Abstract
Pesticide exposure to eyes is a major source of ocular morbidities in adults and children all over the world. Carbofuran (CF), N-methyl carbamate, pesticide is most widely used as an insecticide, nematicide, and acaricide in agriculture, forestry, and gardening. Contact or ingestion of carbofuran causes high morbidity and mortality in humans and pets. Pesticides are absorbed in the eye faster than other organs of the body and damage ocular tissues very quickly. Carbofuran exposure to eye causes blurred vision, pain, loss of coordination, anti-cholinesterase activities, weakness, sweating, nausea and vomiting, abdominal pain, endocrine, reproductive, and cytotoxic effects in humans depending on amount and duration of exposure. Pesticide exposure to eye injures cornea, conjunctiva, lens, retina, and optic nerve and leads to abnormal ocular movement and vision impairment. Additionally, anticholinesterase pesticides like carbofuran are known to cause salivation, lacrimation, urination, and defecation (SLUD). Carbofuran and its two major metabolites (3-hydroxycarbofuran and 3-ketocarbofuran) are reversible inhibitors of acetylcholinesterase (AChE) which regulates acetylcholine (ACh), a neurohumoral chemical that plays an important role in corneal wound healing. The corneal epithelium contains high levels of ACh whose accumulation by AChE inhibition after CF exposure overstimulates muscarinic ACh receptors (mAChRs) and nicotinic ACh receptors (nAChRs). Hyper stimulation mAChRs in the eye causes miosis (excessive constriction of the pupil), dacryorrhea (excessive flow of tears), or chromodacryorrhea (red tears). Recent studies reported alteration of autophagy mechanism in human cornea in vitro and ex vivo post carbofuran exposure. This review describes carbofuran toxicity to the eye with special emphasis to corneal morbidities and blindness.
Keywords: Cornea, Carbofuran, Pesticide, Ocular toxicity, Carbamate, Acetylcholine, Acetylcholinesterase
1. Introduction
Pesticides are widely used in agriculture, horticulture, forestry, gardens, offices and for public and animal health (Gupta, 2006; Gupta and Milatovic, 2012; Sharma and Sharma, 2012). Carbamate (CM) compounds are also used in veterinary medicine as ectoparasiticides and in human medicine for therapeutic interventions of Alzheimer’s disease, myasthenia gravis, glaucoma, and in prophylaxis of organophosphate (OP) nerve gas poisonings (Bajgar et al., 2015; Gupta, 2006; Stojiljković et al., 2020; Woltjer and Milatovic, 2006). The commonly used N-methyl carbamates (aldicarb, carbofuran, carbaryl, methomyl, oxamyl, pirimicarb, propoxur, etc.) are esters of carbamic acid. Carbofuran is a highly toxic chemical commonly used as an insecticide, nematicide and acaricide for agricultural, forestry, and industrial applications, and in accidental and malicious poisonings (Gupta et al., 2018; Khan et al., 2021; Lv et al., 2022; Pivariu et al., 2020; Umeda et al., 2018).
Approximately 5 million pounds of carbofuran is utilized every year in the United States alone, and as a result, soil and water are contaminated significantly. About 45% of urban African American women show a trace amount of carbofuran in their plasma (Bonner et al., 2005). Nitrosated carbofuran shows mutagenic properties (Bonner et al., 2005). The United States Environmental Protection Agency (US EPA) reported several pesticide poisoning incidences from multiple sources including state and federal agencies. These reports indicate that the workers got carbofuran (a) in the eye while spraying in the field, (b) in the face, (c) farmer spilled on hands while spraying, (d) exposed during unloading of carbofuran from truck, splashed in the eyes, (e) person applied carbofuran for the whole day becoming ill, (f) exposed during a spray break, (g) exposed when cleaning up spray (US EPA, 1997).
Carbamate compounds produce toxicity in mammals, birds, fish, and wildlife primarily due to carbamylation (reversible inhibition) of acetylcholinesterase (AChE) and accumulation of ACh) at the synapses in the brain and the neuromuscular junctions in skeletal muscles (Gupta and Milatovic, 2012; Gupta et al., 2018; Mishra et al., 2020; Silberman and Taylor, 2022). This leads to the toxic signs of hypercholinergic preponderance. In humans and animals, carbofuran also exerts toxic effects due to non-cholinergic mechanisms, such as endocrine, reproductive, cytotoxic, genotoxic disorders, etc. (Goad et al., 2004; Gupta and Milatovic, 2012; Gupta et al., 2007; Mishra et al., 2020). In mammals, carbofuran exposure induces apoptosis of the neurons in the hippocampus, oxidative stress, neuroinflammation, memory dysfunction, and chromosomal abnormalities (Gupta and Milatovic, 2012; Gupta et al., 2007; Khan et al., 2021). A recent study reported that carbofuran toxicity increases the cellular aging process and interferes with biological aging in animals with abnormal spns1 (Khan et al., 2021). This review describes carbofuran toxicity in general and ocular toxicity in particular.
2. Carbofuran chemistry
Chemically carbofuran (CAS number, 1563-66-2; EINECS number, 216-353-0; and RTECS number, FB9450000) is 2,3-dihydro-2,2-dimethyl-7-benzofuranol methylcarbamate; 2,2-dimethyl-2,3-dihydro-7-benzofuranyl-N-methylcarbamate; methyl carbamic acid 2,3-dihydro-2,2-dimethyl-7-benzofuranyl ester; 2,2-dimethyl-7-coumaranyl-N-methylcarbamate; or 2,2-dimethyl-2,3-dihydro-1-benzofuran-7-yl methylcarbamate (IUPAC name). The chemical formula of carbofuran is C12H15NO3 and molar mass is 221.256 g·mol−1. Its structural formula is shown in Figure 1. Carbofuran is manufactured by the reaction of methyl isocyanate with 2,3-dihydro-2,2-dimethyl-7-hydroxybenzofuran. Some physical and chemical properties of carbofuran include white, crystalline solid, melting/point 150–152 °C, boiling point 313.3 °C, flash point °143.3C, specific gravity 1.18 at 20 °C, density 1.2 g/cm3, vapor pressure 2 × 10 −5 mm Hg at 33 °C, water solubility 300 ppm at room temperature and 700 ppm at 25 °C, and octanol/water partition coefficient as log Pow 2.32. Carbofuran is highly soluble in N-methyl-2-pyrrolidone, dimethylformamide, dimethyl sulfoxide, acetone, acetonitrile, methylene chloride, cyclohexanone, benzene, and xylene. Evaporation of carbofuran at 20 °C is negligible.
Figure 1.
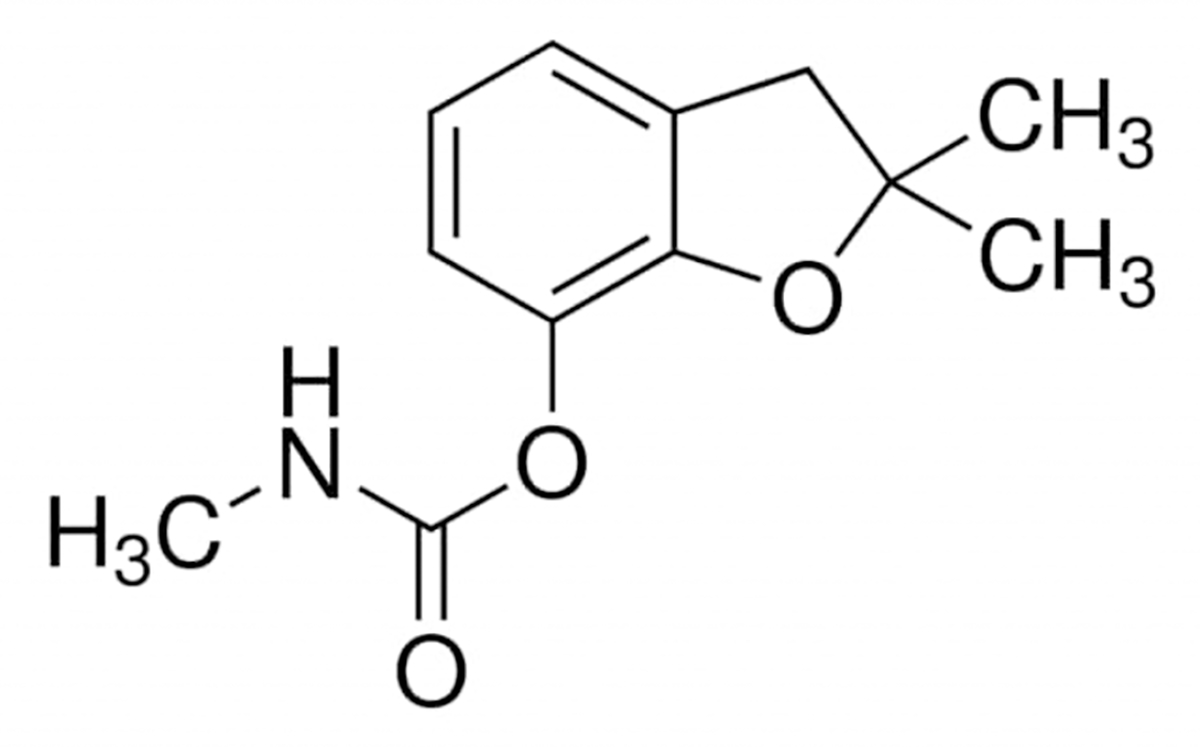
Chemical structure of carbofuran.
3. Carbofuran mode of action
3.1. Cholinergic mechanisms
Carbofuran, like other N-methylarbamate compounds, exerts acute intoxication in humans and animals by virtue of binding and inhibiting (carbamylation) acetylcholinesterase (AChE). The cholinesterases (ChEs) are serine hydrolases that normally catalyze the breakdown of acetylcholine (ACh), a neurohumoral transmitter. Carbofuran binds to the anionic subsite of AChE with high-affinity and makes a carbofuran-AChE complex. The rate of carbamylation is more imprtant than the binding of carbofuran to AChE. Potent AChE inhibition results from rapid carbamylation, as the carbamylation rate constant (ki) is directly correlated with toxicity (Gupta, 1994). Sequestration of the AChE in carbamylated form thus precludes the hydrolysis of ACh, leading to excessive ACh accumulation. Severity of toxicity depends on the speed with which AChE is inhibited and the degree of AChE inhibition. AChE inhibition >70% in discrete brain regions (cortex, amygdala and hippocampus) and in diaphragm muscle leads to a toxic-level accumulation of ACh.
Accumulated ACh overstimulates ACh receptors in the brain, at neuromuscular junctions (NMJs) in skeletal muscles, and in other tissues. Muscarinic AChRs (mAChRs) and nicotinic AChRs (nAChRs) are functionally different. The mAChRs, which are G-protein coupled receptors, mediate a slow metabolic response via second messenger cascades. The nAChRs are ligand-gated ion channels, which mediate a fast-synaptic transmission of the neurotransmitter ACh. Skeletal muscles are enriched with nAChRs and are devoid of mAChRs. During AChE inhibition by OPs and CMs, unhydrolyzed ACh does not diffuse from the cleft, but repeatedly combines with post-synaptic receptors. The prolonged presence of ACh in the synaptic area appears to cause some of the myopathic changes. The common mAChR-associated toxicities include miosis, apprehension, hypersalivation, excessive tracheobronchial secretions, gastrointestinal cramps, broncho- and laryngospasms, dacryorrhea/chromodacryorrhea, urination, defecation, and bradycardia. The nAChR-associated toxicities are muscle fasciculations, tremors, convulsions, weakness of muscles, and seizures.
Evidence suggests that some CMs can directly interact with mAChRs and nAChRs and contribute to their toxicity (Gupta and Milatovic, 2012). In fact, CMs which have a more potent interaction with nAChRs are less potent inhibitors of AChE. The overstimulation of the somatic nervous system usually results in tremors, muscle fasciculations, and piloerection, as well as ataxia and paresis. Following an acute exposure, onset of toxicity signs/symptoms occurs within 10–15 min, maximal severity signs within 30–60 min, and death within one hour. Death ensues due to cardiac arrest and respiratory failure (Gupta, 1994; Gupta and Milatovic, 2012). The respiratory failure is of both central and peripheral origin.
During recovery period, methylcarbamic acid is removed from AChE and the enzyme is reactivated/regenerated by decarbamylation (Gupta, 1994; Timchalk, 2006). The rate of AChE decarbamylation varies among CM compounds. A slower the rate of decarbamylation results in slower recovery in humans and animals from toxicity. It is to note that the carbamylated enzyme half-life (t½) for hydrolysis (~15–30 min) is substantially slower than for deacetylation (turnover time of ACh ~150 μsec). Carbamylated AChE from carbofuran does not ‘age’ as is reported with phosphorylated AChE (t½ ~ days, irreversible inhibition) with OP pesticides or nerve agents (Gupta, 2006, 2020). Since the AChE recovers at almost the same rate as do the AChRs, a balance of AChE to AChRs is maintained over the postsynaptic surface during recovery. Therefore, a relatively constant ratio of AChE to AChRs is very important for maintaining normal neuromuscular function. Surviving humans and animals recover from toxic signs and symptoms of CMs within a few hours to 24 hours. However, the neuropsychological symptoms may persist for weeks or months (Fan, 2011).
3.2. Non-cholinergic mechanisms
In addition to cholinergic mechanisms, multiple non-cholinergic mechanisms are involved in the myriad of toxic effects by CMs/OPs (Gupta and Milatovic, 2012; Gupta et al., 2001a, b; Gupta et al., 2007; Milatovic et al., 2011; Milatovic et al., 2006). Such non-cholinergic targets include various enzymes, N-methyl-D-aspartate (NMDA) receptors, adenosinergic, γ-aminobutergic (GABAergic), and serotonergic systems, signaling molecules (such as nitric oxide) that are reported to be involved in seizures and lethality associated with anti-ChE pesticides and nerve agents (Dekundy and Kaminski, 2011; Gupta, 2020; Gupta and Milatovic, 2012; Gupta et al., 2007; Gupta et al., 2019; Lockridge and Schopfer, 2006). Carbofuran-induced unremitting excitotoxicity for an hour can cause neuronal degeneration in discrete brain areas such as cortex, amygdala, and hippocampus (areas primarily involved in initiation and propagation of convulsions and seizures) (Gupta et al., 2007). The early morphological alterations include dendritic swelling of pyramidal neurons in CA1 region of the hippocampus. The AChEI-induced neuronal cell death is a consequence of a cascade of extra- and intracellular events leading to the intracellular accumulation of Ca2+ ions and the generation of free radicals (Gupta et al., 2001a, b; Gupta et al., 2007). Excessive production of free radicals causes oxidative/nitrosative stress to which the brain is especially vulnerable. This leads to depletion of high-energy metabolites (ATP and phosphocreatine). Lipids are readily attacked by free radicals, resulting in the formation of a number of peroxidation products (formed non-enzymatically), such as F2-isoprostanes, furans, and F4-neuroprostanes (specific markers of oxidative stress to the neurons) (Gupta and Milatovic, 2012; Gupta et al., 2007; Zaja-Milatovic et al., 2009). The cholinergic and non-cholinergic mechanisms involved in CMs/OPs toxicity are depicted in Figure 2.
Figure 2.

Cholinergic and non-cholinergic mechanisms involved in CM/OP toxicity; Courtesy (Gupta and Milatovic, 2012).
3.3. Carbofuran-induced intermediate syndrome
Intermediate syndrome (IMS) was reported for the first time in Sri Lanka, where 10 human patients presented 24–96 hours after acute cholinergic crisis from exposure to OP pesticides, such as methamidophos, fenthion, dimethoate and monocrotophos (Senanayake and Karalliedde, 1987). These patients had acute muscle paralysis and some required ventilator support. Since then IMS has been diagnosed in many countries involving a dozen OPs. IMS appears to be due to insufficient oxime therapy and lack of oxygenation or ventilation. In 2005, carbofuran was also demonstrated to cause IMS in patients accidentally or intentionally exposed to large doses of this insecticide (Paul and Mannathukkaran, 2005). IMS is characterized by acute respiratory paresis and muscular weakness, primarily in the facial, neck and proximal limb muscles. In addition, it is often accompanied by generalized weakness, cranial nerve palsies, depressed deep tendon reflexes, ptosis and diplopia. These symptoms can last for days or weeks.
It has been suggested that the defect in IMS is at the neuromuscular endplate and postsynaptic level, but the effect of neural and central components in producing muscular weakness have not been ruled out. Evidently, some anti-AChE insecticides are greatly distributed to muscles and have a higher affinity for nAChRs. There is no specific antidote for IMS. For further detail on IMS, readers are referred to following research work (De Bleecker et al., 1993).
3.4. Carbofuran phamacokinetics and physiologically based pharmacokinetics
Pharmacokinetics of CM pesticides, including carbofuran, is concerned with the quantitative integration of absorption, distribution, metabolism, and excretion, and associated toxicological responses in several mammalian species (Gupta, 2006; Gupta et al., 1994; Timchalk, 2006). Carbofuran can be absorbed by the oral, inhalation and dermal routes or through eye contact. It is poorly absorbed through intact skin.
The metabolism of carbofuran has a significant impact on its overall toxicity. Following an oral ingestion, 75% of absorbed carbofuran is protein bound. Metabolism of free carbofuran appears to involve hydroxylation and/or oxidation reactions that result in the formation of carbofuran phenol, 3-hydroxycarbofuran, 3-hydroxycarbofuran-7-phenol, 3-ketofuran, and 3-ketofuran-7-phenol. The major metabolites are produced by hydroxylation at the benzylic carbon to give -3-hydroxycarbofuran, which is oxidized to the 3-ketocarbofuran when not blocked by formation of conjugates. The other researchers also identified with certainty: 3-hydroxycarbofuran, 3-ketocarbofuran, and their respective 7-hydroxy hydrolysis products (Metcalf et al., 1968). These phenols, together with carbofuran phenol, are present in the free state and have also been identified as conjugates in various biological systems. In in vitro studies, cytochrome P-450 2E1 is the major isoenzyme responsible for 3-hydroxycarbofuran formation (Usmani et al., 2004).
Several carbofuran metabolites (3-hydroxycarbofuran, 3-ketocarbofuran, 3-ketocarbofuran phenol, carbofuran phenol, and conjugate of 3-ketocarbofuran) in the urine of mice and rats orally dosed with radiolabeled carbofuran (Metcalf et al., 1968). Interestingly, oral exposure of rats to labeled [14C]carbofuran resulted in enterohepatic circulation of some metabolites, such as 3-hydroxycarbofuran (known to inhibit AChE) (Marshall and Dorough, 1979). Most metabolites of carbofuran form glucuronide or sulfate conjugates which are excreted in the urine. The bile contained predominantly 3-hydroxycarbofuran glucuronide, a metabolite having the carbamate ester linkage intact, which may be cleaved to yield a potent anti-AChE aglycone. Since enterohepatic cycling of glucuronides involves cleavage of the conjugate in the gut, biliary excretion may lead to increased systemic activity of toxic carbamate metabolites. The plasma elimination t½ of 3-hydroxycarbofuran (64 min) is more than twice that of carbofuran (29 min). These findings clearly demonstrated that enterohepatic circulation may be a key factor in maintaining anti-AChE activity after carbofuran no longer exists in the body (Ferguson et al., 1984; Marshall and Dorough, 1979).
The majority of carbofuran metabolites (3-hydroxycarbofuran, 3-ketocarbofuran, and their conjugated products) are excreted in the urine, very little in the feces, and trace amounts in the milk (Gupta, 1994).
A PBPK/PD model for carbofuran in Sprague-Dawley rats, and the corresponding human model was derived by replacing the rat physiological structure with that of the human (Zhang et al., 2010; Zhang et al., 2007). The physiological structure included arterial blood, brain, skin, fat, GI tract, kidney, liver, lung, portal blood, and venous blood. A full GI compartment model including stomach, duodenum, lower small intestine, and colon was utilized to better understand carbofuran GI absorption, biliary circulation, and fecal elimination. The advantage of the application of the constructed PBPK/PD model enables the dose-response study and the toxicological endpoints to be estimated in silico, something that cannot be easily achieved by regular bench work. It needs to be mentioned that only when a well-calibrated and validated PBPK/PD model is constructed will these computerized study findings be useful.
Using a generic PBPK model and the IndusChemFate PBPK model, urinary concentration of 3-hydroxycarbofuran after exposure during an 8-hour shift to carbofuran at the level of TLV of 0.1 mg/m3, the model predicted concentration of free-3-hydroxycarbofuran at 0.005 μmol/L=1.1 μg/L. Some models can also evaluate the impact of their variability on model predictions and predict the inhibition of blood AChE and BuChE. PBPK/PD models suggest that there is no interaction among N-methylcarbamates (NMCs) nor is there competition from the NMCs with AChE. For further details on PBPK modeling of CM and OP pesticides, their interactions, and risk assessment from cumulative exposure (Lowit, 2006; Padilla, 2006; Timchalk, 2006).
4. Carbofuran and pesticide toxicity
Pesticides are used in the agricultural industry to protect and improve crops. However, agricultural use of pesticides can have unintended negative effects on the environmental conditions and humans (Sharma et al., 2012a). Although currently many countries banned carbofuran usage, carbofuran is still used as an insecticide in agriculture and households, affecting humans as well as animals because it contaminates the atmosphere, water bodies, and food products (Cinar et al., 2015). Carbofuran is anticholinesterase (inhibits cholinesterase) and metabolites 3-hydroxycarbofuran and 3-ketocarbofuran induce cholinergic and non-cholinergic biochemical, hematological, and immunologic toxicity (Gupta, 1994). Carbofuran and its metabolites can enter the placenta and initiate severe maternal-placental-fetal structural abnormalities (Gupta, 1994).
The rate of carbofuran adsorption and desorption in soils is determined by the type of clay and organic carbon ingredients, but carbofuran can easily contaminate groundwater from pesticide runoff due to its high solubility and high mobility in soils (Bermudez-Couso et al., 2011). Carbofuran runoff can also harm the environment since it induces acute toxic effects on freshwater algae and fish (Anton et al., 1993). Carbofuran pesticide can have a differential influence on microorganisms in the soil as well. Some pesticides influence the growth of microorganisms, but some show suppressive activity or no action on microorganisms. Carbofuran, however, increased the population of Azospirillum and other anaerobic nitrogen fixers in flooded and non-flooded soil conditions (Lo, 2010).
Carbofuran and pesticide exposures occur in occupational and non-occupational workers and has led to homicides and suicides (Sakunthala Tennakoon et al., 2013). Toxic pesticides can enter the body via the ocular route (Jaga and Dharmani, 2006). Repeated exposure to pesticides is a significant health risk to body, specifically to eyes. Aerial spraying of pesticides over plants also increases the risk of ocular exposure in people. Ocular pesticide contact is very common to humans and animals from pesticide manufacturing plant accidents (Jaga and Dharmani, 2006) or its intentional use in wars, conflicts, and terrorism.
Pesticide exposure can show mutagenic effects by inducing chromosomal abnormalities, deoxyribonucleic acid (DNA) damage, micronuclei formation or cytotoxicity in the cells. It has been reported that pesticide toxicity causes abnormal ocular movements and can also lead to ocular pathologies such as visual loss. Pesticide toxicity can cause Saku disease, an optico-autonomic peripheral neuropathy with myopia, astigmatism, vision defect, abnormal eye movement, optic neuronal inflammation, reduced plasma level of ChE, and neurological defects (Jaga and Dharmani, 2006). Increased cellular apoptosis in ocular tissue, especially in corneal cells, is common in pesticide toxicity (Sanyal and Law, 2019). Recent studies found alteration of autophagy mechanism in human cornea in vitro and ex vivo post carbofuran exposure (Kempuraj and Mohan, 2022). Chronic pesticide exposure causes defective cellular responses in the cornea leading to visual defects (Sanyal and Law, 2019). However, the ocular/corneal effects of carbofuran and pesticides in general are poorly understood. It has been our central postulate that carbofuran exposure to eyes causes corneal stromal pathologies and vision loss in humans and companion animals.
4.1. Carbofuran toxicity to non-ocular system
Exposure or ingestion of carbofuran in humans leads to multiple ocular and non-ocular morbidities and can be lethal if not treated immediately. People exposed to carbofuran while mixing with seeds, spraying pesticides, cleaning pesticide sprayers, etc. experience redness of hands, blurred vision, excessive constriction of the pupil, nausea, vomiting, headache, dizziness, tachycardia, tachypnea, salivation, high blood pressure, and fasciculation; and laboratory findings of hyperglycemia (Satar et al., 2005). Typical acute occupational carbofuran poisoning can cause immediate illness that quickly improves. These studies recommend that agricultural workers should be appropriately educated on the toxic effects of carbofuran exposure so that they can reduce exposure-associated toxicity (Satar et al., 2005).
Carbofuran readily pollutes air, food, water bodies, and causes significant ocular defects, vomiting, hypersalivation, diarrhea, respiratory abnormalities, and death (Nguyen et al., 2021). It suppresses the AChE enzyme in the central nervous system (CNS) and inhibits neuronal signals (Purushothaman and Kuttan, 2017). In physiological conditions, AChE inhibits neuronal signal transmission by hydrolyzing ACh in the CNS and peripheral nervous system (Colovic et al., 2013). Carbofuran shows toxic effects on blood vessels as evidenced by significantly reduced cell survival of human umbilical endothelium and upregulated intracellular reactive oxygen species (ROS) levels indicating increased oxidative stress, DNA damage, increased apoptosis, and defective vascular functions including the angiogenesis process (Saquib et al., 2021). Since carbofuran can cause toxic effects on the endothelial cells of the blood vessels, it could also affect corneal endothelium which plays an important in maintaining corneal hydration, homeostasis, and transparency.
The CF toxicity involves oxidative stress, cellular senescence and autophagy. Exposure to CF reduces nuclear erythroid 2-related factor (Nrf2) and glutathione S-transferase Pi isoform 1 (Gstp1) levels while influencing autophagy and senescence (Khan et al., 2021; Oda et al., 2022). CF exposure didn’t increase cancer risk in farmers (Bonner et al., 2005) but caused an adverse effect on cell membranes by altering oxidative balance and membrane stability (Sharma and Sharma, 2012). CF significantly elevated the levels of F2-isoprostanes and F4-neuroprostanes (in vivo biomarkers of lipid peroxidation and generation of ROS), and citrulline (a specific marker of nitric oxide (NO)/nitric oxide synthase (NOS) and reactive nitrogen species (RNS) (Gupta et al., 2007; Milatovic et al., 2006; Zaja-Milatovic et al., 2009). Carbofuran can induce aneuploidy or cell death with high doses, thereby reducing the fertilization and implantation process (Cinar et al., 2015; Shen et al., 2022).
4.2. Carbofuran toxicity to ocular system
Pesticides affect conjunctiva, cornea, lens, retina, and optic nerve, and cause abnormal ocular movement, excessive tearing, and loss of vision (Figure 3). Improved safety procedures and regulations can reduce pesticide-associated eye injury (Food Safety Commission of, 2019; Jaga and Dharmani, 2006).
Figure 3:

Diagrammatic representation of carbofuran toxicity to eye. Carbofuran morbidities to eye include corneal haze/scarring, miosis, lacrimation, dacryorrhea, chromodacryorrhea, cataract, conjunctivitis, retinal degeneration, and vision impairment.
Carbofuran functions as a cholinesterase inhibitor, thereby increasing the levels of ACh in the central and peripheral nervous systems. Ach can influence cell membrane permeability through pores (nicotinic receptors) (Ringvold and Reubsaet, 2016). CF ingestion is known to cause coma, respiratory failure from acute respiratory distress syndrome (ARDS), and cortical blindness from the increased ACh levels in the nervous system (Baban et al., 1998). Corneal epithelium possesses high ACh, but its role is not yet clearly known. Additionally, rabbit corneal epithelium is devoid of cholinergic receptors (Pesin and Candia, 1982). However, mammalian tissue studies report that the corneal epithelium contains the highest concentration of muscarinic receptors (Sloniecka and Danielson, 2020). The corneal epithelium contains large nerve endings but the level of ACh is relatively low compared to junctional tissues. The ACh could regulate water and ion transport into the corneal epithelium (Sloniecka and Danielson, 2020). The role of the cholinergic system on active ionic transport was analyzed using frog corneas. An exogenous ACh (2 mM) showed a moderate inhibition of sodium transport but had no effect on chloride transport. It suggested that only endogenous ACh could stimulate ionic transport but not the exogenous Ach (Pesin and Candia, 1982). Also, ACh influences wound healing in the cornea. ACh inhibited mRNA levels of collagen I, collagen III, collagen V, lumican, fibronectin, and alpha-smooth muscle actin (α-SMA) in quiescent keratocytes. Additionally, decreased gene expression of collagens (I, III, and V), lumican, fibronectin, and α-SMA were seen with ACh treatment during and after the development of fibrosis (Sloniecka and Danielson, 2020). This study suggested that ACh inhibited corneal fibroblasts to develop contractile structures by the activation of muscarinic ACh receptors and consequently fibrosis in corneal stroma (Sloniecka and Danielson, 2020). In another study, ascorbic acid in corneal epithelium is shown to decrease in response to ACh exposure (Ringvold and Reubsaet, 2016). ACh could increase the corneal re-epithelialization process by changing proliferation and apoptosis.
Corneal epithelial cells contain choline acetyltransferase (ChAT), AChE, and two muscarinic and nicotinic ACh receptors (mAChR) subtypes, and several nAChR subtypes. Continuous activation of corneal epithelial cells (CEC) by the muscarinic and nicotinic signaling pathways is important for CEC survival and migration. Additionally, this study exhibited that cholinergic stimulation of CEC increases expression of integrin and cadherin molecules that are involved in the re-epithelialization process (Chernyavsky et al., 2014).
TGF-β1 is a key cytokine in corneal fibrosis development and ACh modulates keratocyte conversion to myofibroblast through TGF-β1 in vitro (Sloniecka and Danielson, 2020). After an insult, TGF-β1 from the epithelium is poured into the stromal extracellular matrix (ECM) in an inactive TGF-β1 form. This inactive form is activated by integrins and proteases and initiates the wound healing process in the stroma. TGF-β1 provokes significant release of ECM components (collagens, fibronectin, etc.) and facilitates myofibroblasts formation which can be inhibited by the ACh (Mohan et al., 2022; Sloniecka and Danielson, 2020). ACh not only can regulate the initiation of corneal fibrosis but may decrease the level of a fibrotic condition from the previous injuries (Sloniecka and Danielson, 2020).
Autophagy regulates corneal function and vision. Our recent studies with human primary corneal fibroblasts and human cornea organ culture found that CF alters expression of autophagy signature genes Beclin 1 and microtubule-associated protein light chain 3 (LC3I)/II mRNA (Kempuraj & Mohan, 2022). This report first time indicated that CF is injurious to autophagy mechanism in the cornea. Furthermore, it suggested that CF exposure to eye, like alkali and warfare agents, could lead to vision impairing corneal pathologies in humans and animals (Fuchs et al., 2021; Gupta et al., 2020; Kamil and Mohan, 2021; Kempuraj and Mohan, 2022; Mohan et al., 2022; Sinha et al., 2021; Tripathi et al., 2020). Therefore, it is imperative to develop a better understanding of molecular mechanisms by which carbofuran and other pesticides compromise normal corneal function and vision.
4.3. Carbofuran toxicological studies in experimental animals
Pesticides enter into eye and damage tissues. Table-1 lists toxic effects of pesticides reported in humans and in various models. Increased pesticide poisoning is due to the increased rate of apoptosis, expression of death receptor (FAS/CD95), and caspase 3 and decreased cellular proliferation and improper wound healing in cornea and other ocular and non-ocular tissues (Ballantyne, 2006, 2009; Jaga and Dharmani, 2006; Sanyal and Law, 2019; Kontadakis et al., 2014). Rats exposed to CF showed a significant reduction in AChE, increase in ROS and RNS in cortex, amygdala, and hippocampus (Gupta et al., 2007), alteration in hypothalamus-hypophysial ovarian axis and hormonal imbalance (Baligar and Kaliwal, 2002), and and increased neuronal susceptibility to glutamate toxicity (Umeda et al., 2018). Also, CF reduced neurogenesis in the early stage of gestation, reduced the neuronal progenitor cells, increased neurodegeneration in the hippocampus, and induced cognitive disorders in rat offspring (Mishra et al., 2012). Acute carbofuran toxicity also included transient endocrine disruption in male rats (Goad et al., 2004). Interestingly, pretreatment of carboxylesterase inhibitor (iso-OMPA; 1 mg/kg, sc) 1 hour before CF exposure significantly mitigated CF’s toxicity (Gupta and Kadel, 1989).
Table 1:
Carbofuran (CF) toxicity and impact on human case reports, in vivo animals, and in vitro cell culture models.
| Clinical studies | ||||||
| Subject | Exposure Route | Molecular Target | Therapeutic Interventions | Symptoms | Toxicity | Ref. No |
| Human | Oral Ingestion | Synaptic-release blockers of the acetylcholinesterase (AChE) | None | Epitaxis, Eyelid Hemorrhage, Hyphema, Congestion, Myocardial Infarct, Edema, Ecchymosis | Respiratory and Cardiac Depression | (Yen et al., 2015) |
| Human | Dermal, Absorption | None | None | Sleeping issues, Headache, lack of appetite | Mental Disorders | (Ong-Artborirak et al., 2022) |
| Human | Oral Ingestion | Blocking the activity of cholinesterase | Multi drug antibiotic therapy | Hypersalivation, Lacrimation, Pupil Constriction, Bronchoconstriction, Convulsions | Respiratory and Cardiac Arrest | (Klatka et al., 2021) |
| Human | Oral Ingestion | None | Atropine, Dopamine, dobutamine | Seizures | Respiratory failure, | (Lamb et al., 2016) |
| Human | Oral Ingestion | Blocking the activity of cholinesterase | Calcium channel blocking drugs and magnesium sulfate | Paresthesia | Sensorimotor Neuropathy | (Yang et al., 2000) |
| Human | Inhalation | None | None | Weakness, Fatigue, Cephalalgia, Disorientation, Abdominal pain, Vomiting | Genotoxicity | (Zeljezic et al., 2008) |
| Human | Oral Ingestion | None | None | Coma, Acute respiratory distress syndrome (ARDS), and Cortical blindness | Respiratory failure | (Baban et al., 1998) |
| Human | Dermal, Oral, Inhalation | None | None | Mutagenic | Lung Cancer | (Bonner et al., 2005) |
| Animal studies | ||||||
| Subject | Exposure Route | Molecular Target | Therapeutic Interventions | Symptoms | Toxicity | Ref. No |
| Dogs | Oral Ingestion | Synaptic-release blockers of the acetylcholinesterase | None | Epitaxis, Eyelid Hemorrhage, Hyphema, Congestion, Myocardial Infarct, Edema, Ecchymosis | Respiratory and Cardiac Depression | (Pivariu et al., 2020) |
| Mice and Rats | Oral Ingestion and IV | Hormonal imbalance, Acetylcholinesterase inhibition, Increase oxidative and LPO stress, | Bridelia tomentosa leaf extract, Curcumin, Kebar grass extract, Curcuma longa | Loss of Body Weight, Loss of liver weight, increased oxidative stress, changes in blood indices, effected estrous cycle and follicles | Hepatic Damage, Neurobehavioral disorders, liver, heart, and brain damage, neuronal vulnerability, dermal toxicity, Reproductive toxicity | (Baligar and Kaliwal, 2002; Ferguson et al., 1984; Gammon et al., 2012; Hossen et al., 2017; Jaiswal et al., 2014; Mondal et al., 2021; Purushothaman and Kuttan, 2017; Rai et al., 2009; Seth et al., 2019; Umeda et al., 2018; Yulitasari et al., 2021) |
| Fishes | Absorption and Ingestion | Acetylcholinesterase inhibition | Lycopene | Increased oxidative stress, Testicular lesions, cardiac edema | Behavioral and Histopathological alterations, reproductive toxicity, Cardiotoxicity | (Anton et al., 1993; Bretaud et al., 2000; Covert et al., 2020; Hamed and Osman, 2017; Oda et al., 2022; Saputra et al., 2021; Singh et al., 2003) |
| Cell culture studies | ||||||
| Subject | Tested Dose | Molecular Target | Therapeutic Interventions | Symptoms | Toxicity | Ref. No |
| Human umbilical vein endothelial cells | 1000 μM | - | - | - | Intracellular ROS, Mitochondrial Membrane Potential, DNA Damage | (Saquib et al., 2021) |
| Human peripheral blood lymphocyte | 5–100 μM | - | Vitamin C and E | - | DNA Damage and cytogenotoxicity | (Das et al., 2007; Naravaneni and Jamil, 2005; Sharma et al., 2012a; Sharma et al., 2012b; Sharma and Sharma, 2012) |
| Bacteria (Sinorhizobium Saheli) | 25–100 μg/mL | - | - | - | Cellular Viability, Cellular Proliferation, Cellular Morphology, Biofilms | (Shahid et al., 2021) |
| Plant (Vigna mungo L). | 25–100 μg/mL | - | - | - | Growth, morphology, survival, cellular respiration, and inner membrane permeability | (Shahid et al., 2021) |
| Human liver microsomes | 2.5–300 μM | - | Recombinant cytochrome P450 enzymes | - | - | (Abass et al., 2022) |
| Mouse oocytes | 10–400 μM | - | - | - | Cell death, Reduced fertilization rates | (Cinar et al., 2015) |
| Hamster ovary cells (CHOK1) | 5–100 μg/mL | - | - | - | Genotoxicity and Cytotoxicity | (Soloneski et al., 2008) |
| Cat fibroblast cells | 0.045–1.08 mM | - | - | - | DNA Damage and cytogenotoxicity | (Chandrakar T. R, 2020) |
| Bull squamous carcinoma cells | 400 ppm | - | - | - | Cellular toxicity on cancer cells | (Amanullah and Hari, 2011) |
5. Carbofuran and pesticide toxicity to civilians, military personnel, and war Veterans
Symptoms of CF toxicity can appear within five to forty-five minutes depending on the amount and location of exposure. These include miosis, bronchospasm, lacrimation, defecation, bronchorrhea, urination, defecation, emesis, and salivation (Nguyen et al., 2021). The underlying mechanisms involves, but not limited to, increase in parasympathetic activation/accumulation of ACh in the central and peripheral nervous system due inhibition of AChE. Further, CF exposure causes a mix of parasympathetic and few adrenergic symptoms of tachycardia, hypertension, and mydriasis, however, parasympathetic symptoms generally prevail (Satar et al., 2005; Silberman and Taylor, 2022). CF can also cause neurotoxicity by crossing the blood-brain barrier (Gupta et al., 2007).
The ocular toxicities of anti-AChE pesticides and chemical warfare agents in humans and animals are documented (Ballantyne, 2006, 2009; McNutt and Hamilton, 2015; McNutt et al., 2020). Miosis is a classical sign of CF toxicity, and it degree can be dose-related. Iritis can occur from CF and is generally associated with conjunctival hyperemia. Application of physostigmine, neostigmine, or diisopropylphosphorofluoridate (DFP) to rabbit eye initially causes hyperemia of the iris, increase in intraocular pressure and increase in capillary permeability, permitting entry of proteins that may cause an aqueous humor flare (Ballantyne, 2006, 2009). The pig, rabbit, and human lens treated with paraoxon results in 50% inhibition of oxygen consumption (Ballantyne, 2006, 2009; Gehring and Smith, 1971; Gupta, 2015; Santolucito and Whitcomb, 1971). Recently, in an intentional CF poisoning in dogs led to 3rd eyelid/conjunctival hemorrhage, diffuse uveal congestion and unilateral or bilateral hyphema (Pivariu et al., 2020). Biomarkers of CF’s ocular toxicity may aid early detection, severity, and progression of CF-induced ailment, as well as can be used predict response to treatment (Kontadakis et al., 2014). High pesticide exposure causes ocular irritation in 99% subjects (Jaga and Dharmani, 2006). It led to degeneration of ciliary body, optic nerve, retina, and extraocular muscles. Clinically, most observed symptoms of ocular pesticide exposure include corneal opacity, conjunctivitis, and conjunctival chemosis/hyperemia (Jaga and Dharmani, 2006). Clinical eye symptoms post CF include pinpoint pupils, blurred vision, and sore eyes (United States Environmental Protection Agency, 1997).
Gulf War Illness (GWI) includes a neuroimmune disorder with chronic multisymptomatic ailments such as fatigue, myalgic encephalomyelitis/chronic fatigue syndrome, headaches, pain, sleep disorder and memory issues in the troops deployed to the Middle East in the Persian Gulf War (Operation Desert Storm/Desert Shield) of 1990–91 (Baksh et al., 2021; Chester et al., 2019; Michalovicz et al., 2020; White et al., 2016). GWI veterans showed blurred/double vision, dry eye symptoms, photophobia, ocular pain, cognitive dysfunction, neuroinflammation, astrocyte and microglial activations, and increased proinflammatory cytokine levels (Attaluri et al., 2022). Although the exact causes of the GWI are poorly known, about 41,000 military personnel could have been over-exposed to pesticides and inhibitory effects against AChE (Chester et al., 2019). Exposure to AChE inhibitors (AChEIs) induces GWI symptoms, mitochondrial dysfunction, and oxidative stress in GWI veterans. Further, AChEIs can cause decreased AChE activity, neuronal degeneration, and neuronal death in animal models of GWI (Chester et al., 2019). The dry eye symptoms and retinal nerve fiber layer thinning could be a biomarker for GWI (Baksh et al., 2021; Sanchez et al., 2022).
Several human epidemiological and clinical studies ocular toxicity from exposure to anti-ChEs (Ballantyne, 2006, 2009). A ocular toxicity was reported in a pesticide manufacturing plant in workers exposed to CM pesticide methomyl (Morse et al., 1979). A cross-sectional study of fenitrothion sprayers in India found macular degeneration in 16% of workers (Misra et al., 1985). School children from Saku region of Japan demonstrated a 65% incidence of optic neuritis and/or chorioretinal atrophy (Oto, 1971).
Anti-AChEs can modify retinal physiology and function. The retina has at least five neurotransmitters (GABA, glycine, dopamine, idolamine, and ACh) and pathogenesis of retinal lesions by anti-AChEs appears complex (Ballantyne, 2006, 2009). Retinal detachment has been noted as a complication of the treatment of glaucoma with anti-AChEs. Histopathological studies demonstrated that diazinon and DFP induced necrotic lesions in retinal cells (Ballantyne, 2006). From a mechanistic standpoint, some studies have suggested that inhibition of retinal AChE may not be a major etiological factor in the causation of retinal toxicity by anti-ChEs (Atkinson et al., 1994). However, the possibility exists that OP/CM-induced retinal degeneration may occur if a substantial inhibition of cyclic guanosine monophosphate (cGMP) occurs apart from inhibition of retinal AChE.
6. Current therapeutics for pesticide/carbofuran toxicity
Presently, the first line of treatment for carbofuran exposed eyes is to immediately flush eyes with copious amounts of water and removing contact lenses (Silberman and Taylor, 2022). In large ingestions of CF, gastric lavage or activated charcoal can be considered. Additionally, if the patient has seizures, benzodiazepines may be given even though there is limited data on their efficacy in CF toxicity (Silberman and Taylor, 2022). Atropine can be given to the patient in the acute setting to stabilize the patient’s cardiorespiratory status and reduce the symptoms of CF toxicity since atropine competitively antagonizes acetylcholine at muscarinic receptors (Silberman and Taylor, 2022). Pretreatment of Atropine and memantine in rats protected animals from hypercholinergic activity such as seizures by blocking pathways associated with neuronal oxidative damage in CF toxicity (Gupta et al., 2007). In addition, rats that were given therapeutic atropine with memantine antagonizes the acute toxicity of CF more than atropine and memantine individually (Gupta and Kadel, 1989). If it is certain that a patient has CF toxicity, pralidoxime may be withheld since studies have shown that pralidoxime may cause complications of increased AChE inactivation after CF dissociates spontaneously (Silberman and Taylor, 2022). Several additional therapeutic evaluations are reported to treat CF-induced toxicity in animals or humans. Curcumin administration significantly prevented CF-induced neurobehavioral disorders, activities of acetylcholinesterase, lactate dehydrogenase, creatine kinase, gamma-glutamyl transferase, and mitochondrial enzyme in animals (Purushothaman and Kuttan, 2017). Vitamin C pretreatment prevented CF induced oxidative stress and erythrocyte fragility in rats (Rai et al., 2009). Likewise, Vitamin E pretreatment showed protective effects in CF-induced oxidative stress (Jaiswal et al., 2014). Turmeric is shown to inhibit CF toxicity by increasing antioxidant enzymes and decreasing lipid peroxidation (Hossen et al., 2017). In an environmental scope, methods to degrade carbofuran include using up-flow anaerobic sludge blankets, modified up-flow anaerobic sludge blankets with tube settlers, and solar-photo-Fenton treatment (Lopez-Alvarez et al., 2012; Madhubabu et al., 2007). We have evaluated various modalities to mitigate chemically induced corneal scars by promoting physiological repair and preventing pathological events (Gupta et al., 2021; Mohan et al., 2021; Mohan et al., 2011; Tripathi et al., 2020).
7. Conclusion
Carbofuran is a highly toxic carbamate pesticide that causes severe toxicity. Following exposure, CF rapidly enters in the eye. Ocular toxicity largely involves cholinergic mechanisms involving AChE inhibition followed by ACh accumulation and unremitting overstimulation of mAChRs and nAChRs. Early symptoms of cholinergic toxidrome include miosis, tearing, and loss of accommodation. The noncholinergic mechanisms include various transmitter systems, oxidative stress, neuroinflammation, apoptosis, autophagy, signaling pathways, etc. Toxic ocular effects unrelated to cholinergic system include dry eye symptoms, neuroimmune disorders, abnormal ocular movement, and blurred or loss of vision. Development of novel animal models and mechanistic understanding is warranted to counter CF toxicity
Highlights.
Carbofuran causes multiple corneal/ocular pathologies and blindness in humans.
Carbofuran exposure modulates wound healing and autophagy mechanism in the cornea.
In the eye, miosis, dacryorrhea, chromodacryorrhea, ocular pain and irritation, and blurred vision are common post carbofuran exposure.
Acknowledgments
This work was mainly supported by the NEI/NIH 1R01EY034319, U01EY031650, R01EY030774, and 5R21EY032742 grants, and in part by the United States Department of Veterans Health Affairs Merit 1I01BX00357 and SRCS IK6BX005646 awards and the Ruth M. Kraeuchi Missouri Endowed Chair Ophthalmology Fund, University of Missouri, Columbia, MO USA (RRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abass K, Reponen P, Alsanie WF, Rautio A, Pelkonen O, 2022. Characterization of furathiocarb metabolism in in-vitro human liver microsomes and recombinant cytochrome P450 enzymes. Toxicol Rep 9, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanullah M, Hari BY, 2011. Evaluation of carbamate insecticides as chemotherapeutic agents for cancer. Indian J Cancer 48, 74–79. [DOI] [PubMed] [Google Scholar]
- Anton FA, Laborda E, Laborda P, Ramos E, 1993. Carbofuran acute toxicity to freshwater algae and fish. Bull Environ Contam Toxicol 50, 400–406. [DOI] [PubMed] [Google Scholar]
- Atkinson JE, Bolte HF, Rubin LF, Sonawane M, 1994. Assessment of ocular toxicity in dogs during 6 months’ exposure to a potent organophosphate. J Appl Toxicol 14, 145–152. [DOI] [PubMed] [Google Scholar]
- Attaluri S, Upadhya R, Kodali M, Madhu LN, Upadhya D, Shuai B, Shetty AK, 2022. Brain-Specific Increase in Leukotriene Signaling Accompanies Chronic Neuroinflammation and Cognitive Impairment in a Model of Gulf War Illness. Front Immunol 13, 853000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban NK, Nunley DL, Borges AS Jr., Roy TM, 1998. Human sequelae of severe carbamate poisoning. Tenn Med 91, 103–106. [PubMed] [Google Scholar]
- Bajgar J, Fusek J, Kassa J, Kuca K, Jun D, 2015. Chapter 66 - Pharmacological Prophylaxis Against Nerve Agent Poisoning: Experimental Studies and Practical Implications, in: Gupta RC (Ed.), Handbook of Toxicology of Chemical Warfare Agents (Second Edition). Academic Press, Boston, pp. 979–987. [Google Scholar]
- Baksh BS, Zayan KL, Goldhardt R, Felix ER, Klimas N, Galor A, 2021. Ocular manifestations and biomarkers of Gulf War Illness in US veterans. Sci Rep 11, 6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baligar PN, Kaliwal BB, 2002. Reproductive toxicity of carbofuran to the female mice: effects on estrous cycle and follicles. Ind Health 40, 345–352. [DOI] [PubMed] [Google Scholar]
- Ballantyne B, 2006. CHAPTER 31 - Local and Systemic Ophthalmic Pharmacology and Toxicology of Organophosphate and Carbamate Anticholinesterases, in: Gupta RC (Ed.), Toxicology of Organophosphate & Carbamate Compounds. Academic Press, Burlington, pp. 423–445. [Google Scholar]
- Ballantyne B, 2009. Ophthalmic Toxicology, General, Applied and Systems Toxicology.
- Bermudez-Couso A, Fernandez-Calvino D, Pateiro-Moure M, Novoa-Munoz JC, Simal-Gandara J, Arias-Estevez M, 2011. Adsorption and desorption kinetics of carbofuran in acid soils. J Hazard Mater 190, 159–167. [DOI] [PubMed] [Google Scholar]
- Bonner MR, Lee WJ, Sandler DP, Hoppin JA, Dosemeci M, Alavanja MC, 2005. Occupational exposure to carbofuran and the incidence of cancer in the Agricultural Health Study. Environ Health Perspect 113, 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretaud S, Toutant JP, Saglio P, 2000. Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus). Ecotoxicol Environ Saf 47, 117–124. [DOI] [PubMed] [Google Scholar]
- Chandrakar TR, S.A.P., Sarkhe BC, Bagchi SN, 2020. In Vitro Cytotoxicity and Genotoxicity Assessments of Carbofuran and Malathion Pesticides on Cat (Felis catus) Fibroblast Cells. Biomed Pharmacol J 13, 1157–1162. [Google Scholar]
- Chernyavsky AI, Galitovskiy V, Shchepotin IB, Jester JV, Grando SA, 2014. The acetylcholine signaling network of corneal epithelium and its role in regulation of random and directional migration of corneal epithelial cells. Invest Ophthalmol Vis Sci 55, 6921–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JE, Rowneki M, Van Doren W, Helmer DA, 2019. Progression of intervention-focused research for Gulf War illness. Mil Med Res 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar O, Semiz O, Can A, 2015. Carbofuran Alters Centrosome and Spindle Organization, and Delays Cell Division in Oocytes and Mitotic Cells. Toxicological Sciences 144, 298–306. [DOI] [PubMed] [Google Scholar]
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM, 2013. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11, 315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covert SA, Shoda ME, Stackpoole SM, Stone WW, 2020. Pesticide mixtures show potential toxicity to aquatic life in U.S. streams, water years 2013–2017. Sci Total Environ 745, 141285. [DOI] [PubMed] [Google Scholar]
- Das PP, Shaik AP, Jamil K, 2007. Genotoxicity induced by pesticide mixtures: in-vitro studies on human peripheral blood lymphocytes. Toxicol Ind Health 23, 449–458. [DOI] [PubMed] [Google Scholar]
- De Bleecker J, Van den Neucker K, Colardyn F, 1993. Intermediate syndrome in organophosphorus poisoning: a prospective study. Crit Care Med 21, 1706–1711. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Kaminski RM, 2011. Central Mechanisms of Seizures and Lethality Following Anticholinesterase Pesticide Exposure.
- Fan AM, 2011. Epidemiology of Anticholinesterase Pesticide Poisoning in the United States.
- Ferguson PW, Dey MS, Jewell SA, Krieger RI, 1984. Carbofuran metabolism and toxicity in the rat. Fundam Appl Toxicol 4, 14–21. [DOI] [PubMed] [Google Scholar]
- Food Safety Commission of J, 2019. Flubenziamide (Pesticides). Food Saf (Tokyo) 7, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Giuliano EA, Sinha NR, Mohan RR, 2021. Ocular toxicity of mustard gas: A concise review. Toxicol Lett 343, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon DW, Liu Z, Becker JM, 2012. Carbofuran occupational dermal toxicity, exposure and risk assessment. Pest Manag Sci 68, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring PJ, Smith RS, 1971. The Cataractogenic Activity Of Chemical Agents. CRC Critical Reviews in Toxicology 1, 93–118. [DOI] [PubMed] [Google Scholar]
- Goad RT, Goad JT, Atieh BH, Gupta RC, 2004. Carbofuran-induced endocrine disruption in adult male rats. Toxicol Mech Methods 14, 233–239. [DOI] [PubMed] [Google Scholar]
- Gupta R, 2015. Handbook Of Toxicology Of Chemical Warfare Agents.
- Gupta RC, 1994. Carbofuran toxicity. J Toxicol Environ Health 43, 383–418. [DOI] [PubMed] [Google Scholar]
- Gupta RC, 2006. CHAPTER 1 - Introduction, in: Gupta RC (Ed.), Toxicology of Organophosphate & Carbamate Compounds. Academic Press, Burlington, pp. 3–4. [Google Scholar]
- Gupta RC, 2020. Chapter Two - Neurotoxicity of organophosphate nerve agents, in: Aschner M, Costa LG (Eds.), Advances in Neurotoxicology. Academic Press, pp. 79–112. [Google Scholar]
- Gupta RC, Goad JT, Kadel WL, 1994. In vivo acute effects of carbofuran on protein, lipid, and lipoproteins in rat liver and serum. J Toxicol Environ Health 42, 451–462. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Kadel WL, 1989. Concerted role of carboxylesterases in the potentiation of carbofuran toxicity by iso-OMPA pretreatment. J Toxicol Environ Health 26, 447–457. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic D, 2012. Chapter 4 Toxicity of Organophosphates and Carbamates, Mammalian Toxicology of Insecticides. The Royal Society of Chemistry, pp. 104–136. [Google Scholar]
- Gupta RC, Milatovic D, Dettbarn WD, 2001a. Depletion of energy metabolites following acetylcholinesterase inhibitor-induced status epilepticus: protection by antioxidants. Neurotoxicology 22, 271–282. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic D, Dettbarn WD, 2001b. Nitric oxide modulates high-energy phosphates in brain regions of rats intoxicated with diisopropylphosphorofluoridate or carbofuran: prevention by N-tert-butyl-alpha-phenylnitrone or vitamin E. Arch Toxicol 75, 346–356. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic S, Dettbarn WD, Aschner M, Milatovic D, 2007. Neuronal oxidative injury and dendritic damage induced by carbofuran: protection by memantine. Toxicol Appl Pharmacol 219, 97–105. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Miller Mukherjee IR, Malik JK, Doss RB, Dettbarn W-D, Milatovic D, 2019. Chapter 26 - Insecticides, in: Gupta RC (Ed.), Biomarkers in Toxicology (Second Edition). Academic Press, pp. 455–475. [Google Scholar]
- Gupta RC, Sachana M, Mukherjee IM, Doss RB, Malik JK, Milatovic D, 2018. Organophosphates and carbamates, in: Gupta RC (Ed.). Elsevier, Amsterdam, Netherlands, pp. 495–508. [Google Scholar]
- Gupta S, Fink MK, Martin LM, Sinha PR, Rodier JT, Sinha NR, Hesemann NP, Chaurasia SS, Mohan RR, 2020. A rabbit model for evaluating ocular damage from acrolein toxicity in vivo. Ann N Y Acad Sci 1480, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Sinha NR, Martin LM, Keele LM, Sinha PR, Rodier JT, Landreneau JR, Hesemann NP, Mohan RR, 2021. Long-Term Safety and Tolerability of BMP7 and HGF Gene Overexpression in Rabbit Cornea. Transl Vis Sci Technol 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HS, Osman AGM, 2017. Modulatory effect of lycopene against carbofuran toxicity in African catfish, Clarias gariepinus. Fish Physiol Biochem 43, 1721–1731. [DOI] [PubMed] [Google Scholar]
- Hossen MS, Tanvir EM, Prince MB, Paul S, Saha M, Ali MY, Gan SH, Khalil MI, Karim N, 2017. Protective mechanism of turmeric (Curcuma longa) on carbofuran-induced hematological and hepatic toxicities in a rat model. Pharm Biol 55, 1937–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaga K, Dharmani C, 2006. Ocular toxicity from pesticide exposure: A recent review. Environ Health Prev Med 11, 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal SK, Siddiqi NJ, Sharma B, 2014. Carbofuran induced oxidative stress mediated alterations in Na(+)-K(+)-ATPase activity in rat brain: amelioration by vitamin E. J Biochem Mol Toxicol 28, 320–327. [DOI] [PubMed] [Google Scholar]
- Kamel F, Boyes WK, Gladen BC, Rowland AS, Alavanja MC, Blair A, Sandler DP, 2000. Retinal degeneration in licensed pesticide applicators. Am J Ind Med 37, 618–628. [DOI] [PubMed] [Google Scholar]
- Kamil S, Mohan RR, 2021. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul Surf 19, 290–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Mohan RR, 2022. Autophagy in Extracellular Matrix and Wound Healing Modulation in the Cornea. Biomedicines 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Fahad TM, Akther T, Zaman T, Hasan MF, Islam Khan MR, Islam MS, Kishi S, 2021. Carbofuran accelerates the cellular senescence and declines the life span of spns1 mutant zebrafish. J Cell Mol Med 25, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatka BZ, Terpiłowski M, Orzeł AK, Janeczko D, Hołowczuk M, Tchórz M, Krajewska A, Szponar J, 2021. Severe carbamates intoxication of 43-year-old farmer - case report. Ann Agric Environ Med 28, 358–360. [DOI] [PubMed] [Google Scholar]
- Kontadakis GA, Plaka A, Fragou D, Kymionis GD, Tsatsakis AM, 2014. Chapter 19 - Ocular biomarkers in diseases and toxicities, in: Gupta RC (Ed.), Biomarkers in Toxicology. Academic Press, Boston, pp. 317–324. [Google Scholar]
- Lamb T, Selvarajah LR, Mohamed F, Jayamanne S, Gawarammana I, Mostafa A, Buckley NA, Roberts MS, Eddleston M, 2016. High lethality and minimal variation after acute self-poisoning with carbamate insecticides in Sri Lanka - implications for global suicide prevention. Clin Toxicol (Phila) 54, 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, 2010. Effect of pesticides on soil microbial community. J Environ Sci Health B 45, 348–359. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM, 2006. CHAPTER 48 - Biomarkers of Organophosphate Exposure, in: Gupta RC (Ed.), Toxicology of Organophosphate & Carbamate Compounds. Academic Press, Burlington, pp. 703–711. [Google Scholar]
- Lopez-Alvarez B, Torres-Palma RA, Ferraro F, Penuela G, 2012. Solar photo-Fenton treatment of carbofuran: analysis of mineralization, toxicity, and organic by-products. J Environ Sci Health A Tox Hazard Subst Environ Eng 47, 2141–2150. [DOI] [PubMed] [Google Scholar]
- Lowit A, 2006. Federal Regulations and Risk Assessment of Organophosphate and Carbamate Pesticides. Toxicology of Organophosphate & Carbamate Compounds, 617–632. [Google Scholar]
- Lv X, Chang Q, Li H, Liang S, Zhe Z, Shen S, Pang G, 2022. Risk assessment of carbofuran residues in fruits and vegetables at the Chinese market: A 7-year survey. Ecotoxicol Environ Saf 239, 113667. [DOI] [PubMed] [Google Scholar]
- Madhubabu S, Kumar M, Philip L, Venkobachar C, 2007. Treatment of carbofuran-bearing synthetic wastewater using UASB process. J Environ Sci Health B 42, 189–199. [DOI] [PubMed] [Google Scholar]
- Marshall TC, Dorough HW, 1979. Biliary excretion of carbamate insecticides in the rat. Pesticide Biochemistry and Physiology 11, 56–63. [Google Scholar]
- McNutt P, Hamilton T, 2015. Chapter 38. Ocular Toxicity of Chemical Warfare Agents. [Google Scholar]
- McNutt PM, Hamilton TA, Lyman ME, Nelson MR, 2020. Chapter 36 - Ocular toxicity of chemical warfare agents, in: Gupta RC (Ed.), Handbook of Toxicology of Chemical Warfare Agents (Third Edition). Academic Press, Boston, pp. 567–588. [Google Scholar]
- Metcalf RL, Fukuto TR, Collins C, Borck K, Abd El-Aziz S, Munoz R, Cassil CC, 1968. Metabolism of 2,2-dimethyl-2,3-dihydrobenzofuran-7-N-methylcarbamate (Furadan) in plants, insects, and mamals. Journal of Agricultural and Food Chemistry 16, 300–311. [Google Scholar]
- Michalovicz LT, Kelly KA, Sullivan K, O’Callaghan JP, 2020. Acetylcholinesterase inhibitor exposures as an initiating factor in the development of Gulf War Illness, a chronic neuroimmune disorder in deployed veterans. Neuropharmacology 171, 108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Aschner M, Gupta RC, Zaja-Milatovic S, Barnes G, 2011. Involvement of Oxidative Stress in Anticholinesterase Pesticide Toxicity, Anticholinesterase Pesticides, pp. 133–147. [Google Scholar]
- Milatovic D, Gupta RC, Aschner M, 2006. Anticholinesterase toxicity and oxidative stress. ScientificWorldJournal 6, 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D, Tiwari SK, Agarwal S, Sharma VP, Chaturvedi RK, 2012. Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol Sci 127, 84–100. [DOI] [PubMed] [Google Scholar]
- Mishra S, Zhang W, Lin Z, Pang S, Huang Y, Bhatt P, Chen S, 2020. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 259, 127419. [DOI] [PubMed] [Google Scholar]
- Misra UK, Nag D, Misra NK, Mehra MK, Ray PK, 1985. Some observations on the macula of pesticide workers. Hum Toxicol 4, 135–145. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Kempuraj D, D’Souza S, Ghosh A, 2022. Corneal stromal repair and regeneration. Prog Retin Eye Res, 101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Martin LM, Sinha NR, 2021. Novel insights into gene therapy in the cornea. Exp Eye Res 202, 108361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC, 2011. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest Ophthalmol Vis Sci 52, 4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M, Hossen MS, Rahman MA, Saha S, Sarkar C, Bhoumik NC, Kundu SK, 2021. Antioxidant mediated protective effect of Bridelia tomentosa leaf extract against carbofuran induced oxidative hepatic toxicity. Toxicol Rep 8, 1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse DL, Baker EL Jr., Kimbrough RD, Wisseman CL 3rd, 1979. Propanil-chloracne and methomyl toxicity in workers of a pesticide manufacturing plant. Clin Toxicol 15, 13–21. [DOI] [PubMed] [Google Scholar]
- Naravaneni R, Jamil K, 2005. Cytogenetic Biomarkers of Carbofuran Toxicity Utilizing Human Lymphocyte Cultures In Vitro. Drug and Chemical Toxicology 28, 359–372. [DOI] [PubMed] [Google Scholar]
- Nguyen HH, Kidron A, Liu T, Nguyen N, Nguyen H, Vo A, 2021. Presentation and Treatment of cholinergic Crisis in the setting of Carbamate poisoning. Clin Case Rep 9, 1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda SS, El-Manakhly EM, Abou-Srag MA, Tohamy HG, 2022. Assessment of reproductive toxicity of carbofuran and copper sulfate in male Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res Int 29, 15896–15904. [DOI] [PubMed] [Google Scholar]
- Ong-Artborirak P, Boonchieng W, Juntarawijit Y, Juntarawijit C, 2022. Potential Effects on Mental Health Status Associated with Occupational Exposure to Pesticides among Thai Farmers. Int J Environ Res Public Health 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oto K, 1971. [Eye disease induced by organic phosphorous insecticides--adult case]. Nippon Ganka Gakkai Zasshi 75, 1944–1951. [PubMed] [Google Scholar]
- Padilla SS, 2006. Cumulative Effects of Organophosphorus or Carbamate Pesticides.
- Paul N, Mannathukkaran TJ, 2005. Intermediate syndrome following carbamate poisoning. Clin Toxicol (Phila) 43, 867–868. [DOI] [PubMed] [Google Scholar]
- Pesin SR, Candia OA, 1982. Acetylcholine concentration and its role in ionic transport by the corneal epithelium. Invest Ophthalmol Vis Sci 22, 651–659. [PubMed] [Google Scholar]
- Pivariu D, Oros AN, Tabaran F, Gal A, Martonos C, Nagy AL, 2020. Intentional Carbofuran poisoning in 7 dogs. BMC Vet Res 16, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman BP, Kuttan R, 2017. Protective Effect of Curcumin Against Carbofuran-Induced Toxicity in Wistar Rats. J Environ Pathol Toxicol Oncol 36, 73–86. [DOI] [PubMed] [Google Scholar]
- Rai DK, Rai PK, Rizvi SI, Watal G, Sharma B, 2009. Carbofuran-induced toxicity in rats: protective role of vitamin C. Exp Toxicol Pathol 61, 531–535. [DOI] [PubMed] [Google Scholar]
- Ringvold A, Reubsaet JL, 2016. The impact of high-dose acetylcholine on bovine corneal epithelium. Acta Ophthalmol 94, 160–164. [DOI] [PubMed] [Google Scholar]
- Sakunthala Tennakoon DA, Karunarathna WD, Udugampala US, 2013. Carbofuran concentrations in blood, bile and tissues in fatal cases of homicide and suicide. Forensic Sci Int 227, 106–110. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Baksh BS, Cabrera K, Choudhury A, Jensen K, Klimas N, Galor A, 2022. Dry Eye Symptoms and Signs in US Veterans With Gulf War Illness. Am J Ophthalmol 237, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolucito JA, Whitcomb E, 1971. Effect of paraoxon on erythrocyte metabolism as measured by oxygen uptake in vitro. Br J Pharmacol 42, 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Law S, 2019. Ocular surface and chronic pesticide exposure: Evaluating the alterations in corneal cellular turnover concerning cell cycle and apoptosis. Exp Eye Res 178, 122–132. [DOI] [PubMed] [Google Scholar]
- Saputra F, Uapipatanakul B, Lee JS, Hung SM, Huang JC, Pang YC, Muñoz JER, Macabeo APG, Chen KH, Hsiao CD, 2021. Co-Treatment of Copper Oxide Nanoparticle and Carbofuran Enhances Cardiotoxicity in Zebrafish Embryos. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saquib Q, Siddiqui MA, Ansari SM, Alwathnani HA, Al-Khedhairy AA, 2021. Carbofuran cytotoxicity, DNA damage, oxidative stress, and cell death in human umbilical vein endothelial cells: Evidence of vascular toxicity. J Appl Toxicol 41, 847–860. [DOI] [PubMed] [Google Scholar]
- Satar S, Satar S, Sebe A, Yesilagac H, 2005. Carbofuran poisoning among farm workers. Mt Sinai J Med 72, 389–392. [PubMed] [Google Scholar]
- Senanayake N, Karalliedde L, 1987. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med 316, 761–763. [DOI] [PubMed] [Google Scholar]
- Seth B, Yadav A, Tandon A, Shankar J, Chaturvedi RK, 2019. Carbofuran hampers oligodendrocytes development leading to impaired myelination in the hippocampus of rat brain. Neurotoxicology 70, 161–179. [DOI] [PubMed] [Google Scholar]
- Shahid M, Manoharadas S, Chakdar H, Alrefaei AF, Albeshr MF, Almutairi MH, 2021. Biological toxicity assessment of carbamate pesticides using bacterial and plant bioassays: An in-vitro approach. Chemosphere 278, 130372. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Jaiswal SK, Siddiqi NJ, Sharma B, 2012a. Effect of carbofuran on some biochemical indices of human erythrocytes in vitro. Cell Mol Biol (Noisy-le-grand) 58, 103–109. [PubMed] [Google Scholar]
- Sharma RK, Rai DK, Sharma B, 2012b. In-vitro carbofuran induced micronucleus formation in human blood lymphocytes. Cell Mol Biol (Noisy-le-grand) 58, 128–133. [PubMed] [Google Scholar]
- Sharma RK, Sharma B, 2012. In-vitro carbofuran induced genotoxicity in human lymphocytes and its mitigation by vitamins C and E. Dis Markers 32, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Long Z, Lu Y, Chen J, 2022. Fluorescence detection of carbofuran in aqueous extracts based on dual-emission SiO2 @Y2 O3 :(Eu(3+),Tb(3+) )@MIP core-shell structural nanoparticles. Luminescence 37, 348–356. [DOI] [PubMed] [Google Scholar]
- Silberman J, Taylor A, 2022. Carbamate Toxicity, StatPearls, Treasure Island (FL). [Google Scholar]
- Singh RK, Singh RL, Sharma B, 2003. Acute Toxicity of Carbofuran to a Freshwater Teleost, Clarias batrachus. Bull Environ Contam Toxicol 70, 1259–1263. [DOI] [PubMed] [Google Scholar]
- Sinha NR, Balne PK, Bunyak F, Hofmann AC, Lim RR, Mohan RR, Chaurasia SS, 2021. Collagen matrix perturbations in corneal stroma of Ossabaw mini pigs with type 2 diabetes. Mol Vis 27, 666–678. [PMC free article] [PubMed] [Google Scholar]
- Sloniecka M, Danielson P, 2020. Acetylcholine decreases formation of myofibroblasts and excessive extracellular matrix production in an in vitro human corneal fibrosis model. J Cell Mol Med 24, 4850–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloneski S, Reigosa MA, Molinari G, González NV, Larramendy ML, 2008. Genotoxic and cytotoxic effects of carbofuran and furadan® on Chinese hamster ovary (CHOK1) cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 656, 68–73. [DOI] [PubMed] [Google Scholar]
- Stojiljković MP, Jokanović M, Lončar-Stojiljković D, Škrbić R, 2020. Chapter 65 - Prophylactic and therapeutic measures in nerve agents poisonings, in: Gupta RC (Ed.), Handbook of Toxicology of Chemical Warfare Agents (Third Edition). Academic Press, Boston, pp. 1103–1119. [Google Scholar]
- Timchalk C, 2006. Physiologically Based Pharmacokinetic Modeling of Organophosphorus and Carbamate Pesticides, pp. 103–125. [Google Scholar]
- Tripathi R, Balne PK, Sinha NR, Martin LM, Kamil S, Landreneau JR, Gupta S, Rodier JT, Sinha PR, Hesemann NP, Hofmann AC, Fink MK, Chaurasia SS, Mohan RR, 2020. A Novel Topical Ophthalmic Formulation to Mitigate Acute Mustard Gas Keratopathy In Vivo: A Pilot Study. Transl Vis Sci Technol 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K, Miyara M, Ishida K, Sanoh S, Ohta S, Kotake Y, 2018. Carbofuran causes neuronal vulnerability to glutamate by decreasing GluA2 protein levels in rat primary cortical neurons. Arch Toxicol 92, 401–409. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency, W., D.C, 1997. Carbofuran - Review of pesticide poisoning incident data
- Usmani KA, Hodgson E, Rose RL, 2004. In vitro metabolism of carbofuran by human, mouse, and rat cytochrome P450 and interactions with chlorpyrifos, testosterone, and estradiol. Chem Biol Interact 150, 221–232. [DOI] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R, 2016. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 74, 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjer RL, Milatovic D, 2006. CHAPTER 3 - Therapeutic Uses of Cholinesterase Inhibitors in Neurodegenerative Diseases, in: Gupta RC (Ed.), Toxicology of Organophosphate & Carbamate Compounds. Academic Press, Burlington, pp. 25–33. [Google Scholar]
- Yang PY, Tsao TC, Lin JL, Lyu RK, Chiang PC, 2000. Carbofuran-induced delayed neuropathy. J Toxicol Clin Toxicol 38, 43–46. [DOI] [PubMed] [Google Scholar]
- Yen CC, Hsieh MC, Tsai MJ, Chen HC, 2015. Human carbofuran intoxication with myocardial injury mimicking acute myocardial infarction. Kaohsiung J Med Sci 31, 112–113. [DOI] [PubMed] [Google Scholar]
- Yulitasari NA, Hidanah S, Widjiati W, Hendrawan VF, Luqman EM, 2021. Effect of kebar grass extract ( Biophytum petersianum Klotzsch) on histopathological changes in liver of mice offspring from the parent exposed to carbofuran during lactation period. Pol J Vet Sci 24, 573–578. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Gupta RC, Aschner M, Milatovic D, 2009. Protection of DFP-induced oxidative damage and neurodegeneration by antioxidants and NMDA receptor antagonist. Toxicol Appl Pharmacol 240, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeljezic D, Vrdoljak AL, Kopjar N, Radic B, Milkovic Kraus S, 2008. Cholinesterase-inhibiting and genotoxic effects of acute carbofuran intoxication in man: a case report. Basic Clin Pharmacol Toxicol 103, 329–335. [DOI] [PubMed] [Google Scholar]
- Zhang X, Knaak JB, Tornero-Velez R, Blancato JN, Dary CC, 2010. Chapter 73 - Application of Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling in Cumulative Risk Assessment for N-Methyl Carbamate Insecticides, in: Krieger R (Ed.), Hayes’ Handbook of Pesticide Toxicology (Third Edition). Academic Press, New York, pp. 1591–1605. [Google Scholar]
- Zhang X, Tsang AM, Okino MS, Power FW, Knaak JB, Harrison LS, Dary CC, 2007. A physiologically based pharmacokinetic/pharmacodynamic model for carbofuran in Sprague-Dawley rats using the exposure-related dose estimating model. Toxicol Sci 100, 345–359. [DOI] [PubMed] [Google Scholar]


