Abstract
Endogenously produced cannabinoids as well as phytocannabinoids broadly exhibit anti-inflammatory actions. Recent emergence of cannabis for multiple medical issues combined with reports on potent immunomodulatory actions of distinct components has underscored the therapeutic potential of cannabis. While synthetic cannabinoids that are based on structural similarities to existing class of cannabinoids have been on the rise, their application in therapeutics have been limited owing to toxicity concerns. Herein, we review the current literature that details the immunomodulatory actions of cannabinoids. Further, we highlight the complexities of cannabinoid biology and examine the potential inflammatory risks associated with use of cannabis including potential for toxic interactions between distinct constituents of cannabis.
Keywords: Cannabis, allergy, cannabinoids, mast cells
INTRODUCTION
Cannabis legal status is constantly evolving worldwide, but it remains the most prevalent drug for illicit use in the United States. Cannabis is not a novel entity and has been cultivated through millennia as a source of fiber, oil, food, and for its medicinal (specifically pain management) and mood-altering properties.1 However, criminalization of cannabis began as early as 1906 with the enacting of poisoning laws, thereby limiting its accessibility and in 1925 the League of Nations plenipotentiary International Opium conference formalized cannabis regulation, but the United States withheld participation. By 1937, the Marihuana Tax Act essentially made possession or transportation of the plant illegal under federal law (with certain exceptions for medical or industrial uses). The Comprehensive Drug Abuse Prevention and Control act of 1970 was passed soon after the SCOTUS found the Marihuana Tax Act to be constitutional (Leary v. United States). Cannabis was assigned Schedule I classification under Title two of this act (aka: Controlled Substances Act). This classification deemed cannabis as having a high potential for abuse with no accepted medical use, thus prohibiting its accessibility. However, the laws pertaining to recreational cannabis use have recently evolved, particularly owing to potential medicinal benefits and evolving social acceptance. Despite its recognition as an illegal substance at the Federal level, many constituent states and cities have increased access to cannabis and/or decriminalized its possession and states/cities have nuanced access to cannabis for medicinal and recreational use.
Over 80% of our present therapeutic armamentarium is derived from herbal based natural compounds2, which underlies the recent resurgence in complementary medicine (particularly cannabis) and their beneficial effects in managing inflammation. In recent years, we have also seen increased clinical interest in understanding cannabis and its physiological and pathophysiological effects. Cannabis has a rich chemical profile including cannabinoids that can affect a wide range of physiologic systems. These cannabinoids are understood to exert their actions primarily through cannabinoid (CB1 and CB2) receptors (although other targets are presumed to exist). Consequently, agonists and antagonists are showing significant clinical value as a potent immunoactive therapy. Concurrently, there has also been increased reporting of immunological impacts of cannabis such as allergy and other adverse drug reactions, including synthetic forms.3, 4 This manuscript explores our current understanding of plant cannabinoids and the endocannabinoid system, reviews the literature on allergenicity and immunotherapeutic/immunomodulatory potential of this emerging class of substances.
General Overview of Cannabinoids
Cannabinoids are a group of closely related compounds that exist in nature in the form of phytocannabinoids (sourced from plants) or naturally occurring in higher animals (endogenous cannabinoids or endocannabinoids). To overcome the limitations of phytocannabinoids (particularly efficacy issues), various strategies were developed to synthesize cannabinoids artificially in the laboratory. Over years these strategies have evolved significantly, and cannabinoids of distinct chemical class have emerged.5 However, synthetic cannabinoids have unraveled multiple safety concerns. Herein, we provide a brief overview of various components of cannabis and how they contribute to allergy and inflammation.
Endocannabinoids and the Endocannabinoid system (ECS)
While phytocannabinoids were first characterized in 1930’s by R.S. Cahn; first chemical synthesis was achieved in 1940 in the laboratories of R. Adams in the US. and Lord Todd in the U.K.6 that assisted in characterizing their effects on human physiology. However, the receptors for these phytocannabinoids remained elusive for many years and were only discovered much later. In 1992, Dr. Raphael Mechoulam and colleagues based in Israel identified the first endogenous cannabinoid called anandamide,7 six decades after the discovery of the key plant phytocannabinnoid structures by Dr. Roger Adams in the U.S.6 Around the same time, the receptors that constitute the ECS were discovered by Dr. Herkenham in 1990 at the US National Institute of Mental Health (NIMH).8
The ECS consists of (i) endogenous lipid-based endocannabinoid ligands; (ii) their receptors such as the G protein-coupled receptors CB1, CB2,9 and transient receptor potential vanilloid 1 (TRPV1) channel; and (iii) the enzymes such as fatty acid amide hydrolyase (FAAH) that regulate the levels of endocannabinoids in vivo.5 In recent years, additional GPCRs and other targets such as peroxisome proliferator-activated receptors (PPARs) have been reported for cannabinoids.10, 11 Both CB1 and CB2 receptors are G protein-coupled receptors (GPCRs) that preferentially couple with heterotrimeric Gi proteins (inhibit adenylyl cyclases (ACs)). Previously, CB1 was also referred to as the ‘central’ cannabinoid receptor because it is mainly expressed in the central nervous system, and CB2 was referred to as the ‘peripheral’ cannabinoid receptors since they were predominantly found on immune cells. It is now well recognized that both receptor subtypes are ubiquitously expressed in many cell types, including both the central nervous system and peripheral tissues, although their relative distribution is varied.
Endocannabinoids are lipid mediators that belong to the N-acylethanolamine family and are synthesized naturally by human cells from membrane glycerophospholipids that demonstrate signaling capabilities.12 They are synthesized by cellular apparatus relevant for synthesis of eicosanoids (prostaglandins and leukotrienes). In 1992, Dr. Devane and colleagues identified the first endogenous cannabinoid neurotransmitter called anandamide, named after the Sanskrit word ‘ananda’ which means ‘joy or bliss’.7 Anandamide is a partial agonist of CB1 and CB2, with stronger affinity to former. Subsequently, the discovery of virodhamine, named from Sanskrit word ‘virodha’ meaning ‘opposition’, acts as an antagonist at CB1 receptor (and possibly a CB2 receptor agonist). Another endocannabinoid 2-arachidonoylglycerol (2-AG) acts as a full agonist at both receptors.13, 14 Anandamide and 2-AG, together are the most well characterized cannabinoids. Other cannabinoid-like compounds have also been isolated and shown to exhibit anti-inflammatory actions and include lipoamino acids such as elmiric acids (EMAs) are endogenously synthesized, although they are unlikely to act through CB receptors.15
Collectively, elevated endocannabinoid activity has been linked with maintenance of homeostatic functions and are generally considered to be anti-inflammatory. In the CNS, endocannabinoid signaling is deemed to have protective effects on neurons, through regulation of anorexic signals, inhibition of γ-aminobutyric acid (GABA) and suppression of nociception.5 Further, the endocannabinoid system also plays an essential role in managing neuropathic pain in peripheral tissues. Endocannabinoids have also been shown to regulate allergic inflammation. Further, anandamide can inhibit proliferation of B and T cell populations.
Phytocannabinoids
As noted earlier, cannabis plant possesses a rich chemical profile consisting of >100 phytocannabinoids. Constituent cannabinoids demonstrate complex pharmacological behavior that is yet to be completely understood. Cannabidiolic acid (the chemical precursor to cannabidiol), the most abundant constituent of Cannabis sativa, was first isolated in 1955 by Krejči and Šantavy and was initially thought to be strongly antibiotic.16 The next major active ingredient of Cannabis sativa, isolated in 1964, was Δ9-tetrahydrocannabinol (THC).17 We now know that cannabis contains at least 108 exogenous cannabinoids, some of which exert opposing pharmacological and physiological effects. Further, cannabis contains over 200 non-cannabinoid substances with unclear effect on human physiology and disease. This said, THC is the only psychoactive cannabinoid with clearly established mood-altering and hallucinogenic effects, while cannabidiol has been attributed with anti-inflammatory and anti-emetic roles.
1). Delta 9 tetrahydrocannabinol (THC) -
THC is a psychoactive component of marijuana that binds to CB1 receptors to evoke the hallucinogenic effects associated with the drug. CB1 receptor is primarily found in the brain and is more closely associated with mood/cognitive change when stimulated while CB2 is primarily found in peripheral tissues, thus explaining the stronger hallucinogenic and mood-altering capabilities of THC. Smoking marijuana has been linked with compromised microbicidal activity in macrophages and decrease in ciliated epithelial cells, both essential for optimal response to inhaled pathogens.18–20 Some of these effects have been attributed to immunosuppressive actions of THC.19 This immunosuppressive disposition brought on by THC have been investigated in seropositive HIV patients that are elevated risk of developing respiratory infections (pneumonia). While some have suggested that chronic marijuana use may increase risk of pneumonia within the HIV cohort,21 others have failed to demonstrate a clear link.22 However, one study demonstrated that while macrophages from heavy cannabis users have impaired nitric oxide, cellular function can be improved through addition of exogenous cytokines, suggesting that cytokine signaling networks are not perturbed by THC (and possibly other cannabinoids).23 Rather the defect is likely in phagolysosomal processing of foreign materials and induction of an effective inflammatory response.
2). Cannabidiol (CBD) -
Cannabidiol (CBD) is a nonpsychotropic component of cannabis that does not cause the typical marijuana-associated hallucinogenic effects and has major potential for use in several therapeutic areas. In contrast to Δ9-THC, CBD binds very weakly to the CB1 and CB2 receptors while showing potent activity at both receptors in in vitro and in vivo assays24–26. CBD has a very low affinity for CB1 and CB2 receptors (100-fold less than Δ9-THC).26 Further, many actions of CBD seem to be mediated by binding to transient receptor potential vanilloid type 1 (TRPV1),27 G protein-coupled receptor 55 (GPR55),14, 28, 5-hydroxytryptamine receptor subtype 1A (5-HT1A)29, and enzymes associated with endocannabinoid breakdown.30 CBD has been shown to exert anti-inflammatory actions in rodent models for various inflammatory diseases including neuropathies.31–33 Specifically, CBD suppresses expression of proinflammatory cytokines such as TNF-α. Although a partial agonist for the vanilloid receptors, CBD has been shown to function by activating TRPV1 in several models of inflammation, although a definitive physiological link remains unclear.32, 33 The anti-inflammatory effects of CBD in experimental hepatitis has been established to be TRPV1-dependent and associated with increased MDSC numbers in liver. However, in mice deficient in Trpv1 (Trpv1−/−), introduction of CBD induced accumulation of CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs) in the peritoneum and was independent of TRPV1.34
Among other cannabinoids, cannabinol, cannabigerol and cannabichromene are the other most commonly isolated phytocannabinoids, however their pharmacological interactions are poorly understood. These compounds may exert anti-inflammatory actions and cannabichromene and cannabigerol have been shown to regulate inflammation in colitis models.35, 36 Among other compounds from cannabis, caryophyllene (a sesquiterpene and a cannabinoid) has also been shown to interact with CB receptors (specifically, CB2) and contribute to anti-inflammatory responses,37
In summary, cannabinoids from exogenous sources typically demonstrate affinity to distinct class of receptors and channels. Cannabis strains with a THC-rich pharmacological profile have gained prominence in recent decade with the concurrent development of synthetic mimics that demonstrate higher affinity to the metabotropic CB receptors. While administration of these cannabinoids could provide a more efficient means to exert desirable (therapeutic) effects, consumption of cannabinoids from such sources could potentially overwhelm the ECS system. Exposure to high levels of these substances is often beyond the physiological capacity of the receptors and raises concerns for non-specific effects or side-effects. The broad immunomodulatory actions of phytocannabinoids (and endocannabinoids) appear to be disposed to regulate inflammation. Therefore, to untap the true therapeutic potential of this system it is essential to develop understanding of the pharmacokinetics/pharmacodynamics of these compounds in addition to potential for interactions, to mitigate the undesirable effects.
Cannabis and allergic inflammation
Cannabis proteins and allergy
As early as 1971 there were official reports of the medical manifestations of cannabis that included allergic manifestations.38, 39 Skin prick testing (SPT) with water-soluble antigen suggested that cannabis allergy is not mediated by THC (owing to its poor solubility in water and requires organic solvents).40 Recent investigations into allergic sensitization to cannabis have focused on identifications cannabis proteins (allergens) that can potentially stimulate an IgE-mediated response. Studies have suggested a role for type I hypersensitivity mechanisms. IgE reactivity to Cannabis sativa proteins in western blots was heterogeneous and ranged from 10 to 70 kDa with the identification of a 23-kDa oxygen-evolving enhancer protein 2 (OEEP2) and a 50-kDa protein identified to be the photosynthetic enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBiSCo).3 In the same study, additional proteins were identified in the proteomic analysis, including those from adenosine triphosphate synthase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, and luminal binding protein (heat shock protein 70), suggesting these proteins are potential allergens. Deglycosylation studies helped refine protein allergen identification and demonstrated significant IgE antibodies against plant oligosaccharides that could help partially explain cross-reactivity.
Studies in Europe have identified lipid transfer protein (LTP, Can s 3) as a major allergen of cannabis.41–46 Moreover, LTP is also understood to be the driver of cross-reactive allergies to other plant foods.47 Reports suggest that C. sativa sensitization may be mediated by 2 mechanisms, cross-reactivity (mainly with LTP and thaumatin-like protein) and exposure-related de novo sensitization.48
Owing to increasing access for cannabis grow options within private settings, there are concerns regarding the allergenicity of cannabis pollen. Studies on allergenic potential of cannabis pollen are limited. Previously, studies have identified a 14 kDa protein fragment (potentially LTP) from C. sativa pollen extract.49 It should be noted though that most cannabis plants that are enriched for THC are female plants and approaches exist to cultivate plants in absence of male plants.
The allergenic potential of the ECS system
Allergic inflammation to cannabis is typically manifested by cutaneous manifestations including urticaria and angioedema as well as nasal, ocular, and even allergic asthma, but it is unclear if cannabinoids can act as low molecular weight (LMW) allergens, thus directly influencing immunological outcomes. In allergy, endocannabinoid release is typically observed with the associated inflammation. For e.g., 2-arachidonoylglycerol (2-AG) levels increase under inflammation induced by oxazolone, a hapten that promotes delayed-type hypersensitivity reactions in skin.50 Further, blocking 2-AG using a CB2 receptor antagonist (and to a lesser extent CB1 antagonist) limits swelling and the expression of proinflammatory cytokines induced by oxazolone. Elsewhere, other endocannabinoids have been shown to mitigate inflammation. In murine dermal allergy models, endocannabinoids such as N-palmitoyl ethanolamine exerted anti-edema and anti-inflammatory effects that are independent of CB1 and CB2 receptors and likely to involve GPR55 (coupling to G protein G12/13) and PPAR-α.11 Similarly, N-stearoyl ethanolamine inhibited edema in a mast-cell mediated allergy model using dermal exposure to 2,4-dinitrophenyl.51 Further, N-stearoyl ethanolamine effects are not mediated by CB1/CB2 receptors but possibly via the heat- and capsaicin-sensitive excitatory TRPV1 channel (aka the vanilloid receptor 1 or capsaicin receptor) that are expressed on sensory neurons and more importantly on mast cells. These findings suggest that endocannabinoids may be directly involved in homeostatic as well as pathological features associated with allergy (particularly in skin) and is dependent on the specific endocannabinoid. Nevertheless, preliminary studies implicate CB2 in the role of endocannabinoids in sensitization and elicitation phases of the reaction. Finally, mice deficient in fatty acid amide hydrolase (FAAH) which elevates circulating anandamide levels are fairly protected from development of allergic inflammation.52
Thus, allergenic potential of endocannabinoids is nuanced. Although the molecular mechanisms are only now being appreciated, it is increasingly becoming clear that cannabinoids may modulate mast cell functions. In contrast, very little is understood about the allergenic potential of synthetic cannabinoids and phytocannabinoids. Highly selective agonists of CB2 receptor may worsen allergic inflammation, whereas non-selective CB1/CB2 agonists are protective.51 The ECS system is fine tuned to respond to changes in endocannabinoid levels and exposure to synthetic cannabinoids and phytocannabinoids (typically a higher pharmacological exposure) may promote dysfunction in the ECS, and additional studies are essential in examining allergenic potential from these sources.
Synthetic- and phyto- cannabinoids in allergy therapeutics
As discussed above, endocannabinoids may act as mediators of allergic inflammation. Indeed, in murine allergy models, targeting of cannabinoid receptors (CB1 and CB2) with antagonists to limit endocannabinoid signaling protects from the development of cutaneous pathology associated with dermal allergy.50 Studies employing synthetic cannabinoids have indicated complex pharmacological behavior of the system in allergy models. While some have suggested that antagonists of CB2 receptor may provide relief from the symptoms of dermal allergic reactions,51 others have contended that antagonists of cannabinoid receptors worsen allergic inflammation while agonists alleviate associated symptoms.52 Thus, whether modulation of ECS components using pharmacological agents would presumably be an attractive approach to mitigate inflammation remains to be clarified and appears to be dependent on the context of allergic inflammation and prevalent circulating endocannabinoids.
Immunodulatory actions of exogenously synthesized cannabinoids are well documented. For e.g., synthetic cannabinoid HU-44 lowers the formation of tumor necrosis factor (TNF)-alpha, a proinflammatory cytokine, and was found to be an oral antiarthritic therapeutic in murine collagen-induced arthritis in vivo.25 Another example of this immunoregulatory action is SMM-187, a CB2 receptor inverse agonist, which was recently shown to have potent anti-inflammatory activity. Specifically, it inhibits production of pro-inflammatory cytokines including IFN-gamma, IL-6, and IL-12p70, while inhibiting chemokines IL-8, MCP-1, MIP-1b, CCL17, MDC, and eotaxin-3 in activated primary human microglia.53, 54 In studies with microglia and traumatic brain injury (TBI), SMM-187’s anti-inflammatory activity is coupled with increased phosphorylation of cAMP response element binding protein (CREB).55
In a recent study, Palomares and colleagues demonstrated that synthetic compound WIN55212-2 (a CB1 agonist) can actively inhibit dendritic cell activation (in vitro) and migration to lymph nodes (in vivo).56 Further, the study indicated reduction in allergen-specific IgE and IgG in mice treated with the synthetic cannabinoid. It was also observed that IL-5 producing T cell numbers are contracted because of WIN55212-2 treatment, while regulatory T cell populations expanded. However, it is unclear if these effects are brought on solely in a CB1 receptor -dependent manner or through other targets such as peroxisome proliferator-activated receptors, PPARα or PPARγ. Collectively, the studies show that the ‘sensitization phase’ as well as the ‘effector phase’ of allergen response are accessible for ligands targeting the ECS.
Investigations into CB receptor biology and function (on agonist or antagonist binding) has yielded some clues into subcellular signaling. The CB2 receptor regulates production of cAMP, mitogen activated protein kinase (MAPK), modulation of intracellular calcium,57 and affects a broad spectrum of signaling pathways including critical transcriptional factors such as NF-kB,58, 59 NFAT,60 and AP-1.59 CB2 receptors are coupled to Gi proteins and consequently agonists decrease intracellular cAMP and inverse agonists increase intracellular cAMP.61 CB2 receptor antagonists may also act as inverse agonists and can suppress inflammatory pathways via activation of ACs, which consequently increases cellular cAMP and subsequently activates protein kinase A (PKA). The activation of PKA results in signaling transduction and phosphorylation of its substrates, the cAMP response binding element binding protein (CREB) and CREB binding protein (CBP) to co-activate cAMP response element (CRE) that leads to anti-inflammatory responses.62, 63
In summary, the modulation of ECS components through pharmacological means could unlock novel therapeutic opportunities. More studies are needed to clarify the role of ECS components. Endogenously produced cannabinoids drive inflammation through CB receptors,50 suggesting blocking of CB receptors may be beneficial in regulating allergy. However, genetic deletion of these receptors appears to exacerbate allergy.52 Other strategies including blocking FAAH have also emerged, but more studies are needed to contextualize their therapeutic benefits.
Mast cells and regulation of allergic inflammation by cannabinoids
In recent years, mast cells have emerged as critical players in allergy mediated by cannabinoids. Experimentally used mast cell lines express both CB1 and CB2 receptors that on activation by CB2 agonists or non-selective agonists induce activation of extracellular signal-regulated kinase (ERK) and AKT.64 (Figure 1) Further, CB1 receptor function appears to be coupled with secretory functions (degranulation) linked to FcεRI on mast cells.65, 66 However, the precise mechanism by which CB1 receptors regulate degranulation in mast cells are poorly defined. Theoretically, these receptors are coupled to heterotrimeric Gi G proteins that suppress cAMP, however elevation of cAMP is also linked with inhibition of secretory function. Thus, cannabinomimetic compounds (i.e., agents able to modulate endocannabinoid function) are considered as an intriguing class of regulators of mast cell behavior. Elsewhere others have shown that Δ9-THC augments β-hexosaminidase release, which is a marker of mast cell activation in rat mast cell line (RBL-2H3), murine mast cell line (MC/9) and isolated primary peritoneal mast cells.67 In mast cell-mediated dermal allergy models of allergy, synthetic cannabinoids such as JWH-133 (selective CB2 agonist) have been shown to promote inflammation, whereas non-selective CB receptor agonists inhibit inflammation.51
Figure 1: Model for CB1 and CB2 receptor signaling and function in mast cells.
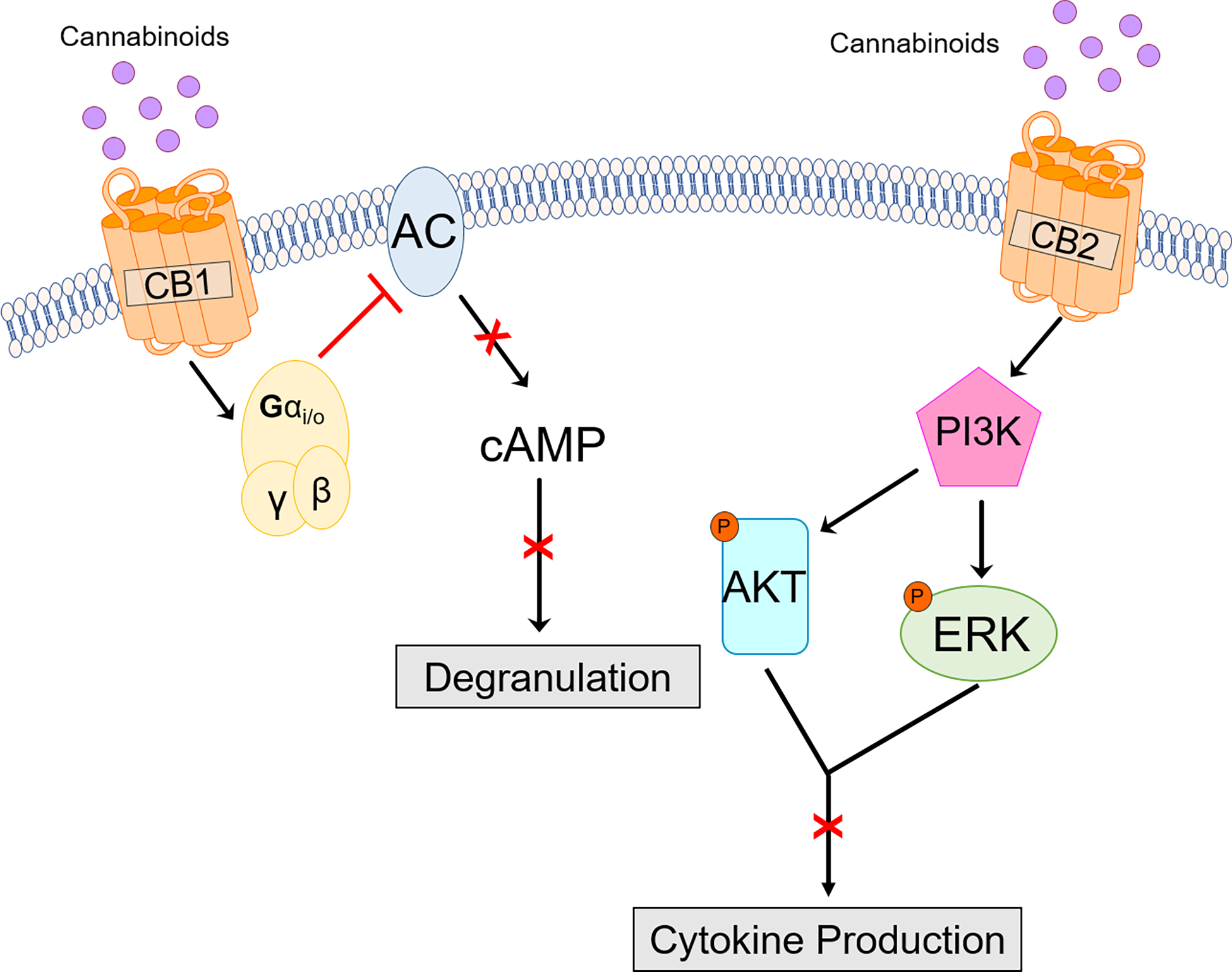
CB1 receptors are G protein-coupled receptors that in theory on activation inhibit adenylyl cyclases (AC) which is essential for generation of second messenger cAMP and consequently regulating degranulation in an FcεRI-dependent manner. CB2 receptors are also GPCRs and may regulate mast cell cytokine release by PI3K-dpeendent mechanisms.
Integrative models of allergic inflammation make it difficult to ascertain the relative contribution of mast cells to the development of allergic reactions owing to presence of CB2 on other local immune cells types such as dermal Langerhans cells.50 Further, there is lack of clarity on whether epidermal keratinocytes express CB2 receptors making it difficult to define the contribution by non-immune cells.50, 52, 68, 69
Cannabinoids in ocular allergy therapy
Cannabinoids are beginning to be investigated in their potential roles in ocular surface inflammation and pain, as CB1 receptors are expressed in the corneal epithelium and endothelium in rodents and primates.70, 71 Human and murine conjunctiva also widely express CB1 and CB2 receptors in the epithelial layers including goblet cells.72
Functional responses were mediated by stimulation of cannabinoid receptors (CB1 and CB2) with CP 55,940. CP 55,940 is a potent cannabinoid analogue that acts on both CB1 and CB2. This resulted in decreased cAMP levels, proliferation, and modulation of stress signaling pathways thus regulating epithelial renewal and inflammatory processes at the ocular surface.72 Most epithelial cells in conjunctiva demonstrate CB1 and TRPV1 co-localization based on overlapping immunostaining.73 Injury to epithelial cells activates TRPV1 inducing proinflammatory cytokines and chemoattractant expression. CB1 activation after epithelial injury leads to reduction of inflammation. Injury also activates a protein-protein interaction between TRPV1 and CB1, which downregulates TRPV1-induced inflammation in an adaptive manner.74
Natural or synthetic cannabinoid extracts delivery to the ocular surface is challenging due to their highly lipophilic profile that would require emulsification in formulating a water-oil formulation. This may be appropriate for the ocular surface disorders but limits its intraocular delivery for the treatment of conditions such as glaucoma. A few approaches were proposed to overcome this challenge. Initially these involved the use of light mineral oil as a vehicle. Low concentrations of THC (0.1%) were dissolved in light mineral oil and applied directly to the eyes of human subjects with high blood pressure. This caused a decrease in systolic blood pressure (pressure in blood vessels felt immediately when the heart contracts and pumps), which can reduce intraocular pressure. But ultimately the diluted THC-infused mineral oil proved to be an irritant to the human eye, which precluded its use as an anti-inflammatory remedy.75, 76
Some studies have shown that different micro-emulsions and cyclodextrins (cyclic sugar molecules) can enhance the epithelial and corneal penetration of endogenous cannabinoids. These formulations were tested for their ability to lower intraocular pressure, with successful results. Perhaps a similar solution could be utilized to deliver CBD and other plant cannabinoids as a treatment for corneal neuropathic pain and ocular surface inflammatory disorders.77
Finally, other strategies that increase the levels of endocannabinoids may have therapeutic potential at least in context of allergic diseases. One approach would be to block the fatty acid amide hydrolase (FAAH), which is involved in breakdown of endocannabinoids, thus sustaining availability of endocannabinoid pool. The potential for such strategy has been discussed in context of pain management and neurological disorders.78
Other cannabis components and their impact on inflammation
In addition to cannabinoids, cannabis also expresses dozens of other pharmacologically active compounds (e.g., terpenes and flavonoids) that interact with multiple tissues systems to mediate a myriad of physiologic and psychologic effects Cannabis plant is a major source of cannabinoids that can have a variety of effects on human health and physiology. However, cannabis contains other substances that carry the potential to impact human health. Herein, we briefly describe these substances and discuss their benefits or harms to human health as it specifically relates to inflammation.
Flavonoids
Flavonoids are secondary polyphenolic metabolites that commonly have a ketone group and yellowish pigments, after which they are named (from the Latin flavus, “yellow”) and have a multitude of functions. These span from regulating plant development, pigmentation, and UV protection, to an array of roles in defense and signaling between plants and microorganisms.
Because of their prevalence in the human diet, many flavonoids constitute important components of medicinal plants and are used in the control of inflammation that naturally occur in fruits, vegetables, tea, and wine. Fruits and vegetables that provide a considerable concentration of flavonoids per 100 g include citrus fruits, blueberries, blackberries, onions, peppers, green tea, as well as dried oregano and parsley.79 Ongoing research has proposed flavonoids have numerous health benefits.
In allergic disorders, with the most well-documented evidence published to date on the inhibitory action of flavonoids on mast cells.34, 80–83 Interestingly, research in this area dates back to reports on the use of the flavonoid also found in extracts of cannabinoids, quercetin, on mast cells.84, 85 Flavonoids also include derivatives of the herbal cannabis plant that include cannflavin A, cannflavin B, cannflavin C, vitexin, isovitexin, apigenin, kaempferol, quercetin, luteolin and orientin.86 Quercetin as noted above was one of the earliest flavonoids to be studied in allergic inflammatory disorders84 and mentioned of its potential use in complementary treatment of allergic conjunctivitis.87 The distribution of these in the plant varies depending on the type of flavonoid, but none have been found in the root system of the Cannabis plant.
Impact on clinical practice
As of 2022, thirty-seven states, three territories and the District of Columbia allow the medical use of cannabis products. Further, in 2012, the states of Colorado and Washington legalized recreational marijuana leading to many other states following their lead to presently 17 states. In many states where recreational use of marijuana is illegal, certain cities have decriminalized possession and use. Thus, trends from the past decade indicate continued expansion of accessibility of cannabis for medical and recreational purposes. Consequently, it is expected that reports of cannabis allergies will become common. The allergy symptoms although usually benign include nasal, ocular, and pulmonary complaints. However, life-threatening anaphylactic reactions are rare and are generally limited to hempseed consumption.88 With the increasing availability, the risk of allergies due to both accidental and prescribed exposures is possible. Physicians treating allergy must keep in mind that their patients could be exposed to this antigen. Recently, The International Cannabis Allergy Collaboration published detailed recommendations for management of patients with symptomatic exposures to cannabis.89 Skin testing is helpful diagnostic tool, but only a fraction of allergists apply this technique.90 Accessibility to hemp (cannabis containing < 0.3% THC) may help overcome the current limitations in clinical evaluation of allergic sensitization, although a standardized extract is not yet available. Further, diagnostic approaches for serological analysis of cannabis allergen specific IgE are in development but are in early stages of development. For most cases, avoidance of cannabis is currently a preferred approach to limit symptomatic outcomes, although this is a challenge in occupational settings.
In summary, owing to the immunomodulatory and allergenic potential of cannabis, engaging with patients about their cannabis use may provide helpful clues in clinical management of therapeutic outcomes.
Conclusions
In the face of growing opioid crisis, cannabis and its constituents have emerged as safer alternatives to manage pain. Consequently, neuromodulatory attributes of cannabinoids and the ECS have been a subject of intense investigations in recent years. However, cannabinoid receptors and relevant enzymes and proteins are ubiquitously expressed in diverse physiological systems underscoring their accessibility as pharmacological targets. Cannabinoids can also have immunomodulatory actions, although very little is understood as to how individual components interact with distinct immunological compartments. Endogenously synthesized cannabinoids have been demonstrated to have immunoregulatory actions. Further, phytocannabinoids such as THC may be immunosuppressive while cannabidiol may drive anti-inflammatory responses. However, receptors of the ECS are broadly tuned and very little is understood about over stimulation of these systems which may be likely in chronic and heavy users of cannabis. Also, proteins from cannabis can also drive allergic sensitization in increasing number of individuals. Increased access to cannabis plant in its various consumer forms will boost research, which is essential since cannabis access is becoming increasingly pervasive. Our collective understanding of the public health implications (benefits and harms) of cannabis is likely fraught with preconceived notions and biases. Cannabis and cannabinoids are primarily consumed by either inhalation, oral or topical administration. There are unique pharmacokinetic and safety profiles endowed to each route.91 Inhalational route results in rapid circulation of cannabinoids and is a preferred route,92 however, concerns remain on inhalation use of natural cannabis plant, which is not a recommended route of administration for medical use of cannabis in some states. Additional studies are needed to clarify the context of exposure to cannabis (including its components) to fine tune the potentially beneficial immunomodulatory effects (improved immune function) from deleterious ones (acute lung injury, compromised immune function). This is a clarion call to strategize a research and clinical practice framework to understand how cannabis and components interact with human physiological systems to enable contextualizing of the beneficial facets from harmful effects.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institute of Allergy and Infectious Diseases/National Institutes of Health (grant no. R21AI140411 to A.P.N.) and from The Lambert Center for the Study of Medicinal Cannabis & Hemp (Thomas Jefferson University, Philadelphia).
Abbreviations:
- 2-AG
2-arachidonoylglycerol
- ACs
adenylyl cyclases
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CBD
cannabidiol
- CBP
CREB binding protein
- CREB
cAMP response element binding protein
- ECS
endocannabinoid system
- EMAs
elmiric acids
- ERK
extracellular signal-regulated kinase
- FAAH
fatty acid amide hydrolyase
- GABA
Gamma aminobutyric acid
- GPCR
G protein-coupled receptor
- LTP
lipid transfer protein
- MDSCs
myeloid-derived suppressor cells
- OEEP
oxygen-evolving enhancer protein
- PKA
protein kinase A
- PPAR
peroxisome proliferator-activated receptor
- RuBiSCo
ribulose-1,5-bisphosphate carboxylase/oxygenase
- SCOTUS
The Supreme Court of United States
- THC
tetrahydrocannabinol
- TNF-alpha
tumor necrosis factor-alpha
- TRPV1
transient receptor potential vanilloid 1 channel
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mechoulam R The most comprehensive history of the recreational and medicinal use of Cannabis throughout the centuries. In: Mechoulam R, editor. Cannabis as Theraputic Agent. Boca Raton, FL: CRC Press; 1986. p. 1–19. [Google Scholar]
- 2.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 2001; 109 Suppl 1:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nayak AP, Green BJ, Sussman G, Berlin N, Lata H, Chandra S, et al. Characterization of Cannabis sativa allergens. Ann Allergy Asthma Immunol 2013; 111:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson C, Opacka-Juffry J, Arevalo-Martin A, Garcia-Ovejero D, Molina-Holgado E, Molina-Holgado F. Spicing Up Pharmacology: A Review of Synthetic Cannabinoids From Structure to Adverse Events. Adv Pharmacol 2017; 80:135–68. [DOI] [PubMed] [Google Scholar]
- 5.Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004; 3:771–84. [DOI] [PubMed] [Google Scholar]
- 6.Adams R, Hunt M, Clark JH. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. J Am Chem Soc 1940; 62:196–200. [Google Scholar]
- 7.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258:1946–9. [DOI] [PubMed] [Google Scholar]
- 8.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 1990; 87:1932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365:61–5. [DOI] [PubMed] [Google Scholar]
- 10.Ambrose T, Simmons A. Cannabis, Cannabinoids, and the Endocannabinoid System-Is there Therapeutic Potential for Inflammatory Bowel Disease? J Crohns Colitis 2019; 13:525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 2005; 67:15–9. [DOI] [PubMed] [Google Scholar]
- 12.Schmid HH. Pathways and mechanisms of N-acylethanolamine biosynthesis: can anandamide be generated selectively? Chem Phys Lipids 2000; 108:71–87. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol 2005; 40:2–14. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 2010; 62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burstein SH, Zurier RB. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J 2009; 11:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelepukha SI, Rabinovich AS, Pochinok PY, Negrash AK, Kudryavtsev VA. Antimicrobial properties of preparations from hemp-cansantin. Mikrob Zh Akad Nauk Ukr 1963; 25:42–6. [Google Scholar]
- 17.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964; 86:1646–7. [Google Scholar]
- 18.Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med 1997; 156:1606–13. [DOI] [PubMed] [Google Scholar]
- 19.Roth MD, Whittaker K, Salehi K, Tashkin DP, Baldwin GC. Mechanisms for impaired effector function in alveolar macrophages from marijuana and cocaine smokers. J Neuroimmunol 2004; 147:82–6. [DOI] [PubMed] [Google Scholar]
- 20.Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013; 10:239–47. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz DR, Uno H, Wolinsky SM, Gabuzda D. Effect of marijuana smoking on pulmonary disease in HIV-infected and uninfected men: a longitudinal cohort study. EClinicalMedicine 2019; 7:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quint JJ, Tashkin DP, McKay HS, Plankey MW, Stosor V, Friedman MR, et al. Marijuana use and pneumonia risk in a cohort of HIV-infected and HIV-uninfected men. Ann Epidemiol 2020; 52:64–70 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shay AH, Choi R, Whittaker K, Salehi K, Kitchen CM, Tashkin DP, et al. Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. J Infect Dis 2003; 187:700–4. [DOI] [PubMed] [Google Scholar]
- 24.Reggio PH. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr Med Chem 2010; 17:1468–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haj CG, Sumariwalla PF, Hanus L, Kogan NM, Yektin Z, Mechoulam R, et al. HU-444, a Novel, Potent Anti-Inflammatory, Nonpsychotropic Cannabinoid. J Pharmacol Exp Ther 2015; 355:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 2007; 150:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol 2004; 143:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pertwee RG. GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol 2007; 152:984–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 2005; 30:1037–43. [DOI] [PubMed] [Google Scholar]
- 30.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001; 134:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumariwalla PF, Gallily R, Tchilibon S, Fride E, Mechoulam R, Feldmann M. A novel synthetic, nonpsychoactive cannabinoid acid (HU-320) with antiinflammatory properties in murine collagen-induced arthritis. Arthritis Rheum 2004; 50:985–98. [DOI] [PubMed] [Google Scholar]
- 32.Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, et al. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol 2004; 369:294–9. [DOI] [PubMed] [Google Scholar]
- 33.Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 2007; 556:75–83. [DOI] [PubMed] [Google Scholar]
- 34.Hegde VL, Singh UP, Nagarkatti PS, Nagarkatti M. Critical Role of Mast Cells and Peroxisome Proliferator-Activated Receptor gamma in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J Immunol 2015; 194:5211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borrelli F, Fasolino I, Romano B, Capasso R, Maiello F, Coppola D, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol 2013; 85:1306–16. [DOI] [PubMed] [Google Scholar]
- 36.Couch DG, Maudslay H, Doleman B, Lund JN, O’Sullivan SE. The Use of Cannabinoids in Colitis: A Systematic Review and Meta-Analysis. Inflamm Bowel Dis 2018; 24:680–97. [DOI] [PubMed] [Google Scholar]
- 37.Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A 2008; 105:9099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tennant FS Jr.,Prendergast TJ. Medical manifestations associated with hashish. JAMA 1971; 216:1965–9. [PubMed] [Google Scholar]
- 39.Liskow B, Liss JL, Parker CW. Allergy to marihuana. Ann Intern Med 1971; 75:571–3. [DOI] [PubMed] [Google Scholar]
- 40.Anibarro B, Fontela JL. Allergy to marihuana. Allergy 1996; 51:200–1. [DOI] [PubMed] [Google Scholar]
- 41.Decuyper II, Faber MA, Lapeere H, Mertens C, Rihs HP, Van Gasse AL, et al. Cannabis allergy: A diagnostic challenge. Allergy 2018; 73:1911–4. [DOI] [PubMed] [Google Scholar]
- 42.Decuyper II, Faber MA, Sabato V, Bridts CH, Hagendorens MM, Rihs HP, et al. Where there’s smoke, there’s fire: cannabis allergy through passive exposure. J Allergy Clin Immunol Pract 2017; 5:864–5. [DOI] [PubMed] [Google Scholar]
- 43.Decuyper II, Van Gasse AL, Cop N, Sabato V, Faber MA, Mertens C, et al. Cannabis sativa allergy: looking through the fog. Allergy 2017; 72:201–6. [DOI] [PubMed] [Google Scholar]
- 44.Decuyper II, Van Gasse AL, Faber MA, Elst J, Mertens C, Rihs HP, et al. Exploring the Diagnosis and Profile of Cannabis Allergy. J Allergy Clin Immunol Pract 2019; 7:983–9 e5. [DOI] [PubMed] [Google Scholar]
- 45.Decuyper I, Ryckebosch H, Van Gasse AL, Sabato V, Faber M, Bridts CH, et al. Cannabis Allergy: What do We Know Anno 2015. Arch Immunol Ther Exp (Warsz) 2015; 63:327–32. [DOI] [PubMed] [Google Scholar]
- 46.Rihs HP, Armentia A, Sander I, Bruning T, Raulf M, Varga R. IgE-binding properties of a recombinant lipid transfer protein from Cannabis sativa. Ann Allergy Asthma Immunol 2014; 113:233–4. [DOI] [PubMed] [Google Scholar]
- 47.Armentia A, Herrero M, Martin-Armentia B, Rihs HP, Postigo I, Martinez-Quesada J. Molecular diagnosis in cannabis allergy. J Allergy Clin Immunol Pract 2014; 2:351–2. [DOI] [PubMed] [Google Scholar]
- 48.Larramendi CH, Lopez-Matas MA, Ferrer A, Huertas AJ, Pagan JA, Navarro LA, et al. Prevalence of sensitization to Cannabis sativa. Lipid-transfer and thaumatin-like proteins are relevant allergens. Int Arch Allergy Immunol 2013; 162:115–22. [DOI] [PubMed] [Google Scholar]
- 49.Choudhary S, Murad S, Hayat MQ, Shakoor Z, Arshad M. Identification of IgE- binding pollen protein from Cannabis sativa in pollen-hypersensitive patients from north Pakistan. Pak J Pharm Sci 2017; 30:37–42. [PubMed] [Google Scholar]
- 50.Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol 2006; 177:8796–805. [DOI] [PubMed] [Google Scholar]
- 51.Dalle Carbonare M, Del Giudice E, Stecca A, Colavito D, Fabris M, D’Arrigo A, et al. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: a neglected story. J Neuroendocrinol 2008; 20 Suppl 1:26–34. [DOI] [PubMed] [Google Scholar]
- 52.Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 2007; 316:1494–7. [DOI] [PubMed] [Google Scholar]
- 53.Presley C, Abidi A, Suryawanshi S, Mustafa S, Meibohm B, Moore BM. Preclinical evaluation of SMM-189, a cannabinoid receptor 2-specific inverse agonist. Pharmacol Res Perspect 2015; 3:e00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reiner BC, Dunaevsky A. Deficit in motor training-induced clustering, but not stabilization, of new dendritic spines in FMR1 knock-out mice. PLoS One 2015; 10:e0126572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiner A, Heldt SA, Presley CS, Guley NH, Elberger AJ, Deng Y, et al. Motor, visual and emotional deficits in mice after closed-head mild traumatic brain injury are alleviated by the novel CB2 inverse agonist SMM-189. Int J Mol Sci 2014; 16:758–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angelina A, Jimenez-Saiz R, Perez-Diego M, Maldonado A, Ruckert B, Akdis M, et al. Cannabinoid WIN55212–2 impairs peanut-allergic sensitization and promotes the generation of allergen-specific regulatory T cells. Clin Exp Allergy 2022; 52:540–9. [DOI] [PubMed] [Google Scholar]
- 57.Demuth DG, Molleman A. Cannabinoid signalling. Life Sci 2006; 78:549–63. [DOI] [PubMed] [Google Scholar]
- 58.Juttler E, Potrovita I, Tarabin V, Prinz S, Dong-Si T, Fink G, et al. The cannabinoid dexanabinol is an inhibitor of the nuclear factor-kappa B (NF-kappa B). Neuropharmacology 2004; 47:580–92. [DOI] [PubMed] [Google Scholar]
- 59.Toguri JT, Lehmann C, Laprairie RB, Szczesniak AM, Zhou J, Denovan-Wright EM, et al. Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis. Br J Pharmacol 2014; 171:1448–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaplan BL, Springs AE, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol 2008; 76:726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kendall DA, Alexander SPH. Behavioral neurobiology of the endocannabinoid system. Berlin: Heidelberg, Springer-Verlag; 2009. [Google Scholar]
- 62.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol 2010; 185:6413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bu W, Ren H, Deng Y, Del Mar N, Guley NM, Moore BM, et al. Mild Traumatic Brain Injury Produces Neuron Loss That Can Be Rescued by Modulating Microglial Activation Using a CB2 Receptor Inverse Agonist. Front Neurosci 2016; 10:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samson MT, Small-Howard A, Shimoda LM, Koblan-Huberson M, Stokes AJ, Turner H. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. J Immunol 2003; 170:4953–62. [DOI] [PubMed] [Google Scholar]
- 65.Nam G, Jeong SK, Park BM, Lee SH, Kim HJ, Hong SP, et al. Selective Cannabinoid Receptor-1 Agonists Regulate Mast Cell Activation in an Oxazolone-Induced Atopic Dermatitis Model. Ann Dermatol 2016; 28:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serra-Pages M, Olivera A, Torres R, Picado C, de Mora F, Rivera J. E-prostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. J Leukoc Biol 2012; 92:1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giudice ED, Rinaldi L, Passarotto M, Facchinetti F, D’Arrigo A, Guiotto A, et al. Cannabidiol, unlike synthetic cannabinoids, triggers activation of RBL-2H3 mast cells. J Leukoc Biol 2007; 81:1512–22. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A 2005; 102:3093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maccarrone M, Di Rienzo M, Battista N, Gasperi V, Guerrieri P, Rossi A, et al. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J Biol Chem 2003; 278:33896–903. [DOI] [PubMed] [Google Scholar]
- 70.Straiker A, Stella N, Piomelli D, Mackie K, Karten HJ, Maguire G. Cannabinoid CB1 receptors and ligands in vertebrate retina: localization and function of an endogenous signaling system. Proc Natl Acad Sci U S A 1999; 96:14565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci 1999; 40:2442–8. [PubMed] [Google Scholar]
- 72.Iribarne M, Torbidoni V, Julian K, Prestifilippo JP, Sinha D, Rettori V, et al. Cannabinoid receptors in conjunctival epithelium: identification and functional properties. Invest Ophthalmol Vis Sci 2008; 49:4535–44. [DOI] [PubMed] [Google Scholar]
- 73.De Filippis D, D’Amico A, Iuvone T. Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J Neuroendocrinol 2008; 20 Suppl 1:20–5. [DOI] [PubMed] [Google Scholar]
- 74.Assimakopoulou M, Pagoulatos D, Nterma P, Pharmakakis N. Immunolocalization of cannabinoid receptor type 1 and CB2 cannabinoid receptors, and transient receptor potential vanilloid channels in pterygium. Mol Med Rep 2017; 16:5285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green K, Roth M. Ocular effects of topical administration of delta 9-tetrahydrocannabinol in man. Arch Ophthalmol 1982; 100:265–7. [DOI] [PubMed] [Google Scholar]
- 76.Jay WM, Green K. Multiple-drop study of topically applied 1% delta 9-tetrahydrocannabinol in human eyes. Arch Ophthalmol 1983; 101:591–3. [DOI] [PubMed] [Google Scholar]
- 77.Jarho P, Urtti A, Jarvinen K, Pate DW, Jarvinen T. Hydroxypropyl-beta-cyclodextrin increases aqueous solubility and stability of anandamide. Life Sci 1996; 58:PL 181–5. [DOI] [PubMed] [Google Scholar]
- 78.Ahn K, Johnson DS, Cravatt BF. Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders. Expert Opin Drug Discov 2009; 4:763–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kozlowska A, Szostak-Wegierek D. Flavonoids--food sources and health benefits. Rocz Panstw Zakl Hig 2014; 65:79–85. [PubMed] [Google Scholar]
- 80.Lau AH, Chow SS. Effects of cannabinoid receptor agonists on immunologically induced histamine release from rat peritoneal mast cells. Eur J Pharmacol 2003; 464:229–35. [DOI] [PubMed] [Google Scholar]
- 81.Jonsson KO, Persson E, Fowler CJ. The cannabinoid CB2 receptor selective agonist JWH133 reduces mast cell oedema in response to compound 48/80 in vivo but not the release of beta-hexosaminidase from skin slices in vitro. Life Sci 2006; 78:598–606. [DOI] [PubMed] [Google Scholar]
- 82.Mainardi T, Kapoor S, Bielory L. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J Allergy Clin Immunol 2009; 123:283–94; quiz 95–6. [DOI] [PubMed] [Google Scholar]
- 83.Schlosburg JE, Boger DL, Cravatt BF, Lichtman AH. Endocannabinoid modulation of scratching response in an acute allergenic model: a new prospective neural therapeutic target for pruritus. J Pharmacol Exp Ther 2009; 329:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Middleton E, Jr., Drzewiecki G, Krishnarao D. Quercetin: an inhibitor of antigen-induced human basophil histamine release. J Immunol 1981; 127:546–50. [PubMed] [Google Scholar]
- 85.Middleton E Jr., Krishnarao DG, Atkins D, Drzewiecki G. The flavonoids: a brief review and study of effects on antigen-induced histamine release from human basophils. Trans Am Clin Climatol Assoc 1981; 92:234–52. [PMC free article] [PubMed] [Google Scholar]
- 86.Andre CM, Hausman JF, Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front Plant Sci 2016; 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: Diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol 2020; 124:118–34. [DOI] [PubMed] [Google Scholar]
- 88.Stadtmauer G, Beyer K, Bardina L, Sicherer SH. Anaphylaxis to ingestion of hempseed (Cannabis sativa). J Allergy Clin Immunol 2003; 112:216–7. [DOI] [PubMed] [Google Scholar]
- 89.Skypala IJ, Jeimy S, Brucker H, Nayak AP, Decuyper II, Bernstein JA, et al. Cannabis-related allergies: An international overview and consensus recommendations. Allergy 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeiger JS, Silvers WS, Naimi DR, Skypala IJ, Ellis AK, Connors L, et al. Impact of cannabis knowledge and attitudes on real-world practice. Ann Allergy Asthma Immunol 2022; 129:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Brien K, Blair P. Routes of administration, pharmacokinetics and safety of medicinal cannabis. In: O’Brien K, Blair P, editors. Medicinal cannabis and CBD in mental healthcare. 1 ed. Switzerland: Springer Cham; 2021. p. 513–57. [Google Scholar]
- 92.Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 2018; 84:2477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


