Abstract
A prospectively followed Danish cohort of 55,756 citizens with an observation time upwards of 25 years was investigated for association between eating raw carrots on a regular basis and developing various adenocarcinoma-dominant cancers and leukemia. Mean age at inclusion was 56.2 years (SD 4.4 years), and 52% were females. A dose-dependent reduction in incidence was seen for cancer of the lung (HR 0.76, CI95% 0.66; 0.87) and pancreas (HR 0.79, CI95% 0.61; 1.03), as well as leukemia (HR 0.91, CI95% 0.68; 1.21). Only for lung cancer was the association significant. In the case of pancreatic cancer, a possible type 1 error was present due to a low number of cancers. In cases of breast and prostate cancer, no association and no dose response were demonstrated. The association seen for lung and pancreatic cancer parallels that earlier demonstrated for large bowel cancer and indicates a cancer-protective effect from daily intake of raw carrots not limited to gastrointestinal adenocarcinomas. Processed carrots exhibited no effect. The preventive effect could be due to the polyacetylenic compounds falcarinol and falcarindiol in carrots, whereas carotene may not have an effect. The polyacetylenes are inactivated by heating, supporting our findings that only raw carrot intake has an effect. Indirect evidence for the cancer preventive effect of carrots in humans has reached a level where a prospective human trial is now timely.
Keywords: falcarinol, falcarindiol, polyacetylenes, carrots, cancer, adenocarcinoma, primary prevention
1. Introduction
The association between consistent intake of fruits and vegetables and a reduced risk of developing some types of cancer has been shown in cohort studies for many decades [1,2,3]. The association between a low risk of bowel cancer and high consumption of carrots has attracted special interest both because the association has been shown repeatedly in very large cohorts with extended confounder control [4,5], as well as because the effect is marked with a preventive potential as high as population-based screening programs. Large bowel cancer is the third most common cause of cancer deaths, and the potential effect on population health of increasing the consumption of raw carrots is marked. Further, some studies have shown a negative correlation between carrot intake and development of other cancers dominated by the adenocarcinoma type such as breast, lung, gastric, and prostate cancers [5]. For many years, the health-promoting effect of carrots has been attributed to a high content of beta-carotene in orange carrots because of its potent antioxidant capacity. This has been disproven by randomized trials administering purified beta-carotene to healthy citizens [6,7,8,9,10,11]. Some studies actually demonstrated increased incidence of lung cancer in those taking beta-carotene [12,13].
Carrots are the major dietary source of the falcarinols—falcarinol and falcarindiol—and their interaction with animals and human cancer cells and enzyme systems have been systematically investigated [14]. Purified falcarinols have been demonstrated to inhibit the growth of human cancer cell lines [15,16,17,18,19] and prevent neoplastic transformation in the large bowel in rats primed to develop bowel cancer [20,21,22]. The effect seen in rats can be reproduced when feeding the rats with raw carrots with purified falcarinols. This cancer preventive effect can be explained by the anti-inflammatory effect of the falcarinols demonstrated in several different experimental set-ups [21,23,24] and is believed to be, at least in part, mediated by an anti-inflammatory effect very similar to the cancer-preventive effect provided by aspirin and other pharmacological inhibitors of inflammation. It therefore seems obvious to investigate an additional effect in other cancers of the adenocarcinoma type. Different cultivars vary markedly in the polyacetylenic content, and awareness of this and a change in carrot cultivar available to the citizens might have a positive effect.
As an extension of our formerly published article on bowel cancer incidence [4], we aimed to estimate the risk of adenocarcinoma-dominated cancer types (lung, breast, prostate, and pancreas) on the basis of carrot intake, and we included leukemia as a control.
2. Materials and Methods
2.1. Population
This was a prospective cohort study investigating risk of leukemia, breast cancer, lung cancer, prostate cancer, and pancreatic cancer in individuals originally included in the Diet, Cancer and Health study (DCH). The cohort was linked with National Danish registers in order to identify diagnoses of the diseases of interest in the years to come.
DCH invited a sample (n = 160,725) of Danish born residents of the Aarhus and Copenhagen areas aged 50 to 64 years of age to participate. Non-responders were reminded after three weeks, and again after six weeks. The inclusion period lasted from 1993 until 1997. All participants (n = 57,053) filled out a validated 192 item food frequency questionnaire, describing their dietary intake during the preceding 12 months [25,26,27]. Additionally, a questionnaire collecting lifestyle data on known cancer risks (smoking, alcohol, physical activity, etc.) was filled in by participants. Individuals underwent an examination in which their height and weight were measured by a lab technician [25]. A group of participants (n = 585) were excluded from the original cohort as they had a prior colorectal cancer diagnosis [4], thereby fulfilling the DCH exclusion criteria. We followed each individual in the national registers from their date of inclusion until death, 31 December 2018, or diagnosis, whichever came first.
2.2. Register Data
The DCH cohort was linked with the Danish National Patient Register [28] in order to identify ICD-8 (1985–1993) and ICD-10 (1994–2018) codes (detailed in Table 1) of the outcomes of interest. ICD-8s were included in order to identify diagnoses prior to inclusion, enabling us to exclude those individuals. The Danish Register of Causes of Death [29] was used to identify dates of death.
Table 1.
ICD-8 and ICD-10 codes utilized to identify diagnoses of outcomes.
| Disease | ICD-8 | ICD-10 |
|---|---|---|
| Breast cancer | 174.00; 174.01; 174.02; 174.08; 174.09 | C50 |
| Lung cancer | 162.09-19 | C33; C34; C45 |
| Prostate cancer | 185.99 | C61 |
| Pancreatic cancer | 157.09; 157.80; 157.81; 157.89; 157.99 | C25 |
| Leukemia | 204.09–207.99 | C91–C95 |
2.3. Exposure
Dietary intake of raw carrot was the main exposure of interest. Additionally, intake of processed carrot was also investigated. Both were estimated from the self-reported data in the food frequency questionnaires. Processed carrots are of less interest as the levels of FaOH and FaDOH in carrots decrease when they are thermally processed. In this cohort, we differentiated between consumption of raw and processed carrots. Falcarinols are sensible to heat treatment, and the activity has been shown to decrease after heating [30]. Thermal processing of carrots at 90 °C for 2 min will reduce biological activity by 25–50% [31], but the minimal active dose necessary to conduct biological effect is largely unknown. Participants would report their frequency of raw and processed carrot intake ranging from never to eight or more times per day. This was translated to gram per day (g/day) using standard portion sizes and categorized into no intake, less than 32 g/day, and more than 32 g/day of raw and processed carrot. This division was chosen on the basis of the evidence available from rodent studies, and it was this categorization used to identify significant differences in risk of colorectal cancer in the cohort as described previously [4].
2.4. Outcomes
Outcomes were defined as any diagnose of leukemia, breast cancer, prostate cancer, pancreatic cancer, or lung cancer registered in the Danish National Patient Register after the individual date of inclusion. Specific ICD-8 and ICD-10 codes are provided in Table 1.
2.5. Statistical Analysis
Baseline characteristics were compared using chi-squared tests. The relative risk of the respective diseases was estimated between groups defined by carrot intake (raw and processed) using Cox proportional hazard regression models adjusting for a number of relevant covariates (detailed in Section 2.6). The level of significance was set at 5%, and 95% confidence intervals (CI95%) were provided. Interactions between raw carrot intake and covariates were tested in all models but were only significant for age group in prostate cancer analyses. Therefore, the regression model for prostate cancer was conducted using age as time, comparing individuals of the same age instead of adjusting for age group at entry as a covariate in the regression model. This method was also performed for the other outcomes as a sensitivity analysis to ensure the conclusions would not change. Cumulative incidence proportion curves stratified by raw carrot intake were created for the outcome of lung cancer. Data management was performed using SAS software version 9.4 (SAS Institute Inc. SAS 9.4. Cary, NC, USA), and statistical analyses were conducted using R statistical software package version 3.6.1 (R Core Team, Vienna, Austria) [32,33,34].
2.6. Covariates
The Cox proportional hazards regression models were adjusted for sex, age group, metabolic equivalents (MET) score, other vegetable intake, other root vegetable intake, intake of non-steroidal anti-inflammatory drugs (NSAID), smoking status, educational level, alcohol consumption, body mass index (BMI), former cerebral or coronary artery thrombosis, and hormone replacement therapy. Except for BMI, all covariates relied on self-reported data.
All covariates were included as categorical variables. Sex was defined as male or female. Age groups were divided as 50–54, 55–59, and 60–65 years of age (at baseline). MET score, other vegetable consumption (spinach, salad, cucumber, bell pepper, eggplant, tomato, squash, avocado, beans, and peas), and other root vegetable consumption (celery, ginger root, and partly frozen carrot from vegetable mix) [4] was divided into quartiles of quantity within the sample. NSAID consumption was divided into consumer or non-consumer. Smoking status was grouped into non-smoker, former smoker, and current smoker. Educational level was grouped as low, medium, and high level of education. Alcohol consumption was determined on the basis of the number of units consumed per week and grouped according to the Danish guidelines on maximum consumption of ten units per week, i.e., none, 1–10 units per week, and more than 10 units per week. BMI was grouped as below 18.5, 18.5–25, and above 25. Former cerebral or coronary artery thrombosis were categorized as yes or no.
3. Results
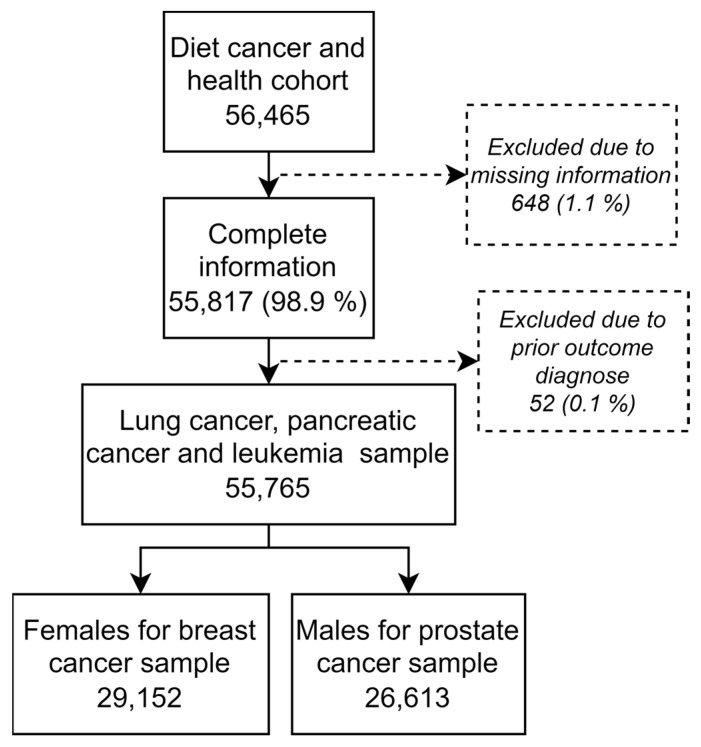
The DCH cohort (n = 56,465) was linked to national registers, and individuals with missing information (n = 648) and individuals with prior outcome diagnosis (n = 52) were excluded. This left 55,765 individuals for analysis of lung cancer risk, pancreatic cancer risk, and leukemia risk, and 29,152 (52%) females for analysis of breast cancer risk and 26,613 (48%) males for prostate cancer risk analysis (Figure 1). Mean age at inclusion was 56.2 years (standard deviation (SD) 4.4 years). The mean follow-up time varied from 20.0 years (SD 4.9) in prostate cancer analyses to 21.1 years (SD 3.9) in lung and pancreatic cancer analyses. Number of cases registered were 542 (1.0%) for leukemia, 2451 (4.4%) for lung cancer, 655 (1.2%) for pancreatic cancer, 2719 (9.3%) for breast cancer, and 2821 (10.6%) for prostate cancer (Table 2). Individuals who died prior to each specific diagnosis and thereby lost to follow-up were 16,625 (29.8%) for leukemia, 15,065 (27.0%) for lung cancer, 16,423 (29.5%) for pancreatic cancer, 6638 (22.8%) for breast cancer, and 8166 (30.7%) for prostate cancer.
Figure 1.
Flow of participants including sample size for each outcome measure.
Table 2.
Baseline carrot intake by outcomes during follow-up.
| No Leukemia, n = 55,223 (%) | Leukemia During Follow-Up, n = 542 (%) | Total, n = 55,765 | p-Value | ||
| Raw carrot intake | None | 7800 (98.8) | 93 (1.2) | 7893 | |
| 1–32 g/day | 31,199 (99.1) | 294 (0.9) | 31,493 | ||
| >32 g/day | 16,224 (99.1) | 155 (0.9) | 16,379 | 0.130 | |
| Processed carrot intake | None | 2240 (98.9) | 24 (1.1) | 2264 | |
| 1–32 g/day | 51,699 (99.0) | 503 (1.0) | 52,202 | ||
| >32 g/day | 1284 (98.8) | 15 (1.2) | 1299 | 0.715 | |
| No Lung Cancer, n = 53,314 (%) | Lung Cancer During Follow-Up, n = 2451 (%) | Total, n = 55,765 | p-Value | ||
| Raw carrot intake | None | 7336 (92.9) | 557 (7.1) | 7893 | |
| 1–32 g/day | 30,097 (95.6) | 1396 (4.4) | 31,493 | ||
| >32 g/day | 15,881 (97.0) | 498 (3.0) | 16,379 | <0.001 | |
| Processed carrot intake | None | 2138 (94.4) | 126 (5.6) | 2264 | |
| 1–32 g/day | 49,930 (95.6) | 2272 (4.4) | 52,202 | ||
| >32 g/day | 1246 (95.9) | 53 (4.1) | 1299 | 0.019 | |
| No Pancreatic Cancer, n = 55,110 (%) | Pancreatic Cancer During Follow-Up, n = 655 (%) | Total, n = 55,765 | p-Value | ||
| Raw carrot intake | None | 7773 (98.5) | 120 (1.5) | 7893 | |
| 1–32 g/day | 31,123 (98.8) | 370 (1.2) | 31,493 | ||
| >32 g/day | 16,214 (99.0) | 165 (1.0) | 16,379 | 0.002 | |
| Processed carrot intake | None | 2236 (98.8) | 28 (1.2) | 2264 | |
| 1–32 g/day | 51,587 (98.8) | 615 (1.2) | 52,202 | ||
| >32 g/day | 1287 (99.1) | 12 (0.9) | 1299 | 0.675 | |
| No Breast Cancer, n = 26,433 (%) | Breast Cancer During Follow-Up, n = 2719 (%) | Total, n = 29,152 | p-Value | ||
| Raw carrot intake | None | 2324 (92.1) | 198 (7.9) | 2522 | |
| 1–32 g/day | 13,825 (90.4) | 1472 (9.6) | 15,297 | ||
| >32 g/day | 10,284 (90.7) | 1049 (9.3) | 11,333 | 0.017 | |
| Processed carrot intake | None | 846 (92.4) | 70 (7.6) | 916 | |
| 1–32 g/day | 24,826 (90.6) | 2572 (9.4) | 27,398 | ||
| >32 g/day | 761 (90.8) | 77 (9.2) | 838 | 0.201 | |
| No Prostate Cancer, n = 23,792 (%) | Prostate Cancer During Follow-Up, 2821 (%) | Total, n = 26,613 | p-Value | ||
| Raw carrot intake | None | 4869 (90.7) | 502 (9.3) | 5371 | |
| 1–32 g/day | 14,433 (89.1) | 1763 (10.9) | 16,196 | ||
| >32 g/day | 4490 (89.0) | 556 (11.0) | 5046 | 0.004 | |
| Processed carrot intake | None | 1228 (91.1) | 120 (8.9) | 1348 | |
| 1–32 g/day | 22,152 (89.3) | 2652 (10.7) | 24,804 | ||
| >32 g/day | 412 (89.4) | 49 (10.6) | 461 | 0.115 |
The disease incidence proportion was significantly higher in those who did not eat any raw carrots for the outcomes of leukemia, lung cancer, pancreatic cancer, breast cancer, and prostate cancer compared to carrot eaters. Regarding processed carrot intake, this was only the case for lung cancer incidence (Table 2). Baseline covariate distributions in the sample has been provided in Supplementary Table S1.
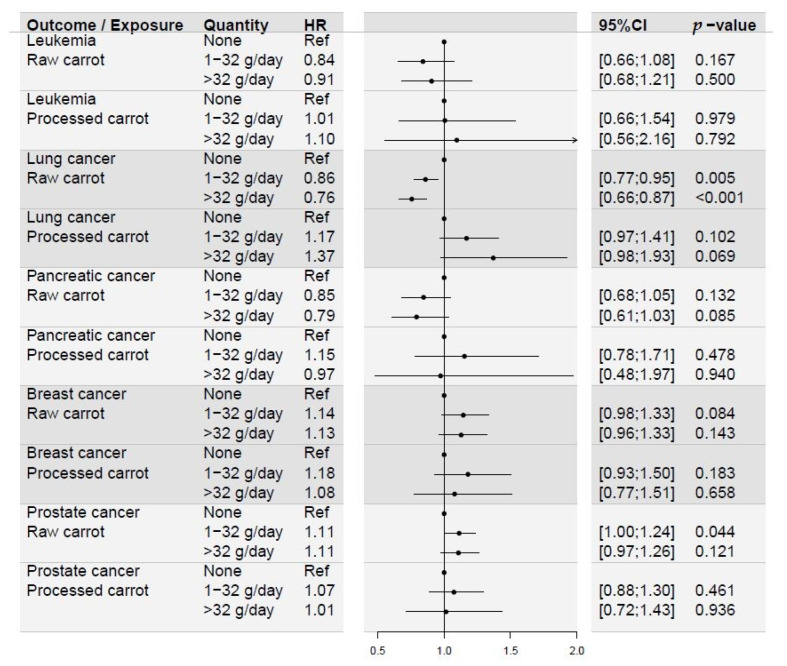
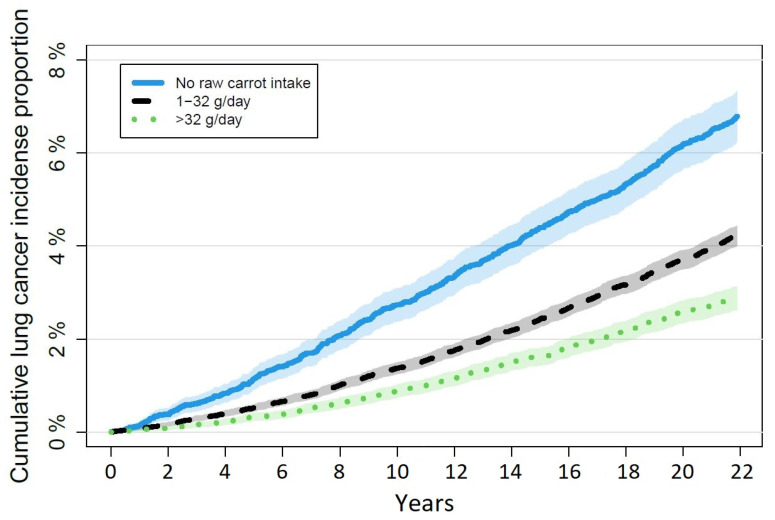
The adjusted hazard ratios (HR) from the Cox proportional hazards regression models revealed significant differences in risk of lung cancer and prostate cancer on the basis of raw carrot intake. The HR for lung cancer were 0.86 (CI95% 0.77; 0.95) in the group eating 1–32 g/day and 0.76 (CI95% 0.66; 0.87) in the group eating more than 32 g/day, compared to those eating no raw carrots. The HR for prostate cancer was significantly increased at 1.11 (CI95% 1.00; 1.24) in the group eating 1–32 g/day, and increased, yet not statistically significantly, at 1.11 (CI95% 0.97; 1.26) in the group eating more than 32 g/day, compared to those eating no raw carrots. Processed carrot intake did not show the same effect (Figure 2). The cumulative incidense proportion curves for lung cancer stratified by raw carrot intake is illustrated in Figure 3. For leukemia, breast cancer, and pancreatic cancer, there were no significant differences in risk of disease during follow-up on the basis of neither raw nor processed carrot intake. Processed carrot intake did not affect prostate cancer risk either. Although the trends showed HR estimates above one for prostate and breast cancer risk according to increasing carrot intake, the pattern in pancreatic cancer and leukemia were similar to that of lung cancer (Figure 2).
Figure 2.
Forest plot illustrating the results from multivariate Cox proportional hazards regression models estimating the risk of the outcomes listed on the basis of carrot intake. All models were adjusted for sex, age group, MET score, other vegetable consumption, other root vegetable consumption, NSAID intake, smoking status, educational level, alcohol consumption, BMI, and former cerebral or coronary artery thrombosis. Breast cancer analysis was additionally adjusted for hormone replacement therapy. Leukemia, lung cancer, and pancreatic cancer, n = 55,765. Breast cancer, n = 29,152. Prostate cancer, n = 26,613.
Figure 3.
Cumulative incidence proportions of lung cancer during follow-up stratified by raw carrot intake. n = 55,765.
The sensitivity analyses conducting the regression models using age as time for outcomes of leukemia, breast cancer, lung cancer, and pancreatic cancer did not alter the conclusion of the main analyses.
4. Discussion
This cohort of 57,000 Danish citizens was followed for more than 20 years through extended questionnaires and clinical controls [25]. We have shown earlier that citizens eating raw carrots have reduced risk of developing colorectal cancer [4]. This was true also after confounder corrections in multivariate test; it was dose dependent and amounted to a 17% risk reduction in the high exposure group. Two hypotheses arose from this observation: (1) Can this preventive effect be reproduced in other types of common human cancer diseases, especially adenocarcinomas as indicated in the literature. (2) Is this effect counterbalanced by a possible carcinogenic effect of beta-carotene in the case of lung cancer. Leukemia was included in the study as a non-carcinomatous cancer control [35]. The design of the study including confounder control is parallel to our earlier published article on colorectal cancer in this population to allow for comparison.
Even though the population is large and the observational period is long, sufficiently high prevalence of cancer diseases allow only for analysis of the more common types. Even in the cases of leukemia and pancreatic cancer, the numbers allow for only limited subgroup analysis.
We find no general pattern of the effect of raw and/or processed carrot intake between the different types of cancer. There is a reduced prevalence in the cancers of the lung and pancreas, as well as leukemia. Although the HR is comparable in these three types, the difference is only significant in the case of lung cancer, which also expresses a convincing dose response, as indicated in Figure 3. The HR of the group of lung cancer patients with high exposure to raw carrots show an even higher preventive effect on cancer prevention than what was seen in colorectal cancer. The dose dependency in both leukemia and pancreatic cancer might indicate a true effect of raw carrots in these cancer types as well, even though the differences are not significant. The total prevalence is much lower in leukemia and pancreatic cancer as compared to lung cancer. This might explain the non-significance in the former two.
The correlation between eating carrots and the incidence of the hormone-influenced cancers of the breast and prostate is different. Overall, there is no significant correlation between eating raw or processed carrots and the incidence. The only significant difference is for the low dose of raw carrots in case of prostate cancer. Indeed, in all groups of raw and processed carrot intake for both prostate and breast cancer, it seems as if carrots increase the risk of cancer. The absence of a positive effect in breast cancer is surprising and in contrast to the general findings in earlier cohort studies [36]. It is not easily explainable, even though we assume that the net effect is a balance between the falcarinols and that the concentration of these substances vary widely between different cultivars. The prevalence of breast cancer is high, and a simple type 2 error seems unlikely.
As discussed previously [4], we estimated the cancer risks on the basis of self-reported recall data with an inherent risk of recall bias and healthy food consumption overestimation. If overestimation of carrot intake systematically (or even if random over- and under-estimation) has occurred, the effects seen in our study are probably underestimated. Further, the type of carrot and specific handling were not registered, and it is possible that even greater effects could be achieved by excluding intake of carrots low in falcarinols, or if we had been able to register the intake throughout follow-up instead of limited to the year prior to inclusion. Individuals eating carrots may also make other healthy lifestyle choices more often than their peers, introducing risk of confounding factors, although the extensive adjustments for health-related covariates should limit this risk. Whether the effects seen in this cohort can be transferred to high-risk individuals, such as those with gene mutations or family history of adenocarcinoma, is unknown. The impact of our findings would increase if such populations benefit in the same way or to an even higher degree. Although cohort studies in general have an inherited risk of confounder-driven misinterpretation, the data confirm other studies from different cohorts. These findings should be confirmed in a prospective randomized trial, but this will be very expensive and time consuming because the number needed to include will be in the thousands and observation period has to be for decades.
5. Conclusions
Our study confirms earlier studies showing that consistent intake of raw carrots protects against cancers of the lung, as it does in the large bowel. We interpret the results as indicative of a similar effect in pancreatic cancer and leukemia.
Acknowledgments
The authors wish to acknowledge and thank the Diet, Cancer and Health study group and the Danish Cancer Society for access to data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15030678/s1, Supplementary Table S1. Baseline covariate distributions in the sample.
Author Contributions
Conceptualization, U.D. and M.K.-L.; methodology, U.D., M.K.-L., and L.K.; validation, M.K.-L. and U.D.; formal analysis, U.D.; resources, U.D., L.K., M.K.-L., and G.B.; data curation, U.D.; writing—original draft preparation, M.K.-L., G.B., and U.D.; writing—review and editing, All authors; visualization, U.D.; supervision, G.B. and M.K.-L.; project administration, M.K.-L. and U.D.; funding acquisition, M.K.-L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the relevant scientific committee [25].
Informed Consent Statement
Written informed consent was obtained from the participants prior to the Diet, Cancer and Health study. Further, individual informed consent for data linkage was obtained from each individual [25].
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by OUH Research Grant, grant number 1021 2520.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vainio H., Weiderpass E. Fruit and vegetables in cancer prevention. Nutr. Cancer. 2006;54:111–142. doi: 10.1207/s15327914nc5401_13. [DOI] [PubMed] [Google Scholar]
- 2.Block G., Patterson B., Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz K.A., Potter J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 4.Deding U., Baatrup G., Christensen L.P., Kobaek-Larsen M. Carrot Intake and Risk of Colorectal Cancer: A Prospective Cohort Study of 57,053 Danes. Nutrients. 2020;12:332. doi: 10.3390/nu12020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Z., Chen H., Li M., Wang W., Fan C., Long F. Association of Dietary Carrot/Carotene Intakes with Colorectal Cancer Incidence and Mortality in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Front. Nutr. 2022;9:888898. doi: 10.3389/fnut.2022.888898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill M.E., Carroll Y., Corridan B., Olmedilla B., Granado F., Blanco I., Van der Berg H., Hininger I., Rousell A.-M., Chopra M., et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. 2001;85:499–507. doi: 10.1079/BJN2000284. [DOI] [PubMed] [Google Scholar]
- 7.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 8.Astorg P. Food carotenoids and cancer prevention: An overview of current research. Trends Food Sci. Technol. 1997;8:406–413. doi: 10.1016/S0924-2244(97)01092-3. [DOI] [Google Scholar]
- 9.Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens F.L., Jr., Valanis B., Williams J.H., Jr., et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg E.R., Baron J.A., Karagas M.R., Stukel T.A., Nierenberg D.W., Stevens M.M., Mandel J.S., Haile R.W. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA. 1996;275:699–703. doi: 10.1001/jama.1996.03530330043027. [DOI] [PubMed] [Google Scholar]
- 11.Albanes D., Heinonen O.P., Taylor P.R., Virtamo J., Edwards B.K., Rautalahti M., Hartman A.M., Palmgren J., Freedman L.S., Haapakoski J., et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 12.Middha P., Weinstein S.J., Männistö S., Albanes D., Mondul A.M. β-Carotene Supplementation and Lung Cancer Incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: The Role of Tar and Nicotine. Nicotine Tob. Res. 2018;21:1045–1050. doi: 10.1093/ntr/nty115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kordiak J., Bielec F., Jabłoński S., Pastuszak-Lewandoska D. Role of Beta-Carotene in Lung Cancer Primary Chemoprevention: A Systematic Review with Meta-Analysis and Meta-Regression. Nutrients. 2022;14:1361. doi: 10.3390/nu14071361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen L.P. Bioactive C(17) and C(18) Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules. 2020;25:2568. doi: 10.3390/molecules25112568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsunaga H., Katano M., Yamamoto H., Fujito H., Mori M., Takata K. Cytotoxic activity of polyacetylene compounds in Panax ginseng C. A. Meyer. Chem. Pharm. Bull. 1990;38:3480–3482. doi: 10.1248/cpb.38.3480. [DOI] [PubMed] [Google Scholar]
- 16.Bernart M.W., Cardellina J.H., II, Balaschak M.S., Alexander M.R., Shoemaker R.H., Boyd M.R. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J. Nat. Prod. 1996;59:748–753. doi: 10.1021/np960224o. [DOI] [PubMed] [Google Scholar]
- 17.Kuo Y.C., Lin Y.L., Huang C.P., Shu J.W., Tsai W.J. A tumor cell growth inhibitor from Saposhnikovae divaricata. Cancer Investig. 2002;20:955–964. doi: 10.1081/CNV-120005911. [DOI] [PubMed] [Google Scholar]
- 18.Young J.F., Duthie S.J., Milne L., Christensen L.P., Duthie G.G., Bestwick C.S. Biphasic effect of falcarinol on caco-2 cell proliferation, DNA damage, and apoptosis. J. Agric. Food Chem. 2007;55:618–623. doi: 10.1021/jf0616154. [DOI] [PubMed] [Google Scholar]
- 19.Purup S., Larsen E., Christensen L.P. Differential effects of falcarinol and related aliphatic C(17)-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009;57:8290–8296. doi: 10.1021/jf901503a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobaek-Larsen M., El-Houri R.B., Christensen L.P., Al-Najami I., Fretté X., Baatrup G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017;8:964–974. doi: 10.1039/C7FO00110J. [DOI] [PubMed] [Google Scholar]
- 21.Kobaek-Larsen M., Baatrup G., Notabi M.K., El-Houri R.B., Pipó-Ollé E., Christensen Arnspang E., Christensen L.P. Dietary Polyacetylenic Oxylipins Falcarinol and Falcarindiol Prevent Inflammation and Colorectal Neoplastic Transformation: A Mechanistic and Dose-Response Study in A Rat Model. Nutrients. 2019;11:2223. doi: 10.3390/nu11092223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobaek-Larsen M., Christensen L.P., Vach W., Ritskes-Hoitinga J., Brandt K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. J. Agric. Food Chem. 2005;53:1823–1827. doi: 10.1021/jf048519s. [DOI] [PubMed] [Google Scholar]
- 23.Metzger B.T., Barnes D.M., Reed J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008;56:3554–3560. doi: 10.1021/jf073494t. [DOI] [PubMed] [Google Scholar]
- 24.Alanko J., Kurahashi Y., Yoshimoto T., Yamamoto S., Baba K. Panaxynol, a polyacetylene compound isolated from oriental medicines, inhibits mammalian lipoxygenases. Biochem. Pharmacol. 1994;48:1979–1981. doi: 10.1016/0006-2952(94)90598-3. [DOI] [PubMed] [Google Scholar]
- 25.Tjønneland A., Olsen A., Boll K., Stripp C., Christensen J., Engholm G., Overvad K. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: A population-based prospective cohort study of 57,053 men and women in Denmark. Scand. J. Public Health. 2007;35:432–441. doi: 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- 26.Overvad K.I.M., JØNneland A.T., HaraldsdÓTtir J., Ewertz M., Jensen O.M. Development of a Semiquantitative Food Frequency Questionnaire to Assess Food, Energy and Nutrient Intake in Denmark. Int. J. Epidemiol. 1991;20:900–905. doi: 10.1093/ije/20.4.900. [DOI] [PubMed] [Google Scholar]
- 27.Tjønneland A., Overvad K.I.M., Haraldsdottir J., Bang S., Ewertz M., Jensen O.M. Validation of a Semiquantitative Food Frequency Questionnaire Developed in Denmark. Int. J. Epidemiol. 1991;20:906–912. doi: 10.1093/ije/20.4.906. [DOI] [PubMed] [Google Scholar]
- 28.Lynge E., Sandegaard J.L., Rebolj M. The Danish National Patient Register. Scand. J. Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 29.Helweg-Larsen K. The Danish Register of Causes of Death. Scand. J. Public Health. 2011;39:26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 30.Rawson A., Brunton N.P., Rai D.K., McLoughlin P., Tiwari B.K., Tuohy M.G. Stability of falcarinol type polyacetylenes during processing of Apiaceae vegetables. Trends Food Sci. Technol. 2013;30:133–141. doi: 10.1016/j.tifs.2013.01.002. [DOI] [Google Scholar]
- 31.Hansen S.L., Purup S., Christensen L.P. Bioactivity of falcarinol and the influenceof processing and storage on its content in carrots (Daucus carota L) J. Sci. Food Agric. 2003;83:1010–1017. doi: 10.1002/jsfa.1442. [DOI] [Google Scholar]
- 32.Team R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2019. [Google Scholar]
- 33.Gerds T.A., Ozenne B. Publish: Format Output of Various Routines in a Suitable Way for Reports and Publication. R Package Version. 2019. [(accessed on 15 November 2022)]. Available online: https://cran.r-project.org/web/packages/Publish/index.html.
- 34.Therneau T. _A Package for Survival Analysis in S_. Version 2.38. 2019. [(accessed on 15 November 2022)]. Available online: https://cranr-project.org/package=survival.
- 35.Tawil M., Bekdash A., Mroueh M., Daher C.F., Abi-Habib R.J. Wild carrot oil extract is selectively cytotoxic to human acute myeloid leukemia cells. Asian Pac. J. Cancer Prev. APJCP. 2015;16:761–767. doi: 10.7314/APJCP.2015.16.2.761. [DOI] [PubMed] [Google Scholar]
- 36.Chen H., Shao F., Zhang F., Miao Q. Association between dietary carrot intake and breast cancer: A meta-analysis. Medicine. 2018;97:e12164. doi: 10.1097/MD.0000000000012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.