Abstract
Synchrotron X‐ray phase‐contrast microtomography (SR‐PhC micro‐CT) is well established, fast and non‐destructive imaging technique for data acquisition that is currently being used to obtain new insights into insect anatomy and function in physiological, morphological and phylogenetic studies. In this study, we described in situ the internal organs of the red flour beetle Tribolium castaneum Herbst 1797, a widespread pest of cereals and stored food causing serious damage to the human economy. Two‐dimensional virtual sections and volumetric reconstructions of the nervous, alimentary and reproductive systems were carried out in both sexes. The results provided a comprehensive overview of the morphological characteristics of this species, such as the different maturation stages of ovarioles and the realistic location, size and shape of internal organs. Given the great interest in this model species in experimental biology and forensic entomology, complete knowledge of the general anatomy is required for future functional applications in pest control and experimental studies. In addition, this study confirms SR‐PhC micro‐CT as a powerful and innovative tool in entomology, particularly suitable for small species and chitinized structures that are difficult to analyse using conventional dissection and histological methods.
Keywords: 3D rendering, abdominal gland, brain, cryptonephridial system, image segmentation, microtomography
This study describes in situ the internal organs of the red flour beetle Tribolium castaneum Herbst, 1797, a common pest of cereals and stored food, using Synchrotron Radiation Phase‐Contrast micro‐CT. Two dimensional virtual dissections and volumetric reconstruction of the nervous, alimentary and reproductive systems were carried out in both sexes. The results provided a comprehensive overview of the morphological characteristics of this species.

1. INTRODUCTION
The use of model organisms in experimental biology is usually required to allow comparison and provide a simpler system to study, transferring the results to humans or more complex systems (Ericsson et al., 2013). Although most of the studies are conducted on model species, such as rats and mice (Ericsson et al., 2013), vertebrates are subject to ethical restrictions regulating their use in laboratory tests, primarily because of the pain, distress and suffering inflicted on the animals (Beauchamp & Morton, 2015). For these reasons, the interest in alternative models has grown. Insects, in particular, represent a suitable and reliable alternative for physiological, biomedical and environmental studies (Adamski et al., 2019; Lagadic & Caquet, 1998; Trevijano‐Contador & Zaragoza, 2014) because they are ubiquitous and represent ecological and environmental diversity worldwide (Wilson & Fox, 2021). In addition, they are not subject to ethical restrictions, except for species on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species, and furthermore, the breeding costs for some model species are comparatively low and they are to be standardised in laboratory tests.
The beetle Tribolium castaneum Herbst, 1797 (Coleoptera: Tenebrionidae) is an important model species for a wide range of researches on population dynamics and genetics, reproduction and sexual selection, interspecific competition, development, behavioural ecology, evolutionary ecology (Brown et al., 2009; Bullock et al., 2020; Campbell et al., 2022 ; Pointer et al., 2021), ecophysiology and ecoimmunology (Futo et al., 2016; Milutinović et al., 2013; Roth et al., 2009; Wang et al., 2020), besides being one of the first species among Coleoptera to have its entire genome sequenced (Campbell et al., 2022). It is also used for addressing questions related to pesticide toxicity as well as insect resistance in crop protection (Campbell et al., 2022; Rösner et al., 2020). The red flour beetle is a cosmopolitan pest of stored foods (Trematerra & Sciarretta, 2004). In the wild, like most tenebrionid species, this beetle lives under tree bark or in dead wood. Over time, a high growth rate, rapid developmental cycle, early maturity and excellent dispersal ability have led to the adaptation of this species to live in stored commodities (Dawson, 1977; Kim et al., 2020). Currently, information on the anatomy and structure of the organs in the red flour beetle is available in old drawings or histological sections (Ameen & Rahman, 1973; Bloch Qazi et al., 1998; Parthasarathy et al., 2010; Sinha, 1958) resulting in insufficient knowledge of the main structures.
Synchrotron Radiation X‐ray phase‐contrast microtomography (SR‐PhC micro‐CT) is a powerful imaging technique for the study of anatomical structures and, when followed by an appropriate volumetric image segmentation, for 3D morphological analyses using an enhanced contrast‐to‐noise ratio in biological tissues, and does not require the use of contrast agents even in samples with weak X‐ray absorption (Donato et al., 2017; Elfarnawany et al., 2017; Endrizzi et al., 2018; Hellerhoff et al., 2019; Lak et al., 2008; Oliva et al., 2020). In previous entomological studies, virtual dissections performed using microtomography (micro‐CT) have provided morphological descriptions of various anatomical parts such as head (Betz et al., 2007; Hörnschemeyer et al., 2002), muscles (Bäumler et al., 2018; Li et al., 2011), brain (Smith et al., 2016), digestive (Alba‐Alejandre et al., 2019; Donato et al., 2021) visual (Giglio et al., 2022), and reproductive (Alba‐Alejandre et al., 2020; Küpper et al., 2019; Vommaro et al., 2022) systems. This non‐invasive method enhances the visualization of structures in situ and avoids all the artefacts associated with dissections and preparation of histological sections using traditional histological techniques, reducing the time of sample preparation protocols (Betz et al., 2007; Donato et al., 2021; Westneat et al., 2008). Three‐dimensional (3D) rendering is useful for studying the volumetric arrangement of organs in miniature insects (Alba‐Alejandre et al., 2019), for toxicological applications (Smith et al., 2020) or for creating data sets of the internal anatomy of model insects (Rother et al., 2021). In terms of cost–benefit analyses, micro‐CT is becoming increasingly accessible to researchers, as it is made available free of charge beamtime at non‐profit scientific organizations available based on peer‐reviewed proposals. However, this technique has some shortcomings related to the cost of analysis, the difficulty of automatically reconstructing organs with complex structures that require the involvement of expert users, and the limitations of the submicron resolution, though modern CT setups are progressively pushing the spatial resolution into the hundreds of nanometer range and even below (Beliaev et al., 2020; Romell et al., 2021). Despite the great interest in T. castaneum in forensic entomology and pest control research, few studies on its anatomy have been published because its small size makes it difficult to dissect and prepare organs for histological analysis. The aim of the present study is, therefore, to use SR‐PhC micro‐CT‐based virtual dissections to: (i) describe the anatomical structures of organs in situ, focusing on the digestive, nervous and reproductive systems in both males and females, and (ii) produce 3D reconstructions and organ segmentations that will provide a base for morphological data, useful for further functional studies and provide insights for teaching purposes in entomology courses.
2. MATERIALS AND METHODS
2.1. Sample collection and preparation
Specimens of T. castaneum belonged to the strain Croatia 1 (CRO1), were collected and isolated from a wild population in Croatia (Milutinović et al., 2013) and reared under laboratory condition over generations in the Animal Evolutionary Ecology lab, University of Muester, Germany. Adult beetles, kept in plastic boxes, were feed with heat‐sterilised (75°C for at least 24 h) organic wheat flour with 5% brewer's yeast powder and reared at 30°C, 70% humidity and a 12:12 h, light: dark cycle.
Males and females from the same 24 h egg lay and were 40 days old were anaesthetized in a cold chamber at 4°C for 3 min and prepared as indicated in Donato et al. (2021). Briefly, beetles were fixed in 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PBS; Electron Microscopy Sciences) overnight at 4°C, washed with PBS and dehydrated in a graded ethanol series. Specimens were then stored and scanned (two males and two females) in absolute ethanol.
2.2. Phase contrast micro‐computed tomography (PhC micro‐CT) data acquisition
Tomographic data acquisition was performed at the SYRMEP (SYnchrotron Radiation for MEdical Physics) beamline of the Elettra Synchrotron facility in Trieste (Italy) illuminating the sample with a polychromatic radiation (Donato et al., 2022). The X‐ray beam available at the beamline is generated by a storage ring‐bending magnet and the energy of photons ranges from 8.5 to 40 keV. To compensate for the effects of beam hardening, we have filtered out the low‐energy components of the X‐ray beam using 1.0 mm of Silicon, thus resulting in beam with an average energy of about 20 keV (storage ring operating mode at 2.0 GeV). Taking into account the natural divergence of the X‐rays produced by the source, the beam cross‐section at the sample position (22.5 m from the source) is almost 150 mm (horizontal) × 5 mm (vertical). The imaging system consisted of a water‐cooled Hamamatsu sCMOS detector, optically coupled to a GGG (Gd3Ga5O12:Eu) scintillator (with a 17 μm thick sensitive layer) utilizing a set of optical lenses that can be used to set different magnification levels. The native pixel size of the detector is 6.5 × 6.5 μm, while the sensor has an area of 2048 × 2048 pixels. The optics coupled with the detector allow the pixel size to be adjusted between 1 and 6.5 μm.
The tomographic images were reconstructed from 1800 evenly spaced projections, spanning 180 degrees and acquired in continuous rotation mode. The projection images were obtained in the propagation‐based phase‐contrast regime (Brombal, 2020; Rigon, 2014) by setting a propagation distance equal to 100 mm between the sample and the detector. The propagation distance was set in order to optimize, in the near‐field regime, the signal‐to‐noise ratio once the pixel size has been set (Donato et al., 2022). Phase‐contrast effects resulting from propagation in free‐space lead to an enhanced contrast arising at the boundaries between details of different composition (the so‐called edge‐enhancement).
The optical magnification of the detecting system was set to 4.3, resulting in a pixel size of 1.5 × 1.5 μm and a lateral field of view of almost 3.1 × 3.1 mm. The exposure time was set to 200 ms/projection. Two vertical scans were required to image both male and female specimens. Considering every auxiliary time (i.e., acquisition of flat and dark current images, vertical step, back‐rotation etc.) the acquisition of each specimen required approximately 10 min.
2.3. Computer‐based 3D‐reconstruction
Digital volumes were obtained from the reconstruction of each acquired dataset. Image reconstruction was performed with a GPU‐based filtered back‐projection (FBP) algorithm using the SYRMEP Tomo Project (STP) software suite (Brun et al., 2017). A Shepp‐Logan filter was used in the FBP reconstruction. Prior to image reconstruction, projections were further processed by conventional flat‐fielding, ring removal and by using a phase‐retrieval algorithm based on the homogeneous transport of intensity equation (TIE‐Hom) (Paganin et al., 2002). The latter algorithm allows to enhance the signal‐to‐noise ratio (when compared to attenuation‐based reconstruction) but at the cost of a loss of the edge‐enhancement signal (Gureyev et al., 2017). The δ/β filter parameter was tuned to effectively regulate the amount of smoothing, as usually used in experimental practice. For this experiment, we used a two‐material delta/beta phase‐retrieval (Beltran et al., 2010) approach, and we set delta/beta = 400. This value was calculated at the average energy of the spectrum considering a soft‐tissue/adipose interface by using a publicly available database (http://ts‐imaging.science.unimelb.edu.au//Services//Simple//ICUtilXdata.aspx). After processing, the final CT reconstruction yields a 3D map that is essentially proportional to the linear attenuation coefficient of the sample (Brombal et al., 2018; Piai et al., 2019).
2.4. Post‐processing, image segmentation and rendering
The full digital volume of each specimen was obtained by concatenating (using linear blending) the two individual stacks of reconstructed images. Post‐processing and segmentation were performed using Avizo® 3D (version 3D; Thermo Fisher Scientific). A male specimen was segmented to obtain a binary representation of testes, nervous system and alimentary canal. For the female specimens, we performed: (i) segmentation of the reproductive organs for the sexually mature sample and (ii) segmentation of the nervous system and the alimentary canal for the sexually immature specimens. For each organ/system, we started by extracting a region of interest (ROI) around it. Then an edge‐preserving filter was applied to the ROI's data followed by an interactive (manual) thresholding. Morphological operators (border kill, closing and/or opening, removal of small structures) were used to remove elements not belonging to the structure we wanted to segment. Finally, by using the “Segmentation Editor” of Avizo, the preliminary segmentation was improved by manual refinements. We used the “Brush” and “Lasso” (with auto‐trace option) tools on a subset of 2D segmented slices to add or remove misclassified elements and then performed interpolation. Volume rendering of different section of the beetles were performed using Avizo® 3D while animations were generated with the scientific visualisation software Drishti (Limaye, 2012).
2.5. Image analyses and measurements
Volumes of each organ were calculated using the “Label Analysis” module and the “Volume3D” measure, while the length of the alimentary canal and nervous systems were obtained from their skeletonization. The “Centerline Tree” module was employed for this procedure by forcing the number of branches to be equal to 1 in order to obtain the longest segment connecting the endpoints of each structure. The skeleton representation is then smoothed (“Smooth Line Set” module) before calculating its curved length (i.e. the true and curved course of a segment) using the “Spatial Graph Statistics” module.
3. RESULTS
3.1. Internal anatomy
SR‐PhC micro‐CT‐based virtual sections, 3D reconstructions and segmentation allowed us to describe the anatomy of T. castaneum in situ (Figures 1, 2, 3, 4, 5, 6; Figure S1 and Video S1 and S2) and estimate the volumetric dimensions of the organs (Table 1), as reported below, for both males and females.
FIGURE 1.
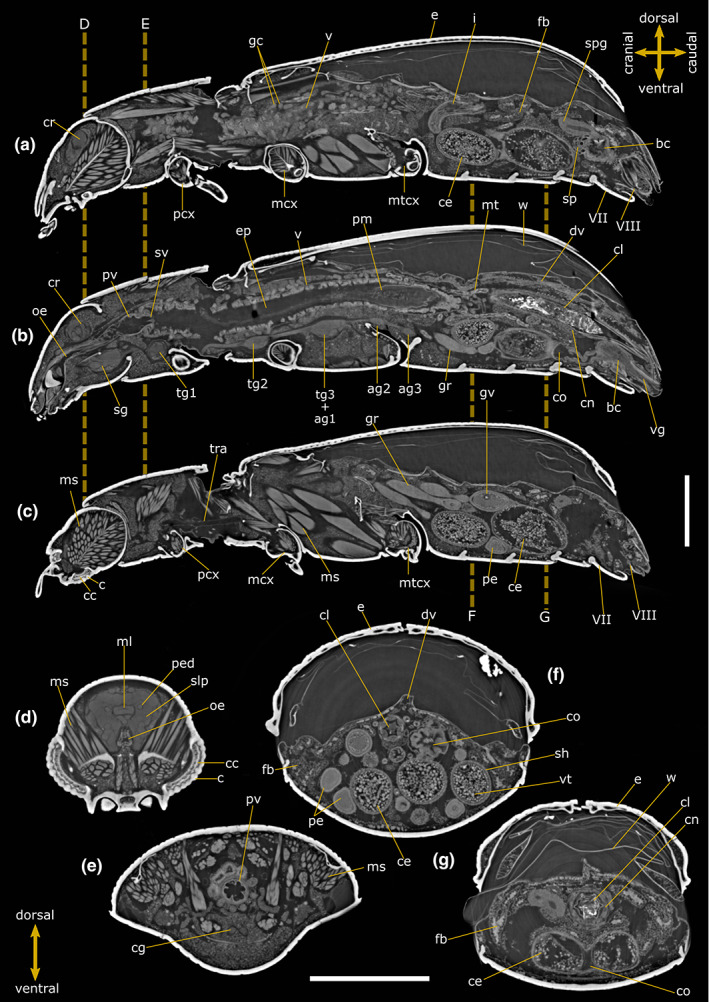
Internal anatomy of Tribolium castaneum female. Two‐dimensional PhC micro‐CT‐based longitudinal (a–c) and cross (d–g) sections. Dashed lines indicate the levels of head (d), pronotum (e) and abdominal (f, g) cross‐sections. VII: Abdominal sternite VII; VIII: Abdominal sternite VIII; ag 2–3: Abdominal ganglia 2 and 3; bc: Bursa copulatrix; c: Cornea; cc: Crystalline cones; ce: Chorionic egg; cg: Connective of ganglia; cl: Colon; cn: Cryptonephridial system; co: Common oviduct; cr: Cerebrum; dv: Dorsal vessel; e: Elytrae; ep: Ectoperitrophic space; fb: Fat bodies; gc: Gastric caeca; gr: Germarium; gv: Germinal vesicle; i: Ileum; mcx: Mesocoxae; ms: Muscle; ml: Median lobe; mt: Malpighian tubules; mtcx: Metacoxae; oe: Oesophagus; pcx: Procoxae; pe: Pre‐vitellogenic egg; ped: Peduncle; pm: Peritrophic matrix; pv: Proventriculus; sg: Suboesophagean ganglion; sh: Epithelial sheath; slp: Superior lateral protocerebrum; spg: Spermathecal gland; sp: Spermatheca; sv: Stomodeal valve; tg1‐2: Thoracic ganglia 1 and 2; tg3 + ag1: Complex of thoracic ganglion 3 and abdominal ganglion 1; tra: Tracheae; v: Ventriculus; vg: Vagina; vt: Vitellum; w: Membranous wings. Scale bars: 500 μm (a–g).
FIGURE 2.

Internal anatomy of Tribolium castaneum male. Two‐dimensional PhC micro‐CT‐based longitudinal (a–c) and cross (d–g) sections. Dashed lines indicate the levels of head (d), pronotum (e) and abdominal (f, g) cross‐sections. VIII: Abdominal sternite VIII; IX: Abdominal sternite IX; ae: Aedeagus; agl: Accessory glands; c: Cornea of compound eye; cc: Crystalline cones; cg: Connective of ganglia; cl: Colon; cn: Cryptonephridial system; cr: Cerebrum; dd: Deferent duct; dv: Dorsal vessel; e: Elytrae; ed: Ejaculatory duct; ep: Ectoperitrophic space; fb: Fat bodies; gc: Gastric caeca; i: Ileum; lb: Lobe of testis; m: Mandible; mcx: Mesocoxae; ml: Median lobe; ms: Muscle; mt: Malpighian tubules; mcx: Mesocoxae; mtcx: Metacoxae; mx: Maxilla; mxp: Maxillary palp; oe: Oesophagus; ol: Optical lobe; p: Pedicel; pcx: Procoxae; ped: Peduncle; py: Pyloric valve; pm: Peritrophic matrix; pv: Proventriculus; r: Rectum; rb: Rhabdoms; sc: Scape of antennae; sg: Subesophagean ganglion; slp: Superior lateral protocerebrum; sv: Stomodeal valve; t: Testis; tg1‐2: Thoracic ganglia 1 and 2; tg3 + ag1: Complex of thoracic ganglion 3 and abdominal ganglion 1; tr: Tritocerebrum; v: Ventriculus; w: Membranous wings. Scale bars: 500 μm (a–g).
FIGURE 3.
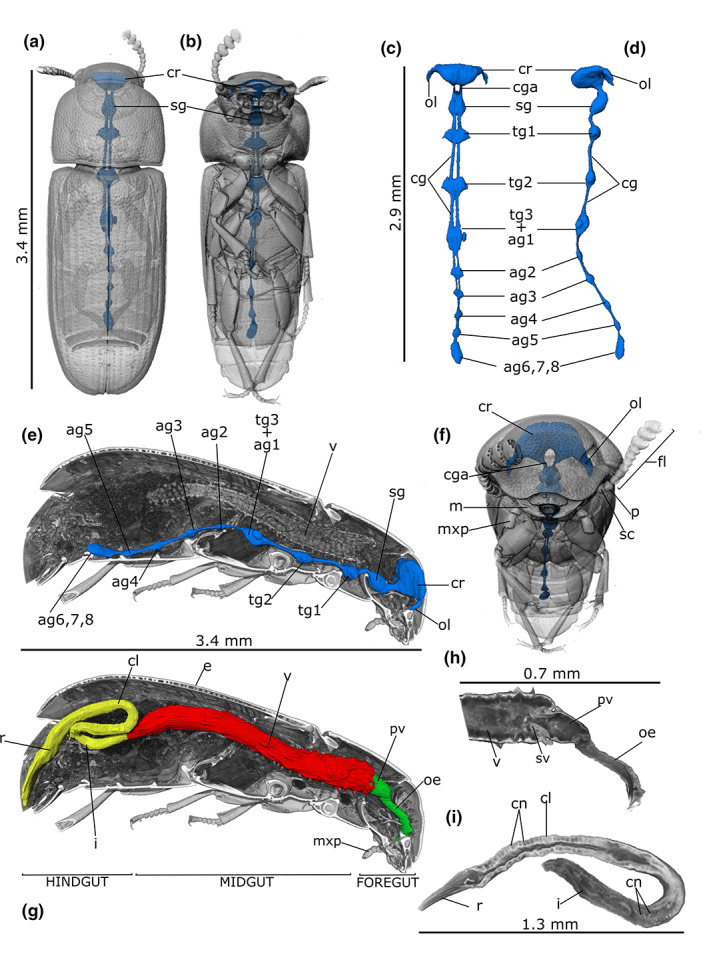
PhC micro‐CT 3D rendering and reconstructions of the nervous (a–f) and alimentary (g–i) systems in Tribolium castaneum male. Localization of nervous system in the transparent rendered body, dorsal (a), ventral (b) and frontal (f) view. Dorsal (c) and lateral (d) view of the segmented nervous system. (e) Longitudinal section of the body showing in situ localization of the cerebrum (cr) connected to the ganglia through the connectives (cg). (g) alimentary canal reconstruction showed in a longitudinal sectioned volume of the rendered body. Detailed rendering images of the foregut (h) and hindgut (i). ag2‐5: Abdominal ganglia 2–5; ag6, 7, 8: Terminal abdominal ganglia fused to form a large caudal ganglion; cga: Circumesophageal connectives loop around the digestive system to link the brain (cr) and subesophageal complex together; cl: Colon; cn: Cryptonephridial system; fl: Antennal flagellum; i: Ileum; m: Mandible; mxp: Maxillary palp; oe: Oesophagus; ol: Optical lobe; p: Pedicel of antenna; pv: Proventriculus; r: Rectum; sc: Scape of antenna; sg: Subesophagean ganglion; sv: Stomodeal valve; tg1‐2: Thoracic ganglia 1–2; tg3 + ag1: Complex of thoracic ganglion 3 and abdominal ganglion 1; v: Ventriculus.
FIGURE 4.

PhC micro‐CT 3D rendering and reconstructions of reproductive system in Tribulium castaneum female. Volume reconstruction of right (blue) and left (light blue) ovaries (ov) showed in the transparent rendered body, dorsal (a) and ventral (B). (c) Segmentation of ovaries showing six ovarioles (ovl) and the apical filaments (f) connecting the gonads to the sospensorium. (d) Reconstruction of the ovaries showed in a longitudinal sectioned volume of the rendered body. (e, f) Longitudinal and lateral rendering of telotrophic ovarioles showing the germinarium (gr) end vitellarium (vr). (g, h) Frontal sections of the abdomen showing in situ oviduct and ovarioles at different level of egg maturation (H) and a detail of the spermatheca (sp) and spermathecal gland (spg) (g). VII: Abdominal sternite VII; VIII: Abdominal sternite VIII; bc: Bursa copulatrix; ce: Chorionic egg; co: Common oviduct; e: Elytrae; gr: Germinarium; gv: Germinal vesicle; nc: Nurse cells; pe: Pre‐vitellogenic egg; sh: Epithelial sheath; spg: Spermathecal gland; sp: Spermatheca; v: Ventriculus; vg: Vagina; vr: Vitellarium; vt: Vitellum.
FIGURE 5.

PhC micro‐CT 3D rendering and reconstructions of reproductive system of Tribolium castaneum male. Right (blue) and left (light blue) testis (t) in rendering image of beetle's body, dorsal (a) and ventral (b) view. (c) Volumetric segmentation of testis. (d) Longitudinal section transparent rendered of the body showing in situ testis. (e) External view of testis showing six lobes (lb). Frontal (f) and sagital (g) sections of the abdominal cavity –showing in situ left and right testis (t), accessory glands (agl) and aedeagus (ae). (h) Longitudinal section of the testis, showing different compartmentalization of spermatic cystis at different degrees of maturation. VIII: Abdominal sternite VIII; IX: Abdominal sternite IX; ae: Aedeagus; agl: Accessory glands; dd: Deferent duct; e: Elytrae; ed: Ejaculatory duct; fo: Foramen of aedeagus; lb: Testis lobes; r: Rectum; t: Testis; v: Ventriculus.
FIGURE 6.

PhC micro‐CT‐based images of T. castaneum female. Two‐dimensional cross (a, b) sections and 3D rendering of the abdomen (c) showing immature ovarioles and abdominal exocrine glands (re). ag2: Abdominal ganglion 2; bc: Bursa copulatrix; cd: Collective duct of abdominal glands; cl: Colon; cn: Cryptonephridial system; dv: Dorsal vessel; e: Elytrae; fb: Fat bodies; gr: Germarium; gv: Germinal vesicle; i: Ileum; pe: Pre‐vitellogenic egg; r: Rectum; v: Ventriculus; w: Membranous wings. Scale bars: 500 μm (a, b).
TABLE 1.
Volumetric measurements of segmented organs in Tribolium castaneum
| Volume (mm3) | Female | Length (mm) | Volume (mm3) | Male | Length (mm) | |||
|---|---|---|---|---|---|---|---|---|
| Alimentary canal a | Foregut | 5.85 × 10−3 | 0.71 | 7.86 × 10−3 | 0.70 | |||
| Midgut | 10.0 × 10−2 | 2.20 | 6.73 × 10−2 | 2.22 | ||||
| Hindgut | 4.95 × 10−2 | 2.77 | 2.06 × 10−2 | 2.49 | ||||
| Total | 15.50 × 10−2 (body: 2.30) | 6.76% d | 5.68 (body: 3.63) | 9.60 × 10−2 (body: 2.47) | 3.88% d | 5.41 (body: 3.40) | ||
| Nervous system b | Cerebrum | 7.17 × 10−3 | 1.07 × 10−2 | |||||
| Sub‐oesophageal ganglion | 1.65 × 10−3 | 2.47 × 10−3 | ||||||
| Ganglia | tg1 | 2.25 × 10−3 | 1.73 × 10−3 | |||||
| tg2 | 1.25 × 10−3 | 1.79 × 10−3 | ||||||
| tg3 + ag1 | 1.85 × 10−3 | 2.42 × 10−3 | ||||||
| ag2 | 2.30 × 10−4 | 3.40 × 10−4 | ||||||
| ag3 | 2.50 × 10−4 | 2.73 × 10−4 | ||||||
| ag4 | 2.60 × 10−4 | 3.13 × 10−4 | ||||||
| ag5 | 2.20 × 10−4 | 2.65 × 10−4 | ||||||
| ag6,7,8 | 8.30 × 10−4 | 11.30 × 10−3 | ||||||
| Total | 1.68 × 10−2 (body: 2.30) | 0.73% | 2.91 (body: 3.63) | 2.24 × 10−2 (body: 2.47) | 0.91% | 3.16 (body: 3.40) | ||
| Gonads c | Right | 6.30 × 10−2 | 1.51 × 10−1 | 1.17 | ||||
| Left | 6.60 × 10−2 | 1.37 × 10−1 | 1.19 | |||||
| Total | 1.29 × 10−1 (body: 2.63) | 4.90% | 2.88 × 10−1 (body: 2.30) | 11.66% |
Alimentary canal measurements are strongly influenced by peristaltic movement and variability can occur between portions and individuals.
The ganglia are reported as thoracic (tg) and abdominal (ag), followed by a progressive number from the cerebrum to the caudal portion.
Gonads were measured in sexually mature individuals.
(Alimentary canal volume / body volume) × 100.
3.2. Central nervous system
The two‐dimensional (2D) virtual sections show the central nervous system in females (Figure 1a,b,d) and males (Figure 2a,b,d), which is divided into two main parts: the cerebrum and the ventral ganglia. The nervous system occupies about 0.73% of the body volume in female and 0.91% in male. Three brain (cerebrum) regions are recognizable: protocerebrum including the optic lobe, deuterocerebrum and tritocerebrum (Figures 1d and 2d). The 3D reconstructions allow visualization of the circumesoephageal connectives that link the brain to the suboesophagean ganglion (Figure 3a–f). The ventral chain consists of three thoracic and five abdominal ganglia (Figure 3c–e; Figure S1). The abdominal ganglia are smaller than those of the thorax (Figure 3a–f). The first abdominal ganglion is fused with the last thoracic ones, while the last abdominal ganglion is the fusion of the sixth, seventh and eighth ganglia. The volumes of the brain and ganglia are listed in Table 1.
3.3. Alimentary canal
The alimentary canal reaches a length of approximately 5.41 mm in males and 5.68 mm in females, which in both cases exceeds the total length of the body (3.63 mm in females and 3.4 mm in males) and filling approximately 3.88% and 6.76% of the body volume, respectively (15.50 × 10−2 mm3 in female and 9.60 × 10−2 mm3 in male) (Table 1). It is divided into three main functional parts: foregut, midgut and hindgut (Figure 3g). The foregut is about 0.7 mm long in both specimens (7.86 × 10−3 mm3 in volume) and consist of the pharynx, oesophagus and proventriculus (Figures 1a,b, 2a,b and 3g; Figure S1). The 2D virtual sections of both females and males show an epithelium of flattened, irregular cells lined by the cuticle of intima (Figures 1a,b and 2a,b). The oesophagus is a tubular structure with a diameter of 58.43 ± 6.59 μm (n = 6) and enlarges in its posterior part to form a very short crop 77.53 ± 8.24 μm (n = 4) in diameter (Figures 1b, 2a and 3h). The diameter of the different portions is strongly influenced by peristaltic movement, as a result, variability can occur between individuals. The proventriculus connects the distal part of the oesophagus to the midgut and has a stomodeal valve distally (Figures 1b,e and 2b). The epithelium is wave‐like folded and internally covered with an intima that bears sclerotized projections. Longitudinal muscles are also visible, arranged in four bundles and surrounded by circular muscles (Figures 1e, 2e and 3h).
The midgut is an elongate tube approximately 2.2 mm long in both samples (volume: 10.0 × 10−2 mm3 in the females and 6.73 × 10−2 mm3 in the males) and the columnar epithelial cells are evident along the lumen wall (Figures 1a,b, 2a,b and 3h). The peritrophic matrix is clearly visible in the 2D slices and envelopes the food in the lumen of the midgut separating them from epithelium. The difference in attenuation present in the midgut lumen makes it possible to clearly distinguish an endoperitrophic portion, characterised by higher attenuation, which comprises the food wrapped by the peritrophic matrix; and the ectoperitrophic space, between the intestinal epithelium and the peritrophic matrix, with lower attenuation. (Figures 1b and 2b). Of notice, the enhanced visibility of the membrane results from the phase‐contrast effect and the application of the phase‐retrieval, which enable, respectively, to detect the edges of the structure and to increase the signal‐to‐noise ratio of image. The midgut wall contains numerous finger‐like gastric caeca, which are enlarged at their bases and protrude into the body cavity as narrow free tubules (Figures 1a,b and 2a,b).
The hindgut, approximately 2.5 and 2.8 mm long and 2.06 × 10−2 mm3 and 4.95 × 10−2 mm3 in volume for male and female, respectively, is folded several times and is divided into ileum, colon and rectum (Figures 1a,b, 2a,b and 3g,i; Figure S1). The ileum is a narrow tube separated from the midgut by a pyloric valve and forms an S‐shaped bend (Figures 1b, 2c and 3h,j). The Malpighian tubules arise from the midgut‐hindgut junction as a long, thin, blind‐ending tube that project into the haemocoelic cavity. Their distal part loops backwards into the cryptonephridial system at the anterior end of the rectum (Figures 1g, 2c, 3i and 6a) while the medial part is free to move. The epithelium of the ileum, lined by the cuticle of intima, projects 6 longitudinal folds into the lumen accompanied by the underlying longitudinal muscles of the wall. The 2D virtual sections discriminate six rectal pads located on the inner surface at the anterior part of the rectum (Figure 2g).
3.4. Female reproductive system
The female reproductive system consists of a pair of ovaries connected with lateral oviducts joining in a common duct, a bursa copulatrix and a vagina (Figures 1a–c,f,g and 4f,g). Each ovary (approximatively 6.30 × 10−2 mm3 in volume), located on the dorso‐lateral side of the alimentary canal, is composed of 6 telotrophic ovarioles (Figure 4a–d). The ovariole, which is enveloped by an outer epithelial sheath, is divided into an anterior terminal filament, a trophic chamber housing nurse cells (germinarium), a vitellarium and pedicel (Figure 4a–g). The terminal filaments join at the mid‐line dorsally to the alimentary canal (Figure 4a,d). In the vitellarium, early oocytes can be seen along the ovariole in 3‐ to 4‐day‐old females (Figure 6a,b). In a 1‐week‐old female, follicle chambers consisting of an oocyte and single‐layer follicular cells are visible (Figure 1a–c,f). Eggs at different stages of maturation are found in the abdomen showing chorion, periplasm, yolk and nucleus (germinal vesicle) in 2D virtual sections (Figure 1a–c,f) and 3D renderings (Figure 4a–h). The chorionic eggs observed in sexually mature females (Figures 1a–c,f and 4e–h) reach a diameter of approximately 225.53 ± 42.52 μm (n = 10), while only previtellogenic eggs, with a smaller diameter and without yolk, are visible in immature females (Figure 6a–c). The ovaries fill a body volume of approximately 4.90% in the sexually mature female. The spermatheca and spermathecal glands (Figure 1a and 4g,h) are located on the dorso‐anterior part of the bursa copulatrix. The spermatheca is a convoluted tube located at the lateral wall of the bursa copulatrix (Figure 4g). The median oviduct, vagina and bursa copulatrix are enveloped by a thick layer of musculature (Figure 1a and 4g,h).
3.5. Male reproductive system
The internal reproductive tract of males consists of a pair of testes, the deferent ducts and two pairs of accessory glands (Figures 2a–c,f and 5a–h). The six lobed testes (Figures 2a,b,f and 5e) occupy most of the dorso‐lateral volume of the male abdomen (about 11.66% of the body; 1.37 × 10−1 mm3 in volume) (Figures 1a and 5a,b,d,f). Each lobe contains testicular follicles with germ cells in various stages of developmental (Figures 2a,f and 5f,g,h). The vas deferens connect the testes to the ejaculatory duct (Figure 2a), which passes through the median foramen of the aedeagus (Figures 2g and 5g). Two pairs of male accessory glands are well recognizable (Figure 2a–c) opening into the ejaculatory duct at the major junction with the vas deferens one pair of long, coiled, tubular glands and one pair of short, finger‐shaped glands (Figures 2a,b,f and 5f,h).
3.6. Other structure
A pair of secretory glands, also called stink glands, are found in the abdomen on the lateral sides of the hindgut (Figure 6b,c). These glands are composed of secretory cells and a sac‐shaped reservoir chamber storing different amounts of secretion. The secretory duct opens at the sides of the last abdominal sternite.
4. DISCUSSION
Virtual dissection and 3D rendering carried out using SR‐PhC micro‐CT allowed us for the first time to describe the spatial organization of T. castaneum organs in both males and females in situ. Our results are consistent with previous studies addressed using histological analyses to describe both the digestive (Ameen & Rahman, 1973; Sinha, 1958) and reproductive (Banu et al., 2006; Bloch Qazi et al., 1998; Takaki et al., 2020) systems. The advantage of SR‐PhC micro‐CT imaging is that it provides more accurate information about the shape, size and location of organs, using a small number of samples and avoiding artefacts in sample dissections and protocol preparations. In addition, the small size of the species analysed would preclude traditional dissection techniques and histology or makes it difficult to maintain integrity during the preparation process. The general morphology of the alimentary canal showed in the renderings and 3D reconstructions is consistent with descriptions reported in previous studies of beetle species (Chapman, 2012; Crowson, 1981; Snodgrass, 1935). Moreover, the morphology is very similar to species with omnivorous feeding habit and adapted to consume stored grain products such as the lesser mealworm Alphitobius diaperinus (McAllister et al., 1995). Sagittal virtual sections and 3D reconstructions of the digestive tract confirmed a large reduction in the length of the crop in this species, as noted in a previous study (Ameen & Rahman, 1973). This reduction, also observed in other tenebrionid species, functionally promotes the transit and accumulation of bacteria towards the midgut (McAllister et al., 1995). The selective pressure of the environment favoured this structural adaptation, likely due to the presence of the peritrophic matrix throughout the intestinal lumen, which prevents pathogens from passing through the intestinal epithelium into the hemocoel and causing infection (Caccia et al., 2019; Terra, 2001). However, this morphological and functional organisation has the potential to compromise the quality of stored foods following infestations with T. castaneum and contribute to their microbiological contamination (Negi et al., 2022), as bacteria are excreted through faeces. This is a health and economic concern, as demonstrated by recent studies, showing that the red flour beetle could act as a vector of important pathogens in poultry and livestock feed and contribute to the spread of antibiotic‐resistant species, such as enterococci (Channaiah et al., 2010).
The midgut ultrastructure of T. castaneum has not been well studied. It has only been described in one study, which described a gut alteration of the intestine induced by a parasitic infection of gregarine (Gigliolli et al., 2015), leading to regeneration of the crypts (caeca) by apoptotic, necrotic and autophagic processes (Malagoli et al., 2010). The density of regenerating crypts in the midgut wall was lower compared to generalist predators previously investigated using SR‐PhC micro‐CT (Donato et al., 2021). The distribution and number of crypts in the midgut wall and the texture of the peritrophic matrix has been demonstrated to be related to the feeding habits of species in Polyphagous beetles (Nardi & Bee, 2012). On the other hand, the ventriculus is more developed in length and represents the longest part of the gut in this species. This could be related to the increased need for water to digest dry plant food, as the gastric caeca provide additional surface area for water absorption and enzymatic digestion (Holtof et al., 2019).
Malpighian tubules are involved in excretion and osmoregulation of insects (Nocelli et al., 2016) and are a useful tool in ecotoxicological studies (Almeida Rossi et al., 2013; Giglio et al., 2017; Giglio & Brandmayr, 2017; Talarico et al., 2014). In T. castaneum, the distal part of the tubules forms a cryptonephridial system that is closely connected to the wall of the colon by a perirectal membrane. T. castaneum is the outstanding model species in which the morphological development and the molecular and physiological functions of the excretory system have been described and compared with those of Drosophila in order to define the selective pressure that has produced this specialised structure from an evolutionary perspective (King & Denholm, 2014). The cryptonephridial system is typically found in species that feed on dry food or live in environmental conditions requiring minimal water loss such as dipterans (Green, 1980), coleopterans (Grimstone et al., 1968; Özyurt Koçakoğlu et al., 2022) and lepidopterans (Kolosov & O'Donnell, 2019; Ramsay, 1976). The high resolution of this structure obtained in our 2D virtual sections and 3D reconstructions suggests that this technique may be useful for further comparative analyses of the cryptonephridial system in insects.
Given the importance to control the dispersal of this species, researchers have focused on the reproductive performance of T. castaneum males and females (Bernasconi & Keller, 2001), including hormonal signal involved in controlling oocyte maturation (Parthasarathy et al., 2010) or male accessory glands (Parthasarathy et al., 2009) and mating behaviour (Boukouvala et al., 2019; Vasudeva et al., 2021). Currently, information on the reproductive tract morphology is fragmentary and has received little attention other than studies focusing on adult irradiation for population control (Banu et al., 2006), hormonal activity during maturation of ovarioles (Takaki et al., 2020) and sperm storage in the female reproductive organ (Bloch Qazi et al., 1998). In addition, detailed knowledge of the female reproductive organs has been helpful in revising the classification of tenebrionid species (Tschinkel & Doyen, 1980) and defining the evolution of teletrophic meroistic ovarioles in insects (Trauner & Büning, 2007). Thus, the new description of male and female reproductive organs in T. castaneum provided in our study by combining virtual dissection and 3D reconstructions can be a useful morphological dataset for further taxonomic and functional studies.
Anatomical descriptions and 3D reconstructions of the brain area in T. castaneum have been performed in previous studies using immunohistochemical protocols in combination with a confocal laser scanning microscope to describe difference between the sexes (Dreyer et al., 2010; Hunnekuhl et al., 2020) and embryonic development (Buescher et al., 2020). The virtual dissections and volumetric reconstruction performed in our study indicated that the same qualitative and quantitative information can be obtained using SR‐PhC micro‐CT imaging, avoiding time‐consuming protocols for sectioning and staining tissues and limiting the inevitable tissue distortion caused by dissection. Body parts as small as the visual system (Giglio et al., 2022) and even the composition of the egg (Vommaro et al., 2022), can be easily studied using this technique, which is an alternative to conventional techniques for small and rare samples.
The exocrine abdominal glands occur in several families of Coleoptera and produce specific defensive secretions with repellent and irritant properties against predators (Blum, 1981; Chapman, 2012; Dettner, 1987). Their morphology and the chemical composition of the secretions have been described comparatively in Carabidae (Giglio et al., 2011; Giglio et al., 2021; Vranić et al., 2021; Will et al., 2000) and Tenebrionidae (Tschinkel & Doyen, 1980). In T. castaneum, several types of highly reactive and toxic benzoquinone compounds have been detected in the secretions of abdominal glands using chemical analyses such as thin‐layer chromatography (Happ, 1968; Unruh et al., 1998) and gas chromatography in combination with mass spectrometry (Villaverde et al., 2007), while 511 gland‐specific genes have been identified by RNA sequencing and transcriptomics in different gland tissues (Li et al., 2013). The interest in these secretions is mainly related to economic concerns because they infest stored products and give them unpleasant organoleptic properties (Hodges et al., 1996). On the other hand, secretions of red flour beetle have been isolated and characterised to define the protective role of chemical defences against natural enemies such as predators and pathogens from an ecological perspective (Sawada et al., 2020).
5. CONCLUSIONS
Our results provide, for the first time, a set of morphological data collected using SR‐PhC micro‐CT and enrich the general knowledge of T. castaneum, a promising and increasingly popular model for experimental biology studies. Micro‐CT imaging facilitated virtual dissection and anatomical reconstruction of the miniaturised beetle T. castaneum, visualising the internal structure of organs in situ without the distortions that occur during dissection. Morphological descriptions of organs are helpful for comparing 3D reconstructions of organs with other insect models, including studies using confocal and histological imaging techniques. In addition, adequate description of anatomical structures in pests of stored products also contributes to physiological and ecological information useful for developing pest management strategies and new targets for more specific and effective insecticides. Virtual sections, videos and 3D reconstructions included in this study could be made available as data for research purposes on a common model, but also as a useful tool for teaching insect anatomy.
AUTHOR CONTRIBUTIONS
Concept/design: A.G., M.L.V.; investigation and acquisition of data: S.D., A.G., L.K.L., M.L.V.; image reconstruction, segmentation and quantification: S.D.; data analysis/interpretation: S.D., A.G., M.L.V.; drafting of the manuscript: A.G. with inputs from S.D., M.L.V.; figures and video preparation: S.D., M.L.V.; funding: A.G.; supervision: A.G.; critical revision of the manuscript and approval of the article: all authors.
FUNDING INFORMATION
S.D. was funded by “AIM: Attraction and International Mobility”‐ PON R&I 2014–2020 Regione Calabria, and “Progetto STAR 2”‐ (PIR01_00008) ‐ Italian Ministry of University and Research.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest
Supporting information
Figure S1.
Video S1.
Video S2.
ACKNOWLEDGMENTS
The authors are grateful to Elettra Synchrotron Trieste for providing access to the SYRMEP beamline and to members of the SYRMEP beamline staff, especially Dr Giuliana Tromba, for help in performing the computerized microtomography experiment. The authors acknowledge Prof. Joachim Kurtz for providing T. castaneum specimens from the population raised in his laboratory.
Vommaro, M.L. , Donato, S. , Lo, L.K. , Brandmayr, P. & Giglio, A. (2023) Anatomical study of the red flour beetle using synchrotron radiation X‐ray phase‐contrast micro‐tomography. Journal of Anatomy, 242, 510–524. Available from: 10.1111/joa.13796
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Adamski, Z. , Bufo, S.A. , Chowański, S. , Falabella, P. , Lubawy, J. , Marciniak, P. et al. (2019) Beetles as model organisms in physiological, biomedical and environmental studies—a review. Frontiers in Physiology, 10, 1–22. Available from: 10.3389/fphys.2019.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba‐Alejandre, I. , Alba‐Tercedor, J. & Hunter, W.B. (2020) Anatomical study of the female reproductive system and bacteriome of Diaphorina citri Kuwayama, (Insecta: Hemiptera, Liviidae) using micro‐computed tomography. Scientific Reports, 10(1), 1–14. Available from: 10.1038/s41598-020-64132-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba‐Alejandre, I. , Alba‐Tercedor, J. & Vega, F.E. (2019) Anatomical study of the coffee berry borer (Hypothenemus hampei) using micro‐computed tomography. Scientific Reports, 9(1), 1–16. Available from: 10.1038/s41598-019-53537-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida Rossi, C. , Roat, T.C. , Tavares, D.A. , Cintra‐Socolowski, P. & Malaspina, O. (2013) Effects of sublethal doses of imidacloprid in malpighian tubules of africanized Apis mellifera (hymenoptera, Apidae). Microscopy Research and Technique, 76(5), 552–558. [DOI] [PubMed] [Google Scholar]
- Ameen, M.U. & Rahman, M.F. (1973) Larval and adult digestive tracts of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). International Journal of Insect Morphology and Embryology, 2(2), 137–152. Available from: 10.1016/0020-7322(73)90014-7 [DOI] [Google Scholar]
- Banu, P. , Ali, I. & Salam, M. (2006) Effects of gamma radiation on the reproductive organs in the red flour beetle Tribolium castaneum (Herbst). University Journal of Zoology, Rajshahi University, 25, 11–14. Available from: 10.3329/ujzru.v25i0.318 [DOI] [Google Scholar]
- Bäumler, F. , Gorb, S.N. & Büsse, S. (2018) Comparative morphology of the thorax musculature of adult Anisoptera (Insecta: Odonata): functional aspects of the flight apparatus. Arthropod Structure and Development, 47(4), 430–441. Available from: 10.1016/j.asd.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Beauchamp, T.L. & Morton, D.B. (2015) The upper limits of pain and suffering in animal research. Cambridge Quarterly of Healthcare Ethics, 24(4), 431–447. Available from: 10.1017/S0963180115000092 [DOI] [PubMed] [Google Scholar]
- Beliaev, M. , Zöllner, D. , Pacureanu, A. , Zaslansky, P. , Bertinetti, L. & Zlotnikov, I. (2020) Quantification of sheet nacre morphogenesis using X‐ray nanotomography and deep learning. Journal of Structural Biology, 209(1), 107432. Available from: 10.1016/j.jsb.2019.107432 [DOI] [PubMed] [Google Scholar]
- Beltran, M.A. , Paganin, D.M. , Uesugi, K. & Kitchen, M.J. (2010) 2D and 3D X‐ray phase retrieval of multi‐material objects using a single defocus distance. Optics Express, 18(7), 6423–6436. Available from: 10.1364/oe.18.006423 [DOI] [PubMed] [Google Scholar]
- Bernasconi, G. & Keller, L. (2001) Female polyandry affects their sons' reproductive success in the red flour beetle Tribolium castaneum . Journal of Evolutionary Biology, 14(1), 186–193. [DOI] [PubMed] [Google Scholar]
- Betz, O. , Wegst, U. , Weide, D. , Heethoff, M. , Helfen, L. , Lee, W. et al. (2007) Imaging applications of synchrotron X‐ray phase‐contrast microtomography in biological morphology and biomaterials science. I. General aspects of the technique and its advantages in the analysis of millimetre‐sized arthropod structure. Journal of Microscopy, 227(1), 51–71. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi, M.C. , Aprille, J.R. & Lewis, S.M. (1998) Female role in sperm storage in the red flour beetle, Tribolium castaneum . Comparative Biochemistry and Physiology—A Molecular and Integrative Physiology, 120(4), 641–647. Available from: 10.1016/S1095-6433(98)10081-8 [DOI] [Google Scholar]
- Blum, M. (1981) Chemical defenses of arthropods. In: Blum, M.S. (Ed.) Chemical defenses of arthropods. New York: Academic Press, p. 561. Available from: 10.1016/B978-0-12-108380-9.50006-5 [DOI] [Google Scholar]
- Boukouvala, M.C. , Romano, D. , Kavallieratos, N.G. , Athanassiou, C.G. , Stefanini, C. , Canale, A. et al. (2019) Asymmetric courtship boosts male mating success in the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Journal of Stored Products Research, 81, 1–6. [Google Scholar]
- Brombal, L . (2020). X‐Ray Phase‐Contrast Tomography: underlying physics and developments for breast imaging http://link.springer.com/10.1007/978‐3‐030‐60433‐2
- Brombal, L. , Donato, S. , Dreossi, D. , Arfelli, F. , Bonazza, D. , Contillo, A. et al. (2018) Phase‐contrast breast CT: the effect of propagation distance. Physics in Medicine & Biology, 63(24), 24NT03. [DOI] [PubMed] [Google Scholar]
- Brown, S.J. , Shippy, T.D. , Miller, S. , Bolognesi, R. , Beeman, R.W. , Lorenzen, M.D. et al. (2009) The red flour beetle, Tribolium castaneum (Coleoptera): a model for studies of development and pest biology. Cold Spring Harbor Protocols, 4(8), pdb‐emo126. Available from: 10.1101/pdb.emo126 [DOI] [PubMed] [Google Scholar]
- Brun, F. , Massimi, L. , Fratini, M. , Dreossi, D. , Billé, F. , Accardo, A. et al. (2017) SYRMEP Tomo project: a graphical user interface for customizing CT reconstruction workflows. Advanced Structural and Chemical Imaging, 3(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher, M. , Oberhofer, G. , Garcia‐Perez, N.C. & Bucher, G. (2020) A protocol for double fluorescent in situ hybridization and immunohistochemistry for the study of embryonic brain development in Tribolium castaneum . In: Brain Development. New York: Springer, Humana Press, pp. 219–232. [DOI] [PubMed] [Google Scholar]
- Bullock, M. , Legault, G. & Melbourne, B.A. (2020) Interspecific chemical competition between tribolium castaneum and tribolium confusum (Coleoptera: Tenebrionidae) reduces fecundity and hastens development time. Annals of the Entomological Society of America, 113(3), 223. Available from: 10.1093/AESA/SAAA006 [DOI] [Google Scholar]
- Caccia, S. , Casartelli, M. & Tettamanti, G. (2019) The amazing complexity of insect midgut cells: types, peculiarities, and functions. Cell and Tissue Research, 377(3), 505–525. [DOI] [PubMed] [Google Scholar]
- Campbell, J.F. , Athanassiou, C.G. , Hagstrum, D.W. & Zhu, K.Y. (2022) Tribolium castaneum: a model insect for fundamental and applied research. Annual Review of Entomology, 67, 347–365. Available from: 10.1146/annurev-ento-080921-075157 [DOI] [PubMed] [Google Scholar]
- Channaiah, L.H. , Subramanyam, B. & Zurek, L. (2010) Survival of enterococcus faecalis OG1RF:pCF10 in poultry and cattle feed: vector competence of the red flour beetle, Tribolium castaneum (Herbst). Journal of Food Protection, 73(3), 568–573. Available from: 10.4315/0362-028X-73.3.568 [DOI] [PubMed] [Google Scholar]
- Chapman, R.F. (2012) The insects: structure and function. Cambridge: Cambridge University Press. [Google Scholar]
- Crowson, R.A. (1981) The biology of the Coleoptera. London, UK: Academic Press. [Google Scholar]
- Dawson, P.S. (1977) Life history strategy and evolutionary history of Tribolium flour beetles. Evolution, 31(1), 226–229. Available from: 10.2307/2407562 [DOI] [PubMed] [Google Scholar]
- Dettner, K. (1987) Chemosystematics and evolution of beetle chemical defenses. Annual Review of Entomology, 32(1), 17–48. Available from: 10.1146/annurev.ento.32.1.17 [DOI] [Google Scholar]
- Donato, S. , Arana Peña, L.M. , Bonazza, D. , Formoso, V. , Longo, R. , Tromba, G. et al. (2022) Optimization of pixel size and propagation distance in X‐ray phase‐contrast virtual histology. Journal of Instrumentation, 17(5), C05021. Available from: 10.1088/1748-0221/17/05/C05021 [DOI] [Google Scholar]
- Donato, S. , Pacilè, S. , Colombo, F. , Garrovo, C. , Monego, S.D. , Macor, P. et al. (2017) Meniscal ossicles as micro‐CT imaging biomarker in a rodent model of antigen‐induced arthritis: a synchrotron‐based x‐ray pilot study. Scientific Reports, 7(1), 1–7. Available from: 10.1038/s41598-017-08025-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato, S. , Vommaro, M.L. , Tromba, G. & Giglio, A. (2021) Synchrotron X‐ray phase contrast micro tomography to explore the morphology of abdominal organs in Pterostichus melas italicus Dejean, 1828 (Coleoptera, Carabidae). Arthropod Structure and Development, 62, 101044. Available from: 10.1016/j.asd.2021.101044 [DOI] [PubMed] [Google Scholar]
- Dreyer, D. , Vitt, H. , Dippel, S. , Goetz, B. , El Jundi, B. , Kollmann, M. , et al. (2010). 3D standard brain of the red flour beetle Tribolium castaneum: a tool to study metamorphic development and adult plasticity. Frontiers in Systems Neuroscience, 4(MAR), 3. 10.3389/neuro.06.003.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfarnawany, M. , Alam, S.R. , Rohani, S.A. , Zhu, N. , Agrawal, S.K. & Ladak, H.M. (2017) Micro‐CT versus synchrotron radiation phase contrast imaging of human cochlea. Journal of Microscopy, 265(3), 349–357. Available from: 10.1111/jmi.12507 [DOI] [PubMed] [Google Scholar]
- Endrizzi, M. , Vittoria, F.A. , Brombal, L. , Longo, R. , Zanconati, F. & Olivo, A. (2018) X‐ray phase‐contrast tomography of breast tissue specimen with a multi‐aperture analyser synchrotron set‐up. Journal of Instrumentation, 13(2), C02004. Available from: 10.1088/1748-0221/13/02/C02004 [DOI] [Google Scholar]
- Ericsson, A.C. , Crim, M.J. & Franklin, C.L. (2013) A brief history of animal modeling. Missouri Medicine, 110(3), 201–205. [PMC free article] [PubMed] [Google Scholar]
- Futo, M. , Armitage, S.A.O. & Kurtz, J. (2016) Microbiota plays a role in oral immune priming in Tribolium castaneum. Frontiers in Microbiology, 6, 1383. Available from: 10.3389/fmicb.2015.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio, A. & Brandmayr, P. (2017) Structural and functional alterations in Malpighian tubules as biomarkers of environmental pollution: synopsis and prospective. Journal of Applied Toxicology, 37(8), 889–894. Available from: 10.1002/jat.3454 [DOI] [PubMed] [Google Scholar]
- Giglio, A. , Brandmayr, P. , Talarico, F. & Brandmayr, T.Z. (2011) Current knowledge on exocrine glands in carabid beetles: structure, function and chemical compounds. ZooKeys, 100, 193–201. Available from: 10.3897/zookeys.100.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio, A. , Cavaliere, F. , Giulianini, P.G. , Mazzei, A. , Talarico, F. , Vommaro, M.L. et al. (2017) Impact of agrochemicals on non‐target species: Calathus fuscipes Goeze 1777 (Coleoptera: Carabidae) as model. Ecotoxicology and Environmental Safety, 142, 522–529. Available from: 10.1016/j.ecoenv.2017.04.056 [DOI] [PubMed] [Google Scholar]
- Giglio, A. , Vommaro, M.L. , Agostino, R.G. , Lo, L.K. & Donato, S. (2022) Exploring compound eyes in adults of four coleopteran species using synchrotron X‐ray phase‐contrast microtomography (SR‐PhC micro‐CT). Life, 12(5), 741. Available from: 10.3390/life12050741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio, A. , Vommaro, M.L. , Brandmayr, P. & Talarico, F. (2021) Pygidial glands in Carabidae, an overview of morphology and chemical secretion. Life, 11(6), 562. Available from: 10.3390/life11060562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliolli, A.A.S. , Lapenta, A.S. , Ruvolo‐Takasusuki, M.C.C. , Abrahão, J. & Conte, H. (2015) Morpho‐functional characterization and esterase patterns of the midgut of Tribolium castaneum Herbst, 1797 (Coleoptera: Tenebrionidae) parasitized by Gregarina cuneata (Apicomplexa: Eugregarinidae). Micron, 76, 68–78. Available from: 10.1016/j.micron.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Green, L.F.B. (1980) Cryptonephric malpighian tubule system in a dipteran larva, the New Zealand glow‐worm, Arachnocampa luminosa (Diptera: Mycetophilidae): a structural study. Tissue and Cell, 12(1), 141–151. Available from: 10.1016/0040-8166(80)90057-9 [DOI] [PubMed] [Google Scholar]
- Gureyev, T.E. , Nesterets, Y.I. , Kozlov, A. , Paganin, D.M. & Quiney, H.M. (2017) On the “unreasonable” effectiveness of transport of intensity imaging and optical deconvolution. JOSA A, 34(12), 2251–2260. [DOI] [PubMed] [Google Scholar]
- Happ, G.M. (1968) Quinone and hydrocarbon production in the defensive glands of Eleodes longicollis and Tribolium castaneum (Coleoptera, Tenebrionidae). Journal of Insect Physiology, 14(12), 1821–1837. [Google Scholar]
- Hellerhoff, K. , Birnbacher, L. , Sztrókay‐Gaul, A. , Grandl, S. , Auweter, S. , Willner, M. et al. (2019) Assessment of intraductal carcinoma in situ (DCIS) using grating‐based X‐ray phase‐contrast CT at conventional X‐ray sources: an experimental ex‐vivo study. PLoS One, 14(1), e0210291. Available from: 10.1371/journal.pone.0210291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges, R.J. , Robinson, R. & Hall, D.R. (1996) Quinone contamination of dehusked rice by Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Journal of Stored Products Research, 32(1), 31–37. Available from: 10.1016/0022-474X(95)00036-7 [DOI] [Google Scholar]
- Holtof, M. , Lenaerts, C. , Cullen, D. & Vanden Broeck, J. (2019) Extracellular nutrient digestion and absorption in the insect gut. Cell and Tissue Research, 377(3), 397–414. Available from: 10.1007/s00441-019-03031-9 [DOI] [PubMed] [Google Scholar]
- Hörnschemeyer, T. , Beutel, R.G. & Pasop, F. (2002) Head structures of Priacma serrata Leconte (Coleptera, Archostemata) inferred from X‐ray tomography. Journal of Morphology, 252(3), 298–314. [DOI] [PubMed] [Google Scholar]
- Hunnekuhl, V.S. , Siemanowski, J. , Farnworth, M.S. , He, B. & Bucher, G. (2020) Immunohistochemistry and fluorescent whole mount RNA In situ hybridization in larval and adult brains of Tribolium. In: Methods in molecular biology, Vol. 2047. New York: Springer, Humana Press, pp. 233–251. Available from: 10.1007/978-1-4939-9732-9_13 [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , Byeon, D. hyeon, Jung, J. M. , Jung, S. , & Lee, W. H. (2020). Spatiotemporal evaluation of red flour beetle (Tribolium castaneum) dispersion under the effect of climate and topography in South Korea. Journal of Stored Products Research, 89, 101735. 10.1016/j.jspr.2020.101735 [DOI] [Google Scholar]
- King, B. & Denholm, B. (2014) Malpighian tubule development in the red flour beetle (Tribolium castaneum). Arthropod Structure & Development, 43(6), 605–613. [DOI] [PubMed] [Google Scholar]
- Kolosov, D. & O'Donnell, M.J. (2019) The Malpighian tubules and cryptonephric complex in lepidopteran larvae. In: Advances in insect physiology, Vol. 56. London: Elsevier, pp. 165–202. [Google Scholar]
- Küpper, S.C. , Klass, K.D. , Uhl, G. & Eberhard, M.J.B. (2019) Comparative morphology of the internal female genitalia in two species of Mantophasmatodea. Zoomorphology, 138(1), 73–83. Available from: 10.1007/s00435-018-0421-z [DOI] [Google Scholar]
- Lagadic, L. & Caquet, T. (1998) Invertebrates in testing of environmental chemicals: are they alternatives? Environmental Health Perspectives, 106(Suppl. 2), 593–611. Available from: 10.2307/3433810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lak, M. , Azar, D. , Nel, A. , Neraudeau, D. & Tafforeau, P. (2008) The oldest representative of the Trichomyiinae (Diptera: Psychodidae) from the lower Cenomanian French amber studied with phase‐contrast synchrotron X‐ray imaging. Invertebrate Systematics, 22(4), 471–478. [Google Scholar]
- Li, D. , Zhang, K. , Zhu, P. , Wu, Z. & Zhou, H. (2011) 3D configuration of mandibles and controlling muscles in rove beetles based on micro‐CT technique. Analytical and Bioanalytical Chemistry, 401(3), 817–825. [DOI] [PubMed] [Google Scholar]
- Li, J. , Lehmann, S. , Weißbecker, B. , Ojeda Naharros, I. , Schütz, S. , Joop, G. et al. (2013) Odoriferous defensive stink gland transcriptome to identify novel genes necessary for quinone synthesis in the red flour beetle, Tribolium castaneum . PLoS Genetics, 9(7), e1003596. Available from: 10.1371/journal.pgen.1003596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye, A. (2012). Drishti: a volume exploration and presentation tool. In Developments in X‐ray tomography VIII (Vol. 8506, p. 85060X). Washington: International Society for Optics and Photonics. [Google Scholar]
- Malagoli, D. , Abdalla, F.C. , Cao, Y. , Feng, Q. , Fujisaki, K. , Gregorc, A. et al. (2010) Autophagy and its physiological relevance in arthropods: current knowledge and perspectives. Autophagy, 6(5), 575–588. [DOI] [PubMed] [Google Scholar]
- McAllister, J.C. , Steelman, C.D. & Carlton, C.E. (1995) Histomorphology of the larval and adult digestive systems of Alphitobius diaperinus (Coleoptera: Tenebrionidae). Journal of the Kansas Entomological Society, 68(2), 195–205. [Google Scholar]
- Milutinović, B. , Stolpe, C. , Peuβ, R. , Armitage, S.A.O. & Kurtz, J. (2013) The red flour beetle as a model for bacterial oral infections. PLoS One, 8(5), e64638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstone, A.V. , Mullinger, A.M. & Ramsay, J.A. (1968) Further studies on the rectal complex of mealworm Tenebrio molitor, L.(Coleoptera, Tenebrioidae). Philosophical transactions of the Royal Society of London Series B, Biological Sciences, 253(788), 343–382. [Google Scholar]
- Nardi, J.B. & Bee, C.M. (2012) Regenerative cells and the architecture of beetle midgut epithelia. Journal of Morphology, 273(9), 1010–1020. [DOI] [PubMed] [Google Scholar]
- Negi, A. , Pare, A. , Manickam, L. & Rajamani, M. (2022) Effects of defect action level of Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae) fragments on quality of wheat flour. Journal of the Science of Food and Agriculture, 102(1), 223–232. [DOI] [PubMed] [Google Scholar]
- Nocelli, R.C.F. , Cintra‐Socolowski, P. , Roat, T.C. , Silva‐Zacarin, E.C.M. & Malaspina, O. (2016) Comparative physiology of Malpighian tubules: form and function. Open Access Insect Physiology, 6, 13–23. [Google Scholar]
- Oliva, P. , Di Trapani, V. , Arfelli, F. , Brombal, L. , Donato, S. , Golosio, B. et al. (2020) Experimental optimization of the energy for breast‐CT with synchrotron radiation. Scientific Reports, 10(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özyurt Koçakoğlu, N. , Candan, S. & Güllü, M. (2022) Anatomy and histology of digestive tract in the red poplar leaf beetle Chrysomela populi Linnaeus, 1758 (Coleoptera: Chrysomelidae). International Journal of Tropical Insect Science, 42(1), 927–939. [Google Scholar]
- Paganin, D. , Mayo, S.C. , Gureyev, T.E. , Miller, P.R. & Wilkins, S.W. (2002) Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. Journal of Microscopy, 206(1), 33–40. Available from: 10.1046/j.1365-2818.2002.01010.x [DOI] [PubMed] [Google Scholar]
- Parthasarathy, R. , Sun, Z. , Bai, H. & Palli, S.R. (2010) Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochemistry and Molecular Biology, 40(5), 405–414. Available from: 10.1016/j.ibmb.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy, R. , Tan, A. , Sun, Z. , Chen, Z. , Rankin, M. & Palli, S.R. (2009) Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mechanisms of Development, 126(7), 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai, A. , Contillo, A. , Arfelli, F. , Bonazza, D. , Brombal, L. , Cova, M.A. et al. (2019) Quantitative characterization of breast tissues with dedicated CT imaging. Physics in Medicine & Biology, 64(15), 155011. [DOI] [PubMed] [Google Scholar]
- Pointer, M.D. , Gage, M.J.G. & Spurgin, L.G. (2021) Tribolium beetles as a model system in evolution and ecology. Heredity, 126(6), 869–883. Available from: 10.1038/s41437-021-00420-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay, J.A. (1976) The rectal complex in the larvae of Lepidoptera. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 274(932), 203–226. [DOI] [PubMed] [Google Scholar]
- Rigon, L. (2014) X‐ray imaging with coherent sources. Comprehensive Biomedical Physics, 2, 193–216. Available from: 10.1016/b978-0-444-53632-7.00209-4 [DOI] [Google Scholar]
- Romell, J. , Jie, V.W. , Miettinen, A. , Baird, E. & Hertz, H.M. (2021) Laboratory phase‐contrast nanotomography of unstained Bombus terrestris compound eyes. Journal of Microscopy, 283(1), 29–40. Available from: 10.1111/jmi.13005 [DOI] [PubMed] [Google Scholar]
- Rösner, J. , Wellmeyer, B. & Merzendorfer, H. (2020) Tribolium castaneum: a model for investigating the mode of action of insecticides and mechanisms of resistance. Current Pharmaceutical Design, 26(29), 3554–3568. Available from: 10.2174/1381612826666200513113140 [DOI] [PubMed] [Google Scholar]
- Roth, O. , Sadd, B.M. , Schmid‐Hempel, P. & Kurtz, J. (2009) Strain‐specific priming of resistance in the red flour beetle, Tribolium castaneum. Proceedings of the Royal Society B: Biological Sciences, 276(1654), 145–151. Available from: 10.1098/rspb.2008.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother, L. , Kraft, N. , Smith, D.B. , el Jundi, B. , Gill, R.J. & Pfeiffer, K. (2021) A micro‐CT‐based standard brain atlas of the bumblebee. Cell and Tissue Research, 386(1), 29–45. Available from: 10.1007/s00441-021-03482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada, M. , Sano, T. , Hanakawa, K. , Sirasoonthorn, P. , Oi, T. & Miura, K. (2020) Benzoquinone synthesis‐related genes of Tribolium castaneum confer the robust antifungal host defense to the adult beetles through the inhibition of conidial germination on the body surface. Journal of Invertebrate Pathology, 169, 107298. Available from: 10.1016/j.jip.2019.107298 [DOI] [PubMed] [Google Scholar]
- Sinha, R.N. (1958) The alimentary canal of the adult of Tribolium castaneum Herbst (Coleoptera, Tenebrionidae). Journal of the Kansas Entomological Society, 31(2), 118–125. [Google Scholar]
- Smith, D.B. , Arce, A.N. , Rodrigues, A.R. , Bischoff, P.H. , Burris, D. , Ahmed, F. et al. (2020) Insecticide exposure during brood or early‐adult development reduces brain growth and impairs adult learning in bumblebees. Proceedings of the Royal Society B: Biological Sciences, 287(1922), 20192442. Available from: 10.1098/rspb.2019.2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D.B. , Bernhardt, G. , Raine, N.E. , Abel, R.L. , Sykes, D. , Ahmed, F. et al. (2016) Exploring miniature insect brains using micro‐CT scanning techniques. Scientific Reports, 6, 21768. Available from: 10.1038/srep21768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass, R.E. (1935) Principles of insect morphology. London: Cornell University Press. [Google Scholar]
- Takaki, K. , Hazama, K. , Yazaki, M. , Kotani, E. & Kaneko, Y. (2020) Maturation of telotrophic ovary accompanied with ecdysteroidogenic activity and contrastive decrease in ecdysteroids in the whole body of red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Applied Entomology and Zoology, 55(3), 299–308. Available from: 10.1007/s13355-020-00682-x [DOI] [Google Scholar]
- Talarico, F. , Brandmayr, P. , Giulianini, P.G. , Ietto, F. , Naccarato, A. , Perrotta, E. et al. (2014) Effects of metal pollution on survival and physiological responses in Carabus (Chaetocarabus) lefebvrei (Coleoptera, Carabidae). European Journal of Soil Biology, 61, 80–89. Available from: 10.1016/j.ejsobi.2014.02.003 [DOI] [Google Scholar]
- Terra, W.R. (2001) The origin and functions of the insect peritrophic membrane and peritrophic gel. Archives of Insect Biochemistry and Physiology, 47(2), 47–61. Available from: 10.1002/arch.1036 [DOI] [PubMed] [Google Scholar]
- Trauner, J. & Büning, J. (2007) Germ‐cell cluster formation in the telotrophic meroistic ovary of Tribolium castaneum (Coleoptera, Polyphaga, Tenebrionidae) and its implication on insect phylogeny. Development Genes and Evolution, 217(1), 13–27. Available from: 10.1007/s00427-006-0114-3 [DOI] [PubMed] [Google Scholar]
- Trematerra, P. & Sciarretta, A. (2004) Spatial distribution of some beetles infesting a feed mill with spatio‐temporal dynamics of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum . Journal of Stored Products Research, 40(4), 363–377. [Google Scholar]
- Trevijano‐Contador, N. & Zaragoza, O. (2014) Expanding the use of alternative models to investigate novel aspects of immunity to microbial pathogens. Virulence, 5(4), 454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel, W.R. & Doyen, J.T. (1980) Comparative anatomy of the defensive glands, ovipositors and female genital tubes of tenebrionid beetles (Coleoptera). International Journal of Insect Morphology and Embryology, 9(5–6), 321–368. Available from: 10.1016/0020-7322(80)90009-4 [DOI] [Google Scholar]
- Unruh, L.M. , Xu, R. & Kramer, K.J. (1998) Benzoquinone levels as a function of age and gender of the red flour beetle, Tribolium castaneum . Insect Biochemistry and Molecular Biology, 28(12), 969–977. Available from: 10.1016/S0965-1748(98)00085-X [DOI] [Google Scholar]
- Vasudeva, R. , Dickinson, M. , Sutter, A. , Powell, S. , Sales, K. & Gage, M.J.G. (2021) Facultative polyandry protects females from compromised male fertility caused by heatwave conditions. Animal Behaviour, 178, 37–48. [Google Scholar]
- Villaverde, M.L. , Juárez, M.P. & Mijailovsky, S. (2007) Detection of Tribolium castaneum (Herbst) volatile defensive secretions by solid phase microextraction‐capillary gas chromatography (SPME‐CGC). Journal of Stored Products Research, 43(4), 540–545. Available from: 10.1016/j.jspr.2007.03.003 [DOI] [Google Scholar]
- Vommaro, M.L. , Donato, S. & Giglio, A. (2022) Virtual sections and 3D reconstructions of female reproductive system in a carabid beetle using synchrotron X‐ray phase‐contrast microtomography. Zoologischer Anzeiger, 298, 123–130. [Google Scholar]
- Vranić, S. , Vesović, N. , Vujisić, L. , Pavlović, D. , Pantelić, D. , Todosijević, M. et al. (2021) Pygidial glands of three ground beetle taxa (Insecta, Coleoptera, Carabidae): a study on their morphology and chemical composition of their secretions. Zoology, 148, 125948. Available from: 10.1016/j.zool.2021.125948 [DOI] [PubMed] [Google Scholar]
- Wang, K. , Liu, M. , Wang, Y. , Song, W. & Tang, P. (2020) Identification and functional analysis of cytochrome P450 CYP346 family genes associated with phosphine resistance in Tribolium castaneum. Pesticide Biochemistry and Physiology, 168, 104622. [DOI] [PubMed] [Google Scholar]
- Westneat, M.W. , Socha, J.J. & Lee, W.‐K. (2008) Advances in biological structure, function, and physiology using synchrotron X‐ray imaging. Annual Review of Physiology, 70, 119–142. [DOI] [PubMed] [Google Scholar]
- Will, K.W. , Attygalle, A.B. & Herath, K. (2000) New defensive chemical data for ground beetles (Coleoptera: Carabidae): interpretations in a phylogenetic framework. Biological Journal of the Linnean Society, 71(3), 459–481. Available from: 10.1006/bijl.2000.0456 [DOI] [Google Scholar]
- Wilson, R.J. & Fox, R. (2021) Insect responses to global change offer signposts for biodiversity and conservation. Ecological Entomology, 46(4), 699–717. Available from: 10.1111/een.12970 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Video S1.
Video S2.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


