Abstract
Background:
We investigated the influence preexisting type 2 diabetes mellitus (T2DM) and antidiabetic drugs have on all-cause and cause-specific mortality among Medicaid-insured women diagnosed with breast cancer.
Methods:
9221 women aged < 64 years diagnosed with breast cancer and reported to the New York State (NYS) Cancer Registry from 2004 to 2016 were linked with Medicaid claims. Preexisting T2DM was determined by three diagnosis claims for T2DM with at least one claim prior to breast cancer diagnosis and a prescription claim for an antidiabetic drug within three months following breast cancer diagnosis. Estimated menopausal status was determined by age (premenopausal age < 50; postmenopausal age ≥50). Hazard ratios (HR) and 95 % confidence intervals (95 %CI) were calculated with Cox proportional hazards regression, adjusting for confounders.
Results:
Women with preexisting T2DM had greater all-cause (HR = 1.40; 95 %CI 1.21, 1.63), cancer-specific (HR = 1.24; 95 %CI 1.04, 1.47), and cardiovascular-specific (HR = 2.46; 95 %CI 1.54, 3.90) mortality hazard compared to nondiabetic women. In subgroup analyses, the association between T2DM and all-cause mortality was found among non-Hispanic White (HR 1.78 95 %CI 1.38, 2.30) and postmenopausal (HR = 1.47; 95 %CI 1.23, 1.77) women, but not among other race/ethnicity groups or premenopausal women. Additionally, compared to women prescribed metformin, all-cause mortality hazard was elevated among women prescribed sulfonylurea (HR = 1.44; 95 %CI 1.06, 1.94) or insulin (HR = 1.54; 95 %CI 1.12, 2.11).
Conclusion:
Among Medicaid-insured women with breast cancer, those with preexisting T2DM have an increased mortality hazard, especially when prescribed sulfonylurea or insulin. Further research is warranted to determine the role antidiabetic drugs have on survival among women with breast cancer.
Keywords: Medicaid, Cancer registry, Diabetes, Breast cancer, Cardiovascular, Mortality, Glucose-lowering drugs
1. Introduction
Breast cancer and type 2 diabetes mellitus (T2DM) are prevalent diseases in the United States (U.S.) and associated with reduced quality of life. Over the past two decades, T2DM has alarmingly increased in adults and is associated with an increased risk of mortality from a wide range of sequelae of T2DM [1,2]. It is estimated that 8 %–20 % of women with breast cancer have comorbid T2DM [3-7].
The relationship between T2DM and breast cancer risk has been well documented in large epidemiological studies including an umbrella review of observational studies and meta-analyses [8]. Several meta-analyses reported that patients with T2DM had greater than a 15 % increased risk of subsequent breast cancer [3,9,10]. Emerging epidemiological studies have also suggested T2DM might contribute to increased mortality among breast cancer patients. Factors suggested to contribute to increased mortality risk include diabetes-related comorbidities, delay in breast cancer treatment, and altered treatment regimens [3,11-14]. Previous meta-analyses observed that patients with diabetes and breast cancer had poorer breast cancer prognosis and elevated risk of mortality [3,15].
Evidence from pharmacotherapy studies suggests the type of T2DM drug prescribed can increase or reduce mortality risk among breast cancer patients. Several meta-analyses found that metformin was protective against all-cause mortality [16,17]. Another study observed insulin was associated with increased risk of all-cause mortality while sulfonylureas had no influence [18]. In contrast, a study evaluating the influence glucose-lowering drugs have on mortality among breast cancer patients found no association for individuals with long-term use of sulfonylurea and insulin, but observed metformin was associated with reduced all-cause mortality [19].
In the general population, cardiovascular disease and cancer are leading causes of death among those diagnosed with T2DM or breast cancer [1,20,21]. However, most studies assessing mortality among women with breast cancer and T2DM examined all-cause mortality. Among the few studies that assessed cause-specific mortality, the majority examined cancer mortality, where findings were inconsistent [13,19,22,23]. These inconsistencies are potentially attributed to differences in how studies accounted for breast cancer treatment, anti-diabetic drugs, comorbidities, and timing of initial T2DM diagnosis in relation to breast cancer diagnosis.
Among the nonelderly population in the U.S., Medicaid-insured individuals have higher breast cancer mortality and elevated risk of T2DM-related complications compared to other insurance types [24-27]. Factors contributing to poorer health outcomes among the Medicaid-insured are lower screening rates, lower probability of having a sole continuous primary care physician, and lower likelihood of receiving recommended treatment [28-30]. For this reason, Medicaid-insured women with T2DM diagnosed with breast cancer are potentially a vulnerable population.
Medicaid provides health insurance coverage to economically disadvantaged individuals at low to no cost and collects complete detailed claims data on an individual’s encounter throughout the healthcare system including prescription claims, which are not available for most insurance types. In the present study, we investigated the association between preexisting T2DM and all-cause, cardiovascular-specific, and cancer-specific mortality among Medicaid-insured women diagnosed with breast cancer. We further assessed the impact the type of anti-diabetic drug prescribed had on mortality. This study presents a unique opportunity to investigate health outcomes among an underrepresented and historically understudied population. New York State (NYS) is the ideal population for this study due to having a large Medicaid-insured population that is racially and ethnically diverse.
2. Methods
2.1. Study design and data sources
The present cohort study was based on linked data from NYS Department of Health Cancer Registry and Medicaid Program. The Cancer Registry-Medicaid linkage allowed for assessment of cancer stage, vital status including causes of death, Medicaid enrollment, and healthcare utilization. Detailed information on the linkage method was previously published [31]. Briefly, individuals were linked to Medicaid enrollment, eligibility, encounter, and claims data by a unique Medicaid identification number. Women with histologically confirmed, first primary, invasive breast cancer (SEER site recode 26000) diagnosed between 2004 and 2016 were eligible. Women were excluded if they were not enrolled in Medicaid for at least 11 out of 12 months following breast cancer diagnosis.
2.2. Study population
We defined exposed individuals as women who had at least three claims containing International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9CM) or Tenth Revision (ICD-10) codes for T2DM (250.0–250.93, E11x) including one claim prior to first breast cancer diagnosis. Women were excluded if they did not have three diagnosis claims for T2DM with at least one claim prior to breast cancer diagnosis or no prescription claim for an antidiabetic drug within three months following breast cancer diagnosis. The unexposed group consisted of women with no ICD-9CM or ICD-10 codes indicating diabetes and no prescription claim for an antidiabetic drug. Comorbidities included as covariates in the analysis were stroke, chronic kidney disease, coronary heart disease, and obesity.
Treatment variables in this analysis included (1) antidiabetic drugs (metformin, sulfonylureas, insulin, other [incretin-based therapies, Meglitinides, Thiazolidinediones, Sodium-glucose co-transporter-2 inhibitors, Alpha-glucosidase inhibitors, Amylin], and combination drugs [two or more separate drugs for specific treatments related to T2DM]); and (2) breast cancer treatment (hormone therapy, surgery, radiation, and chemotherapy). We grouped certain antidiabetic drugs as “other” due to low number of women prescribed these drugs for monotherapy. Antidiabetic drugs were determined by prescription claims within three months after breast cancer diagnosis.
The study population’s demographic characteristics were obtained from the cancer registry and included age at diagnosis in years (continuous), race/ethnicity (Non-Hispanic Black, Non-Hispanic White, Non-Hispanic Asian/Pacific Islander, Non-Hispanic Other, Hispanic, Unknown), marital status (Single, Married/Domestic partner, Divorced/Separated, Widowed, Unknown), and date of death (continuous). Due to lack of information on menopausal status, age was used as a proxy estimate (premenopausal < 50 years of age and postmenopausal ≥50 years of age) [32-34].
Breast cancer characteristics included SEER Summary Stage (local, regional, distant), tumor grade, and molecular subtypes (estrogen receptor (ER), progesterone receptor, (PR), human growth factor-neu receptor (HER2), and unknown). In the present study, molecular subtypes were coded according to the North American Association of Central Cancer Registries standards and grouped by all possible ER, PR, and HER2 combinations. Additionally, ER and PR status were combined as a joint hormone receptor positive (HR+) status which were HR+ (ER−/PR+ or ER+/PR− or ER+/PR+) and HR−(ER−/PR−). The final categories used in the analysis were HR+/HER2−, HR+/HER2+, HR−/HER2+, and HR−/HER2− or “triple-negative”.
Cause-specific mortality was determined by ICD-10 codes for cardiovascular (I00-I99) and cancer (C00-C97) mortality, while all-cause was based on vital status (alive or dead).
2.3. Statistical analysis
Relative frequencies were calculated for categorical variables and means and standard deviations (SD) were calculated for continuous variables. Contingency tables and X2 tests were used to calculate the relationships between categorical variables. T2DM status prior to breast cancer was dichotomized (yes/no) and identified by comparing breast cancer date of diagnosis with first T2DM diagnosis claim date. For all-cause mortality, unadjusted cumulative incidence functions were determined using Kaplan-Meier and compared patients with T2DM and nondiabetics after breast cancer diagnosis using the log-rank test. This was repeated for cause-specific mortality and compared using Gray’s method [35]. Cox proportional hazards regression models were fit to assess the association of T2DM with all-cause and cause-specific mortality, while adjusting for confounders (age at diagnosis, breast cancer date of diagnosis, chemotherapy, coronary heart disease, molecular subtype, chronic kidney disease, marital status at diagnosis, estimated menopausal status, obesity, race/ethnicity, stroke, tumor stage, and days between first T2DM diagnosis claim and breast cancer date of diagnosis). Potential confounders were determined based on being associated with both T2DM and survival. Additionally, confounding variables could not be an intervening variable between T2DM and survival. Cox proportional hazards models were repeated for analyses stratified by race/ethnicity, estimated menopausal status, obesity status, SEER Summary Stage, and molecular subtype. Date of breast cancer diagnosis was used as the time point to estimate adjusted hazard ratios (HR) and 95 % confidence intervals (95 %CI), starting from January 1, 2004 (with independent left truncation on January 1, 2004) until death or right-censoring (December 31, 2016). All statistical tests were two-tailed. Statistical analyses were conducted using SAS software version 9.4.
3. Results
9221 women with breast cancer were included in the study. Of these individuals, 1477 had a diagnosis of preexisting T2DM. Table 1 presents the distributions of women by demographic characteristics and treatment. There were differences in T2DM prevalence across race/ethnicity and marital status categories (all p < 0.0001). Women with T2DM were older and more likely to be postmenopausal and obese (all p < 0.0001). Most women were diagnosed at local stage (p = 0.0008) and were HR+/HER2− (p = 0.0352).
Table 1.
Demographic and health characteristics of Medicaid-insured women diagnosed with breast cancer from 2004 to 2016 in New York State by type 2 diabetes mellitus status.
| Variables | Type 2 diabetes mellitus status | Total (n = 9221) n (%) |
P value c | |
|---|---|---|---|---|
| No (n = 7744) n (%) |
Yes (n = 1477) n (%) |
|||
| Age at diagnosis, mean years (SD) | 48.4 (8.9) | 54.7 (6.9) | 49.4 (8.9) | < 0.0001 |
| Race/Ethnicity | < 0.0001 | |||
| Non-Hispanic Black | 1678 (21.7) | 392 (26.5) | 2070 (22.5) | |
| Non-Hispanic White | 3244 (41.9) | 427 (28.9) | 3671 (39.8) | |
| Non-Hispanic Asian/Pacific Islander | 956 (12.3) | 186 (12.6) | 1142 (12.4) | |
| Non-Hispanic Other | 20 (0.2) | 10 (0.7) | 30 (0.3) | |
| Hispanic | 1825 (23.6) | 455 (30.8) | 2280 (24.7) | |
| Unknown | 21 (0.3) | 7 (0.5) | 28 (0.3) | |
| Marital Status | < 0.0001 | |||
| Single (never married) | 3144 (40.6) | 508 (34.4) | 3652 (39.6) | |
| Married or Domestic Partner | 2669 (34.5) | 480 (32.5) | 3149 (34.1) | |
| Divorced or Separated | 1379 (17.8) | 300 (20.3) | 1679 (18.2) | |
| Widowed | 315 (4.1) | 132 (8.9) | 447 (4.9) | |
| Unknown | 237 (3.0) | 57 (3.9) | 294 (3.2) | |
| Estimated Menopausal Status a | < 0.0001 | |||
| Postmenopausal | 3643 (47.0) | 1151 (77.9) | 4794 (52.0) | |
| Premenopausal | 4101 (53.0) | 326 (22.1) | 4427 (48.0) | |
| Obese | < 0.0001 | |||
| Yes | 1934 (25.0) | 958 (64.9) | 2892 (31.4) | |
| No | 5810 (75.0) | 519 (35.1) | 6329 (68.6) | |
| Hormone Therapy | 0.0355 | |||
| Yes | 3452 (44.6) | 607 (41.1) | 4059 (44.0) | |
| No | 4011 (51.8) | 807 (54.6) | 4818 (52.3) | |
| Unknown | 281 (3.6) | 63 (4.3) | 344 (3.7) | |
| Surgery | 0.0699 | |||
| Yes | 6969 (90.0) | 1346 (91.1) | 8315 (90.2) | |
| No | 728 (9.4) | 117 (7.9) | 845 (9.2) | |
| Unknown | 47 (0.6) | 14 (1.0) | 61 (0.7) | |
| Radiation | 0.5482 | |||
| Yes | 7263 (93.8) | 1395 (94.5) | 8658 (93.9) | |
| No | 155 (2.0) | 24 (1.6) | 179 (1.9) | |
| Unknown | 326 (4.2) | 58 (3.9) | 384 (4.2) | |
| Chemotherapy | < 0.0001 | |||
| Yes | 4612 (59.6) | 770 (52.1) | 5382 (58.4) | |
| No | 2982 (38.5) | 669 (45.3) | 3651 (39.6) | |
| Unknown | 150 (1.9) | 38 (2.6) | 188 (2.0) | |
| Breast Cancer Subtype b | 0.0352 | |||
| HR+/HER2− | 2968 (38.3) | 528 (35.8) | 3496 (37.9) | |
| HR+/HER2+ | 707 (9.1) | 114 (7.7) | 821 (8.9) | |
| HR−/HER2+ | 304 (3.9) | 52 (3.5) | 356 (3.9) | |
| Triple-negative | 702 (9.1) | 146 (9.9) | 848 (9.2) | |
| Unknown | 3063 (39.6) | 637 (43.1) | 3700 (40.1) | |
| SEER Summary Staging | 0.0008 | |||
| Localized | 4101 (53.0) | 819 (55.5) | 4920 (53.4) | |
| Regional | 2952 (38.1) | 546 (37.0) | 3498 (37.9) | |
| Distant | 558 (7.2) | 73 (4.9) | 631 (6.8) | |
| Unknown | 133 (1.7) | 39 (2.6) | 172 (1.9) | |
| Tumor Grade | 0.1324 | |||
| Grade I | 921 (11.9) | 172 (11.6) | 1093 (11.8) | |
| Grade II | 2908 (37.6) | 603 (40.8) | 3511 (38.1) | |
| Grade III | 3294 (42.5) | 583 (39.5) | 3877 (42.1) | |
| Grade IV | 26 (0.3) | 3 (0.2) | 29 (0.3) | |
| Unknown | 595 (7.7) | 116 (7.9) | 711 (7.7) | |
| Age at mortality, mean years (SD) | 51.8 (9.5) | 58.6 (7.6) | 53.2 (9.5) | < 0.0001 |
Estimated menopausal status defined as: Premenopausal < 50 years of age and Postmenopausal ≥50 years of age.
HR+, Estrogen receptor or progesterone receptor positive; HER2, human epidermal growth factor receptor 2.
Significant difference between groups determined by X2 test (all categorical variables).
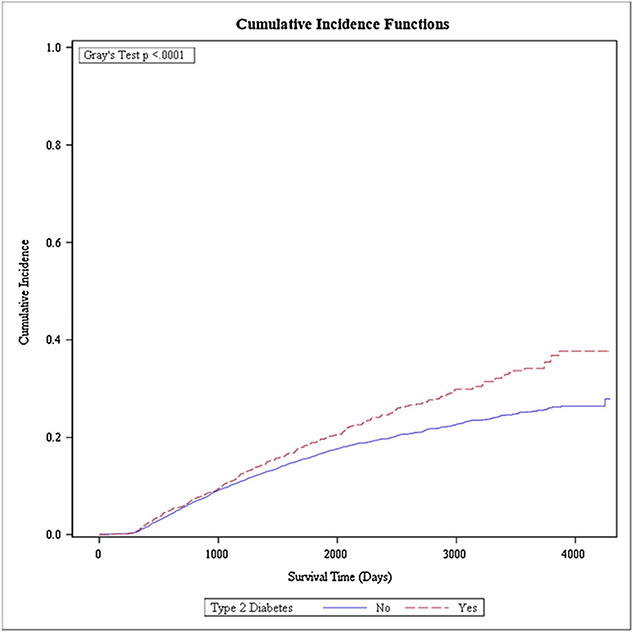
The unadjusted cumulative all-cause mortality by T2DM status after 12 years is shown in Fig. 1. The cumulative all-cause mortality in women with T2DM was 37.7 % (95 %CI 33.0 %, 42.4 %) compared with 27.9 % (95 %CI 24.6 %, 31.2 %) in nondiabetic women (p < 0.001). Supplemental Figs. 1 and 2 present the unadjusted cumulative cancer-specific and cardiovascular-specific mortality after 12 years, respectively. The cumulative cancer-specific mortality was 24.2 % (95 %CI 20.4 %, 28.3 %) in women with T2DM versus 24.0 % (95 %CI % 22.4, 25.6 %) in nondiabetic women (p = 0.4960), while the cumulative cardiovascular-specific morality was 13.6 % (95 %CI 8.9 %, 19.3 %) among women with T2DM compared to 4.1 % (95 %CI 1.3 %, 9.3 %) in nondiabetic women (p < 0.001).
Fig. 1.
Cumulative incidence function for all-cause mortality among Medicaid-insured women diagnosed with breast cancer with preexisting type 2 diabetes mellitus and without diabetes.
Table 2 shows the regression analyses for all-cause and cause-specific morality by preexisting T2DM status. After adjusting for confounders, women with T2DM had an increased all-cause (HR = 1.40; 95 %CI 1.21, 1.63), cancer-specific (HR = 1.24; 95 %CI 1.04, 1.47), and cardiovascular-specific (HR = 2.46; 95 %CI 1.54, 3.90) mortality hazard.
Table 2.
Multivariable-adjusted analysis for preexisting type 2 diabetes mellitus status in relation to mortality among 9221 Medicaid-insured women with breast cancer from 2004-2016 in New York State.
| Variables | Type 2 Diabetes Mellitus Status HR (95 % CI) a |
|
|---|---|---|
| No | Yes | |
| All-cause Mortality | Referent | 1.40 (1.21, 1.63) |
| Cancer Mortality | Referent | 1.24 (1.04, 1.47) |
| Cardiovascular Mortality | Referent | 2.46 (1.54, 3.90) |
Note: Estimated menopausal status defined as: Premenopausal < 50 years of age and Postmenopausal ≥50 years of age.
Abbreviations: HR, Hazard Ratio 95 % CI, 95 % confidence interval.
Adjusted for race/ethnicity, estimated menopausal status, age at breast cancer diagnosis, breast cancer date of diagnosis, marital status at diagnosis, obesity, coronary heart disease, stroke, chronic kidney disease, molecular subtype, chemotherapy, surgery, hormone therapy, SEER Summary Staging.
Table 3 presents stratified multivariable-adjusted analyses assessing all-cause mortality by preexisting T2DM status. Increased risk of all-cause mortality among women with T2DM versus nondiabetics was strongest among non-Hispanic White (HR = 1.78; 95 %CI 1.38, 2.30), postmenopausal (HR = 1.47; 95 %CI 1.23, 1.77), and non-obese (HR = 1.49; 95 %CI 1.22, 1.82) women. When examining differences by stage at diagnosis, the greatest mortality hazard was observed for localized stage (HR = 1.62; 95 %CI 1.23, 2.14).
Table 3.
Multivariable-adjusted analysis assessing the impact of preexisting type 2 diabetes mellitus status on all-cause mortality among 9221 Medicaid-insured women with breast cancer by demographic and clinical subgroup characteristics in New York State, 2004–2016.
| Variables | Type 2 Diabetes Mellitus versus No Diabetes Mellitus HR (95 % CI) a |
|---|---|
| Race/Ethnicity | |
| Non-Hispanic White | 1.78 (1.38, 2.30) |
| Non-Hispanic Black | 1.26 (0.97, 1.64) |
| Non-Hispanic Asian/ Pacific Islander | 0.82 (0.41, 1.61) |
| Non-Hispanic Other | – |
| Hispanic | 1.29 (0.94, 1.78) |
| Estimated Menopausal Status b | |
| Premenopausal | 1.31 (0.99, 1.74) |
| Postmenopausal | 1.47 (1.23, 1.77) |
| Obese | |
| Yes | 1.35 (1.08, 1.69) |
| No | 1.49 (1.22, 1.82) |
| SEER Summary Staging | |
| Localized | 1.62 (1.23, 2.14) |
| Regional | 1.25 (0.99, 1.57) |
| Distant | 1.35 (0.97, 1.87) |
| Molecular Subtype | |
| HR+/HER2− | 1.33 (0.92, 1.93) |
| HR+/HER2+ | 1.40 (0.75, 2.60) |
| HR−/HER2+ | 1.93 (0.70, 5.33) |
| Triple-negative | 1.07 (0.68, 1.69) |
Abbreviations: HR, Hazard Ratio; 95 % CI, 95 % confidence interval; HR+, Estrogen receptor or progesterone receptor positive; HER2, human epidermal growth factor receptor 2.
Note: “− ”indicates not calculable.
Adjusted for race/ethnicity, estimated menopausal status, age at breast cancer diagnosis, breast cancer date of diagnosis, marital status at diagnosis, obesity, coronary heart disease, stroke, chronic kidney disease, molecular subtype, chemotherapy, surgery, hormone therapy, SEER Summary Staging.
Estimated menopausal status defined as: Premenopausal < 50 years of age and Postmenopausal ≥50 years of age.
We further performed multivariable-adjusted analyses restricted to women with T2DM for the association of demographic and clinical characteristics with all-cause mortality (Table 4). Compared to non-Hispanic Whites (referent), reduced mortality hazard was observed among non-Hispanic Asian/Pacific Islanders (HR = 0.45; 95 %CI 0.27, 0.74) and Hispanics (HR = 0.74; 95 %CI 0.55, 0.99). For associations with estimated menopausal status and obesity status, we found postmenopausal women had an increased mortality hazard (HR = 1.60; 95 %CI 1.02, 2.50) compared to premenopausal (referent), and obese women had reduced mortality (HR = 0.65; 95 % CI 0.52, 0.83) compared to non-obese (referent). Additionally, increased mortality was observed for regional (HR = 1.93; 95 %CI 1.48, 2.53) and distant (HR = 8.65; 95 % CI 5.72, 13.07) stage compared to local stage disease (referent). Moreover, triple negative women had an elevated mortality hazard (HR = 1.76; 95 %CI 1.11, 2.80) compared with HR+/HER2−.
Table 4.
Multivariable-adjusted analysis for the association of demographic and clinical characteristics with all-cause mortality among 1477 Medicaid-insured women with preexisting type 2 diabetes mellitus diagnosed with breast cancer in New York State, 2004–2016.
| Variables | HR (95 % CI) a |
|---|---|
| Race/Ethnicity | |
| Non-Hispanic White | Referent |
| Non-Hispanic Black | 1.00 (0.76, 1.33) |
| Non-Hispanic Asian/Pacific Islander | 0.45 (0.27, 0.74) |
| Non-Hispanic Other | 0.38 (0.05, 2.76) |
| Hispanic | 0.74 (0.55, 0.99) |
| Estimated Menopausal Status b | |
| Premenopausal | Referent |
| Postmenopausal | 1.60 (1.02, 2.50) |
| Obese | |
| No | Referent |
| Yes | 0.65 (0.52, 0.83) |
| SEER Summary Staging | |
| Localized | Referent |
| Regional | 1.93 (1.48, 2.53) |
| Distant | 8.65 (5.72, 13.07) |
| Molecular Subtype | |
| HR+/HER2− | Referent |
| HR+/HER2+ | 1.27 (0.76, 2.13) |
| HRȒ/HER2+ | 0.99 (0.46, 2.13) |
| Triple-negative | 1.76 (1.11, 2.80) |
Abbreviations: HR, Hazard Ratio; 95 % CI, 95 % confidence interval; HR+, Estrogen receptor or progesterone receptor positive; HER2, human epidermal growth factor receptor 2.
Adjusted for race/ethnicity, estimated menopausal status, age at breast cancer diagnosis, breast cancer date of diagnosis, marital status at diagnosis, obesity, coronary heart disease, stroke, chronic kidney disease, molecular subtype, chemotherapy, surgery, hormone therapy, SEER Summary Staging, days between first type 2 diabetes mellitus diagnosis claim and breast cancer date of diagnosis.
Estimated menopausal status defined as: Premenopausal < 50 years of age and Postmenopausal ≥50 years of age.
Table 5 presents regression analyses for all-cause and cause-specific morality by antidiabetic drug prescribed after breast cancer diagnosis among women with preexisting T2DM. Compared to women prescribed metformin (referent), an elevated all-cause mortality hazard was observed among women prescribed sulfonylurea (HR = 1.44; 95 %CI 1.06, 1.94) or insulin (HR = 1.54; 95 %CI 1.12, 2.11). We further examined all-cause mortality among women with preexisting T2DM by antidiabetic drug and breast cancer subtype (Table 6). Increased all-cause mortality was observed for women who were HR+/HER2− and prescribed ‘other’ antidiabetic drugs (HR = 3.85; 95 %CI 1.66, 8.91) compared to women prescribed metformin (referent). However, this result was based on a small number of women with documented molecular subtype and was potentially due to chance.
Table 5.
Multivariable-adjusted analysis for the association of antidiabetic drugs prescribed after breast cancer diagnosis and mortality among 1477 Medicaid-insured women with preexisting type 2 diabetes mellitus diagnosed with breast cancer.
| Variables | HR (95 % CI) a | ||
|---|---|---|---|
| All-cause Mortality | Cancer Mortality | Cardiovascular Mortality | |
| Metformin | Referent | Referent | Referent |
| Sulfonylurea | 1.44 (1.06, 1.94) | 1.34 (0.94, 1.94) | 1.69 (0.80, 3.54) |
| Insulin | 1.54 (1.12, 2.11) | 1.25 (0.83, 1.88) | 2.00 (0.98, 4.09) |
| Other | 1.04 (0.72, 1.52) | 0.88 (0.54, 1.44) | 1.21 (0.52, 2.82) |
| Combination b | 0.85 (0.52, 1.38) | 0.78 (0.43, 1.40) | 1.10 (0.36, 3.35) |
Note: Estimated menopausal status defined as: Premenopausal < 50 years of age and Postmenopausal ≥50 years of age.
Abbreviations: HR, Hazard Ratio 95 % CI, 95 % confidence interval.
Adjusted for race/ethnicity, estimated menopausal status, age at breast cancer diagnosis, breast cancer date of diagnosis, marital status at diagnosis, obesity, coronary heart disease, stroke, chronic kidney disease, molecular subtype, chemotherapy, surgery, hormone therapy, SEER Summary Staging, days between first type 2 diabetes mellitus diagnosis claim and breast cancer date of diagnosis.
Combination: Combination of two separate drugs for specific treatments related to diabetes mellitus.
Table 6.
Multivariable-adjusted analysis for the association of antidiabetic drugs prescribed after breast cancer diagnosis and mortality among 1477 Medicaid-insured women with preexisting type 2 diabetes mellitus diagnosed with breast cancer, stratified by breast cancer molecular subtype.
| Variables | HR (95 % CI) a | |||
|---|---|---|---|---|
| HR+/HER2− | HR+/HER2+ | HR−/HER2+ | Triple-negative | |
| Metformin | Referent | Referent | Referent | Referent |
| Sulfonylurea | 2.02 (0.92, 4.47) | – | – | 1.12 (0.31, 4.03) |
| Insulin | 1.14 (0.48, 2.73) | – | – | 2.34 (0.77, 7.13) |
| Other | 3.85 (1.66, 8.91) | 1.94 (0.08, 47.00) | – | 2.64 (0.56, 12.37) |
| Combination b | 1.74 (0.64, 4.75) | 1.37 (0.06, 28.97) | – | 1.01 (0.09, 11.45) |
Abbreviations: HR+, hormone receptor positive; HER2, human epidermal growth factor receptor 2, HR, Hazard Ratio 95 % CI, 95 % confidence interval.
Note: Estimated menopausal status defined as: Premenopausal < 50 years of age and Postmenopausal ≥50 years of age.
“− ”indicates not calculable.
Adjusted for race/ethnicity, estimated menopausal status, age at breast cancer diagnosis, breast cancer date of diagnosis, marital status at diagnosis, obesity, coronary heart disease, stroke, chronic kidney disease, chemotherapy, surgery, hormone therapy, SEER Summary Staging, days between first type 2 diabetes mellitus diagnosis claim and breast cancer date of diagnosis.
Combination: Combination of two separate drugs for specific treatments related to diabetes mellitus.
4. Discussion
In the present study, Medicaid-insured women with preexisting T2DM at breast cancer diagnosis had an elevated risk of all-cause and cause-specific mortality. This was especially pronounced for cardiovascular-specific mortality, where there was greater than a 2-fold increased hazard compared to nondiabetic women. Additionally, our findings suggest that mortality among women with preexisting T2DM is influenced by the type of antidiabetic drug prescribed near the time of breast cancer diagnosis.
Earlier studies reported that all-cause mortality risk was higher in women with breast cancer and T2DM, aligning with our findings among women with preexisting T2DM. In a meta-analysis, women with preexisting diabetes at breast cancer diagnosis had a 49 % increase in all-cause mortality compared to women without diabetes [3]. Another study among patients in the U.S. Military Health System found women with T2DM prior to breast cancer had increased risk of mortality compared to nondiabetic women [7]. However, few studies have examined cause-specific mortality. Of these studies, the majority evaluated breast cancer-specific mortality, where findings were inconsistent [13,19,22,23]. In the present study, we observed preexisting T2DM was associated with cancer-specific mortality. The differences between our results and some prior studies are potentially attributable to differences in receipt of breast cancer treatment and type of treatments adjusted for in statistical models. The relationship between T2DM and cardiovascular-specific mortality among women with breast cancer has not been widely reported. Haukka et al. (2017) reported that diabetes mellitus was strongly associated with increased cardiovascular mortality among patients with breast cancer, aligning with our findings [22].
In analyses stratified by race/ethnicity, estimated menopausal status, obesity status, and staging, the association of T2DM with increased mortality hazard tended to be greater among specific subgroups. Postmenopausal women with T2DM had a 47 % increased mortality hazard than their nondiabetic counterparts [36,37]. Additionally, women with T2DM had an elevated mortality hazard across all molecular subtypes in comparison with nondiabetic women, but results were not statistically significant. When examining mortality among women with preexisting T2DM, we observed that compared to non-Hispanic Whites, non-Hispanic Asian/Pacific Islander and Hispanic groups with T2DM had better overall survival. Previous studies have reported that compared to the general population, Asian/Pacific Islanders in the U.S. have healthier dietary habits, lower rates of heart disease, and lower female smoking rates, particularly among foreign-born individuals, all factors that may contribute to better health outcomes for patients with T2DM and/or cancer [38-44]. This potentially explains our findings as a large proportion of the Asian population in NYS are foreign-born [45]. The health advantage among foreign-born is potentially because healthy immigrants are more likely to migrate to the U.S. or immigrants with more advanced disease tend to return to their country of origin prior to death [46-48]. Prior studies reported that compared to Whites, Hispanics have poorer breast cancer outcomes largely driven by socioeconomic factors [49]. However, Hispanic culture is known for strong social ties and endorsing healthier behaviors, which in the present study where the population is largely economically disadvantaged may have been slightly advantageous [50,51]. Interestingly, we also observed that being obese was protective against mortality in women with preexisting T2DM and breast cancer. Prior studies that observed similar findings referred to this phenomenon as the “obesity paradox”, where being obese was protective against mortality [52,53]. A potential reasoning is that women in the nonobese category could disproportionately include sicker patients who have an elevated mortality risk. Patients with more aggressive cancer at diagnosis often experience weight loss [52]. Obese patients also have higher nutritional reserves, which can be advantageous during periods of acute illness [54].
When examining survival by antidiabetic drug type, our results showed increased all-cause mortality among women with preexisting T2DM prescribed sulfonylurea or insulin compared to women prescribed metformin for monotherapy. Previous studies assessing anti-diabetic drugs on mortality among women with cancer have reported sulfonylureas and insulin were associated with increased mortality [18,55]. We also observed elevated mortality hazard for cancer-specific and cardiovascular-specific mortality among women prescribed sulfonylurea or insulin, though not statistically significant at alpha = 0.05. Literature has reported that compared to metformin, sulfonylureas have greater adverse cardiovascular risk factors that contribute to poorer survival including hypoglycemia, weight gain, and fluid retention [56-58]. Additionally, insulin was suggested to increase risk of vascular damage and major cardiac events among T2DM patients [59-61]. Though metformin is the recommended first-line oral antidiabetic drug for T2DM, patients might be prescribed sulfonylurea or insulin due to contraindications or intolerance to metformin [58,62,63]. Moreover, this is one of few studies assessing the relationship between antidiabetic drugs and molecular subtype on mortality, where findings remain inconsistent [64-68]. In the present study, we observed increased all-cause mortality hazard for women with HR+/HER2− cancers who were prescribed “other” antidiabetic drugs compared to metformin prescribed group. Prior studies have suggested metformin influences inducing apoptosis in HER2+ and triple negative breast cancer cells [69-71]. However, in this study approximately forty percent of women’s breast cancer molecular subtype was unknown making it difficult to interpret findings.
Increased mortality among women with breast cancer and preexisting T2DM can potentially be attributed to receiving less aggressive cancer treatment compared to nondiabetic women [14]. Previous studies reported that physicians might use less aggressive treatment in women with T2DM because of perceived risk of chemotherapy-related toxicity [13]. This is demonstrated in the present study in which women with preexisting T2DM had lower receipt of chemotherapy compared with nondiabetics. In our multivariable analysis, chemotherapy as a potential confounder was adjusted for in our model, thus the impact of treatment on survival was reduced, although there is potential for residual confounding by type of chemotherapy. Additionally, our findings that women with T2DM prescribed sulfonylurea or insulin for monotherapy had poorer breast cancer survival is potentially an indicator for patients with additional comorbid conditions such as severe chronic kidney disease, where mortality risk is greater and metformin is often not prescribed as a result of potentially contributing to poorer clinical outcomes [72,73]. However, in our descriptive analysis for antidiabetic drugs prescribed by medical condition, we observed that the percent distribution of drugs prescribed among patients with chronic kidney disease was similar with all other comorbid conditions (Supplemental Table 1). Another potential explanation is the impact both antidiabetic drugs in combination with breast cancer treatment has on survival. Previous studies have documented that sulfonylureas and insulin are associated with adverse cardiovascular health in comparison with metformin when prescribed for monotherapy [74-76]. Epidemiological studies observed that breast cancer therapy was associated with increased risk of chemotherapy-induced heart disease following completion of cancer treatment [77]. This was specifically observed among women that received combined anthracyclines and new-generation targeted drugs (e.g. trastuzumab) [77-79]. The combined influence of sulfonylureas or insulin prescribed for monotherapy and breast cancer therapy may have a detrimental impact on cardiovascular health, contributing to poorer long-term survival.
Although our study provides new insight on the relationship between preexisting T2DM and antidiabetic drugs on mortality among women with breast cancer, several limitations must be noted. First, we are unable to exclude residual confounding related to breast cancer treatment, such as timing, intensity, duration, and frequency of treatment. Second, this study did not consider specifics of breast cancer treatments, such as type of chemotherapy. Third, menopausal status was determined by age rather than by clinical diagnosis. For this reason, we are unable to account for the potential influence of smoking and obesity on timing of menopause [80,81]. Fourth, identification of antidiabetic drugs was at time of breast cancer diagnosis, failing to consider changes over time. Fifth, there is a possibility that we either under-adjusted or over-adjusted for potential confounders. For instance, we lacked information on dietary habits, physical activity, and smoking frequency, which are associated with both T2DM and mortality, potentially resulting in uncontrolled confounding. Further, obesity status was based on Medicaid claims data which may under-report obesity and result in some misclassification; however, the prevalence of obesity in our population was similar to estimates in comparable populations. Conversely, though we adjusted for known comorbidities in our statistical models, there is a possibility that one or more comorbid condition adjusted for is on the causal pathway between T2DM and mortality resulting in over-adjusting. Finally, this study was only able to assess if a woman filled a prescription for an antidiabetic drug, and we could not determine whether they were adherent to their medication treatment plan.
The present study limitations are offset by its strengths. Most notably, the study consisted of a large, racially and ethnically diverse Medicaid population that was primarily low-income. Additionally, obesity status was based on objective measures obtained during health examinations rather than self-report where individuals tend to over-estimate height and underreport weight [82]. Moreover, this study utilized objective data from NYS cancer registry, which is a gold-rated state cancer registry, and detailed information from Medicaid administrative claims.
5. Conclusion
In conclusion, this study suggests that Medicaid-insured women with preexisting T2DM at breast cancer diagnosis are at greater risk for all-cause, cancer-specific, and cardiovascular-specific mortality, compared to nondiabetic women with breast cancer. Women prescribed sulfonylurea or insulin potentially have greater mortality risk than those prescribed metformin for monotherapy. Additional research is needed to determine the optimal course of treatment for women with preexisting T2DM diagnosed with breast cancer.
Supplementary Material
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors thank Drs. Katheryn Roberson, Erica Tyler, Melissa Noel, Hnin Wai Lwin Myo, Yajaira Cabrera- Tineo, Simone Seward, Ola Kalu, Guillermo J. Escaño in the Center for the Elimination of Minority Health Disparities at the University at Albany, State University of New York for their expert insights.
Footnotes
Disclaimer
The interpretation and reporting of these data are the responsibility of the authors and in no way should be viewed as an official policy or interpretation of the New York State government.
CRediT authorship contribution statement
Wayne R. Lawrence: Writing - original draft. Akiko S. Hosler: Methodology, Writing - review & editing. Margaret Gates Kuliszewski: Writing - review & editing. Matthew C. Leinung: Validation, Writing - review & editing. Xiuling Zhang: Methodology, Software. Maria J. Schymura: Supervision, Project administration. Francis P. Boscoe: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.canep.2020.101710.
References
- [1].Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, Imperatore G, Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data, Lancet (London, England) 391 (2018) 2430–2440, 10.1016/S0140-6736(18)30314-3. [DOI] [PubMed] [Google Scholar]
- [2].Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors Collaboration, Diabetes mellitus, fasting glucose, and risk of cause-specific death, N. Engl. J. Med 364 (2011) 829–841, 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peairs KS, Barone BB, Snyder CF, Yeh H-C, Stein KB, Derr RL, Brancati FL, Wolff AC, Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis, J. Clin. Oncol 29 (2011) 40–46, 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D, Diabetes and cancer: a consensus report, CA Cancer J. Clin 60 (2010) 207–221, 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- [5].Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE, Diabetes mellitus and breast cancer: a retrospective population-based cohort study, Breast Cancer Res. Treat 98 (2006) 349–356, 10.1007/s10549-006-9172-5. [DOI] [PubMed] [Google Scholar]
- [6].Barone BB, Yeh H-C, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL, Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus, JAMA 300 (2008) 2754–2764, 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shao S, Gill AA, Zahm SH, Jatoi I, Shriver CD, McGlynn KA, Zhu K, Diabetes and overall survival among breast cancer patients in the U.S. military health system, Cancer Epidemiol. Biomarkers Prev 27 (2018) 50–57, 10.1158/1055-9965.EPI-17-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA, Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies, BMJ 350 (2015), 10.1136/bmj.g7607 g7607–g7607 [DOI] [PubMed] [Google Scholar]
- [9].Larsson SC, Mantzoros CS, Wolk A, Diabetes mellitus and risk of breast cancer: a meta-analysis, Int. J. Cancer 121 (2007) 856–862, 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- [10].Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, Fairley LL, Boniol M, Zheng T, Zhang Y, Pasterk M, Smans M, Curado MP, Mullie P, Gandini S, Bota M, Bolli GB, Rosenstock J, Autier P, Diabetes and breast cancer risk: a meta-analysis, Br. J. Cancer 107 (2012) 1608–1617, 10.1038/bjc.2012.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beckman TJ, Cuddihy RM, Scheitel SM, Naessens JM, Killian JM, Pankratz VS, Screening mammogram utilization in women with diabetes, Diabetes Care 24 (2001) 2049–2053, 10.2337/diacare.24.12.2049. [DOI] [PubMed] [Google Scholar]
- [12].Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE, The impact of diabetes on survival following breast cancer, Breast Cancer Res. Treat 109 (2008) 389–395, 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]
- [13].Srokowski TP, Fang S, Hortobagyi GN, Giordano SH, Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer, J. Clin. Oncol 27 (2009) 2170–2176, 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van de Poll-Franse LV, Houterman S, Janssen-Heijnen MLG, Dercksen MW, Coebergh JWW, Haak HR, Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis, Int. J. Cancer 120 (2007) 1986–1992, 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- [15].Zhao X-B, Ren G-S, Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta-analysis, Medicine (Baltimore) 95 (2016) e5602, 10.1097/MD.0000000000005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tang GH, Satkunam M, Pond GR, Steinberg GR, Blandino G, Schünemann HJ, Muti P, Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: a GRADE-assessed systematic review and meta-analysis, Cancer Epidemiol. Biomarkers Prev 27 (2018) 627–635, 10.1158/1055-9965.EPI-17-0936. [DOI] [PubMed] [Google Scholar]
- [17].Zhang P, Li H, Tan X, Chen L, Wang S, Association of metformin use with cancer incidence and mortality: a meta-analysis, Cancer Epidemiol. 37 (2013) 207–218, 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- [18].Calip GS, Yu O, Hoskins KF, Boudreau DM, Associations between diabetes medication use and risk of second breast cancer events and mortality, Cancer Causes Control 26 (2015) 1065–1077, 10.1007/s10552-015-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vissers PAJ, Cardwell CR, van de Poll-Franse LV, Young IS, Pouwer F, Murray LJ, The association between glucose-lowering drug use and mortality among breast cancer patients with type 2 diabetes, Breast Cancer Res. Treat 150 (2015) 427–437, 10.1007/s10549-015-3331-5. [DOI] [PubMed] [Google Scholar]
- [20].Troeschel AN, Liu Y, Collin LJ, Bradshaw PT, Ward KC, Gogineni K, McCullough LE, Race differences in cardiovascular disease and breast cancer mortality among US women diagnosed with invasive breast cancer, Int. J. Epidemiol 48 (2019) 1897–1905, 10.1093/ije/dyz108. [DOI] [PubMed] [Google Scholar]
- [21].Kirkham AA, Beaudry RI, Paterson DI, Mackey JR, Haykowsky MJ, Curing breast cancer and killing the heart: a novel model to explain elevated cardiovascular disease and mortality risk among women with early stage breast cancer, Prog. Cardiovasc. Dis 62 (2019) 116–126, 10.1016/j.pcad.2019.02.002. [DOI] [PubMed] [Google Scholar]
- [22].Haukka J, Niskanen L, Auvinen A, Risk of cause-specific death in individuals with cancer-modifying role diabetes, statins and metformin, Int. J. Cancer 141 (2017) 2437–2449, 10.1002/ijc.31016. [DOI] [PubMed] [Google Scholar]
- [23].Lega IC, Austin PC, Fischer HD, Fung K, Krzyzanowska MK, Amir E, Lipscombe LL, The impact of diabetes on breast cancer treatments and outcomes: a population-based study, Diabetes Care 41 (2018) 755–761, 10.2337/dc17-2012. [DOI] [PubMed] [Google Scholar]
- [24].Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E, Risk factors for severe hypoglycemia in black and white adults with diabetes: the atherosclerosis risk in communities (ARIC) study, Diabetes Care 40 (2017) 1661–1667, 10.2337/dc17-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fisher MA, Ma Z, Medicaid-insured and uninsured were more likely to have diabetes emergency/urgent admissions, Am. J. Manag. Care 21 (2015) e312–319 http://www.ncbi.nlm.nih.gov/pubmed/26167779. [PubMed] [Google Scholar]
- [26].Ward MM, Access to care and the incidence of end-stage renal disease due to diabetes, Diabetes Care 32 (2009) 1032–1036, 10.2337/dc09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hsu CD, Wang X, Habif DV, Ma CX, Johnson KJ, Breast cancer stage variation and survival in association with insurance status and sociodemographic factors in US women 18 to 64 years old, Cancer 123 (2017) 3125–3131, 10.1002/cncr.30722. [DOI] [PubMed] [Google Scholar]
- [28].Bickell N, Weidmann J, Fei K, Lin JJ, Leventhal H, Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust, J. Clin. Oncol 27 (2009) 5160–5167, 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Churilla TM, Egleston B, Bleicher R, Dong Y, Meyer J, Anderson P, Disparities in the local management of breast cancer in the US according to health insurance status, Breast J. 23 (2017) 169–176, 10.1111/tbj.12705. [DOI] [PubMed] [Google Scholar]
- [30].Bickell NA, LePar F, Wang JJ, Leventhal H, Lost opportunities: physicians’ reasons and disparities in breast cancer treatment, J. Clin. Oncol 25 (2007) 2516–2521, 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- [31].Boscoe FP, Schrag D, Chen K, Roohan PJ, Schymura MJ, Building capacity to assess cancer care in the medicaid population in New York State, Health Serv. Res 46 (2011) 805–820, 10.1111/j.1475-6773.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thomas F, Renaud F, Benefice E, de Meeüs T, Guegan JF, International variability of ages at menarche and menopause: patterns and main determinants, Hum. Biol 73 (2001) 271–290 http://www.ncbi.nlm.nih.gov/pubmed/11446429. [DOI] [PubMed] [Google Scholar]
- [33].Phipps AI, Ichikawa L, Bowles EJA, Carney PA, Kerlikowske K, Miglioretti DL, Buist DSM, Defining menopausal status in epidemiologic studies: a comparison of multiple approaches and their effects on breast cancer rates, Maturitas 67 (2010) 60–66, 10.1016/j.maturitas.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Morabia A, Flandre P, Misclassification bias related to definition of menopausal status in case-control studies of breast cancer, Int. J. Epidemiol 21 (1992) 222–228, 10.1093/ije/21.2.222. [DOI] [PubMed] [Google Scholar]
- [35].Gray RJ, A class of K-sample tests for comparing the cumulative incidence of a competing risk, Ann. Stat 16 (1988) 1141–1154 https://www.jstor.org/stable/2241622?seq=1#page_scan_tab_contents. [Google Scholar]
- [36].Dibaba DT, Ogunsina K, Braithwaite D, Akinyemiju T, Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype, Breast Cancer Res. Treat 174 (2019) 209–218, 10.1007/s10549-018-5056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bjørge T, Lukanova A, Jonsson H, Tretli S, Ulmer H, Manjer J, Stocks T, Selmer R, Nagel G, Almquist M, Concin H, Hallmans G, Häggström C, Stattin P, Engeland A, Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project, Cancer Epidemiol. Biomarkers Prev 19 (2010) 1737–1745, 10.1158/1055-9965.EPI-10-0230. [DOI] [PubMed] [Google Scholar]
- [38].Danaei G, Rimm EB, Oza S, Kulkarni SC, Murray CJL, Ezzati M, The promise of prevention: the effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States, PLoS Med. 7 (2010) e1000248, , 10.1371/journal.pmed.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huh J, Prause JA, Dooley CD, The impact of nativity on chronic diseases, self-rated health and comorbidity status of Asian and Hispanic immigrants, J. Immigr. Minor. Health 10 (2008) 103–118, 10.1007/s10903-007-9065-7. [DOI] [PubMed] [Google Scholar]
- [40].Acciai F, Noah AJ, Firebaugh G, Pinpointing the sources of the Asian mortality advantage in the USA, J. Epidemiol. Community Health 69 (2015) 1006–1011, 10.1136/jech-2015-205623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pearcey SM, Zhan GQ, A comparative study of American and Chinese college students’ motives for food choice, Appetite 123 (2018) 325–333, 10.1016/j.appet.2018.01.011. [DOI] [PubMed] [Google Scholar]
- [42].Martell BN, Garrett BE, Caraballo RS, Disparities in adult cigarette smoking — United States, 2002–2005 and 2010–2013, MMWR Morb. Mortal. Wkly. Rep 65 (2016) 753–758, 10.15585/mmwr.mm6530a1. [DOI] [PubMed] [Google Scholar]
- [43].Wyatt LC, Trinh-Shevrin C, Islam NS, Kwon SC, Health-related quality of life and health behaviors in a population-based sample of older, foreign-born, Chinese American adults living in New York City, Health Educ. Behav 41 (2014) 98S–107S, 10.1177/1090198114540462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wade B, Lariscy JT, Hummer RA, Racial/ethnic and nativity patterns of U.S. adolescent and young adult smoking, Popul. Res. Policy Rev 32 (2013) 353–371, 10.1007/s11113-013-9275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].United States Census Bureau, New York, (2019) (Accessed 23 January 2019), https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml. [Google Scholar]
- [46].Hallowell BD, Endeshaw M, McKenna MT, Senkomago V, Razzaghi H, Saraiya M, Cancer mortality rates among US and foreign-born individuals: United States 2005–2014, Prev. Med. (Baltim) 126 (2019) 105755, , 10.1016/j.ypmed.2019.105755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Singh GK, Hiatt RA, Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979–2003, Int. J. Epidemiol 35 (2006) 903–919, 10.1093/ije/dyl089. [DOI] [PubMed] [Google Scholar]
- [48].Argeseanu Cunningham S, Ruben JD, Venkat Narayan KM, Health of foreign-born people in the United States: a review, Health Place 14 (2008) 623–635, 10.1016/j.healthplace.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [49].Martínez ME, Gomez SL, Tao L, Cress R, Rodriguez D, Unkart J, Schwab R, Nodora JN, Cook L, Komenaka I, Li C, Contribution of clinical and socioeconomic factors to differences in breast cancer subtype and mortality between Hispanic and non-Hispanic white women, Breast Cancer Res. Treat 166 (2017) 185–193, 10.1007/s10549-017-4389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pruitt S, Tiro J, Xuan L, Lee S, Hispanic and immigrant paradoxes in U.S. breast cancer mortality: impact of neighborhood poverty and hispanic density, Int. J. Environ. Res. Public Health 13 (2016) 1238, 10.3390/ijerph13121238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chiang JJ, Chen E, Leigh AKK, Hoffer LC, Lam PH, Miller GE, Familism and inflammatory processes in African American, Latino, and White youth, Heal. Psychol 38 (2019) 306–317, 10.1037/hea0000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Strulov Shachar S, Williams GR, The obesity paradox in cancer-moving beyond BMI, Cancer Epidemiol. Biomarkers Prev 26 (2017) 13–16, 10.1158/1055-9965.EPI-16-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Han SJ, Boyko EJ, The evidence for an obesity paradox in type 2 diabetes mellitus, Diabetes Metab. J 42 (2018) 179, 10.4093/dmj.2018.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gioulbasanis I, Baracos VE, Giannousi Z, Xyrafas A, Martin L, Georgoulias V, Mavroudis D, Baseline nutritional evaluation in metastatic lung cancer patients: mini nutritional assessment versus weight loss history, Ann. Oncol 22 (2011) 835–841, 10.1093/annonc/mdq440. [DOI] [PubMed] [Google Scholar]
- [55].Evans JMM, Ogston SA, Emslie-Smith A, Morris AD, Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin, Diabetologia 49 (2006) 930–936, 10.1007/s00125-006-0176-9. [DOI] [PubMed] [Google Scholar]
- [56].Tahrani AA, Barnett AH, Bailey CJ, Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus, Nat. Rev. Endocrinol 12 (2016) 566–592, 10.1038/nrendo.2016.86. [DOI] [PubMed] [Google Scholar]
- [57].Ferrannini E, DeFronzo RA, Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes, Eur. Heart J 36 (2015) 2288–2296, 10.1093/eurheartj/ehv239. [DOI] [PubMed] [Google Scholar]
- [58].Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, Featherstone TR, Mortality risk among sulfonylureas: a systematic review and network meta-analysis, Lancet Diabetes Endocrinol. 3 (2015) 43–51, 10.1016/S2213-8587(14)70213-X. [DOI] [PubMed] [Google Scholar]
- [59].Rensing KL, Reuwer AQ, Arsenault BJ, von der Thüsen JH, Hoekstra JBL, Kastelein JJP, Twickler TB, Reducing cardiovascular disease risk in patients with type 2 diabetes and concomitant macrovascular disease: can insulin be too much of a good thing? Diabetes Obes. Metab 13 (2011) 1073–1087, 10.1111/j.1463-1326.2011.01468.x. [DOI] [PubMed] [Google Scholar]
- [60].Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL, Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes, J. Clin. Endocrinol. Metab 98 (2013) 668–677, 10.1210/jc.2012-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Monnier L, Hanefeld M, Schnell O, Colette C, Owens D, Insulin and atherosclerosis: how are they related? Diabetes Metab. 39 (2013) 111–117, 10.1016/j.diabet.2013.02.001. [DOI] [PubMed] [Google Scholar]
- [62].Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020, Diabetes Care 43 (2020) S98–S110, 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- [63].Khunti K, Chatterjee S, Gerstein HC, Zoungas S, Davies MJ, Do sulphonylureas still have a place in clinical practice? Lancet Diabetes Endocrinol. 6 (2018) 821–832, 10.1016/S2213-8587(18)30025-1. [DOI] [PubMed] [Google Scholar]
- [64].Kim HJHJ, Kwon H, Lee JW, Kim HJHJ, Lee SB, Park HS, Sohn G, Lee Y, Koh BS, Yu JH, Son BH, Ahn SH, Metformin increases survival in hormone receptor-positive, HER2-positive breast cancer patients with diabetes, Breast Cancer Res. 17 (2015) 64, 10.1186/s13058-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Xiao Y, Zhang S, Hou G, Zhang X, Hao X, Zhang J, Clinical pathological characteristics and prognostic analysis of diabetic women with luminal subtype breast cancer, Tumor Biol. 35 (2014) 2035–2045, 10.1007/s13277-013-1270-5. [DOI] [PubMed] [Google Scholar]
- [66].Hou G, Zhang S, Zhang X, Wang P, Hao X, Zhang J, Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes, Breast Cancer Res. Treat 137 (2013) 807–816, 10.1007/s10549-012-2404-y. [DOI] [PubMed] [Google Scholar]
- [67].He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee M-H, Yeung S-CJ, Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer, Ann. Oncol 23 (2012) 1771–1780, 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L, Hortobagyi GN, Gonzalez-Angulo AM, Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer, Cancer 118 (2012) 1202–1211, 10.1002/cncr.26439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chen T, Liang Y, Feng D, Tao L, Qi K, Zhang H, Wang H, Lin Q, Kong H, Metformin inhibits proliferation and promotes apoptosis of HER2 positive breast cancer cells by downregulating HSP90, J. BUON 18 (2020) 51–56 n.d. http://www.ncbi.nlm.nih.gov/pubmed/23613388. [PubMed] [Google Scholar]
- [70].Liu B, Fan Z, Edgerton SM, Deng X-S, Alimova IN, Lind SE, Thor AD, Metformin induces unique biological and molecular responses in triple negative breast cancer cells, Cell Cycle 8 (2009) 2031–2040, 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- [71].Vazquez-Martin A, Oliveras-Ferraros C, Del Barco S, Martin-Castillo B, Menendez JA, The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells, Breast Cancer Res. Treat 126 (2011) 355–364, 10.1007/s10549-010-0924-x. [DOI] [PubMed] [Google Scholar]
- [72].Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW, Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease, Ann. Intern. Med 166 (2017) 191, 10.7326/M16-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].American Diabetes Association, 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018, Diabetes Care 41 (2018) S73–S85, 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- [74].Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S, Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes, Ann. Intern. Med 164 (2016) 740, 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- [75].Filion KB, Douros A, Azoulay L, Yin H, Yu OH, Suissa S, Sulfonylureas as initial treatment for type 2 diabetes and the risk of adverse cardiovascular events: a population-based cohort study, Br. J. Clin. Pharmacol 85 (2019) 2378–2389, 10.1111/bcp.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette A-C, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GFM, Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes, JAMA 316 (2016) 313, 10.1001/jama.2016.9400. [DOI] [PubMed] [Google Scholar]
- [77].Rosa GM, Gigli L, Tagliasacchi MI, Di Iorio C, Carbone F, Nencioni A, Montecucco F, Brunelli C, Update on cardiotoxicity of anti-cancer treatments, Eur. J. Clin. Invest 46 (2016) 264–284, 10.1111/eci.12589. [DOI] [PubMed] [Google Scholar]
- [78].Martel S, Maurer C, Lambertini M, Pondé N, De Azambuja E, Breast cancer treatment-induced cardiotoxicity, Expert Opin. Drug Saf 16 (2017) 1021–1038, 10.1080/14740338.2017.1351541. [DOI] [PubMed] [Google Scholar]
- [79].Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W, Munster PN, Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer, J. Am. Coll. Cardiol 73 (2019) 2859–2868, 10.1016/j.jacc.2019.03.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Whitcomb BW, Purdue-Smithe AC, Szegda KL, Boutot ME, Hankinson SE, Manson JE, Rosner B, Willett WC, Eliassen AH, Bertone-Johnson ER, Cigarette smoking and risk of early natural menopause, Am. J. Epidemiol 187 (2018) 696–704, 10.1093/aje/kwx292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tao X, Jiang A, Yin L, Li Y, Tao F, Hu H, Body mass index and age at natural menopause, Menopause 22 (2015) 469–474, 10.1097/GME.0000000000000324. [DOI] [PubMed] [Google Scholar]
- [82].Gorber SC, Tremblay M, Moher D, Gorber B, A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review, Obes. Rev 8 (2007) 307–326, 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.