Abstract
The current review was carried out on the industrial application of fig by-products and their role against chronic disorders. Fig is basically belonging to fruit and is botanically called Ficus carica. There are different parts of fig, including the leaves, fruits, seeds and latex. The fig parts are a rich source of bioactive compounds and phytochemicals including antioxidants, phenolic compounds, polyunsaturated fatty acids, phytosterols and vitamins. These different parts of fig are used in different food industries such as the bakery, dairy and beverage industries. Fig by-products are used in extract or powder form to value the addition of different food products for the purpose of improving the nutritional value and enhancing the stability. Fig by-products are additive-based products which contain high phytochemicals fatty acids, polyphenols and antioxidants. Due to the high bioactive compounds, these products performed a vital role against various diseases including cancer, diabetes, constipation, cardiovascular disease (CVD) and the gastrointestinal tract (GIT). Concussively, fig-based food products may be important for human beings and produce healthy food.
Keywords: fig, bioactive compounds, industrial application, health benefits
1. Introduction
The fig (Ficus carica) is an edible fruit of the Moraceae family. The fig is commonly grown in an area that extends from Asiatic Europe to North India. However, it is also grown as a crop in most Mediterranean countries due to their warm climates [1]. The Mediterranean region is the area that F. carica has steadily migrated to from its origin in western Asia. Worldwide, over a million tons of figs are produced [2]. The common shape of figs is obovoid, turbinate and pear. The color of figs varies according to the variety. Some common hues are creamy, green-yellow, red, copper and florid. The syconium’s walls are thick, pale yellow, golden, pale pink, red or florid. The skin of a fig is fine and thin. About 80% of the overall production of figs took place in Egypt, Israel, Algerian, Iranian, Moroccan, Spain and the United States [3]. The importance of dried fig exchanges is greater. The top producers of dried figs are Turkey (320.0 K metric ton), the United States (27.1 K metric ton), and Spain (59.9 K metric ton) [4]. Furthermore, figs are one of the only five fruit plants mentioned in the Holy Quran [5]. Figs are sweet in taste with high quality fruits and have a high nutritional value [6]. The Moraceae family includes the genus Ficus, which has over 800 varieties [7]. Figs are a great source of antioxidants and phenolic chemicals [8]. Ficus carica is an edible fruit with a substantial commercial value in different forms, including fresh, dried and canned. [9]. It is currently used in many food products such as jams and jellies. The fig fruit has also been used as a filling and garnishing component in baked puddings, cakes and bread. However, biscuits are deficient in several elements, including phenolic compounds, fiber and several of the vitamins and minerals present in fig [10,11]. People continue to utilize fig fruit as a traditional medicine despite its medical benefits being recognized for millennia. Fig contains dietary carbohydrates that have the capacity to postpone digestion, whereas anthocyanins can control hyperglycemia [12]. Consuming dried figs considerably boosts the human plasma antioxidant capability. The number of polyphenols and anthocyanins in fresh figs is related to their overall antioxidant capacity [13]. Free radicals are environmental toxins, including air pollution or cigarette smoke, that affects humans. Antioxidants may help prevent or minimize the cell damage caused by free radicals [14]. The Mediterranean diet is now seen as one which promotes people’s health and wellbeing [15]. The nutritious qualities found in fruits and vegetables are responsible for this effect [16]. These are a great source of phenolic compounds, minerals, amino acids and vitamins [17]. Dried figs have ability to the potentially promote health due to the presence of high levels of polyphenols. [18]. These physiological and nutritional traits are strongly linked to fruit and are typically controlled by the genotype, ripening stage, environmental factors and orchard management techniques [19]. The fig is a nutritious fruit with a large amount of iron and fiber. Eating them as a vegetable may also help individuals to consume enough fiber daily. Fresh or dried fig can be eaten, but people should be aware that dried figs have a higher calorie and sugar content. Because figs are safe to consume, people can utilize them to treat a number of ailments. Ficus carica extract has recently been evaluated for its hepatoprotective effects in rats exposed to the hepatotoxic drug rifampicin [20]. However, many of the assertions made in relation to the purported health advantages of figs are not yet supported by sufficient data. The traditional medical systems such as Ayurvedic, Unani and Siddha have all mentioned the therapeutic benefits of F. carica. [21]. Fresh plant materials, unprocessed extracts and separated Ficus carica components have demonstrated a variety of biological (pharmacological) activity. Fig is valuable against different diseases including disorders of the endocrine (diabetes), respiratory system (liver diseases, asthma and cough), digestive system (ulcer and vomiting) and infectious diseases (skin disease, scabies and gonorrhea) [21]. In the present review, we discussed the consumption of fig and the role of their by-products in food product development against various chronic disorders.
2. Bioactive Compounds and Phytochemicals in Fig
Colorful, delicious and nutrient-dense vegetables and fruits are a wonderful addition to our diets. Bioactive substances (polyphenols, carotene, vitamins and anthocyanins) are present in fruits and vegetables which have gained attention due to their potential health benefits [22]. Plants have been an important source of nutrition that has preserved people’s health and improved individuals’ quality of life since the beginning of civilization on Earth [23]. Different bioactive compounds and phytochemicals are shown in Figure 1 and Table 1, respectively.
Figure 1.
Bioactive compounds and biological properties of fig.
Table 1.
Phytochemical Composition in different parts of fig.
| Different Parts | Extraction Method | Phytochemicals | Solvent | References |
|---|---|---|---|---|
| Fermented fig by-product | High-pressure assisted extraction | Antioxidants, total phenolic, tannin and flavonoid | Ethanol | [24] |
| Fig leaves | Tailor-made deep eutectic solvent (DES) and microwave-assisted extraction | Caffeoylmalic acid, psoralic acid-glucoside, rutin, psoralen and bergapten | Methanol | [25] |
| Whole fig fruit, peel, leaves and pulp | -- | Total phenols and antioxidant | -- | [26] |
| Ficus carica L. latex | Maceration and ultrasound-assisted extraction (UAE) | Total phenolic content (TPC) and antioxidant | Methanol, ethanol, ethyl acetate and n-hexane | [27] |
| Fig leaves | Surfactant-based microwave-assisted extraction | Caffeoylmalic acid, psoralic acid-glucoside, rutin, psoralen and bergapten reached | Ethanol | [28] |
| Fruit, leaves and stembark | Ethanolic extract | Butyl butyrate, 5-hydroxymethyl furfural, 1-butoxy-1-isobutoxy butane, malic acid, tetradecanoic acid, phytol acetate, trans phytol, n-hexadecanoic acid, 9Z,12Z-octadecadienoic acid, stearic acid, sitosterol, 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one and 2,4,5-trimethyl-2,4-dihydro-3H-pyrazol-3-one | Hexane, ethyl acetate, ethanol and water | [29] |
| Ficus carica L. peel | Heat, microwave and ultrasound | Anthocyanin | Ethanol and water | [30] |
| Ficus carica | Ethyl acetate | Phenol groups and amine groups | -- | [31] |
| Leaves | Soxhlet extractor | Quercetin, chlorogenic acid, caffeic acid, syringic acid, coumaric acid, rutin and trans-cinnamic acid | Water, methanol and ethanol | [32] |
| Fig leaf | Ethanolic | Antioxidant | Ethanol | [33] |
| Fruit | Hexane extract | Chemical compounds and antibacterial | Hexane | [34] |
| Ficus carica | Methanol (MeOH) extract | Antimicrobial | Methanol | [35] |
| Black fresh fig | Solvent extraction | Antioxidants and total phenolic contents | Acetone | [36] |
| Leaf | Methanol and water extracts | α-glucosidase and α-amylase | Water | [37] |
| Latex | Methanol extracts | Total phenolic and flavonoid contents | Methanol | [38] |
| Fruit | Ethanol and the temperature of extraction | Total phenolics, total flavonoids and total proanthocyanidins | Ethanol | [39] |
| Fresh fig | Methanol water extraction | Total phenolics and total anthocyanins | Methanol and water | [40] |
| Seed | Solvent extraction | Polyphenol contents and antioxidant | Acetone, methanol and ethanol | [41] |
| Leaves | Solvent extraction | Biological active lipid components | Diethyl ether, petroleum ether, n-hexane, acetone and ethanol | [42] |
2.1. Vitamins
The two main categories of nutrients are macronutrients and micronutrients. Micronutrients are those that the body needs in tiny amounts, whereas macronutrients are those that the body needs in high amounts. Vitamins and minerals are considered micronutrients that aid humans in developing structural and metabolic processes. Ficus carica (fig) contains significant levels of phytochemicals minerals and vitamins and it is prepared in various ways, such as in a fresh, dried, concentrate and paste state. Morgan et al. [43] reported that vitamin C is present in fresh figs from Kadota and Calimyrna. The quantities of vitamin C in fig are near to those of grape and apricots but lower than fresh peach and prunes. The water-soluble vitamin C found in figs is a great natural antioxidant and aids in reducing the non-enzymatic oxidation of vegetables and fruits. It is used to replace artificial antioxidants [44]. The Missions and Adriatic figs contained a minimal quantity of vitamin C. The Black Missions fresh figs have a good source of vitamin A, whereas fresh Kadotas and Calimyrnas contain a low amount of vitamin A. Calimyrnas are essentially light-colored figs. The sulfuring procedure helps Mission and Calimyrnas retain their vitamin A content, but it does not assist the Adriatics variety. Figs have been regarded as one of the healthier fruits with have been linked to a long life, and these fruits also play a significant role in these nations’ traditional Mediterranean cuisine [15]. Antioxidant substances such as polyphenols, organic acids and carotenoids prevent the oxidative processes that may result in degenerative diseases [45].
2.2. Antioxidants
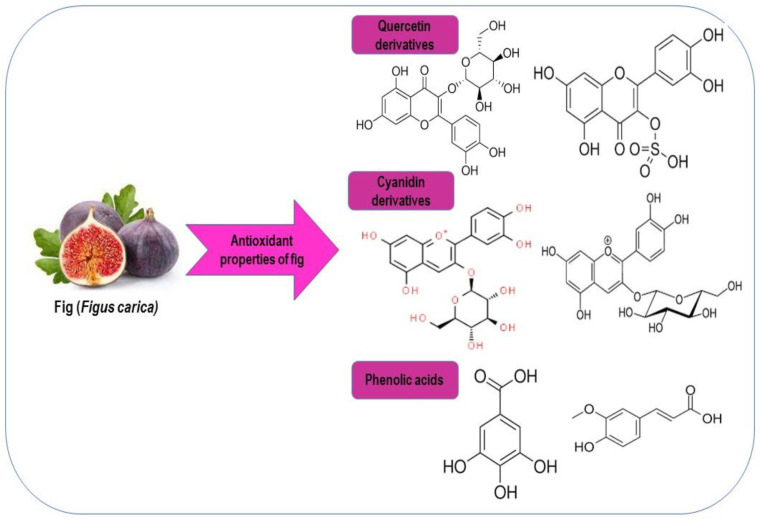
Antioxidants can prevent the generation of reactive oxygen substances. Fig extract contains bioactive compounds including total polyphenols, carotenoids, flavonoids and antioxidant properties. Fig extracts from cultivars of a darker color contained more phytochemicals than the extracts of the lighter color variations. Fruit peel contributes the most polyphenols and antioxidant activity compared to fruit pulp [46]. A significant antioxidant capacity in dried Ficus carica fruit has been reported. Different fig species’ antioxidant profiles were compared by Abdel-Atyet [38]. Ficus carica and Ficussycomorus extracts contain several phytochemical compounds that have therapeutic activities, including anticancer properties and anti-inflammatory and antifungal activity. The number of antioxidants in fig extracts varies, making it a great source of healthy natural antioxidants. Some of these variations differ in the amount of solvent used, phenol present or in the interactions between the various extract constituents. An in vitro study showed that dried figs act as antioxidants after being consumed by humans. According to the study, dried fruits make up a larger portion of the food because these are rich in phenol antioxidants and nutrient (fiber) [47]. The common secondary metabolites of plants, known as phenolic compounds, can serve as antioxidants that have good impacts on human health and their physiological activities in plants. Phenolic compounds contain various antioxidants, including quenching singlet oxygen, scavenging free radicals and reducing or donating hydrogen. [48]. Fresh fruits, vegetables, and their products’ colors, flavors and aromas are significantly influenced by phenolic chemicals. In addition to their antioxidative properties, polyphenols may also have anti-carcinogenic, antitumor, anti-inflammatory and antibacterial effects [49]. Figs contain a high level of natural antioxidant substances in which the polyphenol levels are high [47]. Commercially accessible fruits are a good source of antioxidant vitamins that play a meaningful role in the human diet. According to Solomon et al. [46], the antioxidant activity of fig fruits increases with their polyphenol concentration, such as their anthocyanin level. The results showed that fig antioxidants can greatly increase the plasma antioxidant capacity and shield the plasma phospholipid from oxidation [50]. According to Vinson [47], figs are high in sugars, including fructose, dextrose and glucose [51]. Furthermore, the different parts of fig contain various chemicals compounds including biopolymers [13], anthocyanins, polyphenols, fatty acids, several polyphenolic compounds and polysaccharides [52,53]. The different antioxidants properties of fig are show in Figure 2.
Figure 2.
Antioxidants properties of fig.
2.3. Polyphenols
Phenolic substances can play a key role in neutralizing free radicals, the defense of cellular components against hydrogen peroxide and the defense of organs and tissues against peroxide (the degradation of lipids in unsaturated fatty acids). According to Juániz et al. [54], phenolic chemicals are abundant in the Ficus species. We consume certain plant-based meals which contain polyphenols. Plants contain lots of antioxidants and may be beneficial for human health. Polyphenols are helpful in preventing or treating cardiovascular disease, diabetes, neurodegenerative illness and problems with weight management [55]. The studies showed that the leaf, pulp and peel contained a biochemical profile, antioxidant properties and anthocyanin with different concentrations [46,56]. Numerous fig accessions have not been compared regarding their phytochemical traits such as the total polyphenolics, carotenoids, antioxidant capacity and particular sugars. Typical plant secondary metabolites, termed phenolics, serve as antioxidants by donating an atom of hydrogen, free radical scavengers and quenching singlet oxygen in addition to serving physiological functions in plants [56]. Figs are a good source of phenolic compounds. In fact, red wine and coffee are understood to be sources of phenolic compounds, but both have a lower phenolic content than fig [18]. In regard to the cultivar, the phenolic content of figs usually varies greatly from one fruit section to another [57]. However, these bioactive components are believed to have no effect on a specific tissue or organ. Due to its bioavailability, a substance can be successfully absorbed from the gastrointestinal tract into the blood and transferred to the right place in the body while remaining bioactive. The results of a previous study proved that humans and animal models can be accurately linked with the measurements of bio accessibility made using in vitro models [58]. The bioactive compounds are extracted from the latex of figs including 6-O-acyl-b-D-glucosyl-bsitosterols or AGS (the acyl methyl group: palmitoyl, linoleyl, stearyl and oleyl). Compared to linoleyl, stearyl and oleyl derivatives, the palmitoyl derivative of AGS functions as the most effective inhibitor for various cancer cell lines [58]. An in vitro study shown that AGS suppresses the growth of the cancer cell lines. The finding showed that AGS is the most effective anticancer agent [59].
2.4. Polyunsaturated Fatty Acids
The use of saturated fats, polyunsaturated and monounsaturated fats are regarded as good fats because of their ability to lower the risk of heart disease [14]. Omega-6 fatty acids (31.28%) and omega-3 fatty acids (40.25%) are the two main subgroups of polyunsaturated fatty acids; they are shown in Figure 3. Both of these are crucial healthy fats that the human body requires for both cell development and the brain’s function. However, the human body is unable to produce vital fatty acids. Moreover, these fatty acids can be taken from one’s diet. McCune [60] reported that various areas of a plant or animal contain a different concentration of fatty acids but they do not contain the total levels or proportions in the seeds, flaxseed oil, roots, stems and branches, leaves and blooms tree. Numerous studies have shown that phytosterols lower either the human or animal serum cholesterol levels. The findings showed that fruits, vegetables, seeds and nuts are good sources of phytosterols. The sterols and other biochemical elements discovered in the figs and figs’ leaves which are unsaponifiable have been the focus of a few investigations. The phytosterols present in fig is 433 mg/100 g on a dry basis. Kim et al. [61] found sterol contents in several edible plants, but only three phytosterols were found for figs. (Figure 3).
Figure 3.
Polyphenols and phytosterols of fig.
3. Fig and Fig By-product Applications
The amount of consumers who are aware of nutritional and functional foods is increasing. Customers want to consume safe foods that also provide additional benefits alongside its nutritional value. However, customers are more inclined to buy these functional foods due to positive health benefits. Furthermore, people are becoming increasingly interested in functional foods [62]. Numerous functional foods have been created, including biscuits [63], bread [64], meat [65] and probiotics dairy drinks [66]. The characteristics of functional foods are thought to be high levels of protein, fiber and the total phenolic content [67]. The fig, although regarded as a fruit, is actually a flower turned inside out. In figs, the fruit is actually the seeds. It is only fruit that fully matures and becomes semi-dry on the tree. Fig was originally obtained from Asiatic Turkey; it has now migrated from Northern India to all of the nations encircling the Mediterranean. Today, Greece, Turkey, the United States and Spain are the leading manufacturers of dried figs. Fig trees live for a long time and reach fruit-bearing maturity in just two years. Some fig trees in California planted almost a century ago still produce fruit. Calimyrna is an amber-colored variety. Dried figs are available in a variety of forms including as a paste, concentrate, nuggets, powder and diced forms for industrial products. Dried figs can be preserved from yeast fermentation and the growth of mold with the addition of potassium sorbate. Processing increases the moisture content of dried figs. The Calimyrna variety’s color may be stabilized by applying low levels of sulfur dioxide compared to other dried fruits such as apricots or apple [68] Table 2.
Table 2.
Value addition role of fig in food industries.
| Industry | Food Product | Additive | Function | Reference |
|---|---|---|---|---|
| Food industry | Fig powder co-products (FPC) | Peel and pulp | FPC obtained from peel present higher antioxidant activity than FPC obtained from pulp. | [69] |
| Food industry | Functional food | Leaves, pulp, peels, seeds and latex | It is the high value-added ingredients and their utilization in novel food formulation development. | [70] |
| Food packaging | Fig-based Chitosan film | Leaves | Fig leaves extract incorporated chitosan films can be used for protection of the food items and increase their shelf life. | [71] |
| Confectionery | Doughnut icing and pastry product “beijinhos” | Fig peels | Use of fig peels fruits as natural colorants. | [72] |
| Baking | Cookies | Fresh fig fruit powder | Fig powder-incorporated cookies have rich nutrients as compared to market products. | [73] |
| Snack | Fruit-based snack | Dried fig | In this study, a novel snack based on fig fruit powder was developed. | [74] |
| Dairy | Sugar-free milk-based dessert | Dried figs and CMC | Figs and CMC influenced the dessert’s characteristics. | [75] |
| Oil | Canola oil | Pulp and skin extract | The high efficiency of fig skin extract for oxidative stability is assessed because it is good source of phenolic compounds. | [76] |
| Bakery | Burfi (Indian cookie) | Fig fruit powder | The fig fruit powder-based product was found to be low cost as compared to market products. | [77] |
| Confectionery | Toffee | Fig fruit powder | The toffee products prepared by fig fruit powder were assessed for their physico-chemical and sensory parameters. | [78] |
3.1. Baking Industry
A major portion of the human diet is made up of bakery products because these are prepared from cereal and have a long shelf life. Biscuits are one of the most widely consumed baked goods [79]. Nowadays, most people enjoy consuming bakery products [80]. The world’s rising biscuit consumption is increasing the significance of enhancing cookies [81]. Enriching biscuits with fig seed powder could provide a further pathway for the valorization of food waste. Khapre et al. [73] prepared functional cookies by using fresh Fig (Ficus carica L.) fruit powder. The fig powder was incorporated in the preparation of cookies at levels 0, 6, 12 and 18 %. The results showed that fig powder-enriched cookies have a good nutritional value with acceptable organoleptic qualities. Jung et al. [82] investigated the quality characteristics of sourdough bread using fermented fig. The moisture content increased with the increase in the sourdough, whereas the pH value was found to decrease with the increase in the quantity of the sourdough. A higher volume was attained in the 40 % sourdough sample, whereas the baking loss decreased with the increase in the sourdough. The minimum changes in the hunter color were found with the addition of sourdough. The texture and sensory properties of sourdough are different compared to the control.
3.2. Beverage Industry
Figs are consumed fresh, and customers seek them due to their excellent flavor. Fresh figs have a sweetness alike to honey, a light acidity and a fruity aroma similar to that of berries and grapes. However, figs are primarily used in the dehydrated form or as a component of jams or other sweet goods because figs are extremely perishable fruits. Figs are affected by post-harvest fungal activity. [83]. There have also been attempts to package fresh fruits to preserve them for the purpose of a longer shelf life. A specific packaging is used because fig is a perishable food [84]. Figs share some characteristics with grapes in terms of their composition and aroma; furthermore, figs are grown in the same area as grapes are grown. Figs have not historically been used as extensively as grapes because of the role grapes play in the production of wine. Some supposedly ancient fig wine recipes have been recorded and preserved [85]. The kinds of yeast utilized in the fermentation process has an impact on the antioxidant content of fermented alcoholic beverages in figs. Therefore, Saccharomyces cerevisiae yeasts have a higher fermentation yield and produce a high concentration in alcoholic beverages. Using non-Saccharomyces yeasts (Pichia fermenting, Wickeromomycesanomalus and Hanseniasporauvarum) in fermentation processes gives a higher concentration and antioxidant capacity than the beverages obtained from Saccharomy [86]. Moreover, Ilkin [87] reported that acetic fermentation was carried out to produce certain homemade vinegars based on various recipes including fresh figs, fresh figs that had been dehydrated, fresh figs and apple cider vinegar or fresh figs and grape. Different kinds of raw materials are utilized for the production of fermented alcoholic beverages made from figs. Different products (fresh sliced figs, fig juice and frozen figs) were made by using fermented S. cerevisiae yeast with and without dry fig leaf powder [88]. Numerous Ficus species are used for medical or nutritional purposes in which figs are the most common [89]. Figs have positive effects on human health due to the presence of polyphenols such as flavonoid, rutin and quercetin flavonoid [90,91]. These positive effects are based on their antioxidant capacity [92] and other nutraceutical qualities [93]. These polyphenolic chemicals are present in other fermented beverages as well as beverages made from fruit [94].
3.3. Dairy Industry
Yogurt contains probiotics that improve digestion and absorption while increasing the food security in human nutrition to improve individuals’ health [95]. Probiotic-, prebiotic- and symbiotic-based foods are categorized as functional foods. Despite having a high nutritional value, dairy products (including plain yoghurt) are poor suppliers of phenolic compounds and antioxidants. However, cow milk has low levels of phenolic chemicals and fiber [96]. To satisfy the consumer demand for “clean label” foods, vegetables or fruits are added to dairy products to boost their antioxidant benefits and fiber contents [97]. According to Jeong et al. [38], the fig (Fiscus carica) is a rich source of carbohydrates, acids, minerals (such as manganese, copper, magnesium and calcium), vitamins (such as vitamin K and β-carotenes), polyphenols, flavonoids, fiber and other substances with healthful properties. This study aims to boost the nutritional value of plain yoghurt by adding figs to increase the phenolics, antioxidants, minerals and fiber contents to fulfil human needs. Therefore, the addition of fig may enhance the yoghurt’s probiotic activity for use in human nutrition and medicine that prevents and treats obesity, hypercholesterolemia, hyper-lipemia, hypertension, gastrointestinal diseases and also encourage the development of gut flora. The fig fruit is used to cure inflammation and paralysis because of its antipyretic, purgative and aphrodisiac qualities [98]. Products made from figs are used to treat a variety of illnesses, including dermatological conditions and atopic dermatitis [99]. Fig extracts are also beneficial for reducing atopic dermatitis symptoms when taken with cortisone. The study showed that fig plant extract has the potential to be employed in the prevention and treatment of skin and cervical tumors [100,101].
4. Health Prospective of Fig and Fig-Based By-products
Traditional medical practices have mostly focused on the treatment of dermatological conditions with fig products [102]. Abbasi et al. [103] investigated fig plant extracts’ efficacy in reducing atopic dermatitis symptoms. However, it can also be used in place of corticosteroids. Additionally, another investigation revealed the potential possibility of fig leaf extracts for treating and preventing cervical melanoma [104]. Bergapten and psoralen are two factors of fig leaf extract that have been linked to having anticancer effects. It may be useful in building blocks for creating medicines that inhibit cancer cell proliferation [102]. Furthermore, according to another investigation, figs are useful in cells due to the presence of phenol [105]. Fig extract has shown a potential to be used to develop drugs to treat cardiovascular illnesses because it contains flavone, rutin and quercetin. Additionally, fig leaves and fruits have a significant amount of nutrients and are well-known for having high dietary fiber contents [89]. Fig leaf juice can cure vitiligo due to furanocoumarins, specifically psoralen and daidzein [106] Figure 4, Table 3.
Figure 4.
Health benefits of fig.
Table 3.
Role of different by-product of fig against chronic disorders.
| Fig Part | In vivo/In Vitro | Disease | Action | Reference |
|---|---|---|---|---|
| Leaves and fruit | In vivo and in vitro | Inflammatory and Carcinogenic effects | In vivo and in vitro study illuminates that F. carica leaves and fruits play important role the prevention of inflammatory and carcinogenic effects. | [107] |
| Leaves | In vivo | Kidney | The findings showed that fig extract play important role in the treatment of kidney diseases. | [108] |
| Ficus carica | --- | Anemia, cancer, diabetes, leprosy, liver diseases, paralysis and ulcers | Ficus carica is a good source of traditional medicine for the treatment of anemia, cancer, diabetes, leprosy, liver diseases, paralysis and ulcers. | [21] |
| Ficus carica leaf extracts | In vitro | Cancer, diabetes mellitus and Alzheimer’s disease | The results suggest that F. carica leaves may be valuable source for developing a promising therapeutic agent in cancer, diabetes and Alzheimer’s disease. | [37] |
| Ficus carica polysaccharides (FCPS) | In vivo | Aeromonas hydrophila infection | Results showed that dietary FCPS can be improved the innate immune response, growth performance and disease resistance against A. hydrophila in fish. | [109] |
| Ficus carica stem extract | In vivo | Hepatic oxidative damage | Fig extract played significant role in protecting animals from methanol-induced hepatic oxidative damage. | [110] |
| Ficus carica cell suspension culture extract | In vitro (on keratinocytes cells) and in vivo | Skin | In vitro and in vivo tests demonstrated that the extract from Ficus carica cell cultures alleviated skin damage caused by psychological stress. | [111] |
| Acetonic extract of Ficus carica L. | In vivo | Central nervous system | The outcomes showed that Ficus carica L. can be useful in insomnia, anxiety, schizophrenia, migraine and epilepsy. | [112] |
| Ficus carica (fig) and Olea europaea (olive) | -- | Pro-inflammatory cytokines | Consuming figs and olives can be useful in the prevention or treatment of inflammatory diseases. | [113] |
| Leaves of Ficus carica | In vivo | Hepatotoxicity | Fig leaves extract reduce the risk of hepatotoxicity. | [114] |
| Leaf and fruit | In vitro | Cancer | Results suggest that leaf and fruit have anticancer activity. | [115] |
| Leaves | In vivo | Diabetes | Reduce the risk of diabetes. | [116] |
| Leaf and fruit | -- | Hyperglycemia | The fig fruit or its leaves in food may help to correct the hyperglycemia due to diabetes. | [117] |
4.1. Constipation
Constipation is defined as less than three bowel motions per week [118]. Constipation is assumed to be influenced by psychosocial and behavioral issues including reduced movement, insufficient calorie intake and changes in anorectal feeling. Its multifactorial etiology also includes co-occurring diseases and drug side effects such as pain medications, anticonvulsants, antidepressants and cancer medications [119]. According to the Unani literature, F. carica fruit can cure constipation, dysentery, enteritis and piles because it acts as a laxative, purgative, antipyretic and aphrodisiac. Leukoderma and ringworm can also be treated with roots. Constipation is a highly prevalent health issue. However, laxative foods such as figs and their derivatives may be helpful in treating it [120]. Dietary fiber (12.21 g/100 g) is the most popular functional food component that can treat constipation. The typical fig (Ficus carica L.) is a broadleaf deciduous shrub belonging to the Moraceae family. It is well known for being one of the earliest edible fruits that humans grew in subtropical climates [121]. Different studies have been reported on natural remedies to cure constipation [122]. In the previous study, fresh fig leaves were utilized to treat constipation and similar outcomes were noted in the earlier studies [123]. Furthermore, Ajmal et al. [124] acknowledged the effectiveness of fig leaf extracts in lowering blood sugar levels. However, fig environmental consequences must be reduced and fruit-based goods have a significant benefit as functional additives. Peels and seeds are considered to be less optimal fruits, whereas leaves are a few examples of fruit-based products. Regarding fig by-products, extracts from the peel and leaves may improve the medicinal and nutritious qualities of food items such as sweeteners [125]. The consumption of fig on a regular basis can reduce the incidence of carcinogenesis. Colon cancer can be prevented because the fiber in figs promotes the fast removal of waste from the body. The fig seeds are rich with mucin that binds to waste and fluid in the gut and removes them [126].
4.2. Cardiovascular Diseases
Coronary heart disease (CHD) and strokes are examples of cardiovascular diseases (CVDs) that affecte the heart and blood arteries [127]. A specific diet is the most significant modifiable factor to avoid CVDs. There is proof that consuming a plant-based diet reduces the incidence of CVDs [128]. Fruits are the most fundamental ingredients that can help avoid CVDs because they contain polyunsaturated fatty acids [129]. The demand for fig derivatives/by-products (peel, leaves and oil) and fig-based products have increased because they are composed of bioactive components that are beneficial for heart health. Another study determines the phenolic component of fig products that has been impacted by processing (drying and jam preparation) and storage. Fig can reduce the triglyceride levels in humans which can help to maintain heart health. Blood fat molecules called triglycerides are a major contributor to heart disease. Additionally, the antioxidants in figs eliminate free radicals from body which obstruct coronary arteries and lead to cardiovascular disease. According to Soni et al. [130], the fig extract’s ABTS experiment revealed that its antioxidant capacity was quite good. Additionally, figs contain phenols and heart-healthy essential fats and omega-6 fatty acids.
4.3. Diabetes
Health professionals are quite concerned about the metabolic condition diabetes mellitus because it is becoming more and more common in both developed and developing nations. In 1985, diabetes was declared to affect 30 million individuals according to the World Health Organization. According to the WHO [131], that number rose to 135 million in 1995, and 300 million people are anticipated to be impacted by the year 2025. Worldwide, there is a health concern regarding the attempt to manage diabetes and its consequences. Plant-based medicinal medications for the treatment of diabetic diseases have received a lot of attention in recent years due to its accessibility, affordability and lack of adverse side effects. Different cultures around the world have used a variety of medicinal plants in traditional medicine to stop long-term issues in managing diabetes. About 200 uncontaminated bioactive components have been extracted from therapeutic plants including polyphenols, triterpenoids and starches. These compounds have powerful antidiabetic activities because they control blood glucose levels [132]. The Moraceae family contains approximately 850 plants, including trees and shrubs, vines, epiphytes and the genus Ficus. These trees, bushes, plants and epiphytes are found throughout humid and subtropical regions of Southeast Asia, tropical America and Australia, where there is the highest variety [133]. In Siddha, Ayurvedic and Chinese traditional medicine, multiple Ficus species are utilized for a variety of therapeutic purposes [85]. Originally from Asia Minor, F. carica is a fruit shrub that is common in tropical and subtropical regions. Plants can treat different diseases including diabetes, liver disorders, cough, ulcers, nausea, menstrual cramps, crust conditions and gonorrhea [21]. In Pakistan, leaf decoction is also used to manage diabetes [134]. The Ficus species have important hypoglycemic properties which include improving insulin sensitivity, causing the release of insulin, increasing hepatocellular aerobic glycolysis, decreasing the up-take of carbs, attempting to regulate bowel tract enzyme reactions, increasing the peripheral glucose uptake and boosting the antioxidant status. A previous study showed that the Ficus species are potent in anti-diabetic medications. The in vitro and in vivo studies showed that the essential oil and polyphenol components from many Ficus species have anti-diabetic properties. All diabetes-related problems were significantly reduced by the chemicals extracted from Ficus species in streptozotocin and allowance-induced diabetic mice. The most important antidiabetic effects of the Ficus species have been demonstrated, including an increased insulin awareness, an increase in insulin secretion, encouraged hepatic glycogen synthesis, reduced carbohydrate absorption, controlled intestinal enzyme activities, enhanced hepatocyte gluconeogenesis and enhanced antioxidant potential [135].
5. Conclusions
It is concluded that fig is a rich source of nutrients and bioactive compounds. Different parts of fig (the leaves, seeds and latex) are contained in different phytochemicals. The change in the different concentrations varies based on the part of the fig. Due to the rich source of phytochemicals, fig by-products play an additive role in the development of different food products, including beverage, dairy and bakery industries. Because these products are enriched with bioactive compounds, they are considered to be suitable for various disorders. In the future, fig-based nanoparticles can be used as a coating material for a different food.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial or non-profit sectors.
Author Contributions
Conceptualization, W.K. and A.A. (Afifa Aziz); methodology, W.K.; software, H.K.; validation, A.A. (Anwar Ali) and W.-F.L.; formal analysis, W.K., A.A. (Afifa Aziz), I.F.u.R. and H.K.; investigation, S.A.S. and A.A. (Afifa Aziz); data curation, W.K. and S.A.S.; writing—original draft preparation, I.F.u.R., S.A.S., A.A. (Anwar Ali) and W.K.; writing—review and editing, S.A.S., A.A. (Afifa Aziz), A.A.-F., W.K., W.-F.L. and H.K.; supervision, W.K. and A.A. (Afifa Aziz); project administration, W.K. and A.A.-F.; funding acquisition, W.-F.L. and A.A. (Anwar Ali); All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial or non-profit sectors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Van Noort S., Wang R., Compton S.G. Fig wasps (Hymenoptera: Chalcidoidea: Agaonidae, Pteromalidae) associated with Asian fig trees (Ficus, Moraceae) in southern Africa: Asian followers and African colonists: Insecta: Hymenoptera. Afr. Invertebr. 2013;54:381–400. doi: 10.5733/afin.054.0208. [DOI] [Google Scholar]
- 2.Trad M., Le Bourvellec C., Gaaliche B., Renard C.M., Mars M. Nutritional Compounds in Figs from the Southern Mediterranean Region. Int. J. Food Prop. 2013;17:491–499. doi: 10.1080/10942912.2011.642447. [DOI] [Google Scholar]
- 3.Garibaldi L.A., Schulte L.A., Jodar D.N.N., Carella D.S.G., Kremen C. Time to Integrate Pollinator Science into Soybean Production. Trends Ecol. Evol. 2021;36:573–575. doi: 10.1016/j.tree.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Bose T.K., Mitra S.K., Sanyal D. Fruits: Tropical and subtropical. 3rd ed. Volume 1 Naya Udyog; Kolkata, India: 2001. [Google Scholar]
- 5.Sheikh B.Y. The role of prophetic medicine in the management of diabetes mellitus: A review of literature. J. Taibah Univ. Med. Sci. 2016;11:339–352. doi: 10.1016/j.jtumed.2015.12.002. [DOI] [Google Scholar]
- 6.Kunz T.H., Diaz C.A. Folivory in fruit-eating bats, with new evidence from Artibeusjamaicensis (Chiroptera: Phyllostomidae) Biotropica. 1995;27:106–120. doi: 10.2307/2388908. [DOI] [Google Scholar]
- 7.Frodin D.G. History and concepts of big plant genera. Taxon. 2004;53:753–776. doi: 10.2307/4135449. [DOI] [Google Scholar]
- 8.Mawa S., Husain K., Jantan I. Ficus carica L. (Moraceae): Phytochemistry, Traditional Uses and Biological Activities. Evidence-Based Complement. Altern. Med. 2013;2013:974256. doi: 10.1155/2013/974256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barolo M.I., Mostacero N.R., López S.N. Ficus carica L. (Moraceae): An ancient source of food and health. Food Chem. 2014;164:119–127. doi: 10.1016/j.foodchem.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 10.Akhtar S., Anjum F.M., Anjum M.A. Micronutrient fortification of wheat flour: Recent development and strategies. Food Res. Int. 2011;44:652–659. doi: 10.1016/j.foodres.2010.12.033. [DOI] [Google Scholar]
- 11.Sher H., Bussmann R.W., Hart R., de Boer H.J. Traditional use of medicinal plants among Kalasha, Ismaeli and Sunni groups in Chitral District, Khyber Pakhtunkhwa province, Pakistan. J. Ethnopharmacol. 2016;188:57–69. doi: 10.1016/j.jep.2016.04.059. [DOI] [PubMed] [Google Scholar]
- 12.Adisakwattana S., Yibchok-Anun S., Charoenlertkul P., Wongsasiripat N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011;49:36–41. doi: 10.3164/jcbn.10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X.M., Yu W., Ou Z.P., Ma H.L., Liu W.M., Ji X.L. Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Foods Hum. Nutr. 2009;64:167–173. doi: 10.1007/s11130-009-0120-5. [DOI] [PubMed] [Google Scholar]
- 14.Afzal M.F., Khalid W., Akram S., Khalid M.A., Zubair M., Kauser S., Mohamedahmed K.A., Aziz A., Siddiqui S.A. Bioactive profile and functional food applications of banana in food sectors and health: A review. Int. J. Food Prop. 2022;25:2286–2300. doi: 10.1080/10942912.2022.2130940. [DOI] [Google Scholar]
- 15.Trichopoulou A., Vasilopoulou E., Georga K., Soukara S., Dilis V. Traditional foods: Why and how to sustain them. Trends Food Sci. Technol. 2006;17:498–504. doi: 10.1016/j.tifs.2006.03.005. [DOI] [Google Scholar]
- 16.Widlansky M.E., Duffy S.J., Hamburg N., Gokce N., Warden B.A., Wiseman S., Keaney J., Frei B., Vita J.A. Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease. Free. Radic. Biol. Med. 2005;38:499–506. doi: 10.1016/j.freeradbiomed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Dueñas M., Pérez-Alonso J.J., Santos-Buelga C., Escribano-Bailón T. Anthocyanin composition in fig (Ficus carica L.) J. Food Compos. Anal. 2008;21:107–115. doi: 10.1016/j.jfca.2007.09.002. [DOI] [Google Scholar]
- 18.Vallejo F., Marín J.G., Tomás-Barberán F.A. Phenolic compound content of fresh and dried figs (Ficus carica L.) Food Chem. 2012;130:485–492. doi: 10.1016/j.foodchem.2011.07.032. [DOI] [Google Scholar]
- 19.Behzad B.D. Morphological and pomological characteristics of fig (Ficus carica L.) cultivars from Varamin, Iran. Afr. J. Biotechnol. 2011;10:19096–19105. [Google Scholar]
- 20.Tanwar B., Andallu B., Modgil R. Influence of processing on physicochemical, nutritional and phytochemical composition of Ficus carica L. (fig) products. Asian J. Dairy Food Res. 2014;33:37–43. doi: 10.5958/j.0976-0563.33.1.008. [DOI] [Google Scholar]
- 21.Badgujar S.B., Patel V.V., Bandivdekar A.H., Mahajan R.T. Traditional uses, phytochemistry and pharmacology of Ficus carica: A review. Pharm. Biol. 2014;52:1487–1503. doi: 10.3109/13880209.2014.892515. [DOI] [PubMed] [Google Scholar]
- 22.Sharma B.R., Kumar V., Kumar S., Panesar P.S. Microwave assisted extraction of phytochemicals from Ficusracemosa. Curr. Opin. Green Sustain. Chem. 2020;3:100020. doi: 10.1016/j.crgsc.2020.100020. [DOI] [Google Scholar]
- 23.Jangde R.K. Plant Profile of Ficusreligosa: A Review. J. Sci. Technol. 2015;7:193. [Google Scholar]
- 24.Alexandre E., Araújo P., Duarte M.F., Freitas V., Pintado M.M., Saraiva J.A. High-pressure assisted extraction of bioactive compounds from industrial fermented fig by-product. J. Food Sci. Technol. 2017;54:2519–2531. doi: 10.1007/s13197-017-2697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T., Jiao J., Gai Q.-Y., Wang P., Guo N., Niu L.-L., Fu Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017;145:339–345. doi: 10.1016/j.jpba.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Teruel-Andreu C., Andreu-Coll L., López-Lluch D., Sendra E., Hernández F., Cano-Lamadrid M. Ficus carica Fruits, By-Products and Based Products as Potential Sources of Bioactive Compounds: A Review. Agronomy. 2021;11:1834. doi: 10.3390/agronomy11091834. [DOI] [Google Scholar]
- 27.Shahinuzzaman M., Yaakob Z., Anuar F.H., Akhtar P., Kadir N.H.A., Hasan A.K.M., Sobayel K., Nour M., Sindi H., Amin N., et al. In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Sci. Rep. 2020;10:10852. doi: 10.1038/s41598-020-67765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L., Meng Y., Wang Z.-L., Cao L., Liu C., Gao M.-Z., Zhao C.-J., Fu Y.-J. Sustainable and efficient surfactant-based microwave-assisted extraction of target polyphenols and furanocoumarins from fig (Ficus carica L.) leaves. J. Mol. Liq. 2020;318:114196. doi: 10.1016/j.molliq.2020.114196. [DOI] [Google Scholar]
- 29.Mopuri R., Ganjayi M., Meriga B., Koorbanally N.A., Islam S. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J. Food Drug Anal. 2018;26:201–210. doi: 10.1016/j.jfda.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backes E., Pereira C., Barros L., Prieto M., Genena A.K., Barreiro M.F., Ferreira I.C. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018;113:197–209. doi: 10.1016/j.foodres.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Prabavathy D., Nachiyar C.V. Screening and characterisation of antimicrobial compound from endophytic Aspergillus sp. isolated from Ficus carica. J. Pharm. Res. 2011;4:34–38. [Google Scholar]
- 32.Radwan S., Handal G., Rimawi F., Hanania M. Seasonal Variations in Antioxidant Activity, Total Flavonoids Content, Total Phenolic Content, Antimicrobial Activity and Some Bioactive Components of Ficus carica L. in Palestine. Int. J. PharmTech Res. 2020;13:329–340. doi: 10.20902/IJPTR.2019.130404. [DOI] [Google Scholar]
- 33.Abdel-Rahman R., Ghoneimy E., Abdel-Wahab A., Eldeeb N., Salem M., Salama E., Ahmed T. The therapeutic effects of Ficus carica extract as antioxidant and anticancer agent. South Afr. J. Bot. 2021;141:273–277. doi: 10.1016/j.sajb.2021.04.019. [DOI] [Google Scholar]
- 34.Lazreg-Aref H., Mars M., Fekih A., Aouni M., Said K. Chemical composition and antibacterial activity of a hexane extract of Tunisian caprifig latex from the unripe fruit of Ficus carica. Pharm. Biol. 2012;50:407–412. doi: 10.3109/13880209.2011.608192. [DOI] [PubMed] [Google Scholar]
- 35.Jeong M.R., Kim H.Y., Cha J.D. Antimicrobial activity of methanol extract from Ficus carica leaves against oral bacteria. J. Bacteriol. Virol. 2009;39:97–102. doi: 10.4167/jbv.2009.39.2.97. [DOI] [Google Scholar]
- 36.Bey M.B., Meziant L., Benchikh Y., Louaileche H. Deployment of response surface methodology to optimize recovery of dark fresh fig (Ficus carica L., var. Azenjar) total phenolic compounds and antioxidant activity. Food Chem. 2014;162:277–282. doi: 10.1016/j.foodchem.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 37.Ergül M., Ergül M., Eruygur N., Ataş M., Ucar E. In vitro evaluation of the chemical composition and various biological activities of Ficus carica leaf extracts. Turk. J. Pharm. Sci. 2019;16:401. doi: 10.4274/tjps.galenos.2018.70037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Aty A.M., Hamed M.B., Salama W.H., Ali M.M., Fahmy A.S., Mohamed S.A. Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: Phytochemical screening, antioxidant and cytotoxic properties. Biocatal. Agric. Biotechnol. 2019;20:101199. doi: 10.1016/j.bcab.2019.101199. [DOI] [Google Scholar]
- 39.Bucić-Kojić A., Planinić M., Tomas S., Jokić S., Mujić I., Bilić M., Velić D. Effect of Extraction Conditions on the Extractability of Phenolic Compounds from Lyophilised Fig Fruits (Ficus carica L.) Pol. J. Food Nutr. Sci. 2011;61:195–199. doi: 10.2478/v10222-011-0021-9. [DOI] [Google Scholar]
- 40.Ercisli S., Tosun M., Karlidag H., Dzubur A., Hadziabulic S., Aliman J. Color and Antioxidant Characteristics of Some Fresh Fig (Ficus carica L.) Genotypes from Northeastern Turkey. Plant Foods Hum. Nutr. 2012;67:271–276. doi: 10.1007/s11130-012-0292-2. [DOI] [PubMed] [Google Scholar]
- 41.Nakilcioğlu-Taş E., Ötleş S. Influence of extraction solvents on the polyphenol contents, compositions, and antioxidant capacities of fig (Ficus carica L. ) seeds. An. Acad. Bras. Cienc. 2021;93:e20190526. doi: 10.1590/0001-3765202120190526. [DOI] [PubMed] [Google Scholar]
- 42.Ivanov I., Dincheva I., Badjakov I., Petkova N., Denev P., Pavlov A. GC-MS analysis of unpolar fraction from Ficus carica L.(fig) leaves. Int. Food Res. J. 2018;25:282–286. [Google Scholar]
- 43.Morgan A.F., Kimmel L., Nichols P.F., Field A. The Vitamin Content of Figs. J. Nutr. 1935;9:383–394. doi: 10.1093/jn/9.3.383. [DOI] [Google Scholar]
- 44.Fasakin C.F., Udenigwe C.C., Aluko R.E. Antioxidant properties of chlorophyll-enriched and chlorophyll-depleted polyphenolic fractions from leaves of Vernonia amygdalina and Gongronema latifolium. Food Res. Int. 2011;44:2435–2441. doi: 10.1016/j.foodres.2010.12.019. [DOI] [Google Scholar]
- 45.Silva R., Abílio V., Takatsu A., Kameda S., Grassl C., Chehin A., Medrano W., Calzavara M., Registro S., Andersen M., et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 46.Solomon A., Golubowicz S., Yablowicz Z., Grossman S., Bergman M., Gottlieb H.E., Flaishman M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.) J. Agric. Food Chem. 2006;54:7717–7723. doi: 10.1021/jf060497h. [DOI] [PubMed] [Google Scholar]
- 47.Vinson J.A. The functional food properties of figs. J. Cereal Sci. 1999;44:82–87. [Google Scholar]
- 48.Costa R.M., Magalhães A.S., Pereira J.A., Andrade P.B., Valentão P., Carvalho M., Silva B.M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: A comparative study with green tea (Camellia sinensis) Food Chem. Toxicol. 2009;47:860–865. doi: 10.1016/j.fct.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Eberhardt M.V., Lee C.Y., Liu R.H. Antioxidant activity of fresh apples. Nature. 2000;405:903–904. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- 50.Vinson J.A., Zubik L., Bose P., Samman N., Proch J. Dried fruits: Excellent in vitro and in vivo antioxidants. J. Am. Coll. Nutr. 2005;24:44–50. doi: 10.1080/07315724.2005.10719442. [DOI] [PubMed] [Google Scholar]
- 51.Genna A., De Vecchi P., Maestrelli A., Bruno M. III International Symposium on Fig. Volume 798. ISHS; Leuven, Belgium: 2005. Quality of ‘Dottato’ Dried Figs Grown in the Cosenza Region, Italy. A Sensory and Physical-Chemical Approach; pp. 319–323. [Google Scholar]
- 52.Piga A., Del Caro A., Milella G., Pinna I., Vacca V., Schirru S. III International Symposium on Fig. Volume 798. ISHS; Leuven, Belgium: 2005. Hplc Analysis of Polyphenols in Peel and Pulp of Fresh Figs; pp. 301–306. [DOI] [Google Scholar]
- 53.Jeong W.S., Lachance P.A. Phytosterols and fatty acids in fig (Ficus carica, var. Mission) fruit and tree components. J. Food Sci. 2001;66:278–281. doi: 10.1111/j.1365-2621.2001.tb11332.x. [DOI] [Google Scholar]
- 54.Juániz I., Ludwig I.A., Huarte E., Pereira-Caro G., Moreno-Rojas J.M., Cid C., De Peña M.-P. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016;197:466–473. doi: 10.1016/j.foodchem.2015.10.139. [DOI] [PubMed] [Google Scholar]
- 55.DuPree E.J., Jayathirtha M., Yorkey H., Mihasan M., Petre B.A., Darie C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of this Field. Proteomes. 2020;8:14. doi: 10.3390/proteomes8030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oliveira A.P., Valentão P., Pereira J.A., Silva B.M., Tavares F., Andrade P.B. Ficus carica L.: Metabolic and biological screening. Food Chem. Toxicol. 2009;47:2841–2846. doi: 10.1016/j.fct.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Veberic R., Colaric M., Stampar F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008;106:153–157. doi: 10.1016/j.foodchem.2007.05.061. [DOI] [Google Scholar]
- 58.Bouayed J., Hoffmann L., Bohn T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011;128:14–21. doi: 10.1016/j.foodchem.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 59.El-Sheekh M.M., El-Kassas H.Y. Algal production of nano-silver and gold: Their antimicrobial and cytotoxic activities: A review. J. Genet. Eng. Biotechnol. 2016;14:299–310. doi: 10.1016/j.jgeb.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCune L. Bioactive Food as Dietary Interventions for Cardiovascular Disease: Bioactive Foods in Chronic Disease States. Volume 243 Elsevier; Amsterdam, The Netherlands: 2012. A review of the antioxidant actions of three herbal medicines (Crataegus monogyna, Ginkgo biloba, and Aesculus hippocastanum) on the treatment of cardiovascular diseases. [Google Scholar]
- 61.Kim M.Y., Kim E.J., Kim Y.N., Choi C., Lee B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012;6:21–27. doi: 10.4162/nrp.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bigliardi B., Galati F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013;31:118–129. doi: 10.1016/j.tifs.2013.03.006. [DOI] [Google Scholar]
- 63.Al-Marazeeq K.M., Angor M.M. Chemical Characteristic and Sensory Evaluation of Biscuit Enriched with Wheat Germ and the Effect of Storage Time on the Sensory Properties for this Product. Food Nutr. Sci. 2017;08:189–195. doi: 10.4236/fns.2017.82012. [DOI] [Google Scholar]
- 64.Chareonthaikij P., Uan-On T., Prinyawiwatkul W. Effects of pineapple pomace fibre on physicochemical properties of composite flour and dough, and consumer acceptance of fibre-enriched wheat bread. Int. J. Food Sci. Technol. 2016;51:1120–1129. doi: 10.1111/ijfs.13072. [DOI] [Google Scholar]
- 65.Farouk M.M., Yoo M.J.Y., Hamid N.S.A., Staincliffe M., Davies B., Knowles S.O. Novel meat-enriched foods for older consumers. Food Res. Int. 2018;104:134–142. doi: 10.1016/j.foodres.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 66.Ryan J., Hutchings S.C., Fang Z., Bandara N., Gamlath S., Ajlouni S., Ranadheera C.S. Microbial, physico-chemical and sensory characteristics of mango juice-enriched probiotic dairy drinks. Int. J. Dairy Technol. 2020;73:182–190. doi: 10.1111/1471-0307.12630. [DOI] [Google Scholar]
- 67.Ali A., Rahut D.B. Healthy foods as proxy for functional foods: Consumers’ awareness, perception, and demand for natural functional foods in Pakistan. Int. J. Food Sci. 2019;2019:6390650. doi: 10.1155/2019/6390650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolin H.R., Jackson R. Factors affecting sulfur dioxide binding in dried apples and apricots. J. Food Process. Preserv. 1985;9:25–34. doi: 10.1111/j.1745-4549.1985.tb00705.x. [DOI] [Google Scholar]
- 69.Viuda-Martos M., Barber X., Pérez-Álvarez J.A., Fernández-López J. Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Ind Crop. Prod. 2015;69:472–479. doi: 10.1016/j.indcrop.2015.03.005. [DOI] [Google Scholar]
- 70.Ayuso M., Carpena M., Taofiq O., Albuquerque T.G., Simal-Gandara J., Oliveira M.B.P., Barros L. Fig “Ficus carica L.” and its by-products: A decade evidence of their health-promoting benefits towards the development of novel food formulations. Trends Food Sci. Technol. 2022;127:1–13. doi: 10.1016/j.tifs.2022.06.010. [DOI] [Google Scholar]
- 71.Yilmaz P., Demirhan E., Ozbek B. Development of Ficus carica Linn leaves extract incorporated chitosan films for active food packaging materials and investigation of their properties. Food Biosci. 2022;46:101542. doi: 10.1016/j.fbio.2021.101542. [DOI] [Google Scholar]
- 72.Backes E., Leichtweis M.G., Pereira C., Carocho M., Barreira J.C., Kamal Genena A., José Baraldi I., Filomena Barreiro M., Barros L., Ferreira I.C. Ficus carica L. and Prunus spinosa L. extracts as new anthocyanin-based food colorants: A thorough study in confectionery products. Food Chem. 2020;333:127457. doi: 10.1016/j.foodchem.2020.127457. [DOI] [PubMed] [Google Scholar]
- 73.Khapre A.P., Satwadhar P.N., Deshpande H.W. Studies on standardization of fig fruit (Ficus carica L.) powder enriched cookies and its composition. J. Dairy Foods Home Sci. 2015;34:71–74. [Google Scholar]
- 74.Yeganehzad S., Kiumarsi M., Nadali N., Ashkezary M.R. Formulation, development and characterization of a novel functional fruit snack based on fig (Ficus carica L.) coated with sugar-free chocolate. Heliyon. 2020;6:e04350. doi: 10.1016/j.heliyon.2020.e04350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jahromi M., Niakousari M. Development and characterisation of a sugar-free milk-based dessert formulation with fig (Ficus carica L.) and carboxymethylcellulose. Int. J. Dairy Technol. 2018;71:801–809. doi: 10.1111/1471-0307.12484. [DOI] [Google Scholar]
- 76.Maghsoudlou E., Kenari R.E., Amiri Z.R. Evaluation of Antioxidant Activity of Fig (Ficus carica) Pulp and Skin Extract and Its Application in Enhancing Oxidative Stability of Canola Oil. J. Food Process. Preserv. 2016;41:e13077. doi: 10.1111/jfpp.13077. [DOI] [Google Scholar]
- 77.Khapre A.P., Satwadhar P.N., Syed H.M. Studies on processing technology and cost estimation of fig (Ficus carica L.) fruit powder enriched Burfi (Indian cookie) J. Appl. Nat. Sci. 2015;7:621–624. doi: 10.31018/jans.v7i2.655. [DOI] [Google Scholar]
- 78.Khapre A.P., Satwadhar P.N., Deshpande H.W. Development of technology for preparation of fig (Ficus carica L.) fruit powder and its utilization in toffee. J. Dairy. Foods Home Sci. 2011;30:267–270. [Google Scholar]
- 79.Kumar A., Elavarasan K., Hanjabam M.D., Binsi P., Mohan C., Zynudheen A.A., Kumar A. Marine collagen peptide as a fortificant for biscuit: Effects on biscuit attributes. Lwt. 2019;109:450–456. doi: 10.1016/j.lwt.2019.04.052. [DOI] [Google Scholar]
- 80.Krystyjan M., Gumul D., Ziobro R., Korus A. The fortification of biscuits with bee pollen and its effect on physicochemical and antioxidant properties in biscuits. Lwt. 2015;63:640–646. doi: 10.1016/j.lwt.2015.03.075. [DOI] [Google Scholar]
- 81.Nogueira A.D.C., Steel C.J. Protein enrichment of biscuits: A review. Food Rev. Int. 2018;34:796–809. doi: 10.1080/87559129.2018.1441299. [DOI] [Google Scholar]
- 82.Jung K.T., Park B.G., Lee M.H. Quality characteristics of sourdough bread using fermented fig. Culin. Sci. Hosp. Res. 2017;23:56–65. [Google Scholar]
- 83.Dogan A., Cat A., Catal M., Erkan M. First report of Alternariaalternata causing postharvest decay in fig (Ficus carica L. cv. Bursa Siyahi) fruit in Turkey. J. Biotechnol. 2018;280:S32–S91. doi: 10.1016/j.jbiotec.2018.06.275. [DOI] [Google Scholar]
- 84.Martinez-Damian M.T., Omegar C.A., Oscar C.A. Effect of modified atmosphere packaging on nutraceutical quality and overall appearance of figs stored at 1 C. Not. Bot. Horti Agrobot. 2020;48:2292–2305. doi: 10.15835/nbha48412076. [DOI] [Google Scholar]
- 85.Lansky E.P., Paavilainen H.M., Pawlus A.D., Newman R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008;119:195–213. doi: 10.1016/j.jep.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 86.Liu X., Xu S., Wang M., Wang L., Liu J. Effect of mixed fermentation with Pichia fermentans, Hanseniasporauvarum, and Wickeramomycesanomala on the quality of fig (Ficus carica L.) wines. J. Food Process. Preserv. 2021;45:e15169. doi: 10.1111/jfpp.15169. [DOI] [Google Scholar]
- 87.Ilkin Y.S. Microbiological and chemical properties of fig vinegar produced in Turkey. Afr. J. Microbiol. Res. 2013;7:2332–2338. doi: 10.5897/AJMR12.2275. [DOI] [Google Scholar]
- 88.Jeong M.R., Cha J.D., Yun S.I., Han J.H., Lee Y.E. Manufacturing of wine with Korean figs (Ficus carica L.) and quality improvement by adding fig leaves. J. East Asian Soc. Diet. Life. 2005;15:112–118. [Google Scholar]
- 89.Shi Y., Mon A.M., Fu Y., Zhang Y., Wang C., Yang X., Wang Y. The genus Ficus (Moraceae) used in diet: Its plant diversity, distribution, traditional uses and ethnopharmacological importance. J. Ethnopharmacol. 2018;226:185–196. doi: 10.1016/j.jep.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 90.Weibin J., Kai M., Zhifeng L., Yelin W., Lianju W. II International Symposium on Fig. Volume 605. ISHS; Leuven, Belgium: 2001. The production and research of fig (Ficus carica L.) in China; pp. 191–196. [Google Scholar]
- 91.Watson R., Preedy V.R. Bioactive Foods in Promoting Health: Fruits and Vegetables. Academic Press; Cambridge, MA, USA: 2009. [Google Scholar]
- 92.Arvaniti O.S., Samaras Y., Gatidou G., Thomaidis N.S., Stasinakis A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019;119:244–267. doi: 10.1016/j.foodres.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 93.Cruz J.M.d.A., Corrêa R.F., Lamarão C.V., Kinupp V.F., Sanches E.A., Campelo P.H., Bezerra J.D.A. Ficus spp. fruits: Bioactive compounds and chemical, biological and pharmacological properties. Food Res. Int. 2021;152:110928. doi: 10.1016/j.foodres.2021.110928. [DOI] [PubMed] [Google Scholar]
- 94.Yildirim H.K. Evaluation of colour parameters and antioxidant activities of fruit wines. Int. J. Food Sci. Nutr. 2006;57:47–63. doi: 10.1080/09637480600655993. [DOI] [PubMed] [Google Scholar]
- 95.Sanders M.E. Probiotics, strains matter. Funct. Foods Nutraceuticals Mag. 2007;48:36–41. [Google Scholar]
- 96.O’Connell J.E., Fox P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001;11:103–120. doi: 10.1016/S0958-6946(01)00033-4. [DOI] [Google Scholar]
- 97.Granato D., Nunes D.S., Barba F.J. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017;62:13–22. doi: 10.1016/j.tifs.2016.12.010. [DOI] [Google Scholar]
- 98.Oliveira A.P., Silva L.R., de Pinho P.G., Gil-Izquierdo A., Valentão P., Silva B.M., Andrade P.B. Volatile profiling of Ficus carica varieties by HS-SPME and GC–IT-MS. Food Chem. 2010;123:548–557. doi: 10.1016/j.foodchem.2010.04.064. [DOI] [Google Scholar]
- 99.Ishurd O., Zgheel F., Kermagi A., Flefla M., Elmabruk M., Yalin W., Pan Y. Microbial (1→ 3)-β-D-glucans from Libyan figs (Ficus carica) Carbohydr. Polym. 2004;58:181–184. doi: 10.1016/j.carbpol.2004.06.040. [DOI] [Google Scholar]
- 100.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 101.Ouchemoukh S., Hachoud S., Boudraham H., Mokrani A., Louaileche H. Antioxidant activities of some dried fruits consumed in Algeria. Lwt. 2012;49:329–332. doi: 10.1016/j.lwt.2012.07.022. [DOI] [Google Scholar]
- 102.Abdel-Hameed E.S.S. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009;114:1271–1277. doi: 10.1016/j.foodchem.2008.11.005. [DOI] [Google Scholar]
- 103.Abbasi S., Kamalinejad M., Babaie D., Shams S., Sadr Z., Gheysari M., Rakhshandeh H. A new topical treatment of atopic dermatitis in pediatric patients based on Ficus carica L. (Fig): A randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017;35:85–91. doi: 10.1016/j.ctim.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Ao C., Li A., Elzaawely A.A., Xuan T.D., Tawata S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. Extract. Food con. 2008;19:940–948. doi: 10.1016/j.foodcont.2007.09.007. [DOI] [Google Scholar]
- 105.Zhang Y., Li Y., Ma P., Chen J., Xie W. Ficus carica leaves extract inhibited pancreatic β-cell apoptosis by inhibiting AMPK/JNK/caspase-3 signaling pathway and antioxidation. Biomed. Pharmacother. 2020;122:109689. doi: 10.1016/j.biopha.2019.109689. [DOI] [PubMed] [Google Scholar]
- 106.Ghanbari A., Le Gresley A., Naughton D., Kuhnert N., Sirbu D., Ashrafi G.H. Biological activities of Ficus carica latex for potential therapeutics in Human Papillomavirus (HPV) related cervical cancers. Sci. Rep. 2019;9:19258. doi: 10.1038/s41598-018-37665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slavin J.L. Figs: Past, present, and future. Nutr. Today. 2006;41:180–184. doi: 10.1097/00017285-200607000-00009. [DOI] [Google Scholar]
- 108.Rahmani A.H., Aldebasi Y.H. Ficus carica and Its Constituents Role in Management of Diseases. Asian J. Pharm. Clin. Res. 2017;10:49–53. doi: 10.22159/ajpcr.2017.v10i6.17832. [DOI] [Google Scholar]
- 109.El-Sayed S.M., El-Naggar M.E., Hussein J., Medhat D., El-Banna M. Effect of Ficus carica L. leaves extract loaded gold nanoparticles against cisplatin-induced acute kidney injury. Colloids Surf. B: Biointerfaces. 2019;184:110465. doi: 10.1016/j.colsurfb.2019.110465. [DOI] [PubMed] [Google Scholar]
- 110.Wang E., Chen X., Liu T., Wang K. Effect of dietary Ficus carica polysaccharides on the growth performance, innate immune response and survival of crucian carp against Aeromonas hydrophila infection. Fish Shellfish. Immunol. 2022;120:434–440. doi: 10.1016/j.fsi.2021.12.018. [DOI] [PubMed] [Google Scholar]
- 111.Saoudi M., El Feki A. Protective Role of Ficus carica Stem Extract against Hepatic Oxidative Damage Induced by Methanol in Male Wistar Rats. Evidence-Based Complement. Altern. Med. 2012;2012:150458. doi: 10.1155/2012/150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dini I., Falanga D., Di Lorenzo R., Tito A., Carotenuto G., Zappelli C., Grumetto L., Sacchi A., Laneri S., Apone F. An Extract from Ficus carica Cell Cultures Works as an Anti-Stress Ingredient for the Skin. Antioxidants. 2021;10:515. doi: 10.3390/antiox10040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bhanushali M., Joshi Y., Makhija D. Central nervous system activity of an aqueous acetonic extract of Ficus carica L. in mice. J. Ayurveda Integr. Med. 2014;5:89–96. doi: 10.4103/0975-9476.131734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rezagholizadeh L., Aghamohammadian M., Oloumi M., Banaei S., Mazani M., Ojarudi M. Inhibitory effects of Ficus carica and Olea europaea on pro-inflammatory cytokines: A review. Iran. J. Basic Med. Sci. 2022;25:268. doi: 10.22038/IJBMS.2022.60954.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mohan G.K., Pallavi E., Kumar R., Ramesh M., Venkatesh S. Hepatoprotective activity of Ficus carica Linn leaf extract against carbon tetrachloride-induced hepatotoxicity in rats. DARU J. Pharm. Sci. 2007;15:162–166. [Google Scholar]
- 116.Purnamasari R., Winarni D., Permanasari A.A., Agustina E., Hayaza S., Darmanto W. Anticancer activity of methanol extract of Ficus carica leaves and fruits against proliferation, apoptosis, and necrosis in Huh7it cells. Cancer Inform. 2019;18:1176935119842576. doi: 10.1177/1176935119842576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Canal J., Torres M., Pérez C. Experimental diabetes treated with Ficus carica extract: Effect on oxidative stress parameters. Acta Diabetol. 2003;40:3–8. doi: 10.1007/s005920300001. [DOI] [PubMed] [Google Scholar]
- 118.El-Shobaki F.A., El-Bahay A.M., Esmail R.S.A., El-Megeid A.A.A., Esmail N.S. Effect of figs fruit (Ficus carica L.) and its leaves on hyperglycemia in alloxan diabetic rats. World J. Dairy Food Sci. 2010;5:47–57. [Google Scholar]
- 119.Read N., Timms J., Barfield L., Donnelly T., Bannister J. Impairment of defecation in young women with severe constipation. Gastroenterology. 1986;90:53–60. doi: 10.1016/0016-5085(86)90074-0. [DOI] [PubMed] [Google Scholar]
- 120.Jeong Rea J., Cordes D.H. Disability assessment. J. Fam. Pract. 1985;21:337–338. [PubMed] [Google Scholar]
- 121.Tabrizi A., Dargahi R., Ghadim S.T., Javadi M., Pirouzian H.R., Azizi A., Rad A.H. Functional laxative foods: Concepts, trends and health benefits. J. Nat. Prod. 2020;66:305–330. doi: 10.1016/b978-0-12-817907-9.00011-8. [DOI] [Google Scholar]
- 122.Williams D.C., Sgarbieri V.C., Whitaker J.R. Proteolytic Activity in the Genus Ficus. Plant Physiol. 1968;43:1083–1088. doi: 10.1104/pp.43.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wintola O.A., Sunmonu T.O., Afolayan A.J. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010;10:95. doi: 10.1186/1471-230X-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ajmal M., Arshad M.U., Saeed F., Ahmed T., Khan A.U., Bader-ul-Ain H., Suleria H.A.R. Exploring the Nutritional Characteristics of Different Parts of Fig in Relation to Hypoglycemic Potential. Pak. J. Life Soc. Sci. 2016;14:95. [Google Scholar]
- 125.Al-Ogaili N.A., Osama S., Jazme D., Saad S. In vitro antibacterial investigation and synergistic effect of Ficus carica and oleaEuropaea aqueous extracts. Res. J. Pharm. Technol. 2020;13:1198–1203. doi: 10.5958/0974-360X.2020.00221.8. [DOI] [Google Scholar]
- 126.Aksoy U. V International Symposium on Fig. ISHS; Leuven, Belgium: 2017. The dried fig management and the potential for new products; pp. 377–382. [DOI] [Google Scholar]
- 127.Patil C.R., Thakre S.S., Thakre S.B. A cross-sectional study on the risk factors for cardiovascular disease and risk profiling of adults in central India. J. Clin. Prev. Cardiol. 2017;6:104. [Google Scholar]
- 128.Rodriguez-Monforte M., Flores-Mateo G., Sánchez E. Dietary patterns and CVD: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2015;114:1341–1359. doi: 10.1017/S0007114515003177. [DOI] [PubMed] [Google Scholar]
- 129.Grosso G., Marventano S., Yang J., Micek A., Pajak A., Scalfi L., Galvano F., Kales S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017;57:3218–3232. doi: 10.1080/10408398.2015.1107021. [DOI] [PubMed] [Google Scholar]
- 130.Soni N., Mehta S., Satpathy G., Gupta R.K. Estimation of nutritional, phytochemical, antioxidant and antibacterial activity of dried fig (Ficus carica) Int. J. Pharmacogn. Phytochem. 2014;3:158–165. [Google Scholar]
- 131.World Health Organization . Global NCD Target: Halt the Rise in Diabetes (No. WHO/NMH/NMA/16.186) World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 132.Mahmoudi S., Khali M., Benkhaled A., Benamirouche K., Baiti I. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac. J. Trop. Biomed. 2016;6:239–245. doi: 10.1016/j.apjtb.2015.12.010. [DOI] [Google Scholar]
- 133.Misbah H., Aziz A.A., Aminudin N. Antidiabetic and antioxidant properties of Ficusdeltoidea fruit extracts and fractions. BMC Complement. Med. Ther. 2013;13:118. doi: 10.1186/1472-6882-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mopuri R., Islam M.S. Antidiabetic and anti-obesity activity of Ficus carica: In vitro experimental studies. Diabetes Metab. 2016;42:300. doi: 10.1016/j.diabet.2016.07.020. [DOI] [Google Scholar]
- 135.Deepa P., Sowndhararajan K., Kim S., Park S.J. A role of Ficus species in the management of diabetes mellitus: A review. J. Ethnopharmacol. 2018;215:210–232. doi: 10.1016/j.jep.2017.12.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available.