Abstract
Beta-alanine is an important amino acid involved in several metabolic reactions in the body. The study aimed to investigate the effect of β-alanine supplementation on intestinal development and the immune performance of weaned piglets. Thirty-two 21-day-old healthy weaned piglets (half female and half male; Duroc × Landrace × Yorkshire) with an initial body weight of 8.11 ± 0.21 kg were randomly divided into 4 groups with 8 replicates of 1 pig each. The control group was fed a basal diet and the three experimental treatment groups were fed diets supplemented with 300, 600 and 1,200 mg/kg β-alanine, respectively. The trial lasted 28 days and the diets fed were divided into 2 phases: the late lactation period (day 1 to 14) and the nursery period (day 15 to 28), during which the weaned piglets had free access to food and water. The regulatory effects of β-alanine were further investigated in vitro using organoids obtained from the jejunum of piglets. In vivo, the addition of β-alanine to the diet had no significant effect on the growth performance of weaned piglets (P > 0.05), but significantly reduced serum levels of immunoglobulin G (IgG) (P < 0.01), immunoglobulin M (IgM) (P = 0.005), and complement 3 (C3) (P = 0.017). The serum interleukin- 6 (IL-6) levels (P < 0.01) were significantly reduced in the 1,200 mg/kg treatment group. The addition of β-alanine increased ileal villus height, with the most significant effect at a concentration of 300 mg/kg (P = 0.041). The addition of 600 mg/kg β-alanine significantly up-regulated the expression of superoxide dismutase (SOD) activity (P = 0.020) and the zonula occludens-1 (ZO-1) gene (P = 0.049) in the jejunum. Diets supplemented with 300 mg/kg β-alanine significantly increased the number of Ki67 positive cells in the jejunal crypts (P < 0.01). In vitro, β-alanine increased the organoid budding rates (P = 0.001) and the budding height of the crypt significantly (P = 0.004). In conclusion, β-alanine can improve intestinal morphology and barrier function, reduce inflammatory responses and alleviate the adverse effects of weaning stress on piglet intestinal health.
Keywords: Weaned piglet, Beta-alanine, Intestinal development, Immune performance
1. Introduction
The weaning of piglets is a necessary stage in pig breeding. During this period, piglets are prone to weaning stress, which damages the morphological structure of the piglet intestine, promotes the secretion of inflammatory factors, activates the immune system, and disrupts the intestinal barrier function. This results in lower feed intake and slower growth rate, as well as increased morbidity of piglets. Therefore, how to alleviate weaning stress, promote intestinal development, improve intestinal barrier, and make piglets grow faster and better is a hot area of animal nutrition research.
Currently, research on the regulation of intestinal health by nutrients in feeds is progressing. Amino acids are essential nutrients for healthy animal growth and key regulators of the intestinal metabolic pathway (Stoll et al., 1998), which play an important role in maintaining intestinal health (Wang et al., 2009). Beta-alanine is the only naturally occurring β-amino acid that is not involved in protein synthesis, but it is an important amino acid involved in human metabolism. Beta-alanine relies on a transport carrier to reach the tissues and organs through the blood circulation and can play a functional role by synthesizing sarcosine with histidine, as well as pantothenic acid, calcium pantothenate, coenzyme A, and other substances (Coxon et al., 2005; Drozak et al., 2010). Myostatin, a metabolite of β-alanine (Baguet et al., 2014), traps free radicals and hydrogen peroxide to avoid organismal damage caused by reactive oxygen species (Everaert et al., 2011; Peiretti et al., 2011). Myostatin also effectively inhibits the release of inflammatory factors from LPS-induced astrocytes, thereby suppressing neuroinflammation. In addition to its ability to participate in the synthesis of myostatin, endogenous β-alanine specifically activates Mas-related G protein-coupled receptors (MrgprD) in the organism (Shinohara et al., 2004). Studies have revealed that MrgprD mediates the molecular mechanisms of inflammation triggered by bacterial infection through the regulation of NF-κB (Beaudry et al., 2017). Its agonist β-alanine can also activate the NF-κB signaling pathway involved in the inflammatory response (Lan et al., 2020) and there are also studies that have found a potential relationship between β-alanine and inflammatory response and immune regulation (Hadi et al., 2021; Hoffman et al., 2018; Lan et al., 2020; Zhang et al., 2021). Nowadays, the focus of β-alanine has mainly been in nutritional studies in poultry. Qi et al. (2018) reported that the addition of different levels of β-alanine to the diets of 1-day-old broilers for 42 days significantly increased the average daily gain (ADG) and reduced the feed-to-weight ratio of broilers, while promoting pectoral muscle growth, but had no significant effect on the average daily feed intake (ADFI). It has also been shown that the addition of β-alanine to the diet significantly reduced ADFI and improved feed conversion efficiency in broilers (Jacob et al., 1991). However, some studies indicated that dietary addition of β-alanine had no significant effect on feed conversion in broilers and fattening pigs (Mei et al., 1998; Tomonaga et al., 2012). Lackner et al. (2021) found that dietary addition of 0.5% β-alanine to broiler diets reduced ADFI at both 33 and 53 days and tended to reduce body weight (BW) and ADG, and also reduced breast muscle production at both stages. Tomonaga et al. (2006) fed 1% and 2% β-alanine in the diets of 4-week-old broilers for 4 consecutive weeks, which showed a reduction in pectoral muscle yield compared to the control group. Kralik et al. (2018) found no effect of β-alanine supplementation on pectoral muscle yield in broiler carcasses.
The main target of weaning stress is the intestinal tract of piglets, as it can cause serious damage to intestinal structure. The intestinal epithelium is the focus of research on intestinal development, and weaning stress can seriously affect the process of intestinal epithelial development and renewal. In terms of morphology, the part of the intestinal epithelium of the small intestine protruding from the lumen of the intestine is generally referred to as a villus, and the tubular structure at the bottom of the villi is called a crypt. The villus part and crypt part correspond to the differentiated cell region and stem cell region, respectively (Barker et al., 2008a). Intestinal epithelial cells are constantly proliferating, differentiating and apoptotic in the crypt–villus axis (Clatworthy and Subramanian, 2001). The intestinal epithelium is a rapidly renewing tissue that can be renewed every 3 to 4 days (Gordon, 1989). Weaning stress can increase the apoptosis of intestinal villus epithelial cells, and then cause villus atrophy and shedding, and villus height decrease. Crypt stem cells will accelerate proliferation and differentiation to generate new cells to supplement villus epithelial cells, resulting in increased crypt depth (Pluske et al., 1996a, 1996b, 1997a; van Beers-Schreurs et al., 1998). Therefore, weaning stress tends to damage in intestinal morphology, resulting in a decrease in villus height and an increase in crypt depth.
The traditional intestinal model has high cost, high labor intensity, low survival rate in vitro, and is limited in the study of development and regeneration. The standard transformed cancer cell line, which contains only a single cell type and no intestinal epithelial structure, cannot accurately represent a normal, healthy gut. Therefore, there is a need to develop more appropriate in vitro models. Lgr5-positive cells are recognized as intestinal stem cells (Barker et al., 2007a), and are also the driving force for the rapid renewal of intestinal epithelial cells. There are a large number of stem cells at the bottom of the crypt (Dekaney et al., 1997), and the progeny cells generated after division and proliferation differentiate into progenitor cells (van der Flier and Clevers, 2009), and further differentiate into different types of intestinal epithelial cells.
With the progress of intestinal stem cell isolation technology, Sato et al. (2009) established an in vitro culture method of an intestinal organoid model using single LGR5-labeled stem cells for the first time in 2009. Crypt cells were embedded in matrix gel, and growth factors necessary for the growth of small intestine were added to the medium to make them develop into a three-dimensional intestinal organoid structure composed of epithelium. Isolated intestinal stem cells or crypts can proliferate continuously when cultured in matrix gel and a variety of growth factors, and can differentiate into various mature cells such as Paneth cells, goblet cells, enteroendocrine cells, etc., and self-organize to form cavity-like structures with multiple crypts.
Intestinal organoids are composed of a central globular villus region and an external budding crypt region. Similar to the structure of intestinal tissue in vivo, intestinal organoids contain almost all intestinal epithelial cell types, which can be stably cultivated in vitro for a long time, and have physiological functions such as intestinal absorption and secretion (Sato and Clevers, 2013; Zachos et al., 2016). It has been confirmed that intestinal epithelial stem cells cultured in vitro can be successfully transplanted in vivo (Lei et al., 2014; Yui et al., 2012) and intestinal organoids can also be used to study intestinal diseases (Wehkamp and Stange, 2010). This is helpful to reveal the molecular mechanism of intestinal development and damage repair, showing great application prospects in medical treatment and animal production, and has become a new generation of biological models.
Therefore, the application of β-alanine in livestock farming is still not well studied, and there is a lack of experimental studies on the effect of β-alanine on weaning stress in piglets. This paper aims to investigate the effect of β-alanine on intestinal development and immune performance of weaned piglets and to further verify the effect of β-alanine on the proliferation and differentiation of intestinal stem cells using an intestinal organoid model. It is expected to provide a scientific basis for the rational application of β-alanine in piglet production practice.
2. Materials and methods
2.1. Animal ethics statement
All the experimental procedures applied in this study were reviewed and approved by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China.
2.2. Animals and experimental treatments
The current study was approved by the Animal Protection Committee of Hunan Normal University. Thirty-two 21-day-old healthy weaned piglets (half female and half male; Duroc × Landrace × Yorkshire) with an initial body weight of 8.11 ± 0.21 kg were randomly divided into 4 treatment groups. Each group had 8 replicates, one pig in each replicate. The control group was fed the basal diet (without β-alanine), and the 3 treatment groups were supplemented with 300, 600, and 1,200 mg/kg of β-alanine (provided by Anhui Huaheng Biological Technology Co., Ltd.) on top of the basal diet. The concentrations were designed with reference to the concentration of β-alanine in poultry trials and determined in relation to the body weight and feed intake of piglets. The trial period was 28 days, divided into 2 stages: the late suckling stage (day 1 to 14) and the nursery stage (day 15 to 28). The second phase of the diet gradually replaced the first phase, with a three-day transition period. The base diet was configured to meet the nutritional levels of weaned piglets in NRC (2012).
The ingredients and nutrient composition of the diets are shown in Table 1.
Table 1.
Ingredients and nutrient composition of experimental piglet diets.
| Item | Phase 1 | Phase 2 |
|---|---|---|
| Ingredients, % as fed | ||
| Corn | 40.92 | 40.1 |
| Puffed corn | 20 | 22 |
| Soybean protein concentrate | 8 | 0 |
| Soybean meal, 43% CP | 8.2 | 20.5 |
| Fish meal, 43% CP | 5 | 4 |
| Whey powder, 2% CP | 10 | 5 |
| Glucose | 3 | 3 |
| Soybean oil | 0.5 | 1.5 |
| stone powder | 0.88 | 0.58 |
| Calcium hydrogen phosphate | 0.5 | 0.88 |
| Choline chloride | 0.1 | 0.1 |
| Antioxidants | 0.05 | 0.05 |
| Citric acid | 0.8 | 0.5 |
| Salt | 0.1 | 0.1 |
| Mineral premixes1 | 0.15 | 0.15 |
| Vitamin premixes2 | 0.5 | 0.5 |
| L-Lysine, 98% | 0.64 | 0.53 |
| DL-Methionine | 0.36 | 0.27 |
| L-Threonine | 0.24 | 0.19 |
| L-Tryptophan | 0.06 | 0.05 |
| Total | 100 | 100 |
| Calculated values of nutrient levels, % | ||
| Net energy, Mcal/kg | 2.49 | 2.45 |
| Crude protein | 18.61 | 18.02 |
| Ca | 0.80 | 0.70 |
| Effective phosphorus3 | 0.40 | 0.40 |
| Lysine3 | 1.35 | 1.24 |
| Methionine3 + Cysteine3 | 0.77 | 0.68 |
| Threonine3 | 0.79 | 0.73 |
| Tryptophan3 | 0.22 | 0.22 |
Added per kilogram of basal diet: 150 mg FeSO4, 100 mg ZnSO4, 30 mg MnSO4, 25 mg CuSO4, 0.5 mg KIO3, 0.3 mg CoSO4 and 0.3 mg Na2SeO3.
Added per kilogram of basal diet: 162,500 IU retinyl acetate; 50,000 IU vitamin D3; 400 DL-α- tocopheryl acetate; 50 mg vitamin K3; 50 mg vitamin B1; 120 mg vitamin B2; 75 mg vitamin B6; 0.5 mg vitamin B12; 25 mg folic acid; 600 mg nicotinamide; 350 mg D-calcium pantothenate; 2.5 mg D-biotin.
Standard ileal digestible level.
2.3. Sample collection and measurements
At the end of the feeding cycle at 22:00 on day 28, piglets were fasting (free drinking water) and weighed the next morning. The blood samples were collected from the anterior vena cava of piglets. After 1 h, the samples were centrifuged at 3,000 × g for 12 min, and the supernatant (i.e., serum) was extracted. The piglets were euthanized by injecting 4% pentobarbital sodium solution into the jugular vein. The internal organs were separated along the middle abdominal line of the piglets. After fat removal, the samples of liver, spleen, kidneys and stomach were weighed and the organ index was calculated. After the intestinal sample was taken out, the large intestine and small intestine were separated. The mesentery was removed. Then, the length of the large intestine and small intestine were measured, their weight was recorded, and the intestinal growth index was calculated. The anterior duodenum, the middle jejunum, and the end of the ileum were taken, 3 cm each, and fixed in 10% formaldehyde fixative. The solution was changed 24 h later and stored in a cool place. Another 1.5 cm of jejunum was taken from the middle part of the jejunum, washed with normal saline, wrapped in tin foil, and refrigerated at −80 °C. The instruments used in the processing were sterile.
2.4. Determination of serum biochemical indices
The serum samples were analyzed by a biochemical analyzer (Toshiba 120) for total protein (TP), alanine aminotransferase (ALT), glutathione aminotransferase (AST), glucose (GLU), creatinine (CREA), alkaline phosphatase (ALP), urea nitrogen (BUN), albumin (ALB), globulin (GLO), uric acid (UA), C-reactive protein (CRP), immunoglobulin M (IgM), immunoglobulin G (IgG), complement 3 (C3) and complement 4 (C4); the kits were purchased from Weifang 3D Biological Engineering Co. A chemiluminescence instrument (Siemens Immulite 1,000) was used to detect interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) in the serum, and the test kits were purchased from Siemens Medical Diagnostic Products Co.
2.5. Intestinal morphology
Fixed intestinal tissue samples were removed, embedded and 1.5 cm intestinal segments were cut into 4 μm thick slices. The intestinal sections were dried and stained with hematoxylin-eosin. After that, the intestinal morphology was observed and photographed with an orthochromatic fluorescence microscope (Version 4.12, Leica Imaging Ltd, Cambridge, UK). Villus height (VH) and crypt depth (CD) were measured with Image-Pro Plus 6.0 software, and the villus height to crypt depth ratio (VH:CD) was calculated for each small intestine. At least 20 intact villous crypts were randomly selected and measured for each sample.
2.6. Analysis of digestive enzyme and antioxidant enzyme activities
The jejunal and ileal mucosal tissue samples from piglets were powdered in liquid nitrogen, collected immediately in PBS solution, left to stand for 3 h, and then centrifuged at 3,000 × g at 4 °C for 10 min. Digestive enzymes and antioxidant enzyme assay kits were purchased from Nanjing Jiancheng Company. The digestive enzymes measured were alkaline phosphatase, maltase and sucrase, and the antioxidant enzymes measured were total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-PX), malondialdehyde (MDA) and superoxide dismutase (SOD). The BCA kit (Beyoncé) was used to determine the protein concentration to calibrate the enzyme activities. Detailed procedures and calculation formulae refer to the corresponding kit instructions.
2.7. RNA extraction and cDNA synthesis
Total RNA was isolated from jejunal mucosal tissue using TRIZOL reagent (TaKaRa, Beijing, China). cDNA was synthesized using an RT kit and gDNA Eraser (TaKaRa).
2.8. Quantitative real-time PCR analysis
The primer sequences are shown in Table 2. The gene names and genus design were entered on the NCBI website. The primer sequences were verified by Primer-BLAST for specificity. The primers were finally synthesized by Biotech. The primers and cDNA were diluted according to the recommended ratio in the reagent instructions. Fluorescent PCR was performed by adding the reaction system (Quant-Studio, Thermo Fisher Scientific). The relative expression of target gene mRNA was corrected for β-actin and calculated according to the method of Chen et al. (2019).
Table 2.
Primers used for real-time PCR analysis.
| Gene | Primer | Sequence, 5′–3′ | Product size, bp |
|---|---|---|---|
| β-actin | Forward | AGTTGAAGGTGGTCTCGTGG | 216 |
| Reverse | TGCGGGACATCAAGGAGAAG | ||
| Slc6a14 | Forward | GTGGCTTGGGGTGGTTTAGT | 203 |
| Reverse | AACGCCAAATCGAAACCTGA | ||
| Slc36a1 | Forward | GGTGGAGATGGCAAGGGTT | 256 |
| Reverse | TCAGGGTCTGGAACCATGTTG | ||
| Slc2a2 | Forward | AAGTCGAGGCCTATGATCTGACTAA | 161 |
| Reverse | GGAAGAGGCATATCAGGACTCTACT | ||
| Slc6a1 | Forward | CAACTCCTTCACCACAACGC | 249 |
| Reverse | CAGCCAACACCCTTCCAGAT | ||
| Slc7a9 | Forward | GAAGAAGCCTCCTAGAAGTG | 268 |
| Reverse | CCAGTGTCGCAAGAATCC | ||
| SOD | Forward | GGTGAACCAGTTGTGTTGTCAGG | 114 |
| Reverse | ATGAGGTCCTGCACTGGTACAG | ||
| GPX | Forward | CGCTCTTTACCTTCCTGCGGAA | 120 |
| Reverse | AGTTCCAGGCAATGTCGTTGCG | ||
| CAT | Forward | TGCCCTTGCACAAACAAAACC | 254 |
| Reverse | GCTCAAACACCTTCGCCTTC | ||
| IL-1β | Forward | CCTGGACCTTGGTTCTCT | 123 |
| Reverse | GGATTCTTCATCGGCTTCT | ||
| IL-6 | Forward | GGCAAAAGGGAAAGAATCCAG | 87 |
| Reverse | CGTTCTGTGACTGCAGCTTATCC | ||
| IL-10 | Forward | GGGCTATTTGTCCTGACTGC | 105 |
| Reverse | GGGCTCCCTAGTTTCTCTTCC | ||
| IL-22 | Forward | AGCAAGCGTGAAGGTGCGGTT | 169 |
| Reverse | GCGGACATCTGGGAGCCCTTT | ||
| TNF-α | Forward | ACAGGCCAGCTCCCTCTTAT | 102 |
| Reverse | CCTCGCCCTCCTGAATAAAT | ||
| IFN-γ | Forward | CCATTCAAAGGAGCATGGAT | 146 |
| Reverse | GAGTTCACTGATGGCTTTGC | ||
| TGF-β | Forward | CGAGCCCTGGATACCAACTA | 164 |
| Reverse | AGGCTCCAGATGTAGGGACA | ||
| Muc2 | Forward | AGACGGGCGGAGACTTTGAATC | 102 |
| Reverse | CTTGGATGGGAACGCTGGGATA | ||
| Muc4 | Forward | TTGAAGGCTGGAGATTGCAGAGTC | 113 |
| Reverse | CATTAGCTCATACAGGGCACAGAAGG | ||
| PBD1 | Forward | CCTCCTCCTTGTATTCCTCCTC | 141 |
| Reverse | GTGCCGATCTGTTTCATCTTTG | ||
| PBD2 | Forward | CCTTGTATTCCTCCTCATGGTCC | 136 |
| Reverse | GGTGCCGATCTGTTTCATCTTTG | ||
| TLR2 | Forward | GTTCACGCATTTCCGCAGTT | 224 |
| Reverse | CTTTGTGGACAGCATGGGTCTT | ||
| TLR4 | Forward | AGCACCTATGACGCCTTTGTTA | 229 |
| Reverse | TACACCATCGGCTCTGTATGAA | ||
| TLR5 | Forward | TTAAGCCTTGCGGATAATAACCTGT | 213 |
| Reverse | GAAGCCAAACCCAGAACCCATA | ||
| Claudin-1 | Forward | CTAGTGATGAGGCAGATGAA | 250 |
| Reverse | AGATAGGTCCGAAGCAGAT | ||
| Occludin | Forward | GAGTGATTCGGATTCTGTCT | 181 |
| Reverse | TAGCCATAACCATAGCCATAG | ||
| ZO-1 | Forward | TTGATAGTGGCGTTGACA | 126 |
| Reverse | CCTCATCTTCATCATCTTCTAC |
β-actin = actin, beta 2; Slc6a14 = solute carrier family 6 member 14; Slc36a1 = solute carrier family 36 member 1; Slc2a2 = solute carrier family 2 member 2; Slc6a1 = solute carrier family 6 member 1; Slc7a9 = solute carrier family 7 member 1; SOD = superoxide dismutase; GPX = glutathione peroxidase; CAT = chloramphenicol acetyltransferase; IL-1β = interleukin-1β; IL-6 = interleukin-6; IL-10 = interleukin-10; IL-22 = interleukin-22; TNF-α = tumor necrosis factor-α; IFN-γ = interferon-γ; TGF-β = transforming growth factor-β; Muc2 = mucin 2; Muc4 = mucin 4; PBD1 = porcine β-defensin-1; PBD2 = porcine β-defensin-2; TLR2 = toll like receptor 2; TLR4 = toll like receptor 2; TLR5 = toll like receptor; ZO-1 = zonula occludens-1.
2.9. Immunohistochemical analysis
The ileum and jejunum were paraffin sectioned and antigen repair was performed using 0.01 M sodium citrate buffer. The closure was performed using BSA solution (PhD Bio, Wuhan, China). The primary antibody used was Ki67 antibody (Abcam, item no. ab15580), diluted at a ratio of 1:800 and added to the sample, incubated for 90 min at 37 °C and eluted with PBS solution; the secondary antibody (Zhongshan JinQiao, Beijing, China) was subsequently added to the sample and incubated for 45 min at 37 °C. After blocking, the stained intestinal sections were observed by positive fluorescence microscopy and 20 complete crypt counts of Ki67-positive cells were selected.
2.10. AB-PAS staining
Staining was performed using the AB-PAS kit purchased from Nanjing Jiancheng Biological Company. The stained intestinal sections were observed with an ortho-fluorescence microscope, and 20 intact villi and crypt structures were selected to count the number of goblet cells.
2.11. Intestinal organoid cultures
After euthanasia of weaned piglets, the intestinal segment was removed after dissection of the abdominal cavity. About 5 cm of the anterior portion of the jejunum was cut and the intestinal contents were immediately washed with pre-cooled PBS. Fat and mesenteric tissue was removed using forceps and scissors. The jejunum was cut lengthwise and washed three times with PBS. The intestinal segment was laid flat in a Petri dish and the villi on the inner surface of the jejunum were gently scraped off with a slide. The jejunum was then cut into 3 to 4 mm pieces with a razor blade and washed five times by resuspension in PBS. The washed intestinal tissues were placed in PBS supplemented with 2.5 mmol/L disodium EDTA and incubated for 30 min (100 × g) at 4 °C on a shaker for epithelial cell isolation. After standing for 2 min, the supernatant was discarded and 12 mL of PBS added. Aspirated up and down 20 to 25 times with a 5-mL pipette to completely suspend the tissue. After standing, the supernatant was aspirated and filtered through a 70-μm cell filter and the procedure was repeated 2 to 3 times. To obtain a highly purified crypt, 10% FBS was added to the crypt suspension and spun at 1,200 × g for 5 min. The supernatant was discarded and the crypts were re-suspended in 6 mL of complete medium (DMEM/F12, Hepes buffer, penicillin-streptomycin). Then, the cells were centrifuged at 500 × g for 3 min and the supernatant was discarded. The crypt was resuspended in matrix gel (Corning, Bedford, OH, USA) and 40 μL of resuspension was added to the centre of each well of a 24-well plate pre-warmed at 37 °C. The cells were then incubated in a cell incubator for 20 min. Then, 450 μL of the prepared organoid medium was added to each well. The organoid medium contained Wnt3a, R-spondin1, FBS, B-27, Noggin, N-2, n-acetyl-L-cysteine, and epidermal growth factor. The cell plates were placed in a cell culture incubator and the culture medium was changed every 3 days, and passages were performed every 6 to 7 days. The organoids were treated with β-alanine solution at 0, 0.01, 0.1, 1 and 10 mM, respectively, with 4 replicates of each treatment. On the third and fourth day of β-alanine incubation, pictures of the intestinal organoids were taken by microscopy. The activity of the organoids relied on buddings number per organoid, the budding rates and the budding height of the crypt (Fujii et al., 2018). The germination efficiency of the class organoids was calculated as the ratio of the number of germinated class organs to the total number of class organs. The budding rates of organoids were calculated as the ratio of the number of budding organoids to the total number of organoids. Germinated crypt foci were counted using Image-Pro Plus 6.0 software (Media Cybernetics, San Diego, CA, USA). The number of crypt foci per taxon was expressed by calculating the average number of buds.
2.12. Statistical analysis
Data are expressed as means, and SEM is the total standard error of the overall sample mean. Histograms were produced using GraphPad Prism 6 software. The data were analyzed using SPSS 20.0 software (IBM Corp., Chicago, IL, USA) by first removing intra-group differences from the descriptions, then testing the data for normal distribution, and finally analyzing them by the one-way ANOVA method to determine statistical differences between groups according to the Duncan method, where 0.05 ≤ P < 0.10 was considered a trend of difference, P < 0.05 was considered a significant difference, P < 0.01 was considered a highly significant difference, and P > 0.05 was considered not significant.
3. Results
3.1. Growth performance
In the first week of the trial, one piglet died in the 600 mg/kg β-alanine group, and in the second week, one test piglet died in the control group, both of which were excluded from the trial. The effects of β-alanine addition to the feed on growth performance are presented in Table 3. The different levels of β-alanine had no significant effect on BW, ADG, ADFI, body gain feed intake to body gain ratio (F:G) and diarrhea rates in weaned piglets at both stages, and throughout the trial period (P > 0.05).
Table 3.
Effects of the diet with β-alanine on growth performance in weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1200 | |||
| BW2, kg | ||||||
| 0 d | 8.26 | 8 | 8.26 | 7.98 | 0.211 | 0.945 |
| 14 d | 10.49 | 9.63 | 10.37 | 9.98 | 0.302 | 0.756 |
| 28 d | 14.46 | 13.38 | 14.46 | 14.78 | 0.436 | 0.690 |
| ADFI3, g/d | ||||||
| 0–14 d | 435.58 | 413.21 | 401.85 | 510.94 | 19.984 | 0.223 |
| 15–28 d | 585.92 | 587.12 | 572.54 | 636.96 | 23.044 | 0.796 |
| 0–28 d | 510.75 | 494.28 | 492.2 | 582.91 | 19.267 | 0.317 |
| ADG4, g/d | ||||||
| 0–14 d | 190.48 | 116.07 | 151.02 | 226.19 | 19.577 | 0.213 |
| 15–28 d | 283.67 | 297.96 | 291.84 | 342.86 | 16.573 | 0.589 |
| 0–28 d | 221.43 | 211.22 | 221.43 | 267.35 | 16.059 | 0.632 |
| F:G5 | ||||||
| 0–14 d | 2.58 | 3.66 | 4.82 | 2.17 | 0.528 | 0.323 |
| 15–28 d | 1.98 | 2.02 | 2.14 | 2.02 | 0.073 | 0.883 |
| 0–28 d | 2.44 | 2.49 | 2.78 | 2.28 | 0.147 | 0.705 |
| Diarrhea rate6, % | ||||||
| 0–14 d | 14.29 | 13.28 | 15.18 | 17.97 | 2.54 | 0.927 |
| 15–28 d | 8.16 | 11.61 | 16.33 | 25.89 | 4.13 | 0.465 |
| 0–28 d | 12.25 | 13.39 | 16.84 | 23.21 | 3.08 | 0.597 |
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels, respectively.
BW = body weight.
ADFI = average daily feed intake.
ADG = average daily gain.
F:G = feed intake to body gain ratio.
Diarrhea rate = number of piglets with diarrhoea during the trial period/(total number of pigs × number of trials days) × 100%.
3.2. Serum biochemical indicators
According to Table 4, the addition of β-alanine to the diet significantly reduced serum IgG (P < 0.001) and IgM levels (P = 0.005), significantly reduced serum C3 levels (P = 0.017), and tended to increase the ALB:GLO ratio (AGR) (P = 0.091). At 1,200 mg/kg of β-alanine, there was also a highly significant decrease in IL-6 levels (P < 0.001). No significant effects were shown on other indicators.
Table 4.
Effects of β-alanine on serum biochemical indices of weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1,200 | |||
| Nutrient transfer indicators | ||||||
| ALT, U/L | 51.33 | 53.88 | 49.29 | 52.88 | 2.23 | 0.905 |
| AST, U/L | 95.50 | 83.13 | 107.71 | 101.71 | 7.03 | 0.602 |
| TP, g/L | 53.68 | 47.78 | 54.20 | 52.30 | 1.28 | 0.259 |
| ALP, U/L | 159.50 | 174.38 | 128.71 | 174.25 | 8.23 | 0.158 |
| BUN, mmol/L | 3.29 | 2.82 | 2.78 | 2.93 | 0.81 | 0.805 |
| CREA, μmol/L | 72.83 | 63.50 | 70.00 | 62.50 | 2.16 | 0.286 |
| UA, μmol/L | 6.00 | 4.50 | 6.17 | 7.29 | 0.49 | 0.218 |
| GLU, mmol/L | 4.96 | 4.93 | 4.67 | 4.28 | 0.17 | 0.464 |
| Immunological indicators | ||||||
| CRP, mg/L | 0.022 | 0.025 | 0.023 | 0.024 | 0.002 | 0.925 |
| IgG, g/L | 1.625a | 1.093c | 1.359b | 1.463ab | 0.049 | <0.001 |
| IgM, g/L | 0.238a | 0.175b | 0.167b | 0.149b | 0.010 | 0.005 |
| C3, g/L | 0.023a | 0.016b | 0.016b | 0.016b | 0.001 | 0.017 |
| C4, g/L | 0.038 | 0.030 | 0.030 | 0.031 | 0.002 | 0.232 |
| ALB, g/L | 30.62 | 31.61 | 31.84 | 34.09 | 0.78 | 0.468 |
| GLO, g/L | 23.07 | 16.16 | 22.36 | 18.24 | 1.18 | 0.118 |
| AGR2 | 1.37 | 2.00 | 1.66 | 1.98 | 0.10 | 0.091 |
| IL-6, pg/mL | 4.483a | 4.488a | 3.729a | 2.525b | 0.200 | <0.001 |
| TNF-α, pg/mL | 2.620 | 2.683 | 2.860 | 2.833 | 0.094 | 0.804 |
ALT = alanine aminotransferase; AST = glutathione aminotransferase; TP = total protein; ALP = alkaline phosphatase; BUN = urea nitrogen; CREA = creatinine; UA = uric acid; GLU = glucose; CRP = C-reactive protein; IgG = immunoglobulin G; IgM = immunoglobulin M; C3 = complement 3; C4 = complement 4; ALB = albumin; GLO = globulin; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-α.
a, b Different superscript letters in the same row indicate significant differences (P < 0.05).
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels, respectively.
AGR = ALB/GLO.
3.3. Intestinal morphology
As shown in Table 5, the ileal villus height was significantly increased by the dietary addition of β-alanine compared to the control group (P = 0.041), especially at the dose level of 300 mg/kg. There was no significant effect on the morphological structure of the duodenum, jejunum and colon (P > 0.05).
Table 5.
Effects of β-alanine on intestinal morphology of weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1,200 | |||
| Duodenum | ||||||
| Villus height, μm | 285.42 | 301.26 | 298.28 | 254.16 | 8.08 | 0.149 |
| Crypt depth, μm | 403.88 | 378.75 | 351.54 | 339.94 | 10.85 | 0.165 |
| VH:CD3 | 0.71 | 0.80 | 0.87 | 0.80 | 0.04 | 0.539 |
| Jejunum | ||||||
| Villus height, μm | 297.89 | 296.05 | 271.20 | 286.13 | 54.36 | 0.823 |
| Crypt depth, μm | 336.55 | 361.90 | 293.94 | 303.67 | 64.86 | 0.157 |
| VH:CD 3 | 0.90 | 0.85 | 0.95 | 1.03 | 0.05 | 0.682 |
| Ileum | ||||||
| Villus height, μm | 274.99b | 332.99a | 285.37b | 302.52ab | 7.76 | 0.041 |
| Crypt depth, μm | 284.77 | 295.91 | 290.66 | 292.85 | 5.57 | 0.923 |
| VH:CD 3 | 1.02 | 1.13 | 0.99 | 1.04 | 0.03 | 0.402 |
| Colon | ||||||
| Crypt depth, μm | 485.79 | 467.42 | 429.54 | 481.02 | 13.24 | 0.471 |
a,b Different superscript letters in the same row indicate significant differences (P < 0.05).
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels, respectively.
SEM = standard error of the mean.
VH:CD = villus height to crypt depth ratio.
3.4. Digestive enzyme activities
The effects of β -alanine on intestinal digestive enzyme activities of weaned piglets are shown in Table 6. Dietary β-alanine tended to increase jejunal maltase activity in weaned piglets (P = 0.074), with the highest activity at 600 mg/kg. Dietary β-alanine had a statistically significant effect on the activity of convertase, which was highest in the ileum at 1,200 mg/kg.
Table 6.
Effects of β-alanine on intestinal digestive enzyme activities of weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1,200 | |||
| Jejunum | ||||||
| Alkaline phosphatase, U/mgprot | 8.98 | 10.48 | 8.56 | 9.98 | 0.73 | 0.805 |
| Maltase, U/mgprot | 3.31 | 3.84 | 6.84 | 3.90 | 0.524 | 0.074 |
| Invertase, U/mgprot | 4.03 | 3.48 | 3.75 | 3.97 | 0.18 | 0.688 |
| Ileum | ||||||
| Alkaline phosphatase, U/mgprot | 9.20 | 11.97 | 6.28 | 10.09 | 1.02 | 0.271 |
| Maltase, U/mgprot | 2.10 | 2.78 | 1.19 | 1.60 | 0.35 | 0.495 |
| Invertase, U/mgprot | 2.76ab | 2.76 ab | 2.37b | 3.21a | 0.10 | 0.028 |
a,b Different superscript letters in the same row indicate significant differences (P < 0.05).
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels, respectively.
SEM = standard error of the mean.
3.5. Expression of trans-transport carrier genes
Table 7 presents the effect of β-alanine on the expression of nutrient transport carrier genes in the jejunum of weaned piglets. There was a tendency for β-alanine to increase Slc6a1 expression (P = 0.074), and there was a dose–response effect. There was no significant effect on other indices (P > 0.05).
Table 7.
Effect of β-alanine on the expression of nutrient transport carrier genes in the jejunum of weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1,200 | |||
| Slc36a1 | 1.023 | 0.984 | 0.917 | 0.763 | 0.064 | 0.504 |
| Slc6a14 | 0.895 | 0.824 | 1.373 | 1.135 | 0.137 | 0.509 |
| Slc2a2 | 0.919 | 1.062 | 0.885 | 0.669 | 0.083 | 0.392 |
| Slc6a1 | 1.057 | 1.334 | 1.519 | 1.973 | 0.127 | 0.074 |
| Slc7a9 | 1.084 | 0.790 | 0.856 | 0.677 | 0.063 | 0.120 |
Slc36a1 = solute carrier family 36 member 1; Slc6a14 = solute carrier family 6 member 14; Slc2a2 = solute carrier family 2 member 2; Slc6a1 = solute carrier family 6 member 1; Slc7a9 = solute carrier family 7 member 1.
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels, respectively.
SEM = standard error of the mean.
3.6. Antioxidant enzyme activity and gene expression
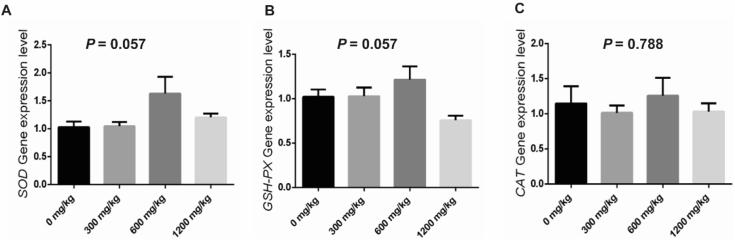
Table 8 shows that the effects of β-alanine on jejunal antioxidant enzyme activity of weaned piglets. Changes in dietary β-alanine content did not significantly affect the T-AOC and GSH-PX activity (P > 0.05), but there was a tendency to reduce MDA enzyme activity (P = 0.095), while the addition of 600 mg/kg of β-alanine increased jejunal SOD activity (P = 0.020). There was a statistically significant trend in the effect of supplementing the diet with β-alanine on jejunal SOD and GSH-PX gene expression compared to the control (P = 0.057, Fig. 1A and B), but no significant effect on CAT gene expression (P = 0.788. Fig. 1C).
Table 8.
Effects of β-alanine on jejunum antioxidant enzyme activity of weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1,200 | |||
| T-AOC, U/mgprot | 0.17 | 0.17 | 0.20 | 0.19 | 0.01 | 0.419 |
| SOD, U/mgprot | 3.73b | 3.76b | 4.65a | 3.48b | 0.15 | 0.020 |
| MDA, nmol/mgprot | 0.27 | 0.09 | 0.05 | 0.08 | 0.03 | 0.095 |
| GSH-PX, mol/L | 16.01 | 7.81 | 6.79 | 7.46 | 1.48 | 0.126 |
T-AOC = total antioxidant capacity; SOD = superoxide dismutase; MDA = malondialdehyde; GSH-PX = glutathione peroxidase.
a,b Different superscript letters in the same row indicate significant differences (P < 0.05).
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels, respectively.
SEM = standard error of the mean.
Fig. 1.
Effect of β-alanine on the expression of antioxidant-related genes in the jejunum of weaned piglets. (A) SOD gene expression levels in jejunum. (B) GSH-PX gene expression levels in jejunum. (C) CAT gene expression levels in jejunum. The data are presented as the mean ± SEM. SOD = superoxide dismutase; GSH-PX = glutathione peroxidase; CAT = chloramphenicol acetyltransferase.
3.7. Expression of inflammation-related genes
The effect of β-alanine on the genes related to jejunal inflammation in weaned piglets is shown in Table 9. The addition of β-alanine to the diet tended to upregulate jejunal IL-10 gene expression compared to the control group (P = 0.080), with the highest IL-10 gene expression at a concentration of 600 mg/kg. However no significant effects were observed for other indicators (P > 0.05).
Table 9.
Effects of β-alanine on jejunal inflammation related gene expression in weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1,200 | |||
| IL-1β | 1.288 | 1.299 | 0.886 | 0.832 | 0.158 | 0.613 |
| IL-6 | 1.146 | 1.319 | 0.862 | 1.194 | 0.127 | 0.688 |
| IL-10 | 0.888 | 1.259 | 2.446 | 1.667 | 0.218 | 0.080 |
| IL-22 | 1.639 | 0.774 | 1.496 | 0.692 | 0.191 | 0.179 |
| TNF-α | 1.051 | 0.815 | 0.614 | 0.958 | 0.075 | 0.223 |
| TGF-β | 0.912 | 0.930 | 0.976 | 0.762 | 0.056 | 0.544 |
| INF-γ | 1.279 | 2.222 | 1.569 | 1.246 | 0.198 | 0.313 |
IL-1β = Interleukin-1β; IL-6 = Interleukin-6; IL-10 = Interleukin-10; IL-22 = Interleukin-22; TNF-α = Tumour necrosis factor-α; TGF-β = Transforming growth factor-β; INF-γ = Interferon-γ.
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels, respectively.
SEM = standard error of the mean.
3.8. Expression of barrier-related genes
As shown in Table 10, there was a significant up-regulation of ZO-1 gene expression (P = 0.049) and a tendency to up-regulate Occludin gene expression (P = 0.051) at a β-alanine level of 600 mg/kg compared to the control group.
Table 10.
Effects of β-alanine on jejunal barrier function related gene expression in weaned piglets.
| Item | β-alanine addition level1, mg/kg |
SEM2 | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 300 | 600 | 1,200 | |||
| Claudin-1 | 1.145 | 1.015 | 1.257 | 1.031 | 0.091 | 0.788 |
| Occludin | 1.020 | 1.026 | 1.215 | 0.777 | 0.056 | 0.051 |
| ZO-1 | 1.028b | 1.042b | 1.630a | 1.209ab | 0.089 | 0.049 |
ZO-1 = zonula occludins-1.
a,b Different superscript letters in the same row indicate significant differences (P < 0.05).
Diets supplemented with 0, 300, 600, and 1,200 mg/kg levels respectively.
SEM = standard error of the mean.
3.9. Immunohistochemistry and goblet cells
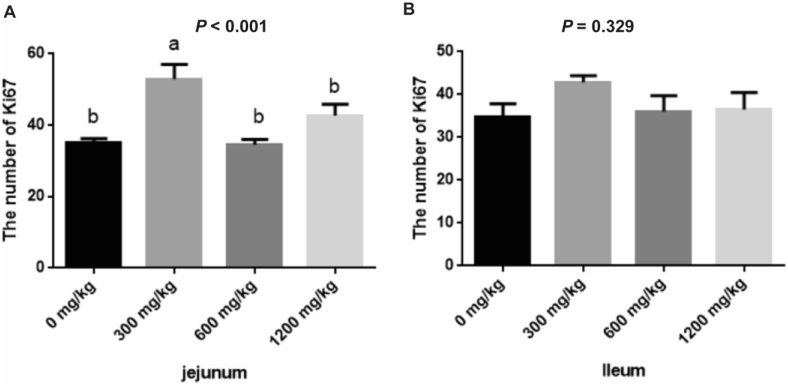
Compared with the control, β-alanine (300 mg/kg) increased the expression of Ki67 positive cells in the jejunal crypts (P < 0.01, Fig. 2A). However, no significant effects were observed in the ileum (P > 0.05, Fig. 2B).
Fig. 2.
Effects of β-alanine on intestinal cell proliferation (Number of Ki67 positive cells) of weaned piglets. (A) The number of jejunal Ki67-positive cells. (B) Number of ileal intestinal Ki67 positive cells. The data are presented as the mean ± SEM. Values marked with letters above the bars indicate significant differences (P < 0.05).
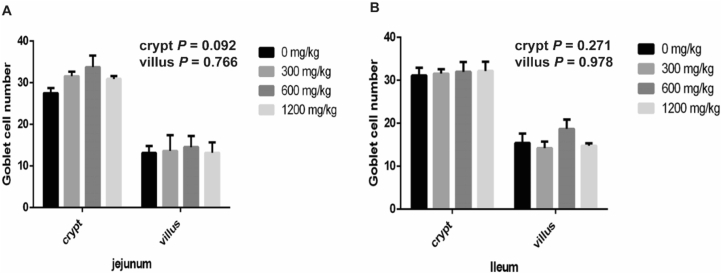
As shown in Fig. 3, the addition of β-alanine to the diet tended to increase the expression of goblet cells in the jejunal crypts (P = 0.092, Fig. 3A), with a maximum at 600 mg/kg. There was significant effect on the number of goblet cells in the jejunal villus and ileal intestinal epithelium.
Fig. 3.
Effect of β-alanine on the number of intestinal goblet cells in weaned piglets. (A) The number of jejunal cupped cells. (B) Number of ileal cupped cells. The data are presented as the mean ± SEM.
3.10. Intestinal organoid
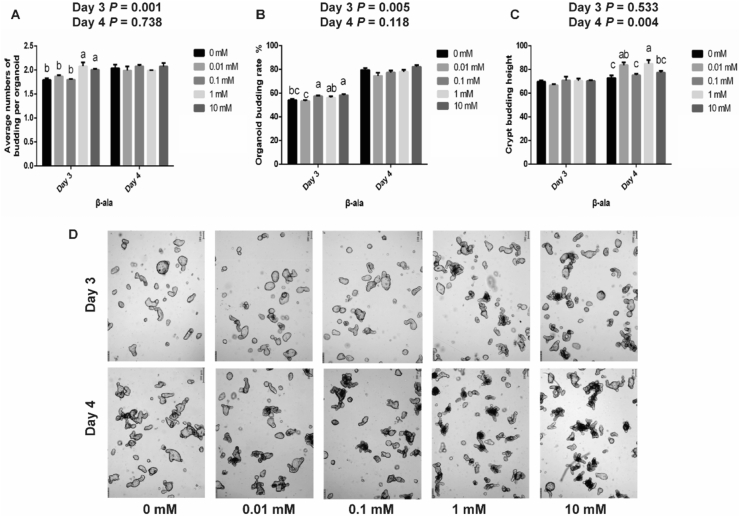
Compared with the control, on day 3, β-alanine at 1 and 10 mM significantly increased the budding number per organoid (P = 0.001, Fig. 4A), and at 0.1 and 10 mM levels, β-alanine significantly increased the budding rates of the organoids (P = 0.005, Fig. 4B), but the crypt budding height was not influenced. On the fourth day, 0.01 and 1 mM of β-alanine significantly increased the crypt germination height (P = 0.004, Fig. 4C), but had no significant effect on the budding rate or the budding number per organoid. Representative pictures of the jejunal organoid treatments with different concentrations of β-alanine are shown in Fig. 4D.
Fig. 4.
Effects of different levels of β -alanine on intestinal organoids of piglets. (A) Average budding number per organoid with different levels of alanine. (B) Organoid budding rate with different levels of alanine. (C) Crypt budding height with different levels of alanine. (D) Representative pictures of intestinal organoids treated with different levels of β-alanine (20× magnification, scale bar = 100 μm). The data are presented as the mean ± SEM. Values marked with different letters above the bars indicate significant differences (P < 0.05).
4. Discussion
In this study, different levels of β-alanine were found to have no significant effect on ADG, ADFI, and F:G in weaned piglets. Tiedje et al. (2010) found that β-alanine acts as a neurotransmitter regulating the secretion of hormones associated with growth and development. Therefore, β-alanine had no significant adverse effect on the growth performance of weaned piglets. Moreover, in this experiment, dietary supplementation of β-alanine significantly decreased serum IgG (P < 0.001), IgM (P = 0.005) and serum C3 (P = 0.017). Beta-alanine decreased serum levels of the pro-inflammatory factor IL-6, especially at a dose of 1,200 mg/kg. Serum biochemical indicators usually reflect the health status and metabolic capacity of piglets. Immunoglobulins (Ig) play an important role in the immune response in humans, with IgA, IgG and IgM being the three main serum Ig (Deng et al., 1998; Fereidan-Esfahani et al., 2019). Usually, the inflammatory response promotes the release of Ig to co-opt the inflammatory response (Ho et al., 2020). In this study, the addition of β-alanine to the diet significantly reduced serum IgG and IgM levels, suggesting that β-alanine may alleviate the inflammatory response generated by weaning stress in piglets and reduce the release of Ig. Complement can participate in the process of elimination of pathogenic microorganisms by Ig and phagocytes, exerting a non-specific anti-infective effect. In the present study, serum C3 levels were significantly lower in the β-alanine group, suggesting that β-alanine may have reduced the body's non-specific immune response. Serum globulin and serum albumin are the main components of serum proteins and their ratio (AGR) is a combined indicator of inflammation and nutrition, with a decrease in AGR representing an increase in the level of the body's specific immune response. The results of this study found a tendency for β-alanine to increase serum AGR, suggesting that β-alanine also decreases the specific immune response. Weaning also causes changes in pro-inflammatory factors (Pié et al., 2004). Immune cells, such as activated T cells and macrophages, produce IL-6 rapidly during the acute response (Van Reeth and Nauwynck, 2000). The immune system activates with the excessive release of pro-inflammatory factors, subsequently allowing the body to induce inflammation and cause damage to intestinal tissues (Lee, 2015; Turner, 2009). Thus, β-alanine may reduce the body's immune response by alleviating the inflammatory response produced by weaning stress in piglets.
In this study, β-alanine was found to increase the height of ileal villi in piglets, with a significant increase in villi height at a dose of 300 mg/kg. This indicates that β-alanine has a beneficial effect on the atrophy of small intestinal villi caused by weaning stress. During the stressful period of weaning, the piglets’ intestines are damaged to varying degrees, manifested by atrophy of the small intestinal villi and hyperplasia of the crypts (Pluske et al., 1997b; Xiong et al., 2015). The morphology of the small intestine reflects the intestinal digestion and absorption capacity of pigs (Varel et al., 1987), and the higher the height of the small intestinal villi and the lower the depth of the crypt represent better intestinal digestion and absorption capacity (Cummings and Macfarlane, 1997). Furthermore, disaccharide digestive enzymes like maltase and sucrose serve as indicators of digestive and catabolic capacity and are positively related to intestinal absorption capacity (Pluske et al., 1997b). In this study, it was found that the addition of β-alanine to the diet tended to increase the jejunal maltase activity in weaned piglets and was highest at 600 mg/kg. This indicates that β-alanine has the ability to improve the intestinal morphological structure and digestive and absorption ability of piglets under weaning stress. Beta-alanine had a tendency to up-regulate the expression of amino acid transporter Slc6a1 and there was a certain dose effect. This result indicates that β-alanine contributes to the expression of amino acids in the body and promotes the digestion and absorption of nutrients to a certain extent.
The jejunal SOD activity was significantly increased at a β-alanine concentration of 600 mg/kg, suggesting that β-alanine has some effect on inhibiting oxidative stress in small intestinal epithelial cells. SOD, CAT, T-AOC and GSH-PX, as important antioxidant enzymes in the body, can play a protective role by scavenging free radicals in the body (Reiter et al., 2007). MDA, as an indicator of the intensity of oxidative stress, can indirectly reflect the degree of oxidative damage in tissues (Gaweł et al., 2004). We examined the mRNA expression of several antioxidant enzyme marker genes in the jejunum and found that 600 mg/kg of β-alanine tended to upregulate the expression of GSH-PX and SOD mRNA in jejunum. Therefore, β-alanine has a certain effect on increasing the expression of antioxidant enzymes and reducing oxidative stress. As carnosine is a recognised antioxidant, the increased antioxidant capacity of the organism in the present results may be due to the promotion of carnosine synthesis by β-alanine in the organism. Therefore, the addition of a certain concentration of β-alanine to the diet may inhibit oxidative stress in the small intestine of piglets and promote intestinal health by increasing the organismal myostatin content (not measured in this study).
IL-10, a potent immunosuppressive cytokine (Suradhat et al., 2003; Suradhat and Thanawongnuwech, 2003), regulates the growth and differentiation of immune cells and inhibits the production of proinflammatory and chemokines, thereby attenuating the inflammatory response (Wannemacher Jr, 1977). Hoffman et al. (2018) showed that continuous intake of 12 g of β-alanine for one week increased IL-10 secretion and enhanced the anti-inflammatory response during high-intensity military training. Similar to this result, the addition of β-alanine to the diet in this study tended to upregulate IL-10 gene expression in the jejunum. Weaning stress causes an increase in the production of pro-inflammatory cytokines, which cause inflammation by redistributing nutrients in the body, reducing nutrients to maintain growth, and strengthening the immune response (Cheng et al., 2018). In combination with the effect of β-alanine on serum immune parameters, it is suggested that the addition of β-alanine to the diet can reduce nutrient depletion due to immune activation, promote nutrient absorption and utilization in the gut and increase the expression of anti-inflammatory factors in piglets by inhibiting serum Ig, complement and pro-inflammatory factors. Beta-alanine may specifically activate the excitability of MrgprD in the body, which is involved in the regulation of inflammation (Beaudry et al., 2017) and reduction of immune stress (Zhang et al., 2021). Therefore, β-alanine may play an indirect immunomodulatory function by enhancing MrgprD expression and the exact mechanism needs to be further investigated.
In the present study, the expression of the ZO-1 gene and occludin gene were significantly upregulated at a β-alanine concentration of 600 mg/kg. When the intestinal epithelial barrier function was impaired, the expression of tight junction proteins such as Claudin, Occludin, and ZO-1 was inhibited. It has been shown that weaning stress in piglets promotes intestinal pro-inflammatory cytokine expression and suppresses occludin expression (Ewaschuk et al., 2011). It is hypothesized that β-alanine promotes the formation of tight junctions between intestinal epithelial cells to a certain extent, preventing the entry of harmful substances and enhancing the function of the intestinal defence barrier.
Three hundred milligrams per kilogram of β-alanine significantly increased the number of Ki67-positive cells in the jejunal crypt, indicating that β-alanine promoted the proliferation and differentiation of intestinal epithelial cells. In this study, the intestinal epithelial goblet cells of the jejunum and ileum were counted, and it was found that the addition of β-alanine in the diet tended to increase the goblet cells of jejunal crypts, but had no significant effect on ileal goblet cells. Since weaning stress seriously affects the development and renewal process of intestinal epithelium, it is easy to cause serious damage to the intestinal structure of piglets. The dietary addition of β-alanine promoted the increase of Ki67-positive cells and goblet cells, which indicated that β-alanine could promote the growth and repair of piglet intestine to some extent. In combination with the intestinal organoid model treatment, high concentrations of β-alanine (1 and 10 mM) significantly increased the budding rate and the average budding number per organoid on day 3 of the treatment. On day 4, 0.01 and 1 mM of β-alanine significantly increased the budding height of crypt organs. Since intestinal stem cells are the driving force for rapid renewal of small intestinal epithelial cells (Barker et al., 2007b), they play an important role in repairing intestinal morphology and promoting intestinal development (Barker et al., 2008b). Crypt outgrowth of organoids may be similar to intestinal stem cell proliferation and crypt fission (Fuller et al., 2012). As with the renewal of intestinal epithelial cells along the crypt–villi axis, the height of crypt outgrowth is positively correlated with intestinal stem cell proliferation and differentiation (Yin et al., 2019). Beta-alanine could promote the proliferation and differentiation of intestinal stem cells to some extent, and may be involved in the regulation of intestinal stem cell activity. Thus, the intestinal organoid results further validate the role of β-alanine in promoting the proliferation and differentiation of intestinal cells.
5. Conclusion
In conclusion, β-alanine reduces some immune markers (IgG, IgM, C3, IL-6), increases the activity of intestinal digestive enzymes (jejunal maltase) and antioxidant enzymes (GSH-PX, SOD), and promotes the expression of intestinal immune barrier genes (ZO-1, Occludin). Moreover, β-alanine could improve the effect of intestinal morphology and structure on weaning stress in piglets by promoting the proliferation and differentiation of intestinal crypt cells. The results in this study showed that the addition of β-alanine to the diet increased the expression of Ki67-positive cells in the jejunal crypts and tended to up-regulate the goblet cells in the jejunal crypts. In the intestinal organoid model, β-alanine treatment at appropriate concentrations significantly increased the budding rate of organoids and the budding number per organoid. Beta-alanine can promote the proliferation and differentiation of intestinal stem cells to a certain extent, which has a greater potential in alleviating weaning stress in piglets, and provides a scientific reference for the use of β-alanine in piglet production practice. According to the present results, the optimal concentration of β-alanine is 600 mg/kg, but large group experiments are still required for further investigation. However, this is only a preliminary experiment, and the specific mechanism of β-alanine on the regulation of intestinal stem cell activity and immune regulation needs further study.
Author contributions
Linlin Chen: Conceptualization, Formal analysis, Data curation, Writing -original draft; Yan Zhong: Methodology, Data curation, Writing-original draft; Xiangqin Ouyang: Methodology, software, Investigation. Chunfeng Wang: Methodology, Software. Lanmei Yin: Data curation, Investigation. Jing Huang: Investigation, Funding acquisition; Yali Li: Methodology, Resources; Qiye Wang: Resources; Junyan Xie: Validation, Funding acquisition; Pengfei Huang: Supervision, Validation; Huansheng Yang: Supervision, Project administration; Yulong Yin: Supervision, Review & editing, Project administration.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was financially supported by Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (No. TSBICIP-CXRC-038) and Scientific Research Project of Hunan Education Department (No. 19C1110).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Pengfei Huang, Email: perfehuang@foxmail.com.
Yulong Yin, Email: yinyulong@isa.ac.cn.
References
- Baguet A., Everaert I., Yard B., Peters V., Zschocke J., Zutinic A., De Heer E., Podgórski T., Domaszewska K., Derave W. Does low serum carnosinase activity favor high-intensity exercise capacity? J Appl Physiol. 2014;116:553–559. doi: 10.1152/japplphysiol.01218.2013. [DOI] [PubMed] [Google Scholar]
- Barker N., Van De Wetering M., Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Van De Wetering M., Clevers H. JG, development. Intestin Stem Cell. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Van Es J.H., Kuipers J., Kujala P., Van Den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N., Van Es J.H., Kuipers J., Kujala P., Van Den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J.J.N. Identification of stem cells in small intestine and colon by marker gene lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Beaudry H., Daou I., Ase A.R., Ribeiro-Da-Silva A., Séguéla P. Distinct behavioral responses evoked by selective optogenetic stimulation of the major trpv1+ and mrgd+ subsets of c-fibers. Pain. 2017;158:2329–2339. doi: 10.1097/j.pain.0000000000001016. [DOI] [PubMed] [Google Scholar]
- Chen C., Wang Z., Li J., Li Y., Huang P., Ding X., Yin J., He S., Yang H., Yin Y. Dietary vitamin e affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets. J Anim Sci. 2019;97:1212–1221. doi: 10.1093/jas/skz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Wei H., Xu C., Xie X., Jiang S., Peng J. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.01047-18. e01047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy J.P., Subramanian V. Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech Dev. 2001;101:3–9. doi: 10.1016/s0925-4773(00)00557-8. [DOI] [PubMed] [Google Scholar]
- Coxon K., Chakauya E., Ottenhof H., Whitney H., Blundell T., Abell C., Smith A. Pantothenate biosynthesis in higher plants. Biochem Soc Trans. 2005;33:743–746. doi: 10.1042/BST0330743. [DOI] [PubMed] [Google Scholar]
- Cummings J.H., Macfarlane G.T. Role of intestinal bacteria in nutrient metabolism. JPEN - J Parenter Enter Nutr. 1997;21:357–365. doi: 10.1177/0148607197021006357. [DOI] [PubMed] [Google Scholar]
- Dekaney C.M., Bazer F.W., Jaeger L.A. Mucosal morphogenesis and cytodifferentiation in fetal porcine small intestine. Anat Rec. 1997;249:517–523. doi: 10.1002/(SICI)1097-0185(199712)249:4<517::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Deng G., Jiang Z., Liu Y., He G., Xu Y. Dietary fiber protects intestinal structure and barrier function. Zhonghua Wai Ke Za Zhi. 1998;36:759–762. [PubMed] [Google Scholar]
- Drozak J., Veiga-Da-Cunha M., Vertommen D., Stroobant V., Van Schaftingen E. Molecular identification of carnosine synthase as atp-grasp domain-containing protein 1 (atpgd1) J Biol Chem. 2010;285:9346–9356. doi: 10.1074/jbc.M109.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert I., Mooyaart A., Baguet A., Zutinic A., Baelde H., Achten E., Taes Y., De Heer E., Derave W. Vegetarianism, female gender and increasing age, but not cndp1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids. 2011;40:1221–1229. doi: 10.1007/s00726-010-0749-2. [DOI] [PubMed] [Google Scholar]
- Ewaschuk J.B., Murdoch G.K., Johnson I.R., Madsen K.L., Field C.J. Glutamine supplementation improves intestinal barrier function in a weaned piglet model of escherichia coli infection. Br J Nutr. 2011;106:870–877. doi: 10.1017/S0007114511001152. [DOI] [PubMed] [Google Scholar]
- Fereidan-Esfahani M., Nayfeh T., Warrington A., Howe C.L., Rodriguez M. IgM natural autoantibodies in physiology and the treatment of disease. Methods Mol Biol. 2019:53–81. doi: 10.1007/978-1-4939-8958-4_3. [DOI] [PubMed] [Google Scholar]
- Fujii M., Matano M., Toshimitsu K., Takano A., Mikami Y., Nishikori S., Sugimoto S., Sato T. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. 2018;23:787–793. doi: 10.1016/j.stem.2018.11.016. e6. [DOI] [PubMed] [Google Scholar]
- Fuller M.K., Faulk D.M., Sundaram N., Shroyer N.F., Henning S.J., Helmrath M. Intestinal crypts reproducibly expand in culture. J Surg Res. 2012;178:48–54. doi: 10.1016/j.jss.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaweł S., Wardas M., Niedworok E., Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004;57:453–455. [PubMed] [Google Scholar]
- Gordon J.I. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989;108:1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi S., Miryan M., Soleimani D., Amani R., Mazaheri Tehrani M., Hadi V., Esmaiil Zali M., Mosalmanzadeh N., Askari G. The effect of food ration bar enriched with β-alanine, l-arginine, and nigella sativa on performance and inflammation following intense military training: a double-blind randomized clinical trial. Food Sci Nutr. 2021;9:3512–3520. doi: 10.1002/fsn3.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Chan H., Liang Y., Liu X., Zhang L., Li Q., Zhang Y., Zeng J., Ugwu F.N., Ho I. Cathelicidin preserves intestinal barrier function in polymicrobial sepsis. Crit Care. 2020;24:1–16. doi: 10.1186/s13054-020-2754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J.R., Gepner Y., Hoffman M.W., Zelicha H., Shapira S., Ostfeld I. Effect of high-dose, short-duration β-alanine supplementation on circulating il-10 concentrations during intense military training. J Strength Cond Res. 2018;32:2978–2981. doi: 10.1519/JSC.0000000000002625. [DOI] [PubMed] [Google Scholar]
- Jacob J., Blair R., Hart L., Gardiner E. The effect of taurine transport antagonists on cardiac taurine concentration and the incidence of sudden death syndrome in male broiler chickens. Poult Sci. 1991;70:561–567. doi: 10.3382/ps.0700561. [DOI] [PubMed] [Google Scholar]
- Kralik G., Sak-Bosnar M., Grčević M., Kralik Z. Effect of amino acids on growth performance, carcass characteristics, meat quality, and carnosine concentration in broiler chickens. J Poult Sci. 2018 doi: 10.2141/jpsa.0170083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner J., Albrecht A., Mittler M., Marx A., Kreyenschmidt J., Hess V., H Sauerwein. Effect of feeding histidine and β-alanine on carnosine concentration, growth performance, and meat quality of broiler chickens. Poult Sci. 2021;100 doi: 10.1016/j.psj.2021.101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L., Xu M., Li J., Liu L., Xu M., Zhou C., Shen L., Tang Z., Wan F. Mas-related g protein-coupled receptor d participates in inflammatory pain by promoting NF-κB activation through interaction with tak1 and ikk complex. Cell Signal. 2020;76 doi: 10.1016/j.cellsig.2020.109813. [DOI] [PubMed] [Google Scholar]
- Lee S. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N.Y., Jabaji Z., Wang J., Joshi V.S., Brinkley G.J., Khalil H., Wang F., Jaroszewicz A., Pellegrini M., Li L., Lewis M., Stelzner M., Dunn J.C., Martín M.G. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L., Cromwell G., Crum A., Decker E. Influence of dietary β-alanine and histidine on the oxidative stability of pork. Meat Sci. 1998;49:55–64. doi: 10.1016/s0309-1740(97)00108-3. [DOI] [PubMed] [Google Scholar]
- Peiretti P.G., Medana C., Visentin S., Giancotti V., Zunino V., Meineri G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food Chem. 2011;126:1939–1947. doi: 10.1016/j.foodchem.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Pié S., LallèS J.P., Blazy F., Laffitte J., Sève B., Oswald I. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Pluske J., Williams I., Aherne F. Villous height and crypt depth in piglets in response to increases in the intake of cows' milk after weaning. Anim Sci. 1996;62:145–158. [Google Scholar]
- Pluske J.R., Hampson D.J., Williams I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51:215–236. [Google Scholar]
- Pluske J.R., Hampson D.J., Williams I. Factors influencing the structure and function of the small intestine in the weaned pig. Review. 1997;51:215–236. [Google Scholar]
- Pluske J.R., Thompson M.J., Atwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows' whole milk after weaning. Br J Nutr. 1996;76:409–422. doi: 10.1079/bjn19960046. [DOI] [PubMed] [Google Scholar]
- Qi B., Wang J., Ma Y.-B., Wu S.-G., Qi G.-H., Zhang H.-J. Effect of dietary β-alanine supplementation on growth performance, meat quality, carnosine content, and gene expression of carnosine-related enzymes in broilers. Poult Sci. 2018;97:1220–1228. doi: 10.3382/ps/pex410. [DOI] [PubMed] [Google Scholar]
- Reiter R.J., Tan D.-X., Terron M.P., Flores L.J., Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54:1–9. [PubMed] [Google Scholar]
- Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., Van De Wetering M., Barker N., Stange D.E., Van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Harada M., Ogi K., Maruyama M., Fujii R., Tanaka H., Fukusumi S., Komatsu H., Hosoya M., Noguchi Y. Identification of a g protein-coupled receptor specifically responsive to β-alanine. J Biol Chem. 2004;279:23559–23564. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- Stoll B., Henry J., Reeds P.J., Yu H., Jahoor F., Burrin D. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr. 1998;128:606–614. doi: 10.1093/jn/128.3.606. [DOI] [PubMed] [Google Scholar]
- Suradhat S., Thanawongnuwech R., Poovorawan Y. Upregulation of il-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003;84:453–459. doi: 10.1099/vir.0.18698-0. [DOI] [PubMed] [Google Scholar]
- Suradhat S., Thanawongnuwech R. Upregulation of interleukin-10 gene expression in the leukocytes of pigs infected with porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003;84:2755–2760. doi: 10.1099/vir.0.19230-0. [DOI] [PubMed] [Google Scholar]
- Tiedje K., Stevens K., Barnes S., Weaver D. Β-alanine as a small molecule neurotransmitter. Neurochem Int. 2010;57:177–188. doi: 10.1016/j.neuint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Tomonaga S., Kaneko K., Kaji Y., Kido Y., Denbow D.M., Furuse M. Dietary β-alanine enhances brain, but not muscle, carnosine and anserine concentrations in broilers. Anim Sci. 2006;77:79–86. [Google Scholar]
- Tomonaga S., Matsumoto M., Furuse M. Β-alanine enhances brain and muscle carnosine levels in broiler chicks. J Poult Sci. 2012 [Google Scholar]
- Turner J. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Van Beers-Schreurs H.M., Nabuurs M.J., Vellenga L., Kalsbeek-Van Der Valk H.J., Wensing T., Breukink H.J. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J Nutr. 1998;128:947–953. doi: 10.1093/jn/128.6.947. [DOI] [PubMed] [Google Scholar]
- Van Der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Van Reeth K., Nauwynck H. Proinflammatory cytokines and viral respiratory disease in pigs. Vet Res. 2000;31:187–213. doi: 10.1051/vetres:2000113. [DOI] [PubMed] [Google Scholar]
- Varel V.H., Robinson I., Pond W. Effect of dietary copper sulfate, aureo sp250, or clinoptilolite on ureolytic bacteria found in the pig large intestine. Appl Environ Microbiol. 1987;53:2009–2012. doi: 10.1128/aem.53.9.2009-2012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Qiao S., Li D. Amino acids and gut function. Amino Acids. 2009;37:105–110. doi: 10.1007/s00726-008-0152-4. [DOI] [PubMed] [Google Scholar]
- Wannemacher Jr R. Key role of various individual amino acids in host response to infection. Am J Clin Nutr. 1977;30:1269–1280. doi: 10.1093/ajcn/30.8.1269. [DOI] [PubMed] [Google Scholar]
- Wehkamp J., Stange E.F. Paneth's disease. J Crohns Colitis. 2010;4:523–531. doi: 10.1016/j.crohns.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H., Wang X., Hu Q., Liu C., Wu X., Deng D., Hou Y., Nyachoti C., Xiao D. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J Anim Sci. 2015;93:1089–1097. doi: 10.2527/jas.2014-7851. [DOI] [PubMed] [Google Scholar]
- Yin Y.-B., Guo S.-G., Wan D., Wu X., Yin Y.-L. Enteroids: promising in vitro models for studies of intestinal physiology and nutrition in farm animals. J Agric Food Chem. 2019;67:2421–2428. doi: 10.1021/acs.jafc.8b06908. [DOI] [PubMed] [Google Scholar]
- Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X., Ichinose S., Nagaishi T., Okamoto R., Tsuchiya K., Clevers H., Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult lgr5⁺ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- Zachos N.C., Kovbasnjuk O., Foulke-Abel J., In J., Blutt S.E., De Jonge H.R., Estes M.K., Donowitz M. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J Biol Chem. 2016;291:3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Edwards T.N., Chaudhri V.K., Wu J., Cohen J.A., Hirai T., Rittenhouse N., Schmitz E.G., Zhou P.Y., Mcneil B. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell. 2021;184:2151–2166. doi: 10.1016/j.cell.2021.03.002. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]