Abstract
Agent Orange, a dioxin-containing toxin, was used as an herbicide during the Vietnam War. Exposures to Agent Orange were initially linked to birth defects among Vietnamese civilians residing near aerially sprayed regions. Years later, returning South Korean and U.S. Veterans exposed to Agent Orange exhibited increased rates of malignancy, cardiovascular disease, diabetes and birth defects in their offspring. Growing evidence that herbicides and pesticides contribute to chronic diseases including neurodegeneration raises concern that Agent Orange exposures may have increased the risk for later development of peripheral or central nervous system (CNS) degeneration. This article reviews published data on the main systemic effects and the prevalence rates, relative risks, characteristics and correlates of Agent Orange-associated peripheral neuropathy and CNS dementia-associated diseases. The critical findings were that relatively high levels of Agent Orange exposure increased risk of developing peripheral neuropathy either alone or as a co-factor complication of diabetes mellitus and likely contributed to the pathogenesis of CNS degenerative diseases, including Alzheimer’s, Parkinson’s and vascular dementias. Given the protracted intervals between the Agent Orange exposures and disease emergence, additional research is needed to identify mechanistic correlates of the related neurological disorders, including lifestyle co-factors.
Keywords: Agent Orange, Veteran, neuropathy, dementia, Vietnam, Korea, herbicide, diabetes, U.S. Military, dioxin
Agent Orange-The Back Story
Agent Orange was a potent synthetic herbicide that was mainly used to defoliate enemy territory during the Vietnam War. Chiefly manufactured by Monsanto and Dow Chemical, the herbicide contained equal proportions of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), together with trace quantities (0.05 to 50 parts per million) of the dioxin, 2,4,7,8-tetrachloro-p-dioxin (TCDD). The health dangers of Agent Orange have been linked to contamination of 2,4,5-T stocks with TCDD. Agent Orange was the most commonly used of the Rainbow Herbicides in the Vietnam war era. Its name stemmed from storage of the herbicide in readily identifiable 55-gallon drums marked with an orange band.
In 1962, the U.S. Air Force initiated the Rainbow Herbicide (green, pink, purple, blue, white orange) spraying missions in South Vietnam. Agent Orange was introduced in 1965 and its use increased between 1966 and 1969. However, reports linking Agent Orange to birth defects (1) halted its use by April, 1970. C-123 aircraft were used for Agent Orange spraying missions because they achieved rapid, broad and intense coverage, i. e. 3 gallons per acre over 340 acres within just a few minutes (1). It is estimated that over eleven million gallons of Agent Orange were sprayed via Operation Ranch Hand, accounting for over 60% of the total recorded herbicide applications during the Vietnam War (1). Between 1965 and 1971, when most of the spraying occurred, 10% of South Vietnam was sprayed once, while 30% or more of the region was sprayed repeatedly (1). After the spraying program was terminated, the nearly 2.2 million remaining gallons of Agent Orange were either incinerated at sea or deposited on Johnston Island, an atoll in the Pacific Ocean.
Among the approximately 3 million Americans who served as military personnel in Vietnam, the majority were men born between 1941 and 1950. More than 85% were White and just over 10% were Black. Recruits from lower socioeconomic and educational backgrounds experienced more direct combat fighting and had higher rates of mortality (1). Allied forces, including approximately 320,000 South Korean military service members (2), served alongside the Americans. U.S. military personnel were exposed to Agent Orange by several means. Approximately 1,250 served in the Operation Ranch Hand branch of the U.S. Air Force and were assigned to aerial spraying of herbicides. Another 950 Army Chemical Corps-trained soldiers sprayed herbicides on the ground (1). Unknown numbers of military personnel participated in small-scale backpack or buffalo turbine spraying (1). A fourth source of exposure occurred from dispensing small buckets of Agent Orange along the perimeters of base camps to curb growth of vegetation. The U.S. soldiers were prohibited from entering zones sprayed by Operation Ranch Hand for at least 1 month to limit risk of enemy fire. However, tens of thousands of troops were positioned within 0.5 km of those regions within days of the spraying mission’s completion, a phenomenon that could account for the bulk of Agent Orange exposures.
Agent Orange Exposure-Related Maternal and Fetal Health Concerns
During the war, laboratory test reports and individual accounts emerged concerning potential dangers of chemical herbicides. Vietnamese civilians were heavily exposed to the herbicides because over 80% of the population resided in rural areas that had been sprayed (1) and the villagers consumed locally grown crops that had been sprayed. For years, the aftermath of the Agent Orange spraying included high rates of birth defects among Vietnamese offspring and alarmingly high rates of malignancies and respiratory ailments in young U.S. veterans. Such reports of human diseases, together with the suspicion that TCDD and possibly the other engineered components of Agent Orange were the culprits, inspired population-based studies to assess correlations, causality and timelines.
In a study of 30 Vietnamese women in whom pre-war pregnancies and deliveries were all normal, most of the post-war pregnancies terminated in stillbirth, pre-term delivery or delivery of infants with major anomalies including deformed immobile limbs, impairments in visual, hearing and other sensory functions and disabilities in learning and cognition. Affected women came from heavily sprayed areas in Vietnam and Agent Orange was deemed the likely cause due to the temporal relationships between the exposures and spikes in birth defects (3). A subsequent meta-analysis of 13 Vietnamese and 9 non-Vietnamese studies linked occurrences of birth defects to Agent Orange exposures with an overall relative risk (RR) of 1.95 (4). Spina bifida was included among the birth defects.
Parental exposures to Agent Orange increased the risk of birth defects in both non-Vietnamese (RR=1.29) and Vietnamese (RR=3.0) civilians (4). Higher rates of maternal exposures led to increased rates of teratogenesis in the offspring, whereas male exposures predominantly led to mutagenesis/malignancies (4). Agent Orange’s toxic effects likely differ in males and females. Independent findings suggest that Agent Orange mediates gender-dependent toxic injury via endocrine system disruption. For example, in a case-control study, saliva and serum samples from North Vietnamese mothers residing in dioxin hotspots had elevated levels of salivary cortisol (1.89 vs. 1.10 ng/ml) and cortisone (10.8 vs. 7.74 ng/ml), and serum cortisol (94.2 vs. 66.3 ng/ml) and cortisone (26.7 vs. 22.0 ng/ml) relative to controls from dioxin-free regions (5). A later cohort study of 51 mother-infant pairs showing altered CNS neuronal activity during the quiet sleep phase and attendant poor communication ability manifested by abnormalities in early childhood gaze behavior highlighted the role of breast milk as a vehicle for perinatal exposures to TCDD (6).
Agent Orange: Increased Rates of Malignancy and Other Chronic Diseases in Veterans
The spikes in rates of malignancies, including increased rates of carcinomas and soft tissue sarcomas, in Vietnam War veterans, drew further attention to the potentially serious adverse health effects of Agent Orange. Systematic investigations led the U.S. Department of Veterans Affairs (VA) to conclude that Agent Orange exposures had either a causal role or increased risk for developing chronic B-cell leukemia, non-Hodgkin’s lymphoma, Hodgkin’s disease, multiple myeloma, prostate cancer, soft tissue sarcomas and chloracne. Skin malignancies including basal cell carcinoma and locally aggressive skin cancers also occurred at higher rates in veterans of Operation Ranch Hand (7). In contrast, the rates of malignant melanoma did not increase. Apart from malignancies, Vietnamese veterans who handled herbicides had higher rates of hypertension, cardiac disease, gastrointestinal ulcers, sexually transmitted diseases, benign fatty tumors and various dermatologic conditions including skin rashes with blisters, changes in skin color and increased sensitivity to light compared with non-herbicide handlers (8). However, those studies have limited value since definitive exposure doses and durations were not documented.
Among enlisted ground personnel, high levels of dioxin exposure via Operation Ranch Hand increased overall mortality due to chronic liver disease and cirrhosis or cardiovascular disease including atherosclerosis and coronary artery occlusion (9), increased rates of blood glucose abnormalities (RR=1.4) and diabetes mellitus (RR = 1.5), and shortened onset times to diabetes (10). The increased rates of cardiovascular mortality were attributable to higher rates of underlying cardiovascular disease and diabetes mellitus. Despite multi-source evidence that Agent Orange exposures via Operation Ranch Hand had broad deleterious effects on health, difficulties with data reproducibility including confirmation of increased morbidity and mortality from lung cancer and birth defects (11) continue to fuel the controversy. More research is needed to confirm and mechanistically understand the role of Agent Orange as a mediator of this diverse array of diseases across the lifespan.
Agent Orange Exposures and Increased Risk of Peripheral Neuropathy
Several large-scale studies provided information about the rates and characteristics of peripheral and central nervous system diseases arising in South Korean and U.S. Vietnam War veterans years after they had been discharged from military service. For the most part, the studies emphasized dose-effects of the exposures and whether the peripheral nerve disease corresponded to mononeuropathy, polyneuropathy or complications of diabetes mellitus. Despite limitations due to their retrospective bases, reliance on subjectively reported exposures, and data acquisition via symptom surveys rather than evidence-based or direct examinations, the studies provided longitudinal assessments and attempted to characterize the nature of peripheral neuropathies based on estimated Agent Orange or TCDD doses. Data extracted from four representative studies are summarized in Figures 1–4.
Figure 1:
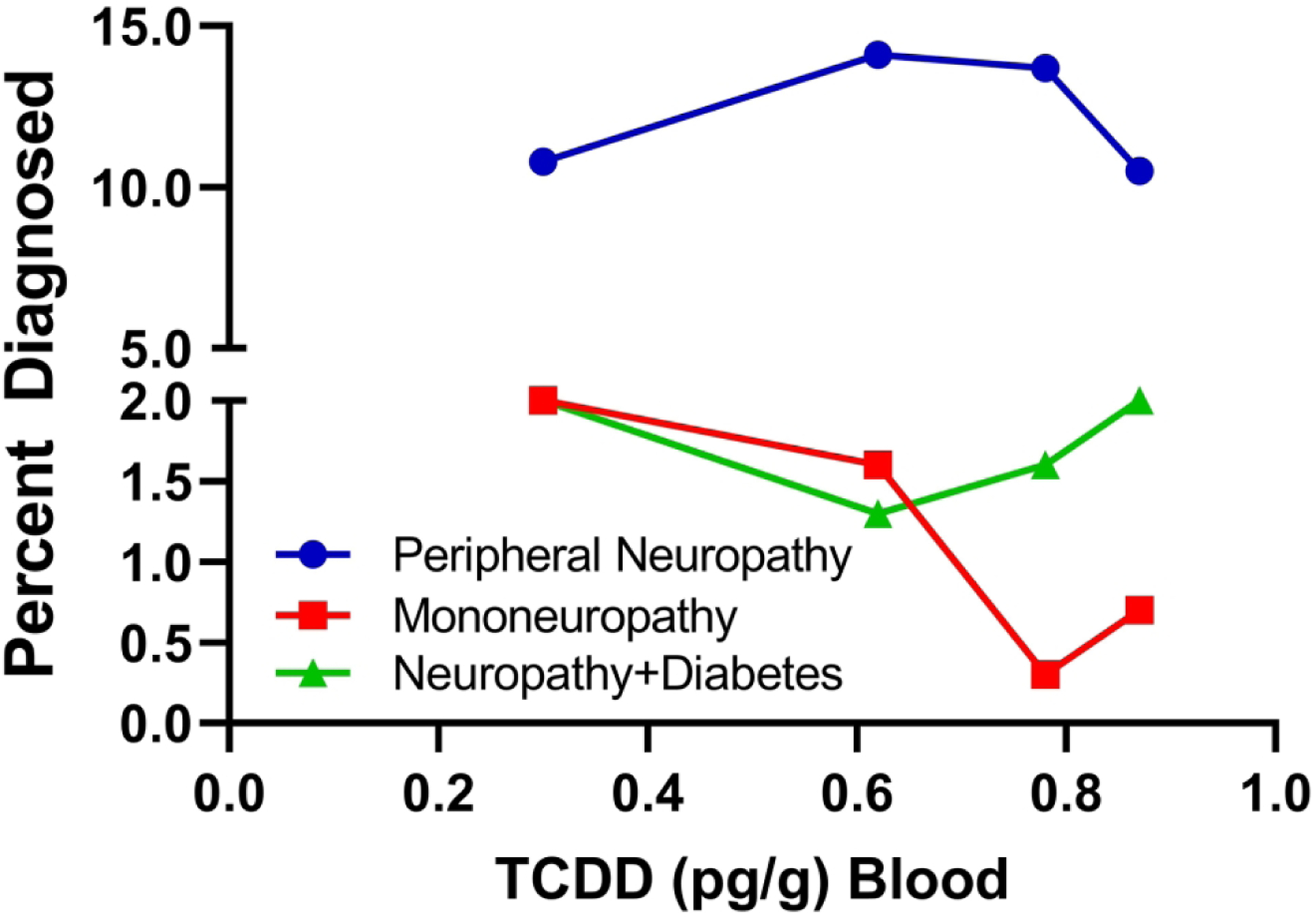
Rates of peripheral neuropathy, mononeuropathy and neuropathy with diabetes in Korean veterans of the Vietnam war (2). Neuropathies were diagnosed clinically 25–30 years after the Agent Orange exposures and TCDD levels were measured in stored blood samples. The graphs depict prevalence rates of the neuropathy subtypes. Statistical comparisons were made with controls. The lack of a TCDD dose-dependent effect argues against a role for that proposed contaminant as a mediator of peripheral neuropathies in soldiers exposed to Agent Orange and instead the data support a pathogenic role for the herbicide constituents.
Figure 4:
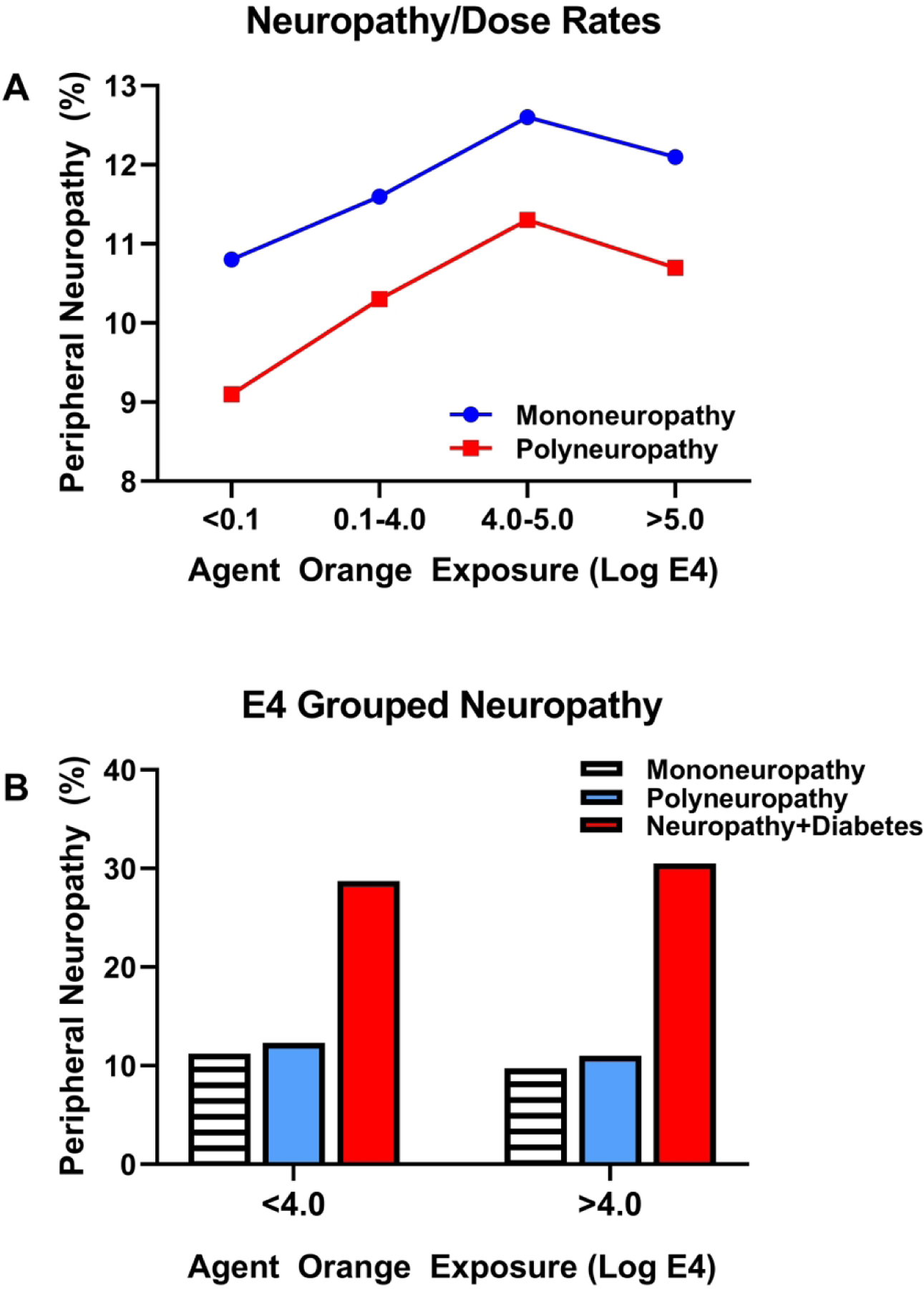
A) Impact of Agent Orange exposure doses on rates of mononeuropathy and polyneuropathy in Korean veterans of the Vietnam war. E4 corresponds to the exposure opportunity model. The line graphs depict parallel exposure dose-dependent increases in rates of both mononeuropathy and polyneuropathy and consistently higher rates of mononeuropathy versus polyneuropathy diagnosed by neurological examination. Note the plateaus at the highest doses for both categories of neuropathy. B) Analysis of grouped data based on threshold Agent Orange Exposures showed additive effects of peripheral neuropathy in veterans with neuropathy and diabetes mellitus but not effect of threshold Agent Orange exposures, i.e. low versus high.
In a study of 1224 middle-aged (45–64) Korean military veterans who served for 7 to 25 months between 1963 and 1973, the rates of all peripheral neuropathies, mononeuropathy and neuropathy with diabetes mellitus were compared with those in 154 control veterans who had not participated in the Vietnam war (2) (Figure 1). Neuropathies were diagnosed by neurologists 25 to 35 years after the reported exposures and the findings were linked to TCDD levels in stored blood samples. The war veterans and controls had similar mean body mass indices and they regularly consumed similar amounts of alcohol and tobacco (heavy use via smoking). The percentage of war-service veterans diagnosed with peripheral neuropathy (12.3%) was significantly higher than in the control group (3.3%; O.R. = 2.39) and over a broad range of TCDD blood levels, the rates of peripheral neuropathy were between 5- and 20-fold higher than for mononeuropathy or neuropathy due to diabetes mellitus (Figure 1). In addition, the rates of diabetes plus peripheral neuropathy were significantly higher in war-serving veterans (1.7%) than controls (0.7%), whereas the rates of mononeuropathy were similar for the two groups (1.1% vs 1.3%). The sub-analysis failed to demonstrate higher rates of peripheral neuropathy, mononeuropathy or neuropathy with diabetes with increasing blood levels of TCDD, suggesting that the 2,4-D and 2,4,5-T exposures via Agent Orange rather than the TCDD dioxin contaminant produced the neurotoxic/degenerative effects on the peripheral nervous system.
Diabetes mellitus is a major cause of peripheral neuropathy. Twenty-five years of Type 1 or Type 2 diabetes leads to symptomatic peripheral neuropathy in up to 50% of afflicted individuals but nearly 7% become symptomatic within the first year of diagnosis. Despite these extraordinary figures, the rates of peripheral neuropathy among diabetics are vastly underestimated since many cases appear to be asymptomatic or else they escape clinical detection. Combining all of its manifestations, the estimated prevalence of neuropathy among diabetic is 90% (12). Since the increased rates of diabetes mellitus among Agent Orange exposed veterans would likely have contributed to any measured increases in peripheral neuropathy rates, studies focusing on Agent Orange-associated neuropathies must weigh the confounding role of Type 2 diabetes mellitus as a causal factor before concluding that Agent Orange had independent neurotoxic effects on the peripheral nervous system.
More definitive insight into the role of Agent Orange as an agent of peripheral neuropathy emerged from a study of U.S. Air Force Vietnam War veterans assigned to Operation Ranch Hand. From 1962 to 1971, that program was responsible for spraying thousands of gallons of Agent Orange in the Vietnamese wilderness. Michalek et al performed longitudinal 5-year check-ups and lifetime analyses of the veterans’ medical records and used formal neurological and electrophysiological testing to characterize the presence and nature of peripheral neuropathy. They also assessed whether the presence of peripheral neuropathy was possible (bilateral symptoms associated with absent Achilles reflex, abnormal ankle vibratory sense or abnormal pin prick) or probable (two or more of the above symptoms) (13). The investigators used stored blood samples to measure dioxin levels. Results were analyzed by sub-dividing the 1000 veteran participants into four exposure-level groups based on blood dioxin levels as follows: no exposure; low-level exposure (<10 parts per trillion (ppt) of dioxin), next highest level (10–94 ppt) and highest level (>94 ppt).
Although the early time period evaluations showed no significant differences in nerve conduction or severity of neuropathy between Agent Orange exposed and control veterans, veterans exposed to the highest or near-highest doses had significantly higher rates of probable symmetric peripheral neuropathy relative to controls and low-exposure veterans (Figure 2). Although there was no evidence of a diabetes co-factor effect on rates of peripheral neuropathy, it is noteworthy that the rates of diabetes (50%) and pre-diabetes (80%) were very high, such that by the last time point of the study, 87.5% of the highest level Agent Orange exposed veterans diagnosed with peripheral neuropathy also had diabetes mellitus. Therefore, it is likely that Agent Orange exposures at high levels caused both peripheral neuropathy and diabetes, either separately or as combined disease processes. Further analysis of the nature of peripheral neuropathies revealed that the Operation Ranch Hand veterans had high rates of small fiber stocking-glove type peripheral neuropathy rather than large fiber neuropathy.
Figure 2:
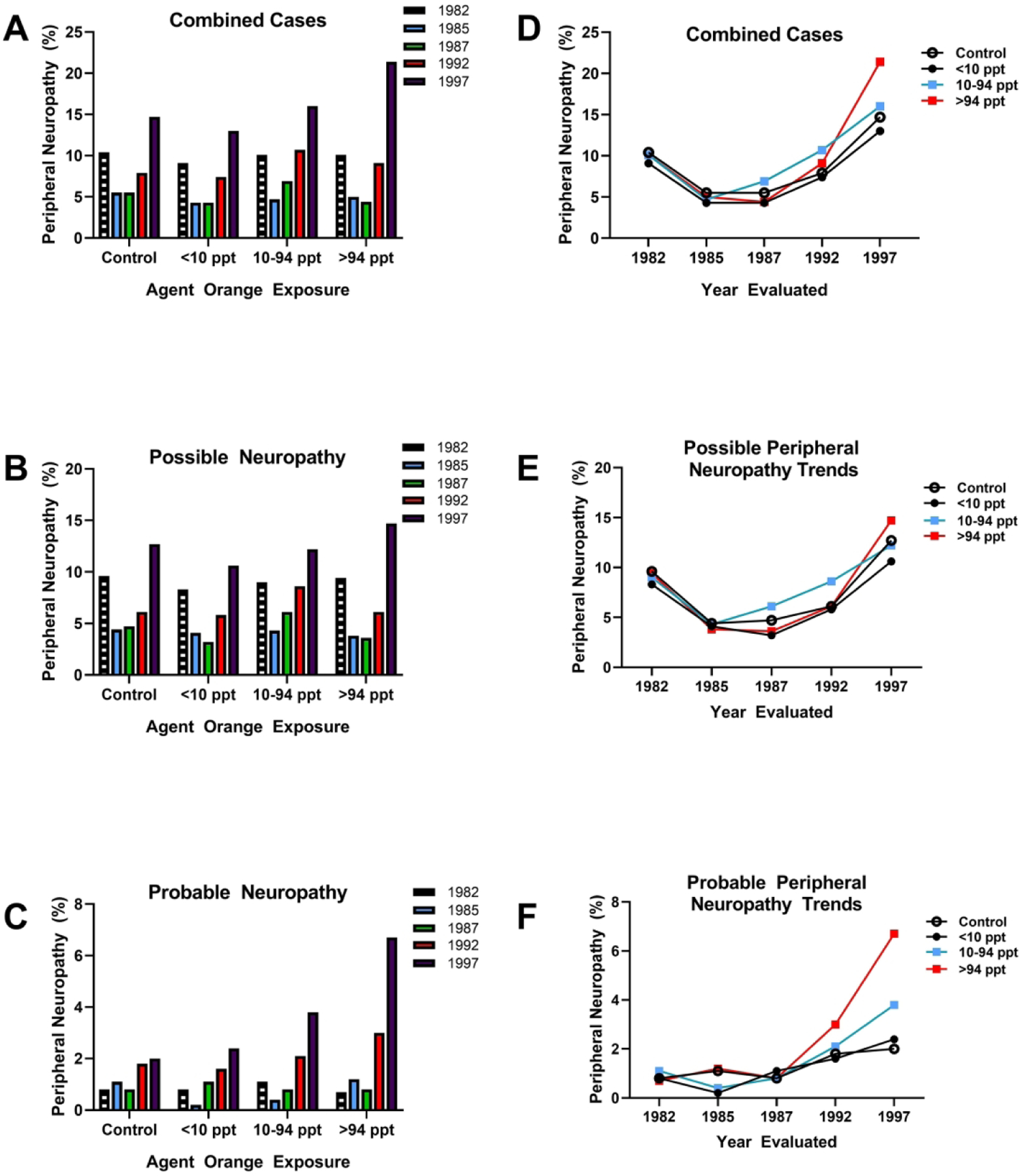
Comparative analysis of Agent Orange/TCDD exposure levels in relation to peripheral neuropathy rates over time. Data were extracted from a study of veterans of Operation Ranch Hand in which peripheral nerve function was assessed over time from 1982 to 1997. Neuropathies were categorized as possible or probable (13). A) Rates of combined (possible+probable) peripheral neuropathy, B) possible neuropathy and C) probable neuropathy diagnosed in 1982, 1985, 1987, 1992 and 1997 in controls and veterans exposed to less than 10 parts per trillion (ppt), between 10–94 ppt or greater than 94 ppt of Agent Orange and TCDD. The largest effect size was detected in veterans diagnosed with (C) probable neuropathy in which dose dependent increases in prevalence were detected at the 10–94 ppt and >94 ppt exposures. Panels D-F display the same data by showing comparative shifts in (D) combined, (E) possible and (F) probably neuropathy over time with comparisons across exposure doses. (F) Clearly with aging, higher exposures doses were associated with higher rates of probable neuropathy.
In 2013 and 2014, Yi, et al cataloged the occurrences and characteristics of self-reported peripheral neuropathy among 114,562 Korean veterans of the Vietnam war (14) (15). In the 2013 study, data pertaining to Agent Orange exposures and perceived symptoms were obtained by questionnaire. The participants were stratified into highest exposures (involved in spraying the herbicide), moderate exposures (present during the spraying), low exposures (indirect contact but present in sprayed regions) and no exposure (no reported contacts). Those categorizations corresponded to proximity-based exposure indices and were based on the E4 exposure opportunity model. The rates of peripheral neuropathy were significantly higher in the high (14.2%) versus low (5.9%) perceived exposure group (Figure 3) and the calculated odds ratios increased with levels of exposure (14). Further comparisons between brigade (narrow group with tactical area responsibility) and battalion (broader company serving at the same time) demonstrated approximately 10% prevalence rates of peripheral neuropathy (Figure 3A), 30% prevalence of neuromuscular diagnoses (Figure 3B) and approximately 20% prevalence of peripheral neuropathy with diabetes mellitus (Figure 3C) for each but no appreciable inter-group differences.
Figure 3:
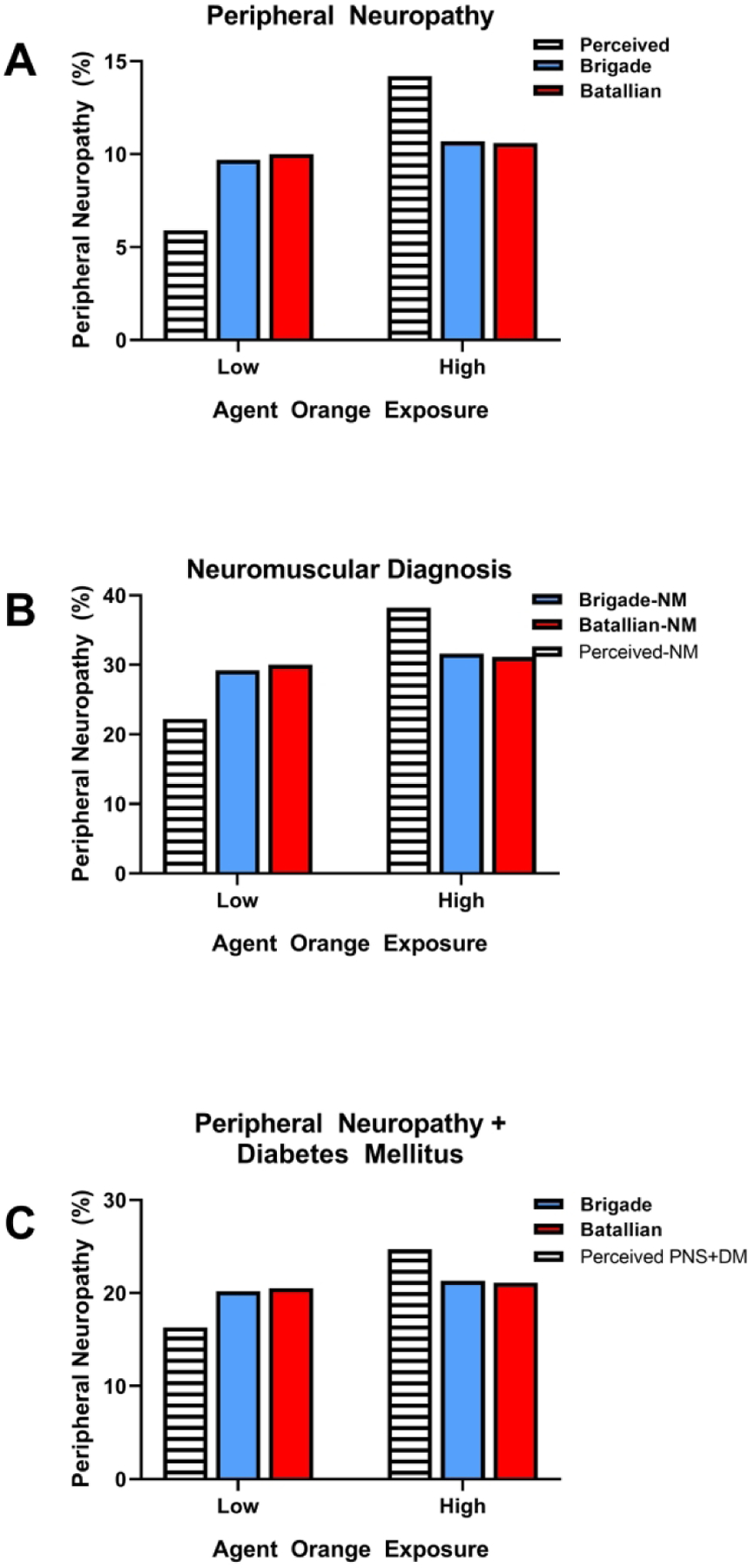
Rates of (A) peripheral neuropathy, (B) neuromuscular diagnoses and (C) peripheral neuropathy with diabetes mellitus among Korean veterans of the Vietnam war. Data were collected via questionnaires and comparisons were made among veterans whose perceived exposures to Agent Orange were low or high. The brigade versus battalion comparisons were made to evaluate rates in veterans who served in tactical units versus the broader population. A) High perceived exposures were associated with higher rates of peripheral neuropathy than low perceived rates, whereas no differences were detected between low and high exposures in brigade and battalion veterans. Rates of B) neuromuscular diagnoses and C) peripheral neuropathy + diabetes mellitus was not established in perceived low versus high Agent Orange exposures. However, rates of neuromuscular and peripheral neuropathy+diabetes diagnoses were similar in brigade and battalion veterans with low or high levels of Agent Orange exposures.
The main findings in this study were that: 1) veterans who had perceived high exposures had a 2.5-fold increase in the odds of developing peripheral neuropathy; 2) the odds of having peripheral neuropathy were 1.8 times higher in the low perceived compared with no perceived exposures; and 3) the odds of having peripheral neuropathy were 2.8 times higher in the moderate and 3.9 times higher in the high perceived compared with the no perceived exposure group. The pitfalls of this study are related to fundamental design flaws (subjective, survey, recall-based), lack of details about the nature of peripheral neuropathies and finding that all of the significant differences were based on self-perceived exposures.
In their 2014 extended study, Yi, et al collected data from 111,726 Korean veterans using a medical database and categorized disease using the International Classification of Diseases (ICD-10) codes rather than self-reported conditions. The peripheral neuropathies were categorized as mononeuropathy (damage to a single nerve) or polyneuropathy (damage to multiple peripheral nerves) (15). The rates of mononeuropathy were consistently higher than polyneuropathy but both increased with Agent Orange exposure levels, although they plateaued at the highest range (Figure 4A). In contrast, when the data were grouped based on a threshold exposure opportunity index (E4) no differences were detected with respect to the rates of mononeuropathy, polyneuropathy or neuropathy with diabetes, although the added impact of diabetes mellitus was evident in cases of mononeuropathy and polyneuropathy (Figure 4B). By combining disease classifications and degrees of exposure, inter-group distinctions regarding risks and rates of peripheral neuropathy were lost. Since data were collected from insurance claim records, granular but significant details about the nature and severity of neuropathy may have been overlooked or unavailable.
Potential Role of Agent Orange in CNS Neurodegenerative Diseases
Recent evidence suggests that Agent Orange exposures may predispose to Alzheimer’s disease (AD) and Parkinson’s Disease (PD) (16, 17), the two most prevalent aging-associated forms of neurodegeneration. Given the fact that the majority of AD and PD cases are considered sporadic in occurrence rather than genetic, the search for environmental and lifestyle co-factors and causal mechanisms continues. This strategy is particularly important because positive outcomes would offer opportunities to prevent or remediate disease. Both PD and AD have been linked to pesticide and other toxin exposures in humans and experimental models (18–22). Therefore, the concept that Agent Orange exposures can increase risk for AD and PD is not farfetched. In light of their strong incident correlations with aging and the fact that Vietnam war veterans are rapidly aging, the potential co-factor role of Agent Orange is a reasonable consideration (23).
Evidence that rates of these diseases may be increased among veterans who had been exposed to Agent Orange was provided by functional neurologic examination studies demonstrating PD-related abnormalities including higher tremor and rigidity scores, altered basal ganglia function and lower facial expression scores relative to controls (17). With regard to AD, plasma amyloid beta levels were found to be elevated in Agent Orange-exposed Korean veterans of the Vietnam war (17). A recent systematic analysis of the U.S. Veterans Health Administration database demonstrated two-fold higher rates of dementia in Agent Orange exposed (5%) compared with random unexposed veterans (2.5%), and 1.25 years earlier onset of disease (24). Apart from its neurotoxic effects, Agent Orange-associated cognitive decline may have been mediated by cerebral infarction due to cardiovascular disease or chronic ischemia secondary to brain microvascular disease and hypoperfusion (25). Both atherosclerosis and small vessel disease are consequences of diabetes mellitus.
Mechanisms of Agent Orange Toxicity in Plants
The two main synthetic herbicide constituents of Agent Orange, 2,4-D and 2,4,5-T are phenoxyalkanoic acids that structurally mimic indole-3-acetic acid (IAA), the most abundant natural plant growth hormone or auxin. 2,4-D has been studied more than 2,4,5-T and was shown to function as an IAA agonist that, when combined with IAA, has dose-dependent differential effects manifested by altered growth, senescence or death of plants. Its high-dose toxic effects are ultimately mediated by altered regulation of auxin-responsive genes such as rate-limiting enzymes involved in the biosynthesis of plant stress hormones. The pathophysiologic effects of 2,4-D are attributable to limited ability for metabolism and degradation which prolong its half-life in cells. Corresponding increases in stress hormones promote cellular injury due to oxidative stress and oxygenation of polyunsaturated fatty acids (lipoxygenases) (26, 27). Inhibition of plant lipoxygenase compromises host defenses and growth and metabolic processes (27). Consequences include cellular build-up of reactive oxidative species and death of the plants (28).
Potential Mechanisms of Agent Orange-Related Nervous System Diseases
The mechanisms of 2,4-D and 2,4,5-T mediated diseases of the PNS and CNS are under investigation. However, limited data from experimental models suggest that 2,4-D’s effects in plants and the PNS are similar and that its cytotoxic effects target supporting epineural, perineural and Schwann cells in a dose-dependent manner. In this regard, using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, investigators demonstrated that 2,4-D exposures increased oxidative stress in peripheral nerve sheath cells by increasing superoxide generation and reducing antioxidant mediators (29). Consequences included G1 cell cycle arrest, restricted growth and increased DNA damage (29).
Regarding the CNS, studies in rats showed that 2,4-D adversely impacts brain structure and function across the lifespan. Exposures in the early postnatal period significantly reduced brain weight along with DNA and protein content by early adolescence. Higher doses and prolonged exposure periods compromised brain lipid expression by reducing gangliosides, which are needed for inter-neuronal connectivity and communication (30) and myelin lipid levels of mono-hexosyl-ceramide and sulfatide. The alterations in myelin lipid composition were associated with white matter fiber loss in the corpus callosum and entorhinal cortex (31). Neurobehavioral consequences of early developmental exposures to 2,4-D include excessive spontaneous motor activity mimicking serotonin syndrome and hyperactivity in the open field (32). In adults, 2,4-D exposures diminished ambulation and rearing and promoted excessive grooming (32). In older rats, 2,4-D reduced locomotion and rearing and increased immobility in the open-field test, effects that were associated with reduced striatal levels of serotonin and reversed by 5-hydroxytryptophan (5-HTP), the precursor of serotonin (33).
Essentially, the neurotoxic effects of 2,4-D differ with developmental stage and aging but one common thread is that it has adverse effects on myelin and related cell types. Myelin is needed for efficient conductivity and neurological function. In addition, damage to myelin exposes axons, rendering them more vulnerable to toxins present in extracellular fluid. Of further interest is that 2,4-D reduces sulfatide in white matter. A similar abnormality occurs in neurodegeneration mediated by other agents and mechanisms (34–37) and importantly, declines in white matter lipid sulfatide due to reduced synthesis or increased degradation correlate with cognitive motor impairments. In addition, ceramide conversion to glucosyl- or galactosylceramide is the intermediary step in generating sphingomyelin. 2,4-D-associated declined in hexosyl (Gluc and Gal)-ceramide reflect build-up in ceramide, which are neurotoxic and promote apoptosis and also compromise the generation of sphingomyelin, which has critical structural and functional roles in the plasma membrane and myelin (36).
2,4-D’s neurotoxic effects mimic those of other chemical herbicides, including paraquat, which is widely used in agriculture. Paraquat causes both CNS and PNS damage by increasing reactive oxidative species and free radicals (38) and like 2,4-D, it damages myelin. Mechanistic studies have shown that paraquat damages myelin by inhibiting myelin gene expression via the Wnt-beta-catenin pathway, reducing promotor activation of myelin protein gene expression (38). Furthermore, paraquat-induced motor impairments in rats have been linked to loss of dopaminergic neurons in the substantia nigra (39). Comparable studies have yet to be performed in relation to 2,4-D and consequently it is not known if the mechanisms of myelin loss resemble those identified for paraquat. Myelin deficiency compromises nerve conductivity and impairs both PNS and CNS functions. These effects could account for the signs and symptoms of peripheral neuropathy and cognitive-motor dysfunctions observed in aging Vietnam war veterans exposed to Agent Orange.AD and PD.
Conclusions
Agent Orange exposures are associated with increased rates of birth defects, malignancies, cardiovascular disease and diabetes mellitus. However, an additional long-term consequence that has received little attention is its potential role as a mediator of chronic degenerative diseases of the PNS and CNS. This review analyzes evidence, that Agent Orange can increase the risk of peripheral neuropathy and also additively impact the neurological effects of diabetes mellitus. Agent Orange’s potential contribution to CNS degenerative diseases, including Alzheimer’s, Parkinson’s and vascular dementias are of particular interest in light of the rapidly aging population of Vietnam war veterans. Given the protracted intervals between the Agent Orange exposures and disease emergence, additional research is needed to identify mechanistic correlates of related neurological disorders.
Acknowledgments
Supported by: R37-AA11431, HD078515, AA019072, GM122732
References
- 1.Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington (DC)1994. [PubMed] [Google Scholar]
- 2.Kim JS, Lim HS, Cho SI, Cheong HK, Lim MK. Impact of Agent Orange exposure among Korean Vietnam veterans. Ind Health. 2003;41(3):149–57. [DOI] [PubMed] [Google Scholar]
- 3.Le TN, Johansson A. Impact of chemical warfare with agent orange on women’s reproductive lives in Vietnam: a pilot study. Reprod Health Matters. 2001;9(18):156–64. [DOI] [PubMed] [Google Scholar]
- 4.Ngo AD, Taylor R, Roberts CL, Nguyen TV. Association between Agent Orange and birth defects: systematic review and meta-analysis. Int J Epidemiol. 2006;35(5):1220–30. [DOI] [PubMed] [Google Scholar]
- 5.Kido T, Dao TV, Ho MD, Duc Dang N, Pham NT, Okamoto R, et al. High cortisol and cortisone levels are associated with breast milk dioxin concentrations in Vietnamese women. Eur J Endocrinol. 2014;170(1):131–9. [DOI] [PubMed] [Google Scholar]
- 6.Pham NT, Nishijo M, Nghiem TTG, Pham TT, Tran NN, Le VQ, et al. Effects of perinatal dioxin exposure on neonatal electroencephalography (EEG) activity of the quiet sleep stage in the most contaminated area from Agent Orange in Vietnam. Int J Hyg Environ Health. 2021;232:113661. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe WH, Michalek JE, Miner JC, Rahe A, Silva J, Thomas WF, et al. Health status of Air Force veterans occupationally exposed to herbicides in Vietnam. I. Physical health. JAMA. 1990;264(14):1824–31. [PubMed] [Google Scholar]
- 8.Stellman SD, Stellman JM, Sommer JF Jr., Health and reproductive outcomes among American Legionnaires in relation to combat and herbicide exposure in Vietnam. Environ Res. 1988;47(2):150–74. [DOI] [PubMed] [Google Scholar]
- 9.Michalek JE, Ketchum NS, Akhtar FZ. Postservice mortality of US Air Force veterans occupationally exposed to herbicides in Vietnam: 15-year follow-up. Am J Epidemiol. 1998;148(8):786–92. [DOI] [PubMed] [Google Scholar]
- 10.Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997;8(3):252–8. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe WH, Michalek JE, Miner JC, Rahe AJ, Moore CA, Needham LL, et al. Paternal serum dioxin and reproductive outcomes among veterans of Operation Ranch Hand. Epidemiology. 1995;6(1):17–22. [DOI] [PubMed] [Google Scholar]
- 12.Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: Where are we now and where to go? J Diabetes Investig. 2011;2(1):18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalek JE, Akhtar FZ, Arezzo JC, Garabrant DH, Albers JW. Serum dioxin and peripheral neuropathy in veterans of Operation Ranch Hand. Neurotoxicology. 2001;22(4):479–90. [DOI] [PubMed] [Google Scholar]
- 14.Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56–65. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Cheon M, Kwak YT. Is Parkinson’s Disease with History of Agent Orange Exposure Different from Idiopathic Parkinson’s Disease? Dement Neurocogn Disord. 2016;15(3):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Giau VV, An SSA, Kim S. Plasma Oligomeric Beta Amyloid in Alzheimer’s Disease with History of Agent Orange Exposure. Dement Neurocogn Disord. 2018;17(2):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrante M, Conti GO. Environment and Neurodegenerative Diseases: An Update on miRNA Role. Microrna. 2017;6(3):157–65. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Santed F, Colomina MT, Herrero Hernandez E. Organophosphate pesticide exposure and neurodegeneration. Cortex. 2016;74:417–26. [DOI] [PubMed] [Google Scholar]
- 20.Modgil S, Lahiri DK, Sharma VL, Anand A. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Transl Neurodegener. 2014;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaganas I, Kapetanaki S, Mastorodemos V, Kanavouras K, Colosio C, Wilks MF, et al. Linking pesticide exposure and dementia: what is the evidence? Toxicology. 2013;307:3–11. [DOI] [PubMed] [Google Scholar]
- 22.Franco R, Li S, Rodriguez-Rocha H, Burns M, Panayiotidis MI. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem Biol Interact. 2010;188(2):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veitch DP, Friedl KE, Weiner MW. Military risk factors for cognitive decline, dementia and Alzheimer’s disease. Curr Alzheimer Res. 2013;10(9):907–30. [DOI] [PubMed] [Google Scholar]
- 24.Martinez S, Yaffe K, Li Y, Byers AL, Peltz CB, Barnes DE. Agent Orange Exposure and Dementia Diagnosis in US Veterans of the Vietnam Era. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han S, Hwang I, Kim SM, Yang YS, Ha S, Han JH, et al. Differences in the clinical manifestations and short-term prognosis of acute cerebral infarction after exposure to Agent Orange. Ann Occup Environ Med. 2016;28:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015;6:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porta H, Rocha-Sosa M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002;130(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol. 2014;56(2):106–13. [DOI] [PubMed] [Google Scholar]
- 29.Sharifi Pasandi M, Hosseini Shirazi F, Gholami MR, Salehi H, Najafzadeh N, Mazani M, et al. Epi/perineural and Schwann Cells as Well as Perineural Sheath Integrity are Affected Following 2,4-D Exposure. Neurotox Res. 2017;32(4):624–38. [DOI] [PubMed] [Google Scholar]
- 30.Rosso SB, Di Paolo OA, Evangelista de Duffard AM, Duffard R. Effects of 2,4-dichlorophenoxyacetic acid on central nervous system of developmental rats. Associated changes in ganglioside pattern. Brain Res. 1997;769(1):163–7. [DOI] [PubMed] [Google Scholar]
- 31.Duffard R, Garcia G, Rosso S, Bortolozzi A, Madariaga M, di Paolo O, et al. Central nervous system myelin deficit in rats exposed to 2,4-dichlorophenoxyacetic acid throughout lactation. Neurotoxicol Teratol. 1996;18(6):691–6. [DOI] [PubMed] [Google Scholar]
- 32.Bortolozzi AA, Duffard RO, Evangelista de Duffard AM. Behavioral alterations induced in rats by a pre- and postnatal exposure to 2,4-dichlorophenoxyacetic acid. Neurotoxicol Teratol. 1999;21(4):451–65. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira GH, Palermo-Neto J. Effects of 2,4-dichlorophenoxyacetic acid (2,4-D) on open-field behaviour and neurochemical parameters of rats. Pharmacol Toxicol. 1993;73(2):79–85. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel S, Merrill AH Jr. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10(12):1388–97. [DOI] [PubMed] [Google Scholar]
- 35.Ariga T, Jarvis WD, Yu RK. Role of sphingolipid-mediated cell death in neurodegenerative diseases. J Lipid Res. 1998;39(1):1–16. [PubMed] [Google Scholar]
- 36.Jana A, Hogan EL, Pahan K. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J Neurol Sci. 2009;278(1–2):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mielke MM, Lyketsos CG. Alterations of the sphingolipid pathway in Alzheimer’s disease: new biomarkers and treatment targets? Neuromolecular Med. 2010;12(4):331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hichor M, Sampathkumar NK, Montanaro J, Borderie D, Petit PX, Gorgievski V, et al. Paraquat Induces Peripheral Myelin Disruption and Locomotor Defects: Crosstalk with LXR and Wnt Pathways. Antioxid Redox Signal. 2017;27(3):168–83. [DOI] [PubMed] [Google Scholar]
- 39.Fahim MA, Shehab S, Nemmar A, Adem A, Dhanasekaran S, Hasan MY. Daily subacute paraquat exposure decreases muscle function and substantia nigra dopamine level. Physiol Res. 2013;62(3):313–21. [DOI] [PubMed] [Google Scholar]


