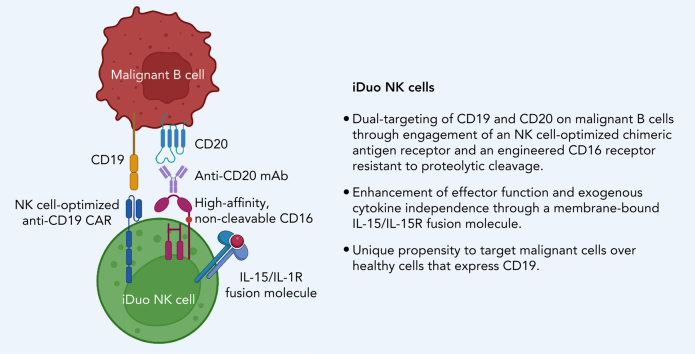
Key Points
-
•
iDuo NK cells prevent antigen escape by targeting both CD19+ and CD19− lymphoma cells.
-
•
iDuo NK cells demonstrate enhanced anti-tumor activity and persistence without the need for cytokine support.
Visual Abstract
Abstract
Substantial numbers of B cell leukemia and lymphoma patients relapse due to antigen loss or heterogeneity after anti-CD19 chimeric antigen receptor (CAR) T cell therapy. To overcome antigen escape and address antigen heterogeneity, we engineered induced pluripotent stem cell-derived NK cells to express both an NK cell-optimized anti-CD19 CAR for direct targeting and a high affinity, non-cleavable CD16 to augment antibody-dependent cellular cytotoxicity. In addition, we introduced a membrane-bound IL-15/IL-15R fusion protein to promote in vivo persistence. These engineered cells, termed iDuo NK cells, displayed robust CAR-mediated cytotoxic activity that could be further enhanced with therapeutic antibodies targeting B cell malignancies. In multiple in vitro and xenogeneic adoptive transfer models, iDuo NK cells exhibited robust anti-lymphoma activity. Furthermore, iDuo NK cells effectively eliminated both CD19+ and CD19− lymphoma cells and displayed a unique propensity for targeting malignant cells over healthy cells that expressed CD19, features not achievable with anti-CAR19 T cells. iDuo NK cells combined with therapeutic antibodies represent a promising approach to prevent relapse due to antigen loss and tumor heterogeneity in patients with B cell malignancies.
Cichocki et al report on a novel CAR-natural killer (CAR-NK) cell therapy for B-cell lymphoma that addresses antigen loss, tumor heterogeneity, and failed CAR-cell persistence. The authors engineered induced pluripotent stem cell (iPSC)-derived NK cells with anti-CD19 CAR and a noncleavable CD16 that augments toxicity in the presence of anti-CD20 therapy and added an IL-15/IL-15R fusion to promote cytokine-independent persistence. In vitro and xenograft models treated with the engineered NK cells plus rituximab had robust activity and may represent a promising “off the shelf” approach to targeting B-cell malignancies.
Introduction
Treatments for B cell leukemia and lymphoma have improved with application of the CD20-specific antibody rituximab and chimeric antigen receptor (CAR) T cells targeting CD19.1, 2, 3, 4, 5, 6, 7 Despite successes, loss of CD20 during rituximab treatment has been reported.8,9 Additionally, up to 50% of all patients receiving anti-CD19 CAR-T cell therapy relapse within the first year, with many patients exhibiting CD19 loss.10, 11, 12 Thus, new therapeutic approaches are needed to prevent tumor antigen escape. To address this issue, we generated a triple gene-modified induced pluripotent stem cell (iPSC)-derived, natural killer “iNK” cell product, collectively termed “iDuo” NK cells, which, through a high-affinity, non-cleavable CD16 (hnCD16), combine the versatility of antibody-dependent cellular cytotoxicity (ADCC)13, 14, 15 with the direct and potent tumor-killing capability of a CAR and a cytokine autonomous persistence provided by a membrane-bound IL-15/IL-15R fusion molecule (IL-15RF).16 Here, we demonstrate the ability of iDuo NK cells to engage in both anti-CD19 CAR-mediated cytotoxicity and ADCC in combination with rituximab. Compared with anti-CD19 CAR-T cells, iDuo NK cells preferentially targeted malignant B cells relative to healthy B cells. Additionally, we demonstrate robust in vivo control of B cell tumors by iDuo NK cells. This preclinical data supports the clinical translation of iDuo NK cells for the treatment of B cell leukemia and lymphoma.
Methods
Mice
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratories) were housed in the institution’s Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal care facility. Experiments were balanced by sex and all experiments were approved by the University of Minnesota Institutional Animal Care Committee under the protocol 1907-37257A.
iNK cell culture and phenotyping
Human iPSC differentiation to iCD34+ cells, differentiation along the NK cell lineage, and expansion of the NK cell population were performed as previously described.16, 17, 18, 19, 20 Briefly, iPSCs were differentiated toward the mesoderm and CD34+ hematopoietic progenitor stages in StemPro34 media (Thermo Fisher) supplemented with BMP4 (Life Technologies) and bFGF (Life Technologies). CD34+ cells were subsequently enriched prior to differentiation into iNK cells. At the beginning of the iNK cell differentiation culture, iCD34+ cells were plated on OP9 cells in B0 media18 to support NK cell differentiation from hematopoietic progenitors. After 20–30 days of culture, iNK cells were harvested and cocultured with irradiated K562 cells transduced with membrane-bound IL-21 and 4-1BB ligand constructs in supplemented B0 media without the addition of cytokines for 2-to-4 weeks. iDuo NK cells were derived from a clonal human iPSC line expressing transgenes encoding hnCD16 and IL-15RF16 and an anti-CD19 CAR construct containing the NKG2D signaling domain coupled to the 2B4 and CD3ζ signaling domains.21 Fluorescently conjugated antibodies used for flow cytometry analyses are listed in the supplemental Methods section. Analysis of FACS data was performed using FlowJo software (FlowJo LLC).
In vitro assays to assess cytotoxicity against B cell leukemia and lymphoma cell lines, healthy B cells, and primary CLL cells
CLL cells from five patients were labeled with CellTrace Violet dye (Thermo Fisher). Expanded PBNK cells, non-transduced iNK cells, and iDuo NK cells were cocultured with labeled CLL cells at a 2:1 E:T ratio for 5 hours with or without rituximab (1 μg/mL) followed by flow cytometry analysis. The percentages of CLL cell-specific killing were determined as [% live CellTrace+ cells cultured alone] – [% live CellTrace+ cells in the treatment condition]. In experiments testing the relative killing of healthy and malignant B cells, CD19+ B cells were isolated using the EasySep Human B Cell Isolation Kit (STEMCELL Technologies) and labeled with CellTrace Violet dye. Healthy B cells were mixed with Nalm6 cells transduced with GFP at a 1:1 ratio, and these cells were cocultured with anti-CD19 CAR-T cells and iDuo NK cells at various E:T ratios for 4 hours. The relative killing of each B cell target population was determined by flow cytometry. In some experiments, an anti-NKG2D blocking antibody (1D11) or isotype IgG1κ antibody (R&D Systems) was added at a concentration of 10 μg/mL.
In vivo xenogeneic adoptive transfer experiments
For the Nalm6 model, NSG mice were injected with 1 × 105 Nalm6 cells expressing firefly luciferase. After allowing the tumors to establish for 1 day, mice either received no treatment or iDuo NK cells. For the wild-type Raji experiments, NSG mice were injected with 1 × 105 Raji cells expressing firefly luciferase. After allowing the tumors to establish for 1 day, mice were either left untreated, received three injections of rituximab (3 μg) alone, iDuo NK cells alone, or iDuo NK cells along with rituximab. In all experiments iDuo NK cells were thawed from cryopreservation and injected immediately. Bioluminescence imaging was performed weekly to monitor tumor progression, and mice were sacrificed when they showed signs of morbidity. For experiments tracking the in vivo persistence, iNK cells were injected into NSG mice, and blood was drawn weekly for assessment of cell counts by flow cytometry to distinguish adoptively transferred cells.
Statistical analysis
Quantified data are expressed as mean ± SD. Statistical significance was assessed using unpaired two-tailed Student’s t-tests with Welch’s correction, one-way ANOVA, two-way ANOVA, and log-rank (Mantel–Cox) tests. Details of “n” values are provided in the figure legends. All quantitative data were analyzed using Prism software (v.9.1.0).
Results
iDuo NK cells exhibit enhanced anti-CD19 CAR-mediated cytotoxicity and hnCD16-mediated ADCC in combination with rituximab
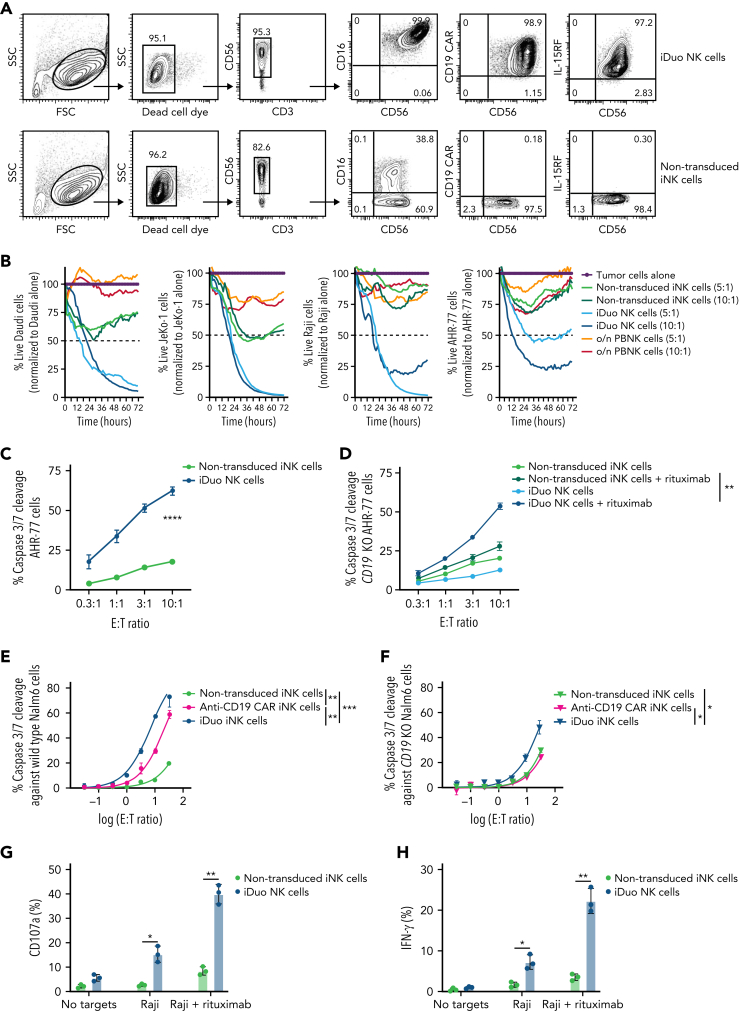
Surface expression of anti-CD19 CAR, hnCD16, and IL-15RF on iDuo NK cells was confirmed by flow cytometry to be uniform and representative of derivation from a clonal engineered iPSC line (Figure 1A). We routinely observed anti-CD19 CAR frequencies above 90% on the surface of iDuo NK cells (supplemental Figure 1, available on the Blood website). CAR function was evaluated in assays where iDuo NK cells, non-transduced iNK cells, and peripheral blood NK (PBNK) cells were cocultured with Daudi, JeKo-1, Raji, and AHR-77 cells to determine the susceptibility of a range of B cell malignancies to iDuo NK cell cytotoxicity. Long-term killing was monitored with live-imaging IncuCyte assays. While non-transduced iNK cells and activated PBNK cells displayed limited activity, iDuo NK cells mediated robust cytotoxicity (Figure 1B). iDuo NK cells and non-transduced iNK cells were also used as effectors in cytotoxicity assays measuring caspase 3/7 cleavage in AHR-77 cells. At all effector/target (E:T) ratios tested, iDuo NK cells exhibited markedly higher rates of cytotoxicity (Figure 1C).
Figure 1.
iDuo NK cells target malignant B cells through anti-CD19 CAR and hnCD16. (A) Representative flow cytometry plots showing surface expression of hnCD16, anti-CD19 CAR, and membrane-bound IL-15RF on non-transduced iNK cells and iDuo NK cells. (B) Non-transduced iNK cells, iDuo NK cells, and PBNK cells activated with 10 ng/mL IL-15 overnight were used as effectors against NLR-transduced Daudi, JeKo-1, Raji, and AHR-77 cells at the indicated E:T ratios in 72-hour IncuCyte assays. Shown are the percentages of live target cells over time normalized to tumor cells alone. Results are representative of two independent experiments. (C) Non-transduced iNK cells and iDuo NK cells were cocultured with NLR-transduced AHR-77 cells labeled with IncuCyte Caspase-3/7 dye at the indicated E:T ratios. Statistical significance was determined by two-way ANOVA. Results are representative of three independent experiments. (D) Non-transduced iNK cells and iDuo NK cells were cocultured with NLR-transduced CD19 knockout AHR-77 cells labeled with IncuCyte Caspase-3/7 dye with or without 1 μg/mL rituximab at the indicated E:T ratios. Statistical significance was determined by two-way ANOVA. Non-transduced iNK cells, anti-CD19 CAR iNK cells, and anti-CD19 CAR/IL-15RF iNK cells were cocultured (E) wild-type Nalm6 cells and (F) CD19 knockout Nalm6 cells labeled with IncuCyte Caspase-3/7 dye at the indicated E:T ratios. Statistical significance was determined by two-way ANOVA. Non-transduced iNK cells and iDuo NK cells were used as effectors and cocultured with Raji cells or Raji cells + 1 μg/mL rituximab at a 2:1 E:T ratio for 5 hours. NK cells were then assessed by flow cytometry for (G) surface CD107a levels and (H) intracellular IFN-γ levels. Results are from three independent experiments. Shown is mean ± standard deviation. Statistical significance was determined by paired two-tailed Student t tests. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
To assess hnCD16-mediated ADCC, we generated CD19 knockout AHR-77 cells. As expected, both non-transduced iNK cells and iDuo NK cells displayed limited cytotoxicity against these targets. Substantial increases in cytotoxicity by iDuo NK cells, but not non-transduced iNK cells, were observed with the addition of rituximab (Figure 1D). To assess the contribution of IL-15RF to cytotoxic function, we generated CD19 knockout Nalm6 cells. Compared with non-transduced iNK cells, anti-CD19 CAR iNK cells demonstrated higher cytotoxicity against wild type but not CD19 knockout Nalm6 cells. These results suggest that, while the addition of the CAR transgene improved CD19-specific targeting, the innate cytotoxicity of the two groups remained comparable. Interestingly, iDuo NK cells not only exhibited higher specific cytotoxicity against CD19+ Nalm6 cells relative to the other two iNK cell groups (Figure 1E), but also demonstrated increased innate cytotoxicity against CD19 knockout Nalm6 cells (Figure 1F). Thus, IL-15RF enhances both the specific and general cytotoxicity of iNK cells. Additionally, iDuo NK cells exhibited significantly higher frequencies of degranulation (Figure 1G) and IFN-γ production (Figure 1H) in response to Raji cells alone and in combination with rituximab. Together, these results demonstrate that iDuo NK cells can efficiently lyse lymphoma cells directly through CAR engagement, in a redirected manner via hnCD16, and generally through innate cytotoxicity.
iDuo NK cells eliminate CD19− B lymphoblasts and lymphoma cells in an in vitro model of tumor heterogeneity and antigen escape
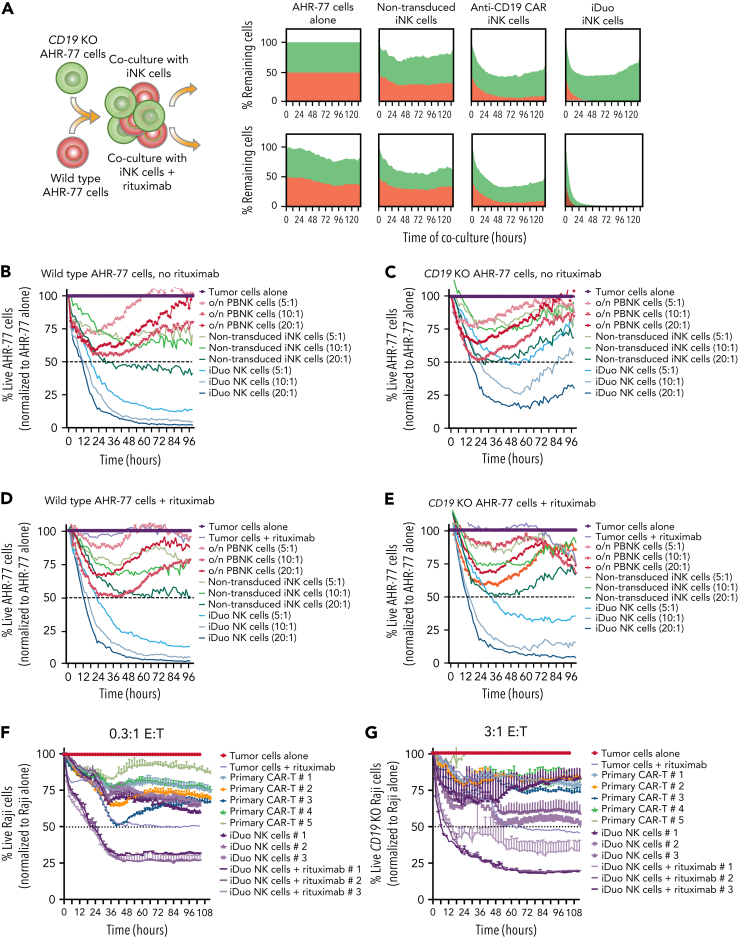
Antigen escape and tumor heterogeneity can render CAR-T cell therapy ineffective.10, 11, 12 To model this in vitro, we established a coculture assay using wild-type CD20+CD19+ AHR-77 cells transduced with NucLightRed and CD20+CD19− AHR-77 cells transduced with NucLightGreen. These two populations of cells were mixed and used as targets with or without rituximab in IncuCyte assays. Rituximab and non-transduced iNK cells mediated a moderate amount of CD20+CD19+ AHR77 and CD20+CD19− AHR77 cell lysis, while anti-CD19 CAR iNK cells preferentially targeted CD20+CD19+ AHR-77 cells. iDuo NK cells alone were highly cytotoxic against CD20+CD19+ AHR-77 cells and eliminated CD20+CD19− AHR-77 cells in the presence of rituximab (Figure 2A). We extended these findings with additional IncuCyte assays where PBNK cells, non-transduced iNK cells, and iDuo NK cells were cocultured with CD20+CD19+ AHR-77 cells (Figure 2B) and CD20+CD19− AHR-77 cells (Figure 2C) in the absence of rituximab. Activated PBNK cells and non-transduced iNK cells exhibited moderate cytotoxicity against CD20+CD19+ AHR-77 cells, while iDuo NK cells mediated efficient and durable killing in a dose-dependent manner (Figure 2B). While iDuo NK cells demonstrated more efficient early CD20+CD19− AHR-77 killing relative to activated PBNK cells and non-transduced iNK cells, tumor control diminished over time (Figure 2C). The same effector populations were also cocultured with CD20+CD19+ AHR-77 cells (Figure 2D) and CD20+CD19− AHR-77 cells (Figure 2E) in the presence of rituximab. Like coculture conditions without rituximab (Figure 2B), only iDuo NK cells mediated efficient and durable cytotoxicity against CD20+CD19+ AHR-77 cells (Figure 2D). iDuo NK cells also mediated strong and sustained cytotoxicity against CD20+CD19− AHR-77 cells with dual targeting enabled by the addition of rituximab (Figure 2E).
Figure 2.
iDuo NK cells eliminate CD19−B cells in models of antigen escape. (A) Wild-type AHR-77 cells transduced with NucLight Red and CD19 knockout AHR-77 cells transduced with NucLight green were mixed at a 1:1 ratio and cultured alone or cocultured with non-transduced iNK cells, anti-CD19 CAR iNK cells, or iDuo NK cells at a 10:1 E:T ratio in the presence or absence of 1 μg/mL rituximab. The percentages of live green and red cells were tracked over time in IncuCyte assays. Shown are overlaid killing curves from a representative experiment. IncuCyte assays were also performed using PBNK cells activated overnight with 10 ng/mL IL-15, non-transduced iNK cells, and iDuo NK cells as effectors at the indicated E:T ratios against (B) wild-type AHR-77 cells in the absence of rituximab, (C) CD19 knockout AHR-77 cells in the absence of rituximab, (D) wild-type AHR-77 cells in the presence of 1 μg/mL rituximab, and (E) CD19 knockout AHR-77 cells in the presence of 1 μg/mL rituximab. The percentages of live AHR-77 cells in each coculture condition were normalized to AHR-77 cells alone and graphed over time. All results are representative of two to three independent experiments. All conditions were run in triplicate. Primary anti-CD19 CAR T cells from five donors and iDuo NK cells from three different expansions were cocultured with NLR-transduced Raji cells at (F) low (0.3:1) and (G) high (3:1) E:T ratios in IncuCyte assays. iDuo NK cells were cocultured with targets in the presence or absence of 1 μg/mL rituximab. The percentages of live Raji cells in each coculture condition were normalized to Raji cells alone and graphed over time. All conditions were run in triplicate. Results are representative of two independent experiments.
In addition to experiments comparing the cytotoxic function of iDuo NK cells and other NK cell products, we performed IncuCyte assays comparing iDuo NK cells from three independent differentiations to primary anti-CD19 CAR-T cells generated from five individuals. First, we performed an in vitro stress test using a low E:T ratio (0.3:1) to assess cytotoxic efficacy in the context of high CD19 antigen availability employing wild-type CD19+CD20+ Raji cells as targets. The killing curves for primary anti-CD19 CAR-T cell and iDuo NK cells were generally overlapping, with moderate killing of wild-type Raji cells. However, iDuo NK cells, when combined with rituximab, mediated superior cytotoxicity even at this low E:T ratio (Figure 2F). Next, we performed an in vitro high-capacity test using a high E:T ratio (3:1) to maximize cytotoxic function in the absence of CD19 antigen availability using CD19 knockout Raji cells as targets. In these assays, both primary anti-CD19 CAR-T cell and iDuo NK cell cytotoxicity was modest because of the lack of CD19 targeting. In contrast, iDuo NK cells maintained robust cytotoxic function when combined with rituximab (Figure 2G). Together, these data show that iDuo NK cells efficiently engage in dual targeting in settings that mimic antigen escape and, unlike anti-CD19 CAR-T cells, can mediate durable cytotoxicity against CD19− tumor cells.
iDuo NK cells effectively kill primary, patient-derived leukemia cells and preferentially target malignant B cells
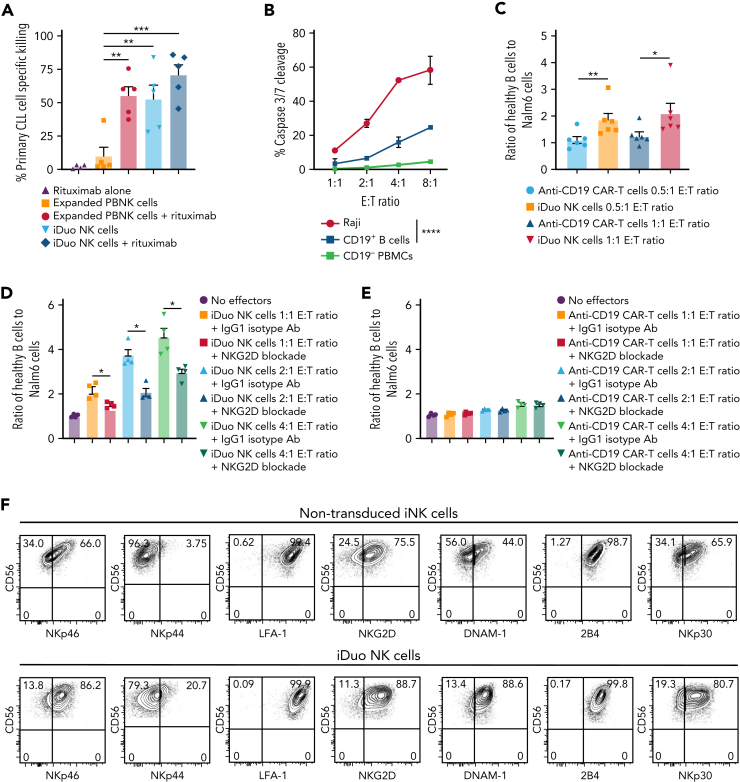
To assess the function of iDuo NK cells against primary leukemia cells, we cocultured expanded PBNK cells, non-transduced iNK cells, and iDuo NK cells at a 2:1 E:T ratio with dye-labeled CLL cells obtained from five different patients with or without the addition of rituximab. All patient samples contained high percentages of CLL blasts that were uniformly positive for CD19 (supplemental Figure 2). As anticipated, PBNK cells alone did not exhibit significant cytotoxicity against CLL targets and required combination with rituximab to induce tumor killing. In contrast, iDuo NK cells mediated high specific killing of CLL cells as a single agent, which was further enhanced with the addition of rituximab to near-elimination of primary CLL targets (Figure 3A).
Figure 3.
iDuo NK cells efficiently kill primary CLL cells and preferentially target malignant B cells. (A) iDuo NK cells and feeder-expanded PBNK cells were cocultured with CellTrace Violet-labeled CLL cells collected from five different patients in the presence or absence of 1 μg/mL rituximab for 5 hours. Percent specific killing was determined by subtracting the percentage of live CLL cells in each condition to CLL cells cultured alone. Results are from two independent experiments. Statistical significance was determined by one-way ANOVA with multiple comparisons. (B) iDuo NK cells were cocultured with IncuCyte Caspase-3/7 dye-labeled Raji cells and peripheral blood mononuclear cells (mixed at a 1:1 ratio) at the indicated E:T ratios for 4 hours. Shown is caspase 3/7 cleavage in target cells as determined by flow cytometry. Results are representative of two independent experiments. (C) iDuo NK cells and primary anti-CD19 CAR T cells were cocultured for 4 hours with CellTrace Violet-labeled CD19+ B cells isolated from five healthy donors and Nalm6 cells expressing GFP at a 1:1 ratio. Shown are the ratios of healthy B cells to Nalm6 cells in each culture condition relative to targets cultured alone. Statistical significance was determined by unpaired two-tailed Student t tests. Results are from two independent experiments. (D) iDuo NK cells and (E) primary anti-CD19 CAR T cells were cocultured for 4 hours with CellTrace Violet-labeled CD19+ B cells isolated from four healthy donors and Nalm6 cells expressing GFP at the indicated E:T ratios in the presence of 10 μg/mL mouse IgG1κ or anti-NKG2D blocking antibody. Shown are the ratios of healthy B cells to Nalm6 cells in each culture condition relative to targets cultured alone. (F) Flow cytometry plots showing surface levels of the indicated activating receptors on non-transduced iNK cells and iDuo NK cells. Results are representative of two independent experiments. Statistical significance was determined by unpaired two-tailed Student t tests. Results are from two independent experiments. Shown is mean ± SD. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
Anti-CD19 CAR-T cell therapy can lead to long durations of B cell aplasia, resulting in lasting damage to the B cell niche well beyond anti-CD19-CAR-T cell persistence.22 NK cells express activating and inhibitory receptors that aid in the discrimination between healthy and malignant cells. We hypothesized that iDuo NK cells, in contrast to anti-CD19 CAR-T cells, may target malignant B cells more efficiently than healthy B cells. To test this hypothesis, iDuo NK cells were cocultured at increasing E:T ratios with a mixture of Raji tumor cells and peripheral blood mononuclear cells collected from healthy donors. Cytotoxicity was measured by flow cytometry analysis of caspase 3/7 cleavage. At all E:T ratios, iDuo NK cells exhibited significantly higher killing of Raji cells compared with healthy B cells, even though both Raji and healthy B cells are CD19+. As expected, iDuo NK cells did not target the CD19 negative PBMC population (Figure 3B). We also performed assays where Nalm6 tumor cells and healthy peripheral blood CD19+ B cells were mixed at a 1:1 ratio and cocultured with iDuo NK cells and primary anti-CD19 CAR-T cells. At both E:T ratios tested, iDuo NK cells exhibited preferential cytotoxicity against Nalm6 cells (Figure 3C).
To further investigate the iDuo NK cell discrimination of healthy versus malignant B cells, we performed additional coculture experiments where iDuo NK cells or anti-CD19 CAR-T cells were cocultured with mixtures of allogeneic B cells from healthy donors and Nalm6 cells at various E:T ratios in the presence or absence of a blocking antibody specific for the activating receptor NKG2D or a control isotype antibody. We found that, at each E:T ratio tested, NKG2D blockade decreased the ratio of healthy B cells to malignant B cells in iDuo NK cell cocultures (Figure 3D) but not anti-CD19 CAR-T cell cocultures (Figure 3E). iDuo NK cells express an array of activating receptors including NKG2D. Activating receptor were observed at higher levels on iDuo NK cells relative to non-transduced iNK cells (Figure 3F). Collectively, these results show that iDuo NK cells mediate robust killing of primary CLL cells and are less cytotoxic against healthy CD19+ B cells relative to anti-CD19 CAR T cells. This discrimination is due, at least in part, to activation through NKG2D.
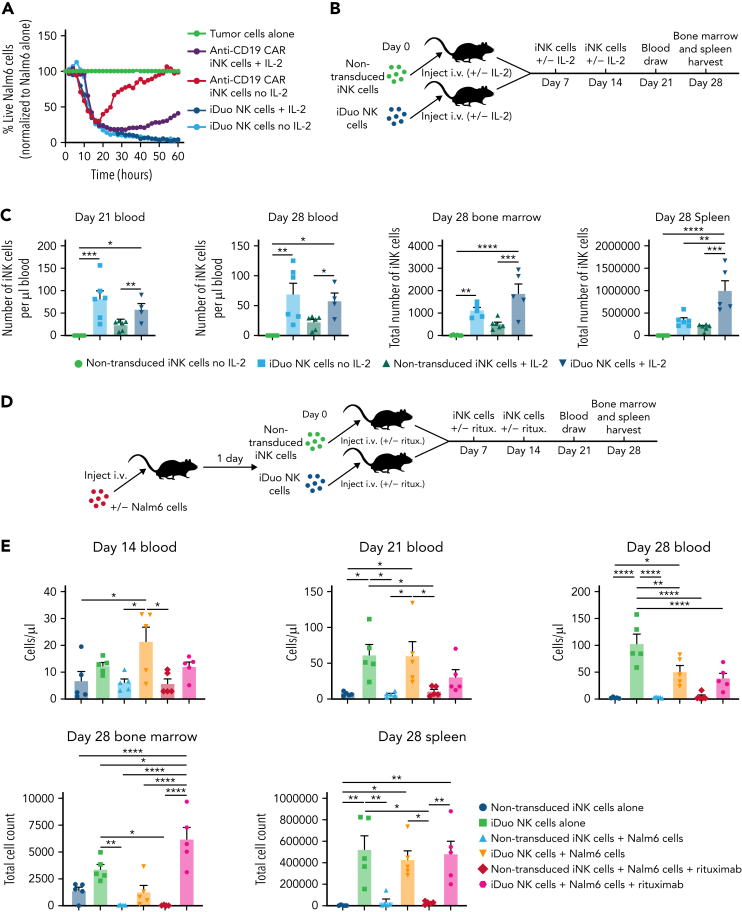
iDuo NK cells persist in the absence of exogenous cytokines
Since IL-15RF enhances iNK cell cytotoxicity (Figure 1E), we next wanted to determine whether the durable response of iDuo NK cells was due to IL-15RF maintaining cell function and persistence. To this end, we performed IncuCyte experiments where anti-CD19 CAR iNK cells and iDuo NK cells were cocultured with Nalm6 cells at a 0.5:1 E:T ratio in the presence or absence of IL-2. Anti-CD19 CAR iNK cells cultured with IL-2 displayed robust killing for the first 3 days of the assay before tumor cell frequencies gradually increased. Anti-CD19 CAR iNK cells cultured without IL-2 also demonstrated initial cytotoxicity, but Nalm6 cell frequencies increased rapidly after 20 hours. In contrast, iDuo NK cells cultured both with and without IL-2 effectively eliminated Nalm6 cells (Figure 4A). This intrinsic ability to maintain function and proliferative capacity absent cytokine support was seen during the expansion of iDuo cells, where they were routinely expanded without supplemental cytokines (supplementary Figure 3). To measure in vivo persistence, NSG mice received three weekly doses of non-transduced iNK cells or iDuo NK cells with or without supplemental IL-2. Blood draws were taken 3 and 4 weeks after the first cell injections, and bone marrow and spleen tissue were harvested at day 28 for assessment of iNK cell numbers by flow cytometry (Figure 4B). At both 3 and 4 weeks, iDuo NK cell numbers were significantly higher in mouse peripheral blood relative to non-transduced iNK cells regardless of whether mice received IL-2. The same trends were observed in bone marrow and spleen tissue. IL-2 supplementation did result in significant elevations in iDuo NK cell numbers in the spleen but not in the blood (Figure 4C).
Figure 4.
iDuo NK cells are cytokine-autonomous. (A) Anti-CD19 CAR iNK cells and iDuo NK cells were cocultured with NLR-transduced Nalm6 cells in the presence or absence of 50 U/mL IL-2. Target cell killing was assessed in IncuCyte assays, and the percentages of live Nalm6 cells in each condition were normalized to Nalm6 cells cultured alone. All conditions were run in triplicate. Results are representative of two experiments. (B) Schematic for the experiments designed to assess the persistence of non-transduced iNK cells and iDuo NK cells in vivo with and without cytokine support. (C) 1 × 107 non-transduced iNK cells or iDuo NK cells were injected IV into NSG mice (n = 6 mice per group) once per week for 3 weeks with or without 5 × 104 U IL-2. One mouse in the iDuo NK cell group with IL-2 died prematurely of causes unrelated to the NK cell transfer. Blood draws were taken at days 21 and 28, and iNK cell counts were determined by flow cytometry using antibodies specific for human CD45 and CD56. Mice were killed at day 28, and bone marrow and spleen were collected for assessment of iNK cell counts. Results are from two independent experiments. Statistical significance was determined using one-way ANOVA. (D) Schematic for experiments designed to assess iNK cell numbers in tissues of mice injected with tumor and rituximab. (E) 1 × 105 Nalm6-luc cells were injected IV into groups of NSG mice (n = 5 per group). The next day, 1 × 107 non-transduced iNK cells or iDuo NK cells were injected IV with or without IP injections of rituximab (2 μg/mouse). Non-tumor–bearing mice also received IV injections of non-transduced iNK cells or iDuo NK cells (n = 5 per group). Mice received subsequent iNK cell and rituximab injections at days 7 and 14. Blood draws were performed at days 14, 21, and 21 for assessment of iNK cell numbers in peripheral blood. Mice were sacrificed at day 28, and bone marrow and spleen tissues were collected for analysis of iNK cell counts. Results are from two independent experiments. Statistical significance was determined using one-way ANOVA. Shown is mean ± standard deviation. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
To investigate iDuo NK cell persistence in a tumor setting, Nalm6 tumor cells were engrafted into NSG mice. After allowing the tumor to establish, groups of mice received three doses of non-transduced iNK cells or iDuo NK cells ± rituximab without the addition of exogenous cytokines. iNK cell numbers in the peripheral blood were determined by weekly blood draws. Mice were sacrificed after 4 weeks for analysis of bone marrow and spleen (Figure 4D). As observed in the previous sets of experiments, iDuo NK cells had a significant persistence advantage relative to non-transduced iNK cells. In mice harboring Nalm6 cells ± rituximab, iDuo NK cell numbers decreased over time. This may be due to the trafficking of the NK cells out of the peripheral blood. In contrast, significant elevations of iDuo NK cells were observed in the bone marrows of mice injected with both Nalm6 cells and rituximab. The presence of tumor cells and rituximab did not impact iDuo NK cell numbers in the spleen (Figure 4E). Together, these data show that iDuo NK cells function and persist in the absence of exogenous cytokines and are found at high numbers in the blood, spleen, and bone marrow.
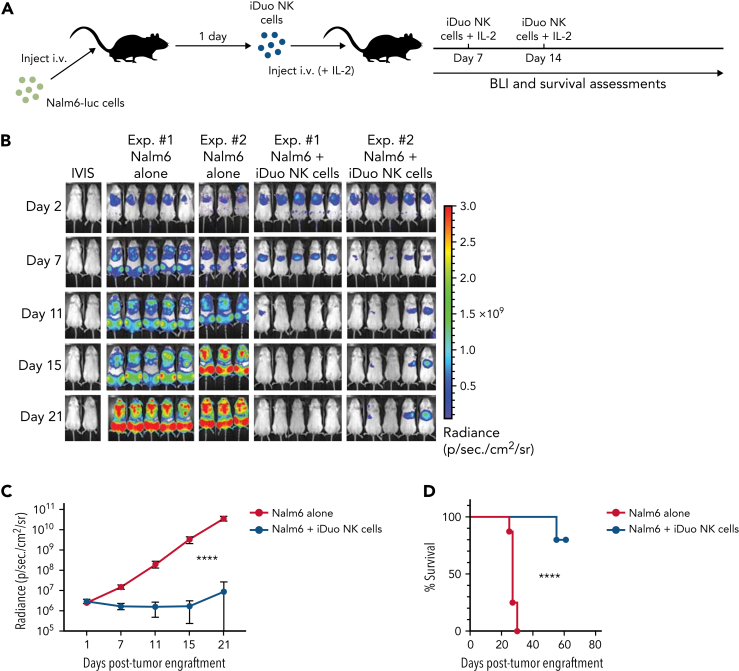
iDuo NK cells exhibit robust and durable in vivo antitumor function in a Nalm6 model of acute lymphoblastic leukemia
We next wanted to test iDuo NK cells as monotherapy in a xenogeneic adoptive transfer model. Nalm6-luc cells were injected into mice, and groups of mice were then untreated or given three weekly injections of iDuo NK cells and IL-2 (Figure 5A). This dosing scheme was chosen to mirror a multi-dosing strategy that would be feasible in a clinical trial setting. In this model, Nalm6 growth rose exponentially in mice receiving tumor alone. In contrast, tumor burden was effectively controlled in mice that received serial doses of iDuo NK cells with little tumor growth out to day 21 (Figure 5B-C). Mice that received adoptive transfer of iDuo NK cells also had a significant survival advantage (Figure 5D). Together, these results demonstrate the efficacy of iDuo NK cells as a monotherapy in a Nalm6 model of acute lymphoblastic leukemia.
Figure 5.
In vivo tumor control by iDuo NK cells. (A) Schematic for experiments assessing iDuo NK cell antitumor function as a single agent in vivo. NSG mice were injected IV with 1 × 105 Nalm6 cells expressing firefly luciferase. After allowing the tumors to establish for 1 day, mice either received no treatment (n = 8) or IV injections of 1 × 107 iDuo NK cells (n = 10) along with 5 × 104 U IL-2 on days 1, 7, and 14. (B) Bioluminescence images throughout the first 3 weeks of the experiment. (C) Graphical representation of cumulative BLI data for the entire 7 weeks of the experiment. Statistical significance was determined using a two-way ANOVA. Shown is mean ± standard deviation. (D) Kaplan–Meier curves for the Nalm6 adoptive transfer experiment. Results are from two independent experiments. Statistical significance was determined using a log-rank (Mantel–Cox) test. ∗∗∗∗P ≤ .0001.
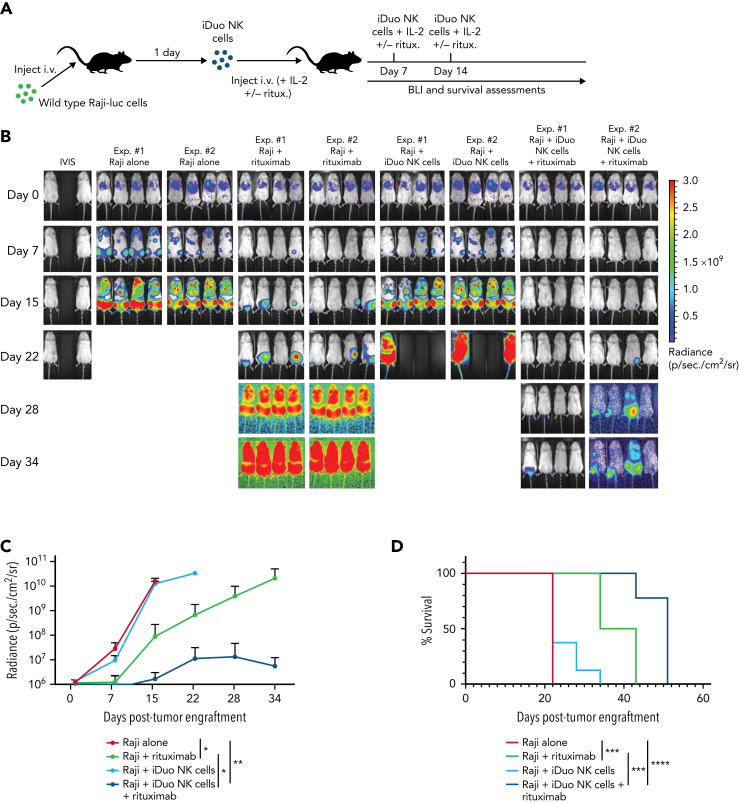
Multi-antigen targeting with iDuo NK cells and rituximab yields durable antitumor responses in Raji models of lymphoma
Because Nalm6 cells were highly sensitive to iDuo NK cell-mediated cytotoxicity in vivo, we next used an aggressive Raji xenograft model to test the efficacy of dual targeting. Raji-luc cells were injected into NSG mice. Groups of mice were then untreated or given three weekly injections of rituximab alone, iDuo NK cells with IL-2, or iDuo NK cells with rituximab and IL-2 (Figure 6A). Tumor burdens grew exponentially in mice that were untreated. Mice that received iDuo NK cells exhibited a modest tumor control at early time points. However, all mice in this group progressively became overwhelmed with tumor. Mice that were given rituximab displayed lower tumor levels at the intermediate stage of the experiments, which is likely due to non-ADCC mechanisms of rituximab action.23 In contrast, the combination of iDuo NK cells and rituximab was associated with durable antitumor responses (Figure 6B-C). Additionally, mice treated with iDuo NK cells and rituximab survived significantly longer (median survival = 51 days) relative to mice treated with rituximab alone (median survival = 38.5 days) (Figure 6D).
Figure 6.
In vivo multi-antigen targeting with iDuo NK cells. (A) Schematic for the experiments to test multi-antigen targeting with iDuo NK cells. NSG mice were injected IV with 1 × 105 Raji cells stably expressing firefly luciferase. After allowing the tumors to engraft for 1 day, mice were either left untreated (n = 8), received three IV injections of rituximab (3 μg) alone (n = 8), 1 × 107 iDuo NK cells alone (n = 8), or 1 × 107 iDuo NK cells along with rituximab (n = 8). Rituximab and cell doses were given on days 1, 4, and 7 post-tumor engraftments. (B) Bioluminescence images through the first 5 weeks of the experiments. (C) Graphical representation of cumulative BLI data. Statistical significance was determined by two-way ANOVA. Shown is mean ± standard deviation. (D) Kaplan–Meier curves for the in vivo Raji experiment. Statistical significance was determined using a log-rank (Mantel–Cox) test. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
Discussion
Several clinical trials have been performed testing adoptive transfer of NK cells for the treatment of patients with advanced B cell lymphoma and leukemia. While some patients achieved partial remissions, the overall efficacy of this approach has been limited.24, 25, 26 To circumvent the limited therapeutic potential, NK cell immunotherapy is now transitioning toward CAR-centric strategies to promote direct engagement and potentiation for enhanced effector function. The use of clonally derived, master iPSC lines is an attractive source for the renewable manufacture of precisely engineered, homogenous NK cell products that can be fully characterized, stored, and administered on-demand for broad patient access. To develop triple gene-modified iDuo NK cells, iPSCs were multiplexed engineered as a one-time event and clonally derived to produce a uniformly engineered population of cells containing a NK cell calibrated CAR for targeting CD19, IL-15RF for enhanced NK cell persistence without the need for exogenous cytokine support, and an hnCD16 for ADCC when combined with a therapeutic mAb. The master multiplexed-engineered iPSC line uniformly incorporating three functional elements was used to continuously produce iDuo NK cells without the need for any further engineering or enrichment steps—thereby improving the consistency of product manufacture, reducing cost, and improving patient access.
Here, we report that triple gene-modified iDuo NK cells exhibit direct targeting of malignant B cell lines and primary CLL cells through a combination of natural cytotoxicity, anti-CD19 CAR signaling, and ADCC mediated by hnCD16 engagement with rituximab. In models of antigen escape, iDuo NK cells, but not activated PBNK cells, non-transduced iNK cells, anti-CD19 CAR iNK cells, or primary anti-CD19 CAR-T cells, were able to eliminate CD19 knockout AHR-77 B lymphoblast cells and Raji lymphoma cells when combined with rituximab, reflective of their unique capability to elicit a durable anti-tumor response. These in vitro results were recapitulated in xenogeneic adoptive transfer models where iDuo NK cells mediated rapid and durable antitumor function against Nalm6 B-ALL cells alone and against Raji lymphoma cells when combined with rituximab. Intriguingly, iDuo NK cells displayed considerably higher levels of specific killing of malignant B cells compared with healthy B cells. This feature may have considerable importance in the clinical setting. Taken together, the preclinical data presented here describe a novel multi-antigen targeting strategy that utilizes unique mechanisms of action to overcome tumor heterogeneity and antigen escape in a cell therapy that is available off-the-shelf and supports further testing of iDuo NK cells in a phase-I clinical trial setting to treat patients with B cell lymphoma (NCT04245722).
Conflict-of-interest disclosure: F.C. and J.S.M. are paid consultants for Fate Therapeutics and they receive research funds from this relationship. J.S.M. serves on the Scientific Advisory Board of OnkImmune, Nektar, Magenta, is a paid consultant consult for GT BioPharma and Vycellix (all unrelated to the content of this manuscript) and receives research funds from these relationships. J.P.G., R.B., S.M., S.G., R.A., B.G., A.W., G.B., J.H., T.D., T.T.L., and B.V. are employees of Fate Therapeutics. Fate Therapeutics owns patents (Methods and Compositions for Inducing Hematopoietic Cell Differentiation; patent no. 10 626 372) covering the iPSC-derived NK cells. The remaining authors declare no competing financial interests.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant R01 HL155150 (F.C.); NIH, National Cancer Institute grants P01 CA111412 (J.S.M.), P01 CA65493 (J.S.M.), R35 CA197292 (J.S.M.), and R01 CA203348 (B.W.); and Fate Therapeutics (J.S.M.).
Authorship
Contribution: F.C., J.P.G., R.B., B.W., B.V., and J.S.M. conceptualized the study and developed the methodology; J.P.G., S.G., S.M., R.A., B.G., A.W., H.W., K.T., B.K., G.B., J.H., T.D., and T.T.L. performed experiments; F.C., J.P.G., R.B., S.G., and Z.B.D. analyzed and interpreted data; F.C. drafted the paper; J.P.G., R.B., B.V., and J.S.M. reviewed and edited the paper; F.C., J.P.G., R.B., Z.B.D., and M.F. coordinated and managed experiments; and F.C., B.V., and J.S.M. supervised the study.
Footnotes
Requests for materials or protocols are available through emails to the corresponding authors.
Presented in oral form at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2019.
Presented in oral form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2021.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Contributor Information
Bahram Valamehr, Email: bob.valamehr@fatetherapeutics.com.
Jeffrey S. Miller, Email: mille011@umn.edu.
Supplementary Material
References
- 1.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maloney DG, Grillo-López AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15(10):3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 5.Davis TA, White CA, Grillo-López AJ, et al. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin’s lymphoma: results of a phase II trial of rituximab. J Clin Oncol. 1999;17(6):1851–1857. doi: 10.1200/JCO.1999.17.6.1851. [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18(17):3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Foran JM, Norton AJ, Micallef IN, et al. Loss of CD20 expression following treatment with rituximab (chimaeric monoclonal anti-CD20): a retrospective cohort analysis. Br J Haematol. 2001;114(4):881–883. doi: 10.1046/j.1365-2141.2001.03019.x. [DOI] [PubMed] [Google Scholar]
- 9.Bellesso M, Xavier FD, Costa RO, et al. Disease progression after R-CHOP treatment associated with the loss of CD20 antigen expression. Rev Bras Hematol Hemoter. 2011;33(2):148–150. doi: 10.5581/1516-8484.20110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalabi H, Kraft IL, Wang HW, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103(5):e215–e218. doi: 10.3324/haematol.2017.183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Edberg JC, Redecha PB, et al. A novel polymorphism of FcγRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100(5):1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing Y, Ni Z, Wu J, et al. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu H, Blum RH, Bjordahl R, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135(6):399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woan KV, Kim H, Bjordahl R, et al. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell. 2021;28(12):2062–2075.e5. doi: 10.1016/j.stem.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valamehr B, Robinson M, Abujarour R, et al. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Reports. 2014;2(3):366–381. doi: 10.1016/j.stemcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cichocki F, Bjordahl R, Gaidarova S, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med. 2020;12(568):eaaz5618. doi: 10.1126/scitranslmed.aaz5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valamehr B, Abujarour R, Robinson M, et al. A novel platform to enable the high-throughput derivation and characterization of feeder-free human iPSCs. Sci Rep. 2012;2(1):213. doi: 10.1038/srep00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsutsui H, Valamehr B, Hindoyan A, et al. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat Commun. 2011;2(1):167. doi: 10.1038/ncomms1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192.e5. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143–155. doi: 10.1182/bloodadvances.2020002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maloney DG. Mechanism of action of rituximab. Anticancer Drugs. 2001;12(suppl 2):S1–S4. [PubMed] [Google Scholar]
- 24.Leemhuis T, Wells S, Scheffold C, et al. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11(3):181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Berdeja JG, Hess A, Lucas DM, et al. Systemic interleukin-2 and adoptive transfer of lymphokine-activated killer cells improves antibody-dependent cellular cytotoxicity in patients with relapsed B-cell lymphoma treated with rituximab. Clin Cancer Res. 2007;13(8):2392–2399. doi: 10.1158/1078-0432.CCR-06-1860. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Lim O, Kim TM, et al. Phase I study of random healthy donor-derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res. 2016;4(3):215–224. doi: 10.1158/2326-6066.CIR-15-0118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.