Abstract
Background
Uterine leiomyosarcoma (uLMS) may show loss of expression of B-cell lymphoma-2 (Bcl-2) protein. It has been suggested that Bcl-2 loss may both be a diagnostic marker and an unfavorable prognostic marker in uLMS.
Objective
To define the diagnostic and prognostic value of Bcl-2 loss in uLMS through a systematic review and meta-analysis.
Methods
Electronic databases were searched from their inception to May 2020 for all studies assessing the diagnostic and prognostic value of Bcl-2 loss of immunohistochemical expression in uLMS. Data were extracted to calculate odds ratio (OR) for the association of Bcl-2 with uLMS vs leiomyoma variants and smooth-muscle tumors of uncertain malignant potential (STUMP), and hazard ratio (HR) for overall survival; a p value < 0.05 was considered significant.
Results
Eight studies with 388 patients were included. Loss of Bcl-2 expression in uLMS was not significantly associated with a diagnosis of uLMS vs leiomyoma variants and STUMP (OR = 2.981; p = 0.48). Bcl-2 loss was significantly associated with shorter overall survival in uLMS (HR = 3.722; p = 0.006). High statistical heterogeneity was observed in both analyses.
Conclusion
Loss of Bcl-2 expression appears as a significant prognostic but not diagnostic marker in uLMS. The high heterogeneity observed highlights the need for further research and larger studies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00404-022-06531-2.
Keywords: bcl2, Immunohistochemistry, Leiomyoma, Prognosis, Smooth-muscle tumor
Introduction
Smooth-muscle tumors are the most common gynecologic neoplasms [1–4]. In most cases, they show that benign histological features, consisting of low mitotic index and absence of necrosis or cytologic atypia, and are labeled as leiomyoma [5, 6]. According to the Stanford criteria, a diagnosis of malignancy should be made in the presence of at least two of the following features: mitotic index > 10/10 high-power fields (HPF), at least moderate cytologic atypia, and coagulative tumor cell necrosis; in such cases, the tumor is labeled leiomyosarcoma [7]. Uterine leiomyosarcoma (uLMS) constitutes 60–70% of all uterine sarcomas [5]. The prognosis of uLMS is very poor, with less of half of patients being alive at 5 years [5]. Treatments for uLMS are non-specific and consist of chemotherapy and/or external beam radiotherapy [8].
The antiapoptotic protein B-cell lymphoma-2 (Bcl-2) has been proposed as diagnostic biomarker to distinguish uLMS from leiomyoma in challenging cases [9]. Indeed, Bcl-2 may show loss of immunohistochemical expression in uLMS, whereas it is almost always expressed in uterine leiomyomas [9]. In this regard, Bcl-2 loss might be a specific marker of malignancy, similarly to what was observed in endometrial pathology [10]. However, the reliability of Bcl-2 loss in differentiating uLMS from leiomyoma variants and smooth-muscle tumors of uncertain malignant potential (STUMP) is less clear. More interestingly, Bcl-2 has been proposed as a possible prognostic marker in uLMS [11–15]. In fact, it has been suggested that Bcl-2 loss identifies a subset of uLMS with more aggressive behavior [9, 11–15].
On this account, the aim of this study was to define whether Bcl-2 loss of expression is a significant diagnostic and prognostic marker in uLMS, by performing a systematic review and meta-analysis.
Materials and methods
Study protocol
This meta-analysis was designed based on previous studies [16, 17]. Each review stage (electronic search, study selection, data extraction, risk of bias within studies assessment, and data analysis) was independently performed by two authors; disagreements, if any, were solved by consensus among all authors. This review was reported following the PRISMA guidelines [18].
Search strategy and study selection
Four electronic databases (Web of Sciences, Scopus, MEDLINE, and Google Scholar) were searched from their inception to May 2020 for all studies assessing the prognostic value of Bcl-2 immunohistochemical expression in uLMS. The following combination of text words was used: (uterine OR uterus OR gynecologic) AND (leiomyosarcoma) AND (immunohistochemistry OR immunohistochemical OR bcl2 OR bcl-2). Reference lists of relevant studies were also searched.
Exclusion criteria, defined a priori, were: sample size < 10, overlapping patient data, reviews.
Data extraction
Data were extracted from primary studies by following the PICO [18]: “P” (population) consisted of patients with uLMS (for prognostic analysis) or with uLMS, leiomyoma variants or STUMP (for diagnostic analysis); “I” (intervention or risk factor) was the loss of Bcl-2 immunohistochemical expression; “C” (comparator) was a retained expression of Bcl-2; “O” (outcome) was the overall survival (for prognostic analysis) or a diagnosis of uLMS (for diagnostic analysis). Further extracted data were: country, period of enrollment, sample size, histological criteria for uLMS diagnosis, and methods for performing and interpreting immunohistochemistry, prognostic data.
Risk of bias within studies assessment
The risk of bias within studies was assessed according to the QUADAS-2 [19]. Four crucial domains were assessed: (1) Patient selection, i.e., if patient selection criteria and period of enrollment were reported; (2) Index test, i.e., if methods for performing and interpreting immunohistochemistry were clearly reported and unbiased; (3) Reference standard, i.e., if diagnostic criteria for uLMS were clearly reported and unbiased (for diagnostic analysis), or if data about prognosis were reported and unbiased (for prognostic analysis); (4) Flow, i.e., if all eligible patients were assessed for Bcl-2 expression and prognosis (the latter one for prognostic analysis). The risk of bias was categorized as “low”, “unclear” or “high” as previously described [20, 21].
Data analysis
Odds ratio (OR) with 95% confidence interval (CI) for the association of Bcl-2 loss with uLMS vs leiomyoma variants and STUMP was calculated for each study. Hazard ratio (HR) with 95% CI for overall survival was extracted or calculated from primary data for each study, as previously described [16]. If the primary study reported HR for Bcl-2 retained expression, we calculated the HR for Bcl-2 loss of expression as 1/HR. Pooled OR and HR were calculated using the random effect model of DerSimonian–Laird. Results were reported on forest plots. Statistical heterogeneity among studies was calculated using inconsistency index (I2) as previously described [22, 23]. The risk of bias across studies (publication bias) was assessed using a funnel plot reporting logarithm of OR/HR values on the x-axis and standard error on the y-axis.
Data analysis was performed using Comprehensive Meta-Analysis (Biostat, 14 North Dean Street, Englewood, NJ 07631, USA).
Results
Study selection and characteristics
Eight studies with a total sample size of 388 patients with uLMS were included [11–15, 24–26]; 64 leiomyoma variants (41 cellular leiomyomas and 23 leiomyomas with bizarre nuclei) and 22 STUMPs were also included. The process of study selection is shown in Supplementary Fig. 1. Four out of 8 studies evaluated the prognostic value of Bcl-2 in uLMS [12–14], 2 studies assessed Bcl-2 as a diagnostic marker of uLMS [25, 26], and 2 studies performed both diagnostic and prognostic analysis [11, 24]. Characteristics of the included studies are shown in Table 1; characteristics of patients and uLMS are shown in Table 2.
Table 1.
Characteristics of the included studies
| Study | Country | Period of enrollment | Sample size | ||
|---|---|---|---|---|---|
| LMS | STUMP | LM-V | |||
| Zhai [11] | Japan | Unclear | 21 | 8 | 23 |
| Bodner [24] | Austria | 1990–2000 | 21 | 14 | 0 |
| Leiser [12] | USA | 1991–2004 | 36 | 0 | 0 |
| Rath-Wolfson [25] | Israel | Unclear | 10 | 0 | 20 |
| D’Angelo [15] | Spain, Canada | 1978–2008 | 84 | 0 | 0 |
| Lusby [13] | USA | 1989–2011 | 157 | 0 | 0 |
| Stanescu [26] | Romania | 2009–2012 | 6 | 0 | 21 |
| Banas [14] | Poland | 2000–2015 | 53 | 0 | 0 |
LMS leiomyosarcoma, STUMP smooth-muscle tumors of uncertain malignant potential, LM-V leiomyoma variants
Table 2.
Characteristics of patients with uterine leiomyosarcoma
| Study | Patient age, mean/median (range) | Menopausal status | Nulliparous, n | Cycle phase | Tumor diameter, cm mean/median (range) |
Tumor stage (n) | Mitotic index, n/10HPF mean (range) |
Adjuvant treatment, n (type) | Follow-up time, mean/median (range) | Bcl-2 antibody, clone (manufacturer) | Criterion to define Bcl-2 loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhai et al. [11] | 52 (32–70) | Not reported | Not reported | Not reported | Not reported | I (12), II (3), III (3), IV (3) | 60 (14–133) | Not reported | 23 (1–101) months | Unclear (Dako) | < 5% |
| Bodner et al. [24] | 51 (36–78) | Not reported | Not reported | Not reported | Not reported | I (13), II (3), III (4), IV (1) | Not reported | 10 (8 RT, 2 CT) | 47 (1–228) months | 124 (Dako) | < 10% |
| Leiser et al. [12] | 53 (23–73) | Not reported | Not reported | Not reported | Not reported | I,II (19), III/IV (15) | Not reported | Not reported | 47 (17–84) months | 124 (Dako) | Weak in ≤ 80% or moderate/strong in ≤ 10% |
| Rath-Wolfson et al. [25] | 61 (50–84) | Not reported | Not reported | Not reported | 9 (4–15) | Not reported | Not reported | Not reported | Not assessed | Unclear | 0% (individual data reported) |
| D’Angelo et al. [15] | 51 (29–67) | Not reported | Not reported | Not reported | 11 (3–35) | I (71), III (3), IV (3) | Not reported (5–19/10HPF) | 31 (13 RT, 18 CT) | 36 (3–96) months | 100/D5 124 (Master Diagnostica) | < 50% |
| Lusby et al. [13] | 52 (19–83) | Not reported | 27 | Not reported | 11 (1–60) | Not reported | 21 (1–83) | 72 (24 RT, 8 CT) | 35 (18–200) months | 100 (Biogenex) | absent-to-weak intensity |
| Stanescu et al. [26] | 55 (not reported) | Not reported | Not reported | Not reported | Not reported | I | 13 (not reported) | Not reported | Not assessed | 124 (Dako) | < 5% |
| Banas et al. [14] | 51 (44–70) |
30 pre- 23 post- |
5 | First phase | 5 (2–15) | I (27), II (5), III (12), IV (9) | ≤ 50 (38 patients), > 50 (15 patients) | 32 (5 RT, 22 CT, 5 RT + CT) | Unclear | Unclear (Leica Biosystems) | < 10% |
RT radiotherapy, CT chemotherapy
Risk-of-bias assessment
For the “Patient selection” domain, unclear risk of bias was assigned to two studies (period of enrollment not reported) [11, 25], while low risk was assigned to the other studies.
For the “Index test” domain, low risk of bias was assigned to all included studies.
For the “reference standard”, unclear risk of bias was assigned to one study (follow-up duration not reported) [14], while low risk was assigned to the other studies.
For the “flow and timing” domain, unclear risk of bias was assigned to one study (only a subset of patients were assessed for the prognostic value of Bcl-2 expression) [13], while low risk was assigned to the other studies. Risk-of-bias results are presented graphically in Supplementary Fig. 2.
Meta-analysis
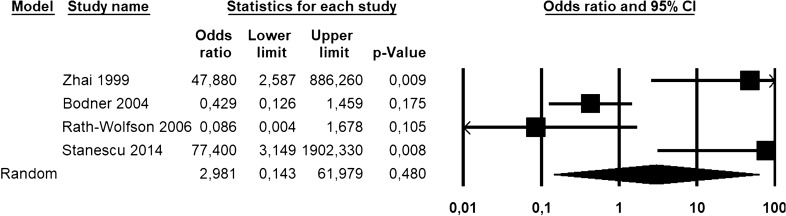
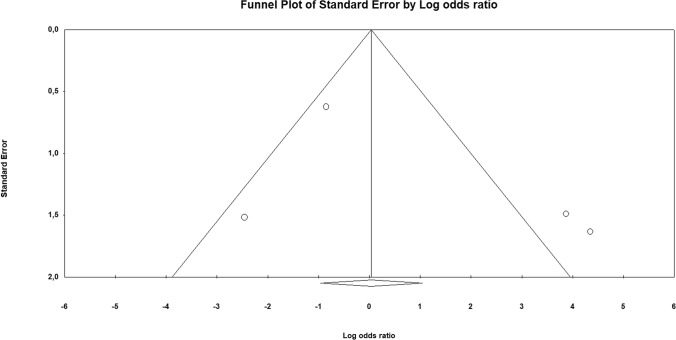
As a diagnostic marker, a loss of Bcl-2 immunohistochemical expression was not significantly associated with a diagnosis of uLMS vs leiomyoma variants and STUMP, with an OR of 2.981 (95% CI 0.143–61.979; p = 0.48) (Fig. 1). The statistical heterogeneity among studies was high (I2 = 83.598%). The funnel plot showed asymmetrical distribution of the primary studies, suggesting the possibility of a publication bias (Fig. 2).
Fig. 1.
Forest plot reporting odds ratio (OR) values with 95% confidence interval (CI), for each study and as pooled estimate, for the association of Bcl-2 loss of immunohistochemical expression with a diagnosis of uterine leiomyosarcoma vs leiomyoma variants and STUMP
Fig. 2.
Funnel plot of logarithm of odds ratio by standard error for the assessment of the risk of bias across studies (publication bias). The asymmetrical distribution of the primary studies suggests the possibility of a publication bias
As a prognostic marker, Bcl-2 loss was significantly associated with a decreased overall survival in uLMS, with an HR of 3.722 (95% CI 1.471–9.416; p = 0.006) (Fig. 3). The statistical heterogeneity among studies was high (I2 = 80.738%) and the funnel plot was asymmetrical (Fig. 4).
Fig. 3.
Forest plot reporting hazard ratio (HR) values with 95% confidence interval (CI), for each study and as pooled estimate, for the association of Bcl-2 loss of immunohistochemical expression with the risk of death in uterine leiomyosarcoma
Fig. 4.
Funnel plot of logarithm of hazard ratio by standard error for the assessment of the risk of bias across studies (publication bias). The asymmetrical distribution of the primary studies suggests the possibility of a publication bias
Discussion
Main findings and interpretation
This study showed that a loss of Bcl-2 immunohistochemical expression was significantly associated with shorter overall survival in uLMS, while it was not able to differentiate uLMS from leiomyoma variants and STUMP.
Bcl-2 protein is encoded by BCL2 gene, a proto-oncogene that promotes tumorigenesis by inhibiting cell death; the antiapoptotic action of Bcl-2 protects cells against the effect of both endogenous and exogenous factors, including chemotherapeutic drugs and glucocorticoids [27]. On the other hand, the loss of Bcl-2 expression might indicate that the tumor shifted toward different pro-survival pathways associated with more aggressive behavior; on this account, Bcl-2 loss has been proposed as a possible marker of tumor aggressiveness [28].
With regard to uterine smooth-muscle tumors, the previous studies showed that the expression of Bcl-2 was higher in uterine leiomyomas than in the normal myometrium [29, 30]. Furthermore, Bcl-2 has been one of the main markers proposed to distinguish between benign and malignant uterine smooth-muscle tumors [9]. Zhai et al. found that Bcl-2 loss was significantly more common in uLMS than in uterine smooth-muscle tumors of uncertain malignant potential (STUMP) [11], while Bodner-Adler et al. found a significant difference between usual leiomyoma and both STUMP and uLMS, but not between STUMP and uLMS [24]. As seen in other human neoplasms, Bcl-2 has also been assessed as a prognostic marker in uLMS. In fact, several studies suggested that a loss of Bcl-2 expression in uLMS was associated with a worst prognosis [9, 11–15]. However, the small size of the individual studies prevents from draw conclusions about the diagnostic and prognostic value of Bcl-2 in uLMS.
Our study found that a loss of Bcl-2 immunohistochemical expression was not significantly associated with a diagnosis of uLMS. Remarkably, we compared uLMS to leiomyoma variants and STUMP, excluding usual leiomyomas. In fact, differentiating between usual leiomyoma and uLMS is not an issue, while leiomyoma variants and STUMP may raise the concern of malignancy [6, 7]. In fact, these lesions may show worrisome features (such as high mitotic index, increased cellularity, infiltrative borders, cytologic atypia, atypical mitoses, or necrosis) which makes it difficult to rule out a uLMS [5–7]; it is in these cases that a reliable diagnostic marker of uLMS should work. Our results do not mean that the expression of Bcl-2 is the same between uLMS, STUMP and leiomyoma variants. Instead, they suggest that a difference may exist (as indicated by the OR > 1), but is not statistically significant, making Bcl-2 inadequate as a diagnostic marker.
Regarding prognosis, we found that Bcl-2 loss was significantly associated with decreased overall survival in uLMS, with a 3.7-fold increase in the hazard of death. This result supports the usefulness of Bcl-2 as an immunohistochemical marker for the prognostic stratification in uLMS. In particular, the assessment of Bcl-2 might be useful in directing the choice of treatment in stage I (when adjuvant treatment is not mandatory) and in stages II-III, where the management of uLMS is not completely defined and includes the possibility of performing systemic therapy and/or external beam radiation therapy (EBRT) [8]. E.g., in patients at stage I, a loss of Bcl-2 expression might indicate the need for a treatment with systemic therapy or EBRT, while observation might be indicated for cases with retained Bcl-2 expression. In patients at stage II-III, Bcl-2 loss might require the combination of EBRT and systemic therapy. It is clear that conclusions cannot be drawn based on a small number of studies. Further studies are necessary to assess the potential role of Bcl-2 in the management of patients with uLMS.
Remarkably, Conconi et al. suggested that, in the STUMP category, Bcl-2 amplification could be associated with aggressive behavior, in contrast with its favorable significance in uLMS [31]. In this regard, it is necessary to correlate Bcl-2 with the whole histomorphologic pattern, to avoid misinterpretation of the immunohistochemical data. Furthermore, D’Angelo et al. found that the combined assessment of tumor size, mitotic index, Bcl-2, and ki67 lead to a still more precise prognostic stratification of uLMS [15]. On the account of these findings, further studies are encouraged to assess Bcl-2 on larger uLMS series, correlating its prognostic value with clinic-pathological data and with further immunohistochemical markers.
Strengths and limitations
To our knowledge, this is the first meta-analysis assessing the diagnostic and prognostic value of Bcl-2 in uLMS. A limitation to our results may be the low number of included studies and patients. Two studies could not be included in the prognostic analysis, because they did not report extractable data [15, 24]; however, since both showed a significant prognostic value for Bcl-2, they would not affect the significance of the results if they could be included in the analysis.
Another limitation may be the high statistical heterogeneity found. Causes for such heterogeneity might lie in different criteria adopted to interpret Bcl-2 immunohistochemistry, with particular regard to the definition of loss of expression. In fact, several different thresholds of intensity and distribution of immunostaining were used. Furthermore, the anti-Bcl-2 antibody was not the same in all studies. Possible confounding factors regarding patients’ characteristics, uLMS features, adjuvant treatment, and immunohistochemical methods are reported in Table 2. Unfortunately, some relevant data, such as menopausal status and menstrual cycle phase, were not provided by most studies.
Finally, a limitation of our meta-analysis may be the possibility of a publication bias, as suggested by the asymmetry of the funnel plots. Therefore, even though the prognostic significance of Bcl-2 was consistent among the published studies, it cannot be excluded that the studies with negative findings were not submitted/published. We hope that our results will encourage further studies to assess this point.
Conclusion
Loss of Bcl-2 immunohistochemical expression does not appear able to differentiate uLMS from leiomyoma variants and STUMP, resulting therefore not useful as a diagnostic marker. By contrast, Bcl-2 loss appears as a significant unfavorable prognostic marker in uLMS. Given the wide availability and low costs of immunohistochemistry, the assessment Bcl-2 expression might easily be introduced in the common practice for the prognostic stratification of uLMS, and might be useful in directing the patient management. However, limitations such as the low number of included studies, the high statistical heterogeneity, and the possibility of a publication bias prevent from drawing conclusions. Further studies are encouraged in this regard.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 Figure 1. Flow diagram of studies identified in the systematic review (Prisma template [Preferred Reporting Item for Systematic Reviews and Meta-analyses]). PNG 18 KB)
Supplementary file 2 Figure 2. Assessment of risk of bias. Summary of risk of bias for each study; Plus sign: low risk of bias; minus sign: high risk of bias; question mark: unclear risk of bias. (PNG 11 KB)
Author contribution
AT, AR: conception, protocol, data extraction, risk of bias assessment, data analysis, manuscript preparation, results interpretation, and disagreement resolutions. DR, AG: protocol, study selection, manuscript preparation, results interpretation, and disagreement resolutions. IE, CG: protocol, manuscript preparation, results interpretation, and disagreement resolutions. FPI, SGV: protocol, electronic search, manuscript preparation, and disagreement resolutions. AM, PC: protocol (revision), results interpretation (revision), and manuscript preparation (revision). RS: protocol, manuscript preparation, results interpretation, disagreement resolutions, and supervision. FZ, LI: conception, protocol, manuscript preparation, results interpretation, disagreement resolutions, and supervision.
Funding
No financial support was received for this study.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 2.De Falco M, Staibano S, D'Armiento FP, et al. Preoperative treatment of uterine leiomyomas: clinical findings and expression of transforming growth factor-beta3 and connective tissue growth factor. J Soc Gynecol Investig. 2006;13(4):297–303. doi: 10.1016/j.jsgi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.De Falco M, Staibano S, Mascolo M, et al. Leiomyoma pseudocapsule after pre-surgical treatment with gonadotropin-releasing hormone agonists: relationship between clinical features and immunohistochemical changes. Eur J Obstet Gynecol Reprod Biol. 2009;144(1):44–47. doi: 10.1016/j.ejogrb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Mazzei P, Piccolo A, Nugnes L, Mascolo M, De Rosa G, Staibano S. Metabolic profile of intact tissue from uterine leiomyomas using high-resolution magic-angle-spinning 1H NMR spectroscopy. NMR Biomed. 2010;23(10):1137–1145. doi: 10.1002/nbm.1540. [DOI] [PubMed] [Google Scholar]
- 5.Kurman R, Carcangiu M, Herrington C, Young R. World Health Organisation classification of tumors of female reproductive organs. 4. Lyon: International Agency for Research on Cancer (IARC) Press; 2014. [Google Scholar]
- 6.Gadducci A, Zannoni GF. Uterine smooth-muscle tumors of unknown malignant potential: a challenging question. Gynecol Oncol. 2019;154(3):631–637. doi: 10.1016/j.ygyno.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth-muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18(6):535–58. doi: 10.1097/00000478-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Rustum NR, Yashar CM, Bean S, et al. NCCN Clinical practice guidelines in oncology (NCCN Guidelines®). Uterine neoplasms version 1.2020 – March 06, 2020
- 9.Rubisz P, Ciebiera M, Hirnle L, et al. The usefulness of immunohistochemistry in the differential diagnosis of lesions originating from the myometrium. Int J Mol Sci. 2019;20(5):1136. doi: 10.3390/ijms20051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travaglino A, Raffone A, Saccone G, et al. Loss of B-cell lymphoma 2 immunohistochemical expression in endometrial hyperplasia: a specific marker of precancer and novel indication for treatment: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(12):1415–1426. doi: 10.1111/aogs.13452. [DOI] [PubMed] [Google Scholar]
- 11.Zhai YL, Nikaido T, Toki T, Shiozawa A, Orii A, Fujii S. Prognostic significance of bcl-2 expression in leiomyosarcoma of the uterus. Br J Cancer. 1999;80(10):1658–1664. doi: 10.1038/sj.bjc.6690578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leiser AL, Anderson SE, Nonaka D, et al. Apoptotic and cell cycle regulatory markers in uterine leiomyosarcoma. Gynecol Oncol. 2006;101(1):86–91. doi: 10.1016/j.ygyno.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 13.Lusby K, Savannah KB, Demicco EG, et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution's experience. Ann Surg Oncol. 2013;20(7):2364–2372. doi: 10.1245/s10434-012-2834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banas T, Pitynski K, Okon K, Czerw A. DNA fragmentation factors 40 and 45 (DFF40/DFF45) and B-cell lymphoma 2 (Bcl-2) protein are underexpressed in uterine leiomyosarcomas and may predict survival. Onco Targets Ther. 2017;10:4579–4589. doi: 10.2147/OTT.S142979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Angelo E, Espinosa I, Ali R, et al. Uterine leiomyosarcomas: tumor size, mitotic index, and biomarkers Ki67, and Bcl-2 identify two groups with different prognosis. Gynecol Oncol. 2011;121(2):328–333. doi: 10.1016/j.ygyno.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Raffone A, Travaglino A, Mascolo M, et al. TCGA molecular groups of endometrial cancer: pooled data about prognosis. Gynecol Oncol. 2019;155(2):374–383. doi: 10.1016/j.ygyno.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Travaglino A, Raffone A, Saccone G, et al. Nuclear expression of β-catenin in endometrial hyperplasia as marker of premalignancy. APMIS. 2019;127(11):699–709. doi: 10.1111/apm.12988. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Travaglino A, Raffone A, Mascolo M, et al. Clear cell endometrial carcinoma and the TCGA classification. Histopathology. 2020;76(2):336–338. doi: 10.1111/his.13976. [DOI] [PubMed] [Google Scholar]
- 21.Travaglino A, Raffone A, Saccone G, et al. Congruence between 1994 WHO classification of endometrial hyperplasia and endometrial intraepithelial neoplasia system. Am J Clin Pathol. 2020;153(1):40–48. doi: 10.1093/ajcp/aqz132. [DOI] [PubMed] [Google Scholar]
- 22.Travaglino A, Raffone A, Saccone G, et al. Significant risk of occult cancer in complex non-atypical endometrial hyperplasia. Arch Gynecol Obstet. 2019;300(5):1147–1154. doi: 10.1007/s00404-019-05299-2. [DOI] [PubMed] [Google Scholar]
- 23.Travaglino A, Raffone A, Mascolo M, et al. TCGA molecular subgroups in endometrial undifferentiated/dedifferentiated carcinoma. Pathol Oncol Res. 2020;26(3):1411–1416. doi: 10.1007/s12253-019-00784-0. [DOI] [PubMed] [Google Scholar]
- 24.Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K, Mayerhofer K. Bcl-2 receptor expression in patients with uterine smooth-muscle tumors: an immunohistochemical analysis comparing leiomyoma, uterine smooth-muscle tumor of uncertain malignant potential, and leiomyosarcoma. J Soc Gynecol Investig. 2004;11(3):187–191. doi: 10.1016/j.jsgi.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Rath-Wolfson L, Rosenblat Y, Halpern M, et al. A new scoring system using multiple immunohistochemical markers for diagnosis of uterine smooth-muscle tumors. J Cell Mol Med. 2006;10(1):197–205. doi: 10.1111/j.1582-4934.2006.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stănescu AD, Nistor E, Sajin M, Stepan AE. Immunohistochemical analysis in the diagnosis of uterine myometrial smooth-muscle tumors. Rom J Morphol Embryol. 2014;55(3 Suppl):1129–1136. [PubMed] [Google Scholar]
- 27.Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43(1):1–67. doi: 10.1080/10408360500295626. [DOI] [PubMed] [Google Scholar]
- 28.Ilyas M, Hao XP, Wilkinson K, et al. Loss of Bcl-2 expression correlates with tumor recurrence in colorectal cancer. Gut. 1998;43(3):383–387. doi: 10.1136/gut.43.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo H, Maruo T, Samoto T. Increased expression of bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82:293–299. doi: 10.1210/jcem.82.1.3650. [DOI] [PubMed] [Google Scholar]
- 30.Khurana KK, Singh SB, Tatum AH, Schulz V, Badawy SZ. Maintenance of increased Bcl-2 expression in uterine leiomyomas after gnrh agonist therapy. J Reprod Med. 1999;44:487–492. [PubMed] [Google Scholar]
- 31.Conconi D, Chiappa V, Perego P, et al. Potential role of BCL2 in the recurrence of uterine smooth-muscle tumors of uncertain malignant potential. Oncol Rep. 2017;37(1):41–47. doi: 10.3892/or.2016.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1 Figure 1. Flow diagram of studies identified in the systematic review (Prisma template [Preferred Reporting Item for Systematic Reviews and Meta-analyses]). PNG 18 KB)
Supplementary file 2 Figure 2. Assessment of risk of bias. Summary of risk of bias for each study; Plus sign: low risk of bias; minus sign: high risk of bias; question mark: unclear risk of bias. (PNG 11 KB)