Summary
Background
Affordability to novel anticancer drugs has become a major health issue in China. It is encouraging to note that China initiated its drug regulatory reform and national price negotiation policies since 2015. As a growing number of domestic within-class targeted anticancer drugs are approved in China, it is expected that this may reduce the price of novel anticancer drugs and improve the affordability of anticancer drugs. This study aimed to evaluate the price, efficacy, and safety of the within-class anticancer drugs between domestic and imported drugs approved in China from 2010 to 2022.
Methods
The domestic and imported within-class targeted drugs for solid cancers approved in China between 2010 and 2022 were extracted. We classified it as a class of anticancer drugs based on the same indication and similar biological mechanism. The published literature derived from pivotal clinical trials of these domestic and imported drugs was identified based on the review report and the latest labels issued by the China National Medical Products Administration. We evaluated the monthly treatment price at launch and the latest (2022), primary efficacy endpoint and safety between domestic and imported anticancer drugs. Meta-analyses were further employed to evaluate the efficacy and safety of the domestic and imported anticancer drugs, including pooled hazard ratios (HR) for progression-free survival (PFS), overall survival (OS), objective response rates (ORR) for solid cancers, and relative risk for serious adverse events (SAE) and Grade ≥3 adverse events (AEs).
Findings
In our cohort study, 12 within-class anticancer drugs with 7 cancer diseases were analyzed, including 18 domestic (21 indications; 21 pivotal trials) and 18 imported (21 indications; 27 pivotal trials) novel anticancer drugs, respectively. The median monthly treatment price of domestic and imported drugs from the years of launch to 2022 had significantly decreased by 71% and 62%, respectively. Moreover, the median monthly treatment price of domestic targeted anticancer drugs on the market at launch ($3786 vs. $5393, P = 0.007) and the latest ($1222 vs. $2077, P = 0.011) was significantly lower than that of imported drugs. No significant differences in median PFS gains (9.0 vs. 11.0 months; P = 0.24), OS gains (9.3 vs 10.6 months; P = 0.66), and ORR (57% vs 62%, P = 0.77) of targeted anticancer drugs in their pivotal trials were observed between the domestic and imported drugs. Additionally, there was no significant difference between domestic and imported drugs in the incidence of SAE (23% vs. 24%; P = 0.41) and Grade ≥3 AEs (59% vs. 57%; P = 0.45). These findings were also further confirmed in the meta-analyses for primary efficacy endpoints and safety outcomes.
Interpretation
The prices of both domestic and imported anticancer drugs significantly decreased after market entry mainly due to the role of national price negotiations. The median monthly treatment price of domestic within-class targeted anticancer drugs was significantly lower than that of imported drugs. Furthermore, the efficacy and safety of domestic anticancer drugs were comparable to that of imported drugs. This evidence implicated that the development of within-class anticancer drugs with national price negotiations in China significantly improved the affordability for patients.
Funding
This study was supported by postdoctoral fellowship from Tsinghua-Peking Joint Centers for Life Sciences (CLS).
Keywords: Targeted anticancer drugs, Pivotal trials, China, Affordability, National drug price negotiation
Research in context.
Evidence before this study
The high expenditure on anticancer drugs has become a great concern and challenge worldwide, showing a rapid annual growth trend. In the past 5 years, spending on anticancer drugs in China has more than doubled, mainly due to the impact of patented drugs. Fortunately, China initiated a package of reforms in 2015 aimed at improving the affordability of anticancer drugs, including drug regulatory reform and national drug price negotiations. Evidence showed the rapid growth in the number of locally developed within-class anticancer drugs approved since 2015 in China. Additionally, the average price of anticancer drugs was reduced by more than 50% in the six rounds (2016–2021) of national drug price negotiations. However, the available evidence of their monthly treatment price between domestic and imported drugs was limited in China. Moreover, it is unclear whether the within-class anticancer drugs developed by local developers can ensure substantial efficacy and safety compared to the imported drugs. In this study, we described and compared the monthly treatment price between domestic and imported within-class targeted anticancer drugs approved in China from 2010 to 2022 at the launch and the latest (2022). Then, the efficacy and safety of these drugs were further evaluated.
Added value of this study
This is the first study to evaluate the differences in price, efficacy and safety between domestic and imported within-class targeted anticancer drugs. The price (at launch and the latest) of within-class targeted anticancer drugs developed by local developers in China were significantly lower than that of the imported drugs without a significant difference in their primary efficacy endpoint and safety. Besides, the implementation of national price negotiations significantly reduced the price of novel anticancer drugs both for domestic and imported within-class targeted drugs after market entry. Our findings supported the significant gains in China had achieved in improving the affordability for patients in recent years.
Implications of all the available evidence
The policy of encouraging research and development (R&D) of novel anticancer drugs and implementing national price negotiations in China had significantly improved the affordability for patients. This pattern may provide a new perspective on international approaches to improve the affordability of targeted anticancer drugs.
Introduction
Cancer has risen to become the second leading cause of death in the world, imposing a significant burden on public health.1,2 Global Cancer Statistics 2020 reported that there were 19.29 million new cancer cases and 9.95 million cancer deaths worldwide in 2020.3 The latest report (2020) of International Agency for Research on Cancer of World Health Organization showed that there were 4.57 million new cancer cases (205/100,000 for the age-standardized incidence rates) and 3.00 million cancer deaths (129.4/100,000 for the age-standardized mortality rates) in China, which was the ranked first in the world, implying that cancer has become a major public health burden and the leading cause of death in China.4 Trends in the cancer spectrum in China have also gradually shifted from the previous burden of liver, stomach, and esophageal cancer to an increased burden of lung, breast, colorectal, and prostate cancers, which is similar to that of developed countries.5 It is foreseeable that China will face a significant burden of cancer treatment in the coming years.
The high expenditure on anticancer drugs has become a great concern and challenge around the world.6, 7, 8, 9 Evidence suggests that global spending on anticancer drugs is up to $150 billion in 2020, with a rapid annual growth trend.6 Also, spending on anticancer drugs in China has more than doubled in the past 5 years largely due to the impact of patented drugs.10 It is well established that being diagnosed with cancer would be considered a catastrophe. In a hospital-based, multicenter, cross-sectional survey of common oncology costs, the average expenditure per patient was $9739, much higher than the average annual household income ($8607).11 Additionally, the previous study reported that China ranked the second-poorest affordability among the six surveyed countries with a median monthly treatment price of $3173 in 2016 for patented anticancer drugs, suggesting a far higher treatment burden in China.12 Therefore, it has been a strong desire and determination of the Chinese government and the public to lower the price of novel anticancer drugs and improve the affordability for patients.
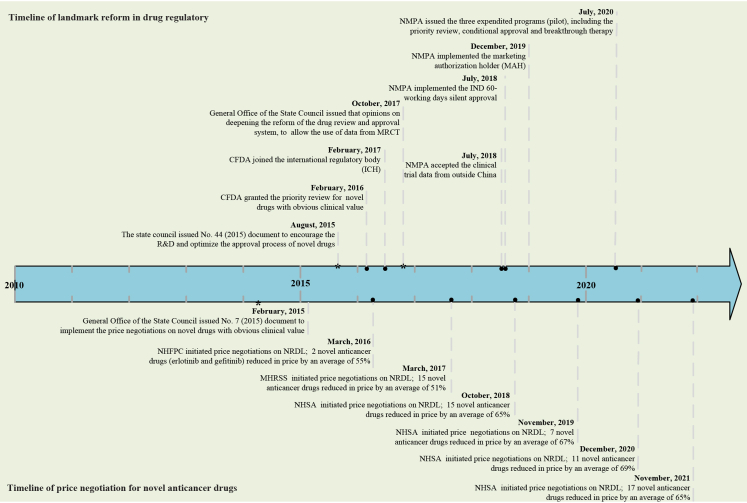
Fortunately, China has initiated a package of reforms aimed at improving the affordability of anticancer drugs, including the drug regulatory reform and national drug price negotiations (Fig. 1). It can be observed that few policies on drug innovation and price negotiations were issued prior to 2015, whereas China embarked on frequent drug reforms after 2015. For the landmark reform in drug regulatory, China National Medical Products Administration (NMPA) has been tackling the drug review backlog by initiating priority reviews, establishing a 60-day silent approval for investigational new drug applications (IND) and encouraging global multi-center clinical trials.13,14 This has greatly increased the incentive for domestic developers to move from generic drugs to innovative drug development.15 Indeed, these reforms have yielded tremendous results, with the number of new molecular entities developed by local developers increasing from less than 5 in 2015 to more than 20 approved by the NMPA in 2021.16 Besides, China issued a document on national drug price negotiations for novel drugs in 2015 and then six rounds of negotiations for novel drugs had been conducted to date (2022). Our preliminary study analyzed the six rounds of successful negotiations on novel anticancer drugs, demonstrating that the novel anticancer drugs had reduced their prices by more than 50% after market entry. If the novel drugs are negotiated successfully, they can be covered in the National Reimbursement Drug List (NDRL), which implies that more patients can be affordable. Previous studies reported a significant increase in the volume of novel anticancer drugs included the NRDL through national drug price negotiations, which could also have a substantial impact on domestic R&D of novel drugs.17,18 These figures suggested that the established drug reforms had brought more treatment options to oncology patients and improved the affordability of novel anticancer drugs.
Fig. 1.
Timeline of landmark reform for drug regulatory and price negotiation for novel anticancer drugs in China. CFDA, China Food and Drug Administration (changed to the National Medical Products Administration in 2018); NMPA, National Medical Products Administration; IND, investigational new drug application; MRCT, multi-regional clinical trial; NHFPC, National Health and Family Planning Commission; MHRSS, Ministry of Human Resource and Social Security; NHSA, National Healthcare Security Administration; NRDL, National Reimbursement Drug List.
It should be acknowledged that the majority of local drug developers in China are now in the "follow up" paradigm of novel drug development. This means structural optimization through new molecular entities already on the market, which can notably reduce the risk of development failure (so-called "me too" or “me better” novel drugs). Based on this development strategy, several novel targeted anticancer drugs developed locally in China have been marketed with similar targets and biological mechanisms to those of imported anticancer drugs. Evidence supports that within-class drugs may contribute to lowering the price of new anticancer drugs and increasing their bargaining power.19,20 However, studies have also shown that more within-class products do not necessarily reduce drug prices, and may even be clinically ineffective in terms of efficacy and safety.21, 22, 23, 24 As an increasing number of targeted within-class anticancer drugs developed by local developers in China, it is unclear whether these drugs can offer lower anticancer drug prices with sufficient evidence of efficacy and safety.
In this study, we first aimed to assess the monthly treatment price of domestic and imported novel within-class anticancer drugs by analyzing the price at launch and the latest (2022) of these agents in China. And then, the efficacy and safety of novel domestic and imported anticancer drugs were evaluated by combining the meta-analyses.
Methods
Data sources
The novel anticancer drugs (New molecular entities, NMEs) for solid cancer approved between January 1, 2010 and August 1, 2022 was included. These agents were derived from the NMPA listing database.25 The price of anticancer drugs was collected from the Insight databases (one of the most widely used commercial databases in China)26 and publicly available data. Taking into account the impact of inflation, we adjusted it based on the annual consumption index published by the National Bureau of Statistics of China.27 The gross domestic product (GDP) per capita and purchasing power parities relative to United States dollars (US$) for China was derived from the public data.28,29 The sources of the meta-analysis were obtained from the published literature (Table S1).
Data extraction
Sample identification
The scope of this study did not include chemotherapy (e.g. eribulin) and cell therapy (e.g. Car-T therapy) considered as non-targeted anticancer agents. The generic or biosimilar of the novel anticancer drugs that had been marketed in China were excluded in this study since these drugs had a significant impact on the price of novel drugs. Besides, the approved but unmarketed novel anticancer drugs (without publishing the price) were also not included in this study. The anticancer drugs introduced from abroad by domestic developers were regarded as imported drugs in this study (e.g. niraparib). The approval date, origin (domestic or imported drugs), indication, biological mechanism, type of cancer, treatment lines, trial participants, and type of approval (regular or conditional approval) were extracted. Additionally, whether these anticancer drugs were included in the National Reimbursement Drug List (NRDL) issued in 2021 was also analyzed. We grouped anticancer drugs within the same class if they shared the same biological mechanism and indications (such as PD1/PDL1 inhibitors for melanoma), similar to the previous study.19 We only included the class that had at least one within-class imported and at least one domestic novel anticancer drug approved by China.
Price data extraction
The Chinese health care system includes the central government, provincial governments, and city, regional, or county governments. Drugs are procured using centralized bidding at the provincial or city level, which may result in prices for drugs varying from different cities.30 Therefore, we extracted the average winning bid prices at launch (defined as the annual average winning bid price of the drug when it enters the market in the first year) and the latest (defined as the average winning bid price of the drug in 2022) for anticancer drugs. We calculated monthly treatment prices for anticancer drugs based on the average winning bid prices using the NMPA-approved labeling, as reported in the previous studies.19,31 Based on the exchange rate between China and the United States on June 20, 2022 (1 USD = 6.17 RMB), we changed the price in China to the US dollar. For some drugs with varying dosage strengths, we chose the lowest price of strength for calculation similar to the study reported by Vokinger KN.19 We determined monthly treatment prices by factoring in the combination of anticancer drugs based on NMPA-approved indications (e.g. dalpiciclib combined with fulvestrant for breast cancer). Drugs that required dosing based on body weight or body surface area (BSA) assumed 70 kg or 1.7 m2, which was consistent with previous studies.31,32 Besides, we calculated the average annual reduction rate (AARR) of monthly treatment prices for each anticancer drug price. The AARR of the monthly treatment price for anticancer drugs was defined as the sum of the reduction rates divided by the number of years. Moreover, similar to the previous study,12 the affordability index of novel anticancer drugs in this study was defined by calculating the percentage of the median monthly treatment price to monthly GDP per capita at purchasing power parities (GDPcap) in China. A smaller affordability index indicates greater affordability of anticancer drugs.
Identification of pivotal trials
We identified all the pivotal clinical trials for each group of anticancer agents from the NMPA review reports (pivotal clinical trials for all drugs are clearly described in the NMPA review report). Considering that drug review reports issued by the NMPA may report only partial efficacy and safety data (e.g. lack of the incidence of serious adverse events), we collected published literature based on the pivotal clinical trials. Owing to the unavailability of review reports for some targeted drugs, we identified their pivotal clinical trial based on the latest drug labels issued by the NMPA through August 2022, which was consistent with the previous study.33 We extracted the pivotal clinical trials involved in the primary efficacy point used to support the approval of the NMPA, including PFS, OS, ORR, disease-free survival (DFS), time to progressive (TTP) and other end outcomes. The evaluation of ORR for solid tumors included partial and complete responses, in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST). Similar to previous studies,34,35 the novel targeted anticancer drug with a PFS benefit of ≥3 months or an OS benefit of ≥2.5 months improvement was deemed clinically significant. Furthermore, to compare the safety differences between domestic and imported novel anticancer drugs, we identified SAE and Grade ≥3 AEs that occurred in pivotal clinical trials.
Two researchers independently extracted these data, and their conclusions following a discussion when produced varying outcomes. The study did not require ethics committee approval because it was based on public data and did not involve patient information.
Statistical analysis
Categorical variables were described as numbers (percentages), while continuous variables were expressed as medians (interquartile range, IQR). For categorical variables (e.g. approval types), the fisher's exact test was employed for comparison. The Kruskal–Wallis test was utilized to determine the differences between imported and domestic anticancer agents in terms of monthly treatment prices at launch and latest (2022), primary efficacy (PFS, OS, and ORR), and safety outcomes (SAE and Grade ≥3 AEs). Additionally, consistent with the previous study,34,36 we performed a pooled analysis of primary efficacy (HR for PFS and OS of randomized controlled trial; ORR for single-arm trial) and safety outcomes (relative risk for SAE and Grade ≥3 AEs of randomized controlled trial) of imported and domestic anticancer drugs. In the case of proportions (ORR for single-arm trial; relative risk for SAE and Grade ≥3 AEs), confidence intervals (CIs) were computed using the Clopper-Pearson method, and the Log transformation was used to stabilize the variance of pooled estimates for proportions. Statistical heterogeneity was estimated by using I2 statistic (calculated by Cochran's Q [100 × (Q–df ÷ Q)]) and χ2 (P < 0.01), which can be employed to assess between-trial heterogeneity.37 I2 values that were ≥50% indicated significant heterogeneity and a random effects model was conducted to calculate the pooled results; otherwise, a fixed-effects model was performed.38 To identify heterogeneity between trials for PFS, OS, ORR, SAE and Grade ≥3 AEs, subgroup analyses were analyzed by drug types, indication and selection of control groups. Statistical analysis was performed using SPSS 20.0 (for data analysis) and R, version 4.1.0 (ggplot2 package for graphs). P < 0.05 was considered a statistically significant difference.
Role of the funding source
The analysis and interpretation of the manuscript was supported by postdoctoral fellowship from Tsinghua-Peking Joint Centers for Life Sciences (CLS). The funder had no role in the study design, data collection, data analysis, interpretation or writing of the paper.
Results
Characteristics of the included anticancer drugs and pivotal trials
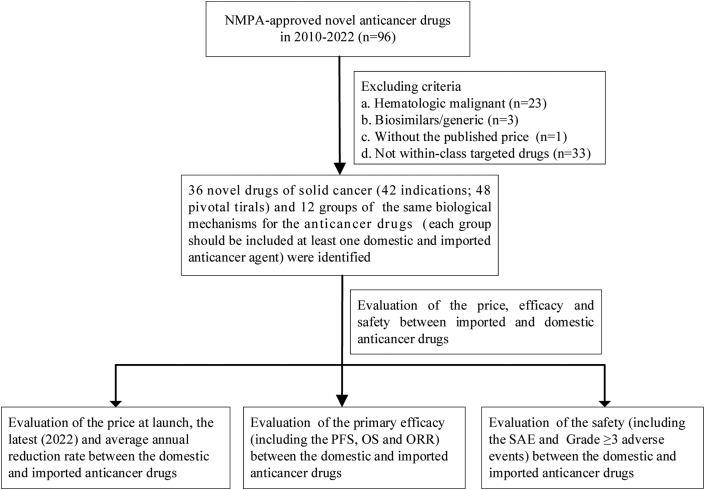
The framework of inclusion for novel anticancer drugs is shown in Fig. 2. Our cohort study included 36 anticancer agents in total (18 domestic drugs with 21 indications; 2 indications for camrelizumab, sintilimab and tislelizumab; 18 imported drugs with 21 indications; 2 indications for regorafenib, pembrolizumab and atezolizumab) and 12 groups (each group had at least one domestic and imported agent), which covered seven cancer diseases (Table 1 and Table S1): breast cancer (CDK4/6 inhibitors, HER2 tyrosine kinase inhibitors and HER2-mab inhibitors), non-small-cell lung cancer (EGFR, ALK, and PD1/PDL1 inhibitors), colorectal cancer (VEGFR inhibitors), ovarian cancer (PARP inhibitors), hepatocellular carcinoma (VEGFR inhibitors), prostate cancer (AR inhibitors) and melanoma (PD1 inhibitors). The number of anticancer agents in each group with the same class included 2 to 6. The majority of anticancer indications were approved in 2016–2022 both for the domestic (20/21) and imported anticancer drugs (18/21). The most common indication was non-small cell lung cancer (n = 17), followed by hepatocellular carcinoma (n = 7) and breast cancer (n = 7). The number of small-molecule drugs included in the study was greater than the number of biologics (26 vs. 10). No significant difference between imported and domestic anticancer agents was found for the number of treatment lines (P = 0.39). The proportion of domestic and imported anticancer drugs with conditional approval was 48% (10/21) and 24% (5/21), respectively. Additionally, the proportion of indications for domestic anticancer drugs entering the 2021 NRDL was 86% (18/21) compared to 71% (15/21) for imported drugs.
Fig. 2.
The framework of inclusion for novel anticancer drugs. OS, overall survival; PFS, progression-free survival; ORR, objective response rate; SAE, serious adverse event.
Table 1.
Characteristics of the included indications between domestic and imported drugs.
| Characteristic | Domestic drugs, N (%) | Imported drugs, N (%) | P value |
|---|---|---|---|
| Included indications | 21 | 21 | NA |
| Cancer type | |||
| Non–small-cell lung cancer | 8 (38) | 9 (43) | 0.94 |
| Hepatocellular carcinoma | 5 (23) | 2 (10) | |
| Breast cancer | 3 (14) | 4 (19) | |
| Ovarian | 2 (10) | 2 (10) | |
| Colorectal | 1 (5) | 1 (5) | |
| Melanoma | 1 (5) | 1 (5) | |
| Prostate cancer | 1 (5) | 2 (10) | |
| Approval date | |||
| 2010–2015 | 1 (5) | 3 (14) | 0.61 |
| 2016–2022 | 20 (95) | 18 (86) | |
| 2021 NRDL | |||
| Included | 18 (86) | 15 (71) | 0.45 |
| Not included | 3 (14) | 6 (29) | |
| Approval types | |||
| Conditional approval | 10 (48) | 5 (24) | 0.20 |
| Regular approval | 11 (52) | 16 (76) | |
| Line of therapy | |||
| Adjuvant therapy | 0 (0) | 2 (10) | 0.39 |
| First-line advanced or metastatic | 9 (43) | 9 (43) | |
| Second-line advanced or metastatic | 9 (43) | 9 (43) | |
| Third- or- later-line advanced or metastatic | 3 (14) | 1 (5) |
NRDL, National Reimbursement Drug List; NA, not available.
A total of 48 pivotal clinical trials were identified to support the approval of 42 indications (Table 2 and Table S1). 21 of these pivotal clinical trials were identified for domestic anticancer drug approval (21 indications), while 27 trials were for imported drug approval (21 indications). 33 (69%) of pivotal clinical trials were derived from NMPA review reports, while 15 (31%) were derived from the latest drug label published by the NMPA (Table S1). All of these pivotal clinical trials were published in the literature (Table S1). The median patient enrolment in each pivotal clinical study for domestic targeted agents was 341 (IQR:217, 412), which was comparable to imported drugs (median: 345; IQR: 206, 588) (Table 2). The number of pivotal clinical trials of imported anticancer drugs was significantly higher than that of domestic drugs (P = 0.04). In regards to clinical trial design, 61% (13/21) of domestic drugs employed RCTs while the proportion of imported anti-oncology drugs was 81% (22/27). The majority of pivotal clinical trials of imported and domestic anticancer drugs were distributed in phase III clinical trials (36/48), followed by phase II clinical trials (10/48). The proportion of methods using open-label was higher than the proportion of double blinding (domestic: 67%; imported: 52%) in cancers. Besides, no significant difference between domestic and imported anticancer drugs was observed in the types of the control group.
Table 2.
Pivotal trials of targeted anticancer drugs between domestic and imported drugs.
| Characteristic | Domestic drugs, N (%) | Imported drugs, N (%) | P value |
|---|---|---|---|
| Total of pivotal trial | 21 | 27 | NA |
| Pivotal trials per indications, median (range) | 1 (1, 1) | 1 (1, 3) | 0.04 |
| Participants per trials, median (IQR) | 341 (217, 412) | 345 (206, 588) | 0.43 |
| Study design | |||
| Randomized | 13 (61) | 22 (81) | 0.19 |
| Single-arma | 8 (38) | 5 (19) | |
| Clinical trial phase | |||
| Phase 3 | 14 (67) | 22 (81) | 0.48 |
| Phase 2 | 6 (29) | 4 (14) | |
| Phase 1 | 1 (4) | 1 (4) | |
| Type of Blinding | |||
| Double | 7 (33) | 13 (48) | 0.38 |
| Open label | 14 (67) | 14 (52) | |
| Type of control | |||
| Active | 6 (29) | 11 (41) | 0.27 |
| Placebo | 6 (28) | 11 (41) | |
| Dose comparison | 1 (5) | 0 (0) | |
| Historical control | 8 (38) | 5 (19) |
IQR, interquartile range; NA, not available.
One of the domestic anticancer drugs for a dose-comparison clinical trial design was considered as single-arm design.
Prices of the anticancer drugs
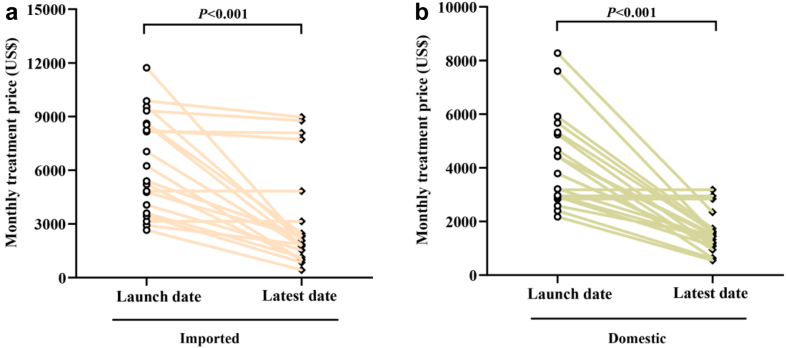
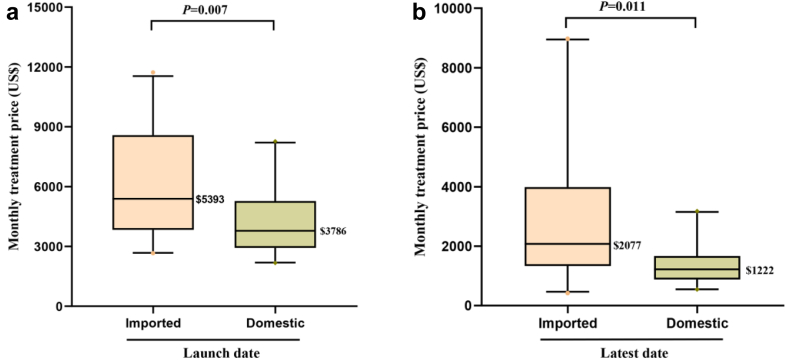
The monthly treatment price change of domestic and imported anticancer drugs in each within-class group are shown in Table 3 and Table S2. Among the novel anticancer drugs included in this study, three domestic drugs and two imported drugs were marketed in 2022 respectively. Thereby, the prices of them remain unchanged (Fig. 3). Other novel anticancer drugs showed a decline in the latest years (2022). Compared to the prices at launch, the domestic and imported anticancer drugs showed significant decreases at the latest (2022) with median price reductions of 71% and 62% (P < 0.001), respectively (Table S2). 86% (18/21) of domestic anticancer drugs and 71% (15/21) of imported anticancer drugs were included by the 2021 NRDL (Table 1). Price reductions for drugs that were included in the 2021 NRDL by the price negotiation were greater than those without in the NRDL, as can be observed in domestic (median: 73% vs. 0%, P < 0.001) and imported oncology drugs (median: 75% vs. 8%, P < 0.001) (Table S2). No significant difference in AARR of monthly treatment price between domestic and imported anticancer drugs (45% vs. 30%, P = 0.06) (Table S2). The median AARR for anticancer drugs approved in 2016–2022 was significantly higher than for drugs approved in 2010–2015 (44% vs. 2%, P < 0.01; data not shown). The median monthly treatment price for the domestic oncology drugs in the year at launch was significantly lower than that of imported drugs ($3786 vs. $5393, P = 0.007) (Fig. 4). Moreover, this difference in the latest (2022) between domestic and imported also can be identified ($1222 vs. $2077, P = 0.011). Since the monthly GDPcap for 2022 in China has not been published yet, we adopted data from 2021 as a surrogate (1617$ for monthly GDPcap in 2021). Thereby, the affordability index for domestic and imported anticancer was estimated as 76% (1222/1617) and 128% (2077/1617), respectively, in 2022.
Table 3.
The trend change of monthly treatment price for within-class targeted anticancer drugs at launch and the latest (2022) between domestic and imported drugs.
| Characteristic | Initial monthly treatment price, median (IQR) | The latest treatment price, median (IQR) | Price reductiona |
|---|---|---|---|
| Domestic anticancer drugs | |||
| Breast cancer (CDK4/6 inhibitors) | 2843 (2843–2843) | 2843 (2843–2843) | 0% |
| Breast cancer (HER2-TKIs) | 4425 (4425–4425) | 1536 (1536–1536) | −65% |
| Breast cancer (HER2 mab inhibitors) | 2920 (2920–2920) | 1089 (1089–1089) | −63% |
| NSCLC (ALK inhibitors) | 5909 (5909–5909) | 1736 (1736–1736) | −71% |
| NSCLC (EGFR inhibitors) | 5235 (3817–6420) | 1075 (844–1337) | −79% |
| NSCLC (PD1/PDL1 inhibitors) | 4872 (4053–5408) | 878 (835–1397) | −81% |
| Hepatocellular carcinoma (PD1/PDL1 inhibitors) | 4663 (4544–6466) | 943 (788–1645) | −80% |
| Hepatocellular carcinoma (VEGFR inhibitors) | 3376 (3171–3581) | 1249 (1213–1286) | −63% |
| Colorectal cancer (VEGFR inhibitors) | 3785 (3785–3785) | 1222 (1222–1222) | −68% |
| Melanoma (PD1 inhibitors) | 2172 (2172–2172) | 543 (543–543) | −75% |
| Ovarian cancer (PAPR inhibitors) | 2897 (2741–3052) | 1439 (1439–1440) | −50% |
| Prostate cancer (AR inhibitors) | 3177 (3177–3177) | 3177 (3177–3177) | 0% |
| Imported anticancer drugs | |||
| Breast cancer (CDK4/6 inhibitors) | 4766 (4766–4766) | 2298 (2298–2298) | −52% |
| Breast cancer (HER2-TKIs) | 4637 (4350–4924) | 1270 (1139–1401) | −73% |
| Breast cancer (HER2-mab-inhibitors) | 7042 (7042–7042) | 1750 (1750–1750) | −75% |
| NSCLC (ALK inhibitors) | 4830 (3141–8614) | 2470 (2077–3141) | −49% |
| NSCLC (EGFR inhibitors) | 3049 (2853–3248) | 633 (528–739) | −79% |
| NSCLC (PD1/PDL1 inhibitors) | 8740 (8449–9031) | 7667 (7503–7831) | −12% |
| Hepatocellular carcinoma (PD1/PDL1 inhibitors) | 9875 (9875–9875) | 8972 (8972–8972) | −9% |
| Hepatocellular carcinoma (VEGFR inhibitors) | 5393 (5393–5393) | 2349 (2349–2349) | −56% |
| Colorectal cancer (VEGFR inhibitors) | 5393 (5393–5393) | 2349 (2349–2349) | −56% |
| Melanoma (PD1 inhibitors) | 8235 (8235–8235) | 7726 (7726–7726) | −6% |
| Ovarian cancer (PAPR inhibitors) | 10,138 (9342–10,934) | 1940 (1823–1996) | −81% |
| Prostate cancer (AR inhibitors) | 4923 (4264–5583) | 1068 (1033–1102) | −78% |
Prices are expressed in inflation-adjusted US dollars of monthly treatment.
AR, androgen receptor; PAPR, poly(ADP-ribose) polymerase; VEGFR, vascular endothelial growth factor receptor; TKIs, tyrosine kinase inhibitors; IQR, interquartile range.
Price reduction is defined as the percentage reduction in the monthly treatment price at the latest relative to the year at launch.
Fig. 3.
Price changes in monthly treatment price of novel anticancer drugs at launch and the latest (2022) in domestic (a) and imported (b) drugs.
Fig. 4.
Comparison of monthly treatment price of novel anticancer drugs between domestic and imported drugs. (a) Comparison of monthly treatment price of novel anticancer drugs at launch between domestic and imported drugs. (b) Comparison of monthly treatment price of novel anticancer drugs at the latest (2022) between domestic and imported drugs. The line indicates the median price; the whiskers indicate the 10th and 90th percentiles of price.
Efficacy and safety of the anticancer drugs
The primary efficacy endpoints of the 48 pivotal clinical trials are shown in Table 4. The most common primary efficacy for domestic agents was ORR (43%), followed by PFS (33%) and OS (14%). For imported anticancer drugs, PFS (41%) as the primary efficacy endpoint was the most popular, followed by ORR (19%) and co-primary of PFS and OS (15%).
Table 4.
Primary endpoint and outcome of the pivotal trials between domestic and imported drugs.
| End Point or Outcome | Domestic, (n = 21) | Imported, (n = 27) | P value |
|---|---|---|---|
| Primary trial end point, No. (%) | |||
| PFS | 7 (33) | 11 (41) | 0.42 |
| OS | 3 (14) | 3 (11) | |
| ORRa | 9 (43) | 5 (19) | |
| PFS and OS | 2 (10) | 4 (15) | |
| DFS | 0 (0) | 2 (7) | |
| MFS | 0 (0) | 1 (4) | |
| TTP | 0 (0) | 1 (4) | |
| Efficacy | |||
| Objective response rate, %b | |||
| Mediana(IQR) | 57 (17, 69) | 62 (47, 62) | 0.77 |
| Pooled estimate (95%CI)d | 37 (22, 64) | 48 (30, 77) | 0.51 |
| Progression-free survivalc | |||
| Gain, months, median (IQR) | 9.0 (7.0, 10.1) | 11.0 (7.9, 16.5) | 0.24 |
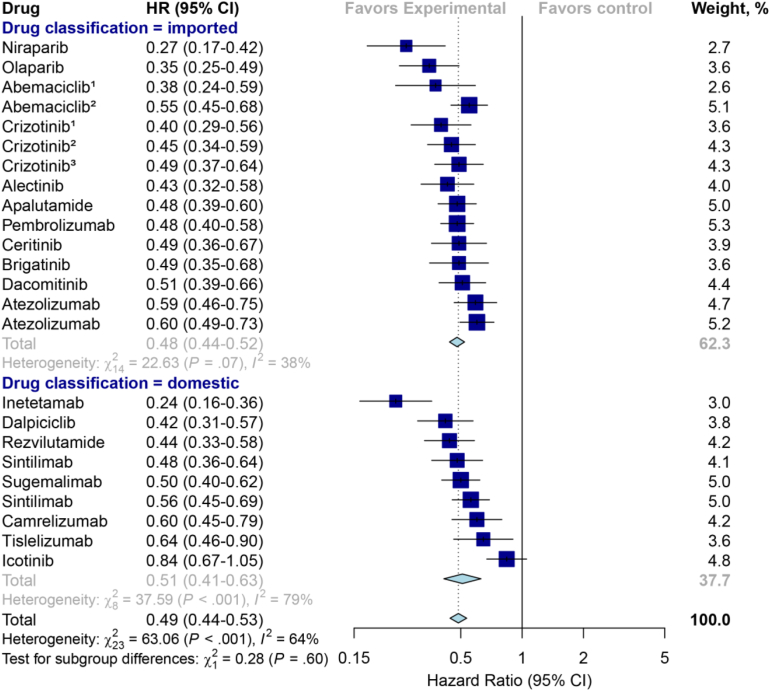
| Pooled hazard ratio (95%CI)d | 0.51 (0.41, 0.63) | 0.48 (0.44, 0.52) | 0.64 |
| Clinically meaningful improvement, No. (%) | 4 (50) | 12 (86) | 0.14 |
| Overall survivalc | |||
| Gain, months, median (IQR) | 9.3 (9.0, 10.7) | 10.6 (8.8, 18.1) | 0.66 |
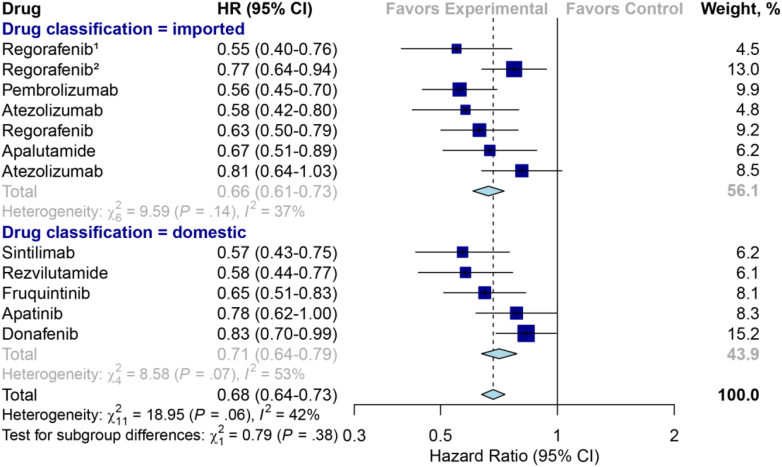
| Pooled hazard ratio (95%CI)d | 0.71 (0.64, 0.79) | 0.66 (0.61, 0.73) | 0.38 |
| Clinically meaningful improvement, No. (%) | 1 (33) | 3 (80) | 0.46 |
| Safetyc | |||
| SAE, No. patients (%) | 646/2783 (23) | 1328/5566 (24) | 0.41 |
| SAE, Pooled Relative Risk (95%CI)d | 1.40 (1.08, 1.83) | 1.18 (1.07, 1.31) | 0.23 |
| Grade≥3 AEs, No. patients (%) | 1480/2504 (59) | 4201/7385 (57) | 0.45 |
| Grade≥3 AEs, Pooled Relative Risk (95%CI)d | 1.61 (1.03, 2.51) | 1.57 (1.21, 2.03) | 0.92 |
IQR, interquartile range; CI, confidence interval; SAE, serious adverse events; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; DFS, disease-free survival; MFS, metastasis-free survival; TTP, time to progress; AE, adverse events.
Only one pivotal clinical trial was a randomized controlled clinical trial design, and all others were single-arm designs.
Objective response rate (ORR) included partial response and complete response. Only ORR reported in single-arm clinical trials were analyzed.
Data for these analyses were derived from randomized controlled clinical trials (not included for single-arm trial designs).
Results from meta-analyses.
No significant differences were observed between the domestic and the imported drugs in terms of median PFS gains (9.0 vs. 11.0 months; P = 0.24), OS gains (9.3 vs. 10.6 months; P = 0.66) and ORR (57% vs. 62%; P = 0.77) derived from their pivotal trials (Table 4). The pooled results also revealed that no significant difference in primary efficacy endpoint between domestic and imported anticancer drugs was found (HR for PFS, 0.51 vs. 0.48, P = 0.60, Fig. 5); HR for OS (0.71 vs. 0.66, P = 0.38, Fig. 6); ORR (37% vs. 48%, P = 0.51, Fig. S1). Besides, the proportion of drugs meeting clinically meaningful improvement criteria between the domestic and imported groups also showed no statistical differences (PFS gains improvement greater than 3 months: 50% vs. 86%, P = 0.14; OS benefits ≥2.5 months: 33% vs 80%, P = 0.46).
Fig. 5.
Hazard ratio forest plot for progression-free survival from the randomized controlled trials for domestic versus imported anticancer drugs. The serial number marked above the drug name in the figure represents the indication that involves multiple pivotal clinical trials.
Fig. 6.
Hazard ratio forest plot for overall survival from the randomized controlled trials for domestic versus imported anticancer drugs. The serial number marked above the drug name in the figure represents the indication that involves multiple pivotal clinical trials.
A pooled analysis of adverse events from the pivotal clinical trials designed as randomized controlled trials (RCT) suggested that the proportion of SAE for domestic anticancer agents was comparable to that of imported drugs (23% vs. 24%, P = 0.41). Similarly, there was no significant difference between domestic and imported anticancer agents in the AEs risk of Grade ≥3 (59% vs. 57%, P = 0.45). The pooled results also confirmed that there was no significant difference between domestic and imported novel anticancer drugs for safety outcomes (relative risk for SAE: 1.40 [95%CI, 1.08–1.83] vs. 1.18 [95%CI, 1.07–1.31], P = 0.23, Fig. S2; relative risk for AEs of Grade ≥3: 1.61 [95%CI, 1.03–2.51] vs. 1.57 [95%CI, 1.21–2.03], P = 0.92, Fig. S3. Subgroup analysis showed no significant differences in PFS, OS, ORR, SAE and Grade ≥3 AEs between domestic and imported anticancer agents (Tables S3–7).
Discussion
In this study, we first evaluated the price of the targeted anticancer drugs, including 12 within the same classes of drugs covering 7 cancer diseases. The results showed an average reduction of 71% and 62% for domestic and imported novel anticancer drugs, respectively, compared to the price at launch. Besides, the AARR of 45% and 30% for monthly treatment price for domestic and imported anticancer drugs, respectively. The affordability index for domestic and imported anticancer drugs was estimated as 76% (1222/1617) and 128% (2077/1617) in 2022, respectively, much lower than 288% for China in 2016 reported by Goldstein DA.12 Besides, the affordability of domestic anticancer drugs was close to some developed countries, e.g. Australia (76% vs. 71%) and the United Kingdom (76% vs. 78%) in 2016, indicating that the affordability of anticancer drugs had been significantly improved in China.12 This is mainly due to the combined results of drug regulatory reform and national price negotiations.
The previous studies indicated that the average monthly treatment price of anticancer drugs in the United States increased from $5790 in 2009–2010 to $14,580 in 2018–2019, which is significantly higher than in other developed nations in Europe, despite the significant increase in the number of novel anticancer drugs approved by the US FDA during this time period.39,40 A recent study also found a 15.31% increase in prices for 12 drug classes covering 9 indications in the United States four years after anticancer drugs entered the market, compared to a 26% and 13% decrease in Germany and Switzerland, respectively.19 This disparity of price change trend could be attributed to price negotiations which were practiced in Germany and Switzerland. Also, China had launched six rounds of national drug price negotiations since 2016 to introduce clinically valuable and unaffordable innovative drugs into the NRDL, with an average price reduction of over 50% from 2016 to 2021. Since China has implemented universal health coverage, there will be a larger market for novel anticancer drugs when they are included in the NRDL due to more patients can afford the copayment of them.
In this study, we noted that 86% (18/21) of novel domestic anticancer drugs included in the 2021 NRDL via price negotiations had significantly reduced their monthly treatment prices when compared to the drugs not included (3/21) in 2021 NRDL (73% vs. 0%, P < 0.001). The three indications were not included in the 2021 NRDL mainly because they were just marketed in 2022. For the imported anticancer drugs, 71% (15/21) of indications entered the 2021 NRDL, which was relatively lower than that of domestic anticancer drugs (81%). The median monthly treatment price reduction for the 15 of imported indications included in the 2021 NRDL was also significantly greater than those not included (75% vs. 3%, P < 0.001). Of the six imported indications that did not enter the 2021 NRDL, two indications were marketed in 2022, while the other four indications were marketed before 2021, indicating that the imported anticancer drugs were less inclined to enter the NRDL through lower prices compared with domestic drugs. Additionally, our findings demonstrated the significantly higher median AARR for approved anticancer drugs in 2016–2022 than in 2010–2015 (44% vs. 2%, P < 0.01). These findings supported that to reduce the price of novel anticancer drugs without national price negotiations may be extremely challenging in China.
We further assessed the difference in the monthly treatment price at launch and the latest between domestic and imported novel anticancer drugs. Notably, the monthly treatment price of domestic anticancer medications was considerably lower than that of imported ($3786 vs. $5393, P = 0.007). Moreover, the monthly treatment price at the latest (2022) of domestic anticancer medications was still significantly lower than that of imports ($1222 vs. $2077, P = 0.011). Moreover, the AARR of domestic anticancer drugs tended to be larger than that of imported drugs although there was no significant difference (45% vs. 30%, P = 0.06). Therefore, it is reasonable to believe that the increasing number of domestic anticancer drugs approved for marketing would contribute to reducing the within-class targeted anticancer drugs. The previous study suggested that locally developed "me-too" anticancer drugs in China, along with price negotiations and health insurance reimbursement policies, could improve the affordability to targeted anticancer drugs, which was consistent with our findings.20 For instance, the most advanced PD1/PDL1 inhibitors have received approval from many regulatory authorities around the world due to their revolutionary therapeutic effects in the treatment of cancer.41 This study included six PD1/PDL1 inhibitors approved for first-line treatment of non-squamous NSCLC, of which three domestic PD1 inhibitors were successfully negotiated and included in the 2021 NDRL, while another two imported PD1 inhibitors were not included for the absence of sufficient price reduction. The lowest monthly price of domestic PD1 inhibitor treatment cost less than one-tenth of imported treatment. It can be estimated that the monthly out-of-pocket price for the patients might be less than $250 when assuming 70% of the price of medical reimbursement in China, making the cost of PD1 inhibitors hopefully the lowest in the world. Therefore, it is highly significant to encourage the R & D of within-class novel anticancer drugs by local developers to improve affordability for patients.
Indeed, the anticancer drugs are less affordable to patients than non-anticancer drugs in China. We further analyzed the prices of 21 novel non-anticancer drugs (only novel drugs that require long-term administration were included) that were successfully negotiated in the national drug price negotiations in 2021. Our results showed that the median monthly treatment price of these novel non-anticancer drugs was $344 (IQR: 121, 621) in 2022, much lower than the median monthly treatment price of the domestic or imported novel anticancer drugs included in this study ($1222 and $2077 for domestic and imported anticancer drugs, respectively). This partly explained the significance of speeding up the R & D of novel anticancer drugs and increasing the national price negotiation of novel anticancer drugs in China.
The characteristics of pivotal clinical trials between domestic and imported anticancer drugs were also analyzed in this study. Our results found no statistically significant difference in the number of patients enrolled in each pivotal clinical trial between domestic and imported drugs. However, the median number of pivotal clinical trials for imported anticancer drugs was significantly higher than that of domestic drugs (P = 0.04). This phenomenon may be partly due to the fact that imported anticancer drugs conducting the multi-regional clinical trial (MRCT) lacked the Chinese population, and therefore some of them require bridging clinical trials to meet the regulatory requirements in China. Besides, the proportion of domestic and imported anticancer drugs granted conditional approval was 48% and 24%, respectively, which reflected the flexibility of China's regulatory review for drugs in serious life-threatening oncology diseases. China implemented the conditional approval program (Pilot) since 2017, indicating that the novel drugs can rely on surrogate endpoints with limited patients marketing in advance. It should be admitted that the majority of imported anticancer drugs usually obtained adequate efficacy and safety trial evidence when submitted for New Drug Application (NDA) or Biologic License Application (BLA), given that the long-standing delay in bringing anticancer drugs to market was observed in China.15 Consequently, the imported anticancer drugs included in this study were primarily regular approval.
It is well known that conditional approval has established itself as a strategy to expedite the launch of novel drugs by primary international regulatory agencies based on the surrogate endpoints (e.g. accelerated approval program in the FDA; conditional marketing authorizations in the EMA), for the treatment of serious life-threatening without available therapy.42 Previous studies have shown that accelerated approval has been used for more than half of the FDA-approved anticancer drug indications from 1992 to 2019, and the majority of anticancer drugs have been converted to traditional approval.43, 44, 45 In an FDA-published article summarizing the accelerated approval of anticancer drugs from 1992 to 2017, the proportion of marketing approvals based on response rate and single-arm trials was 87% and 72%, respectively.46 Only five indications (5%) that received accelerated approval did not observe clinical benefit in post-confirmation clinical trials, indicating that the successful implementation of the accelerated approval program has resulted in more patients experiencing clinical benefit. Therefore, it can be expected that the implementation of conditional approval program in China will contribute to addressing the affordability of drugs for oncology patients effectively. However, the safety and efficacy of these drugs (with conditional approval) should be stringently regulated in China, including the introduction of OS in confirmatory clinical trials and clinical endpoints that directly reflect patient benefit (e.g. health-related quality of life).44,47
This study further evaluated comparative efficacy and safety outcome from pivotal clinical trials of domestic and imported anticancer agents in support of the NMPA marketing approval. The PFS and OS gain results of the RCT revealed no significant differences between domestic and imported drugs. Similarly, no significant difference was found in the ORR analysis of single-arm trials. The meta-analyses provided further evidence that the HR of PFS and OS, and the pooled analysis of ORR, did not significantly vary between imported and domestic anticancer agents. Additionally, we considered OS improvement ≥2.5 months or PFS improvement ≥3 months to be clinically significant for tumor treatment, which were consistent with previous studies.34,35 The findings indicated that no significant differences in OS and PFS improvement between domestic and imported were observed. The safety of domestic and imported anticancer agents was comparable, as shown by the analysis of SAE and Grade ≥3 AEs occurring in pivotal clinical trials. Moreover, it was further confirmed by the meta-analyses. These findings supported that there were no significant differences between domestic and imported in primary efficacy and safety outcomes.
This study confirmed that China had made significant achievements in improving the affordability of anticancer drugs in recent years. However, China also faces a number of challenges, including excessive R & D of the same target and the rationality of the negotiated price of novel drugs.48, 49, 50 The previous study showed that more than 400 me-too drugs were being developed in China, the most common being CD19 (56), EGFR (44) and PD1/PDL1 (32),48 which would result in a serious waste of R&D investment. Therefore, it was recommended that NMPA needs to further optimize the development of clinically value-oriented drugs (e.g. conducting head-to-head RCTs for similar target anticancer drugs). From the societal perspective, when the quality was comparable with no statistical differences, the lower drug price the better. As the price per patient may decrease, the total volume consumed will increase. Hence, China should include more novel anticancer drugs with obvious clinical value for national price negotiations. It can be expected that the close collaboration between NMPA and the National Health Insurance Administration (the department that leads national drug price negotiations) will further accelerate the development of novel drugs faster and at lower prices for more oncology patients to receive novel treatment in China.
Our study has some limitations. First, this study focuses on targeted anticancer agents, therefore generalization to other types of therapeutic agents may not be applicable. Additionally, similar to the earlier study,19 our definition of comparable anticancer agents was broad. For instance, we included first-line and second-line treatments for within-class anticancer drugs, which may have biased the results. Future studies should be more narrowly focused on the same indications. Second, the analysis of anticancer drug prices did not account for the discounting policies of developers, such as free treatment courses for low-income families; however, this percentage is small and has a negligible impact on this price analysis. The monthly treatment price was calculated ideally based on the cheapest per-mg strength in the drug package, which may differ from the actual monthly treatment price. Third, only the primary endpoints were included in this assessment of the efficacy of imported and domestic anticancer drugs, considering that the regulatory agencies approve the new drugs for marketing primarily based on the primary endpoint. In addition, the heterogeneity of some meta-analyses was excessively high, so the interpretation needs to be cautious. Finally, this study did not assess the clinical value of domestic and imported anticancer drugs comprehensively. In recent years, numerous studies assess the clinical value of anticancer drugs using the validated American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) value frameworks, which can serve as a basis for price negotiations.31,39,51, 52, 53 Therefore, these two value frameworks can be utilized further to evaluate the clinical value of anticancer drugs in China.
Conclusion
Overall, the prices of both domestic and imported within-class anticancer drugs significantly decreased after entering the market in China. The median monthly treatment prices of domestic anticancer drugs were significantly lower than that of imported drugs, both at launch and the latest prices. Moreover, no significant differences in the efficacy and safety of anticancer drugs were observed between domestic and imported drugs. Our findings supported that encouraging the development of within-class anticancer drugs and implementing the price negotiation for NRDL were of great importance to reducing patient burden in China. This pattern may provide new perspectives on international approaches to improve the affordability of anticancer medications.
Contributors
XXL contributed to study design, data interpretation, data analysis and drafted the manuscript. XD contributed to study design, data collection, data analysis and data interpretation. LH contributed to data collection, data analysis and data interpretation. QXG contributed to data analysis, data interpretation and figure production. RJT contributed to data collection, data analysis and data interpretation. YZ contributed to data analysis and data interpretation. ZQL contributed to data analysis and data interpretation. XCX contributed to data analysis and data interpretation. TFL contributed to data analysis and data interpretation. KDL contributed to data analysis and data interpretation. FQ contributed to data analysis and data interpretation. SCC contributed to study design, data interpretation and data analysis. YY contributed to study design, data interpretation and data analysis. All authors were involved in each stage of the preparation and revision of the manuscript. XXL, XD and RJT have accessed and verified the data. YY and SCC were responsible for the decision to submit the manuscript.
Data sharing statement
All the data used in this study are from publicly accessible databases.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
This study was supported by postdoctoral fellowship from Tsinghua-Peking Joint Centers for Life Sciences (CLS).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2022.100670.
Contributor Information
Xingxian Luo, Email: luoxingxian@tsinghua.edu.cn.
Xin Du, Email: duxin1220@gmail.com.
Shein-Chung Chow, Email: sheinchung.chow@duke.edu.
Yue Yang, Email: yanghappy@tsinghua.edu.cn.
Appendix A. Supplementary data
References
- 1.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Population fact sheets. https://gco.iarc.fr/today/fact-sheets-populations
- 5.Xia C., Dong X., Li H., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad V., De Jesús K., Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381–390. doi: 10.1038/nrclinonc.2017.31. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H., Rajkumar S.V. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin Proc. 2015;90(4):500–504. doi: 10.1016/j.mayocp.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Prasad V., Wang R., Afifi S.H., Mailankody S. The rising price of cancer drugs-A new old problem? JAMA Oncol. 2017;3(2):277–278. doi: 10.1001/jamaoncol.2016.4275. [DOI] [PubMed] [Google Scholar]
- 9.Fu M., Naci H., Booth C.M., et al. Real-world use of and spending on new oral targeted cancer drugs in the US, 2011-2018. JAMA Intern Med. 2021;181(12):1596–1604. doi: 10.1001/jamainternmed.2021.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Wei Y., Li H., et al. Prices and clinical benefit of national price-negotiated anticancer medicines in China. Pharmacoeconomics. 2022;40(7):715–724. doi: 10.1007/s40273-022-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H.-Y., Shi J.-F., Guo L.-W., et al. Expenditure and financial burden for common cancers in China: a hospital-based multicentre cross-sectional study. Lancet. 2016;388:S10. [Google Scholar]
- 12.Goldstein D.A., Clark J., Tu Y.F., et al. Global differences in cancer drug prices: a comparative analysis. J Clin Oncol. 2016;34(18) [Google Scholar]
- 13.Luo X., Du X., Li Z., Qian F., Yang Y. Assessment of the delay in novel anticancer drugs between China and the United States: a comparative study of drugs approved between 2010 and 2021. Chin Pharmacol Therapeutics. 2022 doi: 10.1002/cpt.2755. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Luo X., Qian F., Yang L., Li Y., Yang Y. Assessment of the breakthrough-therapy-designated drugs granted in China: a pooled analysis 2020-2022. Drug Discov Today. 2022;27(12) doi: 10.1016/j.drudis.2022.103370. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj G., Gupta M., Wang H.H., et al. Challenges and opportunities with oncology drug development in China. Chin Pharmacol Therapeutics. 2019;105(2):363–375. doi: 10.1002/cpt.1017. [DOI] [PubMed] [Google Scholar]
- 16.Luo X., Yang L., Du X., Yang J., Qian F., Yang Y. Analysis of patent and regulatory exclusivity for novel agents in China and the United States: a cohort study of drugs approved between 2018 and 2021. Chin Pharmacol Therapeutics. 2022;112(2):335–343. doi: 10.1002/cpt.2625. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Wushouer H., Han S., et al. The impacts of government reimbursement negotiation on targeted anticancer medication price, volume and spending in China. BMJ Glob Health. 2021;6(7) doi: 10.1136/bmjgh-2021-006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y., Zhu Z., Zhang J., et al. Impacts of national drug price negotiation on expenditure, volume, and availability of targeted anti-cancer drugs in China: an interrupted time series analysis. Int J Environ Res Publ Health. 2022;19(8) doi: 10.3390/ijerph19084578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vokinger K.N., Hwang T.J., Carl D.L., et al. Price changes and within-class competition of cancer drugs in the USA and Europe: a comparative analysis. Lancet Oncol. 2022;23(4):514–520. doi: 10.1016/S1470-2045(22)00073-0. [DOI] [PubMed] [Google Scholar]
- 20.Luo Z., Gyawali B., Han S., Shi L., Guan X., Wagner A.K. Seminars in oncology; 2021. Elsevier; 2021. Can locally developed me-too drugs aid price negotiation? An example of cancer therapies from China; pp. 141–144. [DOI] [PubMed] [Google Scholar]
- 21.Lu S., Wang J., Yu Y., et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512–1522. doi: 10.1016/j.jtho.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Gordon N., Stemmer S.M., Greenberg D., Goldstein D.A. Trajectories of injectable cancer drug costs after launch in the United States. J Clin Oncol. 2018;36(4):319–325. doi: 10.1200/JCO.2016.72.2124. [DOI] [PubMed] [Google Scholar]
- 23.Gagne J.J., Choudhry N.K. How many "me-too" drugs is too many? JAMA. 2011;305(7):711–712. doi: 10.1001/jama.2011.152. [DOI] [PubMed] [Google Scholar]
- 24.Wineinger N.E., Zhang Y., Topol E.J. Trends in prices of popular brand-name prescription drugs in the United States. JAMA Netw Open. 2019;2(5) doi: 10.1001/jamanetworkopen.2019.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Medical Products Administration Listed drug information. https://www.cde.org.cn/main/xxgk/listpage/b40868b5e21c038a6aa8b4319d21b07d
- 26.Insight Price database. https://db.dxy.cn/v5/home
- 27.National Bureau of Statistics Consumer Price Index. https://data.stats.gov.cn/easyquery.htm?cn=C01
- 28.National Bureau of Statistics Per capita gross domestic product. https://data.stats.gov.cn/easyquery.htm?cn=C01
- 29.Organisation for Economic Co-operation and Development Purchasing power parities (PPP) https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm
- 30.Rui M., Li H. Cost-effectiveness of osimertinib vs docetaxel-bevacizumab in third-line treatment in EGFR T790M resistance mutation advanced non-small cell lung cancer in China. Clin Therapeut. 2020;42(11):2159–2170.e6. doi: 10.1016/j.clinthera.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Vokinger K.N., Hwang T.J., Grischott T., et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost-benefit analysis. Lancet Oncol. 2020;21(5):664–670. doi: 10.1016/S1470-2045(20)30139-X. [DOI] [PubMed] [Google Scholar]
- 32.Ratain M.J. Body-surface area as a basis for dosing of anticancer agents: science, myth, or habit? J Clin Oncol. 1998;16(7):2297–2298. doi: 10.1200/JCO.1998.16.7.2297. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Naci H., Wagner A.K., et al. Overall survival benefits of cancer drugs approved in China from 2005 to 2020. JAMA Netw Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang T.J., Franklin J.M., Chen C.T., et al. Efficacy, safety, and regulatory approval of Food and drug administration-designated breakthrough and nonbreakthrough cancer medicines. J Clin Oncol. 2018;36(18):1805–1812. doi: 10.1200/JCO.2017.77.1592. [DOI] [PubMed] [Google Scholar]
- 35.Kumar H., Fojo T., Mailankody S. An appraisal of clinically meaningful outcomes guidelines for oncology clinical trials. JAMA Oncol. 2016;2(9):1238–1240. doi: 10.1001/jamaoncol.2016.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ladanie A., Schmitt A.M., Speich B., et al. Clinical trial evidence supporting US Food and drug administration approval of novel cancer therapies between 2000 and 2016. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ioannidis J.P., Patsopoulos N.A., Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vokinger K.N., Hwang T.J., Daniore P., et al. Analysis of launch and postapproval cancer drug pricing, clinical benefit, and policy implications in the US and Europe. JAMA Oncol. 2021;7(9) doi: 10.1001/jamaoncol.2021.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhodapkar M., Zhang A.D., Puthumana J., Downing N.S., Shah N.D., Ross J.S. Characteristics of clinical studies used for US Food and drug administration supplemental indication approvals of drugs and biologics, 2017 to 2019. JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu J.X., Hodge J.P., Oliva C., Neftelinov S.T., Hubbard-Lucey V.M., Tang J. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat Rev Drug Discov. 2020;19(3):163–164. doi: 10.1038/d41573-019-00182-w. [DOI] [PubMed] [Google Scholar]
- 42.Hwang T.J., Ross J.S., Vokinger K.N., Kesselheim A.S. Association between FDA and EMA expedited approval programs and therapeutic value of new medicines: retrospective cohort study. BMJ. 2020:371. doi: 10.1136/bmj.m3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherla A., Naci H., Kesselheim A.S., Gyawali B., Mossialos E. Assessment of coverage in england of cancer drugs qualifying for US Food and drug administration accelerated approval. JAMA Intern Med. 2021;181(4):490–498. doi: 10.1001/jamainternmed.2020.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen E.Y., Raghunathan V., Prasad V. An overview of cancer drugs approved by the US Food and drug administration based on the surrogate end point of response rate. JAMA Intern Med. 2019;179(7):915–921. doi: 10.1001/jamainternmed.2019.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gyawali B., Hey S.P., Kesselheim A.S. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179(7):906–913. doi: 10.1001/jamainternmed.2019.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beaver J.A., Howie L.J., Pelosof L., et al. A 25-year experience of US Food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–856. doi: 10.1001/jamaoncol.2017.5618. [DOI] [PubMed] [Google Scholar]
- 47.Gyawali B., Rome B.N., Kesselheim A.S. Regulatory and clinical consequences of negative confirmatory trials of accelerated approval cancer drugs: retrospective observational study. BMJ (Clinical research ed) 2021;374:n1959. doi: 10.1136/bmj.n1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G., Qin Y., Xie C., Wu Y.L., Chen X. Trends in oncology drug innovation in China. Nat Rev Drug Discov. 2021;20(1):15–16. doi: 10.1038/d41573-020-00195-w. [DOI] [PubMed] [Google Scholar]
- 49.Liu G.G., Wu J., He X., Jiang Y. Policy updates on access to and affordability of innovative medicines in China. Val Health Reg Issues. 2022;30:59–66. doi: 10.1016/j.vhri.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Huang C., Ung C.O.L., Wushouer H., et al. Health technology assessment-informed pricing negotiation in China: higher negotiated price for more effective targeted anticancer medicines? Health Res Pol Syst. 2022;20(1):3. doi: 10.1186/s12961-021-00810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Paggio J.C. Cancer immunotherapy and the value of cure. Nat Rev Clin Oncol. 2018;15(5):268–270. doi: 10.1038/nrclinonc.2018.27. [DOI] [PubMed] [Google Scholar]
- 52.Vivot A., Jacot J., Zeitoun J.-D., Ravaud P., Crequit P., Porcher R. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000–2015. Ann Oncol. 2017;28(5):1111–1116. doi: 10.1093/annonc/mdx053. [DOI] [PubMed] [Google Scholar]
- 53.Vokinger K., Hwang T., Tibau A., Rosemann T., Kesselheim A. Clinical benefit and prices of cancer drugs in the US and Europe. Ann Oncol. 2019;30:v924. doi: 10.1016/S1470-2045(20)30139-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.