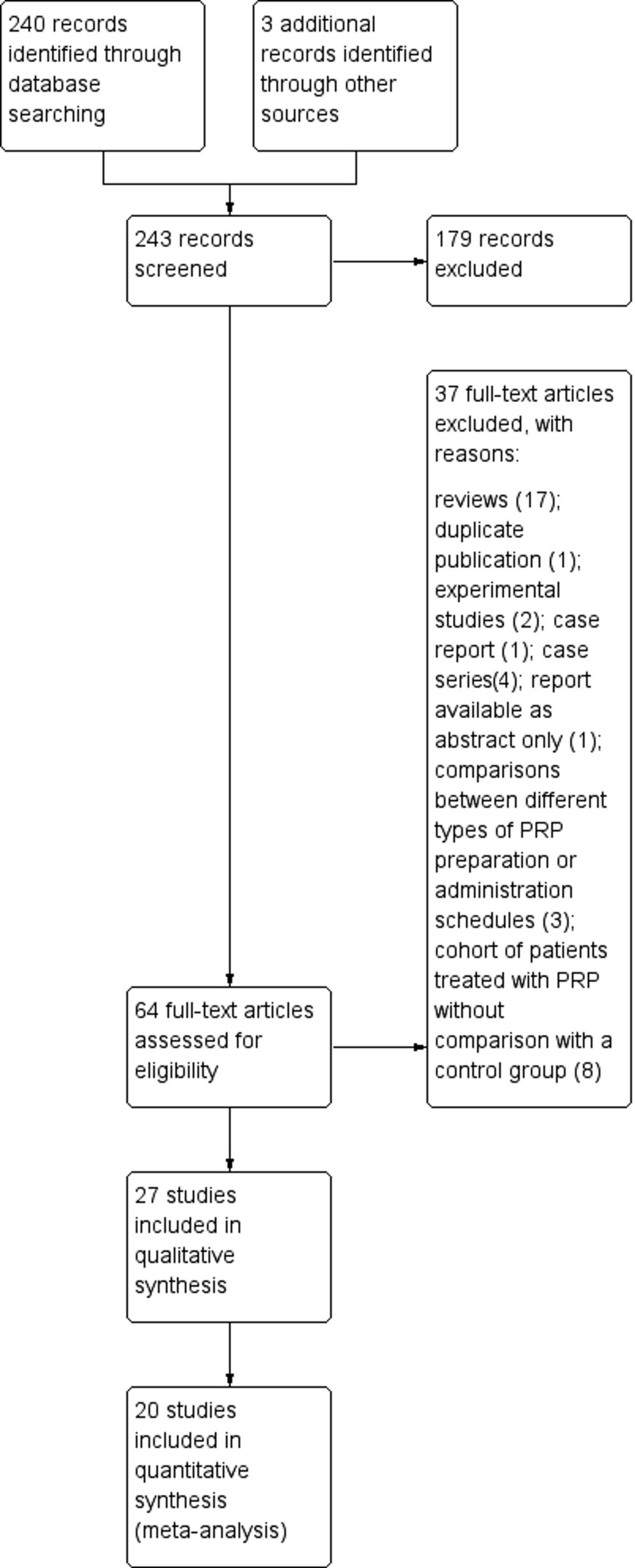
This systematic review was conducted on 243 potentially relevant studies (Figure 1), according to the recommended PRISMA checklist guidelines37.
Figure 1.
Study flow diagram. PRISMA flowchart summarising the inclusion and exclusion of studies
Out of 243 records screened, 27 articles were included in the systematic review and 20 were utilised in the quantitative synthesis. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PRP: platelet-rich plasma.
Search strategy
A computer-assisted literature search of the MEDLINE (through PUBMED), EMBASE, SCOPUS, OVID and Cochrane Library electronic databases was performed (latest search April 30, 2021) to identify clinical trials on the use of PRP for alopecia. A combination of the following text words was used to maximise search specificity and sensitivity: alopecia [MeSH]/androgenetic alopecia/alopecia areata/pattern hair loss/baldness/alopecia AND platelet-rich plasma [MeSH]/PRP/platelet-rich or platelet rich. In addition, we checked the reference lists of the most relevant items (original studies and reviews) in order to identify potentially eligible studies not captured by the initial literature search.
Study selection and inclusion criteria
Studies were selected independently by two reviewers (FM and MC), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (IP). Eligibility was assessed based on the title or abstract and on the full text if required. Articles were eligible if they reported the use of PRP for alopecia either in the title or in the abstract. Studies evaluating AGA and AA were considered. In this review, we included randomised controlled trials or quasi-randomised studies published in full. Studies with a split head design were also included.
Types of interventions
We compared injected PRP preparations with placebo injections, or active medication, by injection or topical administration, as controls. Studies were classified as (i) PRP vs placebo saline; (ii) PRP vs injective steroids; and (iii) PRP vs minoxidil.
Studies evaluating PRP plus other active drug (e.g., PRP injection combined with finasteride, or minoxidil) compared to other drugs alone were also considered.
Outcomes
Primary outcomes were hair density, hair count, hair regrowth, recurrences (such as incomplete remission in the follow-up period) and adverse events. Secondary outcomes included overall patient satisfaction, investigator satisfaction, terminal and vellus hair density, improvement in hair loss and in air thickness. Where available, the outcome measures were reported in different follow-up periods.
Data collection and analysis
For each trial included in the systematic review, the following data were extracted by two reviewers (FM and MC) independently: first author, year of publication, type of alopecia, details of intervention in the study and control groups, sample size, mean age and male/female ratio (PRP and control groups), outcome measurements, follow-up period and main results (Table I). Measures of treatment effect were mean differences (MD) together with 95% confidence intervals (95% CI) for continuous outcome measures (e.g. hair count, hair density) and risk differences (RD) for binary outcomes (e.g., adverse events, patient satisfaction, and improvement in hair count). For continuous measures, the score had to be reported as mean and standard deviation (SD), because it is problematic pooling data provided as medians and ranges, or interquartile intervals, with those provided as means and SD. Methods are available in the literature to convert medians and ranges to means and SD. In the present meta-analysis, we used the method of Hozo et al., which is more reliable for small studies38. We used final scores in preference to change in scores. Disagreement was resolved by consensus and by the opinion of a third reviewer (IP), if necessary.
Table I.
Characteristics and main results of the included studies on the use of platelet-rich plasma in alopecia
| Study (year)ref | Study design | N. of patients (condition) | Male/female | Age in years (range) | Test group (N) | Control group (N) | Outcomes | Follow-up | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Trink (2013) 43 | RCT, split-head design | 45 (AA) | - | - | ILC (15) PRP (15) |
Placebo (15) | Hair regrowth; SALT score; dermoscopic evaluation | 1 year | PRP increased hair regrowth significantly and decreased hair dystrophy |
| Cervelli (2014) 44 | RCT, split-head design | 10 (AA) | 10/0 | 22–60 | PRP (10) | Placebo (10) | Total hair counts; hair density; terminal and vellus hair densities | 6 months | A clinical improvement in mean hair count and mean hair thickness for the PRP group |
| Gentile (2015) 45 | RCT, split-head design | 20 (AGA) | 20/0 | 19–63 | PRP (20) | Placebo (20) | Hair count; hair density; terminal hair density; vellus hair density, microscopic evaluation | 2 years | A significant increase in the mean hair count and terminal hair density for the PRP group |
| Lee (2015) 46 | RCT | 40 (AGA) | 0/40 | 20–60 | PRP + PDRN (20) | PDRN (20) | Hair counts; mean hair thickness | 3 months | PRP + PDRN induced greater improvement in hair thickness than treatment with PDRN therapy alone |
| Mapar (2016) 47 | RCT, split-head design | 17 (AGA) | 17/0 | 25–45 | PRP (17) | Placebo (17) | Terminal and vellus hairs | 6 months | PRP did not improve hair growth |
| Puig (2016) 48 | Non-RCT | 26 (AGA) | 0/26 | - | PRP (15) | Placebo (11) | Hair count; hair mass index; patient-opinion survey | 26 weeks | No statistically significant difference between the two groups |
| Alves (2016) 49 | RCT | 25 (AGA) | 12/13 | 18–65 | PRP (25) | Placebo (25) | Hair count; hair density; terminal hair density | 6 months | A statistically significant increase in mean total hair density for the PRP group |
| El Taieb (2017) 50 | RCT | 90 (AA) | 39/51 | 10–40 | Topical minoxidil 5% (30), PRP (30) | Placebo (30) | Hair growth; dermoscopic evaluation | 3 months | An earlier response in the form of hair regrowth, reduction in short vellus hair and dystrophic hair in the PRP group |
| Shah (2017) 51 | RCT | 50 (AGA) | - | 18–50 | PRP + MN + topical minoxidil 5% (25) | Topical minoxidil 5% (25) | Dermoscopic evaluation | 6 months | A significant improvement in the PRP group |
| Toama (2017) 52 | RCT | 40 (AGA) | 19/21 | 18–45 | PRP (20) | Placebo (20) | Hair count; clinical evaluation; side effects | 6 months | A greater mean number of hairs in the PRP group |
| Kachhawa (2017) 53 | RCT, split-head design | 44 (AGA) | 44/0 | 18–55 | PRP (44) | Placebo (44) | Hair growth; dermoscopic evaluation | 6 months | A significant increase in mean hair thickness/density for the PRP group |
| Tawfik (2017) 54 | RCT, split-head design | 30 (AGA) | 0/30 | 20–45 | PRP (30) | Placebo (30) | Hair density, hair diameter, patient’s satisfaction | 6 months | PRP significantly increased hair density and hair thickness |
| Behrangi (2019) 55 | RCT | 114 (AGA) | 114/0 | 20–40 | Finasteride (28), PRP (26) | Placebo (60) | Hair growth; reduction of hair loss | 6 months | A statistically significant increase in hair growth and hair loss reduction in the PRP group |
| Ranparija (2019) 56 | RCT, split-head design | 30 (AA) | 22/8 | 20–40 | PRP (30) | ILC (30) | Hair regrowth | 3 months | A significant increase in hair regrowth for ILC treatment |
| Rodrigues (2019) 57 | RCT | 26 (AGA) | 26/0 | 18–50 | PRP (15) | Placebo (11) | Hair count; hair density | 2 months | PRP significantly increased hair growth |
| Verma (2020) 58 | Non-RCT | 40 (AGA) | 40/0 | 20–49 | PRP (20) | Topical minoxidil 5% (20) | Hair pull test; hair growth questionnaire; patient’s satisfaction | 6 months | PRP was found to be better than topical minoxidil therapy |
| Albalat (2019) 59 | RCT | 80 (AA) | 68/12 | 17–52 | PRP (40) | ILC (40) | RGS; dermoscopic evaluation; side effects | 6 months | No statistically significant difference between the two groups |
| Aggarwal (2020) 60 | RCT, split-head design | 30 (AGA) | 30/0 | 22–44 | MN + PRP (30) | MN (30) | Hair thickness; hair density; satisfaction score | 3 months | No additional effect in MN + PRP-treated group |
| Balakrishnan (2020) 61 | Non-RCT | 32 (AA) | - | - | PRP (16) | ILC (16) | SALT score; RGS | 12 weeks | No statistically significant difference between the two groups |
| Shapiro (2020) 62 | RCT, split-head design | 35 (AGA) | 18/17 | 18–58 | PRP (35) | Placebo (35) | Hair density; hair diameter; patient’s satisfaction; side effects | 3 months | No significant difference in hair density change between the two groups |
| Dubin (2020) 63 | RCT | 28 (AGA) | 0/28 | 27–85 | PRP (14) | Placebo (14) | Hair density; dermoscopic evaluation; side effects | 24 weeks | A statistically significant increase in mean total hair density for the PRP group |
| Kapoor (2020) 64 | RCT | 40 (AA) | 18/22 | 18–50 | PRP (20) | ILC (20) | SALT score, patient’s satisfaction | 6 months | Reduction in SALT score was greater in the ILC group |
| Hegde (2020) 65 | RCT, split-head design | 50 (AA) | - | 18–60 | PRP (25), ILCs (25) | Placebo (25) | SALT score; dermoscopic evaluation | 5 months | The maximum absolute regrowth occurred in the steroid group followed by the PRP group followed by the placebo group |
| Gressemberger (2020) 66 | RCT | 28 (AGA) | 28/0 | 18–52 | PRP (28) | Placebo (28) | Hair growth; clinical improvement; patient’s satisfaction | 6 months | PRP did not improve hair growth |
| Singh (2019) 67 | RCT | 80 (AGA) | 80/0 | 18–60 | Topical minoxidil 5% (20) PRP (20) PRP + topical minoxidil 5% (20) |
Placebo (20) | Hair density, dermoscopic evaluation | 5 months | PRP with topical minoxidil was the most effective treatment modality while PRP alone and topical minoxidil alone were more effective than placebo |
| Gupta (2021) 68 | RCT, split-head design | 27 (AA) | 13/14 | 18–35 | PRP (27) | Placebo (27) | SALT score; dermoscopic evaluation; side effects | 3 months | PRP showed limited efficacy vs placebo |
| Farid (2016) 69 | RCT | 40 (AGA) | 9/31 | 20–40 | PRP + MN (20) | Topical minoxidil 5% (20) | Hair count; patient’s satisfaction; adverse events | 28 weeks | A statistically comparable efficacy of daily application of 5% topical minoxidil versus PRP + MN |
AGA: androgenetic alopecia; AA: alopecia areata; PRP: platelet-rich plasma; RCT: randomised controlled trial; MN: microneedling; ILC: intralesional corticosteroids; PDRN: polydeoxyribonucleotide injection; RGS: re-growth scale; SALT score: severity of alopecia tool score.
The study weight was calculated using the Mantel-Haenszel method. We assessed statistical heterogeneity using t2, Cochran’s Q and I2 statistics. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error. In the case of no heterogeneity (I2=0), studies were pooled using a fixed-effects model. Where values of I2 were >0, a random-effects analysis was undertaken.
Assessment of risk of bias in included studies
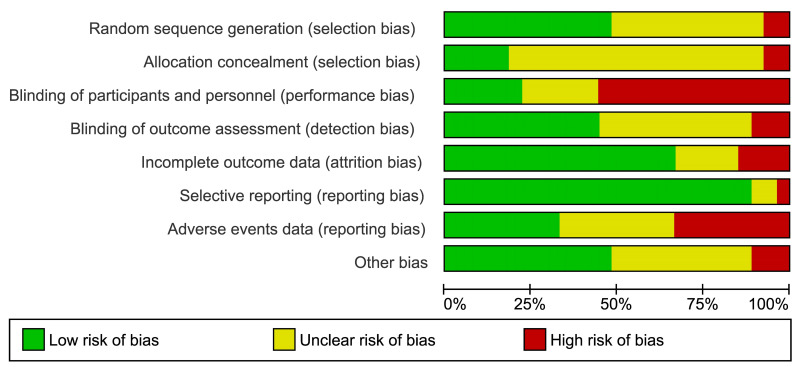
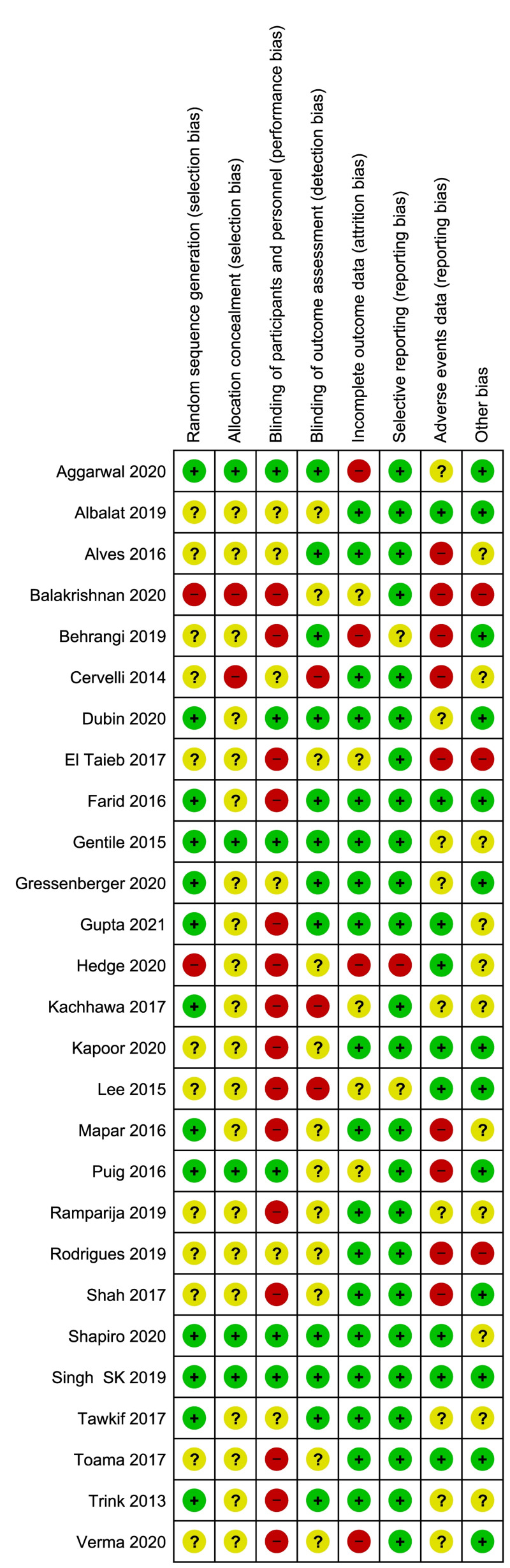
Two review authors (FM, MC) independently assessed the risk of bias of each included study following the domain-based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions39. They discussed any discrepancies and achieved consensus on the final assessment. The Cochrane “Risk of bias” tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues relating to bias. For the selective reporting domain, we added an item for the outcome “adverse events” because reporting was often inadequate for this outcome. We have presented our assessment of risk of bias using two “Risk of bias” summary figures: (i) a summary of bias for each item across all studies; and (ii) a cross-tabulation of each trial by all of the “Risk of bias” items (Figure 2A, B).
Figure 2.
Risk of bias summary and graph
Summary of Cochrane (2A) and cross-tabulation (2B) of risk of bias assessment. Twenty studies (74 %) were at high risk of bias for one or more domains, and 26 studies (96 %) were at unclear risk of bias for one or more domains; only one study was judged at low risk of bias in all the domains.
“Summary of findings” tables
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes, and constructed a “Summary of findings” table (Table II) using REVMAN 5.440. This table presents key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes41. The “Summary of findings” table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias42.
Table II.
Summary of findings
| PRP injections compared with control interventions for alopecia Patients or population: individuals with alopecia (AGA or AA) Settings: outpatients Intervention: PRP injection Comparison: saline placebo or triamcinolone injection for outpatients | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risks* (95 % CI) | Relative effect (95% CI) | N. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | PRP | |||||
| Hair density hairs/cm2 (3–6 months) | The mean hair density ranged across control groups from 32 to 168 hairs/cm2 | The mean hair density in the intervention groups was 145 hairs/cm2 (from 63 to 187) | Mean difference: 25.6 (from 2.62 to 48.57) | 308 (7) | ⊕⊕⊝⊝ low 1 |
On average, use of PRP injection compared with saline injection may increase hair density over a medium-term follow-up period |
| Hair count Hairs/0.65 cm2 | The mean hair count ranged across control groups from 87.9 to 112 hairs/0.65 cm2 | The mean hair count in the intervention groups was 119.6 hairs/0.65 cm2/(from 115 to 123) | Mean difference: 18.4 (from −2.86 to 39.8) | 110 (3) | ⊕⊝⊝⊝ very low2 |
On average, it is unclear whether or not use of PRP injection compared with placebo increases the mean hair count |
| Triamcinolone | PRP | |||||
| Hair regrowth (% of pts) - (rate of subjects with substantial improvement as measured by regrowth grading systems) (from 3 to 12 months) | 61 per 100 | 64 per 100 (from 58 to 65) | Risk difference 0.03 (from −0.35 to 0.42) | 202 (4) | ⊕⊝⊝⊝ very low2 |
On average, it is unclear whether or not use of PRP injection compared with triamcinolone injection increases the rate of subjects with hair regrowht over a follow-up period of 3–12 months |
| Adverse events | In the majority of the included studies, adverse events were not included among the predefined outcomes, and the reporting was incomplete and inadequate. | ⊕⊕⊝⊝ low3 |
No serious adverse events were reported, but the risk of reporting bias and imprecision should be taken into account. For less serious adverse events (e.g., pain at the injection site) it is not clear if their prevalence is increased or not in PRP recipients compared to recipients of injected controls. | |||
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Downgraded for risk of bias and inconsistency (due to heterogeneeity, I2=94).
Downgraded for risk of bias, inconsistency (due to heterogeneeity, I2=90) and imprecision (95% CI includes line of no effect).
Downgraded twice for risk of bias (particularly reporting bias) and for serious imprecision (no events) reflecting the inadequate numbers to detect rare events.
AGA: androgenetic alopecia; AA: alopecia areata; PRP: platelet-rich plasma; CI: confidence interval.
When evaluating the “Risk of bias” domain, we downgraded the GRADE assessment when we classified a study as being at high risk of bias for one or more of the following domains: selection, attrition, performance, detection, reporting, and other bias; or when the “Risk of bias” assessment for selection bias was unclear (this was classified as unclear for either the generation of the randomisation sequence or the allocation concealment domain). For the self-reported outcomes we downgraded for high risk of bias in performance and detection domains, since we judged that these outcomes, self-reported by patients or collected by physicians to help standardise the assessments of patients, are likely to be influenced by lack of blinding. We have presented the following outcomes in the “Summary of findings” table: (i) hair count; (ii) hair density; (iii) hair regrowth; and (iv) adverse events. All calculations were done using REVMAN 5.4.
RESULTS
The search yielded 243 potentially relevant studies (Figure 1) of which 179 articles were excluded after the preliminary screen and 64 were deemed potentially eligible and their full-text was assessed. Thirty-seven studies were then excluded, of which 17 were reviews and 20 were primary publications: these were excluded for various reasons [duplicate publication (n=1), experimental studies (n=2), case report (n=1), case series (n=4), report available as abstract only (n=1), comparisons between different types of PRP preparation or administration schedules (n=3), or cohort of patients treated with PRP without comparison with a control group (n=8). Hence, 27 studies were available for the qualitative synthesis43–69. The main features of the included studies are summarised in Table I.
Overall, 1,117 individuals were enrolled in the 27 randomised controlled trials selected for the review; 11 of these studies had a split head design, and in this case the unit of analysis was each area of the scalp and not the individual43–45,47,53,54,56,60,62,65,68. Of the 27 studies included in the systematic review, 18 (713 individuals) were conducted in patients with AGA45–49,51–55,57,58,60,62,63,66,67,69, and nine (404 individuals) in patients with AA43,44,50,56,59,61,64,65,68. PRP was compared to saline injections (18 studies)43–45,47–50,52–55,57,62,63,65–68, to local steroid injections (4 studies)56,59,61,64, to minoxidil (3 studies)51,58,69, or to others comparators (2 studies)46,60 (Table I). In ten studies non-activated PRP was injected45,48,54,56,60–64,69, and in the remaining 17 studies, activated PRP was injected. Ten studies were conducted in India, five in Egypt, five in Europe (3 in Italy, 1 in Spain and 1 in Austria), three in USA, two in Iran, one in Brazil and one in Korea.
Risk of bias in included studies
Twenty studies (74%) were at high risk of bias for one or more domains, and 26 studies (96%) were at unclear risk of bias for one or more domains; one study67 was judged at low risk of bias in all the domains (Figure 2A, B).
Allocation
We assessed three studies as being at high risk of selection bias, as randomisation was by alternation of the two treatments, or because the generation of the randomisation process was unclear coupled with unbalance between groups at baseline, or because the intervention allocations could have been foreseen in advance41,58,62. The reports of the other 19 studies were unclear regarding the random sequence generation and/or allocation concealment. Only five studies (18 %) were at low risk of selection biases.
Blinding
Performance bias
There were 15 studies (55 %) reported as open label, and they were graded as being at high risk of performance bias (blinding of participants and personnel); six studies (22 %) were graded at unclear risk of performance bias due to the fact that they did not provide information to enable judgement about “high” or “low” risk of bias related to the blinding of participants and personnel. Six studies were reported as double blind.
Detection bias
Twelve studies (44 %) were graded at low risk of detection bias due to the fact that the assessor was blinded to treatment allocation; 12 studies (44 %) were graded at unclear risk of detection bias due to the fact that did not provide information to enable judgement about “high” or “low” risk of bias related to the blinding of outcome assessors; three studies were graded at “high risk” of bias.
Incomplete outcome data
Four studies were judged at high risk of attrition bias because a large proportion of enrolled subjects left the study due to unsatisfactory effect of the initial treatment, or because outcome measures were reported for the PRP group but not for the control group52,55,57,62. Five other studies were judged at unclear risk of bias. The remaining 18 studies (66 %) were judged at low risk of bias.
Selective reporting
Selective reporting bias was low in almost all the included studies for all the outcomes (24 studies, 89 %) but adverse events. For the outcome adverse events nine out of 26 trials (33 %) were judged at high risk of bias, nine studies were judged at unclear risk of bias, and only nine studies were judged at low risk of reporting bias for adverse events.
Other potential sources of bias
We judged three studies to be at high risk for other sources of bias because of unbalance at baseline47,54,58. Moreover we judged the 11 studies with a split-head design at unclear risk of other bias because the analysis was based on individual units (e.g., each area of the scalp) without taking into account that the data were clustered within participants; hence a unit-of-analysis error could occur. Thirteen studies were judged at low risk of other biases.
Effects of interventions
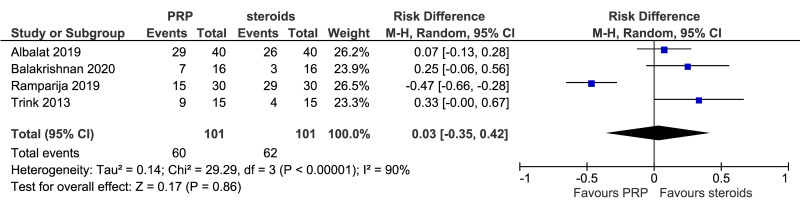
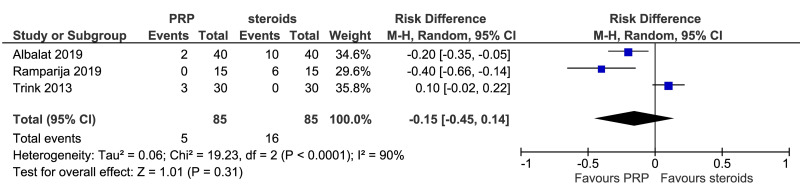
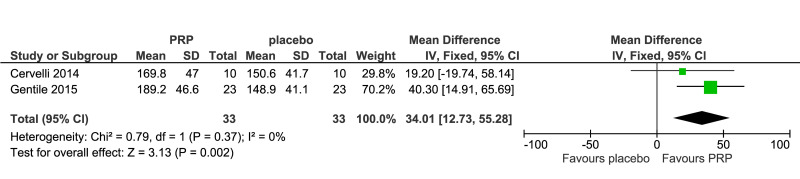
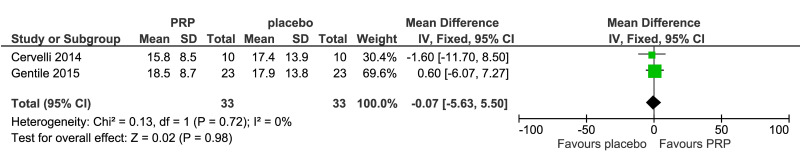
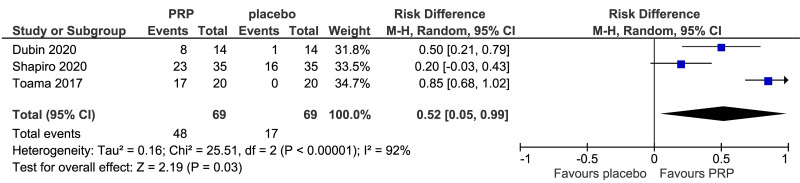
The outcomes more commonly reported were hair density (hairs/cm2) (Figure 3A), hair count (hairs/0.65 cm2) (Figure 3B), hair regrowth (rate of subjects with substantial hair regrowth) (Figure 3C), and recurrences (Figure 3D). Other outcomes reported were terminal and vellus hair density, improvement by investigator assessment, hair thickness, patient self-assessment and physician assessment of efficacy, safety and overall satisfaction, SALT (severity of alopecia tool score), incidence of adverse events and a variety of other outcomes (e.g., hair pull test, cell proliferation as measured by Ki-67 evaluation, terminal and vellus hair count, hair mass index, percentage of anagen hairs, hair cross section). We did not conduct a meta-analysis of all these outcomes because there was heterogeneity in reporting data, and because many outcomes were reported in a single study only.
Figure 3.
Forest plots of comparisons
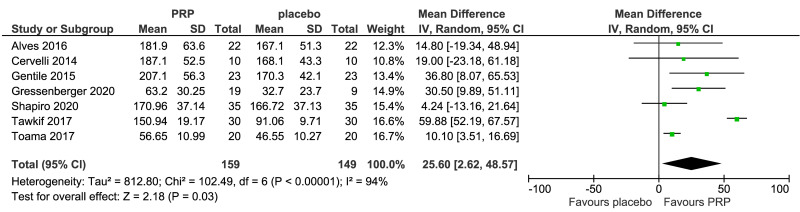
Meta-analysis, conducted for platelet-rich plasma (PRP) vs placebo and PRP vs triamcinolone, for hair density data (A) (7 studies - 6 in AGA and 1 in AA; 308 units of analysis), for hair count (B) (3 studies; 110 units of analysis), for hair regrowth (C) (4 studies; 202 units of analysis) conducted in AA patients, and for recurrences (D) (3 studies; 170 units of analysis). AGA: androgenetic alopecia; AA: alopecia areata; 95% CI: 95 % confidence interval.
Platelet-rich plasma vs placebo
PRP was compared to saline injections in 18 studies. The most commonly reported outcomes were hair density (8 studies, 7 in AGA and 1 in AA; 308 units of analysis) (Figure 3A). The mean increase of hair density was greater in PRP recipients than in placebo recipients (MD 25.6, 95% CI: 2.62 to 48.57; p=0.03); low-quality evidence, downgraded for risk of bias and for inconsistency (due to substantial heterogeneity) (Table II).
Hair count (hairs/0.65 cm2) after 3–6 months was reported in three studies (110 units of analysis) (Figure 3B). PRP recipients showed a slight increase in mean hair count in the treatment area compared to placebo recipients, but the difference was not statistically significant (MD, 18.4, 95% CI: −2.86 to 39.8; p=0.09); very low-quality of evidence, downgraded for risk of bias, inconsistency and imprecision. Other outcomes reported for this comparison were terminal and vellus hair density (2 studies, 66 evaluations) (Figure 4A, B) and investigator-assessed improvement (3 studies, 138 evaluations) (Figure 4C).
Figure 4.
Forest plots of other outcomes
Meta-analysis of terminal hair density (A) (2 studies; 66 evaluations), vellus hair density (B) (2 studies; 66 evaluations), improvement by investigator assessment (C) (3 studies; 138 evaluations). Analysis was conducted for platelet-rich plasma (PRP) vs placebo. 95% CI: 95 % confidence interval.
Platelet-rich plasma vs local steroid injections
This comparison was reported in five studies. Rates of individuals with hair regrowth (as measured by regrowth grading systems, e.g., McDonalds Hull and Norris) was reported in four studies (202 units of analysis) conducted in AA patients (Figure 3C). The mean difference in hair regrowth was similar between PRP and steroid recipients (RD, 0.03, 95 % CI: −0.35 to 0.42; p=0.86); very low-quality of evidence, downgraded for risk of bias, inconsistency and imprecision. Likewise, rate of recurrence did not differ significantly between PRP and triamcinolone recipients with AA (RD, −0.15, 95 % CI: −0.45 to 0.14); very low-quality of evidence (Figure 3D).
Other outcomes and comparisons
A variety of other outcomes and other comparisons (e.g., PRP vs minoxidil, or finasteride) were analysed in the studies included in this systematic review, but most of them were reported in a single study, or two studies at most. Overall, there was no evidence of benefit of PRP compared to controls on these outcomes.
Adverse events
No participant was reported to have developed any serious events in either the PRP or control groups. This comparison was graded as low-quality evidence and downgraded once due to serious risk of bias (especially reporting bias) and once for serious imprecision (no events) reflecting the inadequate numbers of observations to detect rare events. Other less severe, short-term adverse events, mostly post-injection pain, erythema, burning sensation, swelling, redness, minor bleeding in treated areas, and headache were specified in six trials43,49,56,59,61,64. Two studies provided information on pain assessed on a visual analogue scale (VAS): in one study it was reported that the VAS score was significantly lower in triamcinolone recipients than in PRP recipients61, while in another study comparing PRP and placebo59 no differences were found in VAS score between groups. Another study reported that pain was more prevalent among PRP recipients than among triamcinolone recipients (18/25 vs 5/25, respectively)62. In the large majority of evaluated studies, the reporting of adverse events was inadequate (Table I), and where adverse events were reported, these often were limited to short statements of the absence of adverse events in the study results or discussion without indication of systematic recording. Three trials did not mention adverse events at all.
DISCUSSION
The use of PRP, as a treatment in the field of trichology, is aimed at counteracting the progressive thinning of the hair and is based on the possibility of concentrating the platelet content so that the increase in growth factors can accelerate the regeneration of atrophic non-pilo-sebaceous follicles. The advantages of treatment of alopecia with PRP include the autologous nature of the product, low invasiveness, no major side effects and lower costs than hair transplantation. However, it is important to determine the effectiveness of PRP in the field of trichology, on the basis of available evidence.
The differences between the designs of the studies included in our analysis contributed to the difficulty in interpreting results. The studies were stratified considering the use of PRP alone or in combination with other therapeutic treatments, but also by subject sex, severity of alopecia, sample size, randomisation procedures, and drugs in the control groups, further obfuscating PRP treatment results. Each study employed a unique treatment protocol. Although most studies used quantitative and qualitative methods to evaluate measures such as hair count, hair density, and hair thickness, the methods for assessing outcomes varied widely, making it difficult to compare the benefits of treatment on outcomes of AGA and AA.
Our systematic review differs in many aspects from other systematic reviews18,24–33 already published. There was large variability regarding the types and numbers of studies evaluated in these systematic reviews, as well as differences in the eligibility criteria and statistical methods used, and in many instances lack of assessment of the quality of the available evidence.
In the present systematic review and meta-analysis, the largest published so far on this issue, we found a low-quality of evidence that PRP injection, compared with saline injection, may increase hair density over a medium-term follow-up period in individuals with alopecia, mostly AGA. An assessment of low-certainty evidence means that our confidence in the effect estimate is limited, and the true effect may be substantially different from the estimate of the effect. In individuals with AA, it is unclear whether or not use of PRP injection compared with triamcinolone injection increases the rate of subjects with hair regrowth over a follow-up period of 3–12 months, and we graded the available evidence as very low-quality. In other words, these results do not provide a reliable indication of the likely effect, and the possibility that the actual effect will be substantially different is very high.
In most of the other comparisons, the 95% CI crossed the line of no benefit, and at best indicates the possibility of a very marginal clinical benefit. The quantitative analysis conducted in this systematic review does, however, have several limitations which do not allow us to draw definite conclusions on the efficacy of PRP in this setting. The first limitation is certainly related to the heterogeneity of the studies evaluated, particularly in the efficacy outcomes. Another important limitation of this meta-analysis is that we were not able to determine the long-term (>12 months) effect of PRP due to the lack of enough time points in the studies evaluated. Moreover, we highlight the lack of standardisation of PRP production and protocols for clinical application, which makes the PRP products heterogeneous and qualitatively very different from each other, thus limiting the validity of inter-study comparisons.
Injections of PRP involve administration of an individual’s own platelets, and the possibility of systemic adverse reactions to the injections is unlikely; however, patients may have pain, bleeding and local infection at the injection site. In the majority of the studies analysed, adverse events were not included among the predefined outcomes, and reporting was incomplete and inadequate. Data on adverse events (most common adverse events and serious adverse events) produced low certainty evidence, due to imprecision and risk of reporting bias.
Further, adequately powered, randomised trials are needed to better define potential indications, long-term benefit, and optimal treatment protocols of PRP as treatment of AGA and AA. These studies should also perform an adequate cost-benefit analysis of PRP therapy compared with control treatments.
CONCLUSIONS
PRP has been used in a wide array of dermatological applications. Although literature review suggests that PRP is a potential treatment option for AGA and AA, several study design limitations need to be addressed before PRP is widely introduced as a treatment option in the clinical setting. The results of this systematic review and meta-analysis highlight the limited evidence of benefits from PRP for treatment of alopecia; furthermore, most of this evidence is of low-quality. More rigorous study designs, including larger samples, quantitative measurements of effect, and longer follow-up periods, are needed to solidify the utility of PRP for treating AGA and AA. Further studies will be needed to determine whether PRP is a valid treatment in dermatology and whether it can be considered an alternative or adjunct to other therapies.
Footnotes
AUTHORSHIP CONTRIBUTIONS
MC, FM and IP evaluated and analysed the data and prepared the manuscript. All Authors approved the final version of the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739–48. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 2.Wasterlain AS, Braun HJ, Harris AH, et al. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 2013;41:186–93. doi: 10.1177/0363546512466383. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Hussain N, Johal H, Bhandari M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics - a review of the literature. SICOT J. 2017;3:57. doi: 10.1051/sicotj/2017036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez CE, Smith PC, Palma Alvarado VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Fron Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 7.Ferri AL, Ceserani V, Greppi N, et al. Osteogenic differentiation of adipose tissue-derived mesenchymal stem cells cultured on a scaffold made of silk fibroin and cord blood platelet gel. Blood Transfus. 2016;14:206–11. doi: 10.2450/2016.0209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebulla P, Pupella S, Santodirocco M, et al. Italian Cord Blood Platelet Gel Study Group. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016;14:73–9. doi: 10.2450/2015.0122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccin A, Di Pierro AM, Canzian L, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15:333–40. doi: 10.2450/2016.0038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirmani BH, Jones SG, Datta S, et al. A meta-analysis of platelet gel for prevention of sternal wound infections following cardiac surgery. Blood Transfus. 2017;15:57–65. doi: 10.2450/2016.0231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Zapata MJ, Orozco L, Balius R, et al. PRP-RICE group. Efficacy of autologous platelet-rich plasma for the treatment of muscle rupture with haematoma: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Blood Transfus. 2016;14:245–54. doi: 10.2450/2015.0099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchini M, Cruciani M, Mengoli C, et al. Efficacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus. 2018;16:502–13. doi: 10.2450/2018.0111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchini M, Cruciani M, Mengoli C, et al. The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transfus. 2019;17:357–67. doi: 10.2450/2019.0177-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchini M, Cruciani M, Mengoli C, et al. Serum eye drops for the treatment of ocular surface diseases: a systematic review and meta-analysis. Blood Transfus. 2019;173:200–9. doi: 10.2450/2019.0080-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emer J. Platelet-rich plasma (PRP): current applications in dermatology. Skin Therapy Lett. 2019;5:1–6. [PubMed] [Google Scholar]
- 16.Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cervantes J, Perper M, Wong LL, et al. Effectiveness of platelet-rich plasma for androgenetic alopecia: a review of the literature. Skin Appendage Disord. 2018;4:1–11. doi: 10.1159/000477671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun. 2019;98:74–85. doi: 10.1016/j.jaut.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Leo MS, Kumar AS, Kirit R, et al. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14:315–23. doi: 10.1111/jocd.12167. [DOI] [PubMed] [Google Scholar]
- 20.Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29–34. doi: 10.1055/s-0034-1390030. [DOI] [PubMed] [Google Scholar]
- 21.EDQM. Guide to the quality and safety of tissues and cells for human application. 3rd ed. Strasbourg: European Directorate for the Quality of Medicines & HealthCare (EDQM), Council of Europe; 2017. [Accessed on 14/06/2019]. https://register.edqm.eu/freepub . [Google Scholar]
- 22.McElwee KJ, Shapiro J. Promising therapies for treating and/or preventing androgenic alopecia. Skin Therapy Lett. 2012;17:1–4. [PubMed] [Google Scholar]
- 23.Dabek RJ, Roh D, Ozdemir D, et al. Fractional laser-assisted hair regrowth and microneedling for the treatment of alopecia areata: a review. Cureus. 2019;11:e4943. doi: 10.7759/cureus.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein RM, Rassman WR. Follicular transplantation. Patient evaluation and surgical planning. Dermatol Surg. 1997;23:771–84. [PubMed] [Google Scholar]
- 25.Arshdeep, Kumaran MS. Platelet-rich plasma in dermatology: boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5–14. doi: 10.4103/0378-6323.125467. [DOI] [PubMed] [Google Scholar]
- 26.Gkini M-A, Kouskoukis A-E, Rigopoulos D, Kouskoukis K. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichology. 2015;7:54–63. doi: 10.4103/0974-7753.160098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayatollahi A, Hosseini H, Gholami J, et al. Platelet rich plasma for treatment of non-scarring hair loss: systematic review of literature. J Dermatolog Treat. 2017;28:574–81. doi: 10.1080/09546634.2017.1303571. [DOI] [PubMed] [Google Scholar]
- 28.Evans AG, Mwangi JM, Pope RW, et al. Platelet-rich plasma as a therapy for androgenic alopecia: a systematic review and meta-analysis. J Dermatolog Treat. 2020;26:1–14. doi: 10.1080/09546634.2020.1770171. [DOI] [PubMed] [Google Scholar]
- 29.Gentile P, Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil®, finasteride®, and adult stem cell-based therapy. Int J Mol Sci. 2020;21:2702. doi: 10.3390/ijms21082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2020;13:1–11. doi: 10.1080/09546634.2020.1749547. [DOI] [PubMed] [Google Scholar]
- 31.Mao G, Zhang G, Fan W. Platelet-rich plasma for treating androgenic alopecia: a systematic review. Aesthetic Plast Surg. 2019;5:1326–36. doi: 10.1007/s00266-019-01391-9. [DOI] [PubMed] [Google Scholar]
- 32.Gupta AK, Versteeg SG, Rapaport J, et al. The efficacy of platelet-rich plasma in the field of hair restoration and facial aesthetics - a systematic review and meta-analysis. J Cutan Med Surg. 2019;23:185–203. doi: 10.1177/1203475418818073. [DOI] [PubMed] [Google Scholar]
- 33.Giordano S, Romeo M, di Summa P, et al. A meta-analysis on evidence of platelet-rich plasma for androgenetic alopecia. Int J Trichology. 2018;10:1–10. doi: 10.4103/ijt.ijt_74_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ince B, Yildirim MEC, Dadaci M, et al. Comparison of the efficacy of homologous and autologous platelet-rich plasma (PRP) for treating androgenic alopecia. Aesthetic Plast Surg. 2018;42:297–303. doi: 10.1007/s00266-017-1004-y. [DOI] [PubMed] [Google Scholar]
- 35.Dervishi G, Liu H, Peternel S, et al. Autologous platelet-rich plasma therapy for pattern hair loss: a systematic review. J Cosmet Dermatol. 2020;19:827–35. doi: 10.1111/jocd.13113. [DOI] [PubMed] [Google Scholar]
- 36.Gentile P, Cole JP, Cole MA, et al. Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18:408. doi: 10.3390/ijms18020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions – Version 510 [updated March 2011] The Cochrane Collaboration; [Accessed on 01/03/2021]. http://www.cochranehandbook.org . [Google Scholar]
- 40.Schünemann HJ, Oxman AD, Higgins JP, et al. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; [Accessed on 01/03/2021]. http://www.cochranehandbook.org . [Google Scholar]
- 41.Schünemann HJ, Oxman AD, Higgins JP, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; [Accessed on 01/03/2021]. http://www.cochranehandbook.org . [Google Scholar]
- 42.Guyatt GH, Oxman AD, Kunz R, et al. What is ‘quality of evidence’ and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trink A, Sorbellini E, Bezzola P, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169:690–4. doi: 10.1111/bjd.12397. [DOI] [PubMed] [Google Scholar]
- 44.Cervelli V, Garcovich S, Bielli A, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709. doi: 10.1155/2014/760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gentile P, Garcovich S, Bielli A, et al. The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4:1317–23. doi: 10.5966/sctm.2015-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SH, Zheng Z, Kang JS, et al. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyri bonucleotide on female pattern hair loss. Wound Repair Regen. 2015;23:30–6. doi: 10.1111/wrr.12250. [DOI] [PubMed] [Google Scholar]
- 47.Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452–5. doi: 10.1080/14764172.2016.1225963. [DOI] [PubMed] [Google Scholar]
- 48.Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243–7. doi: 10.1097/DSS.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 49.Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491–7. doi: 10.1097/DSS.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 50.El Taieb MA, Ibrahim H, Nada EA, Seif Al-Din M. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther. 2017;30 doi: 10.1111/dth.12437. [DOI] [PubMed] [Google Scholar]
- 51.Shah KB, Shah AN, Solanki RB, Raval RC. A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int J Trichology. 2017;9:14–8. doi: 10.4103/ijt.ijt_75_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toama M, Soliman I. Platelet rich plasma treatment of androgenetic alopecia in men and women. J Clin Investigat Dermatol. 2017;5:5. [Google Scholar]
- 53.Kachhawa D, Vats G, Sonare D, et al. A split head study of efficacy of placebo versus platelet-rich plasma injections in the treatment of androgenic alopecia. J Cutan Aesthet Surg. 2017;10:86–9. doi: 10.4103/JCAS.JCAS_50_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tawfik AA, Osman MAR. The effect of autologous activated platelet-rich plasma injection on female pattern hair loss: a randomized placebo-controlled study. J Cosmet Dermatol. 2018;17:47–53. doi: 10.1111/jocd.12357. [DOI] [PubMed] [Google Scholar]
- 55.Behrangi E, Zamanian A, Ghaffarpour G, et al. Platelet-rich plasma (PRP) effect on androgenetic alopecia and female pattern hair loss. J Skin Stem Cell. 2019;6:e87979. [Google Scholar]
- 56.Ranpariya RH, Gupta SB, Deora MS, et al. Intralesional triamcinolone acetonide versus platelet rich plasma: a comparative study in the treatment of alopecia areata of scalp. Int J Res Dermatol. 2019;5:521–7. [Google Scholar]
- 57.Rodrigues BL, Montalvão SAL, Cancela RBB, et al. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol. 2019;80:694–700. doi: 10.1016/j.jaad.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 58.Verma K, Tegta GR, Verma G, et al. A study to compare the efficacy of platelet-rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichology. 2019;11:68–79. doi: 10.4103/ijt.ijt_64_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albalat W, Ebrahim HM. Evaluation of platelet-rich plasma vs intralesional steroid in treatment of alopecia areata. J Cosmet Dermatol. 2019 May 10; doi: 10.1111/jocd.12858. [DOI] [PubMed] [Google Scholar]
- 60.Aggarwal K, Gupta S, Jangra RS, et al. A dermoscopic assessment of microneedling alone versus microneedling with platelet-rich plasma in cases of male pattern alopecia: a split-head comparative study. Int J Trichology. 2020;12:156–63. doi: 10.4103/ijt.ijt_64_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balakrishnan A, Joy B, Thyvalappil A, et al. A comparative study of therapeutic response to intralesional injections of platelet-rich plasma versus triamcinolone acetonide in alopecia areata. Indian Dermatol Online J. 2020;11:920–4. doi: 10.4103/idoj.IDOJ_6_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro J, Ho A, Sukhdeo K, et al. Evaluation of platelet-rich plasma as a treatment for androgenetic alopecia: a randomized controlled trial. J Am Acad Dermatol. 2020;83:1298–303. doi: 10.1016/j.jaad.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Dubin DP, Lin MJ, Leight HM, et al. The effect of platelet-rich plasma on female androgenetic alopecia: A randomized controlled trial. J Am Acad Dermatol. 2020;83:1294–7. doi: 10.1016/j.jaad.2020.06.1021. [DOI] [PubMed] [Google Scholar]
- 64.Kapoor P, Kumar S, Brar BK, et al. Comparative evaluation of therapeutic efficacy of intralesional injection of triamcinolone acetonide versus intralesional autologous platelet-rich plasma injection in alopecia areata. J Cutan Aesthet Surg. 2020;13:103–11. doi: 10.4103/JCAS.JCAS_16_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hegde P, Relhan V, Sahoo B, Garg VK. A randomized, placebo and active controlled, split scalp study to evaluate the efficacy of platelet-rich plasma in patchy alopecia areata of the scalp. Dermatol Ther. 2020;33:e14388. doi: 10.1111/dth.14388. [DOI] [PubMed] [Google Scholar]
- 66.Gressenberger P, Pregartner G, Gary T, et al. Platelet-rich plasma for androgenetic alopecia treatment: a randomized placebo-controlled pilot study. Acta Derm Venereol. 2020;100:adv00247. doi: 10.2340/00015555-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh SK, Kumar V, Rai T. Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: a randomized, double-blind placebo control trial. Indian J Dermatol Venereol Leprol. 2020;86:150–7. doi: 10.4103/ijdvl.IJDVL_589_18. [DOI] [PubMed] [Google Scholar]
- 68.Gupta V, Parihar AS, Sharma VK, et al. Evaluation of platelet-rich plasma on hair regrowth and lesional T-cell cytokine expression in alopecia areata: a randomized observer-blinded, placebo-controlled, split-head pilot study. J Am Acad Dermatol. 2021;84:1321–8. doi: 10.1016/j.jaad.2020.12.039. [DOI] [PubMed] [Google Scholar]
- 69.Farid CI, Abdelmaksoud RA. Platelet-rich plasma microneedling versus 5% topical minoxidil in the treatment of patterned hair loss. J Egypt Women’s Dermatol Soc. 2016;13:29–36. [Google Scholar]