Abstract
Background
The standard procedure most frequently used for ovarian tissue cryopreservation (OTC) is slow freezing, while vitrification has been proposed as promising alternative and has built an impressive catalog of success in fertility laboratories regarding cryopreservation of oocytes and embryos.
Methods
We developed and evaluated a high-throughput protocol for vitrification of human ovarian tissue suitable for clinical processing. Follicular viability was assessed via calcein staining prior and after cryopreservation analyzing ovarian tissue of a cohort of 30 patients.
Results
We found no significant differences regarding follicular viability between slow frozen and vitrified cortex tissue samples 24 h after thawing and rapid warming. Follicular viability of thawed and rapid warmed samples was not significantly different in comparison to fresh samples, indicating high proportions of follicular survival rates with both methods.
Conclusions
High-throughput vitrification is a promising option in a clinical setting. More research is required to determine the status of other tissue-specific quality indicators potentially influencing on autotransplantation.
Keywords: Ovarian tissue, Slow freezing, Vitrification, Fertility protection, Ovary, Follicle
What does this study add to the clinical work
| In summary, our results indicate that rapid vertical vitrification of ovarian tissue may be equivalent to slow freezing in terms of follicular viability while offering a cost efficient alternative to conventional slow freezing procedures. |
Introduction
Cryopreservation of human oocytes and embryos is a well-established routine method. In many IVF clinics, slow freezing has been superseded by vitrification due to superior survival rates [1–4] accompanied by considerable technical simplification. Additionally, with slow frozen cells, it was shown that applying rapid warming gives superior results compared to standard thawing protocols in terms of survival rates [5–7]. Preservation of oocytes and ovarian tissue cryopreservation (OTC) are two options for fertility protection in female cancer patients [8–10]. Multiple working groups have reported pregnancies and live births after thawing and transplantation of cryopreserved ovarian tissue, substantiating that OTC is a promising alternate method of fertility protection [11–19]. The large majority of OTC data are based on the slow freezing method, that is regarded as well established [20]. Vitrification of ovarian tissue is a promising alternative method with potential major advantages [21–25]. However, the number of reports about pregnancies and live births after retransplantation of vitrified ovarian tissue is still limited [20, 26]. The majority of centers performing OTC use the slow freezing method while the optimal protocol regarding vitrification of ovarian tissue has yet to be determined.
Methods
Ethics
Ethics committee of University Hospital Bonn approved this study (007/09) including the use of 10% of the ovarian tissue for patient related research and quality control analysis. Written, informed consent was obtained individually from each patient.
Statistics
Data were analyzed with SPSS version 25 (IBM) statistical software. Wilcoxon test was used to compare parameters between related samples. Mann–Whitney U test was conducted to determine parameters between groups. Data were tested for normal distribution with Kolmogorov–Smirnov test (Fig. 1).
Fig. 1.
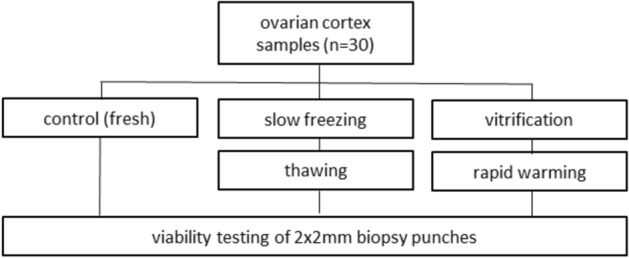
Experimental design of follicular viability measurements
Ovarian tissue
Ovarian tissue was obtained from 30 patients aged 14–41 (Ø 26.7) years prior cryopreservation for fertility protection measures.
Preparation of material for vitrification
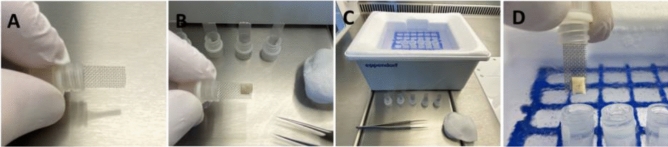
Metal cell meshes (Merck, Darmstadt) with opening sizes of 380 µm were customized to 25 × 8 mm stripes (Fig. 2A). Devices were placed in a sterilization container (Fig. 2B, Euronda Eurobox) prior autoclaving (Tuttnauer, Breda, Netherlands) under humid heat [27, 28] to sterilize the meshes prior further processing. Customized stripes were linked under validated sterile laminar air flow conditions (Kendro Hera Safe) with the caps of 1.8 ml cryovials (Thermo Scientific, Waltham, USA) to obtain thermally conductive loading devices (Fig. 2C).
Fig. 2.
Preparation of material. Metal cell meshes were cut to 25 × 8 mm stripes (A) and prepared for autoclaving (B). After sterilization, meshes were linked with caps of 1.8 ml vials (C) under sterile air conditions
Surgical retrieval and tissue preparation for cryopreservation
Ovarian tissue was surgically removed prior initiation of gonadotoxic cancer treatment or due to a disease that causes premature limitation of the ovarian reserve. Excision of ovarian tissue was performed laparoscopically in an outpatient surgery. Ovarian tissue resection was performed with sharp scissors without any electrocoagulation to protect oocytes. 30–50% (Fig. 3A) of one ovary was resected. Directly after removal, the tissue was transferred to a tube with custodiol solution (Dr. Köhler, Bensheim, Germany) at 4 °C and forwarded to the lab without deviation. A small part of the tissue was conserved in formalin solution and sent to histopathology to investigate for abnormalities, especially for cancer metastases. After ablation of the medulla (Fig. 3B), cortex was processed in stripes measuring 10 × 5 × 1 mm (Fig. 3C, D) under validated sterile laminar air flow conditions to ensure the highest level of safety and quality accompanying the manufacturing process. Cortex preparation was performed in custodiol (Dr. Köhler, Bensheim, Germany) at 4 °C. Additionally, 2 × 2 mm biopsy punches (pfm medical, Köln, Germany) were prepared for standardized viability measurement pre cryopreservation and post thawing/rapid warming. Cryopreservation was defined as exposition of prepared tissue to cryoprotective agents (CPA) and completion of cooling procedure before storing the samples in the cryobank.
Fig. 3.
Preparation of ovarian tissue. Ovarian tissue (A) was processed by removal of the medulla (B) and customized (C) to 10 × 5 × 1 mm stripes (D) for cryopreservation
Decontamination of liquid nitrogen
Liquid nitrogen was sterilized according to established protocols [29–31]. In brief, nitrogen was exposed to UV irradiation at 254 nm (OSRAM Germicidial Puritec, Berlin, Germany) at a distance of 20 cm for 20 min under a validated laminar flow bench.
Vitrification
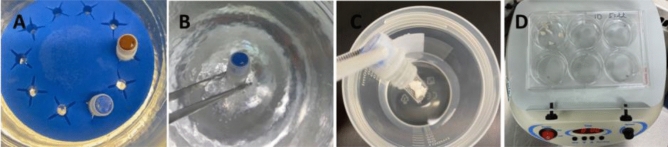
Vitrification and rapid warming media was prepared according to Suzuki and colleagues with modifications [26]. In brief, tissue was equilibrated in GMOPS + (Vitrolife, Göteborg, Sweden) supplemented with 10% serum substitute supplement [SSS] (Fujifilm Irvine scientific, Santa Ana, USA) and 10% ethyleneglycol (Merck, Darmstadt, Germany) for 5 min (step 1), followed by equilibration in GMOPS + (Vitrolife, Sweden) supplemented with 10% SSS (Fujifilm Irvine scientific, Santa Ana, USA) and 20% ethyleneglycol (Merck, Darmstadt, Germany) for 5 min (step 2). Finally, samples were transferred to GMOPS + (Vitrolife, Göteborg, Sweden) supplemented with 10% SSS (Fujifilm Irvine scientific, Santa Ana, USA), 35% ethyleneglycol (Merck, Darmstadt, Germany), 5% polyvinylpyrrolidone [PVP] (Merck, Darmstadt, Germany) and 0.5 mol/L sucrose (Merck, Darmstadt, Germany) [step 3]. After 6 min and within 7 min, tissue pieces were placed on the loading device (Fig. 4A, B), surplus liquid was dabbed away with sterile cellulose material. Samples were vertically plunged in cryovials pre-filled with liquid nitrogen (Fig. 4D) in a setting prepared for high throughput, arranged on a loading grid within a customized cryo dewar vessel (Fig. 4C, Eppendorf, Hamburg, Germany). Equilibration steps 1–3 were performed in a 6-well dish (Sarstedt, Germany) with 5 ml media/well on a rocking shaker at room temperature under validated sterile laminar air flow conditions (Kendro Hera Safe, Heraeus).
Fig. 4.
High-throughput vitrification of ovarian tissue. Ovarian cortex tissue placed on a loading device (A, B) for rapid vertical vitrification (D) in a vial based closed system on a customized, grid based styrofoam device (C), capable of high sample throughput
Rapid warming
Cryovials were placed on a floater (Fig. 5A) in a dewar vessel filled with liquid nitrogen. Vials were opened cautiously (Fig. 5B) and metal meshes with cortex strips were submerged (Fig. 5C) rapidly in 30 ml of prewarmed (37.2 °C) GMOPS + (Vitrolife, Göteborg, Sweden) media supplemented with 0.8 mol/l sucrose (Merck, Darmstadt, Germany) and 10% SSS (Fujifilm Irvine scientific, Santa Ana, USA) for 1 min (step 1) followed by incubation in GMOPS + (Vitrolife, Göteborg, Sweden) media supplemented with 0.4 mol/l sucrose (Merck, Darmstadt, Germany) and 10% SSS (Fujifilm Irvine scientific, Santa Ana, USA) for 3 min (step 2). Finally, 2 washing steps (steps 3–4) with GMOPS + (Vitrolife, Göteborg, Sweden) supplemented with 10% SSS (Fujifilm Irvine scientific, Santa Ana, USA) for 5 min each were conducted. Warming steps 1–4 were performed under validated sterile laminar air flow conditions. Step 1 was performed in a prewarmed sterile cup (Sarstedt, Nümbrecht) at 37.2 °C on a heating plate, while steps 2–4 were conducted in a 6-well dish (Sarstedt, Nümbrecht, Germany) with 5 ml media/well on a rocking shaker (Fig. 5D) at room temperature.
Fig. 5.
Rapid warming. Cryovials arranged on a floater (A), ready for warming. Cryovials were opened cautiously (B) and grids were submerged (C) rapidly in 30 ml of warming solution 1, transferred to warming solution 2 and washing solutions 1 and 2 on a rocking shaker (D)
Slow freezing and thawing
Cryopreservation and thawing of cortex samples were performed according to established protocols with modifications [32–37]. In brief, tissue samples were incubated in L-15 Leibovitz’s medium (Gibco Life technologies, NY, U.S.A.) supplemented with 11% human serum albumin [HSA] (Irvine Scientific, Santa Ana, USA), 10% dimethyl sulfoxide [DMSO] (CryoSure DMSO, WAK Chemie, Steinbach, Germany) and equilibrated for 35 min prior slow freezing procedure. Cortex samples were transferred to 1.8 ml vials (Nunc, Thermo Fisher Scientific, Denmark) and cryopreserved with a cooling rate of − 2 °C per min. After seeding, samples were cooled at a rate of − 0.3 °C per min to − 40 °C and at − 10 °C per min to − 140 °C. For thawing, tissue samples were brought to room temperature for 40 s and kept in a water bath for 130 s at 37.2 °C. Transfer of tissue was conducted in 15-min steps to 3 thawing solutions of decreasing sucrose concentrations based on Dulbecco’s phosphate buffered saline [DPBS] (DPBS CTS, Gibco Life technologies, NY, U.S.A.) supplemented with 11% HSA (Irvine Scientific, Santa Ana, USA) prior 2 washing intervals.
Determination of follicular viability
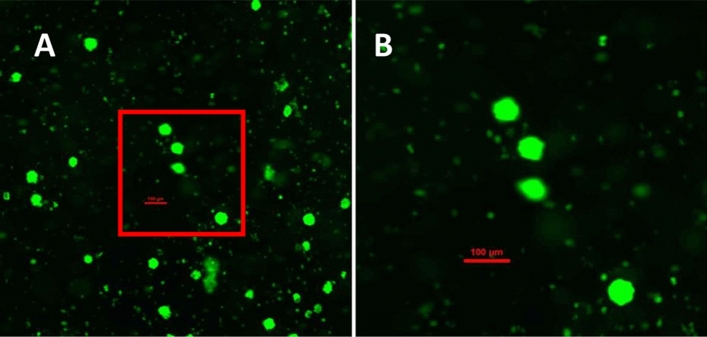
Calcein AM (Merck, Darmstadt, Germany) was dissolved with DMSO (Cryosure DMSO, WAK Chemie, Steinbach, Germany. Follicular viability was determined 24 h after tissue preparation and thawing/rapid warming. Prealiquoted calcein (Merck, Darmstadt, Germany) was dissolved with prewarmed DPBS CTS (Gibco Life technologies, NY, U.S.A.) and transferred to prealiquoted collagenase type 1A (Merck, Darmstadt, Germany) to obtain a viability working solution of 2 µmol/l calcein AM and 1 mg/ml collagenase type 1A. 2 × 2 mm biopsy punches were added to 500 µl of viability working solution and incubated for 90 min at 37.2 °C, protected from light. The solution was resuspended cautiously after 60 and 70 min to enable evenly bottoming of follicles prior fluorescence measurements at room temperature. Number of vital follicles was determined with fluorescence microscopy (Nikon, Ti2, Düsseldorf) as indicated in Fig. 6.
Fig. 6.
Viability staining of ovarian cortex samples after digestion with collagenase. Viable follicles can be identified by a layer of granulosa cells enclosing the oocyte emitting green fluorescence (495 nm) and by their size difference to stroma cells (A, B). Picture with tenfold (A) magnification displaying viable follicles. Zoomed in section of A with 4 viable follicles (B) at 20-fold magnification. Scalebar 100 µm
Results
In all treatment groups, follicular viability was not normally distributed. As indicated in Table 1, thawing results were statistically not significant comparing thawed slow frozen and rapid warmed vitrified samples. High follicular survival rates were obtained with both cryopreservation methods, resulting in statistically non-significant differences in comparison to fresh samples, data shown in Tables 2, 3. Cryopreservation was significantly less time consuming with vitrification compared to slow freezing as indicated in Table 4. In the fresh group and in the thawed after slow freezing group, 2 different cases with absent follicular viability were observed, while in the rapid warmed after vitrification group no samples with absence of follicular viability were detected.
Table 1.
Follicular viability after slow freezing/thawing and vitrification/rapid warming
| Parameter | Thawed after slow freezing [mean] | Interval SD median | Rapid warmed after vitrification [mean] | Interval SD median | n | *P value |
|---|---|---|---|---|---|---|
| Follicular viability count [n] | 68.6 | 0–454 | 59.8 | 0.5–349 | 30 | 0.459 |
| 102.9 | 77.2 | |||||
| 33.8 | 38.5 |
*Wilcoxon test
Table 2.
Follicular viability pre and post vitrification/rapid warming
| Parameter | Fresh [mean] | Interval SD median | Rapid warmed after vitrification [mean] | Interval SD median | n | *P value |
|---|---|---|---|---|---|---|
| Follicular viability count [n] | 72.8 | 0–386 | 59.8 | 0.5–349 | 30 | 0.900 |
| 94.3 | 77.2 | |||||
| 41.5 | 38.5 |
*Wilcoxon test
Table 3.
Follicular viability pre and post slow freezing/warming
| Parameter | Fresh [mean] | Interval SD median | Thawed after slow freezing [mean] | Interval SD median | n | *P value |
|---|---|---|---|---|---|---|
| Follicular viability count [n] | 72.8 | 0–386 | 68.6 | 0–454 | 30 | 0.829 |
| 94.3 | 102.9 | |||||
| 41.5 | 33.8 |
*Wilcoxon test
Table 4.
Cryopreservation time with slow freezing and vitrification
| Parameter | Slow freezing method [mean] | Interval SD median | Vitrification method [mean] | Interval SD median | n | *P value |
|---|---|---|---|---|---|---|
| Cryopreservation time [min] | 188.1 | 186–190 | 17 | 17* | 30 | < 0.001 |
| 1.4 | 0* | |||||
| 187.5 | 17* |
*Mann–Whitney U test. *The vitrification protocol enables exact freezing time with a variation of 60 s in the final step of equilibration. This variation was not recorded and is statistically negligible
Discussion
The aim of our study was to design and validate a high-throughput vitrification approach for human ovarian tissue based on the successful protocol of Suzuki and colleagues reporting two deliveries [26].
The technical advantage of the vitrification procedure is avoidance of ice crystal formation contrary to conventional freezing approaches [38–40]. The avoidance of ice crystal formation prevents the cellular damage, which can otherwise impair tissue integrity or even follicular survival.
Our results indicate that follicular viability rates are comparable to our standard slow freezing approach, suitable for a clinical application As a carrier system, we used cell meshes consisting of metal, linked with caps of 1.8 ml cryovials, suitable for fast loading of tissue fragments prior rapid vertical vitrification in grid embedded cryovials resulting in a high-throughput process. This is important, because in a clinical laboratory application, the amount of tissue and the numbers of tissue fragments for cryopreservation may variate substantially per patient. Processing time per each fragment is critical to minimize prolonged exposition to a high ratio of cryoprotectants in the final step of equilibration solution—resembling the vitrification process of oocytes, pronuclear (PN) stages or embryos. Additionally, the use of meshes may provide faster cooling rates compared to plastic based systems and can be sterilized by autoclavation. Our system is universally applicable for cortex stripes measuring 10 × 5 × 1 mm and for biopsy punches measuring 2 × 2 mm.
The protocol of Suzuki includes a carrier system consisting of four fine stainless needles linked with the cap of a cryogenic vial, before immersion into liquid nitrogen and insertion into cryovials after vitrification [26].
Partially, our handling approach resembles the protocol of Nikiforov, using unlinked metal meshes for carrying ovarian tissue samples with 0.28 mm wire diameter and mesh aperture of 1.31 mm for vitrification and insertion into cryovials after vitrification [41].
Our approach included metal meshes with wire diameters of 0.25 mm and opening sizes of 0.38 mm. This ensures a large contact area of the cortex tissue on a metal carrier as thin as possible, potentially influencing the thermal conductivity while the meshes were pre linked with the caps of the cryovials. This enables the use of grid embedded cryovials, pre-filled with liquid nitrogen ready for fast vertical insertion of loaded meshes into the cryovials directly during the process of vitrification. This results in a standardized, high-throughput vitrification process of ovarian cortex tissue.
One major key aspect to assess the quality of ovarian cortex tissue is follicular viability that can be evaluated with calcein, a well-described fluorescence-based live assay [42–44]. The incubation period of 24 h prior viability measurements of fresh, slow frozen/thawed and vitrified/rapid warmed tissue enables the tissue to express potential damage that could potentially occur with these procedures and may potentially not be observed when analyzed at an earlier stage. The different types of cell death can take minutes to hours [45] and might become visible later than directly after thawing/rapid warming.
Using 2 × 2 mm biopsy samples for evaluation of follicular viability provides valuable, but limited insights due to the fact that follicular density is unevenly [46–48] distributed in the human ovary potentially influencing the results. As indicated by our results, in the rapid warmed after vitrification group, no cases with absent follicular viability were observed while in the fresh and thawed after slow freezing groups 2 different samples with absence of follicular vitality were detected. To minimize these effects, a cohort of 30 patients with individual determination of follicular viability prior cryopreservation, post slow freezing and vitrification was evaluated by two experienced embryologists to mitigate researcher specific bias. In a clinical setting, preparation of at least 2 × 2 mm biopsy samples for individual quality control assessment prior and post freezing is recommended.
The design of our experimental setup is limited by the fact that the amount of tissue per patient variates substantially and our ethical vote restricts research to 10% of the amount of tissue, reflecting the limited access of researchers to ovarian tissue in a routine setting [49]. It will be interesting to investigate other tissue-specific quality markers with potential impact on implantation success like angiopoietic factors after tissue culture in a future study with a larger sample number and selected patients with larger amounts of ovarian tissue available for research purposes.
In terms of economical parameters, our results implicate that vitrification is less cost demanding regarding cryopreservation time that can have a major impact on personnel deployment planning—keeping in mind that in a busy cryobank ovarian tissue from external referrer centers scheduled for, e.g., overnight transportation [50] may occasionally arrive on the next day late in the afternoon during the week, or on saturdays, prior, e.g., a slow freezing process lasting for several hours.
Contrary to vitrification, the time consuming slow freezing cooling process must not be interrupted due to potential technical errors related with the programmable freezer unit, computer or software malfunctions and continuous nitrogen supply that could have a major impact on tissue integrity.
Additionally, slow freezing is cost intensive regarding purchasing and maintenance of equipment like controlled freezing systems with an attached nitrogen supply tank, personal computer, freezing software and requires in contrast to vitrification security of electricity during the cooling procedure.
In summary, our results indicate that rapid vertical vitrification of ovarian tissue may be equivalent to slow freezing in terms of follicular viability while offering a cost-efficient alternative to conventional slow freezing procedures. We follow the argumentation of Suzuki [26], Keros [51], Sugishita [52], Silver [53] and Nikiforov [41] that vitrification of ovarian tissue is a promising alternate approach to conventional slow freezing systems for ovarian tissue.
Author contributions
AS designed the study and was involved in thawing, processing of the samples, viability tests, writing and revision of the manuscript and interpretation of the results. Approval of the ethics committee. RE was involved in viability tests, writing and revision of the manuscript and interpretation of the results. CF processing of the samples, viability tests, writing and revision of the manuscript and interpretation of the results. NE was involved in writing and revision of the manuscript and interpretation of the results. JJ was involved in writing and revision of the manuscript and interpretation of the results. NS funded the study, supervised the project, contributed to the design of the study, manuscript writing, revision and final approvement.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors have not disclosed any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andreas Schallmoser, Email: andreas.schallmoser@ukbonn.de.
Nicole Sänger, Email: nicole.saenger@ukbonn.de.
References
- 1.Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F, Movaghar B. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet. 2009;26(6):347–354. doi: 10.1007/s10815-009-9318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setti PEL, Porcu E, Patrizio P, Vigiliano V, de Luca R, d’Aloja P, Spoletini R, Scaravelli G. Human oocyte cryopreservation with slow freezing versus vitrification results from the National Italian registry data, 2007–2011. Fertil Steril. 2014;102(1):90–95. doi: 10.1016/j.fertnstert.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Glujovsky D, Riestra B, Sueldo C, Fiszbajn G, Repping S, Nodar F, Papier S, Ciapponi A. Vitrification versus slow freezing for women undergoing oocyte cryopreservation. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD010047.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel Hafeza FF, Desai N, Abou-Settab AM, Falconea T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod BioMed Online. 2010;20(2):209–222. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Parmegiani L, Minasi MG, Arnone A, Casciani V, Cognigni GE, Viñoles R, Varricchio MT, Quintero LA, Greco E, Filicori M. Universal warming protocol for vitrified oocytes to streamline cell exchange for transnational donation programs: a multi-center study. J Assist Reprod Genet. 2020;37(6):1379–1385. doi: 10.1007/s10815-020-01798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parmegiani L, Beilby KH, Arnone A, Bernardi S, Maccarini AM, Nardi E, Cognigni GE, Filicori MJ. Testing the efficacy and efficiency of a single “universal warming protocol” for vitrified human embryos: prospective randomized controlled trial and retrospective longitudinal cohort study. Assist Reprod Genet. 2018;35(10):1887–1895. doi: 10.1007/s10815-018-1276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parmegiani L, Tatone C, Cognigni GE, Bernardi S, Troilo E, Arnone A, Maccarini AM, Di Emidio G, Vitti M, Filicori M. Rapid warming increases survival of slow-frozen sibling oocytes: a step towards a single warming procedure irrespective of the freezing protocol? Reprod Biomed Online. 2014;28(5):614–623. doi: 10.1016/j.rbmo.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Jadoul P, Guilmain A, Squifflet J, Luyckx M, Votino R, Wyns C, Dolmans MM. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod. 2017;32(5):1046–1054. doi: 10.1093/humrep/dex040. [DOI] [PubMed] [Google Scholar]
- 9.Wallace WH, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil Steril. 2016;105(1):6–12. doi: 10.1016/j.fertnstert.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Lee Y, Lee S, Kim T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet Gynecol Sci. 2018;61(4):431–442. doi: 10.5468/ogs.2018.61.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 12.Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 13.Donnez J, Dolmans MM, Pellicer A, et al. Fertility preservation for age-related fertility decline. Lancet. 2015;385:506–507. doi: 10.1016/S0140-6736(15)60198-2. [DOI] [PubMed] [Google Scholar]
- 14.Van der Ven H, Liebenthron J, Beckmann M, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31:2031–2041. doi: 10.1093/humrep/dew165. [DOI] [PubMed] [Google Scholar]
- 15.Meirow D, Ra’anani H, Shapira M, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106:467–474. doi: 10.1016/j.fertnstert.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Wallberg KA, Tanbo T, Tinkanen H, et al. Ovarian tissue cryopreservation and transplantation among alternatives for fertility preservation in the Nordic countries—compilation of 20 years of multicenter experience. Acta Obstet Gynecol Scand. 2016;95:1015–1026. doi: 10.1111/aogs.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 Successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnez J, Dolmans M-M. Fertility preservation in women. N Engl J Med. 2017;377:1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 19.Hoekman EJ, Louwe LA, Rooijers M, van der Lucette Westerlaken AJ, Klijn NF, Pilgram GSK, de Kroon CD, Carina Hilders GJM. (2019) Ovarian tissue cryopreservation: low usage rates and high live‐birth rate after transplantation. Acta Obstet Gynecol Scand 00:1–9 [DOI] [PMC free article] [PubMed]
- 20.Anderson RA, Amant F, Braat D, D’Angelo A, de Sousa Lopes SMC, Demeestere I, Dwek S, Frith L, Lambertini M, Maslin C, Moura-Ramos M, Nogueira D, Rodriguez-Wallberg K, Vermeulen N, The ESHRE Guideline Group on Female Fertility Preservation ESHRE guideline: female fertility preservation. Hum Reprod Open. 2020;2020(4):hoaa52. doi: 10.1093/hropen/hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep. 2017;7:8538. doi: 10.1038/s41598-017-09005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23:2256–2265. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Z, Wang Y, Li L, Luo S, Li SW. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010;94:2323–2328. doi: 10.1016/j.fertnstert.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Keros V, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 25.Fabbri R, et al. Good preservation of stromal cells and no apoptosis in human ovarian tissue after vitrification. Biomed Res Int. 2014;2014:673537. doi: 10.1155/2014/673537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Q-Q, Sun W-Q, Connor J. Sterilization of biomaterials of synthetic and biological origin. In: Ducheyne P, Healy KE, Hutmacher DW, Grainger DW, Kirkpatrick CJ, editors. Comprehensive biomaterials. Amsterdam: Elsevier; 2011. pp. 127–144. [Google Scholar]
- 28.Panta G, Richardson AK, Shaw IC. Effectiveness of autoclaving in sterilizing reusable medical devices in healthcare facilities. J Infect Dev Ctries. 2019;13(10):858–864. doi: 10.3855/jidc.11433. [DOI] [PubMed] [Google Scholar]
- 29.Parmegiani L, Accorsi A, Cognigni GE, Bernardi S, Troilo E, Filicori M. Sterilization of liquid nitrogen with ultraviolet irradiation for safe vitrification of human oocytes or embryos. Fertil Steril. 2010;94(4):1525–1528. doi: 10.1016/j.fertnstert.2009.05.089. [DOI] [PubMed] [Google Scholar]
- 30.Gerba CP, Gramos DM, Nwachuku N. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl Env Microbiol. 2002;68:5167–5169. doi: 10.1128/AEM.68.10.5167-5169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srikanth B. (1995) The basic benefits of ultraviolet technology. Water Cond Purif 26–27
- 32.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196° C. Hum Reprod. 1994;9(4):597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 33.Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, Roesing B, van der Ven H. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007;55:261–268. doi: 10.1016/j.cryobiol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Fabbri R, Venturoli S, D’Errico A, Iannascoli C, Gabusi E, Valeri B, Seracchioli R, Grigioni WF. Ovarian tissue banking and fertility preservation in cancer patients: histological and immunohistochemical evaluation. Gynecol Oncol. 2003;89:259–266. doi: 10.1016/s0090-8258(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Madrid B, Dolmans M-M, Van Langendonckt A, Defrère S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82(5):1390–1394. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 36.Leonel ECR, Lucci CM, Amorim CA. Cryopreservation of human ovarian tissue: a review. Transfus Med Hemother. 2019;46:173–181. doi: 10.1159/000499054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebenthron J, Montag M, Reinsberg J, Köster M, Isachenko V, van der Ven K, van der Ven H, Krüssel J-S, von Wolff M. Overnight ovarian tissue transportation for centralized cryobanking: a feasible option. RBMO. 2019;38(5):740–749. doi: 10.1016/j.rbmo.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Amorim CA, Curaba M, Van Langendonckt A, Dolmans M-M, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod BioMed Online. 2011;23:160–186. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Fahy GM. Vitrification: a new approach to organ cryopreservation. In: Meryman HT, editor. Transplantation: approaches to graft rejection. New York, USA: Alan R. Liss; 1986. pp. 305–335. [Google Scholar]
- 40.Liebermann J, Nawroth F, Isachenko V, Isachenko E, Rahimi G, Tucker MJ. Potential importance of vitrification in reproductive medicine. Biol Reprod. 2002;67:1671–1680. doi: 10.1095/biolreprod.102.006833. [DOI] [PubMed] [Google Scholar]
- 41.Nikiforov D, Russo V, Nardinocchi D, Bernabò N, Mattioli M, Barboni B. Innovative multi-protectoral approach increases survival rate after vitrification of ovarian tissue and isolated follicles with improved results in comparison with conventional method. J Ovarian Res. 2018;11(1):65. doi: 10.1186/s13048-018-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schallmoser A, Einenkel R, Färber C, et al. In vitro growth (IVG) of human ovarian follicles in frozen thawed ovarian cortex tissue culture supplemented with follicular fluid under hypoxic conditions. Arch Gynecol Obstet. 2022 doi: 10.1007/s00404-022-06672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;6:1131–1135. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liebenthron J, Reinsberg J, van der Ven K, Saenger N, Kruessel J-S, Von Wolff M. Serum anti-Müllerian hormone concentration and follicle density throughout reproductive life and in different diseases—implications in fertility preservation. Human Reprod. 2019;34(12):2513–2522. doi: 10.1093/humrep/dez215. [DOI] [PubMed] [Google Scholar]
- 45.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45(3):528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt KLT, Byskov AG, Nyboe Andersen A, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Human Reprod. 2003;18(6):1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 47.Qu J, Godin PA, Nisolle M, Donnez J. Distribution and epidermal growth factor receptor expression of primordial follicles in human ovarian tissue before and after cryopreservation. Hum Reprod. 2000;15:302–310. doi: 10.1093/humrep/15.2.302. [DOI] [PubMed] [Google Scholar]
- 48.Kohl J, Dittrich R, Siebzehnrübl E, Wildt L. Determination of follicle numbers in human ovarian biopsies ± a method for estimation of outcome of ovarian cryopreservation. Fertil Steril. 2000;74(Suppl 1):212. [Google Scholar]
- 49.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Human Reprod Update. 2010;16(4):395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Vilela JMV, Dolmans M-M, Amorim CA. Ovarian tissue transportation: a systematic review. Reprod Biomed Online. 2021;42(2):351–365. doi: 10.1016/j.rbmo.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 52.Sugishita Y, Taylan E, Kawahara T, Shahmurzada B, Suzuki N, Oktay K. Comparison of open and a novel closed vitrification system with slow freezing for human ovarian tissue cryopreservation. J Assist Reprod Genet. 2021;38:2723–2733. doi: 10.1007/s10815-021-02297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silber SJ, De Rosa M, Goldsmith S, Fan Y, Castleman L, Melnick J. Cryopreservation and transplantation of ovarian tissue: results from one center in the USA. J Assist Reprod Genet. 2018;35(12):2205–2213. doi: 10.1007/s10815-018-1315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]