Abstract
Sinopodophyllum hexandrum is a perennial alpine herb producing the anti-cancer metabolite podophyllotoxin (PPT). Although the adaptation of S. hexandrum to high altitudes has been demonstrated and the effects of temperature, precipitation, and UV-B light on plant growth and metabolite accumulation have been studied, knowledge on the role of flavonoid biosynthesis in adapting to high altitudes is limited. In this study, light intensity, amount and type of flavonoids, and differentially expressed proteins (DEPs) and genes (DEGs) at 2300 and 3300 m were analyzed by HPLC, proteomic, transcriptomic, and qRT-PCR analysis. We found that higher light intensity correlated with greater flavonoid, flavonol, and anthocyanin content as well as higher anthocyanin to total flavonoid and flavonol ratios observed at the higher altitude. Based on proteomic and transcriptomic analyses, nine DEPs and 41 DEGs were identified to be involved in flavonoid biosynthesis and light response at 3300 m. The relative expression of nine genes (PAL, CHS1, IFRL, ANS, MYB4, BHLH137, CYP6, PPO1, and ABCB19) involved in flavonoid biosynthesis and seven genes (HSP18.1, HSP70, UBC4, ERF5, ERF9, APX3, and EX2) involved in light stress were observed to be up-regulated at 3300 m compared with 2300 m. These findings indicate that light intensity may play a regulatory role in enhancing flavonoid accumulation that allows S. hexandrum to adapt to elevated-altitude coupled with high light intensity.
Keywords: Sinopodophyllum hexandrum, high altitude, flavonoid biosynthesis, gene expression, high light
1. Introduction
Sinopodophyllum hexandrum Royle (family Berberidaceae) is a perennial rhizomatous species that is native to the alpine Himalayan region at altitudes of 2000 to 4500 m above sea level [1,2,3]. The dried fruit is referred to as “xiaoyelian” in China and is used widely as a traditional Tibetan medicine to treat gynecological diseases [4]. The rhizomes are the major source of PPT, which can effectively mitigate specific cancers and heal certain skin lesions [5]. Constituents from dried fruit and rhizomes include lignans, flavonoids, and alkaloids [4,5,6]. The species is currently endangered due to over exploitation of wild plants and limited large-scale artificial cultivation [7,8].
To protect the wild S. hexandrum and to satisfy the commercial demand, preliminary preparations for large-scale cultivation have been performed, including breaking seed dormancy [9], promoting seed germination [10,11], accelerating vegetative propagation by dividing the rhizomes [9], and providing the sustainable cultivation–collection model for optimized PPT production [3]. Currently, large-scale cultivation has yet to be realized, largely because of a failure to identify suitable growth conditions in the field.
Geographical modeling for S. hexandrum predicts that altitude determines plant distribution, with optimal altitudes of 2800 to 3600 m for artificial planting [2]. There is a significant positive correlation of increasing altitude with plant biomass and PPT accumulation [1,3,12,13,14,15]. However, other environmental factors such as temperature, precipitation, and light may also affect plant growth [1,2,3,14]. To dissect the effect of individual environmental components on growth, studies are best executed under controlled laboratory conditions. What is reported thus far is that cool temperatures and reduced water availability are optimal for plant growth [16,17,18,19], while elevated UV-B radiation and high light intensity inhibit growth [20,21].
Flavonoids (i.e., flavonols, flavones, isoflavones, anthocyanidins, flavanones, flavanols, and chalcones) play an essential role in response to elevated light [22,23,24]. Specifically, full sunlight promotes quercetin, kaempferol, and isorhamnetin biosynthesis in ginkgo leaves [25], while petunia leaves and stems under elevated light promote anthocyanin accumulation [26]. Visible light induces proanthocyanidin accumulation in grapes, while UV radiation induces flavonol accumulation in young berries [27]. In fact, anthocyanins can protect plants against light stress by maintaining the higher oxidative levels and minimizing damage to photosynthetic tissues [28].
At the cellular level, flavonoids are synthesized in the cytoplast and then transported into the vacuole to be stored [29]. Generally, flavonoid biosynthesis is regulated by transcription factors (TFs) including MYB, bZIP, bHLH, or their complex, as well as by cytochrome P450s (CYPs) [22,30,31]. Flavonoids are transported through two different transport mechanisms: proton antiport and ABC-type transporter in various plant species [32]. Finally, the stable flavonoids are stored after the modification of acylation (e.g., acetyltransferase, ATs), methylation (e.g., methyltransferases, MTs), and glycosylation (e.g., UDP-glycosyltransferases, UGTs) [29,33].
Previous studies on the developmental adaptation of S. hexandrum have found that anthocyanins in dark spots on the leaf can function as UV protectants, effectively absorbing UV-B radiation at high altitudes [34,35]. In our previous findings, the total flavonoid content was observed in S. hexandrum seedlings under UV-B treatment [21]; a total of 234 characterized genes were differentially expressed at 3300 versus 2300 m, with 22 genes involved in flavonoid biosynthesis and 19 genes involved in light response [36]. To date, the role of flavonoid biosynthesis in adapting to high altitudes is limited. In this study, light intensity and total flavonoids and DEPs were analyzed by spectrophotometer, HPLC, and proteomic approaches. The DEGs were re-analyzed based on our previous transcriptomic data and the expression levels of genes involved in flavonoid biosynthesis and light stress were validated by qRT-PCR. A significant difference in flavonoid accumulation as well as protein and gene expression associated with flavonoid biosynthesis was observed with elevated light.
2. Results
2.1. Differences in Light Intensity and Flavonoid Accumulation
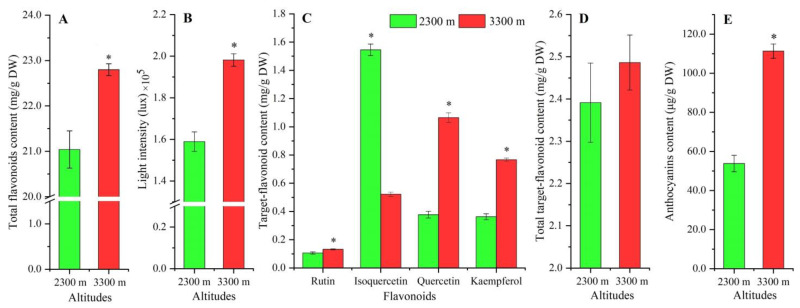
Total flavonoid content was slightly elevated (1.08-fold greater, Figure 1A) for plants grown at 3300 m compared with 2300 m. The light intensity was 1.25-fold greater at the higher altitude (Figure 1B). Specifically, rutin, quercetin, and kaempferol contents were 1.28-, 2.83-, and 2.11-fold greater, while the isoquercetin exhibited a 0.34-fold decrease (Figure 1C). The total content of the four flavonols (rutin, quercetin, kaempferol, and isoquercetin) showed a 1.04-fold increase (Figure 1D) and the anthocyanin content was 2.09-fold greater (Figure 1E).
Figure 1.
The content of total flavonoids (A), light intensity (B), content of target flavonoid (C), total target flavonoid (D), and anthocyanins (E) at 2300 and 3300 m. The * represents a significant difference (p < 0.05) between 2300 and 3300 m (mean ± SD, n = 3).
2.2. Ratio Differences for Anthocyanins and Total Flavonoids
The ratio of anthocyanins to total flavonoid content was 1.91-fold higher (Figure 2A) and the ratio of anthocyanins to four flavonols’ contents was 1.99-fold higher at 3300 m than 2300 m (Figure 2B).
Figure 2.
The ratios of anthocyanins to total flavonoids (A) and flavonol contents (B) at 2300 and 3300 m. The * represents a significant difference (p < 0.05) between 2300 and 3300 m (mean ± SD, n = 3).
2.3. Differentially Expressed Proteins at Higher Elevation
A total of 65 proteins were differentially expressed at 3300 m vs. 2300 m, with 46 over-expressed and 19 down-expressed (Figure S1); among the 46 over-expressed proteins, 30 proteins had known function, while the other 16 proteins had no known function (Table 1). Proteins were classified based on biological function, including primary and secondary metabolism (6), photosynthesis and energy (10), cell morphogenesis (2), transcription (4), and translation (8). Among 30 proteins grouped by biological function, 5 proteins were involved in flavonoid biosynthesis (ANS, CYP6, CYP71BE30, PPO1, and MYB4) and 4 proteins were involved in photosynthesis (ycf3, rbc, rbcL, and petL).
Table 1.
Thirty differentially over-expressed proteins at 3300 vs. 2300 m.
| Genes | Proteins | No. | Nr ID | Spot Ratio (3300 m vs. 2300 m) |
|---|---|---|---|---|
| Metabolism (6) | ||||
| BGLU | Beta-1,3-glucanase | 28 | gi|458601845 | 4.57 |
| ANS | Anthocyanin synthase | 3 | gi|575498405 | 2.51 |
| CYP6 | Cytochrome P450 | 25 | gi|930809490 | 2.40 |
| CYP71BE30 | CYP71BE30 | 31 | gi|441418858 | 6.09 |
| PPO1 | Polyphenol oxidase | 16 | gi|930809512 | 3.30 |
| CS | Corytuberine synthase | 14 | gi|745698910 | 2.44 |
| Photosynthesis and energy (10) | ||||
| ycf3 | Photosystem I assembly protein ycf3 | 9 | gi|1021077178 | 29.30 |
| rbc | Ribulose-1,5-bisphosphate carboxylase/oxygenase | 2 | gi|6513628 | 2.26 |
| rbcL | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit | 5 | gi|825715491 | 11.33 |
| petL | Cytochrome b6-f complex subunit 6 | 22 | gi|1021077124 | 45.76 |
| atpA | ATP synthase CF1 alpha subunit | 24 | gi|918020571 | 54.42 |
| atpB | ATP synthase CF1 beta subunit | 8 | gi|918020573 | 2.39 |
| atpE | ATP synthase CF1 epsilon subunit | 26 | gi|918020601 | 3.41 |
| atpA | ATP synthase subunit alpha | 17 | gi|728802595 | 2.92 |
| matK | Maturase K | 20 | gi|356463749 | 5.73 |
| infA | Translation initiation factor IF-1 | 23 | gi|1025806949 | 2.27 |
| Cell morphogenesis (2) | ||||
| SMCP | Structural maintenance of chromosomes protein 2 | 10 | gi|913341257 | 79.04 |
| ACT | Actin | 15 | gi|307147595 | 9.70 |
| Transcription (4) | ||||
| PI | PISTILLATA-like protein | 1 | gi|186909195 | 2.85 |
| AP3-1 | APETALA3-like protein 1 | 7 | gi|186909191 | 5.54 |
| MYB4 | R2R3-MYB transcription factor MYB4 | 32 | gi|383290957 | 2.49 |
| FUL | FUL-like protein | 18 | gi|371941952 | 7.18 |
| Translation (8) | ||||
| rpl16 | Ribosomal protein L16 | 13 | gi|1025806946 | 4.58 |
| rpl20 | Ribosomal protein L20 | 29 | gi|1021076983 | 4.33 |
| rpl22 | Ribosomal protein L22 | 19 | gi|728802645 | 6.95 |
| rps2 | Ribosomal protein S2 | 21 | gi|1025806873 | 3.47 |
| rps11 | Ribosomal protein S11 | 6 | gi|1025806951 | 3.99 |
| rps12 | Ribosomal protein S12 | 11 | gi|552546114 | 2.33 |
| rps14 | Ribosomal protein S14 | 4 | gi|918020607 | 2.77 |
| rps16 | Ribosomal protein S16 | 27 | gi|728802592 | 4.29 |
Note: the list of proteins is in accordance with the spots in Figure S1.
2.4. Differentially Expressed Genes Based on Elevation Differences
Transcriptomic data of plants at 2300 and 3300 m [36] showed that 22 genes encoding for flavonoid catalysis were differentially expressed, with 16 genes up-regulated 1.4-(C4H, ABCB19, and CYP71CU1) to 4.9-fold (PPO1), while 6 other genes were down-regulated by 1.6- (DTX41) to 5.0-fold (CHS1) (Table 2). In addition, 19 genes involved in light response were differentially expressed, with 9 genes associated with photosynthesis and 10 genes mediated by light stress (Table 3).
Table 2.
Twenty-two DEGs involved in flavonoid biosynthesis at 3300 vs. 2300 m.
| Genes | Proteins | SwissProt ID | log2 FC (3300 vs. 2300 m) |
|---|---|---|---|
| PAL | Phenylalanine ammonia-lyase | A0A059XSS3 | 4.18 |
| C4H | Cinnamic acid hydroxylase | Q43054 | 1.39 |
| CHS1 | Chalcone synthase 1 | P48386 | −5.02 |
| GT6 | UDP-glucose flavonoid 3-O-glucosyltransferase 6 | Q9SZG1 | −1.94 |
| IFRL | Isoflavone reductase-like protein | E1U332 | 2.07 |
| BHLH137 | Transcription factor bHLH137 | Q93W88 | 1.72 |
| DTX41 | Protein DETOXIFICATION 41 | Q9LYT3 | −1.61 |
| ABCB19 | ABC transporter B family member 19 | Q9LJX0 | 1.39 |
| CYP6 | Cytochrome P450 | A0A0N9HQE5 | 3.57 |
| CYP719A23 | CYP719A23 | L7T8H2 | 1.87 |
| CYP71CU1 | Cytochrome P450 family 71 subfamily CU polypeptide 1 | A0A0N9HTU1 | 1.39 |
| CYP734A1 | Cytochrome P450 734A1 | O48786 | 2.64 |
| CYP80B1 | (S)-N-methylcoclaurine 3′-hydroxylase isozyme 1 | O64899 | 3.49 |
| CYP81D11 | Cytochrome P450 81D11 | Q9LHA1 | 3.67 |
| CYP82A2 | Cytochrome P450 82A2 | O81972 | 3.15 |
| CYP82D47 | Cytochrome P450 CYP82D47 | H2DH24 | 2.23 |
| CYP94B3 | Cytochrome P450 94B3 | Q9SMP5 | −1.79 |
| PPO1 | Polyphenol oxidase | A0A0N9HGV8 | 4.87 |
| At5g38780 | S-adenosylmethionine-dependent methyltransferase At5g38780 | Q9FKR0 | −2.26 |
| Csa_1G002880 | Protein-L-isoaspartate O-methyltransferase | A0A0A0LRC6 | 1.51 |
| HMT1 | Homocysteine S-methyltransferase 1 | A4ZGQ8 | −2.78 |
| GXM3 | Glucuronoxylan 4-O-methyltransferase 3 | Q9LQ32 | 1.98 |
Abbreviation: FC—fold change.
Table 3.
Nineteen DEGs involved in light response at 3300 vs. 2300 m.
| Genes | Proteins | SwissProt ID | log2FC (3300 vs. 2300 m) |
|---|---|---|---|
| Photosynthesis (9) | |||
| CAB2 | Chlorophyll a-b binding protein 2 | P0CJ48 | −2.85 |
| CAB3 | Chlorophyll a-b binding protein 3 | P09756 | −2.09 |
| CAB8 | Chlorophyll a-b binding protein 8 | P27490 | 9.20 |
| CAB21 | Chlorophyll a-b binding protein 21 | P27493 | 10.72 |
| RBCS1 | Ribulose bisphosphate carboxylase small chain 1 | P16032 | −1.73 |
| RBCS4 | Ribulose bisphosphate carboxylase small chain 4 | Q39746 | −2.08 |
| RCAB | Ribulose bisphosphate carboxylase/oxygenase activase | Q42450 | −2.40 |
| TPT | Triose phosphate/phosphate translocator | P49132 | −2.52 |
| PSBR | Photosystem II 10 kDa polypeptide | P06183 | 1.93 |
| Light stress (10) | |||
| HSP18.1 | 18.1 kDa class I heat shock protein | P27879 | 7.52 |
| HSP90-1 | Heat shock protein 90-1 | P27323 | 3.08 |
| HSP22.0 | 22.0 kDa class IV heat shock protein | P30236 | 2.28 |
| HSP70 | Heat shock 70 kDa protein | O93866 | 1.54 |
| UBC4 | Ubiquitin-conjugating enzyme E2 4 | P42748 | 1.67 |
| ERF5 | Ethylene-responsive transcription factor 5 | O80341 | 7.18 |
| ERF9 | Ethylene-responsive transcription factor 9 | Q9FE67 | 4.02 |
| TLP | Thaumatin-like protein | Q53MB8 | 9.28 |
| APX3 | L-ascorbate peroxidase 3 | Q42564 | 2.22 |
| EX2 | Protein EXECUTER 2 | Q657X6 | 1.30 |
Abbreviation: FC—fold change.
2.5. Expression Level of Genes Involved in Flavonoid Biosynthesis
Of the five DEPs (Table 1) and 22 DEGs (Table 2) involved in flavonoid biosynthesis, eight genes (PAL, CHS1, GT6, IFRL, BHLH137, ABCB19, CYP6, and PPO1) and four proteins (ANS, MYB4, CYP6, and PPO1) were identified with CYP6 and PPO1, exhibiting overlap for both gene and protein differential expression at the higher altitude. These subsets of differentially expressed genes/proteins were selected to measure relative expression levels (RELs) by qRT-PCR. A 3.3- (ABCB19) to 12.3-fold (PAL) up-regulation and 0.5-fold (GT6) down-regulation were observed at the higher altitude (Figure 3). These RELs were consistent with the fold change (FC) at the two altitudes, except for the CHS1 (Table 2).
Figure 3.
The REL of genes involved in flavonoid biosynthesis at 3300 vs. 2300 m, as determined by qRT-PCR (mean ± SD, n = 3). The dotted line in the image differentiates up-regulation (>1) and down-regulation (<1) at 3300 vs. 2300 m, respectively. The columns highlighted in green represent up-regulation and red represents down-regulation. The same below.
2.6. Expression Level of Genes Involved in Light Stress
To further examine the biological function of genes involved in light stress [36], seven genes (HSP18.1, HSP70, UBC4, ERF5, ERF9, APX3, and EX2) were monitored for the REL by qRT-PCR (Table 3). A 2.03- (UBC4) to 15.47-fold (ERF5) up-regulation was observed at 3300 vs. 2300 m (Figure 4), which was consistent with the FC at the two altitudes, except for the UBC4 (Table 3).
Figure 4.
The REL of genes involved in light stress at 3300 m vs. 2300 m, as determined by qRT-PCR (mean ± SD, n = 3).
3. Discussion
The alpine medicinal plant S. hexandrum has been demonstrated in previous studies to be adapted to high-altitude conditions [1,2,3]. Although anthocyanin dark spots on the leaf surface were proposed to function as UV protection at high altitudes [34,35], the induction of flavonoid biosynthesis by high altitude conditions had not been investigated. Here, we report that higher light intensity is associated with greater flavonoid, flavonol, and anthocyanin content and that higher ratios of anthocyanins to total flavonoids and flavonols are observed for plants grown at 3300 m. Indeed, nine proteins and 41 genes directly involved in flavonoid biosynthesis and light response are differentially expressed in plants grown at the higher altitude.
Previous studies found that climatic factors (e.g., temperature, precipitation, and light) contributed more to elevated lignan and flavonoid content in S. hexandrum than soil elements (e.g., pH, organic matter, and potassium). Specifically, low temperatures (4–15 °C) could enhance plant biomass and accumulation of secondary metabolites, especially in podophyllotoxin (PPT), in S. hexandrum plants by the up-regulation of genes involved in plant growth, PPT biosynthesis, and stress response [16,17,18]; a moderate water deficit could improve PPT accumulation [19]; a moderately shaded habitat could enhance plant growth [20]; UV-B radiation could inhibit plant growth and PPT biosynthesis while improving flavonoid accumulation [21]. Among other effects, the lower temperatures expected in the higher altitude site could probably negatively affect the dark phase of the photosynthesis, therefore exacerbating the photo damage and ROS production caused by high light intensity.
Previous studies have found that higher altitudes (Shangri-La of Yunnan Province and Nyingchi of Tibet) seem to be essential for the accumulation of flavonoids (e.g., quercetin and kaempferol) [14]. Initially, in situ climatic factors including lower annual air temperature, lower annual light duration, and higher annual precipitation at higher elevation growing sites were considered as drivers for robust S. hexandrum growth [3]. However, an alternative driver for growth is significantly higher light intensity at the higher elevation. Indeed, correlation analysis showed a positive correlation between annual light duration and kaempferol and quercetin content [14]. In this study, greater total flavonoids, flavonols, and anthocyanins were observed and correlated with higher light intensity measured at 3300 m than the lower elevation. Light quality and intensity have previously been shown to be a driver of flavonoid biosynthesis in plants [23,24,25,26,27]. In fact, the UV-B radiation enhancing total flavonoid accumulation in S. hexandrum seedlings has been observed [21].
Other climatic factors such as temperature, precipitation, and air pressure may also contribute to flavonoid biosynthesis and/or accumulation at high altitudes [1,2,3]. Several genes involved in upstream (PAL, C4H, and 4CL) and downstream (MTs and UGTs) flavonoid biosynthesis, as well as TFs (MYB, bHLH, and WRKY) and CYPs under low-temperature and water deficit treatments [16,19], have been published; climatic measurements to directly link temperature and/or precipitation with enhanced flavonoid accumulation have yet to be reported.
In this study, five proteins (ANS, CYP6, CYP71BE30, PPO1, and MYB4) and 22 genes (PAL, C4H, CHS1, IFRL, GT6, BHLH137, DTX41, ABCB19, nine CYPs, PPO1, and four MTs) may be involved in flavonoid biosynthesis. Extensive studies have demonstrated that PAL, C4H, CHS1, ANS, and GT6 directly participate in flavonoid biosynthesis, the ABCB19 participates in flavonoid transport, and the MYB4 and BHLH13 participate in the regulation of flavonoid biosynthesis [29,30,37,38,39,40,41,42]. In addition, nine CYPs and four MTs may also participate in flavonoid biosynthesis [31,33,43]. In addition, the PPO1 and IFRL are involved in the biosynthesis of anthocyanins and isoflavanones, respectively [44,45], and the DTX41 acts as a flavonoid/H+-antiporter controlling the flavonoid transport [46]. It is noteworthy that two TFs, MYB4 and BHLH137, may play critical roles in flavonoid biosynthesis. As is known, the R2R3-MYBs participate in plant growth and development, metabolism (e.g., flavonoid biosynthesis), and stress responses [47]. Previous studies have found that MYB4 serves as a repressor for flavonoid biosynthesis and that MYB4 expression is down-regulated and can enhance sinapate ester levels in Arabidospis leaves with exposure to UV-B light [48]. In buckwheat, in response to UV-B, MYB4R1 regulates flavonoid and anthocyanin biosynthesis by binding to L box motifs in the promoter of CHS, FLS, and UFGT [49]. Moreover, the MYB-bHLH-WD40 complex can regulate genes that encode late step enzymes in the pathway, leading to increased flavonoid biosynthesis [50]. Based on the biological function, the roles of identified proteins and genes were mapped in the flavonoid biosynthetic pathway (Figure 5).
Figure 5.
Flavonoid biosynthetic pathway with identified proteins and genes at high altitude shown in red. Enzymatic abbreviates: PAL—phenylalanine ammonia lyase; C4H—cinnamate 4-hydroxylase; 4CL—4-coumarate CoA ligase; CHS—chalcone synthase; CHI—chalcone isomerase; IFRL—isoflavone reductase-like protein; CYP—cytochrome P450; GT—UDP-glycosyltransferase; MT—methyltransferase.
The difference in CHS1 gene expression levels based on transcriptomic and qRT-PCR analysis may result from the annotation of the reference transcriptome, amplification efficiency, and/or individual features. Previous studies have found that there are about 85% genes of RNA sequencing consistent with qRT-PCR data [51]. For the down-regulation of the GT6 gene, previous studies on strawberry found that GT6 might be associated with the glucosylation of flavonols [52]; thus, its down-regulation may inhibit the glucosylation of flavonols, which is in accordance with the results of greater contents of flavonols (rutin, quercetin, and kaempferol) at 3300 m than 2300 m.
Initially, flavonoid biosynthesis is regulated by biotic and abiotic stresses that include light stress [53,54]. Here, 10 DEGs (HSP18.1, HSP90-1, HSP22.0, HSP70, UBC4, ERF5, ERF9, TLP, APX3, and EX2) were identified to be involved in light stress. Generally, the HSPs function to prevent damage under heat, high light, and free radicals [55,56]; meanwhile, the up-regulation of HSPs can increase flavonoid content [57]. UBC4 can enhance the flavonoid accumulation by regulating the expression of the CHS gene [58,59]. For other genes, ERFs are involved in the regulation of gene expression by stress factors [60]; TLP can improve plant tolerance [61]; APX3 may be involved in the detoxification of H2O2 [62]; EX2 together with EX1 enables plants to perceive stress signal [63]. In this study, the up-regulation of selected genes (HSP18.1, HSP70, UBC4, ERF5, ERF9, APX3, and EX2) may play important roles in enhancing flavonoid biosynthesis and protecting plants from abiotic stress (e.g., high light) at high altitudes. In addition, elevated expression CAB8, CAB21, and PSBR, as well as proteins ycf3, rbc, rbcL, and petL, at 3300 m versus 2300 m (Table 1 and Table 3) suggest that flavonoid accumulation can protect PSII and PSI from stronger UV radiation, which could explain greater biomass that is observed for plants grown at the higher elevation [3].
Based on the above results, a model of high-altitude-induced flavonoid biosynthesis in S. hexdanrum is proposed (Figure 6). When plants are exposed to high altitudes and high light intensity, genes involved in light stress (e.g., HSPs, ERFs, and TLP) are up-regulated. Photosynthesis will initially be inhibited, as observed with the down-regulation of genes (e.g., CABs, RBCs, and TPT), and subsequently, flavonoids (e.g., flavonols and anthocyanins) will be synthesized and accumulated with the up-regulation of genes (e.g., PAL, CHS1, and CYPs). Additionally, the ratio of anthocyanins will be increased with the up-regulation of genes (e.g., ANS and MYB) and, finally, the anthocyanin pigments in leaves will play critical roles in absorbing the high light radiation (e.g., UV-B) and removing the free radicals (e.g., H2O2), which confers the ability of the plants to adapt to the high altitude environmental conditions.
Figure 6.
A proposed model of high-altitude-induced flavonoid biosynthesis in Sinopodophyllum hexdanrum adaptation to the high altitude that includes high light intensity.
4. Materials and Methods
4.1. Plant Materials
Mature fruit of Sinopodophyllum hexandrum were harvested in September 2012 from Gannan Tibetan Autonomous Prefecture (3300 m a.s.l.; 34°42′53″ N, 102°53′12″ E) of Gansu province, China. The seeds separated from the fruit were pre-treated with 0.01% Bavistin for 10 min, immersed in sterile 500 mg/L GA3 for 48 h, and then sown at the 3300 m sites in May 2013. Some seedlings were transplanted to Weiyuan (2300 m; 34°58′8″ N, 104°04′52″ E) of Gansu province in May 2014 [3]. The environmental parameters and soil characteristics were shown in Tables S1 and S2, respectively.
S. hexandrum leaves from 3-year-old plants were collected at 12:00 to 14:00 pm on a sunny day from the 2300 and 3300 m sites in July 2016, with 30 leaves at each altitude pooled (×3) and divided into two parts after mixing; one part was rapidly frozen in liquid nitrogen for proteomic partly and the other part was air dried at room temperature for flavonoid analysis (Figure 7). The light intensities at 2300 and 3300 m were measured at 13:00 pm during sunny days (in early July 2016) using the agricultural meteorology monitor (TNHY-7; Zhejiang Tuopu Instrument Co., Ltd., Hangzhou, China).
Figure 7.
Aerial parts of 3-year-old S. hexandrum plants grown at 2300 and 3300 m. Bar represents 2 cm.
4.2. Determination of Total Flavonoid, Flavonol, and Anthocyanin Contents
4.2.1. Extract Preparation
Air-dried leaf powder (0.3 g) was soaked in ethanol (20.0 mL, 95% v/v) at 22 °C for 72 h and then centrifuged at 6000 rpm at 4 °C for 10 min. Following exhaustive extraction (×3), the supernatant was increased to 20 mL with 95% ethanol and concentrated in a rotary evaporator at 55 °C. The concentrated residue was re-dissolved with 9 mL methanol [17,18].
4.2.2. Determination of Total Flavonoid Content
Extracts (150 µL) were added into ddH2O (2 mL) and 5% NaNO2 (0.3 mL). After agitating for 5 min, 10% AlCl3 (0.3 mL) was added and reacted for 1 min and then 1.0 mol/L NaOH (2 mL) was added to stop the reaction. Absorbance was taken at 510 nm [18]. The total flavonoid content was calculated based on the standard curve (Figure S2).
4.2.3. Flavonol Quantification
Extracts filtered with a 0.22 μm Durapore membrane were analyzed at 365 nm using an HPLC (Eclipse Plus C18, 250 mm × 4.6 mm, 5 μm, column temperature 30 °C). Acetonitrile (A): phosphoric acid (0.1%v/v, B) was the mobile phase with gradient elution: 15–40% A (0–8 min), 40–65% A (8–12 min), 65–85% A (12–14 min), 85–15% A (14–16 min), and 15% A (16–20 min) at a flow rate of 1.0 mL/min [64]. The contents of rutin, isoquercetin, quercetin, and kaempferol were calculated based on peak area comparison with reference standards (Figure S3).
4.2.4. Determination of Anthocyanin Content
Air-dried leaf powder (0.5 g) was soaked in methanol (5.0 mL, 0.1% HCL v/v) at 22 °C for 72 h, then centrifuged at 5000 rpm at 4 °C for 30 s. Following exhaustive extraction (×3), the supernatant was increased to 20 mL with methanol (0.1% HCL v/v). Absorbance was taken at 530 nm and the anthocyanin content was calculated based on a relative level compared with the blank control [33,65].
4.3. Transcriptomic Analysis
Transcriptomic analysis was performed as previously described [36]. For the methodology of RNA-seq, total RNA samples were isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and purified using RNase-free DNase I (TakaRa, Dalian, China); poly-A mRNA was enriched and then used to prepare the paired-end cDNA library (2 × 126 nt) (Illumina, San Diego, CA, USA). Sequences were trimmed with 5′ and 3′ prime ends to remove poor-quality reads; paired-end sequencing was performed using an Illumina Hiseq 2000 platform. De novo assembly was carried out using Trinity software (version 2.0.6) [66]. Unigenes were searched against a NR, Swiss-Prot, TrEMBL, Pfam, and KOG using a BLASTx procedure with an e-value ≤ 10−5 [36,67]; DEGs were identified with |log2(fold-change) ≥ 1 and p ≤ 0.05 by DESeq2 software and the edgeR package [68,69].
4.4. Proteomic Analysis
4.4.1. Protein Extraction
Proteins were extracted according to a previously described method [70]. Firstly, frozen leaves were pulverized to a fine powder in liquid nitrogen. Aliquots (200 μL) were then mixed with TCA-2ME-acetone solution (1.8 mL of 10% TCA (w/v), 0.07% 2ME (v/v) in cold acetone) and stored at −20 °C for 1 h. The homogenate was then centrifuged at 10,000× g and 4 °C for 10 min and the precipitate was re-suspended in cold acetone (1.8 mL containing 0.07% 2ME (v/v)). The mixture was then stored at −20 °C for 1 h and then centrifuged at 10,000× g and 4 °C for 15 min. This step was repeated at least three times until the supernatant became colorless. The precipitate was vacuum dried, dissolved in a protein solubilization buffer (1.8 mL of 5 M urea, 2 M thiourea, 2% CHAPS (w/v) with 2% N-decyl-N,N-dimethyl-3-ammonio-1-propane sulfate (w/v), 20 mM DTT, 5 mM phosphine, 0.5% pharmalyte pH 4–6.5 (v/v), and 0.25% pharmalyte pH 3–10 (v/v) in ddH2O) and then vortexed for 1 min. Finally, the solubilized mixture was centrifuged at 10,000× g and 25 °C for 15 min and then the supernatant was centrifuged to remove any cellular debris. Protein amount was quantified using a 2-D Quant kit (GE Healthcare, Wauwatosa, WI, USA) and the protein extracts were stored at −80 °C.
4.4.2. 2-DE Separation
Firstly, protein samples were centrifuged at 10,000× g and 25 °C for 15 min and then diluted in rehydration buffer (8 M urea, 2% CHAPS (w/v), 0.3% DTT (w/v), and 1% IPG buffer (v/v)). Secondly, IEF strips (24 cm, linear pH 4–7) were re-hydrated in protein solution (450 μL at 20 °C) for 12 h in an electrophoresis chamber (Ettan IPGphor 3 system; GE Healthcare, Wauwatosa, WI, USA). Thirdly, the IEF was performed using the following parameters: 200 V for 2 h, 500 V for 2 h, 1000 V for 2 h, and 8000 V for 4 h. Fourthly, after the IEF, strips were incubated twice in equilibration buffer (8 M urea, 1.5 M Tris-HCl buffer (pH 8.8), 30% glycerol (v/v), and 2% SDS (w/v)) with 1% DTT (w/v) for 15 min, followed by 4% (w/v) of iodoacetamide for another 15 min. Fifthly, an Ettan DALTSix system (GE Healthcare, Wauwatosa, WI, USA) was used for 2-DE with the strips placed on a 12.5% SDS-PAGE gel (w/v) and run at 1 W for 1 h and then at 12 W for 5 h per gel. Subsequently, gels were fixed in 40% alcohol (v/v) and 10% acetic acid (v/v) for 30 min, swollen in 10% acetic acid (v/v) for 20 min, and stained with CBB G-250. Finally, the stained gels were rinsed with 10% acetic acid (v/v) until protein spots were distinct from the background and stored in deionized water (Figure S1).
4.4.3. Gel Scanning and Image Analysis
The stained gels were scanned using an ImageScanner III (GE Healthcare, Wauwatosa, WI, USA) and images were analyzed using ImageMaster 2-D Platinum v7.0 software (GE Healthcare, Wauwatosa, WI, USA), with three independent biological replicates for the 2300 or 3300 m samples. Spots were automatically detected and matched; mismatched and unmatched spots were artificially modified through manual editing. Spot densities were expressed as mean normalized volumes, and fold changes were calculated between 2300 and 3300 m. Only spots with intensity ratios over 1.5-fold were selected as differentially expressed proteins (DEPs) and the over-expressed proteins at 3300 m vs. 2300 m were subsequently identified.
4.4.4. Protein Identification
The selected DEP spots were excised from CBB-stained gels and digested with trypsin. Peptides were identified using an AB SCIEX 5800 MALDI-TOF/TOFTM system (AB SCIEX, CA, USA) according to a previously described protocol [71]. The obtained peptide masses were used to search the NCBI database using a MASCOT engine (http://www.matrixscience.com (accessed on 1 June 2020)). Biological functions of identified proteins were classified using the Swiss-Prot database (http://www.uniprot.org (accessed on 6 November 2021)). After removing the repeat proteins by selecting the longer sequencing of amino acids for the same protein, the DEPs involved in light response and flavonoid biosynthesis were further screened and validated in this study.
4.5. qRT-PCR Validation of Genes Involved in Light Stress and Flavonoids Biosynthesis
Based on the biological functions of the DEGs and DEPs, 17 representative genes were selected to validate their expression level by qRT-PCR. The validation of genes was performed according to our previous protocol [21]. The cycle threshold (Ct) values and standard curves of the reference gene actin (ACT) at different concentrations (0.25, 0.5, 1.0, 1.5, 2.0, and 3.0 μL) were performed to correct expression level of selected genes (Figures S4 and S5). The primer sequence (Table S3) was designed using the primer-blast tool in NCBI and synthesized by Sangon Biotech (Shanghai, China). Total RNA samples at 2300 and 3300 m were extracted from the leaves using a Plant RNA Kit (R6827; Omega Bio-Tek, Norcross, GA, USA). cDNA was synthesized using a FastKing RT Kit (KR116; Tiangen, Beijing, China) with 42 °C for 15 min and 95 °C for 3 min for one cycle. The qRT-PCR was carried out using a SuperReal PreMix (FP205; Tiangen, Beijing, China) at 95 °C (15 min) for one cycle, followed by 95 °C (10 s), 60 °C (20 s), and 72 °C (30 s) for 40 cycles. The melting curve was analyzed at 72 °C for 34 s. TheREL was calculated using a 2−∆∆Ct method [72].
| ∆Ct Test gene = Ct Test gene − Ct Reference gene |
| ∆Ct Control gene = Ct Control gene − Ct Reference gene |
| −∆∆Ct (3300 vs. 2300 m) = −(∆Ct 3300 m − ∆Ct 2300 m) |
| REL = 2−∆∆Ct |
4.6. Statistical Analysis
All measurements were performed using three biological replicates. Statistical analysis was conducted via a t-test for independent samples. SPSS 22.0 was the software package used, with p < 0.05 as the basis for statistical differences.
5. Conclusions
From the above observations, greater contents of total flavonoids, flavonols, and anthocyanins in S. hexandrum were observed at higher altitudes, which may be regulated by the up-regulated genes or over-expressed proteins involved in flavonoid biosynthesis and light stress. These findings indicate that light stress may play a determining role in enhancing flavonoid accumulation at high altitudes. In order to exclude the differences in temperature, water, and other factors, the role of light in affecting plant growth and flavonoid biosynthesis will be further studied under controlled laboratory conditions such as shaded habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12030575/s1, Figure S1: 2-DE gels of proteins extracted from leaves of S. hexandrum grown at 2300 and 3300 m; Figure S2: Standard curve of catechin solutions with a range from 25 to 125 μg; Figure S3: Representative HPLC chromatogram of reference standards and samples; Figure S4: The cycle threshold (Ct) values of ACT gene at different volumes (0.25, 0.5, 1.0, 2.0, and 3.0 μL) via PCR amplification with three replications; Figure S5: The standard curve of ACT gene; Table S1: Environmental parameters at the two altitudes over three years; Table S2: Soil characteristics at the two altitudes; Table S3: Primer sequences of selected genes used for qRT-PCR validation.
Author Contributions
Q.Z., formal analysis, investigation and validation; M.D., investigation and writing—original draft; M.L., conceptualization, project administration, supervision and writing—original draft; L.J., resources; P.W.P., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets of transcriptomics are publicly available at NCBI–SRA (http://www.ncbi.nlm.nih.gov/sra (accessed on 1 June 2019)) accession: SRR7196729. The proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org (accessed on 1 March 2017)) with the dataset identifier PXD005640.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the State Key Laboratory of Aridland Crop Science Gansu Agricultural University (GSCS-2021-Z03), Assurance Project of Ecological Planting and Quality of Daodi Herbs (202103003), and National Natural Science Foundation of China (81560617).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Alam M.A., Naik P.K. Impact of soil nutrients and environmental factors on podophyllotoxin content among 28 Podophyllum hexandrum populations of northwestern Himalayan region using linear and nonlinear. Commun. Soil Sci. Plant Anal. . 2009;40:2485–2504. doi: 10.1080/00103620903111368. [DOI] [Google Scholar]
- 2.Wu A.L., Li M., Zhang S.W., Zhao J.F., Liu X., Wang C.H., Wang X.Y., Zhong G.Y. Effect of topographical factors on podophyllotoxin content in Sinopodophyllum hexandrum and study on ecological suitability. China J. Chin. Mater. Med. 2015;40:2299–2303. [PubMed] [Google Scholar]
- 3.Li M.F., Ge L., Kang T.L., Sun P., Xing H., Yang D.L., Zhang J.L., Paré P.W. High-elevation cultivation increases anti-cancer podophyllotoxin accumulation in Podophyllum hexandrum. Ind. Crops Prod. 2018;121:338–344. doi: 10.1016/j.indcrop.2018.05.036. [DOI] [Google Scholar]
- 4.Wang Q.H., Guo S., Yang X.Y., Zhang Y.F., Shang M.Y., Shang Y.H., Xiao J.J., Cai S.Q. Flavonoids isolated from Sinopodophylli fructus and their bioactivities against human breast cancer cells. Chin. J. Nat. Med. 2017;15:225–233. doi: 10.1016/S1875-5364(17)30039-0. [DOI] [PubMed] [Google Scholar]
- 5.Lv M., Xu H. Recent advances in semisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: An update (2008–2010) Mini Rev. Med. Chem. 2011;11:901–909. doi: 10.2174/138955711796575461. [DOI] [PubMed] [Google Scholar]
- 6.Shang M.Y., Xu L.S., Li P., Xu G.J., Wang Y.X., Cai S.Q. Study on pharmacodynamics of Chinese herbal drug Guijiu and its lignan. Chin. Tradit. Herb. Drugs. 2002;33:722–724. [Google Scholar]
- 7.Choudhary D.K., Kaul B.L., Khan S. Cultivation and conservation of Podophyllum hexandrum. An overview. J. Med. Aromat. Plant Sci. 1998;20:1071–1073. [Google Scholar]
- 8.Gupta M.L., Dutta A. Stress-mediated adaptive response leading to genetic diversity and instability in metabolite contents of high medicinal value: An overview on Podophyllum hexandrum. Omics. 2011;15:873–882. doi: 10.1089/omi.2011.0096. [DOI] [PubMed] [Google Scholar]
- 9.Kharkwal A.C., Kushwaha R., Prakash O., Ogra R.K., Bhattacharya A., Nagar P.K., Ahuja P.S. An efficient method of propagation of Podophyllum hexandrum: An endangered medicinal plant of the Western Himalayas under ex situ conditions. J. Nat. Med. 2008;62:211–216. doi: 10.1007/s11418-007-0217-9. [DOI] [PubMed] [Google Scholar]
- 10.Cao X.L., Li M.L., Li J., Song Y.X., Zhang X.N., Yang D.L., Li M.F., Wei J.H. Co-expression of hydrolase genes improves seed germination of Sinopodophyllum hexandrum. Ind. Crops Prod. 2021;64:113414. doi: 10.1016/j.indcrop.2021.113414. [DOI] [Google Scholar]
- 11.Sreenivasulu Y., Chanda S.K., Ahuja P.S. Endosperm delays seed germination in Podophyllum hexandrum Royle—An important medicinal herb. Seed Sci. Technol. 2009;37:10–16. doi: 10.15258/sst.2009.37.1.02. [DOI] [Google Scholar]
- 12.Nadeem M., Palni L.M.S., Kumar A., Nandi S.K. Podophyllotoxin content, above and belowground biomass in relation to altitude in Podophyllum hexandrum populations from Kumaun region of the Indian central Himalaya. Planta Med. 2007;73:388–391. doi: 10.1055/s-2007-967154. [DOI] [PubMed] [Google Scholar]
- 13.Li M.F., Li W., Yang D.L., Zhou L.L., Li T.T., Su X.M. Relationship between podophyllotoxin accumulation and soil nutrients and the influence of Fe2+ and Mn2+ on podophyllotoxin biosynthesis in Podophyllum hexandrum tissue culture. Plant Physiol. Biochem. 2013;71:96–102. doi: 10.1016/j.plaphy.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Liu W., Liu J., Yin D., Zhao X. Influence of ecological factors on the production of active substances in the anti-cancer plant Sinopodophyllum hexandrum (Royle) T.S. Ying. PLoS ONE. 2015;10:e0122981. doi: 10.1371/journal.pone.0122981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey H., Kumar A., Palni L.M.S., Nandi S.K. Podophyllotoxin content in rhizome and root samples of Podophyllum hexandrum Royle populations from Indian Himalayan region. J. Med. Plants Res. 2015;9:320–325. [Google Scholar]
- 16.Kumari A., Singh H.R., Jha A., Swarnkar M.K., Shankar R., Kumar S. Transcriptome sequencing of rhizome tissue of Sinopodophyllum hexandrum at two temperatures. BMC Genom. 2014;15:871. doi: 10.1186/1471-2164-15-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang D.L., Sun P., Li M.F. Chilling temperature stimulates growth, gene overexpression and podophyllotoxin biosynthesis in Podophyllum hexandrum Royle. Plant Physiol. Biochem. 2016;107:197–203. doi: 10.1016/j.plaphy.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Li M.F., Lv M., Yang D.L., Wei J.H., Xing H., Paré P.W. Temperature-regulated anatomical and gene-expression changes in Sinopodophyllum hexandrum seedlings. Ind. Crops Prod. 2020;152:112479. doi: 10.1016/j.indcrop.2020.112479. [DOI] [Google Scholar]
- 19.Kumari A., Dogra V., Joshi R., Kumar S. Stress-responsive cis-regulatory elements underline podophyllotoxin biosynthesis and better performance of Sinopodophyllum hexandrum under water deficit conditions. Front. Plant Sci. 2022;12:751846. doi: 10.3389/fpls.2021.751846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Q.Q., Li H.E., Gao C., Yang R. Leaf traits and photosynthetic characteristics of endangered Sinopodophyllum hexandrum (Royle) Ying under different light regimes in Southeastern Tibet Plateau. Photosynthetica. 2019;57:548–555. doi: 10.32615/ps.2019.080. [DOI] [Google Scholar]
- 21.Lv M., Su H.Y., Li M.L., Yang D.L., Yao R.Y., Li M.F., Wei J.H. Effect of UV-B radiation on growth, flavonoid and podophyllotoxin accumulation, and related gene expression in Sinopodophyllum hexandrum. Plant Biol. 2020;23:202–209. doi: 10.1111/plb.13226. [DOI] [PubMed] [Google Scholar]
- 22.Shen N., Wang T., Gan Q., Liu S., Wang L., Jin B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. doi: 10.1016/j.foodchem.2022.132531. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q.F., Liu M.Y., Ruan J.Y. Metabolomics analysis reveals the metabolic and functional roles of flavonoids in light-sensitive tea leaves. BMC Plant Biol. 2017;17:64. doi: 10.1186/s12870-017-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheynier V., Comte G., Davies K.M., Lattanzio V., Martens S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y., Wang G., Cao F., Zhu C.C., Wang G.Y., El-Kassaby Y.A. Light intensity affects the growth and flavonol biosynthesis of Ginkgo (Ginkgo biloba L.) New For. 2014;45:765–776. doi: 10.1007/s11056-014-9435-7. [DOI] [Google Scholar]
- 26.Albert N.W., Lewis D.H., Zhang H., Irving L.J., Jameson P.E., Davies K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009;60:2191–2202. doi: 10.1093/jxb/erp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama K., Ikeda H., Poudel P.R., Goto-Yamamoto N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry. 2012;78:54–64. doi: 10.1016/j.phytochem.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Merzlyak M.N., Chivkunova O.B. Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J. Photochem. Photobiol. B. 2000;55:155–163. doi: 10.1016/S1011-1344(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 29.Winkel B.S.J. Metabolic channeling in plants. Annu. Rev. Plant Biol. 2004;55:85–107. doi: 10.1146/annurev.arplant.55.031903.141714. [DOI] [PubMed] [Google Scholar]
- 30.Kleindt C.K., Stracke R., Mehrtens F., Weisshaar B. Expression analysis of flavonoid biosynthesis genes during Arabidopsis thaliana silique and seed development with a primary focus on the proanthocyanidin biosynthetic pathway. BMC Res. Notes. 2010;3:255. doi: 10.1186/1756-0500-3-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayabe S.I., Akashi T. Cytochrome P450s in flavonoid metabolism. Phytochem. Rev. 2006;5:271–282. doi: 10.1007/s11101-006-9007-3. [DOI] [Google Scholar]
- 32.Frangne N., Eggmann T., Koblischke C., Weissenbock G., Martinoia E., Klein M. Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H+-Antiport and ATP-binding cassette-type mechanisms1. Plant Physiol. 2002;128:726–733. doi: 10.1104/pp.010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu T.T., Zhang M.H., Su H.Y., Li M.L., Wang Y.Y., Jin L., Li M.F. Integrated metabolomic and transcriptomic analysis reveals differential mechanism of flavonoid biosynthesis in two cultivars of Angelica sinensis. Molecules. 2022;27:306. doi: 10.3390/molecules27010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma S.B., Hu Z.H. Preliminary studies on the distribution pattern and ecological adaptation of Sinopodophyllum hexandrum (Royle) ying (Berberidaceae) J. Wuhan Bot. Res. 1996;14:47–54. [Google Scholar]
- 35.Rajkumar S., Ahuja P.S. Developmental adaptation of leaves in Podophyllum hexandrum for effective pollination and dispersal. Curr. Sci. India. 2010;99:1518–1519. [Google Scholar]
- 36.Li M.F., Sun P., Kang T.L., Xing H., Yang D.L., Zhang J.L., Paré P.W. Mapping podophyllotoxin biosynthesis and growth-related transcripts with high elevation in Sinopodophyllum hexandrum. Ind. Crops Prod. 2018;124:510–518. doi: 10.1016/j.indcrop.2018.08.007. [DOI] [Google Scholar]
- 37.Tohge T., De Souza L.P., Fernie A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017;68:4013–4028. doi: 10.1093/jxb/erx177. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.I., Hidalgo-Shrestha C., Bonawitz N.D., Franke R.B., Chapple C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021;72:3061–3073. doi: 10.1093/jxb/erab055. [DOI] [PubMed] [Google Scholar]
- 39.Santín O., Moncalián G. Loading of malonyl-CoA onto tandem acyl carrier protein domains of polyunsaturated fatty acid synthases. J. Biol. Chem. 2018;293:12491–12501. doi: 10.1074/jbc.RA118.002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yonekura-Sakakibara K., Higashi Y., Nakabayashi R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019;10:943. doi: 10.3389/fpls.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauf A., Imran M., Abu-Izneid T., Patel S., Pan X.P., Naz S., Silva A.S., Saeed F., Suleria H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019;116:108999. doi: 10.1016/j.biopha.2019.108999. [DOI] [PubMed] [Google Scholar]
- 42.Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad N., Liu J.Y., Xu T., Noman M., Jameel A., Yao N., Dong Y.Y., Wang N., Li X.W., Wang F.W., et al. Overexpression of a novel cytochrome P450 promotes flavonoid biosynthesis and osmotic stress tolerance in transgenic Arabidopsis. Genes. 2019;10:756. doi: 10.3390/genes10100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virador V.M., Reyes G.J.P., Blanco-Labra A., Mendiola-Olaya E., Smith G.M., Moreno A., Whitaker J.R. Cloning, sequencing, purification, and crystal structure of Grenache (Vitis vinifera) polyphenol oxidase. J. Agric. Food Chem. 2010;58:1189–1201. doi: 10.1021/jf902939q. [DOI] [PubMed] [Google Scholar]
- 45.Paiva N.L., Edwards R., Sun Y., Hrazdina G., Dixon R.A. Stress responses in alfalfa (Medicago sativa L.) 11. Molecular cloning and expression of alfalfa isoflavone reductase, a key enzyme of isoflavonoid phytoalexin biosynthesis. Plant Mol. Biol. 1991;17:653–667. doi: 10.1007/BF00037051. [DOI] [PubMed] [Google Scholar]
- 46.Debeaujon I., Peeters A.J., Leon-Kloosterziel K.M., Koornneef M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–871. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB tran-scription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Jin H., Cominelli E., Bailey P., Parr A., Mehrtens F., Jones J., Tonelli C., Weisshaar B., Martin C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000;19:6150–6161. doi: 10.1093/emboj/19.22.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu M., Sun W., Ma Z., Guo C., Chen J., Wu Q., Wang X., Chen H. Integrated network analyses identify MYB4R1 neofunctionalization in the UV-B adaptation of tartary buckwheat. Plant Commun. 2022;3:100414. doi: 10.1016/j.xplc.2022.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hichri I., Barrieu F., Bogs J., Kappel C., Delrot S., Lauvergeat V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011;62:2465–2483. doi: 10.1093/jxb/erq442. [DOI] [PubMed] [Google Scholar]
- 51.Everaert C., Luypaert M., Maag J.L.V., Cheng Q.X., Dinger M.E., Hellemans J., Mestdagh P. Benchmarking of RNA-sequencing analysis workflows using whole-transcriptome RT-qPCR expression data. Sci. Rep. 2017;7:1559. doi: 10.1038/s41598-017-01617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griesser M., Vitzthum F., Fink B., Bellido M.L., Raasch C., Munoz-Blanco J., Schwab W. Multi-substrate flavonol O-glucosyltransferases from strawberry (Fragaria × ananassa) achene and receptacle. J. Exp. Bot. 2008;59:2611–2625. doi: 10.1093/jxb/ern117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrussa E., Braidot E., Zancani M., Peresson C., Bertolini A., Patui S., Vianello A. Plant flavonoids--biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013;14:14950–14973. doi: 10.3390/ijms140714950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., Wu Z., Suo Y., Wang J., Zheng L., Wang Y. Gene expression and flavonol biosynthesis are induced by ultraviolet-B and salt stresses in Reaumuria trigyna. Biol. Plant. 2017;61:246–254. doi: 10.1007/s10535-017-0725-8. [DOI] [Google Scholar]
- 55.Kim T., Samraj S., Jiménez J., Gómez C., Liu T., Begcy K. Genome-wide identification of heat shock factors and heat shock proteins in response to UV and high intensity light stress in lettuce. BMC Plant Biol. 2021;21:185. doi: 10.1186/s12870-021-02959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo M., Liu J.H., Ma X., Luo D.X., Gong Z.H., Lu M.H. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant. Sci. 2016;7:114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kittang A.I., Winge P., van Loon J.J.W.A., Bones A.M., Iversen T.H. Adaptation response of Arabidopsis thaliana to random positioning. Adv. Space Res. 2013;52:1320–1331. doi: 10.1016/j.asr.2013.07.001. [DOI] [Google Scholar]
- 58.Kraft E., Stone S.L., Ma L., Su N., Gao Y., Lau O.S., Deng X.W., Callis J. Genome analysis and functional characterization of the E2 and RING–type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol. 2005;139:1597–1611. doi: 10.1104/pp.105.067983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeon E.H., Pak J.H., Kim M.J., Kim H.J., Shin S.H., Lee J.H., Kim D.H., Oh J.S., Oh B.J., Jung H.W., et al. Ectopic expression of ubiquitin-conjugating enzyme gene from wild rice, OgUBC1, confers resistance against UV-B radiation and Botrytis infection in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2012;427:309–314. doi: 10.1016/j.bbrc.2012.09.048. [DOI] [PubMed] [Google Scholar]
- 60.Fujimoto S.Y., Ohta M., Usui A., Shinshi H., Ohme-Takagi M. Arabidopsis ethylene responsive element binding factors act as transcriptional activators or repressors of GCC box mediated gene expression. Plant Cell. 2000;12:393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muoki R.C., Paul A., Kaachra A., Kumar S. Membrane localized thaumatin-like protein from tea (CsTLP) enhanced seed yield and the plant survival under drought stress in Arabidopsis thaliana. Plant Physiol. Biochem. 2021;163:36–44. doi: 10.1016/j.plaphy.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Sharma P., Dubey R.S. Ascorbate peroxidase from rice seedlings: Properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Plant Sci. 2004;167:541–550. doi: 10.1016/j.plantsci.2004.04.028. [DOI] [Google Scholar]
- 63.Lee K.P., Kim C., Landgraf F., Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2007;104:10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu S.Y., Zhang X.Q., Wang H.Y., Yuan Z.X., Lei H.X. Simultaneous determination of five major flavonoids in Tetrastigma hemsleyanum by HPLC. Chin. J. Mod. Appl. Pharm. 2018;35:1878–1880. [Google Scholar]
- 65.Meng X.C., Zhang Y.J., Wang X.Q. Content changes of anthocyanin, reducing sugar and soluble protein during the flower development of Petunia hybrid. J. South China Norm. Univ. 2001;2:96–99. [Google Scholar]
- 66.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conesa A., Götz S., García-Gómez J.M., Terol J., Talón M., Robles M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 68.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNAseq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson M.D., McCarthy D.J., Smyth G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Méchin V., Damerval C., Zivy M. Total protein extraction with TCA-acetone. Methods Mol. Biol. 2007;355:1–8. doi: 10.1385/1-59745-227-0:1. [DOI] [PubMed] [Google Scholar]
- 71.Kosová K., Vítámvás P., Planchon S., Renaut J., Vankova R., Prášil I.T. Proteome analysis of cold response in spring and winter wheat (Triticum aestivum) crowns reveals similarities in stress adaptation and differences in regulatory processes between the growth habits. J. Proteome Res. 2013;18:4830–4845. doi: 10.1021/pr400600g. [DOI] [PubMed] [Google Scholar]
- 72.Willems E., Leyns L., Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008;379:127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of transcriptomics are publicly available at NCBI–SRA (http://www.ncbi.nlm.nih.gov/sra (accessed on 1 June 2019)) accession: SRR7196729. The proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org (accessed on 1 March 2017)) with the dataset identifier PXD005640.