Abstract
Background
The Red blood cell (RBC) storage lesion results in decreased circulation and function of transfused RBCs. Elevated oxidant stress and impaired energy metabolism are a hallmark of the storage lesion in both human and murine RBCs. Although human studies don’t suffer concerns that findings may not translate, they do suffer from genetic and environmental variability amongst subjects. Murine models can control for genetics, environment, and much interventional experimentation can be carried out in mice that is neither technically feasible nor ethical in humans. However, murine models are only useful to the extent that they have similar biology to humans. Hypoxic storage has been shown to mitigate the storage lesion in human RBCs, but has not been investigated in mice.
Materials and methods
RBCs from a C57BL6/J mouse strain were stored under normoxic (untreated) or hypoxic conditions (SO2 ~ 26%) for 1h, 7 and 12 days. Samples were tested for metabolomics at steady state, tracing experiments with 1,2,3-13C3-glucose, proteomics and end of storage post transfusion recovery.
Results
Hypoxic storage improved post-transfusion recovery and energy metabolism, including increased steady state and 13C3-labeled metabolites from glycolysis, high energy purines (adenosine triphosphate) and 2,3-diphospholgycerate. Hypoxic storage promoted glutaminolysis, increased glutathione pools, and was accompanied by elevation in the levels of free fatty acids and acyl-carnitines.
Discussion
This study isolates hypoxia, as a single independent variable, and shows similar effects as seen in human studies. These findings also demonstrate the translatability of murine models for hypoxic RBC storage and provide a pre-clinical platform for ongoing study.
Keywords: erythrocyte; metabolomics; adenosine triphosphate; 2,3-diphosphoglycerate
INTRODUCTION
Refrigerated storage of packed red blood cells (pRBCs) promotes the accumulation of a series of morphological and biochemical alterations, collectively referred to as the storage lesion1. The storage lesion is affected by numerous factors, including (1) biological (donor sex, age, ethnicity, BMI, G6PD status)2–5 and (2) environmental factors that affect the molecular make-up of donated blood, the so-called blood donor exposome6. Xenobiotic and other factors also contribute to the molecular heterogeneity of blood products and the storage lesion: diet (e.g., fatty acid composition)7, consumption of alcohol8, caffeinated beverages9; smoking or exposure to cigarette smoke10; over the counter-drugs that are not ground for blood donor deferral6. A critical factor that is within the ability of blood providers to control is the particulars of storage (i.e., collection and processing, additive solutions11, leukofiltration12, and irradiation13).
Oxidant stress to stored RBCs is a leading driver of the storage lesion1,14–16. Hemoglobin auto-oxidation17 triggers a series of pro-oxidant cascades that target small molecule metabolites18, membrane lipids19, promote vesiculation of oxidized components20, and ultimately compromise RBC morphology21. Yoshida and colleagues have proposed that removal of oxygen from storage bags can mitigate oxidative stress by limiting superoxide generation from hemoglobin autoxidation, which does not occur with deoxyhemoglobin22,23. Indeed, removal of oxygen from blood units does limit oxidant stress to the stored erythrocyte (e.g., lower rates of adenosine deamination, cysteine oxidation and asparagine deamidation in key structural and functional proteins)18,24,25, and better preserves RBC morphology26. Cross-sectional randomized clinical trials have confirmed that hypoxic storage of RBCs results in improved post-transfusion recovery (PTR)27.
Although in vitro hemolysis has been an important metric of storage, the majority of RBC destruction post-transfusion is extravascular consumption by reticuloendothelial cells. It is for this reason that PTR in human trials is the gold standard and is required for FDA approval of new storage systems. Regrettably, human PTR studies can only practically be performed on small numbers of subjects who are heterogeneous regarding genetics and environment, which represents a potential confounder, as it is known that human PTR is impacted by donor factors28. Thus, as an adjuvant to human studies, we described methodologies to study RBC storage in mice, that can control for genetics and environment, as well as provide a platform for interventional experimentation not possible in humans. This approach has led to a number of advances in mechanistic understanding of RBC storage19,29,30.
Despite preliminary studies in rats31, mouse modeling of hypoxic RBC storage has not been fully developed. Hypoxic RBC storage has been shown to confer a significant advantage over conventional blood in resuscitation performances in rodent models of trauma and hemorrhagic shock31, however these studies did not perform a detailed analysis of the storage lesion itself. Despite their advantages, murine models always pose the risk that rodent biology will not translate to humans. As such, as more information about the human storage lesion is learned, it is imperative to juxtapose the current model systems so as to investigate similarities and differences. Herein, we describe a detailed analysis of hypoxic storage in a murine model of RBC storage and demonstrate that it recapitulates the metabolic and PTR benefits of hypoxic storage of human erythrocytes.
MATERIAL AND METHODS
Ethical statement
All experimental protocols were approved by the University of Virginia IACUC on 04/22/2019 (protocol n: 4269).
Mouse blood collection, storage under hypoxic and normoxic conditions and PTR
Murine RBC storage, transfusion, and post-transfusion recovery determinations were carried out as previously described, with minor modifications30. Whole blood was drawn by cardiac puncture under sterile conditions into CPDA-1, was centrifuged, and the hematocrit was adjusted to 75% by removing supernatant. For hypoxic storage, murine RBCs (n=1 from pooled blood samples from 2–3 donor mice, technical triplicate) were brought to 75% hematocrit, and gently bubbled with Argon in an eppendorf tube inside a glove box until SO2 ~ 26% while normoxic counterparts (technical triplicate) were left untreated. No increased hemolysis was observed in blood bubbled with Argon. SO2 was measured by sampling the blood with a Radiometer ABL90 Flex Plus blood gas analyzer (Radiometer America, Brea, CA, USA). Sealed “units” were preserved at 37°C for 1h, prior to storage at 4°C for 7 and 12 days. RBCs from C57BL6/J mice were used as the “test” population and subjected to different storage conditions. Ubi-GFP mice were used as recipients to allow visualization of the test cells in the non-fluorescent gate. To control for differences in transfusion and phlebotomy, RBCs from ROSA26-LCB-mCHERRY mice (mCHERRY) were used as a tracer RBC population (never stored) was added to stored RBCs immediately prior to transfusion. PTR was calculated by dividing the post-transfusion ratio (Test/Tracer) by the pre-transfusion ratio (Test/Tracer)32. At the time of transfusion, blood samples were frozen in liquid nitrogen and stored at −80°C until subsequent analysis.
Generation of a novel mouse with RBC specific expression of mCHERRY
Historically, UbiC-GFP has been the only available mouse expressing a fluorescent protein (in all cells including RBCs). As such, visualization of an RBC tracer population (if GFP was already present) has required either chemical labeling of RBCs with a fluorochrome with a different emission spectrum from GFP, or using transgenic mice that express an antigen on RBCs that can be stained for with antibodies prior to flow cytometry. Both chemical labeling and antibody staining risk altering the RBCs biology and/or properties. To circumvent this concern, a novel mouse strain was generated by targeted transgenesis. The cDNA encoding the fluorescent mCHERRY protein was ligated into a previously described RBC specific expression cassette, and then flanked on both sides with arms homologous to the ROSA26 locus. As ROSA26 has no known function in mice and is highly amenable to homologous recombination, it is commonly used as a “safe-harbor” locus.
Embryonic stem (ES) cells were electroporated with the targeting construct and clones were selected that underwent homologous recombination but not random integration, as determined by Southern Blot. A mouse was generated from a chosen ES clone and the neomycin expression cassette was removed via CRE mediated excision.
Resulting animals had the mCHERRY cDNA driven by an RBC specific beta globin locus control region (ROSA26-LCB-mCHERRY mouse). Expression of mCHERRY was confirmed in RBCs by flow cytometry, and no expression was detected in either platelets or leukocytes (data not shown), confirming RBC specific expression. This mouse is freely available upon request.
Ultra-high-pressure liquid chromatography-mass spectrometry (MS) metabolomics
Frozen RBC aliquots (50 μL) were extracted 1:10 in ice cold extraction solution (methanol:acetonitrile:water 5:3:2 v/v/v)33. Samples were vortexed and insoluble material pelleted, as described34. Analyses were performed using a Vanquish UHPLC coupled online to a Q Exactive mass spectrometer (Thermo Fisher, Bremen, Germany). Samples were analyzed using a 1 min35 and 5 minute gradient-based method34, as described36,37. Data analysis was performed through the auxilium of the software MAVEN38. Graphs and statistical analyses (either two-way ANOVA or repeated measures ANOVA) were prepared with GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA), GENE E (Broad Institute, Cambridge, MA, USA), and MetaboAnalyst 5.039.
RESULTS
Hypoxic storage of murine RBCs significantly improves PTR
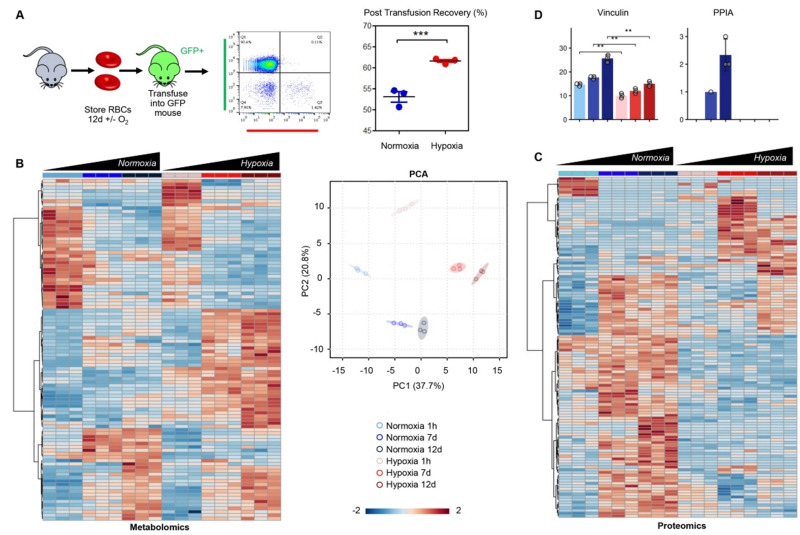
Mouse pRBCs were collected from C57BL6/J mice (technical triplicate) and split in two paired groups for normoxic and hypoxic storage for up to 12 days (Figure 1.A). Compared to normoxic storage, hypoxic storage of murine RBCs was accompanied by significant increases in end of storage PTR (53.1±2.1% for normoxic RBCs vs 61.6±0.6% for hypoxic RBCs – mean ± standard deviation; p<0.001 paired τ-test - Figure 1.A).
Figure 1.
Post-transfusion recovery and omis of normoxic and hypoxic murine RBCs
Hypoxic storage of murine RBCs improves post-transfusion recovery (A) and has a significant effect on the RBC metabolome and proteome. RBCs were stored for 1h, 7 or 12 days under normoxic (shades of blue) or hypoxic (shades of red) conditions. At storage day 12, post-transfusion recovery studies showed a significant increase (p<0.001) in PTR in mouse RBCs stored under hypoxia. Metabolomics analyses showed a significant impact of storage duration and normoxic vs hypoxic storage on mouse RBCs, as gleaned by unsupervised hierarchical clustering analysis of the top 75 metabolites by repeated measures ANOVA (B). Similarly, storage impacted the RBC proteome (C). Bar plots with superimposed dot plots (n = 3) are shown for vinculine and peptidyl-prolyl cis-trans isomerase (PPIA), two of the most significantly impacted proteins in this analysis (mean + standard deviation). Paired t-test between normoxic or hypoxic samples from the same mice are shown for each time point (Welsch τ-test; ns: not significant; *p<0.05).
Metabolomics and proteomics analyses were performed after 1 h, 7 or 12 days of storage (Figure 1.B–C). Results are reported extensively in tabulated form in Online Supplementary Table SI. Unsupervised hierarchical clustering analysis showed a clear separation between normoxic and hypoxic RBC metabolites and proteins, as shown in Figure 1.B–C, respectively. Of note, a subset of proteins accumulated more significantly, or even exclusively in normoxic but not hypoxic RBCs as a function of storage duration (e.g., vinculin and peptidyl-prolyl cis-trans isomerase (PPIA) - Figure 1. D).
Hypoxic storage promotes glycolysis and DPG generation, as gleaned by steady state and metabolic tracing experiments
Hypoxic storage of RBC had a significant effect on glycolysis, as determined by steady state measurements of metabolites in this pathway (Figure 2). Specifically, compared to storage in normoxia, hypoxic RBCs showed significantly higher levels of glyceraldehyde 3-phosphate, DPG, phosphoglycerate (isomers couldn’t be resolved in this analysis), phosphoenolpyruvate and lactate (Figure 2). However, pyruvate levels at baseline and end of storage were significantly lower in hypoxic RBCs, consistent with increased lactate/pyruvate ratios (Figure 2). To follow up on these observations, tracing experiments were performed by incubating murine RBCs for 1h, 7 or 12 days under normoxic (standard) or hypoxic conditions in presence of 1,2,3-13C3-glucose (Figure 3.A). No significant differences were observed between RBCs in the two arms of the study with respect to glucose uptake. However, labeled fructose bisphosphate was significantly higher in hypoxic RBCs at all tested time points, suggestive of increased PFK activity and increased glycolytic fluxes. Indeed, labeled lactate accumulation was significantly higher in hypoxic RBCs compared to normoxic controls at all tested time points (Figure 3.A).
Figure 2.
Impact of normoxic or hypoxic storage on RBC glycolysis and high-energy purinemetabolism
RBCs were stored for 1h, 7 or 12 days under normoxic (shades of blue) or hypoxic (shades of red) conditions. Bar plots with superimposed dot plots (n = 3) are shown for selected metabolites in this pathway (mean + standard deviation). Paired t-test between normoxic or hypoxic samples from the same mice are shown for each time point (Welsch τ-test; ns: not significant; *p<0.05).
Figure 3.
Glucose tracing experiments confirm up-regulation of glycolysis at the expense of PPP in murine RBCs under hypoxic storage
Metabolic tracing experiments were performed by incubating murine RBCs for 1h, 7 or 12 days under normoxic (standard) or hypoxic conditions in presence of 1,2,3-13C3-glucose. Results indicate comparable levels of glucose depletion, but a significantly higher accumulation of labeled glycolytic intermediates (e.g., fructose bisphosphate – FBP) and byproducts (lactate) in hypoxic RBCs (A). Determination of fluxes through the Embden-Meyerhof-Parnas (EMP) glycolytic pathway and the pentose phosphate pathway (PPP – B) can be performed through determination of the levels of lactate isotopologues +2 (from the PPP) and +3 (derived from the EMP – C). In keeping with previous reports in human RBCs, murine hypoxic RBCs have lower levels of PPP-derived lactate +2 and higher levels of EMP derived lactate +3, consistent with increased glycolytic fluxes in hypoxic RBCs.
One of the main advantages of tracing experiments with 1,2,3-13C3-glucose is that it affords determination of glucose oxidation fluxes through glycolysis vs the PPP (Figure 3.B) via the calculation of lactate isotopologues +2 and +324,40: when glucose is oxidized through the PPP, at the hexose to pentose step of the oxidative phase of the PPP, the first carbon atom is lost in the form of 13C-CO2, which results in the generation of lactate labeled only on two carbon atoms after re-entering glycolysis at the crossroads of the non-oxidative phase of PPP and glycolysis (Figure 3.C). Despite total and 13C3-labeled lactate being higher in hypoxic RBCs at all storage time points, PPP-derived 13C2-lactate was significantly lower at storage day 7 and 12 (p<0.01 - Figure 3.C). As such, lactate +3/+2 ratios, a proxy for glycolysis/PPP fluxes, were found to be higher at all tested time points in hypoxic RBCs. Thus, while steady state levels of the PPP intermediate 6-phosphogluconate were significantly higher in hypoxic RBCs, metabolic flux analysis using the isotopic tracer demonstrates decreased PPP activity and decreased end-product ribose phosphate (pentose phosphate isomers unresolved in this analysis). Together, these data indicate less PPP activity during hypoxic storage (Figure 4).
Figure 4.
Impact of normoxic or hypoxic storage on RBC pentose phosphate pathway and glutathione homeostasis
RBCs were stored for 1h, 7 or 12 days under normoxic (shades of blue) or hypoxic (shades of red) conditions. Bar plots with superimposed dot plots (n=3) are shown for selected metabolites in this pathway (mean + standard deviation). Paired t-test between normoxic or hypoxic samples from the same mice are shown for each time point (Welsch τ-test; ns: not significant; *p<0.05).
Hypoxic storage of RBCs impacts glutathione homeostasis, glutaminolysis, carboxylic acid and purine metabolism
Since PPP generates NADPH necessary to recycle oxidized glutathione to GSH, one could predict that GSH would be lower in hypoxic stored RBCs. However, as hypoxic stored RBCs should produce less superoxide, then less GSH should be consumed. To distinguish the net outcome of these opposing events, GSH metabolites were evaluated. The levels of reduced glutathione (GSH) and its byproduct through the gamma-glutamyl-cycle (5-oxoproline) were significantly higher in hypoxic RBCs (Figure 4). Similarly, dehydroascorbate levels were higher in hypoxic RBCs, which were instead characterized by significantly lower levels of glutamine (Figure 4), a substrate for glutathione synthesis via glutaminolysis to glutamate. Other than fueling glutathione synthesis, glutamate is an amine group donor in transamination reactions, which results in the generation of alpha-ketoglutarate. Significant increases in alpha-ketoglutarate in hypoxia were accompanied by similar increases in the levels of several carboxylic acids, including citrate, malate and fumarate, 2-hydroxyglutarate and, only at day 12, succinate (Figure 5). No other significant changes in amino acids were noted between the two groups (Online Supplementary Figure S1), except for lower levels of polyamines (spermidine) and higher levels of arginine catabolites (ornithine) in hypoxic RBCs at storage day 12 (Online Supplementary Figure S2). Together, these data are consistent with less GSH consumption in hypoxic stored RBCs due to less oxidative stress, which utilizes less NADPH, and therefore decreases flux through the PPP. GSH levels are also likely contributed to by ongoing synthesis (contributed to by glutaminolysis), and concordant down stream effects.
Figure 5.
Impact of normoxic or hypoxic storage on RBC carboxylic acid metabolism
To be noted that an incomplete, non-canonical carboxylic acid cycle is present in mature RBCs and here represented as the standard Krebs cycle for simplicity. RBCs were stored for 1h, 7 or 12 days under normoxic (shades of blue) or hypoxic (shades of red) conditions. Bar plots with superimposed dot plots (n = 3) are shown for selected metabolites in this pathway (mean + standard deviation). Paired t-test between normoxic or hypoxic samples from the same mice are shown for each time point (Welsch τ-test; ns: not significant; *p<0.05).
Purine salvage pathway
Carboxylic acid metabolism is intertwined with purine salvage to compensate for purine breakdown and deamination in the stored RBC18. Hypoxic RBCs had significantly lower levels of inosine and hypoxanthine after 1h (the former) and through storage (the latter, Figure 6.A). On the other hand, the antioxidant urate was significantly higher in hypoxic RBCs at day 7 and 12 (Figure 6.A). Xanthine, a precursor to urate, was higher at 1 h, 7 and 12 days in hypoxic RBCs (Figure 6.A).
Figure 6.
A impact of normoxic or hypoxic storage on RBC purine deamination and carboxylic acid metabolism
RBCs were stored for 1h, 7 or 12 days under normoxic (shades of blue) or hypoxic (shades of red) conditions. B Impact of normoxic or hypoxic storage on RBC free fatty acids and acyl-carnitine metabolism. RBCs were stored for 1h, 7 or 12 days under normoxic (shades of blue) or hypoxic (shades of red) conditions. Bar plots with superimposed dot plots (n = 3) are shown for selected metabolites in this pathway (mean + standard deviation). Paired t-test between normoxic or hypoxic samples from the same mice are shown for each time point (Welsch τ-test; ns: not significant; *p<0.05).
Significant increases in the levels of free fatty acids in hypoxic RBCs
Free fatty acids, especially polyunsaturated ones, accumulate in stored units of human and murine pRBCs41. These results were confirmed in this study (Figure 6). However, hypoxic RBCs showed significantly higher levels of almost all free fatty acids tested in this study at baseline and throughout storage, especially monounsaturated palmitoleic (FA 16:1), oleic (18:1), and poly- or highly-unsaturated linoleic (18:2), gamma-linolenic (18:3), docosapentaenoic (22:5) and docosahexaenoic acid (22:6, Figure 6.B). Hypoxic RBCs also showed higher consumption of acetyl-carnitine and accumulation of other acyl-carnitines (especially hexanoyl and decanoyl-carnitine, Figure 6.B).
DISCUSSION
In the present study, we report that hypoxic storage of murine RBCs boosts glycolysis, energy metabolism, and results in significant increases in PTR. These results show a biology that is highly similar to previous metabolomics and PTR studies in human RBCs27. Our results also show an increased flux through glycolysis in hypoxic RBCs, and thus we also recapitulated previous findings in human42 and rodent RBCs43–45 upon exposure to high-altitude hypoxia in vivo, or conditions that mimic it ex vivo. However, in those models, increased fluxes through glycolysis were mechanistically linked to adenosine45 and sphingosine 1-phosphate signaling44, pathways that involve the endothelium and other systems than RBC alone and may thus not be necessarily activated under the artificial conditions of hypoxic storage in the blood bank. On the other hand, other models have been proposed to account for the beneficial effects of hypoxic storage on RBC metabolism, including mitigation of oxidant stress23 and alkalinization of intracellular p H46, which in turn boosts the activity of rate-limiting enzymes of glycolysis (phosphofructokinase) and the Rapoport-Luebering shunt (biphosphoglycerate mutase)47. The two models combine when appreciating that hypoxic storage protects the N-terminus cytosolic domain of band 3 from oxidant damage33, and thus from oxidant stress-induced proteolysis48. This is relevant in that this cytosolic domain of band 3 represents a hub for glycolytic enzyme binding and inhibition at high oxygen saturation; however, under hypoxic conditions, deoxyhemoglobin competes for binding to the band 3 and thus glycolytic enzymes are released in the cytosol, no longer inhibited by their interaction with band 3 and thus promote glycolysis and DPG synthesis to boost oxygen off-loading capacity49,50. This mechanism, which fails as a function of storage duration40, has been shown to play a key role in hypoxic metabolic regulation of human and murine RBCs at the expense of the main antioxidant RBC pathway (PPP)51.
Despite lower fluxes through the PPP, hypoxic RBCs were here observed to preserve reduced glutathione pools, perhaps as a function of decrease in oxidant stress23. However, a limitation of this study is acknowledged in that no direct measures of reactive oxygen or nitrogen species were perforemed here. Interestingly, hypoxic storage was associated with increases in the levels of alpha-ketoglutarate, as well as of other carboxylic acids involved in the homeostasis of reducing equivalents (malate). Similar observations had been reported for short term ex vivo incubation of human RBCs in hypoxia52, along with the appreciation of the presence and activity of cytosolic isoforms of Krebs cycle enzymes in the mature erythrocyte53. This is relevant in that it would suggest a potential alternative mechanism than the PPP by which the hypoxic RBC could generate NADPH necessary to fuel antioxidant pathways e.g., via increased citrate to alpha-ketoglutarate conversion, as previously suggested in the context of supra-physiological stoichiometry of the substrates for this reaction52. Similarly, our studies confirm an increase in glutaminolysis in hypoxic RBCs and provide further rationale for the use of glutamine as an additive in (hypoxic) storage units35,54.
Despite lower levels of hypoxanthine, a purine oxidation product that negatively correlates with PTR, and higher levels of the antioxidant urate, consistent with previous studies in humans18, hypoxic RBCs were here observed to accumulate higher levels of xanthine and free fatty acids, especially polyunsaturated ones. These results were previously associated with increased phospholipase activity (e.g., non-canonical, by peroxiredoxin 655) and increased fatty acid desaturase activity in response to storage-induced oxidant stress and membrane lipid remodeling41. These results are in part divergent from previous reports in human RBCs under hypoxic storage27,35. However, in those studies hypoxia was maintained at SO2<20%, suggesting that non-linear oxygen availability thresholds may explain why some studies have reported an increase in oxidant stress in the mildly hypoxic erythrocyte owing to a higher rate of hemoglobin autoxidation56. To address this point, further studies are necessary to quantify metabolic fluxes in stored RBCs as a function of SO2 levels through a continuum of<5% to 100%. In light of the considerations above, it is worthwhile to remark that PTR was indeed improved in hypoxic RBCs in this paired study, suggestive that the boost in post-transfusion performances of stored RBCs upon removal of oxygen (and CO2)46 may indeed be tied to increased availability of ATP and DPG rather than a beneficial effect on the RBC redox status. These hypotheses are easily testable in follow up studies that will leverage the murine model of (normoxic and hypoxic) RBC storage we described here.
CONCLUSIONS
In conclusion, the data presented herein demonstrate a murine model that recapitulates the major biochemical changes observed with human RBCs under hypoxic storage conditions. This demonstrates a tractable whole-animal experimental system to perform mechanistic elucidations regarding hypoxic storage. Of course, this approach comes with limitations, in that murine models of blood storage may not perfectly recapitulate human RBC storage. For example, unlike human RBCs, murine RBCs were found to be resistant to potential hemolysis induced by deoxygenating via bubbling of noble gas. Still, in future laboratory studies in this space we will test alternatives to bubbling such as the use of dedicated hypoxic chambers (e.g., hypoxystation or similar commercial devices). However, murine models afford the opportunity to perform observational of mechanistic studies that are otherwise impossible to perform in humans owing to ethical or budgetary constraints. For example, we have reported that there is wide variability in PTR of blood stored from genetically distinct strains of mice. Because human PTR studies are limited due to technical57 and budgetary constraints, they will not capture effects of genetic diversity on blood storage systems. Thus, the approach we described in this study affords the opportunity to test how donor genetic differences may affect hypoxic storage. As always, one must be mindful of differences between murine and human biology, as well as an appreciation that both species have a wide genetic diversity, the full spectrum of which should be taken into account. Finally, this approach allows studies of other factors (sex, age)13 and novel storage additives35 that impact post-transfusion performances of the transfused RBC in humans and mice.
Supplementary Information
ACKNOWLEDGMENTS
Research reported in this publication was supported by funds from R01HL146442, R01HL149714, and R01HL148151 (ADA, JCZ) and R21HL150032 (ADA) by the National Heart, Lung and Blood Institutes.
Footnotes
AUTHORS ’ CONTRIBUTIONS
AH and KD performed mouse PTR studies, and along with JCZ interpreted the results. HH and JCZ generated the ROSA26-LCB-mCherry mouse. FG, DN, MD, and AD performed omics analyses. AD performed data analysis and prepared figures and tables. AD wrote the first draft of the manuscript and all the authors contributed to manuscript revisions, editing, and finalization of the manuscript.
DISCLOSURE OF CONFLICT OF INTEREST
The Authors declare that AD is a founder of Omix Technologies Inc. and Altis Biosciences LLC. He is also a consultant for Rubius Inc. Macopharma and Forma Inc. AD is an advisory board member of Hemanext Inc, a company that is developing a product for hypoxic storage of human RBCs. JCZ is a consultant for Rubius Inc. JCZ is a cofounder and the chief scientific officer for Svalinn therapeutics, a company whose focus is unrelated to the current work. All the other Authors disclose no conflicts of interest relevant to this study.
REFERENCES
- 1.Yoshida T, Prudent M, D’Alessandro A. Red blood cell storage lesion: causes and potential clinical consequeces. Blood Transfus. 2019;17:27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzounakas VL, Kriebardis AG, Georgatzakou HT, Foudoulaki-Paparizos LE, Dzieciatkowska M, Wither MJ, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152–165. doi: 10.1016/j.freeradbiomed.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro A, Fu X, Kanias T, Reisz JA, Culp-Hill R, Guo Y, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2021;106:1290–1302. doi: 10.3324/haematol.2020.246603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanias T, Lanteri MC, Page GP, Guo Y, Endres SM, Stone M, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1:1132–1141. doi: 10.1182/bloodadvances.2017004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazegh K, Fang F, Bravo MD, Tran JQ, Muench MO, Jackman RP, et al. Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion. 2021;61:435–448. doi: 10.1111/trf.16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemkov T, Stefanoni D, Bordbar A, Issaian A, Palsson BO, Dumont LJ, et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight. 2021;6:e146175. doi: 10.1172/jci.insight.146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CY, Johnson H, Peltier S, Spitalnik SL, Hod EA, Francis RO, et al. Deuterated Linoleic Acid Attenuates the RBC Storage Lesion in a Mouse Model of Poor RBC Storage. Front Physiol. 2022;13:868578. doi: 10.3389/fphys.2022.868578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessandro A, Fu X, Reisz JA, Stone M, Kleinman S, Zimring JC, et al. Ethyl glucuronide, a marker of alcohol consumption, correlates with metabolic markers of oxidant stress but not with hemolysis in stored red blood cells from healthy blood donors. Transfusion. 2020;60:1183–1196. doi: 10.1111/trf.15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Alessandro A, Fu X, Reisz JA, Kanias T, Page GP, Stone M, et al. Stored RBC metabolism as a function of caffeine levels. Transfusion. 2020;60:1197–1211. doi: 10.1111/trf.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanoni D, Fu X, Reisz JA, Kanias T, Nemkov T, Page GP, et al. Nicotine exposure increases markers of oxidant stress in stored red blood cells from healthy donor volunteers. Transfusion. 2020;60:1160–1174. doi: 10.1111/trf.15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alessandro A, Culp-Hill R, Reisz JA, Anderson M, Fu X, Nemkov T, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2019;59:89–100. doi: 10.1111/trf.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertinhez TA, Casali E, Baroni F, Berni P, Baricchi R, Spisni A. A comparative study of the effect of leukoreduction and pre-storage leukodepletion on red blood cells during storage. Front Mol Biosci. 2016;3:13. doi: 10.3389/fmolb.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roubinian NH, Reese SE, Qiao H, Plimier C, Fang F, Page GP, et al. Donor genetic and nongenetic factors affecting red blood cell transfusion effectiveness. JCI Insight. 2022;7:e152598. doi: 10.1172/jci.insight.152598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–1220. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 15.Tzounakas VL, Dzieciatkowska M, Anastasiadi AT, et al. Red cell proteasome modulation by storage, redox metabolism and transfusion. Blood Transfus. 2020 doi: 10.2450/2020.0179-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–219. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 17.Kanias T, Acker JP. Biopreservation of red blood cells--the struggle with hemoglobin oxidation. Febs J. 2010;277:343–356. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]
- 18.Nemkov T, Sun K, Reisz JA, Song A, Yoshida T, Dunham A, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103:361–372. doi: 10.3324/haematol.2017.178608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howie HL, Hay AM, de Wolski K, Waterman H, Lebedev J, Fu X, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019;3:2272–2285. doi: 10.1182/bloodadvances.2019000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med. 2007;11:148–155. doi: 10.1111/j.1582-4934.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–115. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida T, AuBuchon JP, Tryzelaar L, Foster KY, Bitensky MW. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92:22–31. doi: 10.1111/j.1423-0410.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8(4):220–236. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reisz JA, Wither MJ, Dzieciatkowska M, Nemkov T, Issaian A, Yoshida T, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128:e32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessandro A, Hay A, Dzieciatkowska M, Brown BC, Morrison EJ, Hansen KC, et al. Protein-L-isoaspartate O-methyltransferase is required for in vivo control of oxidative damage in red blood cells. Haematologica. 2021;106:2726–2739. doi: 10.3324/haematol.2020.266676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zolla L, D’Alessandro A. An efficient apparatus for rapid deoxygenation of erythrocyte concentrates for alternative banking strategies. J Blood Transfus. 2013;2013:896537. doi: 10.1155/2013/896537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DAlessandro A, Yoshida T, Nestheide S, Nemkov T, Stocker S, Stefanoni D, et al. Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion. 2020;60:786–798. doi: 10.1111/trf.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 29.Hay AM, Howie HL, Gorham JD, D’Alessandro A, Spitalnik SL, Hudson KE, et al. Mouse background genetics in biomedical research: The devil’s in the details. Transfusion. 2021;61:3017–3025. doi: 10.1111/trf.16628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–148. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AT, Jani VP, Nemkov T, Lucas A, Yoshida T, Dunham A, et al. Transfusion of anaerobically or conventionally stored blood after hemorrhagic shock. Shock. 2020;53:352–362. doi: 10.1097/SHK.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howie HL, Hay AM, de Wolski K, Waterman H, Lebedev J, Fu X, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019;3:2272–2285. doi: 10.1182/bloodadvances.2019000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reisz JA, Nemkov T, Dzieciatkowska M, Culp-Hill R, Stefanoni D, Hill RC, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion. 2018;58:2978–2991. doi: 10.1111/trf.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31:663–673. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemkov T, Yoshida T, Nikulina M, D’Alessandro A. High-throughput metabolomics platform for the rapid data-driven development of novel additive solutions for blood storage. Front Physiol. 2022;13:833242. doi: 10.3389/fphys.2022.833242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemkov T, Reisz JA, Gehrke S, Hansen KC, D’Alessandro A. High-throughput metabolomics: isocratic and gradient mass spectrometry-based methods. Methods Mol Biol. 2019;1978:13–26. doi: 10.1007/978-1-4939-9236-2_2. [DOI] [PubMed] [Google Scholar]
- 37.Reisz JA, Zheng C, D’Alessandro A, Nemkov T. Untargeted and semi-targeted lipid analysis of biological samples using mass spectrometry-based metabolomics. Methods Mol Biol. 2019;1978:121–135. doi: 10.1007/978-1-4939-9236-2_8. [DOI] [PubMed] [Google Scholar]
- 38.Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinformatics. 2012;Chapter 14(Unit 14):11. doi: 10.1002/0471250953.bi1411s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers SC, Ge X, Brummet M, Lin X, Timm DD, d’Avignon A, et al. Quantifying dynamic range in red blood cell energetics: Evidence of progressive energy failure during storage. Transfusion. 2021;61:1586–1599. doi: 10.1111/trf.16395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas T, Cendali F, Fu X, Gamboni F, Morrison EJ, Beirne J, et al. Fatty acid desaturase activity in mature red blood cells and implications for blood storage quality. Transfusion. 2021;61:1867–1883. doi: 10.1111/trf.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Alessandro A, Nemkov T, Sun K, Liu H, Song A, Monte AA, et al. AltitudeOmics: red blood cell metabolic adaptation to high altitude hypoxia. J Proteome Res. 2016;15:3883–3895. doi: 10.1021/acs.jproteome.6b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu P, Chen C, Zhang Y, Dzieciatkowska M, Brown BC, Zhang W, et al. Erythrocyte transglutaminase-2 combats hypoxia and chronic kidney disease by promoting oxygen delivery and carnitine homeostasis. Cell Metab. 2022;34:299–316e296. doi: 10.1016/j.cmet.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun K, Zhang Y, D’Alessandro A, Nemkov T, Song A, Wu H, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun. 2016;7:12086. doi: 10.1038/ncomms12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Zhang Y, Wu H, et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia. Circulation. 2016;134(5):405–421. doi: 10.1161/circulationaha.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, Yoshida T. CO2-dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion. 2016;56:392–403. doi: 10.1111/trf.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56:980–993. doi: 10.1111/trf.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinalducci S, Ferru E, Blasi B, Turrini F, Zolla L. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012;10(Suppl 2):s55–62. doi: 10.2450/2012.009s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci USA. 2009;106:18515–18520. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IIssaian A, Hay A, Dzieciatkowska M, Roberti D, Perrotta S, Darula Z, et al. The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica. 2021;106:2971–2985. doi: 10.3324/haematol.2020.278252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Alessandro A, Xia Y. Erythrocyte adaptive metabolic reprogramming under physiological and pathological hypoxia. Curr Opin Hematol. 2020;27:155–162. doi: 10.1097/moh.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemkov T, Sun K, Reisz JA, Yoshida T, Dunham A, Wen EY, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med (Lausanne) 2017;4:175. doi: 10.3389/fmed.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57:325–336. doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 54.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion. 2011;51:1450–1459. doi: 10.1111/j.1537-2995.2010.03026.x. [DOI] [PubMed] [Google Scholar]
- 55.Fisher AB. The phospholipase A(2) activity of peroxiredoxin 6. J Lipid Res. 2018;59:1132–1147. doi: 10.1194/jlr.R082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balagopalakrishna C, Manoharan PT, Abugo OO, Rifkind JM. Production of superoxide from hemoglobin-bound oxygen under hypoxic conditions. Biochemistry. 1996;35:6393–6398. doi: 10.1021/bi952875+. [DOI] [PubMed] [Google Scholar]
- 57.Francis RO, Mahajan S, Rapido F, La Carpia F, Soffing M, Divgi C, et al. Reexamination of the chromium-51-labeled posttransfusion red blood cell recovery method. Transfusion. 2019;59:2264–2275. doi: 10.1111/trf.15310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.