Abstract
Multiple myeloma (MM) and its precursor monoclonal gammopathy of undetermined significance (MGUS) are distinct disorders that likely originate in the setting of chronic immune activation. Evolution of these lesions is impacted by cross-talk with both innate and adaptive immune systems of the host. Harnessing the immune system may therefore be an attractive strategy to prevent clinical malignancy. While clinical MM is characterized by both regional and systemic immune suppression and paresis, immune-based approaches, particularly redirecting T cells have shown remarkable efficacy in MM patients. Optimal application and sequencing of these new immune therapies and their integration into clinical MM management may depend on the underlying immune status, in turn impacted by host, tumor and environmental features. Immune therapies carry the potential to achieve durable unmaintained responses and cures in MM.
Multiple myeloma (MM) is a plasma cell malignancy, preceded by a precursor phase termed as monoclonal gammopathy of undetermined significance (MGUS)(1). Over the last two decades, there have been major advances in our understanding of the immune system in MM and MGUS patients as well as preclinical disease models(1–4). This has been accompanied by transformative advances in the therapeutic landscape in MM, leading to major improvements in outcomes for patients(5, 6). Therapies that directly or indirectly impact the immune system now form the mainstay of current management of MM and are increasingly being applied earlier in disease course(5). MM patients also exhibit increased susceptibility to infections and reduced responses to vaccines, which has been particularly laid bare during the recent SARS-CoV-2 pandemic(7–9). In addition, as MM is a malignancy of an immune cell (plasma cell) itself, it is increasingly appreciated that the malignancy originates in the setting of chronic immune stimulation(1, 10, 11). Therefore, properties of the immune system impact several aspects of MM biology, from pathogenesis of the malignancy itself, to susceptibility to infections and response to immune-based approaches for prevention or therapy. In this review, I will discuss recent and emerging advances in our understanding of the immune system in MM/MGUS, and offer my perspective on how these insights might shape the application of new / emerging immune-based approaches to prevent or treat MM.
Some principles of immuno-oncology and MM/MGUS as a model:
It is now increasingly appreciated that nearly all human cancers grow in the context of a dynamic cross-talk with the cells of the immune microenvironment; the capacity of tumors to induce immune response and the capacity of immune system to impact tumors forms the basis of the cancer immunity cycle and provides the framework for immune therapy(12). Seminal studies in mouse models established the capacity of the immune system to impact the early growth of tumors, with these interactions characterized by the 3 E’s – elimination, equilibrium and escape(13). Traditionally, immune system been categorized into innate and adaptive immune components, and both of these can play major roles in immune control. Oncogenic mutations can lead to the expression of neoantigens, and pre-existing immune responses to tumors seem to underlie the clinical response to checkpoint blockade in solid tumors(12). MGUS/MM is a useful model to study these interactions in humans as MGUS precedes nearly all cases of MM(1). Further, the preneoplastic phase is readily detected due to the secretion of clonal immunoglobulin (Ig) and is not resectable, thus allowing study of these interactions in patients in vivo. Advances in genomic analysis of MM tumors have shown that the majority of genomic changes found in clinical MM are already detectable before the clinical malignancy develops(11). Indeed, it is now clear that all of the broad genomic subtypes of MM exist as their MGUS counterparts(14). Therefore the immune system is likely exposed to tumor-associated neoantigens for several years before the development of the malignancy. In murine models of chronic viral infection, chronic exposure to antigens was shown to lead to a state of T cell dysfunction termed as T cell exhaustion. The degree to which T cell dysfunction in cancer patients resembles that in the setting of chronic viral infections and can be rejuvenated is a matter of debate and active research. In murine models of chronic viral infection, it has been shown that the exhausted clones are heterogeneous and the capacity to sustain such clones long-term in vivo may depend on the presence of a subset of TCF1/7+ precursor or stem-like memory T cells(15). Immunity within non-lymphoid tissues may depend on a subset of tissue-resident memory (TRM) T cells, with the capacity to persist long-term within tissues(16). Such cells have also been described in both human and murine bone marrow(17, 18) and their contribution to protective immunity in MM is an area of active research. Here again, the degree to which murine models of chronic viral infection recapitulate the biology of tissue residence in human tumors remains an area of debate and ongoing research.
Immune recognition of premalignancy and implications for prevention:
Lesson:
Immune system is capable of recognizing earliest MGUS lesions, which may be targeted for prevention.
MGUS was one of the first human premalignancies wherein the presence of preneoplasia-specific T cell responses in the tumor microenvironment (TME) was documented(19, 20). These responses are specific to each individual patient and consist of both CD4 and CD8+ T cells(19). In addition to adaptive immunity, the microenvironment in preneoplasia also consists of alterations in innate immune cells, with enrichment of subsets of innate lymphoid cells(21). Recent single cell sequencing studies have shown that T cells within MGUS lesions already exhibit markers consistent with T cell dysfunction, although these studies did not evaluate antigen specificity of marrow-infiltrating T cells(22, 23). In particular, TME in clinical MM is associated with reduction in TCF1+ stem-like memory T cells(22). T cell responses against SOX2, an embryonal stem cell-associated antigen have been correlated with reduced risk of progression to clinical MM in the context of a prospective clinical trial(24, 25). Immune profiles have also been linked to outcomes in MM prevention trials(26). In spite of evidence for immune recognition of human MGUS, it is not known however if these lesions undergo immune editing over time. Studies in preclinical MM models, and particularly v-kappa myc mice have provided evidence for immune surveillance, which seems to depend on both T as well as NK cells and is consistent with human data(27). Studies in humanized models have shown that cells from MM precursor states MGUS and smoldering MM (SMM) exhibit progressive growth pattern in humanized mice, supporting the hypothesis that clinical stability of the clone observed in these patients is at least in part due to tumor-extrinsic features in TME(3).
Implications:
The concept of immune recognition of early lesions provides support to strategies to harness the immune system to prevent MM. Two randomized trials have demonstrated that lenalidomide (alone or with dexamethasone) in patients with SMM can reduce the risk of progression to clinical MM(28, 29). However the finding that the hierarchy of T cell exhaustion/dysfunction is established early on in patients with monoclonal gammopathy suggests the need to consider testing immune-based prevention approaches even earlier than “high-risk” SMM(22, 30, 31). In this regard, an important aspect of immune biology may be changes in spatial architecture with evolution to MM. These issues also support consideration of immune redirection therapies in this setting. In addition, there is an unmet need to better understand the antigenic targets and spatial architecture of host response in preneoplasia, as it may allow novel non-toxic approaches for immune prevention.
Origins of MGUS: Role of chronic B cell activation
Lesson:
MGUS originates in the setting of chronic B cell activation.
Several lines of evidence now support the concept that MGUS originates in the setting of chronic B cell activation(1). Epidemiologic studies suggest increased risk of gammopathy in the setting of autoimmune or allergic conditions(32). Genome sequencing studies of the plasma cell clone show mutational signatures of cytidine deaminases (such as activation-induced cytidine deaminase (AID) or APOBEC), which are often associated with B cell activation, starting as early as the third decade of life(10, 33). Clinical setting such as Gaucher’s disease which is characterized by chronic B cell activation due to accumulation of immunogenic lipids may serve as a useful model to understand the role of immune system in early phases of evolution of gammopathies(34). In this setting, chronic B cell activation initially leads to poly- or oligoclonal B cell activation, from which the monoclonal component may emerge. However, even when the monoclonal component is well established or dominant, it remains responsive to antigen-mediated regulation(35). Accordingly, reduction of immunogenic lipids such as with substrate reduction leads to reduction in clonal immunoglobulins, both in mouse models as well as in patients with Gaucher disease(34–36). Reactivity to lysolipids seems to be polyreactive and thus maybe a feature of auto- or poly-reactive B cell receptors (BCRs)(34, 35). Recent clinical studies have begun to provide additional evidence for polyclonal gammopathy preceding MGUS(37). Recent studies in mouse models have also implicated a potential role for microbial stimuli in activating gut Th17 cells and promoting tumor growth(38). Impact of microbiome-derived signals on tumor growth in MM or MGUS patients is an area of ongoing research.
Implications:
The new insights implore the need to better understand the antigenic triggers that lead to gammopathies and eventually MM. Polyreactive BCRs may also be susceptible to regulation by diverse environmental stimuli, including those derived from microbiome or dietary factors. Exposure to pathogens that impact B cells (such as Epstein Barr virus) is greatly impacted by racial and socioeconomic issues(39, 40), peaking nearly a decade earlier in Black children, and may therefore eventually impact the timing / risk of gammopathy in Black patients. Interventions based on diet and lifestyle are also of considerable interest as they may impact the TME and evolution of MGUS.
Immune system as a potent weapon against MM
Lesson:
Tumor cells in advanced or even relapsed MM remain sensitive to immune effectors.
Several studies have shown that immune system is a potent weapon against MM(41). Even in the setting of advanced and relapsed disease, MM cells remain highly susceptible to killing by both innate and adaptive immune cells(41–43). While T cells in the bone marrow of MM patients exhibit features of T cell dysfunction as discussed below, they can be activated to mediate killing of autologous tumor cells(41). It is notable however that while the blood as well as bone marrow in MM patients contains expanded T cell clones(44–47), a proportion of these are likely not tumor-specific but may represent bystander T cell clones associated with aging immune system or carry reactivity against non-tumor (e.g. viral) antigens. Thus while the presence of neoantigen-specific T cells in MM has been demonstrated(48), further studies are needed to better understand the antigen-specificity and function of T cells in MM or MGUS marrow. It is notable that MM tumors cells exhibit considerable heterogeneity with reference to underlying genomic instability and mutational burden, which may translate to neoantigen load. Tumors with high-risk genetics seem to carry higher mutational burden. Human bone marrow has been previously shown to serve as a reservoir for memory T cells with diverse specificities. Therefore T cells in MM marrow could reflect changes associated with aging, prior environmental exposures, as well as those directly or indirectly impacted by tumor cells. Analyses based solely on bone marrow aspirates are also subject to artifact of hemodilution during the process of bone marrow aspiration. Subsets of bone marrow T cells in MM show distinct polarization, particularly towards IL17-producing T cells, which may be linked to MM bone disease(49, 50). Investigators have also tested adoptive transfer of expanded marrow infiltrating T cells with promising early results(51). In addition to T cells, MM cells are also quite sensitive to innate effectors. For example, MM cells commonly express CD1d and can be readily killed by type I natural killer T (NKT) cells(42). Advanced MM is associated with progressive dysfunction of NKT cells in vivo(42). DC-mediated activation of NKT cells in combination with transient low dose lenalidomide led to NKT expansion and tumor regressions in vivo in patients with smoldering MM (SMM)(52). Much of what is currently known about the bone marrow immune microenvironment in MM is based on study of BM aspirates, and data relating to spatial aspects of the immune response in the context of MM biopsies is currently limited.
The capacity of endogenous immune effector T cells to mediate tumor regression has now been translated to the clinic, with the demonstration of high rates of clinical tumor regression following bi-specific antibodies, even in patients with relapsed MM following multiple lines of therapy(53, 54). These data also illustrate the importance of in situ activation / engagement of T cells, as well as the need to better understand spatial aspects of immune response. Recent application of single cell methods has also illustrated considerable inter-patient heterogeneity in terms of immune TME in MM(22, 23). In this regard, immune system in each MM or MGUS patient is likely unique and impacted by host, tumor, therapy and environmental factors (Fig 1). Thus as with tumor genetics, immune profiles may impact outcome in MM and response to immune therapies. Although most of current trials evaluating immune redirection focus on T cells, redirection of innate immune cells may also be worthy of clinical evaluation and is emerging from preclinical studies(55–57).
Fig 1.
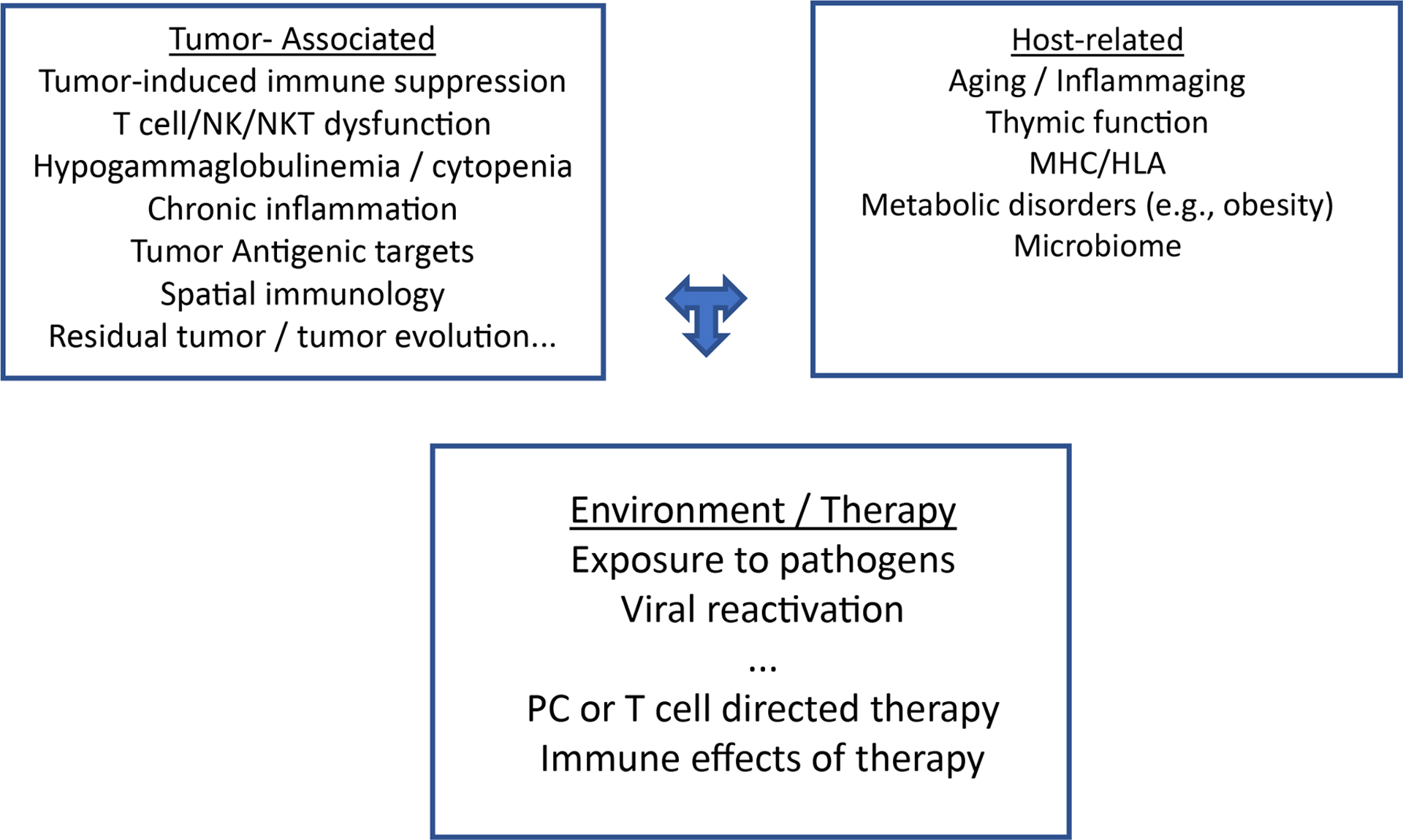
Factors impacting tumor- and pathogen-specific immunity in MM
Tumor and pathogen-specific immunity in MM is likely impacted by several factors that can be broadly classified as tumor, host, environment and therapy related factors.
Implications:
Redirection of effector T cells has shown considerable promise in early clinical trials and suggests that functional properties of effector T cells may be critical determinants of durability of response to these therapies. While much of the work to date has been with redirecting conventional T cells, new approaches redirecting innate immune cells (such as NKT cells) are also emerging and hold promise.
Progressive T cell dysfunction and many paths to immune suppression in MM
Lesson:
Both T cell intrinsic (e.g. costimulation/inhibition, exhaustion, senescence) and extrinsic (e.g. Tregs, MDSCs, stromal inflammation) mechanisms underlie immune suppression in MM(Fig 2).
Fig 2.
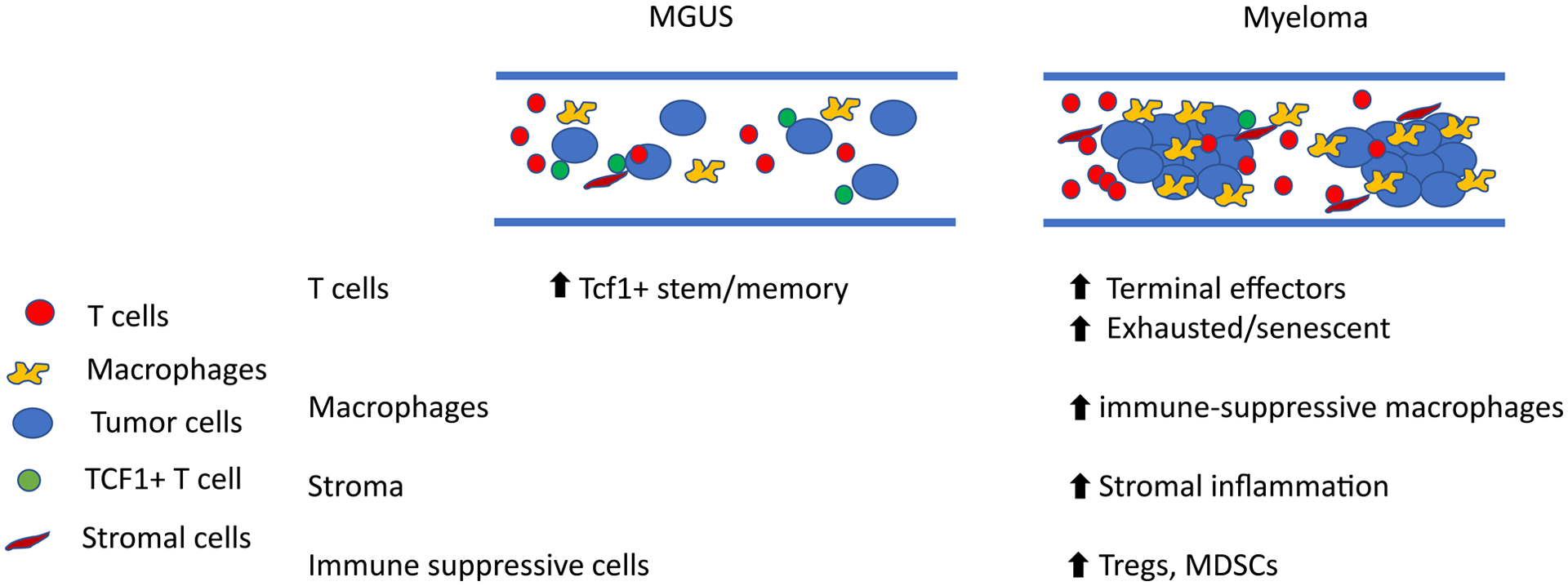
Major changes in T and myeloid composition between MGUS and myeloma
Bone marrow in myeloma is characterized by greater enrichment of terminal effector T cells, along with immune suppressive myeloid cells and stromal inflammation.
Several studies have documented that with disease progression, T cells in MM patients increasingly exhibit features of T cell dysfunction and terminal differentiation(45, 58). These changes persist following therapy and may globally impact T cell function. Some studies have documented the presence of expanded but terminally differentiated and exhausted T cell clones, including those exhibiting features of telomerase-independent T cell senescence(45, 46, 59). The mechanisms underlying T cell dysfunction are diverse and include both T cell-intrinsic as well as T-cell extrinsic factors. T cell intrinsic features have been mostly studied in the context of pathways associated with T cell exhaustion and changes in co-stimulatory/co-inhibitory signaling(60, 61). In the Vkappa-myc model, enhanced costimulatory signaling was shown to mediate protective immunity(62, 63). Analysis of T cells in the human MM marrow have documented the presence of expanded clones with varying degrees of features of T cell exhaustion, and even senescence(22, 59). T cells in the MM marrow are relatively deficient in the population of stem-like memory or precursor exhausted T cells marked by the expression of TCF1(22). Initial studies documenting the expression of PD1 on MM T cells led to extensive evaluation of anti-PD1 therapy in MM, without clear evidence of clinical benefit. More recent studies have explored PD1 blockade in earlier stages such as SMM, however the results are underwhelming(64, 65). Exploration of alternate inhibitory receptors or signaling pathways on MM cells has led to identification of proposed role for TIGIT and Lag-3 axis in immune suppression in MM(60, 61, 66). Strategies to inhibit these pathways in MM patients are currently being explored. Another important feature of T cells in the bone marrow is altered T cell polarization, with reduced Th1 type signaling and instead, polarization towards Th17 cells(50, 67). IL-17 secreted by these cells has been implicated in MM bone disease. It is notable that a major caveat of several of the studies discussed above is that they did not document tumor- or antigen-specificity of T cells. Deeper understanding of specific antigens recognized by tumor-infiltrating T cells in MM is needed to better understand the mechanisms that lead to altered T cell function in these patients.
In addition to T cell intrinsic pathways, several T-cell extrinsic mechanisms including “immune suppressive” cytokines (e.g. IL6, IL10, TGF-beta, IL18) have been implicated in MM-associated immune paresis and promoting tumor growth(68–71). Strategies to block these pathways are therefore being explored in combination therapies. Several potential mechanisms of suppression via immune suppressive cells also deserve specific mention, as these can in principle be directly targeted to harness tumor immunity. Increase in regulatory T cells has been observed in MM patients and mouse models and their depletion shown to enhance anti-MM immunity. The relative proportion of Tregs in MM models seems higher than that seen in MM patients, wherein Tregs may be dysfunctional(72–76). Nonetheless, depletion of Tregs is being explored to harness tumor immunity. An important feature of MM bone marrow is increased infiltration of macrophages with potential suppressive function(77, 78). Although the biology of myeloid cells in MM is complex, with both tumor-promoting and tumor-suppressive function, several studies have documented systemic alterations in myeloid-derived suppressor cells in MM(77–81). These data suggest that targeting such populations may enhance tumor immunity. In addition to Tregs and MDSCs, other populations such as mesenchymal stem cells, cancer-associated fibroblasts, bone marrow stromal cells, as well as osteoclasts have been implicated in mediating suppressive effects on tumor immunity(82, 83). For example, recent studies have suggested an important role for inflammatory stromal phenotype with T cell exhaustion and immune escape(83, 84). Pathways/mechanisms of immune suppression in MM also extend beyond adaptive immunity to include innate immune cells. For example, inhibition of NKG2D by soluble MICA has been implicated in immune suppression(85). In addition to T cells, MM is associated with altered function of both NK as well as NKT cells, which again, may be mediated by both cell-intrinsic as well as extrinsic mechanisms(86).
Implications:
Diverse pathways underlie the T cell dysfunction and immune suppression observed in MM. In the future, it may be important to dissect which of these pathways are regional versus systemic, and impact tumor-specific versus global immune function. Recent application of high-dimensional approaches to study the immune microenvironment to study MM and MGUS have shown that there may be considerable inter-patient heterogeneity as to the nature of immune tumor microenvironment. Therefore optimal strategies to harness tumor immunity may depend in part on the nature of host response to the tumor and the degree to which it may be restored.
Systemic versus Regional Immunity
Lesson:
MM is associated with both regional and systemic alterations in immune cells; both are relevant in different ways and impact protection against tumors and pathogens.
It has been long appreciated since the initial description of MM in that it is “multiple”, in that the tumor cells are found enriched within multiple focal lesions leading to lytic bone disease. However the impact or implications of this multifocal pattern on spatial biology of the microenvironment remains poorly studied. In addition, tumor cells in MM remain localized to the bone marrow, until later in the natural history of the malignancy. This is also potentially relevant to MM immunology, with the appreciation that distinct subsets of immune cells remain resident within the marrow. This issue is likely to become more important in the future, particularly with the appreciation of critical role of tissue-resident memory T cells in the context of tumor immunity in other cancers. While the systemic alterations in immune responses such as depletion of normal B cells and reduction in uninvolved immunoglobulins and alterations in circulating T cells have been extensively studied, our understanding of the dynamic relationship between the circulating and bone-marrow resident compartments remains relatively superficial. Advances in mouse modeling may provide deeper insights into this issue, which is particularly critical due to the emergence of T cell redirection as a clinically effective strategy in MM.
While regional changes are of particular interest for tumor immunity, changes in systemic immune profiles may be of specific relevance for immunity to pathogens and response to vaccines. This has become particularly relevant with the emergence of the SARS CoV-2 pandemic, as MM patients exhibit increased susceptibility to these pathogens and reduced response to current vaccines(87). As we gain deeper insights into the biology of regional versus systemic immunity, particularly at the level of antigen-specific immunity, we are likely to uncover the underlying mechanisms that regulate them. In this regard, it possible and even likely that the pathways that regulate regional immunity differ from those that regulate systemic immunity. For example, what are the properties of tumor-specific T cells within MM lesions? What are the mechanisms that regulate entry and persistence of tumor-specific T cells into MM tumors? Does the spatial biology of naturally occurring T cells differ from synthetic immunity (e.g. redirected T cells with bispecific antibodies or CAR-T cells)? What are the antigens recognized by TCRs that may be critical for long term tumor control or cure?
Implications:
While the study of spatial aspects of immune response in MM as well as mechanistic aspects of how this biology is regulated in vivo remains in its infancy, it is likely that improved understanding of this biology will be critical to understand mechanisms underlying response and resistance to T cell redirection and eventual cure in MM.
Non-immunologic effects of immune cells on MM biology
Lesson:
Tumor-immune interactions may directly impact several aspects of MM biology including tumor growth, bone disease and genomic instability.
While studies of interaction between MM tumors and immune system have understandably focused to date on immune control of tumors, there is also evidence that these interactions also have a more direct effect on tumor biology. This should not be surprising as MM after all is a tumor of an immune cell, i.e. plasma cell. Interactions between DCs and MM cells can directly promote tumor growth, in part by engaging cytokine and Baff-mediated signals(88, 89). Interactions between DCs and tumors may also be critical for activation of cytidine deaminases such as AID within MM cells and may contribute to genomic instability(90). DCs or macrophages in the tumor bed may also contribute more directly to formation of MM bone disease via tumor-induced cell fusion events that lead to the formation of osteoclasts(91). As noted earlier, polarization of T cells to secrete cytokines such as IL17 may further contribute to MM bone disease(49, 50).
Implications:
Strategies to target tumor-associated immune cells may have a more direct impact of MM biology, beyond immune control.
Immune status and implications for immune therapies
Lesson:
Immune system contributes to the mechanism of action of several MM therapies and properties of immune system may impact outcomes following current and emerging immune therapies.
It is now increasingly appreciated that effects on the immune system may contribute to anti-tumor effects of several current MM therapies. Bortezomib can lead to immunogenic death of MM tumors via the expression of heat shock proteins on dying cells and engaging cGAS/STING pathway(92, 93). IMiDs lead to enhanced secretion of IL2 and downstream activation of NK, T and NKT cells via cereblon-dependent degradation of Ikaros, which can persist even with concurrent steroids(26, 94–96). Studies in mouse models have shown that stem cell transplantation can lead to generation of new anti-tumor immune responses and set the stage for future immune combination approaches(61). Anti-CD38 antibodies can impact NK and T cells, and also deplete CD38 expressing immune-regulatory B and myeloid cells(97). Antibody-drug conjugates likely also induce immunogenic cell death and may synergize with other immune approaches. The most direct demonstration of anti-tumor effects of immune system in MM in the clinic has emerged from T cell redirection approaches such as CAR-T or bispecific antibodies(4, 5). Several MM-associated targets such as BCMA or GPRC5D have been effectively targeted by both of these strategies leading to rapid and deep responses. Nature of input T cells is a key determinant of the properties of manufactured CAR-Ts and therefore can differ between individual patients and impacted by the stage of disease(98). Early expansion of CARTs in vivo correlates with initial response to these therapies(99, 100). Recent application of high-dimensional profiling has shown that BCMA CAR-T therapy also leads to major alterations in endogenous T cells(101). Interestingly, while initial response to BCMA CARTs depends in part on the initial expansion of CARTs in vivo(99, 100), properties of pre-existing and endogenous T cells, as well as the nature of myeloid/DC populations in the tumor bed may be critical determinants of the durability of CAR-T-induced tumor regressions in MM(101). Therefore properties of the tumor immune microenvironment may be critical both for patient selection as well as designing future and possibly personalized combination therapies to improve the durability of T cell redirection in MM. In contrast to CAR-T cells, bispecific antibodies rely entirely on endogenous CD3+ cells(53, 54), and accordingly the nature of these T cells would be expected to have a major impact on the likelihood of response as well as durability of remissions following bispecific antibodies(5, 53). It is notable that the effects of these therapies are expected to be systemic and may also target pathogen-reactive T cells, thereby impacting risk of infections. Another critical issue particularly with T cell redirection is the spatial aspects of the immune cells, as discussed above. Deeper understanding of antigen-specificity and spatial aspects of redirected and bystander T cells in the tumor microenvironment (and systemically) both in patients and mouse models will be essential to optimize the clinical benefit of these therapies in MM and develop combination therapies.
Implications:
Rapidly growing body of data now supports an important role for immune microenvironment as a critical determinant of durability of immune therapies, both for redirection therapies, as well as synthetic immunity. Recent studies have demonstrated correlations between immune status and outcome following current therapies, including in long-term survivors(26, 102–106). As these models continue to be refined, it is likely that we may be able to utilize immune status as a tool to select patients for specific immune therapies, as well as optimal sequencing and combinations.
Summary:
In the past decade, appreciation of the role of immune system in MM has undergone a complete transformation, from a largely ignored topic to now integral to all aspects of MM biology. Immune-based approaches also seem poised to transform the therapeutic landscape, both for therapy and prevention of MM. Optimal integration of these approaches into clinical management may depend in part on immune status of the patient, which in turn is impacted by host, tumor, therapy and environmental signals (Fig1). Current treatment paradigms in MM were built on the backbone of direct cytotoxic effects of steroids on MM cells, eligibility for stem cell transplantation, use of plasma-cell directed therapies and have relied on the use of continuous therapy to delay relapses. Immune-based approaches in theory, carry the yet unrealized potential for unmaintained remissions and potential cure or effective prevention. It may be essential to revisit older paradigms in order to fully realize this potential.
Acknowledgments:
MVD is supported in part by funds from LLS Specialized Center for Research, NIH R35 CA197603, U54 CA260563 and Paula and Rodger Riney Foundation. The author acknowledges Kavita Dhodapkar for discussions relating to this review.
Footnotes
Conflict of Interest:
MVD:
Advisory Board: Janssen, Sanofi, Lava Therapeutics
There are no conflicts related to specific content of this review.
conflict of interest disclosure: The author reports no conflict of interest.
References:
- 1.Dhodapkar MV. MGUS to myeloma: a mysterious gammopathy of underexplored significance. Blood. 2016;128(23):2599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. [DOI] [PubMed] [Google Scholar]
- 3.Das R, Strowig T, Verma R, Koduru S, Hafemann A, Hopf S, et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat Med. 2016;22(11):1351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen AD, Raje N, Fowler JA, Mezzi K, Scott EC, Dhodapkar MV. How to Train your T cells: Overcoming Immune Dysfunction in Multiple Myeloma. Clin Cancer Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah N, Aiello J, Avigan DE, Berdeja JG, Borrello IM, Chari A, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of multiple myeloma. Journal for immunotherapy of cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikhael J, Ismaila N, Cheung MC, Costello C, Dhodapkar MV, Kumar S, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol. 2019;37(14):1228–63. [DOI] [PubMed] [Google Scholar]
- 7.Ribas A, Dhodapkar MV, Campbell KM, Davies FE, Gore SD, Levy R, et al. How to Provide the Needed Protection from COVID-19 to Patients with Hematologic Malignancies. Blood Cancer Discov. 2021;2(6):562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhodapkar MV, Dhodapkar KM, Ahmed R. Viral immunity and vaccines in hematologic malignancies: implications for COVID-19. Blood Cancer Discovery. 2021;2(1):9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branagan AR, Duffy E, Gan G, Li F, Foster C, Verma R, et al. Tandem high-dose influenza vaccination is associated with more durable serologic immunity in patients with plasma cell dyscrasias. Blood Adv. 2021;5(5):1535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rustad EH, Yellapantula V, Leongamornlert D, Bolli N, Ledergor G, Nadeu F, et al. Timing the initiation of multiple myeloma. Nat Commun. 2020;11(1):1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maura F, Landgren O, Morgan GJ. Designing Evolutionary Based Interception Strategies to Block the Transition from Precursor Phases to Multiple Myeloma. Clin Cancer Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30. [DOI] [PubMed] [Google Scholar]
- 13.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. [DOI] [PubMed] [Google Scholar]
- 14.Dhodapkar MV, Sexton R, Waheed S, Usmani S, Papanikolaou X, Nair B, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood. 2014;123(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snell LM, MacLeod BL, Law JC, Osokine I, Elsaesser HJ, Hezaveh K, et al. CD8(+) T Cell Priming in Established Chronic Viral Infection Preferentially Directs Differentiation of Memory-like Cells for Sustained Immunity. Immunity. 2018;49(4):678–94 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhodapkar MV, Dhodapkar KM. Tissue-resident memory-like T cells in tumor immunity: Clinical implications. Semin Immunol. 2020:101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okhrimenko A, Grun JR, Westendorf K, Fang Z, Reinke S, von Roth P, et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A. 2014;111(25):9229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boddupalli CS, Nair S, Gray SM, Nowyhed HN, Verma R, Gibson JA, et al. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J Clin Invest. 2016;126(10):3905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy specific effector T cell response in the bone marrow of patients with preneoplastic gammopathy. J Exp Med. 2003;198:1753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn OJ. Premalignant lesions as targets for cancer vaccines. J Exp Med. 2003;198(11):1623–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kini Bailur J, Mehta S, Zhang L, Neparidze N, Parker T, Bar N, et al. Changes in bone marrow innate lymphoid cell subsets in monoclonal gammopathy: target for IMiD therapy. Blood Adv. 2017;1(25):2343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailur JK, McCachren SS, Doxie DB, Shrestha M, Pendleton KE, Nooka AK, et al. Early alterations in stem-like/resident T cells, innate and myeloid cells in the bone marrow in preneoplastic gammopathy. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zavidij O, Haradhvala NJ, Mouhieddine TH, Sklavenitis-Pistofidis R, Cai S, Reidy M, et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nature Cancer. 2020;1(5):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204(4):831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhodapkar MV, Sexton R, Das R, Dhodapkar KM, Zhang L, Sundaram R, et al. Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood. 2015;126(22):2475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paiva B, Mateos MV, Sanchez-Abarca LI, Puig N, Vidriales MB, Lopez-Corral L, et al. Immune status of high-risk smoldering multiple myeloma patients and its therapeutic modulation under LenDex: a longitudinal analysis. Blood. 2016;127(9):1151–62. [DOI] [PubMed] [Google Scholar]
- 27.Guillerey C, Ferrari de Andrade L, Vuckovic S, Miles K, Ngiow SF, Yong MC, et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J Clin Invest. 2015;125(7):2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateos MV, Hernandez MT, Giraldo P, de la Rubia J, de Arriba F, Lopez Corral L, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438–47. [DOI] [PubMed] [Google Scholar]
- 29.Lonial S, Jacobus S, Fonseca R, Weiss M, Kumar S, Orlowski RZ, et al. Randomized Trial of Lenalidomide Versus Observation in Smoldering Multiple Myeloma. J Clin Oncol. 2020;38(11):1126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhodapkar MV, Dhodapkar KM. Moving Immunoprevention Beyond Virally Mediated Malignancies: Do We Need to Link It to Early Detection? Front Immunol. 2019;10:2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaushal A, Nooka AK, Carr AR, Pendleton KE, Barwick BG, Manalo J, et al. Aberrant Extrafollicular B Cells, Immune Dysfunction, Myeloid Inflammation, and MyD88-Mutant Progenitors Precede Waldenstrom Macroglobulinemia. Blood Cancer Discov. 2021;2(6):600–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 2008;111(7):3388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maura F, Rustad EH, Yellapantula V, Luksza M, Hoyos D, Maclachlan KH, et al. Role of AID in the temporal pattern of acquisition of driver mutations in multiple myeloma. Leukemia. 2020;34(5):1476–80. [DOI] [PubMed] [Google Scholar]
- 34.Nair S, Branagan AR, Liu J, Boddupalli CS, Mistry PK, Dhodapkar MV. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N Engl J Med. 2016;374(6):555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair S, Sng J, Boddupalli CS, Seckinger A, Chesi M, Fulciniti M, et al. Antigen-mediated regulation in monoclonal gammopathies and myeloma. JCI Insight. 2018;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair S, Bar N, Xu ML, Dhodapkar M, Mistry PK. Glucosylsphingosine but not Saposin C, is the target antigen in Gaucher disease-associated gammopathy. Mol Genet Metab. 2020;129(4):286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Larson DR, Dispenzieri A, Therneau TM, Murray DL, Leif Bergsagel P, et al. Polyclonal serum free light chain elevation is associated with increased risk of monoclonal gammopathies. Blood Cancer J. 2019;9(6):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calcinotto A, Brevi A, Chesi M, Ferrarese R, Garcia Perez L, Grioni M, et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nature Communications. 2018;9(1):4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhodapkar MV, Sexton R, Hoering A, Van Rhee F, Barlogie B, Orlowski R. Race-Dependent Differences in Risk, Genomics, and Epstein-Barr Virus Exposure in Monoclonal Gammopathies: Results of SWOG S0120. Clin Cancer Res. 2020;26(22):5814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosseboeuf A, Feron D, Tallet A, Rossi C, Charlier C, Garderet L, et al. Monoclonal IgG in MGUS and multiple myeloma targets infectious pathogens. JCI Insight. 2017;2(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong tumor specific cytolytic responses to autologous tumor loaded dendritic cells. Proc Natl Acad Sci. 2002;99:13009–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197(12):1667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noonan K, Matsui W, Serafini P, Carbley R, Tan G, Khalili J, et al. Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer Res. 2005;65(5):2026–34. [DOI] [PubMed] [Google Scholar]
- 44.Raitakari M, Brown RD, Sze D, Yuen E, Barrow L, Nelson M, et al. T-cell expansions in patients with multiple myeloma have a phenotype of cytotoxic T cells. Br J Hematol. 2000;110:203–9. [DOI] [PubMed] [Google Scholar]
- 45.Joshua DE, Vuckovic S, Favaloro J, Lau KHA, Yang S, Bryant CE, et al. Treg and Oligoclonal Expansion of Terminal Effector CD8(+) T Cell as Key Players in Multiple Myeloma. Front Immunol. 2021;12:620596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vuckovic S, Bryant CE, Lau KHA, Yang S, Favaloro J, McGuire HM, et al. Inverse relationship between oligoclonal expanded CD69- TTE and CD69+ TTE cells in bone marrow of multiple myeloma patients. Blood Adv. 2020;4(19):4593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh-Wakefield F, Kruzins A, McGuire HM, Yang S, Bryant C, Fazekas de St Groth B, et al. Mass Cytometry Discovers Two Discrete Subsets of CD39(−)Treg Which Discriminate MGUS From Multiple Myeloma. Front Immunol. 2019;10:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perumal D, Imai N, Lagana A, Finnigan J, Melnekoff D, Leshchenko VV, et al. Mutation-derived Neoantigen-specific T-cell Responses in Multiple Myeloma. Clin Cancer Res. 2020;26(2):450–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116(18):3554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17–1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112(7):2878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noonan KA, Huff CA, Davis J, Lemas MV, Fiorino S, Bitzan J, et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci Transl Med. 2015;7(288):288ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013;121(3):423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen AD, Raje N, Fowler JA, Mezzi K, Scott EC, Dhodapkar MV. How to Train Your T Cells: Overcoming Immune Dysfunction in Multiple Myeloma. Clin Cancer Res. 2020;26(7):1541–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lancman G, Sastow DL, Cho HJ, Jagannath S, Madduri D, Parekh SS, et al. Bispecific Antibodies in Multiple Myeloma: Present and Future. Blood Cancer Discov. 2021;2(5):423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casey M, Tu C, Harrison SJ, Nakamura K. Invariant NKT cells dictate antitumor immunity elicited by a bispecific antibody cotargeting CD3 and BCMA. Blood Adv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lameris R, Shahine A, Pellicci DG, Uldrich AP, Gras S, Le Nours J, et al. A single-domain bispecific antibody targeting CD1d and the NKT T-cell receptor induces a potent antitumor response. Nat Cancer. 2020;1(11):1054–65. [DOI] [PubMed] [Google Scholar]
- 57.Chan WK, Kang S, Youssef Y, Glankler EN, Barrett ER, Carter AM, et al. A CS1-NKG2D Bispecific Antibody Collectively Activates Cytolytic Immune Cells against Multiple Myeloma. Cancer Immunol Res. 2018;6(7):776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung DJ, Pronschinske KB, Shyer JA, Sharma S, Leung S, Curran SA, et al. T-cell Exhaustion in Multiple Myeloma Relapse after Autotransplant: Optimal Timing of Immunotherapy. Cancer Immunol Res. 2016;4(1):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suen H, Brown R, Yang S, Weatherburn C, Ho PJ, Woodland N, et al. Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia. 2016;30(8):1716–24. [DOI] [PubMed] [Google Scholar]
- 60.Guillerey C, Harjunpaa H, Carrie N, Kassem S, Teo T, Miles K, et al. TIGIT immune checkpoint blockade restores CD8(+) T cell immunity against multiple myeloma. Blood. 2018. [DOI] [PubMed] [Google Scholar]
- 61.Minnie SA, Kuns RD, Gartlan KH, Smyth MJ, Hill GR. Myeloma escape after stem cell transplantation is a consequence of T cell exhaustion and is reversed by TIGIT inhibition. Blood. 2018;prepublished online August 28, 2018(doi 10.1182/blood-2018=01-825240). [DOI] [PubMed] [Google Scholar]
- 62.Guillerey C, Ferrari de Andrade L, Vuckovic S, Miles K, Ngiow SF, Yong MC, et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J Clin Invest. 2015;125(5):2077–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guillerey C, Nakamura K, Pichler AC, Barkauskas D, Krumeich S, Stannard K, et al. Chemotherapy followed by anti-CD137 mAb immunotherapy improves disease control in a mouse myeloma model. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bar N, Costa F, Das R, Duffy A, Samur MK, McCachren SS, et al. Differential effects of PD-L1 versus PD-1 blockade on myeloid inflammation in human cancer. JCI Insight. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Costa F, Das R, Kini Bailur J, Dhodapkar K, Dhodapkar MV. Checkpoint Inhibition in Myeloma: Opportunities and Challenges. Front Immunol. 2018;9:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–34. [DOI] [PubMed] [Google Scholar]
- 67.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115(26):5385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura K, Kassem S, Cleynen A, Chretien ML, Guillerey C, Putz EM, et al. Dysregulated IL-18 Is a Key Driver of Immunosuppression and a Possible Therapeutic Target in the Multiple Myeloma Microenvironment. Cancer Cell. 2018;33(4):634–48 e5. [DOI] [PubMed] [Google Scholar]
- 69.Akhmetzyanova I, Aaron T, Galbo P, Tikhonova A, Dolgalev I, Tanaka M, et al. Tissue-resident macrophages promote early dissemination of multiple myeloma via IL-6 and TNFalpha. Blood Adv. 2021;5(18):3592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hayashi T, Hideshima T, Nguyen AN, Munoz O, Podar K, Hamasaki M, et al. Transforming growth factor beta receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res. 2004;10(22):7540–6. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107(6):2432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, et al. Increased Level of both CD4+FOXP3+ Regulatory T Cells and CD14+HLA-DR−/low Myeloid-Derived Suppressor Cells and Decreased Level of Dendritic Cells in Patients with Multiple Myeloma. 2010;72(6):540–7. [DOI] [PubMed] [Google Scholar]
- 73.Guillerey C, Nakamura K, Vuckovic S, Hill GR, Smyth MJ. Immune responses in multiple myeloma: role of the natural immune surveillance and potential of immunotherapies. Cell Mol Life Sci. 2016;73(8):1569–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alrasheed N, Lee L, Ghorani E, Henry JY, Conde L, Chin M, et al. Marrow-Infiltrating Regulatory T Cells Correlate with the Presence of Dysfunctional CD4(+)PD-1(+) Cells and Inferior Survival in Patients with Newly Diagnosed Multiple Myeloma. Clin Cancer Res. 2020;26(13):3443–54. [DOI] [PubMed] [Google Scholar]
- 75.Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107(1):301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tai YT, Lin L, Xing L, Cho SF, Yu T, Acharya C, et al. APRIL signaling via TACI mediates immunosuppression by T regulatory cells in multiple myeloma: therapeutic implications. Leukemia. 2019;33(2):426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asimakopoulos F, Hope C, Johnson MG, Pagenkopf A, Gromek K, Nagel B. Extracellular matrix and the myeloid-in-myeloma compartment: balancing tolerogenic and immunogenic inflammation in the myeloma niche. J Leukoc Biol. 2017;102(2):265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramachandran IR, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, et al. Myeloid-Derived Suppressor Cells Regulate Growth of Multiple Myeloma by Inhibiting T Cells in Bone Marrow. 2013;190(7):3815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121(15):2975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hope C, Foulcer S, Jagodinsky J, Chen SX, Jensen JL, Patel S, et al. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016;128(5):680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tai YT, Cho SF, Anderson KC. Osteoclast Immunosuppressive Effects in Multiple Myeloma: Role of Programmed Cell Death Ligand 1. Front Immunol. 2018;9:1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Jong MME, Kellermayer Z, Papazian N, Tahri S, Hofste Op Bruinink D, Hoogenboezem R, et al. The multiple myeloma microenvironment is defined by an inflammatory stromal cell landscape. Nat Immunol. 2021;22(6):769–80. [DOI] [PubMed] [Google Scholar]
- 84.Wu X, Wang Y, Xu J, Luo T, Deng J, Hu Y. MM-BMSCs induce naive CD4+ T lymphocytes dysfunction through fibroblast activation protein alpha. Oncotarget. 2017;8(32):52614–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A. 2008;105(4):1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neparidze N, Dhodapkar MV. Harnessing CD1d-restricted T cells toward antitumor immunity in humans. Ann N Y Acad Sci. 2009;1174:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nooka AK, Shanmugasundaram U, Cheedarla N, Verkerke H, Edara VV, Valanparambil R, et al. Determinants of Neutralizing Antibody Response After SARS CoV-2 Vaccination in Patients With Myeloma. J Clin Oncol. 2022:JCO2102257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kukreja A, Hutchinson A, Dhodapkar KM, Mazumder A, Vesole D, Angitapalli R, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203(8):1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16(4):309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koduru S, Wong E, Strowig T, Sundaram R, Zhang L, Strout MP, et al. Dendritic cell-mediated activation-induced cytidine deaminase (AID)-dependent induction of genomic instability in human myeloma. Blood. 2012;119(10):2302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: implications for bone disease. Blood. 2009;114(16):3413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC) mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109(Jun 1):4839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gulla A, Morelli E, Samur MK, Botta C, Hideshima T, Bianchi G, et al. Bortezomib induces anti-multiple myeloma immune response mediated by cGAS/STING pathway activation. Blood Cancer Discov. 2021;2(5):468–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sehgal K, Das R, Zhang L, Verma R, Deng Y, Kocoglu M, et al. Clinical and pharmacodynamic analysis of pomalidomide dosing strategies in myeloma: impact of immune activation and cereblon targets. Blood. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164(6):811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pierceall WE, Amatangelo MD, Bahlis NJ, Siegel DS, Rahman A, Van Oekelen O, et al. Immunomodulation in Pomalidomide, Dexamethasone, and Daratumumab-Treated Patients with Relapsed/Refractory Multiple Myeloma. Clin Cancer Res. 2020;26(22):5895–902. [DOI] [PubMed] [Google Scholar]
- 97.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garfall AL, Dancy EK, Cohen AD, Hwang WT, Fraietta JA, Davis MM, et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. 2019;3(19):2812–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384(8):705–16. [DOI] [PubMed] [Google Scholar]
- 101.Dhodapkar KM, Cohen AD, Kaushal A, Garfall AL, Manalo RJ, Carr AR, et al. Changes in Bone Marrow Tumor and Immune Cells Correlate with Durability of Remissions Following BCMA CAR T Therapy in Myeloma. Blood Cancer Discov. 2022:OF1–OF12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parmar H, Gertz M, Anderson EI, Kumar S, Kourelis TV. Microenvironment immune reconstitution patterns correlate with outcomes after autologous transplant in multiple myeloma. Blood Adv. 2021;5(7):1797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kourelis TV, Jevremovic D, Jessen E, Dasari S, Villasboas JC, Dispenzieri A, et al. Mass cytometry identifies expansion of double positive and exhausted T cell subsets in the tumour microenvironment of patients with POEMS syndrome. Br J Haematol. 2020;190(1):79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kourelis TV, Villasboas JC, Jessen E, Dasari S, Dispenzieri A, Jevremovic D, et al. Mass cytometry dissects T cell heterogeneity in the immune tumor microenvironment of common dysproteinemias at diagnosis and after first line therapies. Blood Cancer J. 2019;9(9):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guerrero C, Puig N, Cedena MT, Goicoechea I, Perez C, Garces JJ, et al. A Machine Learning Model Based on Tumor and Immune Biomarkers to Predict Undetectable MRD and Survival Outcomes in Multiple Myeloma. Clin Cancer Res. 2022;28(12):2598–609. [DOI] [PubMed] [Google Scholar]
- 106.Ho CM, McCarthy PL, Wallace PK, Zhang Y, Fora A, Mellors P, et al. Immune signatures associated with improved progression-free and overall survival for myeloma patients treated with AHSCT. Blood Adv. 2017;1(15):1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


