Abstract
Autoimmunity is characterized by self-reactive immune components and autoimmune disease by autoimmunity plus pathology. Both autoimmunity and autoimmune diseases are dramatically increasing in many parts of the world, likely as a result of changes in our exposures to environmental factors. Current evidence implicates the momentous alterations in our foods, xenobiotics, air pollution, infections, personal lifestyles, stress, and climate change as causes for these increases. Autoimmune diseases have a major impact on the individuals and families they affect, as well as on our society and health care costs, and current projections suggest they may soon take their place among the predominant medical disorders. This necessitates that we increase the scope and scale of our efforts, and coordinate our resources and studies, to understand autoimmune disease risk factors and pathogeneses and improve our diagnostic, therapeutic, and preventive approaches, as the costs of inaction will be profound and far greater without such investments.
Keywords: Autoimmunity, Autoimmune diseases, Risk factors, Global efforts, Prevention
Introduction
Autoimmunity can be considered as the presence of self-reactive adaptive immune components, and autoimmune diseases can be thought of as autoimmunity plus clinically apparent pathology [1,2]. Although proposals have been made regarding approaches to define autoimmunity and specific autoimmune diseases, there are still no commonly agreed upon general criteria for these [3,4]. This lack of consensus in autoimmune disease definitions, and even agreement on which specific illnesses constitute the autoimmune diseases, impede research and clinical care, and the development of international stakeholder-defined consensus on these issues would greatly enhance further progress in the field.
The precise mechanisms for the development of autoimmunity and autoimmune diseases remain unclear, however, a growing consensus has developed that they evolve from still-to-be-defined gene-environment interactions [5,6]. As a result of technological advances made possible by the human genome project and related investigations, many genetic risk factors for autoimmune diseases have been identified [7], but there has been relatively little progress in deciphering the even more profound impact of environmental influences [8,9]. This is not surprising given the difficult tasks of assessing the doses, durations, and effects of the myriad combined environmental exposures we experience over a lifetime [10] and the complex impacts they have on the maturation and function of the immune system [11].
Studies have found increased frequencies of autoimmunity and autoimmune diseases over recent decades [12,13]. Yet, there are many challenges in accurately assessing changes in the incidence and prevalence of autoimmunity and autoimmune diseases over time. First, as noted above, there is a lack of universal consensus on definitions of cases and disease criteria [3]. Secondly, many autoimmune disorders individually are rare and heterogeneous conditions that are likely underdiagnosed, with evidence of varying ethnic, racial, and geographic distributions, making current estimates of their actual numbers problematic [14–16]. Third, there are inadequate centralized and standardized national and international databases on which to base these estimates, and there are referral biases to tertiary care centers from which much of our current information arises. Methodologies for autoantibody and other immune assays are constantly evolving, and each varies in accuracy, sensitivity, and specificity [17]. Finally, with increased understanding, classification and diagnostic criteria for some autoimmune diseases have evolved over time [18].
Evidence for increasing autoimmunity
While autoantibodies alone are not diagnostic for disease, they do define the presence of autoimmunity. Some cases can develop transiently after infections, immunizations, drug use, or injuries [6,19,20]. However, in many situations, autoantibodies are persistent, pathogenic, and some of the best predictors of the development of autoimmune disease [21–24]. Investigators have been concerned that autoantibody frequencies have been rising for some time, yet most studies have not been population-based and did not always use validated assays. Recent investigations have studied autoimmunity using population representative samples and carefully assessed and consistent assays to overcome some of these limitations.
The National Health and Nutrition Examination Survey (NHANES) is a Centers for Disease Control and Prevention (CDC) program of studies begun in the 1960s designed to define the health and nutritional status of adults and children in the United States (https://www.cdc.gov/nchs/nhanes/index.htm). The NHANES databases and serum repositories provide unique opportunities to assess the prevalence of autoimmunity in nationally representative samples of the US population across multiple time periods. One NHANES study showed that autoimmunity is common in the US [25]. For example, thyroid autoantibodies alone were present in 18% of US adults, including 10% of younger adults and 25% of older persons. Remarkably, 32% of adults 60 or more years of age had at least one of the four autoantibodies studied: rheumatoid factor (most often associated with rheumatoid arthritis), anti-thyroglobulin or anti-thyroperoxidase autoantibodies (associated with autoimmune thyroid disease), or anti-tissue transglutaminase autoantibodies (associated with gluten-sensitive enteropathies).
Antinuclear antibodies (ANA) are a diverse group of autoantibodies directed against nuclear and other cellular components and a frequent clinical screening assay for autoimmune diseases. NHANES data have shown that ANA prevalence was 11.0% in 1988–1991, 11.4% in 1999–2004, and 16.1% in 2011–2012 (P trend < 0.0001, Figure 1) [12]. This represents 22.3, 26.6, and 41.5 million affected individuals, respectively. The most impressive increase was in adolescents, where ANA prevalence rose steeply, with odds ratios of 2.07 and 2.77 in the second and third time periods compared to the first (P trend = 0.0004). Yet ANA prevalence also significantly increased in both sexes (especially males), older adults, and non-Hispanic whites [12].
Figure 1.
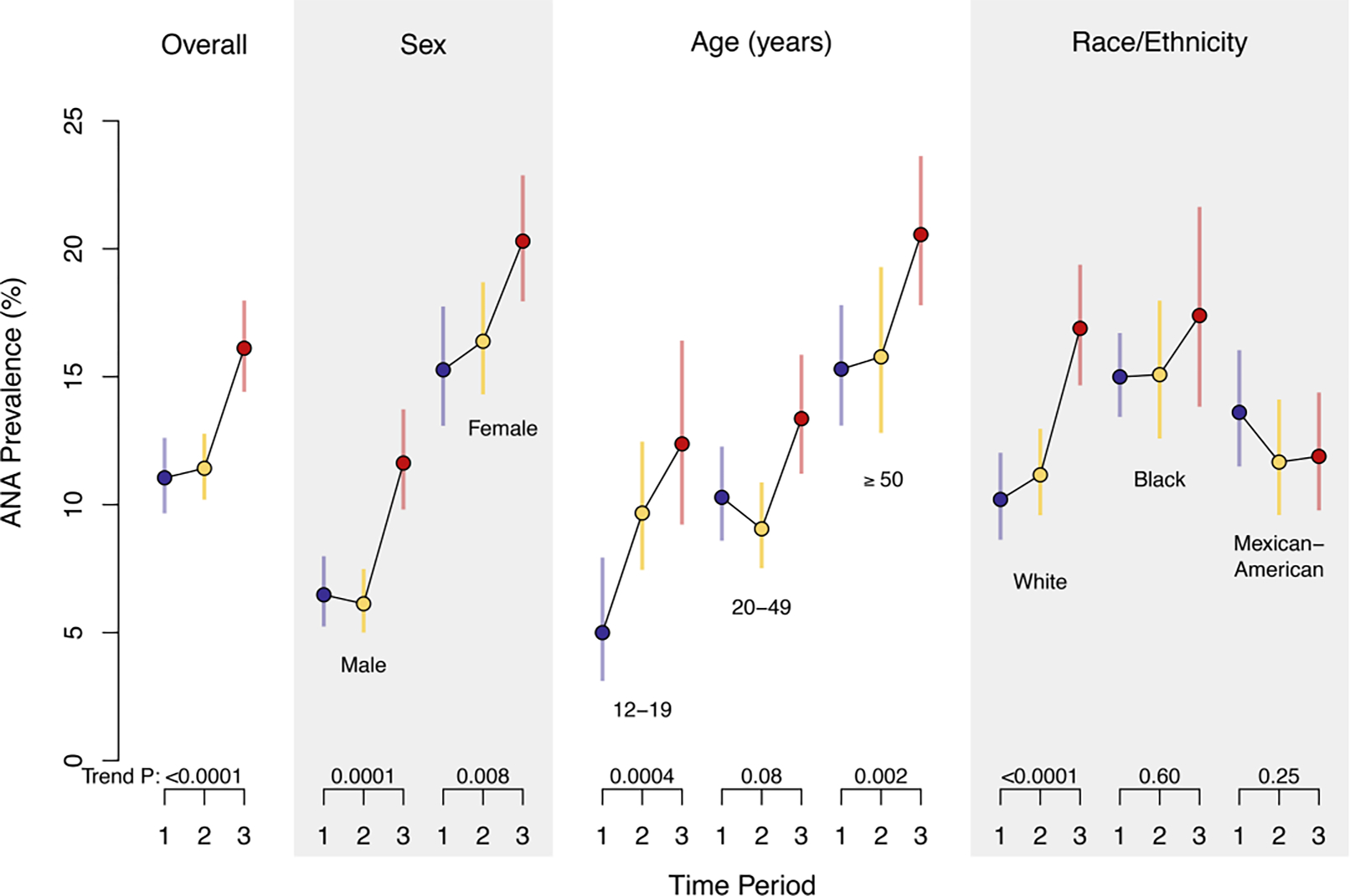
The estimated prevalence of antinuclear antibodies (ANA) in demographic groups in the US population over time. Colored circles represent the weighted estimate of ANA prevalence, and the colored lines show the 95% confidence interval for period 1 (1988–1991, blue), period 2 (1999–2004, yellow), and period 3 (2011–2012, red). P values for ANA time trend are displayed below each category and were derived from a logistic regression model that was adjusted for sex, age, and race/ethnicity (reprinted with permission [12]).
Evidence for increasing autoimmune diseases
Consistent with the ANA findings above, which argue against the notion that increased physician recognition of autoimmune diseases alone may be causing their apparent rises, local, national, and international epidemiologic studies have discovered similar increases in the frequencies of most autoimmune diseases. Estimates of the yearly increases in the overall worldwide incidence and prevalence of autoimmune diseases are 19.1% and 12.5%, respectively [26]. Despite the limitations noted above in obtaining accurate estimates, prevalence data collated from multiple investigations of various autoimmune diseases in many global locations have confirmed an overall rising trend (Figure 2). Perhaps the best studied of these is type 1 diabetes [27], where investigations found a consistent 3–4% annual increase in incidence over the last three decades [28]. Not only are core currently recognized autoimmune diseases increasing, but the range of autoimmune and chronic inflammatory diseases continues to expand in number and scope, as additional disorders are found to have laboratory or clinical features implicating involvement of the immune system and autoimmune signatures [10].
Figure 2.
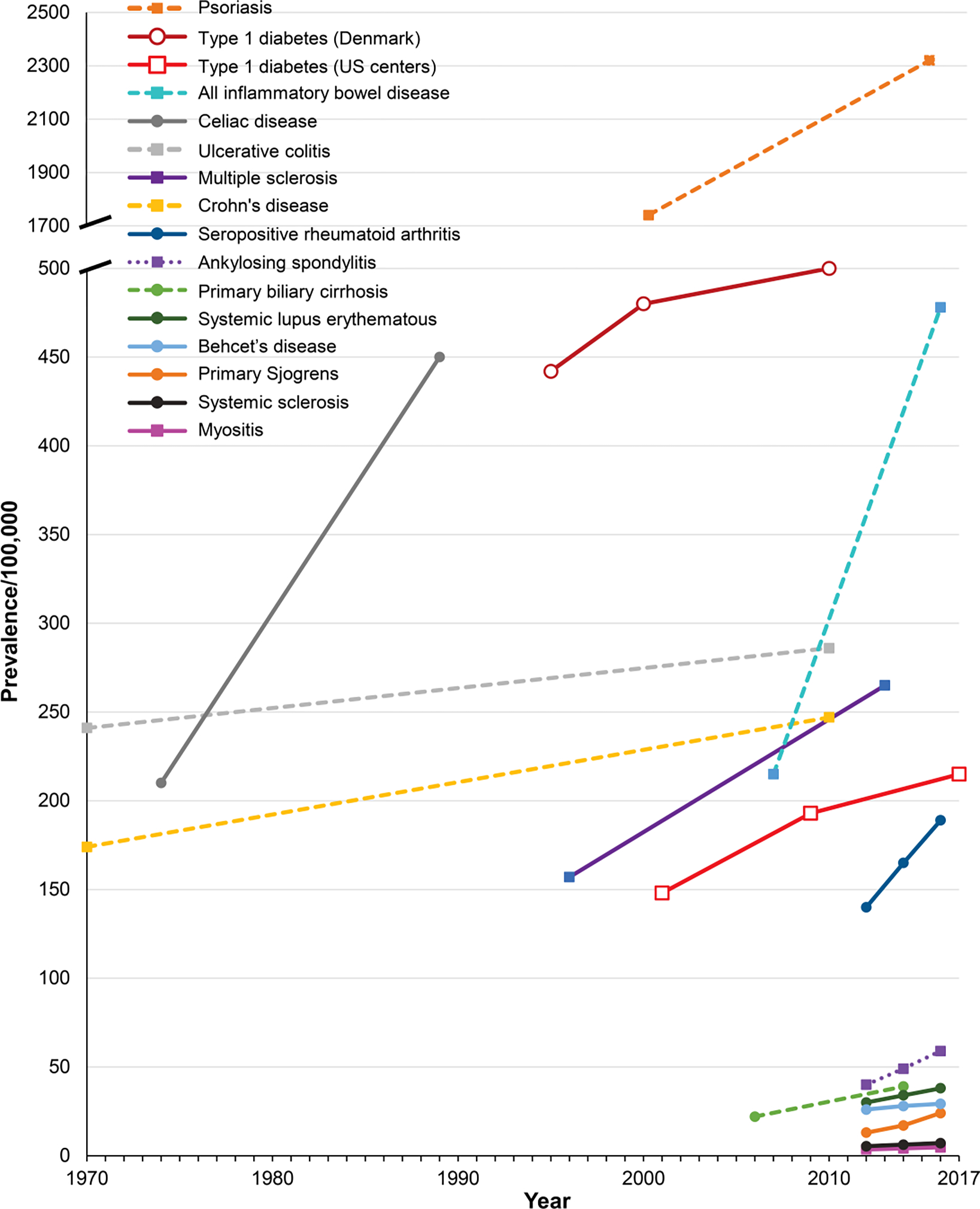
Examples of the increasing prevalence of autoimmune diseases around the world over recent decades. The estimates for the time periods are connected by lines to visualize trends. Data sources are for: psoriasis in Canada [70]; type 1 diabetes in Denmark [71] and in the US [72]; celiac disease in the US [73]; multiple sclerosis in Canada [74]; primary biliary cirrhosis in the US [75]; all inflammatory bowel disease in the US [76]; Crohn’s disease and ulcerative colitis in the US [77]; and seropositive rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, Behçet’s disease, primary Sjogren’s syndrome, systemic clerosis, and myositis in Korea [68].
Understanding the causes
Familial clusterings and recent molecular genomic advances clearly show the importance of genetic factors in the development of autoimmune diseases [7]. Nonetheless, most genes are neither good nor bad, but only their environments make them so. Moreover, our genetic architecture, which has evolved over millennia to allow us to thrive in prior environments, may in many ways be ill-suited to our rapidly changing modern environmental challenges. The contemporary consensus is that in autoimmune disorders, multiple genetic and environmental risk factors interact in complex ways over long time spans to induce disease evolution from the genetic risk factor stage, to subclinical immune activation and autoimmunity, to early clinical signs and symptoms, and to finally result in a phenotype meeting classification or diagnostic criteria [5,29–31].
There are numerous complementary yet intersecting reasons for considering that environmental influences play a crucial role in the development of autoimmunity and autoimmune diseases [32]. These include: low to moderate concordance in monozygotic twins [33]; biologic plausibility from experimental in vitro and animal studies [34]; strong temporal associations with specific exposures and disease onset [35]; clear examples of dechallenge (improvement after suspect agent removal) and re-challenge (recurrence after suspect agent re-exposure), especially for drugs [36,37]; seasonal and geographic clusterings with disease onset [14,38,39]; seasonal associations with birthdates [40–43]; changes in incidence over time [26]; migration studies showing disease increases in groups moving from a low-incidence to a high-incidence region [44]; the major genetic risk factors are environmental response genes [7]; and strong observational epidemiological associations between exposures and certain diseases [9,32].
Taken together, the confluence of growing evidence points to many environmental factors that are possibly related to the development of autoimmunity and autoimmune diseases [45–48]. Of most concern are the major changes in our diets and the effects on microbiomes [49–52], xenobiotic contacts [53,54], infections [55,56], personal lifestyles and attendant increased obesity rates [57] and sleep deprivation [58], stress [59], air pollution [60], and the impacts of climate change [61], as possible contributing factors to these increases.
For example, there are ever-increasing numbers and amounts of xenobiotic chemical pollutants in commercial use [53], most of which have not been studied for their long-term immune effects, and some of which are associated with the development of autoimmunity [62]. It is also of concern that the list of therapeutic drugs implicated in the development of lupus continues to grow [37,63] and that cases of autoimmune diseases following biologic agent treatments are also rapidly escalating [64]. While the specific impacts of climate change on autoimmune diseases have not been well-studied, their many adverse effects on human health -- including increased food-borne, water-borne, vector-borne, and zoonotic infectious diseases, cardiopulmonary, renal, and pregnancy complications, malnutrition, ultraviolet radiation exposure, air and water pollution, toxin exposures, allergies, physical and mental stress, and compromised access to health care services -- are well known [65]. Given the growing evidence of the role of many of these factors in the development of autoimmune diseases, it is reasonable to suspect that climate change is an additional element contributing to their increased prevalence [9,32,45,47,56,59,61].
Implications
These findings have many profound implications. Regarding public health costs and the economy, the total direct and indirect costs of autoimmune diseases are difficult to assess for the reasons mentioned above and due to our fragmented health care systems. Nevertheless, autoimmune diseases clearly cause much personal suffering, high morbidity and mortality, and are a significant driver of health care utilization and costs [66,67]. Direct costs in the US have been estimated at over $100 billion per year, but this is likely a gross underestimate [67]. A study of Korean national databases has estimated direct medical costs of the more common autoimmune diseases as high and rapidly increasing [68] (Figure 3). These estimates do not capture indirect costs, including lost productivity and incomes, and the negative impacts on patients’ health, dependent care, families, and society in general.
Figure 3.
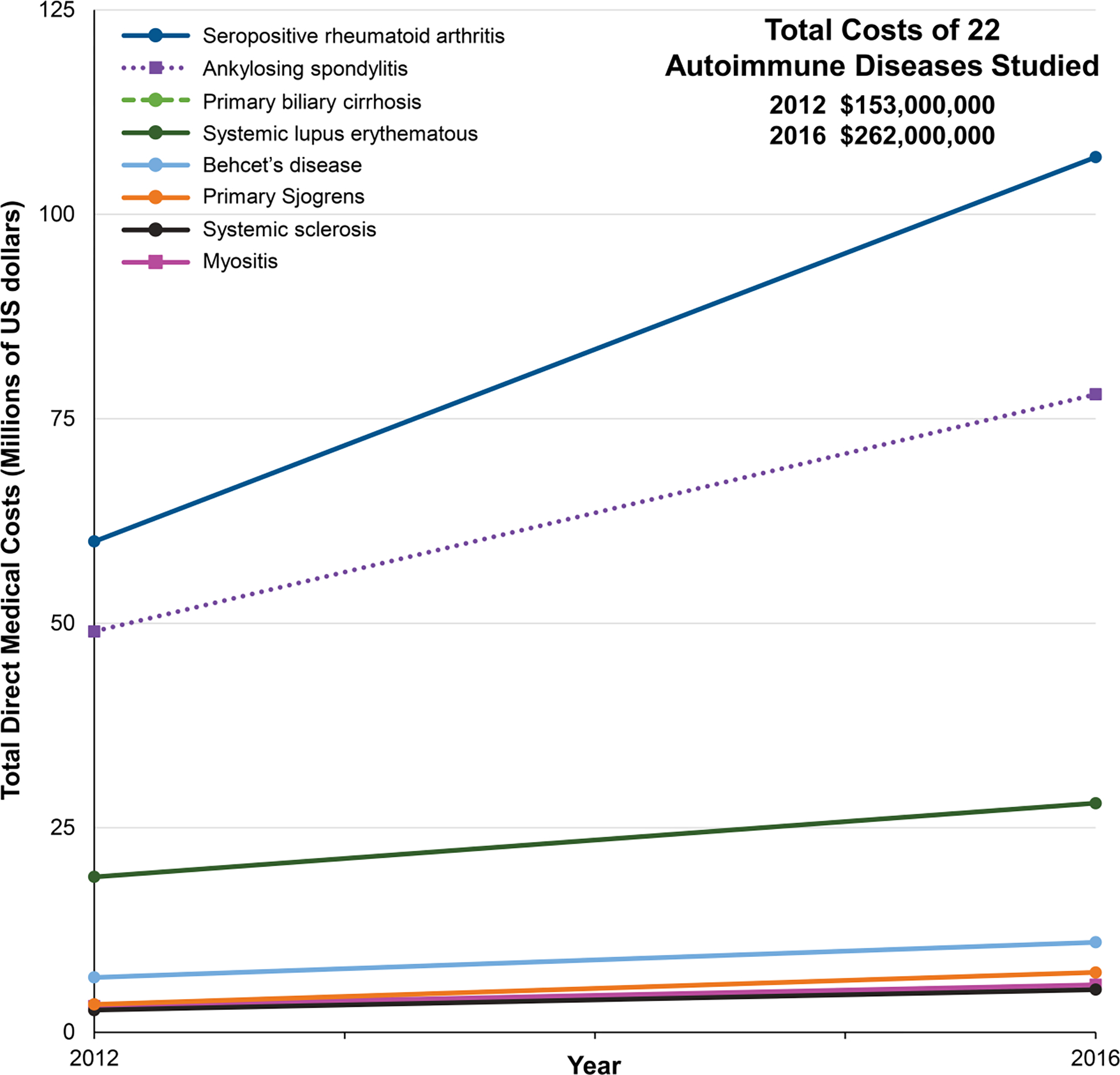
Examples of increasing total direct medical costs of selected autoimmune diseases in Korea [68].
A recent review of NIH’s autoimmune disease research activities by the National Academies of Sciences, Engineering, and Medicine has found a number of gaps and limitations in the approaches that the different NIH institutes pursue, and among their recommendations was the creation of an Office of Autoimmune Disease/Autoimmunity Research within the Office of the NIH Director to enhance research collaboration and coordination in this area [69]. The committee also recommended the establishment of long-term systems to collect and ensure optimum usability of population-based surveillance and epidemiological data [69]. One tactic to increase understanding of the scope, epidemiology, and possible environmental risks would be to establish a central reportable system for autoimmune diseases similar to the NCI Surveillance, Epidemiology, and End Results (SEER) Program, which provides information on cancer statistics in the US population (https://seer.cancer.gov/). These recommendations could certainly aid in achieving some of the many unmet needs in autoimmune disease research in the US, but an even more global approach is needed for the worldwide coordination and enhancement of studies and clinical care in this area.
An urgent call to action
The rapid rise of autoimmune diseases around the world outlined here has had significant impacts on the public health, and which will continue to increase without intervention and change. Overcoming the growing negative personal and societal effects, as well as the rising costs, of this group of increasingly frequent disorders will require systematic planning and action on a global level. Increased research and clinical efforts, resources, and coordination, to intervene on a scale not previously seen, are needed to halt or reverse these trends. Some approaches that could be helpful are outlined below (see Box). Central to these is the integration of the international community of stakeholders – including caregivers, researchers, public health officials, regulatory and funding agencies, pharmaceutical companies, and patient support groups – to pool their expertise and resources and develop an agenda that coordinates global activities to expand current efforts, increase efficiencies, and minimize duplication of efforts.
Box: Possible approaches to address the rapid rise in autoimmune diseases.
Convene international, multidisciplinary, stakeholder consensus conferences to: identify research opportunities and priorities; develop a standardized definition of autoimmune diseases and maintain a comprehensive listing of these; coordinate efforts to improve the integration and prioritization of basic, animal model, and clinical research; define approaches to measure direct and indirect costs to facilitate prioritizations; and assist in the coordination and oversight of other efforts
Establish comprehensive standardized registries and repositories for all autoimmune diseases around the world
Provide resources for well-designed studies of autoimmune diseases with similar features to define common and unique pathogeneses and risk/protective factors for various phenotypes
Assess multiple gene-environment interactions for autoimmune diseases, including those grouped together based on shared mechanisms or risk factors, to allow for preventative strategies
Coordinate efforts to identify a matrix of clinical features, risk factors, and laboratory biomarkers to allow for earlier and more accurate diagnoses that could minimize damage from chronic inflammation
Conduct more efficient “basket/umbrella” investigations and other clinical trials involving multiple autoimmune diseases with similar therapeutic targets (e.g., interferon-targeted therapies for disorders with increased interferon signatures), as are now being performed in cancer studies
Define best practices for medical care, therapies, and access to them
Develop studies and practical guidance on prevention by evaluating a combination of promising biomarkers, lifestyle modifications, avoidance of environmental risk factors, and, particularly for those with major genetic risks, preventative therapies
Conclusions
Autoimmune diseases have had a devastating personal and caregiver impact on our society, with significant health care utilization resulting in high public and private costs, yet current projections suggest they will become even more prominent disorders in the future. This demands that we increase our attention and resources to coordinate and enhance our efforts in understanding their pathogeneses and risk factors, and to improve our diagnostic, therapeutic, and preventative approaches. The costs of inaction will be profound, and only by dedicating additional resources now can we decrease the future frequency, morbidity, mortality, and costs of these conditions.
Highlights.
Autoimmunity is defined by self-reactive components of the adaptive immune system, which cause clinically apparent pathology in the case of autoimmune diseases.
Multiple lines of evidence suggest that autoimmunity and autoimmune diseases are on the rise.
Probable causes for such increases include the major recent changes in our foods, contacts with xenobiotics, air pollution, infections, lifestyles, psychosocial stress, and climate change.
These increases in autoimmune diseases have induced significant upsurges in individual and societal suffering as well as in private and public health care costs.
Increased global-scale multidisciplinary research and coordination, with systematic improvements in our ability to understand, diagnose, treat, and prevent autoimmune diseases, are urgently needed and are likely to be cost-effective.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The author is indebted to Drs. Ejaz Shamim, Terrance O’Hanlon, Adam Schiffenbauer, Lisa Rider, Shepherd Schurman, and Charles Dillon for useful concepts and comments during manuscript preparation. This paper is dedicated to Dr. Noel Rose (1927–2020) in recognition of his groundbreaking discoveries, global vision, and unparalleled leadership in advancing the field of autoimmunity and autoimmune diseases.
Footnotes
Conflict of interest statement
Nothing declared.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Fred Miller
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Rosenblum MD, Remedios KA, Abbas AK: Mechanisms of human autoimmunity. J Clin Invest 2015, 125:2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Wang FS, Gershwin ME: Human autoimmune diseases: a comprehensive update. J Intern Med 2015, 278:369–395. [DOI] [PubMed] [Google Scholar]
- 3.Rose Noel R. and Mackay Ian: The Autoimmune Diseases 5th edn. New York, NY: Elsevier; 2014. [Google Scholar]
- 4.Rose NR, Bona C: Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunology Today 1993, 14:426–430. [DOI] [PubMed] [Google Scholar]
- 5.Ellis JA, Kemp AS, Ponsonby AL: Gene-environment interaction in autoimmune disease. Expert Rev Mol Med 2014, 16:e4. [DOI] [PubMed] [Google Scholar]
- 6.Lleo A, Invernizzi P, Gao B, Podda M, Gershwin ME: Definition of human autoimmunity--autoantibodies versus autoimmune disease. Autoimmun.Rev. 2010, 9:A259–A266. [DOI] [PubMed] [Google Scholar]
- 7. Caliskan M, Brown CD, Maranville JC: A catalog of GWAS fine-mapping efforts in autoimmune disease. Am J Hum Genet 2021, 108:549–563. * A comprehensive synthesis of current genetic locus-by-disease association signals for 15 autoimmune diseases.
- 8.Cooper GS, Miller FW: Environmental influences on autoimmunity and autoimmune diseases. In Immunotoxicology and Immunopharmacology. Edited by Luebke R: CRC Press; 2007:437–454. vol Third.] [Google Scholar]
- 9.Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, De Roos AJ: Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun 2012, 39:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anaya JM, Ramirez-Santana C, Alzate MA, Molano-Gonzalez N, Rojas-Villarraga A: The Autoimmune Ecology. Front Immunol 2016, 7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, et al. : Variation in the human immune system is largely driven by non-heritable influences. Cell 2015, 160:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, Chan EKL, Miller FW: Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol 2022. Aug 26. doi: 10.1002/art.42330. Online ahead of print. https://pubmed.ncbi.nlm.nih.gov/36054084/ ** The first population-representative evaluation of autoimmunity in the US shows significant increases in ANA frequencies in many groups, especially adolescents, over recent decades.
- 13.Bach JF: The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002, 347:911–920. [DOI] [PubMed] [Google Scholar]
- 14.Borchers AT, Uibo R, Gershwin ME: The geoepidemiology of type 1 diabetes. Autoimmun.Rev. 2010, 9:A355–A365. [DOI] [PubMed] [Google Scholar]
- 15.Choung RS, Ditah IC, Nadeau AM, Rubio-Tapia A, Marietta EV, Brantner TL, Camilleri MJ, Rajkumar SV, Landgren O, Everhart JE, et al. : Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol 2015, 110:455–461. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Casals M, Brito-Zeron P, Kostov B, Siso-Almirall A, Bosch X, Buss D, Trilla A, Stone JH, Khamashta MA, Shoenfeld Y: Google-driven search for big data in autoimmune geoepidemiology: analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun Rev 2015, 14:670–679. [DOI] [PubMed] [Google Scholar]
- 17.Claessens J, Belmondo T, De Langhe E, Westhovens R, Poesen K, Hüe S, Blockmans D, Mahler M, Fritzler MJ, Bossuyt X: Solid phase assays versus automated indirect immunofluorescence for detection of antinuclear antibodies. Autoimmun Rev 2018, 17:533–540. [DOI] [PubMed] [Google Scholar]
- 18.Aringer M, Brinks R, Dörner T, Daikh D, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, et al. : European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) SLE classification criteria item performance. Ann Rheum Dis 2021, 80:775–781. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, Gordon T, Hardin JA, Kalden JR, Lahita RG, et al. : Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997, 40:1601–1611. [DOI] [PubMed] [Google Scholar]
- 20. Irure-Ventura J, López-Hoyos M: The Past, Present, and Future in Antinuclear Antibodies (ANA). Diagnostics (Basel) 2022, 12. * An informative review of the role of ANA in assessing and diagnosing autoimmune diseases, as well as novel approaches to utilize artificial intelligence in combining a variety of clinical and laboratory variables to allow for earlier diagnoses in the future.
- 21.Rose NR: Predictors of autoimmune disease: autoantibodies and beyond. Autoimmunity 2008, 41:419–428. [DOI] [PubMed] [Google Scholar]
- 22.Hayter SM, Cook MC: Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev 2012, 11:754–765. [DOI] [PubMed] [Google Scholar]
- 23.Selmi C, Ceribelli A, Generali E, Scirè CA, Alborghetti F, Colloredo G, Porrati L, Achenza MI, De Santis M, Cavaciocchi F, et al. : Serum antinuclear and extractable nuclear antigen antibody prevalence and associated morbidity and mortality in the general population over 15 years. Autoimmun Rev 2016, 15:162–166. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, Komorowski L, Luo J, Cabral-Marques O, Hammers CM, et al. : Mechanisms of Autoantibody-Induced Pathology. Front Immunol 2017, 8:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dillon CF, Weisman MH, Miller FW: Population-based estimates of humoral autoimmunity from the U.S. National Health and Nutrition Examination Surveys, 1960–2014. PLoS One 2020, 15:e0226516. * A summary of autoantibody information from US population-representative surveys emphasizing how common autoimmunity is in the general population.
- 26.Lerner A, Jeremias P, Matthias T: The World Incidence and Prevalence of Autoimmune Diseases is Increasing. International Journal of Celiac Disease 2016, 3:151–155. [Google Scholar]
- 27. Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M: Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect 2020, 10:98–115. ** A carefully performed systematic review of many studies demonstrating the high and ever-increasing incidence and prevalence of type 1 diabetes around the world.
- 28. Norris JM, Johnson RK, Stene LC: Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol 2020, 8:226–238. * A review of the many risk factors and epidemiology for type 1 diabetes, focusing on the consistent 3–4% annual increase in incidence in many parts of the world that suggests preventative efforts are urgently needed to contain this important and costly autoimmune disease.
- 29.Luppi P, Rossiello MR, Faas S, Trucco M: Genetic background and environment contribute synergistically to the onset of autoimmune diseases. Journal of Molecular Medicine 1995, 73:381–393. [DOI] [PubMed] [Google Scholar]
- 30. Choi MY, Costenbader KH: Understanding the Concept of Pre-Clinical Autoimmunity: Prediction and Prevention of Systemic Lupus Erythematosus: Identifying Risk Factors and Developing Strategies Against Disease Development. Front Immunol 2022, 13:890522. ** This article reviews the current data demonstrating a long progression to the development of autoimmune diseases resulting from early gene-environment interactions. The authors propose novel screening and preventative strategies using a combination of promising biomarkers, lifestyle modifications, avoidance of environmental risk factors, and preventative therapies.
- 31.Slight-Webb S, Bourn RL, Holers VM, James JA: Shared and unique immune alterations in pre-clinical autoimmunity. Curr Opin Immunol 2019, 61:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller FW: Environmental agents and autoimmune diseases. Advances in Experimental Medicine and Biology 2011, 711:61–81. [DOI] [PubMed] [Google Scholar]
- 33.Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C, Eric GM: Twin studies in autoimmune disease: Genetics, gender and environment. J.Autoimmun. 2012, 38:J156–J169. [DOI] [PubMed] [Google Scholar]
- 34.Germolec D, Kono DH, Pfau JC, Pollard KM: Animal models used to examine the role of the environment in the development of autoimmune disease: findings from an NIEHS Expert Panel Workshop. J.Autoimmun. 2012, 39:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martirosyan A, Aminov R, Manukyan G: Environmental Triggers of Autoreactive Responses: Induction of Antiphospholipid Antibody Formation. Front Immunol 2019, 10:1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller FW, Pollard KM, Parks CG, Germolec DR, Leung PS, Selmi C, Humble MC, Rose NR: Criteria for environmentally associated autoimmune diseases. J.Autoimmun. 2012, 39:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnaud L, Mertz P, Gavand PE, Martin T, Chasset F, Tebacher-Alt M, Lambert A, Muller C, Sibilia J, Lebrun-Vignes B, et al. : Drug-induced systemic lupus: revisiting the ever-changing spectrum of the disease using the WHO pharmacovigilance database. Ann Rheum Dis 2019, 78:504–508. [DOI] [PubMed] [Google Scholar]
- 38.Schlesinger N, Schlesinger M: Seasonal variation of rheumatic diseases. Discov.Med. 2005, 5:64–69. [PubMed] [Google Scholar]
- 39.Watad A, Azrielant S, Bragazzi NL, Sharif K, David P, Katz I, Aljadeff G, Quaresma M, Tanay G, Adawi M, et al. : Seasonality and autoimmune diseases: The contribution of the four seasons to the mosaic of autoimmunity. J Autoimmun 2017, 82:13–30. [DOI] [PubMed] [Google Scholar]
- 40.Vegosen LJ, Weinberg CR, O’Hanlon TP, Targoff IN, Miller FW, Rider LG: Seasonal birth patterns in myositis subgroups suggest an etiologic role of early environmental exposures. Arthritis Rheum. 2007, 56:2719–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantavou KG, Bagos PG: Season of birth and multiple sclerosis: a systematic review and multivariate meta-analysis. J Neurol 2020, 267:2815–2822. [DOI] [PubMed] [Google Scholar]
- 42.Woodman R, Hakendorf P, Limaye V: Seasonality of birth patterns in an Australian cohort of patients with biopsy-confirmed idiopathic inflammatory myopathy. Intern Med J 2016, 46:619–621. [DOI] [PubMed] [Google Scholar]
- 43.Chowers Y, Odes S, Bujanover Y, Eliakim R, Bar MS, Avidan B: The month of birth is linked to the risk of Crohn’s disease in the Israeli population. American Journal of Gastroenterology 2004, 99:1974–1976. [DOI] [PubMed] [Google Scholar]
- 44.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK: Environmental triggers and determinants of type 1 diabetes. Diabetes 2005, 54 Suppl 2:S125–136. [DOI] [PubMed] [Google Scholar]
- 45.Floreani A, Leung PS, Gershwin ME: Environmental Basis of Autoimmunity. Clin Rev Allergy Immunol 2016, 50:287–300. [DOI] [PubMed] [Google Scholar]
- 46.Powell JJ, Van De WJ, Gershwin ME: Evidence for the Role of Environmental Agents in the Initiation or Progression of Autoimmune Conditions. Environmental Health Perspectives 1999, 107:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agmon-Levin N, Lian Z, Shoenfeld Y: Explosion of autoimmune diseases and the mosaic of old and novel factors. Cell Mol Immunol 2011, 8:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woo JMP, Parks CG, Jacobsen S, Costenbader KH, Bernatsky S: The role of environmental exposures and gene-environment interactions in the etiology of systemic lupus erythematous. J Intern Med 2022, 291:755–778. * A comprehensive review of the known gene-environment interactions with lupus risk, including IL-10, ESR1, IL-33, ITGAM, and NAT2, and observed interactions with smoking, UV exposure, and alcohol. Extensions of this work may facilitate pathogenetic understanding and the development of preventive interventions for modifiable risk factors in susceptible individuals.
- 49.Thorburn AN, Macia L, Mackay CR: Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40:833–842. [DOI] [PubMed] [Google Scholar]
- 50.Powell ES, Smith-Taillie LP, Popkin BM: Added Sugars Intake Across the Distribution of US Children and Adult Consumers: 1977–2012. J Acad Nutr Diet 2016, 116:1543–1550.e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barber TM, Valsamakis G, Mastorakos G, Hanson P, Kyrou I, Randeva HS, Weickert MO: Dietary Influences on the Microbiota-Gut-Brain Axis. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christovich A, Luo XM: Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front Immunol 2022, 13:946248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Persson L, Carney Almroth BM, Collins CD, Cornell S, de Wit CA, Diamond ML, Fantke P, Hassellöv M, MacLeod M, Ryberg MW, et al. : Outside the Safe Operating Space of the Planetary Boundary for Novel Entities. Environ Sci Technol 2022, 56:1510–1521. * The authors propose that the growing levels and extent of the more than 350,000 chemicals (or mixtures of chemicals) on the global market have exceeded our ability to conduct safety-related assessments and monitoring. They recommend that urgent action is needed to reduce the harm associated with these by reducing the production and release of novel chemical entities as much as possible.
- 54.Chemicals I-OPftSMo: Environmental Health Criteria 236: Principles and Methods for Assessing Autoimmunity Associated with Exposure to Chemicals In Environmental Health Criteria Monographs (EHCs). Edited by. Geneva: World Health Organization; 2006:1–330. [Organization WH (Series Editor): [Google Scholar]
- 55.Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G: New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sener AG, Afsar I: Infection and autoimmune disease. Rheumatol Int 2012, 32:3331–3338. [DOI] [PubMed] [Google Scholar]
- 57. Malik VS, Willet WC, Hu FB: Nearly a decade on - trends, risk factors and policy implications in global obesity. Nat Rev Endocrinol 2020, 16:615–616. * This paper reviews the concept that global free trade, with resulting economic growth and urbanization, is the primary driver of obesity trends with the creation of obesogenic environments from unhealthy dietary changes and reductions in physical activity. Much needs to be done to reverse these trends that have major negative health implications.
- 58. Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E: Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol 2021, 4:1304. * This paper emphasizes the many alterations of innate and adaptive immune parameters that can result in chronic inflammation due to increased sleep deprivation in developed countries.
- 59.Song H, Fang F, Tomasson G, Arnberg FK, Mataix-Cols D, Fernandez de la Cruz L, Almqvist C, Fall K, Valdimarsdottir UA: Association of Stress-Related Disorders With Subsequent Autoimmune Disease. JAMA 2018, 319:2388–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao N, Smargiassi A, Jean S, Gamache P, Laouan-Sidi EA, Chen H, Goldberg MS, Bernatsky S: Long-term exposure to fine particulate matter and ozone and the onset of systemic autoimmune rheumatic diseases: an open cohort study in Quebec, Canada. Arthritis Res Ther 2022, 24:151. * This study of over six million adults in Quebec showed associations between exposures to fine particulate matter (PM2.5) and the onset of systemic autoimmune rheumatic diseases, consistent with many other investigations in other locales.
- 61. Dellaripa PF, Bush T, Miller FW, Feldman CH: The Climate Emergency and the Health of Our Patients: The Role of the Rheumatologist. Arthritis Rheumatol 2022. * A summary of the urgent need to address the potentially devastating climate emergency and its possible impacts on patients with autoimmune diseases. The authors are hopeful that the many harmful effects can be minimized via timely and appropriate education, mitigation, and adaptive strategies.
- 62. Dinse GE, Co CA, Parks CG, Weinberg CR, Xie G, Chan EKL, Birnbaum LS, Miller FW: Expanded assessment of xenobiotic associations with antinuclear antibodies in the United States, 1988–2012. Environ Int 2022, 166:107376. * The largest study of xenobiotic associations with ANA ever conducted, analyzing cross-sectional data on 12,058 participants and 253 xenobiotics from three time periods of the National Health and Nutrition Examination Survey. The authors found many significant positive and negative associations with a number of chemicals suggesting important mechanistic, preventive, and treatment implications for various immune-mediated disorders.
- 63.Rubin RL: Evolving and expanding scope of lupus-inducing drugs. Ann Rheum Dis 2019, 78:443–445. [DOI] [PubMed] [Google Scholar]
- 64.Perez-De-Lis M, Retamozo S, Flores-Chavez A, Kostov B, Perez-Alvarez R, Brito-Zeron P, Ramos-Casals M: Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry). Expert Opin Drug Saf 2017, 16:1255–1271. [DOI] [PubMed] [Google Scholar]
- 65. Rocque RJ, Beaudoin C, Ndjaboue R, Cameron L, Poirier-Bergeron L, Poulin-Rheault RA, Fallon C, Tricco AC, Witteman HO: Health effects of climate change: an overview of systematic reviews. BMJ Open 2021, 11:e046333. ** A comprehensive review of the many devastating health effects of climate change on populations around the world.
- 66.Walsh SJ, Rau LM: Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. American Journal of Public Health 2000, 90:1463–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diseases AAR, and AA, Patient NCoA, (NCAPG) G: The Cost Burden of Autoimmune Disease: The Latest Front in the War on Healthcare Spending. 2011. http://www.diabetesed.net/page/_files/autoimmune-diseases.pdf;
- 68. Kim H, Cho SK, Kim JW, Jung SY, Jang EJ, Bae SC, Yoo DH, Sung YK: An increased disease burden of autoimmune inflammatory rheumatic diseases in Korea. Semin Arthritis Rheum 2020, 50:526–533. * A carefully conducted study using Korean national databases that demonstrated the rapid increase in the prevalence and costs of many autoimmune diseases.
- 69. Committee for the Assessment of NIH Research on Autoimmune Diseases; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine: The National Academies Collection: Reports funded by National Institutes of Health. In Enhancing NIH Research on Autoimmune Disease. Edited by: National Academies Press (US). Copyright © 2022, National Academy of Sciences. ** A comprehensive review of autoimmune disease features, epidemiology, and recent NIH research in this area with recommendations to enhance and coordinate these efforts.
- 70.Eder L, Widdifield J, Rosen CF, Cook R, Lee KA, Alhusayen R, Paterson MJ, Cheng SY, Jabbari S, Campbell W, et al. : Trends in the Prevalence and Incidence of Psoriasis and Psoriatic Arthritis in Ontario, Canada: A Population-Based Study. Arthritis Care Res (Hoboken) 2019, 71:1084–1091. [DOI] [PubMed] [Google Scholar]
- 71.Passa P: Diabetes trends in Europe. Diabetes Metab Res Rev 2002, 18 Suppl 3:S3–8. [DOI] [PubMed] [Google Scholar]
- 72.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, et al. : Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Jama 2014, 311:1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Catassi C, Kryszak D, Bhatti B, Sturgeon C, Helzlsouer K, Clipp SL, Gelfond D, Puppa E, Sferruzza A, Fasano A : Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med 2010, 42:530–538. [DOI] [PubMed] [Google Scholar]
- 74.Rotstein DL, Chen H, Wilton AS, Kwong JC, Marrie RA, Gozdyra P, Krysko KM, Kopp A, Copes R, Tu K: Temporal trends in multiple sclerosis prevalence and incidence in a large population. Neurology 2018, 90:e1435–e1441. [DOI] [PubMed] [Google Scholar]
- 75.Kanth R, Shrestha RB, Rai I, VanWormer JJ, Roy PK: Incidence of Primary Biliary Cholangitis in a Rural Midwestern Population. Clin Med Res 2017, 15:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye Y, Manne S, Treem WR, Bennett D: Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates From Large National Databases in the United States, 2007–2016. Inflamm Bowel Dis 2020, 26:619–625. [DOI] [PubMed] [Google Scholar]
- 77.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr.,: Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clin Gastroenterol Hepatol 2017, 15:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]


