Abstract
Plants are affected by various environmental stresses such as high or low temperatures, drought, and high salt levels, which can disrupt their normal cellular functioning and impact their growth and productivity. These stressors offer a major constraint to the morphological, physiological, and biochemical parameters; thereby attributing serious complications in the growth of crops such as rice, wheat, and corn. Considering the strategic and intricate association of soil microbiota, known as plant growth-promoting rhizobacteria (PGPR), with the plant roots, PGPR helps plants to adapt and survive under changing environmental conditions and become more resilient to stress. They aid in nutrient acquisition and regulation of water content in the soil and also play a role in regulating osmotic balance and ion homeostasis. Boosting key physiological processes, they contribute significantly to the alleviation of stress and promoting the growth and development of plants. This review examines the use of PGPR in increasing plant tolerance to different stresses, focusing on their impact on water uptake, nutrient acquisition, ion homeostasis, and osmotic balance, as well as their effects on crop yield and food security.
Keywords: abiotic stress, PGPR, plant productivity, rhizosphere, agricultural sustainability
1. Introduction
The inception of agricultural practices at the interface of human interaction with the environment has merged crop productivity with consistency in the environment [1]. The increase in the human population (projected to be 30% larger by 2030) and decrease in the cultivable land (due to urbanization, industrialization, and increase in the pollution) offer a big challenge in the current day scenario. To this, it is presumed that either area under cultivation should be increased or the productivity of the already cultivable area be increased in order to achieve sustainability in agriculture that can offer a consistent and adequate food supply for the growing population [2]. Enhancement in agricultural productivity to achieve food security for the growing population is greatly influenced by changes in climatic conditions, farming systems, and management techniques [3]. On one side, where food security for the growing population has put forth the need to have a consistent and adequate food supply to meet the demand (almost double), inclement climatic conditions put humans at stake by exacerbating the severity of different stresses that have pronounced effects on crop productivity. In such a scenario, microbial populations inhabiting the rhizosphere (commonly referred to as plant growth-promoting rhizobacteria, PGPR) may offer a solution by assisting plants through the modulation of the developmental activities that affect overall growth and productivity [4]. The microbial populations contribute to a wide range of biological events at the soil root interface including nutrient acquisition and water uptake, maintenance of osmotic balance, and ion homeostasis; thereby helping in the sustenance of crop health and productivity. Additionally, the root-associated microbial population safeguards plants from soil-borne diseases and reinforces stress tolerance to plants in coping with changing climatic conditions such as drought, salinity, etc [5,6].
Considering the knowledge gaps, this study was performed on the available literature of different databases such as PubMed, Web of Sciences, Google Scholar, etc, in order to provide an understanding of the role of the microbiome in assisting the growth of plants under changing climatic conditions. By elucidating the mechanisms of the microbial interactions with plants such as nutrient acquisition, maintenance of ion homeostasis and osmotic balance, regulation of the phytohormone levels, etc, it offers an advantage in mitigating the impact of different environmental factors such as extreme temperature, drought, etc, on productivity and as such improve the resilience of plants to climatic variability. This study covering original research, reviews, opinions, etc, provides adequate information on PGPR functionality and deliberate new ideas and emerging concepts that, if brought to practice, could enhance plant productivity and also make avenues to attain sustainability in agriculture.
2. Soil Fertility and Plant Growth
Soil is generally considered a non-ideal system owing to its complex chemical and mineral status. An increase in the agricultural productivity for demarked cultivable areas largely depends on soil fertility. Soil fertility refers to the ability of soil to provide essential nutrients in suitable proportions and adequate amounts at the right interval of time to meet the nutritional requirement of the plant in order to sustain its growth [7]. Being a vital and important factor in the determination of productivity (both qualitatively and quantitatively), it is necessary to have regular monitoring of activities such as structural arrangements, holding capacity, transformation ability, and nutrient cycling in a continuous and consistent manner. A decrease in soil fertility offers a major hindrance in achieving sustainability in the production towards fulfilling the need of the growing population (Figure 1). Though the application of inorganic fertilizers complemented the loss in agricultural productivity, its continuous use over the years was found to have adverse effects on soil fertility [8]. In addition to their role in regaining soil fertility, the application of organic fertilizers together with inorganic ones was found effective in increasing agricultural productivity [9].
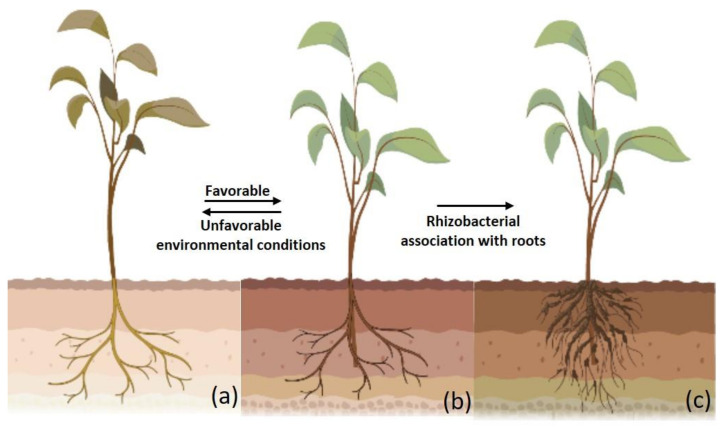
Figure 1.
Plant growth under different environmental conditions: (a) Progression of climatic conditions from favorable to unfavorable that affects overall performance of the plant, (b) Plant growth under normal conditions, and (c) Plant-microbiome interactions that affect overall performance of the plant.
Despite exerting beneficial effects, enhancement in organic fertilizer usage is directly connected with a change in the biological characteristics of the soil; thereby rating soil fertility and long-term enhancement [10]. The lately emerged eco-friendly biofertilizers, which make use of soil microorganisms to decompose the organic matter to readily available nutrients for plant usage, are considered a better alternative to chemical fertilizers for use in sustainable agricultural practices [11]. Improvising soil biological characteristics causes enhancement in the uptake of mineral nutrients (both micro- and macro) to ameliorate the deficiency in the root region and their uptake within the plant system [12]. The microorganisms exhibit both rhizospheric (Azospirillum, Azotobacter, Burkholderia, Enterobacter, Klebsiella, Variovorax, Comamonadaceae, Pseudomonas, Gemmatimonadetes, Streptomyces filamentosus, and Bacillales) and endophytic (Azoarcus spp., Herbaspirillum seropedicae, and Gluconacetobacter diazotrophicus) relationship in the intercellular and apoplastic space of the host plants respectively [13,14]. Besides solubilizing mineral nutrients, microorganisms regulate the production of plant growth hormones (such as indoleacetic acid, IAA; cytokinins, CKs; gibberellins, GA) and inhibitors of ethylene [15], support plant growth; thereby resulting in improved overall productivity.
3. Plant Growth Promoting Rhizobacteria
Plants grown in the field represent a complex community with subtle partner association of a well-structured community of microorganisms referred to as phytomicrobiome [16]. The microbiome (predominantly bacteria and fungi) that colonizes the roots (rhizomicrobiome) represents the most popular association at the rhizospheric plane [17]. Of the different microbial populations that inhabit the root surface, a heterogeneous group of bacteria (preferably free living) exerting beneficial effects on plants either directly and/or indirectly are referred to as plant-growth-promoting rhizobacteria (PGPR) [15]. PGPR include both facultative anaerobes that invade intercellular space and thrive as endophytes and those living outside the host plant but are differentially associated with the rhizospheric plane. The PGPR (including rhizospheric and endophytes) induce their beneficial effects by assisting plants in challenging times, either by increasing their access to nutrients or helping them to overcome stressful conditions and pathogens [18].
3.1. Root Exudates and PGPR
Plants provide a suitable niche for the growth and multiplication of a wide variety of microorganisms. Existing in complex communities, the plant-associated microbiota comprising of bacteria, fungi, etc., manifests a series of beneficial traits such as suppression of plant pathogens, transformation and translocation of essential nutrients in the soil for uptake by plants, that promote plant growth and increase productivity [19]. Necessary for establishing a stable plant-microbe interaction, plant root exudates of variable composition regulate the recruitment and composition of the rhizomicrobiome [20]. The root exudates contain a large proportion of amino acids, sugars, and organic acids, and a small number of secondary metabolites such as flavonoids, phenolic compounds, and terpenes [21]. The composition and amount of plant root exudates vary widely depending on plant species and growth stages, which decisively affect the composition and abundance of root-associated microbiota populations [16]. In addition to the secretion of exopolysaccharides and quorum sensing, different substances in the exudate act as signaling molecules that regulate bacterial chemotaxis [22]. Plant root exudation of signaling molecules such as jasmonic acid and salicylic acid regulates the initial events of microbial colonization of plant roots [23]. Acting as an important part of signaling events at the rhizosphere, root exudates under changing conditions and performs inter-organismal communication (Figure 2). Together, molecules secreted from the root support microbial growth and their activity in the rhizosphere, and in return, they help plants in the acquisition of soil resources, impart protection against different pathogens, and promote growth via increased root hair proliferation, and branching, leaf surface area, besides causing an increase in the indigenous hormone levels and enhancement in the accumulation of carbohydrates that improve vigor and biomass right from germination to the full grown and go up to senescence [24].
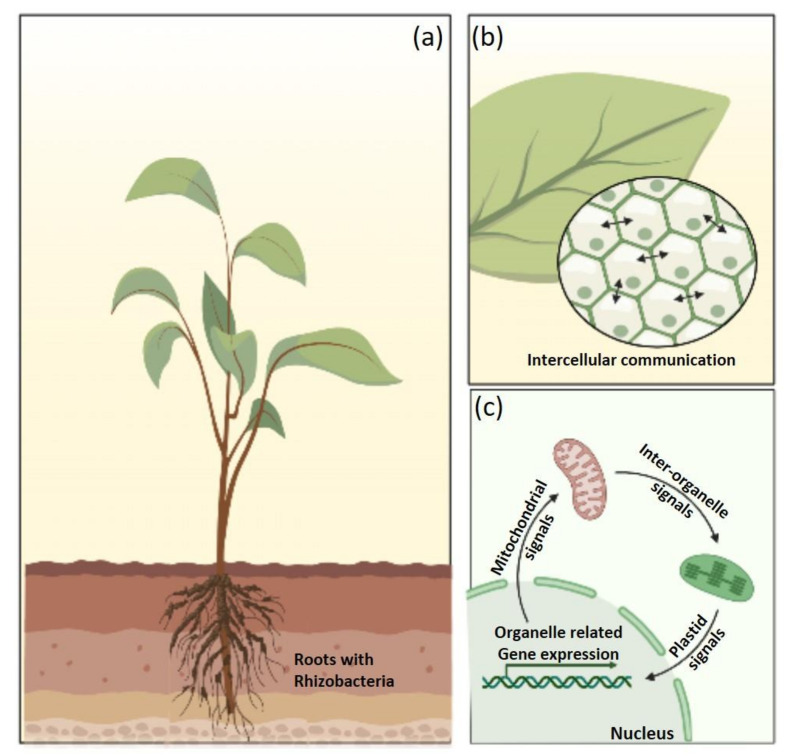
Figure 2.
Plant-microbiome interactions: (a) PGPR association with roots of the plant, (b) Microbial interactions enhancing cellular signaling, and (c) Signaling pathway along different modules affecting gene expression and, as such, overall performance of the plant.
3.2. PGPR in Nutrient Acquisition
Limited nutrient availability and inadequate supply to plants have severe effects on plant growth and productivity. The availability of nutrients in the soil for plants to uptake depends on several parameters such as the composition and moisture content of the soil, its texture, pH, and the existing microflora in the rhizosphere [2]. pH plays an important role in the determination of the sustainability of microbes in the soil, and it is found that the pH ranges from 6.0 to 6.5 favors the beneficiary microbe’s sustainability and ensures healthy plant growth [25]. Though most of the nutrients are available in the pH range of 5–7, their binding to different ions (both cations and anions) in response to changes in the pH makes them less available for uptake by plants. In such a scenario, microbes were found capable of assisting plants in the acquisition of nutrients by employing different mechanisms such as nitrogen fixation, augmentation of surface area accessed by roots, phosphate solubilization, HCN, and siderophore production [26].
Nitrogen—an essential component of the living system is required for nucleic acid and protein synthesis. Microbial inhabitants of soil fix atmospheric nitrogen and thereby make it available for plants in the form of ammonia in the nitrogen fixation process. PGPR is capable of fixing nitrogen in both symbiotic (Azotobacter spp., Bacillus spp., etc) as well as non-symbiotic (free-living diazotrophs, Azospirillum) manner [27]. In leguminous plants, nitrogen fixation is assisted by rhizobia which undergoes a significant transformation from free-living to nitrogen-fixing bacteroid residing in the root nodules [28]. In the early 21st century, research was oriented toward the development of commercial inoculants of free-living bacteria, such as Diazotrophicus sp., Azotobacter sp., and Azospirillium sp., as a medium to provide nitrogen in a wider range of plants [29]. Commercial inoculum of Azospirillium with Rhizobium consortia was found effective in enhancing nodulation and to increase in crop yield [30]. To improve nitrogen uptake, studies are focused on the development of microbes that can facilitate the root systems than finding nitrogen-fixing microbes [31].
The next macronutrient required for the growth and development of plants is phosphorus. Though present in ample amounts in agricultural soils, much of it (30–65%) is present in the non-soluble (organic; phosphates, phosphodiesters, etc) form [32]. As organic forms are not taken up by plants, a significant level of their acquisition is fulfilled by supplementation in the form of fertilizers. Additionally, phosphate-solubilizing bacteria belonging to the genus Pseudomonas, Alcaligenes, Bacillus, Corynebacterium, and others are capable of transforming the recalcitrant forms into easily available forms for uptake by plants [32]. Secretion of organic acids or H+ ions by phosphate solubilizing bacteria promotes the solubilization of inorganic phosphate that has undergone complex formation with calcium, aluminum, and iron [33]. Similarly, the release of phytase by phosphate-solubilizing bacteria promotes the production of its active form from organic moieties.
HCN—a secondary metabolite produced among Gram-negative bacteria, is a product of the oxidative reaction of glycine catalyzed by HCN synthase [34]. Being a flavoenzyme, its reaction is mediated by flavin adenine dinucleotide (FAD), while pyrrolnitrin acts as an inhibitor of the reaction [35]. Though the production of HCN by microbial population was earlier referred to as a plant protective mechanism, it was later recognized to play an active role in enhancing the availability of phosphorus to plants [36]. Iron—a micronutrient, is considered an essential element in terms of its requirement for the growth and development of plants. Despite its abundance in the earth’s crust, scarcity of the bioavailable iron reported in most soils accounted for its occurrence mostly in the ferrous (Fe2+) form [37]. Having greater requirements for ferric (Fe3+) form, bacteria secrete iron-binding ligands (referred to as siderophores) to make Fe3+ available for bacterial use. Siderophore (Extracellular low molecular weight water-soluble compound of microbial origin) production is preferably associated with active sequestration of iron (Fe3+) for its transport into the interior of the cell (ferripyoverdine; Combination of Fe3+ with pyoverdin) for use in nitrogen fixation, respiration, and photosynthesis [38].
3.3. PGPR in Ion Homeostasis and Osmotic Balance
Microbe-mediated siderophore production and pH changes brought about at the rhizospheric plane fulfill the nutritional needs of the plants. In spite of enhancement in the availability and uptake of nutrients, plants are often prone to disturbances that arise due to stress conditions. Under salt stress conditions, an influx of salt at an enhanced rate following nutrient-to-mineral exchange results in a nutritional imbalance in plants. Disturbance in ion homeostasis is often observed in plants that owe the property of being poor excluders of Na+ and having a high affinity for Cl− ions [39]. The ions (both Na+ and Cl−) above their required amount exert toxic effects in terms of reduced growth and senescence following their translocation and accumulation in aerial parts of plants [39]. As only a smaller fraction of Na+ undergoes recirculation, the perturbance of the Na+ to K+ ratio results in the inhibition of the cytosolic activities along with interference in the activities of enzymes associated with respiration and photosynthesis [40]. Microbes reduce the toxic ion uptake through the production of exopolysaccharides (EPS) and regulating the expression of ion affinity transporters. The EPS enables the survival of plants under inhospitable conditions by altering the root structure (developing extensive rhizosheaths) and promoting the trapping of toxic ions in the EPS matrix [18,41,42]. Membrane localized Na+/H+ antiporter (Salt overlay sensitive channel, SOS1) proceeds with efflux of cytosolic Na+, while high-affinity K+ transporters (HKT) enhance uptake of K+ at the root surface [43]. Additionally, sensing of the salt stress signal by calcineurin B-like protein (CBL4; also known as SOS3) makes it undergo interaction and as such complex formation with CBL-interacting protein kinase (CIPK24, also known as SOS2). Phosphorylation of SOS1 by the SOS2-SOS3 complex results in its activation and, thereby, sustenance of the K+-transport for maintaining the Na+/K+ ratio [44].
Accumulation of the salt ions often causes disturbance in the osmotic balance in plants. PGPR improves the plant-water relationship through enhancement in the production of osmolytes for uptake by plants at the root surface [45]. Stimulation for the uptake of compatible solutes (osmolytes) such as proline, glycine, polyamines, etc., followed by accumulation is essential for maintaining osmotic balance to avoid oxidative damage to the cellular components [46]. The compatible solutes affect hydraulic conductivity towards the regulation of the water potential and stomatal opening. Inoculation of Bacillus megaterium causes increased water conductance in maize grown under salt stress via increase in the expression of two plasma membrane aquaporin protein isoforms [47]. Seed inoculation of Bacillus aquimaris causes an increase in the total soluble sugar, shoot biomass, and enhanced NPK accumulation, besides causing a reduction in the Na+ content of the leaves [48]. PGPR-mediated proline accumulation is often correlated with an increase in the expression of pyrroline-5-carboxylate synthase (P5CS), thereby leading to enhancement in the accumulation of free proline in plants [49]. The biofilm formation at the root surface increases stable soil aggregates that often attribute protection of plants against water stress [50].
3.4. PGPR Produced Phytohormones and Plant Growth
PGPR-mediated exogenous release of hormones, metabolites, and enzymes modulate plant hormonal balance, thereby influencing their growth under extreme stress conditions. Capable of altering water and nutrient uptake, morphology and metabolism, and tolerance to different environmental stresses, they play a key role in boosting the growth and development of plants [51]. Indole-3-acetic acid (IAA) production is a relatively common trait of PGPR that increases the fitness of plants growing under stress conditions. PGPR utilizes tryptophan present in the root exudate for inducing IAA synthesis at the rhizospheric plane [52]. IAA produced by PGPR exerts a strong effect on root growth and its architecture [52,53]. Co-inoculation of IAA-producing Azospirillum brasilense Az39 with Bradyrhizobium japonicum E109 causes enhancement in the germination and growth of corn and soybean [53]. Inoculation of Bacillus subtilis GB03 in Arabidopsis regulates the activity of cell wall loosening enzymes, besides promoting growth by having a tight regulation of auxin homeostasis [54]. Similarly, IAA-producing PGPR was found to enhance nutrient uptake under hydroponic conditions and exhibited higher growth of roots and leaves under salinity conditions [55]. PGPR-producing auxin induces a change in the expression of genes pertaining to the cell wall, hormone, and defense-related genes, decreasing stomatal size and density, increasing root biomass, and leading to the activation of auxin response genes associated with the growth and development of plant [56].
Gibberellins are synthesized in the terpenoid pathway and are often found associated with developmental processes such as cell division and elongation, and they have a role in germination following an exit from seed dormancy. PGPR strains such as Bacillus amyloliquefaciens RWL1, Enterococcus faecium LKE12, and others are known to show the production of gibberellins [57,58]. PGPR-mediated production of gibberellins causes enhancement in the hypocotyl and stem growth; thereby assisting in regulating leaf and root meristem size [59]. Cytokinins represent a group of purine-based plant hormones often found associated with regulation cell division and differentiation of the meristematic tissues, chloroplast maturation, and stomatal conductance [60]. Though cytokinin production is a common trait in PGPR, it influences the endogenous cytokinin pool by inducing its synthesis or altering its homeostasis in plants [61]. As the auxin-to-cytokinin ratio plays important role in determining the fate of a cell, inoculation of cytokinin-producing B. subtilis in drought-stressed lettuce plants promotes plant growth via modulation of root-to-shoot signaling [62].
Abscisic acid (ABA)—a stress hormone synthesized in response to stress regulates the expression of genes that impart stress tolerance [18]. Its function is primarily associated with triggering the adaptive response, i.e., the abscission of leaves and retarded shoot growth, with overcoming adverse environmental conditions. Many PGPR such as B. licheniformis, Pseudomonas fluorescens, A. brasilense, and others, are known to show ABA production [63]. PGPR-mediated ABA production modulates ABA biosynthesis and its dependent signaling pathways that cause resistance by mitigating the sensitivity of plants to water scarcity. Its accumulation at the root surface performs the function of enhancing water uptake via an increase in root growth and stimulation of lateral root formation [2]. Under drought conditions, its translocation from root to aerial parts, particularly leaves, causes stomatal closure in order to maintain water balance via a reduction in transpiration activity [64]. PGPR-mediated enhancement in the plant ABA pool relieves plants of the effects of growing under different abiotic stresses.
Ethylene—a gaseous stress hormone effective at extremely low concentrations is produced from its precursor 1-aminocyclopropane-1- carboxylase (ACC) by the ACC oxidase enzyme in plants [2]. PGPR, capable of producing ACC deaminase, reduces ethylene production, thereby enhancing stress tolerance by promoting growth in plants [65]. The ACC deaminase-producing bacteria in the rhizospheric plane degrade ACC from the root exudate, which progresses with a fall in the ACC levels inside the plants. The ACC deaminase converts ACC to ammonia and α-ketoglutarate that keep ethylene at bay from reaching significant levels in order to exert a reduction in plant growth [66].
Jasmonic acid (JA) and Salicyclic acid (SA), having ubiquitous expression in plants, constitute other armories for defense against different stress conditions [67]. They perform definite roles pertaining to different developmental processes such as germination, root and shoot growth, and flowering in plants. PGPR, in particular endophytes, were found capable of synthesizing JA and SA, thereby playing a critical role in regulating the growth and development of plants [68]. JA-mediated induction of the signaling pathways results in the activation of calcium channels that causes enhancement in the levels of cytosolic calcium and by playing an active role in upregulating the expression of genes involved in glutathione (GSH) synthesis for scavenging the ROS as part of the antioxidant response against abiotic stress [68,69]. Inoculation of the plants with P. fluorescens Pf4, B. amyloliquefaciens LJ02, and Burkholderia phytofirmans PsJN results in the enhancement of endogenous levels of SA [58,70,71].
4. PGPR in Abiotic Stress Tolerance
Plants, being immobile, are destined to complete their life cycle in one place. It exposes them to extreme conditions that exert definite pressure on plants to evolve and succeed for traits to withstand the fluctuations in the environmental conditions. The changes in the climatic conditions produce aggravated effects that impose serious effects such as disturbance in genetic makeup, perturbed metabolic activities, and impaired crop yield for a majority of plants worldwide [18]. The selection pressure that confronts the growth and development of plants are categorized into biotic (bacteria, virus to grazing by higher animals) and abiotic (heavy metals, extreme temperature, drought up to salinity) factors [18,72]. Abiotic stresses affecting different aspects of plant growth pose a serious threat to the survival of plants [73]. It progresses with a significant reduction in germination and strong effects on photosynthetic activity, carbon assimilation, and on overall crop productivity [74]. Under such circumstantial conditions, the phytomicrobiome came to the forefront to assist in the survival of holobiont (plants in association with microbes) in extreme environmental conditions [16,17].
4.1. PGPR in Mitigating Heavy Metal Stress
Heavy metals (Mercury, Hg; Arsenic, As; Chromium, Cr; and others) discharged from natural (volcanoes, etc) and anthropogenic (industries, mining, etc) sources result in pollution of the environment [75]. Being recalcitrant to degradation, their persistence in the environment exhibits spatial distribution and, as such higher toxicity across different environmental gradients [76]. Their contamination of the agricultural soils results in their accumulation, and as such, transfer into the food chain and crops, resulting in life-threatening consequences among humans, animals and mankind as a whole [77]. Presence of heavy metals at higher levels in soil results in their uptake and accumulation in different parts of the plant, where they exert serious consequences in terms of interference with the uptake of nutrients, growth parameters via modulation of the enzymatic activities, disruption of the metabolic machinery, etc [77]. Offering a great concern to mankind, their remediation is primarily aimed at physical and chemical clean-up procedures that vary in terms of efficiency and effectiveness [78].
Considering their limitations, plant-based remediation strategy commonly referred as phytoremediation offers an advantage of being an environmental friendly, efficient, and cost-effective strategy to achieve the remediation of heavy metals from the soil [77]. Though it offers a greener approach to achieving remediation, plants employed in the process face extensive stress that causes a reduction in proficiency in extracting heavy metals from the contaminated soils [79]. In such a scenario, microbial strains exhibiting wider resistance to heavy metals offer adaptability to plants in their survival in hostile environments and in leveraging their toxic effects on plants by causing precipitation and as such reduction in metals in soils [80]. They exert a positive influence on plant growth and biomass by providing suitable nutrients for uptake, besides playing an important role in converting toxic forms to less toxic ones, production of chelating agents, modification of phytohormone levels, and the production of biofilms [77,78,79,80]. Association of Bacillus xiamenesis and Bacillus gibsonii with roots of Sesbania sesban commonly inhabiting saline soils and waterlogged conditions, enhances growth and phytoextraction potential via the production of ACC-deaminase that cause a decrease in the production of ethylene, besides enhancing the production of exopolysaccharides (EPS), and phytohormones such as indoleacetic acid (IAA) [79]. Inoculation of cadmium (Cd) and lead (Pb) resistant Bacillus species (QX8 and QX13) of the Solanum nigrum L. roots enhances uptake of nutrients such as phosphorus (P), solubilization of iron (Fe), nitrogen (N2) fixation in addition to increasing in the uptake and accumulation of Cd and Pb in aerial parts of the plant [77]. Similarly, inoculation of Pseudomonas aeruginosa and Burkholderia gladioli to Lycopersicon esculentum seedlings results in a lowering of the expression of metal transporter genes with enhancement in the plant growth (shoot and root length) and an increase in the production of photosynthetic pigments such as chlorophyll, carotenoids, and xanthophylls [80].
Information on the plant growth-promoting rhizobacteria employed in mitigating different stresses is summarized in Table 1. The following sub-sections discuss information on the valuable microbial-based resources that are oriented for the upliftment of the constraints towards achieving sustainability in agriculture.
Table 1.
PGPR mediated promotion of plant growth via, alleviation of abiotic stresses.
| Bacterial Strain(s) | Beneficial Effect | Stress Type | Reference |
|---|---|---|---|
| Abiotic stress (Environmental conditions) | |||
| Pseudomonas monteilii, Cronobacter dublinensis, Bacillus sp. | Enhanced in nutrient and water uptake | Abiotic stress | [81] |
| Achromobacter piechaudi | Enhancement in fresh and dry weight of plant, reduction in ethylene level | Moisture | [82] |
| A. brasilense | Maintenance of Ion homeostasis | Osmotic stress | [83] |
| Azospirillium brasilense Sp245 | Production of phytrochrome | Osmotic stress | [84] |
| A. brasilense Az 39 | Enhancement in osmolyte production accumulation | Osmotic stress | [53] |
| Temperature stress | |||
| Pseudomonas sp. AMK-P6 | Increased expression of stress reliever genes | Heat stress | [85] |
| B. phytofirmans PsJN | Enhancement in ACC deaminase activity | Low temperature | [86] |
| P. putida | Enhancement in ACC deaminase activity | Low temperature | [87] |
| Drought | |||
| Pseudomonas azotoformans | Increase in germination rate, shoot and root length | Drought | [5] |
| Bacillus spp. | Enhanced proline content and nutrient uptake, decreased ascorbate peroxidase and glutathione reductase activity | Drought | [88] |
| Variovorax paradoxus, consortia of Pseudomonas spp. | Increase in root and shoot length, nutrient uptake, and antioxidant activity, improved biomass | Drought | [89] |
| Pseudomonas fluorescens | Increase in germination rate, improved soil adherence to root tissue dry mass | Drought | [90] |
| Rhizobia spp. | Secretion of volatile compounds and EPS, enhanced cytokinin, and ACC deaminase production | Drought | [91] |
| Bacillus subtilis (LDR2) | Enhanced check of regulatory component (CTR1) of the ethylene signaling pathway | Drought | [92] |
| Azosperillum brasilense SP-7 | Increased water uptake and chlorophyl content, improved nitrogen and carbon level, decrease in ethylene and abscisic acid | Drought | [93] |
| Azospirillium spp. | Increase in germination rate and proline content, improved seedling growth and shoot dry weight | Drought | [94] |
| Pseudomonas spp., Enterobacter spp., Bacillus sporothernoduran | Increase in nutrient uptake, chlorophyl content, plant biomass, and siderophore production | Drought | [95] |
| Pseudomonas chlororaphis O6 | Secretion of volatile compounds, osmolyte accumulation, and activity of antioxidants | Drought | [96] |
| Klebsiella sp. IG3 | Enhancement in ACC deaminase activity | Drought | [97] |
|
Achromobacter xylosoxidans, P. oryzihabitans, Variovorax paradoxus |
Increase in auxin production, improved root mass, enhanced ACC-deaminase activity, and decreased ethylene concentration | Drought | [98] |
| P. putida H-2-3 | - | Drought | [57] |
| Pseudomonas aeruginosa | Regulation of relative water content, upregulation of stress-responsive genes, Increased root and shoot length | Drought | [57] |
| Phyllobacterium brassicacearum | Enhancement in abscisic acid production | Drought | [99] |
| Bacillus pumilu | Regulation of plant growth; leaf numbers and, size and number of tubers | Drought | [100] |
| Bacillus licheniformis K11 | Enhancement in ACC deaminase activity, increased expression of stress-responsive genes | Drought | [101] |
| A. brasilense | Enhancement in osmolyte production and accumulation | Drought | [102] |
| Bacillus cereus, Bacillus subtilis, Serratia sp. | Enhanced chlorophyl content, root growth, decreased amount of malondialdehyde content | Drought | [103] |
| Azospirillum sp. | Enhancement in lateral root formation, improved uptake of water and nutrients | Drought | [104] |
| Bacillus spp. | Enhancement in osmolyte production and accumulation | Drought | [105] |
| Azospirillium sp. | Improved uptake of water and nutrient | Drought | [106] |
| P. putida P45 | Enhancement in EPS production | Drought | [46] |
| B. subtilis GB03 | Enhancement in osmolyte production and accumulation | Drought | [107] |
| Pseudomonas putida | Enhanced proline content and water uptake, decrease in production of ROS | Drought | [46] |
| Pseudomonas mendocina | Improvement in antioxidative enzyme production | Drought | [108] |
| Paraphaeosphaeria quadriseptata | Enhancement in the expression of stress reliever genes | Drought | [109] |
| B. subtilis | Volatile compound production | Drought, salinity | [54,110] |
| Rhizobium sp. | Enhancement in EPS production | Drought | [111] |
| Paenibacillus polymyxa | Enhancement in the expression of stress reliever genes | Drought | [112] |
| Salinity | |||
| Pseudomonas | Enhancement in IAA production and ACC deaminase activity | Salinity | [113] |
| Arthrobacter protophormiae | Enhancement in ACC deaminase activity | Salinity | [114] |
| B. subtilis | Regulation of ion homeostasis | Salinity | [115] |
| Alcaligens sp., Bacillus sp., Ochrobactrum sp. | Improved seed germination and seedling growth via enhancement in ACC deaminase activity | Salinity | [116] |
| EPS-producing bacteria | Enhancement in EPS production | Salinity | [117] |
| Pseudomonas pseudoalcaligenes | Enhancement in osmolyte production and accumulation | Salinity | [118] |
| Brevibacterium iodinum | Enhancement in IAA production and ACC deaminase activity, Increase in ammonia and HCN production | Salinity | [119] |
| Pseudomonas aurantiaca, P. extremorientalis | Improved phytrochrome production | Salinity | [120] |
| Rhizobium and Pseudomonas | Enhancement in osmolyte production and accumulation | Salinity | [121] |
| Sinorhizobium meliloti | Improvement in antioxidative enzyme production | Salinity | [122] |
| Piriformospora indica | Improvement in antioxidative enzyme production | Salinity | [123] |
| Acinetobacter, A. faecalis, P. aeruginosa, B. Cereus, Enterobacter hornaechei, Pantoae agglomerans | Enhancement in ACC deaminase activity | Salinity | [124] |
| P. fluorescens | Enhancement in ACC deaminase activity | Salinity | [125] |
| Bradyrhizobium japonicum | Improvement in antioxidative enzyme production | Salinity | [126] |
| A. brasilense | Regulation of ion homeostasis | Salinity | [127] |
| Metal stress | |||
| Brevundimonas, Acaligenes faecalis | Reduced toxicity and enhancement in phytoremediation potential | Mercury | [128] |
| Rhizobium sp., Microbacterium sp. | Reduced toxicity, improvement in nitrogen content | Chromium (VI) | [129] |
| Bradyrhizobium japonicum CB1809 | Enhancement in growth and plant biomass | Arsenic | [130] |
| Bacillus megaterium | Reduced toxicity | Lead | [131] |
| Bacillus | Enhancement in phytoremediation potential | Chromium | [132] |
| Staphylococcus, Aerococcus | Reduced toxicity | Cadmium, copper, lead and zinc | [132] |
| Azotobacter chroococcum HKN-5, Bacillus megaterium HKP-1, B. Mucilaginosus HKK-1, | Reduced toxicity and stimulation of plant growth | Lead, Zinc | [133] |
| Bacillus subtilis SJ-101 | Enhanced nickel accumulation | Nickel | [134] |
4.2. PGPR in Alleviating Effects of Extreme Temperature
Change in climatic conditions has exacerbated the severity of environmental stressors. Having a profound effect on the adaptation and survival of plants, it accounts for a huge loss in overall productivity [18]. Gradual increase in temperature (5–15 °C above the normal) proceeds with a significant effect on the growth and development of plants [135]. The increase in temperature that often proceeds with the decrease in the soil water content led to a significant reduction in the cultivable capacity of the soil i.e., a shift from fertile to marginal [18]. Perceived as an outcome of the climatic conditions, water scarcity adversely affects chlorophyll content and rate of photosynthesis, nutrient acquisition, carbon assimilation, and gaseous exchange at the leaf surface, which is considered critical for the survival of plants [73]. It is well documented that an increase in the temperature in the range of 3–4 °C results in a significant decrease i.e., almost 15–35% in Asia and Africa and by an amount of 25–35% in the middle-east countries. Representing a well-defined heat stress, mechanisms encompassing alteration in the expression of genes encoding heat shock and scavenger proteins, and a trigger for the accumulation of metabolites required for normal cellular metabolism, starts operating as part of the internal defense of plants [103,135].
Though tolerance to heat stress attributed by PGPR largely remains speculative, their ability the produce exogenous polysaccharides attributed it with molecules for use in facilitating their interaction with the root appendages, followed by colonization of roots and in shielding them against desiccation via induction in the formation of biofilms [96]. The exopolysaccharides released into the extracellular matrix provide a range of macromolecules necessary for the growth and development of plants in addition to the interface for improving water uptake through the rhizospheric plane and enhancing its retention by several folds [136]. The ability of P. putida to survive in low-moisture soils promotes its colonization of the wheat and sunflower roots via an increase in the soil adhering capacity and in promoting the formation of stable soil aggregates [50]. Inoculation of potato plants with Burkholderia phytofirmans promotes enhancement in the root and shoot biomass and an increase in the stem length following their growth at high temperatures [137]. Inoculation of plants with P. aeruginosa not only increases its water retention capacity but also contributes to an increase in the total chlorophyll content, length of roots, leaf area, dry matter, and a substantial decrease in the cell membrane injury when grown under high-temperature conditions [138].
Cold stress contributing to slow down or suspension of metabolic activities contributes significantly to a reduction in the yield. Compared to control, inoculation of the explants of Vitis vinifera cv. Chardonnay with Burkholderia phytofirmans PsJN results in improved tolerance of the plantlets to cold [86]. Holding endophytic colonization of the plantlets reduced their sensitivity to cold, thereby improving their growth at an ambient temperature of 26 °C and at a low temperature of 4 °C. With improved growth and physiological activities, the major benefits were observed in the growth of roots and to overall biomass of the platelets. Bacterization of the wheat results in significant improvement in root and shoot length along with their dry mass, an increase in the free proline, starch, chlorophyll, and anthocyanin contents, and a subsequent decrease in the electrolyte leakage and Na+ to K+ ratio [128]. In another study, inoculation of wheat with Pseudomonas spp. PPERs23 results in an improvement in the nutrient content in shoots that improves grain proportion and thereby overall productivity by 13.4% [139].
4.3. PGPR in Alleviating Effects of Drought Stress
A decrease in the soil water level progresses to a change in the land use pattern. In drought, the water potential and turgor pressure are so disturbed that it causes a major hindrance to the flow of nutrients, thereby causing a reduction in the photosynthetic activity [140]. Depending on the duration, drought stress ranges from moderate to prolonged, with implications from interference in the normal cell functions to changes in morphological and physiological traits if prolonged [140]. Suppressing the cellular antioxidant defense, it induces the production of free radicals and reactive oxygen species (ROS) such as superoxide, hydroxyl radical, and hydrogen peroxide, which contributes significantly to the induction of oxidative stress [141]. ROS generation progresses with damage at various levels, such as membrane disintegration and lipid peroxidation, followed by serious damage to proteins and nucleic acids [142]. For growth under drought conditions, plants primarily switch to a drought escape (DE) mechanism that involves growth at a rapid rate and a great reduction in the life span in order to complete the life cycle within the stipulated period of favorable conditions [143]. As part of survival strategies, it proceeds with enhancement in the accumulation of compatible solutes, such as proline, for regulating normal cellular function [144]. In addition to its role in regulating mitochondrial function, proline-mediated activation of stress-responsive genes helps in the regulation of cell proliferation activity. Proline accumulation proceeds with the scavenging of free radicals that decrease the rate of lipid peroxidation, thereby helping to maintain the structural integrity of the membranous structures [145]. In addition to regulation of the physiological function, compatible solutes impart regulation of cellular turgor and stomatal movement towards the regulation of the photosynthetic events that contribute to the overall regulation of growth and development in plants.
Studies performed on the rhizobacteria have suggested their role in alleviating the impact of drought stress that proceeds with enhancement in the growth and overall productivity of the plants [146]. To examine the impact of microbial inoculations, it was found effective in improving nutrient uptake and, as such, the growth of cereals, vegetables, and legumes [40]. PGPR-mediated production of EPS, such as cellulose, and alginate was found effective in mitigating the effects of stress, thereby increasing the tolerance of plants to different environmental stresses [147]. Acting as a barrier around the roots, it regulates bacterial attachment to roots and ensures the movement of water and nutrients toward improving plant growth under drought conditions. PGPR-mediated production of phytohormones adds to the endogenous hormonal pool of plants [148]. Inoculation of plants with IAA-producing bacteria results in increasing root growth via stimulation in lateral root formation and development of root hairs, thereby increasing water and nutrient uptake to cope with the water deficit conditions [120]. IAA-mediated transcription induction of the ACC synthase gene progresses with an increase in the ACC concentration that enhances the production of ethylene. It proceeds with an increase in plant growth mediated by IAA via enhancement in the water uptake and absorption of nutrients [149,150]. Similarly, bacterial isolates belonging to the Bacillus genus (DS4 and DS9) capable of growing under drought conditions were found effective in the production of high levels of IAA (1.61µg/mL and 0.9µg/mL) and gibberellins (49.95µg/mL) as part of an attribute in promoting growth under harsh environmental conditions [151]. PGPR-mediated IAA production promotes plant growth and induces stress tolerance via a reduction in the levels of ethylene. Additionally, PGPR plays a beneficial role in drought stress by mitigating the effect of ABA. Foliar spray of Platycladus orientalis increases the relative water content and stomatal conductance thereby plays a role in the growth of plants under drought conditions [152].
PGPR-mediated accumulation of osmoprotectants and enhancement in the metabolic activity and antioxidant defense contribute significantly to the promotion of plant growth. Inoculation of maize with Herbaspirillum seropedicae and Azospirillum brasilense results in improved water usage efficiency, membrane stability, osmolyte accumulation, and enhanced ACC deaminase and antioxidant enzymes activities [92,153]. Bacillus thuringiensis inoculation of Lavandula dentate overcomes the effect of drought stress via an enhancement in the proline content, nutrient uptake, and increase in metabolic activities [154]. Inoculation of soybean with Pseudomonas sp. results in an increase in chlorophyll content, stem height, and fresh weight when grown under water deficit conditions [155]. Inoculation of maize genotype TP30 with Bacillus spp. causes a significant increase in membrane integrity via an enhancement in the antioxidant defense with a subsequent decrease in electrolyte leakage [94]. PGPR strains such as P. jessenii, Anthrobacter nitroguajacolicus, and P. synxantha inoculation of rice seedlings, P. putida in maize, B. polymyxa in tomato, B. subtilis in mustard, Pseudomonas and Mesorhizobium cicero in green gram, are capable of promoting plants grown under drought stress conditions [46,156,157,158]. Application of E. cloacae improves the growth of the root system and, as such relative water content for plants grown under water stress conditions [159].
4.4. PGPR in the Alleviation of Salt Stress
Soil salinity (electrical conductivity of 4 dSm−1) is a parameter attributed to the presence of high levels of salts such as NaCl in addition to MgSO4, K2SO4, MgCl2, etc., that originates from weathering, irrigation, and evaporation of shallow groundwater [18]. An increase in salt concentration of soil exposes plants to osmotic (accumulation of salts at roots that hampers uptake of water) and ion (surplus accumulation of Na+ in aerial parts of plants especially leaves that cause reduction in photosynthesis and other metabolic activities) stress [160]. Salinity stress causes a decrease in the cultivable land, i.e., almost 1–2% annually, and is projected to show a progressive decrease on account of global climate change [18]. Being deleterious to plant health, it interferes with the physiological processes of plants and exerts a strong impact on important soil parameters such as nitrification, microbial diversity, etc [161]. In addition to its effect on the extraction of water required for optimal growth, it adversely affects flowering and fruiting and causes aberrations to reproductive structures that affect normal cellular functioning and overall yield [18]. Acting as a limiting factor for the growth of salt-sensitive plants and even some halophytes, it causes up to 70% reduction in the yield of some plants such as rice, wheat, maize, and barley [162].
Water scarcity, which encompasses both drought and salinity, presents an unpredictable constraint with a serious impact on morphological, physiological, and biochemical parameters and, thus, on overall plant health [73]. The salt tolerance parameters such as survival, vegetative growth, and harvestable biomass quantified over a period of time are either adopted by inheriting the adaptable genetic traits or through the exclusion of salts at the root surface [124]. In certain plant species, especially halophytes, an enhancement in the conductance of accumulated salts via the xylem to aerial parts, in particular leaves for precipitation or to salt glands (special structures developed in shoots) for excretion at the surface [41]. Additionally, plants develop interactions with the rhizospheric microbiota that range from mutualism to antagonism after successful colonization and establishment at the rhizospheric plane. The prerequisite condition for the successful establishment of PGPR in the rhizospheric region includes the property of being motile and with the ability to adhere to plant roots via pilli or surface localized proteins [18,163]. The root exudates rich in nutrients fulfill the requirement of microbes to flourish while prompting changes such as nutrient availability and defense that elicit strong responses related to stress tolerance and offer a definite mechanism for promoting plant growth [18,120].
An alternative strategy of PGPR makes use of EPS for the successful establishment of bacteria in roots and soil particles, besides playing an intricate role in developing healthy interaction between bacteria of different microbial communities [164]. The application of Marinobacter lypoliticus and B. subtilis on wheat causes a significant decrease in the effects exerted by salt and drought stress [165]. Inoculation of soybean with Bradhrhizobium japonicum 532C and Chenopodium quinoa with Bacillus sp. MN54 led to significant improvement in plant growth under high (36–400 mM NaCl) salt concentrations [166]. Trehalose—a non-reducing disaccharide resistant to high temperature and acidity-prevents protein aggregation and degradation, thereby imparting definite protection to plants against salinity and drought stress [167]. Inoculation of soybean plants with P. putida and tomato plants with Azospirillum brasilense, Chryseobacterium, P. chlororaphis, and streptomyces sp. causes a reduction in the ethylene levels and, as such, imparts tolerance to salinity stress [57,168]. In a study, the presence of the Priestia endophytica SK1 in soil with fenugreek plant (Trigonella foenum-graecum L.) grown was found effective in enhancing nitrogen-fixing ability and improvement in the biosynthesis of trigonelline content [169]. In another study, inoculation of Acinetobacter johnsonii (SUA-14) to maize resulted in enhancement in the enzymatic activity of urease, alkaline phosphatase in soil and a subsequent decrease in catalase (39%), superoxide dismutase (26%) and malondialdehyde (27%); thereby imparts resistance of maize to salinity stress [170]. Though inoculation of B. megaterium regulates phosphate solubilization, inoculation of Pseudomonas sp. to eggplant, Bacillus sp. to alfalfa, B. licheniformis to red pepper, Burkholderia cepacia to cucumber, Phyllobacterium sp. to strawberries and Enterobacter sp. to okra were found attributing them property to tolerance salinity stress [57,119,171,172,173]. The PGPR strains were found to play a vital role in improvising the resistance of plants to salinity stress and in fulfilling the requirements towards enhanced growth and development of different plants.
5. Conclusions and Future Perspectives
The unabated use of chemical fertilizers in agriculture led to unanticipated environmental impacts that exerted serious health complications. Having an adverse effect on the environment and serious human health concerns, it gained momentum for the development of environmentally friendly alternatives as a suitable replacement for chemical-based fertilizers for use in agriculture (Figure 3). As the development of tolerant cultivars by genetic manipulation encounters various hindrances, the exploitation of soil microbiota for uses as biofertilizers and as biocontrol agents sums up the integral component of the organic farming system. The mechanisms elicited by PGPR include the production of biofilm for enhancing soil binding to roots and regulation of relative water content, triggering of osmotic response via production and accumulation of osmolytes, enhancement in nutrient uptake, and maintenance of ion homeostasis, enhancement in the production of plant growth regulators, improved antioxidant defense, chlorophyll, carbon and nitrogen levels, HCN and siderophore production, besides regulating the expression of stress reliever genes and proteins. It induces a protective system to mitigate the existing stresses in a time-sensitive and cost-effective manner. With the rising emphasis on sustainability, environmental safety, and food security, the employment of bio-inoculants will help to overcome the constraints resulting from a persistent change in the climatic conditions and offer a promising alternative to achieve sustainability in agriculture in an environmentally friendly manner.
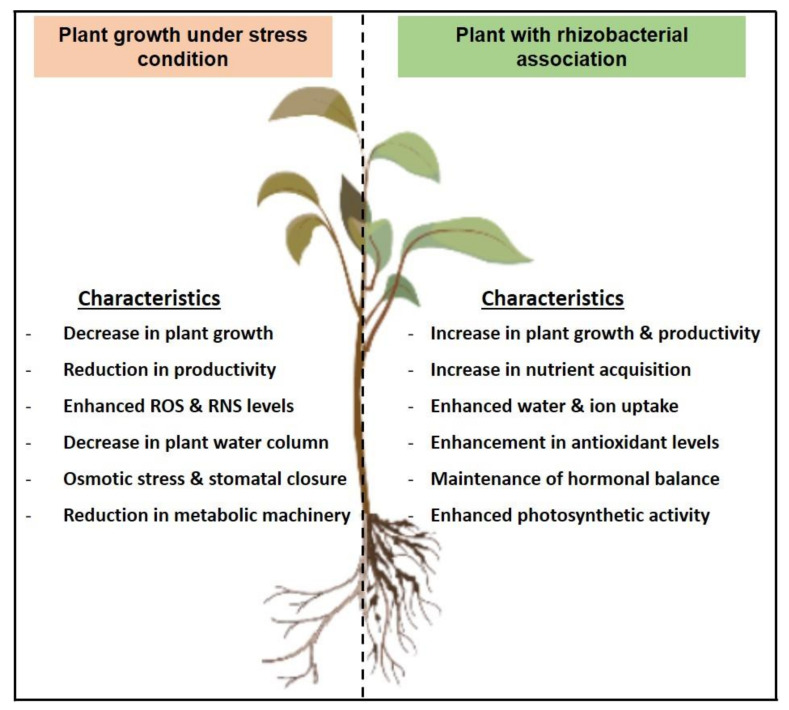
Figure 3.
Summarized view of plant-microbiome interactions exerting beneficial effects in plants.
The PGPR attributing multiple plant-growth-promoting (PGP) traits to plants increases their resilience to overcome the effects of stresses under prevailing environmental conditions. Since the diversity of PGPRs depends on soil dynamics and plant species, studies should be directed towards delineating the community structure in the rhizospheric plane and elucidating their ecological association and mechanisms thereof that contribute to enhancement in the plant growth and tolerance to different stresses. Additionally, knowledge of the endogenous microbial communities will provide insights into designing the inoculant strains with traits that optimize their performance and maximize their benefits on application under field conditions. Delineating their biochemical and physiological profile under conditions of varying pH, extremes of temperature, drought, and salinity will provide important insights into survival under stress conditions and their dependence on the environment for bio-stimulation and efficacy. It will also provide the baseline data for genetic approaches to engineer bacterial strains that address specificity issues and enhance their ability growth of plants under changing paradigm of existing endogenous microbiota. The futuristic approach should also include mechanistic exploration of the basic architecture of regulatory circuits and harness them for improvement in the yield that could serve the purpose of alleviating stress and achieving sustainability in agriculture to fulfill the global needs for food.
Acknowledgments
The authors would like to thank their colleagues, whose suggestions helped in improving the contents of the manuscript. The authors would also like to thank Mohd Saeed, University of Hail, Hail for his help in the designing of illustrations in BioRender.
Author Contributions
Conceptualization, A.A.S., S.R. and A.T.J.; resources, M.A.K. and A.K.M.; investigation, M.A.B. (Mudasir Ahmad Bhat), A.K.M., S.J., M.A.B. (Mujtaba Aamir Bhat), and A.T.J.; validation, A.A.S., S.R. and A.T.J.; writing-original draft preparation, M.A.B. (Mudasir Ahmad Bhat), A.K.M., S.J., M.A.B. (Mujtaba Aamir Bhat), and A.T.J.; writing-review and editing, M.A.K., A.K.M. and A.T.J.; supervision, A.A.S. and A.T.J.; project administration, S.R. and A.T.J.; funding acquisition, A.T.J. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by J&K Science Technology and Innovation Council (J&K ST&IC), India, grant number JK ST&IC/SRE/996-998 and DST SERB, India, grant number CRG/2019/004106.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Robertson G.P., Swinton S.M. Reconciling Agricultural Productivity and Environmental Integrity: A Grand Challenge for Agriculture. Front. Ecol. Environ. 2005;3:38–46. doi: 10.1890/1540-9295(2005)003[0038:RAPAEI]2.0.CO;2. [DOI] [Google Scholar]
- 2.Vaishnav A., Varma A., Tuteja N., Choudhary D.K. Plant Microbe Interaction: An Approach to Sustainable Agriculture. Springer; Singapore: 2016. PGPR-mediated amelioration of crops under salt stress; pp. 205–226. [Google Scholar]
- 3.Coban O., De Deyn G.B., Van Der Ploeg M. Soil microbiota as game changers in restoration of degraded lands. Science. 2022;375:abe0725. doi: 10.1126/science.abe0725. [DOI] [PubMed] [Google Scholar]
- 4.Kumawat K.C., Sharma B., Nagpal S., Kumar A., Tiwari S., Nair R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2023;13:1101862. doi: 10.3389/fpls.2022.1101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari F., Jabeen M., Ahmad I. Pseudomonas azotoformans FAP5, a novel biofilm forming PGPR strain, alleviates drought stress in wheat plant. Int. J. Environ. Sci. Technol. 2021;18:3855–3870. doi: 10.1007/s13762-020-03045-9. [DOI] [Google Scholar]
- 6.Abulfaraj A.A., Jalal R.S. Use of plant growth promoting bacteria to enhance salinity stress in soybean (Glycine max L.) plants. Saudi J. Biol. Sci. 2021;28:3823–3834. doi: 10.1016/j.sjbs.2021.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott L.K., Murphy D.V. Soil Biological Fertility: A Key to Sustainable Land Use in Agriculture. Springer Science and Business Media; Dordrecht, The Netherlands: 2003. [Google Scholar]
- 8.Kumar R., Prakash O. The impact of chemical fertilizers on our environment and ecosystem. Res. Trends Environ. Sci. 2019;5:69–86. [Google Scholar]
- 9.Emmerson M., Morales M.B., Oñate J.J., Batáry P., Berendse F., Liira J., Aavik T., Guerrero I., Bommarco R., Eggers S., et al. How agricultural intensification affects biodiversity and ecosystem Services. Adv. Ecol. Res. 2016;55:43–97. [Google Scholar]
- 10.Bargaz A., Lyamlouli K., Chtouki M., Zeroual Y., Dhiba D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management System. Front. Microbiol. 2018;9:1606. doi: 10.3389/fmicb.2018.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santhosh K.M., Chandramohan G., Mamta P., Santosh K. Role of bio-fertilizers towards sustainable agricultural development: A review. J. Pharmacogn. Phytochem. 2018;7:1915–1921. [Google Scholar]
- 12.Narendra K., Rakesh K., Sudhir K., Vijay S.M., Meena V.S. Agriculturally Important Microbes for Sustainable Agriculture. Springer; Singapore: 2017. Nutrient solubilizing microbes (NSMs): It’s role in sustainable crop production; pp. 25–61. [Google Scholar]
- 13.Qiu Z., Egidi E., Liu H., Kaur S., Singh B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019;37:107371. doi: 10.1016/j.biotechadv.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Afzal I., Shinwari Z.K., Sikandar S., Shahzad S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Tsukanova K.A., Chebotar V.K., Meyer J.J.M., Bibikova T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017;113:91–102. doi: 10.1016/j.sajb.2017.07.007. [DOI] [Google Scholar]
- 16.Sokol N.W., Slessarev E., Marschmann G.L., Nicolas A., Blazewicz S.J., Brodie E.L., Firestone M.K., Foley M.M., Hestrin R., Hungate B.A., et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022;20:415–430. doi: 10.1038/s41579-022-00695-z. [DOI] [PubMed] [Google Scholar]
- 17.Cappelli S.L., Domeignoz-Horta L.A., Loaiza V., Laine A. Plant biodiversity promotes sustainable agriculture directly and via belowground effects. Trend Plant Sci. 2022;27:674–687. doi: 10.1016/j.tplants.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Bhat M.A., Kumar V., Bhat M.A., Wani I.A., Dar F.L., Farooq I., Bhatti F., Koser R., Rahman S., Jan A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front. Microbiol. 2020;11:1952. doi: 10.3389/fmicb.2020.01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upadhyay S.K., Srivastava A.K., Rajput V.D., Chauhan P.K., Bhojiya A.A., Jain D., Chaubey G., Dwivedi P., Sharma B., Minkina T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Front. Microbiol. 2022;13:916488. doi: 10.3389/fmicb.2022.916488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R., Vivanco J.M., Shen Q. The unseen rhizosphere root-soil-microbe interactions for crop production. Curr. Opin. Microbiol. 2017;37:8–14. doi: 10.1016/j.mib.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Musilova L., Ridl J., Polivkova M., Macek T., Uhlik O. Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016;17:1205. doi: 10.3390/ijms17081205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badri D.V., Chaparro J.M., Zhang R., Shen Q., Vivanco J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 2013;288:4502–4512. doi: 10.1074/jbc.M112.433300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doornbos R.F., Geraats B.P., Kuramae E.E., Van Loon L.C., Bakker P.A. Effects of jasmonic acid, ethylene, and salicylic acid signaling on the rhizosphere bacterial community of Arabidopsis thaliana. Mol. Plant Microbe Interact. 2011;24:395–407. doi: 10.1094/MPMI-05-10-0115. [DOI] [PubMed] [Google Scholar]
- 24.Vocciante M., Grifoni M., Fusini D., Petruzzelli G., Franchi E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022;12:1231. doi: 10.3390/app12031231. [DOI] [Google Scholar]
- 25.Berger L.R., Stamford N.P., Santos C.E.R.S., Freitas A.D.S., Franco L.O., Stamford T.C.M. Plant and soil characteristics affected by biofertilizers from rocks and organic matter inoculated with diazotrophic bacteria and fungi that produce chitosan. J. Soil Sci. Plant Nutr. 2013;13:592–603. doi: 10.4067/S0718-95162013005000047. [DOI] [Google Scholar]
- 26.Pii Y., Mimmo T., Tomasi N., Terzano R., Cesco S., Crecchio C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils. 2015;51:403–415. doi: 10.1007/s00374-015-0996-1. [DOI] [Google Scholar]
- 27.Mia M.A.B., Hossain M.M., Shamsuddin Z.H., Islam M.T. Bacteria in Agrobiology: Crop Productivity. Springer; Berlin/Heidelberg, Germany: 2013. Plant-associated bacteria in nitrogen nutrition in crops, with special reference to rice and banana; pp. 97–126. [Google Scholar]
- 28.Oke V., Long S.R. Bacteroid formation in the Rhizobium–legume symbiosis. Curr. Opin. Microbiol. 1999;2:641–646. doi: 10.1016/S1369-5274(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 29.Vessey J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- 30.Bashan Y., de-Bashan L.E. Inoculant preparation and formulations for Azospirillum spp. In: Cassán F., Okon Y., Creus C., editors. Handbook for Azospirillum. Springer; Berlin/Heidelberg, Germany: 2015. pp. 469–485. [Google Scholar]
- 31.Beattie G.A. Microbiomes: Curating communities from plants. Nature. 2015;528:340–341. doi: 10.1038/nature16319. [DOI] [PubMed] [Google Scholar]
- 32.Sindhu S.S., Dua S., Verma M.K., Khandelwal A. Microbes for Legume Improvement. Springer; Vienna, Austria: 2010. Growth promotion of legumes by inoculation of rhizosphere bacteria; pp. 195–235. [Google Scholar]
- 33.Backer R.G., Saeed W., Seguin P., Smith D.L. Root traits and nitrogen fertilizer recovery efficiency of corn grown in biochar-amended soil under greenhouse conditions. Plant Soil. 2017;415:465–477. doi: 10.1007/s11104-017-3180-6. [DOI] [Google Scholar]
- 34.Askeland R.A., Morrison S.M. Cyanide production by Pseudomonas fluorescens and Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1983;45:1802–1807. doi: 10.1128/aem.45.6.1802-1807.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chlumsky L.J., Zhang L., Jorns M.S. Sequence analysis of sarcosine oxidase and nearby genes reveals homologies with key enzymes of folate one-carbon metabolism. J. Biol. Chem. 1995;270:18252–18259. doi: 10.1074/jbc.270.31.18252. [DOI] [PubMed] [Google Scholar]
- 36.Rijavec T., Lapanje A. Hydrogen cyanide in the rhizosphere: Not suppressing plant pathogens, but rather regulating availability of phosphate. Front. Microbiol. 2016;7:1785. doi: 10.3389/fmicb.2016.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neilands J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 38.Saha M., Sarkar S., Sarkar B., Sharma B.K., Bhattacharjee S., Tribedi P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016;23:3984–3999. doi: 10.1007/s11356-015-4294-0. [DOI] [PubMed] [Google Scholar]
- 39.Tester M., Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacoby R.P., Che-Othman M.H., Millar A.H., Taylor N.L. Analysis of the sodium chloride-dependent respiratory kinetics of wheat mitochondria reveals differential effects on phosphorylating and non-phosphorylating electron transport pathways. Plant Cell Environ. 2016;39:823–833. doi: 10.1111/pce.12653. [DOI] [PubMed] [Google Scholar]
- 41.Ilangumaran G., Smith D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017;8:1768. doi: 10.3389/fpls.2017.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morcillo R.J.L., Manzanera M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites. 2021;11:337. doi: 10.3390/metabo11060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodríguez-Navarro A., Rubio F. High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 2006;57:1149–1160. doi: 10.1093/jxb/erj068. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra J., Fatima T., Arora N.K. Plant Microbiome: Stress Response. Springer; Singapore: 2018. Role of secondary metabolites from plant growth-promoting rhizobacteria in combating salinity stress; pp. 127–163. [Google Scholar]
- 46.Sandhya V.S., Ali S.Z., Grover M., Reddy G., Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. On compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010;62:21–30. doi: 10.1007/s10725-010-9479-4. [DOI] [Google Scholar]
- 47.Marulanda A., Azcon R., Chaumont F., Ruiz-Lozano J.M., Aroca R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta. 2010;232:533–543. doi: 10.1007/s00425-010-1196-8. [DOI] [PubMed] [Google Scholar]
- 48.Upadhyay S.K., Singh D.P. Effect of salt-tolerant plant growth promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015;17:288–293. doi: 10.1111/plb.12173. [DOI] [PubMed] [Google Scholar]
- 49.Kumari S., Vaishnav A., Jain S., Varma A., Choudhary D.K. Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill) J. Plant Growth Regulat. 2015;34:558–573. doi: 10.1007/s00344-015-9490-0. [DOI] [Google Scholar]
- 50.Karimi E., Aliasgharzad N., Esfandiari E., Hassanpouraghdam M.B., Neu T.R., Buscot F., Reitz T., Breitkreuz C., Tarkka M.T. Biofilm forming rhizobacteria affect the physiological and biochemical responses of wheat to drought. AMB Expr. 2022;12:93. doi: 10.1186/s13568-022-01432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fahad S., Hussain S., Bano A., Saud S., Hassan S., Shan D., Khan F.A., Khan F., Chen Y., Wu C., et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015;22:4907–4921. doi: 10.1007/s11356-014-3754-2. [DOI] [PubMed] [Google Scholar]
- 52.Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microb. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 53.Cassán F., Perrig D., Sgroy V., Masciarelli P., Penna C., Luna V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L. and soybean (Glycine max L.) Eur. J. Soil Biol. 2009;45:28–35. doi: 10.1016/j.ejsobi.2008.08.005. [DOI] [Google Scholar]
- 54.Zhang H., Kim M.-S., Krishnamachari V., Payton P., Sun Y., Grimson M., Farag M.A., Ryu C.-M., Allen R., Melo I.S., et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta. 2007;226:839–851. doi: 10.1007/s00425-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 55.Saleem S., Iqbal A., Ahmed F., Ahmad M. Phytobeneficial and salt stress mitigating efficacy of IAA producing salt tolerant strains in Gossypium hirsutum. Saudi J. Biol. Sci. 2021;28:5317–5324. doi: 10.1016/j.sjbs.2021.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Llorente B.E., Alasia M.A., Larraburu E.E. Biofertilization with Azospirillum brasilense improves in vitro culture of Handroanthus ochraceus, a forestry, ornamental and medicinal plant. Nat. Biotechnol. 2016;33:32–40. doi: 10.1016/j.nbt.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Kang S.M., Radhakrishnan R., Khan A.L., Kim M.J., Park J.M., Kim B.R., Shin D.H., Lee I.J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Shahzad R., Waqas M., Khan A.L., Asaf S., Khan M.A., Kang S.M., Lee I.J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016;106:236–243. doi: 10.1016/j.plaphy.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Martínez C., Espinosa-Ruiz A., Prat S. Gibberellins and plant vegetative growth. Annu. Plant Rev. 2016;49:285–322. [Google Scholar]
- 60.Cassán F., Vanderleyden J., Spaepen S. Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J. Plant Growth Regulat. 2014;33:440–459. doi: 10.1007/s00344-013-9362-4. [DOI] [Google Scholar]
- 61.Kapoor R., Kaur M. Cytokinins production by fluorescent Pseudomonas isolated from rhizospheric soils of Malus and Pyrus. Afr. J. Microbiol. Res. 2016;10:1274–1279. [Google Scholar]
- 62.Arkhipova T.N., Prinsen E., Veselov S.U., Martinenko E.V., Melentiev A.I., Kudoyarova G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil. 2007;292:305–315. doi: 10.1007/s11104-007-9233-5. [DOI] [Google Scholar]
- 63.Cohen A.C., Bottini R., Pontin M., Berli F.J., Moreno D., Boccanlandro H., Travaglia C.N., Piccoli P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015;153:79–90. doi: 10.1111/ppl.12221. [DOI] [PubMed] [Google Scholar]
- 64.Kaushal M., Wani S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2015;66:35–42. doi: 10.1007/s13213-015-1112-3. [DOI] [Google Scholar]
- 65.Vejan P., Abdullah R., Khadiran T., Ismail S., Nasrulhaq B.A. Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules. 2016;21:573. doi: 10.3390/molecules21050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heydarian Z., Yu M., Gruber M., Glick B.R., Zhou R., Hegedus D.D. Inoculation of soil with plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acds gene in transgenic plants increases salinity tolerance in camelina sativa. Front. Microbiol. 2016;7:1966. doi: 10.3389/fmicb.2016.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmad P., Rasool S., Gul A., Sheikh S.A., Akram N.A., Ashraf M., Gucel S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016;7:813. doi: 10.3389/fpls.2016.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J., Yan Z., Li X. Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol. Environ. Saf. 2014;104:349–356. doi: 10.1016/j.ecoenv.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 69.Sun Q.P., Yu Y.K., Wan S.X., Zhao F.K., Hao Y.L. Is there crosstalk between extracellular and intracellular calcium mobilization in jasmonic acid signaling. Plant Growth Regulat. 2009;57:7–13. doi: 10.1007/s10725-008-9317-0. [DOI] [Google Scholar]
- 70.Bordiec S., Paquis S., Lacroix H., Dhondt S., Ait Barka E., Kauffmann S., Dorey S. Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J. Exp. Bot. 2011;62:595–603. doi: 10.1093/jxb/erq291. [DOI] [PubMed] [Google Scholar]
- 71.Li Y., Gu Y., Li J., Xu M., Wei Q., Wang Y. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front. Microbiol. 2015;6:883. doi: 10.3389/fmicb.2015.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhat M.A., Bhat M.A., Kumar V., Wani I.A., Bashir H., Shah A.A., Rahman S., Jan A.T. The era of editing plant genomes using CRISPR/Cas: A critical appraisal. J. Biotechnol. 2020;20:34–60. doi: 10.1016/j.jbiotec.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Nadeem M., Li J., Yahya M., Sher A., Ma C., Wang X., Qiu L. Research progress and perspective on drought stress in legumes: A review. IJMS. 2019;20:2541. doi: 10.3390/ijms20102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chowdhury J.A., Karim M.A., Khaliq Q.A., Ahmed A.U., Khan M.S.A. Effect of drought stress on gas exchange characteristics of four soybean genotypes. Bang J. Agric. Res. 2016;41:195–205. doi: 10.3329/bjar.v41i2.28215. [DOI] [Google Scholar]
- 75.Chibuike G.U., Obiora S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014;2014:752708. doi: 10.1155/2014/752708. [DOI] [Google Scholar]
- 76.Marchant B.P., Saby N.P.A., Arrouays D. A survey of topsoil arsenic and mercury concentrations across France. Chemosphere. 2017;181:635–644. doi: 10.1016/j.chemosphere.2017.04.106. [DOI] [PubMed] [Google Scholar]
- 77.He X., Xu M., Wei Q., Tang M., Guan L., Lou L., Xu X., Hu Z., Chen Y., Shen Z., et al. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2020;205:111333. doi: 10.1016/j.ecoenv.2020.111333. [DOI] [PubMed] [Google Scholar]
- 78.Deng Z., Cao L. Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere. 2017;168:1100–1106. doi: 10.1016/j.chemosphere.2016.10.097. [DOI] [PubMed] [Google Scholar]
- 79.Zainab N., Khan A.A., Azeem M.A., Ali B., Wang T., Shi F., Alghanem S.M., Hussain Munis M.F., Hashem M., Alamri S., et al. PGPR-Mediated Plant Growth Attributes and Metal Extraction Ability of Sesbania sesban L. in Industrially Contaminated Soils. Agronomy. 2021;11:1820. doi: 10.3390/agronomy11091820. [DOI] [Google Scholar]
- 80.Khanna K., Jamwal V.L., Gandhi S.G., Ohri P., Bhardwaj R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019;9:5855. doi: 10.1038/s41598-019-41899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh R., Soni S.K., Patel R.P., Kalra A. Technology for improving essential oil yield of Ocimum basilicum L. (sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind. Crops Prod. 2013;45:335–342. doi: 10.1016/j.indcrop.2013.01.003. [DOI] [Google Scholar]
- 82.Mayak S., Tirosh T., Glick B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004;42:565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Del Amor F.M., Cuadra-Crespo P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2012;39:82–90. doi: 10.1071/FP11173. [DOI] [PubMed] [Google Scholar]
- 84.Cohen A.C., Bottini R., Piccoli P.N. Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Regul. 2008;54:97–103. doi: 10.1007/s10725-007-9232-9. [DOI] [Google Scholar]
- 85.Ali S.K.Z., Sandhya V., Minakshi G., Kishore N., Rao L.V., Venkateswarlu B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils. 2009;46:45–55. doi: 10.1007/s00374-009-0404-9. [DOI] [Google Scholar]
- 86.Ait-Barka E., Nowak J., Clément C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2007;72:7246–7252. doi: 10.1128/AEM.01047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng Z., Park E., Glick B.R. 1-Aminocyclopropane-1-carboxylate (ACC) deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 2007;53:912–918. doi: 10.1139/W07-050. [DOI] [PubMed] [Google Scholar]
- 88.Silva R., Filgueiras L., Santos B., Coelho M., Silva M., Estrada-Bonilla G., Vidal M., Baldani J.I., Meneses C. Gluconacetobacter diazotrophicus changes the molecular mechanisms of root development in Oryza sativa L. growing under water stress. Int. J. Mol. Sci. 2020;21:333. doi: 10.3390/ijms21010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chandra R., Amit , Ghosh U.K. Effects of various abiotic factors on biomass growth and lipid yield of Chlorella minutissima for sustainable biodiesel production. Environ. Sci. Pollut. Res. 2019;26:3848–3861. doi: 10.1007/s11356-018-3696-1. [DOI] [PubMed] [Google Scholar]
- 90.Niu X., Song L., Xiao Y., Ge W. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Associated with Foxtail Millet in a Semi-arid Agro ecosystem and Their Potential in Alleviating Drought Stress. Front. Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forni C., Duca D., Glick B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410:335–356. [Google Scholar]
- 92.Barnawal D., Bharti N., Pandey S.S., Pandey A., Chanotiya S.C., Kalra A. Plant growth promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017;161:502–514. doi: 10.1111/ppl.12614. [DOI] [PubMed] [Google Scholar]
- 93.Curá J.A., Franz D.R., Filosofía J.E., Balestrasse K.B., Burgueño L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms. 2017;5:41. doi: 10.3390/microorganisms5030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.García J.E., Maronicheb G., Creusb C., Suárez-Rodríguezd R., Ramirez-Trujillod J.A., Groppae M.D. In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol. Res. 2018;202:21–29. doi: 10.1016/j.micres.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Pourbabaee A.A., Bahmani E., Alikhani H.A., Emami S. Promotion of wheat growth under salt stress by halotolerant bacteria containing ACC deaminase. JAST. 2016;18:855–864. [Google Scholar]
- 96.Vurukonda S.S.K.P., Vardharajula S., Shrivastava M. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 97.Gontia-Mishra I., Sapre S., Sharma A., Tiwari S. Amelioration of drought tolerance in wheat by the interaction of plant growth promoting rhizobacteria. Plant Biol. 2016;18:992–1000. doi: 10.1111/plb.12505. [DOI] [PubMed] [Google Scholar]
- 98.Belimov A.A., Dodd I.C., Hontzeas N., Theobald J.C., Safronova V.I., Davies W.J. Rhizosphere bacteria containing 1-aminocyclopropane-1- carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signaling. New Phytol. 2009;181:413–423. doi: 10.1111/j.1469-8137.2008.02657.x. [DOI] [PubMed] [Google Scholar]
- 99.Bresson J., Varoquaux F., Bontpart T., Vile B.T.D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013;200:558–569. doi: 10.1111/nph.12383. [DOI] [PubMed] [Google Scholar]
- 100.Gururani M.A., Upadhyaya C.P., Baskar V., Venkatesh J., Nookaraju A., Park S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2013;32:245–258. [Google Scholar]
- 101.Lim J.H., Kim S.D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013;29:201–208. doi: 10.5423/PPJ.SI.02.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodríguez-Salazar J., Suárez R., Caballero-Mellado J., Iturriaga G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009;296:52–59. doi: 10.1111/j.1574-6968.2009.01614.x. [DOI] [PubMed] [Google Scholar]
- 103.Wang C.-J., Yang W., Wang C., Gu C., Niu D.-D., Liu H.-X., Wang Y.-P., Guo J.-H. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth-Promoting Rhizobacterium Strains. PLoS ONE. 2012;7:e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arzanesh M.H., Alikhani H.A., Khavazi K., Rahimian H.A., Miransari M. Wheat (Triticum aestivum L. growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011;27:197–220. doi: 10.1007/s11274-010-0444-1. [DOI] [Google Scholar]
- 105.Vardharajula S., Zulfikar Ali S., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.; effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011;6:1–14. doi: 10.1080/17429145.2010.535178. [DOI] [Google Scholar]
- 106.Pereyra M.A., García P., Colabelli M.N., Barassi C.A., Creus C.M. A better water status in wheat seedlings induced by Azospirillum under osmotic stress is related to morphological changes in xylem vessels of the coleoptiles. Appl. Soil Ecol. 2012;53:94–97. doi: 10.1016/j.apsoil.2011.11.007. [DOI] [Google Scholar]
- 107.Zhang H., Murzello C., Sun Y., Kim M.S., Xie X., Jeter R.M., Zak J.C., Dowd S.E., Paré P.W. Choline and osmotic-stress tolerance induced in arabidopsis by the soil microbe bacillus subtilis (GB03) Mol. Plant-Microbe Interact. 2010;23:1097–1104. doi: 10.1094/MPMI-23-8-1097. [DOI] [PubMed] [Google Scholar]
- 108.Kohler J., Hernández J.A., Caravaca F., Roldán A. Plant-growth promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water stressed plants. Funct. Plant Biol. 2008;35:141–151. doi: 10.1071/FP07218. [DOI] [PubMed] [Google Scholar]
- 109.McLellan C.A., Turbyville T.J., Wijeratne E.M., Kerschen A., Vierling E., Queitsch C. A rhizosphere fungus enhances Arabidopsis thermotolerance through production of an HSP90 inhibitor. Plant Physiol. 2007;145:174–182. doi: 10.1104/pp.107.101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H., Xie X., Kim M.S., Kormyeyev D.A., Holaday S., Paré P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008;56:264–273. doi: 10.1111/j.1365-313X.2008.03593.x. [DOI] [PubMed] [Google Scholar]
- 111.Alami Y., Achouak W., Marol C., Heulin T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000;66:3393–3398. doi: 10.1128/AEM.66.8.3393-3398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Timmusk S., Wagner E.G.H. The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: A possible connection between biotic and abiotic stress responses. Mol. Plant Microbe Interact. 1999;12:951–959. doi: 10.1094/MPMI.1999.12.11.951. [DOI] [PubMed] [Google Scholar]
- 113.Sen S., Chandrasekhar C. Effect of PGPR on enzymatic activities of rice (Oryza sativa L.) under salt stress. Asian J. Plant Sci. Res. 2015;5:44–48. [Google Scholar]
- 114.Barnawal D., Bharti N., Maji D., Chanotiya C.S., Kalra A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J. Plant Physiol. 2014;171:884–894. doi: 10.1016/j.jplph.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 115.Han Q.Q., Lü X.P., Bai J.P., Qiao Y., Paré P.W., Wang S.M. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014;5:525. doi: 10.3389/fpls.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bal H.B., Nayak L., Das S., Adhya T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil. 2013;366:93–105. [Google Scholar]
- 117.Awad N.M., Turky A.S., Abdelhamid M.T., Attia M. Ameliorate of environmental salt stress on the growth of Zea mays L. plants by exopolysaccharides producing bacteria. J. Appl. Sci. Res. 2012;8:2033–2044. [Google Scholar]
- 118.Jha B., Sharma A., Mishra A. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol. Biol. Rep. 2011;38:4823–4832. doi: 10.1007/s11033-010-0625-x. [DOI] [PubMed] [Google Scholar]
- 119.Siddikee M.A., Glick B.R., Chauhan P.S., Jong Y.W. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011;49:427–434. doi: 10.1016/j.plaphy.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 120.Egamberdieva D., Kucharova Z. Selection for root colonizing bacteria stimulating wheat growth in saline soils. Biol. Fert. Soils. 2009;45:561–573. doi: 10.1007/s00374-009-0366-y. [DOI] [Google Scholar]
- 121.Bano A., Fatima M. Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fert. Soils. 2009;45:405–413. doi: 10.1007/s00374-008-0344-9. [DOI] [Google Scholar]
- 122.Bianco C., Defez R. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 2009;60:3097–3107. doi: 10.1093/jxb/erp140. [DOI] [PubMed] [Google Scholar]
- 123.Waller F., Mukherjee K., Deshmukh S.D., Achatz B., Sharma M., Schäfer P., Kogel K.-H. Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. J. Plant Physiol. 2008;165:60–70. doi: 10.1016/j.jplph.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 124.Egamberdieva D., Kamilova F., Validov S., Gafurova L., Kucharova Z., Lugtenberg B. High incidence of plant growth stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ. Microbiol. 2008;10:1–9. doi: 10.1111/j.1462-2920.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 125.Saravanakumar D., Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbiol. 2007;102:1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 126.Han H.S., Lee K.D. Physiological responses of soybean inoculation of Brady rhizobium japonicum with PGPR in saline soil conditions. Res. J. Agric. Biol. Sci. 2005;1:216–221. [Google Scholar]
- 127.Hamdia A.B.E., Shaddad M.A.K., Doaa M.M. Mechanisms of salt tolerance and interactive effects of Azospirillum brasiliense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 2004;44:165–174. doi: 10.1023/B:GROW.0000049414.03099.9b. [DOI] [Google Scholar]
- 128.Mishra J., Prakash J., Arora N.K. Role of beneficial soil microbes in sustainable agriculture and environmental management. Clim. Chang. Environ. Sustain. 2016;4:137–149. doi: 10.5958/2320-642X.2016.00015.6. [DOI] [Google Scholar]
- 129.Mishra J., Singh R., Arora N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front. Microbiol. 2017;8:1706. doi: 10.3389/fmicb.2017.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yavar A., Sarmani S., Hamzah A., Khoo K.S. Phytoremediation of mercury contaminated soil using Scripus mucronatus exposed by bacteria. Int. Conf. Agric. Ecol. Med. Sci. 2014;7:6. [Google Scholar]
- 131.Reichman S.M. Probing the plant growth-promoting and heavy metal tolerance characteristics of Bradyrhizobium japonicum CB1809. Eur. J. Soil Biol. 2014;63:7–13. doi: 10.1016/j.ejsobi.2014.04.001. [DOI] [Google Scholar]
- 132.Wani P.A., Khan M.S. Bioremediaiton of lead by a plant growth promoting Rhizobium species RL9. Bacteriol. J. 2012;2:66–78. doi: 10.3923/bj.2012.66.78. [DOI] [Google Scholar]
- 133.Wu S.C., Cheung K.C., Luo Y.M., Wong M.H. Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environ. Policy. 2006;140:124–135. doi: 10.1016/j.envpol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 134.Zaidi S., Usmani S., Singh B.R., Musarrat J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991–997. doi: 10.1016/j.chemosphere.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 135.Fragkostefanakis S., Simm S., Paul P., Bublak D., Scharf K.D., Schleiff E. Chaperone network composition in Solanum lycopersicum explored by transcriptome profiling and microarray metaanalysis. Plant Cell Environ. 2015;38:693–709. doi: 10.1111/pce.12426. [DOI] [PubMed] [Google Scholar]
- 136.Chang W.-S., van de Mortel M., Nielsen L., de Guzman G.N., Li X., Halverson L.J. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water limiting conditions. J. Bacteriol. 2007;189:8290–8299. doi: 10.1128/JB.00727-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bensalim S., Nowak J., Asiedu S.K. A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am. J. Potato Res. 1998;75:145–152. doi: 10.1007/BF02895849. [DOI] [Google Scholar]
- 138.Meena H., Ahmed A., Prakash P. Amelioration of heat stress in wheat, Triticum aestivum by PGPR (Pseudomonas aeruginosa strain 2CpS1) Biosci. Biotech. Res. Commun. 2015;8:171–174. [Google Scholar]
- 139.Dardanelli M.S., Carletti S.M., Paulucci N.S., Medeot D.B., Rodriguez-Caceres E.A., Vita F.A., Bueno M., Fumero M.V., Garcia M.B. Benefits of Plant growth-Promoting Rhizobacteria and Rhizobia in Agriculture. In: Maheshwari D.K., editor. Microbiology Monographs Plant Growth and Health Promoting Bacteria. Springer; Berlin/Heidelberg, Germany: 2010. [Google Scholar]
- 140.Rahdari P., Tavakoli S., Hosseini S.M. Studying of salinity stress effect on germination, proline, sugar, protein, lipid and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Stress Physiol. Biochem. 2012;8:182–193. [Google Scholar]
- 141.Jan A.T., Azam M., Siddiqui K., Ali A., Choi I., Haq Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. IJMS. 2015;16:29592–29630. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nair A.S., Abraham T.K., Jaya D.S. Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculata L.) varieties. J. Environ. Biol. 2008;29:689–691. [PubMed] [Google Scholar]
- 143.Farooq M., Farooq M., Hussain M., Siddique K.H.M. Drought stress in wheat during flowering and grain- filling periods. Crit. Rev. Plant Sci. 2014;33:331–349. doi: 10.1080/07352689.2014.875291. [DOI] [Google Scholar]
- 144.Gujjar R.S., Karkute S.G., Rai A., Singh M., Singh B. Proline-rich proteins may regulate free cellular proline levels during drought stress in tomato. Curr. Sci. 2018;14:915–920. doi: 10.18520/cs/v114/i04/915-920. [DOI] [Google Scholar]
- 145.Shinde S., Villamor J.G., Lin W., Sharma S., Verslues P.E. Proline co-ordination with fatty acid synthesis and redox metabolism of chloroplast and mitochondria. Plant Physiol. 2016;172:1074–1088. doi: 10.1104/pp.16.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bhat M.A., Mir R.A., Kumar V., Shah A.A., Zargar S.M., Rahman S., Jan A.T. Mechanistic insights of CRISPR/Cas-mediated genome editing towards enhancing abiotic stress tolerance in plants. Physiol. Plant. 2021;172:1255–1268. doi: 10.1111/ppl.13359. [DOI] [PubMed] [Google Scholar]
- 147.Nadeem S.M., Zahir Z.A., Naveed M., Asghar H.N., Arshad M. Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci. Soc. Am. J. 2010;74:533–542. doi: 10.2136/sssaj2008.0240. [DOI] [Google Scholar]
- 148.Khan N., Bano A., Ali S., Babar M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020;90:189–203. doi: 10.1007/s10725-020-00571-x. [DOI] [Google Scholar]
- 149.Amna S., Sarfraz B., Din Y., Xia M.A., Kamran M.T., Javed T.S., Chaudhary H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing bacillus strains under induced salinity stress. Ecol. Toxicol. Environ. Saf. 2019;183:109466. doi: 10.1016/j.ecoenv.2019.109466. [DOI] [PubMed] [Google Scholar]
- 150.Mohamed I., Eid K.E., Abbas M.H., Salem A.A., Ahmed N., Ali M., Fang C. Use of plant growth promoting Rhizobacteria (PGPR) and mycorrhizae to improve the growth and nutrient utilization of common bean in a soil infected with white rot fungi. Ecotoxicol. Environm. Saf. 2019;171:539–548. doi: 10.1016/j.ecoenv.2018.12.100. [DOI] [PubMed] [Google Scholar]
- 151.Ashry N.M., Alaidaroos B.A., Mohamed S.A., Badr O.A.M., El-Saadony M.T., Esmael A. Utilization of drought-tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR) Saudi J. Biol. Sci. 2022;29:1760–1769. doi: 10.1016/j.sjbs.2021.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu F., Xing S., Ma H. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013;97:9155–9164. doi: 10.1007/s00253-013-5193-2. [DOI] [PubMed] [Google Scholar]
- 153.Xu Z., Pan G., Zhou H., Shen B. Discovery and Characterization of 1-Aminocyclopropane-1-carboxylic Acid Synthase of Bacterial Origin. J. Am. Chem. Soc. 2018;140:16957–16961. doi: 10.1021/jacs.8b11463. [DOI] [PubMed] [Google Scholar]
- 154.Armada E., Probanza A., Roldán A., Azcón R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2016;192:1–12. doi: 10.1016/j.jplph.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 155.Radhakrishnan R., Kang S.M., Baek I.Y., Lee I.J. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J. Plant Interact. 2014;9:754–762. doi: 10.1080/17429145.2014.930524. [DOI] [Google Scholar]
- 156.Bano Q.U., Ilyas N., Bano A., Zafar Z.A., Akram A.B., Hassan F. Effect of azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013;45:13–20. [Google Scholar]
- 157.Gusain Y.S., Singh U.S., Sharma A.K. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.) Afr. J. Biotechnol. 2015;14:764–773. [Google Scholar]
- 158.Shintu P.V., Jayaram K.M. Phosphate solubilising bacteria (Bacillus polymyxa)-an effective approach to mitigate drought in tomato (Lycopersicon esculentum Mill) Trop. Plant Res. 2015;2:17–22. [Google Scholar]
- 159.Casanovas E.M., Barassi C.A., Sueldo R.J. Azospirillum inoculation mitigates water stress effects in maize seedlings. Cereal Res. Commun. 2020;30:343–350. doi: 10.1007/BF03543428. [DOI] [Google Scholar]
- 160.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 161.Schirawski J., Perlin M.H. Plant–microbe interaction 2017-the good, the bad and the diverse. IJMS. 2018;19:1374. doi: 10.3390/ijms19051374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sobhanian H., Aghaei K., Komatsu S. Changes in the plant proteome resulting from salt stress: Toward the creation of salt-tolerant crops? J. Proteom. 2011;74:1323–1337. doi: 10.1016/j.jprot.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 163.Jan A.T., Azam M., Ali A., Haq Q.M.R. Novel approaches of beneficial Pseudomonas in mitigation of plant disease: An appraisal. J. Plant Interact. 2011;6:195–205. doi: 10.1080/17429145.2010.541944. [DOI] [Google Scholar]
- 164.Yang A., Akhtar S.S., Iqbal S., Amjad M., Naveed M., Zahir Z.A. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016;43:632–642. doi: 10.1071/FP15265. [DOI] [PubMed] [Google Scholar]
- 165.Miransari M., Smith D. Alleviating salt stress on soybean (Glycine max L. (Merr.))-Bradyrhizobium japonicum symbiosis, using signal molecule genistein. Eur. J. Soil Biol. 2009;45:146–152. doi: 10.1016/j.ejsobi.2008.11.002. [DOI] [Google Scholar]
- 166.Atouei M.T., Pourbabaee A.A., Shorafa M. Alleviation of salinity stress on some growth parameters of wheat by exopolysaccharide-producing bacteria. Iran. J. Sci. Technol. Trans. A. 2019;43:2725–2733. doi: 10.1007/s40995-019-00753-x. [DOI] [Google Scholar]
- 167.Jalili F., Khavazi K., Pazira E., Nejati A., Rahmani H.A., Sadaghiani H.R., Miransari M. Isolation and characterization of ACC deaminase-producing fluorescent pseudomonads, to alleviate salinity stress on canola (Brassica napus L.) growth. J. Plant Physiol. 2009;166:667–674. doi: 10.1016/j.jplph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 168.Palaniyandi S.A., Damodharan K., Yang S.H., Suh J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014;117:766–773. doi: 10.1111/jam.12563. [DOI] [PubMed] [Google Scholar]
- 169.Sharma K., Sharma S., Vaishnav A., Jain R., Singh D., Singh H.B., Goel A., Singh S. Salt-tolerant PGPR strain Priestia endophytica SK1 promotes fenugreek growth under salt stress by inducing nitrogen assimilation and secondary metabolites. J. Appl Microbiol. 2022;133:2802–2813. doi: 10.1111/jam.15735. [DOI] [PubMed] [Google Scholar]
- 170.Shabaan M., Asghar H.N., Zahir Z.A., Zhang X., Sardar M.F., Li H. Salt-Tolerant PGPR Confer Salt Tolerance to Maize Through Enhanced Soil Biological Health, Enzymatic Activities, Nutrient Uptake and Antioxidant Defense. Front. Microbiol. 2022;13:901865. doi: 10.3389/fmicb.2022.901865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fu Q., Liu C., Ding N., Guo B. Ameliorative effects of inoculation with the plant growth promoting rhizobacterium pseudomonas sp. DW1 on growth of eggplant (Solanum melongena L.) seedlings under salt stress. Agric. Water Manag. 2010;97:1994–2000. doi: 10.1016/j.agwat.2010.02.003. [DOI] [Google Scholar]
- 172.Flores J.D., Silva L.R., Rivera L.P. Plants probiotics as a tool to produce highly functional fruits: The case of Phyllo bacterium and vitamin C in strawberries. PLoS ONE. 2015;10:e012228. doi: 10.1371/journal.pone.0122281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Habib S.H., Kausar H., Saud H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed. Res. Int. 2016;2016:6284547. doi: 10.1155/2016/6284547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.