Abstract
The chemical composition and aroma profile of industrial essential oils (EOs) from species of rose grown in China, including the native Kushui rose (R. sertata × R. rugosa) and R. rugosa Thunb. cv. Plena, and the recently introduced Damask rose (R. damascena Mill.), were studied in comparison by means of GC/MS and GC-FID. More than 150 individual compounds were detected in Chinese rose samples, of which 112 were identified and their quantitative content determined, representing 88.7%, 96.7% and 97.9% of the total EO content, respectively. It was found that the main constituents of the Chinese rose EOs were representatives of terpenoid compounds (mono- and sesquiterpenoids, predominantly) and aliphatic hydrocarbons. Comparative chemical profiling revealed different chemical composition and aroma profiles: while the R. damascena oil showed a balance between the eleoptene and stearoptene fractions of the oil (the average ratio between the main terpene alcohols and paraffins was 2.65), in the Kushui and R. rugosa oils, the odorous liquid phase strongly dominated over the stearopten, with a ratio of 16.91 and 41.43, respectively. The most abundant terpene was citronellol, ranging from 36.69% in R. damascena to 48.32% in R. rugosa oil. In addition, the citronellol enantiomers distribution, which is an important marker for rose oil authenticity, was studied for the first time in R. rugosa oil.
Keywords: R. damascena Mill., R. rugosa, Kushui rose, oil-bearing roses, gas chromatography-mass spectrometry, chemical profiling, citronellol enantiomers separation
1. Introduction
The genus Rosa L. (Rosaceae) comprises 200 species with more than 18,000 cultivars naturally spread throughout the temperate and subtropical regions of the Northern Hemisphere and 95 of them have been found in China [1]. The plant is highly valued for its decorative features, but also has high economic importance due to the essential oil in its petals. Only a few rose species have found industrial-scale applications for their fragrance and flavoring properties: Rosa damascena Mill. var. trigintipetala Dieck., Rosa gallica L., Rosa centifolia L. and Rosa alba L. [2], widely used for the production of rose oil, rose water, absolute and concrete. The most important rose species from a commercial point of view, cultivated for rose oil production, is R. damascena Miller. It is considered superior in terms of essential oil quality and is produced in Bulgaria, Turkey, Iran, Saudi Arabia and India. Rose oil is an important raw material in the flavor and fragrance industry, but it is also widely used in cosmetology, clinical aromatherapy, and medicine due to its benefits to human health. Rose species have a wide spectrum of bio-pharmacological activities such as antidepressant, hypoglycemic, cardiovascular, anti-inflammatory, analgesic, antioxidant and antimicrobial effects [3,4,5,6,7,8].
Roses have been grown in East Asia for thousands of years. The most ancient local species are Rosa chinensis, Rosa multiflora and Rosa rugosa Thunb. [1,9,10]. R. rugosa is an oil-bearing plant with a small number of flower petals, but as a result of the long-term work of Chinese breeders, cultivars with multi-petal blossoms and a high content of essential oil were developed. The most cultivated species is R. rugosa Thunb. cv. plena [1,11], widespread in the eastern provinces, with a planting area more than 4000 hectares. It is known as the Pingyin rose (named after Pingyin county in Jinan, East China’s Shandong province) and has been used as material for industrial rose oil since the beginning of the XXth century. The flowers are large, with thick petals that are violet-pink in color. Its odor is strong and sweet.
Second in importance, but even more famous, is the so-called Kushui rose, which is grown in the central provinces of China (Gansu). It is authorized as a natural hybrid of R. sertata × R. rugosa, but without any direct genetic evidence [1,12]. Its essential oil is highly valued and its annual production averages 600–700 kg [13]. The limited variation in its major components are defined by national and international standards [10,14]. It is worth mentioning that Kushui rose oil is the second rose oil, after R. damascena oil, with an ISO standard for quality and authenticity [14].
With the country’s century-old traditions in rose cultivation, in recent decades, China has started to develop its own rose oil production based on R. damascena [2,10] by introducing the species into provinces with suitable climatic conditions [15,16]. Due to the large-scale development of production technology, the country has earned its place in the world market as one of the producers of R. damascena oil [17].
Although its botanical origin (i.e., rose species genotype) is the main factor determining the quantitative and qualitative characteristics of rose essential oil and its aromatic products, there are many other factors affecting the chemical compositions and quality of the final product: geographical origin (climatic and soil conditions), flower processing technology (storage conditions and production methods) [18], local traditions and even the analytical techniques used are all of significant importance [2,19,20,21,22,23].
Feng et al. studied the chemical composition of R. rugosa Thunb. flowers in different developmental stages [11]. Fifty-three compounds were identified in the flower-bud stage, sixty-five at the early opening stage, sixty-two during half opening, sixty-five at full opening, and fifty-eight at the end of the full opening stage. The main aroma constituents were already formed during the early opening stage, with the majority of them reaching peak concentration between the half and full opening stages, when they should be picked [11,24]. Wua et al. [25] reported that salt intervention during flower processing increases the essential oil yield by 56.52% and the citronellol content by 31.90%, while the geraniol content decreases by 16.34%.
An analysis of the aroma constituents present in the Chinese Kushui-type rose essential oil by GC-FID and GC-MS was performed using three types of capillary columns with different polarities. The main constituents, as determined by GC-FID, were β-citronellol (41.6~46.7%), geraniol (9.7~11.0%), and nerol (3.4~4.5%). In addition, a comparative analysis was conducted using Bulgarian rose (R. damascena Miller) oil and perfume; Bulgarian rose oil showed a richer profile of characteristic aroma constituents than the Chinese Kushui-type essential oil [26]. Double molecular distillation was used to separate different fractions from Kushui rose oil and to evaluate the antioxidant and antimicrobial activity. Citronellol and nerol were considered to be the characteristic aroma components with differences of 3.57–34.9% and 0.29–5.99%, respectively, between the fractions [18].
A comparative analysis of the headspace volatiles of Chinese R. rugosa was performed, in which volatile fractions of 23 rose germplasms were isolated and up to 33 volatile compounds were identified, including 2-phenylethanol, β-citronellol, ethanol, and n-hexane [27]. The chemical composition of R. rugosa Thunb. var. plena Regal oil was studied by Ueyama et al. [23], revealing high citronellol content (60%).
Solid-phase micro extraction (SPME)-headspace analysis with olfactometric evaluation revealed the compounds responsible for the characteristic odor of the Chineese R. damascena oil and the changes in its composition after application on human skin [19].
The key aroma compounds of industrial oils from R. damascena, R. centifolia, R. alba, R. rugosa cv. ‘Plena’ and Rosa xanthina Lindl were studied using gas chromatography–olfactometry (GC–O), gas chromatography–mass spectrometry (GC–MS) and quantitative descriptive analysis (QDA) [28]. Chinese rose concrete was steam-distilled to essential oil and its composition was compared with the rose absolutes from Bulgaria, France and Japanese R. rugosa Thunb [20].
Although there are some data on the chemical composition and olfactometric characteristics of the essential oils from various rose species grown in China, comprehensive chemical profiling of industrial-type essential oils from the main oil-bearing roses has not been performed up to now. It is worth underlining that no data on the enantiomer distribution of the important constituents of rose oil, such as citronellol, were found in the literature.
Therefore, the main aim of the current study was to perform a comparative chemical profiling of essential oils from the main oil-bearing rose species with industrial importance produced in China, revealing new data on the chemical composition and chirality of some important aroma profile components.
2. Results and Discussion
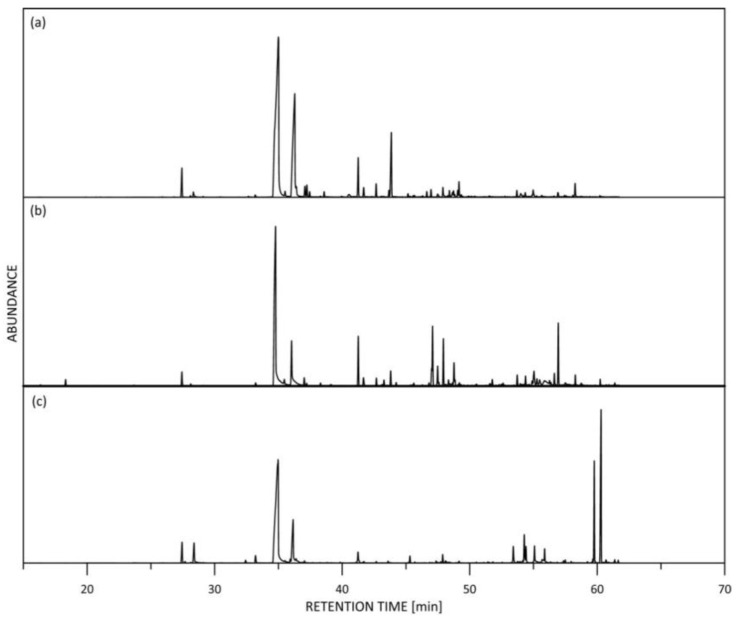
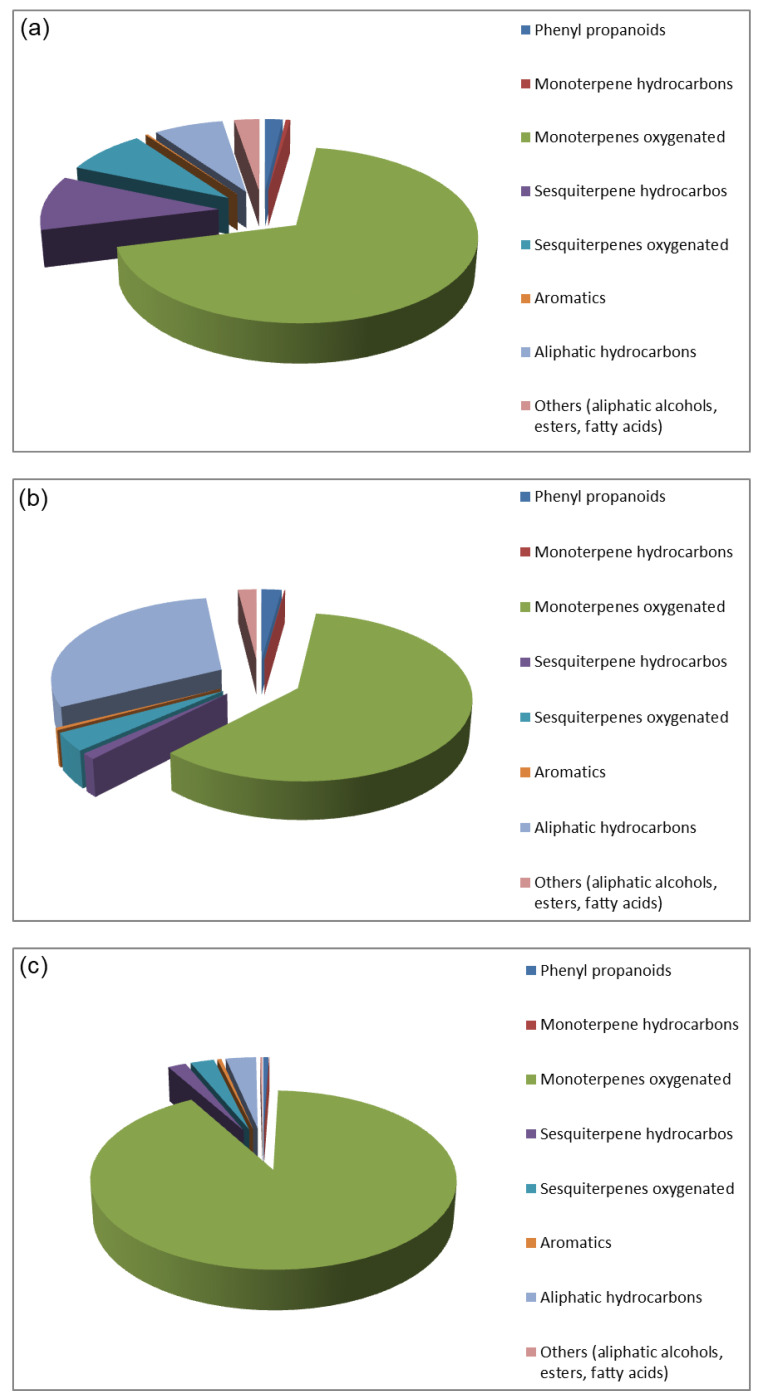
Rose oil is a very complex mixture containing compounds with high structural diversity. More than 150 individual compounds were detected in Chinese rose essential oil samples, 112 of which were identified by GC/MS, and their quantitative content was determined by GC-FID, representing 96.74–99.40% of the total essential oil content in D1-D4 samples, 88.74% in KS- and 96.71% in R-sample. Quantitative data for the components of the Chinese rose oil samples, as determined by GC-FID, with concentrations higher than 0.01%, are summarized in Table 1. As seen from the data in Table 1, the main constituents of the Chinese rose oil samples are representatives of the terpenoids compounds (mono- and sesquiterpenoids, predominantly) and aliphatic hydrocarbons. The representative GC/MS chromatograms in the total ion current (TIC) mode of the samples are shown in Figure 1. The clearly distinguished chromatographic fingerprint of the Chinese rose oil samples can be seen. The distribution in the main chemical classes in the samples is presented in Figure 2.
Table 1.
Chemical composition of the rose oils samples, as determined by GC/MS/FID on a DB-5ms column.
| No | Compounds | Formula | Mw | RIref
1 (DB5) |
RIexp (DB5) |
Rel.%, as Determined by GC-FID | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | KS | R | ||||||
| 1. | Ethanol | C2H6O | 46 | 459 | n.d. | 0.03 | 0.03 | 0.02 | 0.04 | 0.09 | 0.01 |
| 2. | β-Pinene | C10H16 | 136 | 974 | 981 | n.d. 2 | n.d. | n.d. | n.d. | 0.08 | 0.01 |
| 3. | β-Myrcene | C10H16 | 136 | 988 | 987 | 0.1 | tr. 3 | 0.01 | tr. | 0.03 | 0.02 |
| 4. | Furane, 2-pentyl- | C9H14O | 138 | 984 | 988 | 0.1 | tr. | n.d. | n.d. | 0.02 | 0.01 |
| 5. | p-Cymene | C10H14 | 134 | 1022 | 1027 | 0.1 | tr. | tr. | n.d. | 0.02 | tr. |
| 6. | Limonene | C10H16 | 136 | 1024 | 1032 | 0.03 | 0.04 | tr. | tr. | 0.05 | tr. |
| 7. | Benzyl alcohol | C7H8O | 108 | 1026 | 1033 | 0.04 | 0.02 | 0.05 | 0.04 | n.d. | n.d. |
| 8. | α-Ocimene | C10H16 | 136 | 1032 | 1037 | n.d. | n.d. | n.d. | n.d. | 0.11 | n.d. |
| 9. | β-Ocimene | C10H16 | 136 | 1044 | 1045 | n.d. | n.d. | n.d. | n.d. | 0.07 | 0.01 |
| 10. | Linalool oxide | C10H18O2 | 213 | 1067 | 1073 | 0.04 | 0.04 | 0.03 | 0.03 | 0.03 | 0.06 |
| 11. | α-Terpinolene | C10H16 | 136 | 1086 | 1089 | 0.02 | 0.01 | 0.01 | 0.01 | 0.06 | n.d. |
| 12. | Linalool | C10H18O | 154 | 1095 | 1099 | 2.13 | 1.98 | 2.02 | 2.06 | 1.49 | 1.98 |
| 13. | Nonanal | C9H18O | 142 | 1100 | 1104 | 0.17 | 0.16 | 0.16 | 0.16 | 0.06 | n.d. |
| 14. | cis-Rose oxide | C10H18O | 154 | 1106 | 1112 | 0.09 | 0.06 | 0.07 | 0.07 | 0.19 | 0.11 |
| 15. | Phenyl ethyl alcohol | C8H10O | 122 | 1106 | 1116 | 2.13 | 2.07 | 2.09 | 2.13 | 0.05 | 0.47 |
| 16. | trans-Rose oxide | C10H18O | 154 | 1122 | 1129 | 0.04 | 0.05 | 0.04 | 0.04 | 0.07 | 0.06 |
| 17. | Nerol oxide | C10H16O | 152 | 1154 | 1153 | 0.05 | 0.03 | 0.04 | 0.03 | 0.07 | 0.04 |
| 18. | Camphor | C10H16O | 152 | 1141 | 1155 | 0.02 | 0.02 | 0.02 | 0.02 | tr. | 0.01 |
| 19. | 4-Terpineol | C10H18O | 154 | 1174 | 1191 | 0.22 | 0.21 | 0.22 | 0.22 | 0.03 | 0.08 |
| 20. | α-Terpineol | C10H18O | 154 | 1200 | 1200 | 0.63 | 0.61 | 0.62 | 0.63 | 0.36 | 0.16 |
| 21. | α-Ionene | C13H18 | 174 | 1255 | 1220 | 0.04 | tr. | tr. | tr. | tr. | 0.04 |
| 22. | p-Menth-1-en-9-al | C10H16O | 152 | 1232 | 1224 | 0.07 | 0.03 | 0.03 | n.d. | n.d. | 0.02 |
| 23. | Citronellol | C10H20O | 156 | 1223 | 1232 | 39.01 | 38.51 | 36.69 | 39.09 | 39.51 | 48.32 |
| 24. | Nerol 4 | C10H18O | 154 | 1227 | n.d. | 6.35 | 6.77 | 5.94 | 6.36 | 2.97 | 5.97 |
| 25. | Citronellal | C10H18O | 154 | 1165 | 1241 | 0.26 | 0.49 | 0.34 | 0.46 | 1.40 | 0.42 |
| 26. | Neral | C10H16O | 152 | 1235 | 1242 | 0.42 | 0.38 | 0.48 | 0.27 | tr. | tr. |
| 27. | Geraniol | C10H18O | 154 | 1249 | 1249 | 7.55 | 7.59 | 7.64 | 7.88 | 8.66 | 19.88 |
| 28. | Linalyl acetate | C12H20O2 | 196 | 1254 | 1253 | 0.28 | 0.37 | 0.36 | 0.30 | n.d. | n.d. |
| 29. | Phenyl ethyl acetate | C10H12O | 164 | 1258 | 1258 | 0.46 | 0.78 | 1.00 | 0.99 | 053 | 1.78 |
| 30. | Geranial | C10H16O | 152 | 1264 | 1269 | 0.19 | 0.19 | 0.20 | 0.18 | 0.91 | 0.76 |
| 31. | Citronellyl formate | C11H20O2 | 184 | 1271 | 1273 | 0.04 | 0.02 | 0.03 | 0.03 | 0.21 | 0.55 |
| 32. | Neryl formate | C11H18O2 | 182 | 1280 | 1281 | 0.03 | 0.02 | 0.03 | 0.03 | tr. | 0.25 |
| 33. | trans-Anethole | C10H12O | 148 | 1282 | 1291 | 0.03 | 0.02 | 0.02 | 0.02 | tr. | 0.06 |
| 34. | 2-Undecanone | C11H22O | 170 | 1293 | 1292 | 0.01 | 0.01 | 0.01 | 0.01 | 0.27 | tr. |
| 35. | Geranyl formate | C11H18O2 | 182 | 1298 | 1297 | 0.10 | 0.13 | 0.13 | 0.12 | tr. | 0.29 |
| 36. | Citronellyc acid | C12H22O2 | 198 | 1312 | 1302 | 0.14 | tr. | tr. | 0.14 | tr. | tr. |
| 37. | 2-Methylnaphthalene | C11H10 | 142 | 1310 | 1316 | tr. | 0.02 | 0.02 | 0.02 | 0.42 | n.d. |
| 38. | Methyl geranate | C10H16O2 | 168 | 1322 | 1322 | 0.06 | 0.06 | 0.06 | 0.06 | 0.07 | 0.01 |
| 39. | Methylnaphthalene (isomer) | C11H10 | 142 | 1318 | 1324 | tr. | 0.01 | 0.01 | tr. | 0.03 | 0.11 |
| 40. | Citronellyl acetate + Geranic acid 5 | C12H22O2/C10H16O2 | 198/168 | 1350 | 1350 | 1.32 | 1.00 | 1.24 | 1.29 | 3.03 | 1.76 |
| 41. | Eugenol + Neryl acetate | C10H12O2/C12H20O2 | 164/196 | 1356 | 1358 | 0.15 | 0.14 | 0.15 | 0.14 | 0.74 | 0.63 |
| 42. | Geranyl acetate | C12H20O2 | 196 | 1379 | 1378 | 0.11 | 0.11 | 0.11 | 0.11 | 0.58 | 0.66 |
| 43. | α-Copaene | C15H24 | 204 | 1374 | 1381 | 0.02 | 0.02 | 0.02 | 0.02 | 0.13 | 0.06 |
| 44. | Daucene | C15H24 | 204 | 1380 | 1390 | 0.01 | 0.01 | 0.01 | 0.01 | 0.34 | tr. |
| 45. | 2-Dodecanone | C12H24O | 184 | 1388 | 1394 | n.d. | n.d. | n.d | 0.01 | 0.07 | tr. |
| 46. | β-Bourbonene | C15H24 | 204 | 1388 | 1396 | 0.11 | 0.12 | 0.12 | 0.11 | tr. | tr. |
| 47. | β-Elemene | C15H24 | 204 | 1389 | 1398 | tr. | tr. | tr. | tr. | tr. | 0.28 |
| 48. | Methyl eugenol | C11H14O2 | 178 | 1403 | 1402 | 0.03 | 0.03 | 0.03 | 0.03 | 0.81 | 3.11 |
| 49. | α-Bisabolene | C15H24 | 204 | 1500 | 1422 | tr. | tr. | tr. | 0.01 | 0.04 | 0.02 |
| 50. | β-Gurjunene | C15H24 | 204 | 1431 | 1431 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.16 |
| 51. | trans-β-Caryophyllene | C15H24 | 204 | 1455 | 1435 | 0.41 | 0.39 | 0.39 | 0.38 | 0.07 | 0.05 |
| 52. | α-Bergamotene | C15H24 | 204 | 1432 | 1442 | tr. | tr. | tr. | tr. | tr. | 0.10 |
| 53. | Germacrene D | C15H24 | 204 | 1451 | 1444 | 0.06 | 0.07 | 0.06 | 0.06 | n.d. | 0.08 |
| 54. | Neryl acetone | C13H22O | 194 | 1455 | 1450 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 |
| 55. | α-Humulene | C15H22 | 204 | 1464 | 1470 | 0.04 | 0.04 | 0.04 | 0.04 | 0.19 | tr. |
| 56. | γ-Muurolene | C15H24 | 204 | 1478 | 1475 | 0.04 | 0.04 | 0.04 | 0.04 | 0.81 | 0.30 |
| 57. | γ-Cadinene | C15H24 | 204 | 1513 | 1476 | tr. | tr. | tr. | tr. | 3.35 | tr. |
| 58. | Pentadecene | C15H32 | 212 | n.d. | 1483 | 0.12 | 0.12 | 0.13 | 0.12 | n.d. | n.d. |
| 59. | α-Amorphene | C15H24 | 204 | 1483 | 1486 | 0.05 | 0.05 | 0.05 | 0.05 | 1.14 | 0.15 |
| 60. | ar-Curcumene | C15H22 | 204 | 1479 | 1488 | n.d. | n.d. | n.d. | n.d. | 0.18 | n.d. |
| 61. | Phenyl ethyl 2-methyl butanoate | C13H18O2 | 206 | 1490 | 1491 | 0.07 | 0.07 | 0.07 | 0.06 | 0.04 | 0.02 |
| 62. | epi-Cubebol | C15H24O | 220 | 1494 | 1495 | 0.46 | 0.43 | 0.44 | 0.43 | 2.70 | 0.49 |
| 63. | 2-Tridecanone | C13H26O | 198 | 1496 | 1496 | 0.02 | 0.02 | 0.02 | tr. | tr. | 0.06 |
| 64. | Pentadecane | C15H32 | 212 | 1500 | 1500 | 0.07 | 0.13 | 0.13 | 0.12 | 0.12 | 0.04 |
| 65. | Benzyl tiglate | C12H14O2 | 190 | 1497 | 1502 | 0.05 | 0.07 | 0.07 | 0.07 | 0.04 | 0.06 |
| 66. | α-Farnesene | C15H24 | 204 | 1505 | 1506 | 0.05 | 0.05 | 0.06 | 0.05 | 0.61 | 0.06 |
| 67. | γ-Muurolene | C15H24 | 204 | 1478 | 1509 | 0.06 | 0.06 | 0.06 | 0.05 | 0.35 | 0.27 |
| 68. | γ-Bisabolene | C15H24 | 204 | 1515 | 1515 | 0.02 | 0.02 | 0.02 | 0.02 | 0.26 | tr. |
| 69. | iso-Germacrene D | C16H26 | 218 | n.d. | 1516 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.29 |
| 70. | Dauca-8,11-diene (Isodaucene) | C15H24 | 204 | 1500 | 1519 | n.d. | n.d. | n.d. | n.d. | 1.26 | 0.24 |
| 71. | α-Agarofuran | C15H24O | 220 | 1548 | 1522 | 0.03 | 0.02 | 0.02 | 0.02 | 0.28 | 0.10 |
| 72. | α-Muurolene | C15H24 | 204 | 1500 | 1526 | 0.08 | 0.08 | 0.03 | 0.03 | 0.06 | 0.30 |
| 73. | δ-Cadinene | C15H24 | 204 | 1522 | 1529 | 0.01 | 0.09 | 0.09 | 0.08 | 0.15 | 0.52 |
| 74. | Nerolidol | C15H26O | 222 | 1561 | 1565 | 0.06 | 0.07 | 0.07 | 0.07 | 0.22 | 0.04 |
| 75. | Hexadecene | C16H32 | 224 | n.d. | 1581 | 0.04 | 0.04 | 0.04 | 0.04 | 0.02 | 0.02 |
| 76. | Phenyl ethyl tiglate | C13H16O2 | 204 | 1584 | 1590 | 0.05 | 0.05 | 0.05 | 0.03 | 0.05 | |
| 77. | Ethyl laurate | C14H28O2 | 228 | 1594 | 1592 | 0.05 | 0.05 | 0.05 | 0.03 | 0.22 | 0.12 |
| 78. | Aromadendrene epoxide | C15H24O | 220 | 1639 | 1599 | 0.08 | 0.08 | 0.08 | 0.08 | 0.44 | 0.05 |
| 79. | α-Cubenol | C15H26O | 222 | 1645 | 1644 | 0.06 | 0.05 | 0.05 | 0.05 | tr. | 0.05 |
| 80. | α-Eugesmol | C15H26O | 222 | 1649 | 1650 | 0.93 | 0.92 | 0.93 | 0.94 | 0.06 | tr. |
| 81. | Caryophylla-4(12),8(13)-dien-5α-ol | C15H24O | 220 | 1639 | 1659 | 0.14 | 0.15 | 0.16 | 0.14 | 0.60 | 0.31 |
| 82. | α-Copaene-11-ol | C15H24O | 220 | n.d. | 1666 | 0.07 | 0.06 | 0.08 | 0.07 | 0.19 | 0.07 |
| 83. | τ-Muurolol | C15H26O | 222 | 1640 | 1672 | 0.13 | 0.15 | 0.14 | 0.13 | 0.14 | 0.10 |
| 84. | Bisabolol oxide | C15H26O2 | 238 | 1656 | 1671 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.22 |
| 85. | α-Eudesmol | C15H26O | 222 | 1652 | 1676 | 1.44 | 1.43 | 1.42 | 1.46 | 0.10 | tr. |
| 86. | Ledene oxide + Heptadecene | C15H24O/C17H34 | 220/238 | n.d. | 1680 | 0.81 | 0.84 | 0.83 | 0.81 | 0.61 | tr. |
| 87. | α-Bisabolol | C15H26O | 222 | 1701 | 1698 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.48 |
| 88. | 2-Pentadecanone + Heptadecane | C15H30O/C17H36 | 226/240 | 1700 | 1700 | 1.59 | 1.67 | 1.66 | 1.66 | 1.44 | tr. |
| 89. | Nootkatol | C15H22O | 218 | 1714 | 1707 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.08 |
| 90. | trans-β-Farnesol | C15H26O | 222 | 1714 | 1722 | 0.51 | 0.57 | 0.60 | 0.60 | 1.76 | 0.05 |
| 91. | Heptadecadiene | C17H32 | 236 | n.d. | 1729 | 0.98 | 0.93 | 0.92 | 0.93 | n.d. | n.d. |
| 92. | Farnesal | C15H24O | 220 | 1715 | 1745 | 0.07 | 0.08 | 0.08 | 0.07 | 0.36 | tr. |
| 93. | Ylangenal | C15H22O | 218 | 1764 | 1765 | n.d. | n.d. | n.d. | n.d. | 2.96 | tr. |
| 94. | Octadecene | C18H36 | 252 | n.d. | 1779 | 0.11 | 0.11 | 0.11 | 0.11 | 0.06 | 0.04 |
| 95. | Cinnamaldehyde, 3,4-dimethoxy- | C11H12O3 | 192 | 1790 | 1782 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.13 |
| 96. | Benzyl benzoate | C14H12O2 | 212 | 1759 | 1772 | 0.13 | 0.12 | 0.13 | 0.13 | 0.15 | 0.11 |
| 97. | Ethyl myristate | C16H32O | 256 | 1795 | 1792 | 0.02 | 0.02 | 0.02 | 0.02 | 0.11 | n.d. |
| 98. | Octadecane | C18H38 | 254 | 1800 | 1800 | 0.09 | 0.10 | 0.09 | 0.09 | n.d. | tr. |
| 99. | Germazone | C15H22O | 218 | 1746 | 1814 | 0.02 | 0.01 | 0.01 | 0.01 | 0.53 | 0.74 |
| 100. | Phenyl ethyl octanoate | C16H24O2 | 248 | 1847 | 1856 | 0.13 | 0.12 | 0.13 | 0.14 | n.d. | n.d. |
| 101. | Nonadecene | C19H38 | 266 | n.d. | 1879 | 4.51 | 4.28 | 4.27 | 4.38 | 0.34 | 0.03 |
| 102. | n-Nonadecane | C19H40 | 268 | 1900 | 1900 | 11.82 | 11.08 | 10.94 | 10.87 | tr. | tr. |
| 103. | Eicosene | C20H42 | 282 | n.d. | 1976 | 0.11 | 0.12 | 0.12 | 0.11 | tr. | 0.02 |
| 104. | Eicosane | C20H42 | 282 | 2000 | 2000 | 0.84 | 0.80 | 0.79 | 0.80 | 0.11 | 0.02 |
| 105. | Geranic acid, 2-phenyl ethyl ester | C10H16O2 | 168 | 2081 | 2056 | 0.10 | 0.02 | 0.11 | 0.09 | n.d. | 0.08 |
| 106. | Heneicosane | C21H44 | 296 | 2100 | 2100 | 7.05 | 6.71 | 6.56 | 5.63 | 1.49 | 0.49 |
| 107. | Docosane | C22H46 | 310 | 2200 | 2200 | 0.27 | 0.25 | 0.20 | 0.25 | 0.08 | 0.08 |
| 108. | Tricosene | C23H46 | 322 | n.d. | 2289 | 0.02 | 0.02 | 0.04 | 0.02 | 0.31 | 0.09 |
| 109. | Tricosane | C23H48 | 324 | 2300 | 2300 | 2.33 | 2.21 | 1.67 | 2.10 | 1.34 | 1.30 |
| 110. | Tetracosane | C24H50 | 338 | 2400 | 2400 | 0.12 | 0.11 | 0.14 | 0.11 | 0.07 | 0.09 |
| 111. | Pentacosane | C25H52 | 352 | 2500 | 2500 | 0.43 | 0.41 | 0.31 | 0.39 | 0.21 | 0.46 |
| 112. | Heptacosane | C27H56 | 380 | 2700 | 2700 | 0.18 | 0.18 | 0.13 | 0.17 | 0.03 | 0.12 |
| Total Identified compounds | 99.40 | 96.74 | 96.81 | 98.78 | 88.74 | 96.71 | |||||
| Aliphatic hydrocarbons | 31.49 | 30.11 | 29.08 | 28.71 | 6.23 | 2.80 | |||||
| Aliphatics (alcohols, esters, fatty acids) | 1.89 | 1.96 | 1.94 | 1.93 | 2.26 | 0.19 | |||||
| Monoterpene hydrocarbons | 0.25 | 0.05 | 0.02 | 0.01 | 0.42 | 0.04 | |||||
| Monoterpenes oxygenated | 58.84 | 57.64 | 58.38 | 60.82 | 59.58 | 81.39 | |||||
| Aromatic-compounds | 0.44 | 0.42 | 0.47 | 0.46 | 0.22 | 0.38 | |||||
| Sesquiterpenes hydrocarbons | 1.23 | 1.20 | 1.20 | 1.19 | 8.98 | 1.69 | |||||
| Sesquiterpenes oxygenated | 3.51 | 3.57 | 3.62 | 3.62 | 7.36 | 2.19 | |||||
| Phenylpropanoids | 2.34 | 2.26 | 2.29 | 2.32 | 1.60 | 4.27 | |||||
Remarks: 1—according to NIST Chemistry WebBook, SRD 69 (https://webbook.nist.gov (accessed on 5 January 2023)); 2 n.d.—not detected; 3 tr.—traces <0.01%; 4—as determined on DB-17HT column; 5—co-eluting components.
Figure 1.
GC/MS (TIC) chromatograms of the Chinese RO samples: (a) R. rugosa; (b) Kushui rose; (c) R. damascena.
Figure 2.
Main chemical classes distribution of the Chinese rose oil samples: (a) Kushui rose, (b) R. damascena, (c) R. rugosa.
2.1. Terpenes
Terpenes and terpenoids are the main biosynthetic building blocks and important mediators of ecological interactions in plants. Of all the terpenoids, the mono- and sesquiterpenes are the main constituents of the EOs and are most frequently studied due to their abundance.
2.1.1. Monoterpenes
Alcohols. As seen from Table 1, the most abundant class of aroma compounds found in the Chinese rose oils are monoterpene alcohols. β-citronellol, determining the basic rosaceous character of the rose oil, was found in a concentration range of 36.69–39.09% in Damask rose, 37.61% in Kushui rose, and the highest content in R. rugosa oil at 48.32%. These results are in agreement with the literature data, defining R. rugosa oil produced in China as a high citronellol type [6,26,29]. Similar results were also reported for Kushui rose oil at 39.5% [25], while the citronellol content in industrial R. damascena EO was found in a lower concentration in the range 5.04–18.87%, which can be attributed to the ecological conditions [21] or the local production technology features [19].
Geraniol was observed in the range 7.55–8.78% in D1-D4 and KS samples, and in a much higher concentration at 19.88% in R samples, which is in agreement with other data [15,21].
Various pharmacological activities are reported for these two main terpene alcohols, such as antispasmodic, anti-inflammatory, antibacterial and antifungal effect (citronellol), and insecticidal, repellent, acaricidal, antibacterial, and antifungal activity (geraniol) [5].
The concentration of nerol did not show significant differences between R. damascena and R. rugosa samples (5.94–6.77%), while in Kushui rose oil it was only 2.97%.
These three terpene alcohols are responsible for the specific odor of the rose oil: citronellol with a pleasant rose-like odor, geraniol with a flowery rose-like odor (different from that of citronellol), and nerol with a pleasant rose-like odor (different from that of geraniol). The observed high concentration of these important terpene alcohols in Chinese rose EOs reveals their olfactory and health-beneficial potential.
Other monoterpene alcohols found in the Chinese rose oils were linalool at a concentration between 1.49% (KS) and 2.13% (D1), and terpinen-4-ol and α-terpineol, which have been observed in a relatively constant concentration in D1-D4 samples (0.22% and 0.62%, respectively). 4-Terpineol was observed in tr. amount (<0.1%) in KS and R samples.
Oxides. Rose oxide has four isomers, of which the (-)-cis isomer is the most desirable for its fragrance properties. Cis- rose oxide was found in Chinese rose oils in low amounts, with the highest concentration in KS (0.19%) and R (0.11%), and its content complies with the requirements of the ISO 25157:2013 standard (<0.5%) [14]. Trans-rose oxide and nerol oxide was found in trace amounts (<0.1%).
Aldehides. Among the acyclic monoterpene aldehydes, citronellal and citrals are the key aroma compounds in many Eos, with an odor reminiscent of lemon. In Chinese rose oils, citronellal was found in the highest concentration in the KS sample (1.42%).
Monoterpene hydrocarbons are present in very low, trace amounts in all samples, with the highest quantity in Kushui rose oil (0.42%).
2.1.2. Sesquiterpenes
Sesquiterpene hydrocarbons were most abundant in the KS sample (8.98%), with the main representatives γ-cadinene (3.35%), β-cubebene (2.70%), α-amorphene (1.14%), γ-muurolene (0.81%), etc., while the total amount of the sesquiterpene hydrocarbons in other samples were in the range 1.19–1.69%. Daucene (trans-dauca-4,8-diene) and isodaucene (dauca-8,11-diene) are sesquiterpene hydrocarbons that have not been reported in R. damascena oils. In this study, these compounds were found in amounts of 0.34% and 1.26% (KS) and in trace amounts of (<0.01%) and 0.24% (R), respectively, and could be used as marker compounds for the botanical origin of Kushui and R. rugosa oil. This observation is in agreement with the latest studies on Pinguin rose oil [6].
Oxygenated sesquiterpenes. The sesquiterpene alcohol trans-β-farnesol was found in the highest amount in KS (1.76%) in the range 0.51–0.60% (D1-D4), and in trace amounts in R-samples. -A- and β-eudesmols are other sesquiterpene alcohols typically observed in R. damascena oil. In this study, they were found in D1-D4 samples at a concentration of 0.93% and 1.44%, respectively, and only in trace amounts in KS and R samples.
The tricyclic sesquiterpene ketone germazone is another interesting compound previously reported in Geranium macrorrhizum L. [30] that had not been found until now in rose oils. It was observed in amounts of 0.53% in Kushui oil, 0.74% in R. rugosa oil and in trace amounts (0.1%) in D1-D4 samples.
2.2. Aliphatic hydrocarbons (Stearopten)
Aliphatic hydrocarbons (alkanes and alkenes), although odorless, play a significant role in rose oils as compounds responsible for odor stability. The content of heptadecane and nonadecene/nonadecane is considered to be of particular importance for the quality of R. damascena oil. In the Chinese rose oil samples, heptadecane was found in the range 1.44 (KS) and 1.60% (D1-D4 samples), while in R samples, it was detected only in trace amounts (<0.01%). It is interesting to mention that nonadecane/nonadecene was not observed in KS ad R samples. In general, the total amount of aliphatic hydrocarbons was found to be relatively low in Kushui and R. rugosa oils at 6.23% and 2.80%, respectively, in contrast with R. damascena oils (28.71–31.49%).
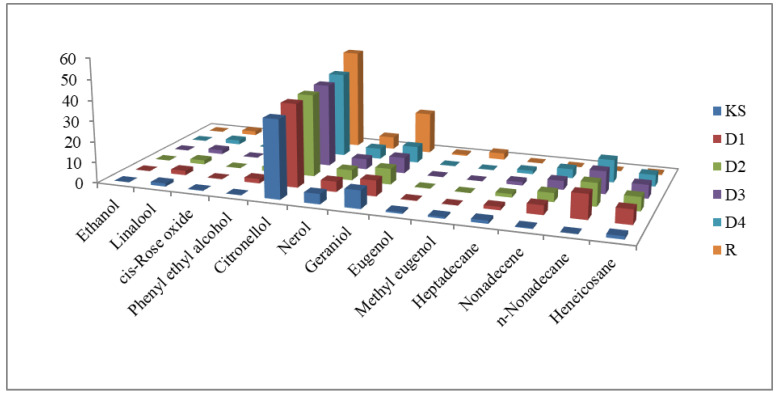
The most important components in rose oil, responsible for rose oil quality and authenticity, are regulated by the International Standard Organization (ISO). Table 2 and Figure 3 present quantitative data determined by GC-FID, together with the ISO 9842:2003 and ISO 25157:2013 specifications. The qualitative and quantitative analyses of the aroma constituents reveal that R. damascena EOs are, in general, not consistent with the International standard ISO 9842:2003 [31]: they do not fit into the parameters for the Bulgarian rose oil, but show similar characteristics to oils originating from Turkey and Morocco. The chemical profiles of Kushui and R. rugosa rose oils differ most significantly.
Table 2.
The most important Chinese rose oil components, together with the ISO 9842:2003 and ISO 25157:2013 specifications.
| No | Compounds | Rel.%, as Determined by GC-FID | ISO 9842:2003 1 | KS | ISO 25157:2013 2 | R | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | StDev(D) | ||||||
| 1. | Ethanol | 0.03 | 0.03 | 0.02 | 0.04 | 0.01 | ≤2.0 | 0.09 | 1.0–3.5 | Tr 3. |
| 2. | cis-Rose oxide | 0.09 | 0.06 | 0.07 | 0.07 | 0.01 | n.d. 4. | 0.19 | ≤0.5 | 0.11 |
| 3. | Linalool | 2.13 | 1.98 | 2.02 | 2.06 | 0.06 | ≤2.0 | 1.49 | 1.0–3.5 | 1.98 |
| 4. | Phenylethanol | 2.13 | 2.07 | 2.09 | 2.13 | 0.03 | ≤3.5 | 0.05 | ≤0.3 | 0.47 |
| 5. | Citronellol | 39.01 | 38.51 | 36.69 | 39.09 | 1.12 | 20.0–34.0 | 39.51 | 40.0–50.0 | 48.32 |
| 6. | Nerol | 6.35 | 6.77 | 5.94 | 6.36 | 0.34 | 5.0–12.0 | 2.97 | 2.0–5.5 | 5.97 |
| 7. | Geraniol | 7.55 | 7.59 | 7.64 | 7.88 | 0.15 | 15.0–22.0 | 8.78 | 6.0–18.0 | 19.88 |
| 8. | Geranyl acetate | 0.11 | 0.11 | 0.11 | 0.11 | 0.00 | -n.d. | 0.58 | 2.5–4.5 | 0.66 |
| 9. | Eugenol | 0.15 | 0.14 | 0.15 | 0.14 | 0.01 | ≤2.0 | 0.74 | n.d. | 0.63 |
| 10. | Methyl eugenol | 0.03 | 0.03 | 0.03 | 0.03 | 0.00 | ≤3.0 | 0.81 | 0.8–2.0 | 3.11 |
| 11. | Heptadecane 5 | 1.59 | 1.67 | 1.66 | 1.66 | 0.04 | 1.0–2.5 | 1.44 | n.d. | tr. |
| 12. | Farnesol | 0.51 | 0.57 | 0.60 | 0.60 | 0.04 | -n.d. | 1.76 | 2.0–3.5 | tr. |
| 13. | Nonadecene | 4.51 | 4.28 | 4.27 | 4.38 | 0.11 | -n.d. | 0.34 | n.d. | tr. |
| 14. | Nonadecane | 11.82 | 11.08 | 10.94 | 10.87 | 0.44 | 8.0–15.0 | n.d. | n.d. | tr. |
| 15. | Eicosane | 0.84 | 0.80 | 0.79 | 0.80 | 0.02 | n.d. | 0.12 | n.d. | tr. |
| 16. | Heneicosane | 7.05 | 6.71 | 6.56 | 5.63 | 0.61 | 3.0–5.5 | 1.49 | 0.6–2.0 | 0.49 |
| 17. | Tricosane | 2.33 | 2.21 | 1.67 | 2.10 | 0.29 | n.d. | 1.54 | 0.6–2.0 | 1.30 |
| 18. | Pentacosane | 0.33 | 0.46 | 0.39 | 0.38 | 0.05 | n.d. | 0.21 | n.d. | 0.46 |
| 19. | Heptacosane | 0.18 | 0.18 | 0.13 | 0.17 | 0.02 | n.d. | <0.05 | n.d. | 0.12 |
Figure 3.
The most important Chinese rose oil components, monitored by the ISO, as determined by GC-FID.
It is interesting to mention that the chemical composition of the KS and R samples do not fully comply with the ISO 25157:2013 standard for Kushui rose oil produced in China [14]. It is noteworthy that the document limits some minor components whose values vary in a narrow interval of concentration; for a natural product such as rose oil, this is difficult to achieve. Therefore, it can be assumed, in general, that the composition of the KS sample fits into the standard (with the exception of geranyl acetate). The other authors also note similar deviations, both for macro and minor constituents [18,25,26]. Regarding the chemical compositions of the R sample, our results are comparable with other investigations of the same genotype [6,23,29]. It is noteworthy that in all studies, the methyl eugenol content is above 3%, which is in agreement with our findings (3.11%) and can be attributed to the genotype specificity.
The relationship between the content of terpene alcohols and aliphatic hydrocarbons can also be easily traced through the standard. This ratio is an important indicator for the perfumery quality of rose oil. The ingredients of the odoriferous liquid part (eleoptene) determine the overall aroma character, while the odorless paraffins are responsible for its durability. In our study, the R. damascena oils revealed a total amount of main terpene alcohols citronellol + nerol + geraniol, on average, of 52.35%, and 19.41% for the main aliphatic hydrocarbons C17 + C19 + C21, with the ratio of 2.65. Compared with the ISO 9842:2003 data, this value is close to Bulgarian rose oil (2.91), but differs from the Turkish and Moroccan oils (with ratio of 4.34 and 3.42, respectively).
In contrast, a low paraffin content was observed in the Kushui and R. rugosa oil samples, demonstrating a terpene alcohols:paraffins ratio of 16.91 and 41.43, respectively (27.69 in the ISO 25157:2013 standard). This fact could be explained by the peculiarities of its blossoms and the thinner wax layers.
2.3. Enantiomers Distribution
It is worth noting that plants produce metabolites in many instances, such as chiral molecules, and enantiomers can differ from one species to another within the same genus. Although presenting the same physicochemical properties, except for their optical activity, enantiomers can exhibit divergent biological activities [32], including different aroma properties. Therefore, it is of crucial importance for essential oil quality and authenticity that the exact enantiomer distribution of important odor-bearing compounds are investigated. Citronellol exists in nature as two enantiomers, namely R-(+) citronellol and S-(–) citronellol. The (–) isomer is naturally presented in R. damascena and the citronellol enantiomer ratio in R. damascena oil is monitored by the European Pharmacopoeia [33], requiring excess S-(–) citronellol of >99% and R-(+) citronellol should be presented in amounts <1%.
According to our observations, D1-D4 and KS samples comply with these requirements.
An interesting hypothesis was drawn by Wu et al. [25], who investigated the influence of salt intervention on the composition, aroma, and antioxidant properties of Kushui rose oil: the authors reported increasing the R-(+) citronellol content when rose petals were processed by salinization, explaining this finding by the higher boiling point of the R-(+) citronellol.
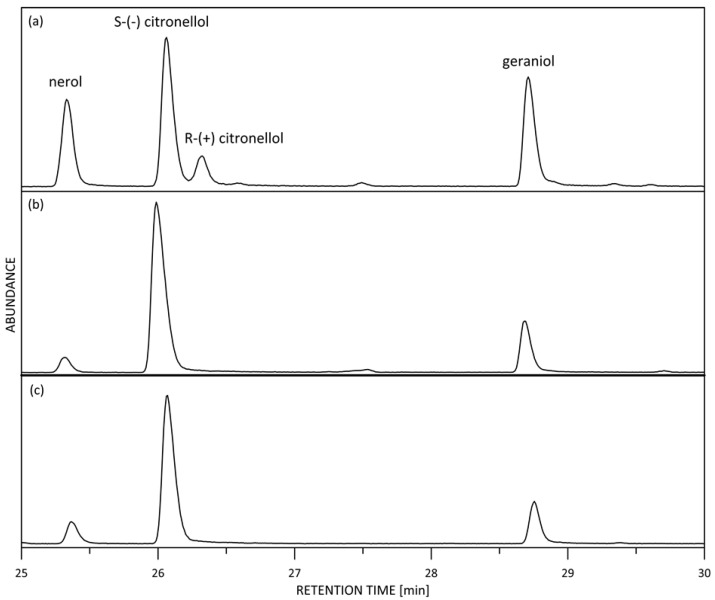
Within the frame of this study, the citronellol enantiomer distribution was studied for the first time in R. rugosa oil, revealing 82.9% S-(–) citronellol and 17.1% R-(+) citronellol content in R samples. Although this observation is very interesting and could be used for botanical origin assessment, more R. rugosa oil samples should be analyzed to confirm these preliminary results.
The GC/MS (TIC) chiral chromatograms of the Chinese rose oil samples are presented in Figure 4.
Figure 4.
GC/MS (TIC) chromatograms on a chiral stationary phase Cyclodex-B: (a) R. rugosa; (b) Kushui rose; (c) R. damascena.
3. Materials and Methods
3.1. Samples
Six samples of industrial-type rose EOs were studied: four rose oil samples of R. damascena Mill. from Uyghur (Xiujiang), provided by Shenzhen BioTech Ltd., a sample of essential oil from the Kushui rose (R. sertata × R. rugosa), provided by the Gansu Agricultural University, Gansu Province, and a sample of essential oil from the Pingyin rose (R. rugosa cv. Plena), provided by Jinan Huinong Rose Essential Oil Co., Ltd. According to the suppliers, the age of the plantations is 5–10 years, cultivated according to the established technology. The Kushui and R. rugosa rose oil samples were derived in 2017 and R. damascene in 2019. In this manuscript, the R. damascena samples are marked as D1-D4 and the Kushui and the R. rugosa samples as KS and R, respectively.
For the citronellol enantiomers study, the following reference materials were used: (R)-(+)-β-citronellol, 97% and citronellol, mixture of isomers, natural, ≥95%, FG (Sigma-Aldrich, Saint Louis, MO, USA).
For the Retention indices calculation, a standard mixture of aliphatic hydrocarbons from C8 to C32, >98%, dissolved in GC grade n-hexane (Sigma-Aldrich, Saint Louis, MO, USA), was used.
3.2. Analytical Methods
Gas chromatography-mass spectrometry (GC/MS) and gas chromatography with flame ionization detection (GC-FID) were used for the chemical profiling. In addition, GC/MS with chiral stationary phases was applied and the distribution of citronellol enantiomers was evaluated.
The GC/MS analysis was performed on an Agilent 7820A GC System gas chromatograph coupled with a 5977B Mass Selective detector and flame-ionization detector (Agilent Technologies, Palo Alto, CA, USA). The ultra-inert non-polar fused silica capillary column DB-5ms UI (J&W Scientific, Folsom, CA, USA) with 30 m column length, 0.25 mm i.d. and 0.25 mm film thickness was used.
The oven temperature was programmed from 60 °C (2.5 min held) to 100 °C at a rate of 5 °C/min, from 100 to 225 °C at a rate of 2.5 °C/min, and from 225 to 275 °C at a rate of 5 °C, 10 min held at the final temperature. Helium (99.999%) was used as a carrier gas at a constant flow rate of 0.8 mL/min. The split ratio was 1:200, the inlet temperature was set to 260 °C and the transfer line temperature was 280 °C. A mass selective detector was operated in electron impact ionization (EI) mode at 70 eV electron energy, the ion source temperature was set to 230 °C, and the quadrupole temperature was 150 °C. The mass scan range was 30–600 m/z.
The GC-FID analysis was performed on the same instrument under the same temperature gradient as described above. The system was equipped with a post-column split of the flow, allowing the simultaneous analysis of both detectors. Instrument control and data collection were carried out using Mass Hunter Workstation Software (Revision B.06.07, Agilent Technologies).
The identification of the compounds was performed using commercial mass spectral libraries (NIST 14, Wiley 7th Mass Spectra Register) and retention times (linear retention indices, LRI). In cases where there was a lack of corresponding reference data, the structures were proposed based on their general fragmentation pattern or using reference literature mass spectra. The quantification of the main compounds was carried out by the internal normalization method, with a response factor equal to unity for all of the sample constituents.
Retention indices were calculated using a mixture of homologues aliphatic hydrocarbons (C8-C32) analyzed under identical conditions, applying the following equation [34]:
| RI (x) = 100 n + 100 [(tRx − tRn)/(tRn+1 − tRn)], |
where: tRx—retention time of the component x; tRn, n+1—retention times of the n-alkanes, eluting before and after the component × (bracketing x); n, n + 1—carbon atom number of the n-alkanes, eluting before and after the component x.
For the enantiomers distribution, chiral stationary phase Cyclodex-B, with 30 m column length, 0.25 mm i.d., 0.25 mm (J&W Scientific, Folsom, CA, USA) was used under the following temperature gradient: from 60 °C (2.5 min held) to 120 °C at a rate of 2.5 °C/min (4.5 min held) and from 100 to 225 °C at a rate of 10 °C/min 10 min isotherm at the final temperature.
4. Conclusions
A comparative study of Chinese rose oils revealed their distinctive chemical composition and aroma profile, both for introduced and native rose genotypes. The main constituents of the Chinese rose oil samples are representatives of terpenoids compounds (mono- and sesquiterpenoids, predominantly) and aliphatic hydrocarbons. Higher amounts of characteristic aroma constituents in the EOs from Damask rose were observed in comparison with the Kushui rose and R. rugosa oils. In addition, the chemical profile of Chinese R. damascena oil samples differs significantly from the Bulgarian one, but is close to the Turkish and Moroccan rose oil types (ISO 9842:2003).
Acknowledgments
The financial support of the project KP-06-OPR-01/5 “BG ROSEnsing: Traditional and e-sensing of Bulgarian Rose aromatic products: towards fast authenticity and quality control”, funded by the National Science Fund (Ministry of Education and Science) of Bulgaria is gratefully acknowledged.
Author Contributions
Conceptualization, D.N.-A. and A.D.; methodology, D.N.-A. and A.D.; investigation, D.N.-A. and A.D; resources, D.N.-A.; data curation, D.N.-A.; writing—original draft preparation, D.N.-A. and A.D.; writing—review and editing, D.N.-A. and A.D.; visualization, D.N.-A.; supervision, D.N.-A.; project administration, D.N.-A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Science Fund (Ministry of Education and Science) of Bulgaria, the project KP-06-OPR-01/5 “BG ROSEnsing: Traditional and e-sensing of Bulgarian Rose aromatic products: towards fast authenticity and quality control”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cui W.-H., Du X.-Y., Zhong M.-C., Fang W., Suo Z.-Q., Wang D., Dong X., Jiang X.-D., Hu J.-Y. Complex and Reticulate Origin of Edible Roses (Rosa, Rosaceae) in China. Hortic. Res. 2022;9:uhab051. doi: 10.1093/hr/uhab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal P.K. Evaluation, Genetic Diversity, Recent Development of Distillation Method, Challenges and Opportunities of Rosa Damascena: A Review. J. Essent. Oil Bear. Plants. 2013;16:1–10. doi: 10.1080/0972060X.2013.764176. [DOI] [Google Scholar]
- 3.Wang Y., Zhao Y., Liu X., Li J., Zhang J., Liu D. Chemical Constituents and Pharmacological Activities of Medicinal Plants from Rosa Genus. Chin. Herb. Med. 2022;14:187–209. doi: 10.1016/j.chmed.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akram M., Riaz M., Munir N., Akhter N., Zafar S., Jabeen F., Ali Shariati M., Akhtar N., Riaz Z., Altaf S.H., et al. Chemical Constituents, Experimental and Clinical Pharmacology of Rosa Damascena: A Literature Review. J. Pharm. Pharmacol. 2020;72:161–174. doi: 10.1111/jphp.13185. [DOI] [PubMed] [Google Scholar]
- 5.Mileva M., Ilieva Y., Jovtchev G., Gateva S., Zaharieva M.M., Georgieva A., Dimitrova L., Dobreva A., Angelova T., Vilhelmova-Ilieva N., et al. Rose Flowers—A Delicate Perfume or a Natural Healer? Biomolecules. 2021;11:127. doi: 10.3390/biom11010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raka R.N., Zhiqian D., Yue Y., Luchang Q., Suyeon P., Junsong X., Hua W. Pingyin Rose Essential Oil Alleviates LPS-Induced Inflammation in RAW 264.7 Cells via the NF-ΚB Pathway: An Integrated in Vitro and Network Pharmacology Analysis. BMC Complement. Med. Ther. 2022;22:272. doi: 10.1186/s12906-022-03748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J., Wang C. Medicinal Components and Pharmacological Effects of Rosa Rugosa. Rec. Nat. Prod. 2018;12:535–543. doi: 10.25135/rnp.60.17.12.191. [DOI] [Google Scholar]
- 8.Boskabady M.H., Shafei M.N., Saberi Z., Amini S. Pharmacological Effects of Rosa Damascena. Iran J. Basic Med. Sci. 2011;14:295–307. [PMC free article] [PubMed] [Google Scholar]
- 9.Scalliet G., Piola F., Douady C.J., Réty S., Raymond O., Baudino S., Bordji K., Bendahmane M., Dumas C., Cock J.M., et al. Scent Evolution in Chinese Roses. Proc. Natl. Acad. Sci. USA. 2008;105:5927–5932. doi: 10.1073/pnas.0711551105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L., Zhang N., Li S., Wang H., Petkova M., Yao L. An Overview of Oil-Bearing Roses in China. Agric. Sci. 2019;XI:21–26. [Google Scholar]
- 11.Feng L., Chen C., Li T., Wang M., Tao J., Zhao D., Sheng L. Flowery Odor Formation Revealed by Differential Expression of Monoterpene Biosynthetic Genes and Monoterpene Accumulation in Rose (Rosa Rugosa Thunb.) Plant Physiol. Biochem. 2014;75:80–88. doi: 10.1016/j.plaphy.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H., Zhao F., Wang Y., Zhang Y., Sun K., Da L.-L. Molecular and Morphological Evidences for Hybrid Origin of Kushui Rose. Res. Sq. 2022 in review . [Google Scholar]
- 13.Wu M., Feng H., Song J., Chen L., Xu Z., Xia W., Zhang W. Structural Elucidation and Immunomodulatory Activity of a Neutral Polysaccharide from the Kushui Rose (Rosa Setate × Rosa Rugosa) Waste. Carbohydr. Polym. 2020;232:115804. doi: 10.1016/j.carbpol.2019.115804. [DOI] [PubMed] [Google Scholar]
- 14.Essential Oil of Rose, Chinese Kushui Type (Rosa Sertata × Rosa Rugosa) ISO; Geneva, Switzerland: 2013. [Google Scholar]
- 15.Wang Y., Tang Y., Wu D., Wu Y., Yao L. Introduction and Cultivation of Rosa Damascena Mill. Var. Kazanlika in Anji Zhejiang. J. Shanghai Jiaotong Univ. 2009;27:226–230. [Google Scholar]
- 16.Wang X., Su C., Peng L., Wang H., Wang H., Liu W., Li P., Fang Y. Ecological Suitability Assessment and Introduction Experiment on Rosa Damascena Trigintipetala in Sichuan Province, China. J. Mt. Sci. 2014;11:805–815. doi: 10.1007/s11629-013-2802-6. [DOI] [Google Scholar]
- 17.Kovacheva N., Rusanov K., Atanassov I. Industrial Cultivation of Oil Bearing Rose and Rose Oil Production in Bulgaria During 21 ST Century, Directions and Challenges. Biotechnol. Biotechnol. Equip. 2010;24:1793–1798. doi: 10.2478/V10133-010-0032-4. [DOI] [Google Scholar]
- 18.Kang Y., Wu K., Sun J., Liu C., Su C., Yi F. Preparation of Kushui Rose (Rosa Setate × Rosa Rugosa) Essential Oil Fractions by Double Molecular Distillation: Aroma and Biological Activities. Ind. Crops Prod. 2022;175:114230. doi: 10.1016/j.indcrop.2021.114230. [DOI] [Google Scholar]
- 19.Jirovetz L., Buchbauer G., Stoyanova A., Balinova A., Guangjiun Z., Xihan M. Solid Phase Microextraction/Gas Chromatographic and Olfactory Analysis of the Scent and Fixative Properties of the Essential Oil OfRosa Damascena L. China Flavour Fragr. J. 2005;20:7–12. doi: 10.1002/ffj.1375. [DOI] [Google Scholar]
- 20.Ohno Y., Tanaka S. On the Constituents of a Chinese Rose Oil. Agric. Biol. Chem. 1977;41:399–401. doi: 10.1080/00021369.1977.10862505. [DOI] [Google Scholar]
- 21.Moein M., Etemadfard H., Zarshenas M.M. Investigation of Different Damask Rose (Rosa Damascena Mill.) Oil Samples from Traditional Markets in Fars (Iran); Focusing on the Extraction Method. Trends Pharm. Sci. 2016;2:51–58. [Google Scholar]
- 22.Shamspur T., Mostafavi A. Chemical Composition of the Volatile Oil of Rosa Kazanlik and Rosa Gallica from Kerman Province in Iran. J. Essent. Oil Bear. Plants. 2010;13:78–84. doi: 10.1080/0972060X.2010.10643794. [DOI] [Google Scholar]
- 23.Ueyama Y., Hashimoto S., Nii H., Furukawa K. The Essential Oil from the Flowers of Rosa rugosa Thunb. var. plena Regel. Flavour Fragr. J. 1990;5:219–222. doi: 10.1002/ffj.2730050406. [DOI] [Google Scholar]
- 24.Liguo F., Xiaodi H. Changes of aromatic components and content during the development of roses. Chin. Agric. Sci. 2008;41:4341–4351. doi: 10.3864/j.issn.0578-1752.2008.12.054. [DOI] [Google Scholar]
- 25.Wu Y., Han X., Yuan W., Wang X., Meng D., Hu J., Lv Z. Salt Intervention for the Diversities of Essential Oil Composition, Aroma and Antioxidant Activities of Kushui Rose (R. Setate×R. Rugosa) Ind. Crops Prod. 2020;150:112417. doi: 10.1016/j.indcrop.2020.112417. [DOI] [Google Scholar]
- 26.Son H.-H., Lee D.-S. Gas Chromatographic Profiles of Rose Essential Oils: A Round-Robin Test on Oil of Rose, Chinese Kushui Type (Rosa Sertata × Rosa Rugosa) Anal. Sci. Technol. 2012;25:207–213. doi: 10.5806/AST.2012.25.4.207. [DOI] [Google Scholar]
- 27.Feng L.-G., Chen C., Sheng L.-X., Liu P., Tao J., Su J.-L., Zhao L.-Y. Comparative Analysis of Headspace Volatiles of Chinese Rosa Rugosa. Molecules. 2010;15:8390–8399. doi: 10.3390/molecules15118390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Z., Luo J., Niu Y., Wu M. Characterization of Key Aroma Compounds from Different Rose Essential Oils Using Gas Chromatography-Mass Spectrometry, Gas Chromatography–Olfactometry and Partial Least Squares Regression. Nat. Prod. Res. 2018;32:1567–1572. doi: 10.1080/14786419.2017.1389933. [DOI] [PubMed] [Google Scholar]
- 29.Farkaš P. The Standard GC Retention Index Library of Flavour Compounds. In: Maarse H., Heij D.G., editors. Trends in Flavour Research: Proceedings of the 7th Weurman Flavour Research Symposium, Noordwijkerhout, The Netherlands, 15–18 June, 1993. Elsevier; Amsterdam, The Netherlands: New York, NY, USA: 1994. pp. 145–149. Developments in Food Science. [Google Scholar]
- 30.Song J., Raka R.N., Yuan Y., Park S., Wang J., Liu F., Wu H., Xiao J., Huang M., Yang S. Potential Anti-Inflammatory Effects of Pingyin Rose Essential Oil on Lipopolysaccharide-Induced HaCaT Cells. Res. Sq. 2022 in review . [Google Scholar]
- 31.Tsankova E., Ognyanov I. Germazone, a Novel Tricyclic Sesquiterpene Ketone in the Essential Oil from Geranium Macrorrhizum L. Tetrahedron Lett. 1976;17:3833–3836. doi: 10.1016/S0040-4039(00)93123-X. [DOI] [Google Scholar]
- 32.Oil of Rose (Rosa × Damascena Miller) ISO; Geneva, Switzerland: 2003. [Google Scholar]
- 33.Do T.K.T., Hadji-Minaglou F., Antoniotti S., Fernandez X. Authenticity of Essential Oils. TrAC Trends Anal. Chem. 2015;66:146–157. doi: 10.1016/j.trac.2014.10.007. [DOI] [Google Scholar]
- 34.European Directorate for the Quality of Medicines & Healthcare . European Pharmacopoeia Supplements 10.6–10.8. 10th ed. European Directorate for the Quality of Medicines & Healthcare; Strasbourg, France: 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.