Abstract
Background and Aims:
Alcohol-associated liver disease (ALD) is a devastating complication of alcohol use disorder (AUD). Once it develops, ALD is exceedingly difficult to treat; it is therefore critical to identify ways to prevent ALD. By treating the causes of elevated alcohol consumption, psychotherapy may offer prophylactic benefit against the development of ALD for patients with AUD.
Methods:
In this retrospective cohort study, we used ICD-9 and 10 codes to identify 9635 patients with AUD in the Mass General Brigham Biobank. The mean follow-up from AUD diagnosis was 9.2 years. We used Cox regression models to generate hazard ratios (HR) for the development of ALD given the receipt or non-receipt of psychotherapy, adjusting for a range of other contributors including the receipt of medication-assisted treatment.
Results:
In our cohort, 60.4% were male, 83.5% were white, median age was 57.0 years, and 3544 patients (36.8%) received psychotherapy. ALD developed in 1135 patients (11.8%). In multivariable analysis, psychotherapy was associated with reduced rate of ALD (HR: 0.59, 95% confidence interval [CI]: 0.50–0.71, p < 0.001). This association held for both individual psychotherapy (HR: 0.70, 95% CI: 0.56–0.86, p < 0.001) and group psychotherapy (HR: 0.76, 95% CI: 0.61–0.94, p = 0.01). Among patients with cirrhosis, psychotherapy was associated with lower rate of hepatic decompensation (HR: 0.68, 95% CI: 0.44–0.95, p = 0.03).
Conclusion:
The receipt of psychotherapy in the setting of AUD is associated with reduced incidence and progression of ALD. Given the safety and potential benefit of psychotherapy, clinicians should consider using it to prevent the development of ALD.
Keywords: Addiction Medicine, Psychosocial Therapy, Behavioral Therapy, Cirrhosis
In recent decades, there has been a concerning increase in the prevalence of alcohol use disorder (AUD) (1–3) and in recent years, a rise in alcohol use associated with the COVID-19 pandemic (4). In parallel, the incidence of alcohol-associated liver disease (ALD), the most common complication of AUD, is also rising (5,6). ALD represents a wide spectrum of liver pathology ranging from bland hepatic steatosis to cirrhosis (7) and accounts for up to 90% of alcohol-related deaths (8). Given the high morbidity and mortality associated with ALD, there are significant efforts to develop novel diagnostic and therapeutic strategies for patients with advanced ALD (9–14). However, less is known about strategies to reduce the risk of developing ALD in patients with AUD.
Psychotherapy for AUD includes clinician-led treatments offered in formats including individual, group, and family therapy, which are distinct from layperson-led mutual help groups such as 12-step facilitation therapy (15). Short-term goals of psychotherapy are to support abstinence or reduction in alcohol use and encourage adherence to treatment; long-term goals are to restore self-esteem, improve physical and social well-being, and achieve durable abstinence. Psychotherapy has been shown to reduce alcohol use in patients with ALD (16–18), chronic hepatitis C virus (HCV) (19–21), and in patients without liver disease (22). In patients with cirrhosis, treatment for AUD with psychotherapy and/or medication-assisted treatment (MAT) is associated with reduced hepatic decompensation (23). However, the effect of psychotherapy on the development of ALD, as well as its ability to modulate long-term risk of hepatic decompensation, is not well studied.
In this work, we sought to assess whether psychotherapy reduces incident ALD in patients with AUD in a large, well-characterized cohort of patients with AUD and long-term follow-up.
EXPERIMENTAL PROCEDURES
Patient cohort
This retrospective cohort study examined patients with AUD who were enrolled in the Mass General Brigham (MGB) Biobank between January 2010 and August 2021. The MGB Biobank is a data repository for consented patients being cared for within the MGB healthcare system which consists of multiple tertiary medical centers and community hospitals in eastern Massachusetts (24). The Biobank integrates and updates data from primary and curated sources, is accessible via a data portal, and can construct customized datasets. For the purposes of this study, AUD was defined by DSM-IV disorders using ICD codes for alcohol abuse (ICD-10: F10.1, ICD-9: 305.0) and alcohol dependence (ICD-10: F10.2, ICD-9: 303). Patients who had ALD at the time of their index AUD diagnosis were excluded from all analyses. Accordingly, all patients enrolled in this study were free of ALD at the time of enrollment. Patients identified in the Biobank cohort were retrospectively reviewed as far back as the earliest AUD diagnosis in 1979. Demographic, clinical, and social information were available for each patient and accessed through an online platform. All patients enrolled in the MGB Biobank provided informed consent.
Demographic and clinical variables
Demographic and clinic data included sex, age, race, ethnicity, history of homelessness, body mass index (BMI), viral hepatitis status, substance use disorder history, mental health disorder history, the receipt of MAT, and liver disease history. Viral hepatitis and BMI status were classified as previously described (25). A full list of the diagnosis codes and test results used to define variables in our multivariable analysis are available in Supplementary Table 1. We constructed our model by selecting these factors a priori because they may contribute to alcohol use, or to liver disease directly.
Alcohol Consumption
We also collected the results of a questionnaire on reported alcohol consumption. From the categorical answers of this questionnaire, we constructed a linear scale to evaluate alcohol consumption according to whether a given patient had already received psychotherapy, would go on to receive psychotherapy, or has not ever received psychotherapy. Patients that completed the questionnaire before AUD diagnosis or after ALD diagnosis were excluded from this analysis. The linear scale of standard drink consumption was as follows: 1. None or less than 1 per month; 2. 1–3 per month; 3. 1 per week; 4. 2–4 per week; 5. 5–6 per week; 6. 1–2 per day; 7. 3–4 per day; 8. 5–6 per day; 9. more than 6 per day. Patients completed the questionnaire only once; thus, the patients completing the questionnaire before therapy are different from those completing the questionnaire after therapy.
Definitions of alcohol-associated liver disease
Patients with ALD were defined as previously described (26). This included patients having an ICD-9 or 10 diagnosis of alcoholic hepatitis (ICD-10: K70.1, ICD-9: 571.1), alcoholic cirrhosis of liver (ICD-10: K70.3, ICD-9: 571.2), alcoholic hepatic failure (ICD-10: K70.4), alcoholic fibrosis and sclerosis (ICD-10: K70.2), other cirrhosis (K74.69, ICD-9: 571.5) or unspecified cirrhosis (K74.60, ICD-9: 571.5). Hepatic decompensation was defined as an ICD-9 or 10 diagnosis of hepatocellular carcinoma (ICD-10: C22.0, ICD-9: 155.0), esophageal varices with bleeding (ICD-10: I85.01, ICD-9: 456.0), secondary esophageal varices with bleeding (ICD-10: I85.11, ICD-9: 456.20), acute and subacute hepatic failure (ICD-10: K72.0, ICD-9: 570), chronic hepatic failure (ICD-10:K72.1), hepatic failure-unspecified (ICD-10: K72.9, ICD-9: 572.2, 572.8), hepatorenal syndrome (ICD-10: K76.7, 572.4), hepatopulmonary syndrome (ICD-10: K76.81, ICD-9: 573.5), or ascites (ICD-10: R18, ICD-9: 789.5).
Definition of addiction treatment
Treatment with psychotherapy was defined using CPT-4 codes taken from validated Healthcare Effectiveness Data and Information Set and included only those specifically referencing psychotherapy. General procedural codes and those referencing psychiatric intervention were excluded. Final CPT-4 codes included were for individual psychotherapy (90804–90815, 90826–90829, 90845, 90875, 90876, 99408), family psychotherapy (90847, 90849), and group psychotherapy (90853, 90857). Patients were considered treated in each analysis if they initiated treatment before the relevant outcome. Specifically, we did not include any patients in the treatment group who started psychotherapy after the prespecified outcome. If patients started psychotherapy after the prespecified outcome, they were included in the non-treatment group.
Statistical analyses
Continuous variables were summarized using means compared using the t-test with Welch correction; categorical variables were expressed as percentages and compared using the Fisher’s exact test. Cox regressions were used to determine the hazard ratios (HR) and 95% confidence interval (CI) of developing ALD and hepatic decompensation, adjusting for covariates listed in Supplementary Table 1, in addition to the time of follow-up. To minimize immortal time bias, in patients treated with psychotherapy, we began counting follow-up from the later of either psychotherapy initiation or inclusion criterion (AUD diagnosis or cirrhosis diagnosis). To further adjust for immortal time bias, these analyses were repeated in landmark analyses. Cox regression models and landmarks at 30, 90 and 360 days after AUD diagnosis were used. Patients that received treatment by the landmark were considered treated, while patients that did not receive treatment or did so only after the landmark were considered untreated. Patients that experienced the relevant outcome before the landmark were excluded from the analysis. Kaplan-Meier analysis was used to examine the proportion of patients over time who developed hepatic decompensation following a patient’s index AUD or cirrhosis diagnosis. Censoring for death was not included due to lack of protected identifiable health information. To compare the reported alcohol consumption of patients that had initiated psychotherapy or not, we used a one-way ANOVA followed by Tukey Tests. An alpha level of 0.05 was considered statistically significant. Statistical analyses were performed in GraphPad Prism (San Diego, CA).
RESULTS
Patient Characteristics
In our cohort of 9365 patients with AUD, 5821 patients (60.4%) were male, 8045 patients (83.5%) were white, and the median age was 57.0 years. The mean follow-up time from AUD diagnosis was 9.2 years. 3544 patients (36.8%) received psychotherapy (Figure 1). Patients who received psychotherapy had significantly different characteristics compared to those that did not receive it (Table 1). Patients receiving psychotherapy were more likely to be younger (51.3 vs. 56.8 years, p < 0.001), female (47.5% vs. 35.0%, p < 0.001), have viral hepatitis (13.6% vs. 11.8%, p = 0.009), homelessness (13.0% vs 8.5%, p < 0.001), co-morbid psychiatric disorder (98.7% vs 77.6%, p < 0.001), nicotine dependence (50.1% vs 40.7%, p < 0.001), non-alcohol substance use disorder (52.4% vs 27.4%, p < 0.001), and receive MAT (54.9% vs 32.1%, p < 0.001).
Figure 1. Flow diagram for patient selection.
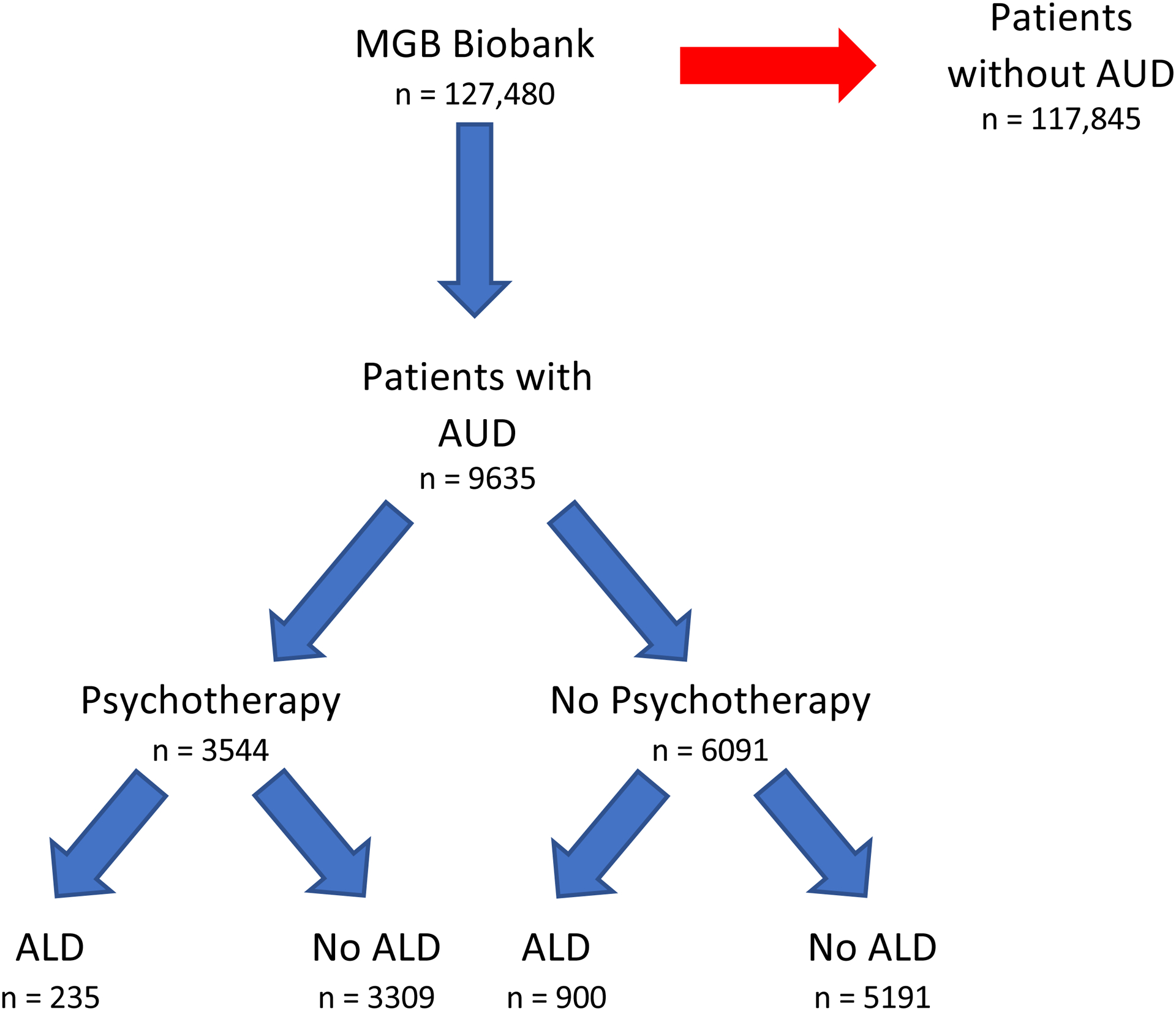
ALD, alcohol-associated liver disease; AUD, alcohol use disorder.
Table 1:
Demographic and social characteristics of patients, by group allocation
| P value† | ||||
|---|---|---|---|---|
| Age, Mean (SD) | 54.8 (16.5) | 51.3 (16.0) | 56.8 (16.5) | <0.001 |
| Female Sex, N (%) | 3814 (39.6) | 1682 (47.5) | 2132 (35.0) | <0.001 |
| Male Sex, N (%) | 5821 (60.4) | 1862 (52.5) | 3959 (65.0) | <0.001 |
| Asian, N (%) | 84 (0.9) | 36 (1.0) | 48 (0.8) | 0.26 |
| Black, N (%) | 720 (7.5) | 224 (6.3) | 496 (8.1) | 0.001 |
| White, N (%) | 8045 (83.4) | 2975 (83.9) | 5070 (83.2) | 0.36 |
| Other, N (%) | 332 (3.4) | 134 (3.8) | 198 (3.3) | 0.18 |
| Unknown, N (%) | 454 (4.7) | 175 (4.9) | 279 (4.6) | 0.43 |
| Hispanic, N (%) | 320 (3.3) | 110 (3.1) | 210 (3.4) | 0.38 |
| BMI, Mean (SD) | 28.7 (6.7) | 28.8 (6.3) | 28.7 (7.0) | 0.63 |
| Viral Hepatitis, N (%) | 1204 (12.4) | 484 (13.6) | 720 (11.8) | 0.009 |
| Homeless, N (%) | 978 (10.1) | 463 (13.0) | 515 (8.5) | <0.001 |
| MAT, N (%) | 3906 (40.5) | 1949 (54.9) | 1957 (32.1) | <0.001 |
| Psychiatric Disorder, N (%) | 8227 (85.3) | 3500 (98.7) | 4727 (77.6) | <0.001 |
| Nicotine Dependence, N (%) | 4260 (44.2) | 1777 (50.1) | 2483 (40.7) | <0.001 |
| Non-alcohol SUD‡, N (%) | 3532 (36.6) | 1860 (52.4) | 1672 (27.4) | <0.001 |
SD: standard deviation, N: number of patients, BMI: body mass index, MAT: medical addiction therapy, SUD: substance use disorder
p values for categorical variables refer to the results of a Fisher exact test comparing patients receiving any psychotherapy to those not receiving psychotherapy. For continuous variables, they refer to the results of a t test with Welch correction.
Non-alcohol SUD refers to one of: cannabis, cocaine, other stimulant, opioid, inhalant, or sedative use disorder.
We also report the multivariable associations with the receipt of psychotherapy (Supplementary Table 2). In this model, patients were more likely to receive psychotherapy if they received MAT ([odds ratio] OR: 1.61, 95% CI: 1.45–1.79, p < 0.001). Patients more frequently received therapy if they had a diagnosed anxiety disorder (OR: 2.56, 95% CI: 2.20–2.99, p < 0.001), specific personality disorder (OR: 2.95, 95% CI: 2.51–3.46, p < 0.001), mood disorder (OR: 5.34, 95% CI: 4.51–6.36, p < 0.001) or schizophrenia (OR: 1.74, 95% CI: 1.52–2.01, p < 0.001). Patients were also more likely to receive therapy if they had a diagnosed cannabis use disorder (OR: 1.40, 95% CI: 1.21–1.61, p < 0.001), inhalant use disorder (OR: 1.45, 95% CI: 1.23–1.72, p < 0.001) or opioid use disorder (OR: 1.21, 95% CI: 1.04–1.40, p = 0.01). AUD class was also relevant, with patients receiving psychotherapy more frequently if they had a diagnosis of alcohol dependence rather than abuse (OR: 1.61, 95% CI: 1.40–1.84, p < 0.001) or diagnoses of both abuse and dependence (OR: 1.74, 95% CI: 155–1.95, p < 0.001). By contrast, age was inversely associated with the receipt of psychotherapy (OR: 0.99, 95% CI: 0.987–0.993, p < 0.001), as was male sex (OR:0.75, 95% CI: 0.68–0.84, p < 0.001), black race (OR: 0.78, 95% CI: 0.64–0.95, p = 0.01) and homelessness (OR: 0.53, 95% CI: 0.44–0.63, p < 0.001). Notably, patients at risk for ALD because of hepatitis C infection were less likely to receive psychotherapy (OR: 0.67, 95% CI: 0.56–0.80, p < 0.001).
Lastly, we compared the reported alcohol consumption of the 2018 patients that completed an alcohol consumption questionnaire after their index AUD diagnosis and that had no evidence of ALD. We compared three groups: those that did not receive psychotherapy (1173 patients), those that completed the alcohol consumption questionnaire before receiving psychotherapy (89 patients), and those that completed the questionnaire after receiving psychotherapy (756 patients). On linear scale of alcohol consumption, we found that patients who completed the questionnaire after therapy reported significantly lower alcohol consumption that either patients that completed the questionnaire before psychotherapy (3.4 vs 4.7, p < 0.001) or those that did not receive psychotherapy (3.4 vs 4.1, p < 0.001). There was no significant difference between patients that completed the questionnaire before receiving psychotherapy and those that did not receive psychotherapy (4.7 vs 4.1, p = 0.13).
Alcohol-associated Liver Disease
We first sought to assess if patients with AUD receiving psychotherapy were less likely to develop ALD than patients that did not receive psychotherapy. We identified 3544 patients that received psychotherapy and compared them to 6091 patients that didn’t receive psychotherapy. In the treated group, 1804 patients received individual therapy, 2586 patients received group therapy and 715 patients received family therapy. Patients receiving psychotherapy did so for a median 1.1 years. The median follow-up from AUD diagnosis was 8.6 years in the group that received psychotherapy and 6.5 years in patients that did not receive psychotherapy (Figure 2a). Patients received psychotherapy a median of 3 days after their index AUD diagnosis. The median time from AUD diagnosis to ALD diagnosis was 4.6 years. Among patients that developed ALD after receiving psychotherapy, the median time from treatment initiation to ALD diagnosis was 5.6 years.
Figure 2. Development of ALD in patients with AUD according to psychotherapy.
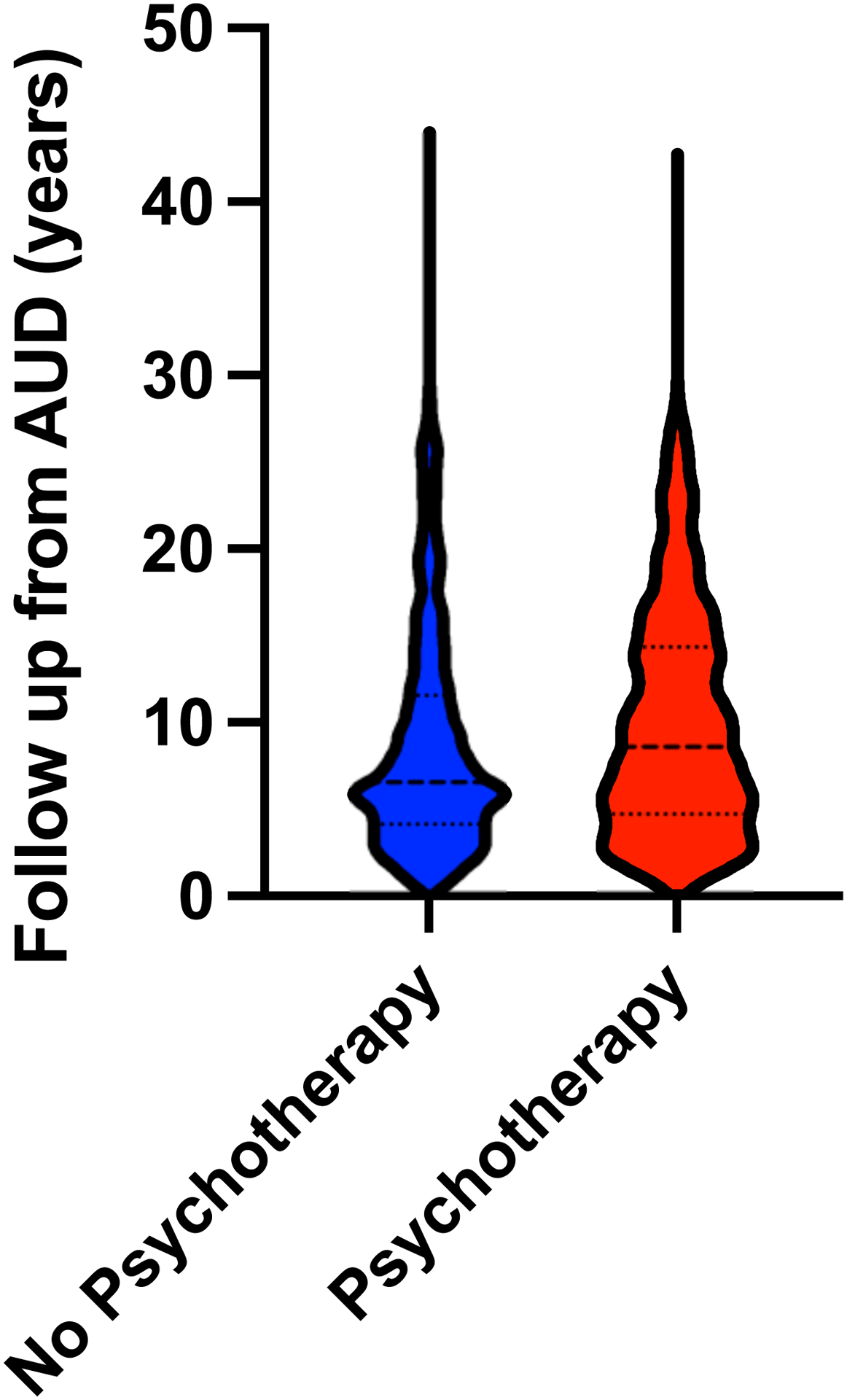
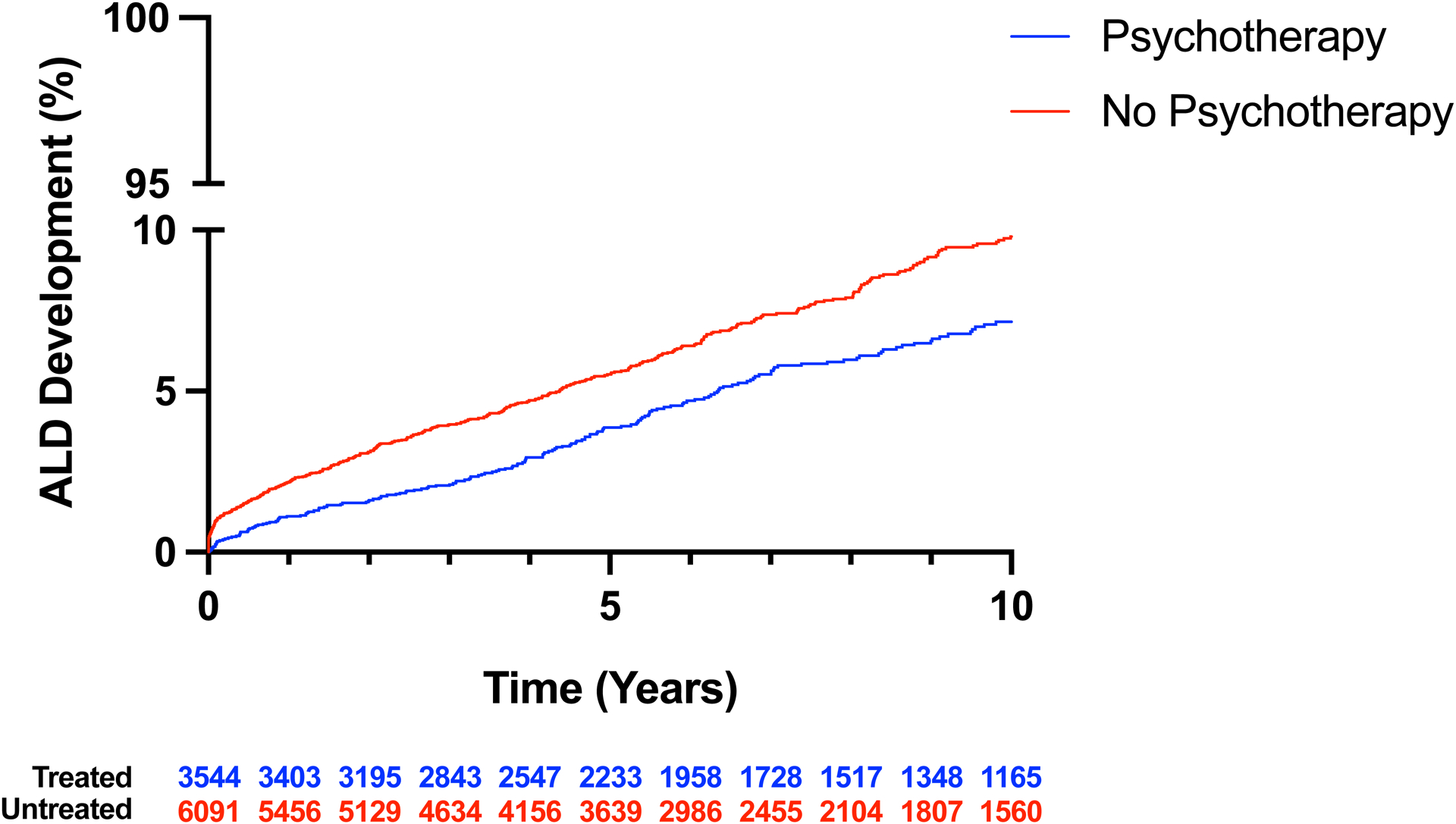
A, Violin plot of follow-up after AUD diagnosis in patients with and without psychotherapy treatment. Thickly dotted line represents the median, while the thinly dotted lines represent the 25th and 75th percentiles. B, Kaplan-Meier curve of ALD development in patients with AUD treated with and without psychotherapy. The incidence of ALD was lower in patients with AUD that received psychotherapy than in patients that did not receive psychotherapy. ALD, alcohol-associated liver disease; AUD, alcohol use disorder.
In a Kaplan-Meier analysis, we found that patients receiving psychotherapy were less likely to develop ALD in the 10 years following AUD diagnosis compared to patients that did not receive psychotherapy (p < 0.001) (Figure 2b). In multivariable analysis, which account for the time of follow-up in addition to the variables in Supplementary Table 1, patients receiving psychotherapy were less likely to develop ALD (HR: 0.59, 95% CI: 0.50–0.71, p < 0.001) (Table 2). When the multivariable model was recreated using specific types of psychotherapy, individual and group therapy were each independently associated with ALD (HR: 0.70, 95% CI: 0.56–0.86, p < 0.001; HR: 0.76, 95% CI: 0.61–0.94, p = 0.01), but family therapy was not (HR: 0.81, 95% CI: 0.56–1.14, p = 0.25). The univariable (Supplementary Table 3) associations for all factors in the model with the outcome of ALD are available in the supplement.
Table 2:
Multivariate associations with the development of ALD
| P value | |||
|---|---|---|---|
| Age | 1.01 | 1.00 to 1.02 | 0.04 |
| Male Sex | 1.20 | 1.01 to 1.43 | 0.04 |
| Asian Race | 2.29 | 0.97 to 4.51 | 0.03 |
| Black Race | 0.83 | 0.61 to 1.09 | 0.20 |
| Other Race | 0.97 | 0.63 to 1.43 | 0.90 |
| Unknown Race | 1.48 | 0.90 to 2.32 | 0.11 |
| Hispanic Ethnicity | 0.60 | 0.35 to 1.00 | 0.053 |
| Overweight | 0.96 | 0.78 to 1.17 | 0.68 |
| Obese Class I | 0.93 | 0.59 to 1.17 | 0.52 |
| Obese Class II | 0.83 | 0.59 to 1.15 | 0.28 |
| Obese Class III | 1.04 | 0.70 to 1.49 | 0.85 |
| Missing Weight | 0.79 | 0.58 to 1.05 | 0.11 |
| Nicotine Dependence | 1.10 | 0.93 to 1.31 | 0.28 |
| Hep B Positive | 1.08 | 0.74 to 1.56 | 0.67 |
| Hep C Positive | 2.17 | 1.79 to 2.63 | <0.001 |
| Homeless | 1.25 | 1.01 to 1.56 | 0.04 |
| Cannabis Use Disorder | 1.18 | 0.95 to 1.45 | 0.13 |
| Cocaine Use Disorder | 0.73 | 0.58 to 0.92 | 0.009 |
| Inhalant Use Disorder | 0.87 | 0.70 to 1.09 | 0.24 |
| Opioid Use Disorder | 1.12 | 0.91 to 1.38 | 0.28 |
| Other Stimulant Use Disorder | 1.00 | 0.73 to 1.35 | 0.99 |
| Sedative Use Disorder | 1.03 | 0.80 to 1.32 | 0.81 |
| Anxiety Disorder | 1.30 | 1.04 to 1.65 | 0.03 |
| Specific Personality Disorder | 0.94 | 0.73 to 1.19 | 0.59 |
| Mood Disorder | 0.98 | 0.78 to 1.23 | 0.84 |
| Schizophrenia | 1.18 | 0.97 to 1.43 | 0.10 |
| Chronic Passive Congestion of Liver | 1.86 | 1.48 to 2.30 | <0.001 |
| Hemochromatosis | 1.75 | 1.16 to 2.53 | 0.005 |
| Autoimmune Hepatitis | 1.05 | 0.33 to 2.69 | 0.92 |
| Primary Biliary Cholangitis | 0.20 | 0.05 to 1.20 | 0.04 |
| Secondary Biliary Cholangitis | 6.528 | 1.05 to 25.68 | 0.02 |
| Biliary Cirrhosis | 3.86 | 1.19 to 10.42 | 0.01 |
| Alpha-1-antitrypsin Deficiency | 3.38 | 0.56 to 10.66 | 0.09 |
| Nonalcoholic Steatohepatitis | 4.04 | 2.93 to 5.46 | <0.001 |
| Alcohol Dependence | 1.18 | 0.81 to 1.69 | 0.37 |
| Both Alcohol Abuse and Dependence | 4.90 | 3.93 to 6.18 | <0.001 |
| Medication-assisted Therapy | 0.56 | 0.47 to 0.66 | <0.001 |
| Psychotherapy | 0.59 | 0.50 to 0.71 | <0.001 |
Odds ratios for racial categories use white race as reference level, while BMI categories use the reference level of the non-overweight BMI class. AUD class (dependence, abuse or both) use alcohol abuse as a reference level.
In landmark analyses using Cox regression models and landmarks at 30, 90, and 360 days, psychotherapy was associated with a reduced risk of ALD (HR: 0.72, 95% CI 0.58–0.89, p = 0.003; HR: 0.70, 95% CI 0.57–0.87, p = 0.002; HR: 0.71, 95% CI: 0.57–0.88, p = 0.002, respectively). Descriptive statistics for these landmark analyses are available Supplementary Table 4.
Decompensation among patients with cirrhosis
Given that psychotherapy was associated with lower risk of developing ALD, we sought to determine if patients with cirrhosis were less likely to experience hepatic decompensation if they had received psychotherapy at any time. Among the 599 patients with cirrhosis, we identified 189 patients that received psychotherapy and compared them to 410 patients that did not receive psychotherapy. In the treated group, 89 patients received individual therapy, 69 patients received group therapy and 19 patients received family therapy. Patients with cirrhosis who had received psychotherapy were treated for a median 1.7 years. The median follow-up from cirrhosis diagnosis was 7.3 years in the psychotherapy-treated group and 5.9 years in patients that did not receive psychotherapy (Figure 3a). Patients that experienced hepatic decompensation did so after a median 0.9 years. Patients with cirrhosis that received psychotherapy initiated it a median of 5.7 years before their index cirrhosis diagnosis. Patients with cirrhosis who experienced a decompensating event initiated psychotherapy 6.73 years before the decompensating event.
Figure 3. Development of hepatic decompensation in a subgroup of patients who develop cirrhosis according to psychotherapy.
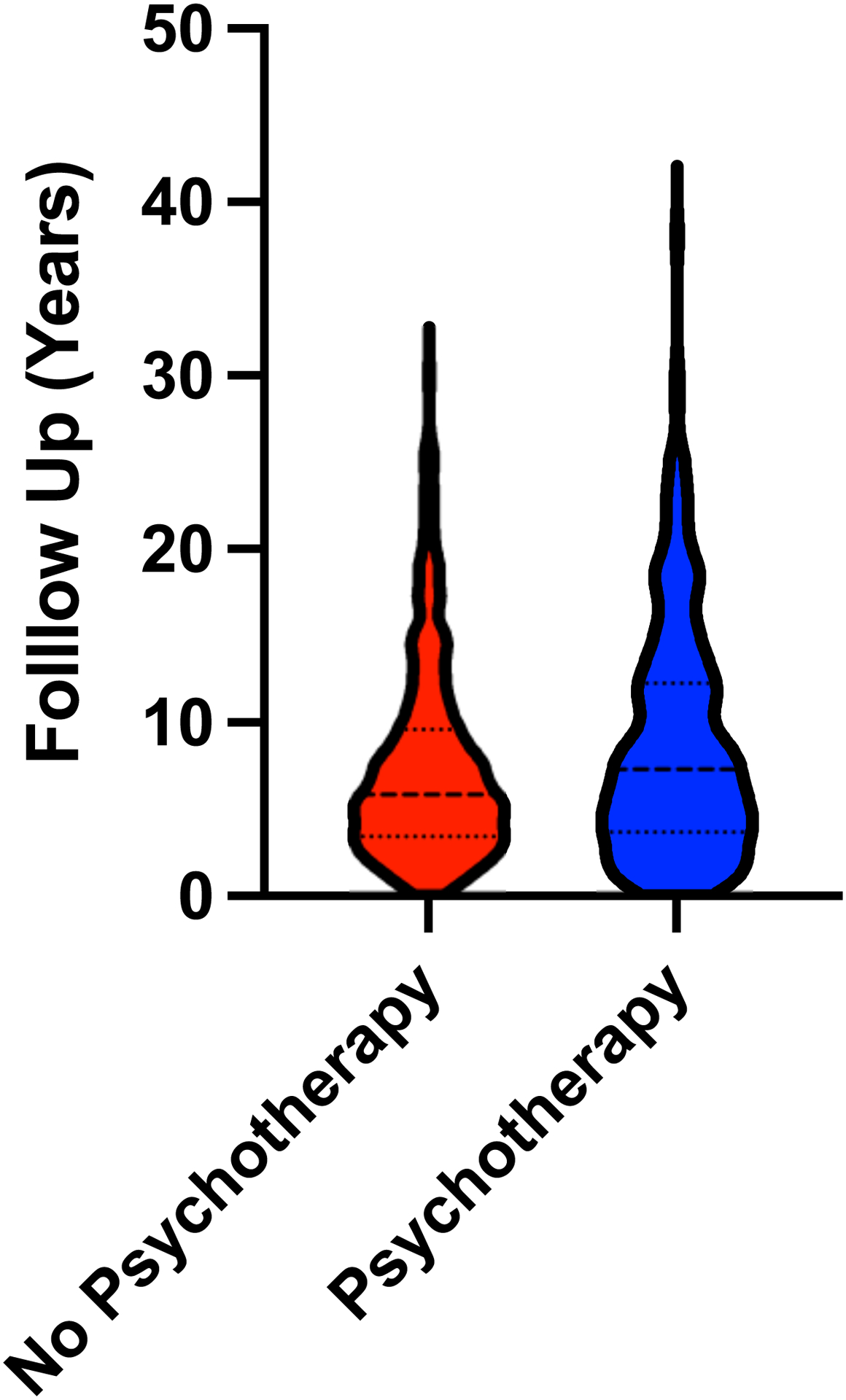
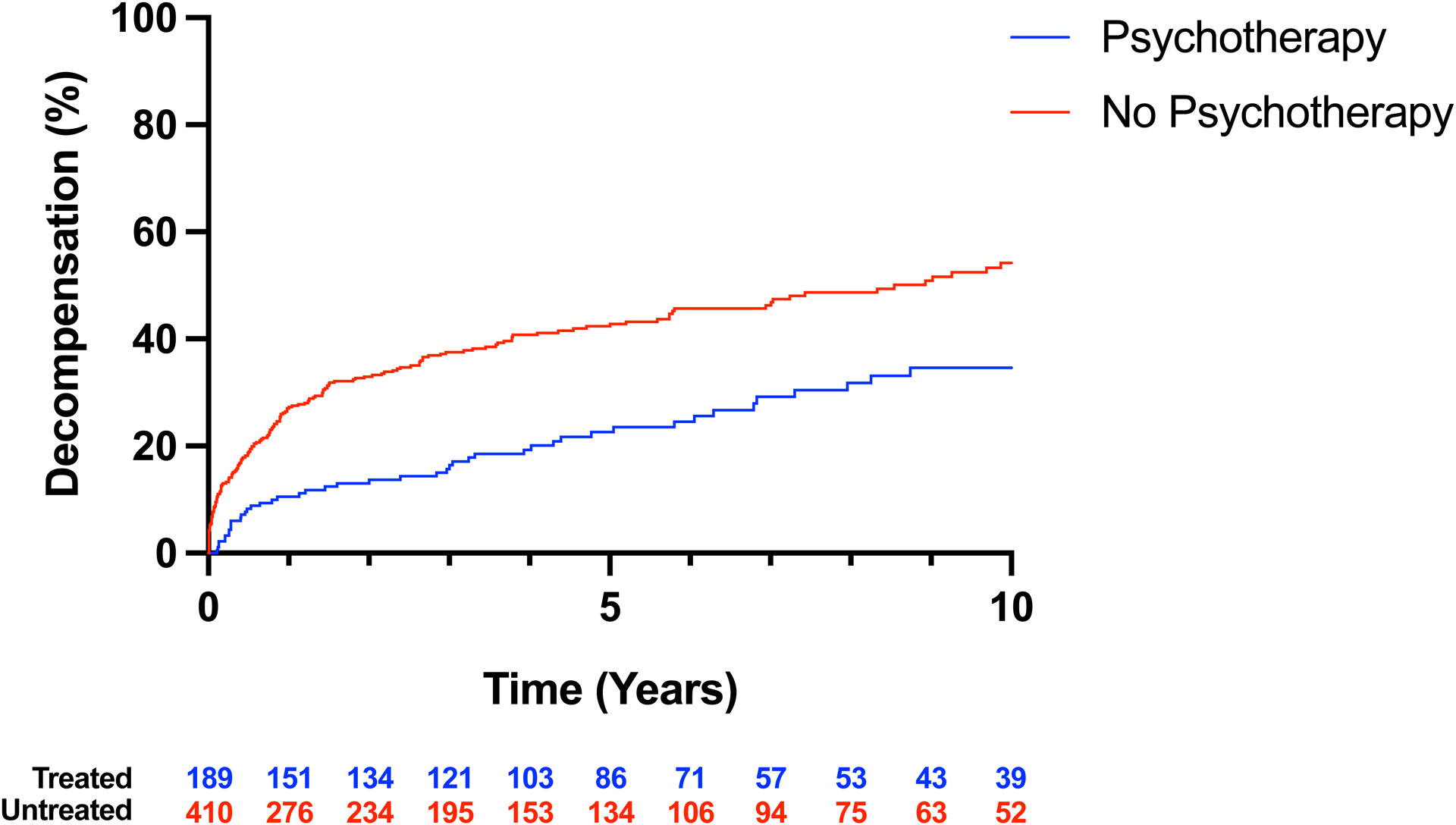
A, Violin plot of follow-up after cirrhosis diagnosis in patients with and without psychotherapy treatment. Thickly dotted line represents the median, while the thinly dotted lines represent the 25th and 75th percentiles. B, Kaplan-Meier curve of decompensation in patients with cirrhosis treated and not treated with psychotherapy. The incidence of hepatic decompensation was lower in patients with cirrhosis that received psychotherapy than in patients that did not receive psychotherapy.
In a Kaplan-Meier analysis, we found that among patients with cirrhosis, those receiving psychotherapy were less likely to experience hepatic decompensation in the 10 years following cirrhosis diagnosis compared to patients that did not receive psychotherapy (p < 0.001) (Figure 3b). In Cox regressions, which account for the time of follow-up in addition to the variables in Supplementary Table 1, patients receiving psychotherapy were found to have lower rate of hepatic decompensation (HR: 0.68, 95% CI: 0.48–0.95, p < 0.001) (Table 3). Among patients that experienced hepatic decompensation after receiving psychotherapy, the median time from treatment initiation to hepatic decompensation was 6.9 years. Patients that received individual psychotherapy had a nonsignificant trend towards reduced likelihood of decompensation (HR: 0.69, 95% CI: 0.44–1.05, p = 0.09). No strong trend was observed for either family or group therapy. The univariable (Supplementary Table 5) associations for all factors in the model with the outcome of hepatic decompensation are available in the supplement.
Table 3:
Multivariate associations with hepatic decompensation
| P value | |||
|---|---|---|---|
| Age | 0.99 | 0.97 to 1.00 | 0.04 |
| Male Sex | 0.93 | 0.68 to 1.27 | 0.63 |
| Asian Race | 0.30 | 0.02 to 1.41 | 0.23 |
| Black Race | 0.42 | 0.18 to 0.81 | 0.02 |
| Other Race | 0.78 | 0.34 to 1.59 | 0.53 |
| Unknown Race | 0.63 | 0.21 to 1.72 | 0.39 |
| Hispanic Ethnicity | 0.63 | 0.23 to 1.48 | 0.32 |
| Overweight | 0.94 | 0.65 to 1.37 | 0.75 |
| Obese Class I | 0.79 | 0.52 to 1.20 | 0.26 |
| Obese Class II | 0.69 | 0.40 to 1.17 | 0.18 |
| Obese Class III | 0.59 | 0.26 to 1.20 | 0.17 |
| Missing Weight | 1.10 | 0.61 to 1.91 | 0.75 |
| Nicotine Dependence | 0.82 | 0.62 to 1.08 | 0.16 |
| Hep B Positive | 0.98 | 0.60 to 1.55 | 0.94 |
| Hep C Positive | 1.00 | 0.73 to 1.35 | 0.98 |
| Homeless | 1.02 | 0.64 to 1.61 | 0.92 |
| Cannabis Use Disorder | 1.38 | 0.87 to 2.14 | 0.16 |
| Cocaine Use Disorder | 0.90 | 0.57 to 1.42 | 0.66 |
| Inhalant Use Disorder | 0.74 | 0.47 to 1.15 | 0.19 |
| Opioid Use Disorder | 0.64 | 0.43 to 0.95 | 0.03 |
| Other Stimulant Use Disorder | 0.96 | 0.45 to 1.87 | 0.90 |
| Sedative Use Disorder | 1.24 | 0.71 to 2.09 | 0.43 |
| Anxiety Disorder | 0.92 | 0.64 to 1.32 | 0.65 |
| Specific Personality Disorder | 0.83 | 0.49 to 1.35 | 0.47 |
| Mood Disorder | 1.24 | 0.88 to 1.77 | 0.23 |
| Schizophrenia | 0.77 | 0.53 to 1.10 | 0.16 |
| Chronic Passive Congestion of Liver | 1.54 | 1.14 to 2.07 | 0.00 |
| Hemochromatosis | 0.78 | 0.45 to 1.26 | 0.34 |
| Autoimmune Hepatitis | 2.19 | 0.67 to 5.80 | 0.15 |
| Primary Biliary Cholangitis | 0.77 | 0.14 to 4.50 | 0.77 |
| Secondary Biliary Cholangitis | 1.84 | 0.29 to 9.16 | 0.50 |
| Biliary Cirrhosis | 1.41 | 0.67 to 2.70 | 0.33 |
| Alpha-1-antitrypsin Deficiency | 0.76 | 0.04 to 4.27 | 0.80 |
| Nonalcoholic Steatohepatitis | 1.74 | 1.14 to 2.56 | 0.01 |
| Alcohol Dependence | 1.01 | 0.64 to 1.57 | 0.02 |
| Both Alcohol Abuse and Dependence | 1.53 | 1.07 to 2.24 | 0.97 |
| Medication-assisted Therapy | 0.48 | 0.36 to 0.64 | <0.001 |
| Psychotherapy | 0.68 | 0.48 to 0.95 | 0.03 |
Odds ratios for racial categories use white race as reference level, while BMI categories use the reference level of the non-overweight BMI class. AUD class (dependence, abuse or both) use alcohol abuse as a reference level.
In landmark analyses using Cox regression models and landmarks at 30, 90, and 360 days psychotherapy was not associated with a reduced risk of decompensated cirrhosis (HR: 0.77, 95% CI 0.47–1.19, p = 0.26; HR: 0.89, 95% CI 0.57–1.35, p = 0.70; HR: 1.02, 95% CI: 0.61–1.62, p = 0.95). Descriptive statistics for these landmark analyses are available in Supplementary Table 6.
We next sought to determine if initiating psychotherapy after a diagnosis of cirrhosis associated with an altered risk of hepatic decompensation. In our cohort, 57 patients received therapy only after their index diagnosis of cirrhosis. For psychotherapy initiated only after a diagnosis of cirrhosis we saw a nonsignificant trend towards lower odds of hepatic decompensation (HR: 0.62, 95% CI: 0.34–1.07, p = 0.10).
DISCUSSION
Preventing the development of ALD is a critically important outcome as this is the most common complication of AUD and is associated with significant morbidity and mortality worldwide. In this large well-characterized cohort of patients with AUD, our analysis suggests that treatment with psychotherapy may reduce the development of ALD in patients with AUD. In multivariable models, psychotherapy was associated with a significantly lower risk of ALD and decompensated cirrhosis. We also found that patients reported lower alcohol consumption after the receipt of psychotherapy than before therapy, suggesting that psychotherapy’s association with lower risk of ALD may be linked to reduced alcohol consumption. For patients with AUD, individual therapy and group therapy, but not family therapy, were independently associated with reduced incidence of ALD. Psychotherapy was shown to be protective against ALD and hepatic decompensation independent of multiple factors including use of MAT with disulfiram, acamprosate, naltrexone, gabapentin, baclofen or topiramate. In landmark analyses, we again found that psychotherapy was independently associated with reduced incidence of ALD at all three landmarks of 30, 90 and 360 days. Thus, the finding was not sensitive to the placement of the landmark. However, landmark analysis of psychotherapy in patients with cirrhosis did not reveal an association with reduced incidence of decompensation using landmarks of 30, 90 or 360 days.
Our finding that psychotherapy may reduce the development of ALD in patients with AUD has important clinical implications. Prevention of ALD and cirrhosis are important goals for reducing morbidity and mortality, particularly in the context of rising rates of liver-related mortality in the United States and limited availability of liver transplantation (5,6). Though this study did not include detailed characterization beyond individual, group, and family psychotherapy, evaluation of various strategies of behavioral change such as brief interventions, mutual help groups (e.g. 12-step programs), cognitive behavioral therapy, and motivational enhancement therapy could better inform this finding. Unfortunately, most patients with AUD are not treated with psychotherapy (23); however, this study supports a growing body of literature which suggests that psychotherapy is effective in preventing meaningful clinical events in patients with AUD including important end-organ sequelae. Prospective randomized trials are warranted to further assess the benefits of various modalities of psychotherapy on liver-related outcomes. Our findings emphasize the importance of screening for AUD, referral for treatment, and multidisciplinary care for patients with AUD. In recent years, novel models of identifying and caring for patients with ALD have emerged including combined psychological and medical care delivery models which have potential to improve care for patients with AUD and ALD (26,27). There are many potential approaches to integrate care for patients with ALD and further study is warranted to develop and understand these multidisciplinary approaches (28).
Our study demonstrated that individual therapy and group therapy, but not family therapy, were independently associated with the development of ALD in patients with AUD. Family therapy engages family members in treatment to address relationships and communication as part of management of substance use disorder and has shown efficacy in the treatment of substance use disorder as a stand-alone intervention and as part of a multicomponent intervention (29). Including family members in the treatment paradigm of substance use disorder has been shown to improve treatment engagement in persons with substance use disorder (30). Superiority of one psychosocial intervention over another has not been clearly demonstrated in clinical trials and selection of psychosocial therapy is often tailored to the individual patient, their preferences, and accessibility.
Our results are consistent with similar findings of reduced alcohol use in patients with AUD treated with psychotherapy (16–22). This study also confirms previous findings showing reduced hepatic decompensation events with psychotherapy and MAT (22). In one study of veterans with cirrhosis in the United States, behavioral therapy and pharmacotherapy for AUD was associated with a significant reduction in hepatic decompensation (23). This study included follow-up for six months and found only 14% of patients received behavioral therapy or pharmacotherapy for AUD. In contrast, our study had a median follow-up of 7.3 years in the patients with cirrhosis receiving psychotherapy and demonstrated broader use of psychotherapy with over a third of patients receiving treatment. Follow-up over many years is critical in patients with AUD who are at risk for liver-related outcomes over the long-term. Pharmacotherapy with MAT is a hallmark of treatment for AUD; how MAT and psychotherapy compare or can be used synergistically remains to be seen and is likely better suited for a prospective trial.
This study is limited in that it is retrospective and may not include all the factors differentiating patients treated with psychotherapy and those who were not. Socioeconomic status was accounted for in a limited capacity and may affect ability to initiate and adhere to psychotherapy. Adherence to treatment was not accounted for in this study and may be a moderator in the association between psychotherapy and liver-related outcomes. Additionally, ALD tends to be diagnosed at more advanced stages compared to other liver diseases (31), which may confound our observed results. Certain gaps exist in this data set including limited data on alcohol consumption over time and the absence of mortality data. These are important limitations of this study and future prospective studies of psychotherapy in patients with AUD should include liver-related outcomes, validated quantification of alcohol consumption, and mortality. The MGB Biobank cohort encompasses a multicenter health system in eastern Massachusetts and is predominantly white. While we account for race and ethnicity in our multivariable analysis, our findings may have limited generalizability. Further investigation in more diverse cohorts is warranted. In addition, the number patients with AUD treated with psychotherapy or MAT is higher than previously reported and may be explained by the availability of multidisciplinary addiction services within the MGB system and by widespread health insurance coverage in Massachusetts.
In conclusion, psychotherapy for patients with AUD was associated with a lower rate of ALD and a lower rate of decompensated cirrhosis. Individual and group therapy, but not family therapy, were associated with a lower rate of ALD. Psychotherapy may prevent liver-related complications for patients with AUD over the long-term.
Supplementary Material
“What You Need to Know”.
Background:
Psychotherapy for alcohol use disorder (AUD) has been shown to reduce alcohol use. In patients with AUD, the effect of psychotherapy on future alcohol-associated liver disease (ALD) and hepatic decompensation is not known.
Findings:
Among patients with AUD, psychotherapy was associated with a reduced rate of ALD when adjusting for multiple confounders including pharmacotherapy for AUD. Among patients with cirrhosis, psychotherapy was associated with a reduced rate of hepatic decompensation.
Implication for patient care:
Psychotherapy for AUD may reduce the incidence and progression of ALD independent of pharmacotherapy for AUD.
FINANCIAL SUPPORT
Eric M. Przybyszewski was supported by a T-32 grant from the National Institutes of Health (5T32DK007191-48).
ABBREVIATIONS
- ALD
Alcohol-associated liver disease
- AUD
alcohol use disorder
- BMI
body mass index
- CI
confidence interval
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HR
hazard ratio
- MAT
medication-assisted treatment
- MGB
Mass General Brigham
- NASH
non-alcoholic steatohepatitis
- OR
odds ratio
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors have no financial or personal conflict of interest to disclose.
STUDY MATERIALS
All data, analytic methods and study materials are available on request.
REFERENCES
- 1.Degenhardt L, Charlson F, Ferrari A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018;5:987–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015;72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbosa C, Cowell AJ, Dowd WN. Alcohol consumption in response to the COVID-19 pandemic in the United States. J Addict Med 2021;15:341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guirguis J, Chhatwal J, Dasarathy J, et al. Clinical Impact of Alcohol-Related Cirrhosis in the Next Decade: Estimates Based on Current Epidemiological Trends in the United States. Alcohol Clin Exp Res 2015;39:2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabb DW, Im GY, Szabo G. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2020;71:306–333. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan R, Sinclair JMA. Alcohol use disorder and the liver. Addiction 2021;116:1270–1278. [DOI] [PubMed] [Google Scholar]
- 9.Arab JP, Díaz LA, Baeza N, et al. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. J Hepatol 2021;75:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan A, Tansel A, White DL, et al. Efficacy of Psychosocial Interventions in Inducing and Maintaining Alcohol Abstinence in Patients with Chronic Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol 2016;14:191–202.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Murad MH, Chandar AK, et al. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology 2015;149:958–970.e12. [DOI] [PubMed] [Google Scholar]
- 12.Singh V, Sharma AK, Narasimhan RL, et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol 2014;109:1417–1423. [DOI] [PubMed] [Google Scholar]
- 13.Singal AK, Kamath PS, Gores GJ, et al. Alcoholic Hepatitis: Current Challenges and Future Directions. Clin Gastroenterol Hepatol 2014;12:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo G Clinical Trial Design for Alcoholic Hepatitis. Semin Liver Dis 2017;37:332–342. [DOI] [PubMed] [Google Scholar]
- 15.Longabaugh R, Wirtz PW, Zweben A, et al. Network support for drinking, Alcoholics Anonymous and long-term matching effects. Addiction 1998;93:1313–1333. [DOI] [PubMed] [Google Scholar]
- 16.Willenbring ML, Olson DH. A Randomized Trial of Integrated Outpatient Treatment for Medically Ill Alcoholic Men. Arch Intern Med 1999;159:1946–1952. [DOI] [PubMed] [Google Scholar]
- 17.Weinrieb RM, Van Horn DHA, Lynch KG, et al. A randomized, controlled study of treatment for alcohol dependence in patients awaiting liver transplantation. Liver Transpl 2011;17:539–547. [DOI] [PubMed] [Google Scholar]
- 18.Khan A, Tansel A, White DL, et al. Efficacy of Psychosocial Interventions in Inducing and Maintaining Alcohol Abstinence in Patients with Chronic Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol 2016;14:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieperink E, Fuller B, Isenhart C, et al. Efficacy of motivational enhancement therapy on alcohol use disorders in patients with chronic hepatitis C: a randomized controlled trial. Addiction 2014;109:1869–1877. [DOI] [PubMed] [Google Scholar]
- 20.Dieperink E, Ho SB, Heit S, et al. Significant Reductions in Drinking Following Brief Alcohol Treatment Provided in a Hepatitis C Clinic. Psychosomatics 2011;51:149–156. [DOI] [PubMed] [Google Scholar]
- 21.Proeschold-Bell RJ, Patkar AA, Naggie S, et al. An Integrated Alcohol Abuse and Medical Treatment Model for Patients with Hepatitis C. Dig Dis Sci 2011;57:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Project MATCH Research Group. J Stud Alcohol 1998;59:631–639. [DOI] [PubMed] [Google Scholar]
- 23.Rogal S, Youk A, Zhang H, et al. Impact of Alcohol Use Disorder Treatment on Clinical Outcomes Among Patients With Cirrhosis. Hepatology 2020;71:2080–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro VM, Gainer V, Wattanasin N, et al. The Mass General Brigham Biobank Portal: an i2b2-based data repository linking disparate and high-dimensional patient data to support multimodal analytics. J Am Med Inform Assoc 2022;29:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannier AGL, Shay JES, Fomin V, et al. Incidence and Progression of Alcohol-Associated Liver Disease After Medical Therapy for Alcohol Use Disorder. JAMA Netw Open 2022;5:e2213014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fomin V, Marshall C, Tsai S, et al. Creation of an Inpatient Alcohol Liver Service Improves Early Liver Disease Detection in Patients with Alcohol Use Disorder. Clin Gastroenterol Hepatol 2022;S1542–3565(22)00382–2. [DOI] [PubMed] [Google Scholar]
- 27.Mellinger JL, Winder GS, Fernandez AC, et al. Feasibility and early experience of a novel multidisciplinary alcohol-associated liver disease clinic. J Subst Abuse Treat 2021;130:108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winder GS, Fernandez AC, Mellinger JL. Integrated Care of Alcohol-Related Liver Disease. J Clin Exp Hepatol 2016;6:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogue A, Schumm JA, MacLean A, et al. Couple and family therapy for substance use disorders: Evidence-based update 2010–2019. J Marital Fam Ther 2022;48:178–203. [DOI] [PubMed] [Google Scholar]
- 30.Kirby KC, Marlowe DB, Festinger DS, et al. Community reinforcement training for family and significant others of drug abusers: a unilateral intervention to increase treatment entry of drug users. Drug Alcohol Depend 1999;56:85–96. [DOI] [PubMed] [Google Scholar]
- 31.Shah ND, Ventura-Cots M, Abraldes JG, et al. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol 2019;17:2320–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


