Abstract
Biocontrol agents (BCA) have been an important tool in agriculture to prevent crop losses due to plant pathogens infections and to increase plant food production globally, diminishing the necessity for chemical pesticides and fertilizers and offering a more sustainable and environmentally friendly option. Fungi from the genus Trichoderma are among the most used and studied microorganisms as BCA due to the variety of biocontrol traits, such as parasitism, antibiosis, secondary metabolites (SM) production, and plant defense system induction. Several Trichoderma species are well-known mycoparasites. However, some of those species can antagonize other organisms such as nematodes and plant pests, making this fungus a very versatile BCA. Trichoderma has been used in agriculture as part of innovative bioformulations, either just Trichoderma species or in combination with other plant-beneficial microbes, such as plant growth-promoting bacteria (PGPB). Here, we review the most recent literature regarding the biocontrol studies about six of the most used Trichoderma species, T. atroviride, T. harzianum, T. asperellum, T. virens, T. longibrachiatum, and T. viride, highlighting their biocontrol traits and the use of these fungal genera in Trichoderma-based formulations to control or prevent plant diseases, and their importance as a substitute for chemical pesticides and fertilizers.
Keywords: Trichoderma, biocontrol agent, bioformulations, mycoparasitism, antibiosis, secondary metabolites
1. Introduction
The continuing and growing world human population is demanding more food, putting enormous pressure on various agricultural production systems. In this sense, producing more requires more significant extensions of open-field cultivation areas, which are generally dedicated to the cultivation of grains and forages; likewise, greater efficiency and investment in producing fruits and vegetables under greenhouse conditions are needed. To increase plant growth, chemical fertilizers have been used to enhance plant production to the limit of its innate capacities [1].
On the other hand, any agricultural system is exposed to the infection of potential pathogens, be they viruses, bacteria, fungi, or other types of macro-organisms [2], causing serious economic losses each year, which is why, again, the use of chemical pesticides is the first option of many agricultural producers. The main advantage of these pesticides is their immediate use and “solution” to the problem. However, the collateral damage caused by the use of fertilizers and pesticides in the environment and human and animal health has been widely documented [3,4,5,6]. In addition, chemical pesticides induce resistance in pathogens, making them challenging to control after years of continuous application [7]. Fortunately, many countries, mainly in North America and Europe, and some Asian countries, are trying to regulate and decrease its use [8,9,10]. Likewise, the mentality of consumers is changing to organic forms of production, leaving aside large fruits and vegetables and excellent aesthetics. Other developing countries are still struggling with these issues [11].
An important part of sustainable agriculture practices is the control or effective management of plant diseases. Fungi belonging to several genera have been widely used as effective biocontrol agents against fungal phytopathogens, such as Alternaria, Penicillium, Pichia, Aspergillus, and Trichoderma, with Trichoderma being the most used in the field [12,13]. The fungi Aspergillus terreus and Penicillium citrinum were able to diminish disease symptoms caused by the pathogen Sclerotium rolfsii, inducing salicylic and jasmonic acid accumulation in sunflower plants [13], proving to be effective biocontrol agents. Ten endophytic fungi, which include Penicillium sp., Guignardia mangiferae, Hypocrea sp., Neurospora sp., Eupenicillium javanicum, Lasiodiplodia theobromae, and Trichoderma sp., showed inhibition under greenhouse conditions against Fusarium oxysporum f.sp. cucumerinum, the main causal agent of cucumber stem rot disease [14]. Among 32 fungal isolates from the plant Brugmansia aurea, A. aculeatus inhibited the growth of F. solani and A. fumigatus, showing potential as BCA [15].
Fungal BCAs are also effective against other kinds of pests, such as insects and nematodes [16,17,18]. Several Trichoderma species have been proven to be effective at controlling pests such as Tetranychus urticae and different insects that affect important crops [17]. Arbuscular mycorrhizal fungi (AMF) have been widely studied because of their positive effects on plant growth promotion; nonetheless, they are also effective against phytopathogens, such as Meloidogyne incognita and other nematodes [18]. The fungus Arthrobotrys oligospora, which forms adhesive structures to capture nematodes, is another potential BCA of phytopathogens [19,20]. This information suggests the versatility of fungal BCA to counteract several types of phytopathogens.
Fungal biocontrol agents can also protect plants against abiotic stresses, such as high temperatures [21,22]. They are also used as plant defense enhancers due to their ability to induce systemic resistance, protecting them against several pathogens, all of which lead to an increase in plant yield. In this regard, fungi also have played important roles in enhancing plant growth and crop production [23]. Fungi that can induce plant growth include species from the genera Trichoderma, Aspergillus, Fusarium, Penicillium, Piriformospora, Rhizoctonia, Colletotrichum, Gliocladium, Phoma, and others [24,25]. The fungus Acremonium sp. showed plant growth-promoting traits on Allium tuberosum plants, increasing root and shoot length, as well as antifungal activity against Botryiosphaeria dothidea and Botrytis cinerea [26]. The fungi Alternaria sp., Phomopsis sp., and Cladosporium sp. increased the biomass of tobacco plants, showing potential as plant growth-promoting fungi [27]. T. virens and T. atroviride can promote secondary root system development and biomass production of Arabidopsis and tomato plants [28,29], being one of the most used genera as plant growth—promoters.
The damage caused by the use of chemical fertilizers and pesticides and the growing use of biocontrol agents presents the need to steer agricultural production systems toward sustainability and stop using synthetic fertilizers and pesticides as much as possible.
An efficient, low-cost, and eco-friendly alternative is the application of microorganisms that promote plant growth and offer protection against pests and pathogens, such as the fungi of the genus Trichoderma [30,31]. The use and application of bioinoculants with Trichoderma as an antagonistic agent is one of the most active biological control strategies in various countries. In fact, between 50 and 60% of the global market for biological control agents (BCAs) around the world is based on the content of several Trichoderma species [32,33]. The controlling action of these Trichoderma-based biopesticides mainly includes fungal and oomycete pathogens, such as Acremonium cucurbitacearum, Alternaria spp., Aphanomyces cochlioides, Aspergillus spp., Lasiodiplodia theobromae, Botrytis cinerea, Botrytis spp., Collisletotnicios spp., Collisletnicios spp., Diplodia natalensis, Fusarium spp., Gaeumannomyces graminis var. tritici, Lasiodiplodia theobroma, Phoma betae, Rhizoctonia solani, Rhizopus oryzae, Pythium spp., Serpula spp., Sclerotium spp., Verticillium dahliae, among others [32].
Trichoderma comprises several species of filamentous fungi that are common inhabitants of the soil, rhizosphere, and endosphere of plants. These fungi have attracted our attention because they can control the growth and infection of potential pathogens such as fungi or nematodes [17]. In this work, these beneficial aspects of different Trichoderma species are reviewed, exhibiting different modes of action that benefit many sustainable agricultural production systems.
2. An Overview of the Genus Trichoderma
The first description of the fungus Trichoderma as a genus was in 1794 by Persoon, while Tulasne and Tulasne suggested the sexual state of a Hypocrea species in 1865 [34]. Likewise, in 1932 Weindling was a pioneer in proposing Trichoderma as a fungus that “parasites” other fungi with the potential to control them [35]. Trichoderma species belong to the Hypocreaceae family. They present filamentous hyphae, with optimal growth temperatures between 25 and 30 °C, and they are widely present in various environments, preferring those where there is a decomposing organic matter [36]. Trichoderma conidiophores are abundant and end in phialides, pyramidal in shape, and their branches grow in pairs [37]. Asexual conidia are formed abundantly, elliptical in shape, and hyaline, which then develop from white to yellow, and then green conidia when completely mature [36].
Enormous advances have been made in the taxonomy of Trichoderma; however, there are still some issues to be resolved when differentiating species within the genus since the vast majority of Trichoderma species are not associated with their sexual state and are therefore handled as monoclonal and mitotic. Recent attempts to improve their classification based on barcode oligonucleotide include online tools such as TrichoKEY [38] and DNA Barcoding markers (TrichoMARK), such as internal transcribed sequences (ITS), tef1, and rpb2 genes, to perform specific BLAST type searches (TrichoBLAST) [39]. Recently, Dou and colleagues [40] proposed a Multilocus Identification System (MIST) online for the identification of Trichoderma and Hypocrea (anamorphs) species for automated detection of 349 Trichoderma possible species, also based on a set of three DNA barcodes. Online websites are https://trichokey.com (accessed on 18 December 2022) and http://mmit.china-cctc.org (accessed on 18 December 2022), respectively.
Genome sequencing techniques allowed for a more in-depth study of the genus Trichoderma, with T. virens, T. atroviride, and T. reesei being the first species among the genus to have their genome sequenced. This allows a better understanding of their lifestyle as mycoparasites and the difference between species [41].
Some examples of species include T. harzianum, T. aggressivum, T. citrinoviride, T. asperellum, T. ghanense, T. hamatum, T. koningii, T. pseudokoningii, T. virens, T. longibrachiatum, T. polysporum, T. tomentosum, T. atroviride, T. gamsii, T. koningii, Hypocrea jecorina/Trichoderma reesei, T. spirale, T. viridescens, T. viride, and T. koningiopsis, which have been found in different ecosystems, such as soils of forests, gardens, decaying wood, cultivated mushroom compost, cereal grains, from various regions of the world, and in marine environments [42,43,44].
Trichoderma spp. has been (mostly) considered as non—pathogenic and opportunistic plant symbionts, which can colonize plant roots, establishing a beneficial interaction with their hosts mediated by Trichoderma effector proteins and hormonal crosstalk in exchange for plant-derived sugars [45,46,47,48].
During the Trichoderma– plant interaction, the benefits received by the plant are not just an increase in biomass and overall nutrition but also protection against several phytopathogens, either by acting directly over the pathogen as a mycoparasite and competing for nutrients or indirectly by inducing the plant defense system [49,50,51,52]. Several species from this genus have been studied and used in field assays as effective biocontrol agents, such as T. harzianum, T. virens, T. atroviride, T. asperellum, T. hamatum, T. gamsii, T. viride, among others [30,51,53,54,55,56]. Some of these species will be reviewed further.
3. Mechanisms for Protecting Plants Exerted by Trichoderma
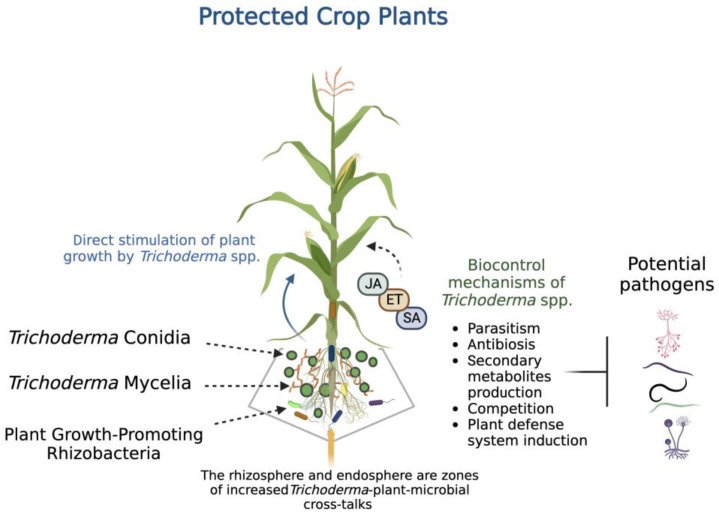
The biocontrol mechanisms exerted by Trichoderma, which lead to efficient plant protection, can be direct when the fungus interacts with the pathogen by mycoparasitism, competition, or antibiosis; and indirect if the fungus enhances plant defense systems so the plant can defend itself against its pathogen [57,58]. Additionally, Trichoderma spp. can exert diverse direct plant growth-promoting activities by producing some molecules, such as phytohormones. Figure 1 depicts the biocontrol properties of Trichoderma, exerting protection on crop plants.
Figure 1.
Direct biostimulation and biocontrol properties of Trichoderma species. Beneficial Trichoderma spp. exert fungal-root communication via diffusible and volatile compounds, regulation of the stress hormone ethylene, and production of phytohormones, such as auxins (indole-3-acetic acid). Some of the plant-protecting mechanisms of Trichoderma include parasitism, antibiotic and secondary metabolites production, or activation of the induced systemic resistance (ISR). Trichoderma can trigger both growth-stimulating effects and plant defense action by the elicitation of salicylic acid (SA), ethylene (ET), and jasmonic acid (JA) dependent pathways against several types of potential plant pathogens such as nematodes and fungi.
3.1. Mycoparasitism
Mycoparasitism is one of the main mechanisms of inhibition of the mycelial growth of fungal pathogens, providing nutrients to the mycoparasite when they kill their prey. In some cases, Trichoderma obtains the nutrients but does not kill the pathogen (biotrophic mycoparasites). In work by Kubicek and collaborators [41], they compared the genome of three Trichoderma species (T. reesei, T. virens and T. atroviride), as well as their respective teleomorph or sexual forms (Hypocrea jecorina, H. virens and H. atroviridis, respectively). The authors found high conservation of the genetic origin (up to 96%), in addition to the fact that several genes that code for antagonistic or mycoparasitic activities are conserved in these species, suggesting that mycotrophy is an ancestral lifestyle in this genus [41,59].
There are three main steps during the act of mycoparasitism. This function can be carried out in the rhizosphere of plants, an ecosystem efficiently colonized by Trichoderma and where the biological control of potential pathogens is important to avoid plant diseases. First, Trichoderma requires recognition of the host (or possible plant pathogenic fungus), where the production of oligochitins has been proposed as sensor molecules [60]. Likewise, it is known that during this previous step, various genes that encode proteases and oligopeptide transporters are expressed before contact with the fungal host. Second, hydrophobin-like proteins may have a relevant function once Trichoderma encounters the plant-pathogenic fungus, which leads to the formation of papillae or appressoria-like structures. The third step occurs when Trichoderma coils around the pathogen hyphae and starts degrading it via the production of cell-wall degrading enzymes, such as cellulases and hemicellulases, chitinases, proteases, and -1,3-glucanases, among other secondary metabolites, that are essential for mycoparasitism [60]. It should be noted that the host that is being parasitized also produces metabolites and reactive oxygen species (ROS) as defense mechanisms in response to the attack, which in turn, Trichoderma turns on genes involved in detoxification and response to stress. Interestingly, these lytic proteins are also produced and purified using Trichoderma as a host for biotechnological purposes [61].
3.2. Antibiosis
The biological control mechanism known as antibiosis involves the production and excretion of secondary metabolites, which include compounds of a different chemical nature with cytotoxic activity, that can limit or inhibit pathogen growth. Antibiosis is one of the main modes of action of Trichoderma and other biological control agents, such as plant growth-promoting bacteria (PGPB) [34,51,62]. In fact, the expression of coding genes to produce antibiotic metabolites is increased in the presence of pathogens and compounds produced by plants, exerting a stimulating effect of protection and fine signaling between the plant, the pathogen, and the biocontrol agent [34,51].
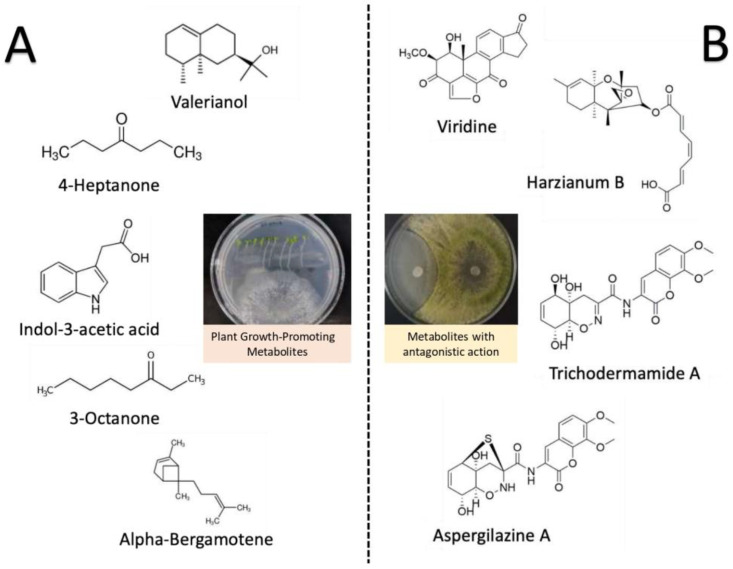
The various species of Trichoderma are a factory of secondary metabolites, as more than 180 different types of compounds have been proposed and can be classified according to their function in competition and iron-quelating metabolites, inducers of plant resistance, plant growth-promoting metabolites, antibiotics, and if the metabolites are volatile or non-volatile [63,64,65]. For example, T. virens species produce trichodermamides, while T. koningii synthesizes Koninginins, both with antimicrobial and antifungal activity [66,67]. Furthermore, in T. harzianum and T. virens, compounds such as azaphilones, viridins, nitrogen heterocyclic compounds (e.g., harzianopyridone and harzianic acid), and volatile terpenes have been characterized, and are involved in the biocontrol of pathogenic fungi [30]. The production of hydrolytic enzymes and proteases, such as exo- and endochitinases, chitinases, xylanases, glucanases, lipases, endo-and exopeptidases, among others with antifungal action, have also been characterized in different Trichoderma spp. [68]. The volatile organic compound (VOC) 6-pentyl-2H-pyran-2-one (6-pentyl-α-pyrone, 6-PP) is the most abundant VOC from T. atroviride, and it enhances plant growth and regulates sugar transport in Arabidopsis roots, along with other VOCs produced by the fungus [69]. Figure 2 shows a glimpse of the metabolite’s arsenal involved in fungal antagonism and some compounds involved in plant growth promotion (e.g., indol-3-acetic acid).
Figure 2.
Examples of Trichoderma secondary metabolites involved in plant interactions with growth-promoting effects (A) and secondary metabolites involved in mycoparasitism with antibiotic effect (B).
3.3. Competition
Bulk and rhizospheric soil are complex ecosystems with a continuous battle to access resources and maintain survival. In the rhizosphere, a much richer environment than bulk soil due to the excretion of nutrients by the plant roots, such as amino acids, vitamins, organic acids, saccharides, etc., competition is an essential strategy for survival [70,71]. For this reason, those organisms residing in the rhizosphere with efficient metabolic and competitive capacities will access the best “sites” where the resources exist. In this sense, Trichoderma species, as mentioned before, are capable of producing a series of antagonistic compounds (e.g., antibiotics or lytic enzymes), which, in conjunction with further rapid growth and colonization strategies (e.g., metabolic versatility), they can occupy spaces in the rhizosphere and, directly, benefit the growth of plants and restricting the development of other potentially pathogenic microorganisms [72,73]. However, this strategy is also employed by PGPB, which exhibits efficient colonization mechanisms to occupy rhizospheric spaces and endophytic regions [74]. Therefore, when selecting Trichoderma biocontrol species (or other biocontrol microorganisms), it is important to perform antagonism tests towards beneficial organisms for plants, such as PGPB [75] to determine their synergistically or detrimental potential among each other.
3.4. Induction of Plant Defense System
When attacked by various pathogens or mechanical damage, plants turn on defense systems that allow them to protect themselves, such as systemic acquired resistance (SAR) [76]. In some other cases, plant-associated microbes can induce the plant defense systems, such as the rhizobacteria-induced systemic resistance (RISR) pathway, which phenotypically resembles SAR [1], in response to the presence of the microorganism. It could be said that Trichoderma-induced systemic resistance (TISR) is very similar to RISR since both are regulated by the jasmonic acid and ethylene (JA/ET) signaling pathway [52,77,78]. However, the plant defense system and the signaling that coordinates the response are highly variable, even within the same plant kingdom. In fact, Bakker et al. [78] mention that in RISR, there is no induction of the expression of pathogenesis-related proteins (PR), as in SAR, which is stimulated by the attack of fungal or herbivore pathogens. Nonetheless, T. hamatum strain Th23 can induce PR-1 and PR-7 expression in tomato plants upon infection with Tobacco Mosaic Virus (TMV)[79]. The overall plant response to pathogens includes the production of antifungal glucanases and chitinases, thaumatins, as well as the synthesis of oxidative enzymes, including peroxidases (POD), polyphenol oxidases (PPO), and lipoxygenases [80] and the activation of several transcription factors involved in the plant immune response to biotic stressors [81]. T. hamatum strain Th23 induces CAT, SOD, and PPO enzymatic activity in tomato plants during infection with TMV and reduces H2O2 and malondialdehyde (MDA) concentrations [79]. One of them is NPR1, a transcription factor that is widely known for its action in modulating both SAR and RISR pathways [82]. T. harzianum TR 274 induces the expression of several defense-related genes in Phaseolus vulgaris plants, such as glu, chit, and pal [83], which are genes related to TISR; and the commercial formulation BIOSPARK™, made from Trichoderma spp. induces resistance in Lansium domesticum plants against the insect Unaspis mabilis [84]. Some TISR elicitor compounds are homologous to those produced by rhizobacteria, such as siderophores, acyl-homoserine lactones, and antimicrobial compounds, among others [85,86]. It should be taken into account that the TISR response has been little studied compared to RISR. Trichoderma elicitors may be regulated in different ways according to the species; for example, SM1/EPL1 from T. virens and T. atroviride induce a defense response in plants, but SM1 from T. harzianum seems to be downregulating plant defense responses, allowing root colonization [83]. This suggests that species and type of elicitor are important factors to consider when inducing TISR in plants, so it is necessary to delve further into the elicitors and induction pathways of plant defense systems.
4. Biocontrol Potential of Registered Trichoderma Species
With over 200 Trichoderma species registered [40,87] and their potential to be used as biocontrol agents and plant growth promoters, it has led to many studies to gain more knowledge about their mechanisms of action, focusing studies on mycoparasitism and competition [60,88], production of secondary metabolites with antagonistic activity [63,68] and induction of plant systemic resistance [52,89].
Among the registered Trichoderma species, T. harzianum, T. asperellum, T. atroviride, T. longibrachiatum, T. viride, and T. virens are the most sampled ones [55]. The first three species are among the most used biocontrol agents, using mycoparasitism and competition as their primary mechanism of action against fungal phytopathogens. Meanwhile, species such as T. virens, T. longibrachiatum, and T. viride use antibiosis as a strong mechanism of action against several plant pathogens [50,88,90,91]. Here, we review the Trichoderma species mentioned above, focusing on their biocontrol traits and the most recent literature on this subject, summarized in Table 1.
Table 1.
Main Trichoderma species used in agriculture and their biocontrol traits.
| Trichoderma Species | Biocontrol Traits | Biocontrol Effect | Reference |
|---|---|---|---|
| T. atroviride | Parasitism and competition | Competition and mycoparasitism inhibit the growth of several plant pathogens | [92] |
| Competition against Ph. cinnamomi inhibits the pathogen growth | [93] | ||
| Competition inhibits the growth of N. parvum | [94] | ||
| Competition and antagonistic activity against F. avenaceum and F. culmorum | [95] | ||
| Secondary metabolites production and antibiosis | Ethyl acetate extract inhibits growth and has antifungal activity against 25 plant pathogens | [92] | |
| Swollenin TaSwo1 confers protection in Capsicum annum plants against A. solani and R. solani | [96] | ||
| Vel1-derived secondary metabolites and parasitism-related enzymatic activity influences mycoparasitic activity against F. graminearum | [97] | ||
| Fungal culture inhibits the growth of pathogen F. avenaceum | [95] | ||
| Fermented culture inhibits the growth of B. cinerea | [98] | ||
| Tal6, a LysM effector, antagonizes several plant pathogens | [99] | ||
| VOCs inhibit the growth of pathogen F. avenaceum | [95] | ||
| 6-PP production under dark conditions enhances antagonistic activity against R. solani and F. oxysporum | [100] | ||
| VOCs inhibit the growth of R. solani, B. cinerea, and F. oxysporum, conferring resistance in Arabidopsis plants | [101] | ||
| Plant defense induction/Priming | SA induced defense response in grapevine Tempranillo cultivar, protecting the plant against N. parvum | [94] | |
| Increasing the defense-related enzymatic activity in tomato plants confers resistance against B. cinerea and diminishes the disease’s symptoms | [98] | ||
| Modification of gene transcripts related to plant defense, and induction of plant-defense VOCs, confer resistance to the moth S. littotalis and the aphid M. euphorbiae in tomato plants | [102] | ||
| Priming JA and SA defense pathways increased gene expression confers resistance against B. cinerea in Arabidopsis plants | [103] | ||
| T. harzianum | Parasitism and competition | Growth inhibition of F. oxysporum in in vitro confrontations | [104] |
| Competition for nutrient and space and mycoparasitism inhibits the growth of F. sudanense | [105] | ||
| Growth inhibition of A. cerealis in in vitro confrontations | [106] | ||
| Mycoparasitism inhibits the growth of F. oxysporum | [107] | ||
| Growth inhibition of several postharvest pathogens of sweet potato in in vitro assays | [108] | ||
| Mycoparasitism inhibits the growth of F. oxysporum, A. alternata, A. flavus, and A. carbonarius | [109] | ||
| Competition for space with the pathogen F. pseudograminearum in the rhizosphere soil of wheat plants | [110] | ||
| Mycoparasitism of F. graminearum inhibits the pathogen growth | [111] | ||
| Growth inhibition of C. truncatum in in vitro confrontations | [112] | ||
| Secondary metabolites production and antibiosis | 6-PP application on maize roots diminishes root damage by the scarab P. vetula | [113] | |
| Secondary metabolite production regulated by the transcriptional coactivator ThMBF-1 is important to inhibit the growth of B. cinerea and F. oxysporum and to confer resistance in tomato plants | [114] | ||
| Reduction of cucumber mosaic virus infection on cowpea plants by three peptaibols: trichorzins HA II, HA V, and HA VI. | [115] | ||
| Aspartic protease P6281 inhibits the growth and spore formation of B. cinerea, M. circinelloides, A. flavus, A. fumigatus and inhibits the growth of R. solani | [116] | ||
| Chitinase activity and hydrophobicity are essential for the mycoparasitism of R. solani | [117] | ||
| SMs from the fungal extract inhibit the growth of F. graminearum | [73] | ||
| Culture filtrate from T. harzianum has antifungal activity against F. oxysporum | [107] | ||
| The enzyme ThLAAO induces the expression of defense-related genes in tobacco plants, conferring resistance against B. cinerea and S. sclerotiorum | [118] | ||
| Epl-1 down-regulates virulence genes in B. cinerea during in vitro confrontations | [119] | ||
| Culture filtrate from T. harzianum inhibits P. ultimum growth | [120] | ||
| SM from culture filtrates reduce the growth of several plant pathogens | [121] | ||
| Metabolites extracts alleviate the symptoms caused in wheat seedlings by the pathogen F. culmorum | [122] | ||
| In planta expression of ThKEL1 induces the expression of genes involved in SA and JA pathways in Arabidopsis and rapeseed plants, conferring resistance against B. cinerea | [123] | ||
| Plant defense induction/Priming | JA signaling induction in tomato against the feeding insect N. viridula | [124] | |
| SA and JA/Et signaling induction in tomato plants upon infection with the nematode M. incognita | [125] | ||
| Strong VOC priming in tomato plants to attract the parasitoid A. ervi to exert biocontrol over the aphid M. euphorbiae | [126] | ||
| Induction of antioxidant enzymes in tomato plants upon F. oxysporum infection | [104] | ||
| Induction of Et, JA, ISR pathways, and isoprenoid biosynthesis in tomato plants upon M. euphorbiae infestation | [127] | ||
| Induction of defense-related enzymes, SA accumulation, and phenolic compounds in wheat, conferring resistance to B. sorokiniana | [128] | ||
| Induction of several plant defense-related compounds in tomato plants upon infection with A. cerealis | [106] | ||
| Increased protection and reduction of cell death in soybean plants upon F. oxysporum infection | [107] | ||
| SA signaling pathway and priming are induced by Epl-1 in tomato plants against B. cinerea | [119] | ||
| Induction of antioxidant activity and redox homeostasis in cucumber plants promotes resistance against F. oxysporum | [129] | ||
| Induction of priming, defense-related enzymatic activity, antioxidant enzymatic activity, and reduction of ROS accumulation in chili pepper plants, protecting and reducing symptoms from C. truncatum disease | [112] | ||
| T. asperellum | Parasitism and competition | Competition and mycoparasitism inhibit the growth of eight phytopathogens | [130] |
| Parasitism of the maize moth pathogen O. furnicalis, inducing enzymatic activity related to plant defense in maize | [131] | ||
| Growth inhibition of C. truncatum in in vitro confrontations | [112] | ||
| Competition and mycoparasitism inhibit the growth of several plant pathogens | [92] | ||
| Growth inhibition of R. solani and A. alternata under salt stress conditions | [132] | ||
| Competition and mycoparasitism inhibit the growth of F. proliferatum f.sp. malus domestica MR5, and other plant pathogens | [133] | ||
| Growth inhibition of the lettuce pathogens C. cassiicola and C. aeria | [134] | ||
| Competition and mycoparasitism inhibit the growth of F. oxysporum f.sp. cucumerinum Owen and F. graminearum | [135] | ||
| Mycoparasitism inhibits the growth of P. noxius and confers resistance in E. japonica plants | [136] | ||
| Secondary metabolites production and antibiosis | Elicitor protein Epl1-Tas induces enzymatic activity related to plant defense response in P. davidiana × P. alba var. pyramidalis, conferring resistance against A. alternata | [137] | |
| Hydrophobin HFBII-4 induces enzymatic activity and gene expression related to plant defense response in P. davidiana × P. alba var. pyramidalis, conferring resistance against A. alternata | [138] | ||
| The fermented broth has antifungal activity against F. oxysporum, F. graminearum, and B. cinerea and increases the resistance of wheat against F. graminearum | [139] | ||
| The crude extract containing peptaibols inhibits spore germination of A. solani, R. solani, and F. moniliforme, and it has antibacterial activity against M. luteus | [140] | ||
| Crude extract and 6-PP inhibit the growth of M. maydis | [141] | ||
| Ethyl acetate extract inhibits growth and has antifungal activity against 25 plant pathogens | [92] | ||
| Liquid fermentation extract inhibits F. proliferatum f.sp. malus domestica MR5 growth and spore germination | [133] | ||
| Filtered fermentation liquor inhibits F. graminearum growth | [135] | ||
| Crude citric extract inhibits F. oxysporum f.sp. lycopersici growth and induces enzymatic activity related to plant defense response in tomato plants | [142] | ||
| Vel1-derived SM induces the expression of defense-related genes in maize plants, conferring resistance against C. herostrophus and F. verticilloides | [143] | ||
| VOCs prevent postharvest rot caused by F. incarnatum in Cucumis melo fruits, and they inhibit pathogen growth | [144] | ||
| VOCs inhibit F. proliferatum f.sp. malus domestica MR5 growth | [133] | ||
| Plant defense induction/Priming | Induction of defense-related genes in tomato plants, granting resistance against A. alternata | [130] | |
| Induction of hypersensitive response in Pisum sativum plants in response to the pathogen E. pisi | [145] | ||
| Induction of systemic resistance and reduction of ROS accumulation in tomato leaves upon infection with F. oxysporum and B. cinerea | [146] | ||
| Induction of priming, defense-related enzymatic activity, antioxidant enzymatic activity, and reduction of ROS accumulation in chili pepper plants, protecting and reducing symptoms from C. truncatum disease | [112] | ||
| Induction of defense-related enzymatic activity in lettuce plants upon infection with C. cassiicola and C. aeria | [134] | ||
| T. virens | Parasitism and competition | Antagonistic and mycoparasitic activity against F. oxysporum f.sp. physalia, diminishing disease severity in Physalis peruviana plants | [147] |
| Mycoparasitic activity against R. solani. | [148] | ||
| Secondary metabolites production and antibiosis | Excess production of secondary metabolites enhances antibiosis and mycoparasitic capacity against P. aphanidermatum and S. rolfsii and confers protection on Cicer arietinum plants against S. rolfsii | [149] | |
| Chitinase and cellulase activity inhibit R. solani growth | [150] | ||
| Secondary metabolites inhibit the growth of R. solani AG2 and induce JA and SA accumulation in A. thaliana plants | [151] | ||
| Non-volatile secondary metabolites inhibit the growth of R. solani and downregulate genes coding for defense enzymatic activity in the pathogen | [148] | ||
| Ferricrocin, a siderophore, is involved in ISR induction in maize against C. heterostrophus | [152] | ||
| Endopolygalacturonase TvPG2 induces resistance in tomato plants against B. cinerea via ISR induction | [153] | ||
| Cell-free supernatant inhibits the growth of F. oxysporum f.sp. physalia, and confers resistance in P. peruviana plants | [147] | ||
| Culture filtrate induces ISR in tomato plants, conferring resistance to F. oxysporum f.sp. lycopersici. Priming and induction of JA defense pathway in tomato plants against F. oxysporum f.sp. lycopersici | [154] | ||
| Volatile secondary metabolites inhibit R. solani growth | [148] | ||
| Plant defense induction/Priming | Induction of defense-related genes confers resistance against R. solani in Vigna radiata susceptible and resistant varieties. | [155] | |
| Induced systemic resistance in maize plant against C. graminicola, via the induction of oxylipins and ketol, as ISR signals | [156,157] | ||
| T. longibrachiatum | Parasitism and competition | Competition and mycoparasitism inhibit the growth of six phytopathogens, being more effective against R. solani and A. solani | [140] |
| Competition and antibiosis inhibit the growth of Sclerotium rolfsii and M. phaseolina | [158] | ||
| Mycoparasitism inhibits the growth of F. pseudograminearum | [159] | ||
| Parasitism of eggs and second-stage juveniles of H. avenae | [160] | ||
| Mycoparasitism inhibits the growth of M. phaseolina | [161] | ||
| Competition diminishes the presence of Magnaporthiopsis maydis in maize plants and its negative effect on plant growth and disease symptoms in field conditions | [162] | ||
| Secondary metabolites production and antibiosis | The crude extract containing peptaibols has antibacterial activity against M. luteus | [140] | |
| Dendrobine has antibacterial properties against plant-pathogenic bacteria | [163] | ||
| Synthetic analogs to the peptaibol Trichogin inhibit the growth of Pyricularia oryzae, reduce disease symptoms in rice and barley plants, and alter the spore and mycelial structure of the pathogen | [164] | ||
| The hydrophobin HYTLO1 induces the expression of defense-related genes in Lotus japonicus plants | [165] | ||
| Metabolites inhibit the growth of M. phaseolina | [161] | ||
| Sesquiterpenes and cyclodepsipeptides inhibit the growth of several plant fungal pathogens and the nematode pathogen M. incognita | [166] | ||
| Culture filtrate and sorbicillinoids inhibit the growth of several plant pathogens and confer resistance in tomato plants against Ph. infestans | [167] | ||
| Ethyl acetate extract has effective toxicity against the cotton aphid A. gossypii | [168] | ||
| Fermentation crude extract and fungicide compounds inhibit the growth of the pathogen V. mali | [169] | ||
| VOCs inhibit the growth of S. rolfsii and M. phaseolina | [158] | ||
| Plant defense induction/Priming | Induction of JA/Et and SA pathways, conferring resistance in cucumber plants against B. cinerea | [77] | |
| Induction of defense-related enzymatic activity and flavonoids and lignin content in wheat roots upon infection with H. avenae | [160] | ||
| T. viride | Parasitism and competition | Competition inhibits S. sclerotiorum growth in dual confrontations | [170] |
| Competition inhibits F. solani, R. solani, and S. rolfsii growth in dual confrontations | [171] | ||
| Secondary metabolites production and antibiosis | VOCs show antibacterial and antifungal activity | [171] | |
| VOCs inhibit the growth of S. rolfsii in soil and in dual confrontations, affecting the mycelial structure. VOCs induce defense-related enzymatic activity in okra plants upon infection with S. rolfsii | [172] | ||
| Crude and ethanol extract show antibacterial and antifungal activity | [171] | ||
| Plant defense induction/Priming | Induction of antioxidant enzymatic activity and reduction of ROS accumulation in Phaseolus vulgaris plants upon infection with S. sclerotiorum | [170] |
4.1. Trichoderma Atroviride
Trichoderma atroviride is a filamentous fungus that can be isolated from soil, mainly in temperate climates, with optimal growth at 25 °C, presenting thin and hyaline colonies and inconspicuous aerial hyphae and gray to dark green conidia after 2 to 7 days [173]. It has a characteristic coconut smell due to the production of the volatile compound 6-pentyl-2H-pyran-2-one, or 6-PP, which also is involved in biocontrol against several plant pathogens, including Cylindrocarpon destructans, Mcrophomina phaseolina, Phytophthora sp., and others, and it is also involved in plant growth-promotion and induction of systemic resistance [174,175,176].
T. atroviride has biocontrol capacity on different plant pathogens, including fungi, oomycetes, and pests, such as nematodes or insects, by exerting different mechanisms of biocontrol [17,31], which are presented below with recent examples from the literature.
4.1.1. Parasitism and Competition
In a competition for space and nutrients, T. atroviride inhibits Phytophthora cinnamomic growth and zoospore formation. In a tripartite interaction with tomato plants, T. atroviride enhances protection against the disease induced by this oomycete [93]. Also, T. atroviride competes with and is capable of antagonizing Fusarium avenaceum and Fusarium culmorum, important maize pathogens [95], and the grapevine pathogen Neofusicoccum parvum [94]. In dual culture assays, T. atroviride can parasitize several fungal pathogens, including Neofusicoccum batangarum, N. parvum, Phytophthora nicotianae, Penicillium digitatum, P. commune, P. roqueforti, P. verrucosum, Aspergillus steynii, Fusarium proliferatum, F. verticilloides, F. sporotrichoides and F. poae [92].
As stated before, the main mechanism used by T. atroviride as a biocontrol agent against fungal pathogens is mycoparasitism [50]. Nonetheless, this fungus also uses other strategies to limit the growth of different plant pathogens.
4.1.2. Secondary Metabolites
The effect of secondary metabolites as a biocontrol trait can be tested as whole fungal cultures or extracts, as individual components, or as a mix of components that had been identified from the whole extract. Whole fungal culture (soluble metabolites) from a local T. atroviride strain BC0584 and its volatile organic compounds (VOCs) are capable of inhibiting the growth of F. avenaceum. In contrast, both soluble metabolites and VOCs did not have any statistical significance in inhibiting the growth of F. culmorum. Nonetheless, in confrontation assays, both pathogens are controlled by T. atroviride BC0584 [95]. The production of VOCs is a characteristic of Trichoderma species, and 6-PP is probably the most characterized VOC from the species that synthesize this compound, such as T. atroviride [175]. The synthesis of 6-PP is regulated by dark conditions when it is produced in more quantities. It enhances the antagonistic activity of T. atroviride P1 and IMI 206,040 strains against R. solani and F. oxysporum [100]. Fermented cultures are used to obtain certain metabolites, such as antibiotics, and are obtained at the end of several days of the fungus growing in a liquid medium [177]. The fermented culture from T. atroviride CCTCCSBW0199 could inhibit the growth of B. cinerea in an in vitro assay to 73% [98], indicating an antibiosis mechanism to control the pathogen.
In a broad-range pathogen study, Stracquadanio and collaborators [92] found that the ethyl acetate extract and the fungal filtrate from T. atroviride (TS) inhibit growth and have strong cytotoxic activity against 25 pathogens, which includes 7 species of Penicillium, 6 species of Aspergillus, 6 species of Fusarium, 2 species of Neofusicoccum, 2 species of Colletotrichum, and 2 species of Phytophthora. The velvet complex proteins in Trichoderma are involved in several physiological processes, including secondary metabolite synthesis [91]. In a study to unravel the role of vel1, a member of the velvet complex in T. atroviride T23, Karuppiah and collaborators [97,143] found that the fungal extract of the wild-type strain and the vel1 overexpressing strain, both alone and in the co-culture with Bacillus amyloliquefaciens 1841, inhibit the growth of the wheat pathogen F. graminearum, and decrease the disease severity in plants treated with those strains; the authors also note that the co-cultures have better inhibition rate over the pathogen, and induce a stronger plant resistance that the single cultures [97].
Swollenins are proteins with similarity to plant expansins and are involved in the remodeling of plant cell walls and colonization [178]. TaSWO1, a swollenin secreted by T. atroviride, can induce resistance in Capsicum annum plants against A. solani and R. solani, reducing the symptoms caused by these pathogens [96]. The LysM effector identified as Tal6 from T. atroviride IMI 206,040 binds fungal chitin, preventing the plant from sensing the BCA, allowing it to establish a beneficial interaction, and enhancing T. atroviride mycoparasitic activity against B. cinerea, Sclerotium cepivorum, Colletotrichum lindemutianum and R. solani AG2 [99].
The capacity of T. atroviride to produce a wide range of volatile and non-volatile secondary metabolites is indicative of its capacity to control different types of phytopathogens, which makes this fungus a capable BCA in many agricultural situations.
4.1.3. Plant Defense Induction and Priming
Trichoderma, colonization of roots, can induce different plant defense responses; for example, T. atroviride SC1 induces SA-mediated defense response in grapevine Tempranillo cultivar, enhancing the plant protection against N. parvum, which is also inhibited in dual cultures with T. atroviride [94]. Leal and collaborators (2021) also performed co-cultures of T. atroviride with B. subtilis PTA-271. They found that co-culture is better at enhancing plant protection than the inoculation of single organisms.
Some of the secondary metabolites produced by T. atroviride can induce plant resistance; for instance, fermented culture from T. atroviride CCTCCSBW0199 alone or in combination with brassinolide increases peroxidase (POD) and superoxide dismutase (SOD) activity in tomato plants, which then increases the plant resistance and reduces the symptoms induced by B. cinerea [98].
Trichoderma species, when colonizing the plant, alter plant transcriptome, modifying gene transcription involved in plant defense responses, such as T. atroviride P1, which modifies gene transcripts related to plant defense, and induces plant-defense related VOCs to attract aphid-predatory wasps Aphidius ervi. They provide a better defense mechanism against the aphid Macrosiphum euphorbiae and the moth Spodoptera littotalis in tomato plants [102], proving that T. atroviride may be able to control pathogens indirectly, modulating plant physiology. Besides modification of the transcription of genes involved in plant defense responses, colonization by T. atroviride also induces Arabidopsis’s sRNA-mediated gene silencing, leading to an increase in gene expression of JA and SA-mediated pathways, which in turn induces priming and increases resistance in the plant against B. cinerea [103].
Plant defense induction by T. atroviride is an important mechanism of action for this BCA due to the span of plant pathogens that can be controlled with it since direct mechanisms such as mycoparasitism may be limited. Still, it can be complemented with indirect mechanisms. Therefore, T. atroviride is an effective antagonist of several fungal, oomycete, insect, and other plant pathogens.
4.2. Trichoderma Harzianum
T. harzianum is frequently found in temperate climates, with optimal growth at 30 °C, but can grow fine at 35 °C; conidiation is presented at day 2 in concentric zones when growing in Petri dishes, changing from green to dark green/brownish in color; unlike T. atroviride, T. harzianum has no particular odor [37,179].
This fungus is found in several substrates such as soil, other fungi, decaying plant material, and as an endophyte of several plants, acting as a biocontrol agent for different soil-borne diseases. It has been used widely in agriculture, being one of the active ingredients of commercial products used to control crop diseases and to promote plant growth and yield [179].
As a biocontrol agent, T. harzianum is efficient at inhibiting plant pathogens such as Fusarium solani or mycotoxin—producing fungi by competition, antibiosis, and inducing plant defense responses [109,180]. It is also a biocontrol agent of pests such as aphids by inducing the plant defense system against them [126]. Examples of direct and indirect mechanisms of biocontrol from T. harzianum are presented below.
4.2.1. Parasitism and Competition
In an in vitro assay of F. oxysporum f.sp. lycopersici in confrontation with five Trichoderma species, all the species were able to inhibit the pathogen’s growth, being both T. harzianum strains tested, BHU-BOT-RYRL4 and MTCC936, the ones that inhibited the pathogen’s growth the most (83.17% and 72.13%, respectively) [104]. In a rhizosphere colonization assay with wheat plants, T. harzianum Tr904, as well as T. gamsii and T. afroharzianum, colonized the rhizosphere and competed for space and nutrients with the pathogen Fusarium pseudograminearum, preventing plant disease caused by this pathogen [110].
In the dual confrontation of T. harzianum against Fusarium sudanense, the BCA parasites the pathogen degrading its hyphae and inhibiting its growth by also competing for space and nutrients, preventing seed rot in wheat plants [105]. T. harzianum has also shown antagonistic ability in vitro during the confrontation with the pathogen Alternaria cerealis, limiting its growth [106], and the strain T. harzianum T-soybean showed mycoparasitic activity against F. oxysporum, reducing its growth by 45.45% [107].
In dual confrontation assays, two strains of T. harzianum, CMML20-26 and CMML20-27, showed strong antagonistic activity against several sweet potato postharvest pathogens, including Fusarium ipomeae, F. oxysporum, F. solani, Penicillium citrinum¸ P. rotoruae, Aspergillus wentii, Mucor variicolumellatus and M. phaseolina [108]. T. harzianum MRI001 can mycoparasite F. oxysporum, A. alternata, Aspergillus carbonarius, and A. flavus, overgrowing the pathogens and reducing the production of the mycotoxins ochratoxin and aflatoxin B1, produced by A. carbonarius and A. flavus respectively [109]. In a confrontation assay, T. harzianum inhibits the growth of the chili pepper pathogen Colletotrichum truncatum, competing for space [112]. Several Egyptian T. harzianum strains showed mycoparasitic activity against F. graminearum, M. phaseolina, and F. solani, the strain T. harzianum Th6, the most effective one against all three pathogens [111,181].
T. harzianum has proved to be an effective mycoparasite, not only by degrading its host hyphae but by competing for space and nutrients against a wide range of fungal plant pathogens. These characteristics make this fungus a competent BCA against soil-borne fungal pathogens, with prominent applications in agriculture.
4.2.2. Secondary Metabolites
T. harzianum is also a good producer of secondary metabolites with important biocontrol traits. Inoculation of T. harzianum induces VOCs production in maize roots, and the exogenous application of 6-PP diminishes the damage caused by the root herbivore Phyllophaga vetula [113], indicating that the volatiles produced by the fungus induces resistance in the plant, even though, no direct biocontrol of P. vetula was observed by T. harzianum. ThMBF1 is a transcriptional coactivator from T. harzianum T34 involved in the synthesis of several secondary metabolites. Its regulation is vital to maintaining biocontrol capability over B. cinerea and F. oxysporum since its overexpression significantly reduced the BCA capacity to inhibit the pathogens’ growth and its capacity to confer resistance in tomato plants [114].
Some important secondary metabolites from Trichoderma species are peptaibols, which are involved in antibiosis activity against several plant pathogens [63]. Three peptaibols from T. harzianum HK-61, named trichorzins HA II, HA V, and HA VI, reduced lesions caused by Cucumber mosaic virus (CMV) up to a 90% (trichorzin HA V) in Vigna sesquipedalis plants [115].
Other important secondary metabolites are proteases. The aspartic protease P6281 from T. harzianum GIM 3.442 significantly reduces the growth of B. cinerea, Mucor circinelloides, A. flavus, A. fumigatus, and R. solani, disrupting the cell wall integrity of B. cinerea, preventing it from causing lesions in fruits such as orange and apples, and inhibiting spore formation in B. cinerea, M. circinelloides, A. flavus, and A. fumigatus [116].
Secondary metabolites can have antibiosis or antimicrobial activity. SM present in the fungal extract of T. harzianum CCTCC-RW0024 strain showed antifungal activity against F. gramineraum, inhibiting the pathogen growth up to 96.3% and conferring resistance in maize plants [73]. The culture filtrate from T. harzianum T-soybean has antifungal activity against F. oxysporum, inhibiting the pathogen growth up to 60.4%, granting resistance in soybean against F. oxysporum [107]. Culture filtrate from T. harzianum can inhibit the growth of the bean pathogen Pythium ultimum and, combined with chamomile extract, reduces disease symptoms in Phaseolus vulgaris seeds caused by this pathogen [120]. Cell-free culture filtrates improved in chitinase activity, showed fungal growth inhibition against the pathogens Dematophora necatrix, F. solani, F. oxysporum, and Pythium aphanidermatum, and the effect is concentration-dependent, were at a concentration of 25% of the filtrate showed the maximum growth inhibition rate for all the pathogens tested [121]. The G-protein signal regulatory mechanism is involved in fungal processes such as pathogenesis and secondary metabolism synthesis [182], and the Thga3 subunit from T. harzianum Th33 is involved in its mycoparasitic ability against R. solani, regulating chitinase activity, hydrophobicity, and growth of the fungus [117].
Some secondary metabolites produced by T. harzianum can induce plant defense mechanisms, such as the flavoenzyme ThLAAO (L-amino acid oxidase) from T. harzianum ETS323, which has antibiotic activity, and when it is expressed in tobacco plants, induces the expression of genes related to SA-, JA- and Et- mediated defense pathways, as well as ROS accumulation, conferring resistance to Sclerotinia sclerotiorum and B. cinerea, and resistance to B. cinerea in cabbage plants [118]. The expression of the gene thkel1 from T. harzianum CECT 2431 in Arabidopsis and Brassica napus plants caused resistance to B. cinerea in Arabidopsis and P. lingam in rapeseed, increasing the expression of genes related to SA- and JA- mediated defense pathways [123]. Other SM can regulate virulence genes from the pathogen, such as Epl-1 from T. harzianum, which represses virulence genes in B. cinerea, and induces SA- mediated defense pathway and priming in tomato plants, conferring resistance to the pathogen [119]. SM from culture filtrates of T. harzianum and the fungus diminished the adverse effects that Fusarium culmorum causes in wheat plants, such as reduced germination or lower plant growth, and modified antioxidant enzymatic activity, overall conferring protection against the pathogen [122].
T. harzianum is a proficient secondary metabolites producer. This ability works in its favor as a BCA, regulating and inhibiting the growth of several phytopathogens and using its SM to induce plant resistance, protecting plants not just in a direct manner but indirectly as well. This makes the study of secondary metabolites produced by Trichoderma an important subject to take advantage of in agriculture.
4.2.3. Plant Defense Induction and Priming
T. harzianum is effective at inducing plant defense systems against insects, such as with the feeding insect Nezara viridula, whose growth is impaired in tomato plants that had been inoculated with T. harzianum T22, also inducing the expression of loxD and PIN2 genes, related to the JA-mediated defense pathway in the plants [124]. T. harzianum T22 also induces a strong plant VOCs priming in tomatoes, attracting the parasitoid A. ervi, so the plants can defend themselves against the aphid M. euphorbiae [126], reprogramming the plant transcriptome and metabolome to favor induction of JA, Et and ISR defense pathways, and increasing isoprenoid biosynthesis, leading to a strong defense response against M. euphorbiae [127].
Besides, T. harzianum can also induce resistance against other pathogens, such as nematodes. T. harzianum diminishes infection symptoms caused by the nematode Meloidogyne incognita in tomato plants, inducing the gene expression of PR1, PR5, JERF3, and ACO, which are related to SA- and JA/Et- mediated defense responses in plants [125].
The production of ROS is one of the defense responses in a plant that can be induced by beneficial microbes, such as T. harzianum induction of accumulation of H2O2 and other important defense-related enzymatic activity, such as SOD, in tomato plants, upon infection with F. oxysporum f.sp. lycopersici [104]. To confer protection against F. oxysporum, T. harzianum colonizes cucumber roots reducing ROS and reactive nitrogen species (RNS) accumulation caused by the pathogen, promoting redox homeostasis, and increasing antioxidant enzymatic activity to enhance plant protection [129]. T. harzianum induced priming, defense-related enzymatic activity (PAL, POX, PPO), as well as antioxidant enzymatic activity (SOD, catalase (CAT), and others) in chili pepper plants upon infection with C. truncatum, also diminishing the symptoms caused by the pathogen and ROS induced accumulation, protecting the plant against its pathogen [112].
A defense-related enzymatic activity such as PAL, POX, CAT, and ascorbate peroxidase is induced by inoculation with T. harzianum UBSTH-501 in wheat plants, conferring resistance against Bipolaris sorokiniana infection, and promoting SA and phenolic compounds accumulation, as well as lignin and suberization in leaves, to reinforce plant defense [128]. Upon infection of tomato plants with A. cerealis, T. harzianum induces the accumulation of different phenolic compounds, such as flavonoids and terpenoids, and increases the plant antioxidant enzymatic activity, diminishing the infection caused by A. cerealis [106]. Soybean plants treated with T. harzianum T-soybean showed less cellular death caused by F. oxysporum and increased protection against the pathogen [107].
It is worth noting that the induction of plant defense systems mediated by T. harzianum causes the plant to reprogram its metabolite synthesis to favor the production of compounds that can help the plant to defend itself against pathogens that cannot be directly attacked by T. harzianum, such as aphids or viruses, conferring this way a whole protection against different kinds of phytopathogens.
4.3. Trichoderma Asperellum
T. asperellum grows well in temperatures ranging from 25 °C to 30 °C, and it is a cosmopolitan species, found frequently in agricultural and undisturbed soils and plant material, with lifestyles ranging from saprotrophy to biotrophy [183]. Conidia appear after 5 days and are dark green in color, forming at the center of the colony in Petri dishes [135].
Along with T. atroviride, T. asperellum is considered a strong mycoparasite of different plant pathogenic fungi by competition, hyperparasitism of host or antibiosis, and it can induce plant resistance [112,135,184]. Below, we present examples of the direct and indirect biocontrol mechanisms used by T. asperellum.
4.3.1. Parasitism and Competition
In dual culture between several strains of Trichoderma, with the phytopathogens Fusarium camptocerus, F. oxysporum, A. alternata, F. solani, Colletotrichum gleosporoides, Ganoderma applanatum, B. cinerea and Cytospora chrysosperma, the Trichoderma strain TaspHu1, identified as T. asperellum, showed better biocontrol traits, inhibiting the growth of the pathogens by showing mycoparasitic activity and competition for space and nutrients [130]. In a dual confrontation assay, T. asperellum inhibits the growth of the chili pepper pathogen Colletotrichum truncatum, competing for space [112].
In dual confrontation assays, T. asperellum IMI393899 showed a mycoparasitic capacity of several fungal pathogens, including Neofusicoccum batangarum, N. parvum, C. gloeosporoides, Phytophthora nicotianae, Phytophthora parvispora, Penicillium digitatum, P. roqueforti, P. verrucosum, Fusarium proliferatum, F. sporotrichoides, F. langsethiae, F. graminearum and F. poae [92]. T. asperellum T1 showed antifungal activity in dual confrontation assays against the pathogens Corynespora cassiicola and Curvularia aeria, the causal agents of leaf spot in lettuce, inhibiting the growth of the pathogens, and overgrowing them in the Petri dish [134].
Confrontation of T. asperellum 6S-2 against the apple pathogen Fusarium proliferatum f.sp. malus domestica MR5 leads to the degradation of the pathogen mycelia during the mycoparasitic interaction [133]. In dual culture antagonism assays, T. asperellum TA showed mycoparasitic activity against the white-rot fungus Phellinus noxius, conferring resistance in Eryobotria japonica plants in an in planta assay, showing fewer symptoms caused by the pathogen [136]. Additionally, T. asperellum GDSF1009 showed biocontrol traits by competing and mycoparasite the pathogens F. oxysporum f.sp. cucumerinum Owen and F. graminearum, reducing their growth [135].
Trichoderma species are also used in agriculture because some of them are resistant to several abiotic stresses. T. asperellum ACCC30536 salt-tolerant mutants T3 and T5 showed antifungal capacity against R. solani and A. alternata under salt stress conditions, conferring resistance in PdPap plants by activating SOD, CAT, and POD enzymatic activities [132]. This shows the potential of isolating strains from extreme environments or conditions to favor plant growth under stressful situations.
Besides parasitizing other fungi, T. asperellum GDFS1009 is capable of parasitizing the moth Ostrinia furnacalis, a maize pest, when it ingests the BCA conidia, and when inoculated in maize plants, T. asperellum GDFS1009 induces POD, SOD, proline, protease, and PPO activities, increasing defense against the moth, and the co-inoculation with the well-known entomopathogen Beauveria bassiana has a better protection effect in the plants [131].
It is noteworthy the potential of T. asperellum as a mycoparasite, showing that it cannot only parasitize in a strong manner fungal pathogens but other plant pests such as moths. Moreover, the resistance of this fungus to abiotic stresses makes it a good candidate for agricultural uses in extreme conditions, protecting and promoting the growth of important crops under these circumstances.
4.3.2. Secondary Metabolites
As mentioned before, vel1 is involved in secondary metabolite biosynthesis. Its overexpression in T. asperellum induces the expression of defense-related genes in maize plants, conferring resistance against the pathogens Cohilohorus herostrophus and Fusarium verticilloides and the co-culture of T. asperellum with B. amyloliquefaciens provides better protection against the pathogens [143].
Secondary metabolites from T. asperellum that can induce defense responses in plants include Epl1-Tas, which induces the expression of genes related to the SA-mediated defense pathway (NPR1, TGA, and PR1), JA-mediated defense pathway (COI1, JAZ, MYC2, and ORCA3) and auxin signaling (TIR1 and ARF1) in Populus davidiana × P. alba var. pyramidalis (PdPap), and increases defense-related enzymatic activity, conferring over 90% more resistance to the pathogen A. alternata [137]. The expression in planta of the class II hydrophobin HFBII-4 from T. asperellum ACCC30536 in PdPap plants alters the expression of genes related to auxin signaling, SA and JA defense pathways, and defense-related enzymatic activity (PAL, POD, PPO enzymes), reducing ROS accumulation and diminishing lesion area caused by A. alternata [138].
Secondary metabolites from T. asperellum GDFS1009 contained in fungal fermented broth, alone or in combination with B. amyloliquefaciens, showed antagonistic activity against F. graminearum, F. oxysporum, and B. cinerea, and conferred resistance to F. graminearum in wheat plants [139]. Ethyl acetate extract and the fungal filtrate from T. asperellum IMI 393,899 showed growth inhibition activity and strong cytotoxic activity against 25 pathogens, including Penicillium spp., Aspergillus spp., Fusarium spp., Neofusicoccum spp., Colletotrichum spp. and Phytophthora spp. [92].
Some of the secondary metabolites from Trichoderma species that have antibiotic activity are peptaibols [63], such as crude fungal extract containing peptaibols from T. asperellum IRAN 3062C, showing the growth inhibition of Micrococcus luteus, R. solani and A. solani, and inhibition of spore germination in A. solani, R. solani and Fusarium monilifome [140]. Another SM, such as the VOC 6-PP and the filtrate from T. asperellum P1, inhibits the growth of the maize pathogen Magnaporthiopsis maydis [141]. In field conditions, T. asperellum P1 confers resistance in maize plants to M. maydis [162]. Filtered fermentation extract from T. asperellum GDFS1009 inhibited the growth of the pathogens F. oxysporum f.sp. cucumerinum Owen and F. graminearum in 67.59% and 100%, respectively, and induced resistance in tobacco and cucumber plants, observed as increased defense-related enzymatic activity [135]. The crude citric acid extract from T. asperellum showed antagonistic capacity against F. oxysporum f.sp. lycopersici, inhibiting its growth and diminishing the severity of the symptoms caused by this pathogen in tomato plants, increasing PPO and POD enzymatic activity [142].
Secondary metabolites such as VOCs can have growth inhibitory effects over pathogens, such as VOCs released by T. asperellum T76-14, which in vitro assays show growth inhibition of the melon pathogen Fusarium incarnatum, also preventing the postharvest rot in melon fruits caused by this pathogen [144]. Both VOCs and liquid fermentation extract from T. asperellum 6S-2 can inhibit the growth of the pathogen Fusarium proliferatum f.sp. malus domestica MR5 and the liquid extract also showed the capacity to inhibit pathogen spore formation [133].
The secondary metabolites, both volatile and non-volatile, produced by T. asperellum show the capacity to inhibit fungal pathogen growth and to induce plant defense systems. This suggests the versatility of the SM from this fungus to act as an important biocontrol mechanism and its potential to be used in agriculture.
4.3.3. Plant Defense Induction and Priming
T. asperellum TaspHu1 increases resistance in tomato plants against A. alternata inducing the plant defense pathways, which was observed by an increase in JAR1, MYC2, NPR1, PR1, and GH3.2 gene expression, genes involved in JA- and SA-mediated defense signaling [130]. T. asperellum T42 induces a hypersensitive response (HR) in Pisum sativum plants upon infection with Erysiphe pisi, observed as an increased antioxidant enzymatic activity and lignin accumulation in the plants, and the co-culture with Pseudomonas fluorescens has a stronger HR induction in the plants [145]. T. asperellum induced priming, increased defense-related enzymatic activity, and antioxidant enzymatic activity in chili pepper plants upon infection with C. truncatum, diminishing the symptoms caused by the pathogen and conferring resistance in the plant [112]. Root dipping with T. asperellum T1 increases β-1,3-glucanase, chitinase, POX, and phenol oxidase activity in lettuce, inducing resistance against the pathogens C. cassiicola and C. aeria [134].
The inoculation of T. asperellum in tomato plants reduces the ROS accumulation caused by the pathogens B. cinerea and F. oxysporum¸. It induces ISR in the plants upon B. cinerea infection, reducing the symptoms caused by the pathogen [146].
Along with the strong mycoparasitic capacity of T. asperellum, its ability to induce plant resistance and confer protection against different pathogens makes this fungus an extraordinary example of an efficient BCA that is already one of the most ubiquitous Trichoderma species. Thus, its application in agricultural fields may be facilitated.
4.4. Trichoderma Virens
T. virens is a ubiquitous fungus isolated from soil and plant matter. In nature, two strains can be identified and distinguished by their secondary metabolite production: strains “Q” and strains “P”. Q strains are characterized by the production of gliotoxin, dimethylgliotoxin, viridiol, and viridin. Meanwhile, P strains produce gliovirin, heptelidic acid, viridiol, and viridin, but no gliotoxin nor dimethylgliotoxin [185].
Gliotoxin and gliovirin are two important metabolites produced by this fungus with strong toxic activity and roles in the establishment of beneficial interactions with plants and in pathogenic interactions with plant pathogens [186,187]. Hence, the strong use of secondary metabolites as a primary mechanism of biocontrol by this fungus. Nonetheless, mycoparasitism is also important for the biocontrol capacity of T. virens, along with the induction of plant defense responses to protect plants against different pathogens.
4.4.1. Parasitism and Competition
T. virens is an effective mycoparasite and antagonist of several plant fungal pathogens, such as F. oxysporum f.sp. physalia, whose growth is inhibited in dual confrontation with T. virens Gl006, alone or in combination with Bacillus velezensis Bs006 supernatant. Nonetheless, the major reduction in the pathogen’s growth is when confronted with the BCA alone (above 70%) [147]. In dual confrontation assays, T. virens ZT05 showed mycoparasitic activity over R. solani, penetrating the pathogen hyphae [148], thus inhibiting its growth. Its mycoparasitic abilities have been known for several decades, when it was first observed as coiling hyphae around R. solani back in 1932 by Weindling, R. [50].
4.4.2. Secondary Metabolites
Secondary metabolites produced by microorganisms can be tested using cell-free supernatants, such as the cell-free supernatant from T. virens Gl006, which alone or in combination with cells or cell-free supernatant from B. velezensis Bs006, has antagonistic activity over F. oxysporum f.sp. physalia, diminishing disease severity caused by this pathogen in Physalia peruviana plants [147]. Culture filtrates from T. virens TriV_JSB100, or the fungus, induces priming in tomato plants upon infection with F. oxysporum f.sp. lycopersici, diminishing the symptoms caused by the pathogen [154]. Jogaiah and collaborators [154] also noted that the inoculation of T. virens induced the JA-mediated defense pathway in the plant. In contrast, the fungal culture filtrate primarily induces the SA-mediated defense pathway, resulting in an overall resistance to the pathogen.
Some secondary metabolites are antibiotics, such as viridin from T. virens IMI 304061, one of the main SMs found in the culture filtrate from an SM-overexpressing strain from T. virens IMI 304061, named G2, which has better antibiosis effect over the pathogen Pythium aphanidermatum and confers greater protection in Cicer arietinum plants against Sclerotium rolfsii [149]. Gliotoxin from T. virens T23 is important to control S. rolfsii, causing structural damage to the pathogen hyphae [188]. The volatile and non-volatile SMs from T. virens ZT05 showed growth inhibition of R. solani at 80.1% and 63.32% respectively. The non-volatile SMs repressed defense-related enzymatic activity in R. solani, indicating that the BCA could regulate the defense mechanism of the pathogen against the mycoparasite [148].
Chitinase and cellulase protein activity are important traits in BCA. Several T. virens mutant strains with enhanced chitinase and cellulase activities showed to be more effective at inhibiting R. solani growth in dual confrontation assays than the T. virens wild-type strain [150].
Secondary metabolites biosynthesis is regulated by different enzymes, such as p450 monooxygenases [189]. Tvcyt2 is a member of the p450 monooxygenases in T. virens and is involved in SM biosynthesis [151]. Ramírez-Valdespino and collaborators [151] found that the overexpression of tvcyt2 results in a higher concentration of SMs, leading to an increased antagonistic activity against R. solani AG2 and a stronger JA- and SA-mediated defense response in Arabidopsis plants.
Some other SMs can induce plant resistance, such as the intracellular siderophore ferricrocin from T. virens, which is involved in inducing ISR in maize upon infection with the pathogen Cochliobolus heterostrophus, since null mutants in the gene tex10, the one coding for ferricrocin, failed to induce ISR in the maze, and were more aggressive at colonizing the plants [152]. TvPG2, a constitutive endopolygalacturonase from T. virens I10, is involved in inducing ISR in tomato plants against B. cinerea, regulating the expression of the inducible tvpg1 gene coding for TvPG1 endopolygalacturonase, which leads to the resistance against the pathogen [153].
4.4.3. Plant Defense Induction and Priming
T. virens can induce plant defense systems, conferring resistance to different pathogens; for example, T. virens IARI-P3 induces PR10 gene expression in susceptible and resistant Vigna radiata plants when infected with R. solani, reducing significatively disease symptoms caused by the pathogen [155]. T. virens induces ISR in maize plants by increasing gene expression of two oxylipins coding genes, 12-OPDA (12-Oxo-10(Z),15(Z)-phytodienoic acid) and an ᵧ-ketol, 9,10-KODA (10-oxo-9-hydroxy- 12(Z), 15(Z)-octadecadienoic acid), granting resistance to the pathogen Colletotrichum graminicola [156,157].
T. virens has been one of the most used BCA among the Trichoderma genus. It is an important secondary metabolite producer with biocontrol activity against many phytopathogens. Its secondary metabolites show great potential to be used in agriculture to control phytopathogens and to induce plant protection against them. Besides, along with other secondary metabolites from Trichoderma spp., compounds such as gliotoxin are showing medical applications as possible treatments against cancer [190,191,192], suggesting the span of applications that this fungus has, not being limited to agricultural uses.
4.5. Trichoderma Longibrachiatum
T. longibrachiatum is frequently isolated from agricultural soils, mushrooms, and marine environments, and it grows better at tropical temperatures rather than in temperate climates [193]. This fungus has been reported to cause cardiac and pulmonary mycoses in immunocompromised humans [194,195,196]. Nonetheless, it has also been reported to be used as an important biocontrol agent [159,169], exerting parasitism and inducing plant defense systems, along with the production of several important secondary metabolites, as shown by the examples described below.
4.5.1. Parasitism and Competition
In dual confrontation assays, T. longibrachiatum EF5 showed mycoparasitic activity against M. phaseolina, showing hyphal entanglement between both fungi [161], and antagonistic activity with mycelia modifications on M. phaseolina and S. rolfsii [158]. T. longibrachiatum (TG1) coils around Fusarium pseudograminearum in a mycoparasitic interaction. In a tripartite interaction with wheat plants under salt stress conditions, the BCA reduces the disease symptoms caused by the pathogen [159]. Tested under field conditions, T. longibrachiatum T7407 had a negative effect in the presence of the pathogen Magnaporthiopsis maydis in soil by competing with it, thus protecting maize plants from this pathogen and diminishing disease incidence [162]. Besides being a mycoparasite, T. longibrachiatum T6 can parasitize eggs and second-stage juveniles from the plant pathogen nematode Heterodera avenae, reducing its viability [160].
The parasitic ability of T. longibrachiatum is a trait that should be exploited more, especially since it is a parasite of important fungal and nematode phytopathogens, but should be taken carefully under field applications, considering that it is the only fungus from the genus Trichoderma to be reported as an opportunistic human pathogen so far.
4.5.2. Secondary Metabolites
Peptaibols are SMs with antibiotic activity, as mentioned before [63]. The crude fungal extract from T. longibrachiatum containing peptaibols showed antibacterial activity against the pathogen M. luteus [140]. Synthetic analogs to the peptaibol Trichogin GA IV from T. longibrachiatum are effective antagonistic compounds to inhibit Pyricularia oryzae, a rice pathogen. They can reduce disease symptoms in barley and rice plants [164], which indicates that those synthetic analogs could be used as biocidal compounds instead of chemical compounds. The crude fungal extract containing peptaibols from T. longibrachiatum IRAN 3067C showed growth inhibition of several plant pathogens, mainly effective against R. solani and A. solani [140].
Other SMs with antibiotic activity includes dendrobine from T. longibrachiatum MD33, which is an endophyte of the plant Dendrobium nobile, known to be the only plant producing dendrobine [163,197]. Sarsaiya and collaborators [163] showed that the T. longibrachiatum MD33 has strong antibacterial activity against Bacillus subtilis, B. mycoides, and Staphylococcus sp., showing the potential of this BCA to inhibit pathogenic bacteria. Three cyclodepsipeptides and six sesquiterpenes compounds identified among the SM produced by T. longibrachiatum showed antibiotic activity against several plant pathogens [166]. Only the three cyclodepsipeptides and two sesquiterpenes identified by Du and collaborators [166] inhibited the growth of the nematode Meloidogyne incognita, and just the remaining four sesquiterpenes were able to inhibit the fungal pathogens Colletotrichum lagrnarium, C. fragariae, B. cinerea PTQ1, and CMQ1, F. oxysporum f.sp. cucumerinum and F. oxysporum f.sp. lycopersici, showing pathogen specificity of the SM tested. Culture filtrate containing 13 SMs, known as sorbicillinoids, from T. longibrachiatum SFC100166 showed in vitro growth inhibition of the pathogens Alternaria brassicola, B. cinerea, Colletotrichum coccodes, Cladosporium cucumerinum, Cylindrocarpon destructans, Magnaporthe oryzae and Phytophthora in festans [167]. When tested separately, eleven of the sorbicillinois identified inhibited the pathogens tested, with P. infestants being the most affected by the compounds, and four sorbicillinois were able to induce resistance in tomato plants against this pathogen [167]. VOCs from T. longibrachiatum EF5 inhibited the growth of the pathogens S. rolfsii (57%) and M. phaseolina (35%) by altering mycelia structure [158], suggesting that these compounds may be important to the biocontrol traits of T. longibrachiatum.
Some secondary metabolites induce plant defense responses, such as the hydrophobin HYTLO1 from T. longibrachiatum MK1, which is perceived by Lotus japonicus, activating the expression of Ca2+-mediated signaling, leading to the induction of defense-related genes in the plant [165].
Some SMs from T. longibrachiatum have biocontrol activity over other organisms, such as the ethyl acetate extract from T. longibrachiatum AUMC 5125 that has effective antibiotic activity over the cotton aphid Aphis gossypii [168]. Fermentation crude extract from T. longibrachiatum T6 showed antagonistic activity against eleven phytopathogens tested, being especially effective against Valsa mali¸ inhibiting up to 95% [169].
As shown by the examples above, secondary metabolites from T. longibrachiatum play important roles in its biocontrol capacity, and considering that this fungus may cause human diseases, SMs could be studied to be used alone, without the need for the microorganism, diminishing health concerns about the use and introduction of microorganisms in the environment.
4.5.3. Plant Defense Induction and Priming
T. longibrachiatum H9, a novel strain, colonized cucumber roots promoting plant growth and inducing JA/Et and SA defense signaling pathways, conferring resistance in cucumber to the pathogen B. cinerea [77]. In a greenhouse experiment, T. longibrachiatum T6 induced flavonoid and lignin content, as well as defense-related enzymatic activity in wheat roots, conferring resistance against the nematode H. avenae [160].
T. longibrachiatum has shown to be a versatile biocontrol agent, parasitizing not only fungi but nematodes as well, and it is a good secondary metabolite producer, being used to obtain important compounds such as peptaibols. This fungus has good potential to be an efficient BCA, and its traits could be exploited in field conditions, considering the potential to use SMs in substitution of synthetic compounds.
4.6. Trichoderma Viride
T. viride has optimal growth at 25 °C and does not grow at 35 °C; it can be isolated from soil and organic matter, some of its strains have a faint coconut smell, and conidia can be observed after 2 days. T. viride is considered the type species of the genus Trichoderma [173,198], and it is one of the most common species found in soil.
T. viride has been used as a biocontrol agent, especially due to its mycoparasitic ability. It has been reported that T. viride can mycoparasite fungal pathogens such as F. moniliforme, Cryphonectria parasitica, and Schizophyllum commune [199,200,201,202]. The use of commercial chitinases derived from T. viride causes damage to the silkworm Bombix mori [203], suggesting the ability of this fungus to degrade chitin from insects that could cause plant diseases. Below, we present recent examples regarding the biocontrol mechanisms of T. viride.
4.6.1. Parasitism and Competition
By competing for space and nutrients in dual culture assays, T. viride showed antagonistic activity against the pathogen Sclerotinia sclerotiorum, presenting a clear zone of inhibition in the culture plates on day 4 of interaction, indicating antibiosis mechanisms exerted by T. viride over the pathogen, and a 67.284% growth inhibition by day 6 of confrontation [170].
In dual confrontation assays, T. viride showed antagonistic activity against the pathogens Fusarium solani, R. solani, and S. rolfsii, limiting their growth by 29.76%, 15.27%, and 19.73%, respectively [171].
4.6.2. Secondary Metabolites
T. viride produces SM with antifungal activity. The crude mycelial extract and the ethanolic extract from this fungus showed antifungal activity against Candida albicans, Fusarium solani, F. oxysporum, R. solani, and Pythium ultimum, and antibacterial activity against Bacillus subtilis, Escherichia coli, and Pseudomonas fluorescens showing inhibition zones in the culture plates. In contrast, the VOCs produced by T. viride showed antibacterial activity against B. subtilis and E. coli and antifungal activity against C. albicans, F. solani, and R. solani [171].
VOCs from T. viride BHU-V2 showed antagonistic activity against S. rolfsii, inhibiting the pathogen growth both in vitro and in soil experiments. It was determined that VOCs are capable of altering the structure of the pathogen hypha, thus limiting its growth [172]. Singh and collaborators [172] also showed that VOCs from T. viride increase PAL, PPO, chitinases, and β-1,3-glucanase activity in okra plants, inducing resistance and diminishing cell death caused by S. rolfsii.
4.6.3. Plant Defense Induction and Priming
In glasshouse experiments, inoculation of T. viride alone or in combination with Trichoderma erinaceum suppressed the disease caused by Sclerotinia sclerotiorum in Phaseolus vulgaris cv. Anupama plants. Nonetheless, the combination of the BCAs had better results [170]. Kumar and collaborators [170] also found that plants pretreated with either Trichoderma species or their combination reduced ROS accumulation induced by the pathogen, enhancing antioxidant activity in the plants.
Despite being the species reviewed here with less recent literature regarding its biocontrol traits, T. viride has been studied as a plant growth promoter or enhancer of desirable traits in plants [204,205] or as an important organism in bioremediation or preparation of surfaces for bioremediation of toxic organic compounds such as toluene [206] or TNT [207] or heavy metals such as lead [208]. These make an interesting Trichoderma species to study further in various possible applications, not limited to agricultural uses.
4.7. Other Trichoderma Species
Besides being the most used Trichoderma species as biocontrol agents and/or plant growth promoters [55], other species are surfacing. T. lignorum, in combination with B. bassiana, was effective at controlling leafcutter ants (Atta cephalotes) populations [209]. T. koningii reduced disease severity in grapevines caused by the pathogens Phaeomoniella chlamydospora and P. minimum [210]. T. erinaceum can suppress an infection caused by S. sclerotiorum in bean plants [170]. It is also capable of overgrowing R. solani and inducing ROS and defense-related enzymatic activity in rice plants [211]. T. citrinoviride resulted in an effective mycoparasite of six pathogens of ginseng plants: R. solani, B. cinerea, Alternaria panax, Cylindrocarpon destructans, Phytophthora cactorum, and Pythium spp. [212].
These studies about other Trichoderma species, and the constant reports of new isolates and their applications in industry and medicine, for example, show the diversity of species belonging to this genus and their potential to be used outside of the traditional plant growth promotion and biocontrol abilities attributed to Trichoderma since it was first described. Hence, not only new isolates or species should be of interest, but additional information about the “classic” Trichoderma species and its applicability in other fields could be addressed.
5. Other Biocontrol Strategies Involving Trichoderma spp.
Besides the direct or indirect use of Trichoderma spp. in the biocontrol of plant pathogens, that is, applied in field or greenhouse conditions as conidia, hyphae, a mix of its metabolites, other new and forthcoming biocontrol strategies have been arising over the past years. These include the combined use or co-inoculation of Trichoderma species with plant growth-promoting bacteria or mycorrhizae, where the co-inoculation of the BCA T. viride and several arbuscular mycorrhizal fungi (AMF) such as Rhizoglomus clarum, Funneliformis monosporum, Acaulospora laevis, and Dentiscutata nigra, had a positive and synergistic effect on the overall health of Allium cepa plants [205], and the co-inoculation of T. harzianum with Bacillus sp. proved effective in controlling the disease caused by F. oxysporum f.sp. capae on shallot plants [213].
Trichoderma species are not only capable of cooperating with other beneficial microbes, but they can even change the plant rhizosphere microbiome and microbial communities, favoring plant resistance against several pathogens. T. harzianum CCTCC-RW0024 modifies the rhizosphere microbiome in maize plants, conferring resistance against F. graminearum [73]. Moreover, T. harzianum changes microbial communities in the rhizosphere of Piper nigrum plants, favoring the presence of other beneficial microbes [214] and diminishing the incidence of Plasmodiophora brassicae, Alternaria sp. and Fusarium sp. pathogens in the rhizosphere of cabbage plants [215]. The presence of T. asperellum M45a in the watermelon rhizosphere modifies microbial composition, increasing the presence of plant-growth-promoting rhizobacteria and reducing the presence of plant pathogenic fungi, and disease incidence caused by Fusarium oxysporum f.sp. niveum in watermelon plants [216]. T. asperellum also modifies endophytic microorganisms’ population in maize stalk, increasing resistance against F. graminearum and F. verticilloides [217]. This information suggests that changes in the microbiome could have a biocontrol effect on important plant pathogens, thanks to the presence of Trichoderma species.
Trichoderma has also been used for the green biosynthesis of nanoparticles and their subsequent use in agriculture. Ag, ZnO, and CuO nanoparticles have been biosynthesized using T. harzianum, and those nanoparticles showed an inhibitory effect over A. alternata, Pyricularia oryzae, and S. sclerotiorum [218], and ZnO nanoparticles showed biocontrol capability against Fusarium sp., R. solani and M. phaseolina, three important pathogens of cotton plants [219]. T. viride has also been used to synthesize TiO2, showing larvicidal and pupicidal effects on Helicoverpa armigera, a pest of important crops such as maize, wheat, and beans [220]. This shows that Trichoderma can be used as a bio-tool to obtain chemical products that are beneficial in agriculture, in substitution of chemical fungicides [221].
These new approaches aim to minimize the harmful effects on health and the environment that chemical fungicides present. Trichoderma seems to have an important role in replacing chemical agricultural products.
6. Trichoderma Bioformulations in Agriculture for Use in Biocontrol
The use of Trichoderma as a biocontrol and plant growth promoter in agriculture is not new. There is significant research about the bioformulations that are most effective in the field, as well as the issues regarding the use and distribution of such products, such as acceptance from the farmers’ community, the introduction of different species in the environment, and their efficacy compared to chemical fertilizers and pesticides [12,222,223,224]. Hence, the importance of producing or manufacturing Trichoderma-based products, alone or in combination with other BCA, is their efficacy when tested so that the consumers will receive them well and would be willing to switch to bioformulations and use fewer chemical products on their crops.
Several Trichoderma-based formulations have been tested under greenhouse or field conditions, showing positive results such as increased plant growth, production, and resistance against diseases.
Wong and collaborators [62] combined T. harzianum CBF2- with Pseudomonas aeruginosa DRB1 using four different formulations: Pesta granules, Talc powder, alginate beds, and liquid formulation, and these bioformulations were tested to biocontrol F. oxysporum f.sp. cubense, the causing agent of banana wilt. Pesta granules and Talc powder were more efficient at diminishing disease symptoms in the plant (66.67% and 58.33%, respectively), followed by alginate beds (46.75%) and liquid formulations (43.06%). The four formulations were better than the application of Benomyl, a known chemical antifungal agent, with only a 37.50% reduction in disease symptoms [62]. As Pesta granules formulation, both BCA were viable for at least 180 days when stored at 4 °C, and the formulation showed better performance in BCA viability and storage [62].
Using five different agro-based wastes (vermicompost, vegetable wastes, used tea leaves, sugarcane bagasse, and cow dung) to grow T. lixii TvR1 and use as bio-products, sugarcane bagasse was the most efficient substrate to grow Trichoderma, and in pot experiments using spinach, promoted the plant growth [225]. Using a formulation of T. asperellum in coconut fiber promoted the growth of tomato plants and conferred resistance against F. oxusporum f.sp. lycopersici in field trials [226]. T. harzianum grown on a spent mushroom substrate (SMS) of Pleurotus ostreatus showed growth promotion in tomato plants and increased disease resistance against F. oxysporum f.sp. lycopersici. The bioformulation used was the best among several substrates, including combinations of SMS with paddy straw [227]. Two formulations from T. citrinoviride, dustable powder and granules, were effective against B. cinerea and C. destructans in vitro. Both were effective at preventing disease caused by A. panax in ginseng plants [213].
Seeds of chickpea and lentils treated with a formulation made with the mutant strain T. virens G2 on tamarind seeds and talcum powder, named TrichoBARC, improved yield and reduced seed mortality in chickpea and lentils in field trials, and induced resistance against S. rolfsii in chickpea plants [149]. Seed-coating with a bioformulation made with T. harzianum and chitosan-PEG as the delivery system showed antagonistic activity against F. oxysporum, M. phaseolina, and Aspergillus niger, the bioformulation also promoted the growth of safflower and groundnut plants, and resistance against M. phaseolina and A. niger [228].
Commercial formulations T34 Biocontrol (Biocontrol Technologies S.L) from T. asperellum T34 and Trianum P (Koppert) from T. harzianum T22 induced systemic resistance in tomato plants, repressed reproduction of the plant nematode Meloidogyne incognita and, T34 Biocontrol also reduced the nematode infectivity [229]. The commercial product Xedavir made with T. asperellum (Xeda International®) was tested in vitro against F. graminearum and F. verticilloides [230]. As spore suspension, Xedavir inhibits germination of F. graminearum up to 53%, F. verticilloides up to 22%, and as the cell-free extract, Xedavir inhibits F. graminearum and F. verticilloides germination up to 82% and 76%, respectively. Xedavir also showed the capacity to inhibit the production of the mycotoxin deoxynivalenol (DON) from F. graminearum [230].
One concern about the use of BCA in the field is precisely the introduction of species in the environment, which is why some studies are focusing on using local BCA strains [231]. Nonetheless, another option to avoid the use of whole microorganisms is the use of elicitor agents. Nandini and collaborators [232] formulated nanoemulsions using total lipids extracted from six Trichoderma species. The nanoemulsion from T. brevicompactum showed a remarkable capacity to induce resistance and hypersensitive response in pearl millet plants against the downy mildew pathogen Sclerospora graminicola, both in vitro and in field conditions [232], showing the feasibility of using elicitors from the microorganisms, without the necessity for the living organism.
The results of field or greenhouse testing of the Trichoderma-based formulations are promising, and studies regarding this issue should complement in vitro assays of its biocontrol capacity, to facilitate the application and distribution of bioformulations.
7. Conclusions
The use of chemical pesticides and fertilizers has been detrimental to human and environmental health. That is why the search for more sustainable and environmentally friendly solutions has led to the research of organisms as biocontrol agents. Such as Trichoderma, which possesses different biocontrol traits, which makes it one of the most effective organisms studied against various types of plant pathogens, not being limited to controlling fungi and oomycetes, but insects, pests, and nematodes as well, either by limiting their growth by competition, antibiosis, or parasitism, or by enhancing plant protection against them, making this fungus a versatile option to control several phytopathogens.
Trichoderma has been used in different types of formulations in agriculture, mainly to promote plant growth and increase crop yield. Nonetheless, the use of Trichoderma-based formulations for the control of pathogens also needs to be considered in studying such products, especially under field conditions, since most of the studies that consider this aspect are done in vitro in dual confrontations.
Another interesting point to remark on is the use of secondary metabolites from Trichoderma or green biosynthesis of nanoparticles using this fungus, which can be used in agriculture to promote plant growth or to inhibit pathogen growth without the fungus per se, or using Trichoderma strains isolated from local environments, eliminating the introduction of foreign strains into the environment.
It is clear that the different Trichoderma species are used as mycoparasites, and specific species such as T. atroviride or T. harzianum are among the strongest and classic mycoparasites. Nonetheless, there are emerging Trichoderma species that have been isolated and applied from local areas and are promising candidates as biocontrol agents. Trichoderma as biocontrol agents started being studied as mycoparasites. Nonetheless, its use against other plant pathogens such as nematodes and insects is gaining notice due to the different mechanisms it has to exert control of such a variety of plant pathogens, regulating both soil and aerial-borne diseases.
There is still much to be done regarding applying Trichoderma-based formulations in field conditions and interaction with other soilborne microorganisms to understand better its interaction within the plant microbiome and its biocontrol traits. This a field to be exploited in depth for further research.
Acknowledgments
G.S. thanks CONACYT-México (Proposal: A1-S-15956) and CIC-UMSNH (2021–2022) for financial support to our research projects.
Author Contributions
P.G.-G.: Conceptualization, Writing—Original draft preparation. A.K.: Writing—Review & Editing. S.d.l.S.-V.: Writing—Review & Editing. F.I.P.-C.: Writing—Review & Editing. M.d.C.O.-M.: Writing—Review & Editing. A.E.F.: Writing—Review & Editing. S.H.: Writing—Review & Editing. O.O.B.: Writing—Review & Editing. G.S.: Conceptualization, Writing- Original draft preparation, Supervision. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by CONACYT-México (Proposal: A1-S-15956) and CIC-UMSNH (2021–2022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Syed Ab Rahman S.F., Singh E., Pieterse C.M.J., Schenk P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018;267:102–111. doi: 10.1016/j.plantsci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Moustafa-Farag M., Almoneafy A., Mahmoud A., Elkelish A., Arnao M.B., Li L., Ai S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules. 2019;10:54. doi: 10.3390/biom10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z., Jennings A. Worldwide Regulations of Standard Values of Pesticides for Human Health Risk Control: A Review. Int. J. Environ. Res. Public Health. 2017;14:826. doi: 10.3390/ijerph14070826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair A., Ritz B., Wesseling C., Freeman L.B. Pesticides and Human Health. Occup. Environ. Med. 2015;72:81–82. doi: 10.1136/oemed-2014-102454. [DOI] [PubMed] [Google Scholar]
- 5.Jepson P.C., Murray K., Bach O., Bonilla M.A., Neumeister L. Selection of Pesticides to Reduce Human and Environmental Health Risks: A Global Guideline and Minimum Pesticides List. Lancet Planet. Health. 2020;4:e56–e63. doi: 10.1016/S2542-5196(19)30266-9. [DOI] [PubMed] [Google Scholar]
- 6.Richardson J.R., Fitsanakis V., Westerink R.H.S., Kanthasamy A.G. Neurotoxicity of Pesticides. Acta Neuropathol. 2019;138:343–362. doi: 10.1007/s00401-019-02033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins N.J., Bass C., Dixon A., Neve P. The Evolutionary Origins of Pesticide Resistance. Biol. Rev. Camb. Philos. Soc. 2018;94:135–155. doi: 10.1111/brv.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijver M.G., Hunting E.R., Nederstigt T.A.P., Tamis W.L.M., van den Brink P.J., van Bodegom P.M. Postregistration Monitoring of Pesticides Is Urgently Required to Protect Ecosystems. Environ. Toxicol. Chem. 2017;36:860–865. doi: 10.1002/etc.3721. [DOI] [PubMed] [Google Scholar]
- 9.Marchand P.A. Synthetic Agrochemicals: A Necessary Clarification about Their Use Exposure and Impact in Crop Protection. Environ. Sci. Pollut. Res. 2019;26:17996–18000. doi: 10.1007/s11356-019-05368-8. [DOI] [PubMed] [Google Scholar]
- 10.Besset-Manzoni Y., Rieusset L., Joly P., Comte G., Prigent-Combaret C. Exploiting Rhizosphere Microbial Cooperation for Developing Sustainable Agriculture Strategies. Environ. Sci. Pollut. Res. 2018;25:29953–29970. doi: 10.1007/s11356-017-1152-2. [DOI] [PubMed] [Google Scholar]
- 11.del Carmen Orozco-Mosqueda M., Flores A., Rojas-Sánchez B., Urtis-Flores C.A., Morales-Cedeño L.R., Valencia-Marin M.F., Chávez-Avila S., Rojas-Solis D., Santoyo G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy. 2021;11:1167. doi: 10.3390/agronomy11061167. [DOI] [Google Scholar]
- 12.Thambugala K.M., Daranagama D.A., Phillips A.J.L., Kannangara S.D., Promputtha I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020;10:604923. doi: 10.3389/fcimb.2020.604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waqas M., Khana A.L., Hamayuna M., Shahzad R., Kang S.M., Kim J.G., Lee I.J. Endophytic Fungi Promote Plant Growth and Mitigate the Adverse Effects of Stem Rot: An Example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015;10:280–287. doi: 10.1080/17429145.2015.1079743. [DOI] [Google Scholar]
- 14.Abro M.A., Sun X., Li X., Jatoi G.H., Guo L.D. Biocontrol Potential of Fungal Endophytes against Fusarium oxysporum f. sp. cucumerinum Causing Wilt in Cucumber. Plant Pathol. J. 2019;35:598–608. doi: 10.5423/PPJ.OA.05.2019.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh G., Katoch A., Razak M., Kitchlu S., Goswami A., Katoch M. Bioactive and Biocontrol Potential of Endophytic Fungi Associated with Brugmansia aurea Lagerh. FEMS Microbiol. Lett. 2017;364:194. doi: 10.1093/femsle/fnx194. [DOI] [PubMed] [Google Scholar]
- 16.Poveda J., Abril-Urias P., Escobar C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020;11:992. doi: 10.3389/fmicb.2020.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poveda J. Trichoderma as Biocontrol Agent against Pests: New Uses for a Mycoparasite. Biol. Control. 2021;159:104634. doi: 10.1016/j.biocontrol.2021.104634. [DOI] [Google Scholar]
- 18.Schouteden N., De Waele D., Panis B., Vos C.M. Arbuscular Mycorrhizal Fungi for the Biocontrol of Plant-Parasitic Nematodes: A Review of the Mechanisms Involved. Front. Microbiol. 2015;6:1280. doi: 10.3389/fmicb.2015.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu X.-M., Zhang K.-Q. Arthrobotrys Oligospora: A Model Organism for Understanding the Interaction between Fungi and Nematodes. Mycology. 2011;2:59–78. doi: 10.1080/21501203.2011.562559. [DOI] [Google Scholar]
- 20.Liang L.-M., Zou C.-G., Xu J., Zhang K.-Q. Signal Pathways Involved in Microbe–Nematode Interactions Provide New Insights into the Biocontrol of Plant-Parasitic Nematodes. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20180317. doi: 10.1098/rstb.2018.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashyap P.L., Rai P., Srivastava A.K., Kumar S. Trichoderma for Climate Resilient Agriculture. World J. Microbiol. Biotechnol. 2017;33:1–18. doi: 10.1007/s11274-017-2319-1. [DOI] [PubMed] [Google Scholar]
- 22.Kashyap P.L., Solanki M.K., Kushwaha P., Kumar S., Srivastava A.K. Biocontrol Potential of Salt-Tolerant Trichoderma and Hypocrea Isolates for the Management of Tomato Root Rot Under Saline Environment. J. Soil Sci. Plant Nutr. 2020;20:160–176. doi: 10.1007/s42729-019-00114-y. [DOI] [Google Scholar]
- 23.Phour M., Sehrawat A., Sindhu S.S., Glick B.R. Interkingdom Signaling in Plant-Rhizomicrobiome Interactions for Sustainable Agriculture. Microbiol. Res. 2020;241:126589. doi: 10.1016/j.micres.2020.126589. [DOI] [PubMed] [Google Scholar]
- 24.Igiehon N.O., Babalola O.O. Biofertilizers and Sustainable Agriculture: Exploring Arbuscular Mycorrhizal Fungi. Appl. Microbiol. Biotechnol. 2017;101:4871–4881. doi: 10.1007/s00253-017-8344-z. [DOI] [PubMed] [Google Scholar]
- 25.Hossain M.M., Sultana F., Islam S. Plant-Microbe Interactions in Agro-Ecological Perspectives. Volume 2. Springer; Singapore: 2017. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. [Google Scholar]
- 26.Khan M.S., Gao J., Munir I., Zhang M., Liu Y., Moe T.S., Xue J., Zhang X. Characterization of Endophytic Fungi, Acremonium Sp., from Lilium Davidii and Analysis of Its Antifungal and Plant Growth-Promoting Effects. BioMed Res. Int. 2021;2021:9930210. doi: 10.1155/2021/9930210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z., Zhang C., Zhou W., Li W., Chu L., Yan J., Li H. Diversity and Plant Growth-Promoting Ability of Endophytic Fungi from the Five Flower Plant Species Collected from Yunnan, Southwest China. J. Plant Interact. 2014;9:585–591. doi: 10.1080/17429145.2013.873959. [DOI] [Google Scholar]
- 28.Contreras-Cornejo H.A., Macías-Rodríguez L., Cortés-Penagos C., López-Bucio J. Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiol. 2009;149:1579–1592. doi: 10.1104/pp.108.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salas-Marina M.A., Silva-Flores M.A., Uresti-Rivera E.E., Castro-Longoria E., Herrera-Estrella A., Casas-Flores S. Colonization of Arabidopsis Roots by Trichoderma atroviride Promotes Growth and Enhances Systemic Disease Resistance through Jasmonic Acid/Ethylene and Salicylic Acid Pathways. Eur. J. Plant Pathol. 2011;131:15–26. doi: 10.1007/s10658-011-9782-6. [DOI] [Google Scholar]
- 30.Sood M., Kapoor D., Kumar V., Sheteiwy M.S., Ramakrishnan M., Landi M., Araniti F., Sharma A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants. 2020;9:762. doi: 10.3390/plants9060762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira F.V., Musumeci M.A. Trichoderma as Biological Control Agent: Scope and Prospects to Improve Efficacy. World J. Microbiol. Biotechnol. 2021;37:90. doi: 10.1007/s11274-021-03058-7. [DOI] [PubMed] [Google Scholar]
- 32.Verma M., Brar S.K., Tyagi R.D., Surampalli R.Y., Valéro J.R. Antagonistic Fungi, Trichoderma Spp.: Panoply of Biological Control. Biochem. Eng. J. 2007;37:1–20. doi: 10.1016/j.bej.2007.05.012. [DOI] [Google Scholar]
- 33.Ketta H.A., Hewedy O.A.E.R. Biological Control of Phaseolus vulgaris and Pisum sativum Root Rot Disease Using Trichoderma Species. Egypt. J. Biol. Pest. Control. 2021;31:1–9. doi: 10.1186/s41938-021-00441-2. [DOI] [Google Scholar]
- 34.Mukhopadhyay R., Kumar D. Trichoderma: A Beneficial Antifungal Agent and Insights into Its Mechanism of Biocontrol Potential. Egypt. J. Biol. Pest. Control. 2020;30:133. doi: 10.1186/s41938-020-00333-x. [DOI] [Google Scholar]
- 35.Mukherjee P.K., Horwitz B.A., Herrera-Estrella A., Schmoll M., Kenerley C.M. Trichoderma Research in the Genome Era. Annu. Rev. Phytopathol. 2013;51:105–129. doi: 10.1146/annurev-phyto-082712-102353. [DOI] [PubMed] [Google Scholar]
- 36.Atanasova L., Druzhinina I.S., Jaklitsch W.M. Two Hundred Trichoderma Species Recognized on the Basis of Molecular Phylogeny. In: Mukherjee P.K., Horwitz B.A., Singh U.S., Mukherjee M., Schmoll M., editors. Trichoderma: Biology and Applications. CABI; Wallingford, UK: 2013. pp. 10–42. [Google Scholar]
- 37.Jaklitsch W.M. European Species of Hypocrea Part I. The Green-Spored Species. Stud. Mycol. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Druzhinina I.S., Kopchinskiy A.G., Komoń M., Bissett J., Szakacs G., Kubicek C.P. An Oligonucleotide Barcode for Species Identification in Trichoderma and Hypocrea. Fungal Genet Biol. 2005;42:813–828. doi: 10.1016/j.fgb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Kopchinskiy A., Komoń M., Kubicek C.P., Druzhinina I.S. TrichoBLAST: A Multilocus Database for Trichoderma and Hypocrea Identifications. Mycol. Res. 2005;109:658–660. doi: 10.1017/S0953756205233397. [DOI] [PubMed] [Google Scholar]
- 40.Dou K., Lu Z., Wu Q., Ni M., Yu C., Wang M., Li Y., Wang X., Xie H., Chen J., et al. MIST: A Multilocus Identification System for Trichoderma. Appl. Environ. Microbiol. 2020;86:1–13. doi: 10.1128/AEM.01532-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubicek C.P., Herrera-Estrella A., Seidl-Seiboth V., Martinez D.A., Druzhinina I.S., Thon M., Zeilinger S., Casas-Flores S., Horwitz B.A., Mukherjee P.K., et al. Comparative Genome Sequence Analysis Underscores Mycoparasitism as the Ancestral Life Style of Trichoderma. Genome Biol. 2011;12:R40. doi: 10.1186/gb-2011-12-4-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubicek C.P., Bissett J., Druzhinina I., Kullnig-Gradinger C., Szakacs G. Genetic and Metabolic Diversity of Trichoderma: A Case Study on South-East Asian Isolates. Fungal Genet. Biol. 2003;38:310–319. doi: 10.1016/S1087-1845(02)00583-2. [DOI] [PubMed] [Google Scholar]
- 43.Błaszczyk L., Popiel D., Chełkowski J., Koczyk G., Samuels G.J., Sobieralski K., Siwulski M. Species Diversity of Trichoderma in Poland. J. Appl. Genet. 2011;52:233. doi: 10.1007/s13353-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sariah M., Choo C.W., Zakaria H., Norihan M.S. Quantification and Characterisation of Trichoderma Spp. from Different Ecosystems. Mycopathologia. 2005;159:113–117. doi: 10.1007/s11046-004-4432-6. [DOI] [PubMed] [Google Scholar]
- 45.Harman G.E., Howell C.R., Viterbo A., Chet I., Lorito M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 46.Guzmán-Guzmán P., Porras-Troncoso M.D., Olmedo-Monfil V., Herrera-Estrella A. Trichoderma Species: Versatile Plant Symbionts. Phytopathology. 2019;109:6–16. doi: 10.1094/PHYTO-07-18-0218-RVW. [DOI] [PubMed] [Google Scholar]
- 47.Gutjahr C., Paszkowski U. Weights in the Balance: Jasmonic Acid and Salicylic Acid Signaling in Root-Biotroph Interactions. Mol. Plant Microbe Interact. 2009;22:763–772. doi: 10.1094/MPMI-22-7-0763. [DOI] [PubMed] [Google Scholar]
- 48.Vargas W.A., Mandawe J.C., Kenerley C.M. Plant-Derived Sucrose Is a Key Element in the Symbiotic Association between Trichoderma virens and Maize Plants. Plant Physiol. 2009;151:792–808. doi: 10.1104/pp.109.141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinale F., Sivasithamparam K., Ghisalberti E.L., Marra R., Woo S.L., Lorito M. Trichoderma-Plant-Pathogen Interactions. Soil Biol. Biochem. 2008;40:1–10. doi: 10.1016/j.soilbio.2007.07.002. [DOI] [Google Scholar]
- 50.Mukherjee P.K., Mendoza-Mendoza A., Zeilinger S., Horwitz B.A. Mycoparasitism as a Mechanism of Trichoderma-Mediated Suppression of Plant Diseases. Fungal Biol. Rev. 2022;39:15–33. doi: 10.1016/j.fbr.2021.11.004. [DOI] [Google Scholar]
- 51.Alfiky A., Weisskopf L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi. 2021;7:61. doi: 10.3390/jof7010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Risoli S., Cotrozzi L., Sarrocco S., Nuzzaci M., Pellegrini E., Vitti A. Trichoderma-Induced Resistance to Botrytis Cinerea in Solanum Species: A Meta-Analysis. Plants. 2022;11:180. doi: 10.3390/plants11020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galletti S., Paris R., Cianchetta S. Selected Isolates of Trichoderma gamsii Induce Different Pathways of Systemic Resistance in Maize upon Fusarium verticillioides Challenge. Microbiol. Res. 2020;233:126406. doi: 10.1016/j.micres.2019.126406. [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee M., Mukherjee P.K., Horwitz B.A., Zachow C., Berg G., Zeilinger S. Trichoderma–Plant–Pathogen Interactions: Advances in Genetics of Biological Control. Indian J. Microbiol. 2012;52:522–529. doi: 10.1007/s12088-012-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubicek C.P., Steindorff A.S., Chenthamara K., Manganiello G., Henrissat B., Zhang J., Cai F., Kopchinskiy A.G., Kubicek E.M., Kuo A., et al. Evolution and Comparative Genomics of the Most Common Trichoderma Species. BMC Genom. 2019;20:485. doi: 10.1186/s12864-019-5680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olowe O.M., Nicola L., Asemoloye M.D., Akanmu A.O., Babalola O.O. Trichoderma: Potential Bio-Resource for the Management of Tomato Root Rot Diseases in Africa. Microbiol. Res. 2022;257:126978. doi: 10.1016/j.micres.2022.126978. [DOI] [PubMed] [Google Scholar]
- 57.Benítez T., Rincón A.M., Limón M.C., Codón A.C. Biocontrol Mechanisms of Trichoderma Strains. Int. Microbiol. 2004;7:249–260. [PubMed] [Google Scholar]
- 58.Cortes-Penagos C., Olmedo-Monfil V., Herrera-Estrella A. Biological Control of Plant Diseases. Haworth Food & Agricultural Products Press; Binghamton, NY, USA: 2007. The Nature of Fungal Mycoparasitic Biocontrol Agents; pp. 327–353. [Google Scholar]
- 59.Druzhinina I.S., Seidl-Seiboth V., Herrera-Estrella A., Horwitz B.A., Kenerley C.M., Monte E., Mukherjee P.K., Zeilinger S., Grigoriev I.v., Kubicek C.P. Trichoderma: The Genomics of Opportunistic Success. Nat. Rev. Microbiol. 2011;9:749–759. doi: 10.1038/nrmicro2637. [DOI] [PubMed] [Google Scholar]
- 60.Viterbo A., Horwitz B.A. Mycoparasitism. In: Borkovich K.A., Ebbole D.J., editors. Cellular and Molecular Biology of Filamentous Fungi. ASM Press; Washington, DC, USA: 2010. pp. 676–693. [Google Scholar]
- 61.Fang H., Li C., Zhao J., Zhao C. Biotechnological Advances and Trends in Engineering Trichoderma reesei towards Cellulase Hyperproducer. Biotechnol. Bioprocess Eng. 2021;26:517–528. doi: 10.1007/s12257-020-0243-y. [DOI] [Google Scholar]
- 62.Wong C.K.F., Saidi N.B., Vadamalai G., Teh C.Y., Zulperi D. Effect of Bioformulations on the Biocontrol Efficacy, Microbial Viability and Storage Stability of a Consortium of Biocontrol Agents against Fusarium Wilt of Banana. J. Appl. Microbiol. 2019;127:544–555. doi: 10.1111/jam.14310. [DOI] [PubMed] [Google Scholar]
- 63.Vinale F., Sivasithamparam K. Beneficial Effects of Trichoderma Secondary Metabolites on Crops. Phytother. Res. 2020;34:2835–2842. doi: 10.1002/ptr.6728. [DOI] [PubMed] [Google Scholar]
- 64.Li M.F., Li G.H., Zhang K.Q. Non-Volatile Metabolites from Trichoderma Spp. Metabolites. 2019;9:58. doi: 10.3390/metabo9030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee S., Hung R., Yap M., Bennett J.W. Age Matters: The Effects of Volatile Organic Compounds Emitted by Trichoderma atroviride on Plant Growth. Arch. Microbiol. 2015;197:723–727. doi: 10.1007/s00203-015-1104-5. [DOI] [PubMed] [Google Scholar]
- 66.Garo E., Starks C.M., Jensen P.R., Fenical W., Lobkovsky E., Clardy J. Trichodermamides A and B, Cytotoxic Modified Dipeptides from the Marine-Derived Fungus Trichoderma virens. J. Nat. Prod. 2003;66:423–426. doi: 10.1021/np0204390. [DOI] [PubMed] [Google Scholar]
- 67.Souza A.D.L., Rodrigues-Filho E., Souza A.Q.L., Pereira J.O., Calgarotto A.K., Maso V., Marangoni S., Da Silva S.L. Koninginins, Phospholipase A2 Inhibitors from Endophytic Fungus Trichoderma koningii. Toxicon. 2008;51:240–250. doi: 10.1016/j.toxicon.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Khan R.A.A., Najeeb S., Hussain S., Xie B., Li Y. Bioactive Secondary Metabolites from Trichoderma Spp. against Phytopathogenic Fungi. Microorganisms. 2020;8:817. doi: 10.3390/microorganisms8060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esparza-Reynoso S., Ruíz-Herrera L.F., Pelagio-Flores R., Macías-Rodríguez L.I., Martínez-Trujillo M., López-Coria M., Sánchez-Nieto S., Herrera-Estrella A., López-Bucio J. Trichoderma atroviride-emitted Volatiles Improve Growth of Arabidopsis Seedlings through Modulation of Sucrose Transport and Metabolism. Plant Cell Environ. 2021;44:1961–1976. doi: 10.1111/pce.14014. [DOI] [PubMed] [Google Scholar]
- 70.Venturi V., Keel C. Signaling in the Rhizosphere. Trends Plant Sci. 2016;21:187–198. doi: 10.1016/j.tplants.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Igiehon N.O., Babalola O.O. Rhizosphere Microbiome Modulators: Contributions of Nitrogen Fixing Bacteria towards Sustainable Agriculture. Int. J. Environ. Res. Public Health. 2018;15:574. doi: 10.3390/ijerph15040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He C., Liu C., Liu H., Wang W., Hou J., Li X. Dual Inoculation of Dark Septate Endophytes and Trichoderma viride Drives Plant Performance and Rhizosphere Microbiome Adaptations of Astragalus Mongholicus to Drought. Environ. Microbiol. 2022;24:324–340. doi: 10.1111/1462-2920.15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saravanakumar K., Li Y., Yu C., Wang Q., Wang M., Sun J., Gao J., Chen J. Effect of Trichoderma harzianum on Maize Rhizosphere Microbiome and Biocontrol of Fusarium Stalk Rot. Sci. Rep. 2017;7:1771. doi: 10.1038/s41598-017-01680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez P.A., Rothballer M., Chowdhury S.P., Nussbaumer T., Gutjahr C., Falter-Braun P. Systems Biology of Plant-Microbiome Interactions. Mol. Plant. 2019;12:804–821. doi: 10.1016/j.molp.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Orozco-Mosqueda M.D.C., Rocha-Granados M.d.C., Glick B.R., Santoyo G. Microbiome Engineering to Improve Biocontrol and Plant Growth-Promoting Mechanisms. Microbiol. Res. 2018;208:25–31. doi: 10.1016/j.micres.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Shoresh M., Harman G.E., Mastouri F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 77.Yuan M., Huang Y., Ge W., Jia Z., Song S., Zhang L., Huang Y. Involvement of Jasmonic Acid, Ethylene and Salicylic Acid Signaling Pathways behind the Systemic Resistance Induced by Trichoderma longibrachiatum H9 in Cucumber. BMC Genom. 2019;20:144. doi: 10.1186/s12864-019-5513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bakker P.A.H.M., Ran L.X., Pieterse C.M.J., van Loon L.C. Understanding the Involvement of Rhizobacteria-Mediated Induction of Systemic Resistance in Biocontrol of Plant Diseases. Can. J. Plant Pathol. 2003;25:5–9. doi: 10.1080/07060660309507043. [DOI] [Google Scholar]
- 79.Abdelkhalek A., Al-Askar A.A., Arishi A.A., Behiry S.I. Trichoderma Hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco Mosaic Virus. J. Fungi. 2022;8:228. doi: 10.3390/jof8030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pieterse C.M.J., Zamioudis C., Berendsen R.L., Weller D.M., Van Wees S.C.M., Bakker P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 81.Amorim L., Santos R., Neto J., Guida-Santos M., Crovella S., Benko-Iseppon A. Transcription Factors Involved in Plant Resistance to Pathogens. Curr. Protein Pept. Sci. 2017;18:335–351. doi: 10.2174/1389203717666160619185308. [DOI] [PubMed] [Google Scholar]
- 82.Kuai X., MacLeod B.J., Després C. Integrating Data on the Arabidopsis NPR1/NPR3/NPR4 Salicylic Acid Receptors; a Differentiating Argument. Front. Plant Sci. 2015;6:235. doi: 10.3389/fpls.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopes da Silva F., Aquino E.N., Costa da Cunha D., Vieira Hamann P.R., Magalhães T.B., Steindorff A.S., Ulhoa C.J., Noronha E.F. Analysis of Trichoderma harzianum TR 274 Secretome to Assign Candidate Proteins Involved in Symbiotic Interactions with Phaseolus vulgaris. Biocatal. Agric. Biotechnol. 2022;43:102380. doi: 10.1016/j.bcab.2022.102380. [DOI] [Google Scholar]
- 84.Silva B.B.I., Bannay C., Salamanez K. Trichoderma-Induced Systemic Resistance against the Scale Insect (Unaspis Mabilis Lit & Barbecho) in Lanzones (Lansium Domesticum Corr.) J. Agric. For. 2019;65:59–78. doi: 10.17707/AgricultForest.65.2.05. [DOI] [Google Scholar]
- 85.Korolev N., Rav David D., Elad Y. The Role of Phytohormones in Basal Resistance and Trichoderma-Induced Systemic Resistance to Botrytis Cinerea in Arabidopsis Thaliana. BioControl. 2008;53:667–683. doi: 10.1007/s10526-007-9103-3. [DOI] [Google Scholar]
- 86.Brotman Y., Lisec J., Méret M., Chet I., Willmitzer L., Viterbo A. Transcript and Metabolite Analysis of the Trichoderma-Induced Systemic Resistance Response to Pseudomonas Syringae in Arabidopsis Thaliana. Microbiology. 2012;158:139–146. doi: 10.1099/mic.0.052621-0. [DOI] [PubMed] [Google Scholar]
- 87.Bissett J., Gams W., Jaklitsch W., Samuels G.J. Accepted Trichoderma Names in the Year 2015. IMA Fungus. 2015;6:263–295. doi: 10.5598/imafungus.2015.06.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reithner B., Ibarra-Laclette E., Mach R.L., Herrera-Estrella A. Identification of Mycoparasitism-Related Genes in Trichoderma atroviride. Appl. Environ. Microbiol. 2011;77:4361–4370. doi: 10.1128/AEM.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nawrocka J., Małolepsza U. Diversity in Plant Systemic Resistance Induced by Trichoderma. Biol. Control. 2013;67:149–156. doi: 10.1016/j.biocontrol.2013.07.005. [DOI] [Google Scholar]
- 90.Mukherjee M., Horwitz B.A., Sherkhane P.D., Hadar R., Mukherjee P.K. A Secondary Metabolite Biosynthesis Cluster in Trichoderma virens: Evidence from Analysis of Genes Underexpressed in a Mutant Defective in Morphogenesis and Antibiotic Production. Curr. Genet. 2006;50:193–202. doi: 10.1007/s00294-006-0075-0. [DOI] [PubMed] [Google Scholar]
- 91.Zeilinger S., Gruber S., Bansal R., Mukherjee P.K. Secondary Metabolism in Trichoderma—Chemistry Meets Genomics. Fungal Biol. Rev. 2016;30:74–90. doi: 10.1016/j.fbr.2016.05.001. [DOI] [Google Scholar]
- 92.Stracquadanio C., Quiles J.M., Meca G., Cacciola S.O. Antifungal Activity of Bioactive Metabolites Produced by Trichoderma asperellum and Trichoderma atroviride in Liquid Medium. J. Fungi. 2020;6:263. doi: 10.3390/jof6040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Macías-Rodríguez L., Guzmán-Gómez A., García-Juárez P., Contreras-Cornejo H.A. Trichoderma atroviride Promotes Tomato Development and Alters the Root Exudation of Carbohydrates, Which Stimulates Fungal Growth and the Biocontrol of the Phytopathogen Phytophthora Cinnamomi in a Tripartite Interaction System. FEMS Microbiol. Ecol. 2018;94:fiy137. doi: 10.1093/femsec/fiy137. [DOI] [PubMed] [Google Scholar]
- 94.Leal C., Richet N., Guise J.-F., Gramaje D., Armengol J., Fontaine F., Trotel-Aziz P. Cultivar Contributes to the Beneficial Effects of Bacillus Subtilis PTA-271 and Trichoderma atroviride SC1 to Protect Grapevine Against Neofusicoccum Parvum. Front. Microbiol. 2021;12:726132. doi: 10.3389/fmicb.2021.726132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coninck E., Scauflaire J., Gollier M., Liénard C., Foucart G., Manssens G., Munaut F., Legrève A. Trichoderma atroviride as a Promising Biocontrol Agent in Seed Coating for Reducing Fusarium Damping-off on Maize. J. Appl. Microbiol. 2020;129:637–651. doi: 10.1111/jam.14641. [DOI] [PubMed] [Google Scholar]
- 96.Sánchez-Cruz R., Mehta R., Atriztán-Hernández K., Martínez-Villamil O., del Rayo Sánchez-Carbente M., Sánchez-Reyes A., Lira-Ruan V., González-Chávez C.A., Tabche-Barrera M.L., Bárcenas-Rodríguez R.C., et al. Effects on Capsicum Annuum Plants Colonized with Trichoderma atroviride P. Karst Strains Genetically Modified in Taswo1, a Gene Coding for a Protein with Expansin-like Activity. Plants. 2021;10:1919. doi: 10.3390/plants10091919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karuppiah V., Li Y., Sun J., Vallikkannu M., Chen J. Vel1 Regulates the Growth of Trichoderma atroviride during Co-Cultivation with Bacillus amyloliquefaciens and Is Essential for Wheat Root Rot Control. Biol. Control. 2020;151:104374. doi: 10.1016/j.biocontrol.2020.104374. [DOI] [Google Scholar]
- 98.Li T., Zhang J., Tang J., Liu Z., Li Y., Chen J., Zou L. Combined Use of Trichoderma atroviride CCTCCSBW0199 and Brassinolide to Control Botrytis Cinerea Infection in Tomato. Plant Dis. 2020;104:1298–1304. doi: 10.1094/PDIS-07-19-1568-RE. [DOI] [PubMed] [Google Scholar]
- 99.Romero-Contreras Y.J., Ramírez-Valdespino C.A., Guzmán-Guzmán P., Macías-Segoviano J.I., Villagómez-Castro J.C., Olmedo-Monfil V. Tal6 From Trichoderma atroviride Is a LysM Effector Involved in Mycoparasitism and Plant Association. Front. Microbiol. 2019;10:2231. doi: 10.3389/fmicb.2019.02231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moreno-Ruiz D., Fuchs A., Missbach K., Schuhmacher R., Zeilinger S. Influence of Different Light Regimes on the Mycoparasitic Activity and 6-Pentyl-α-Pyrone Biosynthesis in Two Strains of Trichoderma Atroviride. Pathogens. 2020;9:860. doi: 10.3390/pathogens9100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Speckbacher V., Ruzsanyi V., Martinez-Medina A., Hinterdobler W., Doppler M., Schreiner U., Böhmdorfer S., Beccaccioli M., Schuhmacher R., Reverberi M., et al. The Lipoxygenase Lox1 Is Involved in Light- and Injury-Response, Conidiation, and Volatile Organic Compound Biosynthesis in the Mycoparasitic Fungus Trichoderma Atroviride. Front. Microbiol. 2020;11:2004. doi: 10.3389/fmicb.2020.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coppola M., Cascone P., Di Lelio I., Woo S.L., Lorito M., Rao R., Pennacchio F., Guerrieri E., Digilio M.C. Trichoderma atroviride P1 Colonization of Tomato Plants Enhances Both Direct and Indirect Defense Barriers Against Insects. Front. Physiol. 2019;10:813. doi: 10.3389/fphys.2019.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rebolledo-Prudencio O.G., Estrada-Rivera M., Dautt-Castro M., Arteaga-Vazquez M.A., Arenas-Huertero C., Rosendo-Vargas M.M., Jin H., Casas-Flores S. The Small RNA-mediated Gene Silencing Machinery Is Required in Arabidopsis for Stimulation of Growth, Systemic Disease Resistance, and Suppression of the Nitrile-specifier Gene NSP4 by Trichoderma atroviride. Plant J. 2021;109:873–890. doi: 10.1111/tpj.15599. [DOI] [PubMed] [Google Scholar]
- 104.Zehra A., Meena M., Dubey M.K., Aamir M., Upadhyay R.S. Synergistic Effects of Plant Defense Elicitors and Trichoderma harzianum on Enhanced Induction of Antioxidant Defense System in Tomato against Fusarium Wilt Disease. Bot. Stud. 2017;58:44. doi: 10.1186/s40529-017-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larran S., Santamarina Siurana M.P., Roselló Caselles J., Simón M.R., Perelló A. In Vitro Antagonistic Activity of Trichoderma harzianum against Fusarium Sudanense Causing Seedling Blight and Seed Rot on Wheat. ACS Omega. 2020;5:23276–23283. doi: 10.1021/acsomega.0c03090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahmoud G.A.-E., Abdel-Sater M.A., Al-Amery E., Hussein N.A. Controlling Alternaria Cerealis MT808477 Tomato Phytopathogen by Trichoderma harzianum and Tracking the Plant Physiological Changes. Plants. 2021;10:1846. doi: 10.3390/plants10091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang F., Chen C., Zhang F., Gao L., Liu J., Chen L., Fan X., Liu C., Zhang K., He Y., et al. Trichoderma harzianum Containing 1-Aminocyclopropane-1-Carboxylate Deaminase and Chitinase Improved Growth and Diminished Adverse Effect Caused by Fusarium Oxysporum in Soybean. J. Plant Physiol. 2017;210:84–94. doi: 10.1016/j.jplph.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 108.Paul N.C., Park S., Liu H., Lee J.G., Han G.H., Kim H., Sang H. Fungi Associated with Postharvest Diseases of Sweet Potato Storage Roots and In Vitro Antagonistic Assay of Trichoderma harzianum against the Diseases. J. Fungi. 2021;7:927. doi: 10.3390/jof7110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Braun H., Woitsch L., Hetzer B., Geisen R., Zange B., Schmidt-Heydt M. Trichoderma harzianum: Inhibition of Mycotoxin Producing Fungi and Toxin Biosynthesis. Int. J. Food Microbiol. 2018;280:10–16. doi: 10.1016/j.ijfoodmicro.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 110.Stummer B.E., Zhang Q., Zhang X., Warren R.A., Harvey P.R. Quantification of Trichoderma Afroharzianum, Trichoderma harzianum and Trichoderma gamsii Inoculants in Soil, the Wheat Rhizosphere and in Planta Suppression of the Crown Rot Pathogen Fusarium Pseudograminearum. J. Appl. Microbiol. 2020;129:971–990. doi: 10.1111/jam.14670. [DOI] [PubMed] [Google Scholar]
- 111.Hewedy O.A., Abdel Lateif K.S., Seleiman M.F., Shami A., Albarakaty F.M., El-Meihy R.M. Phylogenetic Diversity of Trichoderma Strains and Their Antagonistic Potential against Soil-Borne Pathogens under Stress Conditions. Biology. 2020;9:189. doi: 10.3390/biology9080189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yadav M., Dubey M.K., Upadhyay R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi. 2021;7:307. doi: 10.3390/jof7040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Contreras-Cornejo H.A., Macías-Rodríguez L., Real-Santillán R.O., López-Carmona D., García-Gómez G., Galicia-Gallardo A.P., Alfaro-Cuevas R., González-Esquivel C.E., Najera-Rincón M.B., Adame-Garnica S.G., et al. In a Belowground Multitrophic Interaction, Trichoderma harzianum Induces Maize Root Herbivore Tolerance against Phyllophaga Vetula. Pest. Manag. Sci. 2021;77:3952–3963. doi: 10.1002/ps.6415. [DOI] [PubMed] [Google Scholar]
- 114.Rubio M.B., Pardal A.J., Cardoza R.E., Gutiérrez S., Monte E., Hermosa R. Involvement of the Transcriptional Coactivator ThMBF1 in the Biocontrol Activity of Trichoderma harzianum. Front. Microbiol. 2017;8:2273. doi: 10.3389/fmicb.2017.02273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kai K., Mine K., Akiyama K., Ohki S., Hayashi H. Anti-Plant Viral Activity of Peptaibols, Trichorzins HA II, HA V, and HA VI, Isolated from Trichoderma harzianum HK-61. J. Pestic. Sci. 2018;43:283–286. doi: 10.1584/jpestics.D18-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Deng J.-J., Huang W.-Q., Li Z.-W., Lu D.-L., Zhang Y., Luo X. Biocontrol Activity of Recombinant Aspartic Protease from Trichoderma harzianum against Pathogenic Fungi. Enzym. Microb. Technol. 2018;112:35–42. doi: 10.1016/j.enzmictec.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Ding J., Mei J., Huang P., Tian Y., Liang Y., Jiang X., Li M. Gα3 Subunit Thga3 Positively Regulates Conidiation, Mycoparasitism, Chitinase Activity, and Hydrophobicity of Trichoderma harzianum. AMB Express. 2020;10:221. doi: 10.1186/s13568-020-01162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peng K.-C., Lin C.-C., Liao C.-F., Yu H.-C., Lo C.-T., Yang H.-H., Lin K.-C. Expression of L-Amino Acid Oxidase of Trichoderma harzianum in Tobacco Confers Resistance to Sclerotinia Sclerotiorum and Botrytis Cinerea. Plant Sci. 2021;303:110772. doi: 10.1016/j.plantsci.2020.110772. [DOI] [PubMed] [Google Scholar]
- 119.Gomes E.V., Ulhoa C.J., Cardoza R.E., Silva R.N., Gutiérrez S. Involvement of Trichoderma harzianum Epl-1 Protein in the Regulation of Botrytis Virulence- and Tomato Defense-Related Genes. Front. Plant Sci. 2017;8:880. doi: 10.3389/fpls.2017.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ghoniem A.A., Abd El-Hai K.M., El-khateeb A.Y., Eldadamony N.M., Mahmoud S.F., Elsayed A. Enhancing the Potentiality of Trichoderma harzianum against Pythium Pathogen of Beans Using Chamomile (Matricaria chamomilla, L.) Flower Extract. Molecules. 2021;26:1178. doi: 10.3390/molecules26041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohiddin F.A., Padder S.A., Bhat A.H., Ahanger M.A., Shikari A.B., Wani S.H., Bhat F.A., Nabi S.U., Hamid A., Bhat N.A., et al. Phylogeny and Optimization of Trichoderma harzianum for Chitinase Production: Evaluation of Their Antifungal Behaviour against the Prominent Soil Borne Phyto-Pathogens of Temperate India. Microorganisms. 2021;9:1962. doi: 10.3390/microorganisms9091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mironenka J., Różalska S., Bernat P. Potential of Trichoderma harzianum and Its Metabolites to Protect Wheat Seedlings against Fusarium Culmorum and 2,4-D. Int. J. Mol. Sci. 2021;22:13058. doi: 10.3390/ijms222313058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poveda J., Hermosa R., Monte E., Nicolás C. The Trichoderma harzianum Kelch Protein ThKEL1 Plays a Key Role in Root Colonization and the Induction of Systemic Defense in Brassicaceae Plants. Front. Plant Sci. 2019;10:1478. doi: 10.3389/fpls.2019.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alınç T., Cusumano A., Peri E., Torta L., Colazza S. Trichoderma harzianum Strain T22 Modulates Direct Defense of Tomato Plants in Response to Nezara Viridula Feeding Activity. J. Chem. Ecol. 2021;47:455–462. doi: 10.1007/s10886-021-01260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leonetti P., Zonno M.C., Molinari S., Altomare C. Induction of SA-Signaling Pathway and Ethylene Biosynthesis in Trichoderma harzianum-Treated Tomato Plants after Infection of the Root-Knot Nematode Meloidogyne Incognita. Plant Cell Rep. 2017;36:621–631. doi: 10.1007/s00299-017-2109-0. [DOI] [PubMed] [Google Scholar]
- 126.Coppola M., Cascone P., Chiusano M.L., Colantuono C., Lorito M., Pennacchio F., Rao R., Woo S.L., Guerrieri E., Digilio M.C. Trichoderma harzianum enhances Tomato Indirect Defense against Aphids. Insect Sci. 2017;24:1025–1033. doi: 10.1111/1744-7917.12475. [DOI] [PubMed] [Google Scholar]
- 127.Coppola M., Diretto G., Digilio M.C., Woo S.L., Giuliano G., Molisso D., Pennacchio F., Lorito M., Rao R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum Strain T22 Primes and Enhances Defense Responses against Aphids. Front. Physiol. 2019;10:745. doi: 10.3389/fphys.2019.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh U.B., Malviya D., Singh S., Kumar M., Sahu P.K., Singh H.V., Kumar S., Roy M., Imran M., Rai J.P., et al. Trichoderma harzianum- and Methyl Jasmonate-Induced Resistance to Bipolaris Sorokiniana Through Enhanced Phenylpropanoid Activities in Bread Wheat (Triticum aestivum L.) Front. Microbiol. 2019;10:1697. doi: 10.3389/fmicb.2019.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen S.-C., Ren J.-J., Zhao H.-J., Wang X.-L., Wang T.-H., Jin S.-D., Wang Z.-H., Li C., Liu A.-R., Lin X.-M., et al. Trichoderma harzianum Improves Defense Against Fusarium Oxysporum by Regulating ROS and RNS Metabolism, Redox Balance, and Energy Flow in Cucumber Roots. Phytopathology. 2019;109:972–982. doi: 10.1094/PHYTO-09-18-0342-R. [DOI] [PubMed] [Google Scholar]
- 130.Yu Z., Wang Z., Zhang Y., Wang Y., Liu Z. Biocontrol and Growth-Promoting Effect of Trichoderma asperellum TaspHu1 Isolate from Juglans Mandshurica Rhizosphere Soil. Microbiol. Res. 2021;242:126596. doi: 10.1016/j.micres.2020.126596. [DOI] [PubMed] [Google Scholar]
- 131.Batool R., Umer M.J., Wang Y., He K., Zhang T., Bai S., Zhi Y., Chen J., Wang Z. Synergistic Effect of Beauveria Bassiana and Trichoderma asperellum to Induce Maize (Zea mays L.) Defense against the Asian Corn Borer, Ostrinia Furnacalis (Lepidoptera, Crambidae) and Larval Immune Response. Int. J. Mol. Sci. 2020;21:8215. doi: 10.3390/ijms21218215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo R., Wang Z., Huang Y., Fan H., Liu Z. Biocontrol Potential of Saline- or Alkaline-Tolerant Trichoderma asperellum Mutants against Three Pathogenic Fungi under Saline or Alkaline Stress Conditions. Braz. J. Microbiol. 2018;49:236–245. doi: 10.1016/j.bjm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang H., Zhang R., Duan Y., Jiang W., Chen X., Shen X., Yin C., Mao Z. The Endophytic Strain Trichoderma asperellum 6S-2: An Efficient Biocontrol Agent against Apple Replant Disease in China and a Potential Plant-Growth-Promoting Fungus. J. Fungi. 2021;7:1050. doi: 10.3390/jof7121050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baiyee B., Ito S., Sunpapao A. Trichoderma asperellum T1 Mediated Antifungal Activity and Induced Defense Response against Leaf Spot Fungi in Lettuce (Lactuca sativa L.) Physiol. Mol. Plant Pathol. 2019;106:96–101. doi: 10.1016/j.pmpp.2018.12.009. [DOI] [Google Scholar]
- 135.Wu Q., Sun R., Ni M., Yu J., Li Y., Yu C., Dou K., Ren J., Chen J. Identification of a Novel Fungus, Trichoderma asperellum GDFS1009, and Comprehensive Evaluation of Its Biocontrol Efficacy. PLoS ONE. 2017;12:e0179957. doi: 10.1371/journal.pone.0179957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chou H., Xiao Y.-T., Tsai J.-N., Li T.-T., Wu H.-Y., Liu L.D., Tzeng D.-S., Chung C.-L. In Vitro and in Planta Evaluation of Trichoderma asperellum TA as a Biocontrol Agent Against Phellinus Noxius, the Cause of Brown Root Rot Disease of Trees. Plant Dis. 2019;103:2733–2741. doi: 10.1094/PDIS-01-19-0179-RE. [DOI] [PubMed] [Google Scholar]
- 137.Yu W., Mijiti G., Huang Y., Fan H., Wang Y., Liu Z. Functional Analysis of Eliciting Plant Response Protein Epl1-Tas from Trichoderma asperellum ACCC30536. Sci. Rep. 2018;8:7974. doi: 10.1038/s41598-018-26328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang H., Ji S., Guo R., Zhou C., Wang Y., Fan H., Liu Z. Hydrophobin HFBII-4 from Trichoderma asperellum Induces Antifungal Resistance in Poplar. Braz. J. Microbiol. 2019;50:603–612. doi: 10.1007/s42770-019-00083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Karuppiah V., Sun J., Li T., Vallikkannu M., Chen J. Co-Cultivation of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 Causes Differential Gene Expression and Improvement in the Wheat Growth and Biocontrol Activity. Front. Microbiol. 2019;10:1068. doi: 10.3389/fmicb.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tamandegani P.R., Marik T., Zafari D., Balázs D., Vágvölgyi C., Szekeres A., Kredics L. Changes in Peptaibol Production of Trichoderma Species during In Vitro Antagonistic Interactions with Fungal Plant Pathogens. Biomolecules. 2020;10:730. doi: 10.3390/biom10050730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Degani O., Khatib S., Becher P., Gordani A., Harris R. Trichoderma asperellum Secreted 6-Pentyl-α-Pyrone to Control Magnaporthiopsis Maydis, the Maize Late Wilt Disease Agent. Biology. 2021;10:897. doi: 10.3390/biology10090897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Al-Askar A.A., Saber W.I.A., Ghoneem K.M., Hafez E.E., Ibrahim A.A. Crude Citric Acid of Trichoderma asperellum: Tomato Growth Promotor and Suppressor of Fusarium oxysporum f. sp. lycopersici. Plants. 2021;10:222. doi: 10.3390/plants10020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Karuppiah V., Zhixiang L., Liu H., Vallikkannu M., Chen J. Co-Culture of Vel1-Overexpressed Trichoderma asperellum and Bacillus amyloliquefaciens: An Eco-Friendly Strategy to Hydrolyze the Lignocellulose Biomass in Soil to Enrich the Soil Fertility, Plant Growth and Disease Resistance. Microb. Cell Factories. 2021;20:57. doi: 10.1186/s12934-021-01540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Intana W., Kheawleng S., Sunpapao A. Trichoderma asperellum T76-14 Released Volatile Organic Compounds against Postharvest Fruit Rot in Muskmelons (Cucumis melo) Caused by Fusarium Incarnatum. J. Fungi. 2021;7:46. doi: 10.3390/jof7010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel J.S., Kharwar R.N., Singh H.B., Upadhyay R.S., Sarma B.K. Trichoderma asperellum (T42) and Pseudomonas Fluorescens (OKC)-Enhances Resistance of Pea against Erysiphe Pisi through Enhanced ROS Generation and Lignifications. Front. Microbiol. 2017;08:306. doi: 10.3389/fmicb.2017.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Herrera-Téllez V.I., Cruz-Olmedo A.K., Plasencia J., Gavilanes-Ruíz M., Arce-Cervantes O., Hernández-León S., Saucedo-García M. The Protective Effect of Trichoderma asperellum on Tomato Plants against Fusarium Oxysporum and Botrytis Cinerea Diseases Involves Inhibition of Reactive Oxygen Species Production. Int. J. Mol. Sci. 2019;20:2007. doi: 10.3390/ijms20082007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Izquierdo-García L.F., González-Almario A., Cotes A.M., Moreno-Velandia C.A. Trichoderma virens Gl006 and Bacillus Velezensis Bs006: A Compatible Interaction Controlling Fusarium Wilt of Cape Gooseberry. Sci. Rep. 2020;10:6857. doi: 10.1038/s41598-020-63689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Halifu S., Deng X., Song X., Song R., Liang X. Inhibitory Mechanism of Trichoderma virens ZT05 on Rhizoctonia Solani. Plants. 2020;9:912. doi: 10.3390/plants9070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mukherjee P.K., Mehetre S.T., Sherkhane P.D., Muthukathan G., Ghosh A., Kotasthane A.S., Khare N., Rathod P., Sharma K.K., Nath R., et al. A Novel Seed-Dressing Formulation Based on an Improved Mutant Strain of Trichoderma virens, and Its Field Evaluation. Front. Microbiol. 2019;10:1910. doi: 10.3389/fmicb.2019.01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ghasemi S., Safaie N., Shahbazi S., Shams-Bakhsh M., Askari H. The Role of Cell Wall Degrading Enzymes in Antagonistic Traits of Trichoderma virens against Rhizoctonia Solani. Iran. J. Biotechnol. 2020;18:18–28. doi: 10.30498/IJB.2020.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ramírez-Valdespino C.A., Porras-Troncoso M.D., Corrales-Escobosa A.R., Wrobel K., Martínez-Hernández P., Olmedo-Monfil V. Functional Characterization of TvCyt2, a Member of the P450 Monooxygenases From Trichoderma virens Relevant during the Association with Plants and Mycoparasitism. Mol. Plant-Microbe Interact. 2018;31:289–298. doi: 10.1094/MPMI-01-17-0015-R. [DOI] [PubMed] [Google Scholar]
- 152.Mukherjee P.K., Hurley J.F., Taylor J.T., Puckhaber L., Lehner S., Druzhinina I., Schumacher R., Kenerley C.M. Ferricrocin, the Intracellular Siderophore of Trichoderma virens, Is Involved in Growth, Conidiation, Gliotoxin Biosynthesis and Induction of Systemic Resistance in Maize. Biochem. Biophys. Res. Commun. 2018;505:606–611. doi: 10.1016/j.bbrc.2018.09.170. [DOI] [PubMed] [Google Scholar]
- 153.Sarrocco S., Matarese F., Baroncelli R., Vannacci G., Seidl-Seiboth V., Kubicek C.P., Vergara M. The Constitutive Endopolygalacturonase TvPG2 Regulates the Induction of Plant Systemic Resistance by Trichoderma virens. Phytopathology. 2017;107:537–544. doi: 10.1094/PHYTO-03-16-0139-R. [DOI] [PubMed] [Google Scholar]
- 154.Jogaiah S., Abdelrahman M., Tran L.-S.P., Ito S.-I. Different Mechanisms of Trichoderma virens -Mediated Resistance in Tomato against Fusarium Wilt Involve the Jasmonic and Salicylic Acid Pathways. Mol. Plant Pathol. 2018;19:870–882. doi: 10.1111/mpp.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dubey S.C., Tripathi A., Tak R. Expression of Defense-Related Genes in Mung Bean Varieties in Response to Trichoderma virens Alone and in the Presence of Rhizoctonia Solani Infection. 3 Biotech. 2018;8:432. doi: 10.1007/s13205-018-1453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang K.-D., Borrego E.J., Kenerley C.M., Kolomiets M.V. Oxylipins Other than Jasmonic Acid Are Xylem-Resident Signals Regulating Systemic Resistance Induced by Trichoderma virens in Maize. Plant Cell. 2020;32:166–185. doi: 10.1105/tpc.19.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang K.-D., Gorman Z., Huang P.-C., Kenerley C.M., Kolomiets M.V. Trichoderma virens Colonization of Maize Roots Triggers Rapid Accumulation of 12-Oxophytodienoate and Two ᵧ-Ketols in Leaves as Priming Agents of Induced Systemic Resistance. Plant Signal Behav. 2020;15:1792187. doi: 10.1080/15592324.2020.1792187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sridharan A.P., Thankappan S., Karthikeyan G., Uthandi S. Comprehensive Profiling of the VOCs of Trichoderma longibrachiatum EF5 While Interacting with Sclerotium Rolfsii and Macrophomina Phaseolina. Microbiol. Res. 2020;236:126436. doi: 10.1016/j.micres.2020.126436. [DOI] [PubMed] [Google Scholar]
- 159.Boamah S., Zhang S., Xu B., Li T., Calderón-Urrea A. Trichoderma longibrachiatum (TG1) Enhances Wheat Seedlings Tolerance to Salt Stress and Resistance to Fusarium Pseudograminearum. Front. Plant Sci. 2021;12:741231. doi: 10.3389/fpls.2021.741231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang S., Gan Y., Ji W., Xu B., Hou B., Liu J. Mechanisms and Characterization of Trichoderma longibrachiatum T6 in Suppressing Nematodes (Heterodera avenae) in Wheat. Front. Plant Sci. 2017;8:1491. doi: 10.3389/fpls.2017.01491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sridharan A.P., Sugitha T., Karthikeyan G., Nakkeeran S., Sivakumar U. Metabolites of Trichoderma longibrachiatum EF5 Inhibits Soil Borne Pathogen, Macrophomina Phaseolina by Triggering Amino Sugar Metabolism. Microb. Pathog. 2021;150:104714. doi: 10.1016/j.micpath.2020.104714. [DOI] [PubMed] [Google Scholar]
- 162.Degani O., Rabinovitz O., Becher P., Gordani A., Chen A. Trichoderma longibrachiatum and Trichoderma asperellum Confer Growth Promotion and Protection against Late Wilt Disease in the Field. J. Fungi. 2021;7:444. doi: 10.3390/jof7060444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sarsaiya S., Jain A., Fan X., Jia Q., Xu Q., Shu F., Zhou Q., Shi J., Chen J. New Insights into Detection of a Dendrobine Compound From a Novel Endophytic Trichoderma longibrachiatum Strain and Its Toxicity Against Phytopathogenic Bacteria. Front. Microbiol. 2020;11:337. doi: 10.3389/fmicb.2020.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sella L., Govind R., Caracciolo R., Quarantin A., Vu V.V., Tundo S., Nguyen H.M., Favaron F., Musetti R., De Zotti M. Transcriptomic and Ultrastructural Analyses of Pyricularia Oryzae Treated with Fungicidal Peptaibol Analogs of Trichoderma Trichogin. Front. Microbiol. 2021;12:753202. doi: 10.3389/fmicb.2021.753202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moscatiello R., Sello S., Ruocco M., Barbulova A., Cortese E., Nigris S., Baldan B., Chiurazzi M., Mariani P., Lorito M., et al. The Hydrophobin HYTLO1 Secreted by the Biocontrol Fungus Trichoderma longibrachiatum Triggers a NAADP-Mediated Calcium Signalling Pathway in Lotus Japonicus. Int. J. Mol. Sci. 2018;19:2596. doi: 10.3390/ijms19092596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Du F.-Y., Ju G.-L., Xiao L., Zhou Y.-M., Wu X. Sesquiterpenes and Cyclodepsipeptides from Marine-Derived Fungus Trichoderma longibrachiatum and Their Antagonistic Activities against Soil-Borne Pathogens. Mar. Drugs. 2020;18:165. doi: 10.3390/md18030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ngo T., Van Nguyen M., Han J.W., Park M.S., Kim H., Choi G.J. In Vitro and in Vivo Antifungal Activity of Sorbicillinoids Produced by Trichoderma longibrachiatum Men. J. Fungi. 2021;7:428. doi: 10.3390/jof7060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Elbanhawy A.A., Elsherbiny E.A., Abd El-Mageed A.E., Abdel-Fattah G.M. Potential of Fungal Metabolites as a Biocontrol Agent against Cotton Aphid, Aphis Gossypii Glover and the Possible Mechanisms of Action. Pestic. Biochem. Physiol. 2019;159:34–40. doi: 10.1016/j.pestbp.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 169.Zhang S., Xu B., Zhang J., Gan Y. Identification of the Antifungal Activity of Trichoderma longibrachiatum T6 and Assessment of Bioactive Substances in Controlling Phytopathgens. Pestic. Biochem. Physiol. 2018;147:59–66. doi: 10.1016/j.pestbp.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 170.Kumar S., Shukla V., Dubey M.K., Upadhyay R.S. Activation of Defense Response in Common Bean against Stem Rot Disease Triggered by Trichoderma Erinaceum and Trichoderma viride. J. Basic Microbiol. 2021;61:910–922. doi: 10.1002/jobm.202000749. [DOI] [PubMed] [Google Scholar]
- 171.Awad N.E., Kassem H.A., Hamed M.A., El-Feky A.M., Elnaggar M.A.A., Mahmoud K., Ali M.A. Isolation and Characterization of the Bioactive Metabolites from the Soil Derived Fungus Trichoderma viride. Mycology. 2018;9:70–80. doi: 10.1080/21501203.2017.1423126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Singh J., Singh P., Vaishnav A., Ray S., Rajput R.S., Singh S.M., Singh H.B. Belowground Fungal Volatiles Perception in Okra (Abelmoschus esculentus) Facilitates Plant Growth under Biotic Stress. Microbiol. Res. 2021;246:126721. doi: 10.1016/j.micres.2021.126721. [DOI] [PubMed] [Google Scholar]
- 173.Jaklitsch W.M. European Species of Hypocrea Part II: Species with Hyaline Ascospores. Fungal Divers. 2011;48:1–250. doi: 10.1007/s13225-011-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jin X., Guo L., Jin B., Zhu S., Mei X., Wu J., Liu T., He X. Inhibitory Mechanism of 6-Pentyl-2H-Pyran-2-One Secreted by Trichoderma Atroviride T2 against Cylindrocarpon Destructans. Pestic. Biochem. Physiol. 2020;170:104683. doi: 10.1016/j.pestbp.2020.104683. [DOI] [PubMed] [Google Scholar]
- 175.de Aráujo Á.A., Pastore G.M., Berger R.G. Production of Coconut Aroma by Fungi Cultivation in Solid-State Fermentation. Appl. Biochem. Biotechnol. 2002;98–100:747–752. doi: 10.1385/ABAB:98-100:1-9:747. [DOI] [PubMed] [Google Scholar]
- 176.Vinale F., Sivasithamparam K., Ghisalberti E.L., Marra R., Barbetti M.J., Li H., Woo S.L., Lorito M. A Novel Role for Trichoderma Secondary Metabolites in the Interactions with Plants. Physiol. Mol. Plant Pathol. 2008;72:80–86. doi: 10.1016/j.pmpp.2008.05.005. [DOI] [Google Scholar]
- 177.Wai H.H., Pui C.T., Hyde K.D. Induction of Antibiotic Production of Freshwater Fungi Using Mix-Culture Fermentation. Fungal Divers. 2003;12:45–51. [Google Scholar]
- 178.Brotman Y., Briff E., Viterbo A., Chet I. Role of Swollenin, an Expansin-Like Protein from Trichoderma, in Plant Root Colonization. Plant Physiol. 2008;147:779–789. doi: 10.1104/pp.108.116293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chaverri P., Branco-Rocha F., Jaklitsch W., Gazis R., Degenkolb T., Samuels G.J. Systematics of the Trichoderma harzianum Species Complex and the Re-Identification of Commercial Biocontrol Strains Europe PMC Funders Group. Mycologia. 2015;107:558–590. doi: 10.3852/14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Erazo J.G., Palacios S.A., Pastor N., Giordano F.D., Rovera M., Reynoso M.M., Venisse J.S., Torres A.M. Biocontrol Mechanisms of Trichoderma harzianum ITEM 3636 against Peanut Brown Root Rot Caused by Fusarium Solani RC 386. Biol. Control. 2021;164:1049–9644. doi: 10.1016/j.biocontrol.2021.104774. [DOI] [Google Scholar]
- 181.Hewedy O.A., Abdel-Lateif K.S., Bakr R.A. Genetic Diversity and Biocontrol Efficacy of Indigenous Trichoderma Isolates against Fusarium Wilt of Pepper. J. Basic Microbiol. 2020;60:126–135. doi: 10.1002/jobm.201900493. [DOI] [PubMed] [Google Scholar]
- 182.Lei M., Liu J., Fang Y., Shao Y., Li L., Yu J.-H., Chen F. Effects of Different G-Protein α-Subunits on Growth, Development and Secondary Metabolism of Monascus Ruber M7. Front. Microbiol. 2019;10:1555. doi: 10.3389/fmicb.2019.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Samuels G.J., Ismaiel A., Bon M.-C., de Respinis S., Petrini O. Trichoderma asperellum Sensu Lato Consists of Two Cryptic Species. Mycologia. 2010;102:944–966. doi: 10.3852/09-243. [DOI] [PubMed] [Google Scholar]
- 184.El-Komy M.H., Saleh A.A., Eranthodi A., Molan Y.Y. Characterization of Novel Trichoderma asperellum Isolates to Select Effective Biocontrol Agents against Tomato Fusarium Wilt. Plant Pathol. J. 2015;31:50–60. doi: 10.5423/PPJ.OA.09.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Howell C.R., Stipanovic R.D., Lumsden R.D. Antibiotic Production by Strains of Gliocladium Virens and Its Relation to the Biocontrol of Cotton Seedling Diseases. Biocontrol Sci. Technol. 1993;3:435–441. doi: 10.1080/09583159309355298. [DOI] [Google Scholar]
- 186.Vargas W.A., Mukherjee P.K., Laughlin D., Wiest A., Moran-Diez M.E., Kenerley C.M. Role of Gliotoxin in the Symbiotic and Pathogenic Interactions of Trichoderma virens. Microbiology. 2014;160:2319–2330. doi: 10.1099/mic.0.079210-0. [DOI] [PubMed] [Google Scholar]
- 187.Stipanovic R.D., Howell C.R. The Structure of Gliovirin, a New Antibiotic from Gliocladium Virens. J. Antibiot. 1982;35:1326–1330. doi: 10.7164/antibiotics.35.1326. [DOI] [PubMed] [Google Scholar]
- 188.Hua L., Zeng H., He L., Jiang Q., Ye P., Liu Y., Sun X., Zhang M. Gliotoxin Is an Important Secondary Metabolite Involved in Suppression of Sclerotium Rolfsii of Trichoderma virens T23. Phytopathology. 2021;111:1720–1725. doi: 10.1094/PHYTO-09-20-0399-R. [DOI] [PubMed] [Google Scholar]
- 189.Werck-Reichhart D., Feyereisen R. Cytochromes P450: A Success Story. Genome Biol. 2000;1:REVIEWS3003. doi: 10.1186/gb-2000-1-6-reviews3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hubmann R., Schnabl S., Araghi M., Schmidl C., Rendeiro A.F., Hilgarth M., Demirtas D., Ali F., Staber P.B., Valent P., et al. Targeting Nuclear NOTCH2 by Gliotoxin Recovers a Tumor-Suppressor NOTCH3 Activity in CLL. Cells. 2020;9:1484. doi: 10.3390/cells9061484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen J., Lou Q., He L., Wen C., Lin M., Zhu Z., Wang F., Huang L., Lan W., Iwamoto A., et al. Reduced-Gliotoxin Induces ROS-Mediated Anoikis in Human Colorectal Cancer Cells. Int. J. Oncol. 2018;52:1023–1032. doi: 10.3892/ijo.2018.4264. [DOI] [PubMed] [Google Scholar]
- 192.Zhang J.-L., Tang W.-L., Huang Q.-R., Li Y.-Z., Wei M.-L., Jiang L.-L., Liu C., Yu X., Zhu H.-W., Chen G.-Z., et al. Trichoderma: A Treasure House of Structurally Diverse Secondary Metabolites with Medicinal Importance. Front. Microbiol. 2021;12:723828. doi: 10.3389/fmicb.2021.723828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Samuels G.J., Ismaiel A., Mulaw T.B., Szakacs G., Druzhinina I.S., Kubicek C.P., Jaklitsch W.M. The Longibrachiatum Clade of Trichoderma: A Revision with New Species. Fungal Divers. 2012;55:77–108. doi: 10.1007/s13225-012-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Recio R., Meléndez-Carmona M., Martín-Higuera M.C., Pérez V., López E., López-Medrano F., Pérez-Ayala A. Trichoderma longibrachiatum: An Unusual Pathogen of Fungal Pericarditis. Clin. Microbiol. Infect. 2019;25:586–587. doi: 10.1016/j.cmi.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 195.Georgakopoulou V.E., Melemeni D., Mantzouranis K., Damaskos C., Gkoufa A., Chlapoutakis S., Garmpis N., Garmpi A., Sklapani P., Trakas N., et al. Firstcase of Pneumonia-Parapneumonic Effusion Due to Trichoderma longibrachiatum. IDCases. 2021;25:e01239. doi: 10.1016/j.idcr.2021.e01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hatvani L., Homa M., Chenthamara K., Cai F., Kocsubé S., Atanasova L., Mlinaric-Missoni E., Manikandan P., Revathi R., Dóczi I., et al. Agricultural Systems as Potential Sources of Emerging Human Mycoses Caused by Trichoderma: A Successful, Common Phylotype of Trichoderma longibrachiatum in the Frontline. FEMS Microbiol. Lett. 2019;366:246. doi: 10.1093/femsle/fnz246. [DOI] [PubMed] [Google Scholar]
- 197.Sarsaiya S., Jain A., Jia Q., Fan X., Shu F., Chen Z., Zhou Q., Shi J., Chen J. Molecular Identification of Endophytic Fungi and Their Pathogenicity Evaluation Against Dendrobium Nobile and Dendrobium Officinale. Int. J. Mol. Sci. 2020;21:316. doi: 10.3390/ijms21010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jaklitsch W.M., Samuels G.J., Dodd S.L., Lu B.-S., Druzhinina I.S. Hypocrea Rufa/Trichoderma viride: A Reassessment, and Description of Five Closely Related Species with and without Warted Conidia. Stud. Mycol. 2006;56:135–177. doi: 10.3114/sim.2006.56.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ujor V.C., Peiris D.G., Monti M., Kang A.S., Clements M.O., Hedger J.N. Quantitative Proteomic Analysis of the Response of the Wood-Rot Fungus, Schizophyllum Commune, to the Biocontrol Fungus, Trichoderma viride. Lett. Appl. Microbiol. 2012;54:336–343. doi: 10.1111/j.1472-765X.2012.03215.x. [DOI] [PubMed] [Google Scholar]
- 200.Ujor V.C., Monti M., Peiris D.G., Clements M.O., Hedger J.N. The Mycelial Response of the White-Rot Fungus, Schizophyllum Commune to the Biocontrol Agent, Trichoderma viride. Fungal Biol. 2012;116:332–341. doi: 10.1016/j.funbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 201.Arisan-Atac I., Heidenreich E., Kubicek C.P. Randomly Amplified Polymorphic DNA Fingerprinting Identifies Subgroups of Trichoderma viride and Other Trichoderma Sp. Capable of Chestnut Blight Biocontrol. FEMS Microbiol Lett. 1995;126:249–255. doi: 10.1111/j.1574-6968.1995.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 202.Yates I.E., Meredith F., Smart W., Bacon C.W., Jaworski A.J. Trichoderma viride Suppresses Fumonisin B 1 Production by Fusarium Moniliforme. J. Food Prot. 1999;62:1326–1332. doi: 10.4315/0362-028X-62.11.1326. [DOI] [PubMed] [Google Scholar]
- 203.Berini F., Caccia S., Franzetti E., Congiu T., Marinelli F., Casartelli M., Tettamanti G. Effects of Trichoderma viride Chitinases on the Peritrophic Matrix of Lepidoptera. Pest. Manag. Sci. 2016;72:980–989. doi: 10.1002/ps.4078. [DOI] [PubMed] [Google Scholar]
- 204.Guo K., Sui Y., Li Z., Huang Y., Zhang H., Wang W. Colonization of Trichoderma viride Tv-1511 in Peppermint (Mentha × piperita L.) Roots Promotes Essential Oil Production by Triggering ROS-Mediated MAPK Activation. Plant Physiol. Biochem. 2020;151:705–718. doi: 10.1016/j.plaphy.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 205.Metwally R.A. Arbuscular Mycorrhizal Fungi and Trichoderma viride Cooperative Effect on Biochemical, Mineral Content, and Protein Pattern of Onion Plants. J. Basic Microbiol. 2020;60:712–721. doi: 10.1002/jobm.202000087. [DOI] [PubMed] [Google Scholar]
- 206.Wang X., Cheng H., Ye G., Yao F., Wang Y., Jiao Y., Zhu W., Lan B., Huang H., Ye D. Preparation of Porous Carbon Based on Partially Degraded Raw Biomass by Trichoderma viride to Optimize Its Toluene Adsorption Performance. Environ. Sci. Pollut. Res. Int. 2021;28:46186–46195. doi: 10.1007/s11356-021-12796-y. [DOI] [PubMed] [Google Scholar]
- 207.Alothman Z.A., Bahkali A.H., Elgorban A.M., Al-Otaibi M.S., Ghfar A.A., Gabr S.A., Wabaidur S.M., Habila M.A., Hadj Ahmed A.Y.B. Bioremediation of Explosive TNT by Trichoderma viride. Molecules. 2020;25:1393. doi: 10.3390/molecules25061393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Luo D., Geng R., Wang W., Ding Z., Qiang S., Liang J., Li P., Zhang Y., Fan Q. Trichoderma viride Involvement in the Sorption of Pb(II) on Muscovite, Biotite and Phlogopite: Batch and Spectroscopic Studies. J. Hazard. Mater. 2021;401:123249. doi: 10.1016/j.jhazmat.2020.123249. [DOI] [PubMed] [Google Scholar]
- 209.Daza F.F.F., Roman G.R., Rodriguez M.V., Vargas I.A.G., Heano H.C., Cereda M.P., Mulet R.A.C. Spores of Beauveria Bassiana and Trichoderma Lignorum as a Bioinsecticide for the Control of Atta Cephalotes. Biol. Res. 2019;52:51. doi: 10.1186/s40659-019-0259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pilar Martínez-Diz M., Díaz-Losada E., Andrés-Sodupe M., Bujanda R., Maldonado-González M.M., Ojeda S., Yacoub A., Rey P., Gramaje D. Field Evaluation of Biocontrol Agents against Black-foot and Petri Diseases of Grapevine. Pest. Manag. Sci. 2021;77:697–708. doi: 10.1002/ps.6064. [DOI] [PubMed] [Google Scholar]
- 211.Swain H., Adak T., Mukherjee A.K., Mukherjee P.K., Bhattacharyya P., Behera S., Bagchi T.B., Patro R., Shasmita, Khandual A., et al. Novel Trichoderma Strains. Isolated from Tree Barks as Potential Biocontrol Agents and Biofertilizers for Direct Seeded Rice. Microbiol. Res. 2018;214:83–90. doi: 10.1016/j.micres.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 212.Park Y.H., Chandra Mishra R., Yoon S., Kim H., Park C., Seo S.T., Bae H. Endophytic Trichoderma citrinoviride Isolated from Mountain-Cultivated Ginseng (Panax ginseng) Has Great Potential as a Biocontrol Agent against Ginseng Pathogens. J. Ginseng Res. 2019;43:408–420. doi: 10.1016/j.jgr.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Poromarto S.H., Supyani, Supriyadi, Indriani S.A., Hadiwiyono Trichoderma and Bacillus as Combined Biocontrol Agent of Moler Disease on Shallots; Proceedings of the International Seminar on Promoting Local Resources for Sustainable Agriculture and Development (ISPLRSAD 2020); Online Seminar. 8 October 2020; pp. 92–95. [Google Scholar]
- 214.Umadevi P., Anandaraj M., Srivastav V., Benjamin S. Trichoderma harzianum MTCC 5179 Impacts the Population and Functional Dynamics of Microbial Community in the Rhizosphere of Black Pepper (Piper nigrum L.) Braz. J. Microbiol. 2018;49:463–470. doi: 10.1016/j.bjm.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li J., Philp J., Li J., Wei Y., Li H., Yang K., Ryder M., Toh R., Zhou Y., Denton M.D., et al. Trichoderma harzianum Inoculation Reduces the Incidence of Clubroot Disease in Chinese Cabbage by Regulating the Rhizosphere Microbial Community. Microorganisms. 2020;8:1325. doi: 10.3390/microorganisms8091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang Y., Tian C., Xiao J., Wei L., Tian Y., Liang Z. Soil Inoculation of Trichoderma asperellum M45a Regulates Rhizosphere Microbes and Triggers Watermelon Resistance to Fusarium Wilt. AMB Express. 2020;10:189. doi: 10.1186/s13568-020-01126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.He A., Sun J., Wang X., Zou L., Fu B., Chen J. Reprogrammed Endophytic Microbial Community in Maize Stalk Induced by Trichoderma asperellum Biocontrol Agent against Fusarium Diseases and Mycotoxin Accumulation. Fungal Biol. 2019;123:448–455. doi: 10.1016/j.funbio.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 218.Consolo V.F., Torres-Nicolini A., Alvarez V.A. Mycosinthetized Ag, CuO and ZnO Nanoparticles from a Promising Trichoderma harzianum Strain and Their Antifungal Potential against Important Phytopathogens. Sci. Rep. 2020;10:20499. doi: 10.1038/s41598-020-77294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zaki S.A., Ouf S.A., Albarakaty F.M., Habeb M.M., Aly A.A., Abd-Elsalam K.A. Trichoderma harzianum-Mediated ZnO Nanoparticles: A Green Tool for Controlling Soil-Borne Pathogens in Cotton. J. Fungi. 2021;7:952. doi: 10.3390/jof7110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chinnaperumal K., Govindasamy B., Paramasivam D., Dilipkumar A., Dhayalan A., Vadivel A., Sengodan K., Pachiappan P. Bio-Pesticidal Effects of Trichoderma viride Formulated Titanium Dioxide Nanoparticle and Their Physiological and Biochemical Changes on Helicoverpa Armigera (Hub.) Pestic. Biochem. Physiol. 2018;149:26–36. doi: 10.1016/j.pestbp.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 221.Abd-Elsalam K.A., Hashim A.F., Alghuthaymi M.A., Said-Galiev E. Nanobiotechnological Strategies for Toxigenic Fungi and Mycotoxin Control. Food Preserv. 2017:337–364. doi: 10.1016/B978-0-12-804303-5.00010-9. [DOI] [Google Scholar]
- 222.Lewis J.A., Larkin R.P., Rogers D.L. A Formulation of Trichoderma and Gliocladium to Reduce Damping-off Caused by Rhizoctonia Solani and Saprophytic Growth of the Pathogen in Soilless Mix. Plant Dis. 1998;82:501–506. doi: 10.1094/PDIS.1998.82.5.501. [DOI] [PubMed] [Google Scholar]
- 223.Mawar R., Manjunatha B.L., Kumar S. Commercialization, Diffusion and Adoption of Bioformulations for Sustainable Disease Management in Indian Arid Agriculture: Prospects and Challenges. Circ. Econ. Sustain. 2021;1:1367–1385. doi: 10.1007/s43615-021-00089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rocha I., Ma Y., Souza-Alonso P., Vosátka M., Freitas H., Oliveira R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019;10:1357. doi: 10.3389/fpls.2019.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sachdev S., Singh A., Singh R.P. Optimization of Culture Conditions for Mass Production and Bio-Formulation of Trichoderma Using Response Surface Methodology. 3 Biotech. 2018;8:360. doi: 10.1007/s13205-018-1360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hasan Z.A.E., Mohd Zainudin N.A.I., Aris A., Ibrahim M.H., Yusof M.T. Biocontrol Efficacy of Trichoderma asperellum-Enriched Coconut Fibre against Fusarium Wilts of Cherry Tomato. J. Appl. Microbiol. 2020;129:991–1003. doi: 10.1111/jam.14674. [DOI] [PubMed] [Google Scholar]
- 227.Singh G., Tiwari A., Gupta A., Kumar A., Hariprasad P., Sharma S. Bioformulation Development via Valorizing Silica-Rich Spent Mushroom Substrate with Trichoderma asperellum for Plant Nutrient and Disease Management. J. Environ. Manag. 2021;297:113278. doi: 10.1016/j.jenvman.2021.113278. [DOI] [PubMed] [Google Scholar]
- 228.Prasad R.D., Chandrika K.S.V.P., Godbole V. A Novel Chitosan Biopolymer Based Trichoderma Delivery System: Storage Stability, Persistence and Bio Efficacy against Seed and Soil Borne Diseases of Oilseed Crops. Microbiol. Res. 2020;237:126487. doi: 10.1016/j.micres.2020.126487. [DOI] [PubMed] [Google Scholar]
- 229.Pocurull M., Fullana A.M., Ferro M., Valero P., Escudero N., Saus E., Gabaldón T., Sorribas F.J. Commercial Formulates of Trichoderma Induce Systemic Plant Resistance to Meloidogyne Incognita in Tomato and the Effect Is Additive to That of the Mi-1.2 Resistance Gene. Front. Microbiol. 2020;10:3042. doi: 10.3389/fmicb.2019.03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pellan L., Dieye C.A.T., Durand N., Fontana A., Strub C., Schorr-Galindo S. Biocontrol Agents: Toolbox for the Screening of Weapons against Mycotoxigenic Fusarium. J. Fungi. 2021;7:446. doi: 10.3390/jof7060446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dugassa A., Alemu T., Woldehawariat Y. In-Vitro Compatibility Assay of Indigenous Trichoderma and Pseudomonas Species and Their Antagonistic Activities against Black Root Rot Disease (Fusarium solani) of Faba Bean (Vicia faba L.) BMC Microbiol. 2021;21:115. doi: 10.1186/s12866-021-02181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nandini B., Puttaswamy H., Prakash H.S., Adhikari S., Jogaiah S., Nagaraja G. Elicitation of Novel Trichogenic-Lipid Nanoemulsion Signaling Resistance against Pearl Millet Downy Mildew Disease. Biomolecules. 2020;10:25. doi: 10.3390/biom10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.