Abstract
Adolescent stress is a risk factor for schizophrenia. Emerging evidence suggests that age-dependent sensitive windows for childhood trauma are associated more strongly with adult psychosis, but the neurobiological basis and potential sex differences are unknown.
Using in vivo electrophysiology and immunohistology in rats, we systematically compared the effects of two age-defined adolescent stress paradigms, prepubertal (postnatal day [PD] 21-30; PreP-S) and postpubertal (PD41-50; PostP-S) foot-shock and restraint combined stress, on ventral tegmental area (VTA) dopaminergic activity, pyramidal neuron activity in the ventral hippocampus (vHipp) and the basolateral amygdala (BLA), corticoamygdalar functional inhibitory control, and vHipp and BLA parvalbumin interneuron (PVI) impairments. These endpoints were selected based on their well-documented roles in the pathophysiology of psychosis.
Overall, we found distinct sex- and exposure age-dependent stress vulnerability. Specifically, while males were selectively vulnerable to PreP-S-induced adult VTA dopamine neuron and vHipp hyperactivities, females are selectively vulnerable to PostP-S. These male selective PreP-S effects were correlated with stress-induced aberrant persistent BLA hyperactivity, dysfunctional prefrontal inhibitory control of BLA neurons, and vHipp/BLA PVI impairments. In contrast, female PostP-S only produced vHipp PVI impairments in adults, with the BLA structure and functions largely unaffected.
Our results indicated distinct adolescent sensitive periods during which stress can sex-dependently confer maximal risks to corticolimbic systems to drive dopamine hyperactivity, which provide critical insights into the neurobiological basis for sex-biased stress-related psychopathologies emphasizing but not limited to schizophrenia. Furthermore, our work also provides a framework for future translational research on age-sensitive targeted interventions.
Introduction
Exposure to early developmental stress (such as childhood trauma) is strongly associated with adult risks of psychotic disorders (1). In this context, emerging studies implicate age-dependent sensitive periods during which developmental stress specifically associates with later-life psychosis risks (2, 3). Childhood adversity is also associated with the adult manifestation of elevated presynaptic dopamine (DA) functions (4, 5), a pathophysiological hallmark of psychosis in schizophrenia (6). Currently, the mechanisms that link early developmental stress exposure to adult DA dysfunctions are unclear.
Preclinical research indicates that stress exposure can induce various changes in presynaptic DA functions, indexed by abnormal DA release upon stimulation and alterations in the firing of DA neurons in the ventral tegmental area (VTA) (7, 8). Comparatively, more intense research effort has been given to DA release-related measures, and converging microdialysis evidence indicates that most acute stressors can immediately increase extracellular mesolimbic DA levels for tens of minutes [reviewed in (7)]. This finding is consistent with increased DA neuron spontaneous firing during stress sessions or immediately after (9). On the other hand, the effects of chronic and/or repeated stress on DA release (10, 11) and the underlying DA neuron activation appear to be more persistent, lasting for weeks (12, 13), albeit more heterogeneous and stressor-dependent (14, 15). In particular, chronic stress can sensitize DA release to future challenges, although these results are inconsistent and again show stressor dependence (7). Thus, these lines of evidence predict chronic stress to persistently modulate DA neuron firing. However, previous findings on the physiological effects of stress on DA neurons were obtained mainly from adult male animals, with developmental relevance and potential sex difference in stressor responses being largely unknown. In developing brains, although sporadic evidence suggests that early life stress during the first 2-3 postnatal weeks can lead to adult DA release cross-sensitization (16, 17) and/or increased VTA DA neuron excitability (18, 19), the ensuing windows such as during adolescence were largely unexplored. Given that in humans late childhood or adolescent trauma most strongly correlates with adult psychosis risk (2), studying how stress during the corresponding windows in rodents [i.e., postnatal day (PD), 21-60 (20)] modulate DA neuron firing could have substantial translational value.
We have reported recently that, in male rats, chronic stress during PD31-40 induced long-term tonic activation of VTA DA neurons (21). Furthermore, this effect appears to be age- and sex-selective, as PD65-74 adult stress in males (22) and adolescent or adult stress in females (23) did not produce sustained VTA firing abnormalities. Adolescence is a protracted period. Thus, based on behavioral and/or sexual development, rodent adolescence can be further divided into separate windows, defined by chronological age and relative temporal relationship to puberty (i.e., pre-adolescence/prepuberty vs. post-adolescence/postpuberty) (24). Despite our recent substantial findings in PD31-40 rats (22, 23), it was unknown whether stress during other temporally defined adolescent windows may affect adult DA neuron firing.
In summary, based on the emerging human and animal literature linking developmental stress to adult DA dysfunctions, we sought to determine the long-term neurophysiological effects of two adolescent stress paradigms on adult VTA DA neuron firing. In particular, we emphasized age- and sex-dependence of stress exposure, and tested whether a 10-day stress paradigm can lead to distinct later-life schizophrenia-relevant electrophysiological alterations in VTA DA neurons and their afferent regulators, specifically the ventral hippocampus (vHipp) and the basolateral amygdala (BLA) (25). Furthermore, we also determined the age- and sex-specific effects of adolescent stress on the maturation of parvalbumin interneurons (PVIs) in these regions, given their functional importance in regulating regional neurophysiological output and clinical relevance for schizophrenia (26).
Materials and Methods
Male and female Sprague-Dawley rats were weaned at PD21 and randomly assigned to prepubertal stress (PreP-S), postpubertal stress (PostP-S), or their corresponding control groups. Similar to our previous studies (21–23), a 10-day combined footshock and restraint stress protocol started respectively at PD21 or PD41 in PreP-S or PostP-S rats, followed by in vivo extracellular recordings of VTA dopamine (together with behavioral tests), vHipp pyramidal, and BLA pyramidal neurons from different subsets of animals. Trajectories of PVI maturation after stress were determined by immunohistochemistry and fluorescent microscopy. Detailed experimental procedures are provided in Supplementary Methods.
Results
PreP-S and PostP-S produced sex-selective effects on adult VTA DA neuron population activity
We first explored the effects of PD21-30 footshock (FS) and restraint (RS) combined stress, which we define as prepubertal stress (PreP-S), on the adult VTA DA neuron activity and behaviors (Figure 1A). A two-way ANOVA detected a significant sex and PreP-S interaction [F(1,31)=12.30, p<0.01] and a significant main effect of PreP-S [F(1,31)=9.852, p<0.01] on DA neuron population activity. Tukey’s post hoc analyses revealed that PreP-S selectively increased DA population activity in males (p<0.01) (Figure 1B). This effect is limited to the medial aspect of the VTA, a subregion projecting predominantly to the reward- and affect-related ventral striatum (27) [two-way repeated measure (RM) ANOVA; sex:stress condition: F(3,93)=7.165, p<0.001; PreP-S:Males, p<0.05] (Figure 1C). At PD65-71, PreP-S-induced behavioral changes were evaluated using elevated plus maze (EPM), novel object recognition (NOR), and amphetamine-induced hyperlocomotion (AIH). PreP-S:Males selectively exhibited less time spent in the open arms [interaction: F(1,38)=6.228, p<0.05; PreP-S:Males, p<0.05] in EPM (Figure 1D) and reduced discrimination index [interaction, F(1,37)=6.884, p<0.01; PreP-S:Males, p<0.01] in NOR (Figure 1F). These sex differences in stress-induced anxiety-like behaviors are broadly consistent with previous studies (28–31), and the observed male-biased cognitive effects of stress are consistent with accumulating evidence from humans [reviewed in (32)]. Although a two-way RM ANOVA on post-amphetamine locomotion revealed significant main effects of time [F(5.031,181.1)=16.51; p<0.0001] and stress:sex conditions [F(3,36)=9.573, p<0.0001] (Figure 1G), Tukey’s post hoc analysis did not reveal any significant stress-related differences within sex.
Figure 1. Effects of prepubertal stress (PreP-S) and postpubertal stress (PostP-S) on adult ventral tegmental area (VTA) dopamine (DA) neuron activity and behaviors in males vs. females.
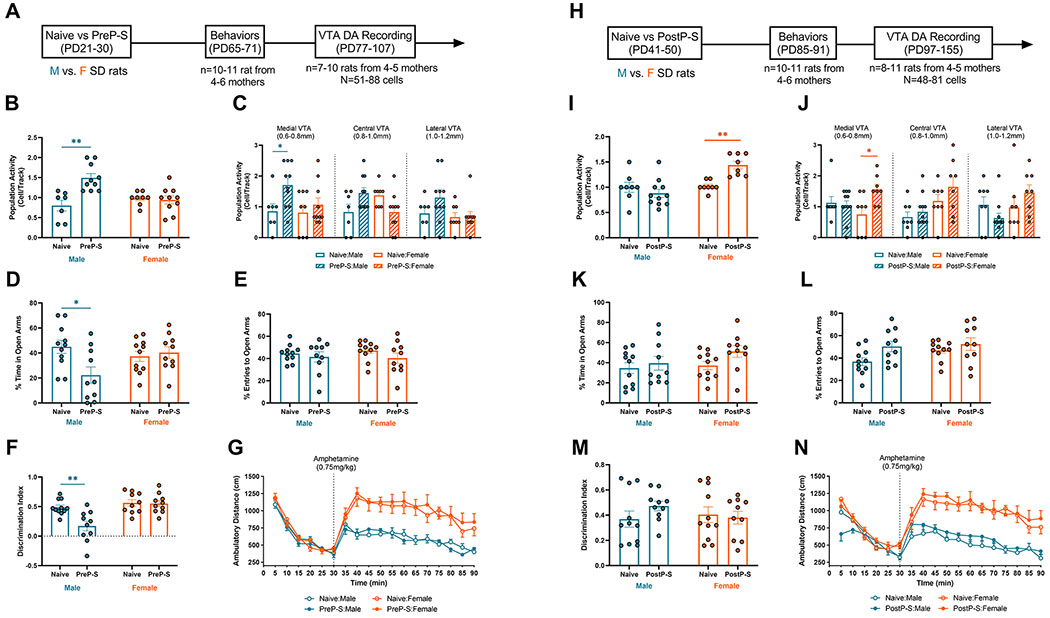
(A) Experimental timeline for PreP-S effects. Male (blue) and female (orange) Sprague-Dawley rats were exposed to repeated footshock and restraint combined stress (see Supplementary Methods for details) during PD21-30. During PD65-71, rats were evaluated using elevated plus-maze (EPM), novel object recognition (NOR) and amphetamine-induced hyperlocomotion (AIH) tests. After at least a week of recovery, rats were subsequently tested for DA neuron activity via in vivo single-unit electrophysiology. (B) PreP-S produced a persistent increase in DA neuron population activity in males (p<0.01) but not in females. (C) The male-specific effect of PreP-S was preferentially in the medial aspect of VTA (i.e., 0.6-0.8 mm from the midline). (D) In addition to DA dysfunction, concomitant anxiety-like behaviors were observed only in PreP-S male rats, indexed by less time spent in the open arms, whereas females displayed resilience to PreP-S in this measure. (E) No significant difference was found in the percentage of open entries. (F) PreP-S:Male rats displayed a reduced discrimination index that reflects memory/cognitive impairments, an effect absent in PreP-S females. (G) In the AIH test, although females showed significantly greater post-amphetamine locomotion (0.75mg/kg; i.p.) than males (main effect: p<0.0001), post hoc Tukey’s tests did not detect any significant differences between stress vs. naïve groups within each sex. This indicates no effects of PreP-S on adult locomotor sensitivity to amphetamine. (H) Experimental timeline for PostP-S effects. (I) PostP-S produced a persistent increase in DA neuron population activity in females (p<0.01) but failed to alter that in males. (J) PostP-S-induced DA population activity increase in females was preferentially in the medial VTA. (K) No behavioral difference was found in EPM and NOR. (I) In AIH, despite significant main effects in the post-amphetamine locomotion, no within-sex PostP-S effect was found. Data are presented as mean ± SEM. *p<0.05; **p<0.01.
Next, we determined the long-term effects of PD41-50 postpubertal stress (PostP-S) (Figure 1H). PostP-S selectively increased DA population activity in females [interaction: F(1,31)=11.47; p<0.01; sex: F(1,31)=13.32, p<0.01; PostP-S:Females, p<0.01] (Figure 1I), an effect again localized to the medial VTA [stress:sex condition: [F(3,93)=6.714, p<0.001; PostP-S:Females, p<0.05] (Figure 1J). Behaviorally, no significant effect was found on EPM or NOR (Figure 1K–M). In AIH, despite significant main effects of time [F(4.326,164.4)=17.93; p<0.0001] and stress:sex conditions [F(3,38)=11.49; p<0.0001], post hoc comparisons did not reveal significant stress-related effects (Figure 1N). Altogether, this dataset indicates marked sex and exposure-time differences in stress-induced adult DA neuron firing alterations.
Neither PreP-S nor PostP-S altered the average firing rate and burst firing of DA neurons in adults (Figure S1). Body weight development was largely unaffected by stress (Table S1), except for a transient body weight decrease measured 10 days after PostP-S. To control for potential confounding factors from behavioral tests and recovery time, we performed DA recordings at 1-2 or 5-6 weeks after stress in another set of behavioral testing-naive rats (Figure S2). Whereas both PreP-S and PostP-S uniformly increased DA neuron population activity in 1-2 weeks (Figure S2A and C), the long-term stress impact was sex- and exposure-age-specific (Figure S2B and D), a pattern similar to the observations from animals previously tested for behaviors. Furthermore, consistent with previous reports (21, 23), we found that prepubertal or postpubertal RS alone did not alter adult DA neuron firing (Figure S3). Interestingly, prepubertal FS alone recapitulated the above described male-selective stress vulnerability to PreP-S (Figure S3). This result contrasted with our previous finding that during PD31-40, only the combined FS and RS are sufficient to alter adult DA neuron firing. Thus, this FS-selective effect could suggest that at younger ages (i.e., PD21-30) male rats are increasingly vulnerable to a broader range of stressor types. Given that prominent sex differences were only observable in the long-term DA neuron responses, in subsequent experiments we focused on the combined stress and adult stress consequences.
Sex and exposure-age differences in adult vHipp pyramidal neuron firing stress responses
VTA DA neuron tonic and phasic firing modes are regulated by distinct afferent circuits. The ventral subiculum (vSub) of the vHipp predominantly controls DA neuron tonic activity (i.e., population activity) via a polysynaptic, disinhibitory circuit that involves the nucleus accumbens (NAc) and the ventral pallidum (VP) (25). Emerging evidence suggests that preweaning early life stress can produce enduring activity changes in hippocampal pyramidal neurons (PN) (33, 34). Consistently, our previous studies show that PD31-40 stress leads to increased PN firing rates in the adult vHipp (22). However, the precise temporal boundaries and sex dependencies of such stress vulnerability in vHipp PNs are unknown. To characterize potential vHipp neuron stress vulnerability, we recorded from PNs in adult PreP-S (age: PD76-124), PostP-S (age: PD92-146), and control rats (age: PD81-131) of both sexes.
Putative vHipp PNs were recorded in adults and identified by electrophysiological signatures as previously described (22) (Figure 2A). No effect of stress on vHipp PN population activity was detected (Figure 2B). Significant group median differences were detected in adult PN firing rate after stress (data non-normally distributed, Kruskal–Wallis test, H=24.80, p<0.001), characterized by a male-selective increase in by PreP-S (p<0.05; Dunn’s test) and a female-selective increase by PostP-S (p<0.01) (Figure 2C). In addition, significant group median differences in bursting firing were also observed (Kruskal-Wallis, H=16.34, p<0.01), with post hoc tests revealing a sex-selective increase only in PreP-S:Males (p<0.05) (Figure 2D). Altogether, this dataset indicates distinct sex- and expose-age-dependent stress effects on adult vHipp PN firing, a vulnerability pattern corresponding highly to VTA DA neurons.
Figure 2. Sex-selective and exposure age-dependent effects of PreP-S vs. PostP-S on adult ventral hippocampus (vHipp) pyramidal neuron activity.
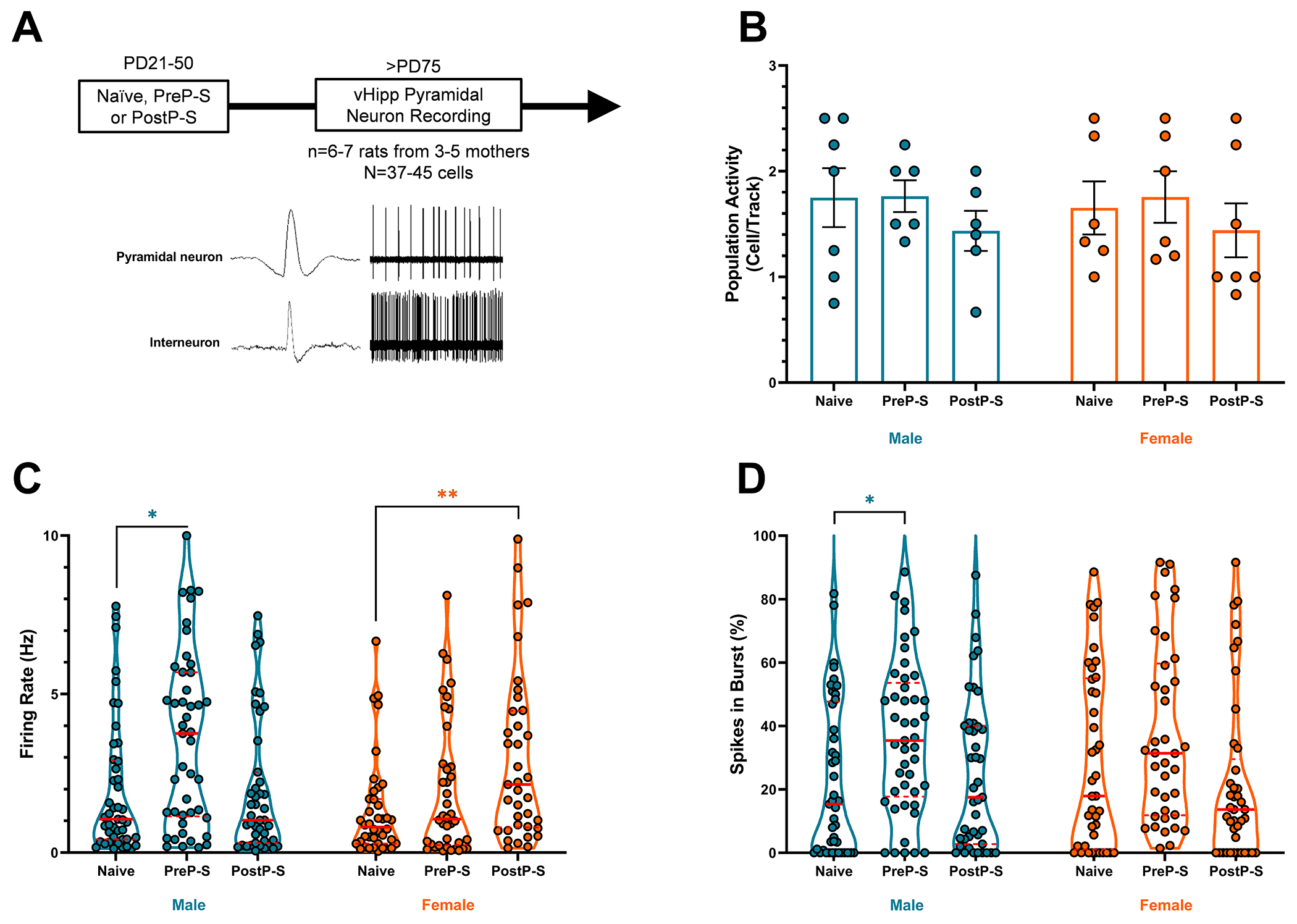
(A) Experimental timeline, representative spike waveform, and 10-sec sample firing of identified vHipp neurons. vHipp pyramidal neurons (PNs) were identified based on low firing rate (<10Hz) and long spike duration (>2.2ms). (B) No change in the number of vHipp putative PNs detected per electrode track. (C) PreP-S selectively increased vHipp PN firing rate in males whereas PostP-S selectively increased PN firing rate in females. (D) PreP-S selectively increased percent of vHipp spikes occurring in bursts (defined by interspike interval <80ms) in males. Violin plots were provided (here and in subsequent figures) to indicate datasets that contain non-normally distributed group data. Accordingly, Kruskal–Wallis analysis of group median differences followed by Dunn’s multiple-comparison test was used. Data are presented as median (solid red line) and quartiles (dashed red line). *p<0.05; **p<0.01.
Sex and exposure-age differences in BLA pyramidal neuron firing response to stress
The BLA sends dense excitatory projections to modulate the vHipp (35), and chronic stress can age-dependently increase BLA PN firing (36) and excitability (37) that persists for days. Notably, aberrant BLA activities can induce deleterious effects on the hippocampus, characterized by interneuron deficits (38) and synaptic plasticity disruptions (39). Moreover, across stress exposure and different exposure time, amygdala alterations from developmental stress tend to precede hippocampal alterations, suggesting that amygdala stress response represents a “temporal prerequisite” for hippocampal alterations [reviewed in (40)]. Thus, we posit that, before the observed adult vHipp PN and VTA DA neuron activation, developmental stress might age- and sex-dependently activate the BLA (26). Prior studies have shown that restraint stress can age-dependently increase BLA PN spontaneous firing rate and excitability (36, 41). Consistently, another study reported that continuous social isolation beginning in adolescence can increase adult BLA excitability (42). However, whether stress in adolescent substages can have persistent effects on the firing of BLA PN and, if so, whether there is sex dependency has not been examined systematically.
To address this, we recorded from putative BLA PNs both 1-2 weeks (short-term effect) and >5 weeks (long-term effect) after stress. BLA PNs were identified based on reported criteria (36, 43) (Figure 3A and B). For the short-term effect of PreP-S, a significant group median difference was detected (Kruskal–Wallis test, H=13.33, p<0.01), and Dunn’s tests revealed a selective increase in PreP-S:Males (p<0.01) (Figure 3C). This effect was persistent, as a significant group median difference was also selectively observed in PreP-S:Males at >5 weeks after stress (Kruskal–Wallis test, H=24.69, p<0.0001; Dunn’s test, PreP-S:Males, p<0.0001) (Figure 3D). In contrast, no stress-related effect was observed in the short-term response to PostP-S. For the long-term PostP-S effect, although significant group median differences were observed (Kruskal–Wallis test, H=13.64, p<0.01), Dunn’s analysis did not reveal any stress-related change. Instead, only between-sex differences were found in PostP-S:Males vs. Naïve:Females (p<0.05) and PostP-S:Males vs. PostP-S:Females (p<0.05) comparisons. Altogether, this dataset indicates that while PreP-S chronically activated the BLA specifically in males, PostP-S failed to affect BLA PN firing in either sex.
Figure 3. Sex differences in amygdala neuron firing rate following stress.
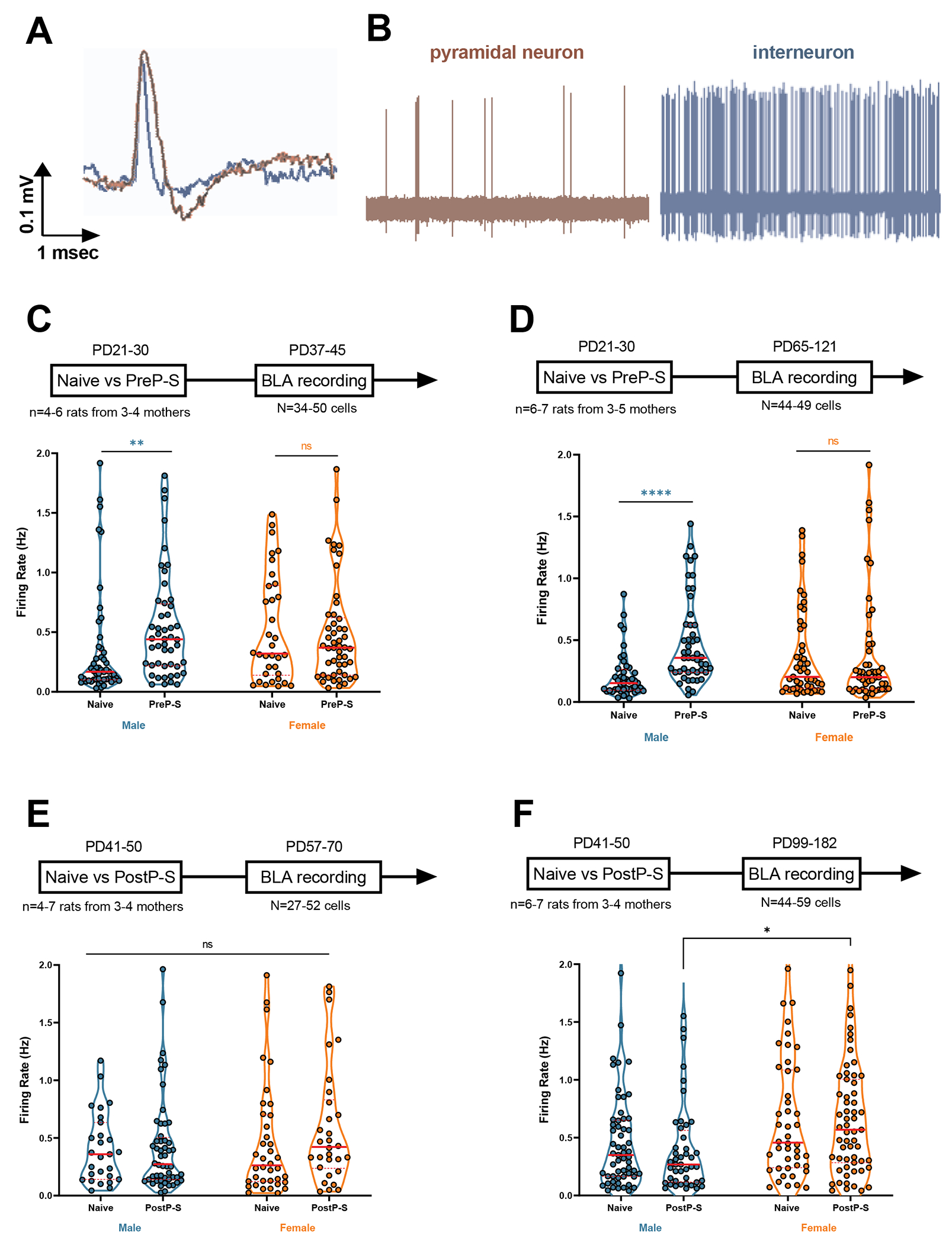
(A) and (B) Representative spike waveform and 10-second sample of spontaneous activity of basolateral amygdala (BLA) neurons. BLA pyramidal neurons were defined by spike duration >2.2ms and mean firing rate <2Hz. (C) PreP-S-induced BLA pyramidal neuron hyperactivity (i.e., increased firing rate), measured 1-2 weeks post-stress, was observed only in males, whereas females showed resilience to these short-term effects. (D) Male-specific, PreP-S-induced BLA hyperactivity persisted into adulthood; no effect was observed in adult females. (E) No effect of sex or PostP-S was found in the 1-2 week responses. (F) No within-sex PostP-S effect was found in the 5-6 week responses, although females showed higher stress activation compared to stressed males. Firing rate data were analyzed by Kruskal-Wallis test followed by Dunn’s post hoc multiple comparisons. Data are presented as median (solid red line) and interquartile range (dashed red line). **p<0.01; ****p<0.0001.
Sex- and exposure age-dependent stress effects on plPFC-BLA inhibitory control
The dorsomedial PFC exerts “top-down” control of stress-induced BLA activation (44, 45). This modulatory action of PFC can be studied by spike plasticity recordings. We have demonstrated previously that for PFC-evoked BLA PN extracellular activity, high-frequency electric stimulation (HFS) in the prelimbic PFC (plPFC) can lead to spike long-term depression (LTD) (46). Such inhibitory plPFC-BLA regulation has been independently demonstrated by chemogenetic activation (47) and attributed to a feedforward inhibition mechanism driven by BLA interneurons (44). Functionally, PFC-BLA control is thought to be adaptive to cope with the stress-induced BLA hyperactivity (46), a mechanism proposed to underlie cognitive regulation of emotion (48). Notably, PFC inhibitory modulation over BLA neurons is prone to severe stress (46, 47) and is not developed at adolescence (PD39) (48). Altogether, these previous findings predict that PFC-BLA inhibitory regulation may have heightened responsivity to stress during early life (e.g., adolescence) and is potentially age- and sex-dependently regulated (49). To test this, we next evaluated the longitudinal impacts of PreP-S and PostP-S stress on the plPFC-BLA inhibitory control pathway.
The impact of plPFC HFS on BLA PN evoked spiking was assessed at <3 weeks or >6 weeks after stress. HFS-induced spike plasticity was recorded as described previously (46) (Figure 4A–B). We did not observe any difference in the baseline evoked firing properties (Table S2). For the short-term PreP-S effect (tested at PD37-51), in the time course response a two-way RM ANOVA in males revealed significant effects of PreP-S [F(1,13)=7.283, p<0.05] and PreP-S and time interaction [F(7,91)=2.269, p<0.05]. Bonferroni’s post hoc analyses indicated significant spike depression at 5 (p<0.01) and 10 minutes (p<0.05) post-HFS, whereas these effects were absent in PreP-S:Females (Figure 4C). Notably, the negative main effect of time suggested an overall immature circuit response to HFS at this age (PD37-51), consistent with previous findings (46) and with the minimal response (<20%) observed in mean post-HFS spike probability alteration (Figure 4D). This male-specific emergence in HFS-induced spike depression was similar to the adult-like response, which we tentatively labeled as precocious maturation of plasticity. For the long-term effects of PreP-S, in the time course response we found significant effects of PreP-S [F(1,9)=28.42, p<0.001], time [F(7,63)=2.574, p<0.05] and interaction [F(7,63)=5.674, p<0.0001] in males, with significant LTD deficits at 10-30 minutes post-HFS. Similar LTD deficits were also observed in females, in which impaired spike depression was observed at 15-30 minutes post-HFS [interaction: F(7,91)=4.773, p<0.001); Figure 4E]. Consistently, significant long-term PreP-S effects were observed in the mean spike response in both sexes [PreP-S: F(1,22)=32.47, p<0.0001)] (Figure 4F).
Figure 4. Sex-specific effects of stress on PFC-BLA inhibitory control.
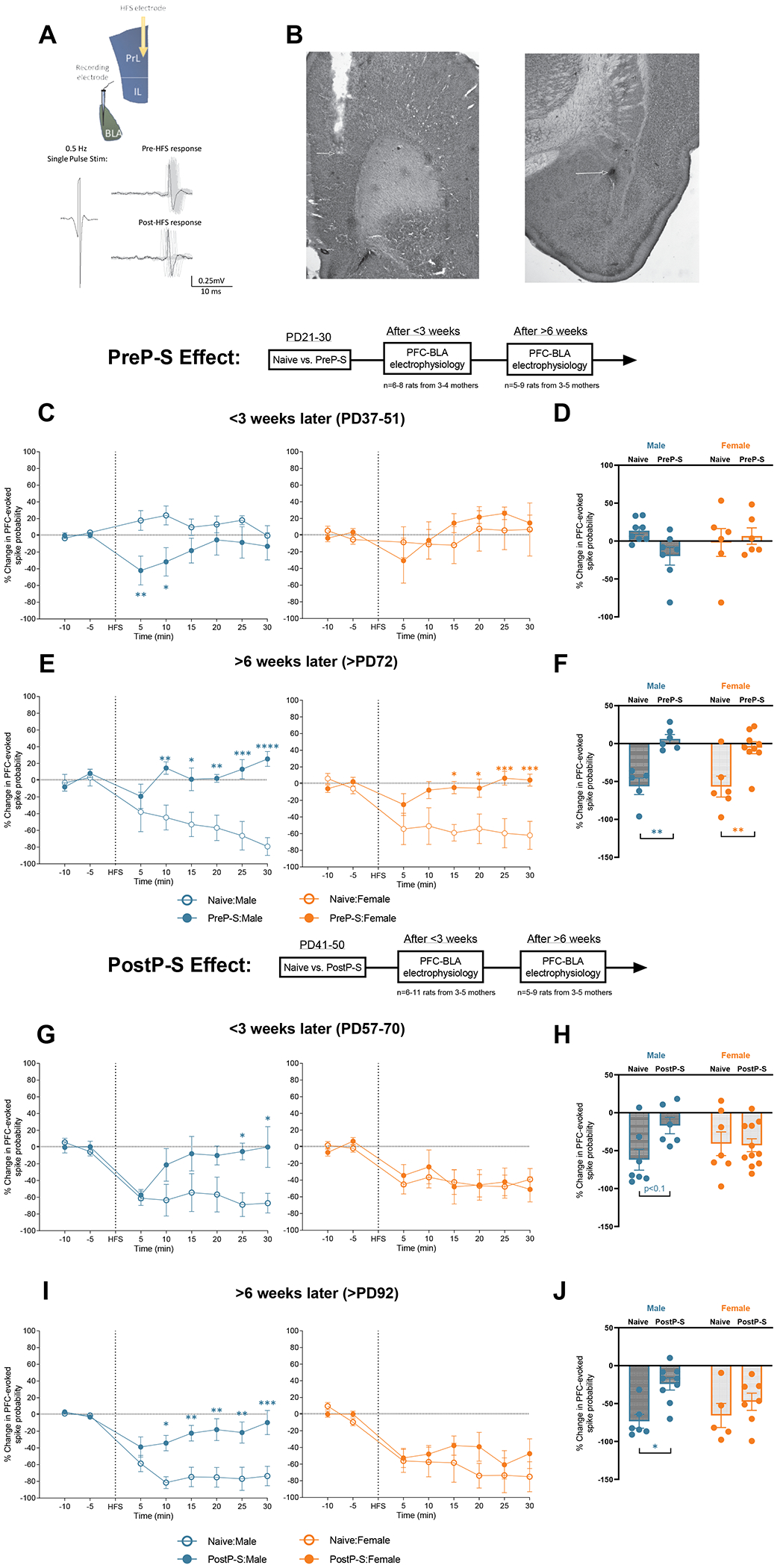
(A, Top) A diagram illustrating the recording paradigm (see Supplementary Methods for details). A stimulating electrode and a recording electrode were implanted in the prelimbic prefrontal cortex (plPFC) and the BLA, respectively. BLA pyramidal neurons responsive to repeated plPFC single-pulse stimulation (0.5 Hz; 0.1-1.5 mA; square wave with 0.25ms duration) were searched and recorded for 10 minutes to obtain baseline measures (spike probability maintained at ~50%). High-frequency stimulation (HFS; 20 Hz; 10s, suprathreshold) was given immediately following the baseline recording, and spike probability response to HFS was recorded for an additional 30 minutes (Post-HFS). (A, Bottom) BLA pyramidal neurons typically respond to plPFC HFS with a long-term depression (LTD) of spiking, i.e., exhibiting fewer action potential responses to PFC stimuli after HFS. (B) Representative photomicrographs of plPFC stimulating electrode and BLA recording electrode placement. (C) In <3 weeks after stress, PreP-S induced a male-specific precocious form of PFC-BLA spike LTD in the first 10 minutes of post-HFS recordings (left), whereas in females PreP-S did not induce an effect (right) (time-course data). (D) No short-term effect of PreP-S was found across the test intervals in the mean Post-HFS spike probability change. (E) At >6 weeks after PreP-S, stressed rats (solid circles) of both sexes failed to show HFS-induced LTD (time-course data). (F) PreP-S induced impaired mean LTD averaged across test intervals in both sexes. (G) In <3 weeks post-stress, PostP-S induced a male-specific LTD impairment at 25-30 minutes post-HFS. (H) When averaged across the entire Post-HFS testing period, there was only a trend towards LTD impairment in males. (I) In >6 weeks post-stress, PostP-S induced a robust LTD deficit in the time course response only in males. (J) In >6 weeks post-stress, averaging across the entire test interval showed significant LTD impairments only in PostP-S males. Time-course data were analyzed by repeated measure two-way ANOVA with Bonferroni tests at each time point. Mean post-HFS response was analyzed by two-way ANOVA with Tukey’s post hoc test. *p<0.05; **p<0.01.
After PostP-S, males displayed LTD deficits in the time course response at <3 weeks [interaction: F(7,77)=2.663, p<0.05; 25 and 30 minutes Post-HFS, p<0.05; Figure 4G] and >6 weeks [interaction: F(7,91)=4.296, p<0.001; 10–30 minutes Post-HFS, p<0.05; Figure 4I]. In contrast, no effect was found in females at both testing ages (Figure 4G and I). Consistently, in the mean spike response, despite a trend for a short-term effect [interaction: F(1,27)=3.698, P=0.0651; Figure 4H] , after 6 weeks post-stress, only PostP-S:Males showed PFC-BLA spike LTD deficits [PostP-S: F(1,23)=9.589, p<0.01; PostP-S:Males>Naïve:Males, p<0.05; Figure 4J].
In conclusion, these findings suggest distinct stress vulnerability at the level of the plPFC-BLA inhibitory control. Whereas females are vulnerable to the long-term PreP-S effects, males displayed pronounced vulnerability to both stress paradigms. Moreover, after PreP-S males displayed an accelerated plPFC-BLA maturation, a putative concomitant adaptive response to BLA hyperactivity (Figure 3C), albeit insufficient.
Age- and sex-dependent stress vulnerability of parvalbumin interneurons in the BLA and the vHipp
Throughout extended adolescence, corticolimbic PVIs adopt perineuronal nets (PNNs), which are extracellular matrix structures that enhance GABAergic inhibition and support fast-spiking ability (50). Developmental stress can induce diverse PVI cellular alterations, including decreased PV or PNN expression and their co-localization (51). Given the critical role of PVIs in regulating PN firing (52), PVI impairments could underlie the observed PN hyperactivity in both the BLA and the vHipp. To explore this, we next determined the longitudinal impact of stress on PVIs, emphasizing co-localization with PNNs.
PV, PNN cell counts and their co-labelling were assessed by immunohistochemistry and fluorescent microscopy in the basal nucleus of BLA (Figure 5A–B). Substantial PVI impairments were found mostly in males after PreP-S (Figure 5C–D). Thus, at PD75 PreP-S:Males displayed reduced PV+ (Welch t-test; t(8.863)=4.349, p<0.01), PNN+ [t(8.913)=2.555, p<0.05], and co-labeled cells counts [t(5.634)=2.934, p<0.05]. Reduced co-labelled cell count was also detected at PD41, suggesting persistent deficits. All these effects were absent in females. Instead, PreP-S:Females displayed transient reductions of PNN+ [t(7.309)=2.819, p<0.01] and PV/PNN co-labeled cells [t(9.997)=2.495, p<0.05] at PD31. Notably, these effects were exposure age-dependent, as PostP-S failed to change these markers in either sex (Figure 5E–F).
Figure 5. Sex differences in the longitudinal impact of PreP-S and PostP-S on BLA parvalbumin (PV) interneurons.
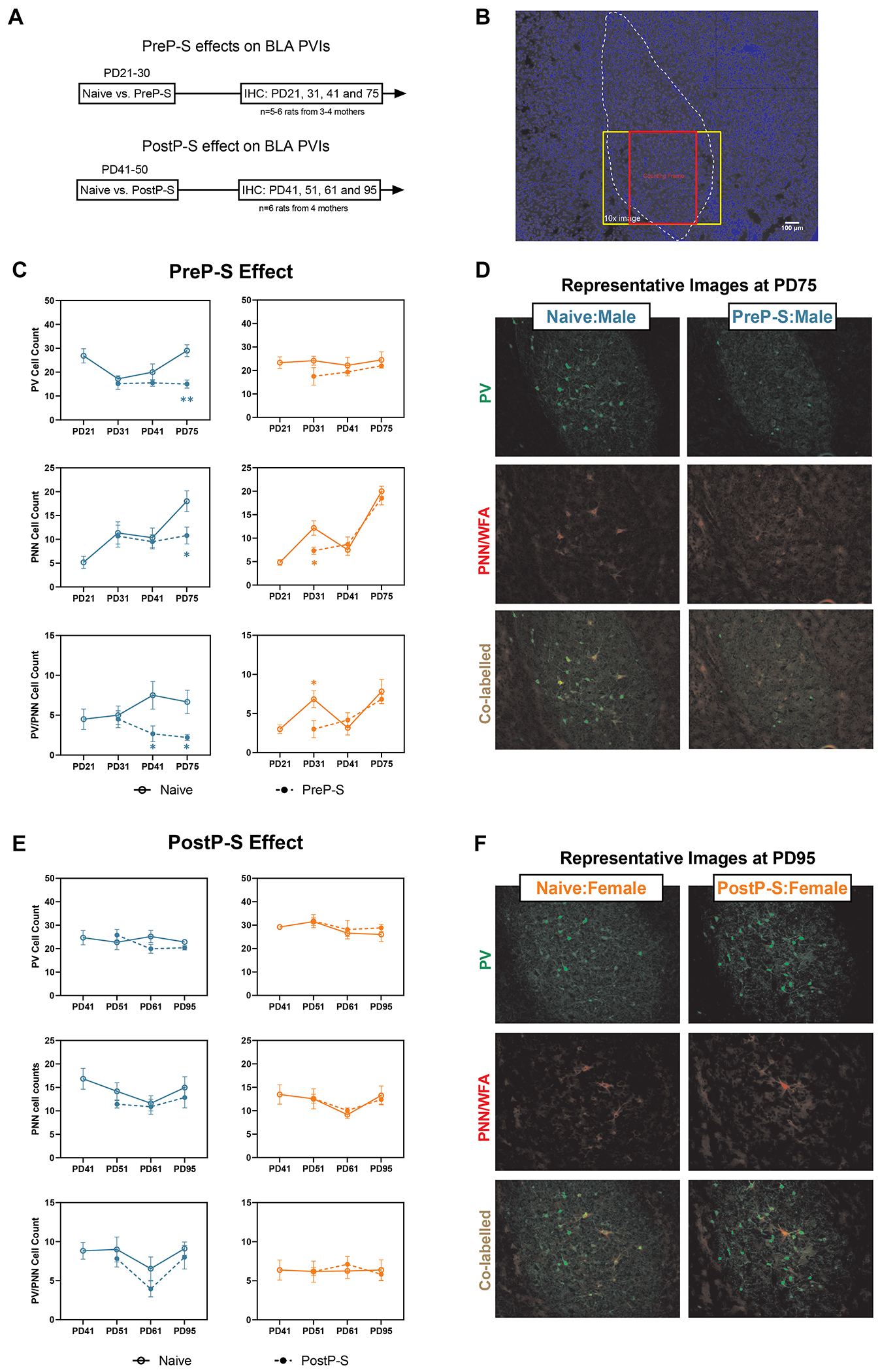
(A) After stress exposure, immunohistochemistry and fluorescent microscopy were performed in the BLA at various post-stress time points. Specifically, the BLA was sampled before the stress paradigms (in naïve rats) and subsequently at 1, 10, or 45 days after stress termination. (B) A 4x image with DAPI staining showing BLA anatomical borders and the counting frame. Cell counts of PV+ neurons, perineuronal-net+ (PNN+, labelled by WFA) neurons, and their co-labelling were measured in a 550 x 690μm counting frame on 10x images (909 × 692μm). (C) PreP-S-induced changes were detected primarily in males, including delayed reductions of PV+ and PNN+ cell counts only at PD75 and chronically reduced PV+/PNN+ co-labeled cell counts at PD41 and PD75. In the female BLA, PreP-S resulted in immediate yet transient reductions of PNN+ and PV+/PNN+ co-labeled cells only at PD31 (i.e., one day after stress), suggesting potential recovery at later time points. (D) Representative 10x images from PreP-S:Males at PD75. (E) PostP-S did not impair BLA PV interneurons in either sex. (F) Representative images from PostP-S:Females at PD95. Data are presented as mean ± SEM. Unpaired t-test with Welch correction was conducted at each post-stress time point. *p<0.05, **p<0.01, indicating stress-related changes.
We next determined vHipp PVI impairment (Figure 6A–B). In males, PreP-S reduced PV+ [t(9.120)=4.939, p<0.001] and co-labelled cell counts [t(5.832)=3.773, p<0.01] only at PD95. In contrast, PreP-S:Females were largely unaffected, except for a transient decrease of PV+ cell count at PD41 [t(9.561)=2.291, p<0.05] (Figure 6C–D). A reversed sex dependence was found after PostP-S. Thus, only females at PD95 displayed significant reductions of PV+ [t(9.573)=6.918, p<0.001] and co-labeled cell counts [t(10.00)=3.845, p<0.01] (Figure 6E–F).
Figure 6. Sex differences in the longitudinal impact of stress on vHipp PV interneurons.
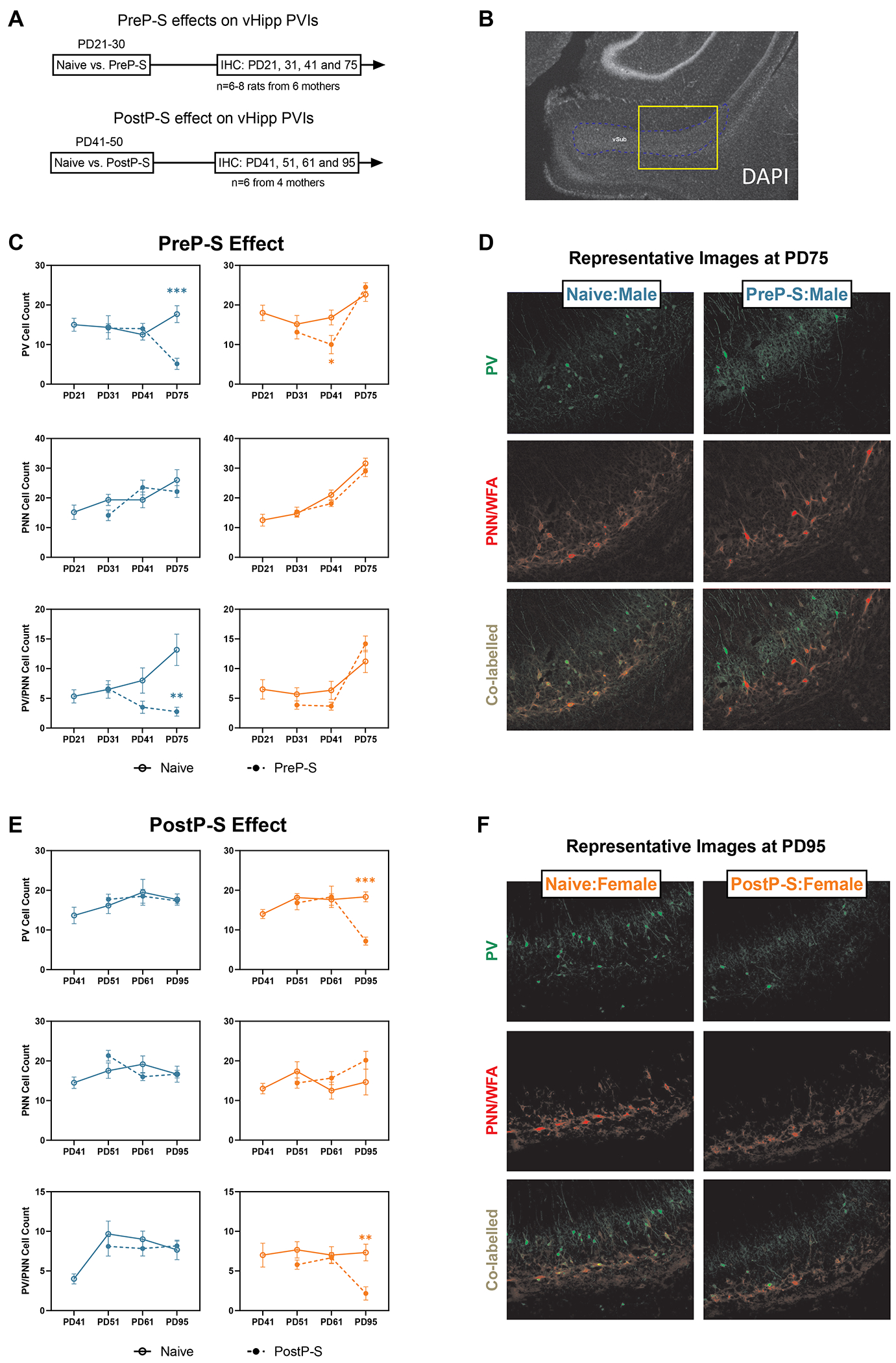
(A) vHipp was sampled at different time points after PreP-S and PostP-S to examine potential stress-induced PV interneuron (PVI) impairments. (B) A 4x image with DAPI staining showing the relative location of the 10x imaging site targeting the proximal portion of the ventral subiculum of the vHipp. (C) PreP-S induced male-dominant PVI impairments in the vHipp. The PreP-S effect in males was delayed, as stress-related reductions of PV+ and PV+/PNN+ co-labeled cell counts were only observed at PD75 (i.e., 45 days after stress). Female vHipp responded to stress with a minor PVI deficit, characterized by a PV reduction at PD41 (i.e., 10 days after stress), which did not persist into PD75. (D) Representative images showing PreP-S effect in males on PVIs measured at PD75. (E) In PostP-S females, stress-related reductions of PV and PV/PNN co-labeled cell count were only observed at PD95 (i.e., 45 days after stress), whereas in males, PostP-S did not induce any change in any examined marker. Data are presented as mean ± SEM. Unpaired t-test with Welch correction was conducted at each post-stress time point. **p<0.01; ***p<0.001, indicating stress-related changes.
Collectively, BLA and vHipp PVIs are sex- and age-dependently disrupted by developmental stress. While vHipp PVI impairments are strongly correlated with sex- and exposure-age-dependent heightened PN activity (Figure 2), in the BLA stress-induced PVI impairments appeared to be male-dominant.
Discussion
Summary of findings
This is the first systematic study to examine sex differences in the electrophysiological effects of two adolescent stress paradigms on VTA DA neurons and related regulatory regions. Our main findings are that adolescent stress during age- and sex-selective sensitive windows is sufficient to persistently increase adult DA system activities, potentially by differentially modulating activity states and PVI maturation of the vHipp and the BLA. See Supplementary Table S3 for a summary of findings.
Age group and stress paradigm
Rodent adolescence is a prolonged period containing several substages (53). In this study, we grouped rats according to chronological age and defined stressors in PD21-30 and PD41-50 as “prepubertal stress” and “postpubertal stress”, respectively. We did not individually control for pubertal onset age, as techniques that reliably measure this (e.g., repeated vaginal lavage) are stressful (54), which would confound our analyses. Notably, our operational definition of “prepuberty” is consistent with several previous reports [for example (55, 56)], and the designation of PD41-50 as “postpuberty” was based on documented average pubertal onset age at PD36 in Sprague-Dawley female rats and at approximately six weeks of age in males (57). Importantly, we selected these temporally distant windows to investigate the exposure-age-dependent effects of adolescent stress. PD21-30 and PD41-50 in rodents correspond approximately to childhood and adolescence in humans (20, 58, 59), during which stress experience age-dependently associates with adult psychopathology risks (e.g., schizophrenia (2, 3)) (60) and with differential structural/functional stress consequences [e.g., (61)]. However, in preclinical research neurophysiological stress impacts during distinct childhood and adolescent windows have been seldom compared systematically. Moreover, we purposefully selected PD21-30 and PD41-50 to complement our recent studies using the same stress protocol during PD31-40 separately in males and females (21, 23), with the goal to generalize conclusions within broader contexts. Noteworthy, recovery duration (i.e., stress recency) determines stress outcomes (62). Thus, to ensure comparable recovery timelines, the age of our adult recordings was different between experiments for PreP-S and for PostP-S, although we intentionally created some overlap. Given that VTA, vHipp, and BLA activities appear overall stable in young and mature adult rats, we do not believe that a 20-day delay in the testing age of PostP-S rats could drive the observed activity differences across multiple regions.
Sensitive periods for stress are defined by regions and outcome measures (63), and here we defined stress vulnerability based on electrophysiological consequences in VTA DA system and its afferent regulators. We used 10-day FS+RS combined stress as reported previously (21), as its duration is suitable for being placed within rodent adolescent substages, allowing us to infer developmental stage specificity. Notably, current literature on adolescent stress impact on adult VTA DA neuron activity has been almost exclusively conducted in social stress models (8, 58), despite in humans childhood adversity with no apparent social element has also been associated with risks for DA-related psychopathologies (64). Childhood adversities are often repeated and involve complex experiences. Considering all these factors, we selected a non-social and heterotypic stress paradigm to study exposure age and sex-dependent effects.
Stress-induced DA neuron hyperactivity: relevance to previous studies
Our VTA recordings showed that stress during chronologically discrete, sex-dependent adolescent sensitive periods induced long-term DA neuron tonic activation in adults, without affecting phasic firing parameters (Figure 1 and S1). Tonic spontaneous DA neuron firing is a physiological prerequisite for phasic, behaviorally relevant DA release (25). Thus, this study complemented the vast microdialysis literature on stress-induced long-term increase in DA release [reviewed in (8, 65)]. Interestingly, although the exact vulnerability windows shifted between sexes, PreP-S and PostP-S appeared to converge to induce persistent VTA hyperdopaminergic activities (Figure 1 and S2). This markedly contrasts with the adult stress responses we recently reported, which are characterized by transient VTA hypodopaminergic activities (22, 23). Furthermore, the chronicity of stress effects on tonic dopaminergic activities itself is a novel finding. This is because, although studies using social defeat stress have shown that that DA neuron phasic activation can last 28 days (12, 13), to our knowledge, no other works have used long post-stress delay or comprehensively assessed both the tonic and the phasic DA neuron activity.
Relevance to schizophrenia
Correlating with hyperdopaminergic states, PreP-S male and PostP-S female rats also exhibited vHipp hyperactivity and PVI impairments. These results are consistent with schizophrenia clinical observations (66), a neurodevelopmental model of schizophrenia risks (i.e., the MAM model (26, 66)), and our previous findings with PD31-40 stress (22). In particular, the stress-induced increase in VTA DA neuron spontaneous firing is consistent with human molecular imaging evidence showing elevated presynaptic striatal DA functions in schizophrenia patients (67), which implicates increased active DA terminals driven by more active neurons. The increased vHipp and BLA PN firing rates are consistent with the increased cerebral perfusion in corresponding regions of schizophrenia patients (68, 69). Therefore, although the exact sensitive period timing is sex-dependent, upon adolescent stress exposure the adult stress outcomes closely recapitulate neurophysiological disruptions observed in schizophrenia. Intriguingly, our previous studies show that adult (PD65-74) rats of both sexes are resilient to stress-induced persistent DA neuron firing alterations (22, 23). Thus, the current study extended our previous findings and further indicated that adult DA neuron activity states are dynamically modulated by adolescent stress history. Notably, PD21-30 male rats were also susceptible to singular FS-induced hyperdopaminergic states (Figure S3), a phenotype reminiscent of early PFC-lesioned rats (21). This may indicate a relatively immature PFC in prepubertal males, which could contribute to increased vulnerability to a broader range of stressor types.
Potential role of vHipp PVIs in age-dependent stress impacts
The vHipp regulates VTA DA neuron tonic activity via a well-established vSub-NAc-VP polysynaptic circuit (25). Thus, the observed vHipp firing increase after sex- and age-selective stress exposure is upstream to stress-induced VTA hyperdopaminergic activities. The observed vHipp PVI impairments are likely an important mediator of the vHipp/VTA pathophysiology (26), as PVIs are a major inhibition source and are highly stress-sensitive (51). Notably, direct vHipp PV knockdown upregulates vHipp PN output and VTA DA neuron population activity (70), similar to the stress effects in the current study. Thus, we posit that the stress-induced vHipp physiological alterations are likely secondary to PVI impairments.
The developmental trajectories of vHipp PVIs could potentially explain stress-sensitive period timing differences. Developmental stress can impair PVIs via activating oxidative stress-related processes (71–73). During early adolescence in rodents, anti-oxidative PNNs are not fully developed (74, 75). Thus, vHipp PVIs might be particularly vulnerable during early adolescence, as demonstrated by stress during PD21-30 (this study) or PD31-40 (22), as well as several other studies [reviewed in (51)]. The present data in male rats demonstrated that only PreP-S was sufficient to reduce adult PV, PNN, and co-labeled cell counts in vHipp, whereas PostP-S failed to do so (Figure 6). PVIs lacking PNNs are incapable of fast spiking inhibitory activities (50). Moreover, enzymatically dissolving vHipp PNNs can activate vHipp PNs and increase downstream VTA DA population activities (76). Considering these previous findings, our results suggest that male PreP-S impairs PVIs by disrupting their PNN association, which could lead to loss of PVI-mediated inhibition and the observed vHipp hyperactivity. Notably, the above-described line of evidence has been almost exclusively established in males. In females, emerging studies (albeit in other brain regions) suggest that PV and PNNs develop following sexually divergent trajectories (77–80), which is proposed to contribute to differential PVI stress vulnerability (81). Whether adolescent vHipp PV/PNN developments are sexually divergent is unknown. Nonetheless, our data clearly indicated that PostP-S females at PD95 showed PV/PNN pathologies similar to those of PreP-S males (Figure 6). Thus, it is possible that instead of a continuous increase in PV/PNN expression as observed in males (82), female vHipp PVIs might develop following a more dynamic trajectory, contributing to the selective postpubertal vulnerability.
Potential involvement of the BLA
Chronic stress can activate BLA PNs (83). Under normal conditions, BLA PNs are potently inhibited by local PVIs, exhibiting low spontaneous firing rates (52, 84). This activity state could be disrupted by BLA PVI impairments. Importantly, the BLA sends dense glutamatergic projections to the vHipp (35), and pharmacological BLA activation (e.g., via picrotoxin infusion) disrupts schizophrenia-related hippocampal GABAergic neurotransmission (68), including reduced PV expression (38). Thus, early BLA GABAergic deficits are proposed to drive hippocampal PVI pathology and VTA hyperactivity in schizophrenia (25, 68). In our male BLA recordings, high spontaneous firing occured within 1-2 weeks after PreP-S (i.e., PD37-45; Figure 3C), which preceded the adult emergence of vHipp PVI impairments (PD75; Figure 6C). Therefore, these male observations supported that vHipp PVI deficits might be consequences of BLA hyperactivity (38, 85). The BLA directly projects to limbic hippocampal PNs but also presumably to PVIs (86, 87). Whether the observed BLA activation directly confers excitotoxicity to PVIs, or by PN activation, is worth future investigations using projection-specific approaches. Since our data found concurrent BLA PVIs impairments (reduced PV/PNN co-labelling at PD41; Figure 5C) and BLA hyperactivity (PD37-45; Figure 3C), it is plausible that persistent BLA PVI disruption drives BLA hyperactivity to further lead to impairments in vHipp PV networks. Alternatively, a direct, long-lasting increase in BLA PN excitability is also possible, which would be consistent with previous findings with continuous social isolation stress beginning in adolescence (42) and with emerging findings that stress can specifically increase glutamatergic neurotransmission in BLA neurons projecting to vHipp (88). Only in PreP-S males, BLA hyperactivity persisted into adulthood, which might directly contribute to the vHipp hyperactivity via monosynaptic projections and in turn cause VTA hyperdopaminergic activity. Whether such male-selective adult BLA hyperactivity is an upstream therapeutic target or if some of the observed changes can mediate the downstream stress sequelae warrants further investigation.
We also tested the effects of PreP-S and PostP-S on plPFC-BLA spike plasticity. Previous studies indicated that mPFC inhibitory control over BLA PNs is immature during rat adolescence (48). In this study, within 3 weeks after PreP-S, in males HFS can already induce an adult-like spike LTD (Figure 4C and D). This male-selective BLA accelerated maturation after PreP-S might emerge as an adaptive process to ameliorate BLA PN hyperactivity. Interestingly, PreP-S did not trigger this adaptive process in females, which is expected due to normal firing of the BLA (Figure 3). In adulthood, while naïve animals of both sexes achieved mature PFC-BLA LTD, both male and female PreP-S rats showed LTD deficits. These adult changes suggest that long after PreP-S, the PFC is still incapable of gating BLA activity, consistent with the observed long-lasting BLA hyperactivity in males (Figure 3C). Intriguingly, despite PFC-BLA control being disrupted (Figure 4C), adult PreP-S females did not display either BLA hyperactivity or PVI deficits. Thus, it appears that PFC-BLA dysfunctional connectivity alone might represent the inability of the PFC to regulate excessive BLA firing rather than a direct cause. On the other hand, we observed a male-selective PostP-S effect on PFC-BLA control within 3 weeks (PD57-70; i.e., early adulthood) and after 6 weeks after stress, with no effect observed in females. Collectively, these results indicated male-dominant vulnerability in PFC-BLA inhibitory control circuit regardless of stress exposure age. The behavioral implications of this sex difference remained to be thoroughly determined by future studies, which putatively pertains to the processing of fear stimuli (46).
Potential mediators of sex differences
The observed resilience of female VTA DA neurons to PreP-S (Figure 1B) extended our previous findings with PD31-40 stress (23). These findings together suggest a sudden emergence of stress vulnerability in the female VTA DA system during late adolescence (i.e., PD41-50). Thus, the temporal profile of female stress vulnerability is distinct from that of males, which encompasses PD21-30 (this study) and PD31-40 (22). Speculatively, the observed female prepubertal resilience after chronic stress is potentially due to more mature neuroendocrine stress responses at this age [characterized by more rapid negative feedback (89)], whereas in males stress hormone (e.g. corticosterone) feedback modulation does not reach adult capacity until PD30-40 (90). Future studies are necessary to determine if the observed electrophysiological stress consequences are linked to sex-biased neuroendocrine responses after stress (91).
Conclusion and translational relevance
Using a rat model, we examined adolescent sensitive periods for early life stress to induce adult hyperdopaminergic states. While males are vulnerable to stress during the early, prepubertal period of adolescence, females are more vulnerable in postpuberty. During these sensitive periods measured by DA system responses, age- and sex-dependent stress exposure also elicits vHipp PN hyperactivity and PVI impairments, which with the DA system dysfunctions collectively recapitulate schizophrenia-related circuit disruptions. Despite the overall sex-convergent activating effects on VTA DA neurons and vHipp PNs, stress-induced changes in BLA spontaneous activity, PFC-BLA functional connectivity, and BLA PVIs structural integrity are primarily observed in PreP-S males. In females, these stress outcome measures were largely unaffected by either stress paradigm. Altogether, these findings are consistent with the emerging clinical evidence that childhood and adolescence may represent distinct sensitive periods for stress to specifically increase psychosis risks (2), and further pinpoint vHipp and VTA DA system hyperactivities as potential neurobiological mediators. In humans, histories of childhood adversity are associated with elevated DA synthesis capacity (4), sensitized DA release to acute amphetamine/stressor challenges (92, 93), and heightened midbrain activation in response to emotional stimuli (94). These observations are consistent with the increased VTA DA population activity observed in the present study, supporting the hypothesis that environmental stressors sensitize the dopamine system which in turn leads to development of psychosis (6, 95). Furthermore, early adversity/trauma can induce differential impairments in human limbic structures based on exposure age and sex (40). Nevertheless, due to methodological and ethical challenges, how these abnormalities interact temporally and/or whether they are due to differential vulnerability in humans are unknown. Thus, the present study offered several novel testable hypotheses for clinical research. Noteworthy, individuals at clinical high risks for schizophrenia tend to experience trauma more likely before early adolescence (before age 12 years) (96), which approximately corresponds to the prepuberty in this study. Thus, the observed male-selective PreP-S effects might explain the well-known male-biased risks for more severe schizophrenia symptoms and earlier psychosis onset (97, 98). Although relatively protected from DA hyperactivity induced by earlier stress, females are vulnerable to stress post-pubertally (this study) and in adulthood (23), which would be consistent with the human observation that adult females are at increased susceptibility to affect-related psychopathologies (99). Therefore, these findings may have strong implications for the etiology and pathophysiology of stress-related developmental disorders, with a particular emphasis on schizophrenia. Beyond this, the discovered sex-specific stress effects on DA neuron firing might provide a framework for understanding other sex-biased, DA-related psychopathologies (60, 63, 100).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant MH57440 (to AAG). We thank Niki MacMurdo and Christy Smolak for their technical assistance. We also thank Madhura Leninakan, Ruth Haider, Junhao Xu, and Dhruti Raghuraman for their assistance in data analysis.
Conflict of Interest
A.A.G received funds from the following organizations: Lundbeck, Pfizer, Otsuka, Asubio, Autofony, Janssen, Alkermes, SynAgile, Merck, and Newron. X.Z reports no conflicts of interest.
Footnotes
Supplementary information is available at MP’s website
References
- 1.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective-and cross-sectional cohort studies. Schizophrenia bulletin. 2012;38(4):661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croft J, Heron J, Teufel C, Cannon M, Wolke D, Thompson A, et al. Association of Trauma Type, Age of Exposure, and Frequency in Childhood and Adolescence With Psychotic Experiences in Early Adulthood. JAMA Psychiatry. 2019;76(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alameda L, Golay P, Baumann PS, Ferrari C, Do KQ, Conus P. Age at the time of exposure to trauma modulates the psychopathological profile in patients with early psychosis. J Clin Psychiatry. 2016;77(5):e612–8. [DOI] [PubMed] [Google Scholar]
- 4.Egerton A, Valmaggia LR, Howes OD, Day F, Chaddock CA, Allen P, et al. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr Res. 2016;176(2-3):171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahoun T, Nour MM, McCutcheon RA, Adams RA, Bloomfield MAP, Howes OD. The relationship between childhood trauma, dopamine release and dexamphetamine-induced positive psychotic symptoms: a [(11)C]-(+)-PHNO PET study. Transl Psychiatry. 2019;9(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. The Lancet. 2014;383(9929):1677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl). 2016;233(2):163–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douma EH, de Kloet ER. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci Biobehav Rev. 2020;108:48–77. [DOI] [PubMed] [Google Scholar]
- 9.Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31(11):4280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577(2):194–9. [DOI] [PubMed] [Google Scholar]
- 11.Miczek KA, Nikulina EM, Shimamoto A, Covington HE, 3rd. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31(27):9848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344(6181):313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao JL, Covington HE 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30(49):16453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CH, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry. 2014;76(3):223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jezierski G, Zehle S, Bock J, Braun K, Gruss M. Early stress and chronic methylphenidate cross-sensitize dopaminergic responses in the adolescent medial prefrontal cortex and nucleus accumbens. J Neurochem. 2007;103(6):2234–44. [DOI] [PubMed] [Google Scholar]
- 17.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19(7):1863–74. [DOI] [PubMed] [Google Scholar]
- 18.Spyrka J, Gugula A, Rak A, Tylko G, Hess G, Blasiak A. Early life stress-induced alterations in the activity and morphology of ventral tegmental area neurons in female rats. Neurobiol Stress. 2020;13:100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh WC, Rodriguez G, Asede D, Jung K, Hwang IW, Ogelman R, et al. Dysregulation of the mesoprefrontal dopamine circuit mediates an early-life stress-induced synaptic imbalance in the prefrontal cortex. Cell Rep. 2021;35(5):109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheth C, McGlade E, Yurgelun-Todd D. Chronic Stress in Adolescents and Its Neurobiological and Psychopathological Consequences: An RDoC Perspective. Chronic Stress (Thousand Oaks). 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes FV, Grace AA. Prefrontal Cortex Dysfunction Increases Susceptibility to Schizophrenia-Like Changes Induced by Adolescent Stress Exposure. Schizophr Bull. 2017;43(3):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes FV, Zhu X, Grace AA. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol Psychiatry. 2020;25(12):3278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinger K, Gomes FV, Rincon-Cortes M, Grace AA. Female rats are resistant to the long-lasting neurobehavioral changes induced by adolescent stress exposure. Eur Neuropsychopharmacol. 2019;29(10):1127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Rev. 2016;70:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes FV, Zhu X, Grace AA. Stress during critical periods of development and risk for schizophrenia. Schizophr Res. 2019;213:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760–73. [DOI] [PubMed] [Google Scholar]
- 28.Brydges NM, Jin R, Seckl J, Holmes MC, Drake AJ, Hall J. Juvenile stress enhances anxiety and alters corticosteroid receptor expression in adulthood. Brain Behav. 2014;4(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barna I, Balint E, Baranyi J, Bakos N, Makara GB, Haller J. Gender-specific effect of maternal deprivation on anxiety and corticotropin-releasing hormone mRNA expression in rats. Brain Res Bull. 2003;62(2):85–91. [DOI] [PubMed] [Google Scholar]
- 30.Prusator DK, Greenwood-Van Meerveld B. Gender specific effects of neonatal limited nesting on viscerosomatic sensitivity and anxiety-like behavior in adult rats. Neurogastroenterol Motil. 2015;27(1):72–81. [DOI] [PubMed] [Google Scholar]
- 31.Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KC. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Res. 2006;1097(1):123–32. [DOI] [PubMed] [Google Scholar]
- 32.Bangasser DA, Eck SR, Telenson AM, Salvatore M. Sex differences in stress regulation of arousal and cognition. Physiol Behav. 2018;187:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali I, O’Brien P, Kumar G, Zheng T, Jones NC, Pinault D, et al. Enduring Effects of Early Life Stress on Firing Patterns of Hippocampal and Thalamocortical Neurons in Rats: Implications for Limbic Epilepsy. PLoS One. 2013;8(6):e66962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy S, Kane GA, Katchur NJ, Lara Mejia PS, Obiofuma G, Buschman TJ, et al. Perineuronal Nets, Inhibitory Interneurons, and Anxiety-Related Ventral Hippocampal Neuronal Oscillations Are Altered by Early Life Adversity. Biol Psychiatry. 2019;85(12):1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403(2):229–60. [PubMed] [Google Scholar]
- 36.Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67(12):1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14(7):876–94. [DOI] [PubMed] [Google Scholar]
- 39.Vouimba RM, Richter-Levin G. Physiological dissociation in hippocampal subregions in response to amygdala stimulation. Cereb Cortex. 2005;15(11):1815–21. [DOI] [PubMed] [Google Scholar]
- 40.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hetzel A, Rosenkranz JA. Distinct effects of repeated restraint stress on basolateral amygdala neuronal membrane properties in resilient adolescent and adult rats. Neuropsychopharmacology. 2014;39(9):2114–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress. J Neurosci. 2015;35(26):9730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Grace AA. Prepubertal Environmental Enrichment Prevents Dopamine Dysregulation and Hippocampal Hyperactivity in MAM Schizophrenia Model Rats. Biol Psychiatry. 2021;89(3):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22(1):324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang WH, Zhang JY, Holmes A, Pan BX. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol Psychiatry. 2021;89(9):847–56. [DOI] [PubMed] [Google Scholar]
- 46.Uliana DL, Gomes FV, Grace AA. Stress impacts corticoamygdalar connectivity in an age-dependent manner. Neuropsychopharmacology. 2021;46(4):731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei J, Zhong P, Qin L, Tan T, Yan Z. Chemicogenetic Restoration of the Prefrontal Cortex to Amygdala Pathway Ameliorates Stress-Induced Deficits. Cereb Cortex. 2018;28(6):1980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selleck RA, Zhang W, Samberg HD, Padival M, Rosenkranz JA. Limited prefrontal cortical regulation over the basolateral amygdala in adolescent rats. Sci Rep. 2018;8(1):17171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrara NC, Trask S, Rosenkranz JA. Maturation of amygdala inputs regulate shifts in social and fear behaviors: A substrate for developmental effects of stress. Neurosci Biobehav Rev. 2021;125:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. Journal of Neuroscience. 2017;37(5):1269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perlman G, Tanti A, Mechawar N. Parvalbumin interneuron alterations in stress-related mood disorders: A systematic review. Neurobiol Stress. 2021;15:100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol. 2007;98(5):2956–61. [DOI] [PubMed] [Google Scholar]
- 53.Green MR, Nottrodt RE, Simone JJ, McCormick CM. Glucocorticoid receptor translocation and expression of relevant genes in the hippocampus of adolescent and adult male rats. Psychoneuroendocrinology. 2016;73:32–41. [DOI] [PubMed] [Google Scholar]
- 54.Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci. 2003;42(1):9–18. [PubMed] [Google Scholar]
- 55.Brydges NM, Wood ER, Holmes MC, Hall J. Prepubertal stress and hippocampal function: sex-specific effects. Hippocampus. 2014;24(6):684–92. [DOI] [PubMed] [Google Scholar]
- 56.Tsoory M, Richter-Levin G. Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. International Journal of Neuropsychopharmacology. 2006;9(6):713–28. [DOI] [PubMed] [Google Scholar]
- 57.McCormick CM, Green MR, Simone JJ. Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiol Stress. 2017;6:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watt MJ, Weber MA, Davies SR, Forster GL. Impact of juvenile chronic stress on adult cortico-accumbal function: Implications for cognition and addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):136–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18(10):1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J, Lowen SB, Anderson CM, Ohashi K, Khan A, Teicher MH. Association of Prepubertal and Postpubertal Exposure to Childhood Maltreatment With Adult Amygdala Function. JAMA Psychiatry. 2019;76(8):843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith KE, Pollak SD. Rethinking Concepts and Categories for Understanding the Neurodevelopmental Effects of Childhood Adversity. Perspect Psychol Sci. 2021;16(1):67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hodes GE, Epperson CN. Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol Psychiatry. 2019;86(6):421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morgan C, Gayer-Anderson C. Childhood adversities and psychosis: evidence, challenges, implications. World Psychiatry. 2016;15(2):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holly EN, DeBold JF, Miczek KA. Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology (Berl). 2015;232(24):4469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38(3):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry. 2016;15(1):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology. 2010;35(1):239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Molecular Psychiatry. 2018;23(8):1764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez SM, Boley A, Lodge DJ. Region specific knockdown of Parvalbumin or Somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Transl Psychiatry. 2019;9(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaletel I, Filipovic D, Puskas N. Chronic stress, hippocampus and parvalbumin-positive interneurons: what do we know so far? Rev Neurosci. 2016;27(4):397–409. [DOI] [PubMed] [Google Scholar]
- 72.Rossetti AC, Paladini MS, Colombo M, Gruca P, Lason-Tyburkiewicz M, Tota-Glowczyk K, et al. Chronic Stress Exposure Reduces Parvalbumin Expression in the Rat Hippocampus through an Imbalance of Redox Mechanisms: Restorative Effect of the Antipsychotic Lurasidone. Int J Neuropsychopharmacol. 2018;21(9):883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol Psychiatry. 2013;73(10):1024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada J, Jinno S. Spatio-temporal differences in perineuronal net expression in the mouse hippocampus, with reference to parvalbumin. Neuroscience. 2013;253:368–79. [DOI] [PubMed] [Google Scholar]
- 75.Lensjo KK, Christensen AC, Tennoe S, Fyhn M, Hafting T. Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro. 2017;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry. 2013;3(1):e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drzewiecki CM, Willing J, Juraska JM. Influences of age and pubertal status on number and intensity of perineuronal nets in the rat medial prefrontal cortex. Brain Struct Funct. 2020;225(8):2495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang N, Yan Z, Liu H, Yu M, He Y, Liu H, et al. Hypothalamic Perineuronal Nets Are Regulated by Sex and Dietary Interventions. Front Physiol. 2021;12(1207):714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Griffiths BB, Madden AMK, Edwards KA, Zup SL, Stary CM. Age-dependent sexual dimorphism in hippocampal cornu ammonis-1 perineuronal net expression in rats. Brain Behav. 2019;9(5):e01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu YC, Du X, van den Buuse M, Hill RA. Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: a role for estradiol. Psychoneuroendocrinology. 2014;45:167–78. [DOI] [PubMed] [Google Scholar]
- 81.Guadagno A, Verlezza S, Long H, Wong TP, Walker CD. It Is All in the Right Amygdala: Increased Synaptic Plasticity and Perineuronal Nets in Male, But Not Female, Juvenile Rat Pups after Exposure to Early-Life Stress. J Neurosci. 2020;40(43):8276–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caballero A, Diah KC, Tseng KY. Region-specific upregulation of parvalbumin-, but not calretinin-positive cells in the ventral hippocampus during adolescence. Hippocampus. 2013;23(12):1331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19(24):11027–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berretta S, Munno DW, Benes FM. Amygdalar activation alters the hippocampal GABA system: “partial” modelling for postmortem changes in schizophrenia. J Comp Neurol. 2001;431(2):129–38. [DOI] [PubMed] [Google Scholar]
- 86.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38(1–2):247–89. [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Barbas H. Specificity of Primate Amygdalar Pathways to Hippocampus. J Neurosci. 2018;38(47):10019–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang JY, Liu TH, He Y, Pan HQ, Zhang WH, Yin XP, et al. Chronic Stress Remodels Synapses in an Amygdala Circuit-Specific Manner. Biol Psychiatry. 2019;85(3):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31(2):232–40. [DOI] [PubMed] [Google Scholar]
- 90.Foilb AR, Lui P, Romeo RD. The transformation of hormonal stress responses throughout puberty and adolescence. J Endocrinol. 2011;210(3):391–8. [DOI] [PubMed] [Google Scholar]
- 91.Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology (Berl). 2014;231(12):2417–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. Journal of Neuroscience. 2004;24(11):2825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicol K, Pope M, Romaniuk L, Hall J. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5(5):e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383(9929):1677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loewy RL, Corey S, Amirfathi F, Dabit S, Fulford D, Pearson R, et al. Childhood trauma and clinical high risk for psychosis. Schizophr Res. 2019;205:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565–71. [DOI] [PubMed] [Google Scholar]
- 98.Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender Differences in Schizophrenia and First-Episode Psychosis: A Comprehensive Literature Review. Schizophrenia Research and Treatment. 2012;2012:916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44(1):111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andersen SL. Stress, sensitive periods, and substance abuse. Neurobiol Stress. 2019;10:100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


