Abstract
The rate of textile waste generation worldwide has increased dramatically due to a rise in clothing consumption and production. Here, conversion of cotton-based, colored cotton-based, and blended cotton-polyethylene terephthalate (PET) textile waste materials into value-added chemicals (bioethanol, sorbitol, lactic acid, terephthalic acid (TPA), and ethylene glycol (EG)) via enzymatic hydrolysis and fermentation was investigated. In order to enhance the efficiency of enzymatic saccharification, effective pretreatment methods for each type of textile waste were developed, respectively. A high glucose yield of 99.1% was obtained from white cotton-based textile waste after NaOH pretreatment. Furthermore, the digestibility of the cellulose in colored cotton-based textile wastes was increased 1.38–1.75 times because of the removal of dye materials by HPAC-NaOH pretreatment. The blended cotton−PET samples showed good hydrolysis efficiency following PET removal via NaOH–ethanol pretreatment, with a glucose yield of 92.49%. The sugar content produced via enzymatic hydrolysis was then converted into key platform chemicals (bioethanol, sorbitol, and lactic acid) via fermentation or hydrogenation. The maximum ethanol yield was achieved with the white T-shirt sample (537 mL/kg substrate), which was 3.2, 2.1, and 2.6 times higher than those obtained with rice straw, pine wood, and oak wood, respectively. Glucose was selectively converted into sorbitol and LA at a yield of 70% and 83.67%, respectively. TPA and EG were produced from blended cotton−PET via NaOH–ethanol pretreatment. The integrated biorefinery process proposed here demonstrates significant potential for valorization of textile waste.
Keywords: Textile waste, Value-added biochemicals, Integrated biorefinery, Bioethanol, Lactic acid
Graphical abstract

Highlights
-
•
An integrated textile waste biorefinery process was successfully demonstrated.
-
•
Textile waste was converted into bioethanol, sorbitol, lactic acid, TPA, and EG.
-
•
The EtOH yield of white T-shirt sample was 3.2 times higher than that of rice straw.
-
•
HPAC-NaOH pretreatment improves the hydrolysis efficiency of the colored textile waste.
-
•
NaOH-ethanol pretreatment enhanced enzymatic hydrolysis of blended textile waste.
1. Introduction
The global clothing production and consumption has increased steadily in the last 50 years. The textile industry continues to grow due to the rise of fast fashion, which is based on inexpensive manufacturing, frequent consumption, and short-lived garment use [1,2]. The global textile industry generated approximately US $920 billion in 2018, and is projected to generate approximately US$1.2 trillion by 2024 at a compound annual growth rate of 5% [3]. However, this growth of the textile industry has also led to a significant increase in textile waste, resulting in considerable economic, environmental, and social problems [4]. Increasing volumes of textile waste are generated as cheaper clothes are consumed more frequently. This waste is usually landfilled or incinerated due to high recycling costs associated with clothing; this also leads to a waste of valuable resources and creates environmental pollution [1]. To this end, strategies are being sought by many researchers for the conversion of textile waste into value-added products.
Cotton-based textile wastes are mainly derived from the cotton. Raw cotton fibers consist of 88–96% cellulose that is a renewable and biodegradable polymer that can be recycled into a variety of useful products [5,6]. Recently, cellulose has gained attention particularly as a potential source for biofuels, bio-based chemicals, and biomedical materials. Lignocellulosic biomass has been considered as an abundant raw material as a source of cellulose. There are some special advantages of the cotton-based textile wastes for biorefineries comparing to lignocellulosic biomass. Lignocellulosic biomass typically contains lignin and hemicellulose as well, in addition to cellulose. Lignocellulosic biomass types generally consist of 35–50% cellulose, 20–35% hemicellulose, and 10–25% lignin (Table 1) [7]. Thus, pretreatment of lignocellulose biomass is often required to separate cellulose from lignin/hemicellulose, to facilitate cellulose utilization. In contrast to those of other raw materials such as hardwood, softwood and agricultural waste, the relatively high cellulose content (88–96%) of cotton-based textile waste renders it a promising source of cellulose (Table 1). Moreover, the contents of lignin and hemicellulose in the cotton is very low. Therefore, the processes for removal of lignin and hemicellulose are not mandatory. However, the conversion of cotton-based textile waste for bio-based materials is still challenging, although the high cellulose content of cotton-based textile waste is a considerable advantage over other types of lignocellulosic biomass, [14]. Cotton cellulose included in cotton-based textile materials has a higher crystallinity (73%) than that of wood cellulose (35%) [15,16], which inhibits enzymatic hydrolysis of the substrate [17,18]. Hence, a pretreatment procedure that reduces crystallinity is necessary to enable efficient enzymatic hydrolysis.
Table 1.
Potential lignocellulosic biomass sources and their compositions (% dry weight).
Color is an important factor in the visual appearance of products. Various types of dyes are widely used in the textile industry in order to color their products [19]. However, dyes used in clothing manufacturing may pose a severe threat to the environment. Moreover, it has been reported that textile reactive dye conjugated on cotton fiber surface significantly impacts the rate of enzymatic hydrolysis [20]. Previous work reports that chemical and combination of physical/chemical pretreatment methods of are methods to treat colored textile wastes prior to its enzymatic hydrolysis [[21], [22], [23], [24]]. Therefore, finding suitable pretreatment methods that can effectively enhance the enzymatic hydrolysis of the colored cotton-based textile wastes is of novelty and importance.
Polyethylene terephthalate (PET) is one of the most widely used textile materials for clothing. PET is a petroleum-based synthetic polymer used in fibers for clothing production due to its low production cost, high durability, and water-resistant [25]. However, PET-based textile waste is non-biodegradable, and therefore, creates environmental pollution. Thus, development of waste management strategies based on recycling is an important issue for PET-based textile waste [26]. Moreover, microplastic pollution caused by the textile washing has recently been identified as a major source of primary microplastics in the oceans [27,28]. Microplastics released from degrading textile waste in water can also impact the life cycles of marine organisms [29]. COVID-19 pandemic has also increased attention on reducing amounts of plastic waste; however, this has not been extended to include PET-based textile waste.
Blended cotton–PET is the most widely used material for clothing production in the textile industry. Blending with PET improves the shrinkage, durability and wrinkling profile of natural cotton. However, recycling of blended fibers is cumbersome, and the shorter fiber length of cotton–PET blends reduces the quality and strength of produced fibers. Hence, these materials cannot be recycled for secondary use as clothing. The well-organized construction of the textiles of cotton and PET also limits the enzymatic hydrolysis [30]. Furthermore, large-scale reuse and recycling of textile waste is also difficult due to the limitations in sorting and recycling technology [31]. Therefore, the recycling of blended cotton–PET textile waste is an active field of research.
The design of integrated biorefineries for the production of value-added materials from textile waste is a huge and active field of research. The value-added materials that can be produced from textile waste include bioethanol, lactic acid (LA), sorbitol, ethylene glycol (EG), and terephthalic acid (TPA). These chemicals can be used as replacements for a large proportion of the industrial chemicals and materials produced using fossil resources [[31], [32], [33], [34]]. Bioethanol can be used as biofuel, and has also been identified as a promising platform chemical as well [31], since ethanol can be converted into various derivatives via different chemical reactions. These reactions include dehydration to produce ethylene as an intermediate chemical for subsequent production of polyethylene (PE), PET, polyvinyl acetate (PVA), and polyvinyl chloride (PVC); esterification to generate green solvents, ethyl lactate and ethyl acetate; and oxidation to produce acetic acid [35]. Current research on bioethanol is driven by the need to reduce its production cost. Using less expensive feedstocks such as textile waste may help reduce bioethanol production costs [36,37]. Textile waste is an attractive and viable feedstock alternative for bioethanol production for both economic and environmental reasons. Year-round supply of textile is also available.
Sorbitol and LA are also building block chemicals that can serve as substrates to produce various other high value-added products. These chemicals are extensively utilized within the food, cosmetic, pharmaceutical, and chemical industries. In particular, sorbitol was chosen as one of the 12 most value-added building block intermediate chemicals that can be produced from renewable biomass resources [38]. Sorbitol is also a platform chemical that can be converted into various other value-added chemicals such as isosorbide, propylene glycol, EG, and short-chain alkanes [34]. LA has also recently found use in food, pharmaceutical, leather, and textile industries [39]. Approximately 40% of produced LA is used to produce of polylactic acid (PLA). In addition, TPA and EG can be used as raw materials in PET production.
Various strategies for the conversion of cotton-based textile waste into new value-added products have been developed over the past decades [40]. However, previous researches mostly focused on the cotton-based textile wastes. Here, a novel textile waste-based biorefinery process for valorization of three types of textile waste is proposed: (i) cotton-based textile wastes, (ii) colored cotton-based textile wastes, and (iii) cotton-PET blended textile wastes (Fig. 1). Especially, an appropriate pretreatment method for each types of textile has been developed. It can be applied to convert various type of textile waste into a bio-based chemical such as bioethanol, lactic acid (LA), sorbitol, ethylene glycol (EG), and terephthalic acid (TPA) through an enzymatic hydrolysis and fermentation process. In addition, this process may contribute to the effective management of textile waste, and thereby to circular economy by promoting recycling and conversion of textile waste into valuable products such as biofuel and platform chemicals.
Fig. 1.
Overall process for value-added material production using various types of textile waste.
2. Materials and methods
2.1. Materials
A white cotton T-shirt, yellow cotton T-shirt, white towel, linen, denim, and blended cotton−PET textiles (cotton/PET ratios of 35:65, 50:50, and 70:30) were purchased from a local store. The cotton-based textile waste was made of 100% cotton. The textile wastes were cut into squares shape within a diameter of 2.0 cm. Sodium hydroxide (NaOH) and ethanol were purchased from Ducksan (Seoul, S. Korea). Sodium borohydride (NaBH4) was purchased from Sigma Aldrich. (St Louis, MO, USA). Analytical reagents including glucose, xylose, galactose, rhamnose, fructose, arabinose, mannose, D-sorbitol, and LA were purchased from Sigma Aldrich. Aqueous solutions were prepared with distilled deionized water. Two enzymes, in-house produced cellulase from Trichoderma reesei RUT C30 and recombinant WCCG, were used for enzymatic hydrolysis. The cellulase was used for the enzymatic hydrolysis of the cotton textile waste, while WCCG was used for the enzymatic hydrolysis of the PET textile waste.
2.2. Pretreatment of the textile waste
2.2.1. NaOH pretreatment of cotton-based textile waste
Cotton-based textile wastes samples (0.2 g) were treated with 20 mL of different concentrations of NaOH solution (0, 5, 10, or 15 wt%) at room temperature for different times (10, 20, 30, 60, or 120 min). NaOH solution of each concentration was prepared in advance before using because dissolution of NaOH in water is exothermic. After preparing the NaOH solution, it was allowed to cool to room temperature. Then, cotton was added to the prepared NaOH solution. After NaOH pretreatment, the treated cotton-samples were then filtered and washed with distilled water until the wash water reached a neutral pH. The pretreated cotton samples were used directly for enzymatic hydrolysis. The pretreatment efficiency was assessed by measuring the sugar yield from enzymatic hydrolysis using high-performance liquid chromatography (HPLC).
2.2.2. Hydrogen peroxide acetic acid pretreatment of colored cotton-based textile waste
The colored cotton-based textile waste samples were first treated with HPAC for decoloration [9]. The colored cotton-based samples (0.2 g) were soaked in acetic acid and 30% (wt%) hydrogen peroxide at 80 °C for 2 h. The ratio of hydrogen peroxide to acetic acid was 1:1 (v/v). The solution was filtered and washed with hot water at least five times. The HPAC-pretreated samples were then subjected to NaOH pretreatment.
2.2.3. NaOH−ethanol pretreatment of blended cotton−PET textile waste
Blended cotton−PET textile waste with three different ratios of cotton and polyester (30:70, 50:50, and 70:30) was tested. First, 0.2 g of each cotton−PET blend was treated with 20 mL of a solution containing 15% NaOH (w/v) and 60% EtOH (v/v) solution at 80 °C for 2 h. The reactor was then cooled with cold water for 5 min. The remaining textile residues were filtered, and the filtrate was precipitated by acidification with H2SO4 to a pH of 2.5. The precipitation was then filtered and produced TPA and EG were analyzed using proton nuclear magnetic resonance (1H NMR; Bruker 400 MHz spectrometer, Biospin, Rheinstetten, German 400 MHz).
2.3. Production of enzymes
2.3.1. Production of in-house cellulase
The T. reesei strain RUT-30 grown on potato dextrose agar plates was added to seven baffle flasks (3 L) each containing 1 L of potato dextrose broth. T. reesei (KCCM 11770) was obtained from the Korean Culture Center of Microorganisms (KCCM; Seoul, South Korea). Cultivation was carried out at 28 °C for 4 d under stirring in an orbital shaker at 150 rpm. The seed culture was transferred to a 100-L fermenter (Kobiotech, Korea) with a working volume of 70 L. The medium used for fermentation contained the following components: 4 g/L glucose, 2.8 g/L KH2PO4, 1.5 g/L MgSO4·7H2O, 3.8 g/L (NH4)2SO4, 2.6 g/L CaCl2, a 0.2% trace element solution (0.5% FeSO4·7H2O, 0.1% MnSO4·H2O, 0.14% ZnSO4·7H2O, and 0.2% CoCl2), 0.2% Tween 80, 20 g/L cellulose powder, and 0.5 g/L corn steep. The agitation speed and flow rate for aeration were set at 400 rpm and 1 vvm, respectively. The culturing temperature was 28°C and the pH was maintained at 4.8 using 30% ammonia water and 2 M phosphoric acid. After culturing for 8 d, the cells were removed using continuous centrifugation at 13,000 rpm and the enzyme activity was assayed based on an IUPAC process. Using the cellulase produced from T. reesei RUT-30, the optimal pH, temperature, and filter paper cellulose units (FPUs) were confirmed to be 5 and 50 °C and 19 FPU/mL, respectively.
2.3.2. Production of recombinant WCCG
The WCCG gene was commercially synthesized with codon optimization for expression in E. coli cells (Bioneer Co). Digested pBHA-WCCG with EcoRI and XbaI was inserted into a pColdI vector. pCold-WCCG was transformed into the E. coli Rosetta. The cells were grown in 3 mL of Luria−Bertaani (LB) broth with 100 μg/mL ampicillin at 37 °C for 14 h with stirring in an orbital shaker at 150 rpm. The seed culture was transferred into 500 mL of LB broth with 100 μg/mL ampicillin at 37 °C in a shaking incubator at 150 rpm for 4−6 h until cells reached an optical density at 600 nm (OD600) of 0.4–0.5. After the addition of isopropyl ß-d-1-thiogalactopyranoside to a final concentration of 1 mM, the culturing temperature was lowered to 15 °C and the cells were cultured for 24 h.
The cultured cells were centrifuged at 3500 rpm for 10 min and suspended in lysis buffer (20 mM Tris-HCl, pH 8.0, 500 mM NaCl). The cells were disrupted using sonication and the lysate clarified using centrifugation (13,000 rpm, 10 min, 4 °C). The soluble fraction was added to Ni-NAT agarose (Qiagen, USA) and unbound proteins were removed with washing buffer (20 mM Tris-HCl, pH 8.0, 500 mM NaCl, 20 mM imidazole). The bound proteins in the resin were eluted with elution buffer (20 mM Tris-HCl, pH 8, 250 mM imidazole). The eluted proteins were dialyzed with 20 mM Tris-HCl buffer (pH 8.0) and then used for analysis. To examine the enzyme purity, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was conducted.
2.4. Enzymatic hydrolysis
2.4.1. Hydrolysis of cotton-based textile waste before and after pretreatment
Enzymatic hydrolysis was conducted at a 1% (w/v) substrate loading in a conical tube (50 mL). The raw and pretreated cotton-based samples were soaked in 0.05 M sodium citrate buffer (pH 5.0) supplemented with 0.01% (w/v) sodium azide. RUT-30 cellulase (20 FPU cellulase per gram of substrate) was then added. The samples were then incubated at 45 °C, 120 rpm for 7 d, and the sugar levels were analyzed at 24-h intervals. The amount of sugar released into the hydrolysate was quantified using HPLC equipped with a refractive index detector (2414, Waters, Milford, CT, USA) and a Rezex RPM column (4.6 × 300 mm; Phenomenex, CA, USA) and a Rezex ROA-Organic acid H+ ion exclusion column (4.6 × 300 mm; Phenomenex, CA, USA). In the RPM column, HPLC-grade water was supplied at a flow rate of 0.6 mL/min as the mobile phase at a controlled temperature of 80 °C. In the ROA column, 0.005 N H2SO4 was supplied at a flow rate of 0.6 mL/min as the mobile phase at a controlled temperature of 80 °C. All enzymatic hydrolysis experiments were conducted in triplicate.
2.4.2. Hydrolysis of blended cotton−PET textile waste
In order to evaluate the enzymatic hydrolysis on blended cotton−PET textile waste, the NaOH−ethanol pretreated cotton−PET sample was loaded into a conical tube (50 mL) containing 20 mL of 0.05 M sodium citrate buffer (pH 5.0) with RUT-30 cellulase and WCCG. The tube was then incubated at 37 °C. Control reactions with PETase were carried out under the same conditions used for each experiment. Commercial Novozym© 51032 was used as the PETase source.
2.5. Production of bioethanol: separate hydrolysis and fermentation
Enzymatic hydrolysis was conducted in a 50 mL conical tube with a total working volume of 10 mL with 2% (w/v) NaOH-pretreated cotton, 0.1% (w/v) yeast extract, 0.2% (w/v) peptone, and 0.05 M citrate buffer (pH 5.0). Saccharification was performed at 45 °C for 7 d with agitation at 200 rpm. After enzymatic hydrolysis, 0.1% (w/v) dry yeast was added as inoculum to 7.0 mL hydrolyzates. Fermentation was performed at 32 °C for 24 h. For comparison, 2% glucose was also fermented under the same conditions. HPLC (Rezex ROA-Organic acid H+ ion exclusion column) was used to analyze the glucose consumption and the ethanol content. The ethanol conversion yield (%) was calculated based on the glucose content by dividing the quantity of ethanol produced by the total amount of glucose.
2.6. Production of lactic acid
2.6.1. Strain and seed culture
For the production of LA, Actinobacillus succinogenes ATCC 55618 was taken from the American Type Culture Collection. A. succinogenes was added to 10 mL trypticase soy broth at 200 rpm and 37 °C for 24 h.
2.6.2. Lactic acid production
LA production was conducted in a 500-mL Erlenmeyer flask with a total working volume of 100 mL of hydrolysate with 1.5% (w/v) yeast extract, 0.3% (w/v) KH2PO4, 0.15% (w/v) K2HPO4, 0.3% (w/v) (NH4)2PO4, 0.1% (w/v) NaCl, 0.03% (w/v) MnCl2∙6H2O and 0.03% (w/v) CaCl2∙2H2O. The pre-cultured cells were added to the LA production medium and suspended at 200 rpm and 37 °C for 24 h. Anaerobic conditions were maintained with the addition of MgCO3 (1:10 solid-to-liquid ratio). LA was analyzed using an HPLC system (Waters, Millipore Co., Milford, MA). A Rezex ROA-organic acid H+ ion exclusion column (4.6 × 300 mm; Phenomenex, CA, USA) was used with 0.005 N H2SO4 as the mobile phase at a flow rate of 0.6 mL/min, while the column temperature was maintained at 80 °C.
2.7. Production of sorbitol
Sorbitol was produced using a chemical method based on the catalytic hydrogenation of glucose [41]. Sodium borohydride (0.2 g) in water (10 mL) was added to the biosugar solution (20 mL) derived from the white t-shirt at room temperature (RT) for 4 h. After completion of the reduction, the reaction mixture was acidified with 3 N HCl to remove the excess sodium borohydride and evaporated to dryness in vacuo. The liquid products were analyzed using HPLC (Waters, Milford, CT, USA) equipped with an RI detector and a ROA column. The eluent was water with a flow rate of 0.6 mL/min. The column was maintained at 80 °C with a column heater.
2.8. Analyses
2.8.1. Nuclear magnetic resonance analysis
Solid state 13C NMR spectroscopy utilizing cross-polarization magic-angle spinning was performed using a Bruker DMX-400 MHz NMR spectrometer.
2.8.2. Microscopic analysis
To observe the differences in the surface morphology between the untreated and NaOH-pretreated cotton samples, a stereomicroscope (Leica, Stereozoom S9D, Switzerland) and optical microscopy (Olympus, Model BX41TF, Japan) were employed.
3. Results and discussion
3.1. Cotton-based textile waste: white cotton samples
3.1.1. NaOH pretreatment of cotton-based textile waste
Alkali pretreatment (often referred to as mercerization) is a well-known method for cellulose modification [42]. The mercerization process affects the twisting and swelling of cellulose fibers [43]. Aqueous alkali-based solvents such as NaOH, potassium hydroxide (KOH), and lithium hydroxide (LiOH) are often used for mercerization of cotton fibers [44,45]. Here, white cotton samples were pretreated with NaOH. The textile wastes were treated at room temperature with 0–15% NaOH for 0–120 m. The treated textile wastes remained solid in the solution, but swelled, where more than 97% of initial materials were recovered after the NaOH pretreatment.
First, the effect of dry and wet NaOH pretreatment procedures on enzymatic hydrolysis was investigated (Fig. S1). The results showed great effects of wet process on the rate of enzymatic hydrolysis. Using the dry process did not lead to any significant difference between the enzymatic hydrolysis of treated and untreated cotton samples. On the other hand, wet pretreatment process proved to be more effective in this regard. Similar results were also found for natural-, heat-, and freeze-dried samples (data not shown). This may be attributed to change in the cellulose structure during the drying process. Upon treatment with NaOH solution, the Na+ ions penetrated the intra-crystalline spaces within cellulose I, and cause the cellulose structure to swell and form Na-cellulose structures (Fig. S1). Following washing and drying, Na-cellulose undergoes irreversible structural transformation to form cellulose II [46]. Hence, wet process after NaOH pretreatment is a key factor in obtaining higher reducing-sugar yields from cotton-based textile wastes.
Next, the effects of NaOH concentration and treatment time on hydrolysis efficiency were investigated. Enzymatic hydrolysis efficiency was found to increase with increasing NaOH concentration (Fig. 2a). The highest reducing-sugar yield was obtained with 15 wt% NaOH. On the other hand, enzymatic hydrolysis efficiency did not improve significantly with treatment times longer than 30 min. Thus, NaOH pretreatment with 15% NaOH at room temperature for 30 min was determined to be optimal for the enzymatic degradation of cotton-based textile waste. A maximum glucose concentration of 19.82 mg/mL was achieved after 5 days of enzymatic hydrolysis. In addition, a percentage of theoretical glucose yield of 99.1% was reached. Jeihanipour and Taherzadeh reported similar results with these results. They obtained a glucose yield of 99.1% after 4 days in 12 wt% NaOH at 0 °C for 3 h [47].
Fig. 2.
(a) Effect of NaOH concentration on hydrolysis efficiency (b) Effect of pretreatment time on hydrolysis efficiency.
3.1.2. Morphological changes in cotton-based textile waste upon NaOH pretreatment
The morphologies of cotton-based textile samples before and after the NaOH pretreatment were investigated using optical microscopy (Fig. 3). Analyses were conducted on five types of cotton-based textile materials: a white T-shirt, a yellow T-shirt, linen, denim, and white towel. All materials featured pores between the yarns (i.e., inter-yarn pore) and pores between the fibers within a yarn (i.e., intra-yarn pore). The length and width of the inter-yarn pores in linen waste were 0.09 mm and 0.06 mm, respectively before NaOH pretreatment. The pores largely disappeared upon NaOH pretreatment. Furthermore, the pore size of the yarn also changed from 0.05–0.07 mm to 0.12–0.14 mm. Other cotton-based samples yielded similar results, which were most likely due to the swelling of the cotton fibers during NaOH pretreatment.
Fig. 3.
(a) Optical microscope images of cotton-based textile waste before and after NaOH pretreatment (N-pretreatment). (b) Inter-and intra-yarn pores in the fabric.
3.1.3. Enzymatic hydrolysis of cotton-based textile waste
High glucose concentrations during enzymatic hydrolysis are necessary to obtain high yields of value-added materials, such as ethanol, sorbitol, and LA. In-house cellulase produced from Trichoderma reesei RUT-C30 was used for the enzymatic hydrolysis of NaOH-pretreated cotton-based samples. Glucose was found to be the main sugar in the hydrolysate of cotton-based samples (Fig. 4a and Fig. S2). A maximum glucose concentration of 19.82 mg/mL was achieved after 5 d of hydrolysis with the white T-shirt sample, followed by white towel (19.68 mg/mL), yellow T-shirt (19.27 mg/mL), linen (18.43 mg/mL), and denim (12.59 mg/mL). Glucose concentrations were found to decrease significantly with the dyed cotton-based samples (yellow T-shirt, linen, and denim). This finding may be attributed to the inhibition effect of the dye molecules on cellulase. Indeed, low levels of cellulase activity due to the presence of dye molecules or interactions between dye molecules and cellulose have been reported in previous studies [48,49].
Fig. 4.
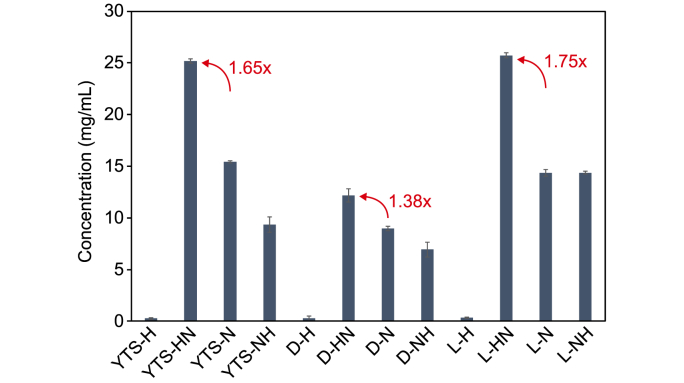
(a) Enzymatic hydrolysis of NaOH-pretreated cotton-based textile waste. (b) Hydrolysis of mixed cotton-based samples. (c) Hydrolysis of colored cotton-based textile waste with different pretreatments procedures. WTS: white T-shirt; YTS: yellow T-shirt; L: linen; D: denim. H: HPAC, N: NaOH, HN: HPAC-NaOH, NH: NaOH-HPAC.
Hydrolysis of mixtures of cotton-based samples was also investigated. Fig. 4b and Fig. S3 show enzymatic hydrolysis results after 24 h for the white T-shirt sample mixed with each of the other three samples in the same amount and ratios, and for all four samples. Accordingly, the hydrolysis efficiency for the mixed textile samples was found to be lower than that of white T-shirt alone. Despite the low yields obtained, these results demonstrated the potential of employing enzymatic hydrolysis on mixed cotton-based textile waste.
3.1.4. Analysis of cotton fiber size during enzymatic hydrolysis
Morphological changes in the white T-shirt sample during enzymatic hydrolysis were observed. First, the size of the sample was monitored after 24 h of cellulase treatment. The size of the sample was found to decrease over time, and the sample reduced to powdery form from its initial square shape (diameter = 2.0 cm) (Fig. 5). Powdered form of hydrolyzed cellulose fibers was observed under an optical microscope, and the size of the fibers was also found to decrease over time. The fiber size was smaller than about 50 μm after 4 d. Thus, in-house cellulase effectively hydrolyzed cotton-based textile waste.
Fig. 5.
Hydrolysis of cotton-based textile samples over time.
3.2. Cotton-based and colored cotton-based textile waste
3.2.1. Pretreatment of colored cotton-based textile waste
As demonstrated in the previous section, the yellow T-shirt sample was found to yield a lower concentration of glucose compare to the white T-shirt sample during enzymatic hydrolysis. HPAC pretreatment was performed to increase the enzymatic hydrolysis efficiency of colored cotton-based textile waste. The effect of HPAC pretreatment applied in different stages of the procedure on the enzymatic hydrolysis of colored cotton-based textile waste was investigated. Fig. 6 shows enzymatic hydrolysis results of yellow T-shirt, linen, and denim samples pretreated with only NaOH, only HPAC, NaOH followed by HPAC (NaOH−HPAC), and HPAC followed by NaOH (HPAC−NaOH). The yellow T-shirt turned white in color after HPAC pretreatment (Fig. S4), whereas the linen and denim samples turned pale blue. Despite the removal of color, cellulose content of the colored cotton-based samples did not readily decompose into glucose upon HPAC pretreatment only. Furthermore, the hydrolysis efficiency of the samples pretreated with NaOH–HPAC was lower than that of NaOH-pretreated samples. However, sugar yield from the HPAC−NaOH-pretreated yellow T-shirt samples was 1.65 times higher than that from the NaOH-pretreated yellow T-shirt samples. Similarly, sugar yield from HPAC−NaOH-pretreated denim and linen samples was 1.38 and 1.75 times higher than their respective NaOH-pretreated counterparts (Fig. S5). Hence, HPAC pretreatment for the removal of dye materials prior to enzymatic hydrolysis of colored cotton-based textile waste is necessary, as it increases the digestibility of the cellulose.
Fig. 6.
Hydrolysis of colored cotton-based textile waste with different pretreatments procedures. WTS: white T-shirt; YTS: yellow T-shirt; L: linen; D: denim. H: HPAC, N: NaOH, HN: HPAC-NaOH, NH: NaOH-HPAC.
HPAC pretreatment also has the advantage of decomposing of dye materials, which are considered the major water contaminants in textile industry. In case of yellow T-shirts, the color of yellow T-shirt was lightened from yellow to white during the HPAC pretreatment. In addition, the color of the reaction solution gradually changed from colorless to light yellow, and it changed again to colorless within about 1 h. It means that the dye is degraded into lighter compounds.
To confirm the degradation of dyeing agents by HPAC pretreatment, indigo carmine (IC) which is widely used in the dyeing of denim was used. The chromophore center of indigo carmine consists of a C C double bond substituted by two NH donor groups and two CO acceptor groups [50]. When the cleavage of the –C C− bonds occurs, the intensity of the absorbance peak of the polluted solution decreases. As shown in Fig. S6, the characteristic peaks of indigo carmine are situated at 610 nm and 332 nm in the UV–visible absorption spectrum [51]. The peak at 610 nm, which accounts for the blue color of indigo carmine, disappeared after the HPAC treatment. The results suggested the cleavage of the chromophoric structure of the dye. Therefore, the results also showed that HPAC pretreatment was very effective in degradation of dye materials.
3.3. Production of value-added products from cotton-based textile waste
3.3.1. Production of bioethanol
We next investigated bioethanol production from sugars derived from cotton-based textile waste. For this purpose, ethanol was produced using a separate hydrolysis and fermentation (SHF) process. After 24 h of fermentation, ethanol conversion yields from the white T-shirt, yellow T-shirt, linen, denim, and white towel samples were 83.5, 78.0, 75.7, 64.0, and 82.3%, respectively. The yields were based on the theoretical maximum based on glucose levels only (Fig. 7a and Fig. S7). These findings indicate that cotton-based textile waste may be a viable biomass source for bioethanol production, as it lowers ethanol production costs. These results were also compared to those obtained using pine wood, oak wood, and rice straw as reference materials (Table 2). The maximum ethanol yield was achieved with the white T-shirt sample (537 mL/kg substrate), which was 3.2, 2.1, and 2.6 times higher than those obtained with rice straw, pine wood, and oak wood, respectively [9]. Thus, cotton-based textile waste can be used to produce bioethanol much more efficiently compared to other lignocellulosic biomasses (see Table 3).
Fig. 7.
(a) Production of bioethanol from NaOH-pretreated cotton-based textile waste. (b) Production of sorbitol from white T-shirt-derived hydrolysate. (c) Production of lactic acid (LA) from white T-shirt-derived hydrolysate.
Table 2.
Pretreatment conditions for a variety of dyed textile wastes.
| Textile waste | Pretreatment condition | Enzymatic hydrolysis Yield (%) | Reference |
|---|---|---|---|
| Dyed cotton (100% cotton) | Ionic liquid [AMIM]Cl, 110 °C, 75min | 80.0 | [21] |
| Blue Jean (100% cotton) | Shredded and grounded + 12% NaOH, 0 °C, 96 h | 85.0 | [22] |
| Blue Jean (blend cotton/PET) | Milled + H3PO4, 85%, 50 °C, 7 h | 79.0 | [23] |
| Dyed blend cotton/PET (60/40) | Shredded + Pressure + Na2CO3, 0.5 M, 150 °C, 72 h | 88.0 | [24] |
Table 3.
Comparison of bioethanol production from various biomass types.
| Biomass | Amount of glucose/1 kg biomass (kg) | Conversion yield of glucose to ethanol (%) | Bioethanol production (from 1 kg biomass) (mL) | Reference |
|---|---|---|---|---|
| Rice straw | 413 | 86.4 | 166 | [9] |
| Pine wood | 458 | 84.8 | 253 | |
| Oak wood |
425 |
83.1 |
204 |
|
| White T-shirt | 991 | 83.5 | 537 | This work |
| Yellow T-shirt | 963 | 78.0 | 402 | |
| Linen | 921 | 75.7 | 487 | |
| Denim | 629 | 64.0 | 411 | |
| White towel | 984 | 82.3 | 502 |
3.3.2. Production of sorbitol
Sorbitol is considered a major building block chemical due to its versatile characteristics. Here, sorbitol was produced via hydrogenation of glucose solutions obtained via hydrolysis of the cotton-based textile waste (Fig. 7b). Glucose was thus selectively converted into sorbitol at a yield of 70% with a glucose conversion rate of 85.3%. The results found here are higher yields than those obtained by Ribeiro et al., which produced sorbitol with 45% of yield from cotton textile using ball-milling pretreatment and a Ru/CNT catalyst [52]. Hence, cotton-based textile waste was demonstrated to show potential for sorbitol production as well.
3.3.3. Production of LA from hydrolysate
The biological synthesis of LA has attracted considerable interest due to its application potential in various industries [53]. LA has been used specifically as a monomer in the production of biodegradable and biocompatible PLA polymers [54]. Despite the advantages of biological LA production, inexpensive substrates are still needed to ensure industrial feasibility [55]. The majority of LA-producing microorganisms belong to the genera Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, and Rhizopus. Actinobacillus succinogenes is the most often used species for the production of succinic acid, but it has also been reported to have high productivity for LA as well [56]. Here, A. succinogenes ATCC 55618 was used to produce LA from the hydrolysate of cotton-based textile waste. The final LA concentration was higher than 12.3 mg/mL after 36 h with a yield of over 83.67% (Fig. 7c). There are many studies on LA production using various biomass such as food waste [57], bakery waste [58], rice straw, and cassava bagasse [59], but there are no studies using textile wastes yet. This finding demonstrates that cotton-based textile waste may also be used for LA production, and enhance the economic feasibility of modern biorefineries.
3.4. Blended cotton–PET textile waste
3.4.1. NaOH−ethanol pretreatment of blended cotton−PET textile waste
NaOH pretreatment was first performed as described above to degrade blended cotton−PET textile waste as well. However, the blended cotton−PET sample showed poor digestibility (Fig. 8 and Fig. S8). Therefore, NaOH−ethanol pretreatment was conducted instead to effectively remove PET from textile waste samples, and thereby to enhance enzymatic hydrolysis. For this purpose, blended cotton−PET samples were pretreated using 15% NaOH and 60% ethanol at 80 °C for 2 h. Rates of solid recovery and loss of lignin were calculated using the following equations:
| (1) |
| (2) |
where w is the dry weight of the pretreated blended cotton−PET textile waste, w0 is the initial dry weight of the untreated blended cotton−PET textile waste, and C is the initial PET content (%) in the untreated blended cotton−PET textile waste.
Fig. 8.
(a) Comparison of the efficiency of NaOH (N) and NaOH–ethanol (NE) pretreatment with blended cotton–polyethylene terephthalate (PET) textile waste. (b) Images of NaOH– and NaOH–ethanol-pretreated cotton–PET samples after enzymatic hydrolysis. (c) Optical microscope images of blended cotton–PET samples before and after NaOH–ethanol pretreatment.
The results indicated that 86.51% of the PET was removed, and the solid recovery yield was 56.74%. The compounds in the reaction solution were also investigated using 1H NMR (Fig. S9). Singlets at 7.6 and 3.6 ppm corresponded to TPA and EG, respectively. Thus, PET taken from the blended cotton−PET samples was degraded into its monomers TPA and EG, which can be repolymerized to obtain PET for reuse as a high value-added material.
The samples were also analyzed using 13C solid-state NMR (Fig. S10) following NaOH−ethanol pretreatment. Both cellulose and PET peaks were observed for untreated cotton−PET samples. However, the peak corresponding to the PET component disappeared after the NaOH−ethanol pretreatment, and only signals corresponding to cellulose were observed between δ 60 and δ 110 ppm. Thus, PET in the raw cotton−PET samples was clearly broke down during NaOH−ethanol pretreatment.
3.4.2. Optical microscope analysis
The removal of PET upon NaOH−ethanol pretreatment was also investigated using an optical microscope. The textile samples were stained with safranin, which is used to detect cellulose. As expected, only cellulose fibers remained in the sample upon NaOH−ethanol pretreatment, confirming the separation of cotton from PET (Fig. 8c). The optical microscope images showed the cellulose in red, whereas PET was colorless. These results clearly indicate that PET was effectively removed by NaOH−ethanol pretreatment.
3.4.3. Enzymatic hydrolysis of NaOH−ethanol pretreated blended cotton−PET textile waste
Cotton−PET samples pretreated with NaOH–ethanol were used as the substrate for enzymatic hydrolysis using the in-house produced cellulase (20 FPU/g substrate). The samples showed good hydrolysis efficiency following PET removal via NaOH–ethanol pretreatment, with a glucose yield of 92.49%. In addition, glucose yields of 17.70% and 74.21% were obtained for cotton−PET ratios of 35:65 and 70:30, respectively. Thus, NaOH−ethanol pretreatment enhanced enzymatic hydrolysis of blended cotton−PET textile waste.
3.5. Biological decomposition of blended cotton−PET textile waste
Biological degradation of blended cotton−PET textile waste is considered more versatile and environment-friendly compared to chemical conversion procedures. Tournier et al. previously developed the WCCG PET hydrolase, which is a leaf−branch compost cutinase (LCC) variant [60]. The WCCG PET hydrolase releases several intermediates and end products including BHET, MHET, TPA, and EG during PET degradation. WCCG hydrolase was first produced to degrade blended cotton−PET textile waste. Then, cellulase and WCCG hydrolase were mixed to investigate the synergetic relationship between these enzymes during the enzymatic hydrolysis of blended cotton−PET textile waste. PETase was also used for comparison purposes. All enzymes were mixed at a ratio of 50:50. The results indicated that hydrolysis efficiency of the blended cotton−PET samples was significantly increased upon addition of WCCG, and a higher glucose yield was obtained (Fig. 9). The most effective enzyme combination was cellulase and PETase. Despite its lower efficiency compared to PETase, WCCG hydrolase was demonstrated to be a suitable alternative for the hydrolysis of blended cotton−PET textile waste.
Fig. 9.
(a) HPLC spectra of mixing enzymes on the enzymatic hydrolysis of untreated and NaOH–ethanol pretreated blended cotton–PET samples. (b) Schematization of the HPLC results.
4. Conclusions
Our results demonstrated that textile waste can be successfully used as a feedstock for the production of several valuable products, including bioethanol, sorbitol, LA, TPA, and EG. The developed NaOH, HPAC–NaOH, and NaOH–ethanol pretreatment were very effective for cotton, colored cotton, and blended cotton–PET, respectively. The sugar content produced via enzymatic hydrolysis was then converted into key platform chemicals (bioethanol, sorbitol, and lactic acid) via fermentation or hydrogenation. TPA and EG were produced from blended cotton−PET via NaOH–ethanol pretreatment. Using textile waste as an alternative feedstock for the production of value-added materials can thus be considered as an alternative strategy for the production of value-added chemicals, and also offers a promising solution to environmental and energy issues related to the generation of textile waste.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education NRF-2022R1A2C10028591140982119420101 and 2020R1I1A1A01061751). This work was also supported by the IBCT project (2021-0083) funded by the Tan Tao Group (TTG), Vietnam.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2023.100238.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bick R., Halsey E., Ekenga C.C. The global environmental injustice of fast fashion. Environ. Health. 2018;17:92. doi: 10.1186/s12940-018-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niinimäki K., Peters G., Dahlbo H., Perry P., Rissanen T., Gwilt A. The environmental price of fast fashion. Nat. Rev. Earth Environ. 2020;1:189–200. [Google Scholar]
- 3.Shani H., Chopra N. In: Crowther D., Quoquab F., editors. vol. 16. Emerald Publishing Limited; Bingley: 2020. CSO and SDG mapping in fashion & textile industry: identifying potential challenges in the wake of isolationism; pp. 221–245. (CSR in an Age of Isolationism (Developments in Corporate Governance and Responsibility). [DOI] [Google Scholar]
- 4.Ütebay B., Çelik P., Çay A. In: . Textile Wastes: Status and Perspective. Köriü A., editor. IntechOpen; 2020. Waste in textile and leather sectors; pp. 39–58.http://ds.doi/10.5772/inrechopen,90014 Chapter 3. [Google Scholar]
- 5.Mather R.R., Wardman R.H. second ed. Royal Society of Chemistry; Cambridge: 2011. The Chemistry of Textile Fibres. [Google Scholar]
- 6.Zhang S., Zhang F., Jin L., Liu B., Mao Y., Liu Y. Preparation of spherical nanocellulose from waste paper by aqueous NaOH/thiourea. Cellulose. 2019;26:5177–5185. [Google Scholar]
- 7.Isikgor F.H., Becer C.R. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015;6:4497–4559. [Google Scholar]
- 8.Cho E.J., Trinh L.T.P., Song Y., Lee Y.G., Bae H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020;298 doi: 10.1016/j.biortech.2019.122386. [DOI] [PubMed] [Google Scholar]
- 9.Wi S.G., Cho E.J., Lee D.S., Lee S.J., Lee Y.J., Bae H.-J. Lignocellulose conversion for biofuel: anew pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol. Biofuels. 2015;8:228. doi: 10.1186/s13068-015-0419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malherbe A., Cloete T.E. Lignocellulose biodegradation: fundamentals and applications. Rev. Environ. Sci. Biotechnol. 2002;1:105–114. [Google Scholar]
- 11.Howard R.L., Abotsi E., Jasen van Rensburg E.L., Howard S. Lignocellulose biotechnology: issues of bioconversion and enzyme production. Aft. J. Biotechnol. 2003;2:602–619. [Google Scholar]
- 12.Wakade G., Lin S., Saha P., Kumari U., Daniell H. Abatement of microfibre pollution and detoxification of textile dye-indigo by engineered plant enzymes. Plant Biotechnol. J. 2022:1–15. doi: 10.1111/pbi.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioelovich M., Leykin A. Structural investigations of various cotton fibers and cotton celluloses. BioResource. 2008;3:170–177. [Google Scholar]
- 14.Yue Y., Han G., Wu Q. Transitional properties of cotton fibers from cellulose I to cellulose II structure. Bioresources. 2013;8:6460–6471. [Google Scholar]
- 15.Cai J., Zhang L. Rapid dissolution of cellulose in LiOH/Urea and NaOH/Urea aqueous solutions. Macromol. Biosci. 2005;5:539–548. doi: 10.1002/mabi.200400222. [DOI] [PubMed] [Google Scholar]
- 16.Sun B., Peng G., Duan L., Xu A., Li X. Pretreatment by NaOH swelling and then HCl regeneration to enhance the acid hydrolysis of cellulose to glucose. Bioresour. Technol. 2015;196:454–458. doi: 10.1016/j.biortech.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Okano T., Sarko A. Mercerization of cellulose II. Alkali-cellulose intermediates and a possible mercerization mechanism. J. Appl. Polym. Sci. 1985;30:325–332. [Google Scholar]
- 18.Nikolić S., Lazić V., Veljović Ð., Mojović L. Production of bioethanol from pre-treated cotton fabrics and waste cotton materials. Carbohydr. Polym. 2017;164:136–144. doi: 10.1016/j.carbpol.2017.01.090. [DOI] [PubMed] [Google Scholar]
- 19.Castro E., Avellaneda A., Marco P. Combination of advanced oxidation processes and biological treatment for the removal of benzidine-derived dyes. Environ. Progress Sustain. Energy. 2014;33:873–885. [Google Scholar]
- 20.Cavaco-Paulo A., Almeida L. Cellulase hydrolysis of cotton cellulose: the effects of mechanical action, enzyme concentration and dyed substrates. Biocatal. Biotransform. 1994;10:353–360. [Google Scholar]
- 21.Guo X., Chen L., Tang J., Jonsson L.J., Hong F.F. Production of bacterial nanocellulose and enzyme from [AMIM]Cl-pretreated waste cotton fabrics: effects of dyes on enzymatic saccharification and nanocellulose production. J. Chem. Technol. Biotechnol. 2016;91:1413–1421. [Google Scholar]
- 22.Jeihanipour A., Taherzadeh M.J. Ethanol production from cotton-based waste textiles. Bioresour. Technol. 2009;100:1007–1010. doi: 10.1016/j.biortech.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Shen F., Xiao W., Lin L., yang G., Zhang Y., Deng S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour. Technol. 2013;130:248–255. doi: 10.1016/j.biortech.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Hasanzadeh E., Mirmohamadsadeghi S., Karimi K. Enhancing energy production from waste textile by hydrolysis of synthetic parts. Fuel. 2018;218:41–48. [Google Scholar]
- 25.Jeyakodi Moses J., Pitchai S. A study on the dyeing of sodium hydroxide treated polyester/cotton blend fabrics. Int. J. Sci. Technol. Soc. 2015;3:1–9. [Google Scholar]
- 26.Park S.H., Kim S.H. Poly (ethylene terephthalate) recycling for high value-added textiles. Fashion Textiles. 2014;1:1–17. http://link.springer.com/article/10.1186/s40691-014-0001-x [Google Scholar]
- 27.De Falco F., Pace E.D., Cocca M., Avella M. The contribution of washing process of synthetic clothes to microplastic pollution. Sci. Rep. 2019;9:6633. doi: 10.1038/s41598-019-43023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu W.-M., Yang J., Criddle C.S. Microplastics pollution and reduction strategies. Front. Environ. Sci. Eng. 2017;11:6. [Google Scholar]
- 29.Almulhim A.I., Ahmad I., Sarkar S., Chavali M. Consequences of COVID-19 pandemic on solid waste management: scenarios pertaining to developing countries. Remediation (NY) 2021;31:111–121. doi: 10.1002/rem.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen F., xiao W., Lim L., yang G., Zhang Y., Deng S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour. Technol. 2013;130:248–255. doi: 10.1016/j.biortech.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z., Qi R., Xiong M., Zhang D., Gu H., Chen W. Conversion of cotton textile waste to clean solid fuel via surfactant-assisted hydrothermal carbonization: mechanisms and combustion behaviors. Bioresour. Technol. 2021;321 doi: 10.1016/j.biortech.2020.124450. [DOI] [PubMed] [Google Scholar]
- 32.Bozell J.J., Petersen G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy's “Top 10” revisited. Green Chem. 2010;12:539–554. [Google Scholar]
- 33.Kusserow B., Schimpf S., Claus P. Hydrogenation of glucose to sorbitol over nickel and ruthenium catalysts. Adv. Synth. Catal. 2003;345:289–299. [Google Scholar]
- 34.Yu S., sun J., Shi Y., Wang Q., Wu J., Liu J. Nanocellulose from various biomass wastes: its preparation and potential usages towards the high value-added products. Environ. Sci. Ecotechnol. 2021;5 doi: 10.1016/j.ese.2020.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi A., Song C.W., Shin J.H., Lee S.Y. Biorefineries for the production of top building block chemicals and their derivatives. Metab. Eng. 2015;28:223–239. doi: 10.1016/j.ymben.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Oke M.A., Annuar M.S.M., Simarani K. Mixed feed stock approach to lignocellulosic ethanol production-Prospects and limitations. Bioenerg. Res. 2016;9:1188–1203. [Google Scholar]
- 37.Pascoli D.U., Suko A., Gustafson R., Gough H.L., Bura R. Novel ethanol production using biomass preprocessing to increase ethanol yield and reduce overall costs. Biotechnol. Biofuels. 2021;14:9. doi: 10.1186/s13068-020-01839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia B., Moreno J., Morales G., Melero J.A., Iglesias J. Production of sorbitol via catalytic transfer hydrogenation of glucose. Appl. Sci. 2020;10:1843. [Google Scholar]
- 39.Mathew A.K., Abraham A., Mallapureddy K.K., Sukumaran R.K. Lignocellulosic biorefinery wastes, or resources?Waste Biorefinery. Potential and Perspectives. 2018:267–297. Chapter 9. [Google Scholar]
- 40.Haslinger S., Wang Y., Rissanen M., Lossa M.B., Tanttu M., Ilem E., Maattanen M., H A., Hummel M., Sixta H. Recycling of vat and reactive dyed textile waste to new colored man-made cellulose fibers. Green Chem. 2019;21:5598–5610. [Google Scholar]
- 41.Abdek-Akher M., Hamilton J.K., Smith F. The reduction of sugars with sodium borohydride. J. Am. Chem. Soc. 1951;73:4691–4692. [Google Scholar]
- 42.Ferro M., Mannu A., Panzeri W., Theeuwen C.H.J., Mele A. An integrated approach to optimizing cellulose mercerization. Polymers. 2020;12:1559. doi: 10.3390/polym12071559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yue Y., Han G., Wu Q. Transitional properties of cotton fibers from cellulose I to cellulose II structure. Bioresources. 2013;8:6460–6471. [Google Scholar]
- 44.Cai J., Zhang L. Rapid dissolution of cellulose in LiOH/Urea and NaOH/Urea aqueous solutions. Macromol. Biosci. 2005;5:539–548. doi: 10.1002/mabi.200400222. [DOI] [PubMed] [Google Scholar]
- 45.Sun B., Peng G., Duan L., Xu A., Li X. Pretreatment by NaOH swelling and then HCl regeneration to enhance the acid hydrolysis of cellulose to glucose. Bioresour. Technol. 2015;196:454–458. doi: 10.1016/j.biortech.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Okano T., Sarko A. Mercerization of cellulose II. Alkali-cellulose intermediates and a possible mercerization mechanism. J. Appl. Polym. Sci. 1985;30:325–332. [Google Scholar]
- 47.Jeihanipour A., Taherzadeh M.J. Ethanol production from cotton-based waste textiles. Bioresour. Technol. 2009;100:1007–1010. doi: 10.1016/j.biortech.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Nikolić S., Lazić V., Veljović Ð., Mojović L. Production of bioethanol from pre-treated cotton fabrics and waste cotton materials. Carbohydr. Polym. 2017;164:136–144. doi: 10.1016/j.carbpol.2017.01.090. [DOI] [PubMed] [Google Scholar]
- 49.Uddin M.G. Effect of biopolishing on dye ability of cotton fabric-A review. Trends Green Chem. 2016;2:1–5. [Google Scholar]
- 50.Vautier M., Guillard C., Herrmann J.M. Photocatalytic degradation of dyes in water: case study of indigo and of indigo carmine. J. Catal. 2001;201:46–59. [Google Scholar]
- 51.Wang J., Lu L., Feng F. Improving the indigo carmine decolorization ability of a bacillus amyloliquefaciens laccase by site-directed mugagenesis. Catalysts. 2017;7:275–285. [Google Scholar]
- 52.Ribeiro L.S., de Melo Órfād J.J., Peeira M.F.R. Direct catalytic production of sorbitol from waste cellulose materials. Bioresour. Technol. 2017;232:152–158. doi: 10.1016/j.biortech.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Panesar P.S., Kennedy J.F., Gandhi D.N., Bunko K. Bioutilisation of whey for lactic acid production. Food Chem. 2007;105:1–14. [Google Scholar]
- 54.Lopes M.S., Jardini A.L., Filho R.M. Poly(lactic acid) production for tissue engineering applications. Procedia Eng. 2012;42:1402–1413. [Google Scholar]
- 55.Lim L.T., Auras R., Rubono M. Processing technologies for poly(lactic acid) Prog. Polym. Sci. 2008;33:820–852. [Google Scholar]
- 56.Wang C., Li Q., Wang D., Xing J. Improving the lactic acid production of Actinobacillus succinogenes by using a novel fermentation and separation integration system. Process Biochem. 2014;49:1245–1250. [Google Scholar]
- 57.Song L., Yang D., Liu R., Liu S., Dai L., Dai X. Microbial production of lactic acid from food waste: latest advances, limits, and perspectives. Bioresour. Technol. 2022;345 doi: 10.1016/j.biortech.2021.126052. [DOI] [PubMed] [Google Scholar]
- 58.Kwan T.H., Hu Y., Lin C.S.K. Valorisation of food waste via fungal hydrolysis and lactic acid fermentation with Lactobacillus casei Shirota. Bioresour. Technol. 2016;217:129–136. doi: 10.1016/j.biortech.2016.01.134. [DOI] [PubMed] [Google Scholar]
- 59.Chen H., Chen B., Su Z., Wang K., Wang B., Wang Y., Si Z., Wu Y., Cai D., Qin P. Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind. Crop. Prod. 2020;146 [Google Scholar]
- 60.Tournier V., Topham C.M., Gilles A., David B., Folgoas C., Moya-Leclair E., Kamionka E., Desrousseaux M.L., Texier H., Gavalda S., Cot M., Guemard E., Dalibey M., Nomme J., Cioci G., Barbe S., Chateau M., Andre I., Duquesne S., Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020;580:216–219. doi: 10.1038/s41586-020-2149-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.