Abstract
As genomic technologies rapidly develop, polygenic scores (PGS) are entering into a growing conversation on how to improve precision in public health and prevent chronic disease. While the integration of PGS into public health and clinical services raises potential benefits, it also introduces potential harms. In particular, there is a high level of uncertainty about how to incorporate PGS into clinical settings in a manner that is equitable, just, and aligned with the long-term goals of many healthcare systems to support person-centered and value-based care. This paper argues that any conversation about whether and how to design and implement PGS clinical services requires dynamic engagement with local communities, patients, and families. These parties often face the consequences, both positive and negative, of such uncertainties and should therefore drive clinical translation. As a collaborative effort between hospital stakeholders, community partners, and researchers, this paper describes a community-empowered co-design process for addressing uncertainty and making programmatic decisions about the implementation of PGS into clinical services. We provide a framework for others interested in designing clinical programs that are responsive to, and inclusive and respectful of, local communities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12687-023-00638-y.
Keywords: Community engagement, Polygenic scores, Precision public health, Program development, Implementation science
Introduction
Polygenic scores (PGS) incorporate genetic information across the genome to provide risk estimates for complex conditions, and have the potential to improve population risk stratification and precision public health management (Adeyemo et al. 2021; Lewis and Green 2021). Currently, however, there is growing concern about the validity, feasibility, and equity of using PGS across populations (Adeyemo et al. 2021; Lewis and Green 2021; Martin et al. 2019). Despite these concerns, PGS-based testing is already available to consumers in much of the Western world (Adeyemo et al. 2021; Lewis and Green 2021). Given the current and potential applications of PGS to clinical and social settings, discussion is needed on how implementation of PGS can be meaningful and equitable at the local and global scale. Local communities, patients, and their families are the ones most likely to shoulder any harms from inadequate implementation of PGS clinical services. Therefore, these parties should be the driving voice of clinical translation.
This case study describes a community empowered design (co-design) framework, which utilizes shared decision-making between healthcare systems and communities, to develop a PGS clinical service (called “Preventive Genomics Program”). In situations like PGS translation where uncertainty is high (Adeyemo et al. 2021), shared decision-making offers a means for arbitrating on hypothetical benefits and risks while aligning healthcare system and community values, interests, and resources. Co-design requires healthcare systems and communities to establish a reciprocal relationship with true partnership, have a commitment to co-learning, and share decision-making power. It is a process for acknowledging community members as experts in their lived experiences and thus healthcare design.
Written as a collaborative effort between healthcare system stakeholders, community partners, and researchers involved in the co-design process, this case study reflects on operationalizing co-design principles with the intent of describing a generalizable approach for clinical program design that is inclusive and respectful of impacted local communities. Critically, this case study focuses on how co-design principles were achieved and experienced by different team members. We do not place emphasis on the programmatic decisions themselves; such decisions are secondary to, or a consequence of, achieving co-design principles and ought to be uniquely tailored to an institution’s relationship with their own communities.
Merging community empowerment with person-centered care
Community engagement is a method for garnering public recognition of the social value of research, respecting study participant and community values, building trust, and empowering those traditionally left out of decision-making processes (Holzer et al. 2014; Sabatello et al. 2022). Grounded in social justice and community change processes, community engagement entails collaborative partnerships with groups on issues that affect their wellbeing (Holzer et al. 2014). Interactions should be respectful, genuine, and trustworthy enough to elicit honest feedback (Edick and Pilditch 2020; PCORI (Patient-Centered Outcomes Research Institute) 2014). Shallower forms of engagement treat community engagement as a checkbox (Mehta and Seim 2023). To distinguish levels of engagement, one must ask if the intention is to have community input, consultation, or collaboration. Collaborative engagement where power is shared is hereafter referred to as “community empowerment” as a distinction from passive and imbalanced forms of community engagement.
Empowered engagement follows principles of participatory-based research, where the research and decision-making is done in partnership, and communities are “naming the problems and solutions for themselves” (Wallerstein and Bernstein 1994). One conceptualization for how to facilitate community empowerment is outlined in the Patient-Centered Research Outcomes Institute (PCORI) network’s four engagement principles (Sheridan et al. 2017): (1) reciprocal relationships; (2) true partnerships with thoughtful timing for feedback, fair compensation, and a commitment to diverse group composition; (3) co-learning approaches with team learning and person-centeredness; and (4) transparency in decision-making, limitations and communication.
While community empowerment is primarily formulated as partnerships between research entities and research populations, it aligns with person-centered care in clinical practice. In person-centered care, provider and patient work together to decide the best course of treatment for that specific patient (The American Geriatrics Society Expert Panel on Person-Centered Care 2016). Similar to community engagement, person-centeredness exists along a spectrum of seeking patient input, consultation or collaboration in care plans. Truly collaborative person-centered care is an intentional and theory-driven process. In a person-centered approach (PCA), the provider-patient relationship is characterized by three principles: (1) genuineness, (2) empathic understanding established through active inquiry and listening, and (3) unconditional positive regard, or acceptance, for a patient’s decisions and values (Rogers 1979). Analogous to community empowered frameworks, PCA views providers as facilitators for patients to realize their own needs and solutions (Rogers 1979); it is in direct contrast to paternalistic medicine.
Theoretical similarities between PCA and PCORI principles suggest that community empowerment has the potential to be useful for describing partnerships between healthcare systems and clinical populations. In other words, using community empowerment to design new clinical programs may lead to community-centered healthcare by design. This was the rationale for this case study, in which we adopted PCORI principles for clinical program design. Figure 1 details our co-design conceptual framework, showing that PCORI principles create the conditions for achieving community empowered co-design, much as Rogers’ three principles have been envisioned to create the conditions for achieving person-centeredness.
Fig. 1.
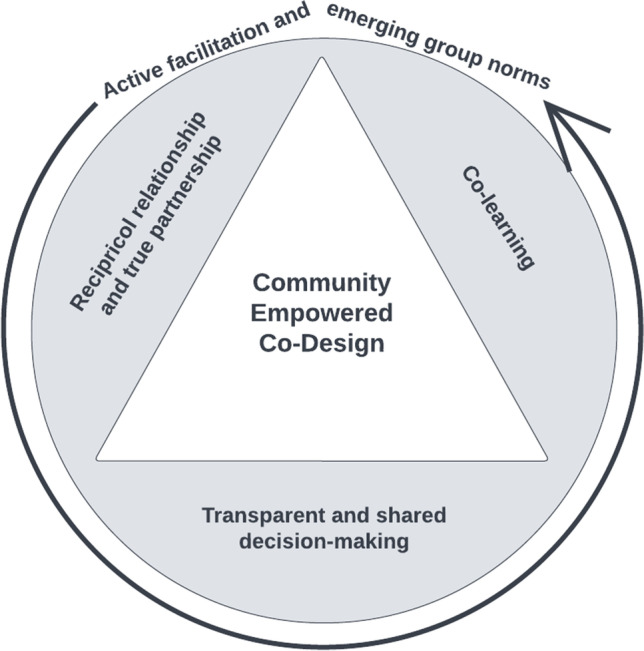
Community empowered co-design model, adapted from Wong-Gates n.d
Methods
Preventive genomics program
The Preventive Genomics Program (PGP) was initiated as an endeavor to support precision public health services and improve prevention of chronic disease. Polygenic scores were one area of interest and exploration of the program in response to recent evidence of improved risk prediction in cardiovascular disease (Aragam and Natarajan 2020) and breast cancer (Carver et al. 2021; Mavaddat et al. 2019). While the limitations and uncertainties of PGS were concerning, program leadership had experience in implementing other population genomics programs and saw this uncertainty as an opportunity to evaluate and incorporate community interest and preferences proactively rather than reactively. Importantly, support for co-design was established by program leadership at the conceptualization of the PGP based on clinical practice values for supporting person-centered care.
Team composition
The co-design team included two facilitators from the Preventive Genomics Program (PGP) and eight community partners. The two facilitators included the PGP Director (HW), who is trained as a genetic counselor, and a bioethicist (DOM) consulting for the program. These facilitators, who had background expertise in PGS and prior experiences in patient care and community engagement, were liaisons to the extended clinical and research team members involved in PGP’s design. The extended PGP team included a multidisciplinary group of individuals from population health, genetics, cardiology, cancer, epidemiology, clinical operations, risk and compliance, legal, bioethics, health IT, bioinformatics, and laboratory services. Members of the extended team met community partners at the introductory session, and later joined ad-hoc for specific call topics in order to keep group size small for discussion.
Community partner recruitment
Community partners were recruited through the Stanford Health Care Patient and Family Partners Program (Stanford Healthcare n.d), which connects Stanford providers with community members on provider-initiated community engagement projects. The Patient and Family Partners program includes self-selected patients and caregivers at Stanford Health Care who have completed basic privacy and confidentiality training prior to volunteering on projects. Community engagement requests are emailed out to volunteers by the volunteer manager, and typically include information on the purpose of the project, meeting time, frequency, and length of commitment. The PGP co-design project requested recruitment of a diverse set of community members of varying ages, races, education levels, prior experiences with genetics, patient vs caregiver status, and insurance types to ensure diverse perspectives on the topic of PGS. Recruitment was facilitated by the volunteer manager who has access to this information; facilitators were blinded to specific demographics and community members were asked only to share their individual demographic information as they felt comfortable doing so.
Box 1 shares community member’s and the co-design facilitators’ motivations for participation in their own words.
Box 1 Motivations for participation in PGP co-design
| E.G. (community partner) — I am a frequent flyer at Stanford Hospital and Clinics for over 37 years. I have Cystic Fibrosis and when I reached end stage, I received a life saving gift of life of a double lung transplant. I am eternally grateful to my medical team and staff at Stanford clinics. This was an opportunity for me to pay it forward. I have always been interested in preventative medicine so this volunteer opportunity seemed very interesting to me. I am glad that I was able to be a part of this project! |
| L.K. (community partner) — I joined because I’m directly affected and wanted to see what the future may hold |
| V.S. (community partner) — My motivation for joining this group comes from my desire to support this type of research, given my own experience with illness /conditions that likely stem from genetics. If my background can support research that might help others, I’m very happy to do so. My other motivation in joining was an opportunity to give back to Stanford |
| J.O. (community partner) — I joined the group to try to give back in helpful ways after receiving so much from so many, including all the talented dedicated health professionals that have helped me navigate breast cancer twice |
| T.P. (community partner) — I am an elderly Chinese American patient and caregiver at Stanford Healthcare. I have been exposed to the genetic factor of personal health. My interest in the project is in the utilization of GWAS to assess polygenetic risk for health problems. The community engagement aspect of the project sets an example for introducing new clinical assessment that are more patient centered |
| S.M. (community partner) — My motivation to join the group was two-fold: one to learn something new about a topic that might have a huge impact on the health of myself, family and community and two: to contribute to the work of others using my experience as a patient with chronic disease, caregiver and healthcare worker who has been committed to developing new ways of providing care |
| A.S. (community partner) — I joined this group out of a general interest in the topic, a deep desire to contribute to the Stanford Health Care community, and, ultimately, it was a volunteer opportunity that aligned with my schedule |
| H.W. (facilitator) — I am a genetic counselor and translational science researcher. In holding these identities, I try to ensure that person-centeredness (and therefore community-centeredness) happens at all levels of healthcare translation. As an individual with chronic disease, I can empathize with disjointed and confusing healthcare experiences. I am motivated to make genomic medicine accessible, equitable, and meaningful for all. As the program’s director, I could not imagine designing a program without the input of its intended audience |
| D.O.M. (facilitator) — I am a bi-racial woman who identifies as African American and who is deeply interested in health equity and socially and ethically responsible genetic research and research communication. My concern about the currently limited utility and validity of PGS applications in clinic and equity concerns about PGS translation motivated my participation in this co-design process. I believe that centering patient and family perspectives in decision-making about whether and how to introduce genomics into clinical settings is critical |
| One community member opted not to disclose a motivation statement |
Meeting logistics
The co-design team met bi-weekly from May to August 2021. Each meeting lasted approximately 90 min. Due to the ongoing COVID-19 pandemic, meetings were held virtually, over Zoom. The first session included an extra 30 min for introductions and setting group expectations and norms (the process of “contracting”). The first three didactic sessions covered background on (1) preventive health and risk stratification, (2) polygenic scores for risk prediction, and (3) data privacy of genetic information. This was followed by three open-ended calls to discuss preferences about (1) informed consent for PGS, (2) equity and access to PGS and genetic services, and (3) PGS results and long-term care. A final session was used to collaboratively summarize community feedback, including whether community members saw value in implementing a PGS program and how community feedback should be integrated into its design. Additionally, the group used this final meeting to agree on their communication preferences for ongoing programmatic updates or feedback requests. The group decided on email communication and ad hoc video conferences. Meeting minutes were taken by a program assistant, and all calls were recorded and transcribed.
In addition to the co-design group call schedule, the two facilitators met weekly to synthesize meetings and adjust call topics in real-time response to group feedback. This allowed call topics to naturally adapt to emerging group interests and questions. Before each co-design call, the facilitators sent an email that (1) summarized the previous session and any key take-aways, questions, and action-items; (2) solicited additional clarification and feedback; (3) outlined the plan and/or provided slides for the upcoming session.
In January 2022, community partners were approached by the facilitators to elicit their interest in documenting the co-design process for learning purposes. Four community partners volunteered and attended an introductory hour-long meeting on academic publishing and ethics standards for authorship and academic publishing. After the introductory session on publishing and authorship, three community volunteers (AS, SM, TP) felt able to commit the time required for co-authorship, which included attending regular meetings to discuss and divide up writing tasks and respond to reviewer feedback. This manuscript is the outcome of that process.
Results
Here, we reflect on the steps taken to operationalize the co-design framework described in Fig. 1, and our successes and challenges in achieving them from the collective perspective of the community, hospital, and research stakeholders involved in PGP co-design.
Reciprocal relationship and true partnership
A major tenet of community empowerment is forming a reciprocal relationship between co-design facilitators (as representatives of the research and clinical teams supporting PGP) and community partners (as representatives of the community intended to be served by PGP). Any reciprocal relationship requires true power sharing built on mutual respect and benefit.
To achieve mutual respect, the call schedule was structured to mimic the stages of developing a trusted and power-balanced relationship:
Introductions and contracting provided an opportunity for the group to share their goals and communication preferences and establish an ethos of trust, inclusivity, and mutual learning. The group regularly revisited and updated these ground rules and group values to maintain rapport (see Supplementary Note 1 for established group norms).
The didactic sessions were designed to bring community partners up to speed on what is known and not known about PGS and their possible implementation. This created a shared knowledgebase for all co-design members to feel informed on a topic and its uncertainties, thus allowing for balanced power on the topic.
Discussion calls were a dedicated space for brainstorming after all individuals felt informed on the topic. Transparency and vulnerability in sharing the uncertainties of PGS translation earlier during didactic sessions invited open-ended brainstorming.
The final summary call reiterated the group’s value in accountability and ensured accurate translation of feedback into program actions.
Calls were summarized and adjusted in real time to demonstrate true listening and power sharing in dictating the group’s direction. From the perspective of the community partners, this attention to reciprocity and intentionality in how PGP facilitators and extended team members communicated and interacted with community members led to partnership instead of unidirectional, hierarchical engagement. Box 2 shares a written perspective from one of the community member co-authors of this manuscript (AS) on this partnership dynamic.
Additionally, a diverse group of individuals of various demographic and health backgrounds were recruited. While implicit diversity was intentional in the engagement design, disclosure was up to individuals. Individuals have a right to confidentiality and to avoid tokenization. Although leaving it up to individuals to decide whether, when, and how to share personal experiences likely helped accelerate rapport, this was not studied or documented systematically.
Box 2 Reciprocal relationship and true partnership.
| The Stanford Preventive Genomic Research Project felt very similar and also vastly different from other community engagement work I’ve done, both inside and outside of the Stanford Health Care system. I was expecting the usual ‘focus group’ types of interactions I’d come to expect from my other PFAC experiences: The institutional representatives ask: Do you like A or B? What are the benefits of A? What are the benefits of B? Oh, is that a suggestion for C? What are the benefits of C? What are the drawbacks? If we are unable to give patrons C, would they prefer A or B? However, Hannah and Daphne began immediately with establishing norms – unwritten but understood agreements in the PFAC world – that allowed for a free-flowing but always respectful discussion. By establishing the boundaries and limits of conversation, trust and respect were ‘baked into’ the following discussions. It helped, I think, that most participants were patients, former patients, or caregivers and were very aware of HIPAA codes; that shared value of privacy enhanced the trust that was necessary to the conversations that occurred over a number of weeks |
| - Annamaria Smitherman, Member of the Preventive Genomics PFAC Workgroup |
To foster mutual benefit, fair compensation must be negotiated by all parties for their time and expertise. This can be difficult to do, and our co-design process was limited in this regard:
Compensation is grounded in personal benefit and interest; it may or may not be perceived as monetary. Therefore, incentives, motivations, and expectations of all group members should be explicitly understood prior to long-term participation. Ethical considerations of monetary compensation are complicated in clinical community engagement projects such as this. Unlike research, community members in the Patient and Family Partners Program are advisors within the healthcare system and considered volunteers. Typically, reimbursement is constrained to gift cards and certain gift amounts. Community members in this co-design process were compensated with $20 gift cards for the entire engagement, as dictated by hospital volunteer reimbursement restrictions. Traditional approaches to compensation, which this co-design process was confined to, can limit participation from patients from underserved groups. Although literature on the effects of compensation on community engagement are limited, undue hardship from financial constraints and demands on time holds the potential to produce disparities in who has the means and resources to participate in community engaged initiatives (Brunton et al. 2017). These constraints are fundamentally at odds with having diverse and inclusive healthcare because it limits whose perspective gets included. This co-design process is not immune to the limitations posed by traditional approaches to compensation, or the challenges community engaged initiatives face more broadly in terms of diverse, inclusive recruitment.
The time commitment for this engagement was significant. As volunteers within the Stanford Patient and Family Partners Program, community members expected to be approached for volunteer opportunities — making the recruitment strategy for this co-design process unique. The initial recruitment email stated the expected time commitments, allowing volunteers to determine their interest and ability to participate. Hours were selected to avoid standard daylight work hours, and program facilitators minimized requests beyond verbal feedback during call hours. In traditional engagement, compensation is balanced with avoiding financial coercion for volunteering; however, it is unclear how this perspective might shift with viewing patients as expert consultants in healthcare system design, rather than volunteers for providing feedback. More thoughtful frameworks for appropriate compensation and diverse recruitment should be considered by hospital systems interested in adopting this model.
Transparent and shared decision-making
Another major tenet of co-design is ensuring transparency and accountability in shared decision-making. This principle was interwoven throughout the entire series, culminating in the final summary session for translating feedback into programmatic decisions. This co-design benefited from several key factors:
Institutional decision-makers and leaders were supportive and involved in the co-design process. The co-design process was co-led by the program’s director with support from hospital leadership — ensuring decisions were advocated for and upheld.
Decisions were considered at the programmatic ecosystem level. This systems approach created flexibility for accommodating community preferences; changes could be made in a particular area by adapting in another. For example, if there was a strong preference for how to display polygenic scores in the electronic health record, this could be implemented by health IT while adjusting processes in lab services, clinical operations and provider training to accommodate the change. Not having a program siloed in a single department or institution is critical to ensure true decisional support.
The current lack of PGS translational guidance is a double-edged sword. While uncomfortable for hospital systems wanting to implement something quickly and confidently, grey areas provide optimal freedom for tailoring decisions and personalizing programs to a community’s interests.
Box 3 provides a written perspective from a community member co-author on shared decision-making in the co-design process.
Box 3 Transparent and shared decision-making.
| Typically, individuals who are involved in community engagement projects are self-selected and have a commitment to assist in research; however, the projects are very prescriptive and already have a vision of the role of the community participant. Projects involve answering predetermined questions or issues of concern—the focus is on the researcher and their project. It is never the community’s project. As an individual with experience in community engagement projects, I initially felt that the Preventive Genomics Program project would be in the typical style of a community engagement project. Questions would be asked, opinions would be sought, and the investigators would utilize what pieces of the group conversation were helpful to them. I was pleasantly surprised to see a different situation emerge. In the Preventive Genomics project, our group members became empowered to make decisions by educating us in-depth on the topic, allowing time to reflect on the implications for ourselves and our community, valuing our intellect, responding to questions about the project and allowing healthy discussion that sometimes went “off track.”. This project has shown that through community empowerment, group members can be actively involved in the development of the project and feel as if they also “own” the success or failure of the project |
| - Sheryl Michelson, Member of the Preventive Genomics PFAC Workgroup |
Co-learning
An additional, critical tenet of the co-design process is to establish co-learning that acknowledges the importance of everyone’s contributions and individual expertise. Successful co-learning relies on having reciprocal relationships, as discussed previously. Several group processes contributed to the development of a shared knowledgebase between group members:
Facilitators synthesized discussions in real-time to ensure correct understanding about what community members were saying. This is similar to a technique called “teachback” used by clinicians to ensure patients correctly understand (Yen and Leasure 2019). In this scenario, however, the facilitators were the learners repeating back to community members.
Facilitators encouraged individuals to elaborate on their statements or respond to each other, often calling individuals by name to allow all voices to be heard while also demonstrating a conversational style of sharing as a group (rather than Q&As or round robin). This encouraged co-learning between community members. As rapport grew, these dynamics became more natural; the group eventually self-regulated discussion such that all members shared their opinions with limited prompting from facilitators.
To protect group rapport, the co-design facilitators liaised with their clinical and research team members separately and reported back to the co-design group. Decisions to invite extended team members to calls ad hoc were initiated by interest from the co-design group to dive into a topic beyond the scope of the facilitators (e.g., having a genomic data privacy expert consult). To preserve the co-learning environment, facilitators briefed invited clinical and research team members on group expectations and communication preferences.
A major challenge for facilitators when supporting the co-learning process was balancing the information level such that it was transparent without being unconstructively overwhelming or uninteresting to community partners. While quality community empowerment reiterates the need to engage the community at the onset of a new initiative, in practice, it would have been difficult to involve community members without preparation and pre-work by the PGP team to present what was known and unknown about PGS in the scientific literature or regulatory landscape of test development.
Box 4 provides the experience of co-learning from the perspective of one of the community member co-authors of this manuscript.
Box 4 Co-learning — reflections from a community partner.
| Those conversations were a give and take, different from my other community engagement experiences. There was an agenda, including a recap of our previous work – pretty typical. But the engagement involved time for the volunteers to process our thoughts from the previous meeting, to report on conversations we’d had with friends and family, to express our new or ongoing concerns around preventive genomics. There was a concerted effort to educate us about things like ethical standards, the likelihood that anyone who received genetic counseling would require interventions, how genomics can—and cannot—enhance the health of patients, and so on. The education, the response, and continued research to questions and concerns, and the detailed follow-up all contributed to a feeling beyond engagement – a sense that the work the volunteers were doing was making a difference in the outcomes |
| - Annamaria Smitherman, Member of the Preventive Genomics PFAC Workgroup |
Outputs of co-design
As an illustration of the programmatic decisions resulting from the community empowered co-design approaches, we provide two demonstrative examples in programmatic decisions regarding service delivery and risk communication in Tables 1 and 2. We focus on service delivery and risk communication because of their importance to a clinical audience implementing PGS. However, data protections and privacy, cost and insurance coverage, and clinical actionability/medical management were other areas for programmatic decision-making in this co-design process. These tables are organized to highlight (1) the contextual uncertainty creating ambivalence about programmatic decisions, (2) what was learned from co-design discussions about the topic and its uncertainty (e.g. preferences, values, assessment of evidence), and (3) subsequent decisions resulting from the co-design approach. These decisions centered community input, rather than the opinions of clinicians and researchers who may otherwise be seen as the experts.
Table 1.
Service delivery* co-design
| Initial considerations — reflected in expert review and literature |
Uncertainty: • PGS may be scaled out and supported through population health services, but specific provider training needs and infrastructural supports for this are unclear • Primary care physicians (PCPs) seem to find risk conveyed by PGS intuitive with current risk prediction and multifactorial disease models (Smit et al. 2021) • PGS is a numerical value grounded in a population distribution and can be incorporated into existing clinical risk calculators used in preventive screening (Smit et al. 2021) • The underlying genomic methodologies and limitations are less familiar for providers, and should be a focus of future training (Smit et al. 2021; Vassy et al. 2018) |
| Co-design considerations — community preferences and local adaptation |
Community preference: • Patients are more inclined to engage with new and possibly sensitive information (like PGS) if they trust the healthcare system • Current uncertainties about PGS limitations and clinical utility require attention to informed choices about PGS testing. PGS cannot simply be offered across a healthcare system by anyone • While PGS is a numerical value, it is still grounded in genetics and feels sensitive or emotionally laden to our group. Thus, provider capacity to support PGS testing decisions should also require skills in assessing personal values and personal utility • Providers with proper training are not helpful if they are not available in a reasonable time. Access is an important dimension of quality care |
| Programmatic decisions |
Co-design lesson: • Service delivery is an opportunity for building trust, and decisions should not solely be driven by logistics • Provide suggested questions and educational materials to the patient pre-visit to prepare patients to think about their values in advance and maximize provider-patient discussion during the visit • Provider training is not just about education; it is also about skills development. Patient advocates can help providers understand the importance of personal utility in test decisions, as well as the emotional nature of genetic information • Care should be coordinated through team-based care and provider handoffs if different specialists are involved. This provides the stability needed for long-term preventive health discussions |
Table 2.
Informed choice and risk communication co-design
| Initial considerations — reflected in expert review and literature |
Uncertainty: • It is not clear how people will understand, misunderstand, or ascribe meaning to PGS • PGS is more deeply engrained in numeracy values than other types of genetic tests. Numerical risk information is notoriously difficult to understand (Davis et al. 2021; Peck et al. 2022) • Risk and risk perceptions may be embedded in lay beliefs or cultural values; thus, provider training in communication and cultural competency is needed (Hong et al. 2020; Hopwood 2000) • Providing genetic risk results for common, complex disease may have unintended consequences or reactions due to “essentialist bias,” i.e., the belief that one’s genes define their essence or identity (Dar-Nimrod and Heine 2011) • Integrating PGS into clinical care requires careful communication to avoid misinformed notions of genetic determinism and essentialism |
| Co-design considerations — community preferences and local adaptation |
Community preference: • Patients want providers to (1) contextualize risk in a personal way and (2) help them make meaning from it • Genetic information has both a cognitive and emotional layer of meaning that need to be attended to • If a person is told PGS is not deterministic, then their other contributory factors should also be explained • There is no right, wrong, or best way to explain complex risk. Everyone has different learning styles and health literacy. Providers should be trained to meet the needs of diverse audiences • Understanding a number is not as important as understanding the implications of it (e.g., personal utility, clinical utility) • The goal of risk communication should be to support informed choice (pre-test) or adaptation (post-test), not education |
| Programmatic decisions |
Co-design lesson: • Provider training should focus on risk communication skills, not just the accurate recall of information • Patients will try to assimilate risk information with their personal and current experiences, as well as their personal and cultural values. Providers might have a duty to bridge these connections or patients may have maladaptive responses to test results • Professional development in these soft skills of assessing patient needs and values align with person-centered care |
*Service delivery includes providers, operations, infrastructure.
This manuscript is also considered an output of the co-design process as a report and reflection on the successes, failures, and challenges of operationalizing co-design. Rarely in research or other types of community engagement is one able to share and iterate on their rationale, design, and conceptual approach alongside participants. Yet, as one co-author and community member reflects, “we started off as two groups (facilitators, community members) and now seem like one.” This document is a testament to that lived experience; as a single co-design group, we present our unified synthesis and impressions of the co-design process. What is presented here was synthesized together, after the conclusion of our design sessions. True to our principles, this manuscript is a product of our true partnership, written as a collective. Documenting and reflecting on the co-design process provided an opportunity to learn from each other about how and why this was done, and an accountability measure for ensuring the hypothetical principles we started with matched lived experience.
Box 5 includes a reflection from the program director and co-facilitator on the experience of debriefing, as it relates to realizing how central active facilitation is to securing the co-design principles adopted from PCORI. As Fig. 1 demonstrates, active facilitation was critical to our co-design conceptual framework.
Box 5 Active facilitation.
| The organic and emergent nature of this type of work is challenging. In reflecting on the co-design process with our community partners, a major learning point for us was realizing how active the facilitation process is. We can now appreciate the influence of our respective backgrounds and training in aiding the process. Between the two of us, we share expertise in risk communication, patient communication, group counseling, adult learning, and patient engagement. These prior experiences in explaining complex concepts to patient and public audiences was critical for establishing a power sharing dynamic when sharing knowledge. It would not have been successful without a close working relationship and willingness to learn from each other as facilitators, and an inherent curiosity and genuineness to learn from our community partners. As a future learning lesson, we advocate that any researchers or clinicians taking on co-design endeavors invest the time in gaining skills for group facilitation, adult learning, and community engagement |
| - Hannah Wand and Daphne Martschenko, Co-Design Facilitators |
Discussion
The current translational environment simultaneously holds the anticipated personal and clinical benefits of PGS in improving complex disease prevention, and very real uncertainties and concerns about balancing benefits and risks in population implementation. This case study highlighted the utility of community empowered co-design in deciding whether and how to translate polygenic scores to clinical care. It is intended as a blueprint for process and not a prescription.
How does or should one evaluate the success of this, or any other co-design process? Currently, there are no agreed upon metrics for doing so. The co-design process necessarily entails a hyper contextual and localized approach. In other words, many metrics for success will be specific to the social, economic, cultural, and political context in which a co-design is situated. Although developing generalizable metrics for evaluation that sit alongside local ones may be challenging, doing will help to determine the long-term outcomes of co-design. Community empowered co-design acknowledges community members as the experts in community, healthcare experiences, and public interest. Thus, one generalizable metric should be the extent to which communities and patients — as the intended beneficiaries of clinical translation — drive decision-making. One potential means for capturing this is the GRIPP2 checklist for reporting patient and public involvement (Staniszewska et al. 2017), which has been previously recommended as a public health utility reporting standard for polygenic scores (Wand et al. 2021).
Unique contextual factors for this co-design and hospital/geographic setting may not generalize to other settings or healthcare systems. For example, the community partners in this co-design already had a strong sense of rapport and familiarity with group feedback due to their prior community engagement activities; other programs might need to invest more time in developing rapport and safety. The sense of rapport and safety that existed among several community partners prior to this co-design also raises an important question about whether co-design processes are best when conducted virtually (e.g., via Zoom) or in-person. Although the COVID-19 pandemic necessitated that our co-design process occur virtually, more needs to be done to understand whether and how developing group expectations and norms are affected by decisions to conduct a co-design virtually or in-person. For instance, Hall et al. (2021) examined the use of distanced-based participatory methods during the COVID-19 pandemic and found that there are benefits and challenges to such approaches. In our case, it is possible that prior in-person engagements involving several community partners cultivated interpersonal relationships that carried over, and were valuable to, this co-design process. Indeed, Hall et al. (2021) found that many of the distanced-based participatory approaches they examined involved parties who had experienced at least some prior in-person exchange. It is possible that trying to build interpersonal relationships may prove more difficult to establish virtually in the absence of any in-person interaction. Additionally, attending online meetings requires that one has reliable access to the internet and a device that can connect to it, which could restrict a person’s ability to participate. Alternatively, however, Zoom meetings may have increased engagement in this co-design by removing the need to travel, reducing the time or financial burdens that come with travel, and providing a more disability-accessible format (Guckenheimer 2020).
An additional contextual factor that cannot be generalized to other co-designs is that our team generally arrived at a consensus. A successful co-design model should help to ensure community needs and preferences are being met by new services. Simultaneously, it should recognize that individual users of a service may have additional or different needs and preferences to those of the community. Thus, any service committed to person-centered care, and any co-design process that leads to its development, will need to successfully respond to both the community and the individual. We were privileged to not have to navigate intractable disagreements. Other programs and topics may encounter higher levels of disagreement, and it takes time to understand and be able to articulate disagreements. Complete consensus may be impossible. Moreover, this work was funded as a clinical program and initiated by that clinical program, and thus is motivated by pragmatism and direct patient care improvement; other PGS programs might have red tape or competing incentives in decision-making, such as securing grant funding.
Furthermore, this work was done in the USA, where healthcare is privatized and new genetic services are typically commercially driven, and not always coverage or evidence based. Thus, decision-making for elective tests like PGS may be more salient than other healthcare systems. However, context and adaptation is the foundation of this work, and any healthcare systems considering PGS implementation will have their unique contextual factors, circumstances and communities. True equity in care requires locally adapting to those needs and resources and adhering to the values and decisions of community members. Thus, all institutions are encouraged to embark on community empowered co-design at the onset, when first exploring the feasibility and pragmatism of services. It is our hope that the general blueprint of a community empowered co-design is generalizable to other new technologies (e.g., machine learning in healthcare) and/or quality improvement processes.
Genuine community empowerment requires a commitment to partnership. Communication and uncovering opinions require active facilitation that adapts to shifting group dynamics and rapport. Given the importance of the facilitator-participant relationship that came out of this writing process, future study should systematically assess the elements of these relationships that drive mutual respect, power-sharing and co-learning. While most hospitals and research centers in the USA have community advisory boards, this article reflects on the investment and resources required to engage community members in ways that are dynamic and in-depth rather than static and shallow. A commitment to community empowerment requires institutional support, facilitator training, community relationship-building, power-sharing, and time. This investment of resources and time to community co-design is aligned with the stated long-term goals of most healthcare systems to support person-centered and value-based care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Preventive Genomics Program Co-Design Working Group comprised of Elyse Goldberg, Linda Knepper, Sheryl Michelson, Jackie Osborne, Ting Pun, Val Sanders, Annamaria Smitherman, and one anonymous member. Facilitators were Daphne Martschenko and Hannah Wand. We thank this working group for their engagement and feedback in the co-design process. The extended Preventive Genomics Program planning committee included Euan Ashley, Philip Chen, Mildred Cho, Shoa Clarke, Rosalie Geronimo, Hannah Ison, Charlene Kell, Alison Kerr, Joshua Knowles, Megan Mahoney, Vasiliki Rahimzadeh, Stuart A. Scott, Sri Seshadri, Sandra Tsai, and John Witte. We thank this committee for their assistance in conceptualizing the program and preparing call topics for the working group. We thank Patient and Family Partner Program managers Sarah Foad and Katie Smith for their assistance in convening the working group and for consultation in preparing the manuscript. We thank Jennifer Siranosian and Dorsa Moslehi for administrative assistance in organizing the working group calls and taking meeting minutes.
Author contribution
All authors contributed to the study conception and editing. Material preparation and content analysis were performed by Hannah Wand and Daphne Martschenko. The first draft of the manuscript was written by Hannah Wand, Daphne Martschenko, Annamaria Smitherman, and Sheryl Michelson. Ting Pun and Jackie Osborne commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This manuscript is supported by the Stanford Training Program in ELSI research grant (T32HG008953) and the National Institutes of Health ClinGen Consortium grant (U41 HG009649).
Declarations
The following disclosures provide transparency about positions held by the authors related to either polygenic score clinical standards development, or community engagement best practices development. Hannah Wand is co-chair of the NIH ClinGen Consortium’s Complex Disease working group, lead author of the National Society of Genetic Counselors practice resource on polygenic scores, and a committee member for the American College of Medical Genetics practice resource and laboratory Q&A on polygenic scores. Daphen Martschenko is a guest editor for this special edition, and a member of the Hastings Center working group for social and behavioral genomics, which looks at the ethical responsibility of polygenic scores. Ting Pun has served on the advisory panel for patient engagement for the Patient Centered Outcomes Research Institute as a patient member.
Conflict of interest
Euan A. Ashley is a co-founder of Deepcell, Personalis, and SVEXA; a board member of AstraZeneca; and an adviser to Apple, Foresite Labs, Nuevocor, and Sequencebio. Stuart A. Scott is a paid consultant of Sema4. John S. Witte is a co-founder of Avail Bio and is paid expert work from Pfizer and Sanofi. Hannah Wand, Daphne O. Martschenko, Annamaria Smitherman, Sheryl Michelson, Ting Pun, and Mildred K. Cho declare no financial conflicts of interest.
Studies with human subjects
This study did not require IRB review. Patient and Family Partners are enrolled and trained as Stanford Health Care volunteers. Participants are considered working group members in working groups with Stanford faculty, rather than serving as research participants. The Patient and Family Partner Program managers were extensively consulted about appropriate permission processes for publication. At the introductory call, during the contracting process, all participants expressed an interest in disseminating this information to the external scientific community (see Supplementary Note 1). Verbal consent was obtained to record and transcribe meetings, and written permission was obtained by email to write and publish this manuscript. In the email requesting permission, community members were informed on the goal of manuscript writing and a general outline of content to help them decide if this manuscript was an appropriate publication on behalf of the group. Four community members volunteered to meet regularly and engage in the manuscript writing process as authors. All community members were sent draft and final versions of the manuscript for approval. Community members were asked to include a motivation statement and their name in acknowledgments at their volition; only one member opted out and is acknowledged as an anonymous member.
Footnotes
Hannah Wand and Daphne O. Martschenko contributed equally to this work.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hannah Wand, Email: hwand@stanfordhealthcare.org.
on behalf of the Preventive Genomics Program Co-Design Working Group:
Elyse Goldberg, Linda Knepper, Sheryl Michelson, Jackie Osborne, and Val Sanders
References
- Adeyemo A, Balaconis MK, Darnes DR, Fatumo S, Granados Moreno P, Hodonsky CJ, Inouye M, Kanai M, Kato K, Knoppers BM, Lewis ACF, Martin AR, McCarthy MI, Meyer MN, Okada Y, Richards JB, Richter L, Ripatti S, Rotimi CN, … Polygenic Risk Score Task Force of the International Common Disease Alliance (2021) Responsible use of polygenic risk scores in the clinic: potential benefits, risks and gaps. Nat Med 27(11), Article 11. 10.1038/s41591-021-01549-6 [DOI] [PubMed]
- Aragam KG, Natarajan P. Polygenic scores to assess atherosclerotic cardiovascular disease risk: clinical perspectives and basic implications. Circ Res. 2020;126(9):1159–1177. doi: 10.1161/CIRCRESAHA.120.315928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton G, Thomas J, O’Mara-Eves A, Jamal F, Oliver S, Kavanagh J. Narratives of community engagement: a systematic review-derived conceptual framework for public health interventions. BMC Public Health. 2017;17(1):944. doi: 10.1186/s12889-017-4958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver T, Hartley S, Lee A, Cunningham AP, Archer S, Babb de Villiers C, Roberts J, Ruston R, Walter FM, Tischkowitz M, Easton DF, Antoniou AC. CanRisk Tool-A web interface for the prediction of breast and ovarian cancer risk and the likelihood of carrying genetic pathogenic variants. Cancer Epidemiol, Biomark Prev: Publ Am Assoc Cancer Res, Cosponsored Am Soc Prev Oncol. 2021;30(3):469–473. doi: 10.1158/1055-9965.EPI-20-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar-Nimrod I, Heine SJ. Genetic essentialism: on the deceptive determinism of DNA. Psychol Bull. 2011;137(5):800–818. doi: 10.1037/a0021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KW, Roter DL, Schmidlen T, Scheinfeldt LB, Klein WMP. Testing a best practices risk result format to communicate genetic risks. Patient Educ Couns. 2021;104(5):936–943. doi: 10.1016/j.pec.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edick M, Pilditch J (2020) Rare Disease and Research Engagement (RaRE). Rare Disease and Research Engagement (RaRE) | PCORI. https://www.pcori.org/research-results/2020/rare-disease-and-research-engagement-rare. Accessed Dec 2022
- Guckenheimer D (2020) Virtual event participation is key for accessibility. Rooted in Rights. https://rootedinrights.org/virtual-event-participation-is-key-for-accessibility/. Accessed Dec 2022
- Hall J, Gaved M, Sargent J. Participatory research approaches in times of Covid-19: a narrative literature review. Int J Qual Methods. 2021;20:16094069211010088. doi: 10.1177/16094069211010087. [DOI] [Google Scholar]
- Holzer JK, Ellis L, Merritt MW. Why we need community engagement in medical research. J Investig Med. 2014;62(6):851–855. doi: 10.1097/JIM.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Goodman M, Kaphingst KA. Relationships of family history-related factors and causal beliefs to cancer risk perception and mammography screening adherence among medically underserved women. J Health Commun. 2020;25(7):531–542. doi: 10.1080/10810730.2020.1788677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P. Breast cancer risk perception: what do we know and understand? Breast Cancer Res: BCR. 2000;2(6):387–391. doi: 10.1186/bcr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ACF, Green RC. Polygenic risk scores in the clinic: new perspectives needed on familiar ethical issues. Genome Med. 2021;13(1):14. doi: 10.1186/s13073-021-00829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ (2019) Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 51(4), Article 4. 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed]
- Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, Tyrer JP, Chen T-H, Wang Q, Bolla MK, Yang X, Adank MA, Ahearn T, Aittomäki K, Allen J, Andrulis IL, Anton-Culver H, Antonenkova NN, Arndt V, … Easton DF (2019) Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Human Genet 104(1):21–34. 10.1016/j.ajhg.2018.11.002 [DOI] [PMC free article] [PubMed]
- Mehta RN, Seim B. Avoiding “checkbox inclusion”: structuring meaningful inclusion of underrepresented groups in policy engagement. PS: Political Sci Politics. 2023;56(1):133–136. doi: 10.1017/S1049096522000841. [DOI] [Google Scholar]
- PCORI (Patient-Centered Outcomes Research Institute) (2014) PCORI Engagement Rubric. https://www.pcori.org/sites/default/files/Engagement-Rubric.pdf. Accessed Dec 2022
- Peck L, Borle K, Folkersen L, Austin J. Why do people seek out polygenic risk scores for complex disorders, and how do they understand and react to results? Eur J Hum Genet. 2022;30(1):81–87. doi: 10.1038/s41431-021-00929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CR. The foundations of the person-centered approach. Education. 1979;100(2):98–107. [Google Scholar]
- Sabatello M, Martschenko DO, Cho MK, Brothers KB. Data sharing and community-engaged research. Science. 2022;378(6616):141–143. doi: 10.1126/science.abq6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan S, Schrandt S, Forsythe L, Hilliard TS, Paez KA. The PCORI engagement rubric: promising practices for partnering in research. Ann Fam Med. 2017;15(2):165–170. doi: 10.1370/afm.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AK, Sharman AR, Espinoza D, Wallingford C, Young M-A, Dunlop K, Tiller J, Newson AJ, Meiser B, Cust AE, Yanes T. Knowledge, views and expectations for cancer polygenic risk testing in clinical practice: a cross-sectional survey of health professionals. Clin Genet. 2021;100(4):430–439. doi: 10.1111/cge.14025. [DOI] [PubMed] [Google Scholar]
- Stanford Healthcare (n.d.) Patient & Family Partner Program. Retrieved June 10, 2022, from https://stanfordhealthcare.org/for-patients-visitors/patient-advisory-councils.html Accessed Dec 2022
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, Altman DG, Moher D, Barber R, Denegri S, Entwistle A, Littlejohns P, Morris C, Suleman R, Thomas V, Tysall C. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. doi: 10.1136/bmj.j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American Geriatrics Society Expert Panel on Person-Centered Care Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15–18. doi: 10.1111/jgs.13866. [DOI] [PubMed] [Google Scholar]
- Vassy JL, Davis JK, Kirby C, Richardson IJ, Green RC, McGuire AL, Ubel PA. How primary care providers talk to patients about genome sequencing results: risk, rationale, and recommendation. J Gen Intern Med. 2018;33(6):877–885. doi: 10.1007/s11606-017-4295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerstein N, Bernstein E. Introduction to community empowerment, participatory education, and health. Health Educ Q. 1994;21(2):141–148. doi: 10.1177/109019819402100202. [DOI] [PubMed] [Google Scholar]
- Wand H, Lambert SA, Tamburro C, Iacocca MA, O’Sullivan JW, Sillari C, Kullo IJ, Rowley R, Dron JS, Brockman D, Venner E, McCarthy MI, Antoniou AC, Easton DF, Hegele RA, Khera AV, Chatterjee N, Kooperberg C, Edwards K, … Wojcik GL (2021) Improving reporting standards for polygenic scores in risk prediction studies. Nature 591(7849), Article 7849. 10.1038/s41586-021-03243-6 [DOI] [PMC free article] [PubMed]
- Wong-Gates R (n.d.) Client centered therapy. Rennet Wong Gates. Retrieved December 30, 2022, from https://www.rennetwonggates.com/client-centered-therapy/ Accessed Dec 2022
- Yen PH, Leasure AR. Use and effectiveness of the teach-back method in patient education and health outcomes. Fed Pract: Health Care Prof VA, DoD, PHS. 2019;36(6):284–289. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


