Abstract
Introduction.
Genetic profiling has caused an explosion in subclassification of sinonasal malignancies. Distinguishing several of these tumor types by histomorphology alone has been quite challenging, and although pathological classification aims to be as specific as possible, it remains to be seen if this recent move toward tumor speciation bears clinical relevance, most particularly focused on subtyping for the sake of prognostication and treatment. One such recently described cohort, predominantly lumped under the moniker of sinonasal undifferentiated carcinoma (SNUC) is IDH2-mutated sinonasal carcinoma, a high-grade carcinoma associated with mutations in the isocitrate dehydrogenase-2 (IDH2) gene. A hotspot mutation in the R172 codon has been described in 50–80% of tumors classified as SNUC, large cell neuroendocrine carcinomas (LCNEC), and rarely in cases classified as olfactory neuroblastoma (ONB). The use of immunohistochemical and molecular approaches are required to correctly identify this subset of sinonasal tumors, with further study necessary to elucidate their unique pathophysiology, ultimately determining whether revision is required toward current therapeutic approach.
Aims.
Here, we provide an overview of the IDH2-mutated sinonasal tumors, discuss histopathologic and clinical features, and focus on molecular diagnostics and novel immunohistochemical (IHC) markers.
Results.
A review of the literature reveals 82 reported cases with IDH2-mutated sinonasal tumors (IST), confirmed either by molecular studies or diagnostic IHC markers. The mean patient age is 60-years (female/male: 1/1.4), the median tumor size is 5 cm (range: 2.5–7.0 cm), and the most common location is the nasal cavity (81%). IST display tumor necrosis and increased mitotes. Histopathologically, IST show SNUC-like, LCNEC-like, or poorly differentiated carcinoma- (PDC)-like features (77%, 12%, and 9% respectively). The molecular hotspot alterations in mitochondrial IDH2 are: R172S (61%), R172T (19%), R172G (7%), and R172M (3%). Sixty-five percent of tumors are surgically resectable and all patients received chemotherapy, radiation therapy or both. Rates of locoregional recurrence and distant metastasis are 60% and 40%, respectively. One-, 3- and 5-year survival rates are 83%, 50% and 43%, respectively. In all but one study, IST are associated with better outcomes than IDH2-wild type tumors and SMARCB1-deficient sinonasal tumors.
Keywords: IDH2, Sinonasal Undifferentiated Carcinoma, SNUC, Molecular Diagnostics
Introduction
Sinonasal undifferentiated carcinoma (SNUC) is an aggressive malignancy of the nasal cavity, paranasal sinuses and skull base, arising from the so-called “Schneiderian epithelium”, respiratory-lined ectodermal derivative epithelium with ciliated respiratory epithelium and scattered goblet cells, and accounts for less than 5% of sinonasal tumors(1). SNUC has been a diagnosis of exclusion since it was described by Frierson et al. in 1986(2). The recent advances in molecular studies have led to a reclassification of undifferentiated carcinomas of the sinonasal tract into their respective genomically-defined entities. In 2022, the World Health Organization (WHO) defined SNUC as a malignant epithelial tumor without any glandular, squamous or neuroendocrine line of differentiation(3). The discovery of IDH2 mutation in SNUC(4) was followed by investigating other high-grade malignancies of the sinonasal tract and led to the detection of this mutation in large cell neuroendocrine carcinoma (LCNEC), some poorly differentiated carcinomas (PDC)(5, 6) and olfactory neuroblastomas(7, 8). With over eighty cases described to date (Table 1), the diagnosis of IDH2 mutated sinonasal tumors (IST) continued to expand, and with the use of inexpensive techniques like immunohistochemical studies (IHC), more cases will be added to this category, now recognized as the most common variant present in SNUC according to the 2022 WHO Classification of Head and Neck Tumors (5th Edition)(3). Given the expanding profile and therapeutic options for tumors with IDH2-mutant profiles, there could be a strong argument to include them as a unique diagnostic entity in subsequent editions of the WHO tumor classifications.
Table 1:
Literature review of IDH2-mutated sinonasal carcinomas, confirmed by molecular or diagnostic immunohistochemical markers and their histopathologic classification.
| Author No. of patients |
SNUC-like | LCNEC-like | PDC-like | HG-Non-ITAC |
|---|---|---|---|---|
| Dogan et al.a 30 patients |
22 | 5 | 3 | - |
| Mito et al. 26 patients |
23b | - | 3c | - |
| Riobello et al. 14 patients |
9 | 2 | 1 | 2 |
| Jo et al. 6 patients |
6 | - | - | - |
| Libera et al. 5 patients |
2 | 3 | - | - |
| Heft Neal et al. 1 patient |
1 | - | - | - |
|
| ||||
| Total (82 patients) |
63 | 13 | 4 | 2 |
Including 4 articles with non-overlapping cases;
14 cases confirmed by immunohistochemical markers only;
1 case confirmed by immunohistochemical markers only; SNUC: sinonasal undifferentiated carcinoma; LCNEC: large cell neuroendocrine carcinoma; PDC: poorly differentiated carcinoma; HG-Non-ITAC: high grade non-intestinal-type adenocarcinoma.
Epidemiology
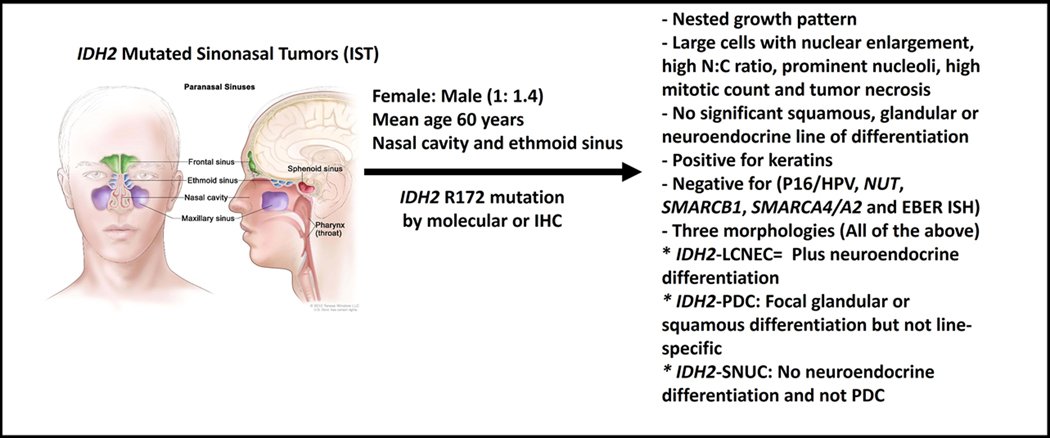
IDH2 mutated sinonasal tumors (IST) account for 20–88% of SNUC, 11–83% of LCNEC, 13–38% of poorly differentiated carcinoma (PDC), 50% of high-grade non-intestinal-type adenocarcinoma (HG-Non-ITAC)(4-6, 9, 10), and a subset controversially classified as ONB(7, 8, 11). Female to male ratio is 1:1.4 with a 22–87-year age range(5, 10, 12, 13). Median age of patients with IDH2 mutated sinonasal tumor is 57 years, compared to 71 years in IDH2 wild-type(6).
Etiology
Molecular profiling identified the hotspot mutation in the mitochondrial IDH2 enzyme of the Isocitrate dehydrogenase family(14), in poorly/undifferentiated tumors of sinonasal tract. The most common alterations are R172S (61%), R172T (19%), R172G (7%), and R172M (3%)(4-6, 10, 12, 13, 15).
Localization
IST size ranges from 2.5 to 7.0 cm(4, 5). IST arise most frequently in the nasal cavity and the ethmoid sinus(4, 5) and 60% of patients present with moderately or very advanced disease(5, 10, 13, 15). In one study of 22 IST patients, 68% present with sinonasal masses involving multiple anatomic sites, typically the nasal cavity and ethmoid sinuses (60%, each) and maxillary, sphenoid and frontal sinuses (32%, 27% and 23%, respectively). Intracranial and intraorbital extension are seen in 23% of patients and skull base involvement is reported in 18% of cases(1). Direct invasion into adjacent structures, like cribriform plate, cranial nerves, dura and brain has also been reported(5).
Clinical Features
Patients with IST can present with wide range of symptoms including eye pain, ptosis, visual changes, orbital pressure sensation, double vision, nasal congestion and obstruction, loss of smell and taste, epistaxis, facial pain and swelling, headache, memory loss and personality changes(5). Twenty-eight percent of patients have metastatic lymph nodes at presentation(13). Patients typically present with tumor-related symptoms within 1–3 months of diagnosis(4).
Histopathology
IST is a group of diseases that are characterized by high-grade morphology (large cells with nuclear enlargement, high nuclear: cytoplasmic (N:C) ratio, prominent nucleoli, high mitotic count and tumor necrosis) and shared molecular profile (IDH2 mutation). Initially, IDH2 mutation was described in SNUC and LCNEC(4), but later, more cases with PDC or high-grade non-ITAC morphology harbored IDH2 mutation(5, 6). Further, the subset of IDH2-mutated ONB has some associated controversy, as histologic review, in some cases, has called into question, especially with keratin staining, the diagnosis of ONB to begin with(8, 11).
The challenging part in diagnosing IST is the diversity of its histopathologic features. Most cases with IDH2 mutation exhibit SNUC-like, LCNEC-like, or PDC-like features (77%, 12%, and 9%, respectively). In one study, IST with SNUC-like and PDC-like, architectures include nested or sheet-like growth patterns (76% and 20%, respectively). Cytologically, tumor cells are moderate-large with rounded to slightly elongated nuclei. Prominent nucleoli and tumor necrosis are present in two-third of cases. Mitotic figures are frequent with median of 37 mitoses/10 high power fields(12). While nested growth pattern, large cells, prominent nucleoli, high mitotic count and necrosis are consistent findings, there is no unique feature that is helpful to differentiate IDH2 mutated from IDH2 wild-type tumors(4-6, 10, 12, 13, 15), thus molecular and immunohistochemical studies are warranted.
Some pathologists may use the term poorly differentiated carcinoma (PDC) to describe a high-grade carcinoma with a glandular and/or squamous component that lacks a specific line of differentiation, by histology or immunohistochemical studies, but does not fit into any specific WHO category. PDC is a purely descriptive term, given glandular or squamous features technically conflict, definitionally, with being undifferentiated. However, sinonasal PDC is not a recognized WHO entity, and its diagnostic use should be avoided. Consideration should be given to a descriptive diagnosis best considered as a stated WHO entity. In one study, IST with so-called PDC-like morphology (IDH2-PDC) demonstrates trabecular or pseudoglandular patterns(12). One case diagnosed as HG-non-ITAC shows high-grade morphology with nested growth pattern, nuclear pleomorphism focal glandular differentiation but was negative for CDX2 and CK20 immunostains(6). Some pathologists may call this case PDC, but again, this terminology is best avoided.
LCNEC is also a high-grade carcinoma with neuroendocrine differentiation. Most of the time, LCNEC cannot be differentiated from SNUC based merely on morphology. The presence of speckled or coarse chromatin, brisk mitosis and extensive necrosis might be an indication to order neuroendocrine markers, which will show evidence of neuroendocrine differentiation(13). LCNEC will show focal positivity for synaptophysin or chromogranin(6). INSM1 nuclear stain can also be used to highlight neuroendocrine differentiation (29438167). LCNEC can have IDH2 mutation in 11–83% of cases(4-6) ). IDH2 with LCNEC-like morphology (IDH2-LCNEC) can only be diagnosed by performing IDH2 studies on tumors with carcinoma differentiation (keratins positive) along with immunohistochemical evidence of neuroendocrine differentiation.
SNUC is defined by the WHO as a high-grade malignancy with no glandular or squamous differentiation. However, minimal squamous or glandular differentiation is allowed, by some pathologists who will label such tumors as SNUC after extensive workup as a diagnosis of exclusion. Significant squamous or glandular differentiation hinder the diagnosis of SNUC. IST accounts for 20–88% of SNUC cases(4-6, 10, 12, 13, 15). IST with SNUC-like morphology (IDH2-SNUC) show large, undifferentiated epithelial tumor cells, display open chromatic, cherry-red nucleoli and high N:C ratio(5). IDH2 mutated with SNUC-like and neuroendocrine differentiation should be called IDH2-LCNEC. Interestingly, IDH2-LCNEC and IDH2-SNUC share the same molecular profile and outcome(13).
Most reported IST have tumor necrosis (66–100%). All IST show frequent mitotic activity and stain positive for keratins (100%)(12, 13)). Seventeen percent of tumors express a weak positive staining for p40, p63 or S100. In tumors with associated TP53 mutation, p53 immunostain can be either diffusely immunopositive or completely negative(5).
Differential Diagnosis
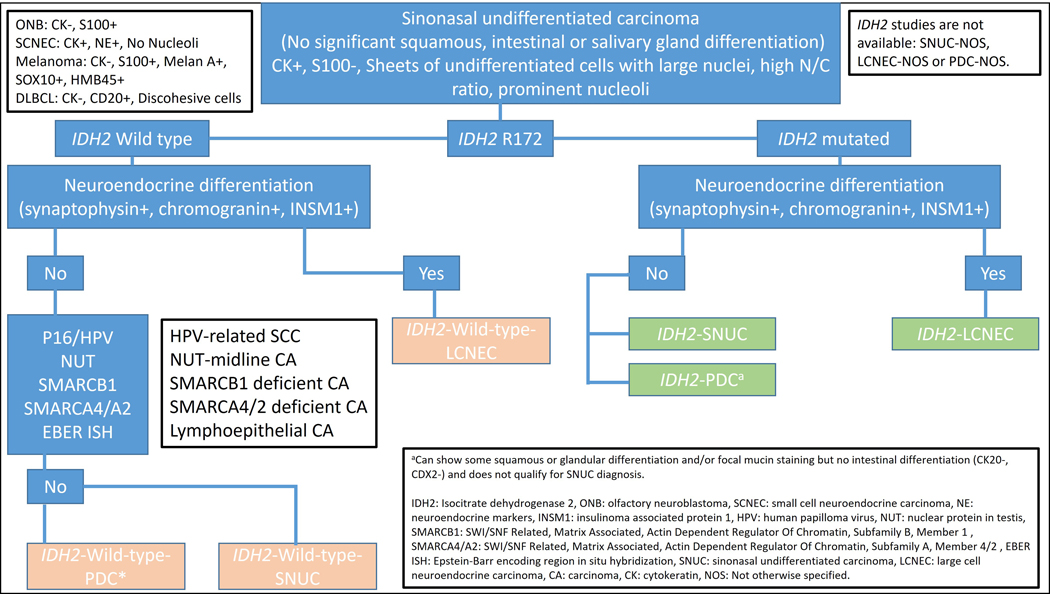
High-grade sinonasal tumors including IST share an overlapping morphology, and histopathology alone is often insufficient to precisely render a clinically meaningful diagnosis. In addition to SNUC, LCNEC and PDC with wild-type IDH2, the differential diagnosis of IST includes poorly differentiated carcinomas like (EBV- or HPV-related), neuroendocrine and neuroectodermal tumors (LCNEC and possibly olfactory neuroblastoma), tumors with defined molecular alterations (NUT, SMARCB1 and SMARCA4/A2), melanoma and high-grade B-cell lymphoma. See Table 3 and Figure 1 for IST work up.
Table 3:
Workup and differential diagnosis of IDH2 mutated sinonasal tumors.
| Diagnosis | Keratins | P63/p40 | S-100 | Neuro-endocrine markers | P16/HPV | EBER ISH | NUT (IHC) | SMARCB1 (INI) (IHC) | SMARCA4 (BRG1) (IHC) | SMARCA2 (BRM) (IHC) | IDH2 R172 (IHC) | Note |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IDH2 Sinonasal tumor | + | − | − | +/− | − | − | − | Retained | Retained | Retained | + | IDH2 R172 hotspot mutation, at 15q26.1 locus. |
| HPV-related sinonasal carcinoma | + | + | − | -a | + | − | − | Retained | Retained | Retained | − | Including multiphenotypic sinonasal carcinoma (HPV33+). |
| Sinonasal lymphoepithelial carcinoma | + | + | − | − | − | + | − | Retained | Retained | Retained | − | Syncytial growth. |
| NUT-midline carcinoma | + | + | − | − | − | − | + | Retained | Retained | Retained |
BRD4-NUT fusion protein, t(15;19)(q14;p13.1) or NUT-variant. Abrupt keratinization. |
|
| SMARCB1 deficient SNC | + | + | − | − | − | − | − | Lost | Retained | Retained | − |
hSNF5/INI1 loss (SMARCB1) at 22q11.2 locus. Cytoplasmic vacuoles. Rhabdoid or plasmacytoid cells. |
| SMARCA4 deficient SNC | + | + | − | − | − | − | − | Retained | Lost | Retainedb | − | BRG1 loss (SMARCA4) at 19p13.2, with or without BRM loss (SMARCA2) at 9p24.3 locus. |
| Olfactory neuroblastoma | − | − | + | + | − | − | − | Retained | Retained | Retained | − | − |
| Small cell neuroendocrine carcinoma | + | − | − | + | − | − | − | Retained | Retained | Retained | − | − |
| Large cell neuroendocrine carcinoma | + | − | − | + | − | − | − | Retained | Retained | Retained | − | − |
| IDH2 wild-type LCNEC | + | − | − | + | − | − | − | Retained | Retained | Retained | − | − |
| IDH2 wild-type SNUC or PDC | + | − | − | − | − | − | − | Retained | Retained | Retained | − | − |
| Melanoma | − | − | + | − | − | − | − | Retained | Retained | Retained | − | Melanin pigment, binucleation or multinucleation. Positive for Melan-A, HMB45, SOX10, etc. |
| Diffuse large B-cell lymphoma | − | − | − | − | − | − | − | Retained | Retained | Retained | − | Discohesive cells. Positive for CD20, PAX5, etc. |
May be focally positive in HPV-SCC and HPV-LCNEC.
May be lost in 13% of cases. SNC: sinonasal carcinoma, SCC: small cell carcinoma, LCNEC: large cell neuroendocrine carcinoma, PDC: poorly differentiated carcinoma, NOS: not otherwise specified.
Figure 1.
Work up for IDH-mutated tumors.
Olfactory neuroblastoma (ONB) is a neuroectodermal tumor arising from the cribriform plate and often shows uniform lobular pattern with neurofibrillary background and Homer-Wright pseudorosettes. High-grade ONB demonstrates nuclear pleomorphism, prominent nucleoli but will be consistently negative for keratins. S100 stains the sustentacular cells and tumor cells are positive for synaptophysin and other neuroendocrine markers. One third of ONB may stain focally for Cam5.2 and CK18(16). In one study, 18% of ONBs harbor IDH2 mutation(17), however, ONBs did not harbor IDH2 mutation in other two studies(6, 12). Small cell neuroendocrine carcinoma (SNEC) is a small round blue cell tumor with high N:C ratio, brisk mitoses, nuclear molding and inconspicuous nucleoli. SNECs should be differentiated from other tumors with neuroendocrine differentiation (ONB, LCNEC) as the former have very poor outcome(13).
Human papillomavirus (HPV)-related sinonasal squamous cell carcinomas are morphologically identical to HPV-related oropharyngeal carcinomas, exhibiting little or no keratinization and diffuse p16 immunopositivity(18). Transcriptionally active HPV can be labeled by high-risk HPV-RNA by in situ hybridization (ISH)(19). HPV-related LCNEC will show similar morphologic features to IDH2-LCNEC, however, positivity for HPV will be diagnostic of the former(20). HPV-related multiphenotypic sinonasal carcinoma (HMSC) is a high-grade non-keratinizing carcinoma with variable growth patterns including cribriform, tubular, sarcomatoid, chondroid, epimyoepithelial or myoepithelial carcinoma-like growth. HPV 33 will be positive in 67% of HMSC(21).
Sinonasal lymphoepithelial carcinoma (SNLEC) is strongly associated with Epstein-Barr virus (EBV)(22, 23). SNLEC is morphologically similar to non-keratinizing nasopharyngeal carcinoma (NK-NPC) undifferentiated subtype and shows syncytial appearance, round to oval vesicular nuclei and large central nucleoli, with variable lymphocytic and plasma cells infiltration. One study shows absence of EBV association (EBER ISH) in all cases of SNUC(24). IST lacks the syncytial growth seen in SNLEC.
Midline carcinoma with NUT rearrangement presents in young adults and children and shows sheets of small to medium-sized poorly differentiated cells with abrupt squamous differentiation. The cells are usually monotonous with round to oval nuclei and fine chromatin, with necrosis and increase mitosis. P63 and p40 are diffusely positive and immunostain for NUT antibody will be positive(25).
SMARCB1-deficient sinonasal carcinoma shows basaloid growth, with nests of basophilic cells, with high N:C ratio, growing in a desmoplastic stroma. Rarely, non-specific cytoplasmic vacuoles are present, and cells may show plasmacytoid or rhabdoid differentiation. SMARCB1 (INI-1) will be lost by immunostaining(26).
SMARCA4-deficient sinonasal carcinoma is heterogeneous, but cases show either LCNEC-like morphology (large round to ovoid nuclei, some with prominent nucleoli) or SNEC (small round blue cells with brisk mitoses, and nuclear molding). SMARCA4 immunostain will be lost(27). A subset of teratocarcinosarcoma (triphasic ectoderm/mesoderm/endoderm differentiation) show loss of SMARC4, but not all, and therefore, are still considered to be a separate, morphologically-defined entity. Unlike other sites, SMARCA2 will be intact in most cases. Rarely, SMARCA4-deficient sinonasal tumors can have concomitant SMARCA2-loss(27, 28). Isolated SMARCA2 sinonasal carcinomas have not been reported.
High-grade morphology and prominent nucleoli should raise the possibility of carcinoma mimickers, like melanoma or diffuse large B-cell lymphoma (DLBCL). Keratins will be negative in both. Melanoma will be positive for S100 and other melanocytic markers and may show melanin pigmentation. DLBCL shows discohesive cells and stains positive for CD45, CD20 and other B-cell markers.
SNUC is morphologically similar to IST, and in fact, in the most recent 2022 WHO, IDH2 mutations are listed as the most commonly identified mutation in what remains of the SNUC category(3). SNUC has typically been thought of as a diagnosis of exclusion, but as the genetic underpinnings have filled in, the category is rapidly shrinking or at least becoming a more specific diagnosis associated with a narrower mutational profile. Cases which lack IDH2 mutation can be signed out as IDH2 wild-type-SNUC/-LCNEC or-PDC and in cases where IDH2 testing is not available, suspicious IST cases can be signed out as SNUC-/LCNEC-/PDC-not otherwise specified (NOS). However, a consensus among pathologist is needed to implement such new names. Further, more recently distinguished categories, such as the SWI/SNF complex-deficient sinonasal carcinomas(29), may or may not stay distinct categories depending on the ability to develop unique therapeutic options.
Genetic Profile
Isocitrate dehydrogenase (IDH) gene family is composed of three genes, IDH1, IDH2 and IDH3. IDH1/2 are known to induce a global hypermethylation phenotype. Located in the mitochondria, IDH2 catalyzes the conversion of alpha-ketoglutarate in the citric acid cycle, to its counterpart oncometabolite, 2-hydroxygluterate (2HG). IDH2-mutated tumors show abnormally high level of 2HG, which inhibits histone demethylase and ten-eleven translocation (TET) family 5-methylcytosine hydroxylases; enzymes responsible for DNA hypomethylation. Hypomethylation is associated with tissue differentiation and gene expression(14). IDH2 mutations have been described in gliomas(30), acute myeloid leukemias(31), cholangiocarcinomas(32), central chondrosarcoma and central and periosteal chondromas(33), solid papillary carcinoma with reverse polarity of the breast(9), medulloblastoma(34) and sinonasal tumors(5, 6, 10, 12, 13),.
Next generation sequencing (NGS) or Sanger sequencing will identify the hotspot mutation affecting amino acid R172 of IDH2 gene. Reported mutations include R172S, R172T, R172G and R172M (61%, 19%, 7%, and 3%, respectively)(4-6, 10, 12, 13, 15, 35).
Molecular profiling of sinonasal tumors regardless of their histologic appearance shows that 40% of those tumors have IDH2 gene mutation(5). This percentage increases to 88% when IDH2 is being investigated in high-grade/undifferentiated tumors(13). In one study, IST is associated with epigenetic dysregulation, including a higher global DNA methylation level compared to IDH2 wild-type (70% vs 55%). In the same study, IDH2 mutation is an independent predictor of worse disease specific survival (DSS)(10). DNA methylation studies found that IST form a single cluster, irrespective of their histologic type and show no overlap with SMARCB1 deficient tumors or those classified as ONB. In this study, however, IDH2 tumors are associated with better disease-free survival (DFS) and less propensity for lung metastasis(13).
Other concomitant genetic alterations in IDH2-mutated sinonasal tumors include TP53 in 50% of cases(9). Less common concomitantly altered genes include ASXL1, B2M, BCOR, BRD4, CCND2, CDKN2A, CDKN2B, CYLD, DDR2, EGFR, ERBB3, FANCA, FOXP1, ID3, KIT, KMT2D, LATS1, MCL1, MGA, MTOR, NSD1, NTRK1, PICTOR, PIK3CA PTEN, PTPRT, SETD2, SPEN, TGFBR2, TSC1, TSC2, ZFHX3(12, 13).
Immunohistochemical studies (IHC) for IDH2 mutated tumors
Different immunostains targeting IST were developed (Table 2). The monoclonal 11C8B antibody is diffusely positive in all cases with R172S and R172T but negative in R172G and R172M(5, 35). 11C8B can stain SNUC-like as well as LCNEC-like IDH2 mutated sinonasal carcinomas. Another monoclonal antibody is MMab1 and is specific for R172M(35). A third monoclonal antibody is 3C11 and is positive in R172G(5).
Table 2:
Novel immunohistochemical studies and their diagnostic utilization in IDH2 mutated sinonasal tumors.
| Mutation | 11C8B (strong diffuse granular cytoplasmic) |
mIDH1/2 (strong granular cytoplasmic) |
MsMab-1 (strong diffuse granular cytoplasmic) |
3C11 (strong diffuse granular cytoplasmic) |
MMab1 (strong diffuse granular cytoplasmic) |
|---|---|---|---|---|---|
| R172S | 16/16 | 4/4 | 7/7 | 0/4 | 0/4 |
| R172T | 2/2 | 3/4b | - | 0/1 | 0/4 |
| R172G | 0/1 | 2/2 | 4/4 | 1/1 | 0/1 |
| R172M | 0/1 | - | 1/1d | 0/1 | 1/1 |
| R132Ca | - | 0/1 | 1/1d | 1/1f | 0/1 |
| Unknown | 15/15c | 3/3 e | - | - |
Bold: strong positive;
R132C of IDH1;
Weak granular in 2 cases and negative in one case;
mIDH1/2 was only used (11 SNUC-cases were strong positive, 3 SNUC were weak granular and 1 PDC was weak granular);
Weak to moderate cytoplasmic;
One case of (SNEC, ITAC and HG-Non-ITAC);
Smooth cytoplasmic in IDH1-mutated intrahepatic cholangiocarcinoma; SNUC: sinonasal undifferentiated carcinoma, PDC: poorly differentiated carcinoma, SNEC: small cell neuroendocrine carcinoma, ITAC: Intestinal-type adenocarcinoma and HG-Non-ITAC: high grade non-intestinal-type adenocarcinoma.
MsMab-1 which targets both IDH1/2 mutations (R172S, R172G and R172M of IDH2 and R132C of IDH1) shows diffuse granular cytoplasmic staining in R172S, R172G and R132C and weak to moderate cytoplasmic multifocal staining in R172M(4, 6). Another immunostain which targets both IDH1/2 proteins is mIDH1/2. It shows strong positivity for R172S and R172G and weak granular positivity in R172T(12) (Figure 2).
Figure 2.
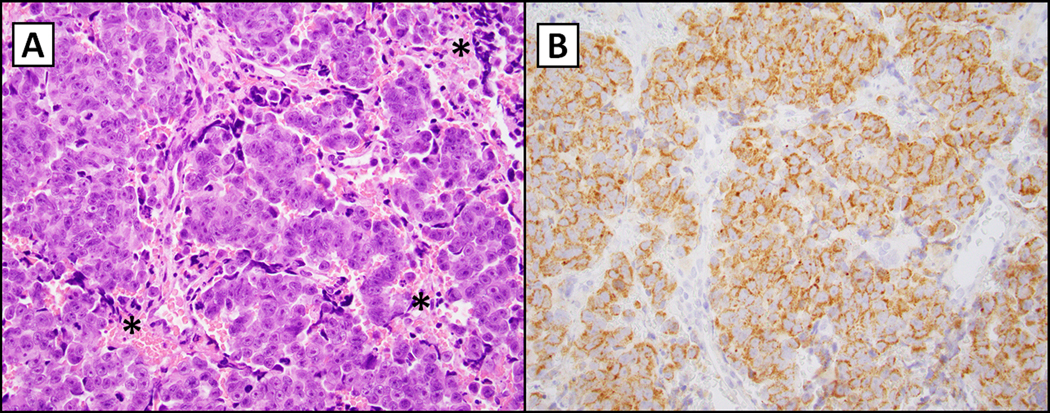
IDH2-mutated sinonasal carcinoma (R172S; 400X magnification). The left panel (hematoxylin and eosin, A) demonstrates a nested population of epithelioid cells with a prominent nucleolus, variable hyperchromasia and dusky blue cytoplasm typically noted in undifferentiated neoplasms in the head and neck. There is focal necrosis indicated by asterisks. The right panel (B) shows immunohistochemistry for multispecific antibody directed against mIDH1/2 with granular cytoplasmic staining (considered positive if noted in greater than 10% of cells (as seen here)(12). Micrographs courtesy of Dr. Vickie Y. Jo, Brigham and Women’s Hospital, Boston, MA.
Of note, the IDH2 protein sequence in proximity to R172 is remarkably similar to IDH1 protein around codon R132 and immunostains like 3C11 can stain both IDH1 and IDH2 mutated tumors(35).
IHC studies may serve as surrogate and inexpensive markers for the presence of IDH2, however, molecular studies are still necessary to identify cases with negative IHC staining.
Prognosis and Predictive Factors
All except one study report better disease-specific survival with IST compared with wild-type IDH2 carcinomas and SMARCB1-deficient tumors(5, 7). One study shows that IST has more favorable prognosis regardless of the histologic classification(6). Another study shows that tumors with IDH2 mutation tend to recur and metastasize less than SMARCB1-deficient sinonasal tumors (56% vs 87%)(5). IDH2 mutation predicts better disease-free survival (DFS) and is associated with lower incidence of lung metastasis(13). On the other hand, one study reports IDH2 mutation to be associated with worse disease specific survival (DSS)(10).
IST is locally invasive and destructive and 40% of patients present with non-resectable disease with treatment options limited to chemotherapy, radiation therapy or both.
Up to 60% of patients experience locoregional recurrence and 40% develop distant metastasis(5, 13). IST can metastasize to bone, liver, abdominal lymph nodes, adrenal gland, and skin(5). Forty percent of patients die of disease within 4–33 months, and 33% may experience remission in up to 144 months(5, 10, 15). One-, 3- and 5-years survival rates are 83%, 50% and 43%, respectively.
Since most of the studies about IST are retrospective studies, no comparison is available in which a particular treatment grants better outcomes. Treatment options include chemotherapy or chemo-radiation, with or without surgery. One clinical trial on Enasidenib (a selective mutant IDH2 inhibitor) studies the response of IDH2 mutated refractory/relapsed acute myeloid leukemia, and shows a clinical response of 40%, however, this study is Phase I and does not include patients with IDH2-mutated sinonasal carcinomas(36). Another study investigated hypomethylation agents like Decitabine, which can induce reactivation of silent genes, shows some efficacy against IDH1-mutated myelodysplastic diseases(37). Finally, preclinical studies shows that poly (adenosine 5’-diphosphate-ribose) polymerase (PARP) inhibitors, can reverse the IDH1-mutated protein, thus facilitate targeting 2-Hydroxyglutarate (implicated in tumor progression) in primary gliomas(38). Discovering IDH2 mutation in sinonasal tumors should encourage further studies on IDH2 targeted therapy and hypomethylation agents with both treatment and control groups.
Conclusion
IST is seen in more than 80% of high-grade sinonasal tumors. Patients are typically middle-aged males, with advanced stage and a wide range of symptoms related to mass effect and/or direct invasion of sinuses and cranial nerves. IDH2 mutated group of tumors include tumors with high-grade morphology. IST share histopathologic features like nested pattern, large nuclei, open chromatin, prominent nucleoli, necrosis and abundant mitotic figures. In addition to keratin stains, neuroendocrine markers, P16 and HPV studies, EBV studies, NUT, SMARCB1 (INI-1), SMARCA4 (BRG1) and SMARCA2 (BRM), the work up of high-grade sinonasal tumors should include IDH2 immunohistochemical and molecular studies (Figure 3). Currently, there is no standard treatment for IST, however, some preclinical studies show promising results in other tumors. In most studies, patients with IST tend to have a better prognosis, however, multi-institutional and prospective studies are needed to better understand and address such a disease. Deep genotyping of IDH2 wild-type tumors may further identify new mutations.
Figure 3.
Summary Figure of IDH-mutated tumors (Published with permission from Terese Winslow LLC. 714 South Fairfax Street, Alexandria, Virginia 22314, terese@teresewinslow.com).
Conflicts of Interest and Source of Funding:
The authors have disclosed that they have no significant relationships with, or financial interest in any commercial companies pertaining to this article.
Dr. Sadow received from funding from the National Cancer Institute of the National Institutes of Health (5P01CA240239–04)
References:
- 1.Llorente JL, Lopez F, Suarez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014;11(8):460–72. [DOI] [PubMed] [Google Scholar]
- 2.Frierson HF Jr., Mills SE, Fechner RE, Taxy JB, Levine PA Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10(11):771–9. [PubMed] [Google Scholar]
- 3.Thompson LDR, Bishop JA. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2022;16(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol. 2017;30(5):650–9. [DOI] [PubMed] [Google Scholar]
- 5.Dogan S, Chute DJ, Xu B, Ptashkin RN, Chandramohan R, Casanova-Murphy J, et al. Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol. 2017;242(4):400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riobello C, Lopez-Hernandez A, Cabal VN, Garcia-Marin R, Suarez-Fernandez L, Sanchez-Fernandez P, et al. IDH2 Mutation Analysis in Undifferentiated and Poorly Differentiated Sinonasal Carcinomas for Diagnosis and Clinical Management. Am J Surg Pathol. 2020;44(3):396–405. [DOI] [PubMed] [Google Scholar]
- 7.Gloss S, Jurmeister P, Thieme A, Schmid S, Cai WY, Serrette RN, et al. IDH2 R172 Mutations Across Poorly Differentiated Sinonasal Tract Malignancies: Forty Molecularly Homogenous and Histologically Variable Cases With Favorable Outcome. Am J Surg Pathol. 2021;45(9):1190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Classe M, Yao H, Mouawad R, Creighton CJ, Burgess A, Allanic F, et al. Integrated Multi-omic Analysis of Esthesioneuroblastomas Identifies Two Subgroups Linked to Cell Ontogeny. Cell Rep. 2018;25(3):811–21 e5. [DOI] [PubMed] [Google Scholar]
- 9.Alsadoun N, MacGrogan G, Truntzer C, Lacroix-Triki M, Bedgedjian I, Koeb MH, et al. Solid papillary carcinoma with reverse polarity of the breast harbors specific morphologic, immunohistochemical and molecular profile in comparison with other benign or malignant papillary lesions of the breast: a comparative study of 9 additional cases. Mod Pathol. 2018;31(9):1367–80. [DOI] [PubMed] [Google Scholar]
- 10.Libera L, Ottini G, Sahnane N, Pettenon F, Turri-Zanoni M, Lambertoni A, et al. Methylation Drivers and Prognostic Implications in Sinonasal Poorly Differentiated Carcinomas. Cancers (Basel). 2021;13(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capper D, Engel NW, Stichel D, Lechner M, Gloss S, Schmid S, et al. DNA methylation-based reclassification of olfactory neuroblastoma. Acta Neuropathol. 2018;136(2):255–71. [DOI] [PubMed] [Google Scholar]
- 12.Mito JK, Bishop JA, Sadow PM, Stelow EB, Faquin WC, Mills SE, et al. Immunohistochemical Detection and Molecular Characterization of IDH-mutant Sinonasal Undifferentiated Carcinomas. Am J Surg Pathol. 2018;42(8):1067–75. [DOI] [PubMed] [Google Scholar]
- 13.Dogan S, Vasudevaraja V, Xu B, Serrano J, Ptashkin RN, Jung HJ, et al. DNA methylation-based classification of sinonasal undifferentiated carcinoma. Mod Pathol. 2019;32(10):1447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bledea R, Vasudevaraja V, Patel S, Stafford J, Serrano J, Esposito G, et al. Functional and topographic effects on DNA methylation in IDH1/2 mutant cancers. Sci Rep. 2019;9(1):16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heft Neal ME, Birkeland AC, Bhangale AD, Zhai J, Kulkarni A, Foltin SK, et al. Genetic analysis of sinonasal undifferentiated carcinoma discovers recurrent SWI/SNF alterations and a novel PGAP3-SRPK1 fusion gene. BMC Cancer. 2021;21(1):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell D. Sinonasal Neuroendocrine Neoplasms: Current Challenges and Advances in Diagnosis and Treatment, with a Focus on Olfactory Neuroblastoma. Head Neck Pathol. 2018;12(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, An J, Liu H. Clinicopathologic Features and Prognosis of Olfactory Neuroblastoma with Isocitrate Dehydrogenase 2 Mutations. World Neurosurg. 2022;159:e23–e31. [DOI] [PubMed] [Google Scholar]
- 18.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen E, Coviello C, Menaker S, Martinez-Duarte E, Gomez C, Lo K, et al. P16 and human papillomavirus in sinonasal squamous cell carcinoma. Head Neck. 2020;42(8):2021–9. [DOI] [PubMed] [Google Scholar]
- 20.Thompson ED, Stelow EB, Mills SE, Westra WH, Bishop JA. Large Cell Neuroendocrine Carcinoma of the Head and Neck: A Clinicopathologic Series of 10 Cases With an Emphasis on HPV Status. Am J Surg Pathol. 2016;40(4):471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop JA, Andreasen S, Hang JF, Bullock MJ, Chen TY, Franchi A, et al. HPV-related Multiphenotypic Sinonasal Carcinoma: An Expanded Series of 49 Cases of the Tumor Formerly Known as HPV-related Carcinoma With Adenoid Cystic Carcinoma-like Features. Am J Surg Pathol. 2017;41(12):1690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zong Y, Liu K, Zhong B, Chen G, Wu W. Epstein-Barr virus infection of sinonasal lymphoepithelial carcinoma in Guangzhou. Chin Med J (Engl). 2001;114(2):132–6. [PubMed] [Google Scholar]
- 23.Wenig BM. Lymphoepithelial-like carcinomas of the head and neck. Semin Diagn Pathol. 2015;32(1):74–86. [DOI] [PubMed] [Google Scholar]
- 24.Cerilli LA, Holst VA, Brandwein MS, Stoler MH, Mills SE. Sinonasal undifferentiated carcinoma: immunohistochemical profile and lack of EBV association. Am J Surg Pathol. 2001;25(2):156–63. [DOI] [PubMed] [Google Scholar]
- 25.Lee T, Cho J, Baek CH, Son YI, Jeong HS, Chung MK, et al. Prevalence of NUT carcinoma in head and neck: Analysis of 362 cases with literature review. Head Neck. 2020;42(5):924–38. [DOI] [PubMed] [Google Scholar]
- 26.Agaimy A, Hartmann A, Antonescu CR, Chiosea SI, El-Mofty SK, Geddert H, et al. SMARCB1 (INI-1)-deficient Sinonasal Carcinoma: A Series of 39 Cases Expanding the Morphologic and Clinicopathologic Spectrum of a Recently Described Entity. Am J Surg Pathol. 2017;41(4):458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakkar A, Ashraf SF, Rathor A, Adhya AK, Mani S, Sikka K, et al. SMARCA4/BRG1-Deficient Sinonasal Carcinoma. Arch Pathol Lab Med. 2022;146(9):1122–30. [DOI] [PubMed] [Google Scholar]
- 28.Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient Sinonasal Carcinoma: A Series of 10 Cases Expanding the Genetic Spectrum of SWI/SNF-driven Sinonasal Malignancies. Am J Surg Pathol. 2020;44(5):703–10. [DOI] [PubMed] [Google Scholar]
- 29.Agaimy A. Proceedings of the North American Society of Head and Neck Pathology, Los Angeles, CA, March 20, 2022: SWI/SNF-deficient Sinonasal Neoplasms: An Overview. Head Neck Pathol. 2022;16(1):168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasinskas AM. Cholangiocarcinoma. Surg Pathol Clin. 2018;11(2):403–29. [DOI] [PubMed] [Google Scholar]
- 33.Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224(3):334–43. [DOI] [PubMed] [Google Scholar]
- 34.Bezerra Salomao K, Cruzeiro GAV, Bonfim-Silva R, Geron L, Ramalho F, Pinto Saggioro F, et al. Reduced hydroxymethylation characterizes medulloblastoma while TET and IDH genes are differentially expressed within molecular subgroups. J Neurooncol. 2018;139(1):33–42. [DOI] [PubMed] [Google Scholar]
- 35.Dogan S, Frosina D, Geronimo JA, Hernandez E, Mohanty A, Bale T, et al. Molecular epidemiology of IDH2 hotspot mutations in cancer and immunohistochemical detection of R172K, R172G, and R172M variants. Hum Pathol. 2020;106:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130(6):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turcan S, Fabius AW, Borodovsky A, Pedraza A, Brennan C, Huse J, et al. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor Decitabine. Oncotarget. 2013;4(10):1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulkowski PL, Corso CD, Robinson ND, Scanlon SE, Purshouse KR, Bai H, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017;9(375). [DOI] [PMC free article] [PubMed] [Google Scholar]