Abstract
The prevalence of inflammatory disease conditions, including allergies, asthma, and autoimmune disorders, increased during the latter half of the twentieth century, as societies transitioned from rural to urban lifestyles. A number of hypotheses have been put forward to explain the increasing prevalence of inflammatory disease in modern urban societies, including the hygiene hypothesis and the “Old Friends” hypothesis. In 2008, Rook and Lowry proposed, based on the evidence that increased inflammation was a risk factor for stress-related psychiatric disorders, that the hygiene hypothesis or “Old Friends” hypothesis may be relevant to psychiatric disorders. Since then, it has become more clear that chronic low-grade inflammation is a risk factor for stress-related psychiatric disorders, including anxiety disorders, mood disorders, and trauma- and stressor-related disorders, such as posttraumatic stress disorder (PTSD). Evidence now indicates that persons raised in modern urban environments without daily contact with pets, relative to persons raised in rural environments in proximity to farm animals, respond with greater systemic inflammation to psychosocial stress. Here we consider the possibility that increased inflammation in persons living in modern urban environments is due to a failure of immunoregulation, i.e., a balanced expression of regulatory and effector T cells, which is known to be dependent on microbial signals. We highlight evidence that microbial signals that can drive immunoregulation arise from phylogenetically diverse taxa but are strain specific. Finally, we highlight Mycobacterium vaccae NCTC 11659, a soil-derived bacterium with anti-inflammatory and immunoregulatory properties, as a case study of how single strains of bacteria might be used in a psychoneuroimmunologic approach for prevention and treatment of stress-related psychiatric disorders.
Keywords: Anxiety, Darwinian medicine, Depression, Gut-brain axis, Hygiene hypothesis, Microbiome, Microbiota, Microbiota-gut-brain axis, Old friends, Posttraumatic stress disorder
1. Introduction
In this chapter, we focus on the potential role of exposures to diverse microbial environments in promotion of stress resilience, particularly in the context of prevention and treatment of stress-related psychiatric disorders, including anxiety disorders, mood disorders, and trauma- and stressor-related disorders such as posttraumatic stress disorder (PTSD) (American Psychiatric Association 2013). One approach to prevention of stress-related mental health disorders is to identify modifiable risk factors (Insel and Scolnick 2006). In this review, we outline evidence supporting the hypothesis that: (1) inappropriate inflammation is a risk factor for the development and persistence of symptoms of stress-related psychiatric disorders; and (2) exposures to diverse microbial environments, including non-pathogenic bacteria found in nature, can induce anti-inflammatory and immunoregulatory responses and thus regulate the inflammatory response to day-to-day stressors and traumatic events, in turn promoting resilience to stress. A failure of immunoregulation, which is defined as a balanced expression of regulatory T cells (Treg) and effector T cells, may be involved in contributing to an overreactive inflammatory stress response thus predisposing individuals to the development of stress-related psychiatric disorders (Langgartner et al. 2018), particularly in urban environments with reduced exposures to diverse microbial environments (Böbel et al. 2018). Promoting stress resilience by increasing contact with microbial “Old Friends,” i.e., microorganisms with anti-inflammatory and immunoregulatory properties, may provide an alternative strategy for prevention and treatment of stress-related psychiatric disorders.
2. Global Incidence and Prevalence of Common Mental Health Disorders
Common mental health disorders include anxiety disorders, depressive disorders, and trauma- and stressor-related disorders, such as PTSD that are classified in ICD-10 as: “neurotic, stress-related and somatoform disorders” and “mood disorders” (Patel and Kleinman 2003; World Health Organization 1992; National Collaborating Centre for Mental Health (UK) 2011). Anxiety and mood disorders are the most prevalent forms of common mental health disorders and substantially contribute to the global burden of disease (Whiteford et al. 2013). The World Health Organization surveyed mental health conditions in 2015 and found an estimated 3.6% of the global population was suffering from anxiety disorders and 4.4% of the global population was suffering from depression, with women being more likely to be affected by these disorders than men (World Health Organization 2017). Depression is ranked as the single largest contributor to global disability while anxiety disorders are ranked as the sixth largest contributor to global disability (World Health Organization 2017). Subsequent to the onset of the COVID-19 pandemic, the global prevalence and burden of anxiety and mood disorders has increased further, particularly among young persons, and again with a stronger impact on females (Santomauro et al. 2021).
While previously classified as an anxiety disorder, and thus included among the common mental health disorders, PTSD is now classified in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as a trauma- and stressor-related disorder (American Psychiatric Association 2013). PTSD is particularly prevalent among military Veterans. Since October of 2001, approximately 2.7 million U.S. troops have been deployed in recent conflicts (Wenger et al. 2018). Findings suggest that approximately 20% of returning service members meet criteria for PTSD or associated mental health conditions (Tanielian et al. 2008). In the USA, many Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans are resistant to engaging in conventional mental health treatments (Kim et al. 2010; Kim et al. 2010), highlighting the importance of exploring alternative interventions (Williams et al. 2011). Moreover, a substantial proportion of individuals are not significantly helped by traditional treatments. Non-response rates in outcome studies for PTSD are often as high as 50% (Schottenbauer et al. 2008). Similar non-response rates have been described in non-military populations (Stein et al. 2006, 2009).
3. A Need for more Effective Therapies with a More Rapid Onset of Action
First-line psychotherapies for generalized anxiety disorder (GAD), as one example of an anxiety disorder, include cognitive behavioral therapy (CBT), cognitive therapy (CT), and applied relaxation, while first-line pharmacotherapies for GAD include selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs) (Anxiety and Depression Association of America 2015). Meanwhile, first-line psychotherapies for major depressive disorder include psychotherapy, including CBT, interpersonal psychotherapy (IPT), and problem-solving therapy (PST), while first-line pharmacotherapies for major depressive disorder include SSRIs, SNRIs, bupropion, mirtazapine, and a number of newer agents (Anxiety and Depression Association of America 2020). However, several limitations affect the efficacy and feasibility of these treatments. These limitations include, but are not limited to, delayed onset of action, adverse effects that impair quality of life, relapse risk upon withdrawal, and non-adherence (Andrews et al. 2011; Freedman 2010; Li et al. 2012; Lin et al. 1995; Mathew et al. 2012; Papakostas and Fava 2009; Rush et al. 2006b).
First-line therapies for the treatment of PTSD are evidence-based cognitive behavioral psychotherapies, for example, CBT and eye movement desensitization and reprocessing (EMDR) (Resick et al. 2017; Department of Veterans Affairs DoD 2017; American Psychological Association 2017; Watkins et al. 2018). These interventions are effective in reducing PTSD symptoms; however, not all persons respond with complete recovery (Steenkamp et al. 2020). For example, approximately two-thirds of US Veterans who complete these treatments continue to meet diagnostic criteria for PTSD (Steenkamp et al. 2020; Stein et al. 2006, 2009) and there are high dropout rates from first-line psychotherapies for PTSD (Kehle-Forbes et al. 2016; Steenkamp et al. 2020).
First-line pharmacotherapies for PTSD include SSRIs (Martin et al. 2021). Unfortunately, pharmacotherapies also have important shortcomings. Indeed, only about half of patients respond to SSRIs and more than a third of SSRI-treated patients fail to respond, do not reach full remission, or even develop SSRI resistance (Bernardy and Friedman 2015; Golden et al. 2002; Rush et al. 2006a; Kemp et al. 2008). Furthermore, most patients who initially respond to SSRI treatment fail to maintain therapeutic gains over time. In particular, Veterans affected by PTSD are generally resistant to SSRI therapy (Schnurr et al. 2007; Prigerson et al. 2001; Friedman et al. 2007).
4. A Need for Approaches to Increasing Stress Resilience: Strategies for Prevention of Common Mental Health Disorders
While it is important to pursue novel, fast acting interventions, including pharmacological interventions, for treatment of stress-related psychiatric disorders, there is also a need for novel approaches to prevention of these common mental health disorders (Insel and Scolnick 2006). When considering approaches to prevention of stress-related psychiatric disorders, one could argue that a reasonable strategy would be to target risk factors for these disorders, focusing on modifiable risk factors (Fig. 1). One factor that seems to increase risk of developing stress-related psychiatric disorders is chronic low-grade inflammation (Rohleder 2014). In the next section, we briefly consider the evidence that inappropriate or excessive inflammation is a risk factor for development of stress-related psychiatric disorders.
Fig. 1.
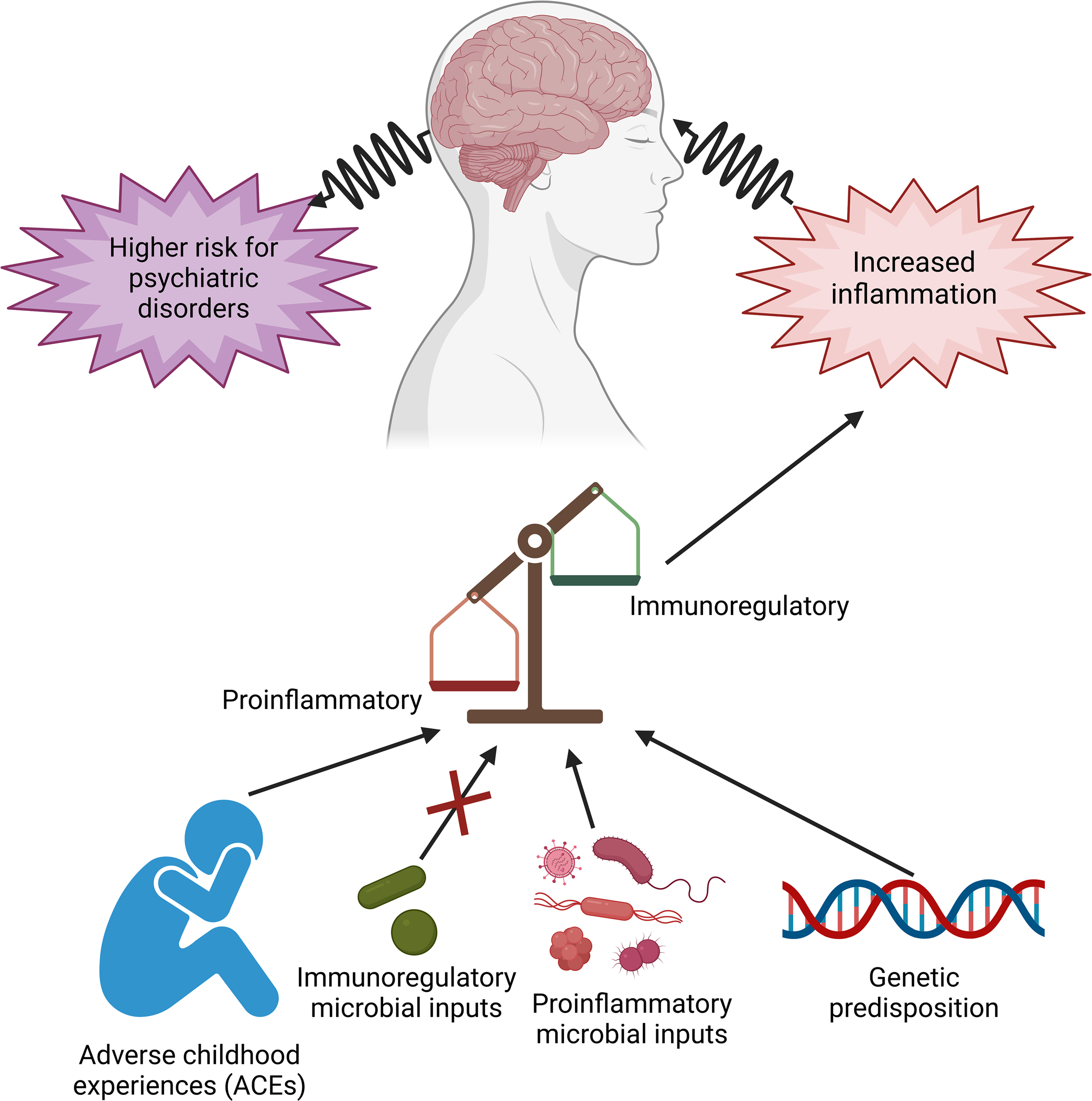
Risk factors for stress-related psychiatric disorders include: (1) genetic predisposition; and (2) environmental influences, including adverse childhood experiences (ACEs), and exposures to diverse microbial inputs. Microbial inputs can be either proinflammatory or anti-inflammatory/immunoregulatory (i.e., resulting in a balanced expression of regulatory T cells (Treg) and effector T cells). A failure of immunoregulation can lead to chronic low-grade inflammation and increased risk of stress-related psychiatric disorders. Figure created with biorender.com
5. Inflammation as a Risk Factor for Common Mental Disorders
Increasing evidence suggests that inflammation plays an important role in determining risk of development of stress-related psychiatric disorders, including anxiety disorders, mood disorders, and trauma- and stressor-related disorders such as PTSD. A connection between increases in cytokine activation, low-grade background inflammation, and stress-related psychiatric disorders has been observed in numerous studies implying a role for chronic low-grade inflammation in both the risk of development of stress-related psychiatric disorders and the persistence of symptoms (Capuron and Dantzer 2003; Dantzer et al. 1998, 1999; Miller and Raison 2016; Michopoulos et al. 2017; Flux and Lowry 2023; Rohleder 2014).
5.1. Inflammation as a Risk Factor for Anxiety Disorders
Anxiety disorders such as GAD, panic disorder (PD), and phobias (agoraphobia, social phobia, etc.) have been shown to be associated with chronic low-grade inflammation. For example, heightened proinflammatory markers, such as C-reactive protein (CRP), have been demonstrated in individuals diagnosed with anxiety disorders (Michopoulos et al. 2017; Vogelzangs et al. 2013; Copeland et al. 2012; Bankier et al. 2008). Other studies have found increased circulating proinflammatory cytokines, such as tumor necrosis factor (TNF), interleukin (IL) 1 beta, and IL-6 among individuals diagnosed with GAD and PD (Vieira et al. 2010; Hoge et al. 2009; Michopoulos et al. 2017; Brambilla et al. 1994; Zou et al. 2020). In line with these findings, there have been reports of high incidences of anxiety disorders and increased levels of emotional reactivity in individuals with inflammatory disease, including asthma, allergies, and autoimmune disorders (Lowry et al. 2016; Stanhope et al. 2022; von Mutius and Vercelli 2010; von Mutius et al. 1994). The weight of evidence is consistent with the hypothesis that inappropriate inflammation plays a role in determining risk of anxiety disorders (Haroon et al. 2012; Michopoulos et al. 2017).
Interestingly, lower levels of interferon gamma (IFNγ), a proinflammatory cytokine, have been found in both GAD and PD patients (Tukel et al. 2012; Vieira et al. 2010). Decreased IFNγ secretion from isolated peripheral blood mononuclear cells (PBMCs) from GAD patients could reflect decreased exposures to diverse microbial environments. For example, administration of the soil-derived bacterium, Mycobacterium vaccae NCTC 11659, or the type strain of M. vaccae, M. vaccae ATCC 15483, increases IFNγ and Th1 signaling in mice (Gong et al. 2020; Lahey et al. 2016; Smith et al. 2020; Zhang et al. 2016). Meanwhile, in humans, immunization with Mycobacterium vaccae NCTC 11659 increases IFNγ responses of PBMCs to subsequent antigen challenge up to a month following treatment (von Reyn et al. 2017). Interestingly, IFNγ expression, possibly arising from meningeal T cells then acting on both microglia and neurons in the brain, is necessary for social behavior (Filiano et al. 2016).
Consistent with a potential dysregulation of immunoregulation in individuals with anxiety disorders, lower phytohemagglutinin (PHA)-stimulated secretion of anti-inflammatory cytokines, including IL-2, IL-4, and IL-10 from isolated PBMCs have been documented in GAD patients (Vieira et al. 2010). Consistent with these findings, Hou et al. 2017 reported decreased circulating concentrations of IL-10, as well as higher TNF/IL-10 and TNF/IL-4 ratios (Hou et al. 2017). Altogether, data are consistent with the hypothesis that persons with a diagnosis of GAD have dysregulation of immunoregulation.
5.2. Inflammation as a Risk Factor for Mood Disorders
Among the common mental health disorders, the strongest case can be made for a role for inappropriate inflammation as a risk factor for development of mood disorders. This has been reviewed extensively elsewhere (Capuron and Dantzer 2003; Dantzer et al. 1998, 1999; Miller and Raison 2016; Michopoulos et al. 2017; Flux and Lowry 2023) and thus will not be reviewed in detail here.
Evidence supports impaired immunoregulation in persons with a diagnosis of mood disorders. Previous studies have demonstrated lower percentages of Treg in association with lower serum concentrations of the anti-inflammatory cytokines IL-10 and transforming growth factor beta 1 (TGFβ-1) in persons with major depressive disorder (Grosse et al. 2016; Li et al. 2010; Chen et al. 2011; Snijders et al. 2016) and increases in percentages of Treg following treatment (Grosse et al. 2016).
Based on the weight of existing evidence, induction of Treg has been proposed as one novel intervention for treatment of major depressive disorder, at least in the subset of patients with inflammatory MDD (Ellul et al. 2018).
5.3. Inflammation as a Risk Factor for Trauma and Stressor-Related Disorders
As mentioned above, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) recategorized PTSD into a new classification of “Trauma- and Stressor-Related Disorders.” In this revision of the DSM, the main diagnostic criterion of this class requires a previous exposure to a traumatic or stressful event (American Psychiatric Association; 2013). The DSM-5 describes clusters of specific behavioral symptoms that accompany PTSD including “re-experiencing,” “avoidance,” “negative alterations in cognitions and mood,” and “hypervigilance” (Reber et al. 2016; American Psychiatric Association 2013). PTSD affects 10–30% of individuals who have experienced a traumatic event (VanElzakker et al. 2014) with the greatest likelihood of PTSD occurring due to traumas involving interpersonal violence such as forms of assault, rape, or abuse (Yehuda and LeDoux 2007). According to the National Center for PTSD, about 6% of the population will experience PTSD at some point in their lives (National Center for PTSD 2022). About 8% of women develop PTSD sometime in their lives compared with about 4% of men (National Center for PTSD 2022). Approximately 60% of men and 50% of women experience at least one traumatic event throughout their lifetime (National Center for PTSD 2022). This suggests that there is an underlying vulnerability to developing PTSD that affects a percentage of the population.
Not everyone develops PTSD following a traumatic event; individual variability based on genetic, epigenetic, and environmental factors contributes to how susceptible an individual is to developing trauma- and stressor-related disorders (Reber et al. 2016; American Psychiatric Association 2013). While PTSD places a significant burden on the individual and society at large due to negative social and psychiatric consequences, it is also correlated with negative somatic consequences including autoimmune disorders, metabolic syndrome, pulmonary disease, and cardiovascular diseases that may be due to underlying mechanisms of low-grade systemic inflammation (Babson et al. 2015; Dennis et al. 2016; Lindqvist et al. 2014; Speer et al. 2018; Wolff et al. 2011). A transdiagnostic meta-analysis (i.e., a meta-analysis that quantitatively integrates the literature on the relationship of inflammatory biomarkers to trauma exposure and related symptomatology) found that trauma exposure was associated with increased circulating CRP and circulating proinflammatory cytokines, including IL-1β, IL-6, and TNF (Tursich et al. 2014), suggesting that trauma exposure may be causal for chronic low-grade inflammation. Conversely, studies indicate that increased biomarkers of inflammation, including increased circulating CRP concentrations, predict future risk of developing PTSD (Eraly et al. 2014; Schultebraucks et al. 2020). Finally, persons with a diagnosis of PTSD have a higher risk of developing an autoimmune disorder. Specifically, Veterans diagnosed with PTSD have a significantly higher risk for diagnosis with any of the autoimmune disorders alone or in combination (i.e., thyroiditis, inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, and lupus) considered individually compared with Veterans with no psychiatric disorders (O’Donovan et al. 2014). This is suggestive of a broad failure of immunoregulation and an inability to suppress inappropriate inflammation in Veterans with a diagnosis of PTSD. Consistent with these findings, those with a PTSD diagnosis have a decreased proportion of peripheral Treg cells (Sommershof et al. 2009), and Treg abundance can be increased following successful treatment using narrative exposure therapy (NET) (Morath et al. 2014).
Recent studies have demonstrated that persons with a diagnosis of PTSD, relative to healthy controls, have elevated circulating concentrations of lipopolysaccharide (LPS) and lipopolysaccharide-binding protein (LBP) (Voigt et al. 2022). LPS is a component of the outer membrane of gram-negative bacteria, while LBP is induced by prolonged elevation of LPS and is considered a biological marker of “leaky gut,” a condition where bacteria and other microorganisms within the lumen of the gut can translocate across the gut mucosa into the body and systemic circulation. Together, these data are consistent with the hypothesis that PTSD is associated with a dysregulated microbiome-gut-brain axis (Hemmings et al. 2017; Loupy and Lowry 2019; Malan-Muller et al. 2018, 2022) and that comprehensive therapy would benefit from stabilizing the dysregulated microbiome and gut mucosal barrier.
6. The Increasing Incidence and Prevalence of Inflammatory Disease in Modern Urban Societies
In 2002, Jean-François Bach published an article in the New England Journal of Medicine reporting an alarming increase in the incidence of immune disorders in the 50-year period from 1950–2000 (Bach 2002). Included were increases in autoimmune disorders, including Crohn’s disease (a form of inflammatory bowel disease (IBD), multiple sclerosis, and type 1 diabetes, as well as asthma, citing data from a number of contemporary original research articles (Pugliatti et al. 2001; Tuomilehto et al. 1999; Dubois et al. 1998; Farrokhyar et al. 2001). These historical trends are consistent with a gradient of the incidence of asthma and atopy based on rural vs. urban living, with, for example, the Amish (who maintain traditional farming practices, including use of large animals for farm work) having the lowest prevalence, Swiss farmers (who have adopted modern farming practices, including the use of tractors instead of animals for farm work) having intermediate levels of prevalence, and Swiss non-farmers having the highest prevalence (Holbreich et al. 2012). These differences have persisted in subsequent studies and mechanistic studies point toward an important role of innate immune signaling, leading the authors to conclude “These findings suggest that in the Amish, intense and presumably sustained exposure to microbes activates innate pathways that shape and calibrate downstream immune responses” (Stein et al. 2016).
7. Urban vs. Rural Upbringing and Mental Health
Although there are many potential confounds, meta-analysis suggests that individuals living in urban areas have an increased risk of developing any psychiatric disorder, and specifically an anxiety disorder or a mood disorder, relative to individuals living in rural areas (Peen et al. 2010; Stamper et al. 2016). This relationship led Böbel et al. (2018) to conduct a study to determine if differences were evident in inflammatory immune responses to a psychosocial stress exposure (the Trier Social Stress Test (TSST)) in healthy young persons that were either: (1) raised on a farm with farm animals for the first 15 years of life; or (2) raised in a city in the absence of daily contact with animals. The two groups did not differ in early life or perceived life stress. However, even though rural participants reported higher levels of anxiety before and after the TSST, urban participants responded with greater stress-induced increases in circulating PBMCs and prolonged increases in circulating IL-6, a proinflammatory cytokine. These data are consistent with the hypothesis that not only does the transition to an urban lifestyle involve increased risk of immune disorders, such as allergic asthma, but that it also involves increased risk of stress-related psychiatric disorders in which psychosocial stress and exaggerated inflammation are thought to be important risk factors (Rohleder 2014).
8. Hypothetical Frameworks Highlighting the Importance of Exposures to Diverse Microbial Environments to Mental Health
A number of hypotheses have been put forward to explain the increasing incidence and prevalence of inflammatory disease in modern urban societies. These include the hygiene hypothesis (Strachan 1989, 2000), the “Farm Effect” (von Mutius 2022), the biodiversity hypothesis (von Hertzen et al. 2011; Haahtela 2022), the disappearing microbiota hypothesis (Blaser and Falkow 2009; Blaser 2015), and the “Old Friends” hypothesis (Rook et al. 2004). What all of these hypotheses share, however, is that they propose, in one manner or another, that reduced exposures to diverse microbial ecosystems are responsible for increases in noncommunicable diseases in modern urban environments. Here we will briefly describe each of these hypotheses in turn, then focus on the “Old Friends” hypothesis, the context in which the most research has been done to evaluate the role of diverse microbial exposures in promotion of stress resilience and mental health.
8.1. The Hygiene Hypothesis
Initially, the term “hygiene hypothesis” was based on observations made by David Strachan, then at the London School of Hygiene and Tropical Medicine, in which a decrease in hay fever was observed in children with multiple older siblings in first world countries (Strachan 1989). This led Strachan to the conclusion that an excess of hygiene and decreases in childhood illness exposures were the causes of the increase in hay fever seen in first world countries (Strachan 1989; Rook and Lowry 2022; Rook et al. 2014a). Media and journalists latched onto this phrase as it was a simplistic concept that determined our excess cleanliness was the cause of our allergies and autoimmune disorders (Rook and Lowry 2022). While hay fever was not a new concept and was first described in the tenth century by Abu Bakr Al-Razi who called it “rose fever,” a connection between wealth, urbanization, and hay fever was observed in the nineteenth century by Dr. Charles Blackley (1873; Azizi 2010; Bungy et al. 1996). Blackley noticed that hay fever was more prevalent in wealthy and urbanized people compared to common people who did not live in the city (Blackley 1873; Rook and Lowry 2022). With this connection and Strachan’s suggestion that allergic diseases were prevented by infections in early childhood, the hygiene hypothesis was developed and initially focused on allergic disorders without taking into consideration humanity’s evolutionary history and dependence on microorganisms during the hunter-gatherer phase (Rook and Lowry 2022). These early childhood infections, which were largely not present during the hunter-gatherer phase of human evolution, are known as crowd infections, which became prevalent during urbanization. Crowd infections either elicit immunity or kill the host, which is why they cannot persist in hunter-gatherer groups but are common in urban environments. Crowd infections are not protective against chronic inflammatory disorders and instead have been shown to worsen them. Hygienic practices have been shown to decrease rates of crowd infections, as repeatedly emphasized by public health agencies during the COVID-19 pandemic, and thus a focus on negative consequences of hygiene, as proposed in the hygiene hypothesis, is somewhat misleading from the perspective of public health.
8.2. The “Farm Effect”
Subsequent studies demonstrated that rural upbringing confers protection against allergic asthma (von Mutius 2022). The protective effect of rural upbringing against allergic asthma is so highly replicated, it is referred to as simply the “Farm Effect” (von Mutius 2022; Genuneit 2012).
8.3. The Biodiversity Hypothesis
The biodiversity hypothesis states that current deficits in the exposure to natural environments and microbial diversity of individuals living in Westernized civilizations have unintended adverse health consequences (von Hertzen et al. 2011; Haahtela 2022; von Hertzen et al. 2015). Lack of exposure to microbial biodiversity especially during early development causes a deficiency in immunoregulatory circuits and leads to increased risk of developing allergic asthma later in life (von Hertzen et al. 2011, 2015; Haahtela 2022).
8.4. The Disappearing Microbiota Hypothesis
The disappearing microbiota hypothesis (Blaser and Falkow 2009; Blaser 2015) postulates that the important factor in increasing prevalence of modern allergic and metabolic diseases might not be decreased exposures to environmental microorganisms but instead could reflect the loss of ancestral microorganisms through vertical transfer, i.e., from one generation to the next, which in turn affects human physiology and disease risk. Notable losses include Helicobacter pylori, and losses of microbiota due to antibiotic use.
8.5. The “Old Friends” Hypothesis
The “Old Friends” hypothesis was formulated by Professor Graham Rook and colleagues in 2004 as a revision of the hygiene hypothesis (Rook et al. 2004). It provides a useful hypothetical framework for explaining the increases in inflammatory diseases in modern urban societies, in part because it highlights the importance of immunoregulation, indicated by a balanced expansion of regulatory T cells (Treg) and effector T cell populations, which are known to be driven by microbial signals. It is also useful in that it takes the focus away from hygiene (i.e., the idea that we are “too clean”), particularly at a time when personal hygiene is important to avoid transmission of communicable disease, including COVID-19, and instead emphasizes the importance of exposures to diverse microbial environments with microbial signals that can drive immunoregulation. Immunoregulation is driven mainly by organisms with which mammals co-evolved, including: (1) the commensal microbiota, which have been altered by the Western lifestyle, including a diet that is commonly low in microbiota-accessible carbohydrates (Sonnenburg and Sonnenburg 2014; von Hertzen et al. 2015); (2) pathogens associated with the “old infections” that were present throughout life in evolving human hunter-gatherer populations (Atherton and Blaser 2009); and (3) organisms from the natural environment with which humans were inevitably in daily contact with (and, consequently, had to be tolerated by the immune system) (Rook et al. 2014a). Immunoregulation is thought to be compromised in modern high-income settings due to reduced contact with these three categories of organisms (Rook et al. 2014a; Ohnmacht et al. 2015; Sefik et al. 2015). Failure of immunoregulation, attributable to reduced exposure to the microbial environment within which the mammalian immune system evolved, is thought to be one factor contributing to recent increases in stress-related and chronic inflammatory disorders in high-income countries (Sonnenburg and Sonnenburg 2014; Atherton and Blaser 2009; Rook et al. 2014a). Finally, and directly relevant to the thesis of this chapter, data from both preclinical and clinical studies are consistent with the hypothesis that inadequate immunoregulation also increases risk for development of stress-related psychiatric disorders (Raison et al. 2010; Rook et al. 2013, 2014a; Rook and Lowry 2008), an idea first put forward by Rook and Lowry in 2008 (Rook and Lowry 2008). Figure 2 illustrates the three categories of “Old Friends,” while Fig. 3 illustrates potential mechanisms underlying induction of immunoregulation by microbial signals.
Fig. 2.
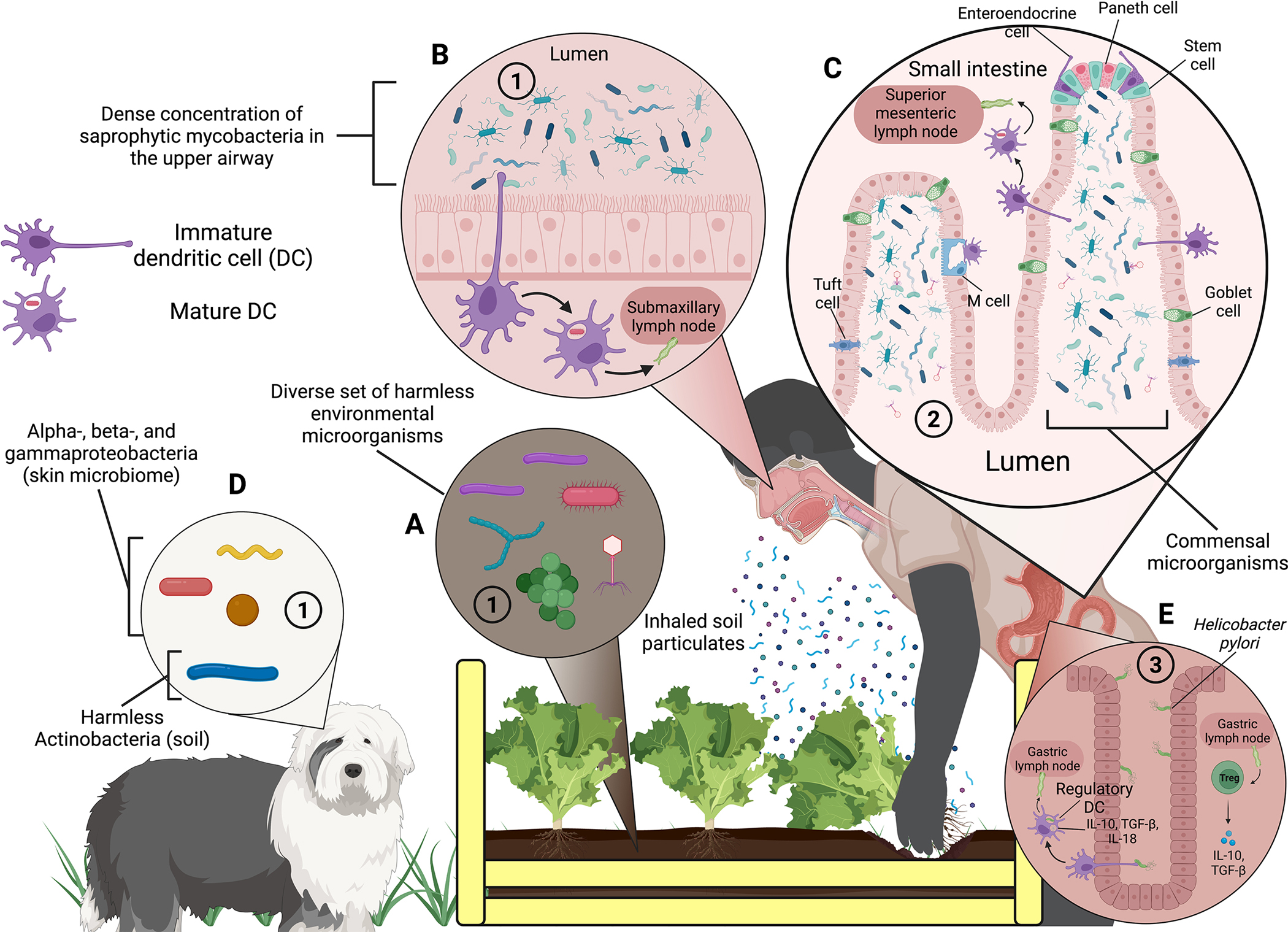
Potential sources of the three categories of “Old Friends” and how they interact with the immune system to induce anti-inflammatory and immunoregulatory effects. The three categories of “Old Friends” are: (1) harmless environmental microorganisms found in mud, untreated water, and fermenting vegetable material that have been depleted during the transition from a rural to an urban lifestyle; (2) organisms that form part of the co-evolved human microbiota (including commensal microorganisms); and (3) “Old Infections,” i.e., infections present in early man that usually do not sterilize or kill the host and that have also been depleted since urbanization. (A) The soil in this diagram depicts a diverse set of microorganisms that live within it, an environment that is rare to find in an urban environment compared to a rural one, thus depicting the first category of “Old Friends.” Here, a person harvesting lettuce they have grown is agitating the soil enough to form soil particulates that they eventually breathe in, exposing themselves to “Old Friends.” (B) One broad subset of soil microbes, Actinobacteria, are commonly found in the upper airway, depicted by mycobacteria from the genus Mycobacterium being inhaled into the nasal cavity (Macovei et al. 2015; Kim et al. 2022). Dendritic cells “sample” the contents of the nasal lumen by extending pseudopods that allow the cell to phagocytize a bacterium. The dendritic cell will then digest it and CD103+, CCR7+ dendritic cells migrate to a nearby lymph node via a lymphatic vessel to present processed antigens of the bacterium to lymphocytes. (C) Dendritic cell sampling is a common theme of the innate immune system – in the lumen of the small intestine, home to part of the gut microbiome, dendritic cells undergo a similar process of phagocytizing microorganisms in the lumen, digesting them, and ultimately presenting the processed antigens to lymphocytes. Unlike in the upper airway, dendritic cells in the small intestine mostly sample microorganisms that form part of the co-evolved human microbiota – the second category of “Old Friends.” The commensal microbes in the small intestine are especially influenced by whether a person was raised in an urban/rural environment as well as diet. (D) Animals that humans are in close contact with, such as dogs, can also influence the composition of the human microbiota. Commensal microbes from a dog’s skin microbiome can be transferred to a person’s skin, where they can colonize to form part of the person’s skin microbiome (Song et al. 2013); further, dogs can expose their owners to “Old Friends” by bringing microbes found in mud and untreated water into the house. (E) The last category of “Old Friends” is depicted by Helicobacter pylori infecting the epithelial cells of the stomach. Unlike a regular infection that produces a robust inflammatory response, if H. pylori is tolerated by the dendritic cells and lymphocytes of the immune system, then an anti-inflammatory and immunoregulatory response (i.e., characterized by a balanced expression of regulatory and effector T cells) arises instead (Arnold et al. 2012; Lundgren et al. 2005). Abbreviations: DC, dendritic cell; IL, interleukin; TGF-β, transforming growth factor beta; Treg, regulatory T cell. Not to scale. Figure created with biorender.com
Fig. 3.
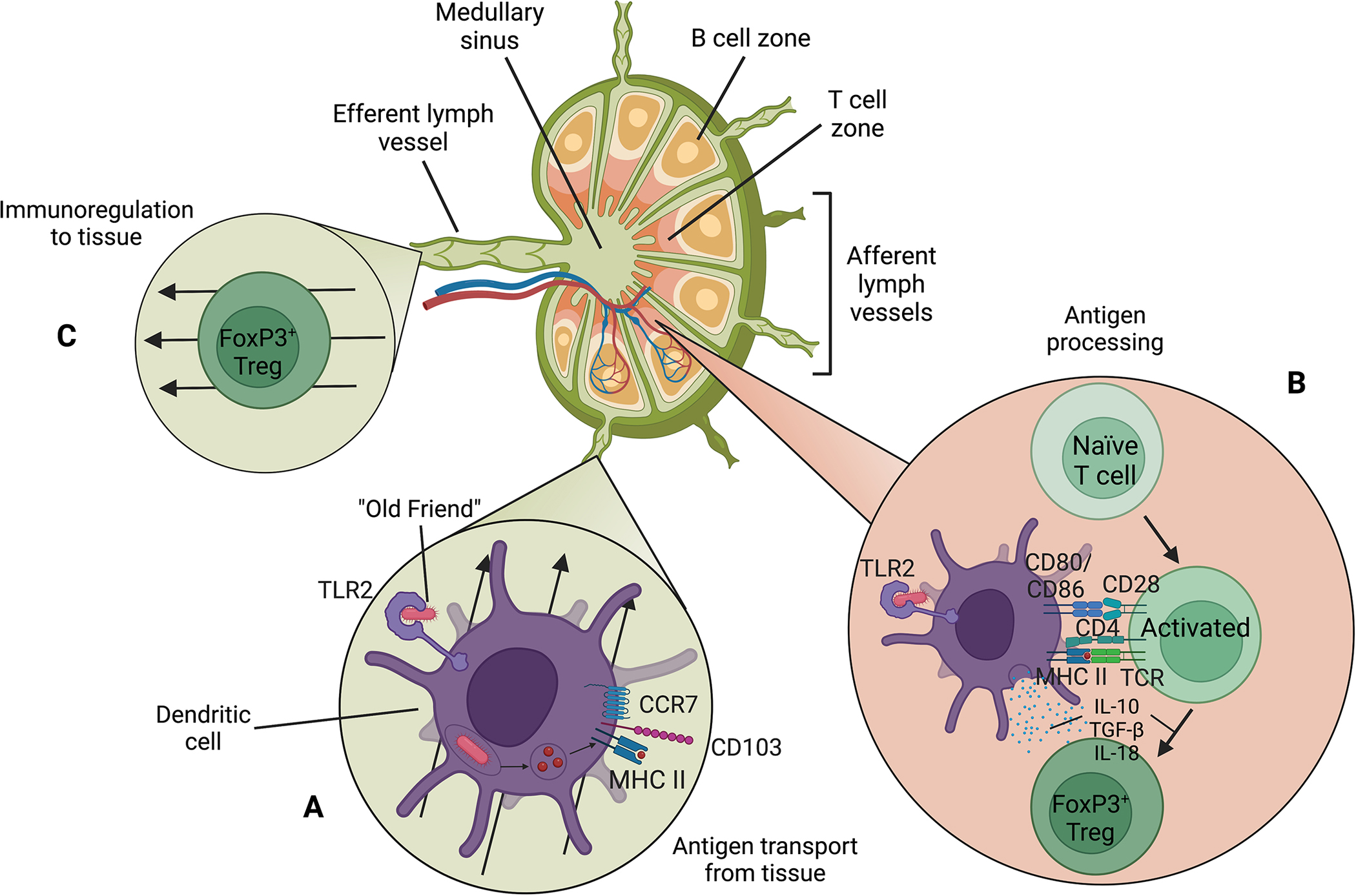
A dendritic cell transports a phagocytized “Old Friend” to a lymph node and presents it to naïve T cells, resulting in differentiation of naïve T cells into regulatory T cells. (A) A mature CD103+, CCR7+ dendritic cell migrates to a lymph node via an afferent lymph vessel. An “Old Friend” is bound to TLR2 and is ultimately phagocytized and bound to MHC II for presentation to a lymphocyte. (B) Activation of a naïve T cell in the presence of IL-10, TGF-β, and IL-18 secreted from a dendritic cell results in differentiation of the naïve T cell to a FoxP3+ regulatory T cell. (C) A FoxP3+ regulatory T cell migrates from the lymph node to tissue via an efferent lymph vessel. Abbreviations: CCR, C-C chemokine receptor; CD, cluster of differentiation; IL, interleukin; MHC, major histocompatibility complex; TCR, T cell receptor; TGF-β, transforming growth factor beta; TLR, toll-like receptor; Treg, regulatory T cell. Not to scale. Figure created with biorender.com
8.5.1. The Phylogenetically Broad But Strain-Specific Nature of Microorganisms That Induce Immunoregulation
Commensal microbes are initially transmitted by mothers and other family members and play an important role in the development of mammalian organ systems including the gut, immune system, and brain (Rook et al. 2014a, b). Germ-free mice have severely deficient numbers of regulatory T cells (Ohnmacht et al. 2015; Sefik et al. 2015). The immunoregulatory potential of single strains of bacteria is highlighted by the fact that inoculation of germ-free mice with single strains of bacteria is sufficient to restore percentages of Rorγ+ Helios− Treg (a distinct population of Tregs in the mouse colon that constrains inflammatory responses) to levels found in specified pathogen-free (SPF) mice. This induction mapped to a broad, but specific, array of individual bacterial species, meaning that bacteria from widely different phyla were capable of inducing these immunoregulatory effects, but that the effects were strain-specific. We have yet to understand the “code” that enables one bacterial strain to induce immunoregulatory responses, while other closely related strains cannot do so. This remains an important objective for future studies.
Given that we do not yet understand fully what enables specific strains of bacteria to drive immunoregulation, a reasonable strategy might be to promote high diversity of the gut microbiota, in hopes that one or more strains could provide the bacterial signals that drive immunoregulation. A number of physiological variables and lifestyle factors that are positively or negatively associated with diversity of the gut microbiota have recently been identified (Manor et al. 2020).
8.5.2. Immunoregulatory Strategies for Prevention of Stress-Related Psychiatric Disorders: Soil-Derived Mycobacterium vaccae NCTC 11659 as a Case Study
Ellul and colleagues recently proposed a path toward induction of Treg to promote psychoneuroimmune resilience with the intention of developing an immunotherapy approach to treatment of major depressive disorder. The strategy proposed involves use of low-dose interleukin 2 (IL-2), which induces Treg and inhibits inflammatory Th17 lymphocytes (Ellul et al. 2018). Another approach might be use of bacterial strains that have demonstrated anti-inflammatory and immunoregulatory effects (for recent review, see Sterrett et al. 2022; Flux and Lowry 2020, 2023; Loupy and Lowry 2019; Lowry et al. 2016).
We have been studying the potential of one such bacterial strain, M. vaccae NCTC 11659, in rodent models in order to test the hypothesis that this strain has potential as an intervention for prevention and treatment of stress-related psychiatric disorders. Although not yet tested in clinical trials for stress-related psychiatric disorders, it has been studied in numerous clinical trials for other conditions (for review, see Amoroso et al. 2021). M. vaccae NCTC 11659 induces regulatory T cells in mice and rats and has shown particular promise for promotion of stress resilience effects in a number of stress models, consistent with prevention of a PTSD-like syndrome (Table 1).
Table 1.
PTSD-relevant findings following immunization with Mycobacterium vaccae NCTC 11659
| PTSD symptom based on DSM-5 | Effects of M. vaccae NCTC 11659 in rodent models |
|---|---|
| Intrusions | N.A. |
| Avoidance (avoiding people, situations, circumstances resembling or associated with the event) | Decreased stress-induced anxiety-like defensive behavioral responses (avoidance) (Amoroso et al. 2019a, b; Frank et al. 2018; Reber et al. 2016; Loupy et al. 2021) Promotion of proactive behavioral responses to stress (Amoroso et al. 2019a, b; Frank et al. 2018; Reber et al. 2016; Loupy et al. 2021) |
| Negative alterations in mood and cognition | Antidepressant-like behavioral effects (Lowry et al. 2007; Siebler et al. 2018) Prevention of surgery-induced microglial priming (Fonken et al. 2018; Frank et al. 2018; Frank et al. 2019) and cognitive impairment (Fonken et al. 2018) |
| Alterations in arousal or reactivity (hypervigilance for threat, exaggerated startle response, irritability, difficulty concentrating, sleep problems) | Enhanced fear extinction in fear-potentiated startle (Fox et al. 2017; Hassell et al. 2019; Loupy et al. 2019) Enhanced fear extinction in models of stress-induced exaggeration of cued fear paradigms (Hassell 2019) Prevention of stress-induced cortical hyperarousal (Bowers et al. 2019) Prevention of stress-induced sleep and behavioral impairments (Bowers et al. 2021) |
9. Conclusions
Interventions that increase immunoregulation and attenuate chronic low-grade inflammation have potential for prevention and/or treatment of stress-related psychiatric disorders in which a failure of immunoregulation and the resulting chronic low-grade inflammation are recognized as risk factors. Increased exposure to immunoregulatory “Old Friends” may provide a novel and promising strategy to promote stress resilience for the purposes of prevention and treatment of stress-related psychiatric disorders, including anxiety disorders, mood disorders, and trauma- and stressor-related disorders, such as PTSD.
10. Future Directions
Although evidence strongly supports the hypothesis that exposures to “Old Friends” can promote stress resilience, the mechanisms involved are not completely understood, particularly in the context of the microbiome-gut-brain axis signaling mechanisms. Thus, future studies should identify mechanisms involved, which will facilitate development of novel interventions.
Acknowledgements
This work is supported by NIH T32 HL149646. Dr. Christopher A. Lowry is supported by the National Center for Complementary and Integrative Health (grant numbers R01AT010005 and R41AT011390), the Colorado Office of Economic Development and International Trade (OEDIT) Advanced Industries Accelerator Program (grant number CTGG1-2020-3064), the Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award (grant number N00014-15-1-2809), and an anonymous donor through Benefunder.
Footnotes
Conflict of Interest CAL serves on the Scientific Advisory Board of Immodulon Therapeutics, Ltd., is a cofounder and Chief Scientific Officer of Mycobacteria Therapeutics Corporation, and is a member of the faculty of the Integrative Psychiatry Institute. The remaining authors have no conflict of interests to report.
Contributor Information
Lamya’a M. Dawud, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA
Evan M. Holbrook, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA
Christopher A. Lowry, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO, USA; Rocky Mountain Mental Illness Research Education and Clinical Center (MIRECC), Rocky Mountain Regional VA Medical Center (RMRVAMC), Aurora, CO, USA; Department of Physical Medicine and Rehabilitation, University of Colorado Anschutz Medical Campus, Aurora, CO, USA; Military and Veteran Microbiome: Consortium for Research and Education (MVM-CoRE), Aurora, CO, USA; Center for Neuroscience, University of Colorado Boulder, Boulder, CO, USA; Center for Microbial Exploration, University of Colorado Boulder, Boulder, CO, USA; inVIVO Planetary Health, Worldwide Universities Network (WUN), West New York, NJ, USA.
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Arlington [Google Scholar]
- American Psychological Association (2017) Clinical practice guideline for the treatment of posttraumatic stress disorder. American Psychological Association [Google Scholar]
- Amoroso M, Bottcher A, Lowry CA, Langgartner D, Reber SO (2019a) Subcutaneous Mycobacterium vaccae promotes resilience in a mouse model of chronic psychosocial stress when administered prior to or during psychosocial stress. Brain Behav Immun 87:309–317 [DOI] [PubMed] [Google Scholar]
- Amoroso M, Kempter E, Eleslambouly T, Lowry CA, Langgartner D, Reber SO (2019b) Intranasal Mycobacterium vaccae administration prevents stress-induced aggravation of dextran sulfate sodium (DSS) colitis. Brain Behav Immun 80:595–604 [DOI] [PubMed] [Google Scholar]
- Amoroso M, Langgartner D, Lowry CA, Reber SO (2021) Rapidly growing Mycobacterium species: the long and winding road from tuberculosis vaccines to potent stress-resilience agents. Int J Mol Sci 22(23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Kornstein SG, Halberstadt LJ, Gardner CO, Neale MC (2011) Blue again: perturbational effects of antidepressants suggest monoaminergic homeostasis in major depression. Front Psychol 2:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anxiety & Depression Association of America (2015) Clinical practice review for GAD
- Anxiety & Depression Association of America (2020) Clinical practice review for major depressive disorder
- Arnold IC, Hitzler I, Muller A (2012) The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JC, Blaser MJ (2009) Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest 119:2475–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi MH (2010) Rhazes and the first clinically exact description of hay fever (seasonal allergic rhinitis). Iran J Med Sci 35:262–263 [Google Scholar]
- Babson KA, Heinz AJ, Ramirez G, Puckett M, Irons JG, Bonn-Miller MO, Woodward SH (2015) The interactive role of exercise and sleep on veteran recovery from symptoms of PTSD. Ment Health Phys Act 8:15–20 [Google Scholar]
- Bach JF (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347:911–920 [DOI] [PubMed] [Google Scholar]
- Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL (2008) Association between C-reactive protein and generalized anxiety disorder in stable coronary heart disease patients. Eur Heart J 29:2212–2217 [DOI] [PubMed] [Google Scholar]
- Bernardy NC, Friedman MJ (2015) Psychopharmacological strategies in the management of posttraumatic stress disorder (PTSD): what have we learned? Curr Psychiatry Rep 17:564. [DOI] [PubMed] [Google Scholar]
- Blackley CH (1873) Experimental researches on the causes and nature of Catarrhus Aestivus (hay-fever or hay-asthma). Baillière, Tindall & Cox, London [Google Scholar]
- Blaser MJ (2015) Missing microbes. How the overuse of antibiotics is fueling our modern plagues. Harper Collins Publishers, Toronto: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ, Falkow S (2009) What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 7:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böbel TS, Hackl SB, Langgartner D, Jarczok MN, Rohleder N, Rook GA, Lowry CA, Gundel H, Waller C, Reber SO (2018) Less immune activation following social stress in rural vs. urban participants raised with regular or no animal contact, respectively. Proc Natl Acad Sci U S A 115:5259–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers SJ, Lambert S, He S, Olker CJ, Song EJ, Wright KP, Fleshner M, Lowry CA, Turek FW, Vitaterna M (2019) Preimmunization with a non-pathogenic bacterium Mycobacterium vaccae NCTC 11659 prevents the development of cortical hyperarousal and PTSD-like sleep phenotype following sleep disruption plus acute stress in mice. Sleep 42:A94–A95 [Google Scholar]
- Bowers SJ, Lambert S, He S, Lowry CA, Fleshner M, Wright KP, Turek FW, Vitaterna MH (2021) Immunization with a heatkilled bacterium, Mycobacterium vaccae NCTC 11659, prevents the development of cortical hyperarousal and a PTSD-like sleep phenotype after sleep disruption and acute stress in mice. Sleep 44(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P (1994) Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry Res 54:135–142 [DOI] [PubMed] [Google Scholar]
- Bungy GA, Mossawi J, Nojoumi SA, Brostoff J (1996) Razi’s report about seasonal allergic rhinitis (hay fever) from the 10th century AD. Int Arch Allergy Immunol 110:219–224 [DOI] [PubMed] [Google Scholar]
- Capuron L, Dantzer R (2003) Cytokines and depression: the need for a new paradigm. Brain Behav Immun 17(Suppl 1):S119–S124 [DOI] [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, Sun Y (2011) Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Res 188:224–230 [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ (2012) Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med 42:2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, Kelley KW (1998) Molecular basis of sickness behavior. Ann N Y Acad Sci 856:132–138 [DOI] [PubMed] [Google Scholar]
- Dantzer R, Wollman EE, Vitkovic L, Yirmiya R (1999) Cytokines, stress, and depression. Conclusions and perspectives. Adv Exp Med Biol 461:317–329 [DOI] [PubMed] [Google Scholar]
- Dennis PA, Weinberg JB, Calhoun PS, Watkins LL, Sherwood A, Dennis MF, Beckham JC (2016) An investigation of vago-regulatory and health-behavior accounts for increased inflammation in posttraumatic stress disorder. J Psychosom Res 83:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Veterans Affairs DoD (2017) VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder
- Dubois P, Degrave E, Vandenplas O (1998) Asthma and airway hyperresponsiveness among Belgian conscripts, 1978–91. Thorax 53:101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul P, Mariotti-Ferrandiz E, Leboyer M, Klatzmann D (2018) Regulatory T cells as supporters of psychoimmune resilience: toward immunotherapy of major depressive disorder. Front Neurol 9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG (2014) Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiat 71:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhyar F, Swarbrick ET, Irvine EJ (2001) A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol 36:2–15 [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, Peerzade SN, Chen H, Lee KS, Scott MM, Beenhakker MP, Litvak V, Kipnis J (2016) Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535:425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flux MC, Lowry CA (2020) Finding intestinal fortitude: integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiol Dis 135:104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flux MC, Lowry CA (2023) Inflammation as a mediator of stress-related psychiatric disorders. In: Zigmond MJ, Wiley CA, Chesselet M-F(eds) Neurobiology of brain disorders: biological basis of neurological and psychiatric disorders. Academic Press, Elsevier, pp 885–911 [Google Scholar]
- Fonken LK, Frank MG, D’Angelo HM, Heinze JD, Watkins LR, Lowry CA, Maier SF (2018) Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction. Neurobiol Aging 71:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Hassell JE Jr, Siebler PH, Arnold MR, Lamb AK, Smith DG, Day HEW, Smith TM, Simmerman EM, Outzen AA, Holmes KS, Brazell CJ, Lowry CA (2017) Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav Immun 66:70–84 [DOI] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Schmidt D, Watkins LR, Maier SF, Lowry CA (2018) Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav Immun 73:352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Watkins LR, Maier SF, Lowry CA (2019) Could probiotics be used to mitigate neuroinflammation? ACS Chem Nerosci 10:13–15 [DOI] [PubMed] [Google Scholar]
- Freedman R (2010) Abrupt withdrawal of antidepressant treatment. Am J Psychiatry 167:886–888 [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM (2007) Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry 68:711–720 [DOI] [PubMed] [Google Scholar]
- Genuneit J (2012) Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with meta-analysis. Pediatr Allergy Immunol 23:509–518 [DOI] [PubMed] [Google Scholar]
- Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dube EM (2002) Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. J Clin Psychiatry 63:577–584 [DOI] [PubMed] [Google Scholar]
- Gong WP, Liang Y, Ling YB, Zhang JX, Yang YR, Wang L, Wang J, Shi YC, Wu XQ (2020) Effects of Mycobacterium vaccae vaccine in a mouse model of tuberculosis: protective action and differentially expressed genes. Mil Med Res 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, Bergink V (2016) Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology (Berl) 233:1679–1688 [DOI] [PubMed] [Google Scholar]
- Haahtela T (2022) Clinical application of the biodiversity hypothesis in the management of allergic disorders. In: Rook GAW, Lowry CA (eds) Evolution, biodiversity and a reassessment of the hygiene hypothesis. Springer, pp 393–414 [Google Scholar]
- Haroon E, Raison CL, Miller AH (2012) Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37:137–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JE Jr (2019) The effects of heat-killed soil-derived saprophytic bacterium Mycobacterium vaccae on stress-induced fear behavior and serotonergic systems. University of Colorado Boulder, pp 1–249 [Google Scholar]
- Hassell JE Jr, Fox JH, Arnold MR, Siebler PH, Lieb MW, Schmidt D, Spratt EJ, Smith TM, Nguyen KT, Gates CA, Holmes KS, Schnabel KS, Loupy KM, Erber M, Lowry CA (2019) Treatment with a heat-killed preparation of Mycobacterium vaccae after fear conditioning enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav Immun 81:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings SMJ, Malan-Müller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, Marotz CA, Siebler PH, Maarten B, Criekinge WV, Hoisington AJ, Brenner LA, Postolache TT, McQueen MB, Krauter KS, Knight R, Seedat S, Lowry CA (2017) The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 79:936–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM (2009) Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 26:447–455 [DOI] [PubMed] [Google Scholar]
- Holbreich M, Genuneit J, Weber J, Braun-Fahrlander C, Waser M, von ME (2012) Amish children living in northern Indiana have a very low prevalence of allergic sensitization. J Allergy Clin Immunol 129:1671–1673 [DOI] [PubMed] [Google Scholar]
- Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L, Baldwin DS (2017) Peripheral inflammatory cytokines and immune balance in generalised anxiety disorder: case-controlled study. Brain Behav Immun 62:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM (2006) Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry 11:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehle-Forbes SM, Meis LA, Spoont MR, Polusny MA (2016) Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma 8:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Gordon E, Rush AJ, Williams LM (2008) Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. CNS Spectr 13:1066–1086 [DOI] [PubMed] [Google Scholar]
- Kim PY, Thomas JL, Wilk JE, Castro CA, Hoge CW (2010) Stigma, barriers to care, and use of mental health services among active duty and National Guard soldiers after combat. Psychiatr Serv 61:582–588 [DOI] [PubMed] [Google Scholar]
- Kim S-O, Son SY, Kim MJ, Lee CH, Park SA (2022) Physiological responses of adults during soil-mixing activities based on the presence of soil microorganisms: a metabolomics approach. J Amer Soc Hort Sci 147:135–144 [Google Scholar]
- Lahey T, Laddy D, Hill K, Schaeffer J, Hogg A, Keeble J, Dagg B, Ho MM, Arbeit RD, von Reyn CF (2016) Immunogenicity and protective efficacy of the DAR-901 booster vaccine in a murine model of tuberculosis. PLoS One 11:e0168521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, Yang H (2010) Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord 124:68–75 [DOI] [PubMed] [Google Scholar]
- Li X, Frye MA, Shelton RC (2012) Review of pharmacological treatment in mood disorders and future directions for drug development. Neuropsychopharmacology 37:77–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EH, Von KM, Katon W, Bush T, Simon GE, Walker E, Robinson P (1995) The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care 33:67–74 [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, Makotkine I, Reus VI, Yan X, Taylor NM, Marmar CR, Dhabhar FS (2014) Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun 42:81–88 [DOI] [PubMed] [Google Scholar]
- Loupy KM, Lowry CA (2019) Posttraumatic stress disorder and the gut microbiome. In: Shepherd G, Byrne J, Chao M, Pfaff D, Kruger L, Kaczmarek L, Menini A (eds) The [Oxford] university handbook of the microbiome-gut-brain axis. Oxford University Press, Oxford [Google Scholar]
- Loupy KM, Arnold MR, Hassell JE Jr, Lieb MW, Milton LN, Cler KE, Fox JH, Siebler PH, Schmidt D, Noronha SISR, Day HEW, Lowry CA(2019) Evidence that preimmunization with a heat-killed preparation of Mycobacterium vaccae reduces corticotropin-releasing hormone mRNA expression in the extended amygdala in a fear-potentiated startle paradigm. Brain Behav Immun 77:127–140 [DOI] [PubMed] [Google Scholar]
- Loupy KM, Cler KE, Marquart BM, Yifru TW, D’Angelo HM, Arnold MR, Elsayed AI, Gebert MJ, Fierer N, Fonken LK, Frank MG, Zambrano CA, Maier SF, Lowry CA (2021) Comparing the effects of two different strains of mycobacteria, Mycobacterium vaccae NCTC 11659 and M. vaccae ATCC 15483, on stress-resilient behaviors and lipid-immune signaling in rats. Brain Behav Immun 91:212–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Hollis JH, de Vries A, Pan B, Brunet LR, Hunt JR, Paton JFR, Van Kampen E, Knight DM, Evans AK, Rook GAW, Lightman SL (2007) Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience 146:756–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA et al. (2016) The microbiota, immunoregulation, and mental health: implications for public health. Curr Environ Health Rep 3:270–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren A, Stromberg E, Sjoling A, Lindholm C, Enarsson K, Edebo A, Johnsson E, Suri-Payer E, Larsson P, Rudin A, Svennerholm AM, Lundin BS (2005) Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun 73:523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovei L, McCafferty J, Chen T, Teles F, Hasturk H, Paster BJ, Campos-Neto A (2015) The hidden ‘mycobacteriome’ of the human healthy oral cavity and upper respiratory tract. J Oral Microbiol 7:26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan-Muller S, Valles-Colomer M, Raes J, Lowry CA, Seedat S, Hemmings SMJ (2018) The gut microbiome and mental health: implications for anxiety- and trauma-related disorders. OMICS 22:90–107 [DOI] [PubMed] [Google Scholar]
- Malan-Muller S, Valles-Colomer M, Foxx CL, Vieira-Silva S, van den Heuvel LL, Raes J, Seedat S, Lowry CA, Hemmings SMJ (2022) Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur Neuropsychopharmacol 56:24–38 [DOI] [PubMed] [Google Scholar]
- Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, Gibbons SM, Magis AT (2020) Health and disease markers correlate wth gut microbiome composition across thousands of people. Nat Commun 11:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Naunton M, Kosari S, Peterson G, Thomas J, Christenson JK (2021) Treatment guidelines for PTSD: a systematic review. J Clin Med 10(18):4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW (2012) Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs 26:189–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017) Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42:254–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morath J, Gola H, Sommershof A, Hamuni G, Kolassa S, Catani C, Adenauer H, Ruf-Leuschner M, Schauer M, Elbert T, Groettrup M, Kolassa IT (2014) The effect of trauma-focused therapy on the altered T cell distribution in individuals with PTSD: evidence from a randomized controlled trial. J Psychiatr Res 54:1–10 [DOI] [PubMed] [Google Scholar]
- National Center for PTSD (2022) How common is PTSD in adults?
- National Collaborating Centre for Mental Health (UK) (2011) NICE Clinical guidelines, no. 123. Common mental health disorders: identification and pathways to care. British Psychological Society (UK) [PubMed] [Google Scholar]
- O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, Neylan TC (2014) Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry 77:365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, Busslinger M, Cerf-Bensussan N, Boneca IG, Voehringer D, Hase K, Honda K, Sakaguchi S, Eberl G (2015) MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349:989–993 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fava M (2009) Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 19:34–40 [DOI] [PubMed] [Google Scholar]
- Patel V, Kleinman A (2003) Poverty and common mental disorders in developing countries. Bull World Health Organ 81:609–615 [PMC free article] [PubMed] [Google Scholar]
- Peen J, Schoevers RA, Beekman AT, Dekker J (2010) The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand 121:84–93 [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Rosenheck RA (2001) Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 189:99–108 [DOI] [PubMed] [Google Scholar]
- Pugliatti M, Sotgiu S, Solinas G, Castiglia P, Pirastru MI, Murgia B, Mannu L, Sanna G, Rosati G (2001) Multiple sclerosis epidemiology in Sardinia: evidence for a true increasing risk. Acta Neurol Scand 103:20–26 [DOI] [PubMed] [Google Scholar]
- Raison CL, Lowry CA, Rook GA (2010) Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry 67:1211–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO et al. (2016) Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A 113: E3130–E3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resick PA, Monson CM, Chard KM (2017) Cognitive processing therapy for PTSD: a comprehensive manual. The Guilford Press, New York [Google Scholar]
- Rohleder N (2014) Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med 76:181–189 [DOI] [PubMed] [Google Scholar]
- Rook GA, Lowry CA (2008) The hygiene hypothesis and psychiatric disorders. Trends Immunol 29:150–158 [DOI] [PubMed] [Google Scholar]
- Rook GAW, Lowry CA (2022) Evolution, biodiversity and a reassessment of the hygiene hypothesis. Springer [Google Scholar]
- Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR (2004) Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol 25:237–255 [DOI] [PubMed] [Google Scholar]
- Rook GA, Lowry CA, Raison CL (2013) Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol Med Public Health 2013:46–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Raison CL, Lowry CA (2014a) Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol 177:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Raison CL, Lowry CA (2014b) Microbiota, immunoregulatory old friends and psychiatric disorders. Adv Exp Med Biol 817:319–356 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M (2006a) An evaluation of the quick inventory of depressive symptomatology and the Hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry 59:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006b) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917 [DOI] [PubMed] [Google Scholar]
- Santomauro DF, Mantilla Herrera AM, Shadid J, Zheng P, Ashbaugh C, Pigott DM, Abbafati C, Adolph C, Amlag JO, Aravkin AY, Bang-Jensen BL, Bertolacci GJ, Bloom SS, Castellano R, Castro E, Chakrabarti S, et al. (2021) Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398:1700–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, Bernardy N (2007) Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA 297:820–830 [DOI] [PubMed] [Google Scholar]
- Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH (2008) Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry 71:134–168 [DOI] [PubMed] [Google Scholar]
- Schultebraucks K, Qian M, Abu-Amara D, Dean K, Laska E, Siegel C, Gautam A, Guffanti G, Hammamieh R, Misganaw B, Mellon SH, Wolkowitz OM, Blessing EM, Etkin A, Ressler KJ, Doyle FJ III, Jett M, Marmar CR (2020) Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: a machine-learning approach for analyzing multivariate predictors. Mol Psychiatry 26(9):5011–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, Ghosh S, Earl A, Snapper SB, Jupp R, Kasper D, Mathis D, Benoist C (2015) MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349:993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebler PH, Heinze JD, Kienzle DM, Hale MW, Lukkes JL, Donner NC, Kopelman JM, Rodriguez OA, Lowry CA (2018) Acute administration of the nonpathogenic, saprophytic bacterium, Mycobacterium vaccae, induces activation of serotonergic neurons in the dorsal raphe nucleus and antidepressant-like behavior in association with mild hypothermia. Cell Mol Neurobiol 38: 289–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZZ, Kubiak RA, Arnold MR, Loupy KM, Taylor JA, Crist TG, Bernier AE, D’Angelo HM, Heinze JD, Lowry CA, Barth DS (2020) Effects of immunization with heat-killed Mycobacterium vaccae on autism spectrum disorder-like behavior and epileptogenesis in a rat model of comorbid autism and epilepsy. Brain Behav Immun 88:763–780 [DOI] [PubMed] [Google Scholar]
- Snijders G, Schiweck C, Mesman E, Grosse L, de WH, Nolen WA, Drexhage HA, Hillegers MHJ (2016) A dynamic course of T cell defects in individuals at risk for mood disorders. Brain Behav Immun 58:11–17 [DOI] [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT (2009) Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun 23:1117–1124 [DOI] [PubMed] [Google Scholar]
- Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, Gordon JI, Fierer N, Knight R (2013) Cohabiting family members share microbiota with one another and with their dogs. elife 2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Sonnenburg JL (2014) Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 20:779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer K, Upton D, Semple S, McKune A (2018) Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res 11:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper CE, Hoisington AJ, Gomez OM, Halweg-Edwards AL, Smith DG, Bates KL, Kinney KA, Postolache TT, Brenner LA, Rook GA, Lowry CA (2016) The microbiome of the built environment and human behavior: implications for emotional health and well-being in post-modern Western societies. Int Rev Neurobiol 131:289–323 [DOI] [PubMed] [Google Scholar]
- Stanhope J, Breed M, Weinstein P (2022) Biodiversity, microbiomes, and human health. In: Rook GAW, Lowry CA (eds) Evolution, biodiversity, and a reassessment of the hygiene hypothesis. Springer, pp 67–104 [Google Scholar]
- Steenkamp MM, Litz BT, Marmar CR (2020) First-line psychotherapies for military-related PTSD. JAMA 323:656–657 [DOI] [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Seedat S (2006) Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2006(1):CD002795. 10.1002/14651858.CD002795.pub2. Update in: Cochrane Database Syst Rev. 2022 Mar 2; 3:CD002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Ipser J, McAnda N (2009) Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines. CNS Spectr 14:25–31 [PubMed] [Google Scholar]
- Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, Ledford JG, Marques dos Santos M, Anderson RL, Metwali N, Neilson JW, Maier RM, Gilbert JA, Holbreich M, Thorne PS, Martinez FD, von Mutius E, Vercelli D, Ober C, Sperling AI (2016) Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med 375:411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrett JD, Andersen ND, Lowry CA (2022) The influence of the microbiota on brain structure and function: implications for stress-related neuropsychiatric disorders. In: Rook GAW, Lowry CA (eds) Evolution, biodiversity and a reassessment of the hygiene hypothesis. Springer, pp 267–345 [Google Scholar]
- Strachan DP (1989) Hay fever, hygiene, and household size. BMJ 299:1259–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP (2000) Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55(Suppl 1):S2–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian T, Jaycox LH, Schell TL, Marshall GN, Burnam MA, Eibner C, Karney BR, Meredith LS, Ringel JS, Vaiana ME, Invisible Wounds Study Team (2008) Invisible wounds of war: summary and recommendations for addressing psychological and cognitive injuries. RAND Corporation, Santa Monica. MG-720/1-CCF [Google Scholar]
- Tukel R, Arslan BA, Ertekin BA, Ertekin E, Oflaz S, Ergen A, Kuruca SE, Isbir T (2012) Decreased IFN-gamma and IL-12 levels in panic disorder. J Psychosom Res 73:63–67 [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Karvonen M, Pitkaniemi J, Virtala E, Kohtamaki K, Toivanen L, Tuomilehto-Wolf E (1999) Record-high incidence of type I (insulin-dependent) diabetes mellitus in Finnish children. The Finnish Childhood Type I Diabetes Registry Group. Diabetologia 42:655–660 [DOI] [PubMed] [Google Scholar]
- Tursich M, Neufeld RW, Frewen PA, Harricharan S, Kibler JL, Rhind SG, Lanius RA (2014) Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry 4:e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM (2014) From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 113:3–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araujo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Linhares UC, Andrade AF, Bento CA (2010) Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol 229:212–218 [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, de JP, Penninx BW (2013) Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry 3:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Zalta AK, Raeisi S, Zhang L, Brown JM, Forsyth CB, Boley RA, Held P, Pollack MH, Keshavarzian A (2022) Abnormal intestinal milieu in post-traumatic stress disorder is not impacted by treatment that improves symptoms. Am J Physiol Gastrointest Liver Physiol 323 (2):G61–G70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen L, Hanski I, Haahtela T (2011) Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep 12:1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen L et al. (2015) Helsinki alert of biodiversity and health. Ann Med 47:218–225 [DOI] [PubMed] [Google Scholar]
- von Mutius E (2022) From observing children in traditional upbringing to concepts of health. In: Rook GAW, Lowry CA (eds) Evolution, biodiversity and a reassessment of the hygiene hypothesis. Springer, pp 1–26 [Google Scholar]
- von Mutius E, Vercelli D (2010) Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 10:861–868 [DOI] [PubMed] [Google Scholar]
- von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann HH (1994) Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med 149:358–364 [DOI] [PubMed] [Google Scholar]
- von Reyn CF, Lahey T, Arbeit RD, Landry B, Kailani L, Adams LV, Haynes BC, Mackenzie T, Wieland-Alter W, Connor RI, Tvaroha S, Hokey DA, Ginsberg AM, Waddell R (2017) Safety and immunogenicity of an inactivated whole cell tuberculosis vaccine booster in adults primed with BCG: a randomized, controlled trial of DAR-901. PLoS One 12:e0175215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LE, Sprang KR, Rothbaum BO (2018) Treating PTSD: a review of evidence-based psychotherapy interventions. Front Behav Neurosci 12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger JW, O’Connell C, Cotrell L (2018) Examination of recent deployment experience across the services and components. RAND Arroyo Center, Santa Monica [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382:1575–1586 [DOI] [PubMed] [Google Scholar]
- Williams JWJ, Gierisch JM, McDuffie J, Strauss JL, Nagi A (2011) An overview of complementary and alternative medicine therapies for anxiety and depressive disorders: supplement to efficacy of complementary and alternative medicine therapies for posttraumatic stress disorder. VA-ESP Project #09–010 [PubMed] [Google Scholar]
- Wolff E, Gaudlitz K, von Lindenberger BL, Plag J, Heinz A, Strohle A (2011) Exercise and physical activity in mental disorders. Eur Arch Psychiatry Clin Neurosci 261(Suppl 2):S186–S191 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992) The ICD-10 classification of mental and behavioural disorders. Geneva [Google Scholar]
- World Health Organization (2017) Depression and other common mental disorders: global health estimates. World Health Organization, Geneva, pp 1–22. Licence: CC BY-NC-SA3.0 IGO [Google Scholar]
- Yehuda R, LeDoux J (2007) Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56:19–32 [DOI] [PubMed] [Google Scholar]
- Zhang L, Jiang Y, Cui Z, Yang W, Yue L, Ma Y, Shi S, Wang C, Wang C, Qian A (2016) Mycobacterium vaccae induces a strong Th1 response that subsequently declines in C57BL/6 mice. J Vet Sci 17:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Zhou B, Huang Y, Wang J, Min W, Li T (2020) Differences in cytokines between patients with generalised anxiety disorder and panic disorder. J Psychosom Res 133:109975. [DOI] [PubMed] [Google Scholar]


