Abstract
OBJECTIVE
To study prognosis after a first-ever myocardial infarction (MI) in type 1 diabetes, as well as how different MI- and diabetes-related factors affect the prognosis and risk of secondary cardiovascular events.
RESEARCH DESIGN AND METHODS
In this observational follow-up study of 4,217 individuals from the Finnish Diabetic Nephropathy (FinnDiane) Study with no prior MI or coronary revascularization, we verified 253 (6.0%) MIs from medical records or death certificates. Mortality from cardiovascular or diabetes-related cause was our main end point, whereas hospitalization due to heart failure, coronary revascularization, and recurrent MI were secondary end points, while accounting for death as a competing risk.
RESULTS
Of the individuals studied, 187 (73.9%) died during the median post-MI follow-up of 3.07 (interquartile range 0.02–8.45) years. Independent risk factors for cardiovascular and diabetes-related mortality were estimated glomerular filtration rate categories grade 3 (G3) (hazard ratio [HR] 3.27 [95% CI 1.76–6.08]), G4 (3.62 [1.69–7.73]), and G5 (4.03 [2.24–7.26]); prior coronary heart disease diagnosis (1.50 [1.03–2.20]); and older age at MI (1.03 [1.00–1.05]). Factors associated with lower mortality were acute revascularization (HR 0.35 [95% CI 0.18–0.72]) and subacute revascularization (0.39 [0.26–0.59]). In Fine and Gray competing risk analyses, kidney failure was associated with a higher risk of recurrent MI (subdistribution HR 3.27 [95% CI 2.01–5.34]), heart failure (3.76 [2.46–5.76]), and coronary revascularization (3.04 [1.89–4.90]).
CONCLUSIONS
Individuals with type 1 diabetes have a high cardiovascular and diabetes-related mortality after their first-ever MI. In particular, poor kidney function is associated with high mortality and excessive risk of secondary cardiovascular events.
Graphical Abstract
Introduction
Type 1 diabetes is accompanied by a markedly increased risk for cardiovascular disease (1). Cardiovascular disease is often a result of atherosclerosis, which is a complex process involving endothelial dysfunction, oxidative stress, and local inflammation. Chronic hyperglycemia accelerates the chain of events in individuals with diabetes (2). The cardiovascular disease observed in type 1 diabetes shows multiple differences compared with the general population. In type 1 diabetes, atherosclerosis occurs at a younger age, and the progression is much more extensive (3). In addition, individuals with the same symptoms tend to have a more severe and diffuse coronary heart disease (4), and asymptomatic myocardial ischemia is a prevalent finding (5). Despite a vast decrease in cardiovascular disease morbidity and mortality during the past five decades in type 1 diabetes (6), there remains a significant excess rate of death from coronary heart disease compared with the general population (7).
The clinical definition of an acute myocardial infarction (MI) requires elevated cardiac biomarkers indicating myocardial injury combined with evidence of myocardial ischemia (8). The typical initiating mechanism for MI is erosion or rupture of a coronary atherosclerotic plaque, often resulting in an intraluminal occlusive thrombus (9). MIs are classified into numerous subtypes based on differences in electrocardiogram (ECG) findings, pathology, and clinical evaluation (8). In type 1 diabetes, the strongest predictors of MI are duration of diabetes, high levels of LDL cholesterol, and the presence of albuminuria (1,10).
Type 1 diabetes is associated with a worse prognosis after first MI in young adults (11). In the general population, factors associated with worse post-MI survival include older age, poor left ventricular ejection fraction (12), anterior location (13), severity of the underlying coronary heart disease (14), and the presence of chronic kidney disease (CKD) (15). In type 2 diabetes, a longer duration of diabetes (16), insulin treatment (17), and the presence of heart failure are associated with higher mortality and risk of adverse cardiovascular events (18). However, in type 1 diabetes, the impact of diabetes- and infarction-related factors on prognosis are still unknown. Thus, our aim was to study prognosis after a first-ever MI and to assess the impact of diabetes- and MI-related factors on prognosis in Caucasian individuals with type 1 diabetes.
Research Design and Methods
Participants
All participants are part of the Finnish Diabetic Nephropathy (FinnDiane) Study, which is a nationwide, multicenter study with extensive clinical and genetic data collected on individuals with type 1 diabetes. A thorough description of the study has been reported (19). In brief, the FinnDiane Study was launched in 1997 with the aim to identify clinical and genetic risk factors and mechanisms for micro- and macrovascular complications of type 1 diabetes. The study includes Caucasian adults recruited at 77 centers throughout Finland (Supplementary Table 1). The only inclusion criterion for participation in the FinnDiane Study is a diagnosis of type 1 diabetes, and the study represents ∼10% of all individuals with type 1 diabetes in Finland. Although the FinnDiane Study is not population based by strict definitions, the geographical distribution of study participants follows well that of the Finnish general population. At the baseline study visit, all participating individuals undergo a full clinical evaluation, including registration of their medical history, current medical condition and medication, and lifestyle, such as smoking. Furthermore, blood samples are drawn and overnight or 24-h urine collections obtained. The study was approved by the ethics committee of Helsinki and Uusimaa Hospital District (Helsinki, Finland), and the study was performed in accordance with the Declaration of Helsinki. Each participant provided written informed consent.
Selection
For this study, we included all 4,217 participants without a previous history of MI or coronary revascularization who participated in the FinnDiane Study between 1995 and 2011 (clinical characteristics listed in Supplementary Table 2). Of these participants, we identified 286 (6.8%) and verified 253 (6.0%) who had their first MI between their first study visit and the end of 2012. The MI events were identified from the Finnish Care Register for Health Care and from the Finnish Cause of Death Register (ICD-10 code I21) and verified from medical records and death certificates. MI was defined as abnormal cardiac biomarkers in the setting of evidence of acute myocardial ischemia or as detected by autopsy examination. Type 1 diabetes was defined as age at diagnosis <40 years and initiation of insulin treatment within 1 year from diagnosis.
Diabetes-Related Factors
From the medical records at the time of the MI, we registered demographic characteristics, current smoking status, and diabetes-related factors, including medication, presence and severity of diabetic kidney disease, and history of coronary heart disease or coronary revascularization before the MI. In addition, we registered the most recent laboratory test results (within 1 year) for HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, and serum creatinine. Moderately increased albuminuria was defined as urinary albumin excretion rate ≥20 μg/min or ≥30 mg/24 h and severely increased albuminuria as ≥200 μg/min or ≥300 mg/24 h (urinary albumin-to-creatinine ratio ≥3 and >30 mg/mmol, respectively). Estimated glomerular filtration rate (eGFR) was evaluated using the Chronic Kidney Disease Epidemiology Collaboration equation (20). CKD was defined and staged in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines into normal or high eGFR (grade 1 [G1] ≥90 mL/min/1.73 m2), mildly decreased eGFR (G2 60–89 mL/min/1.73 m2), moderately decreased eGFR (G3 30–59 mL/min/1.73 m2), severely decreased eGFR (G4 15–29 mL/min/1.73 m2), and kidney failure (G5 <15 mL/min/1.73 m2 or dialysis treatment) (21).
MI-Related Factors
From the medical records, we also registered MI-related factors, such as ECG findings, angiography findings, troponin, and/or creatine kinase-MB concentrations. All the MIs included were verified and categorized by the study team (P.S. and L.M.T.). MIs were categorized on the basis of ECG findings into ST-elevation MIs (STEMIs) and non-STEMIs (NSTEMIs). STEMI was defined as new bundle branch blocks with ischemic repolarization patterns or new J-point ST-elevation in two or more adjacent leads with ≥1 mm in all other leads than V2–V3, where the cut point was ≥2 mm for men aged ≥40 years, ≥2.5 mm for men <40 years, and ≥1.5 mm for women. MIs that did not meet the criteria for STEMI were defined as NSTEMI (9). The MIs were also categorized based on pathological and clinical presentation in accordance with the fourth universal definition of MI (8). In addition, we recorded clinical symptoms (chest pain, dyspnea, and nausea) at the time of MI, as well as acute treatment (within 120 min) and subacute treatment (after 120 min and within 28 days). Acute revascularization was defined as a percutaneous coronary intervention or coronary artery bypass graft performed within 120 min of diagnosis; meanwhile, acute thrombolysis was defined as thrombolysis initiation within the same time. Subacute revascularization was defined as percutaneous coronary intervention or coronary artery bypass graft performed after 120 min of diagnosis (22).
Follow-up Data
We retrieved follow-up data until the end of 2017. Our main end point was death from cardiovascular or diabetes-related causes. Data on mortality were retrieved from the Finnish Cause of Death Register, provided by Statistics Finland. Causes of death were classified based on death certificates into cardiovascular causes (cardio- or cerebrovascular cause as underlying or immediate cause of death), diabetes-related causes (any acute or chronic complication as underlying or immediate cause of death), or other. For our secondary cardiovascular end points, we retrieved data from the Finnish Care Register for Health Care on recurrent MIs (ICD-10 codes I21–I23), hospitalizations due to heart failure (ICD-10 code I11.0 or I50), and coronary revascularizations (Nordic Medico-Statistical Committee [NOMESCO] Classification of Surgical Procedures codes FNA, FNB, FNC, FND, FNE, FNF, FNG, TFN40, FN1AT, FN1BT, or FN1YT for procedures before 2016 and fn1 codes for procedures after 2016) that occurred at least 28 days after the MI.
Statistical Analyses
All variables were tested for normal distribution. Variables with symmetric distribution were analyzed using t tests and presented as mean ± SD. Variables with a skewed distribution were analyzed using the Mann-Whitney U test and presented as median with interquartile range (IQR). Categorical variables were analyzed using χ2 test and presented as percentages. Kaplan-Meier analyses were conducted to assess the cumulative incidence of mortality. Cox regression analyses were performed to assess the independent impact of diabetes- and MI-related variables on prognosis, with cardiovascular or diabetes-related mortality as the end point. Variables with univariable associations at a significance level of 0.20 and clinically relevant variables based on academic literature were selected for the analyses. The first Cox regression model included age at MI, age at onset of diabetes, sex, current smoking status, HbA1c, coronary heart disease, antihypertensive medication, acute treatment, subacute treatment, eGFR category, and calendar year of MI. The second model included in addition HDL and LDL cholesterol, triglycerides, and lipid-lowering medication. The third model included the first model and additional factors with lesser data availability due to prehospital deaths, including chest pain, dyspnea, and ECG category.
To account for early mortality after MI when evaluating risk factors for secondary cardiovascular outcomes (recurrent MI, hospitalization due to heart failure, and coronary revascularization), we considered the competing risk of death. For purposes of the study, subdistribution hazard ratios (SHRs) were estimated with Fine and Gray competing risk models. Variables with univariable associations at a significance level of 0.20 and clinically relevant variables based on academic literature were selected for the multivariable Fine and Gray analyses. The relationship between these and the secondary outcomes of interest were analyzed in a two-step process. First, the associations were tested one variable at a time only, adjusting for nonmodifiable variables (Supplementary Table 3). Second, the nonmodifiable covariates and all variables with significant associations (P < 0.05) at the first stage were combined into a final multivariable model.
We did not replace missing data in any analyses. Analyses were performed using SPSS version 25.0.0 software (IBM Corporation, Armonk, NY) and R open-source software version 4.1.1 (https://www.r-project.org).
Results
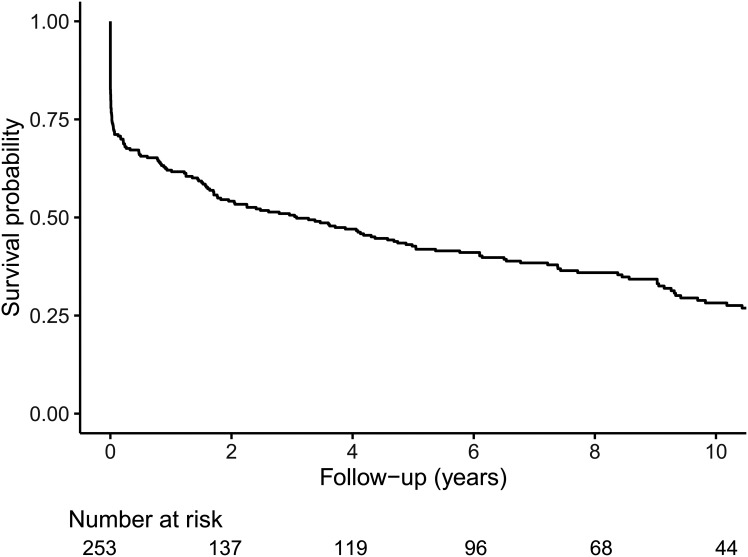
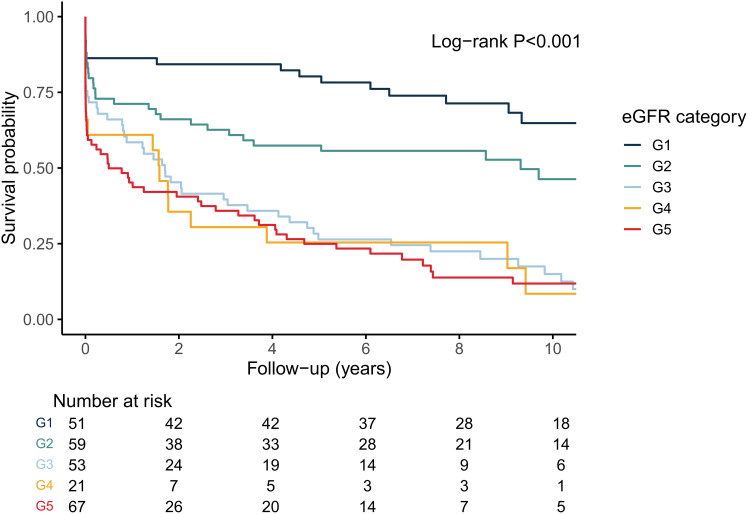
A total of 253 participants had their first-ever MI after inclusion in the FinnDiane Study during the median pre-MI follow-up of 4.69 (IQR 1.61–8.90) years. Mean age at MI was 52.4 ± 9.56 years. During the median post-MI survival time of 3.07 (0.02–8.45) years, 187 participants (73.9%) died. Their overall post-MI survival was 180 of 253 (71.1%) at 30 days, 157 of 253 (62.1%) at 1 year, and 108 of 253 (42.1%) at 5 years after their first-ever MI (Fig. 1). Survival according to eGFR categories for the combined cardiovascular and diabetes-related mortality are presented in Fig. 2. Kidney function at the time of the acute MI affected prognosis and was worse for G2 compared with G1 (P = 0.008) and G3–G5 compared with G2 (P < 0.001) but not for G3, G4, and G5 compared with each other (pairwise comparison P = 0.373–0.916).
Figure 1.
Kaplan-Meier survival probability after first-ever MI (all-cause mortality).
Figure 2.
Kaplan-Meier survival probability after first-ever MI (combined cardiovascular and diabetes-related mortality) according to eGFR category: G1, normal to increased eGFR (≥90 mL/min/1.73 m2); G2, mildly reduced eGFR (60–89 mL/min/1.73 m2); G3, moderately reduced eGFR (30–59 mL/min/1.73 m2); G4, severely reduced eGFR (15–29 mL/min/1.73 m2); and G5, kidney failure (<15 mL/min/1.73 m2 or treated by dialysis).
The baseline clinical characteristics according to cardiovascular or diabetes-related mortality during the post-MI follow-up are presented in Table 1. Participants who died as a result of a cardiovascular or diabetes-related cause during the follow-up period were more often men, had poorer kidney function and more often albuminuria, had higher total cholesterol and triglyceride concentrations, and used antihypertensive medication more frequently. In addition, participants with MI presented less often with chest pain and more often with dyspnea, had an NSTEMI more often, and underwent revascularization less frequently. There were no differences in age at MI, duration of diabetes, HbA1c concentrations, or current smoking status between groups.
Table 1.
Clinical characteristics at the time of the first MI according to cardiovascular or diabetes-related mortality during a median of 3.07 (IQR 0.02–8.45) years of follow-up
| Variable | Cardiovascular or diabetes-related mortality (n = 177) | No cardiovascular or diabetes-related mortality (n = 76) | P |
|---|---|---|---|
| Men, n (%) | 90 (50.8) | 49 (64.5) | 0.046 |
| Age, years | 52.7 (46.2–60.0) | 52.3 (47.0–56.0) | 0.430 |
| Diabetes onset age, years | 12.8 (8.0–18.4) | 14.3 (9.1–21.4) | 0.178 |
| Diabetes duration, years | 40.1 (33.8–45.6) | 38.3 (31.2–44.5) | 0.104 |
| HbA1c | |||
| % | 8.3 (7.3–9.4) | 8.4 (7.4–9.0) | 0.989 |
| mmol/mol | 67 (56–79) | 68 (57–75) | 0.989 |
| Cholesterol, mmol/L | |||
| Total | 4.4 (3.7–5.5) | 4.1 (3.5–5.0) | 0.024 |
| HDL | 1.4 (1.1–1.8) | 1.3 (1.1–1.7) | 0.489 |
| LDL | 2.3 (1.8–3.0) | 2.2 (1.6–2.9) | 0.162 |
| Triglycerides, mmol/L | 1.3 (0.9–1.8) | 1.1 (0.8–1.5) | 0.042 |
| Creatinine, μmol/L | 164 (104–468) | 87 (71–116) | <0.001 |
| eGFR category,* n (%) | <0.001 | ||
| G1 | 18 (10.3) | 33 (43.4) | |
| G2 | 34 (19.4) | 25 (32.9) | |
| G3 | 46 (26.3) | 7 (9.2) | |
| G4 | 18 (10.3) | 3 (3.9) | |
| G5 | 59 (33.7) | 8 (10.5) | |
| Albuminuria category, n (%) | <0.001 | ||
| Normal | 25 (14.2) | 32 (42.1) | |
| Moderately increased | 19 (10.8) | 16 (21.1) | |
| Severely increased | 36 (20.5) | 15 (19.7) | |
| Ongoing dialysis | 51 (29.0) | 5 (6.6) | |
| Functioning kidney transplant | 45 (25.6) | 8 (10.5) | |
| Prior CHD diagnosis, n (%) | 51 (28.8) | 14 (18.4) | 0.083 |
| Prior revascularization, n (%) | 15 (8.5) | 4 (5.3) | 0.368 |
| Antihypertensive medication, n (%) | 164 (93.2) | 62 (81.6) | 0.005 |
| Lipid-lowering medication, n (%) | 94 (55.3) | 47 (61.8) | 0.337 |
| Aspirin, n (%) | 107 (62.6) | 45 (59.2) | 0.616 |
| Current smoking status, n (%) | 37 (21.8) | 17 (23.0) | 0.834 |
| MI data | |||
| Symptoms, n (%) | |||
| Chest pain | 80 (51.9) | 62 (84.9) | <0.001 |
| Dyspnea | 50 (32.5) | 9 (12.3) | 0.001 |
| Nausea | 40 (26.0) | 21 (28.8) | 0.657 |
| ECG category, n (%) | 0.007 | ||
| STEMI | 28 (20.9) | 28 (38.4) | |
| NSTEMI | 106 (79.1) | 45 (61.6) | |
| Clinical category, n (%) | 0.001 | ||
| Type 1 | 139 (78.5) | 73 (96.1) | |
| Type 2 | 3 (1.7) | 1 (1.3) | |
| Type 3 | 34 (19.2) | 0 (0.0) | |
| Type 4a | 1 (0.6) | 2 (2.6) | |
| Type 4b | 0 (0.0) | 0 (0.0) | |
| Type 4c | 0 (0.0) | 0 (0.0) | |
| Type 5 | 0 (0.0) | 0 (0.0) | |
| Acute treatment,† n (%) | 0.001 | ||
| Conservative treatment | 158 (89.8) | 56 (73.7) | |
| Revascularization | 9 (5.1) | 15 (19.7) | |
| Thrombolysis | 9 (5.1) | 5 (6.6) | |
| Subacute treatment,† n (%) | <0.001 | ||
| Conservative treatment | 142 (80.7) | 37 (48.7) | |
| Revascularization | 34 (19.3) | 39 (51.3) | |
| Calendar year, n (%) | <0.001 | ||
| 1998–2003 | 78 (44.1) | 15 (19.7) | |
| 2004–2007 | 59 (33.3) | 28 (36.8) | |
| 2008–2012 | 40 (22.6) | 33 (43.4) | |
| Hospitalization time, days | 8 (1–15) | 6 (4–10) | 0.798 |
Data are median (IQR) unless otherwise indicated. CHD, coronary heart disease.
G1, normal to increased eGFR (≥90 mL/min/1.73 m2); G2, mildly reduced eGFR (60–89 mL/min/1.73 m2); G3, moderately reduced eGFR (30–59 mL/min/1.73 m2); G4, severely reduced eGFR (15–29 mL/min/1.73 m2); G5, kidney failure (<15 mL/min/1.73 m2 or treated by dialysis).
Acute treatment defined as within 120 min of MI diagnosis and subacute treatment as after 120 min but within 28 days of MI diagnosis.
In Cox regression analysis, albuminuria categories were not independently associated with cardiovascular and diabetes-related mortality after adjustment for eGFR categories, and thus, we excluded albuminuria categories from further analyses. After adjustment for other confounders, independent risk factors for cardiovascular or diabetes-related mortality were eGFR categories G3 (hazard ratio [HR] 3.27 [95% CI 1.76–6.08]; P < 0.001), G4 (3.62 [1.69–7.73]; P = 0.001), and G5 (4.03 [2.24–7.26]; P < 0.001), as well as older age at MI (1.03 [1.00–1.05]; P = 0.035) and prior coronary heart disease diagnosis (1.50 [1.03–2.20]; P = 0.034). Acute revascularization (HR 0.35 [95% CI 0.18–0.72]; P = 0.004) and subacute revascularization (0.39 [0.26–0.59]; P < 0.001) decreased the risk of mortality. For the subacute revascularization, this was true also when participants who died within 30 days of their MI were excluded (HR 0.41 [95% CI 0.25–0.68]; P < 0.001). In our second Cox regression model, HDL and LDL cholesterol, triglycerides, and lipid-lowering medication were not associated with cardiovascular or diabetes-related mortality. In our third Cox regression model, younger age at diabetes onset (HR 0.96 [95% CI 0.93–0.98]; P = 0.006) and the absence of chest pain (0.57 [0.38–0.86]; P = 0.008) were also associated with an increased risk of cardiovascular or diabetes-related mortality, while the acute treatment modality was no longer significant.
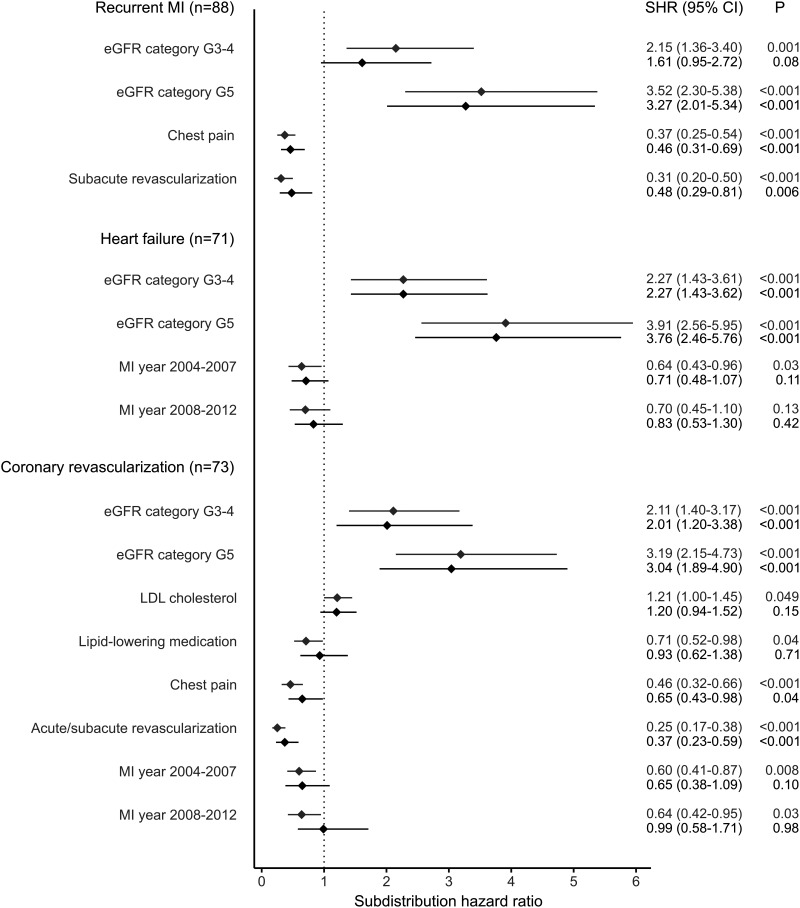
During post-MI follow-up, 128 participants (50.6%) experienced a secondary event. Of them, 88 (68.8%) had a recurrent MI, 71 (55.5%) were hospitalized for heart failure, and 73 (57.0%) underwent coronary revascularization. Of those with a secondary event, 54 (42.2%) had only one event, 44 (34.4%) had two separate events, and 30 (23.4%) experienced all three separate events (Supplementary Fig. 1). Results from the Fine and Gray competing risk analyses are illustrated in Fig. 3. Kidney failure (eGFR category G5 or dialysis treatment) was an independent risk factor for recurrent MI (SHR 3.27 [95% CI 2.01–5.34]; P < 0.001), while the presence of chest pain (0.46 [0.31–0.69]; P < 0.001) and subacute revascularization (0.48 [0.29–0.81]; P = 0.006) were associated with a lower risk of recurrent MI. With respect to heart failure, the only independent risk factors were eGFR categories G3 and G4 (SHR 2.27 [95% CI 1.43–3.61]; P < 0.001) and G5 (3.76 [2.46–5.76]; P < 0.001). For coronary revascularization, eGFR categories G3 and G4 (SHR 2.01 [95% CI 1.20–3.38]; P < 0.001) and G5 (3.04 [1.89–4.90]; P < 0.001) were independent risk factors, while the presence of chest pain (0.65 [0.43–0.98]; P = 0.037) and acute or subacute revascularization (0.37 [0.23–0.59]; P < 0.001) were associated with a lower risk.
Figure 3.
Forest plot of the Fine and Gray competing risk analyses for secondary cardiovascular outcomes. The first SHR per variable (gray color) is adjusted for nonmodifiable covariates (duration of diabetes and sex for recurrent MI; age and sex for hospitalization due to heart failure; and duration of diabetes, age at diabetes onset, and sex for coronary revascularization). The second SHR (black color) is adjusted for the covariates in the first adjustment model, as well as for all significant variables from the first step. eGFR categories were as follows: G3–4, eGFR 15–59 mL/min/1.73 m2, and G5, eGFR <15 mL/min/1.73 m2 or treated by dialysis. Acute revascularization defined as within 120 min of MI diagnosis and subacute revascularization as after 120 min but within 28 days of MI diagnosis. G1–2 (≥60 mL/min/1.73 m2) and MI years 1998–2003 served as reference categories.
Conclusions
In this nationwide study consisting of 4,217 individuals with type 1 diabetes, we identified 253 with their first-ever MI during follow-up. The overall post-MI prognosis was poor, the 30-day mortality was as high as 29%, and only one in four was alive at the end of follow-up. The main factor behind the poor prognosis was reduced kidney function. The MI-related factors associated with poor prognosis were prior coronary heart disease diagnosis and older age at MI, while presence of chest pain and acute and subacute revascularization were associated with a more favorable prognosis. As a novel finding, we observed that the diabetes- and MI-related factors differed in their impact on our secondary outcomes, that is, recurrent MI, hospitalization due to heart failure, and coronary revascularization.
In the current study, the MIs occurred at a relatively young age (mean 52.4 years) and were associated with a poor prognosis. The mortality within 30 days (29%) was comparable to population-based studies of individuals with and without diabetes but of clearly older age at MI (23,24). The 5-year mortality in our study was in line with data from individuals with MI between 1995 and 1999 but worse than for individuals with MI between 2000 and 2009 (24). We observed no association between calendar year of MI and prognosis. Furthermore, the 5-year mortality in our study was substantially higher compared with studies including individuals hospitalized for MI (17,25), and thus, unable to consider prehospital mortality related to MI. The one other study assessing the post-MI prognosis in type 1 diabetes included only individuals aged <40 years at MI, and in this study, the survival was clearly higher: 95% at 5 years (11).
In the current study, the main diabetes-related factor affecting prognosis was impaired kidney function. This was apparent already at moderately decreased eGFR (G3, 30–59 mL/min/1.73 m2), while further impairment of kidney function (G4 and G5) did not have any major additional impact, in contrast to what we expected. There could, for example, be some pre-MI survival bias in those with G4 and G5 eGFR that could explain this finding, but to explore this would warrant further studies.
Impaired kidney function is, though, one of the most important risk factors for MI in individuals with type 1 diabetes (1,10). Furthermore, impaired kidney function is associated with high post-MI mortality both in type 2 diabetes (17) and in the general population (15). Traditional factors that are associated with higher mortality after an MI in the general population, such as smoking, high levels of LDL cholesterol, and albuminuria (26–28), did not affect prognosis in our study. Interestingly, even though higher HbA1c is a risk factor for MI in type 1 diabetes (10), HbA1c did not affect prognosis in our study, similar to that observed in individuals with type 2 diabetes (17). The FinnDiane Study has previously shown that the mortality rates from ischemic heart disease are similar between men and women (29). Our finding here was comparable, as there was no difference in prognosis between men and women, underlining the loss of cardioprotection in women with type 1 diabetes. In addition, the FinnDiane Study showed earlier that the mortality rates from ischemic heart disease were higher in individuals with early-onset diabetes (29). This finding was also comparable to the current analysis, as younger age at diabetes onset was associated with a worse prognosis.
We found no association between type of MI and long-term prognosis. In the general population, STEMI is associated with a worse short-term prognosis (30); meanwhile, its impact on long-term prognosis is controversial and depends on length of follow-up (30–32). The 1-year mortality between STEMI and NSTEMI is comparable (31), while studies with longer follow-up showed worse prognosis of NSTEMI (30,32). Poor long-term survival in NSTEMI is associated with higher prevalence of comorbidities, such as dyslipidemia and diabetes (30,32). We observed that treatment of MI affected the prognosis, and both acute and subacute revascularizations were associated with a more favorable prognosis, in line with that observed in studies of individuals with type 2 diabetes (17,33). Our results are observational; thus, it is not possible to exclude bias based on how individuals were selected for revascularization. Those with diffuse coronary artery disease would, of course, not be candidates for invasive treatment to the same extent, and thus, absence of interventions could simply reflect a worse clinical picture of coronary artery disease. Another factor that could affect prognosis is cardiovascular autonomic neuropathy. In our study, individuals with registered chest pain had a better outcome with regard to mortality and recurrent MI. In contrast, absence of symptoms or atypical symptoms was associated with a worse prognosis and could indicate the presence of cardiovascular autonomic neuropathy, which is associated with both silent myocardial ischemia and a higher overall mortality and mortality after an MI (34). This is, however, speculative, since we did not register cardiovascular autonomic neuropathy in our study.
For our secondary end points, we found that CKD increased the risk of all studied end points and was the only factor that increased the risk of hospitalization due to heart failure. In the general population, CKD is strongly associated with congestive heart failure after an MI. This relationship is believed to be caused by complex neurohumoral damages resulting in hemodynamic changes and reduced kidney perfusion (15). For the other secondary end points of recurrent MI and coronary revascularization, the risk factors were similar, which was expected because MIs are an indication for revascularization (22). In the general population, CKD is also associated with a higher risk of these end points (15), in line with that observed in our study. Additionally, we observed that the presence of chest pain and revascularizations were associated with a lower risk of recurrent MI and new coronary revascularizations in type 1 diabetes. Chest pain after an MI is, interestingly, a risk factor for recurrent MI in the general population (35). This discrepancy could be due to chest pain being present in more severe coronary heart disease in the general population (36), whereas the lack of chest pain could be associated with cardiovascular autonomic neuropathy in type 1 diabetes (34). For coronary revascularizations, our results were in the same direction as in individuals with diabetes with nonfatal MI (37).
The strengths of our study are its nationwide design, including a thoroughly characterized cohort of individuals with type 1 diabetes. In addition, the study cohort included individuals with a fatal prehospital MI, which enabled us to assess the true post-MI mortality rate. Since every MI was identified from death certificates and hospital registers, we cannot rule out that those without MI could have had a silent or unregistered MI; however, for those with MI, the MI was verified and thoroughly characterized. Our study also has some limitations. Due to the observational study setting, all data from the time of the MI were reviewed from medical files. This restricted the factors we could consider, and no centrally measured laboratory data were thus available. The MIs occurred over a long period, from 1998 to 2012, during which the treatment modalities also improved significantly. Yet importantly, all analyses were adjusted for the calendar year of the MI, which was not an independent risk factor for poor prognosis.
In conclusion, our study shows that cardiovascular and diabetes-related mortality is high after a first-ever MI in type 1 diabetes. We also found that CKD is strongly associated with high mortality and that it is a risk factor of great importance for secondary cardiovascular events. Prevention of CKD is consequently crucial to improve prognosis in individuals with type 1 diabetes both before and after an MI.
Article Information
Acknowledgments. The authors are indebted to the late Carol Forsblom (1964–2022), the international coordinator of the FinnDiane Study, a brilliant researcher, as well as a person with type 1 diabetes, for his considerable contribution throughout the years and for this specific study. The authors are also grateful to biostatistician Paula Bergman from the University of Helsinki for helpful advice. The authors acknowledge A. Sandelin, J. Tuomikangas, and M. Korolainen (Folkhälsan Research Center, Helsinki, Finland) for technical assistance, as well as all the physicians and nurses at each center participating in the collection of the study population (Supplemental Table 1).
Funding. This research was funded by grants from Folkhälsan Research Foundation, Suomen Akatemia (316664), Wilhelm och Else Stockmanns Stiftelse, Sigrid Juséliuksen Säätiö, Liv och Hälsa Society, Finska Läkaresällskapet, Diabetestutkimussäätiö, Novo Nordisk Fonden (NNF OC0013659), Päivikki ja Sakari Sohlbergin Säätiö, EVO governmental grants (TYH2018207), Stiftelsen Dorothea Olivia, Karl Walther och Jarl Walther Perkléns Minne, and Aarno Koskelon Säätiö.
Duality of Interest. F.J.S. reports receiving a lecture honorarium from AstraZeneca. P.-H.G. reports receiving lecture fees from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Elo Water, Genzyme, Medscape, Merck Sharp & Dohme, Mundipharma, Novartis, Novo Nordisk, PeerVoice, Sanofi, and Sciarc and being an advisory board member for AbbVie, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Medscape, Merck Sharp & Dohme, Mundipharma, Nestlé, Novartis, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.S., F.J.S., and L.M.T. were responsible for the statistical analyses. P.S. and L.M.T. were responsible for the acquisition of the clinical data and preparation of the first draft of the manuscript. A.Y., N.E., and V.H. contributed to the acquisition of the clinical data. V.H. and L.M.T. were responsible for the study design. All authors interpreted the results, contributed to the critical revision of the manuscript, and approved the final version. P.-H.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 33rd Annual General Meeting of the European Diabetic Nephropathy Study Group, Virtual, 21–22 May 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21396999.
This article is featured in a podcast available at diabetesjournals.org/care/pages/diabetes_care_on_air.
A complete list of the FinnDiane Study Group can be found in the supplementary material online.
References
- 1. Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol 1987;59:750–755 [DOI] [PubMed] [Google Scholar]
- 2. King RJ, Grant PJ. Diabetes and cardiovascular disease: pathophysiology of a life-threatening epidemic. Herz 2016;41:184–192 [DOI] [PubMed] [Google Scholar]
- 3. Dahl-Jørgensen K, Larsen JR, Hanssen KF. Atherosclerosis in childhood and adolescent type 1 diabetes: early disease, early treatment? Diabetologia 2005;48:1445–1453 [DOI] [PubMed] [Google Scholar]
- 4. Valsania P, Zarich SW, Kowalchuk GJ, Kosinski E, Warram JH, Krolewski AS. Severity of coronary artery disease in young patients with insulin-dependent diabetes mellitus. Am Heart J 1991;122:695–700 [DOI] [PubMed] [Google Scholar]
- 5. Koistinen MJ. Prevalence of asymptomatic myocardial ischaemia in diabetic subjects. BMJ 1990;301:92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harjutsalo V, Pongrac Barlovic D, Groop PH. Long-term population-based trends in the incidence of cardiovascular disease in individuals with type 1 diabetes from Finland: a retrospective, nationwide, cohort study. Lancet Diabetes Endocrinol 2021;9:575–585 [DOI] [PubMed] [Google Scholar]
- 7. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418 [DOI] [PubMed] [Google Scholar]
- 8. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation 2018;138:e618–e651 [DOI] [PubMed] [Google Scholar]
- 9. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;368:2004–2013 [DOI] [PubMed] [Google Scholar]
- 10. Rawshani A, Rawshani A, Sattar N, et al. Relative prognostic importance and optimal levels of risk factors for mortality and cardiovascular outcomes in type 1 diabetes mellitus. Circulation 2019;139:1900–1912 [DOI] [PubMed] [Google Scholar]
- 11. Fournier JA, Cabezón S, Cayuela A, Ballesteros SM, Cortacero JAP, Díaz De La Llera LS. Long-term prognosis of patients having acute myocardial infarction when ≤40 years of age. Am J Cardiol 2004;94:989–992 [DOI] [PubMed] [Google Scholar]
- 12. Ahnve S, Gilpin E, Dittrich H, et al. First myocardial infarction: age and ejection fraction identify a low-risk group. Am Heart J 1988;116:925–932 [DOI] [PubMed] [Google Scholar]
- 13. Stone PH, Raabe DS, Jaffe AS, et al. Prognostic significance of location and type of myocardial infarction: independent adverse outcome associated with anterior location. J Am Coll Cardiol 1988;11:453–463 [DOI] [PubMed] [Google Scholar]
- 14. Sanz G, Castañer A, Betriu A, et al. Determinants of prognosis in survivors of myocardial infarction: a prospective clinical angiographic study. N Engl J Med 1982;306:1065–1070 [DOI] [PubMed] [Google Scholar]
- 15. Anavekar NS, McMurray JJV, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 2004;351:1285–1295 [DOI] [PubMed] [Google Scholar]
- 16. Afanasiev SA, Garganeeva AA, Kuzheleva EA, Andriyanova AV, Kondratieva DS, Popov SV. The impact of type 2 diabetes mellitus on long-term prognosis in patients of different ages with myocardial infarction. J Diabetes Res 2018;2018:1780683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold SV, Spertus JA, Jones PG, et al. Predicting adverse outcomes after myocardial infarction among patients with diabetes mellitus. Circ Cardiovasc Qual Outcomes 2016;9:372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Backhaus SJ, Kowallick JT, Stiermaier T, et al. Cardiac magnetic resonance myocardial feature tracking for optimized risk assessment after acute myocardial infarction in patients with type 2 diabetes. Diabetes 2020;69:1540–1548 [DOI] [PubMed] [Google Scholar]
- 19. Thorn LM, Forsblom C, Fagerudd J, et al.; FinnDiane Study Group . Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 2005;28:2019–2024 [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Supp 2013;3:1–150 [Google Scholar]
- 22. O’Gara PT, Kushner FG, Ascheim DD, et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–e425 [DOI] [PubMed] [Google Scholar]
- 23. Kannel WB, Sorlie P, McNamara PM. Prognosis after initial myocardial infarction: the Framingham study. Am J Cardiol 1979;44:53–59 [DOI] [PubMed] [Google Scholar]
- 24. Icks A, Claessen H, Kirchberger I, et al. Mortality after first myocardial infarction in diabetic and non-diabetic people between 1985 and 2009. The MONICA/KORA registry. Eur J Epidemiol 2014;29:899–909 [DOI] [PubMed] [Google Scholar]
- 25. Donahue RP, Goldberg RJ, Chen Z, Gore JM, Alpert JS. The influence of sex and diabetes mellitus on survival following acute myocardial infarction: a community-wide perspective. J Clin Epidemiol 1993;46:245–252 [DOI] [PubMed] [Google Scholar]
- 26. Steg PG, James SK, Atar D, et al.; Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology (ESC) . ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–2619 [DOI] [PubMed] [Google Scholar]
- 27. Schubert J, Lindahl B, Melhus H, et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J 2021;42:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berton G, Citro T, Palmieri R, Petucco S, De Toni R, Palatini P. Albumin excretion rate increases during acute myocardial infarction and strongly predicts early mortality. Circulation 1997;96:3338–3345 [DOI] [PubMed] [Google Scholar]
- 29. Harjutsalo V, Maric-Bilkan C, Forsblom C, Groop PH. Impact of sex and age at onset of diabetes on mortality from ischemic heart disease in patients with type 1 diabetes. Diabetes Care 2014;37:144–148 [DOI] [PubMed] [Google Scholar]
- 30. Park HW, Yoon CH, Kang SH, et al.; KAMIR/KorMI Registry . Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Int J Cardiol 2013;169:254–261 [DOI] [PubMed] [Google Scholar]
- 31. Montalescot G, Dallongeville J, Van Belle E, et al.; OPERA Investigators . STEMI and NSTEMI: are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur Heart J 2007;28:1409–1417 [DOI] [PubMed] [Google Scholar]
- 32. Terkelsen CJ, Lassen JF, Nørgaard BL, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 2005;26:18–26 [DOI] [PubMed] [Google Scholar]
- 33. Norhammar A, Malmberg K, Diderholm E, et al. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol 2004;43:585–591 [DOI] [PubMed] [Google Scholar]
- 34. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–397 [DOI] [PubMed] [Google Scholar]
- 35. Gilpin E, Ricou F, Dittrich H, Nicod P, Henning H, Ross J Jr. Factors associated with recurrent myocardial infarction within one year after acute myocardial infarction. Am Heart J 1991;121:457–465 [DOI] [PubMed] [Google Scholar]
- 36. Califf RM, Mark DB, Harrell FE Jr, et al. Importance of clinical measures of ischemia in the prognosis of patients with documented coronary artery disease. J Am Coll Cardiol 1988;11:20–26 [DOI] [PubMed] [Google Scholar]
- 37. Herlitz J, Wognsen GB, Emanuelsson H, et al. Mortality and morbidity in diabetic and nondiabetic patients during a 2-year period after coronary artery bypass grafting. Diabetes Care 1996;19:698–703 [DOI] [PubMed] [Google Scholar]