Abstract
Mutant EGFR/BRAF pathways are thought to be crucial targets for the development of anticancer drugs since they are over-activated in several malignancies. We present here the development of a novel series of 5-chloro-indole-2-carboxylate 3a–e, 4a–c and pyrrolo[3,4-b]indol-3-ones 5a–c derivatives as potent inhibitors of mutant EGFR/BRAF pathways with antiproliferative activity. The cell viability assay results of 3a–e, 4a–c, and 5a–c revealed that none of the compounds tested were cytotoxic, and that the majority of those tested at 50 µM had cell viability levels greater than 87%. Compounds 3a–e, 4a–c, and 5a–c had significant antiproliferative activity with GI50 values ranging from 29 nM to 78 nM, with 3a–e outperforming 4a–c and 5a–c in their inhibitory actions against the tested cancer cell lines. Compounds 3a–e were tested for EGFR inhibition, with IC50 values ranging from 68 nM to 89 nM. The most potent derivative was found to be the m-piperidinyl derivative 3e (R = m-piperidin-1-yl), with an IC50 value of 68 nM, which was 1.2-fold more potent than erlotinib (IC50 = 80 nM). Interestingly, all the tested compounds 3a–e had higher anti-BRAFV600E activity than the reference erlotinib but were less potent than vemurafenib, with compound 3e having the most potent activity. Moreover, compounds 3b and 3e showed an 8-fold selectivity index toward EGFRT790M protein over wild-type. Additionally, molecular docking of 3a and 3b against BRAFV600E and EGFRT790M enzymes revealed high binding affinity and active site interactions compared to the co-crystalized ligands. The pharmacokinetics properties (ADME) of 3a–e revealed safety and good pharmacokinetic profile.
Keywords: indole, pyrrole, mutant EGFR, BRAFV600E, melanoma, anticancer
1. Introduction
Cancer has been a major public health issue around the world, with an increasing number of patients diagnosed each year [1]. Unfortunately, chemotherapy’s effectiveness as a primary mode of cancer treatment is hampered by drug resistance, severe side effects, and poor selectivity [2,3]. Thus, recently, immunotherapy and newly combined, multi-targeted therapies have been recommended [4,5,6]. Kinase activation in various cell signaling pathways has been linked to cancer cell survival, invasiveness, and drug resistance [7,8]. As a result, anticancer drugs that target kinases, such as the epidermal growth factor receptor (EGFR) and serine/threonine kinases, such as BRAF, are gaining popularity [9,10].
RAF mutations are found in roughly 70% of melanoma, 100% of hairy cell leukemia, and 41% of hepatocellular carcinoma. Meanwhile, EGFR mutations such as T790M and C797S have been identified as important therapeutic targets in lung, breast, and epithelial cancers [11,12,13]. Mutant RAF/EGFR pathways are over-activated in a variety of cancers, and they are regarded as critical targets for anti-cancer drug development [14,15,16]
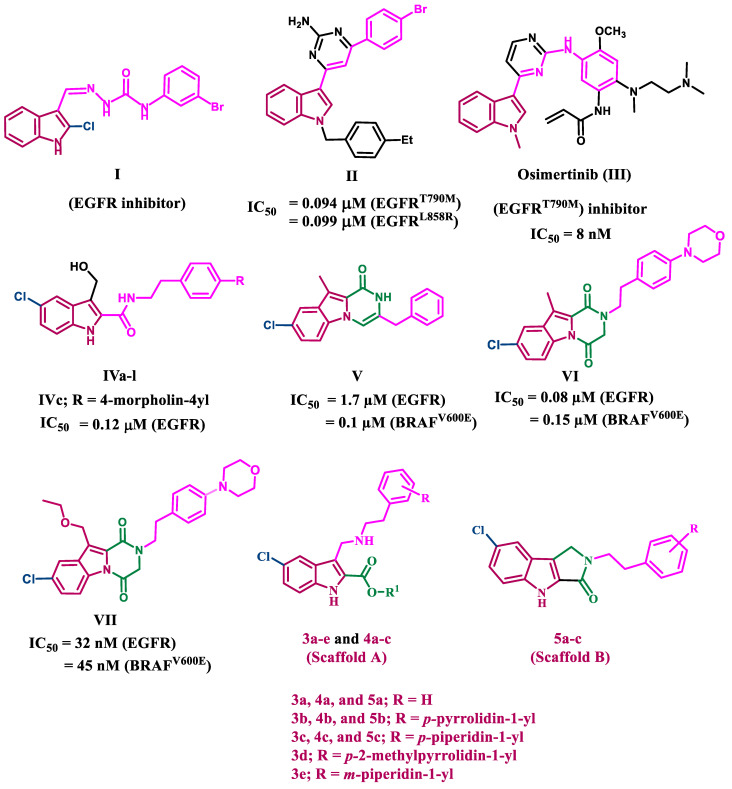
On the other hand, one of the most well-known structures with robust anticancer activity is the indole skeleton, which is present in many active substances and natural products [17]. To date, numerous indole derivatives have been discovered to be effective anticancer agents; some of them have even been used in clinics [18,19,20]. In the literature research, several indole-based compounds with tyrosine kinase inhibitory action have been reported [21,22,23]. Compound I (Figure 1) was reported to have potent anticancer activity against four cancer cell lines, as well as promising EGFR inhibitory activity [21]. Compound II has been identified as a dual EGFRT790M/c-MET inhibitor capable of targeting resistant NSCLC [22]. Compound II had IC50 values of 0.094, 0.099, and 0.595 µM for EGFRT790M, EGFRL858R, and c-MET, respectively (Figure 1). Osimertinib, an indole-based drug, (III, Figure 1) is an EGFR TKI with a 200-fold selectivity index toward EGFR T790M/L858R protein over wild-type EGFR [23]. Osimertinib was approved by the FDA in 2015 to treat EGFR T790M-positive NSCLC [23]. Recently [24], we reported on the development of a novel series of 5-chloro-3-hydroxymethyl-indole-2-carboxamides IVa-l (Figure 1) as EGFR-TK antiproliferative agents. Compound IVc (R= 4-morpholin-4-yl) was the most potent EGFR inhibitor, with an IC50 value of 0.12 µM.
Figure 1.
Structures of compounds I–VII and new targets 3a–e, 4a–c, and 5a–c.
A series of pyrazino[1,2-a]indol-1(2H)-ones has been reported [25] as antiproliferative agents targeting EGFR and BRAFV600E. Compound V (Figure 1) inhibits both EGFR and BRAFV600E with IC50 values of 1.7 µM and 0.1 µM, respectively [25]. Following this, a series of structural modifications to our lead compound V to design and synthesize a new series of pyrazino[1,2-a]indol-1(2H)-ones [26]. Compound VI (Figure 1) was the most effective derivative, with a GI50 value of 1.107 µM against four cancer cell lines. VI inhibited EGFR with an IC50 of 0.08 µM but only moderately inhibited BRAFV600E with an IC50 of 0.15 µM. In another study [27], we describe our efforts to synthesize and optimize a novel class of potent antiproliferative agents VII (Figure 1). The antiproliferative activity of the target compounds is impressive. These compounds have a dual inhibitory effect on EGFR and BRAFV600E, with IC50 values of 32 nM and 45 nM, respectively.
Motivated by the data presented above, and as part of our ongoing efforts to identify promising lead compounds for dual or multi-targeted anticancer agents [28,29,30], we present herein the design and synthesis of a novel class of indole-2-carboxylates, compounds 3a–e and 4a–c (Scaffold A), as well as 1,2-dihydropyrrolo[3,4-b]indol-3(4H)-ones, compounds 5a–c (Scaffold B) (Figure 1), as dual EGFR/BRAFV600E inhibitors with antiproliferative activity. The new compounds will be evaluated for their safety profile by assessing their effect on the viability of human normal cell lines, while their antiproliferative activity will be evaluated against a panel of four cancer cell lines. The most potent compounds will be evaluated for their ability to inhibit wild-type EGFR (EGFRWT) and BRAFV600E as a potential mechanistic target for their antiproliferative effects. Furthermore, the most potent EGFR inhibitors will be tested for their inhibitory effect against mutant-type EGFR (EGFRT790M), and the most potent anti-BRAF agents will be tested for their anticancer effect against the LOX-IMVI melanoma cell line, which has BRAFV600E kinase overexpression. Finally, docking studies will be performed to investigate these compounds’ binding interactions within the active sites of target enzymes.
2. Results and Discussion
2.1. Chemistry
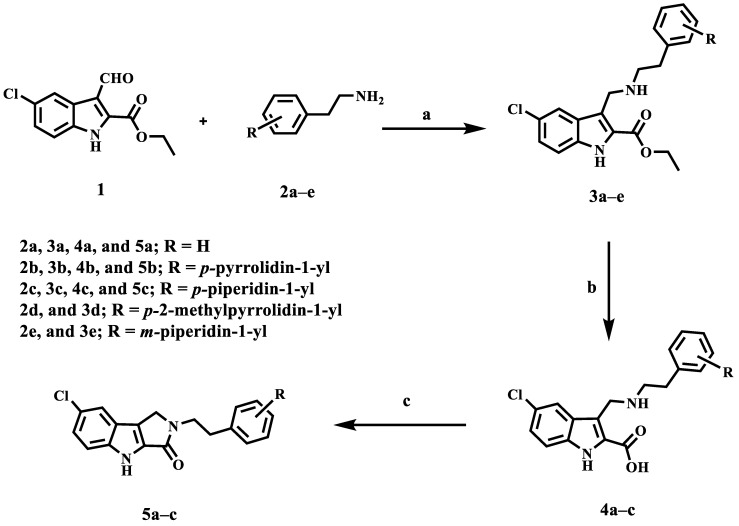
The synthesis of target compounds 3a–e, 4a–c, and 5a–c is depicted in Scheme 1. 5-chloro-3-formyl indole-2-carboxylate 1 [31] was reacted with amines 2a–e [32] through reflux in ethanol followed by reduction of the intermediate imine with NaBH4 under reductive-amination conditions to yield secondary amines 3a–e which subjected to saponification with LiOH to afford a carboxylic acids 4a–c. The structures of compounds 3a–e and 4a–c were confirmed using 1H NMR, 13C NMR, and HRESI-MS spectroscopy (Varian Inova, University of Aberdeen, Meston Building, Aberdeen AB24 3UE, UK). 1H NMR spectrum of compound 3c revealed the presence of a singlet signal δ 9.12 ppm of indole NH, the characteristic signals of ethyl group in the form of quartet at δ 4.33 ppm (2H) and triplet at δ 1.35 ppm (3H), a singlet signal at δ 4.18 ppm (2H) of CH2NH- group, and two triplets (each of 2H) at δ 2.88 and 2.74 ppm of NHCH2CH2 group. Moreover, the spectrum revealed the presence of the characteristic signals of both piperidine and aromatic protons. HRESI-MS m/z of 3c calcd for [M + H]+ C25H31ClN3O2: 440.2099, found: 440.2100. The disappearance of the characteristic signals of the ethyl group in the form quartet at δ 4.33 ppm (2H) and triplet at δ 1.35 ppm (3H) and the appearance of a singlet signal at δ 3.43 ppm (2H) corresponding to COOH and NHCH2 characterize the 1H NMR spectrum of 4c.
Scheme 1.
Synthesis of compounds 3a–e, 4a–c, and 5a–c. Reagents and conditions: (a) NaBH4, EtOH, reflux, 12 h to rt, 1 h, 78%; (b) LiOH, THF/ H2O, 40 °C, 90%; (c) BOP, DIPEA, DMF, rt, overnight, 70%.
Intramolecular coupling of the carboxylic acids 4a–c using BOP as the coupling reagent in the presence of DIPEA in DMF provided target compounds 5a–c. 1H NMR spectrum of compound 5c revealed the presence of a singlet signal δ 12.01 ppm of indole NH, a singlet siganl at δ 4.34 ppm (2H) of CH2N-group, and two triplets (each of 2H) at δ 3.66 and 2.79 ppm of NHCH2CH2 group. Furthermore, the disappearance of the singlet signal at 3.43 ppm (2H) corresponding to COOH and NHCH2 confirms cyclization.
2.2. Biology
2.2.1. Cell Viability Assay
The viability test was performed on a normal human mammary gland epithelial cell line (MCF-10A). The MTT test was used to assess the viability of compounds 3a–e, 4a–c, and 5a–c [33,34]. After four days of incubation with MCF-10A cells, the results showed that none of the substances tested were cytotoxic, and that the majority of those tested at 50 µM had cell viability levels greater than 87%.
2.2.2. Antiproliferative Assay
Using the MTT assay [35,36] and erlotinib as the reference drug, compounds 3a–e, 4a–c, and 5a–c were tested for antiproliferative efficacy against four human cancer cell lines: Panc-1 (pancreatic cancer cell line), MCF-7 (breast cancer cell line), HT-29 (colon cancer cell line), and A-549 (epithelial cancer cell line). The median inhibitory concentration (IC50) calculated (Graph Pad Software, San Diego, CA, USA) is shown in Table 1. For ease of manipulation, the average (GI50) versus the four cancer cell lines was used.
Table 1.
IC50 of compounds 3a–e, 4a–c, 5a–c, and erlotinib.
| Comp. | Cell Viability % | Antiproliferative Activity IC50 ± SEM (nM) | ||||
|---|---|---|---|---|---|---|
| A-549 | MCF-7 | Panc-1 | HT-29 | Average (GI50) |
||
| 3a | 87 | 33 ± 3 | 35 ± 3 | 36 ± 3 | 36 ± 3 | 35 |
| 3b | 91 | 30 ± 3 | 32 ± 3 | 30 ± 3 | 32 ± 3 | 31 |
| 3c | 89 | 41 ± 4 | 45 ± 4 | 42 ± 4 | 44 ± 4 | 42 |
| 3d | 90 | 37 ± 3 | 40 ± 4 | 38 ± 3 | 38 ± 3 | 38 |
| 3e | 92 | 27 ± 2 | 30 ± 3 | 29 ± 3 | 30 ± 3 | 29 |
| 4a | 91 | 75 ± 7 | 79 ± 7 | 78 ± 7 | 78 ± 7 | 78 |
| 4b | 89 | 65 ± 6 | 69 ± 6 | 68 ± 6 | 68 ± 6 | 68 |
| 4c | 87 | 69 ± 7 | 73 ± 7 | 72 ± 7 | 75 ± 7 | 72 |
| 5a | 89 | 46 ± 4 | 48 ± 5 | 50 ± 5 | 49 ± 5 | 48 |
| 5b | 89 | 58 ± 5 | 61 ± 6 | 64 ± 6 | 66 ± 6 | 62 |
| 5c | 91 | 51± 5 | 53 ± 5 | 55 ± 5 | 57 ± 5 | 54 |
| Erlotinib | - | 30 ± 3 | 40 ± 3 | 30 ± 3 | 30 ± 3 | 33 |
When compared to the reference drug erlotinib, which had a GI50 of 33 nM, compounds 3a–e, 4a–c, and 5a–c all had substantial antiproliferative activity with GI50 values ranging from 29 nM to 78 nM. According to Table 1’s findings, 3a–e were superior to 5a–c and 4a–c in their inhibitory actions against the tested cancer cell lines.
Compared to erlotinib (GI50 = 33 nM), the indole-2-carboxylate 3a–e had the most antiproliferative effects, with GI50 values between 29 nM and 42 nM. Compound 3e (R = m-piperidin-1-yl) was the most potent derivative, with a GI50 of 29 nM, outperforming the reference erlotinib, which had a GI50 of 33 nM. Compound 3e was found to be more effective than erlotinib against Panc-1 (pancreatic cancer cell line), MCF-7 (breast cancer cell line), and A-549 (epithelial cancer cell line), Table 1. The substitution of the m-piperidine moiety in compound 3e with the p-piperidine moiety in compound 3c (R = p-piperidin-1-yl) resulted in a significant decrease in the antiproliferative activity of compound 3c, which has a GI50 of 42 nM and is 1.5-fold less potent than 3e, indicating the importance of the substitution position on the antiproliferative activity, where the meta position is better tolerated than the para one. Compound 3b (R = p-pyrrolidin-1-yl) is the second most active antiproliferative compound, with a GI50 value of 31 nM, which is also higher than the reference compound erlotinib (GI50 = 33). Compound 3b is more effective than erlotinib against the MCF-7 cancer cell line, with an IC50 value of 32 nM compared to 40 nM for erlotinib. With a mean GI50 value of 35 nM, the unsubstituted derivative 3a (R = H) ranks third in activity against the four cancer cell lines and is even more potent than erlotinib against the MCF-7 cancer cell line, Table 1. The antiproliferative activity of the 2-methylpyrrolidine derivative 3d (R = p-2-methylpyrrolidin-1-yl) was promising, with a GI50 of 38 nM, which is 1.3-fold less potent than 3e. These findings demonstrated the importance of the substitution pattern on the phenyl group of the phenethyl moiety, with activity increasing in the order m-piperidine > p-pyrrolidine > H > p-2-methylpyrrolidine > p-piperidine.
Compounds 5a–c had lower antiproliferative activity than compounds 3a–e, with GI50 values of 48 nM, 62 nM, and 54 nM, respectively, compared to their congeners 3a–c, which had GI50 values of 35 nM, 31 nM, and 42 nM, indicating that cyclization has a significant decrease in antiproliferative action.
Finally, the carboxylic acid derivatives 4a (R = H), 4b (R = p-pyrrolidin-1-yl), and 4c (R = p-piperidin-1-yl) were the least potent against the four cancer cell lines, with GI50 values of 78 nM, 68 nM, and 72 nM, respectively, indicating the importance of the ethyl group at position two of indole nucleus for the antiproliferative action.
Panc-1 (pancreatic cancer cell line), MCF-7 (breast cancer cell line), HT-29 (colon cancer cell line), and A-549 (epithelial cancer cell line)
2.2.3. EGFR Inhibitory Assay
The most effective antiproliferative derivatives 3a–e were evaluated for their ability to inhibit EGFR using the EGFR-TK assay [37], and the findings are displayed in Table 2. The IC50 range for compounds 3a–e inhibitions of EGFR were 68 to 89 nM. The m-piperidinyl derivative 3e (R = m-piperidin-1-yl) was found to be the most potent of all synthesized derivatives, with an IC50 value of 68 nM, which was 1.2-fold more potent than erlotinib (IC50 = 80 nM). Compound 3b (R = p-pyrrolidin-1-yl) is the second most active compound, with an IC50 value of 74 nM, and it is also more potent than erlotinib. Compounds 3a, 3c, and 3d showed comparable inhibitory activity against EGFR to erlotinib, with IC50 values of 85, 89, and 82 nM, respectively. These results are consistent with the antiproliferative assay results and show that EGFR-TK is a possible target for the antiproliferative effects of compounds 3a–e, and that compounds 3b and 3e were more potent against EGFR-TK than the reference erlotinib.
Table 2.
IC50 of compounds 3a–e against EGFR and BRAFV600E.
| Compound | EGFR Inhibition IC50 ± SEM (nM) |
BRAFV600E Inhibition IC50 ± SEM (nM) |
EGFRT790M Inhibition IC50 ± SEM (nM) |
|---|---|---|---|
| 3a | 85 ± 6 | 43 ± 4 | -- |
| 3b | 74 ± 5 | 39 ± 3 | 9.2 ± 2 |
| 3c | 89 ± 6 | 67 ± 6 | -- |
| 3d | 82 ± 7 | 54 ± 5 | -- |
| 3e | 68 ± 5 | 35 ± 3 | 8.6 ± 2 |
| Erlotinib | 80 ± 5 | 60 ± 5 | -- |
| Vemurafenib | ND | 30 ± 3 | -- |
| Osimertinib | -- | -- | 8 ± 2 |
2.2.4. BRAFV600E Inhibitory Assay
The in vitro anti-BRAFV600E activity of compounds 3a–e was further investigated [38] using erlotinib and vemurafenib as reference compounds and results are shown in Table 2. The enzyme assay revealed that the five compounds tested significantly inhibited BRAFV600E, with IC50 ranges from 35 to 67 nM, Table 2. Interestingly, all the tested compounds 3a–e had higher anti-BRAFV600E activity than the reference erlotinib (IC50 = 60 nM) but were less potent than vemurafenib (IC50 = 30 nM). Once again, compounds 3b (R = p-pyrrolidin-1-yl) and 3e (R = m-piperidin-1-yl) had nearly the same inhibitory efficacy as BRAFV600E, with IC50 values of 39 and 35 nM, respectively, and were shown to be effective inhibitors of cancer cell proliferation (GI50 = 31 and 29 nM) as well as potent inhibitors of EGFR (IC50 = 74 and 68 nM). The unsubstituted derivative 3a (R = H) demonstrated promising BRAFV600E inhibitory activity, with an IC50 value of 43 nM, which was more potent than erlotinib but 1.4-fold less potent than vemurafenib. The findings of the study show that the tested compounds have potent antiproliferative activity and are effective at inhibiting both EGFR and BRAFV600E.
2.2.5. EGFRT790M Inhibitory Assay
The HTRF KinEASE-TK assay [39] was used to assess the inhibitory activity of the most potent compounds, 3b and 3e, against mutant-type EGFR (EGFRT790M). Osimertinib served as the positive control. As shown in Table 2, compounds 3b and 3e had excellent inhibitory activity against EGFRT790M, with IC50 values of 8.6 ± 2 and 9.2 ± 2 nM, respectively, equivalent to the reference osimertinib (IC50 = 8 ± 2 nM), which may explain their potent antiproliferative activity. Compounds 3b and 3e demonstrated 8-fold selectivity index toward EGFRT790M protein over wild-type EGFR. These findings suggested that the phenethyl moiety’s m-piperidine and p-pyrrolidine substitutions are required for the inhibitory effect on EGFRT790M.
2.2.6. LOX-IMVI Melanoma Cell Line Cytotoxicity Assay
The MTT assay was used to assess the anticancer activity of 3b and 3e, the most potent BRAFV600E inhibitors, against the LOX-IMVI melanoma cell line, which has BRAFV600E kinase overexpression [40,41]. The IC50 values of the test compounds were determined at 5-dose concentrations, with staurosporine serving as a control. Table 3 shows that the indole-2-carboxylate derivatives 3b and 3e have a high ability to reduce the viability of the LOX-IMVI cell line. Compound 3e showed potent antiproliferative activity against the LOX-IMVI melanoma cell line with an IC50 value of 0.96 µM, followed by compound 3b with an IC50 value of 1.12 µM in comparison to staurosporine (IC50 = 7.10 µM). The results of this assay add to the evidence that these compounds have potent antiproliferative activity as BRAFV600E inhibitors.
Table 3.
IC50 of compounds 3b and 3e against LOXIMVI melanoma cell line.
| Compound | LOX-IMVI Melanoma IC50 ± SEM (µM) |
|---|---|
| 3b | 1.12 ± 0.01 |
| 3e | 0.96 ± 0.01 |
| Staurosporine | 7.10 ± 0.05 |
2.3. Molecular Modeling
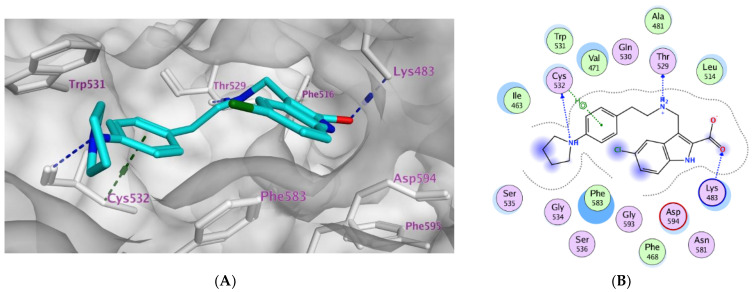
The most active antiproliferative compounds (3a–e) were subjected to in silico docking study in order to investigate their binding modes within BRAFV600E active site. Molecular Operating Environment (MOE) software [42] was used as well as the crystal structure of the BRAFV600E in complex with vemurafenib (PDB: 3OG7) [43]. The accuracy of docking simulation within the protein binding site was validated via redocking the co-crystallized ligand showing S score of −11.78 kcal/mol with RMSD value of 0.96 Å, (S1), Table 4. Docking score analysis of the examined compounds revealed that compounds 3b and 3e showed the highest negative scores (−10.12 and −10.40 kcal/mol, respectively) which are compatible with their in vitro BRAFV600E inhibitory effects. Investigation of the compounds’ binding mode revealed that merely compound 3e with (R = m-piperidin-1-yl) moiety extended comfortably along the large active site (Figure 2C,D). The compound probes the space of the active site in a manner analogous to that of the co-crystalized ligand, vemurafenib. The ligand 5-chloro-indole moiety stacks between the amino acid residues Trp531 and Phe583 inside the hydrophobic pocket forming pi-H interaction with Val471 (4.09 Å) as well as hydrophobic interactions with Trp531, Phe583, Cys532, Ile463, Thr592, and Val471. In addition, the chlorine atom forms halogen bond interaction with the key amino acid residue Cys532 (3.27 Å) at the site gate. Moreover, the ligand indole-2-carboxylate moiety forms ionic as well as H-bond interactions (3.13 Å) with the key amino acid Lys483. Additionally, the ligand stabilizes its complex within the active site by means of donating two H bond interactions with Thr529 (3.49Å), and Gly596 (3.41Å). On the other hand, the para-amino substitution in compounds 3b–d did not allow the ligand to bind deeply inside the pocket compared with the m-piperidine moiety in compound 3e. The latter finding confirms that the active site tolerates the meta substitution rather than the para one. Compound 3b with (R = p-pyrrolidin-1-yl) forms multiple interactions although exhibiting another bent conformation within the active site relative to compound 3e. The ligand indole-2-carboxylate moiety accepts a H-bond interaction from Lys483 (3.03 Å) as well as forming ionic interaction with Lys483 (3.03 Å). Moreover, the compound aminoethyl linker donates H bond to Thr529 (3.07 Å) while the (p-pyrrolidin-1-yl) moiety donates H-bond to Cys532 (3.28) at the gate of binding site. Furthermore, the (p-pyrrolidin-1-yl) moiety forms additional pi-H interaction with Cys532 (3.75 Å). (Figure 2A,B). The binding modes of compound 3c and 3d with R = p-piperidin-1-yl and R = p-2-methylpyrrolidin-1-yl, respectively, resemble that of compound 3b, however they are neither interacting with Cys532 at the gate of active site nor Lys483 at the pocket hinge. Furthermore, the unsubstituted derivative 3a probes the space of active site in an analogous pattern to that of compound 3e while missing interactions with the amino acid residues Cys532, Thr531, and Val471 at the binding site. Other ligands interactions within the active site include hydrophobic ones with Phe583, Cys532, Thr529, Val471, Lys483, and Leu514.
Table 4.
Ligand–protein complex interactions of the tested compounds 3a–e within the active site of BRAFV600E.
| Compd. | MOE Score kcal/mol |
Hydrogen Bond Interactions | Hydrophobic Interactions |
Other Interactions |
|---|---|---|---|---|
| Vemurafenib | −11.78 | Thr529 Gln530 Cys532 Asp594 Gly596 |
Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | Lys483 (ionic) |
| 3a | −8.08 | Lys483 | Phe583, Cys532, Thr592, val471, Lys483, Leu514 | Lys483 (Pi-cation) |
| 3b | −10.12 | Thr529 Cys532 Lys483 |
Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | Lys483 (ionic) Cys532 (Pi-H) |
| 3c | −9.27 | Thr529 Asp594 |
Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | Lys483 (ionic) |
| 3d | −9.14 | Thr529 | Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | Lys483 (ionic) |
| 3e | −10.40 | Thr529 Gly596 Cys532 Lys483 |
Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514, Gly596 | Val471 (Pi-H) Lys483 (ionic) Lys483 Pi-cation) |
Figure 2.
Docking representation models for compounds 3b and 3e; (A): 3D-docked model of compound 3b (cyan) within the active site of BRAFV600E showing the protein surface; (B): 2D-docked model of compound 3b within the active site of BRAFV600E; (C): 3D-docked model of compound 3e (cyan) within the active site of BRAFV600E showing the protein surface; (D) 2D-docked model of compound 3e within the active site of BRAFV600E.
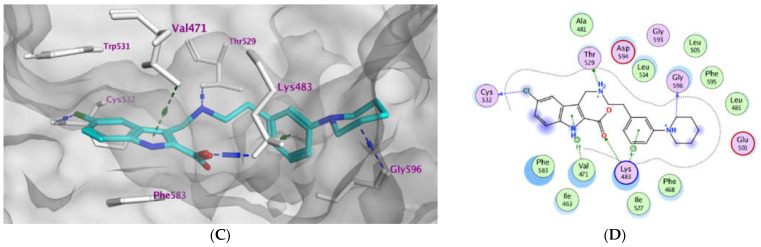
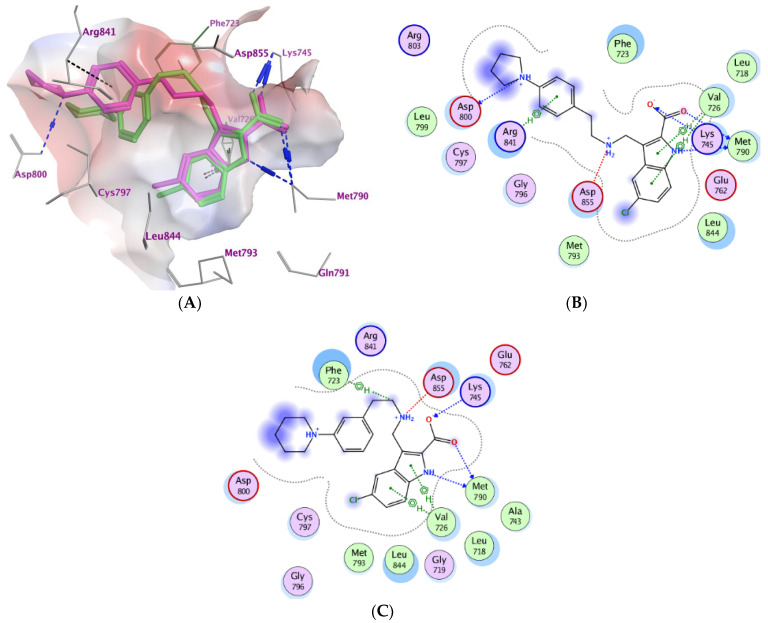
Moreover, the most potent compounds 3b and 3e were subjected to docking study within the active pocket of the EGFR mutant type T790M (PDB: 5J9Z) [44]. The docking protocol was validated by redocking the co-crystallized ligand that exhibiting S score of −10.42 kcal/mol with RMSD value of 0.88 Å, (S2), Table 5. Compounds 3b and 3e exhibited comparable binding modes within the protein binding site (Figure 3). The ligand indole-2-carboxylate moiety binds deeply inside the hydrophobic pocket forming multiple hydrogen bond interactions with Met790 and Lys745 as well as pi-H interactions with Val726. In addition, the compounds form ionic bond interactions with Lys745 and Asp855. In addition, the p-pyrrolidin-1-yl moiety of compound 3b forms ionic bond (3.74 and 3.62 Å) as well as H-bond interactions with Asp800 (3.62 Å) at the gate of the binding site. Moreover, the phenyl moiety of compound 3b forms additional pi-H with Arg841 (4.82 Å). (Figure 3A,B). The ligand protein complexes are stabilized via other hydrophobic interactions with Asp800, Phe723, Leu844, Cys797, Leu718, Val726, Met790, and Lys745. Results of the docking simulations attributed to explaining the biological effects of the compounds 3a–e relative to their binding affinity within the active site of BRAFV600E as well as EGFR mutant type T790M and confirmed the dual kinase targets for the anti-proliferative activity of compounds 3b and 3e.
Table 5.
Ligand–protein complex interactions of the tested compounds 3b and 3e within the active site of EGFRT790M.
| Compd. | MOE Score kcal/mol |
Hydrogen Bond Interactions | Hydrophobic Interactions |
Other Interactions |
|---|---|---|---|---|
| Co-crystalized ligand (6HJ) a | −10.42 | Met790 Gln791 Met793 |
Leu844, Cys797, Leu718, Val726, Met790 | Val726 (Pi-H) Cys797 (covalent) |
| 3b | −6.71 | Met790 Asp800 Lys745 |
Asp800, Phe723, Leu844, Cys797, Leu718, Val726, Met790, Lys745. | Lys745 (Ionic) Asp855 (Ionic) Asp800 (Ionic) Val726 (pi-H) Arg841 (pi-H) |
| 3e | −6.64 | Met790 Lys745 |
Asp800, Phe723, Leu844, Cys797, Leu718, Val726, Met790 | Lys745 (Ionic) Asp855 (Ionic) Phe723 (Pi-H) Val726 (pi-H) |
a (R)-1-(3-(4-amino-3-(1-methyl-1H-indol-3-yl)-1H-pyrazolo [3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one.
Figure 3.
Docking representation models for compounds 3b and 3e; (A): 3D-docked models of compound 3b (purple) aligned with 3e (green) within the active site of EGFRT790M showing the interaction surface of the protein (electrostatics; green: hydrophobic, blue: positive, and red: negative); (B): 2D-docked model of compound 3b within the active site of EGFRT790M; (C): 2D-docked model of compound 3e within the active site of EGFRT790M.
2.4. In Silico ADME/Pharmacokinetics Studies
The most active antiproliferative compounds 3a–e were subjected to in silico ADME studies using the web tools SwissADME [45] as well as ADMETlab [46] by entering a list of two compounds’ SMILES (Simplified Molecule Input Line Entry Specification) provided by ChemDraw software. The in silico pharmacokinetic data (Table 6) showed that all compounds are orally active as they obey Lipinski’s rules of five with zero violation. All compounds are more likely to be a P-gp non-substrate. They exhibit high intestinal absorbance in the range of 88.9–90.5 %. They are capable of crossing BBB with logBB ranging from 0.22 to 0.31. According to Lipinski’s rules, logP should be ≤5. Thus, all compounds exhibited good permeability as indicated by logP values in the range of 4.23– 4.82. Regarding CYP inhibition, all compounds are considered inhibitors with probability exceeding 0.5 as shown in Table 7. The results predict that compounds 3a–e exhibit good pharmacokinetics and ADME properties (Table 6 and Table 7).
Table 6.
Physicochemical and pharmacokinetic properties (Lipinski parameters) of compounds 3a–e.
| Compd. | MW | ROTB | HBA | HBD | Violations | MR | TPSA | Log P |
|---|---|---|---|---|---|---|---|---|
| 3a | 357 | 8 | 3 | 2 | 0 | 101 | 54.12 | 4.23 |
| 3b | 426 | 9 | 3 | 2 | 0 | 126 | 57.36 | 4.59 |
| 3c | 440 | 9 | 3 | 2 | 0 | 131 | 57.36 | 4.82 |
| 3d | 440 | 9 | 3 | 2 | 0 | 131 | 57.36 | 4.79 |
| 3e | 440 | 9 | 3 | 2 | 0 | 131 | 57.36 | 4.76 |
Table 7.
ADME properties of compounds 3a–e.
| Compd. | GI Abs. | BBB | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor |
|---|---|---|---|---|---|---|---|---|
| 3a | 88.904 | 0.312 | −−− | +++ | ++ | ++ | ++ | ++ |
| 3b | 90.505 | 0.239 | −−− | ++ | ++ | + | + | ++ |
| 3c | 90.116 | 0.252 | −−− | ++ | ++ | + | + | ++ |
| 3d | 90.12 | 0.252 | −−− | + | ++ | + | + | ++ |
| 3e | 89.884 | 0.223 | −−− | ++ | ++ | + | + | ++ |
Probability: 0–0.1 (−−−); 0.5–0.7 (+); 0.7–0.9 (++); 0.9–1.0 (+++).
3. Experimental
3.1. Chemistry
General Details: refer to Appendix SA (Supplementary Materials).
Compounds 1 [31] and 2a–e [32] were prepared according to previously reported procedures.
3.1.1. General Method for the Synthesis of Compounds 3a–e
A mixture of compound 1 (0.73 g, 2.90 mmol, 1 equiv) and 2a–e (1.2 equiv) in absolute ethanol (35 mL) was refluxed overnight with stirring. After cooling, NaBH4 (0.13 g, 1.2 equiv) was added portion wise over a period of 20 min with stirring for further 30 min at rt. H2O (30 mL) was added and the reaction mixture was concentrated in vacuo to a minimum, extracted with EtOAc (80 mL), dried over MgSO4, and concentrated in vacuo to give oil which was re-dissolved in EtOAc (30 mL) and treated with 3 M HCl till formation of white precipitate. The precipitate formed was filtered, washed with EtOAc, and dried to give secondary amine as its hydrochloride salt. The hydrochloride salt was dissolved in water/methanol 1:1 (70 mL) and treated with saturated solution of 5% NaOH till alkaline to liberate free amine. The resulting mixture was concentrated in vacuo to a minimum and extracted twice with EtOAc. The organic layer was dried under MgSO4, and evaporated under reduced pressure to give 3a–e.
Ethyl 5-chloro-3-((phenethylamino)methyl)-1H-indole-2-carboxylate (3a)
Yield % 85; mp 104–105 °C. ν max (KBr disc)/cm−1 3296 (NH), 3063, 2950, 2845, 1693 (C=O), 1538, 1451, 1320, 1208, 1087, 895, 799, 748, 699. 1H NMR (400 MHz, Chloroform-d): δ 9.23 (s, 1H, indole NH), 7.65 (s, 1H, Ar-H), 7.25–7.12 (m, 8H, Ar-H, NHCH2), 4.29 (q, J = 6.8 Hz, 2H, OCH2CH3), 4.16 (s, 2H, CH2NHCH2CH2), 2.92 (q, J = 6.8 Hz, 2H, NHCH2CH2), 2.81 (t, J = 6.8 Hz, 2H, NHCH2CH2), 1.31 (t, J = 7.2 Hz, 3H, OCH2CH3). 13C NMR (101 MHz, Chloroform-d): δ 161.71 (C=O), 139.78, 133.90, 128.83, 128.63, 128.40, 126.18, 126.14, 126.09, 125.19, 121.31, 120.06, 112.93, 61.22, 50.22, 42.82, 36.18, 14.26. HRESI-MS m/z calcd for [M + H]+ C20H22ClN2O2: 357.1364, found: 357.1362.
Ethyl 5-chloro-3-((4-(pyrrolidin-1-yl)phenethylamino)methyl)-1H-indole-2-carboxylate (3b)
Yield % 74; mp 173–175 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.68 (d, J = 1.9 Hz, 1H, Ar-H), 7.30–7.18 (m, 2H, Ar-H), 7.01 (d, J = 8.5 Hz, 2H, Ar-H), 6.48 (d, J = 8.5 Hz, 2H, Ar-H), 4.33 (q, J = 7.2 Hz, 2H, OCH2CH3), 4.19 (s, 2H, CH2NHCH2CH2), 3.28–3.20 (m, 4H, pyrrolidin-H), 2.90 (t, J = 7.1 Hz, 2H, NHCH2CH2), 2.75 (t, J = 7.1 Hz, 2H, NHCH2CH2), 2.02–1.94 (m, 4H, pyrrolidin-H), 1.36 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (101 MHz, Chloroform-d) δ 161.79, 146.58, 133.95, 129.30, 128.88, 126.16, 126.11, 126.05, 125.26, 121.31, 120.15, 112.96, 111.75, 61.24, 50.68, 47.68, 42.84, 35.13, 25.43, 14.32. HRESI-MS m/z calcd for [M + H]+ C24H29ClN3O2: 426.1943, found: 426.1944.
Ethyl 5-chloro-3-((4-(piperidin-1-yl)phenethylamino)methyl)-1H-indole-2-carboxylate (3c)
Yield % 78; mp 75–78 °C. ν max (KBr disc)/cm−1 3312 (NH), 2932, 2850, 1705, 1612, 1540, 1514, 1451, 1380, 1236, 1131, 1024, 860, 800, 780. 1H NMR (400 MHz, Chloroform-d) δ 9.12 (s, 1H, indole NH), 7.70 (d, J = 1.8 Hz, 1H, Ar-H), 7.30–7.19 (m, 2H, Ar-H), 7.03 (d, J = 8.5 Hz, 2H, Ar-H), 6.84 (d, J = 8.6 Hz, 2H, Ar-H), 4.33 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.18 (s, 2H, CH2NHCH2CH2), 3.13–3.05 (m, 4H, piperidin-H), 2.88 (t, J = 7.1 Hz, 2H, NHCH2CH2), 2.74 (t, J = 7.1 Hz, 2H, NHCH2CH2), 1.72–1.65 (m, 4H, piperidin-H), 1.58–1.52 (m, 2H, piperidin-H), 1.35 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (101 MHz, Chloroform-d) δ 161.74, 150.73, 133.92, 130.52, 129.14, 128.96, 126.17, 126.08, 125.20, 121.74, 120.21, 116.77, 112.89, 61.20, 50.96, 50.50, 42.90, 35.37, 25.91, 24.28, 14.33. HRESI-MS m/z calcd for [M + H]+ C25H31ClN3O2: 440.2099, found: 440.2099.
Ethyl 5-chloro-3-((4-(2-methylpyrrolidin-1-yl)phenethylamino)methyl)-1H-indole-2-carboxylate (3d)
Yield % 75; mp 158–160 °C. 1H NMR (400 MHz, Chloroform-d) δ 9.25 (s, 1H, indole NH), 7.70 (d, J = 1.7 Hz, 1H, Ar-H), 7.24–2.23 (m, 2H, Ar-H), 7.01 (d, J = 8.5 Hz, 2H, Ar-H), 6.50 (d, J = 8.6 Hz, 2H, Ar-H), 4.33 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.19 (s, 2H, CH2NHCH2CH2), 3.84–3.80, (m, 1H, pyrrolidin-H), 3.44–3.34 (m, 1H, pyrrolidin-H), 3.17–3.06 (m, 1H, pyrrolidin-H), 2.89 (t, J = 7.1 Hz, 2H, NHCH2CH2), 2.74 (t, J = 7.1 Hz, 2H, NHCH2CH2), 2.10–1.90 (m, 3H, pyrrolidin-H), 1.73–1.63 (m, 1H, pyrrolidin-H), 1.36 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.15 (d, J = 6.2 Hz, 3H, CHCH3). 13C NMR (101 MHz, Chloroform-d) δ 161.83, 145.77, 133.97, 129.33, 128.96, 126.11, 126.09, 126.00, 125.24, 121.69, 120.21, 112.90, 111.81, 61.19, 53.66, 50.76, 48.32, 42.90, 35.24, 33.13, 23.32, 19.50, 14.31. HRESI-MS m/z calcd for [M + H]+ C25H31ClN3O2: 440.2099, found: 440.2092.
Ethyl 5-chloro-3-((3-(piperidin-1-yl)phenethylamino)methyl)-1H-indole-2-carboxylate (3e)
Yield % 73; oil. 1H NMR (400 MHz, Chloroform-d) δ 9.04 (s, 1H, indole NH), 7.71 (d, J = 1.8 Hz, 1H, Ar-H), 7.32–7.21 (m, 2H, Ar-H), 7.13 (t, J = 7.8 Hz, 1H, Ar-H), 6.80–6.69 (m, 2H, Ar-H), 6.62 (d, J = 7.5 Hz, 1H, Ar-H), 4.34 (q, J = 7.1 Hz, 2H, OCH2CH3), 4.19 (s, 2H, CH2NHCH2CH2), 3.13–3.05 (m, 4H, piperidin-H), 2.91 (t, J = 7.1 Hz, 2H, NHCH2CH2), 2.78 (t, J = 7.2 Hz, 2H, NHCH2CH2), 1.72–1.63 (m, 4H, piperidin-H), 1.59–1.52 (m, 2H, piperidin-H), 1.36 (t, J = 7.1 Hz, 3H, OCH2CH3). 13C NMR (101 MHz, Chloroform-d) δ 161.67, 152.40, 140.63, 133.89, 128.99, 128.97, 126.22, 126.13, 125.21, 121.73, 120.21, 119.51, 116.95, 114.28, 112.88, 61.22, 50.63, 50.34, 42.86, 36.68, 25.87, 24.31, 14.33. HRESI-MS m/z calcd for [M + H]+ C25H31ClN3O2: 440.2099, found: 440.2093.
3.1.2. General Method for the Synthesis of Compounds 4a–c
Compounds 3a–e (0.68 mmol) in THF: H2O (5:1, 12 mL), LiOH (0.1 g, 4.09 mmol) was added. The reaction mixture was kept at 40 °C with stirring overnight. The residue after removal of solvent under reduced pressure was partitioned between Et2O/H2O (1:1) and the separated aqueous layer was acidified with 5% HCl. The formed precipitate was filtered and dried under vacuum to give 4a–c.
5-Chloro-3-((phenethylamino)methyl)-1H-indole-2-carboxylic acid (4a)
Yield % 87, mp 192–193 °C. ν max (KBr disc)/cm−1 3206 (br, OH and NH), 1693 (C=O), 1538, 1450, 1330, 1200, 1136, 802, 750, 699. 1H NMR (400 MHz, DMSO-d6) δ 11.92 (s, 1H, indole NH), 10.26 (s, 1H, COOH), 7.89 (d, J = 2.0 Hz, 1H, Ar-H), 7.44 (d, J = 8.7 Hz, 1H, Ar-H), 7.36–7.18 (m, 6H, Ar-H), 4.49 (s, 2H, CH2NHCH2CH2), 3.16 (t, J = 6.2 Hz, 2H, NHCH2CH2), 2.99 (t, J = 6.2 Hz, 2H, NHCH2CH2). 13C NMR (101 MHz, DMSO-d6) δ 164.11, 137.82, 133.76, 133.23, 129.06, 129.01, 128.49, 127.15, 124.84, 124.25, 119.43, 114.52, 108.74, 47.43, 41.07, 32.23. HRESI-MS m/z calcd for [M + H]+ C18H18ClN2O2: 329.1051, found: 329.1052.
5-Chloro-3-((4-(pyrrolidin-1-yl)phenethylamino)methyl)-1H-indole-2-carboxylic acid (4b)
Yield % 85, mp 189–190 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.04 (s, 1H, indole NH), 7.55 (s, 1H, Ar-H), 7.27 (d, J = 9.2 Hz, 1H, Ar-H),6.95 (m, 1H, Ar-H), 6.88 (d, J = 8.0 Hz, 2H, Ar-H), 6.34 (d, J = 8.4 Hz, 2H, Ar-H), 4.02 (s, 2H, CH2NHCH2CH2), 3.55 (t, J = 8.8 Hz, 2H, NHCH2CH2), 3.08 (t, J = 6.8 Hz, 4H, pyrrolidin-H), 2.45 (t, J = 2.0 Hz, 2H, NHCH2CH2), 1.87–1.82 (m, 4H, pyrrolidin-H). 13C NMR (101 MHz, DMSO-d6) δ 166.30, 146.76, 140.14, 136.77, 133.07, 130.16, 129.70, 127.25, 125.03, 123.34, 122.03, 113.90, 112.24, 47.99, 45.13, 40.33, 34.20, 25.54. HRESI-MS m/z calcd for [M + H]+ C22H25ClN3O2: 399.1635, found: 399.1638.
5-Chloro-3-((4-(piperidin-1-yl)phenethylamino)methyl)-1H-indole-2-carboxylic acid (4c)
Yield % 90, mp 176–178 °C. ν max (KBr disc)/cm−1 3200 (br, OH and NH), 1690 (C=O), 1530, 1450, 1320, 1210, 1130, 802, 750, 699. 1H NMR (250 MHz, DMSO-d6): δ 11.32 (s, 1H, indole NH), 7.65 (s, 1H, Ar-H), 7.34 (d, J = 8.5 Hz, 1H, Ar-H), 7.15–6.90 (m, 3H, Ar-H), 6.79 (d, J = 3.5 Hz, 2H, Ar-H), 4.21 (s, 2H, CH2NHCH2CH2), 3.43 (s, 2H, OH, NHCH2), 3.02 (t, J = 4. 8 Hz, 4H, piperidin-H), 2.81 (t, J = 6. 8 Hz, 2H, NHCH2CH2), 2.68 (t, J = 6.5 Hz, 2H, NHCH2CH2), 1.65–1.39 (m, 6H, piperidin-H). 13C NMR (62.5 MHz, DMSO-d6): δ 165.3 (C=O), 150.2, 136.1, 132.6, 129.2, 129.0, 123.1, 122.0, 118.1, 116.1, 115.6, 113.6, 110.5, 49.9, 48.6, 40.9, 38.5, 33.5, 25.3. HRESI-MS m/z calcd for [M + H]+ C23H27ClN3O2: 412.1782, found: 412.1780.
3.1.3. General Method for the Synthesis of Compounds 5a–c
A mixture of 4a–c (0.73 mmol, 1 equiv), BOP (0.45 g, 1.5 equiv), and DIPEA (0.24 mL, 2 equiv) in 20 mL DMF was stirred overnight at rt. The reaction mixture was diluted with EtOAc (50 mL) and successively washed with H2O, 5% HCl, saturated solution of NaHCO3, and finally with brine. The organic phase was dried over MgSO4 and concentrated under reduced pressure to yield a crude product which was purified by flash chromatography on silica gel using EtOAc/ hexane (2:1) as an eluent to give 5a–c.
7-Chloro-2-phenethyl-1,2-dihydropyrrolo [3,4-b]indol-3(4H)-one (5a)
Yield % 79, mp 245–247 °C. ν max (KBr disc)/cm−1 3148 (NH), 1659, 1451, 1320, 1250, 840, 808. 1H NMR (400 MHz, DMSO-d6) δ 12.04 (s, 1H, indole NH), 7.72 (d, J = 2.1 Hz, 1H, Ar-H), 7.45 (d, J = 8.8 Hz, 1H, Ar-H), 7.33–7.15 (m, 6H, Ar-H), 4.37 (s, 2H, CH2NCH2CH2), 3.75 (t, J = 7.3 Hz, 2H, NCH2CH2), 2.93 (t, J = 7.3 Hz, 2H, NCH2CH2). 13C NMR (101 MHz, DMSO-d6) δ 162.02, 139.96, 139.48, 136.57, 129.06, 128.83, 126.67, 124.85, 124.49, 124.08, 122.81, 119.65, 115.28, 46.45, 44.47, 34.80. HRESI-MS m/z calcd for [M + H]+ C18H16ClN2O: 311.0946, found: 311.0944.
7-Chloro-2-(4-(pyrrolidin-1-yl)phenethyl)-1,2-dihydropyrrolo [3,4-b]indol-3(4H)-one (5b)
Yield % 75, mp 163–164 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.99 (s, 1H, indole NH), 7.67 (d, J = 1.6 Hz, 1H, Ar-H), 7.39 (d, J = 8.8 Hz, 1H, Ar-H), 7.18 (dd, J = 8.8, 2.0 Hz, 1H, Ar-H), 6.98 (d, J = 8.4 Hz, 2H, Ar-H), 6.39 (d, J = 8.8 Hz, 2H, Ar-H), 4.29 (s, 2H, CH2NCH2CH2), 3.61 (t, J = 8.8 Hz, 2H, NCH2CH2), 3.10 (t, J = 6.4 Hz, 4H, pyrrolidin-H), 2.73 (t, J = 7.6 Hz, 2H, NCH2CH2), 1.88–1.84 (m, 4H, pyrrolidin-H). 13C NMR (101 MHz, DMSO-d6) δ 162.17, 146.94, 140.14, 136.91, 129.79, 125.75, 125.03, 124.63, 124.25, 123.03, 119.89, 115.49, 112.31, 47.93, 46.71, 45.13, 34.20, 25.56. HRESI-MS m/z calcd for [M + H]+ C22H23ClN3O: 398.1630, found: 398.1626.
7-Chloro-2-(4-(piperidin-1-yl)phenethyl)-1,2-dihydropyrrolo [3,4-b]indol-3(4H)-one (5c)
Yield % 70, mp 207–209 °C. ν max (KBr disc)/cm−1 3150 (NH), 2936, 1655 (C=O), 1530, 1500, 1450, 1310, 1260, 1150, 842. 1H NMR (400 MHz, DMSO-d6) δ 12.01 (s, 1H, indole NH), 7.70 (d, J = 2.1 Hz, 1H, Ar-H), 7.43 (d, J = 8.8 Hz, 1H, Ar-H), 7.21 (dd, J = 8.8, 2.2 Hz, 1H, Ar-H), 7.05 (d, J = 8.5 Hz, 2H, Ar-H), 6.81 (d, J = 8.5 Hz, 2H, Ar-H), 4.34 (s, 2H, CH2NCH2CH2), 3.66 (t, J = 7.3 Hz, 2H, NCH2CH2), 3.07–2.99 (m, 4H, piperidin-H), 2.79 (t, J = 7.3 Hz, 2H, NCH2CH2), 1.62–1.42 (m, 6H, piperidin-H). 13C NMR (101 MHz, DMSO-d6) δ 161.99, 150.62, 139.95, 136.66, 129.49, 129.16, 124.83, 124.47, 124.05, 122.82, 119.65, 116.41, 115.28, 50.18, 46.46, 44.69, 33.92, 25.72, 24.32. HRESI-MS m/z calcd for [M + H]+ C23H25ClN3O: 394.1681, found: 394.1673.
3.2. Biology
3.2.1. Cell Viability Assay and Evaluation of IC50
MTT Assay
The MTT assay was used to determine how the synthesized compounds affected the viability of mammary epithelial cells (MCF-10A) [33,34]. See Appendix SA (Supplementary Materials).
Antiproliferative Test
To investigate the antiproliferative potential of 3a–e, 4a–c, and 5a–c, the MTT assay was carried out using various cell lines in accordance with previously reported procedures [35,36]. See Appendix SA (Supplementary Materials).
EGFR Inhibitory Assay
The EGFR-TK assay was used to evaluate the EGFR inhibitory effectiveness of 3a–e [37]. See Appendix SA (Supplementary Materials).
BRAF Kinase Assay
The activity of 3a–e against BRAF was investigated using a V600E mutant BRAF kinase assay [38]. See Appendix SA (Supplementary Materials).
In Vitro Cytotoxicity of LOX-IMVI Melanoma Cell Line
The anticancer activity of the synthesized derivatives was determined using the MTT cytotoxicity assay on LOX-IMVI melanoma cell line [40,41]. See Appendix SA (Supplementary Materials).
4. Conclusions
A new series of 5-chloro-indole-2-carboxylate and pyrrolo [3,4-b]indol-3-one was synthesized and structurally characterized using various spectroscopic methods. The new compounds had no cytotoxic effects on human normal cell lines but demonstrated potent antiproliferative activities against four human cancer cell lines. Some of the compounds tested were found to be dual inhibitors of both wild type and mutant type EGFR and BRAFV600E. Molecular docking attempted to investigate the binding mode of the most active antiproliferative compounds 3a–e within the binding site of BRAFV600E in comparison with vemurafenib. Results proved that compound 3e, with m-piperidinyl substitution at the phenethyl amine moiety, was found to fit more tightly within the active site than the other derivatives with para-amine substituents. Moreover, docking results of compounds 3b and 3e against EGFRT790M concludes that the ligand indole-2-carboxylate scaffold binds intensely forming a combination of H-bond as well as hydrophobic interactions at the hydrophobic pocket of active site. In silico ADME and pharmacokinetic prediction revealed that compounds 3a–e have good pharmacokinetic and ADME properties. Compounds 3b and 3e may act as anticancer agents targeting the EGFRT790M and BRAFV600E pathways after structural modifications, but more in vitro and in vivo testing is needed.
Acknowledgments
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R3), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031269/s1.
Author Contributions
M.H.A. and L.T.: conceptualization, writing, and editing, B.G.M.Y.: biology, methodology, writing, and editing; L.H.A.-W.: editing and revision; A.F.M.: modeling, writing, and editing; B.G.M.Y.: conceptualization, methodology, writing the draft, and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be provided upon request.
Conflicts of Interest
The authors reported no potential conflict of interests.
Sample Availability
Samples of the compounds 3a–e, 4a–c, and 5a–c are available from the authors.
Funding Statement
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R3), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Al-Sanea M.M., Khan M.S.A., Abdelazem A.Z., Lee S.H., Mok P.L., Gamal M., Shaker M.E., Afzal M., Youssif B.G.M., Omar N.N. Synthesis, and In Vitro Antiproliferative Activity of New 1-Phenyl-3-(4-(pyridin-3-yl)phenyl)urea Scaffold-Based Compounds. Molecules. 2018;23:297–309. doi: 10.3390/molecules23020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelbaset M.S., Abdelrahman M.H., Bukhari S.N.A., Gouda A.M., Youssif B.G.M., Abdel-Aziz M., Abuo-Rahma G.E.A. Design, Synthesis, and biological evaluation of new series of pyrrol-2(3H)-one and pyridazin-3(2H)-one derivatives as tubulin polymerization inhibitors. Bioorg. Chem. 2021;107:104522. doi: 10.1016/j.bioorg.2020.104522. [DOI] [PubMed] [Google Scholar]
- 3.Rashid H., Xu Y., Muhammad Y., Wang L., Jiang J. Research advances on anticancer activities of marine and its derivatives: An updated overview. Eur. J. Med. Chem. 2019;161:205–238. doi: 10.1016/j.ejmech.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 4.El-Sherief H.A.M., Youssif B.G.M., Abdelazeem A.H., Abdel-Aziz M., Abdel-Rahman H.M. Design, Synthesis and Antiproliferative Evaluation of Novel 1,2,4-Triazole/Schiff Base Hybrids with EGFR and B-RAF Inhibitory Activities. Anti-Cancer Agents Med. Chem. 2019;19:697–706. doi: 10.2174/1871520619666181224115346. [DOI] [PubMed] [Google Scholar]
- 5.Mohassab A.M., Hassan H.A., Abdelhamid D., Gouda A.M., Youssif B.G.M., Tateishi H., Fujita M., Otsuka M., Abdel-Aziz M. Design and Synthesis of Novel quinoline/chalcone/1,2,4-triazole hybrids as potent antiproliferative agent targeting EGFR and BRAFV600E kinases. Bioorg. Chem. 2021;106:104510. doi: 10.1016/j.bioorg.2020.104510. [DOI] [PubMed] [Google Scholar]
- 6.Al-Wahaibi L.H., Youssif B.G.M., Taher E.S., Abdelazeem A.H., Abdelhamid A.A., Marzouk A.A. Design, Synthesis, Biological Evaluation, and Computational Studies of Novel Tri-Aryl Imidazole-Benzene Sulfonamide Hybrids as Promising Selective Carbonic Anhydrase IX and XII Inhibitors. Molecules. 2021;26:4718. doi: 10.3390/molecules26164718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin H.-L., Leng J., Youssif B.G.M., Amjad M.W., Raja M.A.J., Hussain M.A., Hussaine Z., Kazmi S.N., Bukhari S.N.A. Synthesis, and mechanistic studies of curcumin analogs-based oximes as potential anticancer agents. Chem. Biol. Drug Des. 2017;90:443–449. doi: 10.1111/cbdd.12964. [DOI] [PubMed] [Google Scholar]
- 8.Zha G.-F., Qin H.-L., Youssif B.G.M., Amjad M.W., Raja M.A.J., Abdelazeem A.H., Bukhari S.N.A. Discovery of potential anticancer multi-targeted ligustrazine based cyclohexanone and oxime analogs overcoming the cancer multidrug resistance. Eur. J. Med. Chem. 2017;135:34–48. doi: 10.1016/j.ejmech.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud M.A., Mohammed A.F., Salem O.I.A., Gomaa H.A.M., Youssif B.G.M. New 1,3,4-oxadiazoles linked 1,2,3-triazole moiety as antiproliferative agents targeting EGFR-TK. Arch. Pharm. 2022;355:e2200009. doi: 10.1002/ardp.202200009. [DOI] [PubMed] [Google Scholar]
- 10.Youssif B.G.M., Gouda A.M., Moustafa A.M., Abdelhamid A.A., Gomaa H.A.M., Kamal I., Marzouk A.A. Design and synthesis of new triarylimidazole derivatives as dual inhibitors of BRAFV600E/p38α with potential antiproliferative activity. J. Mol. Struct. 2022;1253:132218. doi: 10.1016/j.molstruc.2021.132218. [DOI] [Google Scholar]
- 11.Yu Z., Ye S., Hu G., Lv M., Tu Z., Zhou K., Li Q. The RAF-MEK-ERK pathway: Targeting ERK to overcome obstacles to effective cancer therapy. Future Med. Chem. 2015;7:269–289. doi: 10.4155/fmc.14.143. [DOI] [PubMed] [Google Scholar]
- 12.Hrustanovic G., Olivas V., Pazarentzos E., Tulpule A., Asthana S., Blakely C.M., Okimoto R.A., Lin L., Neel D.S., Sabnis A., et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat. Med. 2015;21:1038–1047. doi: 10.1038/nm.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frejat F.O.A., Mostafa Y.A., Gomaa H.A.M., Youssif B.G.M., Wu C. Novel indazole derivatives as potent apoptotic antiproliferative agents by multi-targeted mechanism: Synthesis and biological evaluation. Bioorg. Chem. 2022;126:105922. doi: 10.1016/j.bioorg.2022.105922. [DOI] [PubMed] [Google Scholar]
- 14.McCarroll J.A., Gan P.P., Erlich R.B., Liu M. TUBB3/betaIII-tubulin acts through the PTEN/AKT signaling axis to promote tumorigenesis and anoikis resistance in nonsmall cell lung cancer. Cancer Res. 2015;75:415–425. doi: 10.1158/0008-5472.CAN-14-2740. [DOI] [PubMed] [Google Scholar]
- 15.El-Sherief H.A., Youssif B.G., Bukhari S.N.A., Abdel-Aziz M., Abdel-Rahman H.M. Novel 1,2,4-triazole derivatives as potential anticancer agents: Design, synthesis, molecular docking, and mechanistic studies. Bioorg. Chem. 2018;76:314–325. doi: 10.1016/j.bioorg.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Abourehab M.A.S., Alqahtani A., Youssif B.G.M., Gouda A.M. Globally approved EGFR inhibitors: Insights into their syntheses, targeted kinases, biological activity, receptor interactions and metabolism. Molecules. 2021;26:6677. doi: 10.3390/molecules26216677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra R.D.L.A. Anticancer potential of plants and natural products: A review. J. Ethnopharmacol. 2013;1:622–628. [Google Scholar]
- 18.Dhimany A., Sharmay R., Singh R.K. Target-based anticancer indole derivatives and insight into structure-activity relationship: A mechanistic review update (2018–2021) Acta Pharm. Sin. B. 2022;12:3006–3027. doi: 10.1016/j.apsb.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajaniradje S., Kumar M.K., Radhakrishanan R., Sufi S.A., Subramaniam S., Anaikutti P. Indole curcumin reverses multidrug resistance by reducing the expression of ABCD1 and COX2 in induced multidrug resistant human lung cancer cells. Lett. Drug Des. Discov. 2020;17:1146–1154. doi: 10.2174/1570180817666200402124503. [DOI] [Google Scholar]
- 20.Song J., Yoo J., Kwon A., Kim D., Nguyen H.K., Lee B.-Y., Suh W., Min K.H. Structure-Activity Relationship of Indole-Tethered Pyrimidine Derivatives that Concurrently Inhibit Epidermal Growth Factor Receptor and Other Angiokinases. PLoS ONE. 2015;10:e0138823. doi: 10.1371/journal.pone.0138823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W., Qi Y.-Y., Wang Y.-Y., Gan Y.-Y., Shao L.-H., Zhang L.-Q., Tang Z.-H., Zhu M., Tang S.-Y., Wang Z.-C., et al. Design, synthesis, and biological evaluation of sorafenib derivatives containing indole (ketone) semicarbazide analogs as antitumor agents. J. Heterocycl. Chem. 2020;57:2548–2560. doi: 10.1002/jhet.3972. [DOI] [Google Scholar]
- 22.Singh P.K., Silakari O. Molecular dynamics guided development of indole based dual inhibitors of EGFR (T790M) and c-MET. Bioorg. Chem. 2018;79:163–170. doi: 10.1016/j.bioorg.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H. Three generations of epidermal growth factor receptor tyrosine kinase inhibitors developed to revolutionize the therapy of lung cancer. Drug Des. Dev. Ther. 2016;10:3867–3872. doi: 10.2147/DDDT.S119162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed F.A.M., Gomaa H.A.M., Hendawy O.M., Ali A.T., Farghaly H.S., Gouda A.M., Abdelazeem A.H., Abdelrahman M.H., Trembleau L., Youssif B.G.M. Design, synthesis, and biological evaluation of novel EGFR inhibitors containing 5-chloro-3-hydroxymethyl-indole-2-carboxamide scaffold with apoptotic antiproliferative activity. Bioorg. Chem. 2021;112:104960. doi: 10.1016/j.bioorg.2021.104960. [DOI] [PubMed] [Google Scholar]
- 25.Youssif B.G.M., Abdelrahman M.H., Abdelazeem A.H., Abdelgawad M.A., Ibrahim H.M., Salem O.I.A., Mohamed M.F.A., Treamblu L., Bukhari S.N.A. Design, synthesis, mechanistic and histopathological studies of small-molecules of novel indole-2-carboxamides and pyrazino[1,2-a]indol-1(2H)-ones as potential anticancer agents effecting the reactive oxygen species production. Eur. J. Med. Chem. 2018;146:260–273. doi: 10.1016/j.ejmech.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Al-Wahaibi L.H., Gouda A.M., Abou-Ghadir O.F., Salem O.I.A., Ali A.T., Farghaly H.S., Abdelrahman M.H., Trembleau L., Abdu-Allah H.H.M., Youssif B.G.M. Design, and synthesis of novel 2,3-dihydropyrazino[1,2-a]indole-1,4-dione derivatives as antiproliferative EGFR and BRAFV600E dual inhibitors. Bioorg. Chem. 2020;104:104260. doi: 10.1016/j.bioorg.2020.104260. [DOI] [PubMed] [Google Scholar]
- 27.Gomaa H.A.M., Shaker M.E., Alzarea S.I., Hendawy O.M., Mohamed F.A.M., Gouda A.M., Ali A.T., Morcoss M.M., Abdelrahman M.H., Trembleau L., et al. Optimization and SAR investigation of novel 2,3-dihydropyrazino[1,2-a]indole-1,4-dione derivatives as EGFR and BRAFV600E dual inhibitors with potent antiproliferative and antioxidant activities. Bioorg. Chem. 2022;120:105616. doi: 10.1016/j.bioorg.2022.105616. [DOI] [PubMed] [Google Scholar]
- 28.Al-Wahaibi L.H., Mahmoud M.A., Mostafa Y.A., Raslan A.E., Youssif B.G.M. Novel piperine-carboximidamide hybrids: Design, synthesis, and antiproliferative activity via a multi-targeted inhibitory pathway. J. Enzyme Inhib. Med. Chem. 2023;38:376–386. doi: 10.1080/14756366.2022.2151593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abou-Zied H.A., Beshr E.A.M., Gomaa H.A.M., Mostafa Y.A., Youssif B.G.M., Hayallah A.M., Abdel-Aziz M. Discovery of new cyanopyridine/chalcone hybrids as dual inhibitors of EGFR/BRAFV600E with promising antiproliferative properties. Arch. Pharm. 2023. in press . [DOI] [PubMed]
- 30.Tawfeek H.N., Hassan A.A., Bräse S., Nieger M., Mostafa Y.A., Gomaa H.A.M., Youssif B.G.M., El-Shreef E.M. Design, Synthesis, Crystal Structures and Biological Evaluation of Some 1,3-Thiazolidin-4-ones as Dual CDK2/EGFR Potent Inhibitors with Potential Apoptotic Antiproliferative Effects. Arab. J. Chem. 2022;15:104280. doi: 10.1016/j.arabjc.2022.104280. [DOI] [Google Scholar]
- 31.Abdelrahman M.H., Aboraia A.S., Youssif B.G.M., Elsadek B.E.M. Design, Synthesis and Pharmacophoric Model Building of New 3-Alkoxymethyl / 3-Phenyl indole-2-carboxamides with Potential Antiproliferative Activity. Chem. Biol. Drug Des. 2017;90:64–82. doi: 10.1111/cbdd.12928. [DOI] [PubMed] [Google Scholar]
- 32.Abdelrahman M.H., Youssif B.G.M., Abdelgawad M.A., Abdelazeem A.H., Ibrahim H.M., Moustafa A.A., Treamblu T., Bukhari S.N.A. Synthesis, Biological Evaluation, Docking Study and Ulcerogenicity Profiling of Some Novel Quinoline-2-Carboxamides as Dual COXs/LOX Inhibitors Endowed with Anti-Inflammatory Activity. Eur. J. Med. Chem. 2017;127:972–985. doi: 10.1016/j.ejmech.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Gomaa H.A.M., El-Sherief H.A.M., Hussein S., Gouda A.M., Salem O.I.A., Alharbi K.S., Hayallah A.M., Youssif B.G.M. Novel 1,2,4-triazole derivatives as apoptotic inducers targeting p53: Synthesis and antiproliferative activity. Bioorg. Chem. 2020;105:104369. doi: 10.1016/j.bioorg.2020.104369. [DOI] [PubMed] [Google Scholar]
- 34.Youssif B.G.M., Mohamed A.M., Osman E.E.A., Abou-Ghadir O.F., Elnaggar D.H., Abdelrahman M.H., Treamblu L., Gomaa H.A.M. 5-chlorobenzofuran-2-carboxamides: From allosteric CB1 modulators to potential apoptotic antitumor agents. Eur. J. Med. Chem. 2019;177:1–11. doi: 10.1016/j.ejmech.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Zied H.A., Youssif B.G.M., Mohamed M.F.A., Hayallah A.M., Abdel-Aziz M. EGFR inhibitors and apoptotic inducers: Design, synthesis, anticancer activity and docking studies of novel xanthine derivatives carrying chalcone moiety as hybrid molecules. Bioorg. Chem. 2019;89:102997. doi: 10.1016/j.bioorg.2019.102997. [DOI] [PubMed] [Google Scholar]
- 36.Marzouk A.A., Abdel-Aziz S.A., Abdelrahman K.S., Wanas A.S., Gouda A.M., Youssif B.G.M., Abdel-Aziz M. Design, and synthesis of new 1,6-dihydropyrimidin-2-thio derivatives targeting VEGFR-2: Molecular docking and antiproliferative evaluation. Bioorg. Chem. 2020;102:104090. doi: 10.1016/j.bioorg.2020.104090. [DOI] [PubMed] [Google Scholar]
- 37.Hisham M., Hassan H.A., Gomaa H.A.M., Youssif B.G.M., Hayallah A.M., Abdel-Aziz M. Structure-based design, synthesis and antiproliferative action of new quinazoline-4-one/chalcone hybrids as EGFR inhibitors. J. Mol. Struct. 2022;1254:132422. doi: 10.1016/j.molstruc.2022.132422. [DOI] [Google Scholar]
- 38.Alshammari M.B., Aly A.A., Youssif B.G.M., Bräse S., Ahmad A., Brown A.B., Ibrahim M.A.A., Mohamed A.A. Design and synthesis of new thiazolidinone/uracil derivatives as antiproliferative agents targeting EGFR and/or BRAFV600E. Front. Chem. 2022;10:1076383. doi: 10.3389/fchem.2022.1076383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B., Liu Z., Xia S., Liu Q., Gou S. Design, synthesis, and biological evaluation of sulfamoyl phenyl quinazoline derivatives as potential EGFR/CAIX dual inhibitors. Eur. J. Med. Chem. 2021;216:113300. doi: 10.1016/j.ejmech.2021.113300. [DOI] [PubMed] [Google Scholar]
- 40.Hoeflich K.P., Gray D.C., Eby M.T., Tien J.Y., Wong L., Bower J., Gogineni A., Zha J., Cole M.J., Stern H.M., et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66:999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 41.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague T., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 42.Frejat F.O.A., Cao Y., Zhai H., Abdel-Aziz S.A., Gomaa H.A.M., Youssif B.G.M., Wu1 C. Novel 1,2,4-oxadiazole/pyrrolidine hybrids as DNA gyrase and Topoisomerase IV inhibitors with potential antibacterial activity. Arab. J. Chem. 2022;15:103538. doi: 10.1016/j.arabjc.2021.103538. [DOI] [Google Scholar]
- 43.Bollag G., Hirth P., Tsai J., Zhang J., Ibrahim P.N., Cho H., Spevak W., Zhang C., Zhang Y., Habets G., et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engel J., Becker C., Lategahn J., Keul M., Ketzer J., Mühlenberg T., Kollipara L., Schultz-Fademrecht C., Zahedi R.P., Bauer S., et al. Insight into the Inhibition of Drug-Resistant Mutants of the Receptor Tyrosine Kinase EGFR. Angew. Chem. Int. Ed. 2016;55:10909–10912. doi: 10.1002/anie.201605011. [DOI] [PubMed] [Google Scholar]
- 45.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness, and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong J., Wang N.-N., Yao Z.-J., Zhang L., Cheng Y., Ouyang D., Lu A.-P., Cao D.-S. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018;10:29. doi: 10.1186/s13321-018-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be provided upon request.