Abstract
In recent years, there has been a significant increase related to pesticide residues in foods, which may increase the risks to the consumer of these foods with the different quality and concentrations of pesticide residues. Pesticides are used for controlling pests that reduce yields. On the other hand, it has become a major public health concern due to its toxic properties. Thus, the objective of the current study employed the application of Quick Easy Cheap Effective Rugged Safe (QuEChERS) method, in combination with gas and liquid chromatography-tandem mass spectrometric detection (GCMSMS, LCMSMS) in order to determine 137 pesticide residues (63 insecticides, 41 acaricides, 40 herbicide, 55 fungicide, nematicide, growth regulator, Chitin synthesis inhibitors, and Juvenile hormone mimics), in 801 vegetables such as 139 tomatoes, 185 peppers, 217 squash, 94 eggplants, and 166 cucumbers from different locations in Hail and Riyadh cities. The results showed that the majority of pesticide residues were detected for each of the following pesticides: acetaimpride, metalaxyl, imidaclopride, bifenthrin, pyridaben, difenoconazole, and azoxystrobien, which were repeated in the samples studied 39, 21, 11, 10, 8, 7, and 5, respectively. In addition, results observed that the tomato was the most contaminated with pesticide residues; it was contaminated with 19 compounds and was followed by pepper, cucumber, and squash, and the last commodity in the contaminated ranking was eggplant. The highest calculated estimated daily intakes (EDIs) were recorded for tomatoes which were estimated between 0.013 to 0.516 mg/kg of body weight per day (bw/day) while the lowest EDIs value was between 0.000002 to 0.0005 mg/kg of bw/day for cucumber. Results indicated that the EDIs values were lower than the acceptable daily intake (ADI) values. Results observed that the most of pesticide residues exposure in food consumption in Saudi Arabia were lower than ADIs. In addition, the highest value for health risk index (HRI) was recorded with Ethion residue in tomato, but in sweet pepper, the highest value for HRI was 127.5 in the form of fipronil residue. On the other hand, results found that the highest values of HRI were 1.54, 1.61, and 0.047 for difenoconazole, bifenthrin, and pyridaben residues in squash, eggplant, and cucumber.
Keywords: pesticide residues, risk assessment, QuEChERS, EDIs, ADI, HRI, GC–MS/MS, LC-MSMS
1. Introduction
In recent years, we have observed a substantial increase in the importance placed on aspects related to pesticide residues and a growing demand for better agricultural practices, transparency, and traceability in the production and marketing of conventional food. On one hand, pesticides make it possible to increase food production by destroying weeds and pests that attack cultivable plants and agricultural crops, and they also limit losses sustained during the transport and storage of food. On the other hand, pesticides are one of the most dangerous chemical compounds due to their toxic properties, environmental persistence, and bioaccumulation capability. Thus, the presence of pesticide residues in food commodities is a source of great worry; what makes it more complex is that some of these vegetables are consumed fresh or semi-processed, which may contain elevated levels of chemicals compared to other food crops of plant origin. Exposure to pesticides through diet is thought to be five orders of magnitude higher than other exposure routes, for example, air and drinking water [1,2,3]. The level of pesticide residues in foodstuffs is generally legislated so as to minimize consumers’ exposure to harmful or unnecessary pesticide intakes, their maximum concentrations are controlled by the European Union Council Directive 91/414/EEC [4], and established maximum residue limits (MRLs) for pesticides in foodstuffs and animal feed in Directive No. 396/2005 (Regulation2005) [5].
LC and GC coupled to MS/MS detection provides accurate methods of identifying and quantifying numerous pesticides in food extracts. Several articles have recently been published where these techniques were successfully utilized for the analysis of pesticides in fruits and vegetables. Due to the high selectivity provided by MS/MS detection, simple extraction techniques with little cleanup are employed [6].
In the last few years, the so-called QuEChERS (Quick, easy, cheap, effective, rugged, and safe) sample preparation procedure has become a widely used technique because of its applicability on a wide range of pesticides [7,8,9,10]. It has several advantages over traditional methods of pesticide residue analysis, for example, high recoveries (>85%) are achieved for a wide polarity, very accurate (true and precise) results are achieved, solvent usage and waste are very small, and the MeCN is added by dispenser to an unbreakable vessel that is immediately sealed, thus, solvent exposure to the worker is minimal, the method is very inexpensive [11,12,13,14,15].
In this study, we aimed to apply the QuEChERS methodology in combination with gas and liquid chromatography-tandem mass spectrometric detection (GC-MS/MS, LC-MS/MS), for the analysis of 137 pesticides, to determine residues of chemical pesticides (Organophosphates, OPs; acaricides, ACs; fungicides, FUs and insecticides of biological origin, INsB) used in vegetable farming in Hail and Riyadh cities; and to assess the health risk of adults due to the ingestion of pesticides in and on their vegetables.
2. Results and Discussion
2.1. Pesticide Residues in Raw Foods
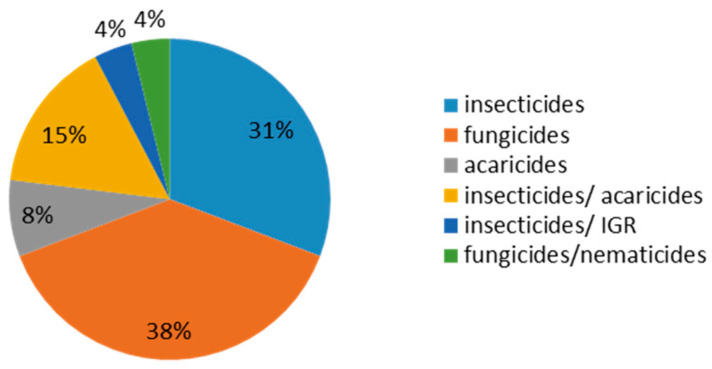
A wide range of pesticide residues (63 insecticides, 41 acaricides, 40 herbicide, 55 fungicide, nematicides growth regulators Chitin synthesis inhibitors and Juvenile hormone mimics) in 801 vegetables such as 139 tomatoes, 185 peppers, 217 squash, 94 eggplants, and 166 cucumbers from different locations in Hail and Riyadh cities were detected in the Kingdom of Saudi Arabia during 2020. Regarding the pesticides that were screened, results showed that most of the pesticide groups that were detected belonged to different groups as fungicides (10 compounds), insecticides (8 compounds), acaricides (2 compounds), and multifunctional groups, such as insecticides/acaricides (4 compounds), insecticides/IGR (1 compound), and insecticides/nematicides (one compound). These compounds belong to many chemicals groups, as we found that the most frequent chemical group was the Triazole chemical group which has three compounds (penconazole, propiconazole, and triadmenol) as a fungicide with a percentage to reach 38%. Following this, each of the other groups (carbamate, dicarboximide, neonicotinoid, organophosphate and pyrethroid) were repeated twice. On other hand, the other remaining groups (phenylpyrazoles, chlorophenyl, dioxolanes, Hydrazine carboxylate, hydroxyanilides, methoxyacrylates, oxadiazine, phenylamide, pyridazinone, quinazoline, tetronic acid, triazolinthione, and unclassified) were repeated one time. All of these chemical groups use 31% insecticides. Following in the most frequency is the mixed group of insecticides/acaricides with 15%. After that, there is the acaricides group with 8%, and the last groups both insecticides/acaricides and fungicides/nematicides have 4% for both. Figure 1. Data was mentioned previously partially in agreement with [1,2,3].
Figure 1.
Illustration of the most important chemical groups and their percentages.
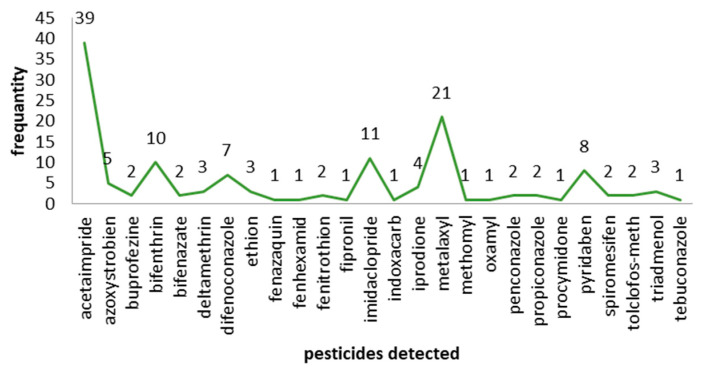
In our study, we observed the represented data in Table 1 and Figure 1, Figure 2 and Figure 3 and the majority of residue compound was detected to be acetaimpride, followed with metalaxyl, imidaclopride, bifenthrin, pyridaben, difenoconazole, and azoxystrobien with a frequency of 39, 21, 11, 10, 8, 7, and 5, respectively. After that, triadmenol, ethion, deltamethrin, tolclofos-meth, spiromesifen, propiconazole, penconazole, fenitrothion, bifenazate, and buprofezine had frequencies of 4, 3, 3, 3, 2, 2, 2, 2, 2, 2, and 2. Finally, tebuconazole, procymidone, oxamyl, methomyl, indoxacarb, fipronil, fenhexamid, and fenazaquin had frequencies of 1 for all previous compounds, respectively.
Table 1.
Demonstrates the frequency of occurrence of pesticides.
| Pesticide | Tomato | Pepper | Squash | Eggplantt | Cucumber | Frq. |
|---|---|---|---|---|---|---|
| Acetaimprid | (0.017–0.347) | (0.011–0.358) | (0.011–0.118) | (0.008–0.085) | (0.018–0.209) | 39 |
| Azoxystrobien | (0.17 9–0.318) | 0.216 | 0.39 | 0.054 | 5 | |
| Buprofezine | 0.056 | 0.827 | 2 | |||
| Bifenthrin | (0.03–0.362) | (0.064–0.145) | (0.01–0.125) | 0.23 | 10 | |
| Bifenazate | 0.1 | 0.052 | 2 | |||
| Deltamethrin | (0.155–0.016) | 0.16 | 3 | |||
| Difenoconazole | (0.07–0.261) | (0.158–0.178) | (0.058–0.188) | 0.012 | 7 | |
| Ethion | (0.125–0.137) | 0.044 | 3 | |||
| Fenazaquin | 0.037 | 1 | ||||
| Fenhexamid | 0.017 | 1 | ||||
| Fenitrothion | (0.159–0.161) | 2 | ||||
| Fipronil | 0.45 | 1 | ||||
| Imidaclopride | (0.076–0.38) | (0.018–0.721) | 11 | |||
| Indoxacarb | 0.382 | 1 | ||||
| Iprodione | (0.178–0.0305) | 0.086 | 4 | |||
| Metalaxyl | (0.04–0.117) | (0.08–0.52) | (0.007–0.03) | 0.005 | (0.007–0.267) | 21 |
| Methomyl | 0.026 | 1 | ||||
| Oxamyl | 0.007 | 1 | ||||
| Penconazole | 0.058 | 0.209 | 2 | |||
| Propiconazole | (0.047–0.107) | 2 | ||||
| Procymidone | 0.053 | 1 | ||||
| Pyridaben | 0.378 | (0.018–0.36) | (0.08–0.328) | 8 | ||
| Spiromesifen | (0.06–0.196) | 2 | ||||
| Tolclofos-meth | 0.198 | 0.096 | 2 | |||
| Triadmenol | (0.011–0.116) | 3 | ||||
| Tebuconazole | 0.309 | 1 | ||||
| Frq. | 19 | 16 | 6 | 4 | 8 |
Figure 2.
Frequency of occurrence of pesticides.
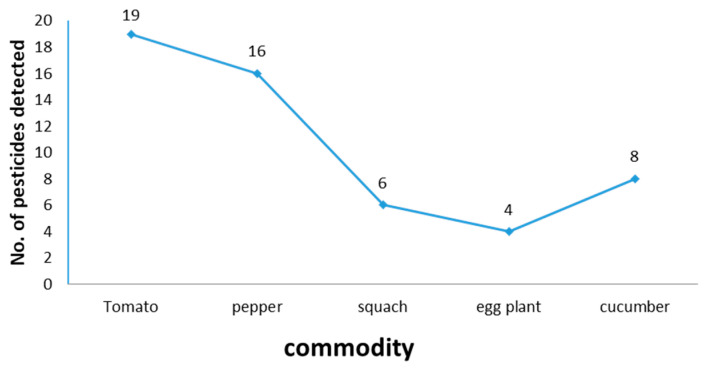
Figure 3.
Demonstrates the contamination with pesticide residues.
On the other hand, we observed that the commodity in our study most contaminated with pesticide residues was tomato, as it is contaminated with acetaimpride, azoxystrobien, bifenthrin, bifenazate, deltamethrin, difenoconazole, ethion, fenazaquin, fenhexamid, fenitrothion, imidaclopride, indoxacarb, iprodione, metalaxyl, oxamyl, propiconazole, pyridaben, tolclofos-meth, and triadmenol with a frequency of 19 times followed by the frequency of 16 times for pepper, which was contaminated with acetaimpride, azoxystrobien, buprofezine, bifenthrin, bifenazate, deltamethrin, difenoconazole, ethion, fipronil, imidaclopride, iprodione, metalaxyl, pyridaben, spiromesifen, tolclofos-meth and tebuconazole after that in ranking cucumber was contaminated with acetaimpride, azoxystrobien, difenoconazole, metalaxyl, methomyl, penconazole, procymidone, and pyridaben. Following with a frequency of 8 times, squash was contaminated with acetaimpride, azoxystrobien, bifenthrin, metalaxyl and penconazole. With a frequency of 6 times, the last commodity in contaminated rankings was eggplant with a frequency of 4 times for acetaimpride, buprofezine, bifenthrin, and metalaxyl. Table 1, Figure 2 and Figure 3. Overall, the pesticide residues which were found in this study were approximately similar to other studies [8,9,11].
2.2. Estimation of Dietary Intake
The objective of risk assessment from the point of view of food safety is, to ensure that in order to evaluate a dietary risk assessment, the ADI values were determined by summing the quotes of the pesticide ingested from various alimentary sources (i.e., vegetables and fruits). The Codex Alimentarius Commission of the FAO of the United Nations and WHO (FAO/WHO 2004) (17) recommended abiding with MRLs in fruits and vegetables. Monitoring of pesticide residues is a key tool for ensuring conformity with regulations and providing a check on compliance with good agricultural practice. The consideration of possible exposure to pesticide residues is an integral part of the risk assessment process to ensure that the ADI of the pesticides are not exceeded. As long as the residue of the pesticides ingested by consumers does not exceed the corresponding ADI, consumers are considered to be adequately protected. This is useful for assessing human exposure to pesticides through the food supply and for understanding the magnitude of health risks.
Additionally, the annual disappearance figures for a food commodity can be divided by the national population and by 365 days to obtain a “per capita” estimate of the food that is available for consumption per day expressed as grams per person per day (g/p/d). Disappearance data cannot be used to estimate intake for targeted sub-populations (e.g., young children, diabetics, or specific age-sex groups). The levels of contaminant pesticide residues used to estimate dietary intake of those substances can be obtained by combining the analytical results with amounts of food consumed reported in national food consumption surveys (Table 2).
Table 2.
Estimated food consumption rate (g/day) in food basket: The Global Environment Monitoring System/Food Contamination Monitoring and Assessment Program (GEMS/Food).
| Commodity | Consumption in the Middle East Grams per Person per Day |
|---|---|
| Tomato | 81.5 |
| Sweet Pepper | 3.4 |
| Squash | 10.5 |
| Eggplant | 6.3 |
| Cucumber | 4.8 |
2.3. Estimation of Pesticide Exposure
The estimated daily intake for each monitoring pesticide residue was calculated with the next formula:
| EDI = (commodity consumption × pesticide residue concentration)/body weight |
2.4. Estimation of Health Risks from Pesticides
Estimation of the exposure risk to an adult person based on potential health risk by using the following formula:
| HRI = EDI/ADI |
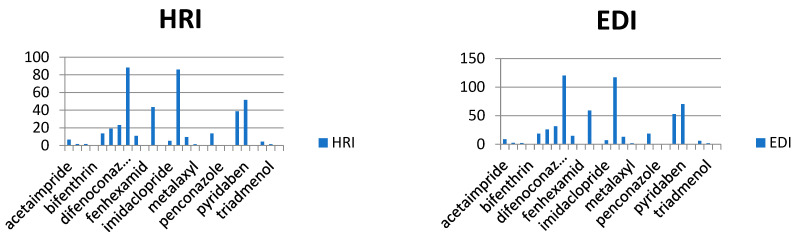
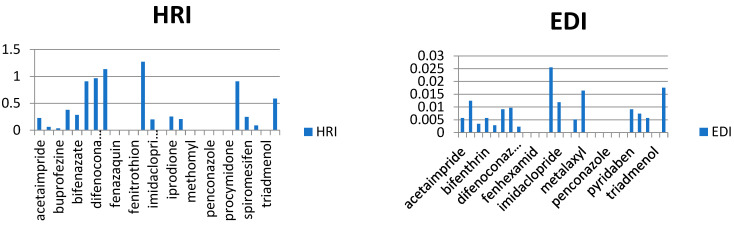
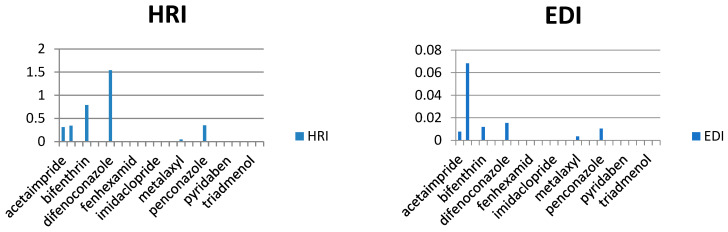
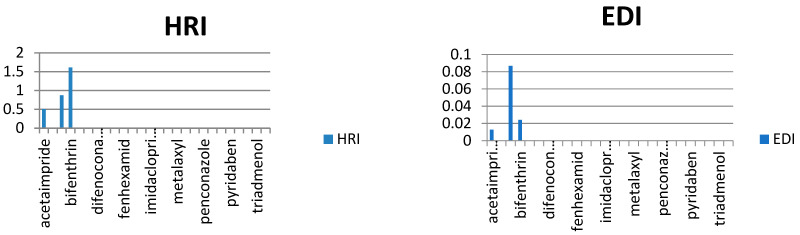
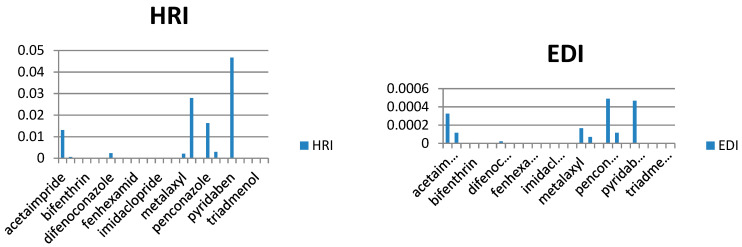
In our study, the authors compiled the available data on pesticide residues in different plants that generate food commodities, such as vegetables. On the basis of previously conducted studies in different cities of Saudi Arabia, it was possible to conduct a human risk assessment using the hazard risk index (HRI). The results are summarized in Table 2, for HRI assessment, the estimated daily intake (EDI) (mg/kg/day) and acceptable daily intake (ADI) values (mg/kg/day) were taken and calculated by following international guidelines [16,17,18,19,20], where EDI is the estimated average daily intake (mg/kg/day), C is pesticide residue concentration (mg/kg) multiplying by the food consumed, and W is the average weight of an adult. Reference values for the food consumption rate of vegetables and fruits were taken from literature as 0.3 kg/person/day for vegetables and 0.4 kg/person/day of fruits, respectively, while 60 kg was considered an average adult weight [21,22,23,24,25]. The HRI value for the risk estimation of different toxic metals and pesticides via food consumption was calculated, and the general consumption rates were used (regardless of seasonal and generic wise consumption) due to data scarcity (Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8).
Figure 4.
Estimation of EDI and HRI for pesticide residues detected in tomatoes.
Figure 5.
Estimation of EDI and HRI for pesticide residues detected in sweet peppers.
Figure 6.
Estimation of EDI and HRI for pesticide residues detected in squash.
Figure 7.
Estimation of EDI and HRI for pesticide residues detected in eggplant.
Figure 8.
Estimation of EDI and HRI for pesticide residues detected in cucumber.
As we observed in Table 3, the calculated EDIs of tomatoes had been estimated between 0.013 to 0.516 mg/kg of bw/day. For sweet pepper, the EDIs value was between 0.0028 to 0.025 mg/kg of bw/day. However, in squash, the EDIs value was between 0.004 to 0.015 mg/kg of bw/day and in eggplant, the EDIs value was between 0.001 to 0.086 mg/kg of bw/day. Lastly, the EDIs value was between 0.000002 to 0.0005 mg/kg of bw/day in the cucumber. We observed that the EDIs values were lower than the ADI values. We reported that most pesticide residue exposure was lower than ADIs, and this depends on style of food consumption in Saudi Araba (Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8).
Table 3.
Estimation of EDI and HRI for pesticide residues detected in Tomato, Sweet pepper, Squash and Cucumber samples.
| Detected Pesticide | ADI | Tomato | Sweet Pepper | Squash | Eggplant | Cucumber | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRL | Mean | EDI | HRI | MRL | Mean | EDI | HRI | MRL | Mean | EDI | HRI | MRL | Mean | EDI | HRI | MRL | Mean | EDI | HRI | ||
| acetaimpride | 0.025 | 0.5 | 0.12 | 0.163 | 6.52 | 0.1 | 0.1 | 0.0056 | 0.226 | 0.2 | 0.044 | 0.008 | 0.309 | 0.2 | 0.12 | 0.013 | 0.504 | 0.3 | 0.14 | 0.0003 | 0.013 |
| azoxystrobien | 0.2 | 3 | 0.25 | 0.34 | 1.697 | 3 | 0.22 | 0.0125 | 0.062 | 1 | 0.39 | 0.068 | 0.341 | - | - | - | - | 1 | 0.05 | 0.000117 | 0.001 |
| buprofezine | 0.1 | 0.3 | 0.12 | 0.163 | 1.63 | 0.5 | 0.06 | 0.0034 | 0.034 | - | - | - | - | 0.3 | 0.827 | 0.086 | 0.868 | - | - | - | - |
| bifenthrin | 0.015 | - | - | - | - | 0.5 | 0.1 | 0.0056 | 0.378 | 0.01 | 0.068 | 0.012 | 0.787 | 0.3 | 0.23 | 0.024 | 1.61 | - | - | - | - |
| bifenazate | 0.01 | 0.5 | 0.1 | 0.134 | 13.58 | 3 | 0.05 | 0.0028 | 0.283 | - | - | - | - | - | - | - | - | - | - | - | |
| deltamethrin | 0.01 | 0.07 | 0.14 | 0.19 | 19.02 | 0.7 | 0.16 | 0.0091 | 0.907 | - | - | - | - | - | - | - | - | - | - | ||
| difenoconazole | 0.01 | 2 | 0.17 | 0.23 | 23.09 | 0.9 | 0.17 | 0.0096 | 0.963 | 0.2 | 0.088 | 0.015 | 1.54 | - | - | - | - | 0.3 | 0.01 | 2.33 × 10−5 | 0.002 |
| ethion | 0.002 | 0.01 | 0.13 | 0.176 | 88.29 | 0.01 | 0.04 | 0.0023 | 1.13 | - | - | - | - | - | - | - | - | - | - | - | |
| fenazaquin | 0.005 | 0.5 | 0.04 | 0.054 | 10.86 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| fenhexamid | 0.2 | 2 | 0.02 | 0.027 | 0.135 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| fenitrothion | 0.005 | 0.01 | 0.16 | 0.217 | 43.46 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| fipronil | 0.0002 | 0.005 | - | - | - | - | 0.45 | 0.025 | 127.5 | - | - | - | - | - | - | - | - | - | - | - | - |
| imidaclopride | 0.06 | 0.5 | 0.23 | 0.312 | 5.206 | 1 | 0.21 | 0.0119 | 0.198 | - | - | - | - | - | - | - | - | - | - | - | - |
| indoxacarb | 0.006 | 0.5 | 0.38 | 0.516 | 86.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| iprodione | 0.02 | 5 | 0.14 | 0.19 | 9.508 | 7 | 0.09 | 0.0051 | 0.255 | - | - | - | - | - | - | - | - | - | - | - | |
| metalaxyl | 0.08 | 0.3 | 0.08 | 0.108 | 1.358 | 0.5 | 0.29 | 0.0164 | 0.205 | 0.01 | 0.02 | 0.004 | 0.044 | 0.01 | 0.005 | 0.001 | 0.007 | 0.01 | 0.071 | 0.0002 | 0.002 |
| methomyl | 0.0025 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.04 | 0.03 | 0.0001 | 0.028 | ||
| oxamyl | 0.001 | 0.01 | 0.01 | 0.013 | 13.58 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| penconazole | 0.03 | - | - | - | - | - | - | - | - | 0.1 | 0.06 | 0.011 | 0.35 | - | - | 0 | 0 | 0.1 | 0.21 | 0.0005 | 0.016 |
| propiconazole | 0.04 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | 0.05 | 0.000117 | 0.003 |
| procymidone | 0.0028 | 0.01 | 0.08 | 0.108 | 38.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| pyridaben | 0.01 | 0.15 | 0.38 | 0.516 | 51.61 | 0.5 | 0.16 | 0.009 | 0.906 | - | - | - | - | - | - | - | - | 0.15 | 0.2 | 0.0005 | 0.047 |
| spiromesifen | 0.03 | - | - | 0.13 | 0.007 | 0.246 | - | - | - | - | - | - | - | - | - | ||||||
| tolclofos-meth | 0.064 | 0.2 | 0.271 | 4.244 | 0.1 | 0.006 | 0.088 | - | - | - | - | - | - | - | - | - | |||||
| triadmenol | 0.05 | 0.05 | 0.067 | 1.358 | - | - | - | - | - | - | - | - | - | - | - | ||||||
| tebuconazole | 0.03 | - | - | 0.31 | 0.018 | 0.585 | - | - | - | - | - | - | - | - | - | ||||||
Furthermore, the EDIs values were used to estimate the hazard index (HRI) for each corps. We found a higher value for HRI for Ethion residue in tomato, but in sweet pepper, the higher value for HRI 127.5 was to fipronil residue. On the other hand, we found that the high value of HRI was 1.54 for difenoconazole residue in squash and 1.61 for bifenthrin residue in eggplant. Lastly, in cucumber, the high value was HRI 0.047 for pyridaben residue. We noticed all estimated data of the Hazard Index were exceeding the value of MRL, which may indicate a bad use of pesticides and failure to follow the application rates and the pre-harvest interval, which leads to exposure to health risks.
3. Materials and Methods
3.1. Chemicals and Reagents
Ultra-gradient HPLC-grade acetonitrile was purchased from J.T. Baker (Deventer, The Netherlands). Deionized water was obtained from a Milli-Q SP Reagent Water System (Millipore; Bedford, MA, USA). Formic acid (98% purity) and anhydrous magnesium sulfate were ordered from Fluka–Sigma–Aldrich (Steinheim, Germany). Each sample was filtered through a 13 mm × 0.45 um PTFE filter before injection, whilst PSA-bonded (primary secondary amine) silica was used as a sample clean-up step—both of them were from Supelco (Bellefonte, PA, USA). Acetic acid (Merck; Darmstadt, Germany) and sodium acetate-3-hydrate (Panreac; Castellonde Valles, Barcelona, Spain) were used for the sample preparation procedure. All certified pesticide standards obtained from Dr. Ehrenstorfer (Augsburg, Germany) were of 95 % or higher purity.
3.2. Study Area
This study was conducted in the Hail and Riyadh regions, which lie between longitude and latitude (43 N and 26 E and 34 N and 46 E), respectively. The city of Riyadh is characterized by a high population density, which is approximately six million people. On the contrary, the Hail region is characterized by a low population density, which reaches one and a half million people. These areas are dominated by a hot summer climate where temperatures reach 48 °C, and in winter, the average temperature drops to 9 °C.
3.3. Collection of Samples and Pretreatment
A total of 801 vegetable samples (139 tomatoes, 185 peppers, 217 squash, 94 eggplants, and 166 cucumber) from five local markets (three from Hail and two from Riyadh) were collected during the different seasons of 2019. Altogether, 2–3 units of fresh vegetables were collected from each local market (>1 kg) in accordance with the procedures described in the FAO, (1999). Samples were not rinsed. A portion of each sample, without tops such as the sepal and peduncle, was prepared according to annex I of European Commission regulation, 396/2005 EU (2010) using a knife and a chopping board and then thoroughly mixed. Two hundred gram of each sample were kept in a separate plastic bag at −20 °C until pesticide extraction and analysis could be carried out.
3.4. Extraction of Pesticide Residues by QuEChERS and Cleanup of Vegetables Samples
Vegetable samples were purchased from a local market and the preparation procedure was the same as the well-known and accepted QuEChERS (16), sample preparation procedure was applied to all the samples. After homogenization with the stainless-steel cutter (Sammic, Azpeitia, Spain), a 15 g portion of the homogenized sample was weighed in a 50 mL PTFE centrifuge tube. Then, 15 mL of acetonitrile were added with 6 g MgSO4and 2.5 g sodium acetate-3-hydrate and the samples were shaken vigorously by hand for 4 min. The extract was then centrifuged (3700 rpm) for 5 min. A 5 mL volume of the supernatant was removed to a 1-mL PTFE centrifuge tube containing 750 mg of MgSO4 and 250 mg of PSA. The extract was shaken in a vortex intensively for 20 s and centrifuged again (3700 rpm) for 5 min. Following this, an aliquot of the supernatant was evaporated under a nitrogen stream and reconstituted with acetonitrile/water (20/80) for LC analysis. Prior to injection into the LC–MS system, the sample was filtered through a 0.45-um PTFE filter. With this treatment, a 1 mL sample extract represents 1 g of sample.
3.5. Standard Preparation
A standard stock solution of each pesticide was prepared in acetonitrile at a concentration of 2000 µg/mL. A mixed standard solution was prepared at a concentration of 10 µg/mL from the individual stock solutions. The calibration curve for the LC measurements was prepared by diluting 10 µg/mL of the mixed standard solution to achieve final concentrations of 0.25, 0.5, 1, 2.5, 5, 10, 25, and 100 ng/mL in a mixture of acetonitrile and water (1:1, v/v). Stock and working solutions were stored at 4 °C until use. Pesticides were analyzed through Liquid Chromatography Triple Quadrupole Mass Spectrometry (LC-MSMS) and Gas Chromatography Triple Quadrupole Mass Spectrometry (GC-MSMS).
3.6. Analytical Techniques by Liquid Chromatography Triple Quadrupole Mass Spectrometry (LC-MSMS)
An ultra-high performance liquid chromatography (ACQUITY) coupled with a tandem quadrupole MS (XEVO TQD) was used with Mass Lynx 4.1 software (Waters Corporation, Milford, MA, USA). For the chromatographic separation, a reversed phase column, Atlantis T3 (100 × 3 mm, 5 µm), was used. The mobile phases (A and B) were water: methanol (98:2, v/v), and methanol, respectively, with 0.1% formic acid (FA) in each. The flow rate was maintained at 0.45 mL/min. The gradient program was initially set at 5% B (1 min), then linearly increased over the next 7.75 min to 100% and kept constant until 8.50 min. Thereafter, it was linearly decreased to 5%, and maintained for another 3.50 min (a total run time of 12 min). The MS was operated with Electrospray Ionization (ESI+). The optimized parameters included desolvation temperature (450 °C), desolvation gas flow rate (1000 L/hour), cone gas flow rate (50 L/hour), ion source temperature (120 °C), and capillary voltage (1 kV). The MS parameters are presented Table 4.
Table 4.
LC-MS/MS retention times and multi reaction monitoring MRM transitions for the LC amenable pesticides.
| Pesticide | Application | Parent | CV (V) | Product 1 | CE (eV) | Product 2 | CE (eV) | RT |
|---|---|---|---|---|---|---|---|---|
| 3,4,5-Trimethacarb | Insecticide | 194.1 | 22 | 137.1 | 12 | 122.1 | 26 | 5.41 |
| Acephate | Insecticide | 184.1 | 17 | 143.0 | 8 | 125.1 | 18 | 1.47 |
| Acetamiprid | Insecticide | 223.0 | 34 | 126.0 | 20 | 56.1 | 15 | 3.41 |
| Alachlor | Herbicide | 271.1 | 28 | 162.1 | 20 | 238.1 | 11 | 6.30 |
| Aldicarb | Acaricide | 213.1 | 30 | 89.1 | 16 | 116.1 | 11 | 3.98 |
| Aldicarb sulfone | Metabolite | 223.0 | 31 | 148.0 | 10 | 86.0 | 14 | 2.04 |
| Aldicarb sulfoxide | Metabolite | 207.0 | 22 | 89.0 | 14 | 132.0 | 10 | 1.91 |
| Ametryn | Herbicide | 228.1 | 38 | 186.1 | 18 | 68.1 | 36 | 4.88 |
| Anilazine | Fungicide | 274.9 | 46 | 153.0 | 26 | 178.0 | 24 | 5.98 |
| Anilofos | Herbicide | 367.9 | 30 | 124.9 | 34 | 198.9 | 15 | 6.57 |
| Atraton | Herbicide | 212.0 | 40 | 170.1 | 18 | 100.0 | 28 | 3.96 |
| Atrazine | Herbicide | 216.1 | 39 | 174.1 | 18 | 96.1 | 23 | 5.20 |
| Atrazine-desethyl | Metabolite | 188.0 | 34 | 146.0 | 16 | 78.9 | 26 | 3.69 |
| Azaconazole | Fungicide | 300.0 | 34 | 159.0 | 28 | 231.1 | 18 | 5.44 |
| Azinphos-ethyl | Insecticide | 346.0 | 16 | 132.0 | 16 | 77.1 | 36 | 6.20 |
| Azinphos-methyl | Insecticide | 318.0 | 20 | 160.0 | 8 | 261.0 | 8 | 5.56 |
| Azoxystrobin | Fungicide | 404.0 | 28 | 372.0 | 15 | 329.0 | 30 | 5.73 |
| Benalaxyl | Fungicide | 326.1 | 26 | 148.0 | 20 | 91.0 | 34 | 6.62 |
| Bendiocarb | Insecticide | 224.1 | 26 | 167.0 | 8 | 109.0 | 18 | 4.62 |
| Benfluralin | Herbicide | 336.0 | 34 | 57.0 | 18 | 236.0 | 15 | no |
| Benfuracarb | Insecticide | 411.1 | 23 | 195.0 | 23 | 190.0 | 13 | 7.07 |
| Benomyl | Fungicide | 291.0 | 22 | 160.0 | 28 | 192.0 | 16 | 5.50 |
| Boscalid | Fungicide | 342.9 | 41 | 307.0 | 20 | 139.9 | 20 | 5.90 |
| Buprofezin | Insecticide | 306.1 | 31 | 201.0 | 12 | 57.4 | 20 | 6.96 |
| Butachlor | Herbicide | 312.2 | 26 | 57.3 | 22 | 238.2 | 12 | 7.17 |
| Cadusafos | Insecticide | 271.1 | 28 | 159.0 | 16 | 131.0 | 22 | 6.88 |
| Carbaryl | Insecticide | 202.0 | 28 | 145.0 | 22 | 117.0 | 28 | 4.86 |
| Carbendazim | Fungicide | 192.1 | 33 | 160.1 | 18 | 132.1 | 28 | 2.20 |
| Carbofuran | Insecticide | 222.1 | 34 | 165.1 | 16 | 123.0 | 16 | 4.63 |
| Carbosulfan | Insecticide | 381.0 | 40 | 118.0 | 22 | 76.0 | 34 | 7.89 |
| Carboxin | Fungicide | 236.0 | 34 | 143.0 | 16 | 87.0 | 22 | 4.79 |
| Chlorfenvinphos | Acaricide | 358.9 | 28 | 155.0 | 12 | 99.0 | 30 | 6.65 |
| Chlorpropham | Herbicide | 214.1 | 18 | 172.0 | 8 | 154.0 | 18 | 6.01 |
| Chlorpyrifos | Insecticide | 349.9 | 36 | 97.0 | 32 | 198.0 | 20 | 7.35 |
| Chlorpyriphos-methyl | Insecticide | 321.8 | 34 | 125.0 | 20 | 289.9 | 16 | 6.87 |
| Clethodim | Herbicide | 360.0 | 32 | 164.0 | 18 | 268.1 | 12 | 7.02 |
| Coumaphos | Insecticide | 363.0 | 32 | 307.0 | 16 | 289.0 | 24 | 6.60 |
| Cyanazine | Herbicide | 241.0 | 41 | 214.0 | 17 | 96.0 | 25 | 4.39 |
| Cyanofenphos | Insecticide | 304.0 | 34 | 157.0 | 22 | 276.0 | 12 | 6.57 |
| Cymoxanil | Fungicide | 199.0 | 23 | 128.0 | 8 | 111.0 | 18 | 3.58 |
| Deltamethrin | Insecticide | 505.9 | 28 | 280.9 | 12 | 93.2 | 46 | 7.64 |
| Desmetryn | Herbicide | 214.1 | 38 | 172.1 | 20 | 82.1 | 30 | 4.26 |
| Diazinon | Insecticide | 305.1 | 31 | 169.0 | 22 | 96.9 | 35 | 2.55 |
| Dichlorvos | Acaricide | 221.0 | 34 | 109.0 | 22 | 79.0 | 34 | 4.53 |
| Dicrotophos | Insecticide | 238.0 | 28 | 112.0 | 10 | 193.0 | 10 | 2.97 |
| Diethofencarb | Fungicide | 268.0 | 28 | 226.0 | 10 | 124.0 | 40 | 5.71 |
| Difenoconazole | Fungicide | 406.0 | 46 | 251.1 | 25 | 111.1 | 60 | 6.90 |
| Dimethoate | Acaricide | 230.1 | 24 | 125.0 | 20 | 199.0 | 10 | 3.32 |
| Diniconazole | Fungicide | 326.1 | 46 | 70.2 | 25 | 159.0 | 34 | 6.87 |
| Disulfoton | Acaricide | 274.9 | 16 | 89.0 | 20 | 61.1 | 35 | 6.80 |
| Disulfoton-sulfone | Metabolite | 307.1 | 24 | 97.1 | 28 | 153.1 | 12 | 5.16 |
| Disulfoton-sulfoxide | Metabolite | 291.0 | 24 | 185.0 | 14 | 97.0 | 31 | 5.08 |
| Diuron | Herbicide | 233.0 | 34 | 72.1 | 18 | 46.3 | 14 | 5.37 |
| Epoxiconazole | Fungicide | 330.0 | 34 | 121.0 | 22 | 101.0 | 50 | 6.27 |
| Ethion | Acaricide | 284.9 | 25 | 199.1 | 10 | 97.0 | 46 | 5.22 |
| Famphur | Insecticide | 326.0 | 32 | 93.0 | 31 | 217.0 | 20 | 5.19 |
| Fenamiphos | Nematicide | 304.1 | 36 | 217.1 | 24 | 202.1 | 36 | 6.39 |
| Fenarimol | Fungicide | 331.0 | 46 | 268.0 | 22 | 81.0 | 34 | 6.26 |
| Fenazaquin | Acaricide | 307.2 | 36 | 57.2 | 25 | 161.0 | 19 | 7.70 |
| Fenhexamid | Fungicide | 302.1 | 41 | 97.2 | 22 | 55.3 | 38 | 6.22 |
| Fenitrothion | Insecticide | 278.0 | 38 | 109.1 | 20 | 79.1 | 34 | 6.06 |
| Fenobucarb | Insecticide | 208.0 | 22 | 94.9 | 14 | 152.0 | 8 | 5.72 |
| Fenoxycarb | Insecticide | 302.1 | 28 | 88.0 | 20 | 116.1 | 11 | 6.45 |
| Fenpropathrin | Insecticide | 350.1 | 24 | 125.0 | 14 | 97.0 | 34 | 7.51 |
| Fenthion | Insecticide | 279.1 | 36 | 169.1 | 16 | 247.1 | 13 | 6.57 |
| Fonofos | Insecticide | 247.1 | 24 | 109.0 | 20 | 137.0 | 10 | 6.60 |
| Heptenophos | Insecticide | 251.0 | 26 | 127.0 | 14 | 125.0 | 14 | 5.43 |
| Hexaconazole | Fungicide | 314.0 | 40 | 70.1 | 22 | 159.0 | 28 | 6.74 |
| Imazalil | Fungicide | 297.0 | 40 | 159.0 | 22 | 69.0 | 22 | 5.03 |
| Imidacloprid | Insecticide | 256.1 | 34 | 175.1 | 20 | 209.1 | 15 | 3.08 |
| Indoxacarb | Insecticide | 528.0 | 34 | 150.0 | 22 | 203.0 | 40 | 6.91 |
| Iprobenphos | Fungicide | 289.0 | 18 | 91.0 | 20 | 205.0 | 10 | 6.47 |
| Iprodione | Fungicide | 330.0 | 21 | 244.7 | 16 | 288.0 | 15 | 6.40 |
| Isocarbofos | Insecticide | 291.1 | 21 | 121.1 | 30 | 231.1 | 13 | 5.39 |
| Kresoxim-methyl | Fungicide | 314.1 | 24 | 116.0 | 12 | 206.0 | 7 | 6.50 |
| Linuron | Herbicide | 249.1 | 31 | 160.1 | 18 | 181.1 | 16 | 5.75 |
| Malathion | Acaricide | 331.0 | 20 | 127.0 | 12 | 99.0 | 24 | 5.95 |
| Metalaxyl | Fungicide | 280.1 | 26 | 220.1 | 13 | 192.1 | 17 | 6.27 |
| Metamitron | Herbicide | 203.1 | 34 | 175.1 | 16 | 104.0 | 22 | 3.25 |
| Methacrifos | Acaricide | 241.1 | 20 | 125.0 | 20 | 209.1 | 8 | 5.47 |
| Methidathion | Insecticide | 303.0 | 18 | 85.1 | 20 | 145.0 | 10 | 5.45 |
| Methiocarb | Acaricide | 226.0 | 28 | 121.0 | 22 | 169.0 | 10 | 5.83 |
| Methomyl | Insecticide | 163.0 | 26 | 88.0 | 10 | 106.0 | 10 | 2.34 |
| Metolachlor | Herbicide | 284.1 | 26 | 176.1 | 25 | 252.1 | 15 | 6.33 |
| Metolcarb | Insecticide | 166.0 | 20 | 109.0 | 12 | 94.1 | 27 | 4.29 |
| Metribuzin | Herbicide | 215.0 | 41 | 131.0 | 18 | 89.0 | 20 | 4.53 |
| Mevinphos | Acaricide | 225.1 | 24 | 127.1 | 15 | 193.1 | 8 | 3.37 |
| Monocrotophos | Acaricide | 224.1 | 26 | 127.1 | 16 | 98.1 | 12 | 2.71 |
| Myclobutanil | Fungicide | 289.1 | 34 | 70.2 | 18 | 125.1 | 32 | 6.08 |
| Omethoate | Acaricide | 214.1 | 26 | 125.1 | 22 | 183.1 | 11 | 1.76 |
| Oxadixyl | Fungicide | 279.0 | 40 | 219.0 | 10 | 132.0 | 34 | 4.32 |
| Oxamyl | Insecticide | 237.0 | 21 | 72.0 | 10 | 90.0 | 10 | 2.13 |
| Paclobutrazol | Growth Regulator | 294.1 | 36 | 125.1 | 38 | 70.2 | 20 | 5.95 |
| Penconazole | Fungicide | 284.0 | 34 | 70.1 | 16 | 159.0 | 34 | 7.35 |
| Pendimethalin | Herbicide | 282.2 | 21 | 212.2 | 10 | 194.1 | 17 | 8.04 |
| Phenmedipham | Herbicide | 301.0 | 34 | 168.0 | 10 | 136.0 | 22 | 5.57 |
| Phenthoate | Insecticide | 321.0 | 18 | 163.0 | 12 | 135.0 | 20 | 6.47 |
| Phorate | Insecticide | 261.0 | 17 | 75.0 | 12 | 97.0 | 32 | 6.74 |
| Phorate sulfone | Metabolite | 293.0 | 24 | 96.9 | 30 | 115.0 | 24 | 5.20 |
| Phosmet | Insecticide | 318.0 | 28 | 160.0 | 22 | 77.0 | 46 | 4.22 |
| Phosphamidon | Insecticide | 300.1 | 28 | 174.1 | 14 | 127.1 | 25 | 4.40 |
| Phoxim | Insecticide | 299.0 | 22 | 129.0 | 13 | 153.0 | 7 | 6.69 |
| Pirimicarb | Insecticide | 239.1 | 34 | 72.0 | 18 | 182.1 | 15 | 3.55 |
| Pirimiphos-ethyl | Insecticide | 334.1 | 42 | 198.1 | 23 | 182.1 | 25 | 7.09 |
| Probenazole | Fungicide | 224.0 | 22 | 41.5 | 10 | 196.1 | 13 | 4.38 |
| Procloraz | Fungicide | 376.0 | 22 | 307.1 | 16 | 70.1 | 34 | 6.53 |
| Procymidone | Fungicide | 284.1 | 42 | 67.1 | 28 | 256.1 | 17 | 8.13 |
| Profenofos | Insecticide | 372.9 | 36 | 302.6 | 20 | 127.9 | 40 | 7.12 |
| Promecarb | Insecticide | 208.1 | 26 | 151.0 | 9 | 109.0 | 15 | 5.94 |
| Propachlor | Herbicide | 212.1 | 31 | 170.1 | 14 | 94.1 | 25 | 5.31 |
| Propetamphos | Insecticide | 282.0 | 17 | 138.0 | 20 | 156.0 | 12 | 6.07 |
| Propham | Herbicide | 180.0 | 14 | 138.0 | 8 | 120.0 | 16 | 5.15 |
| Propiconazole | Fungicide | 342.0 | 46 | 69.0 | 22 | 159.0 | 34 | 6.65 |
| Propoxur | Insecticide | 210.0 | 21 | 111.0 | 16 | 168.0 | 10 | 4.58 |
| Pyracarbolid | Fungicide | 218.1 | 32 | 125.1 | 18 | 97.1 | 28 | 4.66 |
| Pyraclostrobin | Fungicide | 388.1 | 31 | 163.0 | 25 | 193.9 | 12 | 6.70 |
| Pyrazophos | Fungicide | 374.0 | 44 | 222.1 | 22 | 194.0 | 32 | 6.75 |
| Pyroquilon | Fungicide | 174.0 | 41 | 132.0 | 23 | 117.0 | 30 | 4.49 |
| Quinalphos | Acaricide | 299.0 | 24 | 162.9 | 24 | 96.9 | 30 | 6.47 |
| Quinmerac | Herbicide | 222.2 | 28 | 204.2 | 15 | 141.1 | 30 | 3.36 |
| Rotenone | Insecticide | 395.0 | 46 | 213.1 | 24 | 192.1 | 24 | 6.39 |
| Simazine | Herbicide | 202.0 | 40 | 124.0 | 16 | 96.0 | 22 | 4.57 |
| Simetryn | Herbicide | 214.0 | 41 | 124.0 | 20 | 95.9 | 25 | 4.27 |
| Spiromesifen | Insecticide | 371.1 | 16 | 273.1 | 10 | 255.1 | 24 | 7.43 |
| Spiroxamine | Fungicide | 298.0 | 38 | 144.0 | 20 | 100.0 | 32 | 5.44 |
| Sulfotep | Insecticide | 323.0 | 28 | 97.0 | 32 | 171.0 | 15 | 6.51 |
| Terbutryn | Herbicide | 242.1 | 40 | 186.1 | 20 | 91.0 | 28 | 5.49 |
| Thiacloprid | Insecticide | 253.0 | 41 | 126.0 | 20 | 90.1 | 40 | 3.76 |
| Thiamethoxam | Insecticide | 292.0 | 28 | 211.2 | 12 | 132.0 | 22 | 2.56 |
| Thiophanate | Fungicide | 371.0 | 28 | 151.0 | 22 | 93.1 | 50 | 5.37 |
| Tolcofos methyl | Fungicide | 301.1 | 41 | 125.0 | 17 | 174.9 | 29 | 6.8 |
| Triadimefon | Fungicide | 294.1 | 31 | 69.3 | 20 | 197.2 | 15 | 5.94 |
| Triadimenol | Fungicide | 296.1 | 21 | 70.2 | 10 | 99.1 | 15 | 6.15 |
| Triazophos | Acaricide | 314.1 | 31 | 161.9 | 18 | 118.9 | 35 | 6.12 |
| Vamidothion | Acaricide | 288.0 | 28 | 146.0 | 10 | 118.0 | 28 | 3.38 |
| Vernolat | Herbicide | 204.1 | 28 | 128.1 | 11 | 86.1 | 14 | 6.83 |
CV = cone voltage; CE = collision energy; Rt: Retention Time.
3.7. Compound Identification
Identification and confirmation of the target compounds on GC-MSMS, was performed by using the software (TraceFinder and Xcalibar) with an updated pesticides library consisting of a more than 900 pesticides and endocrine disruptors. The software incorporates the data such as retention time (with RT< ± 0.1 min), the parent/target ion (used for quantification), and 2 other ions (as qualifiers), for all the isomers, metabolites for almost all the included compounds (in the database).
MS analysis was carried out on a TSQ 8000 EVO GC triple stage quadrupole mass spectrometer. (Thermo Fisher Scientific, San Jose, CA). The MS conditions were as follows: Ionization mode: EI positive ion. Emission current: 50 μA. Ion source temperature: 220 °C. Scan type: SRM and Scan time: 0.02 s.
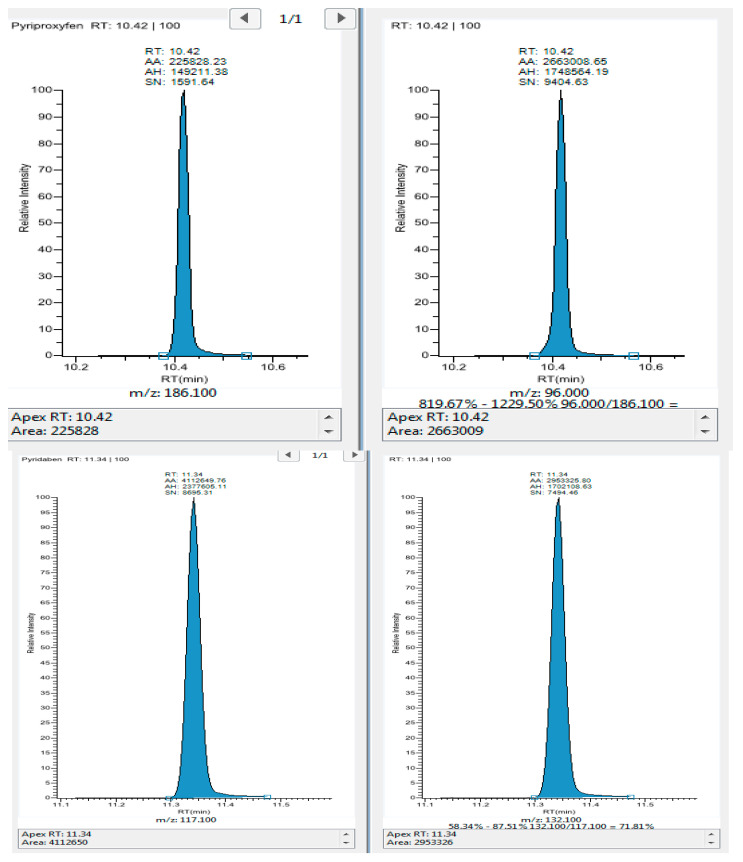
On the other hand, identification and confirmation of the target compounds on LC-MSMS, and two MRM transitions for each pesticide were generated using QUANPEDIA, ™. The data were acquired using MassLynx Software and processed using TargetLynx Application Manager. Peak shapes were adequate in most cases as shown in Figure 9.
Figure 9.
Representative multi-reaction monitoring SRM chromatograms for (1) Pyriprofexen and Pyridaben, spiked at a level of 0.100 µg/g in tomato and extracted using the sample preparation protocol reported (GC MSMS peaks).
3.8. Validation Design
The optimized analytical method was validated to ensure that it was fit for the intended purpose. The method was validated in terms of accuracy (mean recovery of the spiked samples at three different spiking levels), precision (intra-day and inter-day repeatability in terms of percent relative standard deviation, %RSD), selectivity, sensitivity (limit of quantitation (LOQ) and linearity (or linear range of measurement). The LOQ was calculated as the lowest concentration at which the recovery and precision was within the acceptable limits (recovery: 70–120%, precision: RSD < ±20%) (SANTE, 2019).
The calibration curve is determined by the analysis of each of the analysts at 6 calibration levels within the range of 0.25, 0.5, 1, 2.5, 5, 10, 25, and 100 ng/mL. The calibration curves were, in general, best fitted to a linear curve. The quantification was performed from the mean of three bracketing calibration curves. Most of the correlation coefficients (R2) were higher than or equal to 0.99.
For each level, three genuine replicates were performed. The method’s acceptance criteria were accuracy, precision, sensitivity, and the qualifier/quantifier ion ratio of the detected pesticides in real samples (to be <30%). The ion ratio was calculated as: ‘the mean ratio of the qualifier to quantifier ions for a pesticide calculated from an MMS batch’ subtracted from ‘the ion ratio for that pesticide in the positive sample’, and then dividing the resultant by the mean ion ratio calculated for the MMS of the same batch, the value thus obtained was multiplied by 100 to get the percentage value (SANTE, 2019).
To ensure the quality of the analytical work, the analytical batch was designed every time in a way to include a solvent/reagent blank, one matrix blank, and three replicates for all the three spiking levels. The solvent/reagent blanks were processed according to the complete extraction procedure under investigation, to eliminate any chances of laboratory and glassware contamination. One sample as matrix blank (extract of the sample viz. free of the targeted pesticides and which was used in the validation of the method) was also analyzed, and three replicates for all the three, i.e., highest spiking level (HSL), medium spiking level (MSL), and lowest spiking level (LSL), were also run in the same batch. The instrumental samples’ sequence was designed to be in the following order: reagent/solvent blank, then calibration standards in pure solvent, followed by matrix-matched standards (at the same concentration range as that of the standards-in-solvent) and then the real samples, bracketed by the standards-in-solvent, at the end. To eliminate the chances of carryover from previous samples’ injections, the instrument was also configured at back-flush settings, supported by an additional post-run column flushing of one minute.
Uncertainty (U) of the proposed multi-residue method was calculated by bottom-up empirical model in accordance with the ISO 21748. Uncertainty of the method’s repeatability, reproducibility, and trueness estimated was calculated as mentioned previously, partially in [5,10,13,15].
3.9. Pesticide Residue Analysis by Gas Chromatography Mass Spectrometry (GC–MSMS)
A Thermo Scientific TRACE 1310 Gas Chromatography coupled with TSQ 8000 Evo Triple Quadrupole Detector and AI 1310 Auto Sampler was used with Thermo Xcalibur 2.2 mass spectrometry data system (Software). For the chromatographic separation, a Thermo Scientific™ Trace GOLD™ TG-5SilMS 30 m × 0.25 mm I.D. × 0.25 µm film capillary column was used. The flow rate was maintained at constant flow 1.2 mL/min (He, inert carrier gas). The GC oven program was initially set at 70 °C (2 min), then increased 25 °C/min to 180 °C, 5 °C/min to 200 °C, and 10 °C/min to 280 °C, kept constant 5 min. The MS was operated with electrospray ionization (ESI+). The optimized parameters included transfer line (280 °C), electron energy(eV) 70, acquisition mode (SRM), and ion source temperature (320 °C). The Thermo ScientificTM TraceFinderTM software was used for method setup and data processing. For all pesticide compounds two SRM transitions were chosen for the overall MRM acquisition method. The first transition was used for quantitation, the second transition for confirmation. Table 5 and Figure 9, lists the SRM parameters for the compounds analyzed in this method.
Table 5.
GC-MS/MS retention times and multi reaction monitoring SRM transitions for the LC amenable pesticides.
| Pesticide | Application | Quantitation m/z | CE (eV) | Confirmation m/z | CE (eV) | RT (min) |
|---|---|---|---|---|---|---|
| Acephate | Insecticide | 136.01 > 42.00 | 10 | 136.01 > 94.01 | 15 | 7.42 |
| Alachlor | Herbicide | 161.07 > 146.06 | 12 | 188.08 > 160.07 | 10 | 12.58 |
| Atrazine | Herbicide | 215.09 > 173.08 | 10 | 215.09 > 200.09 | 10 | 10.65 |
| Azinphos-ethyl | Acaricide | 132.01 > 77.01 | 20 | 160.02 > 132.01 | 5 | 19.18 |
| Benfluralin | Herbicide | 292.10 > 160.05 | 21 | 292.10 > 264.09 | 10 | 9.54 |
| Bifenthrin | Acaricide | 181.05 > 153.05 | 6 | 181.05 > 166.05 | 15 | 17.86 |
| Boscalid | Fungicide | 342.03 > 140.01 | 15 | 344.03 > 142.01 | 15 | 20.95 |
| Bromophos-ethyl | Acaricide | 358.89 > 302.91 | 20 | 358.89 > 330.90 | 10 | 14.58 |
| Buprofezin | Chitin synthesis inhibitors | 172.09 > 57.03 | 10 | 249.13 > 193.10 | 10 | 15.88 |
| Butralin | Herbicide | 266.14 > 190.10 | 15 | 266.14 > 220.11 | 15 | 13.56 |
| Cafenstrole | Herbicide | 100.04 > 72.03 | 15 | 188.08 > 119.05 | 15 | 20.21 |
| Carbaryl | Acaricide | 144.06 > 115.05 | 20 | 144.06 > 116.05 | 20 | 12.54 |
| Chlordane | Insecticide | 372.81 > 265.87 | 18 | 374.81 > 267.87 | 15 | 14.67 |
| Chlorpropham | Herbicide | 213.00 > 127.00 | 5 | 213.00 > 171.00 | 5 | 9.66 |
| Cyfluthrin | Insecticide | 163.02 > 91.01 | 12 | 163.02 > 127.02 | 10 | 20.08 |
| Cypermethrin | Acaricide | 163.03 > 127.02 | 10 | 181.03 > 152.03 | 25 | 20.66 |
| Cyprodinil | Fungicide | 224.13 > 208.12 | 20 | 225.13 > 210.12 | 18 | 14.08 |
| Deltamethrin | Insecticide | 252.99 > 93.00 | 18 | 252.99 > 173.99 | 18 | 22.19 |
| Diazinon | Acaricide | 137.05 > 84.03 | 10 | 304.10 > 179.06 | 15 | 10.09 |
| Dimethachlor | Herbicide | 197.08 > 148.06 | 10 | 199.08 > 148.06 | 10 | 12.06 |
| Diniconazole | Fungicide | 268.06 > 232.05 | 15 | 270.06 > 234.05 | 15 | 16.18 |
| Dioxathion | Acaricide | 125.00 > 97.00 | 15 | 125.00 > 141.00 | 15 | 10.78 |
| Edifenphos | Fungicide | 173.01 > 109.01 | 15 | 310.03 > 173.01 | 10 | 16.77 |
| Ethion | Acaricide | 230.99 > 202.99 | 15 | 383.99 > 230.99 | 10 | 16.18 |
| Ethoprophos | Insecticide | 158.00 > 80.90 | 15 | 158.00 > 114.00 | 5 | 9.58 |
| Fenarimol | Fungicide | 139.01 > 111.01 | 15 | 219.02 > 107.01 | 15 | 19.26 |
| Fenobucarb | Insecticide | 121.07 > 77.05 | 15 | 150.09 > 121.07 | 10 | 9.18 |
| Fenpropathrin | Acaricide | 181.09 > 152.07 | 23 | 265.13 > 210.10 | 15 | 18.06 |
| Fipronil | Acaricide | 212.97 > 177.98 | 16 | 366.95 > 212.97 | 25 | 13.94 |
| Fluopicolide | Fungicide | 208.80 > 182.00 | 20 | 261.00 > 175.00 | 24 | 16.94 |
| Formothion | Acaricide | 126.00 > 93.00 | 8 | 172.00 > 93.00 | 5 | 11.88 |
| Imazalil | Fungicide | 173.03 > 145.02 | 20 | 215.04 > 173.03 | 15 | 18.22 |
| Iprodione | Fungicide | 187.02 > 124.01 | 20 | 187.02 > 159.02 | 40 | 17.58 |
| Isoprothiolane | Fungicide | 290.06 > 118.03 | 15 | 290.06 > 204.05 | 15 | 15.28 |
| Kresoxim-methyl | Fungicide | 206.09 > 116.05 | 15 | 206.09 > 131.06 | 15 | 15.34 |
| Lactofen | Herbicide | 344.04 > 223.02 | 15 | 344.04 > 300.03 | 15 | 18.88 |
| Malathion | Acaricide | 127.01 > 99.01 | 10 | 173.02 > 127.01 | 10 | 13.05 |
| Mecarbam | Acaricide | 226.04 > 198.03 | 5 | 329.05 > 160.03 | 10 | 14.23 |
| Mepanipyrim | Fungicide | 222.11 > 207.10 | 15 | 223.11 > 208.10 | 15 | 14.26 |
| Metalaxyl | Fungicide | 249.13 > 190.10 | 10 | 249.13 > 249.13 | 5 | 12.56 |
| Metamitron | Herbicide | 202.09 > 174.07 | 5 | 202.09 > 186.08 | 10 | 10.42 |
| Methabenzthiazuron | Herbicide | 164.05 > 136.04 | 12 | 164.05 > 164.05 | 10 | 9.84 |
| Methamidophos | Acaricide | 141.00 > 95.00 | 10 | 141.00 > 126.00 | 5 | 5.77 |
| Methidathion | Insecticide | 124.98 > 98.99 | 22 | 144.98 > 84.99 | 10 | 14.65 |
| Methiocarb | Acaricide | 168.06 > 109.04 | 15 | 168.06 > 153.06 | 15 | 12.98 |
| Metribuzin | Herbicide | 198.08 > 82.03 | 20 | 198.08 > 110.05 | 20 | 12.46 |
| Mevinphos | Acaricide | 127.03 > 109.02 | 10 | 192.04 > 127.03 | 12 | 7.32 |
| Monocrotophos | Acaricide | 127.03 > 95.03 | 20 | 127.03 > 109.03 | 25 | 9.94 |
| Omethoate | Acaricide | 110.01 > 79.01 | 15 | 156.02 > 110.01 | 10 | 9.05 |
| Penconazole | Fungicide | 248.06 > 157.04 | 25 | 248.06 > 192.04 | 15 | 14.09 |
| Pendimethalin | Herbicide | 252.12 > 162.08 | 12 | 252.12 > 191.09 | 12 | 13.86 |
| Phosalone | Acaricide | 181.99 > 111.00 | 15 | 181.99 > 138.00 | 10 | 18.56 |
| Phosphamidon | Insecticide | 227.05 > 127.03 | 15 | 264.06 > 193.04 | 15 | 11.88 |
| Pirimicarb | Insecticide | 166.10 > 96.06 | 10 | 238.14 > 166.10 | 15 | 11.95 |
| Probenfos | Insecticide | 204.07 > 122.04 | 15 | 218.89 > 182.91 | 15 | 11.72 |
| Procymidone | Fungicide | 283.02 > 96.01 | 15 | 283.02 > 255.02 | 10 | 14.56 |
| Profenofos | Insecticide | 138.98 > 96.98 | 8 | 338.94 > 268.95 | 20 | 15.37 |
| Propachlor | Herbicide | 176.06 > 120.04 | 10 | 196.07 > 120.04 | 10 | 9.45 |
| Propanil | Herbicide | 217.01 > 161.00 | 10 | 219.01 > 163.00 | 10 | 12.16 |
| Propargite | Acaricide | 135.06 > 107.05 | 15 | 350.16 > 201.09 | 10 | 17.24 |
| Propoxur | Acaricide | 110.06 > 64.03 | 10 | 152.08 > 110.06 | 10 | 9.02 |
| Pyrimethanil | Fungicide | 198.11 > 158.09 | 30 | 198.11 > 183.10 | 15 | 11.28 |
| Pyriproxyfen | Juvenile hormone mimics | 226.10 > 186.10 | 12 | 136.10 > 96.00 | 10 | 10.45 |
| Pyridaben | Acaricide | 147.10 > 117.10 | 20 | 147.10 > 132.10 | 12 | 11.35 |
| Quinalphos | Acaricide | 146.03 > 118.02 1 | 15 | 157.03 > 129.02 | 13 | 14.29 |
| Spiromesifen | Insecticide | 371.24 > 273.15 | 15 | 371.24 > 255.64 | 25 | 18.42 |
| Spiroxamine | Fungicide | 100.09 > 58.05 | 15 | 100.09 > 72.06 | 15 | 12.89 |
| Tefluthrin | Insecticide | 177.02 > 127.02 | 20 | 197.03 > 141.02 | 15 | 11.27 |
| Tetradifon | Acaricide | 226.93 > 198.94 | 18 | 353.88 > 158.95 | 15 | 18.56 |
| Tolclofos-methy | Fungicide | 264.96 > 92.99 | 20 | 264.96 > 249.96 | 15 | 12.34 |
| Triazophos | Acaricide | 161.03 > 134.03 | 10 | 257.05 > 162.03 | 10 | 16.55 |
| Trifluralin | Herbicide | 264.09 > 160.05 | 15 | 306.10 > 264.09 | 15 | 9.87 |
| Vinclozolin | Fungicide | 100.09 > 58.05 | 15 | 100.09 > 72.06 | 15 | 12.35 |
CV = cone voltage; CE = collision energy; Rt: Retention Time.
4. Conclusions
High consumption of fruits and vegetables contaminated with pesticide residues above the MRL leads to a threat to the population’s health, and this is due to the poor handling practices for pests and disease control that also do not follow the pre-harvest interval (PHI) for pesticides. Therefore, it is important to update the data on the population’s real consumption value to obtain a true estimate of the risk of actual exposure to pesticides. It is impotent to continue with the pesticide residues program to reduce exposure to residues that cause long-term effects or immediate serious illness.
Acknowledgments
The researchers extend their thanks to the Deputy-Ministry of Agriculture, Ministry of Environment, Water and Agriculture, Kingdom of Saudi Arabia for their support of this research, which greatly impacted its completion and publication in a peer-reviewed scientific prestigious journal.
Author Contributions
Each author participated actively in conducting analyses, editing, and approving the final, submitted version. Conceptualization; methodology and software, M.H.E.-S.; validation, M.T.S., M.M.A. and H.I.S.; formal analysis, M.H.E.-S.; investigation, M.M.A. and H.I.S.; resources, M.H.E.-S., M.T.S. and M.M.A.; data curation, All authors writing original draft preparation, M.H.E.-S. writing—review and editing, M.T.S., M.M.A. and H.I.S.; visualization and supervision, M.M.A.; administration. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Funding Statement
This research was supported by the Deputy-Ministry of Agriculture, Ministry of Environment, Water and Agriculture, Kingdom of Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen C., Qian Y., Chen Q., Tao C., Li C., Li Y. Evaluation of pesticide residues in fruits and vegetables from Xiamen, China. Food Control. 2011;22:1114–1120. doi: 10.1016/j.foodcont.2011.01.007. [DOI] [Google Scholar]
- 2.Claeys W.L., Schmit J.F., Bragard C., Maghuin-Rogister G., Pussemier L., Schiffers B. Exposure of several Belgian consumer groups to pesticide residues through fresh fruit and vegetable consumption. Food Control. 2011;22:508–516. doi: 10.1016/j.foodcont.2010.09.037. [DOI] [Google Scholar]
- 3.Na T.W., Rahman M.M., Park J.H., Yang A., Park K.H., Abd El-Aty A.M., Shim J.-H. Residual pattern of acequinocyl and hydroxyl acequinocyl in perilla leaf grown under greenhouse conditions using ultra performance liquid chromatography–photo diode array detector with tandem mass confirmation. J. Korean Soc. Appl. Biol. Chem. 2012;55:657–662. doi: 10.1007/s13765-012-2101-x. [DOI] [Google Scholar]
- 4.European Commission Directive 91/414/EEC, concerning the placing of plant protection products on the market. Off. J. Eur. Commun. 1991;230 [Google Scholar]
- 5.Erney D.R., Gillespie A.M., Gilvydis D.M., Poole C.F. Explanation of the matrix-induced chromatographic response enhancement of organophosphorus pesticides during open tubular column gas chromatography with splitless or hot on-column injection and flame photometric detection. J. Chromatogr. A. 1993;638:57–63. doi: 10.1016/0021-9673(93)85007-T. [DOI] [Google Scholar]
- 6.Regulation (EC) No 396/2005 of the European Parliament and the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. [(accessed on 14 April 2014)]. Available online: http://ec.europa.eu/sanco_pesticides/public/?event=commodity.resultat.
- 7.Hernando M.D., Ferrer C., Ulaszewska M., García-Reyes J.F., Molina-Díaz A., Fernández-Alba A.R. Application of high-performance liquid chromatography–tandem mass spectrometry with a quadrupole/linear ion trap instrument for the analysis of pesticide residues in olive oil. Anal. Bioanal. Chem. 2007;389:1815–1831. doi: 10.1007/s00216-007-1464-z. [DOI] [PubMed] [Google Scholar]
- 8.Díez C., Traag W., Zommer P., Marinero P., Atienza J. Comparison of an acetonitrile extraction/partitioning and “dispersive solid-phase extraction” method with classical multi-residue methods for the extraction of herbicide residues in barley samples. J. Chromatogr. A. 2006;1131:11–23. doi: 10.1016/j.chroma.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 9.Anastassiades M., Lehotay S.J., Štajnbaher D., Schenck F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003;86:412–431. doi: 10.1093/jaoac/86.2.412. [DOI] [PubMed] [Google Scholar]
- 10.Hajšlová J., Zrostlíková J. Matrix effects in (ultra)trace analysis of pesticide residues in food and biotic matrices. J. Chromatogr. A. 2003;1000:181–197. doi: 10.1016/S0021-9673(03)00539-9. [DOI] [PubMed] [Google Scholar]
- 11.Anastassiades M., Tasdelen B., Scherbaum E., Stajnbaher D. Recent developments in QuEChERS methodology for pesticide multiresidue analysis. In: Ohkawa H., Miyagawa H., Lee P.W., editors. Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety. Wiley-VCH; Weinheim, Germany: 2007. [Google Scholar]
- 12.Foods of Plant Origin: Determination of Pesticide Residues Using GC–MS and/or LC–MS (/MS) Following Acetonitrile Extraction/Partitioning and Cleanup by Dispersive SPE–QuEChERS Method. European Committee for Standardization; Brussels, Belgium: 2007. [Google Scholar]
- 13.Pizzutti I.R., Kok A., Zanella R., Adaime M.B., Hiemstra M., Wickert C., Prestes O.D. Method validation for the analysis of 169 pesticides in soya grain, without clean up, by liquid chromatography-tandem mass spectrometry using positive and negative electrospray ionization. Chromatogr. A. 2007;1142:123. doi: 10.1016/j.chroma.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Luke M., Froberg J.E., Masumoto H.T. Extraction and Cleanup of Organochlorine, Organophosphate, Organonitrogen, and Hydrocarbon Pesticides in Produce for Determination by Gas-Liquid Chromatography. J. AOAC Int. 1975;58:1020–1026. doi: 10.1093/jaoac/58.5.1020. [DOI] [PubMed] [Google Scholar]
- 15.Specht W., Pelz S., Gilsbach W. Gas-chromatographic determination of pesticide residues after clean-up by gel-permeation chromatography and mini-silica gel-column chromatography. Fresenius’ J. Anal. Chem. 1995;353:183–190. doi: 10.1007/BF00322956. [DOI] [PubMed] [Google Scholar]
- 16.Fillion J., Sauvé F., Selwyn J. Multiresidue Method for the Determination of Residues of 251 Pesticides in Fruits and Vegetables by Gas Chromatography/Mass Spectrometry and Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2000;83:698–713. doi: 10.1093/jaoac/83.3.698. [DOI] [PubMed] [Google Scholar]
- 17.Cook J., Beckett M.P., Reliford B., Hammock W., Engel M. Quick, Easy, Cheap, Effective, Rugged, and Safe Approach for Determining Pesticide Residues. J. AOAC Int. 1999;82:1419–1435. doi: 10.1093/jaoac/82.6.1419. [DOI] [PubMed] [Google Scholar]
- 18.Andersson A., Palsheden H. Comparison of the efficiency of different GLC multi-residue methods on crops containing pesticide residues. Fresenius’ J. Anal. Chem. 1991;339:365–367. doi: 10.1007/BF00322349. [DOI] [Google Scholar]
- 19.Lehotay S.J., Son K.A., Kwon H., Koesukwiwat U., Fu W., Mastovska K., Hoh E., Leepipatpiboon N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A. 2010;1217:2548–2560. doi: 10.1016/j.chroma.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 20.European Commission . Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed, Document SANTE/11813/2017, 21–22 November 2017 Rev.0. European Commission; Brussels, Belgium: 2019. [Google Scholar]
- 21.FAO/WHO Codex Alimentarius Commission. 2004. [(accessed on 23 January 2023)]. Available online: http://app.fao.org.
- 22.FAO (Food and Agriculture Organization) Submission and Evaluation of Pesticide Residues Data for the Estimation of Maximum Residue Levels in Food and Feed. FAO; Rome, Italy: 2002. pp. 1–279. [Google Scholar]
- 23.Farag R.S., Latif A.M.S., El-Gawad A.E., Dogheim S.M. Monitoring of pesticide residues in some Egyptian herbs, fruits and vegetables. Int. Food Res. J. 2011;18:659–665. [Google Scholar]
- 24.Arain M., Kazi T., Baig J.A., Jamali M., Afridi H., Shah A., Jalbani N., Sarfraz R. Determination of arsenic levels in lake water, sediment, and foodstuff from selected area of Sindh, Pakistan: Estimation of daily dietary intake. Food Chem. Toxicol. 2009;47:242–248. doi: 10.1016/j.fct.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Amin N.-U., Hussain A., Alamzeb S., Begum S. Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan, Pakistan. Food Chem. 2013;136:1515–1523. doi: 10.1016/j.foodchem.2012.09.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.