Abstract
Background
Genomic testing has enhanced pre-surgical decision making for cytologically indeterminate thyroid nodules, but there remains uncertainty regarding RAS mutations. The addition of extra genetic alterations to previous driver mutation panels has been shown to improve predictive value. This study aims to evaluate the relationship between the mutant allele frequency (AF) and likelihood of malignancy in thyroid nodules with RAS mutations.
Methods
A retrospective cohort review was performed evaluating patients with indeterminate cytology (Bethesda categories III, IV and V) and ThyroSeq® v3 testing demonstrating a RAS mutation, who underwent surgery. Univariate and multivariate regression analyses were used to evaluate relationships between AF, other genetic alterations, and malignancy.
Results
Thirty-nine patients met criteria, 77% of the thyroid nodules (30/39) were found to be malignant. None demonstrated aggressive pathology. On univariate regression, there was no relationship between AF and likelihood of malignancy. There was, however, a significant correlation between AF and the rate of an additional genetic alteration. Multivariate analysis found a trend between RAS, a second genetic alteration and malignancy, but it did not reach statistical significance.
Conclusions
There was no direct relationship between the level of allelic frequency in thyroid nodules expressing RAS mutations and the likelihood of malignancy. There was a statistically significant relationship between increasing AF and the presence of a second genetic abnormality, suggesting a possible progression from initial driver mutation and then a second genetic alteration prior to malignant transformation.
Graphical abstract
Keywords: Thyroid cancer, Genetics, Cytology, Allele frequency, Thyroid nodules
Background
Thyroid cancer is currently estimated to be the 9th most common cancer diagnosis worldwide [1], with females being more frequently affected than males (lifetime risk of 1.7%) [2]. Thyroid malignancies are most often well differentiated and prognosis is overall excellent, with 5 year survival approaching 98% [2]. One of the current leading challenges in thyroid cancer care is accurate preoperative diagnosis.
Thyroid fine needle aspiration cytology (FNAC) by its nature is limited by a lack of tissue architecture and small volume sample size, and therefore often cannot definitively distinguish between benign and malignant thyroid pathologies. For this reason, the widely accepted Bethesda reporting system for thyroid FNAC [3] has been designed with six possible diagnostic categories to define the implied risk of malignancy: unsatisfactory (5–10%), benign (0–3%), Atypia of undetermined significance (AUS, 10–30%), follicular neoplasm (FN, 25–40%), suspicious for malignancy (SUS, 50–75%), and malignant (97–99%). Previous meta-analysis has shown that the three indeterminate categories (AUS, FN, SUS) represent between 20 and 25% of all thyroid FNAC results [4], leaving a challenging decision for many patients and thyroid specialists who must balance treating malignancy while avoiding unnecessary surgery in a nodule of uncertain nature.
To help improve pre-surgical diagnosis, several thyroid molecular tests are currently available. These include (with their estimated sensitivity and specificity (Sn, Sp)) the Afirma® gene sequencing classifier [5] (Sn 91%, Sp 68%), combination ThyGenNEXT® and ThyraMIR® [6] (Sn 95%, Sp 90%), and ThyroSeq® v3 [7, 8] (Sn 94%, Sp 82%). Most evaluate cancer risk by testing for known driver mutations—including BRAF, NRAS, HRAS, EIF1AX and KRAS. Additionally, some complement with their own genomic panel, evaluating other features such as gene fusions, copy-number alterations, and/or gene expression alterations. Although presence of the more common BRAF V600E driver mutations have been shown to be essentially diagnostic for thyroid cancer [9], RAS mutations are not quite as definitive as they can be found in the whole spectrum of follicular-pattern thyroid neoplasms including follicular adenoma, non-invasive follicular neoplasm with papillary-like nuclear features (NIFTP), follicular carcinoma, follicular variant of papillary thyroid carcinoma and poorly differentiated thyroid carcinoma. Therefore, RAS mutations are associated with a likelihood of malignancy ranging from 37 to 85% [10–12], resulting in a significant proportion of ultimately benign nodules subject to surgical resection (diagnostic lobectomy).
One of the molecular tests that is currently available, ThyroSeq® v3, reports a genetic quantity known as the mutant allelic frequency (AF), which is the proportion of mutant to normal DNA in a cytology sample [13]. It is noted to be a significant component in its cancer prediction algorithm, yet there is a lack of available data in the literature on the relationship between AF and the diagnosis of thyroid cancer. This data may be useful in better diagnosing and managing cytologically indeterminate thyroid nodules with RAS mutations. This study aims to evaluate specifically the relationship between the mutant allele frequency (AF) and likelihood of malignancy in thyroid nodules with RAS mutations.
Methods
Study design
A retrospective cohort study was performed evaluating patients with cytologically indeterminate thyroid nodules who underwent ThyroSeq® v3 testing from January 2017 to March 2020. The study was approved by the research ethics committees at the McGill University Health Centre and the Jewish General Hospital in Montreal, Quebec.
Patient selection
Included patients were ≥ 18 years with at least one indeterminate thyroid nodule observed on cytology (AUS, FN, SUS), ThyroSeq® v3 testing demonstrating a RAS mutation (NRAS, HRAS, KRAS), and subsequently underwent thyroid surgery. Excluded patients were those awaiting thyroid surgery or with incomplete data at the time of collection.
The lead surgeon followed American Thyroid Association (ATA) guidelines [14] in the workup of said nodules. Patients with indeterminate nodules were presented the options of diagnostic lobectomy, thyroid molecular testing, or observation. They were encouraged to make the final decision based on their own preferences. In the event of indeterminate molecular results, the patient similarly was presented with diagnostic surgery or observation and encouraged to decide.
Data collection
For each patient, the following data was reviewed: patient demographics, cytopathologic results, molecular test results, extent of surgery performed and results from final pathology. Genomic test results included the driver mutation, allele frequency and other genetic features when available. Note that ThyroSeq® divides other genetic features into either gene fusions (GF), copy-number alterations (CNA) or gene expression alterations (GEA).
Ultrasound guided FNA was performed by trained physicians with appropriate patient consent. Samples sent for genomic testing were prepared and shipped according to the provided ThyroSeq® protocol. All FNAC and surgical pathologic samples were analyzed internally by experienced thyroid pathologists, and were reported using the second edition of the Bethesda System for Reporting Thyroid Cytopathology [3] and the World Health Organization (WHO) classification of thyroid tumors [15], respectively. Pathologists were not blinded to molecular genomic test results.
Statistical analysis
All statistical analysis was performed in R v4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). The primary analysis aimed to evaluate relationship between allele frequency and malignancy in all RAS tumors, which was done using logistic regression. Subgroups based on RAS subtype (HRAS, KRAS, NRAS) were analyzed using logistic regression, and subgroups based on AF ranges (e.g. < 10%, 10–20%, etc.) were analyzed using a Chi-squared test. In a secondary analysis, both univariate and multivariate logistic regressions were used to evaluate relationship between AF and other genetic features (CNA, GEA), along with their combined risk of malignancy. These were the only pre-planned analyses; no others were attempted and omitted.
Results
A total of 1066 surgical cases were screened between January 2017 and March 2020 inclusive, 39 of which met all criteria. Baseline characteristics, including Bethesda classification and RAS subtype, are shown in Table 1.
Table 1.
Baseline Characteristic (n = 39)
| Age (mean) | 50.8 |
| Male | 7 |
| Female | 32 |
| Bethesda category | |
| 3 (AUS) | 14 |
| 4 (FN) | 21 |
| 5 (SUS) | 4 |
| RAS mutation | |
| NRAS | 20 |
| HRAS | 18 |
| KRAS | 1 |
| Surgical management | |
| Total thyroidectomy | 5 |
| Hemithyroidectomy | 34 |
Final surgical pathology, shown in Table 2, demonstrated that 77% (30/39) of the RAS-positive nodules were malignant. There were three microcarcinomas that were found outside the sampled nodule (i.e. incidental), accordingly these nodules were counted as negative for malignancy. Of the remaining malignant cases, the majority were the follicular variant of papillary thyroid cancer (FV-PTC, 25/39), either fully encapsulated (4/39), with focal capsular invasion or partially encapsulated (13/39), or unencapsulated/invasive (8/39). The remainder were either the encapsulated solid variant of PTC (1/39) or encapsulated oncocytic (Hürthle) cell carcinoma with no vascular invasion (2/29). Note that there were none of the well recognized aggressive subtypes of PTC (e.g. Tall cell, hobnail/micropapillary, columnar cell, diffuse sclerosing) and none of the tumors had extra-thyroidal extension or other American Thyroid Association high risk features [14]. There were 10% (4/39) benign cases and 5% (2/39) NIFTP.
Table 2.
Pathologic results
| Diagnosis | n |
|---|---|
| Benign | 7* |
| NIFTP | 2 |
| PTC – Follicular Variant | 25 |
| Fully encapsulated | 4 |
| Partially encapsulated | 13 |
| Unencapsulated/invasive | 8 |
| PTC – Oncocytic Variant | 2 |
| PTC – Solid Variant (encapsulated) | 1 |
| Hurthle Cell Carcinoma | 2 |
PTC Papillary thyroid cancer, NIFTP Non-invasive thyroid neoplasm with papillary-like nuclear features. *Note that in three cases there was an incidental finding of microcarcinoma separate from the biopsied nodule, and therefore were considered benign
The distribution of allele frequencies is shown in Fig. 1. The mean, median, and standard deviation of allele frequency in malignant cases were 23.2%, 23%, and 11%, respectively, and of non-malignant cases 20.1%, 17%, and 13%. In regression analysis, there was no significant relationship between allele frequency and malignancy in the full data set (p > 0.05). Dividing based on subtype of RAS (NRAS, HRAS) additionally did not show statistical significance between AF and likelihood of cancer. Subgrouping AF over the entire sample by value of < 10%, 10–19%, 20–29%, and 30 + % demonstrated a rate of malignancy of 57% (4/7), 80% (8/10), 86% (1/7), and 80% (12/15), respectively. Although the < 10% subgroup showed a lower rate of malignancy compared to the combined other cases (57% vs. 81%), this did not achieve significance in Chi-squared analysis (p > 0.05). The lowest AF in a malignant nodule was 2% and the highest AF in a benign nodule was 39%.
Fig. 1.
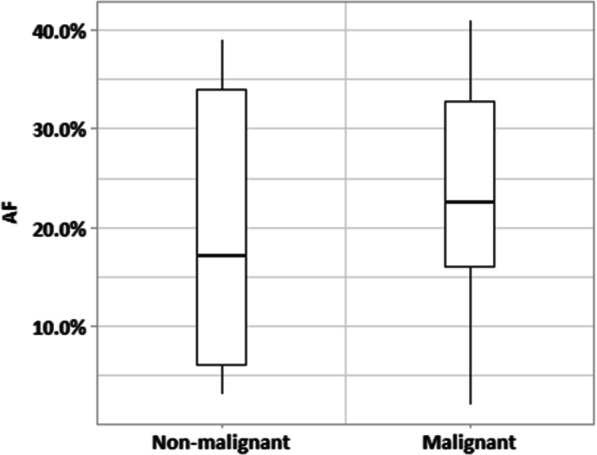
Distribution of allele frequency (AF) among studied tumors
On secondary analysis, evaluation of the relationship between driver mutation allele frequency and second genetic alteration (CNA and/or GEA) revealed a significant correlation (Odds ratio [OR] = 1.082, [Confidence Interval (CI) = 1.018–1.164], p = 0.0199). This is illustrated in Fig. 2, where there is a trend of increasing frequency of additional genetic alterations with increasing allele frequency. On multivariate analysis, there was a positive trend between second genetic alteration and malignancy (r = 1.97), but no statistically significant relationship was found between allele frequency, other genetic alterations, and cancer risk (all p > 0.05). Two of the nodules had a concurrent EIF1AX mutation, both of which were malignant. No other mutations were seen in this sample.
Fig. 2.
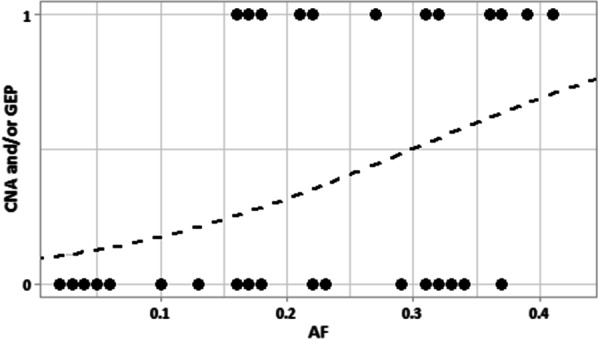
Presence of second genetic alteration in relation to allele frequency (CNA Copy-number alterations, GEA Gene expression profile alterations, AF Allele frequency). Dotted line represents probability fit by linear regression
Discussion
The RAS proto-oncogene encodes three highly homologous membrane-associated proteins: NRAS, HRAS, and KRAS. Under normal circumstances, these proteins are activated by the exchange of GDP for GTP by Grb2/SOS proteins, and then stimulate cellular pathways such as Raf-MAPK and PI3K-AKT. These pathways are known to be regulators of many fundamental cellular functions including growth and survival [16–19]. Certain point mutations on RAS genes, for example at the NRAS and HRAS codon 61, commonly implicated in RAS-associated PTC [16], result in inhibition of the GTP hydrolysis step required for RAS self-deactivation, and therefore leads to constitutional activation of the pathway. This mechanism has previously been shown to cause many forms of human cancer including pancreas, colon, lung, and differentiated thyroid cancer (DTC) [20, 21]. Although the BRAF V600E mutation is more common to DTC and essentially diagnostic for malignancy, RAS mutations remain less definitive, with a significant proportion of resected tumors yielding benign pathology or NIFTP [10, 16, 22].
Recent advances in thyroid genomic testing have gone beyond checking for presence of driver mutations alone, and now evaluate other genetic features including copy number alterations (CNA), single-nucleotide variants and alterations to the gene expression profile (GEA) [7]. The present study examined the allele frequency (AF), or the proportion of mutant DNA affected by the driver mutation to normal DNA, and evaluated its relationship to rate of malignancy among RAS tumors. In this sample, there was no significant relationship found between AF and thyroid cancer. This is in keeping with one previous analysis [23] where they categorically compared tumors with AF < 10% or ≥ 10% against presence of malignancy and did not find a relationship.
In the secondary analysis above, there was a significant relationship found when comparing RAS AF and presence of additional genetic alterations (CNA and/or GEA). Previous literature has suggested a stepwise RAS-mediated oncogenesis in which an initial driver mutation leads to further molecular alterations that promote development of initial cancer [16], and later de-differentiation into poorly differentiated [24] and anaplastic [25, 26] cancers. Indeed, the result above supports this notion, suggesting a possible progression starting with a first mutation (RAS driver), developing increased AF over time, and then acquiring a second alteration on a path to malignancy. Although the multivariate analysis could not confirm a relationship between additional genetic abnormalities and cancer, there was a positive trend in this relatively small sample, and a larger data set could evaluate this further.
Surgical pathology in this sample was largely in keeping with reports from the literature, where RAS mutations have been shown to be associated with thyroid neoplasms that are characterized by a follicular growth pattern, including follicular variant PTC. They additionally tend to be encapsulated and have a low probability of concerning histologic characteristics such as extrathyroidal extension, lymph node metastasis and vascular invasion [27–29], which again was seen in the present study. A few cases in our study were oncocytic or solid variant of PTC, but these cases were also mainly encapsulated and there is currently no evidence that these uncommon variants represent more aggressive tumors in the absence of other adverse features. This reinforces the idea that lobectomy or hemithyroidectomy would be an appropriate surgical choice for RAS positive tumors given proper pre-operative selection criteria (e.g. nodule < 4 cm, no radiotherapy history, clinically lymph node negative, etc.) [23].
Limitations of this study include the retrospective review of electronic medical records. It evaluated data from only two tertiary care centres in one city and would not account for geographic variations in thyroid cancer. Neither the surgeons nor the pathologists were blinded to results from cytology or genomic testing, which could introduce bias in decision making and analysis. The genomic test was mostly not covered by the government public health insurance plan, which likely added bias in patient demographics and selection. Lastly, the study was limited to surgical cases only, and therefore did not account for RAS tumors that were not resected.
Conclusions
In this study, there was no direct relationship between the level of allelic frequency and the likelihood of malignancy in a sample of cytologically indeterminate thyroid nodules with a RAS driver mutation. There was a statistically significant relationship between AF and the presence of a second genetic abnormality (CNA and/or GEA), suggesting a possible progression from initial driver mutation, increasing AF, and then a second genetic alteration prior to malignant transformation.
Acknowledgements
Not applicable
Abbreviations
- AF
Allele frequency
- FNAC
Fine needle aspirate cytology
- AUS
Atypia of undetermined significance
- FN
Follicular neoplasm
- SUS
Suspicious for malignancy
- Sn
Sensitivity
- Sp
Specificity
- NIFTP
Non-invasive follicular neoplasm with papillary-like nuclear features
- CNA
Copy-number alterations
- GEA
Gene expression alterations
- WHO
World health organization
- PTC
Papillary thyroid carcinoma
- FV
Follicular variant
- DTC
Differentiated thyroid cancer
Author contributions
TH: Data collection, analysis and drafting of the manuscript. MP: Pathologic review, study conception and design. MH: Study conception and design. VF: Study conception and design. JY: Study conception and design. RP: Study conception and design, oversee each step of the project. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was evaluated and approved by the research ethics board of the McGill University Health Centre and the research review office of the Jewish General Hospital.
Consent for publication
Not applicable.
Competing interests
There are no conflicts of interest or financial relationships to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clinic 2018;68(6):394–424. [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA: Cancer J Clinic. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346. doi: 10.1089/thy.2017.0500. [DOI] [PubMed] [Google Scholar]
- 4.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 2012;56(4):333–339. doi: 10.1159/000339959. [DOI] [PubMed] [Google Scholar]
- 5.Patel KN, Angell TE, Babiarz J, Barth NM, Blevins T, Duh Q-Y, et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg. 2018;153(9):817–824. doi: 10.1001/jamasurg.2018.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupo MA, Walts AE, Sistrunk JW, Giordano TJ, Sadow PM, Massoll N, et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagnostic Cytopathol. 2020;48(12):1254. doi: 10.1002/dc.24564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikiforova MN, Mercurio S, Wald AI, Barbi de Moura M, Callenberg K, Santana-Santos L, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124(8):1682–1690. doi: 10.1002/cncr.31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol. 2019;5(2):204–212. doi: 10.1001/jamaoncol.2018.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikiforov YE. Molecular analysis of thyroid tumors. Mod Pathol. 2011;24(S2):S34. doi: 10.1038/modpathol.2010.167. [DOI] [PubMed] [Google Scholar]
- 10.Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96(11):3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinkscales W, Ong A, Nguyen S, Harruff EE, Gillespie MB. Diagnostic value of RAS mutations in indeterminate thyroid nodules: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2017;156(3):472–479. doi: 10.1177/0194599816685697. [DOI] [PubMed] [Google Scholar]
- 12.Marotta V, Bifulco M, Vitale M. Significance of RAS mutations in thyroid benign nodules and non-medullary thyroid cancer. Cancers. 2021;13(15):3785. doi: 10.3390/cancers13153785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikiforov YE, Baloch ZW. Clinical validation of the ThyroSeq v3 genomic classifier in thyroid nodules with indeterminate FNA cytology. Cancer Cytopathol. 2019;127(4):225–230. doi: 10.1002/cncy.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd R, Osamura R, Kloppel G, Rosai J. Tumours of the thyroid gland. World health organization classification of tumours of endocrine organs. USA: IARC; 2017. [Google Scholar]
- 16.Howell GM, Hodak SP, Yip L. RAS mutations in thyroid cancer. Oncologist. 2013;18(8):926–932. doi: 10.1634/theoncologist.2013-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- 18.Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE. Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol. 1999;50(4):529–535. doi: 10.1046/j.1365-2265.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- 19.Soares P, Póvoa AA, Melo M, Vinagre J, Máximo V, Eloy C, et al. Molecular pathology of non-familial follicular epithelial–derived thyroid cancer in adults: from RAS/BRAF-like tumor designations to molecular risk stratification. Endocr Pathol. 2021;32(1):44–62. doi: 10.1007/s12022-021-09666-1. [DOI] [PubMed] [Google Scholar]
- 20.Bos JL. Ras oncogenes in human cancer: a review. Can Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 21.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 22.Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95(3):1365–1369. doi: 10.1210/jc.2009-2103. [DOI] [PubMed] [Google Scholar]
- 23.Patel SG, Carty SE, McCoy KL, Ohori NP, LeBeau SO, Seethala RR, et al. Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery. 2017;161(1):168–175. doi: 10.1016/j.surg.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang S-H, Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Investig. 1993;91(1):179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Rostan G, Tallini G, Herrero A, Thomas G, Carcangiu ML, Rimm DL. Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Can Res. 1999;59(8):1811–1815. [PubMed] [Google Scholar]
- 26.García-Rostán G, Costa AM, Pereira-Castro I, Salvatore G, Hernandez R, Hermsem MJ, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Can Res. 2005;65(22):10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- 27.Yip L, Nikiforova MN, Yoo JY, McCoy KL, Stang MT, Armstrong MJ, et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: a study of 1510 patients. Ann Surg. 2015;262(3):519. doi: 10.1097/SLA.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta N, Dasyam AK, Carty SE, Nikiforova MN, Ohori NP, Armstrong M, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98(5):E914–E922. doi: 10.1210/jc.2012-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan H, Toraldo G, Cerda S, Godley FA, Rao SR, McAneny D, et al. Utilities of RAS mutations in preoperative fine needle biopsies for decision making for thyroid nodule management: results from a single-center prospective cohort. Thyroid. 2020;30(4):536–547. doi: 10.1089/thy.2019.0116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during the current study are available from the corresponding author on reasonable request.