Abstract
Background
Extranodal extension (ENE) is an adverse prognostic factor for oral squamous cell carcinoma (OSCC), and patients with OSCC along with ENE require neck dissection. In this study, we developed a novel ENE histology-based pathological predictor using MMP14 expression patterns in small biopsy specimens.
Methods
A total of 71 surgically resected tissue, 64 dissected lymph node (LN), and 46 biopsy specimens were collected from 71 patients with OSCC. Immunohistochemical analyses of total MMP14 expression in the tumour nest and cancer-associated fibroblasts (CAFs) were performed using the MMP14 co-scoring system (high- or low-risk). The association analysis of MMP14 expression in metastatic LNs was performed with respect to the presence and absence of ENE. Clinicopathological analyses and multivariate examinations were performed to assess the risks of metastasis and ENE presence. The predictive value of ENE and the impact of ENE and MMP14 expression on 5-year overall survival were examined.
Results
High-risk MMP14 expression was detected in metastatic LN specimens with ENE. MMP14 expression in tumour nests and CAFs and its overexpression at the tumour–stromal interface significantly correlated with the presence of ENE. The MMP14 co-scoring system was an independent risk predictor for ENE, with sensitivity, specificity, and accuracy of over 80% in biopsy samples; patients with a high risk in the MMP14 co-scoring system had significantly worse prognoses in both resections and biopsies.
Conclusion
The MMP14 co-scoring system accurately predicted ENE presence and poor prognosis via immunohistochemical evaluation of small biopsies. This system is a simple, accurate, and inexpensive immunohistochemical approach that can be used in routine pathological diagnosis for effective treatment planning.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10595-x.
Keywords: Extranodal extension, Oral squamous cell carcinoma, Matrix metalloproteinase 14, Membrane-type 1 matrix metalloproteinase, Cancer-associated fibroblast, Predictor
Background
An extranodal extension (ENE) is an extension of tumour cells through the lymph node (LN) capsule into the surrounding connective tissue [1, 2]. It is the most important prognostic factor in human papillomavirus-positive and -negative head and neck squamous cell carcinoma (HNSCC) and is associated with increased locoregional recurrence, distant metastasis, and decreased survival [3–6]. Patients with pathological ENE (pENE) need high-dose chemoradiotherapy [7]; however, this is only diagnosed by post-operative pathological examination. Clinical and radiological examinations do not satisfactorily evaluate ENE presence (ENE+), and accuracy ranges from 7.0 to 85.0% [8–10].
Oral squamous cell carcinoma (OSCC) is a type of HNSCC [1, 7]. For patients with OSCC, with even minor ENE (< 2.0 mm), the 5-year overall survival is poorer than that of patients without ENE (30.4% vs. 63.1%) [11], and 40% of them experience occult LN metastasis [8–10]. Therefore, OSCC is more strongly recommended for neck dissection than other HNSCCs, even if there are no clinically observed LN metastases [12, 13]. However, neck dissection may negatively affect host immunity and tumour response to immune checkpoint inhibitors [14] as well as incur post-operative cosmetic and functional problems. Thus, an accurate pre-operative prediction method is needed for identifying patients at high risk of ENE+—those who truly need cervical dissection.
Some histological predictive features of ENE + include tumour budding (TB), tumour-infiltrating lymphocytes (TILs), and desmoplastic reaction (DR) [15–22]. TB, TILs, and DRs in the tumour microenvironment (TME) at the tumour–stromal interface (TSI) in primary OSCC reflect tumour TME remodelling ability and are highly concordant with TB, TILs, and DRs at the ENE site [15]. However, histological predictive methods are not useful for biopsies with little or no TSI, wherein the accuracy remains below 80% [15].
Matrix metalloproteinases (MMPs), especially MMP2, 3, 9 and 14, derived from cancer-associated fibroblasts (CAFs) and tumour cells, are powerful TME remodelling factors, which enhance tumour invasiveness and metastasis in OSCC [16, 20, 22–24]. MMP2, 3, and 9 are expressed within the tumour cell cytoplasm, MMP2 and 9 are gelatinases [25, 26], while MMP3 is a stromelysin [26]. MMP14 is a membrane-type-1 MMP expressed at the tumour membrane and cleaves gelatine, fibronectin, and laminin and regulates invadopodium development [27, 28].
Through this study, we aimed to develop a histology-based ENE + prediction method for application to small pre-operative biopsy specimens. Our newly developed MMP14 scoring system is a novel ENE + prediction method with more accuracy than those for TB, TILs, and DRs, and is applicable to biopsies with over 80% accuracy.
Methods
Patients and case selection
This retrospective study included 71 patients with OSCC who underwent surgical resection between 2011 and 2020 (Additional File 1).
Histopathological evaluations
The histological assessments conducted and definitions of TSI, CAFs, TB [16–19], TILs [20, 21], DR [22], and clinicopathological features such ENE are presented in Additional File 2. Histological assessments of TB [16–19], TILs [20, 21], DR [22] and CAFs at the TSI and clinicopathological evaluations were performed based on haematoxylin and eosin (H&E) staining of biopsies, resected primary sites, and LNs from patients with OSCC.
Immunohistochemistry and immunohistochemical scoring of resections, biopsies, and LN dissections
Immunohistochemical staining of MMPs and evaluation of MMP expression in tumour biopsy, surgically resected specimens, and intranodal metastatic areas and in the ENE area in dissected LNs were performed. The evaluation methods for MMP2, 3, 9, and 14 expression are described in Additional Files 3 and 4. The MMP14 expression evaluation method is shown in Fig. 1.
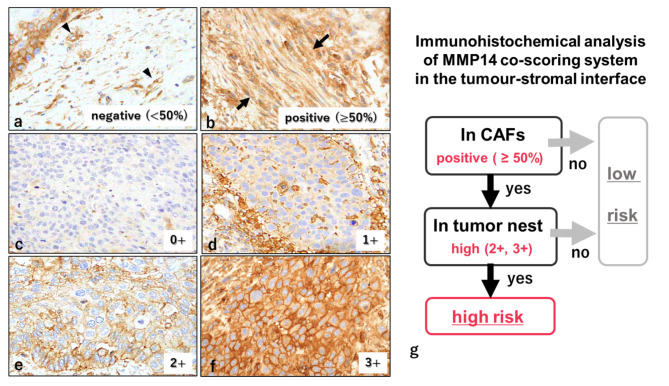
Fig. 1.
Immunohistochemical analysis of MMP14 expression in tumour nests and CAFs at the primary TSI.
Immunohistochemical staining of MMP14 of OSCC resected specimens in TSI. MMP14 expression in CAFs: negative, CAFs < 50% (a); positive, CAFs ≥ 50% (b). MMP14 expression at the tumour nest in the TSI (c−g). MMP14 score at the tumour nest: 0, none (c); 1, weak cytoplasmic expression without membrane expression (d); 2, moderate cytoplasmic expression or incomplete membrane expression (e); and 3, strong cytoplasmic expression or complete membrane expression (f). Assessment of MMP14 established ranges from 0 to 3+; sample scoring of 2 + to 3 + was ‘high’ and 0 to 1 + was ‘low’. Flowchart of the MMP14 co-scoring system (g). Total MMP14 expression in tumours and CAFs was examined as follows: high-risk (cases that were CAFs-positive and whose tumour scores were high) and low-risk (cases that were not high-risk). Original magnification: a−f, 400× HPF. CAFs, cancer-associated fibroblast; TSI, tumour–stromal interface
Statistical analysis
This information is provided in Additional File 5.
Results
Clinicopathological features of 71 matched resections and 46 biopsy specimens from patients with OSCC
In our study, we included 71 surgically resected tissue, 64 matched-neck dissection, and 46 matched biopsy specimens from 71 patients with OSCC (Table 1).
Table 1.
Clinicopathological features of matched 71 resection and 46 biopsy specimens from patients with OSCC
| All patients (n = 71) | Patients with biopsies (n = 46) | |||
|---|---|---|---|---|
| Characteristics | No. of patients | % | No. of patients | % |
| Age | Range = 39–91 | Range = 39–91 | ||
|
(median = 69, mean = 67.8) |
(median = 68.5, mean = 67.5) |
|||
| > 65 | 24 | 34% | 18 | 39% |
| ≤ 65 | 47 | 66% | 28 | 61% |
| Sex | ||||
| Male | 43 | 61% | 26 | 57% |
| Female | 28 | 39% | 20 | 43% |
| Location | ||||
| Buccal mucosa | 8 | 11% | 4 | 9% |
| Gingiva | 15 | 21% | 7 | 15% |
| Tongue | 48 | 68% | 35 | 76% |
| pT | ||||
| 1,2 | 17 | 24% | 14 | 30% |
| 3,4 | 54 | 76% | 32 | 70% |
| LN metastasis | ||||
| Absent | 26 | 37% | 23 | 50% |
| Present |
45 (27 cases were ENE+) |
63% |
23 (10 cases were ENE+) |
50% |
| pN | ||||
| 0,1 | 38 | 54% | 32 | 70% |
| 2,3 | 33 | 46% | 14 | 30% |
OSCC, oral squamous cell carcinoma; ENE, extranodal extension; pT, pathological T; pN, pathological N; LN, lymph node; ENE+, presence of extranodal extension
LN dissection was performed for 64 patients, LN metastasis (pLN+) was observed for 45, 27 cases displayed ENE (pLN+/pENE+), and 18 did not exhibit pENE (pLN+/pENE-). A pLN+/pENE- case was excluded from the analyses owing to the poor FFPE quality; 44 pLN+/pENE cases were available for immunohistochemical examination. Of the 28 cases without LN metastasis (pLN-), LN dissection was conducted in 21 cases and not in the remaining 7 cases, where clinical LN metastasis had not yet occurred. For the 71 cases, 46 matched biopsy specimens were available for analysis, as summarised in Table 1. Of these, 23, 10, and 23 cases were pLN+, pLN+/pENE+, and pLN-/ENE-, respectively.
Comparison of MMP expression at the dissected metastatic LN sites with and without ENE and MMP expression at the ENE site
MMP expression data are described in Additional File 6. MMP2, 3, 9, and 14 expression in the tumour nest and CAFs was more profound at the ENE site than in the intranodal component (p < 0.01; Figs. 2 and 3a and Additional File 7). In the 44 cases of LN metastasis, there was no association between MMP2, 3, and 9 expression and ENE+/- (all p > 0.05, Table 2). Only MMP14 expression in CAFs and the tumour nest was associated with ENE+ (all p < 0.05). High-risk cases according to the MMP14 co-scoring system, including the total MMP14 enzymatic activity derived from tumour nest and CAFs, were associated with ENE+; 83% of them had ENE+ (p < 0.05).
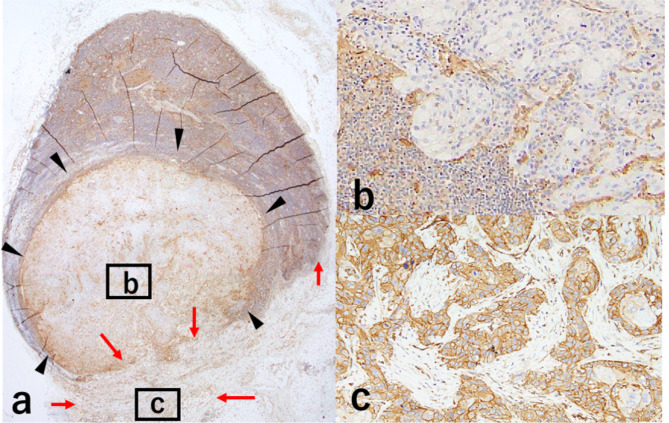
Fig. 2.
Immunohistochemical analysis of MMP14 expression in the tumour nest and CAFs at the metastatic LN.
MMP14 expression in LNs with ENE (a−c). At the ENE site, MMP14 expression in the tumour nest is higher than that in the intranodal tumour nest (a−c: black arrowheads show cancer nest inside the LN; red arrows show cancer nest at the ENE site). Intranodal tumour nest score was 0+; there was no CAFs (b). At the ENE site, the tumour nest had a score of 3+; CAFs was observed; and MMP14 was expressed in CAFs (c). Original magnification: a, 5× high power field (HPF); b and c, 20× HPF. ENE, extranodal extension; CAFs, cancer-associated fibroblasts; LN, lymph node
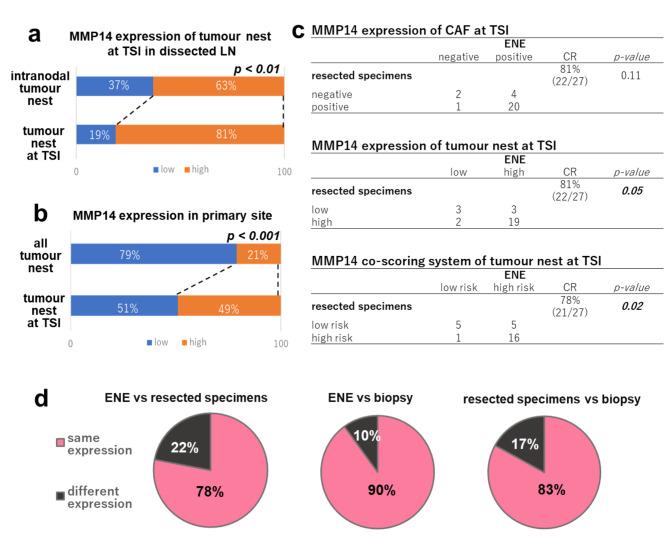
Fig. 3.
MMP14 expression at the TSI in the primary site and dissected LNs.
High MMP14 expression in tumour nest is more at the ENE site than at the intranodal site (a, 63% vs. 81%). High MMP14 expression in the tumour nest is more at the TSI than at the tumour nest in the primary site (b, 21% vs. 49%). The high CR of MMP14 expression at the TSI is detected in CAFs (c, ENE vs. resected specimens, 81%, p = 0.11), tumour nest (c, 81%, p = 0.05), and co-scoring system (c and d, left, 78%, p = 0.02). The CR of MMP14 co-scoring expression between the ENE and biopsy (d, middle, 90%) and the resected specimens and biopsy (d, right, 83%). CAFs, cancer-associated fibroblasts; CR, concordance rate; ENE, extranodal extension; LN, lymph node; TSI, tumour–stromal interface
Table 2.
Association between MMP expression profiles and ENE presence in metastatic LNs
| LN(+)/ENE(-) | LN(+)/ENE(+) | p-value | |
|---|---|---|---|
| Intranodal MMP14 expression | |||
| CAFs | < 0.01 | ||
| Negative | 11 (65%) | 6 (35%) | |
| Positive | 6 (22%) | 21 (78%) | |
| Tumour nest | 0.03 | ||
| Low | 12 (55%) | 10 (45%) | |
| High | 5 (23%) | 17 (77%) | |
| MMP14 co-scoring system | 0.01 | ||
| Low-risk | 14 (54%) | 12 (46%) | |
| High-risk | 3 (17%) | 15 (83%) | |
| Intranodal MMP2 expression | |||
| CAFs | 0.98 | ||
| Negative | 7 (39%) | 11 (61%) | |
| Positive | 10 (38%) | 16 (62%) | |
| Tumour nest | 0.51 | ||
| Low | 9 (35%) | 17 (65%) | |
| High | 8 (44%) | 10 (56%) | |
| Intranodal MMP3 expression | |||
| CAFs | 0.8 | ||
| Negative | 12 (38%) | 20 (63%) | |
| Positive | 5 (42%) | 7 (58%) | |
| Tumour nest | 0.58 | ||
| Low | 13 (39%) | 20 (61%) | |
| High | 4 (36%) | 7 (64%) | |
| Intranodal MMP9 expression | |||
| CAFs | 0.49 | ||
| Negative | 10 (43%) | 13 (57%) | |
| Positive | 7 (33%) | 14 (67%) | |
| Tumour nest | 0.5 | ||
| Low | 15 (38%) | 25 (63%) | |
| High | 2 (50%) | 2 (50%) | |
LN, lymph node; LN+, presence of lymph node metastasis; ENE, extranodal extension; ENE-, absence of extranodal extension; ENE+, presence of extranodal extension; CAFs, cancer-associated fibroblasts. Boldface indicates statistically significant values
Association of MMP expression at the TSI between the resected specimens and the ENE site of metastatic LN specimens
High MMP2, 3, 9, and 14 expression in the tumour nest was more predominant at the TSI than in the tumour nest in primary resected specimens (p < 0.001, Fig. 3b; Additional File 7). The concordance rate (CR) was highest for MMP14 expression, compared to that of other MMPs (CAFs, 81%, p = 0.11; tumour nest, 81%, p = 0.05; co-expression system, 78%, p < 0.01) (Fig. 3c, d, and Additional File 6).
Association of clinicopathological features with MMP14 and other MMPs at the TSI of primary tumour specimens
We determined if the expression pattern of MMPs in the primary resected specimens was associated with pENE presence (pENE+) and TME activity-related features (TB, DR, and TILs). The clinicopathological features of all 71 patients with OSCC are compiled in Additional File 6.
MMP14 expression in CAFs and the tumour nest at the TSI were significantly associated with pENE + and TME features, such as TB, pLN+, pN2/3, lymphatic invasion (Ly), and perineural invasion (Pn) (p < 0.05, Table 3). Additionally, MMP14 expression in CAFs was associated with pT, pDOI > 10 mm, invasion pattern, DR, TILs, and V (p < 0.05, Table 3). The high-risk group of the MMP14 co-scoring system was significantly associated with pENE + and TB and also pN+, pN2/3, pT, invasion pattern, Ly, and Pn (p < 0.05, Table 3). Age, sex, and locations were not significantly associated with MMP14 expression in the CAFs, tumour nest and co-scoring system (data not shown). Moreover, MMP2 expression in CAFs and MMP9 expression in the tumour nest at the TSI was significantly associated with ENE + but not with TME activity-related features (TB, DR, and TILs) (Additional Files 8 and 9). No associations were found between pENE + and the expression of other MMPs in the tumour nest and CAFs at the TSI (Additional Files 8–10) and the expression of all MMPs in all tumour nests (MMP2, 3, 9; data not shown; MMP14, Additional File 11). Therefore, we considered MMP14 to be relevant.
Table 3.
Association of clinicopathological features with MMP14 expression in the tumour nest and CAFs of 71 surgically resected OSCC specimens
| MMP14 expression in CAFs at the TSI |
MMP14 expression in the tumour nest at the TSI | MMP14 co-scoring system | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | p | Low | High | p | Low- risk | High- risk | p | |
| pT | < 0.001 | 0.44 | < 0.01 | ||||||
| 1.2 | 16 | 1 | 10 | 7 | 17 | 0 | |||
| 3.4 | 16 | 38 | 26 | 28 | 30 | 24 | |||
| pDOI | < 0.001 | 0.22 | 0.22 | ||||||
| ≤ 10 mm | 17 | 4 | 13 | 8 | 13 | 8 | |||
| > 10 mm | 15 | 35 | 23 | 27 | 23 | 27 | |||
| Lymph node metastasis | < 0.001 | 0.02 | < 0.001 | ||||||
| (-) | 21 | 5 | 18 | 8 | 25 | 1 | |||
| (+) | 11 | 34 | 18 | 27 | 22 | 23 | |||
| pN | < 0.001 | 0.02 | < 0.01 | ||||||
| 0,1 | 24 | 14 | 24 | 14 | 31 | 7 | |||
| 2,3 | 8 | 25 | 12 | 21 | 16 | 17 | |||
| ENE | < 0.01 | < 0.001 | < 0.001 | ||||||
| (-) | 26 | 18 | 30 | 14 | 37 | 7 | |||
| (+) | 6 | 21 | 6 | 21 | 10 | 17 | |||
| Differentiation | 0.37 | 0.37 | 0.58 | ||||||
| Well | 17 | 28 | 24 | 21 | 27 | 18 | |||
| Moderate | 13 | 11 | 12 | 12 | 18 | 6 | |||
| Poor | 2 | 0 | 0 | 2 | 2 | 0 | |||
| Invasion pattern | 0.03 | 0.06 | 0.047 | ||||||
| 1.2 | 6 | 1 | 6 | 1 | 7 | 0 | |||
| 3.4c.4d | 26 | 38 | 30 | 34 | 40 | 24 | |||
| DR | < 0.001 | 0.12 | 0.21 | ||||||
| Mature | 17 | 6 | 22 | 15 | 27 | 10 | |||
| Immature | 15 | 33 | 14 | 20 | 20 | 14 | |||
| TB | 0.02 | < 0.01 | < 0.01 | ||||||
| Low | 19 | 12 | 23 | 8 | 28 | 3 | |||
| High | 13 | 27 | 13 | 27 | 19 | 21 | |||
| TILs | 0.02 | 0.54 | 0.08 | ||||||
| High | 19 | 12 | 17 | 14 | 24 | 7 | |||
| Low | 13 | 27 | 19 | 21 | 23 | 17 | |||
| Ly | < 0.001 | 0.01 | 0.01 | ||||||
| (-) | 15 | 5 | 15 | 5 | 15 | 5 | |||
| (+) | 17 | 34 | 21 | 30 | 21 | 30 | |||
| V | < 0.001 | 0.05 | 0.06 | ||||||
| (-) | 16 | 1 | 12 | 5 | 12 | 5 | |||
| (+) | 16 | 38 | 24 | 30 | 24 | 30 | |||
| Pn | < 0.001 | 0.04 | 0.048 | ||||||
| (-) | 16 | 6 | 15 | 7 | 15 | 7 | |||
| (+) | 16 | 33 | 21 | 28 | 21 | 28 | |||
CAFs, cancer-associated fibroblasts; OSCC, oral squamous cell carcinoma; TSI, tumour–stromal interface; pT, pathological T; pDOI, pathological depth of invasion; pN, pathological N; DR, depth of invasion; TB, tumour budding; TILs, tumour-infiltrating lymphocytes; Ly, lymphatic invasion; V, vascular invasion; p, p-value; Pn, perineural invasion. Boldface indicates statistically significant values
Association between MMP14 expression at the TSI in biopsy specimens and the ENE site
At the ENE site, the CR of MMP14 expression in the tumour nest, CAFs, and the risk of the co-scoring system at the TSI in biopsy specimens was 90% (9/10; p < 0.05 Figs. 3d and 4a–f, and Additional File 12). Moreover, a high CR of MMP14 expression between the resected and biopsy specimens was found for the tumour nest (65%), CAFs (74%), and co-scoring system (83%; p < 0.01, Fig. 3d and Additional File 12).
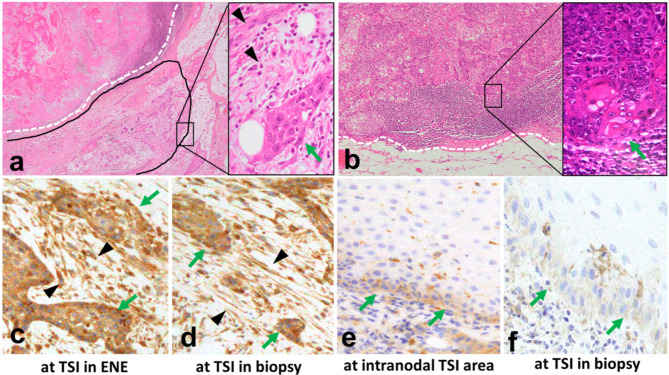
Fig. 4.
Association between MMP14 at the TSI in biopsy and the ENE site
MMP14 expression in the tumour nest (black arrowhead) and CAFs (green arrows) in metastatic LNs with ENE (a, c) and without ENE (b, e), and the matched biopsy (d, f) is consistent. MMP14 expression in the tumour nest (3+, c and d) and CAFs (positive, c and d). MMP14 expression in the tumour nest (1+, e and f) and CAFs (negative, e and f). Original magnification, a and b, left, 5× high power field (HPF); a and b, right, c–f, 40× HPF. TSI, tumour–stromal interface; ENE, extranodal extension; CAFs, cancer-associated fibroblasts; LN, lymph node
Association of clinicopathological features with MMP14 at the TSI in biopsy
Clinicopathological analysis of MMP14 expression in the biopsy samples was performed. CAFs and tumoural MMP14 expression and the MMP14 co-scoring system were significantly associated with pENE+, pN+, pN2/3, and TILs (p < 0.05, Table 4). Furthermore, association between MMP14 expression in CAFs and clinical depth of invasion (cDOI) > 10 mm was detected (p < 0.05). MMP14 expression in tumour nest and the MMP14 co-scoring system were also associated with DR (p < 0.05).
Table 4.
Association of clinicopathological features with MMP14 expression in the tumour nest and CAFs of 46 OSCC biopsy specimens
| MMP14 expression in CAFs at the TSI (biopsies) |
MMP14 expression in the tumour nest at the TSI (biopsies) |
MMP14 co-scoring system (biopsies) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | p | Low | High | p | Low- risk | High- risk | p | |
| pT | 0.01 | 0.01 | 0.05 | ||||||
| 1.2 | 12 | 2 | 12 | 2 | 13 | 1 | |||
| 3.4 | 15 | 17 | 15 | 17 | 21 | 11 | |||
| cDOI | 0.01 | 0.17 | 0.08 | ||||||
| ≤ 10 mm | 14 | 3 | 12 | 5 | 15 | 2 | |||
| > 10 mm | 13 | 16 | 15 | 14 | 19 | 10 | |||
| Lymph node metastasis | < 0.01 | < 0.01 | < 0.001 | ||||||
| (-) | 19 | 4 | 19 | 4 | 23 | 0 | |||
| (+) | 8 | 15 | 8 | 15 | 11 | 12 | |||
| pN | < 0.01 | 0.03 | < 0.001 | ||||||
| 0,1 | 23 | 9 | 22 | 10 | 28 | 4 | |||
| 2,3 | 4 | 10 | 5 | 9 | 6 | 8 | |||
| ENE | < 0.001 | < 0.01 | < 0.001 | ||||||
| (-) | 26 | 10 | 25 | 11 | 32 | 4 | |||
| (+) | 1 | 9 | 2 | 8 | 2 | 8 | |||
| Differentiation | 0.58 | 0.58 | 0.58 | ||||||
| Well | 20 | 14 | 14 | 14 | 19 | 9 | |||
| Moderate | 6 | 5 | 11 | 5 | 13 | 3 | |||
| Poor | 1 | 0 | 2 | 0 | 2 | 0 | |||
| Invasion formation | 0.11 | 0.45 | 0.28 | ||||||
| 1.2 | 4 | 0 | 3 | 1 | 4 | 0 | |||
| 3.4c.4d | 23 | 19 | 24 | 18 | 30 | 12 | |||
| DR | 0.33 | 0.04 | 0.04 | ||||||
| Mature | 10 | 5 | 12 | 3 | 14 | 1 | |||
| Immature | 17 | 14 | 15 | 16 | 20 | 11 | |||
| TB | 0.24 | 0.11 | 0.09 | ||||||
| Low | 14 | 7 | 15 | 6 | 18 | 3 | |||
| High | 13 | 12 | 12 | 13 | 16 | 9 | |||
| TILs | 0.03 | 0.01 | 0.01 | ||||||
| High | 14 | 4 | 15 | 3 | 17 | 1 | |||
| Low | 13 | 15 | 12 | 16 | 17 | 11 | |||
CAFs, cancer-associated fibroblasts; OSCC, oral squamous cell carcinoma; TSI, tumour–stromal interface; pT, pathological T; pDOI, pathological depth of invasion; pN, pathological N; DR, depth of invasion; TB, tumour budding; TILs, tumour-infiltrating lymphocytes; Ly, lymphatic invasion; V, vascular invasion; p, p-value; Pn, perineural invasion. Boldface indicates statistically significant values
Predictive factor for ENE + in resection and biopsy specimens
Bivariate analysis revealed a significant association between ENE + and c/pDOI > 10 mm and between ENE + and MMP14 expression in the tumour nest (high) and CAFs (positive); the analysis was based on the co-scoring system (high risk) in biopsies and resected specimens (all p < 0.05, Table 5). However, no association was found between ENE + and TME activity-related histological factors (DR-I, TB-H, and TILs-L).
Table 5.
Bivariate and multivariate logistic regression analyses of ENE using surgically resected and biopsy specimens
| Bivariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | SE | Odds ratio | 95% CI | p-value | |
| Resection specimens (n = 71) | |||||||
| pT3,4 | 6.466 | 1.346–31.058 | 0.011 | - | - | - | 0.230 |
| pDOI > 10 mm | 3.620 | 1.066–12.299 | 0.033 | - | - | - | 0.508 |
| Invasion pattern | 1.603 | 0.288–8.906 | 0.587 | - | - | - | - |
| DR-I | 3.046 | 0.972–9.543 | 0.050 | - | - | - | - |
| TB-H | 3.429 | 1.205–9.753 | 0.018 | - | - | - | - |
| TILs-L | 1.552 | 0.583–4.135 | 0.378 | - | - | - | - |
| MMP14 at the tumour nest (high) | 7.500 | 2.479–22.691 | < 0.001 | - | - | - | 0.058 |
| MMP14 in CAFs (positive) | 5.056 | 1.703–15.011 | < 0.01 | - | - | - | 0.536 |
| MMP14 co-scoring system (high-risk) | 8.986 | 2.921–27.642 | < 0.001 | 0.573 | 8.986 | 2.921–27.642 | < 0.001 |
| Biopsy specimens (n = 46) | |||||||
| cDOI > 10 mm | 7.200 | 0.824–62.937 | 0.046 | - | - | - | 0.183 |
| DR-I | 5.727 | 0.653–50.258 | 0.09 | - | - | - | - |
| TB-H | 2.000 | 0.458–8.725 | 0.35 | - | - | - | - |
| TILs-L | 0.250 | 0.047–1.344 | 0.09 | - | - | - | - |
| MMP14 at the tumour nest (high) | 9.091 | 1.654–49.965 | < 0.01 | - | - | - | 0.458 |
| MMP14 in CAFs (positive) | 23.400 | 2.626-209.279 | < 0.001 | - | - | - | 0.289 |
| MMP14 co-scoring system (high-risk) | 32.0000 | 4.953-206.762 | < 0.001 | 0.952 | 32.000 | 4.953-206.761 | < 0.001 |
CI, confidence interval; SE, standard error; pT, pathological T; pDOI, pathological depth of invasion; DR-I, immature desmoplastic reaction; TB-H, high tumour budding; TILs-L, low-grade tumour-infiltrating lymphocytes; CAFs, tumour-associated fibroblasts; cDOI, clinical depth of invasion. Boldface indicates statistically significant values
Multivariate logistic regression analysis revealed that the MMP14 co-scoring system was an independent factor of ENE + both in resected (odds ratio [OR] = 8.986, 95% confidence interval [CI] = 2.921−27.642; p < 0.001) and biopsy specimens (OR = 32.0, 95% CI = 4.953−206.761; p < 0.001).
Predictive value of MMP14 expression for ENE + risk factors
In the resected specimens, the MMP14 co-scoring system (high risk) showed high specificity (sensitivity, 63%; specificity, 84%; positive predictive value [PPV], 71%; NPV, 79%; accuracy, 76%; AUC = 0.735; 95% CI, 0.628–0.843; Table 6). In the biopsy specimens, the MMP14 co-scoring system (high risk) showed a high predictive value for ENE+ (sensitivity, 80%; specificity, 89%; PPV, 67%; NPV, 94%; accuracy, 87%; AUC = 0.844; 95% CI, 0.704–0.985). In addition, MMP14 expression in the tumour nest of biopsy specimens showed a high predictive value for predicting ENE+ (sensitivity, 80%; specificity, 72%; PPV, 42%; NPV, 93%; accuracy, 72%; AUC = 0.811; 95% CI, 0.688–0.934).
Table 6.
Performance of MMP14 expression as a predictor of extranodal extension
| MMP14 expression | Sensitivity | Specificity | PPN | NPV | Accuracy | AUC | 95% CI | p-value | Cohort proportion (no.; yes:no) |
|---|---|---|---|---|---|---|---|---|---|
| Surgically resected specimens | |||||||||
|
CAFs (positive) |
78% | 59% | 46% | 81% | 66% | 0.684 | 0.576–0.793 | < 0.001 | (39:32) |
| Tumour nest (high) | 78% | 68% | 60% | 83% | 72% | 0.730 | 0.6238–0.8358 | < 0.01 | (35:36) |
| Co-scoring system (high risk) | 63% | 84% | 71% | 79% | 76% | 0.735 | 0.628–0.843 | < 0.01 | (24:47) |
| Biopsy specimens | |||||||||
| CAFs (positive) | 90% | 72% | 47% | 96% | 76% | 0.811 | 0.688–0.934 | < 0.001 | (19:27) |
| tumour nest (high) | 80% | 72% | 42% | 93% | 72% | 0.747 | 0.596–0.899 | 0.01 | (19:27) |
| Co-scoring system (high-risk) | 80% | 89% | 67% | 94% | 87% | 0.844 | 0.704–0.985 | < 0.01 | (12:34) |
PPV, positive predictive value; NPV, negative predictive value; AUC, area under the roc curve; CI, confidence intervals; CAFs, cancer-associated fibroblasts. Boldface indicates statistically significant values
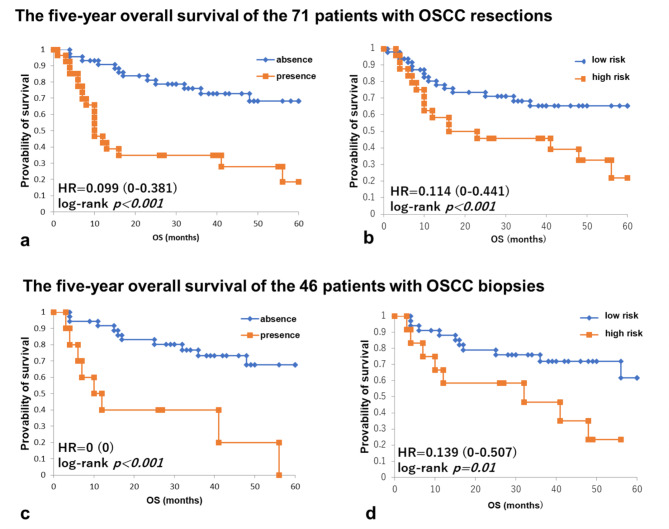
Five-year overall survival of patients with OSCC who underwent 71 resections and 46 biopsies in whom ENE was absent or present and for whom MMP14 co-scoring system indicated high or low risk
In 71 patients with surgically resected OSCC, patients with ENE had a significantly worse overall survival (OS) compared to patients without ENE (HR, 0.099, CI: 0–0.381, p < 0.001, Fig. 5a). Moreover, among the 71 patients for whom MMP14 co-scoring system was evaluated at the TSI in resection, the high-risk patients had a significantly worse OS compared to the low-risk patients (HR 0.114, CI: 0–0.441, p = 0.01, Fig. 5b).
Fig. 5.
Five-year overall survival of patients with OSCC who underwent 71 resections and 46 biopsies in whom ENE was absent or present and for whom the MMP14 co-scoring system indicated high risk or low risk
In 71 resected patients with or without ENE (a) and at high risk or low risk according to the MMP14 co-scoring system (b). In 46 patients also evaluated by biopsies with or without ENE (c) and at high risk or low risk according to the MMP14 co-scoring system (d). Notably, patients with ENE and at high-risk according to the MMP14 co-scoring system had significantly worse prognosis in both resection and biopsies
In 46 patients for whom biopsies were also evaluated, patients with ENE had a significantly worse OS than patients without ENE (HR, 0, CI: 0–0, p < 0.001, Fig. 5c). Furthermore, among the 46 patients for whom the MMP14 co-scoring system was evaluated at the TSI in biopsies, the high-risk patients had a significantly worse OS than the low-risk patients (HR, 0.139, CI: 0–0.507, p = 0.01, Fig. 5d).
Discussion
This study indicated that MMP14 expression in metastatic LNs is associated with ENE + and that MMP14 levels in tumours and CAFs at the TSI are highly concordant with the ENE sites. In biopsies and resections, associations of MMP14 expression level with ENE + and TME remodelling features were detected, along with clinicopathological metastasis and invasive features. High risk was determined by the MMP14 co-scoring system as an independent factor for ENE + and a poor prognosis factor for 5-year OS in both biopsy and resected specimens; notably, the system was highly accurate for detecting ENE + in biopsies. Moreover, MMP14 expression in tumour nests was a reliable predictor in small specimens without connective tissues. Immunohistochemical analysis of MMP14 expression level is simple, easy, and useful in assessing the ENE risk of patients with OSCC without any clinical data. Thus, the developed method can be used in routine pathological diagnoses for planning pre-operative treatment.
MMP14 overexpression in tumour nests and CAFs was more often observed at metastatic LNs with ENE than in those without ENE and at the TSI in the primary and ENE sites; the expression at these sites was similar, even in biopsies. These results indicate that MMP14 expression in tumour nests and CAFs is associated with ENE development. MMP14 localises at the surface-membrane expression in OSCC, particularly in the invasive area, and promotes extracellular matrix degradation [28–31]. In addition, MMP14 derived from tumour cells activates mesenchymal cells and CAFs in the TME [32–36]. CAF-derived MMP14 releases the matrix, promoting OSCC invasion [28–31, 37]. As enzymes and tumour cells demonstrate the most activity at the TSI [19, 22–24], it is reasonable to assume that ENE develops significantly in LNs involved in OSCC metastasis, wherein MMP14 is overexpressed. To date, no studies have assessed the expression of MMPs at the TSI and ENE sites nor the immunohistochemical expression of membranous MMP14 [38]. Considering the high CR of MMP14 at the TSI at primary and ENE sites, the MMP14 co-scoring system could predict tumour invasiveness at the ENE site by assessing it at the primary TSI.
MMP14 expression in tumour nests and CAFs in primary TSI showed a significant association with clinicopathological pENE+, TME activity-related features (TB, DR, and TILs), and invasiveness and metastatic features. MMP2 and MMP9 were also associated with pENE + but did not show any association with TME activity-related features. MMP2, 3, 9, and 14, derived from CAFs and tumour cells in OSCC, are important enzymes for metastasis [38–42]; however, their role in ENE development has not been examined. In vivo studies have revealed that MMP14 in the TME creates a suitable primary and pre-metastatic niche in the LN for tumour survival during epithelial–mesenchymal transition (EMT) [32–36], which may be attributable for the similar expression profiles of MMP14 at TSI and ENE sites. In summary, MMP14, particularly at the TSI, is the most predominant enzyme in OSCC for ENE + and metastasis.
MMP14 expression in resected specimens, particularly CAFs, was associated with TME activity and remodelling factors, namely, TB-high, DR-immature, and TILs-low [21, 23]. TB and DR are important features of EMT, which is a fundamental process for cancer metastasis at the TSI [43]. TILs are histological features relating to tumour immunity; TILs-low is associated with the presence of LN metastasis and the pN stage [15]. In combination with previous studies, our present results indicate a positive correlation between MMP14 expression at the TSI and clinicopathological features of metastasis, which may be linked to EMT, and that tumour immunity might affect the metastatic process. Therefore, a co-scoring system based on MMP14 expression in the tumour and CAFs at the TSI comprehensively demonstrates the response of the TME activity. MMP14 expression might be useful for evaluating the ENE + risk and metastatic and invasion ability.
Similar to resected specimens, in biopsies, MMP14 expression in CAFs and tumour nests and its expression based on the co-scoring system revealed high concordance with MMP14 expression at the ENE site. Furthermore, in biopsies, MMP14 expression in tumour nests and CAFs and the co-scoring system were associated with ENE + and TME activity-related features, such as DR and TILs. Supported by high CR, MMP14 expression level might be useful for indicating ENE+, even in biopsies, which indicate fewer TME components than resections.
Multivariate analysis showed that the MMP14 co-scoring system is an independent ENE-predictor for biopsy and resected specimens. Moreover, high-risk cases according to the MMP14 co-scoring system had significantly worse prognosis in both resections and biopsies. Additionally, the system is a novel and accurate method for detecting ENE and determining poor prognoses based on histology alone. This approach showed higher sensitivity and a PPV in biopsy specimens than previously used methods that evaluated DR-H/TILs-L/cDOI > 10 mm (sensitivity 70%, specificity 77%) [15]. The significant association among DR, TILs, and MMP14 co-expression may explain the high sensitivity and specificity of the MMP14 co-scoring system. A previous study that detected ENE based on immunohistochemistry using OSCC resected specimens showed high specificity; however, it required two different antibodies, was not useful for biopsies, and did not evaluate impact as a prognosis marker [44]. Moreover, in one of our approaches, MMP14 expression in tumour nests could be used for ENE prediction in biopsies without stroma; this is an extremely important feature as OSCC biopsy specimens often lack connective tissues, as suggested by the analysis of patient prognoses.
Our study had some limitations. First, the number of included cases was limited; thus, a more detailed examination with a larger sample size including LN metastasis and ENE is needed. Second, the usefulness of the MMP14 co-scoring system employing immunohistochemistry may also be limited in OSCC cases that require demineralisation treatment and patients undergoing pre-operative treatment were not examined. Third, the present study focused on MMP proteins at TSI using immunohistochemistry, which can be evaluated in general hospitals. Further study is needed, including a comprehensive analysis using a large database of genes to clarify the significance of MMP14 in ENE development from biological and clinical perspectives [45–48].
Conclusion
The MMP14 co-scoring system is a novel ENE-prediction method using biopsy specimens and applies to many OSCC cases. It is highly accurate and can be conducted using a basic, rapid pathological analysis. We strongly believe that our approach is applicable to routine pathological analysis and can be included in patient reports, thereby helping healthcare professionals determine surgical methods and plan pre-operative treatments, such as determining whether patients with OSCC require neck surgery or chemoradiotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
List of abbreviations
- AJCC
American Joint Committee on Cancer
- AUC
Area under the ROC curve
- CAFs
Cancer-associated fibroblasts
- cDOI
Clinical depth of invasion
- cENE
Clinically defined extranodal extension
- CI
Confidence interval
- DOI
Depth of invasion
- DR
Desmoplastic reaction
- DR-I
Immature desmoplastic reaction
- DR-M
Mature desmoplastic reaction
- EMT
Epithelial–mesenchymal transition, ENE:extranodal extension
- H&E
Haematoxylin and eosin
- HR
Hazard ratio
- HNSCC
Head and neck squamous cell carcinoma
- MMP
Matrix metalloproteinases
- NPV
Negative predictive value
- OR
Odds ratio
- OS
Overall survival
- OSCC
Oral squamous cell carcinoma
- pDOI
Pathological depth of invasion
- PPV
Positive predictive value
- pT
Pathological T
- SCC
Squamous cell carcinoma
- SE
Standard error
- TB
Tumour budding
- TB-H
High tumour budding
- TB-L
Low tumour budding
- TME
Tumour microenvironment
- TILs
Tumour-infiltrating lymphocytes
- TILs-L
Low-grade tumour-infiltrating lymphocytes
- TILs-H
High-grade tumour-infiltrating lymphocytes
- TSI
Tumour–stromal interface.
Authors’ contributions
All authors contributed to the study’s conception and design. YN developed the theory and performed the computations. YN and MI conceived the idea and evaluated the histological risk factors listed in this study. RY prepared immunohistochemical staining. YU, TS, TF, and HI helped with the clinical information collection. MI and KT supervised the study findings. YN wrote the first draft of the manuscript and all authors commented on the later versions. All authors have read and approved the final manuscript.
Funding
This study received no funding from any governmental or private institution.
Availability of data and materials
All data analysed during this study are included in this published article and its additional files.
Declarations
Ethics approval and consent to participate
This study was conducted following the Declaration of Helsinki principles and was approved by the Institutional Review Board of the Kansai Medical University Hospital (Approval # 2020289). Informed consent was obtained from patients using the opt-out methodology, owing to the study’s retrospective design with no new risk to the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuri Noda, Email: nodayuridesu@yahoo.co.jp.
Mitsuaki Ishida, Email: mitsuaki.ishida@gmail.com.
Ryosuke Yamaka, Email: yamakary@hirakata.kmu.ac.jp.
Yasuhiro Ueno, Email: uenoy@hirakata.kmu.ac.jp.
Tomofumi Sakagami, Email: sakegami1981@yahoo.co.jp.
Takuo Fujisawa, Email: fujisawt@hirakata.kmu.ac.jp.
Hiroshi Iwai, Email: iwai@takii.kmu.ac.jp.
Koji Tsuta, Email: tsutakoj@hirakata.kmu.ac.jp.
References
- 1.El-Naggar AK, Chan JKC. In: Grandis JR, Takata T, Grandis J, Slootweg PJ, editors. WHO classification of head and neck tumours. 4 ed. Lyon: IARC; 2017.
- 2.Shaw RJ, Lowe D, Woolgar JA, Brown JS, Vaughan ED, Evans C, et al. Extracapsular spread in oral squamous cell carcinoma. Head Neck. 2010;32:714–22. [DOI] [PubMed]
- 3.Abdel-Halim CN, Rosenberg T, Larsen SR, Høilund-Carlsen PF, Sørensen JA, Rohde M, et al. Histopathological definitions of extranodal extension: a systematic review. Head Neck Pathol. 2021;15:599–607. [DOI] [PMC free article] [PubMed]
- 4.Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. 2016;62:60–71. [DOI] [PubMed]
- 5.Greenberg JS, Fowler R, Gomez J, Mo V, Roberts D, El Naggar AK, et al. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003;97:1464–70. [DOI] [PubMed]
- 6.Coatesworth AP, MacLennan K. Squamous cell carcinoma of the upper aerodigestive tract: the prevalence of microscopic extracapsular spread and soft tissue deposits in the clinically N0 neck. Head Neck. 2002;24:258–61. [DOI] [PubMed]
- 7.Koyfman SA, Ismaila N, Crook D, D’Cruz A, Rodriguez CP, Sher DJ, et al. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol. 2019;37:1753–74. [DOI] [PMC free article] [PubMed]
- 8.Chiu K, Hosni A, Huang SH, Tong L, Xu W, Lu L, et al. The potential impact and usability of the eighth edition TNM staging classification in oral cavity cancer. Clin Oncol (R Coll Radiol). 2021;33:e442–9. [DOI] [PubMed]
- 9.Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39:130–7. [DOI] [PubMed]
- 10.Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers-major changes in the american Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–37. [DOI] [PubMed]
- 11.Best DL, Jazayeri HE, McHugh JB, Udager AM, Troost JP, Powell C et al. Extent of extranodal extension in oral cavity squamous cell carcinoma is not independently associated with overall or disease-free survival at a 2.0-mm threshold.J Oral Maxillofac Surg. 2022. [DOI] [PubMed]
- 12.Amini A, Jasem J, Jones BL, Robin TP, McDermott JD, Bhatia S, et al. Predictors of overall survival in human papillomavirus-associated oropharyngeal cancer using the National Cancer Data Base. Oral Oncol. 2016;56:1–7. [DOI] [PubMed]
- 13.Kuo P, Mehra S, Sosa JA, Roman SA, Husain ZA, Burtness BA, et al. Proposing prognostic thresholds for lymph node yield in clinically lymph node-negative and lymph node-positive cancers of the oral cavity. Cancer. 2016;122:3624–31. [DOI] [PubMed]
- 14.Saddawi-Konefka R, O’Farrell A, Faraji F, Clubb L, Allevato MM, Jensen SM, et al. Lymphatic-preserving treatment sequencing with immune checkpoint inhibition unleashes cDC1-dependent antitumor immunity in HNSCC. Nat Commun. 2022;13:4298. [DOI] [PMC free article] [PubMed]
- 15.Noda Y, Ishida M, Ueno Y, Fujisawa T, Iwai H, Tsuta K. Novel pathological predictive factors for extranodal extension in oral squamous cell carcinoma: a retrospective cohort study based on tumor budding, desmoplastic reaction, tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer. 2022;22:402. [DOI] [PMC free article] [PubMed]
- 16.Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor ’budding’ in patients with colorectal cancer. Dis Colon Rectum. 1993;36:627–35. [DOI] [PubMed]
- 17.Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299 – 311. [DOI] [PubMed]
- 18.Almangush A, Pirinen M, Heikkinen I, Mäkitie AA, Salo T, Leivo I. Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br J Cancer. 2018;118:577–86. [DOI] [PMC free article] [PubMed]
- 19.Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. Tumor budding: the name is EMT. Partial EMT. J Clin Med. 2016;5:51. [DOI] [PMC free article] [PubMed]
- 20.Heikkinen I, Bello IO, Wahab A, Hagström J, Haglund C, Coletta RD, et al. Assessment of tumor-infiltrating lymphocytes predicts the behavior of early-stage oral tongue cancer. Am J Surg Pathol. 2019;43:1392–6. [DOI] [PubMed]
- 21.Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, et al. Desmoplastic pattern at the tumor front defines poor-prognosis subtypes of colorectal cancer. Am J Surg Pathol. 2017;41:1506–12. [DOI] [PubMed]
- 22.Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral Dis. 1998;4:70–7. [DOI] [PubMed]
- 23.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018;11:dmm029447. [DOI] [PMC free article] [PubMed]
- 24.Mughees M, SenGupta A, Khowal S, Wajid S. Mechanism of tumour microenvironment in the progression and development of oral cancer. Mol Biol Rep. 2021;48:1773–86. [DOI] [PubMed]
- 25.Okazaki I, Nabeshima K, Introduction. MMPs, ADAMs/ADAMTSs research products to achieve big dream. Anti Cancer Agents Med Chem. 2012;12:688–706. [DOI] [PubMed]
- 26.Murphy G, Crabbe T, Gelatinases A. and B Methods Enzymol. 1995;248:470–84. [DOI] [PubMed]
- 27.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–5. [DOI] [PubMed]
- 28.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. [DOI] [PMC free article] [PubMed]
- 29.Caldieri G, Ayala I, Attanasio F, Buccione R. Cell and molecular biology of invadopodia. Int Rev Cell Mol Biol. 2009;275:1–34. [DOI] [PubMed]
- 30.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–206. [DOI] [PMC free article] [PubMed]
- 31.Yan T, Lin Z, Jiang J, Lu S, Chen M, Que H, et al. MMP14 regulates cell migration and invasion through epithelial-mesenchymal transition in nasopharyngeal carcinoma. Am J Transl Res. 2015;7:950–8. [PMC free article] [PubMed]
- 32.Fujita S, Sumi M, Tatsukawa E, Nagano K, Katase N. Expressions of extracellular matrix-remodeling factors in lymph nodes from oral cancer patients. Oral Dis. 2020;26:1424–31. [DOI] [PubMed]
- 33.Hu C, Huang Q, Sun Q. The regulation of lymph node pre-metastatic niche formation in head and neck squamous cell carcinoma. Front Oncol. 2022;12:852611. [DOI] [PMC free article] [PubMed]
- 34.Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. 2021;38:3. [DOI] [PubMed]
- 35.Takeuchi A, Ozawa M, Cui G, Ikuta K, Katakai T. Lymph node stromal cells: diverse meshwork structures weave functionally subdivided niches. Curr Top Microbiol Immunol. 2021;434:103–21. [DOI] [PubMed]
- 36.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. [DOI] [PMC free article] [PubMed]
- 37.Shan Q, Takabatake K, Kawai H, Oo MW, Inada Y, Sukegawa S, et al. Significance of cancer stroma for bone destruction in oral squamous cell carcinoma using different cancer stroma subtypes. Oncol Rep. 2022;47:81. [DOI] [PMC free article] [PubMed]
- 38.Miguel AFP, Mello FW, Melo G, Rivero ERC. Association between immunohistochemical expression of matrix metalloproteinases and metastasis in oral squamous cell carcinoma: systematic review and meta-analysis. Head Neck. 2020;42:569–84. [DOI] [PubMed]
- 39.Wiegand S, Dünne AA, Müller HH, Mandic R, Barth P, Davis RK, et al. Metaanalysis of the significance of matrix metalloproteinases for lymph node disease in patients with head and neck squamous cell carcinoma. Cancer. 2005;104:94–100. [DOI] [PubMed]
- 40.Kawano K, Yanagisawa S. Predictive value of laminin-5 and membrane type 1-matrix metalloproteinase expression for cervical lymph node metastasis in T1 and T2 squamous cell carcinomas of the tongue and floor of the mouth. Head Neck. 2006;28:525–33. [DOI] [PubMed]
- 41.Myoung H, Kim MJ, Hong SD, Lee JI, Lim CY, Hong SP. Expression of membrane type I-matrix metalloproteinase in oral squamous cell carcinoma. Cancer Lett. 2002;185:201–9. [DOI] [PubMed]
- 42.Kurahara S, Shinohara M, Ikebe T, Nakamura S, Beppu M, Hiraki A, et al. Expression of MMPS, MT-MMP, and TIMPs in squamous cell carcinoma of the oral cavity: correlations with tumor invasion and metastasis. Head Neck. 1999;21:627–38. [DOI] [PubMed]
- 43.Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49:361–74. [DOI] [PMC free article] [PubMed]
- 44.Dhanda J, Triantafyllou A, Liloglou T, Kalirai H, Lloyd B, Hanlon R, et al. SERPINE1 and SMA expression at the invasive front predict extracapsular spread and survival in oral squamous cell carcinoma. Br J Cancer. 2014;111:2114–21. [DOI] [PMC free article] [PubMed]
- 45.Wang J, Wang Y, Kong F, Han R, Song W, Chen D, et al. Identification of a six-gene prognostic signature for oral squamous cell carcinoma. J Cell Physiol. 2020;235:3056–68. [DOI] [PubMed]
- 46.Li H, Zhang X, Yi C, He Y, Chen X, Zhao W, et al. Ferroptosis-related gene signature predicts the prognosis in oral squamous cell carcinoma patients. BMC Cancer. 2021;21:1–16. [DOI] [PMC free article] [PubMed]
- 47.Han Y, Wang X, Xia K, Su T. A novel defined hypoxia-related gene signature to predict the prognosis of oral squamous cell carcinoma. Ann Transl Med. 2021;9:1565. [DOI] [PMC free article] [PubMed]
- 48.Lv S, Qian Z, Li J, Piao S, Li J. Identification and validation of a Hypoxia-Immune-Based prognostic mRNA signature for oral squamous cell carcinoma. J Oncol. 2022;2022:5286251. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are included in this published article and its additional files.