Abstract
With the prohibition of antibiotics in feed, plant functional substances have been widely studied as feed additives. Resveratrol, a natural stilbene, and a non-flavonoid polyphenol found in plants, possesses antioxidant, anti-inflammatory, and metabolic regulatory features. Resveratrol generated intense scientific and public interest, primarily due to its widely reported ability to prevent cancer, delay aging and alleviate related metabolic diseases. Recently, resveratrol has been studied and applied as a feed additive in animal production. This review focuses on the outline of the absorption and metabolism and biological functions of resveratrol and summarizes the application of dietary resveratrol in animal production up to the present, including pigs, poultry, and ruminants. In pigs, dietary resveratrol improved intestinal health, mitochondrial function, meat quality, and more. In poultry, studies have shown that dietary resveratrol improves growth performance and meat and egg quality and alleviates heat stress induced adverse effects. There are few studies on dietary resveratrol in ruminants; however previous studies have indicated that dietary resveratrol increases nutrient digestibility and reduces methane emissions in sheep. It is hoped that this review could provide a specific theoretical basis and research ideas for the research and application of resveratrol.
Keywords: Animal production, Biological function, Health, Pigs, Poultry, Resveratrol, Ruminants
Background
Natural product compounds have recently drawn significant attention from the scientific community for their potent effects on oxidative stress and inflammation. Resveratrol (3,4',5-trihydroxystilbene), a natural stilbene and a nonflavonoid polyphenol, possesses antioxidant [1], anti-inflammatory [2], cardioprotective [3], antimicrobial [4], and anti-cancer qualities [5]. Resveratrol is naturally found in numerous species of plants, including peanuts, grapes, pines, and berries, and assists in responding to pathogen infections. Interestingly, Chinese traditional medicine also contains it in the form of extracts such as those acquired from Polygonum cuspidatum.
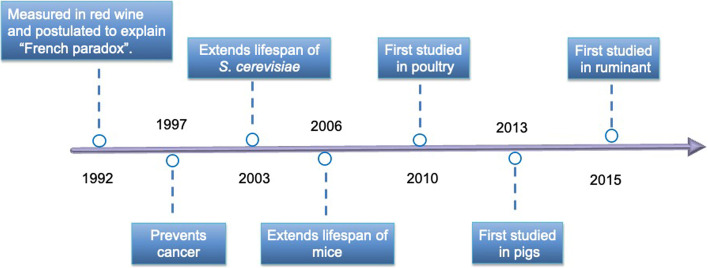
The research on this chemical started through the “French paradox”, a term generated in 1992, which describes French people with a low incidence of coronary heart diseases despite consuming a diet with high saturated fat [6, 7]. Since then, resveratrol has been broadly studied and demonstrated to have antioxidant, anti-inflammatory, antimicrobial, anticancer, and antiangiogenic effects, with those on oxidative stress possibly being the most important and underlying the others. Accumulating reports have indicated that resveratrol could prevent or slow the progression of numerous diseases, including cardiovascular disease, cancer, and aging, and improve stress resistance ability [8, 9]. The most striking effect of resveratrol is the resistance to cancer and aging. In 1997, resveratrol was shown to have cancer chemo-preventive activity in assays representing three main stages of carcinogenesis, owing to its antioxidant, antimutagen and anti-inflammatory effects [10]. In 2003, Howitz et al. [11] first, demonstrated that resveratrol is an activator of sirtuin deacetylases and could extend the lifespan of Saccharomyces cerevisiae. Then in 2006, two critical studies were successively published, and it was found that resveratrol produces changes associated with longer lifespan, including improved insulin sensitivity, peroxisome proliferators-activated receptor gamma coactivator-1 alpha (PGC-1α) activity, mitochondrial function, and motor function [12, 13]. These essential findings further drew the attention of researchers worldwide. Until now, more than 11,000 papers on resveratrol have been indexed by the Web of Science Core Collection.
Recently, researchers in the animal production area have explored the nutrition regulation effects of dietary resveratrol in animal production (Fig. 1), including pigs [14], poultry [15], and ruminants [16]. Because of the qualities of antioxidant, antimicrobial, anti-inflammatory and metabolic regulation, studies have demonstrated that dietary resveratrol has therapeutic effects on the oxidative stress and inflammation induced by early weaning [17, 18], heat stress [19, 20], mycotoxins [21] and bacterial diseases [14], and beneficial effects on growth development and product quality of animals [22]. This review will focus on the absorption and metabolism and biological functions of resveratrol and summarize the application of dietary resveratrol in animal production up to the present, including pigs, poultry, and ruminants, which may provide essential references for the application and research of resveratrol.
Fig. 1.
The important research process of resveratrol
Structure, source, and absorption of resveratrol
Structure and source
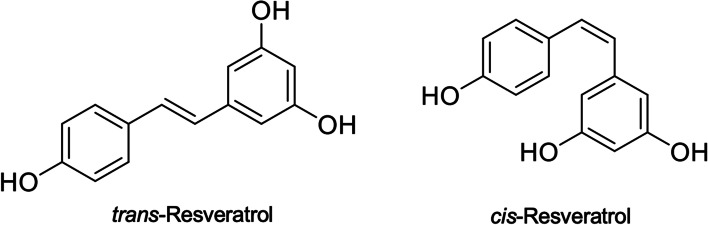
The structure of resveratrol is stilbene-based and consists of two phenolic rings connected by a styrene double bond to produce 3,4',5-trihydroxystilbene (molecular weight 228.25 g/mol), which occurs in both the trans- and cis-isoforms (Fig. 2). The chemical structure of resveratrol was characterized in 1940 by Takaoka, who isolated it from the root of the Veratrum grandiflorum [23]. Although resveratrol exists in two forms, trans-resveratrol is the predominant seen in dietary sources and supplements [23]. As a phytoalexin, resveratrol accumulation in plants is manufactured by a mechanism of resistance to parasites and other adverse conditions, like fungal infection, UV radiation, chemical substances, and generally, stressful factors for the plant [24]. More than 70 species of plants have been discovered to produce resveratrol responding stressful conditions [25]. Additionally, resveratrol is also found in some fruits, such as grapes, blueberries, blackberries, and peanuts. Red wine is the primary source of resveratrol in the Mediterranean diet, which comes from grape skin, seeds, petioles, and woody parts. Polygonum cuspidatum, plays a crucial role in Japanese and Chinese traditional medicine and is the richest source of resveratrol [26]. Furthermore, the cultivars of grapes significantly influence the resveratrol content in the skins, leaves and canes of the grapes [27, 28]. For example, Zhang et al. [28] showed that Vitis vinifera possessed a high number of trans-resveratrol levels than Vitis labrusca or the hybrids of Vitis vinifera and Vitis labrusca, while the grapes used for wine had higher content than that for the table.
Fig. 2.
The chemical structure of resveratrol
Absorption and metabolism
Despite its well-established beneficial effects, resveratrol has limited bioavailability, and the levels of this molecule found in plasma are very low [29]. The primary reason for low bioavailability of resveratrol is induced by extensive phase-II metabolism after oral administration [29, 30]. In enterocytes and hepatocytes, resveratrol is metabolized to glucuronides and sulfates through UDP-glucuronosyltransferases and sulfotransferases [30]. Resveratrol-3-O-glucuronide, resveratrol-3-O-sulfate, and resveratrol-4'-O-glucuronide were discovered as the most abundant metabolites in humans [31]. Azorín-Ortuño et al. [32] characterized the metabolic profile and pharmacokinetics of resveratrol in the plasma of pigs and identified resveratrol diglucuronide, resveratrol sulfoglucuronide isomers, resveratrol glucuronide isomer, resveratrol-3-O-glucuronide, resveratrol sulfate, and resveratrol, identifying that the most abundant metabolite is resveratrol-3-O-glucuronide. Although the systemic bioavailability of resveratrol is very low, the accumulation of resveratrol in epithelial cells along the aerodigestive tract and potentially active resveratrol metabolites may still have beneficial effects [8, 29]. Additionally, recent studies have shown that the biological effects triggered by resveratrol could be partly explained by modulating gut microbiota composition, especially regarding metabolic syndrome, oxidative stress, and inflammation [33, 34]. Some in vitro and in vivo studies have been performed to clarify the absorption, metabolism, and bioavailability of resveratrol. Intestinal absorption due to a rapid passive diffusion process observed in Caco-2 cells is estimated at 46% in isolated perfused rat small intestines, 77%–80% in vivo in rats, and at least 70% in humans [35]. In humans, resveratrol is rapidly absorbed with the plasma resveratrol concentration peaking approximately 30 min after oral consumption, and about 70%–75% of the absorption is by transepithelial diffusion [4]. Extensive metabolism in the intestine and liver causes oral bioavailability of considerably less than 1%. Henry et al. [36] reported that the uptaking of trans-resveratrol is mediated by two transport systems, including passive diffusion and active transport through sodium-dependent glucose transporter 1, and that multidrug resistance protein (MRP)2 seems to be involved in their efflux. Within enterocytes, resveratrol is rapidly metabolized to resveratrol glucuronides and resveratrol sulfates. These metabolites can also effuse back into the small intestine through MRP2, and breast cancer resistance protein 1 and resveratrol conjugates can also efflux through the basal side of the enterocytes through MRP3 [37]. After resveratrol is metabolized by intestinal epithelial cells, it can be transported to the liver and enters hepatocytes through passive and carrier-mediated transport [38]. Resveratrol and its conjugates can be recycled back to the small intestine through the bile or excreted through urine, as reviewed by Nunes et al. [37].
Biological functions of resveratrol
In this paper, the main biological functions of resveratrol are described, including the antioxidant qualities, anti-inflammatory function, and metabolic regulation function, which have been shown in animals or in vitro models.
Antioxidant
Oxidative stress is a state of imbalance in which the production of reactive oxygen species (ROS) overwhelms the intrinsic antioxidant capacity defense. ROS are highly reactive and toxic molecules generated from the respiratory chain during mitochondrial oxidative metabolism and the endoplasmic reticulum. Antioxidant system in the body can be divided into enzymatic groups, including glutathione peroxidase (GPX), superoxide dismutase (SOD), catalase (CAT), glutathione reductase, and non-enzymatic groups, including reduced glutathione (GSH), total thiols, ascorbic acid, carotenoids, and α-tocopherol. A plethora of health-beneficial effects of resveratrol occur through its antioxidant qualities. Resveratrol has been indicated to prevent and treat oxidative stress associated with different diseases, including type 2 diabetes [39], hyperglycemia [40], tissue injury [41, 42], Parkinson’s disease [43], neurodegenerative disorders [44], metabolic syndrome [45], and hazardous substance, including ethanol [46], hydrogen peroxide [47], pesticides [48], and mycotoxins [49]. The antioxidant qualities of resveratrol can be explained through its ability to either directly neutralize ROS or indirectly upregulate the expression of cellular defensive pathways and genes. Resveratrol has been shown to be a very effective scavenger of different oxidants, including superoxide anion, hydrogen peroxide, hydroxyl radical, singlet oxygen, nitrogen oxide, and peroxynitrite, associated with the presence of phenolic rings with three hydroxyl groups in positions 3, 4, and 5, and conjugated double bond, and the potential for electron delocalization in the structural molecule as reviewed by Truong et al. [1]. Additionally, extensive research has shown that resveratrol increases the activities of endogenous antioxidant enzymes including CAT, GPX, and SOD, which constitute the primary part of the enzymatic antioxidant defense system against oxidative stress in vitro or in vivo models [46, 50, 51].
As a direct antioxidant agent, resveratrol scavenges diverse ROS/reactive nitrogen species and secondary organic radicals with mechanisms of hydrogen atom transfer and sequential proton loss electron transfer, thereby protecting cellular biomolecules from oxidative damage [1]. Nuclear factor-erythroid 2-related factor 2 (NRF2) is a transcription factor that plays a crucial role in the transcriptional regulation of antioxidant response element-dependent defense genes. In vitro oxidative stress models, previous studies have demonstrated that resveratrol alleviates oxidative stress by activating/regulating the NRF2 signaling pathway in human lung epithelial cells [52], Hep G2 cells [53], PC12 cells [54], endothelial cells [55] and intestinal-epithelial cell [56]. Additionally, in vivo oxidative stress in rats [57] and mice [58], resveratrol was also shown to regulate the NRF2 pathway and prevent the oxidative state. However, resveratrol may behave as an antioxidant or pro-oxidant depending on many parameters, including the dose and microenvironment [59]. Some studies have shown that resveratrol has biphasic concentration-dependent effects, being an antioxidant at low doses and pro-oxidant at high doses [60, 61]. Further studies on the pro-oxidant activity of resveratrol are indicated.
Anti-inflammatory
The anti-inflammatory qualities of resveratrol have been shown in various animal and in vitro models and contribute to the therapeutic and alleviating effects on disease [8]. Inflammation is a series of cellular and molecular events that help defend the body against infection. Previous studies have reported that resveratrol decreases the production of pro-inflammatory cytokine and inhibits the gene expression associated with inflammation. In a DSS-induced colitis model, dietary resveratrol was shown to reduce the systemic inflammation markers, colonic mucosa prostaglandin E2, cycloxygenase (COX)-2, prostaglandin E synthase and nitric oxide levels in rats [62]. Resveratrol was shown to inhibit the proinflammatory factors interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), and IL-6 in rats [63, 64]. The significant anti-inflammatory effects of resveratrol have been observed in animal production, especially when responding to oxidative stress [65], mycotoxins [21] or heat stress [66]. Resveratrol shows its anti-inflammatory properties by regulating various pathways. COX is the enzyme in the rate-limiting step of the pathway that manufactures mediators of inflammation. Resveratrol was shown to decrease the prostaglandin E-2 production by inhibiting COX-2 expression in human fibroblast-like synoviocytes [67]. Additionally, resveratrol could inhibit COX-2 expression through upstream inhibition of the activity of nuclear factor kappa B (NF-κB) and I-κB kinase [68]. Additionally, anti-inflammatory qualities of resveratrol may also be related to mitogen-activated protein kinases (MAPK) and activator protein-1 pathway as reviewed by Meng et al. [69]. Moreover, resveratrol shows antimicrobial activity against bacteria and fungi, which are undesired in food and plant [70] and are pathogens to animals [4] and humans [71]. The antibacterial activity of resveratrol, impacted by the hydroxy groups at position 4’ [70], is associated with the bacterial species, including Gram-positive bacteria, more sensitive to the resveratrol, whereas the complex structure causes Gram-negative bacteria to show tolerance against it [71]. Previous studies have proved that resveratrol treatment has a therapeutic effect on the inflammation induced by pathogenic bacteria [14, 72]. Therefore, the underlying correlation between resveratrol’s antimicrobial and anti-inflammatory effects should be further investigated.
Metabolic regulation
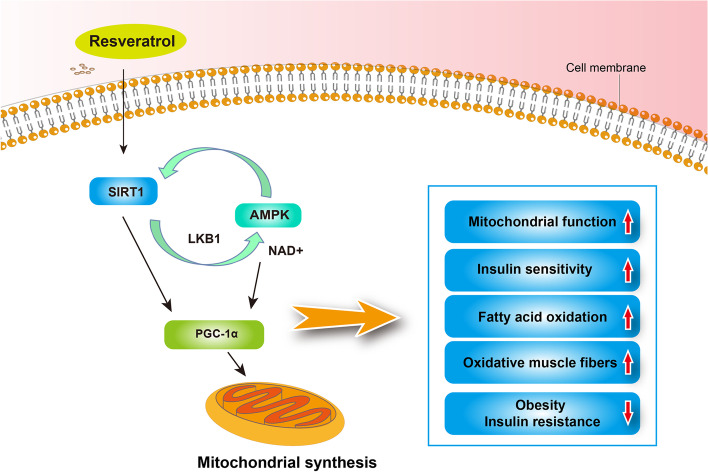
The metabolic regulation effect of resveratrol has been demonstrated, and it shows beneficial effects on metabolic syndrome and related disorders, including obesity and insulin resistance [73, 74]. The anti-obesity effect of resveratrol has been shown in different animal obesity or high-fat models and humans as indicated by reduced fat deposition and related disorders [75, 76]. In vitro, resveratrol could increase the triacylglycerol breakdown triggered by β-adrenergic activation and impair lipogenesis in human fat cells [77]. In freshly isolated rat adipocytes, resveratrol could decrease basal and insulin-induced lipogenesis from glucose and increase epinephrine-induced lipolysis [78]. Additionally, resveratrol was indicated to influence the viability of adipocytes. Bai et al. [79] reported that resveratrol inhibits pig preadipocyte proliferation and differentiation in vitro by modulation of sirtuin 1 (SIRT1). Rayalam et al. [80] also showed that resveratrol reduces adipogenesis and viability and causes apoptosis in maturing preadipocytes by down-regulating adipocyte-specific transcription factors and enzymes. Resveratrol has been shown to promote fatty acid oxidation. In 3T3-L1 adipocytes and adipocytes obtained from primary mouse embryonic fibroblasts, resveratrol could decrease triacylglycerol content and lipogenic genes, resulting in increased carnitine palmitoyl transferase 1 (a rate-limiting enzyme in fatty acid oxidation), reduced receptor interacting protein 140 (a suppressor of oxidative metabolism), and signs of enhanced flux via the fatty acid beta-oxidation pathway [81]. TNF-α is chronically elevated in the adipose tissues of obese rodents and humans and could induce the production of atherogenic adipokines. Resveratrol exerts a beneficial effect on adipocytes to prevent obesity-induced metabolic alterations through the inhibition of TNF-α-induced changes in atherogenic adipokines in vitro, including plasminogen activator inhibitor-1 and IL-6 [82]. In animal models, resveratrol attenuates insulin resistance and improves insulin sensitivity as well as metabolic complications. Notably, Baur et al. [13] discovered that resveratrol shifts the physiology of middle-aged mice on a high-calorie diet toward that of mice on a standard diet and significantly improves their survival. Resveratrol also produces changes associated with a longer lifespan, including improved insulin sensitivity, decreased insulin-like growth factor-1 levels, increased AMP-activated protein kinase (AMPK) and PGC-1α activity, increased mitochondrial number, and improved motor function [13]. Diminished mitochondrial oxidative phosphorylation and aerobic capacity are associated with decreased longevity [6]. Resveratrol is known to impact mitochondrial function. Lagouge et al. [12] reported that treatment of mice with resveratrol increased their aerobic capacity, as evidenced by their increased running time and consumption of oxygen in muscle fibers, which is because of resveratrol-mediated reduction in PGC-1α acetylation and an increase in PGC-1α activity through activating SIRT1. PGC-1α controls mitochondrial biogenesis and function, which can contribute to fiber-type switching in the muscle. They also found that resveratrol increased fast oxidative fiber percentage in the muscle of mice through regulation of PGC-1α. SIRT1, a member of the sirtuin gene family, encodes the most conserved mammalian NAD+ -dependent deacetylase enzyme responsible for removing acetyl groups from numerous proteins [83]. Price et al. [84] further showed that mice treated with resveratrol indicated increased mitochondrial biogenesis and function, AMPK activation, and increased NAD+ levels in skeletal muscle, whereas SIRT1 knockouts indicated none of these benefits, and they also found that resveratrol causes a SIRT1-dependent shift toward more oxidative muscle fibers. Calorie restrictions have the potential to extend the lifespan of model organisms and protect against aging-related diseases. In obese humans, resveratrol significantly minimizes sleeping and resting metabolic rates as well as induces metabolic changes, mimicking the effects of calorie restriction [85]. In muscle, resveratrol activates AMPK, increases SIRT1 and PGC-1α protein levels in muscle, and improves mitochondrial muscle respiration on a fatty acid-derived substrate [85]. Thus, resveratrol shows its metabolic regulation primarily though the PGC-1α, SIRT1, and AMPK pathways (Fig. 3).
Fig. 3.
The metabolic regulation effects of resveratrol via SIRT1, AMPK, and PGC-1α [12, 13, 84]. SIRT1, sirtuin 1; AMPK, AMP-activated protein kinase; PGC-1α, peroxisome proliferators-activated receptor gamma coactivator-1 alpha
Application of resveratrol in swine production
In swine production, the research on dietary resveratrol has mainly been in weaned piglets, finishing pigs, and sows, as shown in Table 1. In this section, we will review the effects of dietary resveratrol based on the growth performance, health, meat quality, and reproductive performance of the pigs.
Table 1.
Application of resveratrol in swine production
| Animal | Dosage | Stress model/ study design |
Beneficial effects | Reference |
|---|---|---|---|---|
|
Weaned piglets 21-day-old |
300 mg/kg | 7-d feeding | Increased villus height in the jejunum; Increased mitochondrial electron transport chain complex I activity, GRP78 and ATF4 protein expression level, and phosphorylation level of IRE1α in the liver | [86, 87] |
|
Weaned piglets 28-day-old |
300 mg/kg | 28-d feeding | Increased apparent digestibility of crude fat; improved villus height, digestive enzyme activities, antioxidant capacity, and intestinal barrier function; decreased m6A enrichment of tight junction protein and antioxidant enzyme-related genes in the jejunum and ileum | [88, 89] |
|
Weaned piglets 28-day-old |
150, 300 mg/kg | 42-d feeding | Increased IgG level and GPX activity while decreased MDA levels in the serum; enhanced villus and crypt morphology, and mRNA expression of ZO-1 and IL-10 in the jejunum | [17] |
|
Weaned piglets 28-day-old |
0.1, 0.33, 1.0 g/kg/d resveratrol dry suspension (3% resveratrol) |
14-d feeding | Increased peripheral blood lymphocytes, splenic lymphocytes, white cells, neutrophils, lymphocytes, and hemocytes; improved antioxidant enzyme activities, inflammatory response, and antibody levels | [90] |
|
Weaned piglets 28-day-old |
300 mg/kg | 28-d feeding | Increased IgA and IgG levels in the plasma; increased IL-10 mRNA expression and Lactobacillus copies while decreased IL-1β and TNF-α mRNA expression in the jejunum and ileum | [91] |
|
Weaned piglets 21-day-old |
300 mg/kg |
IUGR, 14-d feeding |
Increased jejunal villus height; alleviated intestinal oxidative stress by activating NRF2 pathway, inhibited enterocyte apoptosis and decreased intestinal barrier permeability; improved intestinal microbiota composition and increased butyrate production | [92] |
|
Suckling piglets 7-day-old |
80 mg/kg BW/d |
IUGR, 14-d feeding |
Decreased free fatty acid and alanine aminotransferase in serum; increased mRNA expression levels of LPL, GPX1, MCP1, and TNF-α while decreased total triglyceride, free fatty acid, protein carbonyl and MDA levels in the liver | [93] |
|
Suckling piglets 4-day-old |
600 mg/kg milk |
IUGR, 21-d feeding |
Increased CAT activity in the serum and liver; increased hepatic ATP production, and NRF1 mRNA expression | [94] |
|
Suckling piglets 7-day-old |
1 g/kg milk |
IUGR, 21-d feeding |
Increased feed efficiency; increased mitochondrial swelling, complex activities, antioxidant enzyme activities, ATP production, mitochondrial biogenesis, NAD + and NAD+/NADH ratio while decreased AMP/ATP ratio in the liver | [95] |
|
Weaned piglets 21-day-old |
300 mg/kg |
Diquat challenge, 15-d feeding |
Increased SOD activity while decreasing Chiu’s score and cell apoptosis percentage in the jejunum | [41, 96] |
|
Weaned piglets 35-day-old |
100 mg/kg |
Diquat challenge, 14-d feeding |
Increased ADG and ADFI; increased mitochondrial membrane potential, protein expression of PINK1, Parkin, LC3-II, and tight junction protein-related genes while decreased intestinal permeability and ROS production in the jejunum | [97] |
|
Weaned piglets 28-day-old |
10, 30, 90 mg/kg |
Diquat challenge, 21-d feeding |
Improved growth performance; enhanced villus and crypt morphology, antioxidant enzyme activities, and gene expression of antioxidant enzyme in the ileum and jejunum. Increased expression of antioxidant enzyme-related genes and decreased gene expression related to liver inflammation | [65, 98–100] |
|
Weaned piglet 21-day-old |
300 mg/kg |
DON challenge, 28-d feeding |
Improved growth performance; enhanced intestinal morphology, increased goblet cells, and mRNA and protein expression levels of tight junction protein while decreased pro-inflammatory factors levels and mRNA expression in the jejunum; alleviated the negative effects on mRNA and protein expression of mitophagy-related genes in the intestine of piglets challenged with DON | [21, 101] |
|
Weaned piglets 28-day-old |
300 mg/kg |
DON challenge, 28-d feeding |
Increased ADFI; increased villus height and villus height/crypt depth ratio, antioxidant enzyme activities, and mitochondrial membrane potential while decreased MDA and mitochondrial ROS levels in the jejunum | [102] |
|
Weaned piglets 28-day-old |
0.2% Respig® (containing resveratrol) |
E. coli and Salmonella enterica challenge, 28-d feeding |
Increased ADG and ADFI; increased serum IgG level; decreased fecal S. typhimurium and E. coli counts | [14] |
|
Weaned piglets 32-day-old |
3, 10, 30 mg/kg/d, resveratrol dry suspension |
Rotavirus challenge, 25-d feeding |
Increased CD4+ /CD8+ ratio; decreased diarrhea index; increased SOD and GPX while decreased MDA, TNF-α and IFN-γ levels in the serum | [103] |
|
Weaned piglets 35-day-old |
10, 30, 90 mg/kg BW |
Pseudorabies virus challenge, 29-d feeding |
Increased survival rate, BW, and serum concentrations of IFN-α, IFN-γ, TNF-α, and IL-12; decreased Pseudorabies virus copies and lesional scores of brains, lung, kidney, liver, spleen, and heart | [104] |
|
Weaned piglets 28-day-old |
150, 300 mg/kg | 42-d feeding | Increased expression of slow MyHC, SDH, and MDH activity of and type I fiber proportion; decreased LDH activity and type II fiber proportion | [105] |
| Growing-finishing pigs, 78.1 kg | 300, 600 mg/kg | 49-d feeding | Increased pH24h, a*, crude protein and myoglobin content and decreased L*, shear force, drip loss in the LM; Reduced backfat depth and visceral adipose tissue weight; Decreased PPARγ and FAS mRNA levels while increased HSL, ATGL and CPT-1 mRNA levels in the adipose tissue; decreased PPARγ, FAS, ACC, and LPL mRNA levels while increased HSL mRNA levels in the muscle | [22, 106] |
|
Growing-finishing pigs, 65.0 kg |
200, 400, 600 mg/kg feed | 41-d feeding | Increased MyHC I and MyHC IIa and decreased MyHC IIb mRNA expression; increased SDH and MDH activities in the LM; increased AdipoR1, AdipoR2, AMPK, and PGC-1α expression in LM | [107] |
| Growing pigs, 24.67 kg | 600 mg/kg | 119-d feeding | Increased intramuscular fat content and mRNA expression of PPARγ, FAS, ACC, and LPL while reduced mRNA expression of CPT-1, and PPARα in the LM; increased expression of ssc-miR-181a, ssc-miR-370, and ssc-miR-21 and reduced expression of ssc-miR-27a in the LM | [108] |
|
Newborn piglets 7-day-old |
80 mg/kg BW (7–21 days old) 300 mg/kg (21–150 days old) |
143-d feeding (NBW vs. IUGR) |
Increased GPX activity and MyHC I gene expression, reduced protein carbonyl and MDA contents, enhanced fatty acid oxidation via upregulated PPARα and targeted genes expression, thereby improving drip loss and b* | [109] |
|
Sows, average parity 4.4 |
300 mg/kg | Gestation and lactation | Increased weaning weights and ADG 7 d before and after weaning; Increased lactose content in the colostrum and fat content in the milk; alleviated weaning-associated intestinal inflammation and diarrhea and improved intestinal morphology; reduced drip loss and lactic acid level and increased pH24h in the LM of finishing offspring | [18, 110–112] |
|
Sows, average parity 3 |
300 mg/kg |
High temperatures, Gestation and lactation |
Increased the number of piglets born alive in both temperature; increased litter weight gain in high temperature; increased antioxidant ability in the plasma and colostrum in both temperature; increased the IgA, IgG, and IgM levels in colostrum under high temperature; Increased Lactobacillus and Alloprevotella and decreased Escherichia-shigella in the faeces of piglets under high temperature | [113] |
SDH succinate dehydrogenase, MDH malate dehydrogenase, LDH lactate dehydrogenase, AdipoR1 adiponectin receptor 1, PPAR peroxisome proliferator activated receptor γ, FAS fatty acid synthase, LPL lipoprotein lipase, HSL hormone sensitive lipase, AMPK AMP-activated protein kinase, PGC-1α peroxisome proliferators-activated receptor gamma coactivator-1 alpha, ACC acetyl-CoA carboxylase, ATGL adipose triglyceride lipase, CPT-1 carnitine palmitoyl transferase-1, CAT catalase, GRP78 glucose-regulated protein 78, ATF4 activating transcription factor 4, IRE1α inositol-requiring enzyme 1 alpha, Ig immunoglobulin G, GPX glutathione peroxidase, SOD superoxide dismutase, MDA malondialdehyde, ZO-1 zonula occludes 1, IL interleukin-10, TNF-α tumor necrosis factor α, NRF2 nuclear factor erythroid 2-related factor 2, GSH glutathione, MCP1 monocyte chemotactic protein 1, NAD + nicotinamide adenine dinucleotide, NADH reduced form of nicotinamide adenine dinucleotide, PINK1 PTEN-induced putative kinase 1, LC3-II microtubule-associated protein light chain 3-II, DON deoxynivalenol, ROS reactive oxygen species, IFN interferon-α, ADG average daily gain, ADFI average daily feed intake, BW body weight, NRF1 nuclear respiratory factor 1, MyHC myosin heavy chain, LM longissimus muscle, b* yellowness, a* redness, L* lightness
Growth performance
Under normal physiological conditions, most previous studies failed to observe the improved effects of dietary resveratrol on the growth performance of pigs. However, dietary resveratrol benefits the growth performance of pigs under special physiological conditions, stress, and infection status. Intrauterine growth retardation (IUGR) is usually ascribed to “uteroplacental insufficiency”, a common pregnancy complication that induces low birth weight and retards the growth development of piglets after birth. IUGR newborn piglets ingested 1.0 g resveratrol per kg of milk dry matter from 7 to 21 days of age showed improved average daily milk dry matter intake and feed efficiency. Still, dietary resveratrol failed to improve the average daily gain (ADG) of IUGR piglets [95]. Additionally, dietary resveratrol alleviates the adverse effects of oxidative stress, mycotoxin, or pathogens on growth performance in weaned piglets. In diquat-induced oxidative stress models, the supplementation of resveratrol alleviated harmful effects caused by oxidative stress, increasing the ADG and average daily feed intake (ADFI) and reduced feed to gain ratio (F/G) of weaned piglets [65, 97]. Deoxynivalenol (DON), a significant mycotoxin found in food crops and livestock feed, usually induces oxidative stress, inflammation, and growth inhibition. In a recent study, Qiu et al. [21] found that dietary resveratrol increased the final body weight (BW) and ADG, decreased F/G and tended to increase the ADFI of weaned piglets fed normal and DON diets (3.8 mg DON/kg diet). However, the study by Hong et al. [102] failed to observe ADG and F/G of piglets after the DON challenge but found that dietary resveratrol increased the ADFI of DON-challenged piglets. For pathogens, supplementation of additive based on resveratrol increased ADG and ADFI in a piglet model infected with E. coli and Salmonella enterica, while the increased survival rate and BW were observed when the resveratrol was supplemented at 10–90 mg/kg BW in a weaned piglet model infected with Pseudorabies virus [14, 104]. These studies reported that dietary resveratrol supplementation is conducive for piglets to resist adverse stress factors and infection status and plays a beneficial role in growth performance.
Meat quality and fat deposition
In pigs, previous studies have discovered that dietary resveratrol showed significant improvement in meat quality. Zhang et al. [22] reported that dietary resveratrol increased the pH24h and meat color redness (a*) and reduced the meat color lightness (L*), shear force, and drip loss of longissimus muscle. IUGR, which always causes to abnormal growth and metabolism and reduces meat quality [114]. In a recent study, Cheng et al. [109] discovered that dietary resveratrol supplementation in IUGR pigs from the sucking period to the marked age reduced the drip loss at 24 h and tended to reduce the drip loss at 48 h and yellowness (b*) in the longissimus muscle. Meat purchasing decisions are influenced by color more than any other quality factor because consumers use discoloration as an indicator of freshness and wholesomeness [115]. Usually, meat with a high drip loss percentage has an unattractive appearance and therefore has low consumer acceptance, which leads to loss of sales [116]. Thus, the beneficial effects of resveratrol on the meat quality have certain economic value. The meat quality of animals is closely related to muscle fiber type and oxidative stress state. The increased effects of resveratrol on the meat quality of pigs are majorly due to its antioxidant property and muscle fiber-regulating abilities. The antioxidant function of dietary resveratrol in muscle has been shown, as indicated by the improved antioxidant enzyme activities, and related gene expression and reduced oxidative stress markers, including malondialdehyde (MDA) and protein carbonyl [22, 109]. Notably, the muscle fiber-regulating qualities of dietary resveratrol were found in pigs. Muscle fiber type, which is often defined using the isoforms of the myosin heavy chain (MyHC), was closely related to postmortem metabolic rate and meat quality traits. According to the main MyHC isoforms found in adult mammalian skeletal muscles, four single MyHC isoforms have been identified in ATPase-based fiber types: MyHC I in slow-oxidative type I, MyHC IIa in fast oxido-glycolytic type IIa, MyHC IIb in fast glycolytic type IIb and MyHC IIx in fast glycolytic type IIx [117, 118]. Previous studies have demonstrated that higher proportion of IIb fibers causes poor quality of pork and is related to higher L* and lower water-holding capacity and increased the rate and extent of postmortem pH decline [119, 120]. In weaned piglets, a recent study discovered that dietary resveratrol supplementation increased the expression of slow MyHC and the proportion of type I fiber, and reduced the proportion of type II fiber, proposing that resveratrol promotes muscle fiber-type transformation from type II to type I in piglets [105]. In growing-finishing pigs, dietary resveratrol also increases the mRNA expression of MyHC IIa and reduces the mRNA expression of MyHC IIb, with reduced myofiber cross-sectional area of the longissimus muscle. Our previous studies found that maternal dietary resveratrol supplementation increased the mRNA and protein expression of MyHC I and reduced the mRNA and protein expression of MyHC IIb in the longissimus muscle of finishing pigs. The drip loss and lactic acid level were reduced, and the pH24h of longissimus muscle was increased by maternal dietary resveratrol supplementation [111]. Type IIb fibers have a more glycolytic potential than other types of fibers. Additionally, previous studies have shown that dietary resveratrol in rats increased the ratio of oxidative to glycolytic type muscle fibers and reduced MyHC IIb expression [12]. The reduced lactate content and glycolytic potential in the longissimus muscle of pigs and reduced lactate dehydrogenase activity in mouse C2C12 myotubes induced by dietary resveratrol were also observed [22]. The mechanism of muscle fiber-regulating qualities of dietary resveratrol may be because of the regulation of the mitochondrial function and AMPK/SIRT1/PGC-1α pathway [12, 105, 121].
In pigs, resveratrol supplementation in the diets of growing-finishing pigs were shown to decrease the backfat depth at the first rib and the last lumbar vertebra and average backfat depth [22]. Similarly, Zhang et al. [106] reported that resveratrol supplementation reduced the levels of triacylglycerol, total cholesterol, and leptin in serum and the weight of visceral adipose tissue of pigs. Additionally, they also found that the mRNA expression of peroxisome proliferator activated receptor (PPAR)γ and fatty acid synthase (FAS), and the activities of FAS and lipoprotein lipase (LPL) were reduced by resveratrol, while the mRNA expression of hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL) and carnitine palmitoyl transferase-1 (CPT-1), and activities of HSL and CPT-1 were increased by resveratrol [106]. In vitro, resveratrol was found to reduce lipid accumulation and no-esterified fatty acid release in porcine preadipocytes, with increased SIRT1 expression [122]. ATGL is an essential triglyceride hydrolase that promotes the catabolism of stored fat in adipose tissues, which catalyzes the initial step in triglyceride hydrolysis in adipocyte lipids.In vitro exposure of cultured adipocytes to resveratrol for 24 h increased the mRNA levels of ATGL and reduced the lipid accumulation [123]. Interestingly, long-term dietary resveratrol supplementation (119-d feeding) increased intramuscular fat content in pigs; moreover, dietary resveratrol upregulated the mRNA expression of PPARγ, FAS, acetyl-CoA carboxylase (ACC), and LPL and downregulated the mRNA expression of CPT-1, SIRT1, and PPARα in longissimus muscle, which may be related to enhanced expression of ssc-miR-181a, ssc-miR-370, and ssc-miR-21 and reduced expression of ssc-miR-27a [108]. Similarly, another study performed by Cheng et al. [109] explored the long-term influence of dietary resveratrol from the sucking period (7 d) to slaughter (150 d) on normal birth weight (NBW), and IUGR pigs observed a numerical increase in total triglycerides content in longissimus muscle of NBW pigs (159.21 μmol/g protein vs. 174.94 μmol/g protein), but no significant difference was found. However, they found a significant interaction between dietary resveratrol and birth weight, which indicated that dietary resveratrol reduced the total triglycerides content in the longissimus muscle of IUGR pigs, which is vary in NBW pigs and showed that the regulatory effect of dietary resveratrol on fat deposition in muscle vary between NBW pigs and IUGR pigs [109]. It seems that a tissue-specific manner of dietary resveratrol on fat deposition in muscle and adipose tissue of pigs existed. But the underlying molecular mechanisms of dietary resveratrol on fat deposition need further studies.
Health
Intestinal morphology and barriers
Intestinal health is critical for the growth and development of weaned piglets with immature digestive systems [124]. A previous study reported that dietary supplementation of resveratrol increased the villus height, villus height/crypt depth ratio, and the mRNA expression levels of zonula occludens 1 (ZO-1) and IL-10 and reduced the crypt depth in the jejunum of weaned piglets, and the supplementation concentration at 300 mg/kg was more effective than that at 150 mg/kg [17]. Gan et al. [91] also discovered that resveratrol supplementation reduced IL-1β and TNF-α levels and mRNA expression levels of IL-1β, TNF-α, and Toll-like receptor 4 (TLR4) and increased IL-10 mRNA expression levels and Lactobacillus copies in the jejunum and ileum of weaned piglets. Furthermore, resveratrol supplementation could reduce diamine oxidase (DAO) and D-lactate contents, increase the antioxidant activity and mRNA expression levels of antioxidant enzymes CAT and SOD1 and tight junction protein Occludin (OCLN) and Claudin 1 (CLDN1) in the jejunum and ileum, and this study also showed that methylations of ZO-1, heme oxygenase 1, OCLN, and CLDN1 are essential for the resveratrol to have positive effects on intestinal health [88]. For IUGR piglets, resveratrol supplementation increases the villus height and elevates the antioxidant capacity by activating the NRF2 pathway and decreases the intestinal permeability and apoptosis index in the jejunum [92]. Meanwhile, the shift in gut microbiota was also observed when resveratrol supplementation at 300 mg/kg in the diet of IUGR piglets, including enrichment of Bacteroidetes, Provotella, Faecalibaterium and Parabacterioides and reduction in Proteobacteria, Escherichia and Actinobacillus [92].
The antioxidant potential of resveratrol may be the essential reason for its regulation of intestinal health. The intestinal protective effect of dietary resveratrol was shown in oxidative stress models of piglets. For example, resveratrol supplementation at a 100 mg/kg concentration after diquat treatment for 13 d could decrease the accumulation of MDA, hydrogen peroxide, and ROS and increase the total antioxidant capacity (T-AOC) in the jejunum of weaned piglets [97, 125]. Resveratrol supplementation at a 300 mg/kg concentration for 14 d before diquat treatment improved the jejunal SOD activity and reduced cell apoptosis percentage in the jejunum without alleviating the adverse effects induced by diquat on intestinal morphology and tight junction protein expression, showing that more time might be necessary for resveratrol to show the beneficial effects [41]. Resveratrol supplementation at 10–90 mg/kg concentrations for 14 d before and for 6 d after diquat treatment effectively improved mRNA and protein expression levels of tight junction proteins, alleviated oxidative stress and inflammation by activating NRF2/aryl hydrocarbon receptor pathways, increased villus height and villus height/crypt depth ratio in the jejunum, and reduced DAO and D-lactate levels in the plasma of weaned piglets [98, 99]. Therefore, the effectiveness of resveratrol on the intestinal health of piglets suffering oxidative stress caused by diquat might depend on the supplementation phase, and resveratrol supplementation before and after diquat treatment seems to be more effective. Additionally, DON, a toxic metabolite produced by Fusarium graminearum, induces severe oxidative stress, which shows toxicity against piglets [126]. Resveratrol supplementation elevated the jejunal mRNA and protein expression levels of ZO-1 and OCLN, increased villus height, villus height/crypt depth ratio, and goblet cells, and reduced serum D-lactate and DAO levels when the 21 d weaned piglets were exposed to DON [21]. In contrast, the enhancement of jejunal morphology, mitochondrial potential, and antioxidant enzyme activity with no effect on barrier permeability was observed when DON-exposed piglets weaned on d 28 were treated using resveratrol supplementation at the same concentration. This difference might be from the various physiological conditions of piglets weaned on d 21 and 28 and the various application treatment levels of DON, which should be further investigated. For the inflammatory response caused by DON, resveratrol supplementation in the diets of piglets weaned on d 21 and 28 could decrease the mRNA expression of proinflammatory factors, reducing the adverse effects on intestinal health [21, 102]. Additionally, in the diets of weaned pigs, resveratrol supplementation could alleviate the mitochondria damage in the ileum caused by DON and minimize mitophagy-related gene expression [101].
Mitochondrial function
Redox status equilibrium is mediated by ROS production and antioxidant system [127]. The mitochondrial involves the regulation of redox homeostasis, and resveratrol potentially benefits the mitochondrial function [128]. For example, resveratrol supplementation at a concentration of 300 mg/kg diet of early weaning piglets enhanced hepatic mitochondrial electron transport chain complex I activity [87]. Furthermore, previous studies showed that resveratrol supplementation promoted hepatic mitochondrial biogenesis and ATP production in the suckling IUGR piglets, enhancing the energy metabolism [93, 94]. Zhang et al. [95] also reported that resveratrol supplementation in artificial milk of the suckling IUGR piglets improved the hepatic mitochondrial biogenesis by mediating the SIRT1/PGC-1α axis, improved ATP production by upregulating the mRNA expression of ATP synthase alpha subunit and ATP synthase beta polypeptide and improved the activity of complexes III. The improvement of phosphorylation of AMPK and liver kinase B1, mRNA level of acyl-CoA synthetase long-chain family member 1, CPT-1A and PPARα also contributed to mitochondrial fatty acid oxidation stimulated by resveratrol in the liver of the suckling IUGR piglets [95]. In some oxidative stress models, resveratrol supplementation could maintain normal mitochondrial function. For example, the resveratrol treatment could improve the mitochondrial structure and function with a decrease in ROS production and increase in membrane potential, mitochondrial DNA content, and electron transport chain complex I-IV activities by mitophagy improvement in an oxidative stress model of piglets induced by diquat [97]. However, dietary resveratrol could not affect hepatic redox status, mitochondrial biogenesis, and complex activity when the resveratrol was added for 14 d before the oxidative stress treatment [41, 96].
Inflammation and immunity
The host immunity system, comprising the innate and adaptive immune systems, is a critical barrier against pathogen invasion [129]. Resveratrol has been proven to exhibit immunoregulation capability. For example, a previous study showed that supplementation of dry suspension containing resveratrol stimulated the proliferation of peripheral blood and splenic lymphocytes, enhanced the host immune responses of classical swine fever and foot-and-mouth disease vaccines, promoted immunoglobulin (Ig)G production, regulated the release of interferon (IFN)-γ, and downregulated the release of TNF-α [90]. Pathogenic bacteria and virus infection can stimulate the cell, activating the immune system and releasing inflammatory signals [130, 131]. Ahmed et al. [14] showed that dietary resveratrol improved the IgG content in weaned piglets challenged with Salmonella typhimurium and E. coli. In vitro resveratrol alleviated E. coli K88 infection-induced damage, including decreased cell viability, reduced tight junctions, mitochondrial dysfunction, and autophagy in the porcine intestinal epithelial cell by activating SIRT1 signaling [132]. For viruses, resveratrol treatment relieved the inflammation response by inhibiting the TNF-α production and inhibited rotavirus infection by enhancing the IFN-γ content and CD4+/CD8+ ratio, decreasing the diarrhea index [103]. Pseudorabies virus infection results in severe disruption of the pig production and massive economic loss [133]. Zhao et al. [104] showed that resveratrol treatment for 7 d before and for 21 d after Pseudorabies virus infection could reduce the lesional scores of organs (brain, lung, kidney, liver, spleen and heart), increase the levels of TNF-α, IFN-α, IFN-γ, and IL-12 of the serum in weaned piglets, decreasing the inflammation, viral reproduction and lethality.
Reproductive performance and maternal regulation
Because of its remarkable antioxidant, anti-inflammatory, and metabolic regulation features, resveratrol has been indicated as a therapeutic agent for pregnancy complications in rats, mice, and Japanese macaque models of complicated pregnancy (maternal dietary manipulations, gestational diabetes and maternal hypoxia) and shows beneficial effects on maternal, embryo and offspring (as reviewed in [134, 135]). Meng et al. [110] reported that dietary resveratrol supplementation in sows’ diets during gestation and lactation increases the antioxidant status of sows and piglets and regulates the antioxidant gene expression and Kelch-like ECH-associated protein 1 (KEAP1)-NRF2 pathway in the placenta. They also discovered that maternal dietary resveratrol increases the weaning weight and litter weaning weight of offspring [110]. Similarly, a recent study also reported that dietary resveratrol increases the litter weight gain at high summer temperature and increases the number of piglets born alive at both high temperature and moderate temperature [113]. The improved effects of dietary resveratrol on the milk were observed, including the increased lactose and fat contents and increased IgA, IgG, and IgM contents [112, 113]. Furthermore, Meng et al. [18] discovered that maternal dietary resveratrol increases the ADG of piglets during weaning and alleviates weaning-associated diarrhea and intestinal inflammation in porcine offspring during weaning and postweaning, possibly owing to the increased proportion of butyrate-producing bacteria in piglets. Zhao et al. [113] also observed that maternal dietary resveratrol increases the abundances of Lactobacillus and Alloprevotella and decreases the abundance of Escherichia-shigella in the faces of piglets under high summer temperature. Additionally, Sun et al. [112] showed that maternal dietary resveratrol improved the enzyme activity and gene expression related to lipolysis, fatty acid uptake from circulating triacylglycerols and lipogenesis in the adipose tissue of weaning piglets, which indicated that the fat metabolism of weaning offspring was improved by resveratrol. In the continuity study, the same batch of weaning piglets was fed until the finish stage. The backfat thickness of finishing pigs was increased by maternal dietary resveratrol supplementation [111]. The beneficial effects of resveratrol have been demonstrated to be linked with epigenetic regulatory regulation [136]. Previous studies found that maternal dietary resveratrol showed beneficial regulatory effects on offspring via epigenetic modification [137]. Thus, the maternal effects of dietary resveratrol in pigs may mediate through epigenetic mechanisms, which need to be further studied in the future.
Application of resveratrol in poultry production
In poultry production, the effects of dietary resveratrol have been studied in quails, ducks, broilers, and hens, as indicated in Table 2. In this section, the effects of dietary resveratrol on the growth performance, meat and egg quality and health state of poultry will be reviewed.
Table 2.
Application of resveratrol in poultry production
| Animal | Dosage | Stress model/ study design |
Main beneficial effects | Reference |
|---|---|---|---|---|
|
Quails 5-week-old |
200, 400 mg | 12-week feeding | Exhibited a linear decrease in serum and egg yolk MDA and a linear increase in serum vitamin E | [15] |
|
Laying hens 60-week-old |
0.5, 1.0, 2.0, and 4.0 g/kg | 8-week feeding | Reduced FCR; improved Haugh unit and albumen height of eggs and decreased contents cholesterol in the yolk | [138] |
|
Laying hens 210-day-old |
200, 400, and 800 mg/kg | 1-month feeding | Increased egg production rate and ADFI and decreased feed-to-egg ratio; reduced egg cholesterol content; extended egg shelf life and improved egg sensory scores and yolk index | [139] |
|
Ducks 1-day-old |
150, 300, and 450 mg/kg | 21-d feeding | Improved meat quality in leg muscle, including meat color, pH, drip loss and shear force | [140] |
|
Broilers 21-day-old |
400 mg/kg | 21-d feeding | Decreased L*, pH decline, drip loss, and lactate content in pectoralis major muscle; increased antioxidant state and PGC-1α and nuclear NRF1 mRNA expression and citrate synthase activity | [141] |
|
Broilers 21-day-old |
400 mg/kg |
Transport stress, 21-d feeding |
Improved feed conversion ratio and tended to improve final BW; increased glycogen content, LDH activity, and drip loss in the muscle of transported broilers | [142] |
|
Broilers 1-day-old |
300, 600 mg/kg |
E. coli, 42-d feeding |
Increased ADG and decreased FCR of broilers challenged with E. coli; increased villus length to crypt depth and decreased E. coli number in challenged chickens | [143] |
|
Ducks 1-day-old |
500 mg/kg |
Aflatoxin B1, 42-d feeding |
Increase II-phase enzyme activity, activate NRF2 signal pathway, and protect oxidative stress and inflammatory reaction in the liver induced by AFB1 | [144] |
|
Ducks 1-day-old |
500 mg/kg |
Aflatoxin B1, 70-d feeding |
Alleviated ileum injury induced by AFB1, decreased the production of AFB1-DNA adducts and reduced DNA damage and oxidative stress via NRF2/KEAP1 and NF-κB/NLRP3 signaling pathways | [145] |
|
Chicken 1-day-old |
200, 400 and 800 mg/kg |
Conventional vaccinations, 40-d feeding |
Increased ADG and antibody titers against Newcastle disease virus and avian influenza viruses H5 and H9 | [146] |
|
Ducks 1-day-old |
400 mg/kg |
LPS treatment, 28-d feeding |
Improved final BW and ADG before LPS treatment; alleviated LPS-induced inflammatory response in liver and intestine and increased tight junction protein expression | [147, 148] |
|
Ducks 1-day-old |
300, 400 and 500 mg/kg | 70-d feeding | Increased pH and reduced MDA, carbonyl contents, and shear force, thereby improving water mobility and distribution, drip loss and cooking loss of duck meat; inhibited the formation of carbonyl and dityrosine and reduced the loss of sulfhydryl in frozen-thawed duck breast meat | [149, 150] |
|
Quails 55-day-old |
200, 400 mg/kg |
Heat stress, 12-week feeding |
Increased ADFI and egg production; increased hepatic SOD, CAT, and GPX activities as well as NRF2 expression, decreased hepatic MDA concentrations and Hsp70, Hsp90, and NF-κB expressions | [151] |
|
Black-boned chickens 42-day-old |
200, 400 and 600 mg/kg |
Heat stress, 15-d feeding |
Increased feed intake and BW gain; improved villus morphology, numbers of goblet cells and lymphocytes and regulated heat shock protein expression in the thymus and intestine | [19, 152] |
|
Broilers 21-day-old |
400 mg/kg |
Heat stress, 21-d feeding |
Increased final BW of heat-stressed broilers; decreased crypt depth and E. coli populations, and increased villus height, goblet cells numbers, populations of Lactobacillus and Bifidobacterium; Increased a*, pH24h, and decreased L*, drip loss and muscle MDA content of heat-stress broilers | [20, 153] |
|
Broilers 28-day-old |
200, 350, and 500 mg/kg |
Heat stress, 14-d feeding |
Improved ADG and decreased rectal temperature of heat-stressed broilers; lowered contents of corticosterone and adrenocorticotropic hormone | [154] |
|
Broilers 28-day-old |
500 mg/kg |
Heat stress, 14-d feeding |
Increased ADFI and ADG under heat stress; decreased crypt depth, increased villus height; increased gene expression related to gut barrier and decreased gene expression related to inflammation; improved immune organ index and inhibited the NF-κB, MAPK, and PI3K/AKT signaling pathway in spleen | [66, 155] |
|
Broilers 21-day-old |
400 mg/kg |
Heat stress, 21-d feeding |
Increased BW and ADG under heat stress; increased relative jejunum weight, relative length of ileum, jejunal villus height, activities of GPX and GST, and mRNA levels of NRF2 and SOD1 under heat stress | [156] |
|
Ducks 60-day-old |
400 mg/kg |
Heat stress, 15-d feeding |
Increased the ratio of villus height to crypt depth and the number of goblet cells and reduced the histopathological damage of jejunum; activated SIRT1-NRF1/NRF2 signaling pathways, improved ATP level of jejunum | [157] |
|
Broilers 28-day-old |
500 mg/kg |
Heat stress, 14-d feeding |
Decreased FCR under heat stress; decreased levels of corticosterone and adrenocorticotropic hormone caused and reduced heat stress-induced apoptotic cells and apoptosis genes | [158] |
MDA malondialdehyde, PGC-1α peroxisome proliferators-activated receptor gamma coactivator-1 alpha, ADFI average daily feed intake, ADG average daily gain, FCR feed conversion ratio, BW body weight, NRF1 nuclear respiratory factor 1, CAT catalase, GPX glutathion peroxidase, NRF2 nuclear factor erythroid 2-related factor 2, NF-κB nuclear factor κB, KEAP1 kelch-like ECH-associated protein 1, SOD superoxide dismutase, MAPK mitogen-activated protein kinases, LPS lipopolysaccharides, GST glutathione S-transferase, SIRT1 sirtuin 1, LDH lactate dehydrogenase, AFB1 aflatoxin B1, Hsp heat-shock protein 70, PI3K phosphoinositide-3 kinases, AKT protein kinase B, NLRP3 NOD-like receptor protein 3, a* redness, L* lightness
Growth performance
In poultry, accumulating studies have explored the impacts of dietary resveratrol supplementation on the growth performance of broilers, ducks, and quails. In broilers, Zhang et al. [142] discovered that supplementation of 400 mg/kg resveratrol increased the final BW and reduced the feed conversion ratios (FCR) of broilers. Feng et al. [138] explored the influence of various levels of dietary resveratrol (0, 0.5, 1.0, 2.0, and 4.0 g/kg) on the laying hens, discovering that dietary 2.0 g/kg of resveratrol decreased the FCR. In ducks, Yang et al. [147] reported that dietary 400 mg/kg resveratrol increased the final BW and ADG after 28 days of feeding. Additionally, dietary resveratrol exhibits obvious improved effects on the growth performance of poultry in some adverse factor challenges and stress models. Mohebodini et al. [143] studied the effects of dietary resveratrol supplementation in broiler chickens challenged with E. coli and discovered that dietary resveratrol increased BW gain and reduced the FCR of broiler chickens. They also discovered that dietary 600 mg/kg resveratrol has a similar effect to colistin sulfate, and both resulted in similar growth performance to that of the unchallenged broiler chickens. The previous study also explored the effects of dietary resveratrol (0, 200, 400, and 800 mg/kg) on chickens who received conventional vaccinations and discovered that the ADG of chickens quadratically increased with increasing resveratrol supplementation [146]. Heat stress is considered the most significant challenge in poultry production worldwide, always causing increased mortality and reduced growth performance. In poultry, supplementation of resveratrol in diets increases the feed intake [19, 66, 151], ADG [19, 66, 154, 156], and BW [20, 156] and minimizes FCR [158], which shows that dietary resveratrol could effectively alleviate growth inhibition caused by heat stress.
Meat quality and egg quality
Similar to pigs, dietary resveratrol increases the meat quality of poultry. In Pekin ducks, Yu et al. [140] studied the influence of four levels of resveratrol (0, 150, 300, and 450 mg/kg) on the meat quality and reported that dietary resveratrol increased the a*24h and b*24h of breast muscle and a*45min of leg muscle, also reduced shear force, and L*45min of breast muscle and drip loss, shear force, and L*45min of leg muscle. Similarly, Jin et al. [149] also note that dietary resveratrol improves the meat quality of ducks, as demonstrated by increased post-slaughter pH, and decreased shear force, drip loss, and cooking loss, which may be because of the enhanced antioxidant capacity and inhibited lipid and protein oxidation. In broilers, dietary resveratrol decreased L*45min, pH decline, drip loss, and MDA content, and increased the T-AOC and CAT activity in the pectoralis major muscle [141]. Moreover, increased PGC-1α and nuclear respiratory factor 1 (NRF1) mRNA expression and citrate synthase activity were found in the broilers’ muscle [141]. Moreover, dietary resveratrol improves the meat quality of broilers under heat stress. Zhang et al. [153] reported that dietary resveratrol supplementation in broilers alleviated heat stress induced reduction in meat quality, increased the a*, pH24h, T-AOC and CAT activities, and reduced the L*24h, drip loss, and MDA content in muscle.
Additionally, the egg quality was observed to be regulated by dietary resveratrol. Sahin et al. [15] discovered that egg yolk MDA concentration reduced linearly in response to increasing dietary resveratrol level, which may prolong shelf life and benefit consumers; Moreover, the Haugh unit tended to be increased, and the diameter and width of yolk were linearly reduced by dietary resveratrol supplementation [15]. Feng et al. [138] also found that dietary 2.0 g/kg of resveratrol improved the egg quality with increased Haugh unit and albumen height and reduced egg yolk cholesterol. Zhang et al. [139] discovered that 200 mg/kg resveratrol increased egg yield and reduced the feed to egg ratio of laying hens, whereas 400 mg/kg dose was associated with better lipid metabolism (reduced serum cholesterol and triglycerides), decreased egg cholesterol content, extended egg shelf life and improved yolk index and egg sensory scores. Haugh unit and albumen height are important indicators for evaluating freshness and quality of eggs [159]. These results supported that dietary resveratrol supplementation could be beneficial to laying hens for extending the shelf life and reducing the cholesterol of the eggs.
Health
Immune function
Commercial poultry is vaccinated routinely to protect the birds against infection or disease caused by many pathogens to decrease the mortality rate and infection susceptibility. In chickens under normal conventional vaccinations, dietary resveratrol decreases inflammation-related genes in the liver and spleen and increase CD4+ cell and CD4+/CD8+ ratio in peripheral blood and IgM content in serum [146]. Lipopolysaccharides (LPS) are endotoxins, hazardous and toxic inflammatory stimulators released from the outer membrane of Gram-negative bacteria. In poultry, dietary resveratrol was shown to alleviate the inflammatory reactions induced by LPS in vitro and in vivo. Chicken peripheral blood lymphocytes treated with resveratrol showed reduction in the activity of NF-κB, levels of TNF-α and ROS and apoptosis-related protein expression, increased the viability of lymphocytes and reduced the apoptotic rate after continuous stimulation by LPS [160]. In ducks, a recent study reported that dietary resveratrol alleviated LPS-induced inflammatory response with a decrease in the levels of IL-1β and IL-6 in the plasma and liver through TLR4/NF-κB signaling pathways [147]. Yang et al. [148] also discovered that dietary resveratrol could alleviate LPS-induced intestinal dysfunction and increase mRNA levels of CLDN1, OCLN, ZO-1 and protein expression of CLDN1, which may be related to the regulation of TLR4/NF-κB signaling pathway and its downstream genes.
Health-related to heat stress
Heat stress has strong adverse effects on the growth performance, intestinal morphology, mortality, and welfare of broilers, which can be alleviated by nutrition regulation. Because of its antioxidant and anti-inflammatory qualities, dietary resveratrol alleviates heat stress in quails [151], chickens [19, 152], broilers [20, 66, 153–156, 158], and ducks [157]. Previous studies discovered that dietary resveratrol increased SOD, CAT, and GPX enzyme activity and GSH content and reduced MDA levels in the liver and serum in heat-stressed chickens [19, 151]. Additionally, hepatic NRF2 protein expression in heat-stressed quails was increased, and heat shock protein (Hsp)70, Hsp90, and NF-κB protein expressions were reduced with increasing resveratrol supplementation levels [151]. In chickens, heat stress-induced overexpression of Hsp27, Hsp70, and Hsp90 mRNA in the bursa of Fabritius and spleen was attenuated, and low expression of Hsp27 and Hsp90 mRNA in the thymus upon heat stress was increased by dietary resveratrol [19]. In the spleen of broilers, dietary resveratrol reduced heat stress-induced apoptotic cells number and mRNA expression levels of genes involved in apoptosis induced by heat stress, including B-cell lymphoma-2, apoptotic protease activating factor-1, and murine double minute 2 [158]. He et al. [154] and Meng et al. [158] observed that dietary resveratrol reduced rectal temperature and lowered the contents of corticosterone and adrenocorticotropic hormone in heat-stressed broilers, showing that the stress state was alleviated by dietary resveratrol.
Additionally, heat stress usually causes impaired intestinal health in poultry. Recent research found that dietary resveratrol supplementation shows beneficial improvements in the intestinal health of heat-stressed poultry, as shown in intestinal morphology, oxidative stress, and inflammatory state, and intestinal barrier function. In intestinal morphology, studies have reported that dietary resveratrol increased the numbers of goblet cells and lymphocytes, villus height, and villus height/crypt depth ratio and decreased the crypt depth and histopathological damage in the intestine of heat-stressed poultry [20, 152, 157]. The increased mRNA expression of mucin glycoproteins 2, secreted immunoglobulin, OCLN, and CLDN1 in the intestine of heat-stressed broilers was also observed [66], indicating that gut barrier function was improved by dietary resveratrol. The heat shock protein expression in the gut of heat-stressed poultry was also regulated by dietary resveratrol, including Hsp60, Hsp70, and Hsp90 [66, 152, 157]. The antioxidant and anti-inflammatory qualities of resveratrol mainly contribute to the beneficial roles of heat stress, which has been shown by the regulation of related pathways and genes. Yang et al. [157] found that dietary resveratrol activated the SIRT1-NRF1/NRF2 signaling pathway and improved ATP level. This may contribute to the increased SOD and CAT antioxidant enzyme activities in the jejunum of ducks exposed to acute heat stress. Wang et al. [156] observed that the activities of GPX and glutathione S-transferase and mRNA levels of NRF2 and SOD1 were increased, and KEAP1 mRNA levels were reduced in the jejunum of broilers under heat stress. Alternatively, dietary resveratrol could attenuate heat stress-impaired intestinal microbiota balance, as indicated by reduced the population of E. coli and increased the population of Lactobacillus and Bifidobacterium in the intestine [20]. The beneficial role of dietary resveratrol was also observed in the laying performance of heat-stressed poultry. In the heat-stressed quail, Sahin et al. found that egg production, hepatic SOD, CAT, and GPX activities and NRF2 expression were linearly increased while hepatic MDA concentrations and Hsp70, Hsp90, and NF-κB expressions were linearly reduced with increasing supplemental resveratrol levels [151].
Response to mycotoxin
Mycotoxins are secondary metabolites of different species of fungi that could cause chronic or acute toxicity in animals [161]. In ducks, supplementation with 500 mg/kg resveratrol significantly ameliorates aflatoxin B1-induced oxidative stress, inflammation, mitochondrial dysfunction, and DNA damage in the ileum, which may be related to the reduced mRNA expression of cytochrome P4501A1 and cytochrome P4501A2 and NRF2/KEAP1 and NF-κB/NOD-like receptor protein 3 (NLRP3) signaling pathway [145]. Liu et al. [144] also indicated that dietary resveratrol in ducks inhibited acute liver injury induced by aflatoxin B1, regulating phase-II metabolism enzymes, NRF2, SIRT1, NF-κB, and cell apoptosis pathway.
Application of resveratrol in ruminant production
Few studies have investigated the effects of dietary resveratrol on ruminants, as summarized in Table 3. In ruminants, previous studies have failed to observe the improved effects of dietary resveratrol on the growth performance of calves [162]. In sheep, dietary resveratrol was shown to enhance the nutrient digestibility of dry matter, organic matter, neutral detergent fiber, acid detergent fiber, and nitrogen [164]. In fattening goats, a recent study indicated that dietary 150 mg/kg resveratrol increased ADG, final weight, and hot carcass weight, while dietary 600 mg/kg resveratrol exhibited the opposite effect, as evidenced by lower ADFI and ADG and higher F/G [165]. They also found that dietary resveratrol improved the impact on meat quality, as evidenced by increased intramuscular fat content and redness and reduced shear force in muscle [165]. Additionally, a previous study reported that dietary resveratrol reduced CH4 output scaled in sheep [16]. Dietary resveratrol was also shown to regulate the rumen microbiota composition. Zhang et al. [162] discovered that feeding 4 mg/kg BW resveratrol increased the population of Desulfovibrio and reduced the methanogenic archaea population in Holstein’s calves for a short period. Ma et al. [16] discovered that 0.25 g/d resveratrol increased the ruminal populations of Fibrobacter succinogenes, Ruminococcus albus, and Butyrivibrio fibrisolvens and reduced protozoa and methanogens in the rumen of sheep. Because of the anti-inflammatory features, resveratrol was also shown to inhibit inflammation in sheep. The study by Liang et al. [166] reported that injected intravenously with resveratrol attenuated the LPS-evoked inflammatory responses in lambs by inhibiting expression levels of inflammatory cytokines and blocking NF-κB and MAPK signaling pathways. However, the effects of dietary supplementation and injection vary. Thus, dietary resveratrol’s effect on the inflammatory state of ruminants needs further research in the future.
Table 3.
Application of resveratrol in ruminant production
| Animal | Dosage | Stress model/ study design |
Beneficial effects | Reference |
|---|---|---|---|---|
|
Calves 7-day-old |
4 mg/kg BW | 173-day-feeding | Increased Desulfovibrio population; decreased methanogenic archaea population and pH in remen | [162] |
|
Sheep 60 kg |
0.125, 0.25, 0.5, 1.0, and 2.0 g/d | 8-day-feeding | Reduced CH4 and CO2 emission in sheep | [163] |
|
Sheep 60 kg |
0.25 g/d | 16-day-feeding | Reduced CH4 and CO2 output scaled; improved apparent digestibility of DM, OM, NDF, ADF, nitrogen, and ME; reduced energy losses in CH4 output | [164] |
|
Ewes 12-month-old |
0.25 g/d | 29-day-feeding | Improved the apparent digestibility of OM, N, NDF, and ADF | [16] |
|
Ewes 18-month-old |
0.25 g/d | 42-day-feeding | Increased ruminal population of Fibrobacter succinogenes, Ruminococcus albus, and Butyrivibrio fibrisolvens; decreased protozoa and methanogens; decreased CO2 and CH4 output scaled | [16] |
|
Fattening goats 28.25 kg |
0, 150, 300 and 600 mg/kg |
120-day-feeding | 150 mg/kg resveratrol increased ADG, final BW, and hot carcass weight, while 600 mg/kg resveratrol exhibited opposite effect; increased intramuscular fat content and redness and reduced shear force in the muscle | [165] |
DM dry matter, OM organic matter, NDF neutral detergent fiber, ADF acid detergent fiber, ME metabolic energy, N nitrogen, ADG average daily gain, BW body weight
Conclusions and perspectives
This review summarized and reviewed the research on resveratrol in pigs, poultry, and ruminant. The research so far suggested that owing to the antioxidant, anti-inflammatory and metabolic regulation qualities, dietary resveratrol improves the health state and growth performance of animals under adverse or stress states, especially in poultry, and shows beneficial effects on the reproductive performance of sows. Dietary resveratrol could enhance the meat and fat quality in pigs, poultry, and ruminants and show beneficial roles in egg quality. In ruminants, dietary resveratrol could reduce methane emissions, which is conducive to environmental protection (as summarized in Fig. 4). However, the specific supplementation dose in various animals and mechanism of action, especially the gut microbiota interaction behind the effects of resveratrol, should be further studied in the future. Additionally, more studies with many animals are required to study its impact on the growth performance and reproductive performance of animals. In the background of feed antibiotics prohibition, resveratrol, as a plant functional substance, deserves further research and application in animal production. We hoped that this review could provide theoretical basis and research ideas for the research and application of resveratrol.
Fig. 4.
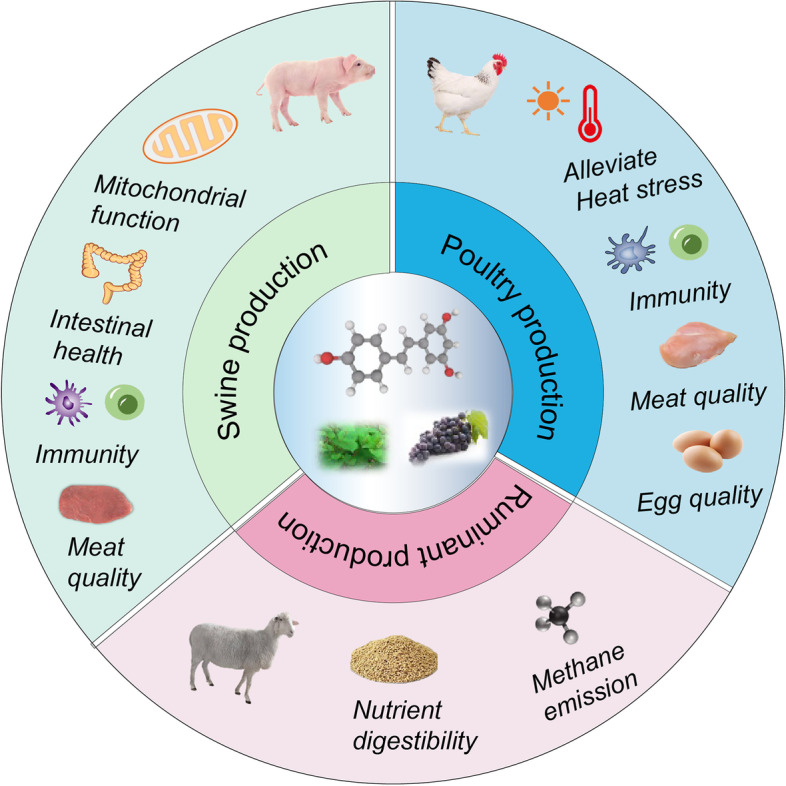
The summary of beneficial effects of dietary resveratrol in animal production
Abbreviations
- a*
Redness
- ACC
Acetyl-CoA carboxylase
- ADFI
Average daily feed intake
- ADG
Average daily gain
- AMPK
AMP-activated protein kinase
- ATGL
Adipose triglyceride lipase
- b*
Yellowness
- BW
Body weight
- CAT
Catalase
- CD4+/CD8+
Cluster of differentiation 4/cluster of differentiation 8
- CLDN1
Claudin 1
- COX
Cyclooxygenase
- CPT-1
Carnitine palmitoyl transferase-1
- DAO
Diamine oxidase
- DON
Deoxynivalenol
- FAS
Fatty acid synthase
- FCR
Feed conversion ratios
- F/G
Feed to gain ratio
- GPX
Glutathione peroxidase
- GSH
Reduced glutathione
- HSL
Hormone sensitive lipase
- Hsp70
Heat shock protein (Hsp)70
- IFN-γ
Interferon (IFN)-γ
- IgG
Immunoglobulin (Ig)G
- IL-1β
Interleukin (IL)-1β
- IUGR
Intrauterine growth retardation
- KEAP1
Kelch-like ECH-associated protein 1
- L*
Lightness
- LPL
Lipoprotein lipase
- LPS
Lipopolysaccharides
- MAPK
Mitogen-activated protein kinases
- MDA
Malondialdehyde
- MRP
Multidrug resistance protein
- MyHC
Myosin heavy chain
- NBW
Normal birth weight
- NF-κB
Nuclear factor kappa B
- NLRP3
NOD-like receptor protein 3
- NRF1
Nuclear respiratory factor 1
- NRF2
Nuclear factor-erythroid 2-related factor 2
- OCLN
Occludin
- PGC-1α
Peroxisome proliferators-activated receptor gamma coactivator-1 alpha
- PPARγ
Peroxisome proliferator activated receptor (PPAR)γ
- ROS
Reactive oxygen species
- SIRT1
Sirtuin 1
- SOD
Superoxide dismutase
- T-AOC
Total antioxidant capacity
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-alpha
- ZO-1
Zonula occludens 1
Authors' contributions
AS and QM conceived the review. QM, JL, and CW wrote the manuscript. AS and QM revised and finalized the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Heilongjiang Province (TD2019C001), the National Natural Science Foundation of China (32002209), the Natural Science Foundation of Heilongjiang Province (YQ2021C017), the Postdoctoral Foundation in Heilongjiang Province (LBH-Z19005), the Academic Backbone Project of Northeast Agricultural University, and Heilongjiang Touyan Innovation Team Program.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There were no conflicts of interest.
Footnotes
Qingwei Meng and Jiawei Li contributed to the work equally and should be regarded as co-first authors.
References
- 1.Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors. 2018;44:36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Zhou J, Jiang B, Miao M. Resveratrol and inflammatory bowel disease. Ann N Y Acad Sci. 2017;1403:38–47. doi: 10.1111/nyas.13426. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefont-Rousselot D. Resveratrol and Cardiovascular Diseases. Nutrients. 2016;8:250. doi: 10.3390/nu8050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattio LM, Dallavalle S, Musso L, Filardi R, Franzetti L, Pellegrino L, et al. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci Rep. 2019;9:19525. doi: 10.1038/s41598-019-55975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, et al. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol. 2012;3:141141. doi: 10.3389/fphar.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 8.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. 10.1038/nrd2060. [DOI] [PubMed]
- 9.Novelle MG, Wahl D, Diéguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 11.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 12.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed ST, Hossain ME, Kim GM, Hwang JA, Ji H, Yang CJ. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. Asian-Australas J Anim Sci. 2013;26:683–690. doi: 10.5713/ajas.2012.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin K, Akdemir F, Orhan C, Tuzcu M, Hayirli A, Sahin N. Effects of dietary resveratrol supplementation on egg production and antioxidant status. Poult Sci. 2010;89:1190–1198. doi: 10.3382/ps.2010-00635. [DOI] [PubMed] [Google Scholar]
- 16.Ma T, Chen DD, Tu Y, Zhang NF, Si BW, Deng KD, et al. Effect of dietary supplementation with resveratrol on nutrient digestibility, methanogenesis and ruminal microbial flora in sheep. J Anim Physiol Anim Nutr. 2015;99:676–683. doi: 10.1111/jpn.12264. [DOI] [PubMed] [Google Scholar]
- 17.Chen XL, Zeng ZY, Huang ZQ, Chen DW, He J, Chen H, et al. Effects of dietary resveratrol supplementation on immunity, antioxidative capacity and intestinal barrier function in weaning piglets. Anim Biotechnol. 2021;32:240–245. doi: 10.1080/10495398.2019.1683022. [DOI] [PubMed] [Google Scholar]
- 18.Meng Q, Sun S, Luo Z, Shi B, Shan A, Cheng B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019;10:5626–5643. doi: 10.1039/c9fo00637k. [DOI] [PubMed] [Google Scholar]
- 19.Liu LL, He JH, Xie HB, Yang YS, Li JC, Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Zhao XH, Yang L, Chen XY, Jiang RS, Jin SH, et al. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- 21.Qiu YQ, Yang J, Wang L, Yang XF, Gao KG, Zhu C, et al. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J Anim Sci Biotechnol. 2021;12:71. doi: 10.1186/s40104-021-00596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Luo JQ, Yu B, Zheng P, Huang ZQ, Mao XB, et al. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015;102:15–21. doi: 10.1016/j.meatsci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Gambini J, Ingles M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev. 2015;2015:837042. 10.1155/2015/837042. [DOI] [PMC free article] [PubMed]
- 24.Jeandet P, Douillet-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M. Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem. 2002;50:2731–2741. doi: 10.1021/jf011429s. [DOI] [PubMed] [Google Scholar]
- 25.Adrian M, Jeandet P, Veneau J, Weston LA, Bessis R. Biological activity of resveratrol, a stilbenic compound from grapevines, against botrytis cinerea, the causal agent for gray mold. J Chem Ecol. 1997;23:1689–1702. doi: 10.1023/B:JOEC.0000006444.79951.75. [DOI] [Google Scholar]
- 26.Tian BR, Liu JY. Resveratrol: a review of plant sources, synthesis, stability, modification and food application. J Sci Food Agric. 2020;100:1392–1404. doi: 10.1002/jsfa.10152. [DOI] [PubMed] [Google Scholar]
- 27.Wang LJ, Xu M, Liu CY, Wang JF, Xi HF, Wu BH, et al. Resveratrols in grape berry skins and leaves in vitis germplasm. PLoS ONE. 2013;8:e61642. doi: 10.1371/journal.pone.0061642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang A, Fang YL, Li XA, Meng JF, Wang H, Li H, et al. Occurrence and estimation of trans-resveratrol in one-year-old canes from seven major chinese grape producing regions. Molecules. 2011;16:2846–61.10.3390/molecules16042846. [DOI] [PMC free article] [PubMed]
- 29.Walle T, Hsieh F, Delegge MH, Oatis JE Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377. 10.1124/dmd.104.000885. [DOI] [PubMed]
- 30.Ge S, Yin T, Xu B, Gao S, Hu M. Curcumin affects phase II disposition of resveratrol through inhibiting efflux transporters MRP2 and BCRP. Pharm Res. 2016;33:590–602. doi: 10.1007/s11095-015-1812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, et al. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- 32.Azorín-Ortuño M, Yañéz-Gascón MJ, Pallarés FJ, Vallejo F, Larrosa M, García-Conesa MT, et al. Pharmacokinetic study of trans-resveratrol in adult pigs. J Agric Food Chem. 2010;58:11165–11171. doi: 10.1021/jf102799m. [DOI] [PubMed] [Google Scholar]
- 33.Chaplin A, Carpene C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10:1651. doi: 10.3390/nu10111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu YL, Chen DW, Zheng P, Yu J, He J, Mao XB, et al. The bidirectional interactions between resveratrol and gut microbiota: an insight into oxidative stress and inflammatory bowel disease therapy. Biomed Res Int. 2019;2019:5403761. doi: 10.1155/2019/5403761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J Control Release. 2012;158:182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 36.Henry C, Vitrac X, Decendit A, Ennamany R, Krisa S, Merillon JM. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J Agric Food Chem. 2005;53:798803. 10.1021/jf048909e. [DOI] [PubMed]
- 37.Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev. 2018;31:85–97. doi: 10.1017/s095442241700021x. [DOI] [PubMed] [Google Scholar]
- 38.Lançon A, Delmas D, Osman H, Thénot JP, Jannin B, Latruffe N. Human hepatic cell uptake of resveratrol: involvement of both passive diffusion and carrier-mediated process. Biochem Biophys Res Commun. 2004;316:1132–1137. doi: 10.1016/j.bbrc.2004.02.164. [DOI] [PubMed] [Google Scholar]
- 39.Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Brit J Nutr. 2011;106:383–389. doi: 10.1017/s0007114511000316. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Nie L, Yin YG, Tang JL, Zhou JY, Li DD, et al. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259:395–401. doi: 10.1016/j.taap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Chen YA, Zhang H, Ji SL, Jia PL, Chen YP, Li Y, et al. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radical Bio Med. 2021;177:1–14. doi: 10.1016/j.freeradbiomed.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Cheng L, Jin ZX, Zhao R, Ren K, Deng C, Yu SQ. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int J Clin Exp Med. 2015;8:10420–10428. [PMC free article] [PubMed] [Google Scholar]
- 43.Kung HC, Lin KJ, Kung CT, Lin TK. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in parkinson’s disease. Biomedicines. 2021;9:918. 10.3390/biomedicines9080918. [DOI] [PMC free article] [PubMed]
- 44.Pourhanifeh MH, Shafabakhsh R, Reiter RJ, Asemi Z. The effect of resveratrol on neurodegenerative disorders: possible protective actions against autophagy, apoptosis, inflammation and oxidative stress. Curr Pharm Des. 2019;25:2178–2191. doi: 10.2174/1381612825666190717110932. [DOI] [PubMed] [Google Scholar]
- 45.Demirtas CY, Bircan FS, Pasaoglu OT, Turkozkan N. The effects of resveratrol on hepatic oxidative stress in metabolic syndrome model induced by high fructose diet. Bratisl Med J. 2018;119:36–40. doi: 10.4149/bll_2018_008. [DOI] [PubMed] [Google Scholar]
- 46.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, May MEI, Gharbi N, et al. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80:1033–9. 10.1016/j.lfs.2006.11.044. [DOI] [PubMed]
- 47.Kao CL, Chen LK, Chang YL, Yung MC, Hsu CC, Chen YC, et al. Resveratrol protects human endothelium from H2O2-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb. 2010;17:970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 48.Li HH, Shen YJ, Xiao H, Sun W. Resveratrol attenuates rotenone-induced inflammation and oxidative stress via STAT1 and Nrf2/Keap1/SLC7A11 pathway in a microglia cell line. Pathol Res Pract. 2021;225:153576. doi: 10.1016/j.prp.2021.153576. [DOI] [PubMed] [Google Scholar]
- 49.Virk P, Al-Mukhaizeem NAR, Bin Morebah SH, Fouad D, Elobeid M. Protective effect of resveratrol against toxicity induced by the mycotoxin, zearalenone in a rat model. Food Chem Toxicol. 2020;146:111840. doi: 10.1016/j.fct.2020.111840. [DOI] [PubMed] [Google Scholar]
- 50.Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60:634–643. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Gu L, Ma Q, Zhu D, Huang X. Resveratrol attenuates hydrogen peroxide-induced apoptosis in human umbilical vein endothelial cells. Eur Rev Med Pharmacol Sci. 2013;17:88–94. doi: 10.3892/mmr.2017.8124. [DOI] [PubMed] [Google Scholar]
- 52.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol-lung C. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 53.Cheng AS, Cheng YH, Chiou CH, Chang TL. Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J Agric Food Chem. 2012;60:9180–9187. doi: 10.1021/jf302831d. [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Tang CY, Luo C, He HX, Zhou YD, Yu WH. Resveratrol attenuates the cytotoxicity induced by amyloid-beta(1–42) in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res. 2018;43:297–305. doi: 10.1007/s11064-017-2421-7. [DOI] [PubMed] [Google Scholar]
- 55.Csiszar A, Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, et al. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci. 2015;70:303–313. doi: 10.1093/gerona/glu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Zhu C, Ye J, Lv Y, Wang L, Chen Z, et al. Protection of porcine intestinal-epithelial cells from deoxynivalenol-induced damage by resveratrol via the Nrf2 signaling pathway. J Agric Food Chem. 2019;67:1726–1735. doi: 10.1021/acs.jafc.8b03662. [DOI] [PubMed] [Google Scholar]
- 57.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. BBA-Mol Basis Dis. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol-Heart C. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential adverse effects of resveratrol: a literature review. Int J Mol Sci. 2020;21:2084. doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rocha KK, Souza GA, Ebaid GX, Seiva FR, Cataneo AC, Novelli EL. Resveratrol toxicity: effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem Toxicol. 2009;47:1362–1367. doi: 10.1016/j.fct.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Posadino AM, Cossu A, Giordo R, Zinellu A, Sotgia S, Vardeu A, et al. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem Toxicol. 2015;78:10–16. doi: 10.1016/j.fct.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 62.Larrosa M, Yanez-Gascon MJ, Selma MV, Gonzalez-Sarrias A, Toti S, Ceron JJ, et al. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agr Food Chem. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 63.Xiao B, Ma W, Zheng Y, Li Z, Li D, Zhang Y, et al. Effects of resveratrol on the inflammatory response and renal injury in hyperuricemic rats. Nutr Res Pract. 2021;15:26–37. doi: 10.4162/nrp.2021.15.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibrahim KA, Abdelgaid HA, Eleyan M, Mohamed RA, Gamil NM. Resveratrol alleviates cardiac apoptosis following exposure to fenitrothion by modulating the sirtuin1/c-Jun N-terminal kinases/p53 pathway through pro-oxidant and inflammatory response improvements: In vivo and in silico studies. Life Sci. 2022;290:120265. 10.1016/j.lfs.2021.120265. [DOI] [PubMed]
- 65.Xun WJ, Fu QY, Hou GY, Shi LG, Cao T. Protective effects of dietary resveratrol supplementation against oxidative stress in diquat-challenged piglets. Ital J Anim Sci. 2020;19:1513–1522. doi: 10.1080/1828051x.2020.1851148. [DOI] [Google Scholar]
- 66.He S, Chen L, He Y, Chen F, Ma Y, Xiao D, et al. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, barrier integrity and inflammation in yellow-feather broilers. Anim Prod Sci. 2020;60:1547–1556. doi: 10.1071/an19218. [DOI] [Google Scholar]
- 67.Tsai MH, Hsu LF, Lee CW, Chiang YC, Lee MH, How JM, et al. Resveratrol inhibits urban particulate matter-induced COX-2/PGE(2) release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-kappa B. Int J Biochem Cell B. 2017;88:113–123. doi: 10.1016/j.biocel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Kumar KJ, Kee SY, Hoon KS, Young-Joon S. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking IκB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 69.Meng TT, Xiao DF, Muhammed A, Deng JY, Chen L, He JH. Anti-inflammatory action and mechanisms of resveratrol. Molecules. 2021;26:229. doi: 10.3390/molecules26010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chalal M, Klinguer A, Echairi A, Meunier P, Vervandier-Fasseur D, Adrian M. Antimicrobial activity of resveratrol analogues. Molecules. 2014;19:7679–7688. doi: 10.3390/molecules19067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paulo L, Ferreira S, Gallardo E, Queiroz JA, Domingues F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J Microb Biot. 2010;26:1533–1538. doi: 10.1007/s11274-010-0325-7. [DOI] [Google Scholar]
- 72.Euba B, Lopez-Lopez N, Rodriguez-Arce I, Fernandez-Calvet A, Barberan M, Caturla N, et al. Resveratrol therapeutics combines both antimicrobial and immunomodulatory properties against respiratory infection by nontypeable Haemophilus influenzae. Sci Rep. 2017;7:12860. doi: 10.1038/s41598-017-13034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diaz-Gerevini GT, Repossi G, Dain A, Tarres MC, Das UN, Eynard AR. Beneficial action of resveratrol: How and why? Nutrition. 2016;32:174–178. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Galiniak S, Aebisher D, Bartusik-Aebisher D. Health benefits of resveratrol administration. Acta Biochim Pol. 2019;66:13–21. 10.18388/abp.2018_2749. [DOI] [PubMed]
- 75.Fernandez-Quintela A, Carpene C, Fernandez M, Aguirre L, Milton-Laskibar I, Contreras J, et al. Anti-obesity effects of resveratrol: comparison between animal models and humans. J Physiol Biochem. 2017;73:417–429. doi: 10.1007/s13105-016-0544-y. [DOI] [PubMed] [Google Scholar]
- 76.Wang P, Li DT, Ke WX, Liang D, Hu XS, Chen F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int J Obesity. 2020;44:213–225. doi: 10.1038/s41366-019-0332-1. [DOI] [PubMed] [Google Scholar]
- 77.Gomez-Zorita S, Treguer K, Mercader J, Carpene C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J Physiol Biochem. 2013;69:585–93. 10.1007/s13105-012-0229-0. [DOI] [PubMed]
- 78.Szkudelska K, Nogowski L, Szkudelski T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2009;113:17–24. doi: 10.1016/j.jsbmb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Bai L, Pang WJ, Yang YJ, Yang GS. Modulation of Sirt1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. Mol Cell Biochem. 2008;307:129–140. doi: 10.1007/s11010-007-9592-5. [DOI] [PubMed] [Google Scholar]
- 80.Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 81.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and retinol-binding protein 4 expression in white adipocytes. J Nutr Biochem. 2011;22:828–834. doi: 10.1016/j.jnutbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Ahn J, Lee H, Kim S, Ha T. Resveratrol inhibits TNF-α-induced changes of adipokines in 3T3-L1 adipocytes. Biochem Bioph Res Co. 2007;364:972–977. doi: 10.1016/j.bbrc.2007.10.109. [DOI] [PubMed] [Google Scholar]
- 83.Kim DH, Jung IH, Kim DH, Park SW. Knockout of longevity gene Sirt1 in zebrafish leads to oxidative injury, chronic inflammation, and reduced life span. PLoS ONE. 2019;14:e0220581. doi: 10.1371/journal.pone.0220581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, Chen Y, Chen Y, Ji S, Jia P, Li Y, et al. Comparison of the protective effects of resveratrol and pterostilbene against intestinal damage and redox imbalance in weanling piglets. J Anim Sci Biotechnol. 2020;11:52. doi: 10.1186/s40104-020-00460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H, Chen Y, Li Y, Jia P, Ji S, Chen Y, et al. Protective effects of pterostilbene against hepatic damage, redox imbalance, mitochondrial dysfunction, and endoplasmic reticulum stress in weanling piglets. J Anim Sci. 2020;98:skaa328. 10.1093/jas/skaa328. [DOI] [PMC free article] [PubMed]
- 88.Gan Z, Wei W, Wu J, Zhao Y, Zhang L, Wang T, et al. Resveratrol and curcumin improve intestinal mucosal integrity and decrease m(6)A RNA methylation in the intestine of weaning piglets. ACS Omega. 2019;4:17438–17446. doi: 10.1021/acsomega.9b02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morosanu M. Prevention of infection and of tuberculosis in 0-1-year-old infants. Viata Med Rev Inf Prof Stiint Cadrelor Medii Sanit. 1980;28:153–156. [PubMed] [Google Scholar]
- 90.Fu Q, Cui Q, Yang Y, Zhao X, Song X, Wang G, et al. Effect of resveratrol dry suspension on immune function of piglets. Evid Based Complement Alternat Med. 2018;2018:595270. doi: 10.1155/2018/5952707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gan ZD, Wei WY, Li Y, Wu JM, Zhao YW, Zhang LL, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules. 2019;24:1220. doi: 10.3390/molecules24071220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen YN, Zhang H, Chen YP, Jia PL, Ji SL, Zhang YY, et al. Resveratrol and its derivative pterostilbene ameliorate intestine injury in intrauterine growth-retarded weanling piglets by modulating redox status and gut microbiota. J Anim Sci Biotechnol. 2021;12:70. doi: 10.1186/s40104-021-00589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng K, Ji S, Jia P, Zhang H, Wang T, Song Z, et al. Resveratrol improves hepatic redox status and lipid balance of neonates with intrauterine growth retardation in a piglet model. Biomed Res Int. 2020;2020:7402645. doi: 10.1155/2020/7402645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hermann RE, Esselstyn CB Jr, Crile G Jr, Cooperman AM, Antunez AR, Hoerr SO. [Long-term results of modified radical and conservative operations of breast cancer]. Chirurg. 1984;55:193–9. https://www.ncbi.nlm.nih.gov/pubmed/6723455Chirurg. [PubMed]
- 95.Zhang H, Li Y, Su W, Ying Z, Zhou L, Zhang L, et al. Resveratrol attenuates mitochondrial dysfunction in the liver of intrauterine growth retarded suckling piglets by improving mitochondrial biogenesis and redox status. Mol Nutr Food Res. 2017;61:5. doi: 10.1002/mnfr.201600653. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H, Chen YN, Chen YP, Jia PL, Ji SL, Xu JX, et al. Comparison of the effects of resveratrol and its derivative pterostilbene on hepatic oxidative stress and mitochondrial dysfunction in piglets challenged with diquat. Food Funct. 2020;11:4202–4215. doi: 10.1039/d0fo00732c. [DOI] [PubMed] [Google Scholar]
- 97.Cao S, Shen Z, Wang C, Zhang Q, Hong Q, He Y, et al. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets(1) Food Funct. 2019;10:344–354. doi: 10.1039/c8fo02091d. [DOI] [PubMed] [Google Scholar]
- 98.Fu Q, Shi L, Zhou H, Cao T, Ma Z, Jiang H, et al. Effects of resveratrol on ileal mucosal morphology, antioxidant capacity, tight junction protein and inflammatory factor mrna expression in oxidative stress piglets. Chin J Anim Nutr. 2021;33:1163–1172. doi: 10.3969/j.issn.1006-267x.2021.02.057. [DOI] [Google Scholar]
- 99.Xun W, Fu Q, Shi L, Cao T, Jiang H, Ma Z. Resveratrol protects intestinal integrity, alleviates intestinal inflammation and oxidative stress by modulating AhR/Nrf2 pathways in weaned piglets challenged with diquat. Int Immunopharmacol. 2021;99:107989. doi: 10.1016/j.intimp.2021.107989. [DOI] [PubMed] [Google Scholar]
- 100.Ji M, Xun W, Hou G, Shi L, Hou X, Yang W. Effects of resveratrol on liver antioxidant enzyme, cytokine mrna expression and nuclear factor erythroid 2 related factor 2 signaling pathway in oxidative stress piglets. Chin J Anim Nutr. 2022;34:1230–1239. doi: 10.3969/j.issn.1006⁃267x.2022.02.054. [DOI] [Google Scholar]
- 101.Huang Y, Zheng C, Song B, Wang L, Xiao H, Jiang Z. Resveratrol ameliorates intestinal damage challenged with deoxynivalenol through mitophagy in vitro and in vivo. Front Vet Sci. 2021;8:807301. 10.3389/fvets.2021.807301. [DOI] [PMC free article] [PubMed]
- 102.Hong QH, Li X, Lin Q, Shen ZJ, Feng J, Hu CH. Resveratrol improves intestinal morphology and anti-oxidation ability in deoxynivalenol-challenged piglets. Animals. 2022;12:311. doi: 10.3390/ani12030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui Q, Fu Q, Zhao X, Song X, Yu J, Yang Y, et al. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS ONE. 2018;13:e0192692. doi: 10.1371/journal.pone.0192692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao X, Tong W, Song X, Jia R, Li L, Zou Y, et al. Antiviral effect of resveratrol in piglets infected with virulent pseudorabies virus. Viruses. 2018;10:457. doi: 10.3390/v10090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeng ZY, Chen XL, Huang ZQ, Chen DW, He J, Chen H, et al. Effects of dietary resveratrol supplementation on growth performance and muscle fiber type transformation in weaned piglets. Anim Feed Sci Tech. 2020;265:114499. doi: 10.1016/j.anifeedsci.2020.114499. [DOI] [Google Scholar]
- 106.Zhang C, Luo JQ, Yu B, Chen JL, Chen DW. Effects of resveratrol on lipid metabolism in muscle and adipose tissues: A reevaluation in a pig model. J Funct Food. 2015;14:590–595. doi: 10.1016/j.jff.2015.02.039. [DOI] [Google Scholar]
- 107.Huang Y, Xia Q, Cui Y, Qu Q, Wei Y, Jiang Q. Resveratrol increase the proportion of oxidative muscle fiber through the AdipoR1-AMPK-PGC-1α pathway in pigs. J Funct Food. 2020;73:104090. doi: 10.1016/j.jff.2020.104090. [DOI] [Google Scholar]
- 108.Zhang HZ, Chen DW, He J, Zheng P, Yu J, Mao XB, et al. Long-term dietary resveratrol supplementation decreased serum lipids levels, improved intramuscular fat content, and changed the expression of several lipid metabolism-related miRNAs and genes in growing-finishing pigs1. J Anim Sci. 2019;97:1745–1756. doi: 10.1093/jas/skz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng K, Yu C, Li Z, Li S, Yan E, Song Z, et al. Resveratrol improves meat quality, muscular antioxidant capacity, lipid metabolism and fiber type composition of intrauterine growth retarded pigs. Meat Sci. 2020;170:108237. doi: 10.1016/j.meatsci.2020.108237. [DOI] [PubMed] [Google Scholar]
- 110.Meng Q, Guo T, Li G, Sun S, He S, Cheng B, et al. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J Anim Sci Biotechnol. 2018;9:34. doi: 10.1186/s40104-018-0248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meng QW, Sun SS, Bai YS, Luo Z, Li ZY, Shi BM, et al. Effects of dietary resveratrol supplementation in sows on antioxidative status, myofiber characteristic and meat quality of offspring. Meat Sci. 2020;167:108176. doi: 10.1016/j.meatsci.2020.108176. [DOI] [PubMed] [Google Scholar]
- 112.Sun S, Meng Q, Luo Z, Shi B, Bi C, Shan A. Effects of dietary resveratrol supplementation during gestation and lactation of sows on milk composition of sows and fat metabolism of sucking piglets. J Anim Physiol Anim Nutr. 2019;103:813–821. doi: 10.1111/jpn.13064. [DOI] [PubMed] [Google Scholar]
- 113.Zhao Y, Huang YJ, Gao KG, Wen XL, Hu SL, Wang L, et al. Maternal resveratrol regulates the growth performance, antioxidant capacity, and intestinal health of suckling piglets through intestinal microorganisms at high summer temperatures. Front Nutr. 2022;9:971496. doi: 10.3389/fnut.2022.971496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006;84:2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- 115.Mancini RA, Hunt MC. Current research in meat color. Meat Sci. 2005;71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 116.Huff-Lonergan E, Lonergan SM. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 117.Chang KC, da Costa N, Blackley R, Southwood O, Evans G, Plastow G, et al. Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs. Meat Sci. 2003;64:93–103. doi: 10.1016/S0309-1740(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 118.Wimmers K, Ngu NT, Jennen DGJ, Tesfaye D, Murani E, Schellander K, et al. Relationship between myosin heavy chain isoform expression and muscling in several diverse pig breeds. J Anim Sci. 2008;86:795–803. doi: 10.2527/jas.2006-521. [DOI] [PubMed] [Google Scholar]
- 119.Ryu YC, Kim BC. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 120.Kim GD, Jeong JY, Jung EY, Yang HS, Lim HT, Joo ST. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 2013;94:267–273. doi: 10.1016/j.meatsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 121.Wen W, Chen X, Huang Z, Chen D, Chen H, Luo Y, et al. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1alpha pathway. J Nutr Biochem. 2020;77:108297. doi: 10.1016/j.jnutbio.2019.108297. [DOI] [PubMed] [Google Scholar]
- 122.Shan T, Wang Y, Wu T, Liu C, Guo J, Zhang Y, et al. Porcine sirtuin 1 gene clone, expression pattern, and regulation by resveratrol. J Anim Sci. 2009;87:895–904. doi: 10.2527/jas.2008-1344. [DOI] [PubMed] [Google Scholar]
- 123.Shan T, Wang Y, Wu T, Guo J, Liu J, Feng J, et al. Porcine adipose triglyceride lipase complementary deoxyribonucleic acid clone, expression pattern, and regulation by resveratrol. J Anim Sci. 2008;86:1781–1788. doi: 10.2527/jas.2007-0659. [DOI] [PubMed] [Google Scholar]
- 124.Wang CX, Zhang BR, Zhang HY, Yang W, Meng QW, Shi BM, et al. Effect of dietary pyrroloquinoline quinone disodium in sows on intestinal health of the offspring. Food Funct. 2020;11:7804–7816. doi: 10.1039/d0fo01403f. [DOI] [PubMed] [Google Scholar]
- 125.Li M, Yuan D, Liu Y, Jin H, Tan B. Dietary puerarin supplementation alleviates oxidative stress in the small intestines of diquat-challenged piglets. Animals. 2020;10:631. doi: 10.3390/ani10040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pinton P, Braicu C, Nougayrede JP, Laffitte J, Taranu I, Oswald IP. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J Nutr. 2010;140:1956–1962. doi: 10.3945/jn.110.123919. [DOI] [PubMed] [Google Scholar]
- 127.Cheng K, Zhang M, Huang X, Zheng X, Song Z, Zhang L, et al. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can J Anim Sci. 2017;98:187–193. doi: 10.1139/cjas-2017-0040. [DOI] [Google Scholar]
- 128.Cheng K, Jia PL, Ji SL, Song ZH, Zhang H, Zhang LL, et al. Improvement of the hepatic lipid status in intrauterine growth retarded pigs by resveratrol is related to the inhibition of mitochondrial dysfunction, oxidative stress and inflammation. Food Funct. 2021;12:278–290. doi: 10.1039/d0fo01459a. [DOI] [PubMed] [Google Scholar]
- 129.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF Jr., Paul WE. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16:1051–9. 10.1038/ni.3244. [DOI] [PMC free article] [PubMed]
- 130.Ni J, Shen TD, Chen EZ, Bittinger K, Bailey A, Roggiani M, et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017;9:416. 10.1126/scitranslmed.aah6888. [DOI] [PMC free article] [PubMed]
- 131.Benard A, Jacobsen A, Brunner M, Krautz C, Klosch B, Swierzy I, et al. Interleukin-3 is a predictive marker for severity and outcome during SARS-CoV-2 infections. Nat Commun. 2021;12:1112. doi: 10.1038/s41467-021-21310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luo X, Wu S, Jia H, Si X, Song Z, Zhai Z, et al. Resveratrol alleviates enterotoxigenic Escherichia coli K88-induced damage by regulating SIRT-1 signaling in intestinal porcine epithelial cells. Food Funct. 2022;13:7346–7360. doi: 10.1039/d1fo03854k. [DOI] [PubMed] [Google Scholar]
- 133.Wang G, Zha Z, Huang P, Sun H, Huang Y, He M, et al. Structures of pseudorabies virus capsids. Nat Commun. 2022;13:1533. doi: 10.1038/s41467-022-29250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Darby JRT, Mohd Dollah MHB, Regnault TRH, Williams MT, Morrison JL. Systematic review: Impact of resveratrol exposure during pregnancy on maternal and fetal outcomes in animal models of human pregnancy complications-Are we ready for the clinic? Pharacol Res. 2019;144:264–278. doi: 10.1016/j.phrs.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 135.Zheng S, Feng Q, Cheng J, Zheng J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci Rep. 2018;38:Bsr20171741. 10.1042/BSR20171741. [DOI] [PMC free article] [PubMed]
- 136.Fernandes GFS, Silva GDB, Pavan AR, Chiba DE, Chin CM, Dos Santos JL. Epigenetic regulatory mechanisms induced by resveratrol. Nutrients. 2017;9:1201. doi: 10.3390/nu9111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Izquierdo V, Palomera-Avalos V, Pallas M, Grinan-Ferre C. Resveratrol supplementation attenuates cognitive and molecular alterations under maternal high-fat diet intake: epigenetic inheritance over generations. Int J Mol Sci. 2021;22:1453. doi: 10.3390/ijms22031453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feng ZH, Gong JG, Zhao GX, Lin X, Liu YC, Ma KW. Effects of dietary supplementation of resveratrol on performance, egg quality, yolk cholesterol and antioxidant enzyme activity of laying hens. Brit Poultry Sci. 2017;58:544–549. doi: 10.1080/00071668.2017.1349295. [DOI] [PubMed] [Google Scholar]
- 139.Zhang C, Kang X, Zhang T, Huang J. Positive effects of resveratrol on egg-laying ability, egg quality, and antioxidant activity in hens. J Appl Poultry Res. 2019;28:1099–1105. doi: 10.3382/japr/pfz072. [DOI] [Google Scholar]
- 140.Yu Q, Fang C, Ma Y, He S, Ajuwon KM, He J. Dietary resveratrol supplement improves carcass traits and meat quality of Pekin ducks. Poult Sci. 2021;100:100802. doi: 10.1016/j.psj.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang C, Yang L, Zhao X, Chen X, Wang L, Geng Z. Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers. J Sci Food Agric. 2018;98:1216–1221. doi: 10.1002/jsfa.8576. [DOI] [PubMed] [Google Scholar]
- 142.Zhang C, Wang L, Zhao XH, Chen XY, Yang L, Geng ZY. Dietary resveratrol supplementation prevents transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Poult Sci. 2017;96:2219–2225. doi: 10.3382/ps/pex004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mohebodini H, Jazi V, Bakhshalinejad R, Shabani A, Ashayerizadeh A. Effect of dietary resveratrol supplementation on growth performance, immune response, serum biochemical indices, cecal microflora, and intestinal morphology of broiler chickens challenged with Escherichia coli. Livest Sci. 2019;229:13–21. doi: 10.1016/j.livsci.2019.09.008. [DOI] [Google Scholar]
- 144.Liu F, Wang Y, Zhou X, Liu M, Jin S, Shan A, et al. Resveratrol relieved acute liver damage in ducks (Anas platyrhynchos) induced by AFB1 via modulation of apoptosis and Nrf2 signaling pathways. Animals. 2021;11:3516. doi: 10.3390/ani11123516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang H, Wang Y, Yu C, Jiao Y, Zhang R, Jin S, et al. Dietary resveratrol alleviates AFB1-induced ileum damage in ducks via the Nrf2 and NF-κB/NLRP3 signaling pathways and CYP1A1/2 expressions. Agriculture. 2022;12:54. doi: 10.3390/agriculture12010054. [DOI] [Google Scholar]
- 146.Zhang C, Tian Y, Yan F, Kang X, Han R, Sun G, et al. Modulation of growth and immunity by dietary supplementation with resveratrol in young chickens receiving conventional vaccinations. Am J Vet Res. 2014;75:752–759. doi: 10.2460/ajvr.75.8.752. [DOI] [PubMed] [Google Scholar]
- 147.Yang H, Wang Y, Liu M, Liu X, Jiao Y, Jin S, et al. Effects of dietary resveratrol supplementation on growth performance and anti-inflammatory ability in ducks (Anas platyrhynchos) through the Nrf2/HO-1 and TLR4/NF-kappaB signaling pathways. Animals. 2021;11:3588. doi: 10.3390/ani11123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang H, Wang Y, Jin S, Pang Q, Shan A, Feng X. Dietary resveratrol alleviated lipopolysaccharide-induced ileitis through Nrf2 and NF-kappaB signalling pathways in ducks (Anas platyrhynchos) J Anim Physiol Anim Nutr. 2022;106:1306–1320. doi: 10.1111/jpn.13657. [DOI] [PubMed] [Google Scholar]
- 149.Jin S, Pang Q, Yang H, Diao X, Shan A, Feng X. Effects of dietary resveratrol supplementation on the chemical composition, oxidative stability and meat quality of ducks (Anas platyrhynchos) Food Chem. 2021;363:130263. doi: 10.1016/j.foodchem.2021.130263. [DOI] [PubMed] [Google Scholar]
- 150.Jin S, Wang M, Yang H, Shan A, Feng X. Dietary supplementation of resveratrol improved the oxidative stability and spatial conformation of myofibrillar protein in frozen-thawed duck breast meat. Food Biosci. 2021;43:101261. doi: 10.1016/j.fbio.2021.101261. [DOI] [Google Scholar]
- 151.Sahin K, Orhan C, Akdemir F, Tuzcu M, Iben C, Sahin N. Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J Anim Physiol Anim Nutr. 2012;96:66–74. doi: 10.1111/j.1439-0396.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 152.Liu L, Fu C, Yan M, Xie H, Li S, Yu Q, et al. Resveratrol modulates intestinal morphology and HSP70/90, NF-kappaB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016;7:1329–1338. doi: 10.1039/c5fo01338k. [DOI] [PubMed] [Google Scholar]
- 153.Zhang C, Zhao X, Wang, L Yang L, Chen X, Geng Z. Resveratrol beneficially affects meat quality of heat-stressed broilers which is associated with changes in muscle antioxidant status. Anim Sci J. 2017;88:1569–74. 10.1111/asj.12812. [DOI] [PubMed]
- 154.Keshri J, Chen YR, Pinto R, Kroupitski Y, Weinberg ZG, Saldinger SS. Bacterial dynamics of wheat silage. Front Microbiol. 2019;10:161532. doi: 10.3389/fmicb.2019.01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He S, Yu Q, He Y, Hu R, Xia S, He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang C, Zhao F, Li Z, Jin X, Chen X, Geng Z, et al. Effects of resveratrol on growth performance, intestinal development, and antioxidant status of broilers under heat stress. Animals. 2021;11:1427. doi: 10.3390/ani11051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang C, Luo P, Chen SJ, Deng ZC, Fu XL, Xu DN, et al. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult Sci. 2021;100:101459. doi: 10.1016/j.psj.2021.101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meng T, Deng J, Xiao D, Arowolo MA, Liu C, Chen L, et al. Protective effects and potential mechanisms of dietary resveratrol supplementation on the spleen of broilers under heat stress. Front Nutr. 2022;9:821272. doi: 10.3389/fnut.2022.821272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nguyen XH, Nguyen HT, Morgan NK. Dietary soluble non-starch polysaccharide level and xylanase supplementation influence performance, egg quality and nutrient utilization in laying hens fed wheat-based diets. Anim Nutr. 2021;7:512–520. doi: 10.1016/j.aninu.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang CY, Huang J, Kang XT. Resveratrol Attenuates LPS-induced apoptosis via inhibiting NF- κ B activity in chicken peripheral lymphocyte cultures. Braz J Poultry Sci. 2018;20:747–752. doi: 10.1590/1806-9061-2017-0720. [DOI] [Google Scholar]
- 161.Liu M, Zhao L, Gong G, Zhang L, Shi L, Dai J, et al. Invited review: Remediation strategies for mycotoxin control in feed. J Anim Sci Biotechnol. 2022;13:19. doi: 10.1186/s40104-021-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang R, Zhang WB, Bi YL, Tu Y, Ma T, Dong LF, et al. Sanguinarine and resveratrol affected rumen fermentation parameters and bacterial community in calves. Anim Feed Sci Tech. 2019;251:64–75. doi: 10.1016/j.anifeedsci.2019.03.004. [DOI] [Google Scholar]
- 163.Chen D, Tu Y, Ma T, Lou C, Jiang C, Diao Q. Effects of mulberry leaf flavonoids and resveratrol on gas metabolism and methane emission in mutton sheep. Chin J Anim Nutr. 2014;26:1221–1228. doi: 10.3969/j.issn.1006-267x.2014.05.012. [DOI] [Google Scholar]
- 164.Chen D, Chen X, Tu Y, Wang B, Lou C, Ma T, et al. Effects of mulberry leaf flavonoid and resveratrol on methane emission and nutrient digestion in sheep. Anim Nutr. 2015;1:362–367. doi: 10.1016/j.aninu.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shen Y, Jiang Y, Zhang S, Zou J, Gao X, Song Y, et al. The effect of dietary supplementation with resveratrol on growth performance, carcass and meat quality, blood lipid levels and ruminal microbiota in fattening goats. Foods. 2022;11:598. doi: 10.3390/foods11040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liang Y, Zhou J, Ji K, Liu H, Degen A, Zhai M, et al. Protective effect of resveratrol improves systemic inflammation responses in LPS-injected lambs. Animals. 2019;9:872. doi: 10.3390/ani9110872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.