Abstract
Background:
The MS community is highly interested in diet as a potential protective factor against disability, but empirical evidence remains limited.
Objective:
Evaluate associations between patient-reported Mediterranean diet alignment and objective disability in a real-world MS cohort.
Methods:
Data were analyzed from persons with MS, aged 18–65, who completed the Mediterranean Diet Adherence Screener (MEDAS), MS Functional Composite (MSFC; primary disability metric), and patient-reported outcomes (PROs; disability, gait disturbance, fatigue, anxiety, depression) as part of our Comprehensive Annual Assessment Program. Multiple regression predicted MSFC (and PROs) with MEDAS after adjusting for demographic (age, sex, race, ethnicity, socioeconomic status) and health-related (BMI, exercise, sleep disturbance, hypertension, diabetes, hyperlipidemia, smoking) covariates.
Results:
Higher MEDAS independently predicted better outcomes across MSFC (z-score, B=0.10 [95%CI: 0.06, 0.13], β=0.18, p<0.001), MSFC components, and PROs in 563 consecutive patients. Each MEDAS point was associated with 15.0% lower risk for MSFC impairment (≤5th percentile on ≥2 tasks; OR=0.850; 95%CI: 0.779, 0.928). Higher MEDAS attenuated effects of progressive disease and longer disease duration on disability.
Conclusion:
With robust control for potential confounds, higher Mediterranean diet alignment predicted lower objective and patient-reported disability. Findings lay the necessary groundwork for longitudinal and interventional studies to guide clinical recommendations in MS.
Keywords: multiple sclerosis, diet, Mediterranean, disability, depression
INTRODUCTION
The multiple sclerosis (MS) community is very interested in the potential for diet to modify disease and/or help manage symptoms.1 In addition to its effects on cardio-metabolic comorbidities already linked to MS outcomes, preclinical research suggests direct immunomodulatory and neuroprotective mechanisms of diet in MS.2 Metabolites derived from the diet and those produced by gut microbiota in response to dietary intake influence immune system patterning, diffuse into the peripheral circulation to activate numerous downstream pathways, and can even cross the blood-brain-barrier.2
Translating this research for clinical use is challenging, but must move forward to provide people living with MS evidence-based answers for their questions. Though experts tend to agree on general healthy eating principles (e.g. limiting processed foods),3 rigorous clinical research in MS is needed to 1) inform more targeted dietary recommendations, 2) motivate clinicians to incorporate discussions about diet into clinical practice, and 3) justify reimbursement of dietitian services for people living with MS. There are a number of epidemiological studies4 and small interventional studies2, 5 linking diet to patient-reported outcomes, but research including objectively-measured disability outcomes is quite limited. High quality observational studies investigating links to disability are necessary to justify and inform the design of larger interventional studies evaluating the potential of diet as an adjunct to traditional disease-modifying therapy in MS.
Interest in further investigating Mediterranean dietary patterns for MS stems from established benefits for general health6 and in cognitive aging, inclusion of foods suggested to be beneficial in preclinical and epidemiologic MS research,2, 4, 7, 8 and relative ease of implementation and adherence.9 Here we investigated whether Mediterranean diet score is associated with preserved objectively-measured cognitive and sensorimotor function assessed with the Multiple Sclerosis Functional Composite (MSFC) in a large real-world sample of people with MS. We also examined relationships with patient-reported outcomes.
METHODS
Sample.
A retrospective chart review captured data from patients with MS aged 18 to 65 years who underwent neurobehavioral monitoring between 2018 and 2021. Our center introduced neurobehavioral screenings into clinical practice in 2018 in the form of a Comprehensive Annual Assessment Program, to monitor functional status and modifiable risk and protective factors (including diet) in all patients regardless of reported functional status. Data were excluded from patients with another primary neurologic condition, serious psychiatric illness (e.g., schizophrenia), clinical relapse within six weeks, or missing data (e.g., diet, exercise, patient-reported outcomes, etc.). The Institutional Review Board at the Icahn School of Medicine approved the study and determined that written informed consent was not required for this retrospective review.
Diet Assessment.
The Mediterranean Diet Adherence Screener (MEDAS)10 is a well-established 14-item questionnaire assessing typical consumption of healthful foods (e.g., oil olive, vegetables) and avoidance of unhealthy foods (e.g., red meat, butter). Patients receive a score of 1 or 0 for each item based on validated rubrics (Table 1), which are summed to derive a MEDAS score. A higher score indicates that the individual’s dietary habits more closely align with a Mediterranean pattern as compared to someone with a lower score. After reviewing several dietary screeners, the American Heart Association recently selected the MEDAS as the optimal dietary screening tool for use in clinical practice, which they advise should become common practice in primary and specialty care settings.11 To be thorough we also we also conducted an IRB exempt anonymous survey via REDCap to characterize MEDAS scores among healthy persons demographically similar to our MS cohort (see MEDAS Scores in healthy controls, Supplemental Methods and Results).
Table 1.
Sample Characteristics
| Total | Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|---|
| n (%) | 563 (100) | 133 (23.6) | 167 (29.7) | 160 (28.4) | 103 (18.3) |
| Age, mean (SD), y | 44.2 (11.3) | 43.4 (11.0) | 42.2 (11.4) | 45.9 (11.6) | 45.9 (10.5) |
| Sex, n (%) | |||||
| Women | 398 (70.7) | 91 (68.4) | 107 (64.1) | 121 (75.6) | 79 (76.7) |
| Men | 165 (29.3) | 52 (31.6) | 60 (35.9) | 39 (24.4) | 24 (23.3) |
| Race and Ethnicity, n (%) | |||||
| Black (non-Hispanic) | 101 (17.9) | 46 (34.6) | 20 (12) | 26 (16.3) | 9 (8.7) |
| Hispanic | 90 (16.0) | 33 (24.8) | 34 (20.4) | 15 (9.4) | 8 (7.8) |
| White (non-Hispanic) | 357 (63.4) | 51 (38.3) | 108 (64.7) | 115 (71.9) | 83 (80.6) |
| Other / Unknown | 15 (2.7) | 3 (2.3) | 5 (3) | 4 (2.5) | 3 (2.9) |
| Socioeconomic Status Index, mean (SD) | 53.6 (18.6) | 43.6 (18.0) | 53.1 (19.2) | 58.4 (16.0) | 60.0 (16.9) |
| Educational Attainment, aggregate years | 15.1 (2.0) | 14.5 (2.0) | 15.0 (2.1) | 15.4 (1.8) | 15.5 (2.) |
| WTAR raw | 37.5 (8.6) | 33.2 (9.6) | 37.4 (7.9) | 39.5 (7.2) | 40.2 (8.0) |
| AHRQ Index | 56.1 (2.8) | 55.0 (2.5) | 56.0 (2.8) | 56.7 (2.7) | 56.8 (2.7) |
| Time since diagnosis, mean (SD), years | 8.89 (7.80) | 9.47 (7.89) | 8.93 (7.79) | 8.23 (7.85) | 9.09 (7.66) |
| Disease Course, n (%) | |||||
| Relapsing Remitting | 441 (78.3) | 94 (70.7) | 137 (82) | 130 (81.3) | 80 (77.7) |
| Primary Progressive | 35 (6.2) | 10 (7.5) | 6 (3.6) | 11 (6.9) | 8 (7.8) |
| Secondary Progressive | 87 (15.5) | 29 (21.8) | 24 (14.4) | 19 (11.9) | 15 (14.6) |
| Disease Modifying Therapy type, n (%) | |||||
| None | 95 (16.9) | 23 (17.3) | 23 (13.8) | 32 (20) | 17 (16.5) |
| Injection | 75 (13.3) | 14 (10.5) | 24 (14.4) | 15 (9.4) | 22 (21.4) |
| Oral | 172 (30.6) | 38 (28.6) | 49 (29.3) | 57 (35.6) | 28 (27.2) |
| Infusion | 221 (39.3) | 58 (43.6) | 71 (42.5) | 56 (35) | 36 (35) |
| Health-Related Covariates, n (%) | |||||
| BMI, mean (SD) | 27.1 (5.9) | 28.3 (6.0) | 27.0 (5.8) | 26.9 (6.1) | 25.8 (5.3) |
| MVPA, mean (SD) | 21.7 (22.2) | 12.1 (19.0) | 21.1 (22.4) | 24.7 (20.5) | 30.2 (23.8) |
| Sleep Disturbance | 251 (44.6) | 67 (50.4) | 75 (44.9) | 66 (41.3) | 43 (41.7) |
| Hypertension | 98 (17.4) | 25 (18.8) | 31 (18.6) | 29 (18.1) | 13 (12.6) |
| Diabetes | 23 (4.1) | 7 (5.3) | 11 (6.6) | 4 (2.5) | 1 (1) |
| Hyperlipidemia | 102 (18.1) | 23 (17.3) | 37 (22.2) | 25 (15.6) | 17 (16.5) |
| Smoking | 179 (31.8) | 48 (36.1) | 44 (26.3) | 49 (30.6) | 38 (36.9) |
| Unadjusted Outcomes, mean (SD) | |||||
| MSFC z-score | −1.12 (1.22) | −1.75 (1.35) | −1.07 (1.14) | −0.90 (1.14) | −0.72 (0.97) |
| SDMT raw | 51.1 (12.0) | 45.9 (13.2) | 51.5 (11.5) | 53.2 (11.3) | 53.7 (10.2) |
| NHPT raw | 23.4 (5.5) | 25.8 (6.1) | 23.6 (5.2) | 22.5 (5.3) | 21.7 (4.6) |
| T25FW raw | 4.99 (1.34) | 5.64 (1.44) | 4.85 (1.33) | 4.83 (1.16) | 4.61 (1.20) |
| MSISphys raw | 39.71 (18.2) | 46.2 (19.6) | 39.2 (19.0) | 38.2 (16.7) | 34.6 (14.7) |
| FSS raw | 3.67 (1.92) | 4.15 (1.86) | 3.79 (2.00) | 3.53 (1.91) | 3.09 (1.72) |
| MSWS raw | 40.5 (23.9) | 49.6 (24.9) | 39.0 (23.9) | 39.0 (22.9) | 33.7 (21.1) |
| PDQ raw | 1.43 (0.99) | 1.67 (1.05) | 1.49 (0.99) | 1.34 (0.98) | 1.20 (0.88) |
| HADS-D raw | 4.44 (3.64) | 5.76 (3.74) | 4.36 (3.83) | 4.28 (3.39) | 3.15 (3.05) |
| HADS-A raw | 7.16 (4.13) | 7.80 (4.51) | 7.31 (4.29) | 7.02 (3.83) | 6.30 (3.66) |
| Mediterranean Diet Adherence | |||||
| MEDAS, mean (SD) | 6.25 (2.35) | 3.08 (1.01) | 5.54 (0.50) | 7.46 (0.50) | 9.61 (0.81) |
| MEDAS, minimum, maximum | 1, 12 | 1, 4 | 5, 6 | 7, 8 | 9, 12 |
| Component criteria met, n (%) | |||||
| Olive oil is primary cooking fat | 373 (66.3) | 44 (33.1) | 107 (64.1) | 131 (81.9) | 91 (88.3) |
| Olive oil consumption ≥4 tbsp. / day | 36 (6.4) | 2 (1.5) | 9 (5.4) | 10 (6.3) | 15 (14.6) |
| Vegetable serving (200g/d) ≥2 / day | 279 (49.6) | 18 (13.5) | 59 (35.3) | 103 (64.4) | 99 (96.1) |
| Fruit serving (80g) ≥3 / day | 91 (16.2) | 4 (3) | 12 (7.2) | 34 (21.3) | 41 (39.8) |
| Red meat, sausage (100–150g) <1 / day | 345 (61.3) | 36 (27.1) | 104 (62.3) | 118 (73.8) | 87 (84.5) |
| Butter (12g) <1 / day | 384 (68.2) | 48 (36.1) | 11 (65.9) | 133 (83.1) | 93 (90.3) |
| Sugary drinks (12oz) <1 / day | 430 (76.4) | 64 (48.1) | 119 (71.3) | 144 (90) | 103 (100) |
| Wine (125ml) ≥7 / week | 100 (17.8) | 4 (3) | 19 (11.4) | 31 (19.4) | 46 (44.7) |
| Legumes (150g) ≥3 / week | 89 (15.8) | 2 (1.5) | 11 (6.6) | 27 (16.9) | 49 (47.6) |
| Fish (100–150g) ≥3 / week | 91 (16.2) | 2 (1.5) | 22 (13.2) | 29 (18.1) | 38 (36.9) |
| Commercial pastries <1 / week | 272 (48.3) | 30 (22.6) | 65 (38.9) | 97 (60.6) | 80 (77.7) |
| Nuts (30g) ≥2 / week | 236 (41.9) | 18 (13.5) | 55 (32.9) | 91 (56.9) | 72 (69.9) |
| Eat chicken or turkey more than pork, hamburger, or sausage | 456 (81.0) | 81 (60.9) | 140 (83.8) | 143 (89.4) | 92 (89.3) |
| Vegetables, pasta, or rice with garlic, tomato, or onion ≥2 / week | 334 (59.3) | 55 (41.4) | 93 (55.7) | 102 (63.8) | 84 (81.6) |
Neurobehavioral Evaluation.
The MS Functional Composite (MSFC)12, 13 is a validated quantitative disability outcome in MS clinical practice and clinical trials that includes sensitive screeners of cognition (Symbol Digit Modalities Test, SDMT), upper extremity coordination (Nine Hole Peg Test, NHPT), and gait speed (Timed 25-Foot Walk, T25FW). The MSFC was our primary functional outcome. Raw scores for SDMT, NHPT, and T25FW were converted to norm-referenced z-scores13 and averaged into an MSFC composite. Persons who could not complete a task (e.g., T25FW due to severe gait disability) were assigned a score matching the lowest performer. Patient Reported Outcomes (PROs): PROs assessed physical disability (MS Impact Scale, physical component, MSISphys, fatigue (Fatigue Severity Scale, FSS), gait disturbance (MS Walking Scale, MSWS), cognitive dysfunction (Perceived Deficits Questionnaire, PDQ), depression (Hospital Anxiety and Depression Scale, HADS-D), and anxiety (HADS-A). Patient-reported outcomes and other questionnaires (e.g., MEDAS, exercise, etc.) were completed in a single electronic capture survey completed within one week of objective assessments, with very rare exceptions still completed within one month.
Demographic Covariates.
Demographic covariates included: AGE (continuous), biological SEX (female, male), RACE (White, non-White), ETHNICITY (Hispanic, non-Hispanic), and socioeconomic status (SES). SES is an important covariate when investigating modifiable lifestyle factors such as diet. Our index of SES consisted of patient and parental educational attainment, literacy as a proxy of educational quality, and neighborhood deprivation (See supplemental methods).
Health-Related Covariates.
Body mass index (BMI, continuous) was assessed as a marker of adiposity and overall health and wellness. Patient-reported frequency of moderate-to-vigorous physical activity (MVPA) was assessed with the health contribution score (HCS) from the Godin Leisure Time Exercise Questionnaire14 (GLTEQ, continuous), which has convergent validity with accelerometer-measured MVPA in persons with MS.15 Sleep disturbance is common in MS, and there is some evidence linking sleep to dietary patterns in other populations. Sleep disturbance was assessed with the Insomnia Severity Index16 (SLEEP, dichotomous) using the validated cutoff (raw ≥10), which we have previously used in MS.16, 17 Medical history forms completed by patients documented dichotomous histories (ever vs never) of hypertension (HTN), diabetes (DM), hyperlipidemia (HLD), and smoking (SMOKE).
Statistical Analysis.
All variables with skewedness ≥1.0 were log-transformed and converted back to original units. Extreme values were winsorized (<Q1−1.5*IQR or >Q3+1.5*IQR) before calculating the MSFC composite to avoid undue influence of outliers. Preliminary analyses characterized the sample and correlations among predictors. The primary analysis used multiple regression to assess the association between MSFC and MEDAS accounting for covariates including AGE, SEX, RACE (White, non-White), ETHNICITY, (Hispanic/Latino, non-Hispanic/Latino), SES, BMI, MVPA, ISI, HTN, DM, HLD, and SMOKE. The same model predicted individual MSFC components (SDMT, NHPT, T25FW) and PROs (MSISphys, FSS, MSWS-12, PDQ, HADS-D, HADS-A). Secondary analyses characterized differences across quartiles of MEDAS scores, adjusting for the same covariates. Sensitivity analyses examined whether relationships between MEDAS and outcomes were moderated by demographic and disease variables (relapsing vs progressive course, time since diagnosis, disease-modifying therapy [DMT]). Finally, we explored relationships among the 14 individual MEDAS components and outcomes related to the total MEDAS score.
Data Availability:
Anonymized data not published within this article will be made available by request from any qualified investigator, as permitted according to institutional regulations.
RESULTS
Preliminary Analyses.
Chart review identified 608 patients with MS; data from 31 patients were excluded due to another neurologic condition (n=24), serious psychiatric illness (n=5), or recent relapse (n=2). Of those remaining, 13 patients (2.3%) were excluded for missing data (i.e., electronic capture survey containing all questionnaires), yielding a final sample of 563 patients with complete neurobehavioral data (Table 1). This final sample of 563 that completed neurobehavioral assessments did not differ demographically or clinically from typical patients at our MS Center (supplemental results). MEDAS scores (mean ± SD: 6.25 ± 2.35) ranged from 1 to 12 with normal skewness (−0.095) and kurtosis (−0.494), and matched that of (a) healthy European adults (n=402, 6.22 ± 2.03)18 and local healthy adults stratified to demographically match our MS cohort (n=140, 6.29 ± 2.01; supplementary results). MEDAS was not related to time since diagnosis (r= −0.02 [95%CI: −0.11, 0.07]), disease course (relapsing: 6.31 [6.10, 6.52]); progressive: 6.02 [5.55, 6.50]), or type of DMT (none, injection, oral, infusion; p=0.299, ηp2=.007). As expected, MEDAS was related to demographic and health-related covariates (Table 2), but relationships were not large enough to raise concern for multicollinearity.
TABLE 2.
Unadjusted correlations among MEDAS and covariates.
| AGE | SEX | RACE | ETHN | SES | BMI | MVPA | SLEEP | HTN | DM | HLD | SMOKE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEX | .05 [−.03, .13] |
|||||||||||
| RACE | .17*** [.09, .25] |
−.06 [−.13, .02] |
||||||||||
| ETHN | −.08 [−.16, .00] |
.03 [−.05, .10] |
−.35*** [−.43, −.27 |
|||||||||
| SES | .08 [.00, .16] |
.00 [−.08, .08] |
.39*** [.31, .47] |
−.25*** [−.33, −.17] |
||||||||
| BMI | .09* [.00, .18] |
−.09* [−.17, −.02] |
−23*** [−.31, −.14] |
.08 [−01, .16] |
−22*** [−29, −.14] |
|||||||
| MVPA | −.10* [−.19, −.01] |
−11* [−.20, −.02] |
.08 [−.01, .15] |
−.02 [−.10, .07] |
.17*** [.09, .25] |
−.09* [−.17, −.01] |
||||||
| SLEEP | .04 [−.05, .12] |
.08* [.00, .16] |
−.13** [−.21, −.05] |
.06 [−.03, .14] |
−.06 [−.14, .02] |
.11* [.03, .19] |
−.10* [−.18, −.01] |
|||||
| HTN | .26*** [.18, .34] |
−.08 [−.17, .01] |
−.11** [−20, −.02] |
.03 [−.06,.12] |
−12** [−.21, −.04] |
.31*** [.23, .39] |
−.05 [−.13, .03] |
.19* [.01, .18] |
||||
| DM | .12** [.05, .19] |
−.10* [−.20, −.01] |
−.03 [−.12, .05] |
.13** [.02, .25] |
−.04 [−.11, 02] |
.17*** [.09, .26] |
−.10* [−.15, −.03] |
−.01 [−.09, .09] |
.21*** [.10, .32] |
|||
| HLD | 29*** [.21, .36] |
−.04 [−.13, .05] |
.04 [−.04, .13] |
−.03 [−.11, .05] |
01 [−.07, .09] |
.15*** [.06, .23] |
−.02 [−.10, .06] |
.09* [.01, .18] |
.30*** [.19, .40] |
.16*** [.05, .27] |
||
| SMOKE | .18*** [.11, .26] |
−.021 [−.10, .06] |
.10* [.03, .18] |
.00 [−.07, .08] |
−.09* [−.17, .00] |
−.01 [−.09, .07] |
−.06 [−.14, .03] |
.09* [.01, .17] |
.01 [−.08, .09] |
−.05 [−.11, .03] |
.12** [.03, .20] |
|
| MEDAS | .09* [.02, .17] |
.08 [−.01, .16] |
.30*** [.22, .38] |
−.17*** [−.25, −.10] |
.33*** [.25, .41] |
−.16*** [−.24, −.08] |
.27*** [.19, .35] |
−.08 [−.16, .00] |
−.08 [−.16, .00] |
−.08* [−.15, −.02] |
−.04 [−.13, .05] |
.01 [−.08, .10] |
Pearson correlations [95% CI] among MEDAS and all covariates are presented. Asterisks represent p-values:
p<0.05,
p<0.01,
p<0.001
Primary Analyses.
MSFC was one standard deviation (SD) below normative expectations (mean z ± SD: −1.12 ± 1.22). Higher MEDAS independently predicted better MSFC performance across models adjusting for (a) age and sex, (b) all demographic covariates, and (c) all demographic and health-related covariates (Table 3, Figure 1). Each MEDAS point increase corresponded to a +0.10 SD increase in MSFC performance (95%CI: 0.06, 0.13). MEDAS was by far the best health-related predictor of MSFC. Higher MEDAS predicted better performance on each MSFC component (SDMT, NHPT, T25FW) and less disability (better function) across all PROs independently of demographic and health-related covariates (Table 3). Among PROs, depression (HADS-D) had the strongest relationship to MEDAS (Figure 1). Relationships between MEDAS and all functional outcomes except anxiety withstood multiple comparison corrections (Bonferroni-corrected p= 0.05/10 = 0.005).
TABLE 3.
Relationship between Mediterranean Diet Adherence (MEDAS) and Outcomes
| A. Hierarchical Regression Predicting MSFC with MEDAS and Covariates | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Results are presented across models predicting MSFC with MEDAS adjusting for age and sex alone (F[3, 559]=57.08, p<0.001, R2=0.234), all demographic covariates (F[6, 556]=46.24, p<0.001, R2=0.333), and all demographic and health-related covariates (F[13, 549]=22.80, p<0.001, R2=0.351). | |||||||||||||||
| Predictor | B | 95% CI | β | p | B | 95% CI | β | p | B | 95% CI | β | p | |||
| Constant | −0.797 | −1.308 | −0.286 | 0.002 | −1.552 | −2.074 | −1.030 | <0.001 | −1.655 | −2.368 | −0.941 | <0.001 | |||
| MEDAS | 0.164 | 0.126 | 0.202 | 0.32 | <0.001 | 0.100 | 0.062 | 0.138 | 0.19 | <0.001 | 0.095 | 0.055 | 0.134 | 0.18 | <0.001 |
| AGE | −0.041 | −0.049 | −0.033 | −0.38 | 0.001 | −0.045 | −0.052 | −0.037 | −0.41 | <0.001 | −0.046 | −0.054 | −0.038 | −0.43 | <0.001 |
| SEX | 0.279 | 0.083 | 0.474 | 0.10 | 0.005 | 0.330 | 0.146 | 0.514 | 0.12 | <0.001 | 0.381 | 0.194 | 0.569 | 0.14 | <0.001 |
| RACE | 0.390 | 0.182 | 0.598 | 0.15 | <0.001 | 0.422 | 0.209 | 0.634 | 0.16 | <0.001 | |||||
| ETHN | 0.135 | −0.109 | 0.379 | 0.04 | 0.278 | 0.151 | −0.095 | 0.396 | 0.05 | 0.228 | |||||
| SES | 0.017 | 0.012 | 0.022 | 0.27 | <0.001 | 0.017 | 0.012 | 0.022 | 0.26 | <0.001 | |||||
| BMI | 0.004 | −0.011 | 0.020 | 0.02 | 0.570 | ||||||||||
| MVPA | 0.002 | −0.002 | 0.006 | 0.04 | 0.235 | ||||||||||
| SLEEP | −0.187 | −0.358 | −0.017 | −0.08 | 0.032 | ||||||||||
| HTN | 0.212 | −0.034 | 0.458 | 0.07 | 0.091 | ||||||||||
| DM | −0.090 | −0.531 | 0.350 | −0.02 | 0.687 | ||||||||||
| HLD | 0.135 | −0.099 | 0.368 | 0.04 | 0.258 | ||||||||||
| SMOKE | −0.142 | −0.327 | 0.043 | −0.05 | 0.131 | ||||||||||
| B. Fully-Adjusted Outcomes across (a) MEDAS scores and (b) MEDAS quartiles | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multiple regression results (a) report relationships between MEDAS scores and outcomes adjusting for all demographic and health-related covariates. Analysis of covariance results (b) report differences in outcomes across MEDAS quartiles adjusting for all demographic and health-related covariates. | ||||||||||||
| (a) Multiple Regression Results | (b) Analysis of Covariance Results | |||||||||||
| Outcome | B | 95% CI | β | p | F3,547 | ηp2 | p | Q1: M [95% CI] | Q2: M [95% CI] | Q3: M [95% CI] | Q4: M [95% CI] | |
| MSFC | 0.095 | 0.055 | 0.134 | 0.18 | <0.001 | 7.86 | 0.041 | <0.001 | −1.48 [−1.67, −1.30] | −1.15 [−1.30, −1.00] | −0.96 [−1.12, −0.80] | −0.84 [−1.04, −0.64] |
| SDMT | 0.869 | 0.458 | 1.279 | 0.17 | <0.001 | 5.44 | 0.029 | 0.001 | 47.9 [46.0, 49.8] | 50.8 [49.2, 52.4] | 52.8 [51.1, 54.4] | 53.1 [51.0, 55.2] |
| NHPT | −0.392 | −0.584 | −0.199 | −0.17 | <0.001 | 5.78 | 0.031 | <0.001 | 24.9 [24.0, 25.8] | 23.7 [23.0, 24.5] | 22.8 [22.0, 23.6] | 22.2 [21.2, 23.1] |
| T25FW | −0.077 | −0.121 | −0.033 | −0.14 | 0.001 | 5.28 | 0.028 | 0.001 | 5.34 [5.14, 5.55] | 4.95 [4.77, 5.12] | 4.89 [4.71, 5.06] | 4.75 [4.53, 4.97] |
| MSISphys | −0.993 | −1.609 | −0.377 | −0.13 | 0.002 | 2.71 | 0.015 | 0.044 | 42.8 [40.0, 45.7] | 39.7 [37.3, 42.1] | 39.3 [36.8, 41.8] | 36.4 [33.3, 39.5] |
| FSS | −0.102 | −0.170 | −0.034 | −0.13 | 0.003 | 2.70 | 0.015 | 0.045 | 3.91 [3.60, 4.22] | 3.80 [3.53, 4.06] | 3.61 [3.34, 3.88] | 3.25 [2.91, 3.60] |
| MSWS | −1.312 | −2.108 | −0.516 | −0.13 | 0.001 | 3.70 | 0.020 | 0.012 | 45.4 [41.7, 49.1] | 40.0 [36.9, 43.1] | 40.1 [36.9, 43.3] | 35.8 [31.8, 39.9] |
| PDQ | −0.056 | −0.091 | −0.022 | −0.13 | 0.001 | 2.27 | 0.012 | 0.079 | 1.55 [1.39, 1.71] | 1.51 [1.38, 1.65] | 1.37 [1.23, 1.51] | 1.26 [1.09, 1.44] |
| HADS-D | −0.234 | −0.362 | −0.105 | −0.15 | <0.001 | 4.53 | 0.024 | 0.004 | 5.23 [4.63, 5.82] | 4.39 [3.89, 4.89] | 4.45 [3.94, 4.97] | 3.51 [2.86, 4.16] |
| HADS-A | −0.190 | −0.338 | −0.042 | −0.11 | 0.012 | 2.43 | 0.013 | 0.064 | 7.64 [6.95, 8.32] | 7.38 [6.80, 7.95] | 7.11 [6.52, 7.70] | 6.26 [5.51, 7.01] |
Figure 1: MEDAS and MS Metrics.
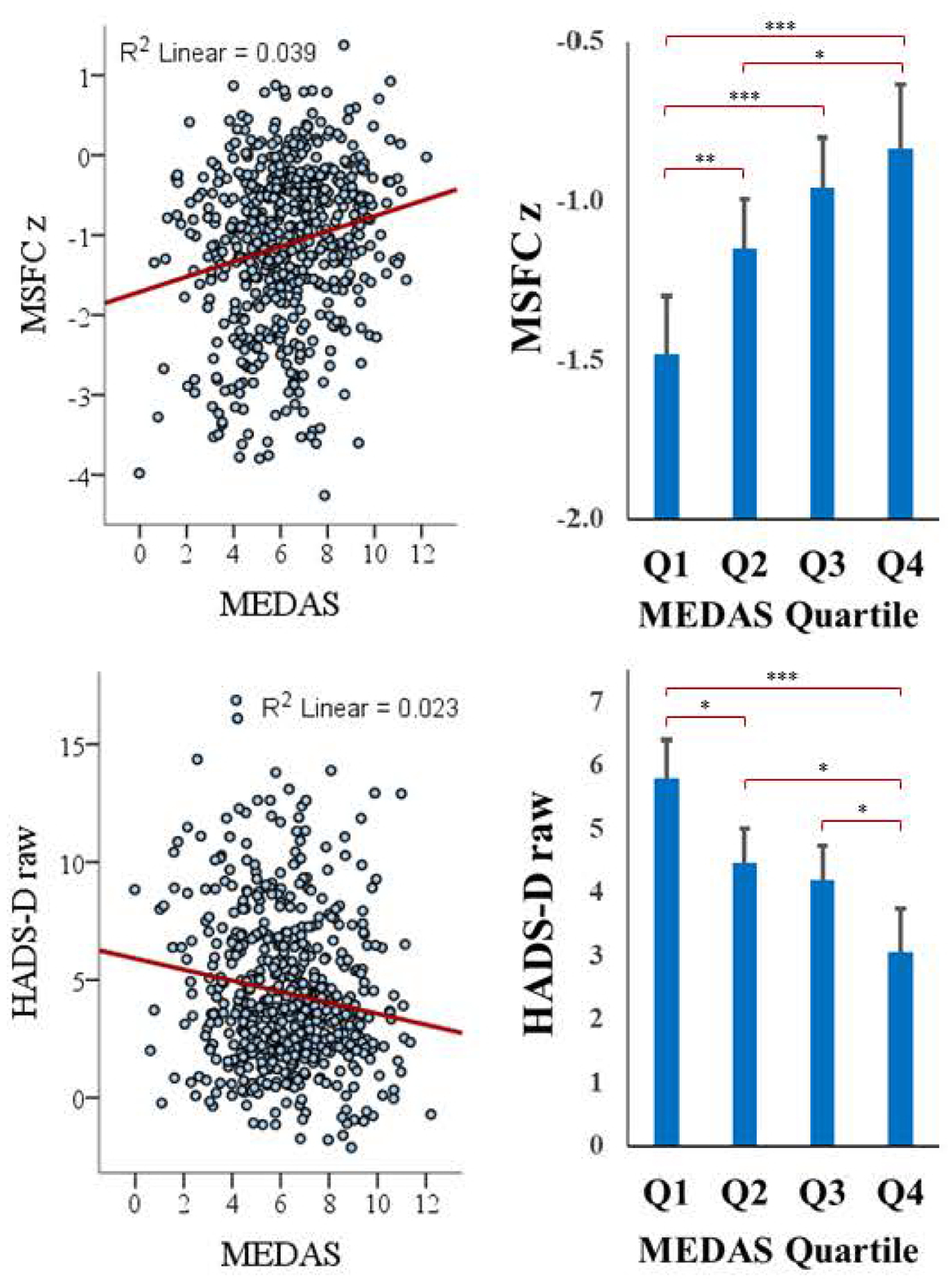
Relationships between MEDAS and 1) MSFC, 2) HADS-D. Panels on the left illustrate regression and on the right by MEDAS quartile. Higher MSFC indicates better performance. Levels of statistical significance between MEDAS quartiles are indicated as: *p<0.05, **p<0.01, ***p<0.001.
MEDAS Quartiles.
To provide an additional perspective, patients were divided into MEDAS quartiles (Table 1) and we assessed differences in outcomes across MEDAS quartiles adjusting for the same covariates. As shown (Table 3, Figure 1), MSFC and most other outcomes differed across MEDAS quartiles. When controlling for multiple comparisons (i.e., Bonferroni-corrected p <0.005), higher MEDAS quartiles were associated with better MSFC, SDMT, NHPT, and T25FW performances, and lower depression.
MSFC Impairment.
Primary analyses treated MSFC as a continuous outcome, but we also examined risk for MSFC impairment (≤5th percentile on ≥2 of 3 demographically-adjusted MSFC components: SDMT, NHPT, and T25FW raw scores were adjusted for demographic covariates using GLM and converted to norm-referenced z-scores).13 Adjusting for health-related covariates, logistic regression predicting impairment (0=no, 1=yes [n=149/563, 26.5%]) showed a protective effect of higher MEDAS (Wald[1]=13.32, p<0.001), with each MEDAS point associated with 15.0% lower risk for impairment (OR=0.850; 95%CI: 0.779, 0.928).
We also considered risk for clinically-meaningful disability with two benchmarks of T25FW walking time: (a) ≥6.0 seconds has been linked to occupational disability, cane use, and needing some help with instrumental activities of daily living [IADLs]), (b) ≥8.0 seconds has been linked to government assistance, wheelchair use, and inability to perform IADLs).19 Adjusting for all covariates, logistic regression associated higher MEDAS with lower risk for T25FW ≥6.0 seconds (n=111/563, 19.7%; Wald[1]=6.66, p=0.010; OR=0.859; 95%CI: 0.765, 0.964) and T25FW ≥8.0 seconds (n=48/563, 8.5%; Wald[1]=8.26, p=0.004; OR=0.798; 95%CI: 0.684, 0.931). We found similar results for validated cutoff scores for patient-reported gait disturbance (MSWS);20 controlling for all covariates, higher MEDAS was associated with lower risk of at least moderate (raw ≥50; n=163; Wald[1]=3.69, p=0.020; OR=0.889; 95%CI: 0.805, 0.982) and severe (raw ≥75; n=79; Wald[1]=5.53, p=0.019; OR=0.860; 95%CI: 0.758, 0.975) gait disturbance. (There are no validated cross-sectional cutoff scores for SDMT or NHPT.)
Effect Modification.
The association between MEDAS and MSFC (adjusted for demographic and health-related covariates) was not reliably modified by age, sex, race, ethnicity, SES, or DMT (Ps > 0.10). There were, however, interactions with disease course (relapsing vs progressive (β= 0.27, p=0.02) and time since diagnosis (β= 0.24, p=0.03) whereby higher MEDAS scores attenuated the negative association of progressive course and longer time since diagnosis on MSFC (Figure 2). Most notably, for those in the 3rd and 4th MEDAS quartiles MSFC scores were quite similar between the longer and shorter disease duration groups.
Figure 2: MEDAS and MSFC: Disease Course and Duration.
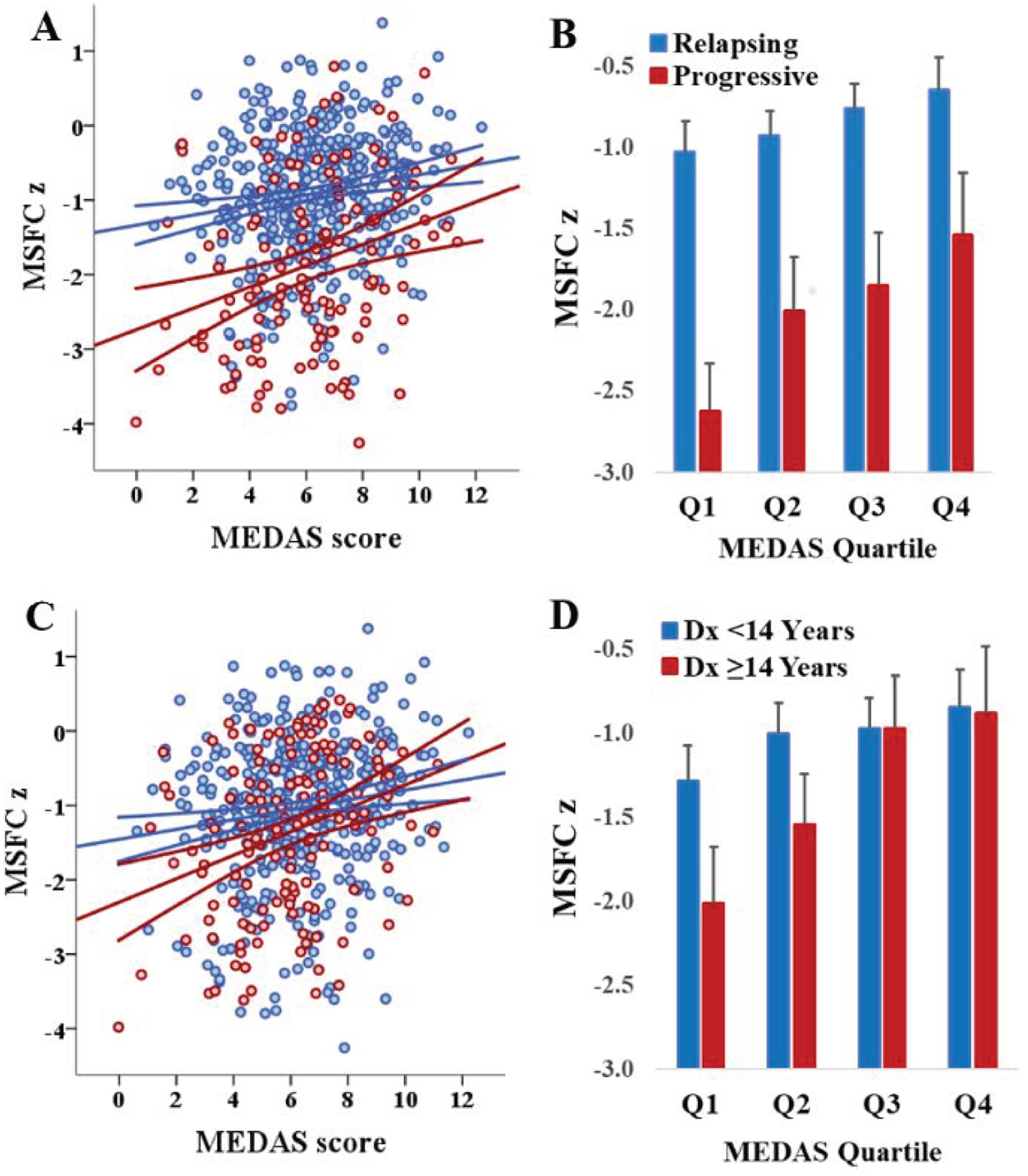
Panels A and B illustrate the interaction between disease course and the MEDAS-MSFC relationship. Higher MEDAS score attenuates the negative impact of progressive disease course on MSFC. Panels C and D illustrate the relationship between MEDAS score, MSFC, and disease duration (≥14 years represents the top quartile of time since diagnosis). High MEDAS score attenuates the negative impact of disease duration on MSFC.
MEDAS Components.
Higher MEDAS scores were robustly related better MSFC and lower depression (HADS-D) across analytic approaches. To explore associations with individual MEDAS components (e.g., vegetables, fish), Welch tests assessed differences in MSFC and HADS-D (adjusted for all covariates with GLM) between patients who did and did not meet consumption criteria for each MEDAS item (Table 4). Better MSFC was most associated with higher olive oil consumption, but also to greater consumption of nuts and wine, and lower consumption of pastries and sugary beverages. Depression was worse among patients who use butter, drink sugary beverages, and consume less fish.
TABLE 4.
Outcomes in Patients Adherent versus Non-Adherent to Individual MEDAS Items
| MSFC | HADS-D | |||||
|---|---|---|---|---|---|---|
| MEDAS Item | ||||||
| Olive Oil, Cooking |
−1.01
[−1.11, −0.91] |
−1.33
[−1.48, −1.17] |
<0.001 | 4.28 [3.96, 4.6] |
4.77 [4.29, 5.26] |
0.094 |
| Olive Oil, Consume |
−0.78
[−1.02, −0.53] |
−1.14
[−1.23, −1.05] |
0.006 | 4.00 [2.86, 5.14] |
4.47 [4.2, 4.75] |
0.421 |
| Vegetables | −1.05 [−1.16, −0.94] |
−1.19 [−1.31, −1.06] |
0.107 | 4.20 [3.85, 4.55] |
4.69 [4.28, 5.09] |
0.071 |
| Fruit | −1.02 [−1.22, −0.83] |
−1.14 [−1.23, −1.04] |
0.288 | 4.20 [3.58, 4.81] |
4.49 [4.2, 4.79] |
0.394 |
| Red Meat | −1.10 [−1.20, −1.00] |
−1.15 [−1.3, −1.01] |
0.535 | 4.40 [4.05, 4.74] |
4.52 [4.09, 4.95] |
0.660 |
| Butter | −1.09 [−1.19, −1.00] |
−1.17 [−1.33, −1.01] |
0.421 |
4.24
[3.93, 4.56] |
4.87
[4.38, 5.37] |
0.036 |
| Sugary Drinks |
−1.07
[−1.16, −0.98] |
−1.27
[−1.46, −1.09] |
0.048 |
4.25
[3.95, 4.55] |
5.06
[4.48, 5.65] |
0.016 |
| Wine |
−0.93
[−1.12, −0.75] |
−1.16
[−1.25, −1.07] |
0.032 | 4.00 [3.39, 4.6] |
4.54 [4.24, 4.84] |
0.113 |
| Beans | −1.08 [−1.27, −0.88] |
−1.13 [−1.22, −1.03] |
0.662 | 3.99 [3.37, 4.6] |
4.53 [4.23, 4.83] |
0.119 |
| Fish | −0.99 [−1.19, −0.79] |
−1.14 [−1.23, −1.05] |
0.178 |
3.85
[3.3, 4.41] |
4.56
[4.26, 4.86] |
0.028 |
| Pastry |
−1.02
[−1.13, −0.90] |
−1.21
[−1.33, −1.09] |
0.020 | 4.32 [3.93, 4.7] |
4.56 [4.19, 4.94] |
0.374 |
| Nuts |
−0.99
[−1.11, −0.88] |
−1.21
[−1.32, −1.09] |
0.011 | 4.27 [3.86, 4.68] |
4.57 [4.21, 4.92] |
0.280 |
| Chicken vs Beef | −1.08 [−1.18, −0.99] |
−1.26 [−1.46, −1.07] |
0.106 | 4.44 [4.13, 4.74] |
4.48 [3.92, 5.05] |
0.883 |
| Garlic, leeks, etc. | −1.09 [−1.20, −0.98] |
−1.16 [−1.28, −1.03] |
0.438 | 4.43 [4.09, 4.77] |
4.46 [4.03, 4.9] |
0.905 |
Differences in mean [95% CI] MSFC and HADS-D are reported for patients who met the consumption criterion for individual MEDAS items (Adherent) versus patients who did not (Non-Adherent). Specific criteria for each item are presented in Table 1. Significant differences are in bold font.
Additional Sensitivity Analyses.
Given that fatigue, depression, and anxiety (a) were lower at higher MEDAS scores, and (b) may influence MSFC performance, we re-examined the link between MSFC and MEDAS controlling for all covariates as well as FSS, HADS-D, and HADS-A. MSFC remained better among patients with higher continuous MEDAS scores (B=0.08 [95%CI: 0.04, 0.18], β=0.15, p<0.001) and within higher MEDAS quartiles (F[3, 544]=5.96, p<0.001, ηp2=.032; Q1 mean [95%CI]: −1.43 [−1.61, −1.25]); Q4: −0.90 [−1.10, −0.71]) even after adjusting for these additional potential covariates.
DISCUSSION
Summary and context of results.
In our real-world sample of people with MS, higher degree of dietary alignment with Mediterranean-style pattern was associated with lower risk for objective disability on the gold-standard MSFC disability outcome as well as patient-reported symptomatology. These relationships remained after rigorously controlling for demographic and health-related covariates and adjusting for multiple comparisons. Primary analyses used multiple regression with continuous MEDAS and outcome variables, and secondary analyses added perspective by demonstrating (a) differences in outcomes across MEDAS quartiles, which may help inform cutoff scores for entry into diet-based clinical trials, and (b) differential risk for MSFC impairment across MEDAS scores, which provides clinical context. For instance, each MEDAS point is associated with 15% lower risk for impairment, e.g., person with MEDAS of 9 (75th percentile) had a 75% lower risk for impairment versus person with MEDAS score of 4 (25th percentile).
Observed effect modifications suggest that the link between higher Mediterranean diet adherence and better MSFC was strongest for persons with progressive disease and/or longer disease duration. This is consistent with our hypothesis that better diet supports brain maintenance,21 which should be strongest among patients at greatest risk for neurodegeneration (i.e., greater disease-related change to moderate). Findings are not explained by aging, as the effect of diet on MSFC was not reliably modified by age, and our age cutoff of 65 avoided life stages when dietary modification of age-related changes are most prominent (i.e., 70’s and 80’s22–24). Nor are findings explained by MS disability leading to poorer diet, as MEDAS scores were extremely well-matched with published data in healthy European adults18 and an independent demographically-matched local sample of healthy adults (supplementary results). If MS disability led to poorer diet, MEDAS would have been lower in our MS cohort performing >1 SD below healthy normative MSFC data.
Further favoring a disease-related rather than age-related explanation for these findings, Mediterranean diet adherence in healthy older adults is associated with larger medial temporal volumes (areas vulnerable to age-related change) but not thalamic volume.25 This is in contrast to the observed association between higher Mediterranean diet score and larger thalamic volume in a cohort of young people with early MS,26 important because thalamic atrophy is among the earliest detectable changes on MRI and correlates with future more widespread brain atrophy and clinical disability in MS.27, 28
Mediterranean diet and depression.
Among PROs, the strongest relationship with MEDAS was depression. Multiple studies have evaluated the relationship between diet and depression in non-MS populations; a recent meta-analysis concluded the highest level of evidence was for Mediterranean diet score.29 Proposed involved pathways include significant overlap with pathways of importance in MS including inflammation, oxidative stress, and neuroplasticity.30 This aligns with inflammatory models of depression associated with lower reward responsiveness via downregulation of dopamine in the ventral striatum,31 which may have important implications for initiation and drive. Future work is needed to understand biological mediators of relationships among diet, depression, and functional outcomes in the context of MS pathophysiology.
MEDAS component drivers of observed effects.
Analysis of individual MEDAS responses yielded information on the most significant drivers of observed effects, the largest of which was olive oil. Both the use of olive oil in cooking and the consumption of olive oil were significantly related to both MSFC. The precise composition of olive oil differs based on factors related to harvesting and extraction, however, it is primarily composed of the monounsaturated fat oleic acid along with much smaller amounts of polyunsaturated (linoleic acid) and saturated (palmitic acid) fats. It also contains a number of phenolic compounds, including flavonoids. Olive oil is postulated to be an important contributor toward the associations between Mediterranean diet and cognition in older adults; olive oil consumption has been linked with verbal memory32 and additional extra virgin olive oil improved cognitive outcomes in a sub-study of PREDIMED-NAVARRA.33 Preclinical and epidemiologic work in MS has focused more on polyunsaturated fatty acids, including several studies suggesting a beneficial effect of fish intake on incident MS.2 Here, we note an association between the fish intake item and HADS-D, but not MSFC. We are not able to drill down to the nutrient level to look specifically at fatty acids in this study. However, our currently enrolling longitudinal study includes a more extensive dietary inventory that will allow for more detailed analyses of different patterns, foods, and nutrients.
Rationale for evaluating Mediterranean diet.
Among the multiple dietary patterns currently being evaluated for MS, Mediterranean-style diets are appealing to study for several reasons. First, Mediterranean-style diets are associated with improved general health outcomes,6 including the prevention of cardio-metabolic conditions that are already adversely linked with prognosis in MS.34 General health considerations are important as we aim to treat people holistically rather than attempting to treat a disease process in isolation. Next, preclinical studies suggest key components of Mediterranean-style diets such as marine-derived omega-3-fatty acids and antioxidant-containing foods have potential neuroprotective benefits in MS.7, 8 Telomere length, which is an indicator of biological aging and interestingly associated with adherence to Mediterranean dietary pattern,35 is also associated with disability independent of chronological age in MS36 and may contribute mechanistically. Finally, from a clinical standpoint, a pilot randomized controlled trial of a modified Mediterranean dietary program in MS showed feasibility of implementation as well as improvements in fatigue and impact of MS symptoms on daily life, and stabilization of disability in those receiving the dietary intervention compared to controls.9 All of these points encouraged us to focus on this pattern, readily measured in a clinical program with the use of a short well-validated questionnaire. The current study provides firm justification for moving forward with longitudinal and interventional studies focused on Mediterranean-style patterns.
Limitations and strengths.
Our study makes important contributions to the literature on diet in MS, including the large real-world sample of MS patients, use of the gold-standard MSFC objective disability outcome, and rigorous statistical control for potential confounds (e.g., SES, BMI, exercise, etc.). Demographically, our sample represents the racial and ethnical diversity of our urban landscape and the MS population,37 which increases generalizability of our results. The biggest limitation of the study is its cross-sectional and observational design which prohibit causal statements. However, the current work is essential for informing longitudinal and intervention studies to determine whether changing one’s diet will mitigate MS disease worsening or treat symptoms. Use of the well-validated MEDAS to assess Mediterranean diet alignment is both a limitation and a strength. MEDAS provides fewer nutritional details than full food frequency questionnaires (FFQ) or repeated 24-hour dietary recalls; however, FFQs and 24-hour recalls require more time and effort by patients, which would introduce selection bias into the data. In contrast, use of the 14-item MEDAS avoided selection bias (97.7% response rate). Also, the MEDAS is a well-established instrument with clear scoring rubrics, which provides methodological transparency and supports replication (versus FFQ scoring rubrics which often vary). Finally, the MEDAS has a limited range of possible scores and was only measured once in this cross-sectional study. This may have limited statistical power; however, our findings were robust enough to withstand this limitation.
Conclusions.
Formal guidance regarding lifestyle modifications with prognostic implications for people living with MS should be based on interventional clinical trials, the design of which must be informed by observational data that establish links between diet and MS outcomes. Prior research on diet in MS has been preclinical, epidemiologic, survey-based, or has utilized very limited clinical tools. Here we demonstrate a robust association between objective, clinically-captured, MS-related disability and diet in a relatively large real-world cohort of people living with MS. We note a strong link between Mediterranean diet score and MSFC, its individual components, and multiple patient-reported instruments, nearly all of which withstood rigorous control for demographic and health-related covariates as well as correction for multiple comparisons in analyses. We anticipate these results will inform the design of longitudinal studies and interventional clinical trials on this crucially important topic going forward.
Supplementary Material
Acknowledgements:
Funding Statement:
This study was funded in part by an Irma T. Hirschl/Monique Weill-Caulier Research Award to Dr. Katz Sand. Time spent by Dr. Katz Sand and Dr. Sumowski on this topic was also funded by National Multiple Sclerosis Society RG-190133374 to Dr. Katz Sand and NIH R01 HD082176 to Dr. Sumowski. Time spent by Dr. Fitzgerald was funded by the National Multiple Sclerosis Society TA-1805-31136 and NIH K01-MH121582.
The authors would like to thank all of the attending neurologists, neuropsychology fellows, MS fellows, nurse practitioners, and administrative staff at the CGD MS Center at Mount Sinai for their roles in data collection for this study. Most importantly, the authors would like to thank the people living with MS who have participated and made this work possible.
Footnotes
Declaration of Conflicting Interests:
The authors have no potential conflicts of interest relevant to this manuscript.
References
- 1.Brenton JN. A study of dietary modification: Perceptions and attitudes of patients with multiple sclerosis. Multiple sclerosis and related disorders. 2016. 8:54–57. doi: 10.1016/j.msard.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 2.Katz Sand I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Current nutrition reports. 2018;7(3):150–160. doi: 10.1007/s13668-018-0236-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. https://www.nationalmssociety.org/Living-Well-With-MS/Diet-Exercise-Healthy-Behaviors/Diet-Nutrition.
- 4.Fitzgerald KC. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology. 2018. 90(1):e1–e11. doi: 10.1212/WNL.0000000000004768 [DOI] [PubMed] [Google Scholar]
- 5.Evans E, Levasseur V, Cross AH, Piccio L. An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult Scler Relat Disord. Nov 2019;36:101393. doi: 10.1016/j.msard.2019.101393 [DOI] [PubMed] [Google Scholar]
- 6.Martínez-González MA. Benefits of the Mediterranean diet beyond the Mediterranean Sea and beyond food patterns. BMC Med. Oct 14 2016;14(1):157. doi: 10.1186/s12916-016-0714-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torkildsen O, Brunborg LA, Thorsen F, et al. Effects of dietary intervention on MRI activity, de and remyelination in the cuprizone model for demyelination. Experimental neurology. 2009;215(1):160–166. doi: 10.1016/j.expneurol.2008.09.026 [doi] [DOI] [PubMed] [Google Scholar]
- 8.Fonseca-Kelly Z, Nassrallah M, Uribe J, et al. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Frontiers in neurology. 2012;3:84. doi: 10.3389/fneur.2012.00084 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz Sand I Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: A pilot study. Multiple sclerosis and related disorders. 2019. 36doi: 10.1016/j.msard.2019.101403 [DOI] [PubMed] [Google Scholar]
- 10.Schröder H, Fitó M, Estruch R, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. Jun 2011;141(6):1140–5. doi: 10.3945/jn.110.135566 [DOI] [PubMed] [Google Scholar]
- 11.Vadiveloo M, Lichtenstein AH, Anderson C, et al. Rapid Diet Assessment Screening Tools for Cardiovascular Disease Risk Reduction Across Healthcare Settings: A Scientific Statement From the American Heart Association. Circ Cardiovasc Qual Outcomes. Sep 2020;13(9):e000094. doi: 10.1161/hcq.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 12.Goldman MD, LaRocca NG, Rudick RA, et al. Evaluation of multiple sclerosis disability outcome measures using pooled clinical trial data. Neurology. Nov 19 2019;93(21):e1921–e1931. doi: 10.1212/wnl.0000000000008519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake AS, Weinstock-Guttman B, Morrow SA, Hojnacki D, Munschauer FE, Benedict RH. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: replacing the PASAT with the Symbol Digit Modalities Test. Multiple sclerosis (Houndmills, Basingstoke, England). 2010;16(2):228–237. doi: 10.1177/1352458509354552; 10.1177/1352458509354552 [DOI] [PubMed] [Google Scholar]
- 14.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciencesJournal canadien des sciences appliquees au sport. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 15.Motl RW, Bollaert RE, Sandroff BM. Validation of the Godin Leisure-Time Exercise Questionnaire classification coding system using accelerometry in multiple sclerosis. Rehabil Psychol. Feb 2018;63(1):77–82. doi: 10.1037/rep0000162 [DOI] [PubMed] [Google Scholar]
- 16.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. May 1 2011;34(5):601–8. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumowski JF, Horng S, Brandstadter R, et al. Sleep disturbance and memory dysfunction in early multiple sclerosis. Ann Clin Transl Neurol. Jun 2021;8(6):1172–1182. doi: 10.1002/acn3.51262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Conesa MT, Philippou E, Pafilas C, et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients. Sep 27 2020;12(10)doi: 10.3390/nu12102960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman MD, Motl RW, Scagnelli J, Pula JH, Sosnoff JJ, Cadavid D. Clinically meaningful performance benchmarks in MS: timed 25-foot walk and the real world. Neurology. Nov 19 2013;81(21):1856–63. doi: 10.1212/01.wnl.0000436065.97642.d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman MD, Ward MD, Motl RW, Jones DE, Pula JH, Cadavid D. Identification and validation of clinically meaningful benchmarks in the 12-item Multiple Sclerosis Walking Scale. Mult Scler. Sep 2017;23(10):1405–1414. doi: 10.1177/1352458516680749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandstadter R. Beyond rehabilitation: A prevention model of reserve and brain maintenance in multiple sclerosis. Multiple sclerosis. 2019. 25(10):1372–1378. doi: 10.1177/1352458519856847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. Dec 2013;84(12):1318–25. doi: 10.1136/jnnp-2012-304792 [DOI] [PubMed] [Google Scholar]
- 23.Morris MC. MIND diet slows cognitive decline with aging. Alzheimer’s & dementia. 2015. 11(9):1015–1022. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu YY. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015. 85(20):1744–1751. doi: 10.1212/WNL.0000000000002121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballarini T, Melo van Lent D, Brunner J, et al. Mediterranean Diet, Alzheimer Disease Biomarkers and Brain Atrophy in Old Age. Neurology. May 5 2021;96(24):e2920–32. doi: 10.1212/wnl.0000000000012067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz Sand IB, Fitzgerald KC, Gu Y, et al. Dietary factors and MRI metrics in early Multiple Sclerosis. Mult Scler Relat Disord. May 19 2021;53:103031. doi: 10.1016/j.msard.2021.103031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azevedo CJ. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Annals of neurology. 2018;83(2):223–234. doi: 10.1002/ana.25150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocca MA. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology. 2010. 257(2):463–469. doi: 10.1148/radiol.10100326 [DOI] [PubMed] [Google Scholar]
- 29.Lassale C, Batty GD, Baghdadli A, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. Jul 2019;24(7):965–986. doi: 10.1038/s41380-018-0237-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. Nov 2017;76(4):427–436. doi: 10.1017/s0029665117002026 [DOI] [PubMed] [Google Scholar]
- 31.Treadway MT, Cooper JA, Miller AH. Can’t or Won’t? Immunometabolic Constraints on Dopaminergic Drive. Trends Cogn Sci. May 2019;23(5):435–448. doi: 10.1016/j.tics.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29(4):773–82. doi: 10.3233/jad-2012-111799 [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Lapiscina EH, Clavero P, Toledo E, et al. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging. 2013;17(6):544–52. doi: 10.1007/s12603-013-0027-6 [DOI] [PubMed] [Google Scholar]
- 34.Marrie RA. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010. 74(13):1041–1047. doi: 10.1212/WNL.0b013e3181d6b125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojeda-Rodríguez A, Zazpe I, Alonso-Pedrero L, Zalba G, Martínez-González MA, Marti A. Higher adherence to an empirically derived Mediterranean dietary pattern is positively associated with telomere length: the Seguimiento Universidad de Navarra (SUN) project. Br J Nutr. Aug 28 2021;126(4):531–540. doi: 10.1017/s0007114520004274 [DOI] [PubMed] [Google Scholar]
- 36.Krysko KM, Henry RG, Cree BAC, et al. Telomere Length Is Associated with Disability Progression in Multiple Sclerosis. Ann Neurol. Nov 2019;86(5):671–682. doi: 10.1002/ana.25592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langer-Gould A. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. 2013. 80(19):1734–1739. doi: 10.1212/WNL.0b013e3182918cc2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator, as permitted according to institutional regulations.


